Abstract
Background
Postoperative cognitive dysfunction (POCD) is a common complication in elderly patients following surgery. The preventive and/or treatment strategies for the incidence remain limited.
Objective
This study aimed to investigate the preventive effect of perioperative probiotic treatment on POCD in elderly patients undergoing hip or knee arthroplasty.
Methods
After obtaining ethical approval and written informed consent, 106 patients (age ≥60 years) were recruited, who scheduled elective hip or knee arthroplasty, from 16 March 2021 to 25 February 2022 for this randomized, double-blind, and placebo-controlled trial. They were randomly assigned with a 1:1 ratio to receive either probiotics or placebo treatment (four capsules, twice/day) from hospital admission until discharge. Cognitive function was assessed with a battery of 11 neuropsychological tests on the admission day and the seventh day after surgery, respectively.
Results
A total of 96 of 106 patients completed the study, and their data were finally analyzed. POCD occurred in 12 (26.7%) of 45 patients in the probiotic group and 29 (56.9%) of 51 patients in the placebo group (relative risk [RR], 0.47 [95% confidence interval [CI], 0.27 to 0.81]; P = 0.003). Among them, mild POCD occurred in 11 (24.4%) in the probiotic group and 24 (47.1%) in the placebo group (RR, 0.52 [95% CI, 0.29 to 0.94]; P = 0.022). No significant difference in severe POCD incidence was found between the two groups (P = 0.209). Compared with the placebo group, the verbal memory domain cognitive function was mainly improved in the probiotic group.
Conclusion
Probiotics may be used perioperatively to prevent POCD development and improve verbal memory performance in elderly patients receiving hip or knee arthroplasty.
Clinical trial registration
www.chictr.org.cn, identifier: ChiCTR2100045620.
Keywords: probiotics, postoperative cognitive dysfunction (POCD), elderly patients, cognitive function, hip or knee arthroplasty
1. Introduction
Postoperative cognitive dysfunction (POCD), characterized by memory, attention, and executive ability impairment (Hood et al., 2018), is highly prevalent in the elderly following orthopedic surgery and is associated with poor clinical outcomes and worst quality of life (Moller et al., 1998; Needham et al., 2017; Deiner et al., 2021). Preventive and/or treatment strategies for POCD development included cognitive and physical exercise (O'Gara et al., 2020; Duan et al., 2022), appropriate depth of anesthesia (Chan et al., 2013), goal-directed fluid therapy (Zhang et al., 2018), effective postoperative analgesia (Kristek et al., 2019), and pharmacologic interventions [e.g., edaravone (Zhang et al., 2020), methylene blue (Deng et al., 2021), dexmedetomidine (Su et al., 2016), and stains (Alam et al., 2018)]. The incidence of POCD in orthopedic patients remains as high as 24.6–75% (Rodriguez et al., 2005; Koch et al., 2007; Ji et al., 2013; Li et al., 2019); thus studying new preventive strategies is urgently needed.
Probiotics are widely used in public and clinically for general health supplements and disease conditions to improve immune function and brain function (Mohajeri et al., 2018; Suez et al., 2019). Recent studies have performed a new insight into the effect of probiotics on postoperative brain function because probiotics have the potential anti-inflammatory capabilities (Zhan et al., 2018; Jiang et al., 2019) and reduce levels of systemic pro-inflammatory cytokines (such as IL-1β, TNF-α, IL-6, IL-10, and IFN-γ) (Schachter et al., 2018; Choi et al., 2020). Our previous study showed that perioperative probiotic treatment has an anti-inflammatory effect and could prevent postoperative cognitive impairment development assessed with Mini-Mental State Examination (MMSE) in the elderly following non-cardiac surgery (Wang et al., 2021). However, MMSE is a broad screening tool and is commonly criticized for its low sensitivity in the diagnosis of POCD. The score of MMSE can be influenced by the education level, leading to false positive indications when patients with a low level of education, or false negative indications when patients with a high level of education (Newman et al., 2007; Malek-Ahmadi et al., 2012). A neuropsychological test battery is wildly recommended to improve study quality (Evered et al., 2018; Borchers et al., 2021). In this study, we further carried out this randomized, double-blind, placebo-controlled trial to investigate the preventive effect of perioperative administration of probiotics on POCD incidence in the elderly undergoing hip or knee arthroplasty using comprehensive neuropsychological battery tests as a cognitive evaluation tool.
2. Methods
2.1. Study design and participants
This prospective trial protocol was established with the compliance of the CONSORT Statement, approved (R21010) by the Ethics Committee, Third Xiangya Hospital, Central South University, Changsha, China, and registered in the Chinese Clinical Trial Registry (ChiCTR2100045620). After the written informed consent was obtained, elderly patients (age ≥60 years) admitted to the Department of Orthopedics, Third Xiangya Hospital, from 16 March 2021 to 25 February 2022, who met the inclusion criteria, were enrolled and randomly assigned to receive either probiotics or placebo (four capsules, twice/day) during the whole hospitalization period. The incidence of POCD was evaluated by a battery of 11 neuropsychological tests on the admission day and the seventh day after surgery.
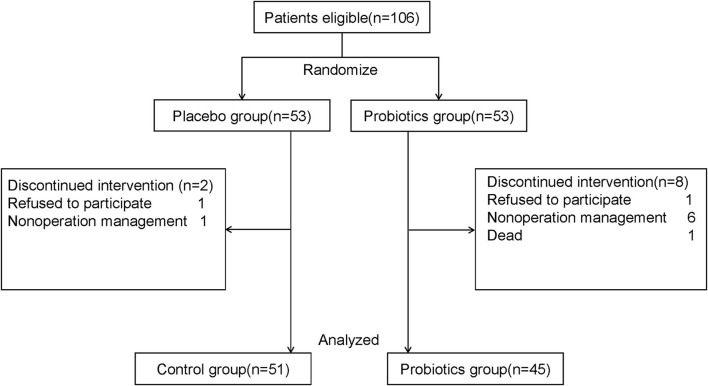
Patients, who had no history of immune system diseases, psychiatric diseases, and neurodegenerative diseases and were scheduled for elective hip or knee arthroplasty, were eligible to be enrolled in this trial (Figure 1). Patients were excluded when they met any of the following criteria: (1) history of communication disorders (such as severe impairment in speaking, hearing, and vision); myocardial infarctions or poor cardiac function; cerebral hemorrhage, cerebral infarction, brain tumor, and stroke; (2) received more than one surgery during hospital stay; (3) used antibiotics, probiotics, or gastro dynamic drug within 10 days before admission; (4) used complete postoperative parenteral nutrition; (5) mental illness or family history of mental illness; alcoholic or drug addicts; (6) postoperative hospital stay duration was < 7 days; (7) participating in other clinical trials; refuse to join the study; not cooperative with the treatment; and (8) for any other reason that is not suitable for this study.
Figure 1.
Flow chart of study: Participant eligibility and enrollment are performed.
2.2. Blinding and treatment allocation
Patients were randomly divided into the probiotic or placebo groups with concealed allocation by generating random numbers in a 1:1 ratio with SPSS 25.0. Patients and clinicians including surgeons and anesthesiologists and all researchers for pre- and postoperative assessments and data collection were blinded with the trial protocol. However, doctors, who closely looked after patients, can request the unmasking of the treatment assignment or terminate patients' participation if the condition of patients was needed.
2.3. Interventions
Patients were randomly assigned to receive either probiotic or placebo treatment (four capsules, twice a day) during the whole hospitalization period and underwent a battery of 11 neuropsychological tests on the admission day and the seventh day after surgery. The probiotic group received four probiotic capsules (0.84 g) twice a day, from hospital admission until discharge. Each probiotic capsule (BIFICO, Sine Pharmaceuticals, Shanghai, China) contained Bifidobacterium longum (>107 colony-forming unit [CFU]/210mg), Lactobacillus acidophilus (>107CFU/210 mg), and Enterococcus faecalis (>107CFU/210 mg). The placebo capsule (also provided by Sine Pharmaceuticals) contained all ingredients except probiotics with an identical size, shape, and smell as probiotic capsules were given to patients in the placebo group in the same way during hospitalization. To fully guarantee the medical treatment and safety of the patients, we did not limit the other clinical treatments of patients.
2.4. Cognitive function assessment
All patients were subjected to cognitive assessments with a battery of 11 neuropsychological tests on the hospital administration (baseline) and the seventh day after surgery by the same assessor who was specifically trained by psychiatrists. The battery tests included the following: Hopkins verbal learning test-revised, delayed recall test, and discrimination index for verbal memory (Lacritz and Cullum, 1998); brief visuospatial memory test-revised (BVMT-R), BVMT-R delayed recall test, and BVMT-R discrimination index for visuospatial memory (Tam and Schmitter-Edgecombe, 2013); number connection test and Benton judgment of line orientation for visuospatial abilities and spatial orientation (Amodio et al., 1999; Boeve et al., 2012); digit span test for attention (Leung et al., 2011); digit symbol substitution test and verbal fluency test for executive function (Jaeger, 2018; Sutin et al., 2019; Juan et al., 2022).
Mild POCD and severe POCD were defined as a decrease of 1–2 standard deviation (SD) or more than 2 SD of two or more neuropsychological tests from the admission baseline to the seventh day after surgery (Sachdev et al., 2014; Borchers et al., 2021). The specific value of the standard deviation is shown in Supplementary Table S1. The change scores of each neuropsychological test were calculated by subtracting the postoperative score from the preoperative score, except for the number connection test in which the change scores were defined as a postoperative score minus the baseline score.
2.5. Outcomes and data collection
The primary outcome was the incidence of POCD on the seventh day after surgery. The secondary outcomes included the length of hospital stay, the incidence of hospital death, and 30-day post-hospital death.
The patients' clinical characteristics including laboratory measurements and parameters during anesthesia and surgery including demographics, such as age, sex, height, weight, education, body mass index (BMI), type of operation, American Society of Anesthesiologists (ASA) classification, intraoperative blood loss, type of anesthesia, length of operation, total intraoperative infusion, and type of antibiotics, were collected.
2.6. Sample size
The sample size was determined assuming a POCD rate of 40% in the placebo group and 20% in the probiotic group. The POCD rate of 40% in the control group was based on previous studies (Rodriguez et al., 2005; Wu et al., 2018). Given a significance set at the level of 0.5, power at 70%, and a loss to follow-up rate of 10%, a total of 106 patients (n1= n2 = 53) are required to detect a difference, according to the formula as follows:
n1 and n2 represent the sample size of two groups, Zα and Zβ represent the standard normal deviate values of α and β, and P1 and P2 represent the incidence of two groups, = (P1+ P2)/2.
2.7. Statistical analyses
Normality was tested with the Shapiro–Wilk test. Patients' general characteristics were presented as mean ± standard deviation (SD), or number and percentage, or the median and interquartile range wherever appropriate. Quantitative data with a normal distribution were presented as mean ± standard deviation (SD), or otherwise as the median and interquartile range. Qualitative variables were analyzed with Pearson's chi-square test or Fisher's exact test, and quantitative data were analyzed with a t-test or Mann–Whitney U-test where appropriate. The per-protocol (PP) population consisted of all patients who completed the study according to the protocol (Seino et al., 2014). The primary analysis was based on the PP population. The intention-to-treat (ITT) analyses included all randomized patients (Supplementary Tables S2–S6) (Sun et al., 2018). The missing data were calculated using the last observation carried forward imputation method (Lv et al., 2015). All statistical analyses were performed with SPSS software (version 25.0, SPSS, Chicago, United States).
3. Results
3.1. General characteristics of patients studied
A total of 106 patients were enrolled and randomly assigned to receive either probiotic (n = 53) or placebo (n = 53) treatment; of those, two patients (2 of 53[3.7%] in the placebo group) and eight patients (8 of 53[15.1%] in the probiotic group) were excluded for various reasons, including refusal to continue participating or cancelation of operations, or one death in the probiotic group; the data from 96 patients were included in the final data analysis (n = 51 in the placebo group; n = 45 in the probiotic group) (Figure 1). There were no statistical differences in the demographics and clinical characteristics of patients (Table 1) and their baseline scores of neuropsychological tests (Table 2) between the two groups.
Table 1.
Basic characteristics.
| Placebo (n = 51) | Probiotics (n = 45) | P-value | |
|---|---|---|---|
| Age (yr) | 70 (64,75) | 68 (65,73.5) | 0.64 |
| Sex | 0.55 | ||
| Male | 20 (39.2) | 15 (33.3) | |
| Female | 31 (60.8) | 30 (66.7) | |
| Height (cm) | 159.76 ± 8.58 | 159.69 ± 7.11 | 0.96 |
| Weight (kg) | 61.16 ± 10.30 | 59.90 ± 9.20 | 0.53 |
| BMI (kg/m2) | 23.97 ± 3.69 | 23.46 ± 3.06 | 0.47 |
| Statins | 1 (1.96) | 2 (4.4) | 0.91 |
| Type of operation | 0.61 | ||
| Knee arthroplasty | 23 (45.1) | 18 (40) | |
| Hip arthroplasty | 28 (54.9) | 27 (60) | |
| ASA classification | 0.203 | ||
| II | 26 (51.0) | 16 (35.6) | |
| III | 25 (49.0) | 28 (62.2) | |
| IV | 0 | 1 (2.2) | |
| Education | 0.132 | ||
| Illiteracy | 4 (7.8) | 7 (15.6) | |
| Elementary school | 21 (41.2) | 15 (33.3) | |
| Middle school | 13 (25.5) | 5 (11.1) | |
| High school | 11 (21.6) | 12 (26.7) | |
| University | 2 (3.9) | 6 (13.3) |
BMI, body mass index; ASA, American Society of Anesthesiologists. All data are presented as number (%), median (first quartile, third quartile), or mean ± SD.
Table 2.
Results of neuropsychological assessment at baseline.
| Placebo (n = 51) | Probiotics (n =45) | P-value | |
|---|---|---|---|
| HVLT-R | 10.02 ± 3.11 | 9.29 ± 3.79 | 0.30 |
| HVLT-R delayed recall test | 2 (1,3) | 2 (0,3.5) | 0.78 |
| HVLT-R discrimination index | 20 (18,22) | 20 (17.5,22) | 0.78 |
| BVMT-R | 6 (3,10) | 5 (3.5,11) | 0.99 |
| BVMT-R delayed recall test | 2 (1,3) | 2 (1,4) | 0.79 |
| BVLT-R discrimination index | 11 (10,12) | 10 (10,12) | 0.10 |
| Number connection test | 431 (360,502) | 427 (322,534) | 0.83 |
| Benton judgment of line orientation | 14 (12,16) | 14 (12,15) | 0.57 |
| Digit span test | 16.29 ± 3.05 | 16.31 ± 3.92 | 0.98 |
| Digit Symbol Substitution Test | 16 (11,22) | 15 (12,24) | 0.87 |
| Verbal fluency test | 39.84 ± 10.67 | 39.33 ± 11.41 | 0.82 |
HVLT-R, Hopkins verbal learning test-revised; BVMT-R, brief visuospatial memory test-revised. All data are presented as number (%), median (first quartile, third quartile), or mean ± SD.
3.2. Parameters during anesthesia and surgery
Parameters during anesthesia and surgery, including operating time, intraoperative infusion volume, intraoperative blood loss, type of anesthesia, postoperative analgesia regime, perioperative dexmedetomidine use, and intraoperative and postoperative antibiotic treatment, were not statistically different between the placebo and probiotic groups (P > 0.05, Table 3).
Table 3.
Parameters during anesthesia and surgery.
| Placebo (n = 51) | Probiotics (n = 45) | P-value | |
|---|---|---|---|
| Intraoperative blood loss (ml) | 100 (50,200) | 100 (50,200) | 0.06 |
| Total intra-operative infusion (ml) | 1,200 (1,100, 1,700) | 1,300 (1,050,1,700) | 0.69 |
| Dexmedetomidine | 15 | 12 | 0.77 |
| Type of anesthesia | 0.36 | ||
| intravertebral | 27 (52.9) | 28 (62.2) | |
| General | 24 (47.1) | 17 (37.8) | |
| Length of operation (h) | 120.0 (110.0,155.0) | 125.00 (95.0,145.0) | 0.74 |
| Intra-operative antibiotic | 1 | ||
| β-lactam | 47 (92.2%) | 42 (93.3%) | |
| Quinolones | 1 (1.1%) | 1 (0.9%) | |
| Polypeptide | 3 (2.7%) | 2 (2.3%) | |
| Postoperative antibiotic | 0.87 | ||
| β-lactam | 47 (92.2%) | 40 (88.9%) | |
| Quinolones | 1 (2.0%) | 2 (4.4%) | |
| Polypeptide | 3 (5.9%) | 3 (6.7%) | |
| Postoperative analgesia regime | 0.21 | ||
| Sufentanil | 30 (58.8%) | 32 (71.1%) | |
| Sufentanil and dezocine | 21 (41.2) | 13 (28.9%) |
All data are presented as number (%), median (first quartile, third quartile), or mean ± SD.
3.3. Probiotics decreased the incidence of POCD following surgery
Postoperative cognitive dysfunction occurred in 12 (26.7%) of 45 patients in the probiotic group and 29 (56.9%) of 51 patients in the placebo group (RR, 0.47 [95% CI, 0.27 to 0.81]; P = 0.003, Table 4). Among them, mild POCD occurred in 11 (24.4%) in the probiotic group and 24 (47.1%) in the placebo group (RR, 0.52 [95% CI, 0.29 to 0.94]; P = 0.022, Table 4). No significant differences in severe POCD incidence were found between the two groups (P = 0.209). The mild or severe decline of score mainly occurred in the Hopkins verbal learning test-revised test, Hopkins verbal learning test-revised delayed recall test, and Hopkins verbal learning test-revised discrimination index test in two groups (Table 5).
Table 4.
Probiotics decrease the incidence of POCD in elderly patients after joint arthroplasty.
| Incidence of cognitive impairment, No./Total (%) | Placebo (n = 51) | Probiotics (n = 45) | RR (95% CI) | P-value |
|---|---|---|---|---|
| Total | 29/51 (56.9) | 12/45 (26.7) | 0.47 (0.27–0.81) | 0.003** |
| Mild | 24/51 (47.1) | 11/45 (24.4) | 0.52 (0.29–0.94) | 0.022* |
| Severe | 5/51 (9.8) | 1/45 (2.2) | 0.23 (0.03–1.87) | 0.209 |
All data are presented as number (%), median (first quartile, third quartile), or mean ± SD; *p < 0.5, **p < 0.01.
Table 5.
Incidence of mild and severe decline in each neuropsychological test in different groups.
| Incidence of mild and severe decline, No./Total (%) | Mild decline | Major decline | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 51) | Probiotics (n = 45) | P-value | Placebo (n = 51) | Probiotics (n = 45) | P-value | |
| HVLT-R | 17/51 (33.3) | 5/45 (11.1) | 0.01* | 2/51 (3.9) | 1/45 (2.2) | 1.000 |
| HVLT-R delayed recall test | 20/51 (39.2) | 6/45 (13.3) | 0.004** | 0 | 0 | |
| HVLT-R discrimination index | 8/51 (15.7) | 3/45 (6.7) | 0.166 | 9/51 (17.6) | 1/45 (2.2) | 0.018* |
| BVMT-R | 6/51 (11.8) | 5/45 (11.1) | 0.920 | 0 | 0 | |
| BVMT-R delayed recall test | 9/51 (17.6) | 7/45 (15.6) | 0.784 | 1/51 (2.0) | 1/45 (2.2) | 1.000 |
| BVLT-R discrimination index | 6/51 (11.8) | 4/45 (8.9) | 0.746 | 5/51 (9.8) | 3/45 (6.7) | 0.719 |
| Number connection test | 0 | 1/45 (2.2) | 0.469 | 0 | 0 | |
| Benton judgment of line orientation | 9/51 (17.6) | 7/45 (15.6) | 0.784 | 1/51 (2.0) | 0 | 1.000 |
| Digit span test total | 4/51 (7.8) | 3/45 (6.7) | 1.000 | 1/51 (2.0) | 1/45 (2.2) | 1.000 |
| Digit symbol substitution test | 4/51 (7.8) | 2/45 (4.4) | 0.681 | 0 | 0 | |
| Verbal fluency test | 6/51 (11.8) | 6/45 (13.3) | 0.817 | 5/51 (9.8) | 0 | 0.058 |
HVLT-R, Hopkins verbal learning test-revised; BVMT-R, brief visuospatial memory test-revised. All data are presented as number (%), median (first quartile, third quartile), or mean ± SD; *p < 0.5, **p < 0.01.
3.4. Probiotics improve performance in verbal tests
To determine which domain of brain function was mainly improved by probiotics, we further compared the incidence of mild and severe decline in each neuropsychological test between the probiotic and placebo groups. The result showed that compared to the placebo group, the incidence of the mild decline of the Hopkins verbal learning test-revised test (11.1 vs. 33.3%, P = 0.01) and the Hopkins verbal learning test-revised delayed recall test (13.3 vs. 39.2%, P = 0.004) in the probiotic group was significantly lower (Table 5). The incidence of severe decline of the Hopkins verbal learning test-revised test discrimination index in the probiotic group was also lower than that in the placebo group (2.2 vs. 17.6%, P = 0.018). These results suggested that probiotics may improve performance mainly in verbal memory.
3.5. Other clinical outcomes
The length of hospital stays, the incidence of hospital death and 30-day post-hospital death, the level of C reactive protein and leukocytes, and the neutrophil percentage did not differ significantly between the placebo and probiotic groups (P > 0.05, Table 6).
Table 6.
Other clinical outcomes.
| Placebo (n = 51) | Probiotics (n = 45) | P-value | |
|---|---|---|---|
| Length of hospital stays (d) | 13 (9,15) | 11 (10,14) | 0.46 |
| Incidence of hospital death | 0 | 0 | 1 |
| Incidence of 30-days post-hospital death | 0 | 0 | 1 |
| Leukocyte (x10 9 /L) | |||
| Baseline | 6.28 (4.56,7.96) | 6.05 (5.28,7.24) | 0.88 |
| Postoperative day 1 | 9.95 (2.90) | 9.85 (2.55) | 0.86 |
| C reactive protein (mg/L) | |||
| Baseline | 5.00 (5.00,16.8) | 6.64 (5.00,41.97) | 0.23 |
| Postoperative day 1 | 52.11 (22.97,64.19) | 47.97 (30.97,77.65) | 0.89 |
| Neutrophils (%) | |||
| Baseline | 62.61 (11.80) | 63.47 (13.20) | 0.74 |
| Postoperative day 1 | 81.50 (78.10,87.40) | 81.00 (78.50,87.85) | 0.85 |
| Neutrophil count (x10 9 /L) | |||
| Baseline | 3.91 (2.78,5.86) | 4.00 (3.02,5.23) | 0.83 |
| Postoperative day 1 | 8.19 (2.76) | 8.12 (2.33) | 0.89 |
All data are presented as number (%), median (first quartile, third quartile), or mean ± SD.
4. Discussion
In the current randomized, double-blind, and placebo-controlled trial, perioperative probiotic treatment significantly reduced the incidence of POCD in patients who underwent elective hip or knee arthroplasty, which is in line with our previous study of the preventive effect of probiotics on POCD development assessed with MMSE (Wang et al., 2021). The length of hospital stays, the incidence of hospital death and 30-day post-hospital death, the level of C reactive protein, and blood cell counts were not significantly different between the placebo and probiotic groups. Furthermore, ITT analyses yielded the same conclusions (Supplementary Tables S2–S6). Our study suggests that perioperative probiotic supplements may be potential strategies for preventing POCD development in elderly patients.
Previous studies reported that perioperative peripheral inflammatory responses act as a major mechanism in the pathogenesis of POCD via inducing neuroinflammation and, as a result, damaging synapses connectivity (Tanabe et al., 2020; Zhu et al., 2021; Chen et al., 2022) and triggering cognitive decline. Limiting perioperative peripheral inflammatory responses may significantly alleviate POCD in the pre-clinical setting (Cibelli et al., 2010; Terrando et al., 2010). Pharmacologic interventions such as anti-inflammatory drugs, dexmedetomidine (Su et al., 2016), and statins (Alam et al., 2018) have also been reported to have certain effects in preventing POCD clinically. However, preventing POCD remains a clinical challenge. Accumulating evidence showed that gut microbial dysbiosis can affect peripheral inflammation (Fung et al., 2017), the pathogenesis of psychological diseases (Cryan et al., 2019), neurodegenerative diseases (Sun and Shen, 2018), and cognitive impairment following surgery (Xu et al., 2020). Probiotic supplements can significantly alleviate gut microbial dysbiosis and its related pathological effects (O'Mahony et al., 2005; Chunchai et al., 2018). Previous studies also demonstrated that gut microbial dysbiosis promoted peripheral inflammatory response via damaging the intestinal wall, changing peripheral metabolites' levels, and modulating HPA axis response (Fung et al., 2017), while probiotic supplements negated all these changes effectively (Suez et al., 2019; Juan et al., 2022). It has been reported that antibiotic administration affects the intestinal microbiota (Dethlefsen and Relman, 2011), which may result in antibiotic-related diarrhea and intestinal complications, such as Clostridium difficile-related colitis (De La Cochetière et al., 2005). To exclude the effects of antibiotics on the composition of gut microbiota, subjects who reported antibiotics treatments 10 days before admission were excluded (Wang et al., 2021). No differences were found with respect to the type of intraoperative antibiotic and the type of postoperative antibiotic between the two groups. In this study, we found that perioperative probiotic supplements significantly reduced the incidence of POCD in elderly patients, which further supports our previous findings (Wang et al., 2021) and provides a new strategy for preventing POCD in elderly patients. Further mechanistic investigation showed that perioperative probiotic supplements accelerated the postoperative decrease of inflammatory cytokines and glucocorticoids in peripheral blood (Wang et al., 2021). It is likely that probiotic supplements offered multi-benefits to our surgical patients, and the underlying mechanisms need to be studied further.
Our study had several limitations. First, as a single-center study with a small sample size and simple surgical population, the enrolled patients may not fully represent the patient population. Second, long-term follow-up was not done, and hence whether the treatment improves long-term outcomes remain unknown. Third, the underlying mechanism for the prevention of POCD using probiotic supplements remains elusive.
5. Conclusion
Our study indicated that a convenient perioperative supplement of probiotics can effectively mitigate postoperative cognitive impairment and improve performance mainly in verbal memory in elderly patients following hip or knee arthroplasty. Furthermore, a large sample-size trial is needed before the strategy can be used clinically to tackle the development of POCD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Third Xiangya Hospital of Central South University, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL designed the study. LH, ML, HH, and LW conceived the original data. LH and HH performed the statistical analysis. LH wrote the manuscript. YL, WO, and JT reviewed the manuscript. All authors agree to be accountable for the content of the work.
Acknowledgments
We authors thank Prof. Daqing Ma, MD, PhD, FRCA, MAE for his critical comments during manuscript preparation. We are grateful to all the participating patients and their families for their contributions during the study.
Funding
This work was supported by the National Natural Science Foundation of China (81771169, 82271235, and 81971028) and the Natural Science Foundation of Hunan Province (2021JJ31008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1037904/full#supplementary-material
References
- Alam A., Hana Z., Jin Z., Suen K. C., Ma D. (2018). Surgery, neuroinflammation and cognitive impairment. EBioMedicine 37, 547–556. 10.1016/j.ebiom.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio P., Del Piccolo F., Marchetti P., Angeli P., Iemmolo R., Caregaro L., et al. (1999). Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology 29, 1662–1667. 10.1002/hep.510290619 [DOI] [PubMed] [Google Scholar]
- Boeve B. F., Boylan K. B., Graff-Radford N. R., DeJesus-Hernandez M., Knopman D. S., Pedraza O., et al. (2012). Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135, 765–783. 10.1093/brain/aws004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers F., Spies C. D., Feinkohl I., Brockhaus W. R., Kraft A., Kozma P., et al. (2021). Methodology of measuring postoperative cognitive dysfunction: a systematic review. Br. J. Anaesth. 126, 1119–1127. 10.1016/j.bja.2021.01.035 [DOI] [PubMed] [Google Scholar]
- Chan M. T., Cheng B. C., Lee T. M., Gin T. (2013). BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J. Neurosurg. Anesthesiol. 25, 33–42. 10.1097/ANA.0b013e3182712fba [DOI] [PubMed] [Google Scholar]
- Chen J., Liu S., Wang X., Huang J., Phillips J., Ma D., et al. (2022). HDAC6 Inhibition Alleviates Anesthesia and Surgery-Induced Less Medial Prefrontal-Dorsal Hippocampus Connectivity and Cognitive Impairment in Aged Rats. Mol. Neurobiol. 59, 6158–6169 10.1007/s12035-022-02959-4 [DOI] [PubMed] [Google Scholar]
- Choi S. H., Oh J. W., Ryu J. S., Kim H. M., Im S. H., Kim K. P., et al. (2020). IRT5 probiotics changes immune modulatory protein expression in the extraorbital lacrimal glands of an autoimmune dry eye mouse model. Invest. Ophthalmol. Vis. Sci. 61, 42. 10.1167/iovs.61.3.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunchai T., Thunapong W., Yasom S., Wanchai K., Eaimworawuthikul S., Metzler G., et al. (2018). Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 15, 11. 10.1186/s12974-018-1055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M., Fidalgo A. R., Terrando N., Ma D., Monaco C., Feldmann M., et al. (2010). Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 68, 360–368. 10.1002/ana.22082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J. F., O'Riordan K. J., Cowan C. S. M., Sandhu K. V., Bastiaanssen T. F. S., Boehme M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- De La Cochetière M. F., Durand T., Lepage P., Bourreille A., Galmiche J. P., Dor,é J. (2005). Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J. Clin. Microbiol. 43, 5588–5592. 10.1128/JCM.43.11.5588-5592.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner S., Liu X., Lin H.-. M., Jacoby R., Kim J., et al. (2021). Does postoperative cognitive decline result in new disability after surgery? Ann. Surg. 274, e1108–e1114. 10.1097/SLA.0000000000003764 [DOI] [PubMed] [Google Scholar]
- Deng Y., Wang R., Li S., Zhu X., Wang T., Wu J., et al. (2021). Methylene blue reduces incidence of early postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery: Aa open-label randomized controlled clinical trial. J. Clin. Anesth. 68, 110108. 10.1016/j.jclinane.2020.110108 [DOI] [PubMed] [Google Scholar]
- Dethlefsen L., Relman D. A. (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U. S. A. 108, 4554–4561. 10.1073/pnas.1000087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S., Liao Y., Tang Y., Zhang B., Peng M., Tong J., et al. (2022). Short-term perioperative cognitive therapy combined with rehabilitation exercise reduces the incidence of neurocognitive disorder in elderly patients: a randomized controlled trial. Minerva Anestesiol. 88, 145–155. 10.23736/S0375-9393.21.15877-8 [DOI] [PubMed] [Google Scholar]
- Evered L., Silbert B., Knopman D. S., Scott D. A., DeKosky S. T., Rasmussen L. S., et al. (2018). Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 121, 1005–1012. 10.1016/j.bja.2017.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T. C., Olson C. A., Hsiao E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. 10.1038/nn.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood R., Budd A., Sorond F. A., Hogue C. W. (2018). Peri-operative neurological complications. Anaesthesia 73 Suppl 1, 67–75. 10.1111/anae.14142 [DOI] [PubMed] [Google Scholar]
- Jaeger J. (2018). Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 38, 513–519. 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M. H., Yuan H. M., Zhang G. F., Li X. M., Dong L., Li W. Y., et al. (2013). Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J. Anesth. 27, 236–242. 10.1007/s00540-012-1506-3 [DOI] [PubMed] [Google Scholar]
- Jiang X. L., Gu X. Y., Zhou X. X., Chen X. M., Zhang X., Yang Y. T., et al. (2019). Intestinal dysbacteriosis mediates the reference memory deficit induced by anaesthesia/surgery in aged mice. Brain Behav. Immun. 80, 605–615. 10.1016/j.bbi.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Juan Z., Chen J., Ding B., Yongping L., Liu K., Wang L., et al. (2022). Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 161, 10–22. 10.1016/j.ejca.2021.11.006 [DOI] [PubMed] [Google Scholar]
- Koch S., Forteza A., Lavernia C., Romano J. G., Campo-Bustillo I., Campo N., et al. (2007). Cerebral fat microembolism and cognitive decline after hip and knee replacement. Stroke 38, 1079–1081. 10.1161/01.STR.0000258104.01627.50 [DOI] [PubMed] [Google Scholar]
- Kristek G., Rado,š I., Kristek D., Kapural L., Neškovi,ć N., Škiljić S., et al. (2019). Influence of postoperative analgesia on systemic inflammatory response and postoperative cognitive dysfunction after femoral fractures surgery: a randomized controlled trial. Reg. Anesth. Pain Med. 44, 59–68. 10.1136/rapm-2018-000023 [DOI] [PubMed] [Google Scholar]
- Lacritz L. H., Cullum C. M. (1998). The Hopkins Verbal Learning Test and CVLT: a preliminary comparison. Arch. Clin. Neuropsychol. 13, 623–628. 10.1093/arclin/13.7.623 [DOI] [PubMed] [Google Scholar]
- Leung J. L., Lee G. T., Lam Y. H., Chan R. C., Wu J. Y. (2011). The use of the Digit Span Test in screening for cognitive impairment in acute medical inpatients. Int. Psychogeriatr. 23, 1569–1574. 10.1017/S1041610211000792 [DOI] [PubMed] [Google Scholar]
- Li W. X., Luo R. Y., Chen C., Li X., Ao J. S., Liu Y., et al. (2019). Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin. Med. J. (Engl) 132, 437–445. 10.1097/CM9.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q. W., Zhang W., Shi Q., Zheng W. J., Li X., Chen H., et al. (2015). Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann. Rheum. Dis. 74, 1078–1086. 10.1136/annrheumdis-2013-204807 [DOI] [PubMed] [Google Scholar]
- Malek-Ahmadi M., Davis K., Belden C., Laizure B., Jacobson S., Yaari R., et al. (2012). Validation and diagnostic accuracy of the Alzheimer's questionnaire. Age Ageing 41, 396–399. 10.1093/ageing/afs008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri M. H., La Fata G., Steinert R. E., Weber P. (2018). Relationship between the gut microbiome and brain function. Nutr. Rev. 76, 481–496. 10.1093/nutrit/nuy009 [DOI] [PubMed] [Google Scholar]
- Moller J. T., Cluitmans P., Rasmussen L. S., Houx P., Rasmussen H., Canet J., et al. (1998). Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet 351, 857–861. 10.1016/S0140-6736(97)07382-0 [DOI] [PubMed] [Google Scholar]
- Needham M. J., Webb C. E., Bryden D. C. (2017). Postoperative cognitive dysfunction and dementia: what we need to know and do. Br. J. Anaes. 119, i115–i125. 10.1093/bja/aex354 [DOI] [PubMed] [Google Scholar]
- Newman S., Stygall J., Hirani S., Shaefi S., Maze M., Warltier D. C. (2007). Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology 106, 572–590. 10.1097/00000542-200703000-00023 [DOI] [PubMed] [Google Scholar]
- O'Gara B. P., Mueller A., Gasangwa D. V. I., Patxot M., Shaefi S., Khabbaz K., et al. (2020). Prevention of early postoperative decline: a randomized, controlled feasibility trial of perioperative cognitive training. Anesth. Analg. 130, 586–595. 10.1213/ANE.0000000000004469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony L., McCarthy J., Kelly P., Hurley G., Luo F., Chen K., et al. (2005). Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128, 541–551. 10.1053/j.gastro.2004.11.050 [DOI] [PubMed] [Google Scholar]
- Rodriguez R. A., Tellier A., Grabowski J., Fazekas A., Turek M., Miller D., et al. (2005). Cognitive dysfunction after total knee arthroplasty: effects of intraoperative cerebral embolization and postoperative complications. J. Arthroplasty 20, 763–771. 10.1016/j.arth.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Sachdev P. S., Blacker D., Blazer D. G., Ganguli M., Jeste D. V., Paulsen J. S., et al. (2014). Classifying neurocognitive disorders: the DSM-5 approach. Nat. Rev. Neurol. 10, 634–642. 10.1038/nrneurol.2014.181 [DOI] [PubMed] [Google Scholar]
- Schachter J., Martel J., Lin C. S., Chang C. J., Wu T. R., Lu C. C., et al. (2018). Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 69, 1–8. 10.1016/j.bbi.2017.08.026 [DOI] [PubMed] [Google Scholar]
- Seino Y., Takami A., Boka G., Niemoeller E., Raccah D. (2014). Pharmacodynamics of the glucagon-like peptide-1 receptor agonist lixisenatide in Japanese and Caucasian patients with type 2 diabetes mellitus poorly controlled on sulphonylureas with/without metformin. Diabetes Obes. Metab. 16, 739–747. 10.1111/dom.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Meng Z. T., Wu X. H., Cui F., Li H. L., Wang D. X., et al. (2016). Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 388, 1893–1902. 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- Suez J., Zmora N., Segal E., Elinav E. (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729. 10.1038/s41591-019-0439-x [DOI] [PubMed] [Google Scholar]
- Sun H. B., Li Y., Liu X. B., Zhang R. X., Wang Z. F., Lerut T., et al. (2018). Early oral feeding following mckeown minimally invasive esophagectomy: an open-label, randomized, controlled, noninferiority trial. Ann. Surg. 267, 435–442. 10.1097/SLA.0000000000002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M. F., Shen Y. Q. (2018). Dysbiosis of gut microbiota and microbial metabolites in Parkinson's Disease. Ageing Res. Rev. 45, 53–61. 10.1016/j.arr.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Sutin A. R., Stephan Y., Terracciano A. (2019). Verbal fluency and risk of dementia. Int. J. Geriatr. Psychiatry 34, 863–867. 10.1002/gps.5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. W., Schmitter-Edgecombe M. (2013). The role of processing speed in the Brief visuospatial memory test - revised. Clin. Neuropsychol. 27, 962–972. 10.1080/13854046.2013.797500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Mohanty R., Lindroth H., Casey C., Ballweg T., Farahbakhsh Z., et al. (2020). Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J. Anaesth. 125, 55–66. 10.1016/j.bja.2020.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N., Monaco C., Ma D., Foxwell B. M., Feldmann M., Maze M., et al. (2010). Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. U. S. A. 107, 20518–20522. 10.1073/pnas.1014557107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yin X., Chen G., Li L., Le Y., Xie Z., et al. (2021). Perioperative probiotic treatment decreased the incidence of postoperative cognitive impairment in elderly patients following non-cardiac surgery: A randomised double-blind and placebo-controlled trial. Clin. Nutr. 40, 64–71. 10.1016/j.clnu.2020.05.001 [DOI] [PubMed] [Google Scholar]
- Wu Z., Zhang M., Zhang Z., Dong W., Wang Q., Ren J., et al. (2018). Ratio of β-amyloid protein (Aβ) and Tau predicts the postoperative cognitive dysfunction on patients undergoing total hip/knee replacement surgery. Exp. Ther. Med. 15, 878–884. 10.3892/etm.2017.5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Hu Y., Yan E., Zhan G., Liu C., Yang C., et al. (2020). Perioperative neurocognitive dysfunction: thinking from the gut? Aging (Albany NY) 12, 15797–15817. 10.18632/aging.103738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G., Yang N., Li S., Huang N., Fang X., Zhang J., et al. (2018). Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 10, 1257–1267. 10.18632/aging.101464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Liang M., Zhang D. D., Xiao Y. R., Li Y. Z., Gao Y. G., et al. (2018). Effect of goal-directed fluid therapy on early cognitive function in elderly patients with spinal stenosis: A Case-Control Study. Int. J. Surg. 54, 201–205. 10.1016/j.ijsu.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Zhang N. N., Sun L., Chen W. T., Yang Y. L., Wu Y. M. (2020). Effects of edaravone on postoperative cognitive function in elderly patients undergoing hip joint. replacement surgery: a randomized controlled trial. Int. J. Surg. 80, 13–18. 10.1016/j.ijsu.2020.05.092 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhou M., Jia X., Zhang W., Shi Y., Bai S., et al. (2021). Inflammation disrupts the brain network of executive function after cardiac surgery. Ann. Surg. 10.1097/SLA.0000000000005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.