Abstract
The mechanism of symptom amplification, developed in the study of somatization, may be helpful in caring for patients with symptoms that, while they have a demonstrable medical basis, are nonetheless disproportionately severe and distressing. Amplified medical symptoms are marked by disproportionate physical suffering, unduly negative thoughts and concerns about them, and elevated levels of health-related anxiety. They are accompanied by extensive and sustained illness behaviors, disproportionate difficulty compartmentalizing them and circumscribing their impact, and consequent problems and dissatisfaction with their medical care. A distinction has long been made between “medically explained” and “medically unexplained” symptoms. However, a more comprehensive view of symptom phenomenology undermines this distinction and places all symptoms along a smooth continuum regardless of cause: Recent findings in cognitive neuroscience suggest that all symptoms—regardless of origin—are processed through convergent pathways. The complete conscious experience of both medically “explained” and “unexplained” symptoms is an amalgam of a viscerosomatic sensation fused with its ascribed salience and the patient’s ideas, expectations, and concerns about the sensation. This emerging empirical evidence furnishes a basis for viewing persistent, disproportionately distressing symptoms of demonstrable disease along a continuum with medically unexplained symptoms. Thus, therapeutic modalities developed for somatization and medically unexplained symptoms can be helpful in the care of seriously ill medical patients with amplified symptoms. These interventions include educational groups for coping with chronic illness, cognitive therapies for dysfunctional thoughts, behavioral strategies for maladaptive illness behaviors, psychotherapy for associated emotional distress, and consultation with mental health professionals to assist the primary care physician with difficulties in medical management.
KEY WORDS: Symptom palliation, Somatization, Amplified medical symptoms
Viscerosomatic amplification is a process whereby patients’ thoughts, emotions, and concerns heighten uncomfortable bodily sensations and symptoms, making them more salient, intense, unpleasant, noxious, disturbing, and distressing. The construct emerged from the study of somatization, where empirical support was found for its role in the development of medically unexplained symptoms.1–4 In this model, somatized symptoms are understood as the product of a self-perpetuating and self-validating cycle of cognition and perception whereby benign bodily sensations become more intrusive and distressing once they are thought to be medically serious and misattributed to disease.2,3,5 Worrisome ideas about their cause, ominous expectations about their future course, negative assumptions about their significance, and threatening prior illness experiences all amplify the symptom and heighten its noxious, bothersome, and distressing quality. This in turn further substantiates the most worrisome and alarming thoughts, thereby perpetuating a vicious cycle. Somatization may thus be thought of as a diathesis towards symptom amplification due to a top-down tendency to misinterpret and misunderstand bothersome and uncomfortable bodily sensations that are medically unexplained.
Three mechanisms drive this cycle of symptom amplification: more intensive monitoring of one’s bodily sensations; increased bodily scrutiny in search of corroborative evidence of disease; and disconfirmatory bias that causes the individual to ignore evidence that contradicts his/her suspicion that something serious is wrong. Misattributing a bothersome but benign sensation to serious disease causes the individual to attend to it more closely and monitor it more consistently, thereby intensifying it.1,6,7 In addition, misinterpreting its medical significance launches a search for ancillary symptoms to corroborate the suspicion of disease.1,6,7 This increased bodily vigilance results in an awareness of other ambiguous, diffuse, or transient symptoms that were previously ignored, minimized, or dismissed as insignificant, but are now mistaken as further evidence of seriousness.6,8–10 This seeming emergence of “new” symptoms is accompanied by disconfirmatory bias—ignoring or dismissing observations that contradict the belief about the presence of disease.1,3,11 Thus, for example, a patient worried about the medical significance of orthostatic dizziness notices every dizzy episode but ignores the instances in which he/she stands up without feeling dizzy. Finally, the escalating cycle of amplification causes anticipatory anxiety, further heightening alarm. Anxiety is accompanied by the symptoms of autonomic arousal (e.g., tachycardia, sweating, dyspnea), which further confound the picture. The net result is an increase in symptom intensity; more numerous symptoms; symptoms that are now more bothersome, distressing, and intrusive; and the strengthened conviction that one is sick.
SYMPTOM AMPLIFICATION IN THE MEDICALLY ILL
Amplification initially appeared to be most helpful in understanding and treating somatized symptoms, i.e., symptoms without a demonstrable medical basis. This phenomenon of somatization is prevalent in medical practice: Indeed, more than one-third of the symptoms reported by outpatients remain medically unexplained after adequate evaluation,12,13 and somatizing patients account for 10–20% of total medical care expenditures in the USA.14,15 In these cases, symptom etiology may be understood as an amplification of benign bodily symptoms and minor, self-limited ailments. Such bothersome symptoms are ubiquitous, arising and subsiding in the daily lives of healthy non-patients,16 and they constitute a reservoir of uncomfortable bodily sensations that are available for amplification. Community survey respondents report a median of five symptoms in the past week and 23% of them reported 10 or more symptoms17; headache is reported by 45%, back pain by 37%, insomnia by 24%, fatigue by 19%, dizziness by 17%, and abdominal pain by 11%.18 These background symptoms may result from transient, benign, self-limited ailments (e.g., colds, tinnitus, rashes, or epistaxis); normal physiology (e.g., orthostatic dizziness); the somatic and autonomic concomitants of emotions such as depression and anxiety; aging (balance problems, diminished visual and auditory acuity); lack of exercise (deconditioning and fatigue); dietary indiscretion (cramps, bloating, diarrhea); or inadequate sleep (fatigue and musculoskeletal pain). This reservoir of discomfort and distress in daily life serves as the substrate for somatization and when the symptoms are misattributed to serious disease, they constitute the sensory component of “medically unexplained” symptoms.
Significant medical disease also causes symptoms that can be amplified by the patients’ ideas, worries, beliefs, and suspicions, leading to a mismatch between the extent and severity of disease on the one hand, and the magnitude and multiplicity of symptoms on the other. Three clinical examples of this mismatch occur in patients with extreme degrees of physical suffering, those complaining of non-specific medication side effects, and patients with symptoms that persist despite “successful” medical and surgical treatment.
Patients with disproportionate symptom distress. Among patients with the same medical condition, subjective symptom severity is only loosely associated with objective measures of disease severity. Clinicians are frequently struck by the wide inter-individual variability in symptom magnitude among patients with comparable disease severity and extent. Thus, for example, knee pain is poorly correlated with the presence of meniscal damage19; self-reported dyspnea corresponds poorly to objective measures of airway obstruction in asthma20,21 and COPD22; fatigue ratings do not correlate significantly with hemoglobin levels in patients with mild to moderate anemia23; urinary obstructive symptoms are not significantly associated with measures of urodynamic obstruction in benign prostatic hypertrophy24; and there is only a weak association between self-reported palpitations and objective measures of arrhythmia.25,26 In short, some patients are much more symptomatic than others with comparable structural disease.
Non-specific medication side effects. Patients frequently develop non-specific medication side effects that are not attributable to the pharmacological activity of the drug.27,28 They are idiosyncratic, not reliably reproducible, not dose dependent, and closely resemble the symptoms reported in comparable populations not taking medication.29 The nocebo phenomenon, in which 20–25% of patients taking placebos in clinical trials report side effects,27 likely accounts for a sizeable fraction of these non-specific side effects reported by patients taking active medications.27,29
Symptoms that persist despite “successful” medical treatment. A lack of correspondence between symptoms and pathology is also evident in cases when treatment corrects the disease process but fails to alleviate its symptoms. For example, 41% of patients undergoing cholecystectomy for gallstones reported persistent abdominal pain at long-term follow-up after surgery,30 and up to one-half of successfully revascularized coronary artery disease patients continue to experience their original chest pain.31 Forty-eight percent of patients with visual evidence of peptic ulcer healing on antacid therapy nonetheless remained symptomatic.32 Likewise, plasma exchange for rheumatoid arthritis reduced inflammatory markers but did not reduce pain33; significant improvement in hemoglobin levels in patients with iron deficiency anemia failed to reduce symptoms such as headache, dizziness, and fatigue23; and MRI findings were not associated with symptomatic outcome in long-term follow-up after surgery for lumbar disc herniation.34 These residual symptoms may persist because of an incorrect diagnosis in the first place, or to adverse effects of the treatment itself; but they may also suggest a role for symptom amplification.
THE CARDINAL FEATURES OF AMPLIFIED SYMPTOMS
Amplified symptoms in medically ill patients are characterized by the clinical features described below. The presence of these features suggests that the patients’ beliefs, ideas, and concerns about the symptoms may be aggravating their physical distress and discomfort. Assessing them generates a profile of the domains of somatic distress, which can then guide and direct palliative interventions.
Disproportionate physical distress. The patient’s bodily experience is disproportionately distressing, excessively bothersome, and unduly impairing. The symptoms are experienced as exceptionally noxious, aversive, disruptive, and intrusive. If serious medical disease is present, the distress is disproportionate to its severity and extent. Multiplicity of symptoms is characteristic.12
Cognitions. Amplified symptoms are accompanied by unnecessarily negative expectations, unduly alarming suspicions, troubling interpretations, and worrisome beliefs about their significance and cause. Patients with significant medical comorbidity are firmly convinced that their condition is more serious, and their symptoms more ominous, than medically indicated. Those without serious medical morbidity often have a persistent, unassuageable conviction that an undiagnosed or inadequately diagnosed disease is present.8
Health-related anxiety. Amplified symptoms are accompanied by undue health-related anxiety, disease fear, a lowered threshold for alarm about disease, and prominent and intrusive health worries that persist despite appropriate reassurance.1,35
Illness behaviors. Extensive and sustained illness and sick role behaviors tend to accompany amplified symptoms. These patients have difficulty coping with, tolerating, and compensating for the somatic distress, manifested in disproportionate impairment of physical, social, family, or role function.36,37 Prominent illness behaviors may include elevated rates of medical care utilization; excessive information seeking and researching of their condition; repeated self-examination; excessive reassurance seeking; and avoidance of activities suspected of worsening the condition despite medical assurance to the contrary.1,7
Pervasiveness. Concerns about amplified symptoms defy the patients’ attempts to compartmentalize and circumscribe them. These patients become preoccupied with their symptoms and their health concerns, and their symptoms tend to pervade many domains of daily life, becoming a prominent part of self-identity, a vocabulary for interpersonal communication, and a lens through which to view the future.38
Unsatisfactory medical care. Amplified symptoms tend to persist despite appropriate reassurance, explanation, and information, and to resist attempts to reduce anxiety and routine symptomatic treatment.1,8,39,40 This may provoke physician irritation or frustration. The symptoms do not respond to reassuring information and explanation, and palliative attempts may result in a paradoxical worsening, undue side effects, adverse reactions, or new symptoms to replace the old.
THE NEUROBIOLGY OF AMPLIFICATION: TOP-DOWN AND BOTTOM-UP SYMPTOM PROCESSING
Although a distinction has long been made between “medically unexplained” and “medically explained” symptoms, between those that are “functional” and those that are “organic,” a more comprehensive view of symptom phenomenology undermines this distinction and places all symptoms along a smooth continuum regardless of cause. Recent advances in cognitive psychology and neuroscience suggest that all viscerosomatic symptoms, regardless of source, are processed through convergent pathways and point to a continuity and commonality in the cortical processing of all symptoms. This suggests that some of what has been learned about amplification in somatizing patients may be applied to medically ill patients with persistent symptoms that are unduly distressing and exceptionally bothersome. Although seeking the medical basis of every symptom is obviously the physician’s first task, when it comes to symptom palliation and treatment, this dichotomous distinction between symptoms with and without a demonstrable medical cause may not be conducive to optimal understanding and care.
There is accumulating empirical evidence that the conscious experience of a symptom is an amalgam of a physical sensation and the interpretations and inferences that the brain makes about it.41,42 That is, there is both an ascending, afferent stimulus (bottom-up component), and a psychological, cognitive interpretation of that stimulus (top-down component).41–44 The brain constantly compares visceral sensory input with beliefs, assumptions, and expectations about that input. All bodily sensations, both medically explained and unexplained, are subject to the same cognitive processes of appraisal and interpretation41,42,45–47 in which the possible cause and significance of the sensation is inferred, and this inference affects the physical experience of the symptom. Attention mediates between the ascending viscerosomatic stimulus and the descending cognitive appraisal of it. This process is bidirectional: top-down direction to devote more attention to a sensation can amplify it, and conversely the bottom-up sensation can elicit greater attention and ascription of salience.41,43,45,48–50
Top-down: the Cognitive Psychology of Active Perception
Our subjective experience of the internal milieu is an active interpretation and reconstruction of that world, not a passive, automatic registration of it.51 Perception is an active process that interprets and imparts meaning to raw sensory inputs. We infer what we are perceiving in light of our expectations, knowledge, emotions, and prior experiences.41,43,45,48–50 Visceral and somatic stimuli are filtered, interpreted, appraised, and evaluated in light of preconceptions, expectations, assumptions, and beliefs about them.41,42,45,47,52 Physical symptoms, whether medically explained or unexplained, are processed in the same fashion: the sensory stimulus is embellished and modulated to produce the final conscious experience. The relative weighting of the bottom-up and top-down components can vary.41–43,47 The ascending sensory component makes a relatively greater contribution to the overall conscious experience when it is acute, localized, and intense, and when the symptom results from major, serious pathology.41–43 The cognitive/evaluative component makes a relatively greater contribution to the experience when the ascending sensory stimulus is more diffuse, poorly localized, and less intense, and has poorer on/off boundaries. This is the case in somatization, when interpretation and inference make a relatively greater contribution.41–43 The strength of the cortical contribution to symptoms is exemplified by the phantom limb phenomenon, in which a central representation of bodily pain persists in the absence of the bodily part.53
The same processing that occurs with medically unexplained symptoms can amplify the symptoms of demonstrable disease, making them more intense, disturbing, noxious, and intrusive.43 Thus, for example, a tendency towards bodily amplification is associated with a greater disparity between subjective symptoms and objective measures of asthmatic severity,20 with increased symptoms in upper respiratory tract infections,54 and is a significant predictor of reported side effects to antihypertensive medications.55 Because this inferential process occurs outside of conscious awareness, all symptoms, regardless of whether or not they result from serious disease, have the same compelling sense of being real and are experienced as accurate reports of bodily processes.41,42,44,47
Bottom-up: the Neuroscience of Interoception
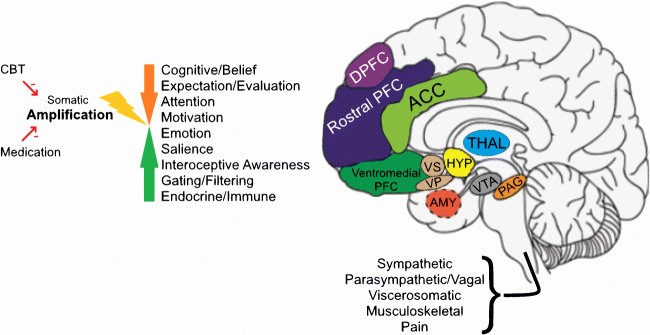
The same afferent pathways mediate viscerosomatic sensation whether or not caused by demonstrable disease (Fig. 1), though the amount and balance of activity at different levels of the system may vary.
Fig. 1.
Neural circuitry of symptom perception. The figure illustrates key levels of nervous system processing of viscerosomatic and musculoskeletal experience and function. The internal organs, skin, and musculoskeletal system are innervated by a distributed neural network, with nodes and afferent and efferent connections, as well as autonomic (sympathetic and parasympathetic) modulation. The processes mediated include sensation, pain, nausea, blood flow, and movement, as well as contraction/expansion/motility-related functions. Sensory-motor signals from these viscerosomatic pathways are evaluated and modulated at more automatic, primitive, reflexic levels in the brainstem and subcortical structures, and at higher levels in specialized and associative areas of the neocortex. Evaluative and behavioral functions include affective/emotional processing and cognitive processing of bodily stimuli. The hypothalamic-pituitary axis mediates evolutionarily conserved responses, both appetitive and aversive. The insula is involved in mediating bodily awareness, the amygdala and related limbic structures are involved in triggering fight-or-flight responses, the nucleus accumbens/ventral striatum is involved in motivated behavior, the orbito-medial prefrontal cortex is involved in the integration of viscerosomatic information with socio-emotional context for decision-making and the orchestration of behavior, guided by higher order prefrontal cortex, and the anterior cingulate cortex is involved in the allocation of attentional resources and the detection and resolution of conflicting inputs and outputs. Beliefs and expectations about symptoms and illness occur at the level of multi-modal neocortex. Somatic amplification can occur through top-down and/or bottom-up amplification. Psychotherapy, pharmacotherapy, and brain stimulation target specific nodes and functions for somatic symptom and anxiety reduction. DLPFC, dorsolateral prefrontal cortex; rostral PFC, rostral prefrontal cortex; ACC, anterior cingulate cortex; ventromedial PFC, ventromedial prefrontal cortex, including orbitofrontal cortex; VS, ventral striatum; VP, ventral pallidum; HYP, hypothalamus, pituitary not shown; THAL, thalamus; AMY, amygdala; VTA, ventral tegmental area; PAG, periaqueductal gray; Insula, hippocampus, not shown.
Interoception has been studied in healthy volunteers and, to a lesser extent, in clinical populations. Studies of healthy volunteers suffer from methodological difficulties, but there is a convergence of some major findings. They demonstrate that interoceptive stimuli are processed in brain regions that impart cognitive and affective dimensions to physical sensations.48,51,56 Affectively, limbic and paralimbic regions such as the amygdala and insula provide salience and a sense of subjective experience to the ascending sensory stimuli.57 In experimental work, bladder distention,58 rectal and esophageal distention,59,60 respiratory airway resistance,61 and cardiac contraction62 all activate the insula, anterior cingulate cortex (ACC), and medial and ventral prefrontal cortices (PFC). At these sites, ascending interoceptive stimuli are integrated with salience, attention, emotion, and cognition, to produce the complete conscious experience of the symptoms.48,56,58,59,62 Experimental pain stimuli are relayed to the thalamus, PFC, posterior and anterior portions of the insula, the ACC, and the periaqueductal gray.41–43 These regions modulate the nociceptive input, imparting the cognitive/affective component that amplifies or diminishes the pain experience and can impart its noxious, distressing, affective quality.46 It is notable that the pathways that mediate physical pain overlap with those that mediate mental pain/suffering.63
Early studies of clinical populations generally corroborate these findings, though further work is necessary. In fibromyalgia pain, an increase in somatosensory cortex-insula connectivity has been reported and is thought to reflect the hyperalgesia to experimental pain exhibited by these patients.64 Studies of pain in irritable bowel syndrome implicate activation of the insula, ACC, and prefrontal cortices65,66—regions associated with attention and cognitive and affective processing of sensation. Pain catastrophizing in patients with diverticulitis correlates with thickness in the anterior cingulate and mid-prefrontal cortices.67 Studies suggest that migraine pain may include a component of disordered sensory processing,68 and that in chronic low back pain, enhanced responses are found in emotion and cognitively related cortical areas.69 Dyspnea in COPD patients has been associated with increased activity in anterior cingulate and related medial prefrontal attentional processing regions,70 and with activation of the thalamus, ACC, insula, and the PFC.71 In preliminary and exploratory work, the same centers appear to be involved in the perception of cough,72 nausea,73 pruritus,73 and dyspnea.61,74
Neuroimaging studies of medically unexplained symptoms are still at a very early stage. However, a recent meta-analysis concluded that, when compared to healthy controls, somatoform disorder patients demonstrate differences in activation in the dorsal posterior cingulate cortex (dPCC), the anterior prefrontal cortex (aPFC), the anterior cingulate cortex (ACC), and the insula.75 These centers overlap with the centers identified in the studies discussed above and support a commonality in the way all symptoms are processed. However, studies also suggest that resting default and attentional and salience networks are more activated and functionally connected in patients with somatic symptom disorder.76,77
MANAGING AMPLIFIED SYMPTOMS
Since cognition is a critical aspect of the symptom experience, changes in the patients’ thinking about amplified symptoms can be ameliorative. The therapeutic goal is not to eliminate the bottom-up sensation, but rather to moderate the top-down cognitive processing, thereby moderating the symptom’s salience and its intrusive, disturbing, aversive quality. Assessing each of the six clinical features of amplified symptoms described above can be helpful in guiding treatment. While symptom palliation is a foundational aspect of all medical practice, the therapeutic approaches below may be helpful in especially difficult cases. This is outlined in Table 1.
Table 1.
Treatment Schema
| Domain | Therapeutic modality |
|---|---|
| Physical sensations are disproportionately noxious, aversive, intrusive, and disturbing |
Chronic disease management program (educational and behavioral techniques) Consider pharmacotherapy |
| Maladaptive health beliefs, ideas, assumptions, expectations | Cognitive behavior therapy |
| Health-related anxiety |
Cognitive behavior therapy Consider pharmacotherapy |
| Undue, extensive illness and sick role behaviors; undue impairment resulting from symptoms; difficulty coping | Cognitive behavior therapy |
|
Prominent emotional distress Apparent psychogenic factors |
Individual psychotherapy |
| Prominent interpersonal miscommunication, family dysfunction |
Family therapy Interpersonal therapy |
| Difficulties with medical care process | Consultation with mental health specialist |
When the physical sensation itself is the most prominent clinical feature—when patients are exceptionally troubled by the noxious, intrusive quality of their bodily experience—disease management programs for coping with chronic illness are helpful. They employ educational and behavioral techniques to minimize impairment and disability and improve function in patients with conditions such as congestive heart failure and migraines. These programs include stress reduction, relaxation training, mindfulness and meditation, yoga, biofeedback, psychoeducation, and complementary and alternative therapies (such as massage and acupuncture) that are not harmful and do not contradict or substitute for medical treatment. These programs often employ a group format. Pharmacotherapy may have an ancillary role in some of these programs, since a limited literature suggests that medication (particularly SSRI antidepressants) may possibly have some benefit for “medically unexplained” bodily symptoms.78,79 The action of antidepressants has been reported to involve the same circuits that have been implicated in the neurologic pain signature,80 and the medication may act by modifying mid-level limbic/paralimbic emotional and evaluative processing.
For amplifying patients with prominent health-related anxiety, dysfunctional disease beliefs, or extensive and maladaptive illness behavior, cognitive behavior therapy (CBT) may be beneficial. CBT helps patients to understand the amplification process and its role in their distress. Symptom misattributions are corrected; misunderstandings about diagnosis are revised; unrealistically fearsome future expectations are moderated; confirmatory bias is modulated with cognitive reframing; maladaptive illness behaviors (e.g., repeated, excessive internet searches) are progressively curtailed; bodily hypervigilance is reduced; and selective attention is moderated with distraction strategies. CBT has been shown to be beneficial in treating health-related anxiety in medical patients.81,82,81
Though evidence is limited, CBT has also been found to be modestly effective in palliating the symptoms of functional somatic syndromes,83–85 chronic pain,86 and chronic illnesses such as rheumatoid arthritis, cancer, and inflammatory bowel disease.87,88 In one study of patients with chronic pain, effective CBT normalized resting-state connectivity between orbitofrontal cortex and key regions of the dorsal attention network and the sensorimotor network.86 Newer forms of CBT, including mindfulness-meditation89 and exposure therapy,90 also appear promising. Prominent and disproportionate health-related anxiety may also benefit from pharmacotherapy (particularly SSRI antidepressants).78,79
For amplifying patients with prominent emotional distress or apparent psychogenic factors, individual psychotherapy may be beneficial.91 Family therapy can be helpful when disturbed interpersonal dynamics and miscommunication about symptoms and illness are occurring in the patient’s family.92 Psychiatric comorbidity, particularly depressive and anxiety disorder, is always a concern and should be sought out and treated. The presence of pychiatric comorbidity is predicted by the total number of all somatic symptoms, both those with and without a medical basis.36,93,94 In particular, the presence of five or more current bodily symptoms substantially increases the likelihood of clinically significant psychiatric disorder.36,95,96
Finally, when the medical care process itself has become unusually difficult and problematical, behavioral health consultation may be beneficial. Physicians are encouraged to clarify their patients’ ideas about the cause and significance of their symptoms, to explain the process of viscerosomatic amplification and its role in their physical distress, to explicitly discuss the goals and limits of their medical care, to provide limited reassurance, and to maintain diagnostic and therapeutic conservatism.97
Acknowledgements
Natalia Santana contributed substantially to the preparation of the manuscript.
No additional contributors. Dr. Barsky received consulting fees from Leidos Corp., and royalty payments from McGraw Hill Publishers and from Mass General Brigham Inc. He is an equity owner in Buoyant Health. There is no overlap the work in this article. Dr. Silbersweig is co-founder and chair of the SAB of Ceretype Neuromedicine, and has equity in Buoyant health. He receives honoraria for grand rounds, and royalties from McGraw-Hill and American Psychiatric Press. There is no overlap with the work in this article.
Funding
Funding comes from Brigham & Women’s Hospital.
Declarations
Conflict of Interest
Dr. Barsky received consulting fees from Leidos Corp., and royalty payments from McGraw Hill Publishers and from Mass General Brigham Inc. He is an equity owner in Buoyant Health. There is no overlap the work in this article. Dr. Silbersweig is co-founder and chair of the SAB of Ceretype Neuromedicine, and has equity in Buoyant health. He receives honoraria for grand rounds, and royalties from McGraw-Hill and American Psychiatric Press. There is no overlap with the work in this article.
Footnotes
The work has not been presented previously.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rief W, Broadbent E. Explaining medically unexplained symptoms-models and mechanisms. Clin Psychol Rev 2007; 27:821-841. [DOI] [PubMed]
- 2.Koteles F, Witthoft M. Somatosensory amplification - an old construct from a new perspective. J Psychosom Res 2017;101:1-9. [DOI] [PubMed]
- 3.Duddu V, Isaac K, Chaturvedi S. Somatization, somatosensory amplification, attribution styles and illness behaviour: a review. Int Rev Psychiat 2006;18:25-33 [DOI] [PubMed]
- 4.Köteles F, Doering B. The many faces of somatosensory amplification: the relative contribution of body awareness, symptom labeling, and anxiety. J Health Psychol. 2016;21(12):2903–2911. doi: 10.1177/1359105315588216. [DOI] [PubMed] [Google Scholar]
- 5.Rief W, Barsky A. Psychobiological perspectives on somatoform disorders. Psychoneuroendocrinology. 2005;30:996–1002. doi: 10.1016/j.psyneuen.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Pennebaker J. The psychology of physical symptoms. New York, NY: Springer-Verlag Publishing; 1982. [Google Scholar]
- 7.Brown R J. Psychological Mechanisms of Medically Unexplained Symptoms: an Integrative Conceptual Model. Psychol Bull. BMJ Open 2014;4:e005374. 10.1136/bmjopen-2014-005364. [DOI] [PubMed]
- 8.Pennebaker J, Watson D. The psychology of somatic symptoms. In L. J. Kirmayer & J. M. Robbins (Eds.). Current Concepts of Somatization: Research and Clinical Perspectives. Washington, DC: American Psychological Association:1991;24-35.
- 9.Schmidt AJM, Wolfs-Takens DJ, Oosterlaan J, van den Hout M. A. Psychological mechanisms in hypochondriasis: attention-induced physical symptoms without sensory stimulation. Psychother Psychosom. 1994;61(1-2):117–120. doi: 10.1159/000288876. [DOI] [PubMed] [Google Scholar]
- 10.Bayer T, Coverdale J, Chiang E, Bangs M. The role of prior pain experience and expectancy in psychologically and physically induced pain. Pain. 1998;74(2-3):327–331. doi: 10.1016/S0304-3959(97)00196-6. [DOI] [PubMed] [Google Scholar]
- 11.Parees I, Saifee T, Kassavetis P, Kojovic M, Rubio-Agusti I, Rothwell JC, Edwards MJ, Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor Brain. 2012;135(Pt 1):117–123. doi: 10.1093/brain/awr292. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K. A practical and evidence based approach to common symptoms. Ann Intern Med 2014;161:579-586. [DOI] [PubMed]
- 13.Steinbrecher N, Koerber S, Frieser D, Hiller W. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263–271. doi: 10.1016/j.psym.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Bermingham S, Cohen A, Hague J, Parsonage M. The cost of somatisation among the working-age population in England for the year 2008-2009. Ment Health Fam Med. 2010;7(2):71–84. [PMC free article] [PubMed] [Google Scholar]
- 15.Barsky A, Orav E, Bates D. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiat. 2005;62:903–910. doi: 10.1001/archpsyc.62.8.903. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Price R. Symptoms in the community. Arch Intern Med. 1993;153:2474–2480. doi: 10.1001/archinte.1993.00410210102011. [DOI] [PubMed] [Google Scholar]
- 17.Petrie K, Faasse K, Crichton F, Grey A. How Common Are Symptoms? Evidence from a New Zealand National Telephone Survey. BMJ Open. 2014;4:e005374. doi: 10.1136/bmjopen-2014-005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rief W, Barsky A, Glombiewski J, Nestoriuc Y, Glaesmer H, Brahler E. Assessing general side effects in clinical trials: reference data from the general population. Pharmacoepidemiology & Drug Safety. 2011;20:405–415. doi: 10.1002/pds.2067. [DOI] [PubMed] [Google Scholar]
- 19.Katz JN, Brophy RH, Chaisson CE, deChaves L, Cole BJ, Dahm D, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. New Engl J Med. 2013;368(18):1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciccone D, Chandler H, Pate-Carolan L, Janal M, Lavietes M. A test of the symptom amplification hypothesis in patients with asthma. J Nerv Ment Dis. 2007;195(2):119–124. doi: 10.1097/01.nmd.0000254731.68430.a9. [DOI] [PubMed] [Google Scholar]
- 21.Rubinfeld A, Pain M. Perception of asthma. Lancet. 1976;1:882–884. doi: 10.1016/S0140-6736(76)92097-3. [DOI] [PubMed] [Google Scholar]
- 22.Agusti A, Calverley P, Celli B, Coxson H, Edwards L, Lomas D, Vestbo J. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood M, Elwood P. Symptoms of iron deficiency anaemia. A community survey. Br J Prev Soc Med. 1996;20:117–121. doi: 10.1136/jech.20.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frimodt-Moller P, Jensen K, Iversen P, Madsen P, Bruskewitz R. Analysis of presenting symptoms in prostatism. J Urol. 1984;132:272–276. doi: 10.1016/S0022-5347(17)49587-5. [DOI] [PubMed] [Google Scholar]
- 25.Atarashi H, Ogawa S, Inoue H. Relationship between subjective symptoms and trans-telephonic ECG findings in patients with symptomatic paroxysmal atrial fibrillation and flutter. J Cardiol. 2008;52(2):102–110. doi: 10.1016/j.jjcc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Strickberger S, Ip J, Saksena S, Curry K, Bahnson T, Ziegler P. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2(2):125–131. doi: 10.1016/j.hrthm.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Barsky A, Saintfort R, Rogers M, Borus J. Non-specific medication side effects and the nocebo phenomenon. JAMA. 2005;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 28.Mahr A, Golmard C, Pham E, Iordache L, Deville L, Faure P. Types, frequencies, and burden of nonspecific adverse events of drugs: analysis of randomized placebo-controlled clinical trials. Pharmacoepidemiol Drug Saf. 2017;26(7):731–741. doi: 10.1002/pds.4169. [DOI] [PubMed] [Google Scholar]
- 29.Reidenberg M, Lowenthal D. Adverse non-drug reactions. New Engl J Med. 1968;279:678–679. doi: 10.1056/NEJM196809262791304. [DOI] [PubMed] [Google Scholar]
- 30.Thistle J, Longstreth G, Romero Y, Arora A, Simonson J, Diehl N, Zinsmeister A. Factors that predict relief from upper abdominal pain after cholecystectomy. Clin Gastro Hepatol. 2011;9(10):891–896. doi: 10.1016/j.cgh.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 31.McGillion M, Arthur H, Natarajan M, Cook A, Gunn E, Watt-Watson J, Cosman T. Nonischemic chest pain following successful percutaneous coronary intervention at a regional referral centre in southern Ontario. Can J Cardiol. 2012;28(2 Suppl):S60–S69. doi: 10.1016/j.cjca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Peterson W, Sturdevant R, Frankl H, Richardson C, Isenberg J, Elashoff J, Fordtran J. Healing of duodenal ulcer with an antacid regimen. New Engl J Med. 1977;297:341–345. doi: 10.1056/NEJM197708182970701. [DOI] [PubMed] [Google Scholar]
- 33.Dwosh I, Giles A, Ford P, Pater J, Anastassiades T. Plasmapheresis therapy in rheumatoid arthritis. New Engl J Med. 1983;308:1124–1129. doi: 10.1056/NEJM198305123081903. [DOI] [PubMed] [Google Scholar]
- 34.el Barzouhi A, Vleggeert-Lankamp C, Lycklama á Nijeholt G, Van der Kallen B, van den Hout W, Jacobs W, Peul W. Magnetic resonance imaging in follow-up assessment of sciatica. New Engl J Med. 2013;368(11):999–1007. doi: 10.1056/NEJMoa1209250. [DOI] [PubMed] [Google Scholar]
- 35.Rief W, Heitmüller A, Reisberg K, Rüddel H. Why Reassurance Fails in Patients with Unexplained Symptoms--an Experimental Investigation of Remembered Probabilities. PLoS Med 2006. 10.1371/journal.pmed.0030269 [DOI] [PMC free article] [PubMed]
- 36.Simon G, Gater R, Kisely S, Piccinelli M. Somatic symptoms of distress: an international primary care study. Psychosom Med. 1996;58:481–488. doi: 10.1097/00006842-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Kisely S, Simon G. An international study comparing the effect of medically explained and unexplained somatic symptoms on psychosocial outcome. J Psychosom Res. 2006;60:125–130. doi: 10.1016/j.jpsychores.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 38.Ford C.The Somatizing Disorders. Illness As a Way of Life New York: Elsevier Biomedical, 1983.
- 39.Escobar J, Burnham A, Karno M, Forsythe A, Golding J. M. Somatization in the community. Arch Gen Psychiat 1987;44:713-718. [DOI] [PubMed]
- 40.Lin E, Katon W, Von Korff M, Bush T, Lipscomb P, Russo J, Wagner E. Frustrating patients. J Gen Intern Med. 1991;6:241–246. doi: 10.1007/BF02598969. [DOI] [PubMed] [Google Scholar]
- 41.Van den Bergh O, Witthoft M, Petersen S, Brown R. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev. 2017;74(Pt A):185–203. doi: 10.1016/j.neubiorev.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Henningsen P, Gundel H, Kop W, Lowe B, Martin A, Rief W, Van den Bergh O. Persistent physical symptoms as perceptual dysregulation: a neuropsychobehavioral model and its clinical implications. Psychosom Med. 2018;80(5):422–431. doi: 10.1097/PSY.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 43.Ongaro G, Kaptchuk T. Symptom perception, placebo effects, and the Bayesian brain. Pain. 2018;160:1–4. doi: 10.1097/j.pain.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaman J, Vlaeyen J, Van O, Wiech K, Van D. Associative fear learning and perceptual discrimination: a perceptual pathway in the development of chronic pain. Neurosci Biobehav Rev. 2015;51:118–125. doi: 10.1016/j.neubiorev.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Barrett L, Simmons W. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tracey I, Mantyh P. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Henningsen P. The body in the brain: towards a representational neurobiology of somatoform disorders. Acta Neuropsychiat. 2003;15(4):157–160. doi: 10.1034/j.1601-5215.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 48.Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 49.Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 50.Clark A. Surfing Uncertainty: Prediction, Action, and the Embodied Mind. Oxford: Oxford University Press; 2015. [Google Scholar]
- 51.Wiech K. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science. 2016;354(6312):584–587. doi: 10.1126/science.aaf8934. [DOI] [PubMed] [Google Scholar]
- 52.Hechler T, Endres D, Thorwart A. Why Harmless Sensations Might Hurt in Individuals with Chronic Pain: About Heightened Prediction and Perception of Pain in the Mind. Frontiers in Psychol 2016. 10.3389/fpsyg.2016.01638 [DOI] [PMC free article] [PubMed]
- 53.Giummarra M, Gibson S, Georgiou-Karistianis N, Bradshaw J. Central mechanisms in phantom limb perception: the past, present and future. Brain Res Rev. 2007;54(1):219–232. doi: 10.1016/j.brainresrev.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Barsky A, Goodson J, Lane R, Cleary P. The amplification of somatic symptoms. Psychosom Med. 1988;50:510–519. doi: 10.1097/00006842-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Doering B, Szecsi J, Bardos G, Koteles F. Somatosensory amplification is a predictor of self-reported side effects in the treatment of primary hypertension: a pilot study. Int J Behav Med. 2016;23(3):327–332. doi: 10.1007/s12529-016-9536-0. [DOI] [PubMed] [Google Scholar]
- 56.Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol. 2000;17(6):604–612. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Hall G, Kamath M, Collins S, Ganguli S, Spaziani R, Miranda K, Bienenstock J. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22(3):276–e280. doi: 10.1111/j.1365-2982.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- 58.Jarrahi B, Mantini D, Balsters J, Michels L, Kessler T, Mehnert U, Kollias S. Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI TIME-series. Hum Brain Map. 2015;36(11):4438–4468. doi: 10.1002/hbm.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubio A, Van O, Pellissier S, Ly H., Dupont P, Lafaye de M, Bonaz B. Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system--a fMRI study in healthy volunteers. Neuroimage 2015;107:10-22. [DOI] [PubMed]
- 60.Hobson A, Aziz Q. Brain processing of esophageal sensation in health and disease. Gastroenterol Clin North Am. 2004;33(1):69–91. doi: 10.1016/S0889-8553(03)00132-8. [DOI] [PubMed] [Google Scholar]
- 61.Faull O, Hayen A, Pattinson K. Breathlessness and the body: neuroimaging clues for the inferential leap. Cortex. 2017;95:211–221. doi: 10.1016/j.cortex.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khalsa S, Rudrauf D, Feinstein J, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12(12):1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez D, Barsky A, Vago D, Baslet G, Silbersweig D. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiat Clin Neurosci. 2015;27:40–50. doi: 10.1176/appi.neuropsych.13070170. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Loggia M, Cahalan C, Harris R, Beissner F, Garcia R, Napadow V. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67(5):1395–1405. doi: 10.1002/art.39043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ke J, Qi R, Liu C, Xu Q, Wang F, Zhang L, Lu G. Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol Motil. 2015;27(12):1796–1803. doi: 10.1111/nmo.12692. [DOI] [PubMed] [Google Scholar]
- 66.Hubbard C, Hong J, Jiang Z, Ebrat B, Suyenobu B, Smith S, Labus J. Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterol Motil. 2015;27(9):1282–1294. doi: 10.1111/nmo.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitiot A, Smith J, Humes D, Garratt J, Francis S, Gowland P, Marciani L. Cortical differences in diverticular disease and correlation with symptom reports. Neurogastro Motil. 2018;30(7):e13303. doi: 10.1111/nmo.13303. [DOI] [PubMed] [Google Scholar]
- 68.Goadsby P, Holland P, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hashmi J, Baliki M, Huang L, Baria A, Torbey S, Hermann K, Apkarian A. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(Pt 9):2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herigstad M, Hayen A, Evans E, Hardinge F, Davies R, Wiech K, Pattinson K. Dyspnea-related cues engage the prefrontal cortex: evidence from functional brain imaging in COPD. Chest. 2015;148(4):953–961. doi: 10.1378/chest.15-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esser R, Stoeckel M, Kirsten A, Watz H, Taube K, Lehmann K, von Leupold A. Brain activation during perception and anticipation of dyspnea in chronic obstructive pulmonary disease. Front Physiol. 2017;8:617. doi: 10.3389/fphys.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ando A, Farrell M, Mazzone S. Cough-related neural processing in the brain: a roadmap for cough dysfunction? Neurosci Biobehav Rev. 2014;47:457–468. doi: 10.1016/j.neubiorev.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Spiegel D, Pattison A, Lyons A, Ansari U, McCroskey A, Luehrs E, Le S. The role and treatment implications of peripheral and central processing of pain, pruritus, and nausea in heightened somatic awareness: a review. Innov Clin Neurosci. 2017;14(5-6):11–20. [PMC free article] [PubMed] [Google Scholar]
- 74.Peiffer C, Poline J, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med. 2001;163(4):951–957. doi: 10.1164/ajrccm.163.4.2005057. [DOI] [PubMed] [Google Scholar]
- 75.Boeckle M, Schrimpf M, Liegl G, Pieh C. Neural correlates of somatoform disorders from a meta-analytic perspective on neuroimaging studies. Neurolimage Clin. 2016;11:606–613. doi: 10.1016/j.nicl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S, Hong J, Min KJ, Han D. Brain functional connectivity in patients with somatic symptom disorder. Psychosom Med. 2019;81(3):313–318. doi: 10.1097/PSY.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 77.Delvecchio G, Rossetti M, Caletti E, Arighi A, Galimberti D, Basilico P, Brambilla P. The neuroanatomy of somatoform disorders: a magnetic resonance imaging study. Psychosomatics. 2019;60(3):278–288. doi: 10.1016/j.psym.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Fallon B, Ahern D, Pavlicova, M, Slavov I, Skritskya N, Barsky A. A randomized controlled trial of medication and cognitive-behavioral therapy for hypochondriasis. Am. J Psychiat. 2017;174(8):756-764. [DOI] [PMC free article] [PubMed]
- 79.Kleinstauber M, Witthoft M, Steffanowski A, van M Hiller W, Lambert M. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev 2014;(11):CD010628. [DOI] [PMC free article] [PubMed]
- 80.Sheline Y, Yu M. Linking antidepressant performance with pain network connectivity. The Lancet Psychiatry. 2019;6:635–639. doi: 10.1016/S2215-0366(19)30250-0. [DOI] [PubMed] [Google Scholar]
- 81.Axelsson E, Andersson E, Ljotsson B, Bjorkander D, Hedman-Lagerlof M, Hedman-Lagerlof E. Effect of internet vs face-to-face cognitive behavior therapy for health anxiety: a randomized noninferiority clinical trial. JAMA Psychiat. 2020;77:915–924. doi: 10.1001/jamapsychiatry.2020.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tyrer P, Wang D, Crawford M, Dupont S, Cooper S, Nourmand S, Tyrer H. Sustained benefit of cognitive behaviour therapy for health anxiety in medical patients (CHAMP) over 8 years: a randomised-controlled trial. Psychol Med. 2021;51(10):1714–1722. doi: 10.1017/S003329172000046X. [DOI] [PubMed] [Google Scholar]
- 83.Thomson A, Page L. Psychotherapies for hypochondriasis (Review). Cochrane Library 2007;(4). [DOI] [PMC free article] [PubMed]
- 84.Cooper K, Gregory J, Walker I, Lambe S, Salkovskis P. Cognitive behaviour therapy for health anxiety: a systematic review and meta-analysis. Behav Cog Psychother. 2017;45:110–123. doi: 10.1017/S1352465816000527. [DOI] [PubMed] [Google Scholar]
- 85.Jackson J, O’Malley P, Kroenke K. Antidepressants and cognitive behavioral therapy for symptom syndromes. CNS Spectr. 2006;11:212–222. doi: 10.1017/S1092852900014383. [DOI] [PubMed] [Google Scholar]
- 86.Yoshino A, Okamoto Y, Okada G, Takamura M, Ichikawa N, Shibasaki C, Yamawaki S. Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychol Med. 2018;48(7):1148–1156. doi: 10.1017/S0033291717002598. [DOI] [PubMed] [Google Scholar]
- 87.Taylor R.Cognitive behavioral therapy for chronic illness and disability. New York, NY: Springer Science & Business Media, 2010
- 88.Halford J, Brown T. Cognitive -behavioural therapy as an adjunctive treatment in chronic physical illness. Advances Psychiatric Treat. 2009;15:306–317. doi: 10.1192/apt.bp.107.003731. [DOI] [Google Scholar]
- 89.Lakhan S, Schofield K. Mindfulness-based therapies in the treatment of somatization disorders: a systematic review and meta-analysis. PLoS One. 2013;8(8):13. doi: 10.1371/journal.pone.0071834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weck F, Neng M. Response and remission after cognitive and exposure therapy for hypochondriasis. J Nerv Ment Dis. 2015;203:835–883. doi: 10.1097/NMD.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 91.Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Psychother Psychosom. 2009;78:265–274. doi: 10.1159/000228247. [DOI] [PubMed] [Google Scholar]
- 92.McDaniel S, Hepworth J, Doherty W. Medical Family Therapy and Integrated Care. Washington DC: American Psychological Association; 2014. [Google Scholar]
- 93.Jackson J, Kroenke K. Prevalence, impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med. 2008;70:430–434. doi: 10.1097/PSY.0b013e31816aa0ee. [DOI] [PubMed] [Google Scholar]
- 94.Creed F, Barsky A. A systemic review of the epidemiology of somatization disorder and hypochondriasis. J Psychosom Res. 2004;56:391–408. doi: 10.1016/S0022-3999(03)00622-6. [DOI] [PubMed] [Google Scholar]
- 95.Kroenke K, Spitzer R, Williams J, Linzer M, Hahn S, de Grny PV, Brody D. Physical symptoms in primary care: predictors of psychiatric disorders and functional impairment. Arch Family Med. 2014;3:774–779. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]
- 96.Katon W, Lin E, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiat. 2007;29:147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Barsky A. A comprehensive approach to the chronically somatizing patient. J Psychosom Res. 1998;45:301–306. doi: 10.1016/s0022-3999(98)00051-8. [DOI] [PubMed] [Google Scholar]