Abstract
Vertical global health programmes often evaluate success with a narrow focus on programmatic outcomes. However, evaluation of broader patient-centred and unintended outcomes is critical to assess impacts on patient choice and autonomy. Here, we evaluate the effects of a postpartum intrauterine device (PPIUD) intervention on outcomes related to contraceptive method choice. The stepped-wedge cluster randomized contolled trial (RCT) took place in five Tanzanian hospitals. Hospitals were randomized to receive immediate (Group 1; n = 11 483 participants) or delayed (Group 2; n = 8148 participants) intervention. The intervention trained providers on PPIUD insertion and counselling. The evaluation surveyed eligible women (18+, resided in Tanzania, gave birth at a study hospital) on provider postpartum contraceptive counselling during pregnancy or immediately postpartum. In our completed study, participants were considered exposed (n = 9786) or unexposed (n = 10 145) to the intervention based on the location and timing of their birth (no blinding). Our secondary analysis examined differences by intervention exposure on the likelihood of being counselled on IUD only, multiple methods, multiple method durations, a broad method mix; and on the number of methods women were counselled across two samples: all eligible women, and only women who reported receiving any contraceptive counselling. Among all eligible women, counselling on the IUD alone was 7% points higher among the exposed (95% confidence interal (CI): 0.02, 0.12). Among women who received any counselling, those exposed to the intervention were counselled on 1.12 fewer contraceptive methods (95% CI: 0.10, 2.34). The likelihood of receiving counselling on any non-IUD method decreased among those exposed, while the likelihood of being counselled on an IUD alone was 14% points higher among the exposed (95% CI: 0.06, 0.22), suggesting this intervention increased IUD-specific counselling but reduced informed contraceptive choice. These findings underscore the importance of broad metrics that capture autonomy and rights (in addition to programmatic goals) at all stages of health programme planning and implementation.
Keywords: Vertical health programmes, programme evaluation, Tanzania, family planning, quality of care, sexual and reproductive health and rights
Key messages.
Vertical global health programmes often evaluate success with a narrow focus on programmatic outcomes, leaving broader impacts on unintended outcomes under-examined.
Using data from a postpartum IUD (PPIUD) intervention in Tanzania, we explore the effect of this programme on access to a broad contraceptive method mix and other outcomes related to reproductive health and rights.
Women exposed to the PPIUD intervention in Tanzania were counselled on fewer contraceptive methods and were more likely to receive counselling on just the IUD (and no other methods) compared to women in the control group.
A narrow focus on the programmatic outcome of interest (PPIUD counselling and uptake) in this programme ended up reducing access to person-centred and rights-based reproductive health care.
Introduction
In 1997, a group of scholars dubbed vertical health programmes the ‘donors’ dilemma’ (Cairncross et al., 1997). In contrast to horizontal programmes dedicated to strengthening health systems, vertical programmes are topic-specific initiatives focusing on a single programmatic interest (such as HIV or family planning). Vertical programmes are popular among global health donors and non-governmental organizations for a number of reasons: they are more easily implemented within the bureaucracy of existing health systems than larger systemic reform, and they allow donors to target funding to specific topics of urgent interest. Critics have expressed concern that vertical programmes are less sustainable than those focused on overall health systems, and can draw resources away from more holistic approaches such as primary health care (Keshavjee and Farmer, 2012; Jayaraman and Vermund, 2015). Despite these concerns, however, vertical programmes remain a staple of global health service provision.
As billions of dollars are spent yearly on vertical programmes, the best way to evaluate their success remains an open question. The vast majority of evaluation studies focuses on assessing the intended outcomes of programmatic interest to donors and other stakeholders (Panter-Brick et al., 2014; Speizer et al., 2014; van de Ruit 2019). There is a clear and intuitive logic to this approach to evaluation, for example, measuring the success of an HIV prevention programme by the number of HIV infections it prevents. However, vertical health programmes may also have considerable unintended impacts that go unrecorded in conventional approaches to programme evaluation. A range of qualitative studies and commentaries have been published in the scientific literature, providing both theoretical grounding and small-scale evidence for these concerns (Yamin and Boulanger, 2013; Panter-Brick et al., 2014; van de Ruit, 2019). But very few large-scale quantitative evaluation studies have sought to document the impacts of vertical health programmes on outcomes beyond the scope of their programmatic goals.
Postpartum IUD and person-centred reproductive health
The focus on vertical programming is particularly evident in the global reproductive health sphere, where each topic, from cervical cancer screening to post-abortion care, has its own dedicated funding stream (Suh, 2019). Global family planning, in particular, has long been funded through vertical programmes, although there have been considerable efforts over the past several decades to better integrate contraceptive service provision with HIV services and maternal health care (Lindegren Lou et al., 2012; USAID, 2014). In the past decade, vertical reproductive health programmes have paid increasing attention to contraception in the postpartum period, emphasizing that perinatal care may often be the only facility-based care many women in low-resource settings receive (Cleland et al., 2012; 2015; Pfitzer et al., 2015; Tran et al., 2019). Postpartum family planning programmes often centre on the provision of long-acting reversible contraceptive (LARC) methods, which are highly effective, are less susceptible to user error compared to shorter-acting methods, and can be used for extended durations (Pfitzer et al., 2015; Stanback et al., 2015). Many global family planning experts consider postpartum LARC programmes to be a synergistic way to combine highly effective methods with a crucial time in the reproductive life-course (Morroni and Glasier, 2020).
As a result of mounting enthusiasm for postpartum LARC use, a growing number of global family planning programmes have been dedicated to the postpartum intrauterine device or postpartum IUD (PPIUD). The PPIUD can be safely inserted between 10 min and 48 h after the delivery of the placenta or during caesarean delivery and provides highly effective contraception for at least 10 years (de Caestecker et al., 2018). The World Health Organization has approved the non-hormonal copper intrauterine device (IUD) for postpartum use, as it does not interfere with breastfeeding and is otherwise safe for use in the immediate postpartum period (World Health Organization, 2012). Because PPIUD helps meet so many of the family planning field’s current goals [e.g. expanding modern contraceptive uptake, promoting LARC use, increasing birth spacing, and meeting the need for postpartum family planning, among others (Secura et al., 2010; Sridhar and Salcedo, 2017; Cahill et al., 2018; Mogeni et al., 2019)], PPIUD initiatives have become popular among global reproductive health non-governmental organisations throughout the Global South (PSI, 2015; Makins and Arulkumaran, 2018).
PPIUD initiatives have been most frequently evaluated by their impact on the availability and uptake of the method of interest (Cleland et al., 2012; Pfitzer et al., 2015; Stanback et al., 2015; Tran et al. 2019; Morroni and Glasier 2020). PPIUD uptake is an important measure for programme implementers to understand, but given family planning’s complex history intertwined with population control (Connelly, 2008), it is equally critical to evaluate a broader set of patient-centred outcomes that emphasize autonomy and freedom of choice (Senderowicz, 2020). Throughout both the Global North and the Global South, women from minoritized racial/ethnic groups, the physically and/or intellectually disabled, and many other marginalized groups have been targeted for fertility control, including through forced contraceptive use and coercive sterilizations (Connelly, 2008; Bashford and Levine, 2010). More recently, reproductive justice advocates have expressed concern that providers may be differentially targeting people from these same marginalized groups for LARC uptake (Christopherson, 2016), and there is emerging evidence of LARC coercion from all over the world (Higgins et al., 2016; Senderowicz, 2019; Yirgu et al., 2020; Britton et al., 2021; Senderowicz and Kolenda, 2022). Contraceptive uptake (and LARC uptake, more specifically) therefore presents a particularly problematic outcome to pursue, with uptake goals found to incentivize a spectrum of coercive counselling strategies (Senderowicz, 2019).
The importance of developing and measuring rights-based contraceptive measures has been frequently affirmed by experts in the field, but just how to measure freedom of choice in family planning has been subject to considerable debate (Brown et al., 2014; Barot et al., 2015). Existing frameworks for standardizing definitions and measurement of patient-centred outcomes in family planning vary, but all emphasize the importance of access to a broad mix of contraceptive methods (Dehlendorf et al., 2018; Sudhinaraset et al., 2018; Holt et al., 2019; Senderowicz, 2020). Patients should be offered an informed choice of multiple methods, and the methods offered should represent a broad range of contraceptive attributes so that women can choose the method that meets their individual needs (Senderowicz, 2020). These attributes may include duration of use (short-acting, medium-acting, long-acting and permanent), presence of hormones (hormonal and non-hormonal), provider dependence (provider dependent and independent) and locus of control (female- and male-controlled) (Festin et al., 2016; Senderowicz, 2020).
In this article, we seek to evaluate a vertical family planning programme implemented in Tanzania, looking beyond the intended programmatic outcomes of interest—PPIUD counselling and uptake—to examine the programme’s impact on other reproductive health outcomes related to informed, full contraceptive choice.
Materials and methods
Overview of study and description of the intervention
We performed a secondary analysis of data from a large cluster-randomized stepped-wedge PPIUD trial in Tanzania to evaluate the impact of the PPIUD intervention on outcomes related to freedom of informed contraceptive method choice. Since the intervention was primarily focused on training providers in hospitals, it was cluster randomized to avoid contamination, while the stepped-wedge design allowed the intervention to eventually reach all study hospitals.
The research described here is part of a broader evaluation of the PPIUD Project implemented by the International Federation of Gynacology and Obstetrics (FIGO) (de Caestecker et al., 2018). This initiative began in 2013, with the goal ‘to address the gap in the continuum of maternal health care and to provide for the postpartum contraceptive needs of women by increasing the capacity of healthcare professionals to offer PPIUDs by training community midwives, health workers, doctors, and delivery unit staff, as appropriate, in counselling and insertion of PPIUD’ (de Caestecker et al., 2018). In 2016, FIGO brought this programme to Tanzania via their national affiliate, the Association of Gynaecologists and Obstetricians of Tanzania (AGOTA), with the goal of introducing and institutionalizing PPIUD for those seeking antenatal care and facility-based births. AGOTA implemented the FIGO PPIUD Project at six public referral hospitals throughout Tanzania (Dodoma General Hospital in Dodoma, Muhimbili National Hospital in Dar es Salaam, Mt. Meru Hospital in Arusha, Tumbi-Pwani Regional Referral Hospital in Kibaha, Mbeya Zonal Referral Hospital in Mbeya and Sekou-Toure Regional Referral Hospital in Mwanza). Doctors and nurses were trained in counselling and insertion. Hospitals were selected to receive the intervention by AGOTA, the implementer of the intervention, in order to provide coverage of PPIUD services for different geographic regions of Tanzania among tertiary care facilities. The size of facilities varied, with smaller referral hospitals such as Mbeya having fewer providers (58 combined junior doctors and trained Ob/Gyns) than the larger Muhimbili National Hospitals (240 combined junior doctors and trained Ob/Gyns).
The FIGO/AGOTA programme trained providers on cadre-appropriate skills and knowledge to support the implementation of PPIUD. AGOTA organized a series of trainings in the six referral hospitals and surrounding satellite antenatal care clinics whose patients deliver at the referral hospitals. Hospital doctors were trained on PPIUD insertion and removal, while nurses and midwives in the satellite clinics were trained to integrate PPIUD counselling into routine antenatal family planning counselling. The FIGO/AGOTA initiative identified and trained a cascade of master trainers to carry out a ‘training the trainer’ approach for counselling and insertion. Counselling training sessions included ‘information on the advantages of PPIUD’, presentation of visual aids and role-playing of potential counselling scenarios (de Caestecker et al., 2018). The training was also designed to include content on method removal. Trained nurses and midwives were then expected to provide family planning counselling to women seeking antenatal care that included information about the PPIUD as part of a wide range of contraceptive methods.
The central FIGO team in London maintained a data ‘dashboard’ to provide real-time feedback to clinicians and project leaders from the hospitals included in the PPIUD Project in Tanzania and the other intervention countries. The dashboard reported numbers of: (1) deliveries; (2) PPIUD insertions; (3) women counselled on PPIUD; (4) PPIUD removals; (5) women followed-up; (6) PPIUD expulsions; (7) providers trained to insert PPIUD; and (8) providers trained to counsel on PPIUD. FIGO coordinators in London regularly communicated dashboard statistics with each other, Tanzanian affiliate staff and providers. Providers were given real-time feedback about their progress towards meeting project goals, all tied to PPIUD counselling and insertion, with the primary marker of success being a calculation from dashboard numbers of the percent of all deliveries leading to a PPIUD insertion. A detailed description of the intervention can be found in de Caestecker et al. (2018).
This analysis is part of a mixed-methods sequence of learning motivated by the qualitative portion nested within this larger RCT. As results from in-depth interviews with women who received antenatal care under PPIUD intervention conditions began to reveal a tendency for counselling to focus on the IUD to the exclusion of other methods (Senderowicz et al., 2021), we turned to the large quantitative dataset to explore how widespread this phenomenon was among the broader study population. Our objective was to estimate the impact of the PPIUD intervention on individual-level overall access to information about contraceptive methods and a broad contraceptive method mix during antenatal, peripartum and immediate postpartum contraceptive counselling.
Research ethics
The study received human subjects research approval from the National Institute of Medical Research (NIMR) in Tanzania (protocol number: NIMR/HQ/R.8a/Vol.IX/2006), and ethical approval as exempt by the institutional review board at Harvard University (protocol number: IRB15–1605). Respondents provided written informed consent to be interviewed, or thumbprints and a witness’s signature if they could not sign their names.
Trial design
A cluster-randomized stepped-wedge trial approach with a 1:1 allocation ratio for clusters was designed to evaluate FIGO intervention sites in Nepal, Sri Lanka and Tanzania. A detailed description of that overarching trial design can be found in Canning et al., 2016. The trial is registered with clinicaltrials.gov NCT02718222. Because of substantial differences between the implementation of both intervention and study procedures between countries, results are presented here for Tanzania alone.
Cluster selection, randomization, and steps
In Tanzania, AGOTA selected six large public referral hospitals across different regions to receive the PPIUD intervention. Each hospital served as a cluster. There was no blinding. Given the range of hospital sizes, we employed a strategy of block randomization, in which clusters were matched on annual obstetric caseload into blocks of n = 2 clusters. Using Stata v14, EP generated a random number for each cluster and assigned the lower number within each pair to Group 1 (Dodoma General Hospital in Dodoma, Muhimbili National Hospital in Dar es Salaam, and Mbeya Zonal Referral Hospital in Mbeya) and the higher number to Group 2 (Mt. Meru Hospital in Arusha, Sekou-Toure Referral Hospital in Mwanza and Tumbi-Pwani Regional Referral Hospital in Kibaha). According to the stepped-wedge design, Group 1 hospitals received the early intervention, and Group 2 hospitals received the late intervention (Figure 1). After randomization, evaluators learned of a pre-existing PPIUD intervention at Sekou-Toure hospital in Mwanza (Group 2), and this hospital was dropped from the study.
Figure 1.
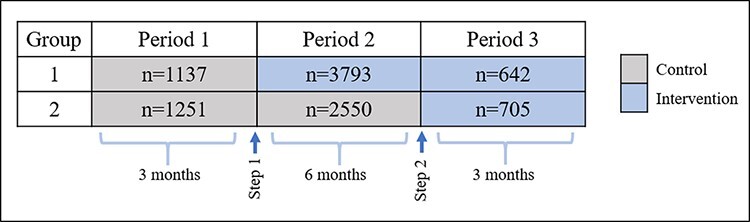
Stepped-wedge design
Data collection began on 15 January 2016. Group 1 hospitals were scheduled to begin PPIUD trainings after 3 months of baseline data collection, while Group 2 hospitals were scheduled to receive the intervention after 9 months. Due to delays in training implementation and other logistical challenges, the actual timing of the rollout to each hospital varied slightly, and these delays are accounted for in the data analysis. The intervention began in Group 1 on 15 April 2016. Group 2 was due to begin on 15 September 2016, but project implementation actually began on 17 November 2016, approximately 2 months later than planned. The present analysis uses quantitative data from the first contact with respondents in the immediate period following delivery of their index pregnancy.
Data collection and participants
Women were eligible to participate if they had given birth at a study hospital during data collection, resided in Tanzania, and were over age 18. All eligible women were invited to participate. Trained data collectors collected survey data in the postnatal wards of study hospitals, administering preprogrammed tablet-based questionnaires to all women who provided informed consent. Consent to participate in the evaluation was sought at the individual level after cluster-level randomization. The survey included questions on fertility desires; experiences with family planning counselling during the antenatal period, peripartum and immediate postpartum periods; perceptions of PPIUD and contraceptive intentions.
Outcomes of interest
The predefined primary outcome of interest to this evaluation was the percent uptake of PPIUD, defined as the proportion of all women who received a PPIUD divided by the number of women who delivered in one of the study hospitals over the course of the study period. No subgroups were excluded from this end-point. Key predefined secondary outcomes as defined at the outset of the study were also focused on PPIUD-related outcomes (e.g. the percentage of women who receive PPIUD counselling and the percentage of PPIUD acceptors who have PPIUD expulsions). Predefined primary and secondary outcomes pertained to the cluster level. Analyses of these outcomes have been performed and reported on elsewhere (Huber-Krum et al., 2019; Hackett et al., 2020; Pearson et al., 2020).
The current secondary data analysis builds on the results of our concurrent nested qualitative study to expand the scope of this inquiry for this study. Results from semi-structured in-depth interviews with women at antenatal clinics exposed to the PPIUD intervention suggested that antenatal family planning counselling was directive and biased to focus on the IUD to the exclusion of other methods. Here, we have developed a set of quantitative measures to test whether these qualitative results can be expanded to the study population more generally. As such, the purpose of this analysis is to examine the effect of the intervention on a set of person-centred family planning outcomes related to method mix and availability of choice (World Health Organization, 2021). The five outcomes of interest are measured on the individual level among women who reported having received any perinatal family planning counselling and include: (1) likelihood of being counselled on the IUD alone (and no other contraceptive methods); (2) likelihood of being counselled on multiple (more than one) methods; (3) likelihood of being counselled on multiple method durations of use; (4) likelihood of being counselled on a broad contraceptive method mix; and (5) number of methods counselled on. Additionally, to assess whether losses to the number of methods counselled were compensated for by gains in counselling under the PPIUD intervention, we explored the total number of contraceptive methods on which women received counselling among all women in the study, including those who reported not receiving any family planning counselling.
For all outcomes, women reported on their contraceptive counselling throughout the antenatal, perinatal and immediate postpartum continuum. Women reported whether they had received counselling on each of the following contraceptive methods at any point throughout the perinatal period: female or male sterilization, injectables, implants, oral contraceptives, condoms, emergency contraceptives, diaphragm, cervical mucus observation, calendar-based methods, lactational amenorrhoea, withdrawal, or another method.
We calculated Outcome 1 as a binary variable (received counselling at any time on any method other than or in addition to the IUD = 0, received counselling on only the IUD and no other method = 1). Outcome 2 was a binary variable (received counselling on only one method = 0, received counselling on more than one method = 1). Outcome 3 was calculated as a binary variable [received counselling on methods from one duration group (long-acting, only short-acting, only medium-acting or only-permanent) = 0, received counselling on methods from two or more durations groups = 1]. Outcome 4 was calculated as a binary variable (not receiving counselling on at least one method from each contraceptive attribute group = 0, receiving counselling on a method from each attribute group = 1) (Senderowicz, 2020). A more detailed explanation of the derivation of Outcomes 3 and 4 is presented in Appendix A. Finally, Outcome 5 was calculated as an ordinal count variable, which was the sum of the number of methods on which each woman received counselling.
Analytic approach
The outcomes of interest for this analysis are focused on the content of the contraceptive counselling received. In addition to affecting the content of counselling, however, we also expected the intervention to affect the proportion of women who receive counselling at all. As a result, except where otherwise noted, we ran all analyses among two samples: (1) the sample of all respondents; and (2) the subsample of respondents who reported receiving any counselling. We used difference-in-difference linear probability models to estimate the effect of the intervention on the outcomes of interest, controlling the time period as a fixed effect and the hospital as a random effect. Coefficients for binary outcomes (IUD only; counselled on >2 methods; counselled on >2 methods durations; and counselled on a broad mix of methods) can be interpreted as the percentage point increase or decrease in the probability of the outcome associated with the intervention. Coefficients for the models for which the outcome was number of methods counselled can be interpreted as the difference in the number of methods counselled on associated with the intervention. We use an intent-to-treat approach, classifying women who received any maternity services at hospitals where the intervention had taken place as exposed, and women who received services when the hospitals had not yet received the intervention as unexposed.
We present results for unadjusted and adjusted models. Adjusted models controlled for sociodemographic characteristics including women’s age, educational attainment, parity, marital status, religion and ‘fast track’ hospital service (a premium service at some hospitals that offers patients better amenities and a lower provider-to-patient ratio for a higher cost) as fixed effects. Results are presented with P-values associated with standard errors adjusted using cluster wild bootstrapping with Rademacher weights. This method is designed to correct for the inflation of precision associated with replications based on a small number of clusters. We present intraclass correlation coefficients (ICC) with associated standard errors from logistic models.
To assess sensitivity to model specification, we also conducted multilevel mixed-effects regression models on the same outcomes of interest. We present the detailed methods and results of these analyses in Appendix C.
Analytic sample
A total of 22 691 women who delivered during the study period (15 January 2016–15 January 2017) in five hospitals were screened for eligibility (Figure 2). Of these, 21 033 met the inclusion criteria. Of those eligible, 1031 women (4.9%) declined to participate. Among eligible women who agreed to participate, 371 (1.9%) were missing data on primary study variables and were dropped from analyses in the full analytic sample. A total of N = 19 631 women were included in the full analytic sample. In the full analytic sample, 9305 (47.4%) reported that they did not receive any contraceptive counselling at all during their antenatal and perinatal care and were dropped for analyses to create the counselled analytic sample. The counselled analytic sample included N = 10 078 women. For Group 1, n = 5771 (n = 1874 at Dodoma General Hospital in Dodoma; n = 1220 at Muhimbili National Hospital in Dar es Salaam and n = 2478 at Mbeya Zonal Referral Hospital in Mbeya), and for Group 2, n = 4555 (n = 3153 at Mt. Meru Hospital in Arusha and n = 1353 at Tumbi-Pwani Regional Referral Hospital in Kibaha). In the counselled analytic sample, 5198 women (51.6%) were exposed to the intervention, while the remaining 4880 constitute our control group. The trial was planned for 1 year and was stopped when the expected duration was complete. Sample size calculations were performed for primary study end-points but were not performed post hoc for the secondary outcomes employed in this analysis. Given that our analytic samples were quite large (full analytic sample = 19 631 and counselled analytic sample= 10 078 observations), we operated under the assumption that these sample sizes were pragmatic for the purposes of our secondary analysis.
Figure 2.
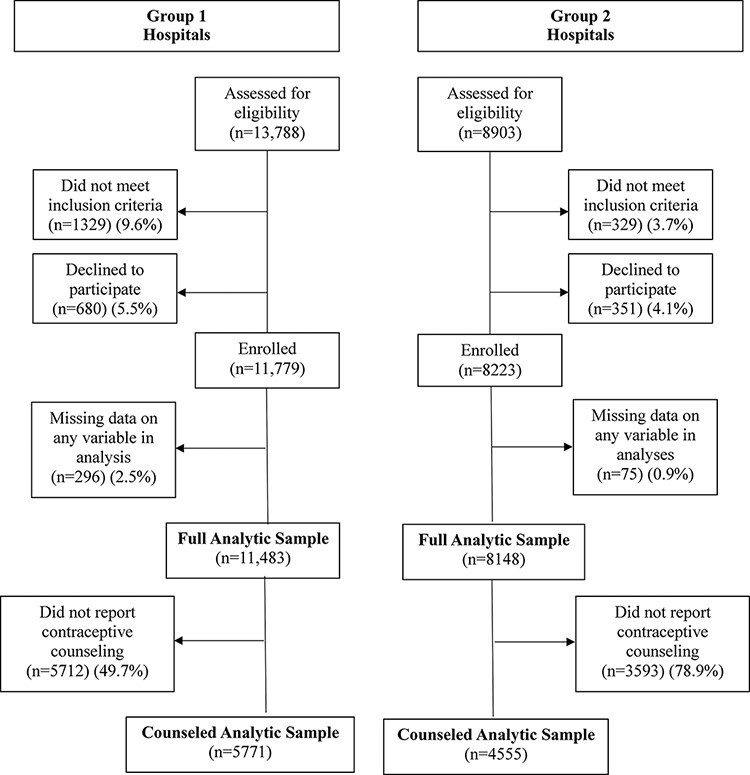
Participant inclusion
Table 1 describes the characteristics of study participants in the full analytic sample. Examining only women who reported ever receiving any contraceptive counselling perinatally, the intervention and control groups remained similar in terms of their sociodemographic characteristics and were not perfectly balanced. Those in the control group were, on average, 0.30 years younger, more likely to have at least a primary education, less likely to be married/cohabitating, more likely to be Catholic, Muslim or Protestant and less likely to be Evangelical Christian compared to their counterparts in the intervention group. There were no statistically significant differences between the respondents in Groups 1 and 2 (Pearson et al., 2020).
Table 1.
Characteristics of respondents by intervention status, all enrolled participantsa
| Control (n = 10 145) | Intervention (n = 9786) | |||||
|---|---|---|---|---|---|---|
| n | % | Missing | n | % | Missing | |
| Background characteristics (covariates) | ||||||
| Education | ||||||
| <Primary education | 89 | 0.89 | 10 | 118 | 1.23 | 4 |
| Primary education | 4798 | 47.86 | 10 | 5423 | 56.45 | 4 |
| Secondary education | 3704 | 36.95 | 10 | 2758 | 28.71 | 4 |
| >Secondary education | 1434 | 14.30 | 10 | 1307 | 13.61 | 4 |
| Religion | ||||||
| Catholic | 2337 | 23.31 | 14 | 1722 | 17.93 | 34 |
| Muslim | 2671 | 26.64 | 14 | 2200 | 22.90 | 34 |
| Protestant | 3152 | 31.44 | 14 | 2763 | 28.76 | 34 |
| Other Christian | 1862 | 18.57 | 14 | 2914 | 30.34 | 34 |
| Other religion | 3 | 0.03 | 14 | 7 | 0.07 | 34 |
| Fast track | 1320 | 13.17 | 31 | 943 | 9.82 | 10 |
| Index birth is first birth | 4429 | 44.18 | 0 | 4298 | 44.74 | 0 |
| Married or cohabitating | 9332 | 93.12 | 3 | 9171 | 95.53 | 6 |
| Mean | SD | Missing | Mean | SD | Missing | |
| Age | 26.65 | 5.88 | 0 | 26.8 | 6.28 | 0 |
| Parity | 2.15 | 1.41 | 0 | 2.28 | 1.65 | 0 |
| Outcome variables | ||||||
| n | % | Missing | n | % | Missing | |
| Ever counselleda | 4969 | 48.98% | 20 | 5357 | 54.74% | 51 |
| Counselled on | ||||||
| IUD only | 92 | 0.92% | 66 | 836 | 8.70% | 136 |
| ≥2 Methods | 4627 | 46.15% | 66 | 4107 | 42.75% | 136 |
| ≥2 Methods durations | 4576 | 45.65% | 5242 | 4020 | 41.85% | 4565 |
| Broad methods mix | 2898 | 28.91% | 0 | 1744 | 18.16% | 0 |
| Mean | SD | Mean | SD | Missing | ||
| Number of methods counselled on | 2.09 | 2.42 | 66 | 1.81 | 2.07 | 136 |
| Number of method durations counselled on | 1.36 | 1.47 | 5242 | 1.31 | 1.39 | 4565 |
Means and frequencies among subsample of women who reported receiving any counselling only reported in Appendix B.
Role of the funding source
The funder played no role in the study design; the collection, analysis or interpretation of data; the writing of the report; or the decision to submit for publication. All authors had full access to all the data in the study and accept the responsibility to submit for publication.
Results
Descriptive results
Table 1 shows means and percentages for control variables and key outcomes between the intervention and control group in the full analytic sample. Some differences in education, religion and hospital track were observed. Overall, a larger proportion of the intervention group received any contraceptive counselling (54.7% vs 49.0%). However, this appears to have been largely driven by counselling on IUD only (8.7% of participants who received any counselling in the intervention group vs 0.9% in the control group). A larger proportion of participants in the control group received counselling on multiple contraception methods (46.1% vs 42.8%), multiple contraceptive method durations (45.7% vs 41.9%) and a broad method mix (28.9% vs 18.2%). Among participants who received any counselling (Supplementary Appendix B Table 2), a greater proportion of the control group received counselling on multiple contraceptive methods (94.8% vs 79.0%), multiple methods durations (93.77% vs 77.34%) and a broad method mix (59.39% vs 33.55%). Including participants who did not receive any counselling at all, both groups received counselling on approximately the same average number of methods (2.09 in the control group, 2.07 in the intervention group) and methods durations (1.36 vs 1.31) (Table 1). However, among only participants who received any contraceptive counselling, the control group received counselling on a greater number of methods (4.29 methods vs 3.35 methods) but approximated the same number of methods durations (2.8 durations vs 2.4 durations) (Supplementary Appendix B Table 2). In the counselled group, the two groups were counselled on approximately the same number of methods durations.
Figure 3 shows that a greater proportion of women in the intervention group received counselling on the IUD than in the control group (76.47% vs 61.07%; P < 0.0001), meeting the PPIUD Project’s aim of increasing counselling on PPIUD (Figure 1). However, women exposed to the PPIUD intervention had lower rates of counselling on every other method, including oral contraceptive pills (67.95% in the intervention group vs 88.59% in the control group; P < 0.0001), condoms (33.28% vs 59.73%; P < 0.0001), fertility awareness-based methods, (5.68% vs 30.02%; P < 0.0001), injectables (74.03% vs 90.45%; P < 0.0001) and implants (67.74% vs 88.59%; P < 0.0001).
Figure 3.
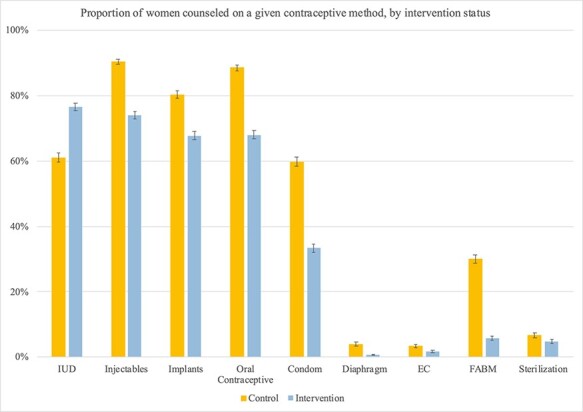
Proportion of women counselled on a given contraceptive method, by intervention status
Difference-in-difference estimates of the effect of the intervention on counselling
Table 2 shows the results from a series of difference-in-difference regression analyses of the effect of the intervention on contraceptive counselling and method mix among women who received contraceptive counselling, with wild cluster bootstrapped P-values. Among women who received any counselling, those exposed to the intervention saw a significant reduction in the number of methods on which they were counselled. Women in the control group reported receiving counselling on an average of 4.29 methods overall. In our unadjusted model, this number is reduced by 19% in the intervention group, to 3.48 methods. In our adjusted model, women’s choice set is reduced by 26% in the intervention group, to 3.17 methods.
Table 2.
Impact of intervention on study outcome variables in both samples, difference-in-difference models
| Unadjusted | Adjusteda | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full sample | Counselled sample | Full sample | Counselled sample | |||||||||
| Coef | 95% CI | P-valueb | Coef | 95% CI | P-valueb | Coef | 95% CI | P-valueb | Coef | 95% CI | P-valueb | |
| Counselled on | ||||||||||||
| IUD only | 0.06 | (0.03, 0.09) | <0.001 | 0.12 | (0.05, 0.20) | 0.002 | 0.07 | (0.02, 0.12) | 0.003 | 0.14 | (0.06, 0.22) | <0.001 |
| ≥2 methods | 0.00 | (−0.14, 0.14) | 0.999 | −0.16 | (−0.27, −0.05) | 0.004 | −0.10 | (−0.28, 0.08) | 0.267 | −0.16 | (−0.27, −0.06) | 0.002 |
| ≥2 methods durations | 0.00 | (−0.14, 0.13) | 0.947 | −0.16 | (−0.31, −0.01) | 0.037 | −0.11 | (−0.26, 0.05) | 0.175 | −0.17 | (−0.29, −0.05) | 0.007 |
| Broad mix of methods | −0.08 | (−0.36, 0.21) | 0.591 | −0.31 | (−0.69, 0.07) | 0.113 | −0.17 | (−0.40, 0.05) | 0.134 | −0.30 | (−0.70, 0.09) | 0.134 |
| Number of methods counselled on | −0.19 | (−1.15, 0.77) | 0.702 | −0.81 | (−1.96, 0.34) | 0.165 | −0.66 | (−1.52, 0.20) | 0.133 | −1.12 | (−2.34, 0.10) | 0.072 |
Adjusted models controlled for maternal marital/cohabiting status, parity, educational attainment, religion, hospital track and age as fixed effects.
P-values calculated using Wild Cluster Bootstrap method.
In the full sample, the intervention increased exclusive counselling on the IUD (and no other method) by 6% points in the unadjusted model, and seven in the adjusted model. Among women who received any counselling, the intervention increased exclusive counselling on the IUD (and no other method) by 12% points in the unadjusted model and 14 in the adjusted model (P < 0.001). In this sample, in the adjusted and unadjusted models, we observed a 16% point decrease in the probability of receiving counselling on multiple contraceptive methods associated with the intervention (P < 0.001). The intervention also decreased counselling on multiple methods durations in this sample by 16% points in the unadjusted model (P < 0.001) and 17% points in the adjusted model (P = 0.01). For the measure of broad contraceptive method mix, the size and the direction of the effect are consistent with other results (around a 30% point reduction associated with the intervention), however this relationship was not statistically significant at alpha level 0.05 (P = 0.11 and 0.13 in adjusted and unadjusted models, respectively). In the full sample, there were no differences between the control and intervention groups on receiving counselling on multiple methods, multiple methods durations or a broad mix of methods in unadjusted models. However, in adjusted models, in the full sample, the intervention was associated with a 16% point reduction in counselling on multiple methods, and a 17% point reduction on counselling on multiple methods durations.
Results from the mixed-effects models shown in Appendix C yielded similar results, with, for example, women exposed to the intervention having more than five times the odds of receiving counselling exclusively on the IUD and no other method compared to women in the control group (Supplementary Appendix C Table 3) among women who reported receiving any counselling. In the full sample, women exposed to the intervention having nearly five times the odds of receiving counselling exclusively on the IUD and no other method compared to women in the control group.
Discussion
Dedicated PPIUD programmes have repeatedly been demonstrated to increase PPIUD uptake, expanding access to this highly effective method at a crucial period in the reproductive life-course (Pleah et al., 2016; Karra et al., 2019; Pradhan et al., 2019; Pearson et al., 2020). In this study, we explore the effects of a targeted PPIUD intervention on a broader set of person-centred outcomes related to family planning and health. We find strong evidence that intervention resulted in increased postpartum counselling on the IUD but reduced counselling on all non-IUD related methods, and reduced the set of contraceptive methods from which women could choose. While the intervention increased contraceptive counselling overall, this increase was attributable nearly entirely to counselling on IUD only, as indicated by analyses performed among all women (including those who reported receiving no counselling) showing no differences in the number of methods or methods durations counselled on.
Our results indicate that the PPIUD intervention resulted in a stark reduction in access to information about a wide method mix in contraceptive counselling. Women exposed to the intervention experienced between an 18% and 26% reduction in the number of methods they were counselled on, and the proportion receiving counselling exclusively on the IUD (and no other methods) was more than nine times greater in the intervention group compared to the control. Women exposed to the intervention were also substantially less likely to receive counselling on multiple methods, multiple method durations, or a broad contraceptive mix with a variety of contraceptive attributes.
Strengths of this study include a large sample size and a rigorous cluster-randomized stepped-wedge design as part of a broader, mixed-methods approach (Hussey and Hughes, 2007). Our large and diverse sample across five geographic regions in Tanzania provides strong support for generalizability to the postpartum population of Tanzania with facility-based births. Results were robust to multiple model specifications and similar in adjusted and unadjusted models. The fact that qualitative in-depth interviews were conducted concurrently to the quantitative data collection allows us to draw on the strengths of both qualitative and quantitative methods. Here, we used qualitative results for hypothesis generation, complementarity, expansion and triangulation of findings (Greene et al., 1989).
The study also had several limitations. The dataset was not originally intended to focus on person-centred outcome or contraceptive decision-making and thus does not comprehensively measure all dimensions of contraceptive decision-making, including measures of access. Recall bias is a concern when relying on retrospective reporting via survey methods, and here it may be possible that respondents may be more likely to recall their counselling on the PPIUD than other methods. Data on the implementation of the intervention is only available at the cluster level, leaving us unable to confirm that individual respondents were exposed to intervention activities in their respective satellite clinics. There were some differences between the intervention and control groups on certain demographic variables; however, randomization ensures exchangeability of treatment and control groups, and any differences are therefore attributable to random chance. Given that randomization was performed by computer software at the cluster level, is extremely unlikely to have been altered, either systematically or otherwise, by these characteristics. Finally, while the small number of clusters (5) may have distorted estimates of precision, we were able to produce robust standard errors using cluster wild bootstrapping.
The PPIUD intervention was designed with the goal of increasing women’s access to IUDs in the immediate postpartum period, and these goals were largely met. PPIUD counselling increased in the intervention group by nearly 20% points, and insertion increased by 6% points (Pearson et al., 2020). Our analysis suggests that despite this success, the programme did not increase women’s access to comprehensive contraceptive care. Rather, while women in the intervention group were more likely to have been counselled overall, those who were counselled were less were less likely to have been counselled on any non-IUD methods, multiple methods or a broad mix of methods. This reflects an overall poorer quality of person-centred care in the intervention group. The design, implementation and monitoring of the PPIUD Project may explain these effects. The intervention began in 2016 as a collaboration between AGOTA and FIGO, with the goal of addressing unmet need for family planning in the postpartum period for women seeking antenatal care and having facility-based births. However, despite this broader-stated goal, programmatic goals and specific vertical activities were more narrowly tailored around promoting and monitoring the PPIUD introduction.
Findings from this analysis corroborate the findings from a qualitative analysis conducted among a subset of women exposed to this PPIUD intervention. Respondents reported being counselled that PPIUD was unequivocally the best contraceptive method, with no side effects or downsides (Senderowicz et al., 2021). Women also reported being counselled on the PPIUD exclusively, or on fewer methods than they had been counselled on in past pregnancies and births. Taken with the present findings, these data suggest that this intervention led to a reduction in the breadth and quality of contraceptive counselling.
These results underscore the importance of evaluating not only the intended goals of vertical global health programmes but also their unintended consequences as well. In order to avoid this type of unintended consequences in the future, it will be important that family planning programmes in particular be broadly conceived and implemented to explicitly focus on offering a wide range of contraceptive methods and informed choice. It is clearly important to continue innovating new contraceptive methods and implement strategies to increase access to these new methods to all who wish to use them. However, the introduction of new methods or programmatic strategies must be done with care, and consideration of the ways in which novel approaches complement and fit within existing programmatic strengths. This includes ensuring that women are given the same level of access to and information on existing methods of contraception as they are on new methods. It is also critical that monitoring and evaluation of family planning interventions reflect the overall goals of the programme by measuring and reporting on a range of outcomes related to full choice and broader contraceptive autonomy, rather than on statistics that may drive providers to encourage use of any given method.
Quantitative measurement, like any other form of knowledge production, is socially mediated and constructed, and as such, can hold and promote the tacit ideologies and biases of those who create and deploy it (Merry, 2011). The quantitative metrics used for programme evaluation often serve as goal posts and benchmarks for success, especially when delivered to providers in real time. Given this important feedback loop, it is critical that measurement and evaluation take a holistic view of health and rights, and a narrow focus on any given outcome should be avoided. It is as crucial to measure and monitor the potential unintended consequences of our programmes as it is to measure and monitor the ones we intend.
Supplementary Material
Acknowledgements
The authors are grateful to Russell Diamond at the Social Science Computing Collaborative at the University of Wisconsin-Madison for his support of this work. The authors are also grateful to our colleagues at FIGO and AGOTA for their support and collegiality throughout this evaluation.
Contributor Information
Leigh Senderowicz, Department of Gender and Women’s Studies, University of Wisconsin—Madison, 475 North Charter Street, Madison, WI 53706, USA; Department of Obstetrics and Gynecology, University of Wisconsin—Madison, 610 Walnut Street, Madison, WI 53726, USA; Department of Global Health and Population, Harvard T.H. Chan School of Public Health, 677 Huntington Ave., Boston, MA 02115, USA.
Natasha Sokol, Center for Alcohol and Addiction Studies, Department of Behavioral and Social Sciences, School of Public Health, Brown University, 121 South Main St., Providence, RI 02903, USA.
Erin Pearson, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, 677 Huntington Ave., Boston, MA 02115, USA; Department of Technical Excellence, Ipas, P.O. Box 9990, Chapel Hill, NC 27515, USA.
Joel Francis, Department of Family Medicine and Primary Care, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, 29 Princess of Wales Terrace, Parktown, Johannesburg 2193, South Africa; Management and Development for Health, P.O Box 79810. Plot #802, Mwai Kibaki Road, Mikocheni, Dar es Salaam, Tanzania.
Nzovu Ulenga, Management and Development for Health, P.O Box 79810. Plot #802, Mwai Kibaki Road, Mikocheni, Dar es Salaam, Tanzania.
Till Bärnighausen, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, 677 Huntington Ave., Boston, MA 02115, USA; Heidelberg Institute of Global Health (HIGH), University of Heidelberg, Im Neuenheimer Feld 130.3. Marsilius Arkaden—6. Stock, Heidelberg 69120, Germany.
Supplementary data
Supplementary data is available at Health Policy and Planning online.
Data availability
Due to the terms of our ethics approval, the individual participant data used in this analysis are not currently publicly available.
Funding
This study was funded by an anonymous donor. LS’ contribution was supported by the Ruth L. Kirschstein National Research Service Award (T32HD049302) and a Population Research Infrastructure grant (P2C HD047873). NAS’s contribution was supported by NIAAA T32 AA007459. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy National Institute of Child Health and Human Development, the National Institute on Alcohol Abuse and Alcoholism, or the National Institutes of Health. Funding bodies played no role in the study design, data analysis or preparation of this manuscript for publication.
Author’s contributions
L.S., E.P., J.F. and T.B. contributed to the conception and design of the study. L.S., E.P., J.F. and N.U. contributed to project administration and the collection/acquisition of data. L.S. and N.S. contributed to the data analysis, data interpretation, and manuscript writing. All authors reviewed and commented on the manuscript and approved the final version
Reflexivity statement
The authorship team for this study consists of three women and three men. Two of us are Tanzanian, one of us is German, and three of us are from the United States. Two of us are early career scholars, two of us are mid-career scholars, and two of us are senior scholars in leadership positions at our respective institutions. Our mix of training and research skills includes epidemiology, biostatistics, clinical medicine, gender analysis, and overall experience in global health.
Meeting presentations
Preliminary versions of this work have been presented to the Population Association of America and to the African Population Conference.
Clinical trial registration
This trial was registered at clinicaltrials.gov: NCT02718222.
Trial registration
The trial is registered with clinicaltrials.gov NCT02718222.
Ethical approval
The study received ethical approval from the National Institute of Medical Research (NIMR) in Tanzania (protocol number: NIMR/HQ/R.8a/Vol.IX/2006). The study received a human subjects exemption from the institutional review board at Harvard University (protocol number IRB15–1605)
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
References
- Barot S, Cohen S, Darroch J. et al. 2015. Sexual and reproductive health and rights indicators for the SDGs. https://www.guttmacher.org/pubs/SRHR-Indicators-Post-2015-Recommendations.pdf.
- Bashford A, Levine P. 2010. The Oxford Handbook of the History of Eugenics. Oxford: Oxford University Press. [Google Scholar]
- Britton LE, Williams CR, Onyango D. et al. 2021. “When it comes to time of removal, nothing is straightforward”: a qualitative study of experiences with barriers to removal of long-acting reversible contraception in Western Kenya. Contraception: X 3: 100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W, Druce N, Bunting J. et al. 2014. Developing the “120 by 20” goal for the Global FP2020 Initiative. Studies in Family Planning 45: 73–84. [DOI] [PubMed] [Google Scholar]
- Cahill N, Sonneveldt E, Stover J. et al. 2018. Modern contraceptive use, unmet need, and demand satisfied among women of reproductive age who are married or in a union in the focus countries of the Family Planning 2020 initiative: a systematic analysis using the Family Planning Estimation Tool. The Lancet 391: 870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairncross S, Periès H, Cutts F. 1997. Vertical health programmes. The Lancet 349: S20–1. [Google Scholar]
- Canning D, Shah IH, Pearson E. et al. 2016. Institutionalizing postpartum intrauterine device (IUD) services in Sri Lanka, Tanzania, and Nepal: study protocol for a cluster-randomized stepped-wedge trial.. BMC Pregnancy and Childbirth 16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson S. 2016. “NWHN-SisterSong Joint Statement of Principles on LARCs.” National Women’s Health Network. https://www.nwhn.org/nwhn-joins-statement-principles-larcs/.
- Cleland J, Conde-Agudelo A, Peterson H. et al. 2012. Contraception and health. The Lancet 380: 149–56. [DOI] [PubMed] [Google Scholar]
- Cleland J, Shah IH, Benova L, Fresh A. 2015. Look at the level of unmet need for family planning in the postpartum period, its causes and program implications. International Perspectives on Sexual and Reproductive Health 41: 155–62. [DOI] [PubMed] [Google Scholar]
- Connelly MJ. 2008. Fatal Misconception: The Struggle to Control World Population. Cambridge: Belknap Press of Harvard University Press. [Google Scholar]
- de Caestecker L, Banks L, Bell E. et al. 2018. Planning and implementation of a FIGO postpartum intrauterine device initiative in six countries. International Journal of Gynecology & Obstetrics 143: 4–12. [DOI] [PubMed] [Google Scholar]
- Dehlendorf C, Henderson JT, Vittinghoff E. et al. 2018. Development of a patient-reported measure of the interpersonal quality of family planning care. Contraception 97: 34–40. [DOI] [PubMed] [Google Scholar]
- Festin MPR, Kiarie J, Solo J. et al. 2016. Moving towards the goals of FP2020—classifying contraceptives. Contraception 94: 289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JC, Caracelli VJ, Graham WF. 1989. Toward a conceptual framework for mixed-method evaluation designs. Educational Evaluation and Policy Analysis 11: 255–74. [Google Scholar]
- Hackett K, Huber-Krum S, Francis JM. et al. 2020. Evaluating the implementation of an intervention to improve postpartum contraception in Tanzania: a qualitative study of provider and client perspectives. Global Health: Science and Practice 8: 270–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JA, Kramer RD, Ryder KM. 2016. Provider bias in Long-Acting Reversible Contraception (LARC) promotion and removal: perceptions of young adult women. American Journal of Public Health 106: 1932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt K, Zavala I, Quintero X. et al. 2019. Development and validation of the client‐reported quality of contraceptive counseling scale to measure quality and fulfillment of rights in family planning programs. Studies in Family Planning 50: 137–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Krum S, Hackett K, Senderowicz L. et al. 2019. Women’s perspectives on postpartum intrauterine devices in Tanzania. Studies in Family Planning 50: 317–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey MA, Hughes JP. 2007. Design and analysis of stepped wedge cluster randomized trials. Contemporary Clinical Trials 28: 182–91. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Vermund SH. 2015. Trauma research—a field without a home base. Science Translational Medicine 7: 302ed11. [DOI] [PubMed] [Google Scholar]
- Karra M, Pearson E, Pradhan E. et al. 2019. The effect of a postpartum IUD intervention on counseling and choice: evidence from a cluster-randomized stepped-wedge trial in Sri Lanka. Trials 20: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavjee S, Farmer PE. 2012. Tuberculosis, drug resistance, and the history of modern medicine. New England Journal of Medicine 367: 931–6. [DOI] [PubMed] [Google Scholar]
- Lindegren Lou M, Kennedy CE, Bain-Brickley D. et al. 2012. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. The Cochrane Database of Systematic Reviews 9: CD010119. [DOI] [PubMed] [Google Scholar]
- Makins A, Arulkumaran S. 2018. Institutionalization of postpartum intrauterine devices. International Journal of Gynecology & Obstetrics 143: 1–3. [DOI] [PubMed] [Google Scholar]
- Merry SE. 2011. Measuring the world: indicators, human rights, and global governance. Current Anthropology 52: S83–95. [Google Scholar]
- Mogeni R, Mokua JA, Mwaliko E. et al. 2019. Predictors of contraceptive implant uptake in the immediate postpartum period: a cross-sectional study. The European Journal of Contraception & Reproductive Health Care 24: 438–43. [DOI] [PubMed] [Google Scholar]
- Morroni C, Glasier A. 2020. Increasing the use of effective postpartum contraception: urgent and possible. The Lancet Global Health 8: e316–7. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C, Eggerman M, Tomlinson M. 2014. How might global health master deadly sins and strive for greater virtues?. Global Health Action 7: 23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson E, Senderowicz L, Pradhan E. et al. 2020. Effect of a postpartum family planning intervention on postpartum intrauterine device counseling and choice: evidence from a cluster-randomized trial in Tanzania. BMC Women’s Health 20: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzer A, Mackenzie D, Blanchard H. et al. 2015. A facility birth can be the time to start family planning: postpartum intrauterine device experiences from six countries. International Journal of Gynecology & Obstetrics 130: S54–61. [DOI] [PubMed] [Google Scholar]
- Pleah T, Hyjazi Y, Austin S. et al. 2016. Increasing use of postpartum family planning and the postpartum IUD: early experiences in West and Central Africa. Global Health: Science and Practice 4: S140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan E, Canning D, Shah IH. et al. 2019. Integrating postpartum contraceptive counseling and IUD insertion services into maternity care in Nepal: results from stepped-wedge randomized controlled trial. Reproductive Health 16: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PSI . 2015. Enabling the healthy spacing and limiting of pregnancies: programmatic approaches to expand postpartum IUD access. https://www.psi.org/publication/enabling-the-healthy-spacing-of-pregnancy-programmatic-approaches-to-expand-postpartum-iud-ppiud-access/.
- Secura GM, Allsworth JE, Madden T. et al. 2010. The contraceptive CHOICE project: reducing barriers to long-acting reversible contraception. American Journal of Obstetrics and Gynecology 203: 115.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderowicz L. 2019. “I was obligated to accept”: a qualitative exploration of contraceptive coercion. Social Science & Medicine 239: 112531. [DOI] [PubMed] [Google Scholar]
- Senderowicz L. 2020. Contraceptive autonomy: conceptions and measurement of a novel family planning indicator. Studies in Family Planning 51: 161–76. [DOI] [PubMed] [Google Scholar]
- Senderowicz L, Kolenda A. 2022. “She told me no, that you cannot change”: understanding provider refusal to remove contraceptive implants. SSM—Qualitative Research in Health 2: 100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderowicz L, Pearson E, Hackett K. et al. 2021. “I haven’t heard much about other methods”: quality of care and person-centeredness in a program to promote the postpartum intrauterine device in Tanzania. BMJ Global Health 6: e005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speizer IS, Corroon M, Calhoun L. et al. 2014. Demand generation activities and modern contraceptive use in urban areas of four countries: a longitudinal evaluation. Global Health: Science and Practice 2: 410–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar A, Salcedo J. 2017. Optimizing maternal and neonatal outcomes with postpartum contraception: impact on breastfeeding and birth spacing. Maternal Health, Neonatology and Perinatology 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanback J, Steiner M, Dorflinger L. et al. 2015. WHO tiered-effectiveness counseling is rights-based family planning. Global Health: Science and Practice 3: 352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhinaraset M, Afulani PA, Diamond-Smith N. et al. 2018. Development of a person-centered family planning scale in India and Kenya. Studies in Family Planning 49: 237–5. [DOI] [PubMed] [Google Scholar]
- Suh S. 2019. Metrics of survival: post-abortion care and reproductive rights in Senegal. Medical Anthropology 38: 152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NT, Seuc A, Coulibaly A. et al. 2019. Post-partum family planning in Burkina Faso (Yam Daabo): a two group, multi-intervention, single-blinded, cluster-randomised controlled trial. The Lancet Global Health 7: e1109–17. [DOI] [PubMed] [Google Scholar]
- USAID . 2014. USAID family planning program timeline: before 1965 to the present.
- van de Ruit C. 2019. Unintended consequences of community health worker programs in South Africa. Qualitative Health Research 29: 1535–48. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2012. Programming Strategies for Postpartum Family Planning. Geneva. [Google Scholar]
- World Health Organization . 2021. WHO Guideline on Health Workforce Development, Attraction, Recruitment and Retention in Rural and Remote Areas. Geneva: World Health Organization. [PubMed] [Google Scholar]
- Yamin AE, Boulanger VM. 2013. Embedding sexual and reproductive health and rights in a transformational development framework: lessons learned from the MDG targets and indicators. Reproductive Health Matters 21: 74–85. [DOI] [PubMed] [Google Scholar]
- Yirgu R, Wood SN, Karp C. et al. 2020. “You better use the safer one… leave this one”: the role of health providers in women’s pursuit of their preferred family planning methods. BMC Women’s Health 20: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the terms of our ethics approval, the individual participant data used in this analysis are not currently publicly available.


