Highlights
-
•
Treatment with immune checkpoint inhibitors can cause serious adverse events.
-
•
Myositis combined with myocarditis ad adverse event have a mortality for 50%.
-
•
Early diagnosis is important for better outcome.
-
•
Employing MRI STIR can rapidly diagnose, guide biopsy, and classify cases of immune-related myositis.
1. Introduction
Cancer therapy with immune checkpoint inhibitors (ICI) has improved the treatment efficacy of several cancer types and their indications continues to expand. A downside to treatment with ICIs is a wide spectrum of immune-related adverse events (irAEs) associated with a high morbidity and mortality [1]. One of the most dreaded irAEs with a mortality rate of 50% is myopathy combined with myocarditis [2]. Rapid diagnosis is mandatory for sufficient treatment of this irAE. However, the pathophysiology and response to treatment could differ depending on underlying immunological mechanisms. A possible method to aid in rapid diagnosis, guide biopsy, and perhaps classify cases of immune-related myositis (irMyositis) is by employing Magnetic resonance imaging (MRI) with Short Tau Inversion Recovery sequences (STIR).
2. Case report
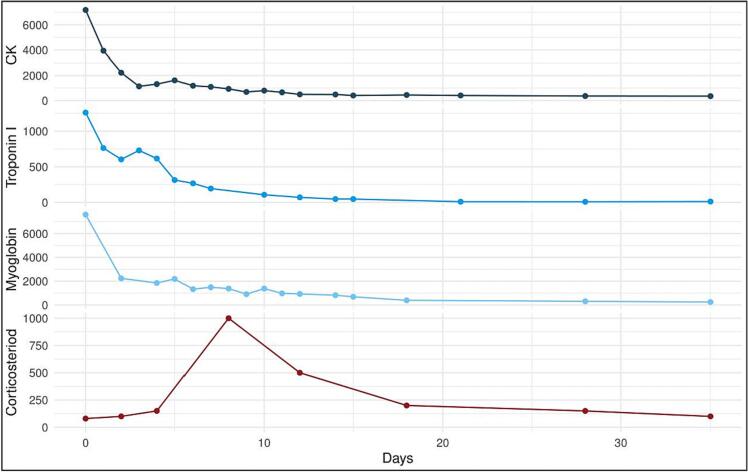
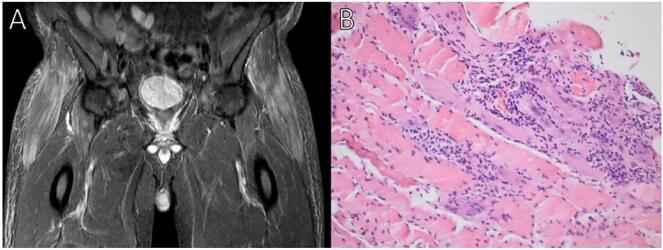
A 49-year-old man with hypertension was diagnosed with renal cell carcinoma (RCC) in 2020. After nephrectomy, the tumor metastasized to the lungs, brain, and lymph nodes. The patient was started on combined ICI treatment with ipilimumab and nivolumab. Two weeks after the second series the patient developed non-fluctuating weakness, myalgia, fatigue, and ptosis which lead to admission. The electrocardiogram showed a new right bundle branch block and laboratory tests revealed highly elevated creatinine phosphokinase (CPK, 7190 U/L; reference values 40–280 U/L), myoglobin (7590 μg/L; 24–77 μg/L), and Troponin I (1,260 ng/L; <45 ng/L). The patient did not have any cardiac complains, no arrythmias, and normal left ventricular ejection fraction throughout the admission. Cardiac MRI was not performed. He started treatment with 100 mg prednisolone and CPK declined. Despite this, the neurological symptoms worsened, with double vision, headdrop, and increasing difficulty swallowing and breathing. On clinical examination he had bilateral ptosis, ophthalmoplegia, a moderate bulbar paresis, and symmetrical proximal paresis of his limbs. There was no muscle fatigability present and Simpsons test was negative. He was treated with high dose methylprednisolone (1 g intravenous for 3 days) with a gradual taper over 4 months (Fig. 1). Mycophenolatemofetil 1 g twice daily was added after 24 days. MRI STIR of his hips and tights 15 days after admission showed diffuse edema in the gluteus minimus and tensor fasciae latae muscles on both sides as well as discrete edema in both distal iliopsoas muscles compatible with myositis (Fig. 2A). Electromyography (EMG) showed myopathtic motor unit potentials with spontaneous muscle fiber activity. Nerve conduction velocities were normal and no decrement was found on repetitive nerve stimulation Muscle stritional, acetylcholine receptor, muscle-specific kinase, anti-signal recogntion particle and anti 3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies were all negative. Eighteen days after steroids was begun a targeted muscle biopsy in tensor fasciae latae muscle showed findings compatible with immune-mediated necrotizing myopathy (IMNM) but with more lymphocytic infiltrates (CD3+ and CD8+) than is usually seen in IMNM (Fig. 2B). All muscle enzymes as well as almost all symptoms had normalized after 4 months and a follow up CT-scan of his thorax and abdomen 4 months later showed no cancer progression.
Fig. 1.
Timeline of blood samples showing the course from admission (day 0).
Fig. 2.
A. MRI STIR reveals featherlike oedema-type T2-hyperintensity enhancement of both tensor fascie latae muscles and distal psoas (mainly on the right side). B. Muscle biopsy revealed necrotic and regenerating muscle fibers. The numerous necrotic fibers appeared both scattered and in clusters. Lymphocyte infiltrates (CD3 and CD8 positive confirmed by immunohistochemistry staining) were mainly observed in relation to necrotic muscle fibers. There was upregulation of human leukocyte antigen class 1 in many fibers. The upregulation was patchy, not generalized.
3. Discussion
IrMyositis represents a new, treatable type of myositis. It is characterized by generalized proximal and axial muscle weakness, and also often in contrast to idiopathic or antibody associated IMNM involves the oculobulbar muscles either alone or in combination with generalized involvement [3,4]. The symptoms often develop within the first three months following initiation of ICI and some patients have concurrent myocarditis [3]. Although CPK often is elevated it can be normal. EMG show a myopathic pattern and muscle biopsy reveal multifocal clusters of necrotic fibers [3,5]. Early recognition is important in order to start immunosuppressive treatment and discontinue ICI, both of which lead to a better outcome [6].
MRI STIR can show inflammation in muscle tissue [7]. We believe that this imaging method can play an important role in rapid identification of irMyositis. It is non-invasive, easily accessible in most centers and compared with an invasive biopsy, it can look at multiple muscles simultaneously instead of just a small fraction of a single muscle. MRI STIR has some limitations since muscle edema is not specific for myositis, but can be seen in other conditions [8]. In our case MRI STIR showed featherlike inflammation in tensor fasciae latae muscles and was able to guide the site of biopsy. Future research should investigate if this featherlike pattern corresponds to the characteristic multifocal clusters of necrotic fibers that seems to distinguish irMyositis from IMNM on pathological grounds [3].
A recent consensus paper defined the criteria for definite irMyositis that does not include biopsy but instead rely on specific imaging findings [9]. A supportive history, positive clinical examination, timing of ictus related to initiation of ICI, muscle edema on MRI STIR, and myopathic motor units on EMG is suggested as enough for a definite diagnosis of irMyositis. Furthermore, elevated CPK supports the diagnosis. Our case meets the new criteria for definite myositis before biopsy. This case report and this recent article support the fact that biopsy might not be necessary in all cases and we can spare the patient an invasive procedure. Conversely, muscle biopsy is necessary to determine the underlying immunological pathology and should be opt for whenever possible. We encourage further studies examining the role of how muscle MRI correlates with specific pathologic findings in irMyositis.
CRediT authorship contribution statement
Mai Erritzøe-Jervild: Conceptualization, Writing – original draft, Project administration, Visualization. David Scheie: Resources. Christian Stenør: Supervision, Writing – review & editing.
Declaration of Competing Interest
We hereby declare that we have no conflicts of interest.
References
- 1.Wang D.Y., Salem J.-E., Cohen J.V., Chandra S., Menzer C., Ye F., Zhao S., Das S., Beckermann K.E., Ha L., Rathmell W.K., Ancell K.K., Balko J.M., Bowman C., Davis E.J., Chism D.D., Horn L., Long G.V., Carlino M.S., Lebrun-Vignes B., Eroglu Z., Hassel J.C., Menzies A.M., Sosman J.A., Sullivan R.J., Moslehi J.J., Johnson D.B. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allenbach Y., Anquetil C., Manouchehri A., Benveniste O., Lambotte O., Lebrun-Vignes B., Spano J.-P., Ederhy S., Klatzmann D., Rosenzwajg M., Fautrel B., Cadranel J., Johnson D.B., Moslehi J.J., Salem J.-E. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun. Rev. 2020;19:102586. doi: 10.1016/j.autrev.2020.102586. [DOI] [PubMed] [Google Scholar]
- 3.Shelly S., Triplett J.D., Pinto M.V., Milone M., Diehn F.E., Zekeridou A., Liewluck T. Immune checkpoint inhibitor-associated myopathy: a clinicoseropathologically distinct myopathy. Brain Commun. 2020;2:1–16. doi: 10.1093/braincomms/fcaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liewluck T., Kao J.C., Mauermann M.L. PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J. Immunother. 2018;41:208–211. doi: 10.1097/CJI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 5.Touat M., Maisonobe T., Knauss S., Salem O. Ben Hadj, Hervier B., Auré K., Szwebel T.-A., Kramkimel N., Lethrosne C., Bruch J.-F., Laly P., Cadranel J., Weiss N., Béhin A., Allenbach Y., Benveniste O., Lenglet T., Psimaras D., Stenzel W., Léonard-Louis S. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91:e985–e994. doi: 10.1212/WNL.0000000000006124. [DOI] [PubMed] [Google Scholar]
- 6.Dubey D., David W.S., Reynolds K.L., Chute D.F., Clement N.F., Cohen J.V., Lawrence D.P., Mooradian M.J., Sullivan R.J., Guidon A.C. Severe neurological toxicity of immune checkpoint inhibitors: growing Spectrum. Ann. Neurol. 2020;87:659–669. doi: 10.1002/ana.25708. [DOI] [PubMed] [Google Scholar]
- 7.Farrow M., Biglands J.D., Grainger A.J., O’Connor P., Hensor E.M.A., Ladas A., Tanner S.F., Emery P., Tan A.L. Quantitative MRI in myositis patients: comparison with healthy volunteers and radiological visual assessment. Clin. Radiol. 2021;76(81):e1–81.e10. doi: 10.1016/j.crad.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Pipitone N. Value of MRI in diagnostics and evaluation of myositis. Curr. Opin. Rheumatol. 2016;28:625–630. doi: 10.1097/BOR.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 9.Guidon A.C., Burton L.B., Chwalisz B.K., Hillis J., Schaller T.H., Amato A.A., Betof Warner A., Brastianos P.K., Cho T.A., Clardy S.L., Cohen J.V., Dietrich J., Dougan M., Doughty C.T., Dubey D., Gelfand J.M., Guptill J.T., Johnson D.B., Juel V.C., Kadish R., Kolb N., LeBoeuf N.R., Linnoila J., Mammen A.L., Martinez-Lage M., Mooradian M.J., Naidoo J., Neilan T.G., Reardon D.A., Rubin K.M., Santomasso B.D., Sullivan R.J., Wang N., Woodman K., Zubiri L., Louv W.C., Reynolds K.L. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002890. [DOI] [PMC free article] [PubMed] [Google Scholar]