Abstract
Background
There are now many biological therapies to treat severe asthma. To assess which work best for which patient, we need to develop definitions of response. This narrative review aims to capture severe asthma patients’ perceptions about non-response and response to biological therapy.
Methods
Four bibliographic databases were searched from inception to September 2021. Grey literature was searched with the involvement of patient representatives. A thematic approach was used for synthesis. No qualitative studies specifically explore patients’ perspectives on response to biological therapy for severe asthma. Three papers and one published asthma patient interview were included. Relevant grey literature was included from online discussion forums, blogs and social media websites.
Results
Adult patients framed positive response to biological therapy in terms of reduced burden of disease and treatment. Both were multifaceted. Some patients experienced reduced benefit from biological therapy over time. There was a group of patients who described a limited response or non-response to biological therapy. This was framed within the context of continuing hospitalisation and oral corticosteroid treatment. The speed of onset of benefit was felt to be important by some.
Conclusions
Definitions of non-response and response need to be patient-centred, yet there is a complete lack of qualitative research focused on this topic. By combining relevant published and grey literature we have provided a description of adult patients’ perceptions of response to biological therapy in severe asthma. We now need to understand the views of children and adolescents with severe asthma and their carers, and diverse patient experiences in real-world settings.
Short abstract
When considering response and non-response to biological therapy, people with severe asthma value participation in everyday activities, reduced exacerbations and reduced exposure to oral corticosteroids https://bit.ly/3Cix28n
Introduction
There is no universally accepted definition of non-response and response to biological therapy for severe asthma [1]. To date, clinical trials for new biological therapies have set their own outcome measures, including oral corticosteroid (OCS) use, blood eosinophil count, asthma control, lung function, hospitalisation and exacerbations. Trials tend to use minimal clinical important difference or minimal important difference to define treatment response where available, prioritising reproducibility and distinguishing treatment from placebo participant cohorts. National healthcare regulators across Europe use a range of outcome criteria to determine treatment access and reimbursement policies [2, 3]. Over the last 20 years, measures of health-related quality of life (QoL) were not included as clinical trial end-points in over a third (37%) of randomised controlled trials (RCTs) of severe asthma treatments, despite a majority of patients considering improved QoL as the most important outcome for biological therapy [4, 5].
Biological therapies have revolutionised the treatment landscape for severe asthma and improved the lives of many patients, although questions remain around how treatment success has been defined. People living with severe asthma are arguably the principal stakeholders within efforts to define response and non-response to biological therapies; however, their views have not been integrated to date. A recent consensus-based “super-responder” definition did not include QoL measures due to a lack of agreement among participants and did not include input from people living with severe asthma [6]. Research has shown discordance between patients’ and clinicians’ views when selecting relevant outcomes to measure response to biological therapies and thus defining “super-responders” [7]. There is recognition within the severe asthma research community that more research is needed to understand patient perspectives and priorities around response to biological therapies [6–8]. While biological therapies can be life-changing for some, they are high-cost, lifelong treatments, and health regulators and payers must balance a range of factors when determining which patients can access and should continue biological therapies.
Developing a consensus definition of non-response and response to biological therapy for severe asthma will inform future policy, research and clinical decision making by offering a consistent approach to defining treatment response, while taking into account the priorities of all stakeholders. When developing a definition of response for future research and practice, it is crucial to understand how those at the centre, i.e. people living with severe asthma, perceive and define treatment success. A future consensus definition of non-response and response must therefore take account of the factors which patients consider important when weighing up the benefits of biological therapy, alongside more traditional measures such as exacerbations, in order to meet the needs of the wider scientific, clinical, regulatory and severe asthma patient communities.
The aim of this narrative review is to synthesise evidence about patient perceptions and opinions of non-response and response to biological therapy for severe asthma, as part of the Innovative Medicines Initiative 3TR multinational consensus study (www.3tr-imi.eu). The findings from this review will help to inform a multi-stakeholder study to agree on definitions of non-response and response to biological therapy for children, adolescents and adults with severe asthma.
Methods
Data sources and search strategy
Four bibliographic databases were searched (Embase (OVID), MEDLINE (OVID), CINAHL (EBSCOhost, Cumulative Index to Nursing and Allied Health Literature) and PsycINFO (EBSCOhost)). The search strategy was developed on Embase (OVID) and subsequently adapted for other databases. Databases were searched from inception to 1 September 2021.
During preliminary searches to optimise the search strategy, it became clear that there was little published literature on the topic. The study team therefore decided to include grey literature within the review (online discussion forums, blogs, news articles, social media and patient organisation websites). The grey literature search strategy was developed with input from adult, youth and parent/carer members of the 3TR Respiratory Patient Working Group (RPWG) to translate scientific terminology into words and phrases patients use to describe the outcomes of interest, e.g. patient-centred search terms for “deleterious response” included “did nothing”, “made worse”, “didn't work” and “pain”. To capture patient experiences from different European healthcare settings, two RPWG members (B.F. and H.N.) were recruited to conduct grey literature searches in their own languages (Dutch and Swedish, respectively), in addition to the study team searching in English. Training and support were provided to the RPWG members to assist with the search. The grey literature search revealed several European online communities related to biological therapy for severe asthma and these were included in the review.
Inclusion criteria
1) Patient characteristics: adults and children with a diagnosis of severe asthma from 6 years of age and their parents and carers. Criteria for severe asthma diagnosis were set by each study. 2) Phenomenon of interest: non-response and response to biological therapy for severe asthma. Biological therapies for severe asthma included mepolizumab, reslizumab, benralizumab, dupilumab, omalizumab, brodalumab, pitrakinra, tralokinumab, lebrikizumab, tezepelumab, ligelizumab and their associated commercial names. 3) Design: qualitative studies including focus groups, semistructured and unstructured interviews, and quantitative studies with a qualitative element including surveys and RCTs published in English were eligible for inclusion. 4) Evaluation: views, attitudes, beliefs, perceptions and experiences. 5) Research type: qualitative and mixed methods.
Exclusion criteria
The following were excluded: systematic reviews and meta-analyses, narrative reviews, discussion papers, editorials, commentaries, case studies, animal studies, conference abstracts, studies not available in full form, unpublished material, non-asthma studies (e.g. viral bronchiolitis or viral-associated wheeze) and studies conducted with exclusively mild or moderate asthma populations.
Data extraction, synthesis and analysis
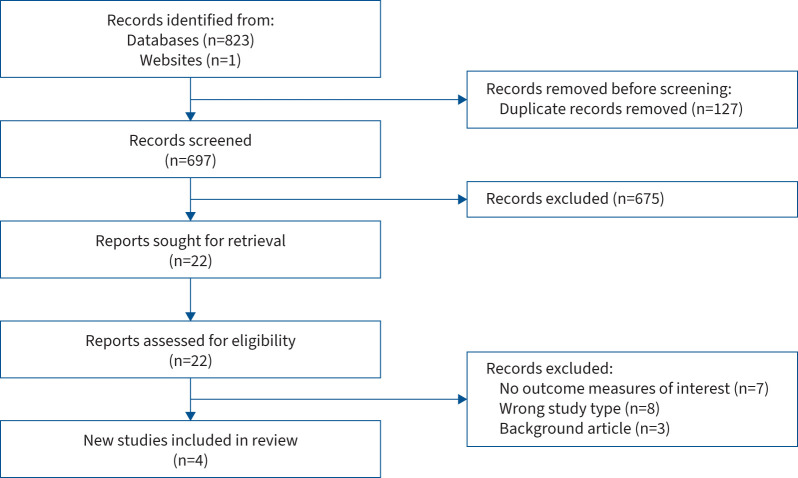
Data extraction was completed by one reviewer (C.C.) and cross-checked by a second reviewer (C.W.). The following data were extracted: country, patient characteristics, number of participants, study design, asthma definition and severity, experience of biological therapy, and outcomes of interest (experience of response to treatment). The outcomes of interest were explored individually within each included study according to the theoretical thematic analysis approach of Braun and Clarke [9]. In order for the results of this narrative review to easily inform the development of definitions of pre-selected treatment response options, we chose to group themes around three broad categories: mainly positive response to treatment, neutral or minimal response and limited or no response. One additional theme, time to onset of efficacy, was selected to distinguish this as an important but separate area of patient concern. Themes were developed by one reviewer (C.C.) and discussed with a second reviewer (C.W.). The findings are described narratively and key themes are summarised. The study selection process is summarised in a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart (figure 1) [10].
FIGURE 1.
PRISMA 2020 flow diagram for systematic reviews.
Results
Search results
The systematic literature search produced 696 papers after duplicates were removed and one additional asthma patient interview conducted by the Health Experiences Research Group at Oxford University (Oxford, UK) using qualitative research methods was included [11], resulting in a total of 697 articles screened. Screening was completed independently by four reviewers (C.C., C.W., E.K. and A.R.). After screening titles and abstracts, 22 papers were deemed relevant and included in the full-text screening stage, completed by C.C. and C.W. Three papers and one patient interview met the inclusion criteria and were included in the review.
Characteristics of included studies
The included studies aimed, through qualitative interviews, to understand the patient experience of living with severe asthma and treatment with biological therapies, where this included patient views on response to biological therapy. At the time the search was conducted, no studies aimed to specifically explore patients’ views on response to therapy. 78 patients (age 18–81 years) were included across all studies [11–14], with additional patient views from the literature also included in De Graaff et al. [13]. The characteristics of the included studies are presented in table 1. The criteria for severe asthma diagnosis varied across all studies and were not reported in two studies [11, 14].
TABLE 1.
Characteristics of included studies
| Reference (year) | Country |
Number of participants
(age range) |
Biological therapy of interest (number of patients) and duration | Study design | Asthma definition and severity |
| Healthtalk.org, Health Experiences Research Group [11] | UK | 1 (34 years) |
Omalizumab (1); treatment duration not reported | Qualitative, semistructured interview | Severe, brittle asthma under consultant care; criteria for severe asthma diagnosis not reported |
| Clark et al. [12] (2021) | Australia | 20 (21–81 years) |
Omalizumab (5), mepolizumab (15), azithromycin as add-on to either biological therapy (5); treatment duration at least 4 months | Qualitative, semistructured interviews | Severe asthma according to the 2014 ATS/ERS definition [15] and prescribed add-on therapy |
| De Graaff et al. [13] (2022) | The Netherlands | 10 (48–69 years) |
Benralizumab (8) (3 with previous experience of different biological therapy: omalizumab, mepolizumab), dupilumab (1), no biological therapy experience (1); treatment duration not reported | Qualitative exploratory study in two steps: 1) patient experience stories from previously published reports and 2) unstructured interviews based on “life histories” | Severe asthma according to the 2014 ATS/ERS definition [15] and treated at a tertiary severe asthma referral centre |
| Gelhorn et al. [14] (2019) | USA | 47 (18–79 years) |
Specific biological therapies not reported; current biological therapy (29, including 4 with previous experience of different biological therapy) for at least 3 months, previous biological therapy, discontinued within last 18 months (6), recommended for biological therapy but declined and biologic-naïve at time of study participation (12) | Quantitative survey and qualitative telephone interviews | Diagnosis of severe asthma for at least 3 years; criteria for severe asthma diagnosis not reported |
ATS/ERS: American Thoracic Society/European Respiratory Society.
Characteristics of grey literature
Patient experiences of biological therapy response and non-response were identified within online discussion forums, blogs and social media websites. Patients had experience of the following biological therapies for severe asthma: benralizumab, dupilumab, mepolizumab and omalizumab [16–21]. Although individuals may access discussion forums and social media sites from anywhere in the world, sites were hosted in the following countries: Austria [18], the Netherlands [20], Sweden [21] and the UK [16, 17, 19].
Patient perspectives about response to biological treatment
Patient perspectives on the following four major themes were identified: positive response to biological therapy, reduced or diminishing response to biological therapy, limited response and non-response to biological therapy, and time to perceive a change in severe asthma (onset of efficacy).
Positive response to biological therapy
In the published literature, patients frame a “positive response” to biological therapy in relation to two domains: a reduced burden of disease and a reduced burden of treatment. Many factors contribute to patients’ experiences of reduced burden of disease, including reduced symptoms, improved lung function, reduced severity of asthma exacerbations and less recovery time following an exacerbation, fewer difficulties with social interaction, greater ability to participate in life, increased energy, and reduced impact on mental health [11–13]:
“In terms of impacting my life, well it means that I can live a normal life, essentially.” [12]
This was echoed in the grey literature. Patients consider a range of measures, such as lung function measures (forced expiratory volume in 1 s (FEV1), fractional exhaled nitric oxide (FENO) and peak expiratory flow (PEF)), as well as the ability to increase their level of activity as a measure of positive treatment response to biological therapy. When describing their response to treatment, patients framed a positive response around the specific activities which are important to them as an individual:
“It took some years before I saw a difference. But after that I even had the strength to walk up 4 flights of stairs.” [21]
“For me it is a difference of day and night. In the last 2 years I had almost always troubled lungs, many medicines needed but still coughing and every 6 weeks an exacerbation and 10 days prednisolone use to calm down my lungs. Now this year I got 3 injections of Dupilumab, barely side-effects and completely calm lungs. Coughing is completely disappeared, I am not breathless anymore. I can exercise well to gain condition and no need for prednisolone or other medicines. I feel a bit as I felt before having asthma.” [20]
Some patients take account of objective lung function measures to better understand what is happening in their lungs and to assess their response to treatment, valuing the ability to compare results before and after biological therapy:
“And then you just see your lung values going up. Once you see more lung capacity without having increased my medication, I think: ‘hey, that's funny stuff. It works!’” [13]
“I feel so much better. Before I had 36% lung capacity.” [21]
“My eosinophils have shot back up and my FeNO is back up in the 100's.” [19]
Patients differentiate degrees of response level when considering their overall response to treatment as positive:
“Unfortunately this medication didn't magically make everything better, neither will it. My lungs still enjoy misbehaving, I sleep with an oxygen tube wrapped around my face and probably need more rest than a baby. But I'm managing to go to University and was able to reduce my steroid dose without my lungs having a complete tantrum.” [18]
Similarly, reduced burden of treatment is multifaceted, including reduced medication use and dependency, fewer treatment side-effects, less need for interactions with health professionals, and fewer hospital stays [12, 13]:
“This year I have only had one hospital admission, whereas, at this time last year, I'd already had five; so, one admission in six months will do me fine, thank you very much. Also my consultant has now said that if I can remain stable for six months, we can A, possibly start reducing some of my other medications but also B, he might even think about letting me work part-time, which is an amazing step forward.” [11]
Patients especially value reducing or stopping OCS as a result of biological therapy [12, 13, 18, 19, 21]:
“I used to have Prednisone [all the time]; like the last few months I really haven't – I haven't had as many – near as many the number of issues [of Prednisone] as I did have with, say, for six to 10 months previous.” [12]
“I'm managing to go to University and was able to reduce my steroid dose without my lungs having a complete tantrum. […] Yes I'm still far away of a good age appropriate general health, but I don't feel like I'm stuck in a constant down spiral anymore.” [18]
“It has transformed my life. I've not had a chest infection/40 mgs steroid course for over three months.” [19]
However, many patients must continue maintenance OCS treatment alongside biological therapy and consider it a “necessary evil” [12].
“Successful” treatment reduces the impact of both disease and treatment on the individual's life. Patients frame this as “regaining what has been lost” as a result of their severe asthma [12]. Clark et al. [12] reported that overwhelmingly, participants talked about how add-on therapy had positively improved their lives.
Even when they experience a positive response, patients remain cautiously hopeful. Biological treatment is an add-on therapy and many patients continue to make lifestyle adjustments to accommodate their severe asthma. Ongoing OCS burden, alongside successful biological therapy, is an area of concern for many patients [12, 13]:
“I still have flare-ups. The Nucala has helped with the thick mucus and inflammation. I still need the dreaded steroids.” [19]
Patients are also aware of the lack of scientific data on the long-term effects of biological therapy and some express concern about not knowing how long they must take the medication for in the future, although this was not a major anxiety [12, 13, 19]. On online forums, patients seek information and discuss side-effects of biological therapies, including fatigue, headache, hair loss, back pain, urinary tract infections, joint stiffness, and tingling in the arms and legs [17, 19, 20]. Patients may find it difficult to assess whether they experience side-effects from biological therapy or as a result of other medications or health conditions [19]. The side-effect profile of biological medications is one of the elements patients weigh up when considering the benefits and risks of biological therapy and whether the burden justifies continuation:
“I haven't got any side effects, maybe the odd headache sore throat but I would gladly suffer a multitude of side effects to have the benefits it gives me. I've gone from not much I can do to not much I can't do.” [19]
Reduced or diminishing response to biological therapy
Some patients experience a reduced benefit from biological therapy over time. Patients frame this experience as the medications “stopped working”, “plateauing”, developing “resistance”, or noticing a gradual increase in symptoms such as chest tightness or a reduction in their QoL [12, 17, 19, 21]:
“I think, for the first eight months, I think it changed a lot. I was quite able to – I felt that I was kind of living a normal life, which was amazing. But in the recent three, four months, I don't think it's helped me a lot at all.” [12]
“For six months or so things were good, I had less time off sick and I had fewer hospital admissions. The problem was though that I felt […] my asthma beginning to plateau, I was having fewer really bad days but was also having fewer symptom free days.” [17]
For these patients, the gains made during treatment, e.g. being able to go on holiday, are then lost and they resume a day-to-day life “dominated by severe asthma” [13]:
“I wasn't particularly happy about this change [reduced treatment efficacy] as it made planning any kind of life virtually impossible.” [17]
This return to pre-biological life may increase the experienced disease and treatment burdens.
Some patients live with a level of “hopelessness, worry about the future” [12] that the biological therapies were losing or would lose their effectiveness:
“I'm sure at some stage I will develop a total – it will become inefficient at combatting what – the job it's doing now. So – but – I'm hoping I run out of time before that happens.” [12]
“I'm all for giving everything a go, definitely. I had really high hopes for the mepo [Mepolizumab], I still do, but I have lost a lot of hope, and my quality of life has definitely dropped in the last four months.” [12]
For some, concern about the future of their severe asthma is limiting to their current life. On the other hand, some patients express hope for the future and the possibility of new treatment discoveries [12].
In the grey literature, patients recognise that staying on a therapy which is not “the right fit” for their asthma may prevent further investigative tests or trials of other more effective treatment options [16, 19].
Limited response and non-response to biological therapy
Within the published literature, most patients responded to biological therapy to some extent. Patients describe limited and non-response as frustrating, and frame it around frequent hospitalisations and the ongoing need for regular OCS treatment.
“I get so frustrated as my life has changed dramatically …” [19]
“Inhalers and the other medication did not do much anymore, so then we searched for another possibility. [Omalizumab] came into the picture, so for five years I had that, but I was admitted to the hospital quite a few times […] And that was also the only biological so far, because I have had five or six, which I think helped me […] but good, in November I will start a new one.” [13]
“No. That's [the problem]. We haven't been able to reduce it [the prednisone]. We tried to not long ago [to] step down. I – we tried – as far as I got was 20 milligrams from 25 but I got sick, so we had to put it back up.” [12]
The lack of response to biological therapy and the unlikelihood of new medication discoveries impacts patients’ mental health and can lead to hopelessness [12, 17]:
“But it is fact that there doesn't seem to be a suitable drug available at the moment, to break the cycle I'm stuck in. Which makes me wonder if hamsters ever get frustrated that they don't move forward despite running.” [17]
Time to perceive a change in severe asthma (onset of efficacy)
Time to onset of efficacy was ranked within the top three most important attributes or features of biological medications by 39.1% of patients in the Gelhorn et al. [14] study. For patients this was defined as: “speed of onset – how long it takes when you first start taking the medication until you notice an improvement in your asthma symptoms” [14]. However, improvement in asthma symptoms is not further quantified or defined, so may have been open to interpretation by each patient and we cannot assess from this study which symptoms are considered more important to patients in terms of treatment response. Some patients who had not yet started biological therapy had concerns around how quickly the treatment would start to work:
“I just want to feel it – I want to feel it – relief in like 5 minutes, I just want to know it's working fast. I like the medicine to work fast most of the time.” [14]
Biological therapies can take several months to reach full efficacy [22]. Patients with severe asthma may anticipate a faster response due to their experience with other treatments, such as inhaled bronchodilators, which provide more immediate symptom relief. In discussion forums, patients seek information and manage expectations about how quickly a treatment response can be expected [19, 20]. Some patients may take the time to onset of efficacy into account when assessing their own response to biological therapy and may perceive a shorter time to equate to a more positive response. A summary of the key findings is in table 2.
TABLE 2.
Key themes: patients’ perceptions and opinions about non-response and response to biological treatment for severe asthma
| • Positive response to biological therapy reduces both disease and treatment burdens for a person with severe asthma. Patients frame this in relation to the specific activities which are meaningful to them as an individual. |
| • Patients may continue to make lifestyle adjustments even when responding to biological therapy. |
| • Patients differentiate the degree of response on a continuum from positive to negative, as well as changes in response over time. Response is fluid for some individuals. |
| • Side-effect profile, time to onset of efficacy, access and cost, and practicalities of treatment administration are important factors when patients consider the benefits and risks of biological therapy. |
| • An individual's mental health can be impacted by limited response to therapy or when an initial response diminishes over time. This may increase their experience of disease and treatment burden. |
| • Patients recognise the importance of stopping ineffective biological therapy in order to trial new therapies or allow further investigative tests. |
Discussion
Severe asthma patients weigh up a range of factors when considering whether or not they have “responded” to a biological therapy. This may include consideration of objective test results (e.g. spirometry, PEF), as well as asthma symptoms, number and severity of exacerbations, exercise capacity, and their ability to participate in family activities and wider society. A reduction in medication usage, particularly reduced OCS use, is an important marker of response for patients.
Patients recognise the degree of response to biological therapy on a continuum from “positive” to “negative” and response is fluid for some individuals. Small reductions in exacerbations or OCS use may be classified as positive responses by an individual patient but may not achieve the level of response they hoped for. Patients do not perceive their response to biological therapy as “fixed”, but recognise that it can vary over time, sometimes resulting in a plateauing or return to life before biological therapy. Reduced, limited and non-response to biological therapy can impact the mental health of patients; however, patients recognise the value in stopping ineffective biological therapy in order to try alternatives or undergo further tests with the hope of finding a treatment which works in the future.
Patients are often uncertain about what to expect when starting biological therapy [19, 20]. Some may reach out to their peers, including through online forums, to help answer questions and manage expectations. Health professionals also play a role in setting and managing expectations, and may influence a patient's perception of their response to treatment and their ongoing adherence when faced with the practical challenges and any side-effects from injected therapies [11, 23, 24]. When supporting patients to understand their potential response to biological treatment, professionals should work closely with each patient to develop shared decision making, allowing them to understand the patient's treatment goals and to note the language the individual uses to frame their goals [23–26].
Clark et al. [12] note that while QoL improvements were modest in RCTs of biological therapies, the experiential description from patients suggests a far greater improvement. This indicates that QoL questionnaires used in trials are not tailored to the aspects most important to patients, and the underreporting of QoL subscale data in the majority of RCTs (70%) risks obscuring specific factors which affect QoL in severe asthma by reporting only the global score [4, 12]. There is also recognition that future research must focus on improving outcome measures to assess QoL within biological therapy studies and in clinical practice [4, 6]. This is consistent with our findings from the Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA) study and the systematic review of definitions of response and non-response to biological therapy [27, 28].
Cost, variation in health insurance and reimbursement of treatment costs were a major concern for patients in the Gelhorn et al. [14] study. Some patients decided not to start biological therapy because they could not afford the costs. As a US-based study, the health funding landscape is markedly different to Europe, so other themes or factors may have been more important to a different patient population. Nevertheless, in the grey literature patients also commented on treatment access and reimbursement criteria:
“Because things have become out of control so quickly they are not waiting for the benra[lizumab] to flush out the system they are going straight over to mepo[lizumab]. I am grateful that it can be done quickly and not to have to [go through] the rigmarole of panels, etc., to be approved.” [19]
“It's a game changer if you have eosinophilic asthma and get through the list of criteria to qualify!” [19]
“I was able to receive Mepolizumab or how I like calling it, the “magic potion” through a Named Patient Programme – which means I don't have to wait until this particular medication gets approved and available here in Austria. This only was possible because I had a doctor standing behind me, pushing and really fighting to find a treatment for me.” [18]
Patients also consider other practical factors including travel distance to access treatment, frequency of injections and appointment duration when weighing up the benefits of treatment [14]. Patients may be willing to tolerate a higher degree of practical inconvenience if they feel a biological therapy significantly improves their QoL:
“Difficult to take distance into consideration for a treatment. I am so happy I can inject myself [at] home shortly and go on holiday whenever I like and no need to take my injections into account.” [20]
“It is so worth it, the struggle was that I for 5 years got them at the hospital, so it has taken a lot of time, I have just started to take them at home so finally it is more convenient.” [21]
This narrative review identifies key areas which patients consider when assessing their own response to biological therapy and which should be taken into account when developing a consensus definition of response. Figure 2 presents the key themes identified by this review.
FIGURE 2.
Patient considerations about biological therapy in severe asthma.
Strengths and limitations
This is the first narrative review of patient perceptions and opinions of non-response and response to biological therapy for severe asthma. It combines a comprehensive search of the published literature with additional patient considerations from non-academic sources. By including grey literature within our review, we searched some of the forums where patients informally share their experiences and seek support, allowing us to identify additional factors which patients consider important.
There are some limitations to this review. First, the literature search was restricted to articles published in English. However, experts in the field were consulted so it is unlikely that any relevant articles were missed. Patients treated within European health systems, apart from the Netherlands and the UK, were not represented in the included published studies and this may limit the generalisability of these findings. This may be due to a lack of research or to the search being limited to the English language. In order to address this limitation in part, the published literature was supplemented by grey literature in two additional languages to provide a broader European perspective.
There is no single definition of severe asthma used across the included studies. Studies were included where the researchers defined participants as having severe asthma and two studies refer to the American Thoracic Society/European Respiratory Society (ATS/ERS) 2014 guidelines on definition, evaluation and treatment of severe asthma [15]. The criteria used by the team from Healthtalk.org [11] and Gelhorn et al. [14] were not fully reported. Across the included studies, patients had different experiences of biological therapy (current, previous and no biological experience) and were limited to the biological therapies licensed for severe asthma in that setting at the time. Some patients only had experience of biological therapy in an experimental setting and real-world experiences may differ [13]. A wider variety of biological therapy exposure and response experiences would be beneficial.
Concerns about treatment side-effects were more prominent in the grey literature. This should be interpreted with some caution in light of the tendency for those with overtly positive or negative experiences to want to share their perspective, resulting in less visibility of the moderate middle ground [29].
Both the published and grey literature lacked patient experiences from children, adolescents and carers. Further research is needed to understand the unique experiences of biological therapy among children and adolescents with severe asthma, and their parents and loved ones.
Implications for research and next steps
This narrative review highlights that there are limited data on severe asthma patient views about response to biological therapy and provides an important synthesis of previous research. The findings reported here, along with results from the systematic review on response and non-response to biological therapies [28], will be discussed in a multi-stakeholder consensus process with representatives from patient and carer, clinician and researcher, pharmaceutical industry, and health regulatory stakeholder groups to agree on patient-centred definitions of non-response and response to biological therapy for use in severe asthma research and clinical practice.
Within the published and grey literature some patients report a reduced or diminishing response to biological therapy over time; however, this has not been reported in clinical research studies, which typically report average response and may not capture the heterogenous nature of individual treatment responses. This is an important area and further research should be directed at this reduced treatment response.
Conclusions
People with severe asthma describe a multifaceted “response” to biological therapy encompassing a wide range of personal, social and health domains, including increased involvement in everyday activities, significant life events, reduced healthcare utilisation and reduced need for OCS. Across the published and grey literature, patients value increased participation in life, reduced exacerbations and reduced OCS exposure as important treatment outcomes. Practical considerations, including time to onset of efficacy and cost of treatment, may also play a role in how patients assess their response to biological therapy. Patients in the published literature expressed low levels of concern regarding the short- and long-term side-effects of biological therapies; however, patients discuss a range of side-effects in online discussion forums, indicating that some patients may find this more worrying. Overall, there is a lack of qualitative data to understand patient experiences and perspectives around response to biological therapy for severe asthma. More research is needed to better understand patient experiences across all age groups, all available biological therapies and in different real-world health systems. Research is also needed to explore patient experiences of switching from one biological therapy to another.
Shareable PDF
Acknowledgements
We would like to acknowledge the support of 3TR in funding the development of this narrative review. We would like to thank Paula Sands (University of Southampton, Southampton, UK) for her assistance in optimising the search strategy. We would like to thank Theo Schilpzand (respiratory representative on the Patient Advisory Board for 3TR) for reviewing the manuscript from a patient's perspective.
Footnotes
Conflict of interest: All authors report that funding was received to support this work by the European Lung Foundation (ELF) from European Commission's Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 831434 (3TR). C. Coleman and C. Williams are employees of the ELF. There are no further disclosures.
Support statement: The 3TR project is funded by the Innovative Medicines Initiative (IMI) 2 Joint Undertaking (JU) under grant agreement number 831434. The JU receives support from the European Union's Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). Disclaimer: Content of this publication reflects only the authors' view and the JU is not responsible for any use that may be made of the information it contains. The funder had no role in the design, conduct or write up of the narrative review or decision to publish. Further details about the 3TR project and IMI funding programme are available on their websites: www.3tr-imi.eu and www.imi.europa.eu. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Rogliani P, Calzetta L, Matera MG, et al. Severe asthma and biological therapy: when, which, and for whom. Pulm Ther 2020; 6: 47–66. doi: 10.1007/s41030-019-00109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porsbjerg CM, Menzies-Gow AN, Tran TN, et al. Global variability in administrative approval prescription criteria for biologic therapy in severe asthma. J Allergy Clin Immunol Pract 2022; 10: 1202–1216. doi: 10.1016/j.jaip.2021.12.027 [DOI] [PubMed] [Google Scholar]
- 3.Santos-Valente E, Buntrock-Döpke H, Abou Taam R, et al. Biologicals in childhood severe asthma: the European PERMEABLE survey on the status quo. ERJ Open Res 2021; 7: 00143-2021. doi: 10.1183/23120541.00143-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanario J, Burns L. Use of health related quality of life in clinical trials for severe asthma: a systematic review. J Asthma Allergy 2021; 14: 99–1010. doi: 10.2147/JAA.S320817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark VL, Gibson PG, McDonald VM. What matters to people with severe asthma? Exploring add-on asthma medication and outcomes of importance. ERJ Open Res 2021; 7: 00497-2020. doi: 10.1183/23120541.00497-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract 2021; 9: 3997–4004. doi: 10.1016/j.jaip.2021.06.041 [DOI] [PubMed] [Google Scholar]
- 7.McDonald VM, Clark VL, Gibson PG. “Nothing about us without us” – what matters to patients with severe asthma? J Allergy Clin Immunol Pract 2022; 10: 890–891. doi: 10.1016/j.jaip.2021.11.035 [DOI] [PubMed] [Google Scholar]
- 8.Upham JW, Le Lievre C, Jackson DJ, et al. Reply to “Nothing about us without us” – what matters to patients with severe asthma? J Allergy Clin Immunol Pract 2022; 10: 891. doi: 10.1016/j.jaip.2021.12.036 [DOI] [PubMed] [Google Scholar]
- 9.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3: 77–101. doi: 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an update guideline for reporting systematic reviews. PLoS Med 2021; 18: e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthtalk.org . Asthma. www.healthtalk.org/asthma/overview Date last updated: August 2017. Date last accessed: 16 December 2021.
- 12.Clark VL, Gibson PG, McDonald VM. The patients’ experience of severe asthma add-on pharmacotherapies: a qualitative descriptive study. J Asthma Allergy 2021; 14: 245–258. doi: 10.2147/JAA.S296147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Graaff MB, Bendien SA, van de Bovenkamp HM. “Like a fish on dry land”: an explorative qualitative study into severe asthma and the impact of biologicals on patients’ everyday life. J Asthma 2022: 59; 980–988. doi: 10.1080/02770903.2021.1888976 [DOI] [PubMed] [Google Scholar]
- 14.Gelhorn HL, Balantac Z, Ambrose CS, et al. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence 2019; 13: 1253–1268. doi: 10.2147/PPA.S198953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 16.Ain't Easy Being Wheezy . Re-evaluating Xolair. http://ainteasybeingwheezy.blogspot.com/2019/03/re-evaluating-xolair.html Date last updated: 5 March 2019. Date last accessed: 3 February 2022.
- 17.Asthma Blog 1971 . https://asthmablog1971.com/2018/10/05/xolair-a-love-and-hate-relationship, https://asthmablog1971.com/2017/09/03/xolair-update Date last updated: October 2018. Date last accessed: 3 February 2022.
- 18.Breathe Easy, Easy Wheezy . http://breatheeeasy-easywheezy.blogspot.com/2015, http://breatheeeasy-easywheezy.blogspot.com/2017 Date last updated: 30 August 2017. Date last accessed: 3 February 2022.
- 19.HealthUnlocked . Asthma UK community forum. https://healthunlocked.com/asthmauk/posts/144137362/biological-therapies, https://healthunlocked.com/asthmauk/posts/140962292/my-sisters-asthma, https://healthunlocked.com/asthmauk/posts/145507431/benralizumab, https://healthunlocked.com/livingwithasthma/posts/142621469/im-doing-better, https://healthunlocked.com/asthmauk/posts/146044746/mepolizumab-injections, https://healthunlocked.com/livingwithasthma/posts/145437499/nucala-and-back-pain, https://healthunlocked.com/asthmauk/posts/138749572/reslizumab, https://healthunlocked.com/asthmauk/posts/145943650/another-little-bump Date last accessed: 3 February 2022.
- 20.Long Forum [Lung Forum] . Astma medicijnen [Asthma medicines]. www.longforum.nl/categories/astma-medicijnen Date last accessed: 3 February 2022.
- 21.Vi med astma och allergi [We with asthma and allergies] . Facebook Group. www.facebook.com/groups/570925839636640 Date last accessed: 21 February 2021.
- 22.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020; 55: 1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 23.Blaiss MS, Steven GC, Bender B, et al. Shared decision making for the allergist. Ann Allergy Asthma Immunol 2019; 122: 463–470. doi: 10.1016/j.anai.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 24.Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010; 181: 566–577. doi: 10.1164/rccm.200906-0907OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stacey D, Légaré F, Lweis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017; 4: CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kew KM, Malik P, Aniruddhan K, et al. Shared decision-making for people with asthma. Cochrane Database Syst Rev 2017; 10: CD012330. doi: 10.1002/14651858.CD012330.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaleva E, Rattu A, Brightling C, et al. Development of Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA). Eur Respir J 2022; in press [ 10.1183/13993003.00606-2022]. doi: 10.1183/13993003.00606-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khaleva E, Rutta A, Brightling C, et al. Definitions of nonresponse and response to biological therapy for severe asthma: a systematic review. ERJ Open Res 2022; in press [ 10.1183/23120541.00444-2022]. doi: 10.1183/23120541.00444-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed F, Burt J, Roland M. Measuring patient experience: concepts and methods. Patient 2014; 7: 235–241. doi: 10.1007/s40271-014-0060-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00837-2022.Shareable (331.8KB, pdf)