Abstract
Mycobacterium tuberculosis is a facultative intracellular pathogen that has evolved the ability to survive and multiply within human macrophages. It is not clear how M. tuberculosis avoids the destructive action of macrophages, but this ability is fundamental in the pathogenicity of tuberculosis. A gene previously identified in M. tuberculosis, designated eis, was found to enhance intracellular survival of Mycobacterium smegmatis in the human macrophage-like cell line U-937 (J. Wei et al., J. Bacteriol. 182:377–384, 2000). When eis was introduced into M. smegmatis on a multicopy vector, sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed the appearance of a unique 42-kDa protein band corresponding to the predicted molecular weight of the eis gene product. This band was electroeluted from the gel with a purity of >90% and subjected to N-terminal amino acid sequencing, which demonstrated that the 42-kDa band was indeed the protein product of eis. The Eis protein produced by M. tuberculosis H37Ra had an identical N-terminal amino acid sequence. A synthetic polypeptide corresponding to a carboxyl-terminal region of the deduced eis protein sequence was used to generate affinity-purified rabbit polyclonal antibodies that reacted with the 42-kDa protein in Western blot analysis. Hydropathy profile analysis showed the Eis protein to be predominantly hydrophilic with a potential hydrophobic amino terminus. Phase separation of M. tuberculosis H37Ra lysates by the nonionic detergent Triton X-114 revealed the Eis protein in both the aqueous and detergent phases. After fractionation of M. tuberculosis by differential centrifugation, Eis protein appeared mainly in the cytoplasmic fraction but also in the membrane, cell wall, and culture supernatant fractions as well. Forty percent of the sera from pulmonary tuberculosis patients tested for anti-Eis antibody gave positive reactions in Western blot analysis. Although the function of Eis remains unknown, evidence presented here suggests it associates with the cell surface and is released into the culture medium. It is produced during human tuberculosis infection and therefore may be an important M. tuberculosis immunogen.
Mycobacterium tuberculosis, the etiological agent of tuberculosis, is the leading cause of death worldwide due to a single infectious agent and is responsible for as many as 26% of all avoidable adult deaths (20, 36, 44, 60). M. tuberculosis is highly infectious; once inhaled into the lungs, the bacilli are engulfed by aveolar macrophages. The microbes are resistant to killing by host macrophages and multiply within these phagocytes. Internalized M. tuberculosis may avoid death by several proposed mechanisms, which include inhibiting phagosome-lysosome fusion (2, 17), inhibiting phagosome acidification (14, 52), and scavenging toxic oxygen products produced by macrophages (9). Various mycobacterial cell wall components may be involved in these processes (49). Classic studies by Goren et al. (28) suggest a possible role for mycobacterial sulfatides in the inhibition of phagosome-lysosome fusion, while the cell wall-associated glycolipid lipoarabinomannan of M. tuberculosis has been shown to scavenge toxic oxygen products produced by macrophages (9, 10). With the publication of the complete genome sequence of M. tuberculosis (13), additional genes and protein products of the pathogen have been identified as potentially playing roles in pathogenesis. These include the mce (3), erp (4), acr (62) and sigF (12) gene products, although their modes of action remain speculative.
Recently the eis gene of M. tuberculosis was shown to enhance Mycobacterium smegmatis intracellular survival in the human macrophage cell line U-937 (56). M. smegmatis containing eis on a multicopy plasmid exhibited a 5- to 10-fold-enhanced intracellular survival over a 2-day infection period. The eis gene corresponds to Rv2416c of the M. tuberculosis genome, but this open reading frame (ORF) has no significant homology to other known genes. Southern blot analysis showed eis present only in members of the M. tuberculosis complex and absent from numerous other mycobacterial species (56). The putative Eis proteins from M. tuberculosis H37Rv and H37Ra had the same electrophoretic mobility as the Eis protein from M. smegmatis(p69) and reacted with the antibody to the Eis protein in Western blots. The N-terminal amino acid sequence of the Eis proteins from M. smegmatis(p69) and M. tuberculosis H37Ra were identical. It was concluded that the Eis proteins from the three mycobacteria are probably the same. The N-terminal sequencing of Eis protein revealed that the translation start site is different from the ones initially reported (13, 56). To help in assigning a function to Eis, cell fractionation experiments were performed to delineate the location of Eis within M. tuberculosis H37Ra.
MATERIALS AND METHODS
Strains and growth media.
M. smegmatis 1-2c (63) was grown in Middlebrook 7H9 broth (Difco) as previously described (56). Shaking liquid cultures of M. tuberculosis H37Ra were grown in Middlebrook 7H9 with or without albumin dextrose catalase (ADC) plus 0.05% Tween 80 (64) or in glycerol-alanine-salts (GAS) medium (53). When required, the antibiotics hygromycin B (200 μg/ml for Escherichia coli; 50 μg/ml for mycobacteria) and kanamycin (25 μg/ml for mycobacteria) were used.
Production of anti-Eis antibody and other antibodies.
Anti-Eis polyclonal antibodies were produced by Quality Controlled Biochemicals, Inc. (Hokinton, Mass.). A potentially immunogenic peptide sequence was selected from the deduced Eis protein amino acid sequence, and an 18-mer oligopeptide (AAANRLRTKDSQLLRRLD) corresponding to a C-terminal domain was synthesized. The peptide was conjugated to keyhole limpet hemocyanin, mixed with Freund's adjuvant, and injected subcutaneously into rabbits. Booster injections were given at least five times, with test bleeds taken at each stage for enzyme-linked immunosorbent assay of antibody titers against the Eis peptide bound to bovine serum albumin (BSA). The synthetic polypeptide proved immunogenic, and the resulting antibody was affinity purified by passing rabbit serum over an agarose-based column with the peptide thiol coupled to the resin. The column was washed, and bound antibodies were eluted using a low-pH glycine buffer before dialysis in phosphate-buffered saline (PBS). The affinity-purified anti-Eis antibody was stored frozen until used in Western blots at a 1:1,250 dilution.
Monoclonal antibodies specific for M. tuberculosis H37Rv antigens were provided by John Belisle, Colorado State University, Fort Collins, through the National Institutes of Health (NIH, Bethesda, Md.) contract entitled “Tuberculosis Research Material and Vaccine Testing.” The M. tuberculosis antigens (antibodies and dilutions) used in Western blots were lipoarabinomannan (LAM) (CS-35; diluted 1:2,000), α-crystallin (CS-49; diluted 1:500), 38-kDa lipoprotein (IT-23; diluted 1:500), and the 45-kDa secreted glycoprotein (CS-93; diluted 1:50).
Sera from human tuberculosis patients.
Sera from 15 sputum smear acid-fast bacterium-positive cavitary tuberculosis patients and 5 purified protein derivative (PPD)-positive healthy controls were used in Western blot analysis for detection of anti-Eis antibody. Electropurified Eis protein (see below) was used to test these sera for Eis-specific antibody. Human sera were used at 1:1,000 dilution to bind with 5-μg aliquots of electropurified Eis transblotted to nitrocellulose.
Construction of M. smegmatis with a chromosomal copy of eis.
Insertion of eis as a single copy into the chromosome of M. smegmatis [M. smegmatis(c69)] was carried out as described by Lee et al. (40), using the integrative plasmid vector pMV306 (41). The eis gene was cloned into this integrative vector by inserting the 1.6-kb Clal-HindIII fragment from p62-97 (56) into Clal-HindIII digested pMV306. The recombinant plasmid was electroporated into M. smegmatis as previously described (6), and transformants were selected by growth on 7H10 plates with kanamycin. The appearance of eis in the M. smegmatis chromosome was validated by Southern blot analysis as previously described (56).
Electroelution and N-terminal sequencing of the Eis protein.
M. smegmatis(p69), which contains the eis gene in the multicopy plasmid pOLGY (56), was grown to late log phase and resuspended in PBS containing protease inhibitor cocktail (Sigma P-8465). Cells were lysed using a Branson Sonifier 450, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (37) using 1.5-mm-thick, 12% resolving gels with 4% stackers. A small central region of the gel was sliced off and stained with GelCode Blue (Pierce) to quickly visualize the location of the Eis protein band for excision with a razor blade. The Eis protein was electroeluted out of gel slices using a Centilutor Micro-Electroeluter (Millipore) according to the manufacturer's directions. Electroeluted protein was concentrated 10-fold with Centicon Centrifugal filter devices (Amicon) with a 10,000-molecular-weight (MW) cutoff. Purity of stained, electroeluted Eis was determined with an AlphaImager 2000 densitometer (Alpha Innotech Corp). N-terminal peptide sequencing was performed by Wallace Clark, University of Arizona Laboratory for Protein Sequence Analysis. Peptide sequencing was performed on electropurified Eis recovered from both M. smegmatis(p69) and M. tuberculosis H37Ra. These preparations were resolved by SDS-PAGE, transblotted onto Immobilon-PSQ membranes (Millipore), and stained with Coomassie brilliant blue R-250 to identify the bands for sequencing.
Western blot analysis.
Mycobacterial lysates for Western blot analysis were prepared as follows. M. smegmatis(pOLYG) and M. smegmatis(p69) were grown on agar plates and harvested; bacterial lysates were prepared using 0.1- to 0.15-mm-diameter glass beads and a Turbomix device (VWR Scientific) attached to a vortexer and mixed for 30 min. Lysates of M. tuberculosis H37Rv and H37Ra were also prepared as described above. H37Rv cells inactivated by gamma irradiation were obtained via NIH contract with Colorado State University as mentioned above.
Western blot analysis was performed as previously described (54). Protein sample concentrations were determined by the bicinchoninic acid colorimetric assay (Pierce) with BSA standards as instructed by the manufacturer. Samples were separated by SDS-PAGE (12% gel) along with prestained MW standards (Gibco BRL), containing BSA (68 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), β-lactoglobulin (19 kDa), and lysozyme (14 kDa). Electrophoresis was done at 250 V and 90 mA for 4 to 5 h. Proteins from gels were transblotted overnight onto nitrocellulose membranes (Protran), which were then blocked for 1 h with PBS containing 5% powdered skim milk and 0.02% sodium azide. All antibodies used were diluted into PBS containing 0.25% powdered skim milk with 0.02% sodium azide. Membranes were rocked at 23°C for 5 h with primary antibodies, washed, and then incubated with secondary antibodies overnight at 4°C. Secondary antibodies conjugated to alkaline phosphatase, specific for Fc regions of either mouse (Bio-Rad catalog no. 170-6520), rabbit (Zymed catalog no. 62-6122), or human (Zymed catalog no. 62-8322) antibody were used at 1:2,000 dilutions. Membranes were washed with PBS and developed with chromogenic substrates as described by Sambrook et al. (51). Densitometry measurements of developed protein bands on Western blots were made using an Alphalmager 2000 (Alpha Innotech Corp.) and assigned arbitrary integrated density values (IDV).
Glycoprotein staining.
Purified Eis protein was analyzed for glycosylation using GelCode Glycoprotein stain (Pierce) as instructed by the manufacturer. The GelCode Glycoprotein stain is specific for proteins with the sugar moieties that include galactose, mannose, glucose, N-acetylglucosamine, N-acetylgalactosamine, sialic acid, fucose, and xylose. Glycoproteins appear as magenta bands against a colorless or pink background. The sensitivity of the stain was tested using avidin and horseradish peroxidase, with amounts of 0.625 ng and 0.16 μg, respectively, detected. One gel was stained for total protein with GelCode Blue and compared with an identical gel stained with GelCode Glycoprotein stain as instructed by the manufacturer.
Preparation of mycobacteria cellular fractions by differential centrifugation partitioning.
Subcellular fractionation was performed essentially as described by Ortalo-Magné et al. (46), with minor modifications. Briefly, 1 liter of M. tuberculosis H37Ra was grown to mid-log phase (3 weeks) in GAS medium (53) at 37°C with shaking before harvesting. By using GAS medium, which does not contain BSA, potential problems in concentration and analysis of the supernatant were alleviated. Cells were collected by a 30-min centrifugation at 10,000 × g, and the culture supernatant was saved for concentration following a procedure modified from Dubos et al. (19). The culture supernatant was passed through a 0.22-μm-pore-size bottle filter, and the filtrate was concentrated 100-fold in a Centricon Plus-80 concentrator with a 10,000-MW cutoff membrane (Amicon, Inc.). The concentrate was saved as the culture filtrate protein (CFP) fraction. Pelleted cells were washed, resuspended in PBS with protease inhibitor cocktail, and lysed using glass beads as described above. Lysates were centrifuged twice at 11,000 × g for 5 min at room temperature to remove unbroken cells and insoluble material. Lysate supernatants were centrifuged at 27,000 × g for 1 h at 4°C to pellet cell walls (34). The supernatant was collected and ultracentrifuged at 100,000 × g for 4 h at 4°C to separate cell membranes (pellet) from the cytoplasmic fraction (supernatant) (39). The cell wall and cell membrane fractions were each washed three times with PBS and resuspended in 500 μl of PBS. The cytoplasmic fraction was ultracentrifuged three more times for 4 h at 100,000 × g to ensure that all residual membranes were removed. The final volume of the cytoplasmic fraction was 3.5 ml, seven times the volumes of the cell wall and cell membrane fractions.
Triton X-114 detergent-phase partitioning.
M. tuberculosis H37Ra was grown in 7H9 with ADC and Tween 80 to late log phase before cells were pelleted, washed, and resuspended in PBS with protease inhibitors. Cells were lysed by vortexing with glass beads, and whole cells were removed as described above. The clarified lysate was centrifuged at 47,000 × g for 1 h at 4°C to produce soluble and insoluble fractions. The insoluble fraction was washed three times with PBS and then resuspended in PBS to a volume equivalent to that of the soluble fraction. Both the soluble and insoluble fractions were subjected to Triton X-114 (TX-114)-phase separation as described by Bordier (5). The soluble and insoluble fractions were separately treated with TX-114 (Sigma) at a final concentration of 2%. Samples were vortexed for 30 s and then placed on ice overnight. Samples were allowed to equilibrate to 37°C for 10 min before centrifugation at room temperature for 5 min at 10,000 × g to produce an upper aqueous phase and a lower detergent phase. All phase fractions were separated and backwashed four times (46). After backwashing, all phases were equilibrated with PBS and TX-114 before separation by SDS-PAGE and analysis by Western blotting.
Accumulation of Eis protein in culture supernatants.
A liquid culture of M. tuberculosis H37Ra grown in GAS medium was shaken at 37°C for 36 days. At various time points (3, 6, 12, 18, 24, 30, and 36 days), 15-ml aliquots were harvested. A sample was removed and used to determine CFU per milliliter by viable plate count determinations. Culture supernatants were then centrifuged to remove bacterial cells and passed through 0.22-μm-pore-size filters, and the filtrates were concentrated 100-fold using Millipore spin columns with a 10,000-MW cutoff. Levels of Eis and other selected M. tuberculosis proteins in the concentrated culture filtrate were analyzed by Western blotting as described above and compared over time to CFU. Data shown are representative of two separate experiments.
RESULTS
Expression of Eis protein in M. tuberculosis.
The eis gene is present only in members of the M. tuberculosis complex (M. tuberculosis strains H37Rv, H37Ra, and Erdman and M. bovis BCG), but its protein product has been previously shown to occur only in M. smegmatis containing eis on plasmid pOLYG (56). For detection of Eis in mycobacterial lysates by Western blot analysis, rabbit anti-Eis antibody was produced by using a synthetic peptide corresponding to the C-terminal domain of Eis as described in Materials and Methods.
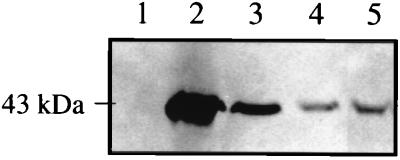
Results of Western blot analysis are presented in Fig. 1. The anti-Eis antibody detected the presence of the 42-kDa Eis protein in lysates of M. smegmatis(p69) (lane 2) and as a single chromosomal copy in M. smegmatis(c69) (lane 3) but not in lysates of M. smegmatis(pOLYG) (lane 1) since this mycobacterial species does not contain an eis homolog. The Eis protein was readily detected in lysates of both M. tuberculosis H37Ra (lane 4) and H37Rv (lane 5). The eis gene product was clearly overexpressed in M. smegmatis(p69) (lane 2) and probably in M. smegmatis(c69) (lane 3). These results demonstrate that the Eis protein is indeed produced in M. tuberculosis.
FIG. 1.
Western blot analysis of lysates of M. smegmatis transformants and M. tuberculosis for presence of the Eis protein. Mycobacterial lysates (47 μg of total protein per lane) were subjected to SDS-PAGE, transblotted onto nitrocellulose, and analyzed by Western blotting using anti-Eis antibody. Lanes: 1, M. smegmatis(pOLYG); 2, M. smegmatis(p69); 3, M. smegmatis(c69); 4, M. tuberculosis H37Ra; 5, M. tuberculosis H37Rv. The position of a 43-kDa protein molecular size standard is shown on the left.
Purification and N-terminal sequencing of the Eis protein.
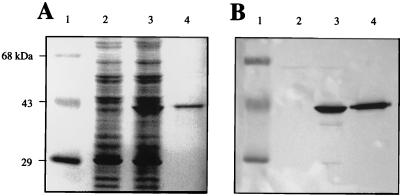
Numerous attempts to purify Eis from overproducing M. smegmatis(p69) with ion-exchange, size exclusion, and antibody-affinity columns all proved unsuccessful. This suggests that Eis may be associated with other components in a stable fashion, thus preventing easy separation by chromatography. Electroelution from SDS-polyacrylamide gels proved successful in purifying Eis (Fig. 2). The vector control M. smegmatis(pOLYG) lysate does not produce Eis (Fig. 2A, lane 2), in contrast to the M. smegmatis(p69) lysate containing the overexpressed 42-kDa Eis (lane 3). In comparison, lane 4 shows electropurified Eis. The electroeluted protein was greater than 90% pure, as determined by densitometry scan analysis on a GelCode Blue-stained gel (Fig. 2A). Western blot analysis using anti-Eis antibody confirmed that the electropurified protein was indeed Eis (Fig. 2B).
FIG. 2.
Purification of the Eis protein. Mycobacterial lysates prepared by sonication were subjected to SDS-PAGE, and proteins were detected by either GelCode Blue protein staining (A) or Western blot analysis using anti-Eis antibody (B). Lanes: 1, prestained MW protein standards; 2, M. smegmatis(pOLYG) (47 μg of total protein); 3, M. smegmatis(p69) (47 μg of total protein); 4, electropurified Eis protein (5 μg of total protein).
This purified protein was N-terminally sequenced (Fig. 3). The N terminus was established as TVTLCS. This demonstrates that the start of translation of Rv2416c is different from the MPQSDS N terminus described by Cole et al. (13) and the MFLLAA N terminus proposed by this laboratory (56). This modification does not alter the previously predicted promoter region for eis, which appears to be a strong promoter since its -35 and -10 promoter regions are in close agreement with the consensus sequences of TTGACA and TATAAT, respectively. The predicted strength of the promoter is in good agreement with the high levels of protein expression observed during in vitro mycobacterial growth (Fig. 1).
FIG. 3.
N-terminal sequencing of the Eis protein. Polypeptide sequence was determined by N-terminal Edman degradation of purified Eis protein. The putative amino acids are shown with N-terminal sequenced residues in bold. The putative −35 and −10 promoter elements and the Shine-Dalgarno (S.D.) sequence are underlined, with bases matching the consensus sequences for these regions in bold. Two previously reported start codons are boxed (13, 56) and the start codon based on this peptide sequence is double-boxed.
Hydropathy analysis of Eis.
The DAS (density alignment surface) (15) and TMpred (35) programs were used to analyze the Eis protein sequence for the presence of possible transmembrane domains. Most of the protein appears hydrophilic, the exception being the N terminus, where 10 uncharged residues from positions 78 to 89 are flanked by proline residues, suggesting a transmembrane domain (data not shown).
Eis is not a glycoprotein.
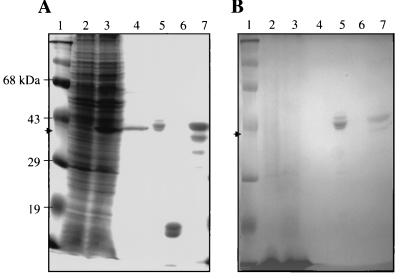
Because numerous secreted and exported M. tuberculosis proteins have been shown or suggested to be glycosylated (33), analysis for glycosylation of Eis was done with a stain that reacts specifically with glycoproteins (GelCode Glycoprotein stain) (Fig. 4). Two positive controls, horseradish peroxidase and the M. tuberculosis 45-kDa secreted glycoprotein (19), and a negative control, soybean trypsin inhibitor, were included in the experiment. Horseradish peroxidase and the 45-kDa glycoprotein both reacted with the stain (Fig. 4B, lanes 5 and 7, respectively). Ample amounts of soybean trypsin inhibitor were loaded (Fig. 4A, lane 6), but this protein did not react with the stain (Fig. 4B, lane 6). Neither Eis in the M. smegmatis(p69) lysate nor electropurified Eis (Fig. 4B, lanes 3 and 4, respectively) reacted with the stain. Similar results were obtained using Eis produced in M. tuberculosis H37Ra (data not shown). These results suggest that Eis is not a glycoprotein.
FIG. 4.
Eis protein is not glycosylated. Proteins subjected to SDS-PAGE were stained with either GelCode Blue (A) or GelCode Glycoprotein (B). Lanes: 1, prestained MW protein standards; 2, M. smegmatis(pOLYG) lysate (200 μg of total protein); 3, M. smegmatis(p69) lysate (200 μg of total protein); 4, electropurified Eis (7 μg of total protein); 5, horseradish peroxidase (4 μg of total protein); 6, soybean trypsin inhibitor (4 μg of total protein); 7, M. tuberculosis 45-kDa secreted glycoprotein (4 μg of total protein). Arrows denote locations of Eis.
M. tuberculosis H37Ra fractionation by differential centrifugation.
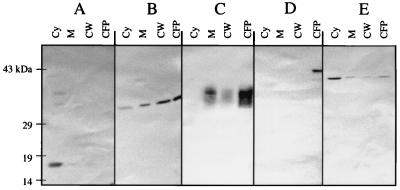
Subcellular fractions of M. tuberculosis H37Ra were separated by differential centrifugation and probed by Western blot analysis for the presence of Eis protein and other M. tuberculosis antigens used as specific markers for various subcellular fractions (Fig. 5). α-Crystallin was predominantly in the cytoplasmic fraction (Fig. 5A, lane Cy). The 45-kDa secreted glycoprotein, conversely, was predominantly seen in the extracellular environment (panel D, lane CFP). LAM is a set of membranous glycophospholipids anchored in the plasma membrane of M. tuberculosis and extending outward through the peptidoglycan, arabinogalactan, mycolic acids, and free lipids of the cell envelope (50). Consequently, it is difficult to isolate LAM into discrete subcellular fractions (Fig. 5C). The only location from which LAM was clearly absent was the cytoplasm (Fig. 5C, lane Cy), which agrees with its site of assembly in the cell wall (50). The 38-kDa lipoprotein was present in all four fractions (Fig. 5B), with increasing amounts found, moving out of the cell from the cytoplasm to the culture supernatant. Like the 38-kDa lipoprotein, Eis was found in all four subcellular fractions (Fig. 5E). However, unlike the 38-kDa lipoprotein, Eis lacks anything resembling the consensus signal sequence of lipoprotein precursors in bacteria (61). Eis was found primarily in the cytoplasmic fraction, with reduced levels in the membrane, cell wall, and culture supernatant fractions.
FIG. 5.
Localization of the Eis protein in M. tuberculosis H37Ra by differential centrifugation partitioning. H37Ra grown to mid-log phase was lysed and divided into the following fractions using differential centrifugation: Cy (cytoplasm; 44 μg of total protein), M (cell membrane; 5 μg of total protein), CW (cell wall; 10 μg of total protein), and CFP (26 μg of total protein). These fractions were subjected to SDS-PAGE, electroblotted onto nitrocellulose, and reacted with anti-Eis polyclonal antibodies or with monoclonal antibodies specific for the various localized M. tuberculosis proteins. The Western blots show primary antibodies binding to α-crystallin (A), 38-kDa lipoprotein (B), LAM (C), 45-kDa secreted glycoprotein (D), and Eis protein (E).
X-114 detergent-phase partitioning fractionation of M. tuberculosis H37Ra.
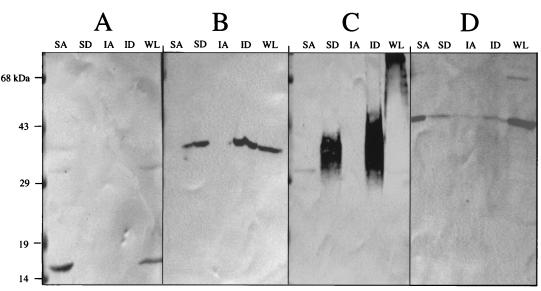
To further test the possibility that Eis might be associated with the hydrophobic environment of the plasma membrane and cell wall, H37Ra lysates were subjected to TX-114-phase fractionation and probed with the antibody for Eis (Fig. 6). Phase partitioning with TX-114 is a strategy for isolating integral membrane proteins that has been used in M. tuberculosis and other bacteria (5, 7, 47, 59, 61). Both soluble and insoluble portions of the bacterial lysates were treated with TX-114 to produce both aqueous and detergent soluble fractions. Antibodies specific for α-crystallin, 38-kDa lipoprotein, LAM, and Eis were used to analyze the various fractions. The hydrophobicities of these antigens match well with their subcellular locations shown in Fig. 5. The α-crystallin showed a preference for the aqueous phase (Fig. 6A, lane SA), while the 38-kDa lipoprotein (Fig. 6B, lanes SD and ID) and LAM (Fig. 6C, lanes SD and ID) partitioned preferentially into the detergent phase. LAM was observed as a diffuse band consistent with reported Western blot analysis of this glycolipid (39). LAM in the detergent fractions was significantly smaller than LAM in the whole-cell lysate (Fig. 6C, lane WL). This may be explained by the physical breakage of LAM during repeated vortexing of the detergent-phase samples as they were back washed to remove aqueous-phase antigens. Eis was found predominantly in the aqueous fraction of the soluble material of the lysate (Fig. 6D, lane SA) just as it was predominantly in the cytoplasmic fraction produced by differential fractionation (Fig. 5E).
FIG. 6.
Localization of the Eis protein in M. tuberculosis H37Ra by TX-114 detergent-phase partitioning. A lysate of M. tuberculosis H37Ra, with whole cells removed, was subjected to ultracentrifugation. Both the supernatant and the insoluble pellet were subjected to TX-114 partitioning into aqueous and detergent phases before Western blot analysis with antibodies specific for α-crystallin (A), the 38-kDa lipoprotein (B), LAM (C), and the Eis protein (D). Lanes: SA, supernatant aqueous phase; SD, supernatant detergent phase; IA, insoluble pellet aqueous phase; ID, insoluble pellet detergent phase; WL, whole-cell lysate not subjected to TX-114 partitioning. Each lane contained 100 μg of total protein.
Release of Eis into the culture supernatant.
The presence of Eis in the culture supernatant was analyzed to determine if the protein was being released by autolysis of cells or if it was naturally exported out of intact cells (61). Analysis of the eis gene, Rv2416c ORF, using a bioinformatic approach described by Gomez et al. (27; http://tbsp.phri.nyu.edu/) reveals a Signal P score of 0.341 and a SPScan score of 6.1. Both values fall below cutoff values established from scores based on known M. tuberculosis secreted proteins (27). Although there is no recognizable N-terminal secretion sequence for Eis, there are numerous examples of M. tuberculosis proteins without secretion sequences that have been identified in culture filtrates that include l-alanine dehydrogenase (57), glutamine synthetase (29), superoxide dismutase (1), catalase (48), and GroES (57). To address the question of whether Eis is released from intact cells, we grew M. tuberculosis H37Ra in liquid media and analyzed culture filtrates at various time points. Western blot analysis was used to detect the presence of Eis as well as a secreted M. tuberculosis antigen (45-kDa secreted glycoprotein) (38), a cytoplasmic protein (α-crystallin) (62), and a surface-associated protein that is known to be released into the culture supernatant (38-kDa lipoprotein) (24).
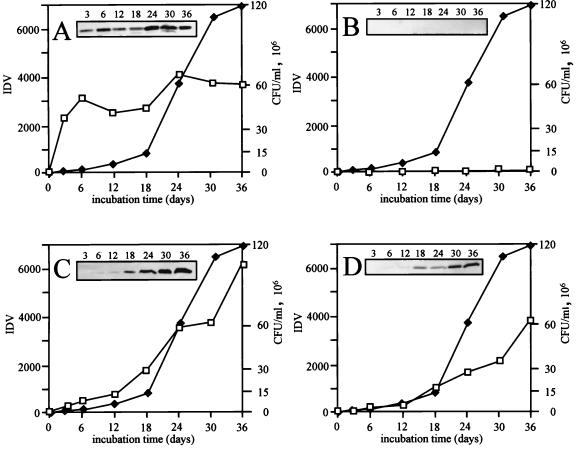
The 45-kDa secreted glycoprotein appeared in culture supernatant as early as 3 days in log-phase growth (Fig. 7A), whereas α-crystallin did not appear in the culture filtrate throughout the 36 days of growth (Fig. 7B). The α-crystallin protein was produced, but it remained intracellular during these stages of cell growth. This is indicated by detection of the antigen in mid-log-phase cell lysates analyzed in Fig. 5 and 6. These results suggest that no significant bacterial cell lysis occurred over the 36 days of the experiment. The 38-kDa lipoprotein, beginning at 18 days, was increasingly released throughout the remainder of the growth curve (Fig. 7C). This is apparently due to temporary acylated association of the antigen with the cell wall before its release. The appearance of the Eis protein in the culture supernatant (Fig. 7D) followed a pattern similar to that of the 38-kDa lipoprotein, with Eis being detected by 18 days of growth. By 36 days of growth, the levels of Eis released into the culture supernatant were comparable to the 3,800 IDV levels observed for the 45-kDa secreted glycoprotein. This suggests that Eis was steadily released across the plasma membrane during growth in liquid culture.
FIG. 7.
Detection of the Eis protein in M. tuberculosis culture supernatants. Aliquots from M. tuberculosis H37Ra GAS liquid cultures were recovered at various time points, and a portion was removed for viable plate count determinations. The remaining samples were centrifuged to remove cells, and the supernatant was passed through 0.22-μm-pore-size filters and concentrated 100-fold before being subjected to Western blot analysis with antibodies specific for 45-kDa glycoprotein (A), α-crystallin (B), 38-kDa lipoprotein (C), and Eis (D). Results of Western blots are shown as insets and represent CFPs collected on the indicated days. Densitometry readings were assigned arbitrary IDV units and were graphed (boxes) and compared to bacterial numbers (CFU per milliliter) (diamonds) during culture growth.
Detection of antibody to Eis protein in sera from human tuberculosis patients.
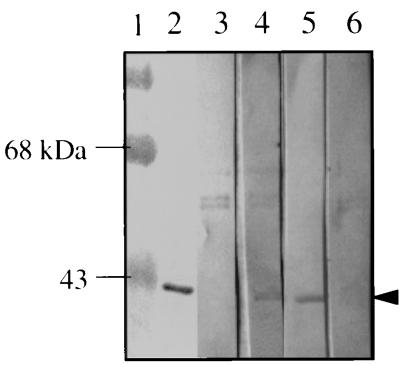
A small-scale study was done to screen sera from human pulmonary tuberculosis patients for the presence of antibodies to the Eis protein by using Western blot analysis (Fig. 8). Detection of antibody would suggest that Eis is produced during an active human infection. Electropurified Eis was transblotted onto nitrocellulose membranes, and strips were incubated with sera from 15 cavitary tuberculosis patients and from 5 PPD-positive healthy controls. Among tuberculosis patient sera, 40% gave clear positive reactions (6 out of 15 patients tested). Lane 2 in Fig. 8 shows a positive reaction using rabbit anti-Eis antibody which readily detects the electropurified Eis, while lanes 4 and 5 are representative of positive patient sera reactions to Eis. Lane 3 represents a tuberculosis patient who was not generating antibody for Eis. None of the five PPD-positive healthy control sera tested gave positive reactions (lane 6).
FIG. 8.
Detection of antibodies to Eis in sera from human tuberculosis patients. Electropurified Eis was analyzed by Western blotting using rabbit anti-Eis antibody (lane 2) or representative sera from tuberculosis patients (lanes 3 to 5). Prestained MW standards are shown in lane 1. Lane 3 is representative of patient serum which did not react with Eis, while lanes 4 and 5 are representative patient sera which demonstrate positive reactions to Eis. Lane 6 is a representative serum from a PPD-positive, sputum-negative healthy individual. The arrowhead shows the position of the Eis protein.
DISCUSSION
It is unlikely that the prevention, control, and ultimate eradication of tuberculosis will be achieved without better methods of diagnosis, treatment, and vaccination. To this end, the search continues for virulence factors of M. tuberculosis that might enable it to survive in the macrophage. One such potential virulence factor is the Eis protein (56). The work presented here was aimed at establishing the subcellular location of Eis as a first step in elucidating its function.
It was first critical to show that M. tuberculosis produces the Eis protein, as the previous study regarding eis dealt with the expression of the gene in M. smegmatis only (56). Eis is indeed produced in M. tuberculosis, where it has the same N terminus as in M. smegmatis(p69). Examination of the predicted amino acid sequence of Eis with appropriate computer programs predicted that the Eis protein would be mainly hydrophilic, with possibly one or two transmembrane regions in its N terminus, and that it would not contain a secretion signal sequence.
Two procedures were used in the subcellular localization studies: differential centrifugation and detergent-phase partitioning. In both techniques, it was essential to use monoclonal antibodies specific for M. tuberculosis antigens known to be associated with particular subcellular fractions to establish the cleanness of separation of the various cellular components. One such antibody was specific for α-crystallin, which was designated a cytoplasmic antigen. The actual subcellular location of α-crystallin in M. tuberculosis is unknown, although it has been proposed by several groups to reside in the cell envelope and outside the bacillus (16, 39). Others, however, have used TX-114-phase partitioning to show that the protein resides in the cytoplasmic fraction (62). Chang et al. (11) showed that the protein is capable of functioning as a multisubunit complex of 149 ± 6 kDa. Since reports of the protein as being cell wall associated are largely based on observations of the protein pelleting at 100,000 × g, it is possible the protein's appearance in the cell periphery is due to the large mass of the oligomeric state of the protein. Claims that the α-crystallin homolog is secreted by M. tuberculosis should be evaluated with caution since the protein is abundantly expressed in older organisms (62) and surface association or release into the environment could be due to older bacilli autolysing in mixed cultures. We found that α-crystallin is produced in the mid-logrithmic phase of growth and that at this stage the protein cannot be detected in the cell envelope or culture supernatant.
Another control antibody used in the subcellular localization studies was specific for the 38-kDa lipoprotein. Immunogold labeling showed this antigen is localized to the cell surface of M. tuberculosis (24). Ortalo-Magné et al. (46) obtained subcellular fractionation results for the 38-kDa protein similar to those presented here in Fig. 5, with the antigen found in the cytosol, on the cell surface, and in 7-day culture filtrates. Young and Garbe (61) found TX-114 detergent-phase partitioning of the 38-kDa protein similar as to that shown in Fig. 6. Unlike Eis, the 38-kDa protein contains a lipoprotein consensus sequence and is believed to attach to the cell envelope via acylation to lipids (22, 32). Antibody specific for LAM, an abundant cell wall component, was used as an additional control for cell surface association. Like the 38-kDa protein, the 45-kDa antigen of M. tuberculosis contains a typical N-terminal signal sequence used for translocation across the plasma membrane by the Sec-dependent pathway (38). However, the 45-kDa protein does not form an acyl bond with the cell surface and is readily released into the culture supernatant, as is evident in Fig. 5 and 7.
The fractionation studies described here suggest that Eis appears primarily in the cytoplasm and in modest amounts in the cell envelope and in the culture supernatant. This pattern is very similar to the subcellular distribution of l-alanine dehydrogenase, another reported M. tuberculosis CFP protein that lacks a signal sequence (46). Eis lacks an N-terminal lipoprotein consensus element but it does appear to interact with the cell envelope, as shown in Fig. 5 and by its ability to exist in the detergent phase (Fig. 6) despite its hydrophilic appearance.
It was interesting to test for glycosylation of Eis protein since its cellular localization pattern is similar to the glycosylated 19- and 38-kDa lipoproteins. Also, Eis protein is found in the CFP fraction of M. tuberculosis which has been shown to contain as many as 21 individual glycoproteins (8). Traditionally glycoproteins of mycobacteria have been identified using Western blot analysis with peroxidase-conjugated concanavalin A as the probe (23, 26). An alternative method was use of GelCode Glycoprotein stain (Pierce), which specifically stains for the carbohydrate moieties on the 45-kDa glycoprotein of M. tuberculosis. Eis protein does not appear to be glycosylated, as tested by this staining procedure. This is not surprising because most of the glycosylated CFPs of M. tuberculosis have typical N-terminal sequences involved in Sec-dependent translocation, which Eis does not contain.
The lack of an N-terminal secretion sequence raises the question of how Eis appears in the CFP fraction in the absence of autolysis. Almost nothing is known about how M. tuberculosis proteins that lack a signal sequence are secreted. Often these proteins are normally cytosolic enzymes (58). Like the Eis protein, GroES lacks a signal sequence but has been found in the culture filtrate as described previously (57). The potential biological significance of secreted or exported proteins from M. tuberculosis is that these proteins may play a role in bacterial pathogenesis by directly interacting with the host. The Erp protein is exported by M. tuberculosis. An erp mutant is significantly impaired for survival in macrophages and in immunocompetent mice (4). Like the Eis protein, Erp has no homology to proteins of known function, and its mode of action remains unclear.
The eis gene has been identified only in M. tuberculosis and in M. bovis BCG (56), and so antibodies to its protein product might prove useful in the development of a serological diagnosis of tuberculosis infections. Evidence presented here shows that 40% of sera from patients with tuberculosis generated antibody to Eis, whereas none of the sera from five PPD-positive individuals did. A histidine-tagged translation fusion of Eis has been purified by nickel affinity chromatography, and affinity-purified His-tagged Eis will be used in enzyme-linked immunosorbent assays to test a large battery of pulmonary and miliary tuberculosis patient sera for Eis-specific antibody.
It is not claimed that Eis is a secreted protein, only that it may be released from intact M. tuberculosis cells. This possibility will be investigated by use of a secretion reporter system. The use of alkaline phosphatase (PhoA) translational fusions in studying M. tuberculosis secreted proteins is limited to use with surrogate hosts such as M. smegmatis which may differ in its secretory methods. However, Staphylococcus aureus nuclease fusions have been shown by Downing et al. to identify proteins secreted directly from M. tuberculosis (18). Immunogold staining of M. tuberculosis for Eis will be done to verify that the protein is truly surface associated. An additional test to determine if Eis is specifically exported to the culture filtrate rather than by autolysis will be to compare supernatant protein profiles with those of whole cell pellets, a technique employed by Vanet and Labique (55).
ACKNOWLEDGMENTS
We thank Clifton E. Barry III for invaluable assistance and technical suggestions, and we thank Esteban Roberts for critical review of the paper. We also thank Amy Carlson for technical assistance.
This work was supported by National Institutes of Health grants AI45537 and AI45537-01A2 to R.L.F.
REFERENCES
- 1.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong J A, Hart P D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes and phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley L W. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. Science. 1993;261:1454–1457. doi: 10.1126/science.8367727. [DOI] [PubMed] [Google Scholar]
- 4.Berthet F-X, Lagranderie M, Gounon P, Laurent-Winter C, Ensergueix D, Chavarot P, et al. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science. 1998;282:759–762. doi: 10.1126/science.282.5389.759. [DOI] [PubMed] [Google Scholar]
- 5.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 6.Bose M, Chander A, Das R H. A rapid and gentle method of the isolation of genomic DNA from mycobacteria. Nucleic Acids Res. 1993;21:2529–2530. doi: 10.1093/nar/21.10.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt M E, Riley B S, Radolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brounstein M, Belisle J T. Genetics of protein secretion. In: Hatfull G F, Jacobs W R Jr, editors. Molecular genetics of mycobacteria. Washington, D.C.: ASM Press; 2000. pp. 203–220. [Google Scholar]
- 9.Chan J, Fujiwara T, Brennan P J, McNeil M, Turco S J, Sibille J-C, Snapper M, Aisen P, Bloom B R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci USA. 1989;86:2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J, Fan X, Hunter S W, Brennan P J, Bloom B R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chin W, Gilbert H F, Quiocho F A. Mycobacterium tuberculosis 16-kDa antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7228. [PubMed] [Google Scholar]
- 12.Chen P, Ruiz R E, Li Q, Silver R F, Bishai W R. Construction and characterization of a Mycobacterium tuberculosis mutant lacking the alternative sigma factor gene, sigF. Infect Immun. 2000;68:5575–5580. doi: 10.1128/iai.68.10.5575-5580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Delvin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skeleton J, Squares R, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 14.Crowle A J, Dahl R, Ross E, May M H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991;60:2160–2165. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham A F, Spreadbury C L. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deretic V, Fratti R A. Mycobacterium tuberculosis phagosome. Mol Microbiol. 1999;31:1603–1609. doi: 10.1046/j.1365-2958.1999.01279.x. [DOI] [PubMed] [Google Scholar]
- 18.Downing K J, McAdam R A, Mizrahi V. Staphylococcus aureus nuclease is a useful secretion reporter for mycobacteria. Gene. 1999;239:293–299. doi: 10.1016/s0378-1119(99)00408-4. [DOI] [PubMed] [Google Scholar]
- 19.Dubos K M, Swiderek K, Khoo K-H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dye C, Scheele S, Pathania V, Raviglione M C. Global burden of tuberculosis: estimated increase, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 21.Erickson P R, Herzberg M C. Evidence of covalent linkage of carbohydrate polymers to a glycoprotein from Streptococcus sanguis. J Biol Chem. 1993;268:23780–23783. [PubMed] [Google Scholar]
- 22.Espita C, Cervera I, Gonzalez R, Mancilla R. A 38-kDa Mycobacterium tuberculosis antigen associated with infection. Its isolation and serological evaluation. Clin Exp Immunol. 1989;77:373–377. [PMC free article] [PubMed] [Google Scholar]
- 23.Espitia C, Mancilla R. Identification, isolation, and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin Exp Immunol. 1989;77:378–383. [PMC free article] [PubMed] [Google Scholar]
- 24.Espitia C, Elinos M, Hernandeez-Pando R, Mancilla R. Phosphate starvation enhances expression of the immunodominant 38-kilodalton protein antigen of Mycobacterium tuberculosis: demonstration by immunogold electron microscopy. Infect Immun. 1992;60:2998–3001. doi: 10.1128/iai.60.7.2998-3001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fifis T, Costopoulos C, Radford A J, Bacic A, Wood P R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991;59:800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garbe T, Harris D, Vordermeir M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez M, Johnson S, Gennaro M L. Identification of Mycobacterium tuberculosis secreted proteins by a bioinformatic approach. Infect Immun. 2000;68:2323–2327. doi: 10.1128/iai.68.4.2323-2327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goren M B, Brokl O, Schaefer W B. Lipids of putative relevance to virulence of Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic acids. Infect Immun. 1974;9:142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harth G, Clemens D L, Horwitz M A. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci USA. 1994;91:9342–9346. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harth G, Lee B Y, Horwitz M A. High-level heterologous expression and secretion in rapidly growing nonpathogenic mycobacteria of four major Mycobacterium tuberculosis extracellular proteins considered to be leading vaccine candidates and drug targets. Infect Immun. 1994;65:2321–2328. doi: 10.1128/iai.65.6.2321-2328.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harth G, Horwitz M A. Export of recombinant Mycobacterium tuberculosis superoxide dismutase is dependent upon both information in the protein and mycobacterial export machinery: a model for studying export of leaderless proteins by pathogenic mycobacteria. J Biol Chem. 1999;274:4281–4292. doi: 10.1074/jbc.274.7.4281. [DOI] [PubMed] [Google Scholar]
- 32.Haslov K, Andersen A B, Ljungqvist L, Weis Bentzon M. Comparison of the immunological activity of five defined antigens from Mycobacterium tuberculosis in seven inbred guinea pig strains: the 38-kDa antigen is immunodominant. Scand J Immunol. 1990;31:503–507. doi: 10.1111/j.1365-3083.1990.tb02798.x. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann J L, O'Gaora P, Gallagher A, Thole J E R, Young D B. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996;15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschfield G R, McNeil M, Brennan P J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990;172:1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann K, Stoffel W. TMbasc—a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 36.Kaufmann S H E, van Embden I D A. Tuberculosis: a neglected disease strikes back. Trends Microbiol. 1993;1:2–5. doi: 10.1016/0966-842x(93)90015-j. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee B-Y, Hefta S A, Brennan P J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee M H, Pascopella L, Jacobs W R, Jr, Hatfull G F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and Bacille Calmette-Guérin. Proc Natl Acad Sci USA. 1991;88:3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mdluli K, Sherman D R, Hickey M J, Kreswirth B N, Morris S, Stover C K, Barry C E., III Biochemical and genetic data suggests that InhA is not the primary target for activated isoniazid in Mycobacterium tuberculosis. J Infect Dis. 1996;174:1085–1090. doi: 10.1093/infdis/174.5.1085. [DOI] [PubMed] [Google Scholar]
- 42.Mescher M F, Strominger J L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci USA. 1976;73:2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milhoc A, Kluepfel D. Purification and characterization of a β-glucosidase from Streptomyces lividans 66. Can J Microbiol. 1990;36:53–56. [Google Scholar]
- 44.Murray C J, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- 45.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1998;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortalo-Magné A, Dupont M-A, Lemassu A, Andersen A B, Gounon P, Daffe M. Molecular composition of the outer most capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 47.Radolf J D, Chamberlain N R, Clausell A, Norgard M V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent Triton X-114. Infect Immun. 1988;56:490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raynaud C, Etienne G, Peyron P, Lanéelle M A, Daffé M. Extracellular enzyme activities potentially involved in the pathogenicity of Mycobacterium tuberculosis. Microbiology. 1998;144:577–587. doi: 10.1099/00221287-144-2-577. [DOI] [PubMed] [Google Scholar]
- 49.Riley L W. Determinants of cell entry and intracellular survival of Mycobacterium tuberculosis. Trends Microbiol. 1995;3:27–31. doi: 10.1016/s0966-842x(00)88865-4. [DOI] [PubMed] [Google Scholar]
- 50.Salman M, Lonsdale J T, Besra G S, Brennan P J. Phosphatidylinositol synthesis in mycobacteria. Biochim Biophys Acta. 1999;1436:437–450. doi: 10.1016/s0005-2760(98)00151-9. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Sturgill-Koszychi S, Schlesinger P H, Chakraborty P, Haddix P L, Collings H L, Fok A F, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification of Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 53.Takayama K, Schnoes H K, Armstrong E L, Boyle R W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J Lipid Res. 1975;16:308–317. [PubMed] [Google Scholar]
- 54.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4356. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanet A, Labique A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei J, Dahl J L, Moulder J W, Roberts E A, O'Gaora P, Young D B, Friedman R L. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J Bacteriol. 2000;182:377–384. doi: 10.1128/jb.182.2.377-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weldingh K, Rosenkrands I, Jacobsen S, Rasmussen P B, Elhay M J, Andersen P. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect Immun. 1998;66:3492–3500. doi: 10.1128/iai.66.8.3492-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiker H G, Michell S L, Hewinson R G, Spierings E, Nagai S, Harboe M. Cloning, expression and significance of MPT53 for identification of secreted proteins of Mycobacterum tuberculosis. Microb Pathog. 1999;26:207–219. doi: 10.1006/mpat.1998.0267. [DOI] [PubMed] [Google Scholar]
- 59.Wise K S, Kim M F. Major membrane surface proteins of Mycoplasma pneumoniae selectively modified by covalently bound lipids. J Bacteriol. 1987;169:5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. Tuberculosis control and research strategies for the 1990s: memorandum from a W.H.O. meeting. Bull W H O. 1992;17:17–21. [PMC free article] [PubMed] [Google Scholar]
- 61.Young D B, Garbe T R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991;142:55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- 62.Yuan Y, Crane D B, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Lathigra R, Garbe T, Catty D, Young D B. Genetic analysis of superoxide dismutase, the 23-kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991;5:381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Heym B, Allen B, Young D B, Cole S T. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]