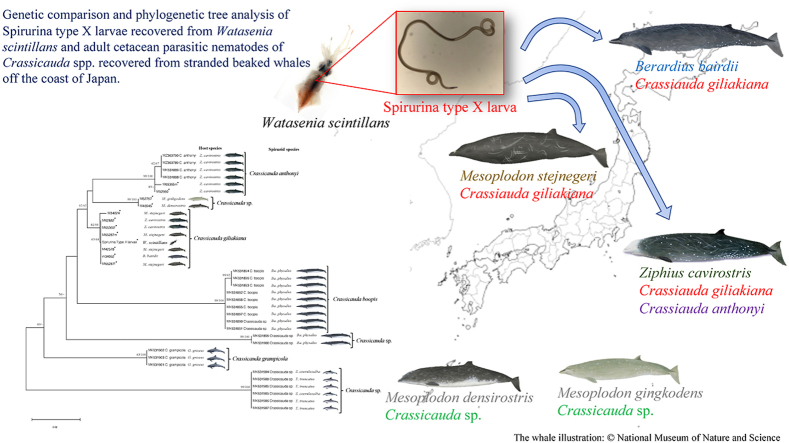
Abstract
The Spirurina type X larvae, which infect firefly squid (Watasenia scintillans), are known to cause cutaneous creeping eruption and intestinal obstruction in humans. Although it has been reported that the adult of this larva is Crassicauda giliakiana, which was recovered from a Baird's beaked whale (Berardius bairdii), it is not well known internationally. In this study, to reconfirm the identification of this species, we determined the mitochondrial cox1 gene and the partial sequence of 18S–28S ribosomal DNA from larvae recovered from firefly squid. As the results, we confirmed that the larvae were C. giliakiana, and partial ribosomal DNA sequences were also performed for phylogenetic analysis. Furthermore, to determine the distribution of the genus Crassicauda in Japan as a definitive host, DNA was extracted from archival specimens of adult worms recovered from the kidneys of family Ziphiidae that had stranded on the coast in Japan, and phylogenetic analysis using ITS2 region was conducted. As a result, C. giliakiana were detected from not only B. bairdii but also Mesoplodon stejnegeri, and Ziphius cavirostris, and C. anthonyi was also detected in Z. cavirostris. Furthermore, the kidney parasitic nematoda Crassicauda sp., which is not registered in the database, was found in both M. densirostris and M. gingkodens. This study provides new insights into the distribution and the lifecycle of genus Crassicauda in Japan based on the phylogenetic relationship between larvae and adults.
Graphical abstract
Highlights
-
•
Spirurina type X larvae parasitic on Watasenia scintillans were identified as Crasicauda giliakiana parasitic on beaked whales.
-
•
A phylogenetic tree was constructed using cox1 and ITS2 sequences of adult Crasicauda spp. recovered from the beaked whales from the sea around Japan.
-
•
C. giliakiana was found in Berardius bairdii, Mesoplodon stejnegeri, Ziphius cavirostris, and C. anthonyi was also found in Z. cavirostris.
-
•
Crassicauda sp. was found in both M. densirostris and M. gingkodens.
1. Introduction
Fourteen species of the genus Crassicauda Leiper and Atkinson, 1914 have been described in the order Spiruria (Nematoda): C. anthonyi, C. bennetti, C. boopis, C. carbonelli, C. costata, C. crassicauda, C. delamureana, C. duguyi, C. fuelleborni, C. giliakiana, C. grampicola, C. magna, C. pacifica, C. tortilis (Jabbar et al., 2015; Marcer et al., 2019). However, C. duguyi and C. pacifica has been shown to be a synonym of C. magna and C. boopis, respectively (Lambertsen, 1985; Jabbar et al., 2015). They are known to infect different species of cetaceans, both odontocetes and mysticetes. Each Crassicauda is a tissue-specific parasite of the kidneys, genitalia, intracranial sinuses and subcutaneous tissue, causing pathogenicity in each of these tissues. Along with C. boopis in fin whale, C. giliakiana in Baird's beaked whale (Berardius bairdii) and beluga (Delphinapterus leucas), and C. anthonyi in Cuvier's beaked whale (Ziphius cavirostris) infect the kidneys, they shed their eggs in the urine, especially with severe and widespread chronic renal granulomatous and fibrotic interstitial nephritis with kidney atrophy and loss due to nematode infection (Marcer et al., 2019; Burek-Huntington et al., 2015; Febronio et al., 2021; Jerdy et al., 2022).
In Japan, Spirurina type X larvae of parasitic nematoda is known to cause cutaneous creeping eruption and intestinal obstruction in humans (Makino et al., 2014; Miyake et al., 2004). The main source of infection is firefly squids (Watasenia scintillans), caused by parasitic larval migration (Ando et al., 1992). Although the final host of the parasite had been unknown, it was found to match the mitochondrial cox1 sequence of adult C. giliakiana collected from the B. bairdii and, Spirurina type X larvae was finally confirmed to be C. giliakiana (Sugiyama et al., 2007). However, little is known about the route of transmission from firefly squids to B. bairdii or the whole life cycle of C. giliakiana. Unfortunately, no sequence information or data on phylogenetic analysis of C. giliakiana was presented in that report.
In this study, we confirmed the DNA sequence of larvae recovered from the firefly squids and followed up on the sequence being identical to that of C. giliakiana. Furthermore, based on some reports that the ribosomal DNA of ITS2 region in nuclear DNA is useful for phylogenetic analysis of the genus Crassicauda (Marcer et al., 2019; Febronio et al., 2021), we determined sequenced partial regions from 18S to 28S ribosomal DNA of C.giliakiana. Furthermore, we obtained adult Crassicauda spp. from the archival collection of the National Museum of Nature and Science (NMNS), which was recovered from beaked whales that had stranded on the coast in Japan. DNA was extracted from these nematodes and phylogenetic analysis including species identification was conducted. In particular, detailed analysis of adult worms of Crassicauda spp. recovered from the family Ziphiidae other than B. bairdii, including Z. cavirostris, Mesoplodon densirostris, M. gingkodens, and M. stejnegeri, was performed. These results provide new insights into the distribution and hosts of genus Crassicauda.
2. Materials and methods
2.1. Epidemiology and biologic data of investigated animals
Spirulina X type larvae were collected by dissolving the pooled internal organs of more than 1800 Watasenia scintillans collected in Toyama Prefecture by commercial fishing in an artificial digestive solution. All collected larvae were stored in 70% ethanol.
Adults of the genus Crassicauda with 70% ethanol fixation stored at NMNS were used in this study (14 samples; 2005–2021). The host whales belonged to the family Ziphiidae (Berardius bairdii, Mesoplodon densirostris, M. gingkodens, M. stejnegeri, and Ziphius cavirostris). The host whales were either dead individuals or individuals that had been stranded alive but were subsequently confirmed dead. The information of the host whales is listed in Table 1 and their stranding location are shown in Fig. 1. The beaked whales were autopsied under the approval of land administrators or local governments. At the necropsy, the kidneys were split after the gross observation and the nematodes were excised from the surface of the sliced kidneys. All the adult Crassicauda samples were preserved in ethanol. The archival specimens of adult worms are referred to the same number of its host afterward in this study. Most of them were decomposed due to the post-mortem changes and had been torn off somewhere in their long bodies or fragmented. Therefore, it was difficult to make morphological observations, and only analysis by DNA extraction was performed. For worms with identifiable tail structures, DNA extraction was performed by each sex to confirm the combination of male-female validity.
Table 1.
List of the stranded beaked whale hosts which adult specimens of Crassicauda nematode were analyzed in this study.
| Museum No. | species | sex | body length *1 | found date *2 | stage of decomposition *3 | locality | infected site | number of analyzed worm |
|---|---|---|---|---|---|---|---|---|
| M34024 | Mesoplodon stejnegeri | female | 487.5 | 2005/5/2 | fresh | Murakami city, Niigata prefecture | kidney | 1 |
| M34052 | Berardius bairdii | male | 1040 | 2005/7/27 | fresh | Yokosuka city, Kanagawa prefecture | kidney | 1 |
| M42578 | Mesoplodon stejnegeri | male | 466 | 2014/3/25 | fresh | Sakaiminato city, Tottori prefecture | kidney | 1 |
| M46945 | Mesoplodon densirostris | male | 377 | 2015/7/26 | advanced | Nichinan-shi, Miyazaki prefecture | kidney | 1 |
| M52660 | Ziphius cavirostris | female | 616.4 | 2016/7/18 | advanced | Kitaibaraki city, Ibaraki prefecture | kidney | 1 |
| M62797 | Mesoplodon gingkodens | male | 480 | 2019/5/30 | fresh | Tateyama city, Chiba prefecture | kidney | 1 |
| M62881 | Ziphius cavirostris | female | 561.4 | 2019/9/18 | moderate | Shizuoka city, Shizuoka prefecture | kidney | 1 |
| M62882 | Ziphius cavirostris | female | 565 | 2019/9/17 | advanced | Shizuoka city, Shizuoka prefecture | kidney | 1 |
| M65287 | Mesoplodon stejnegeri | male | 514.4 | 2020/11/27 | fresh | Numadu city, Shizuoka prefecture | kidney | 2*** |
| M65290 | Berardius bairdii | female | 878.5 | 2020/12/3 | fresh | Rausu town, Hokkaido | kidney | 1 |
| M65363 | Mesoplodon stejnegeri | female | 476.3 | 2021/5/19 | fresh | Fukushima town, Hokkaido | kidney | 1 |
| M65365 | Ziphius cavirostris | female | 548.5 | 2021/5/31 | advanced | Atami city, Shizuoka prefecture | kidney | 2*4 |
*1 Units were centimeter unless otherwise stated.
*2 Year/Month/Day.
*3 The condition of carcasses was divided into three categories: fresh (no swelling and epidermis removal), moderate (begin to swelling), advanced (swelling to ruptured).
*4 Both male and female worms were analyzed.
Fig. 1.
Loca lity of the stranded beaked whales and firefly squids which the Crassicauda spp. recovered. The whale illustration: © National Museum of Nature and Science.
2.2. Morphologic analysis
Morphological observations of the 70% ethanol-fixed Spirurina type X larvae were taken using an iPhone 8 with an attachment (i-INTER LENS, Funakoshi, Tokyo, Japan) to a light microscope (BX43, Olympus, Tokyo, Japan).
2.3. Molecular analysis
DNA was extracted from Spirurina type X larvae recovered from firefly squid using a Get pure DNA Kit (DOJINDO, Kumamoto, Japan). PCR was performed with a set of primers (SSU18A; 5’ – AAAGATTAAGCCATGCATG - 3′, SSU26R; 5’ – CATTCTTGGCAAATGCTTTCG – 3′) (Floyd et al., 2002) that amplify the region of 18S ribosomal DNA, and with a set of primers (nematoda-18S–2F; 5’ – ACGGACGGGGGCATTCGTAT – 3′, nematoda-28S-2R; 5’ – CGGTACTTGTTTGCTATCGG - 3’) that amplify the regions of ITS1 - 5.8S - ITS2 - partial 28S ribosomal DNA. Cycle conditions by Taq polymerase (KOD-Dash, TOYOBO, Osaka, Japan) consisted of an initial 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 10 s, and 74 °C for 1 min, and a final extension at 74 °C for 2 min. The amplicons of PCR product after gel extraction were direct sequenced at BigDye™ Terminator v3.1 Cycle Sequencing Kit using PCR primers. Finally, the two sequences were combined into one as the partial 18S–28S ribosomal DNA sequence.
One section (1 cm) was cut from adult worms of Crassicauda spp. collected from the stranded whales for DNA extraction. DNA extraction was carried out using a simple heat-alkaline method (Kumagai et al., 2010). In brief, adult worms and larvae were placed in 1 ml of 50 mM NaOH and treated at 95 °C for 1 h. After centrifugation at 15,000 rpm for 5 min, 100 μL of the supernatant was collected. This solution was used as a direct template for PCR. PCR was carried out using adult DNA solutions with primers for the cox1 gene of mitochondrial DNA (cox-1-F (JB3); 5’ – TTTTTTGGGCATCCTGAGGTTTAT – 3′, cox-1-R (JB4.5); 5’ – TAAAGAAAGAACATAATGAAAATG – 3′) (Marcer et al., 2019) and primers for the ITS2 region of ribosomal DNA (crassicauda-5.8S–F 5’ – TACTCTTAGCGGTGGATCAC – 3′, crassicauda-28S-R; 5’ – AATCACGACTGAGCTGAGGT – 3’), respectively. Cycle conditions by PrimeSTAR® GXL DNA Polymerase (TAKARA, Shiga, Japan) consisted of an initial 98 °C for 2 min, followed by 35 cycles of 98 °C for 10 s, 55 °C for 15 s, and 68 °C for 1 min. The amplicons of PCR product after gel extraction were direct sequenced as same protocol. As the ITS2 region of only C. giliakiana was difficult to sequence due to its repetitive sequences, the PCR products were inserted into the pGEM-T vector (Promega, Madison, WI) for cloning. The most frequent sequence from four or more colonies was used as the ITS2 sequence of each sample.
2.4. Phylogenic analysis
The phylogenetic analysis of the genus Crassicauda was based on previous studies and was generated using sequences from the cox1 and ITS2 regions (Marcer et al., 2019; Febronio et al., 2021). In particular, the orthologue sequences of cox1 (C. giliakiana: LC057235-46, LC597810-1; C. boopis: MK621823-31; C. anthonyi: MK621821-2; C. magna: MZ222136; C. grampicola: MK621834-36; Crassicauda sp.: MK621823–4, MK621837-9; Habronema muscae: KJ819944) and ITS2 (C. anthonyi: MK631888-89, MZ363789-90; C. boopis: MK631892-8; C. grampicola: MK631901-3; Crassicauda sp.: MK631890–1, MK631899–900, MK631904-9) sequences were obtained from GenBank, respectively. The multiple alignments were performed with MAFFT, using the G–INS–i iterative refinement method (Katoh and Standley, 2013). Phylogenetic analysis was conducted by neighbor joining (NJ) following the K2P model and pairwise deletion, with 1000 bootstrap replicates inferred for each region using MEGA11 software. Moreover, the same data was computed according to the maximum likelihood (ML) method and the Bayesian inference (BI) method. Both methods used the Hasegawa-Kishino-Yano nucleotide substitution model with a gamma rate, which was selected according to the Bayesian Information Criterion (BIC) by ModelTest-NG (Darriba et al., 2020). ML was performed using MEGA11 software with 1000 bootstrap replicates and selecting complete deletion for the gaps/missing data treatment. After the alignment adjusted by TrimAl (Capella-Gutiérrez et al., 2009), the BI method was performed with MrBayes 3.2.7a (Ronquist et al., 2012) with posterior probability (PP) values calculated by four simultaneously running Markov chains using 2,000,000 (cox1) or 1,200,000 (ITS2) generations, respectively. Trees were sampled every 1000th generation, and an average standard deviation of split frequencies <0.01 was used as an indication that convergence had been achieved. The first 500 (cox1) or 300 (ITS2) trees for each run were discarded, respectively, and the consensus tree and PP were computed from the remaining trees. The obtained trees were edited by FigTree 1.4.4 (available at http://tree.bio.ed.ac.uk/software/figtree/). Pairwise genetic distances were calculated using the Tamura 3-parameter model implemented in MEGA11.
3. Results
3.1. Morphological and sequence analysis of Spirurina type X larvae recovered from firefly squid
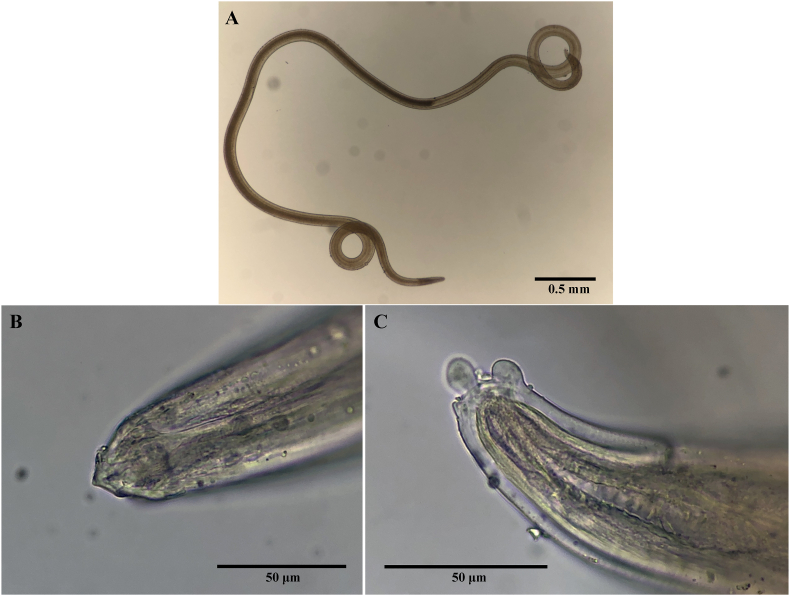
The nematode larvae recovered by artificial digestion of firefly squid were of the Spirurina type X, which has the same morphology as those previously reported (Hasegawa, 1978; Ando et al., 1992). The infection rate in firefly squid was approximately 0.5% (9/1800). In particular, the larvae were about 5 mm–10 mm long, with a protruding mouth tip and two spherical projections at the tail end (Fig. 2). DNA was extracted from the larvae, and partial region of the cox1 sequence of the mitochondrial DNA was sequenced (accession number: LC701532). The pairwise genetic distances indicate that C. giliakiana, including Spirurina type X, shows intraspecific variation (p-distances ranging between 0% and 1.9%) but was clearly a distinct species from C. anthonyi and other Crassicauda spp. (p-distances ranging between 6.4% and 11.7%) (Table S1). This confirms that the Spirurina type X larvae parasitizing the firefly squid are C.giliakiana, as in previous reports (Sugiyama et al., 2007). Next, to determine the sequence of 18S–28S ribosomal DNA in the nuclear DNA, sequencing was determined using common primers that amplify 18S ribosomal DNA. After that, the regions containing ITS1, 5.8S, and ITS2 were determined by direct sequencing by designing novel primers from sequences common to parasitic nematodes in 28S ribosomal DNA and performing PCR with some of the determined 18S ribosomal DNA sequences. In particular, the ITS2 region of C. giliakiana showed repeated A and T sequences and was difficult to sequence directly, so the major nucleotide sequences were confirmed by bi-directional sequencing analysis (accession number: LC700444). Finally, the sequencing analysis of the ITS2 region of C. giliakiana confirmed that the sequence is different from other nematodes of the genus Crassicauda that have been reported (Fig. S1).
Fig. 2.
Typical Spirurina type X larva. (A) whole body, (B) head, and (C) tail.
3.2. Analysis of adult Crassicauda spp. recovered from stranded beaked whales off the coast of Japan
3.2.1. Sequence analysis of adult worms with cox1 gene
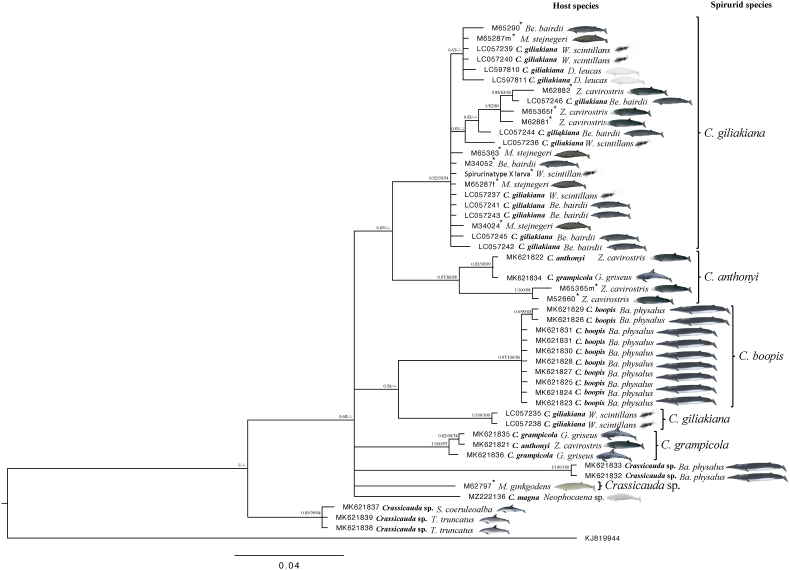
To investigate the distribution of the genus Crassicauda around Japan, adults were recovered from the kidneys of stranded beaked whales (family Ziphiidae) and sequence analysis was carried out from the DNA of each adult worm (Table 2). The phylogenetic trees produced by the three models, NJ, ML, and BI, had nearly identical structures but different bootstrap values and PP (Fig. 3). Phylogenetic analysis using the mitochondrial cox1 gene showed that adults recovered from Baird's beaked whale (Berardius bairdii), Stejneger's beaked whale (Mesoplodon stejnegeri) and Cuvier's beaked whale (Ziphius cavirostris), and larvae recovered from firefly squid (Watasenia scintillans) formed the same cluster within the C. giliakiana group as all registered GenBank sequences (Fig. 3). The C. giliakiana group had a low bootstrap value in the ML model (54%), but a high value in the NJ model (93%), and BI method (0.92) which we considered appropriate for analysis in this gene. Therefore, these results indicate that cox1 is useful for species identification of C. giliakiana using BI method. On the other hand, In addition, the species of C. giliakiana (LC057235, LC057238) from the firefly squid in the existing database formed a distinct cluster. It was considered highly likely that this was a separate species. In addition, MK621821 and MK621834 in the database are consistent with our and previous phylogenetic analyses (Marcer et al., 2019), but the species name and registered sequence may be reversed.
Table 2.
List of Crassicauda nematoda (adult) recovered from stranded whales in this study.
| nematoda ID | sex | identified species name | host species | cox1: Gene ID | ITS2: Gene ID |
|---|---|---|---|---|---|
| M34024 | – | Crassicauda giliakiana | Mesoplodon stejnegeri | LC700442 | LC700447 |
| M34052 | – | Crassicauda giliakiana | Berardius bairdii | LC700443 | LC700448 |
| M42578 | – | Crassicauda giliakiana | Mesoplodon stejnegeri | – | LC700449 |
| M46945 | – | Crassicauda sp. | Mesoplodon densirostris | – | LC700450 |
| M52660 | – | Crassicauda anthonyi | Ziphius cavirostris | LC700440 | LC700451 |
| M62797 | – | Crassicauda sp. | Mesoplodon gingkodens | LC700439 | LC700452 |
| M62881 | – | Crassicauda giliakiana | Ziphius cavirostris | LC700438 | – |
| M62882 | – | Crassicauda giliakiana | Ziphius cavirostris | LC700437 | LC700453 |
| M65287m | male | Crassicauda giliakiana | Mesoplodon stejnegeri | LC700435 | LC700446 |
| M65287f | female | Crassicauda giliakiana | Mesoplodon stejnegeri | LC700436 | LC700447 |
| M65290 | – | Crassicauda giliakiana | Berardius bairdii | LC700434 | – |
| M65363 | – | Crassicauda giliakiana | Mesoplodon stejnegeri | LC700433 | – |
| M65365m | male | Crassicauda anthonyi | Ziphius cavirostris | LC700431 | LC700456 |
| M65365f | female | Crassicauda giliakiana | Ziphius cavirostris | LC700432 | LC700455 |
Fig. 3.
Phylogenetic analysis identified the specimens stored at the National Museum of Nature and Science (12 samples; 2005–2021), based on the cox1 gene, and supported by Bayesian inference (BI) tree. Analysis was performed by MrBayes 3.2.7a for BI and MEGA11 using both the neighbor joining (NJ) amd the maximum likelihood method (ML) (1000 bootstrap replicates) and included Habronema muscae as outgroup. GenBank accession numbers are listed along the species names. Branches with posterior probability and bootstrap values (BI/NJ/ML) support lower than 0.5 or 50% were collapsed, respectively. *Sequences obtained in this study. The whale illustration: © National Museum of Nature and Science.
3.2.2. Phylogenic analysis of adult worms with ITS2 region
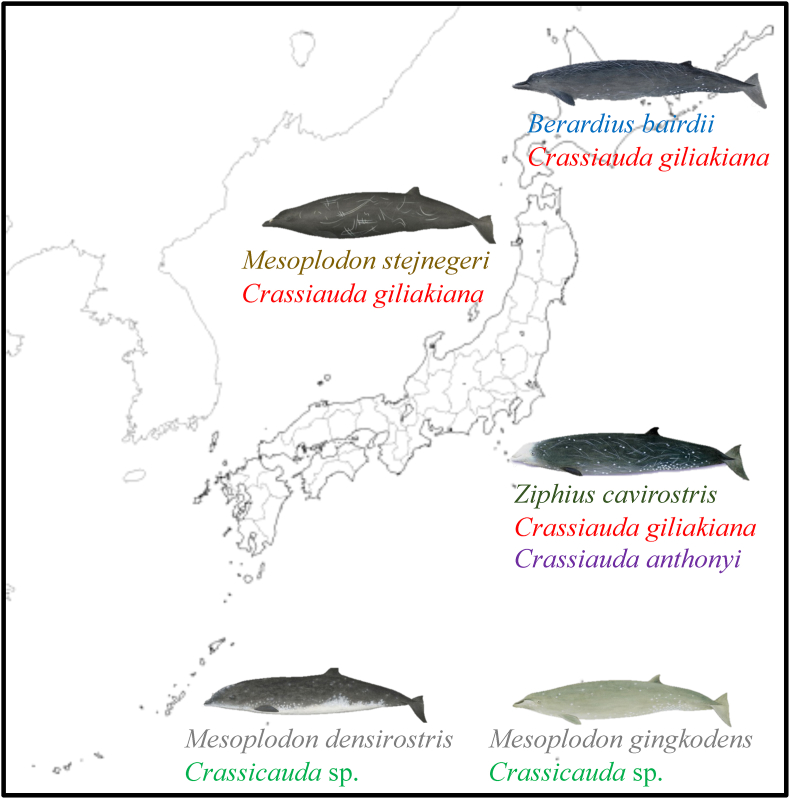
In previous studies, it has been reported that the ITS2 region of ribosomal DNA is more appropriate for phylogenetic tree construction than the cox1 gene. In this study, we constructed a phylogenetic tree using the partial ITS2 region of the obtained samples (Table 2, Fig. S2). As a result, a phylogenetic tree was similar to the analysis using cox1 (Fig. 4). However, the bootstrap value using the ML method was higher than that using cox1, and a more accurate phylogenetic tree was produced. Furthermore, the almost identical values of PP by BI also support this result. As expected, larvae recovered from firefly squid formed the same cluster as adult C. giliakiana recovered from B. bairdii, M. stejnegeri, and Z. cavirostris. C. giliakiana around Japan is abundant not only in Baird's beaked whales but also in other beaked whales, suggesting that this species is commonly distributed in the sea around Japan (Fig. 5). On the other hand, two adult worms (M52660, M65365m) from only Z. cavirostris were identified to be C. anthonyi. The adult worms recovered from M. densirostris (M46945) and M. gingkodens (M62797) formed a separate cluster close to C. giliakiana and C. anthonyi, which have not been registered in the database yet, although all species parasitize in the kidney of the family Ziphiidae.
Fig. 4.
Phylogenetic analysis identified the specimens stored at the National Museum of Nature and Science (9 samples; 2005–2021), based on the ribosomal DNA ITS2 region, and supported by Bayesian inference (BI) tree. Analysis was performed by MrBayes 3.2.7a for BI and MEGA11 using both the neighbor joining (NJ) amd the maximum likelihood method (ML) (1000 bootstrap replicates) and the tree was rooted on midpoint. The sample ID sequenced in this study are listed with the species name. Branches with posterior probability and bootstrap values (BI/NJ/ML) support lower than 0.5 or 50% were collapsed, respectively. *Sequences obtained in this study. The whale illustration: © National Museum of Nature and Science.
Fig. 5.
Relationship between whale habitat and infected Crassicauda spp. The whale illustration: © National Museum of Nature and Science.
4. Discussion
In Japan, the Spirurina type X larvae that infect humans from firefly squid (Watasenia scintillans) have been well studied, and there are many reports on the morphology, other characteristics, and the their host of the larvae (Hasegawa, 1978; Ando et al., 1992; Hasegawa, 1993; Akao, 1994; Otaki et al., 1995; Miyake et al., 2004; Makino et al., 2014). In 2007, the species of this parasitic larva was confirmed by a report that the adult larva is Crassicauda giliakiana, a parasite of the kidney of the Baird's beaked whale (Berardius bairdii) (Sugiyama et al., 2007). However, because this was a short Japanese report without detailed data, this report is not well known internationally and is not well understood by cetacean parasite researchers in other countries. In this study, DNA was extracted from larvae recovered from firefly squid, and homology analysis using the mitochondrial cox1 gene was performed again to reconfirm the species. We also sequenced the ribosomal DNA for further phylogenetic analysis.
The morphology of the larvae was identical to that already reported, with the characteristic two spherical projections at the tail end (Hasegawa, 1978; Ando et al., 1992). In a previous report on C. boopis, the morphology of Crassicauda larvae detected in the intestinal lumen of a newborn was larger than the present larvae and did not show the characteristic small projection on the tail (Marcer et al., 2019). We speculated that this was because the reported larvae of C. boopis had already developed after molting. Indeed, the outermost skin membrane of the larvae detected in the firefly squid was sheath-like (Fig. 2), suggesting that the spherical projections at the tail may also be lost during molting in the digestive tract of the definitive host. Therefore, the larvae detected in the firefly squid are infected larvae (L3) and is in the developmental stage of infection from intermediate or paratenic hosts to the definitive host, the cetacean.
In this study, the Spirurina type X larva in firefly squid was identical to the cox1 sequence of C. giliakiana adults in B. bairdii and D. leucas in the database, which was followed up as a larval form of C. giliakiana. In addition, the partial sequence of ribosomal DNA from 18S to 28S, was also determined, and homology analysis confirmed 99% identity with the 18S ribosomal sequence of C. anthonyi. We thought this would make it difficult to perform phylogenetic analysis using 18S ribosomal DNA, so we decided to use the ITS2 region for phylogenetic analysis, as has been done in previous reports (Marcer et al., 2019; Febronio et al., 2021). In fact, DNA was extracted from some Crassicauda adult worms recovered from the family Ziphiidae that had stranded on the coast around Japan, and the cox1 and ITS2 genes were used for phylogenetic analysis.
Phylogenetic analysis using the mitochondrial cox1 and ribosomal DNA ITS2 region showed that the adult samples collected from stranded whales were within the same cluster for both genes. C. giliakiana was detected not only in B. bairdii, but also in Z. cavirostris and M. stejnegeri, confirming that C. giliakiana group widely infects the family Ziphiidae. Although Crassicauda sp. has been reported from M. stejnegeri (Savage et al., 2021), this is the first time, to our knowledge, that it has been identified as C. giliakiana. C. giliakiana also infects D. leucas (Burek-Huntington et al., 2015), suggesting that C. giliakiana is distributed from northern Japan to the Bering Sea. On the other hand, C. anthonyi was detected only from Z. cavirostris. These results, together with other reports (Febronio et al., 2021; Jerdy et al., 2022), strongly suggest that C. anthonyi only infects to Z. cavirostris and is distributed worldwide. Interestingly, Crassicauda sp. was detected for the first time in both M. densirostris (M46945) and M. gingkodens (M62797). Since these adult worms were detected in the kidneys of odontoceti, it may be a separate species closely related to C. giliakiana and C. anthonyi. In particular, given the distribution range of this host, it was expected to infect family Ziphiidae in South Pacific Ocean (Nishiwaki, 1958)(Borsa, 2006; Yamada et al., 2012; Abecassis et al., 2015; Rosso et al., 2021). Phylogenetic analysis using the ITS2 region rather than the cox1 gene was found to give a higher bootstrap value using ML model. In fact, in previous reports, the bootstrap values for C. giliakiana in cox1 was 72% (Marcer et al., 2019). With the larger sample size this time around, that number was even weaker. This suggests that ML and BI method using ITS2 can show realistic phylogenetic tree.
C. giliakiana larvae were found parasitizing firefly squid, but are they really the source of infection of family Ziphiidae? There have been reports on the stomach contents of several stranded whales, but no mention of feeding on firefly squid can be found. Most of the cephalopods in the stomach contents are in the Gonatidae, Octopoteuthidae, Cranchiidae, and Vampyroteuthidae etc., and many fish belonging to Gadiformes have also been reported (West et al., 2017; Blanco and Raga, 2000; Santos et al., 2001; Walker et al., 2002). Since larvae of C. giliakiana are frequently infecting Theragra chalcogramma (Ando et al., 1992), it is highly likely that fish are contributing to the infection of odontocetes. We look forward to future reports of the detection of genus Crassicauda larvae in cephalopods in the deep sea and in fish.
In conclusion, the Spirurina type X detected in firefly squid was reconfirmed to be a larva of C. giliakiana. Phylogenetic analysis of the ribosomal DNA ITS2 region of adult Crassicauda spp. recovered from the kidneys of adult worms of the family Ziphiidae in the sea around Japan revealed C. giliakiana in B. bairdii, M. stejnegeri, and Z. cavirostris, and C. anthonyi was found in only Z. cavirostris. Furthermore, Crassicauda sp. which is not registered in the database was detected in both M. densirostris and M. gingkodens. The updated information on C. giliakiana larvae and adults will further develop the study of nematodes of the genus Crassicauda in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by JSPS KAKENHI Grant Number 22K19645. We would like to thank the people, institutes, and organizations which cooperated to investigate the stranded cetacean carcasses: 6DORSALS KAYAK SERVICES; Biology, Faculty of Education, University of Miyazaki; Fuji Stranding Network; Mr. Hajime Ishikawa; Prof. Hiroshi Ohizumi and Dr. Yayoi Yoshida (Tokai University); Ibaraki Prefectural Oarai Aquarium; Izu Mito Sea Paradise; Ms. Junko Ogasawara; Kanagawa Prefectural Museum of Natural History; Dr. Kazuo Nagasawa (Yamagata Prefectural Museum); Dr. Kei Ichisawa (Tottori Prefectural Museum); Mr. Kosuke Hayashi; Miyazaki cetacean study group; Natural History Museum and Institute; Chiba Scuba Diving Club Manatees; Stranding Network Hokkaido; Yokohama Hakkeijima Sea Paradise.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2023.01.001.
Contributor Information
Takashi Kumagai, Email: tkuma.vip@tmd.ac.jp.
Akira Shiozaki, Email: a.shiozaki@kahaku.go.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- Abecassis M., Polovina J., Baird R.W., Copeland A., Drazen J.C., Domokos R., Oleson E., Jia Y., Schorr G.S., Webster D.L., Andrews R.D. Characterizing a foraging hotspot for short-finned pilot whales and blainville's beaked whales located off the west side of Hawai‘i Island by using tagging and oceanographic data. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao N. A parasitological survey of the spiruroid type-X larvae in firefly squids captured in the costal waters of the Hokuriku District. Hokuriku J. Public. Heal. 1994;21:56–59. [Google Scholar]
- Ando K., Sato Y., Miura K., Chinzei Y., Ogawa S. Further observation on the larva of the suborder spirurina suspected as the causative agent of creeping eruption. Jpn. J. Parasitol. 1992;41:p384–p389. [Google Scholar]
- Blanco C., Raga J.A. Cephalopod prey of two Ziphius cavirostris (Cetacea) stranded on the western Mediterranean coast. J. Mar. Biol. Assoc. U. K. 2000;80:381–382. doi: 10.1017/S0025315499002064. [DOI] [Google Scholar]
- Borsa P. Marine mammal strandings in the New Caledonia region, Southwest Pacific. C. R. Biol. 2006;329:277–288. doi: 10.1016/j.crvi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Burek-Huntington K.A., Dushane J.L., Goertz C.E.C., Measures L.N., Romero C.H., Raverty S.A. Morbidity and mortality in stranded Cook Inlet beluga whales Delphinapterus leucas. Dis. Aquat. Org. 2015;114:45–60. doi: 10.3354/dao02839. [DOI] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Posada D., Kozlov A.M., Stamatakis A., Morel B., Flouri T. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020;37:291–294. doi: 10.1093/molbev/msz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febronio A.M.B., Boos G.S., Batista R.L.G., Amorim D.B., Guimarães J.P., Bianchi M.V., Mariani D.B., Koproski L., Mari C., Parente J.E.V., Sonne L., Werneck M.R., Marques S.M.T., Driemeier D., Kolesnikovas C.K.M., Groch Karina R., Sobotyk C., Verocai G.G., Groch Kátia R., Díaz-Delgado J. Crassicaudiasis in three geographically and chronologically distant Cuvier's beaked whales (Ziphius cavirostris) stranded off Brazil. Int. J. Parasitol. Parasites Wildl. 2021;16:262–269. doi: 10.1016/j.ijppaw.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Abebe E., Papert A., Blaxter M. Molecular barcodes for soil nematode identification. Mol. Ecol. 2002;11:839–850. doi: 10.1046/j.1365-294X.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa H. Larval nematodes of the superfamily Spiruroidea : a description, identification and examination of their pathogenicity. Acta Med. Biol. 1978;26:79–116. [Google Scholar]
- Hasegawa H. A case of cutaneous larva migrans due to spirurin nematode found in Niigata Prefecture, Japan, with special references to the significance of cuticular morphology on identification. Jpn. J. Parasitol. 1993;42:12–17. [Google Scholar]
- Jabbar A., Beveridge I., Bryant M.S. Morphological and molecular observations on the status of Crassicauda magna, a parasite of the subcutaneous tissues of the pygmy sperm whale, with a re-evaluation of the systematic relationships of the genus Crassicauda. Parasitol. Res. 2015;114:835–841. doi: 10.1007/s00436-014-4245-6. [DOI] [PubMed] [Google Scholar]
- Jerdy H., Werneck M., Barbosa L., Hauser-Davis R.A., De-Oliveira-Nogueira C.H., da Silveira L.S. First report on Phyllobothrium delphini infection and Crassicauda sp. parasitism resulting in osseous metaplasia in a Cuvier's beaked whale (Ziphius cavirostris) from the Brazilian region. Int. J. Parasitol. Parasites Wildl. 2022;17:60–64. doi: 10.1016/j.ijppaw.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai T., Furushima-Shimogawara R., Ohmae H., Wang T.P., Lu S., Chen R., Wen L., Ohta N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am. J. Trop. Med. Hyg. 2010;83:542–548. doi: 10.4269/ajtmh.2010.10-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen R.H. Taxonomy and distribution of a Crassicauda species (Nematoda: spirurida) infecting the kidney of the common fin whale (Balaenoptera physalus Linné, 1758) J. Parasitol. 1985;71:485–488. [PubMed] [Google Scholar]
- Makino T., Mori N., Sugiyama H., Mizawa M., Seki Y., Kagoyama K., Shimizu T. Creeping eruption due to Spirurina type X larva. Lancet. 2014;384:2082. doi: 10.1016/S0140-6736(14)61644-5. [DOI] [PubMed] [Google Scholar]
- Marcer F., Negrisolo E., Franzo G., Tessarin C., Pietrobelli M., Marchiori E. Morphological and molecular characterization of adults and larvae of Crassicauda spp. (Nematoda: spirurida) from Mediterranean fin whales Balaenoptera physalus (Linnaeus, 1758) Int. J. Parasitol. Parasites Wildl. 2019;9:258–265. doi: 10.1016/j.ijppaw.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Ikoma H., Hoshima M., Yamane E., Hasegawa H., Arizono N. Case of acute ileus caused by a spirurina larva. Pathol. Int. 2004;54:730–733. doi: 10.1111/j.1440-1827.2004.01687.x. [DOI] [PubMed] [Google Scholar]
- Nishiwaki M. A beaked whale Mesoplodon stranded at Oiso Beach, Japan. Sci Rep Whales Res Inst. 1958;13 Pl-1. [Google Scholar]
- Otaki N., Fukushima K., Takuno T., Ando K., Kagei N. Two cases of creeping diseases due to spirurina larva with a review of recorded cases. Jpn J. Clin. Dermatol. 1995;44:88–94. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso M., Lin M., Caruso F., Liu M., Dong L., Borroni A., Lin W., Tang X., Bocconcelli A., Li S. First live sighting of Deraniyagala's beaked whale (Mesoplodon hotaula) or ginkgo-toothed beaked whale (Mesoplodon ginkgodens) in the western Pacific (South China Sea) with preliminary data on coloration, natural markings, and surfacing patterns. Integr. Zool. 2021;16:451–461. doi: 10.1111/1749-4877.12507. [DOI] [PubMed] [Google Scholar]
- Santos M.B., Pierce G.J., Herman J., López A., Guerra A., Mente E., Clarke M.R. Feeding ecology of Cuvier's beaked whale (Ziphius cavirostris): a review with new information on the diet of this species. J. Mar. Biol. Assoc. U. K. 2001;81:687–694. doi: 10.1017/S0025315401004386. [DOI] [Google Scholar]
- Savage K.N., Burek-Huntington K., Wright S.K., Bryan A.L., Sheffield G., Webber M., Stimmelmayr R., Tuomi P., Delaney M.A., Walker W. Stejneger's beaked whale strandings in Alaska, 1995–2020. Mar. Mammalian Sci. 2021;37:843–869. doi: 10.1111/mms.12780. [DOI] [Google Scholar]
- Sugiyama H., Morishima Y., Arakawa K., Kishiro T., Kawanaka M. Recent advances in the studies on larval spirurin nematode. Jpn. Soc. Syst. Parasitol. Circular. 2007;25:4–7. [Google Scholar]
- Walker W., Mead J., Brownell R. Diets of baird's beaked whales, Berardius bairdii , in the southern sea of okhotsk and off the pacific coast of honshu. Japan. Mar. Mammal Sci. 2002;18:902–919. doi: 10.1111/j.1748-7692.2002.tb01081.x. [DOI] [Google Scholar]
- West K.L., Walker W., Baird R., Mead J.G., Collins P.W. Diet of Cuvier's beaked whales Ziphius cavirostris from the North Pacific and a comparison with their diet world-wide. Mar. Ecol. Prog. Ser. 2017;574 doi: 10.3354/meps12214. L. [DOI] [Google Scholar]
- Yamada T.K., Tajima Y., Yatabe A., Kitamura S., Isobe T., Brownell R.L., Jr. 2012. Summary of Current Knowledge on Mesoplodon stejnegeri Mainly from the Seas Around Japan and Review of Data from North America. Document Submitted to the Scientific Committee, the 64th IWC, Panama City. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.