Abstract
Cystic fibrosis is an autosomal recessive genetic disorder that damages the exocrine function of the body, resulting in alterations of multiple organs. In the respiratory system, it is known to cause bronchiectasis, recurrent bronchitis, and pneumonia; however, to the best of our knowledge, there are no reported cases of pulmonary arteriovenous malformations associated with this disease. Herein, we report a case of cystic fibrosis with multiple pulmonary arteriovenous malformations. A 16-year-old girl, who has been monitored since childhood for pancreatitis of unknown cause, experienced respiratory symptoms and hypoxemia (PaO2 = 57 mmHg). At 13 years of age, chest computed tomography revealed bronchiectasis, bronchial wall thickening, and tree-in-bud sign. Genetic testing was performed, and the patient was diagnosed with cystic fibrosis. However, the computed tomography scan also showed incidental nodular lesions in the left superior and both the inferior pulmonary lobes, suggesting multiple arteriovenous malformations. Dynamic computed tomography was performed which, confirmed the presence of 3 pulmonary arteriovenous malformations. Coil embolization was performed on all lesions, and the hypoxemia was corrected. Marked hypoxemia in a patient with cystic fibrosis may not be explained only by the presence of bronchiectasis and/or bronchial wall thickening; in such cases, it may be necessary to examine possible additional findings on computed tomography images, such as arteriovenous malformations.
Keywords: Cystic fibrosis, Pulmonary arteriovenous malformations, Hypoxemia, Dynamic CT
Introduction
Cystic fibrosis is caused by an autosomal recessive mutation, impairing the passage of chloride ions (Cl−) through the membranes of epithelial cells in the ductal lumen of various organs. Mucus and secretions in the lumens of the airways, intestinal tract, pancreatic ducts, bile ducts, sweat ducts, and infusion ducts become excessively viscous, causing the lumens to become obstructed and susceptible to infection [1]. In typical cystic fibrosis, the patient develops a fetid ileus after birth, dyspepsia due to pancreatic exocrine insufficiency, and recurrent respiratory tract infections. It is associated with a high rate of sinusitis and male infertility due to congenital tubal defects. The frequency of cystic fibrosis varies widely by race. While Caucasians are commonly affected with an incidence of approximately 1 in 3000 births, the incidence in Japan and other Asian countries is extremely low [2].
Repeated infections and bronchiectasis are associated with decreased airway clearance. Other complications, such as pneumonia, pulmonary hemorrhage, and pneumothorax, are known to occur in these patients; in contrast, there are no previous reports of pulmonary arteriovenous malformations (AVMs) associated with cystic fibrosis. Here, we describe a case of cystic fibrosis with marked hypoxemia; the patient presented with multiple pulmonary AVMs, in addition to bronchiectasis, and bronchial wall thickening.
Case report
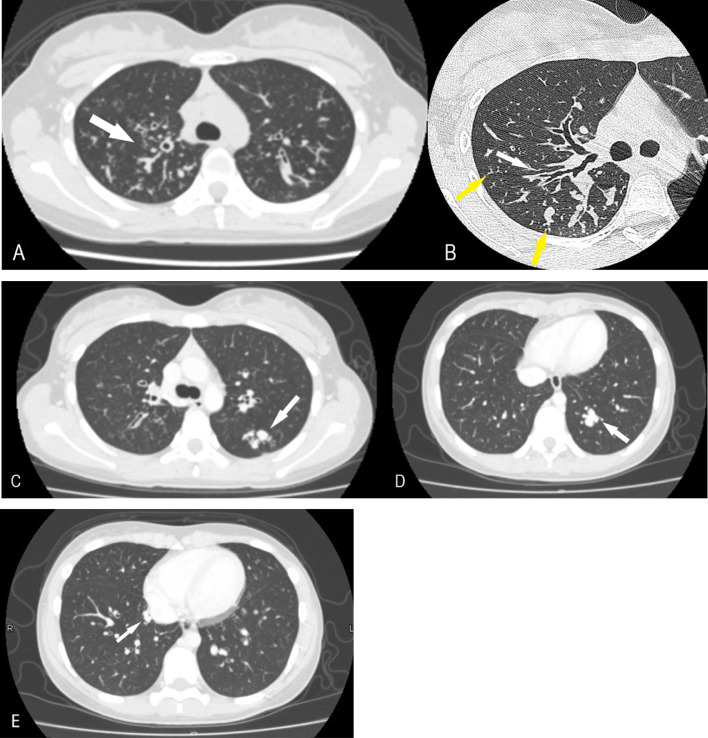
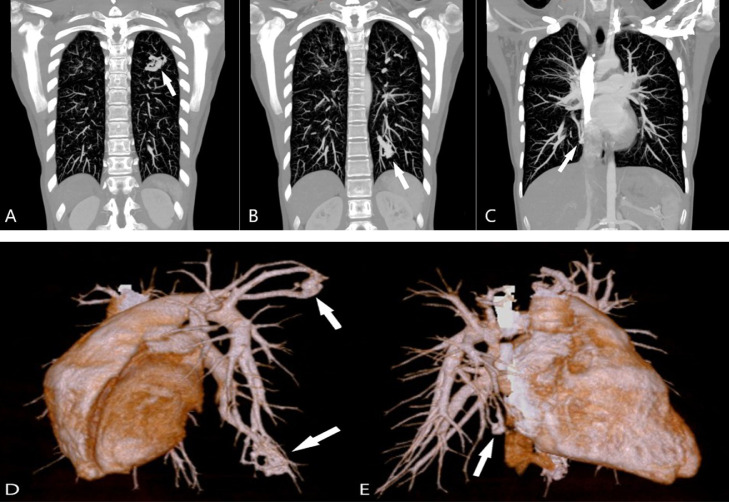
A 4-year-old girl was initially presented with fever, abdominal pain, and vomiting. No alterations were detected on abdominal ultrasound and computed tomography (CT) scans; however, the patient was suspected to have pancreatitis because of hyperamylasemia. She was subsequently followed up for pancreatitis of unknown cause. At 10 years of age, she developed febrile convulsions and was diagnosed with Chiari type I malformation based on magnetic resonance imaging. Respiratory symptoms began to appear approximately at 13 years of age, and chest radiography revealed a lung opacity. Chest CT showed bronchiectasis, bronchial wall thickening, and tree-in-bud sign, predominantly in the right superior pulmonary lobe (Figs. 1A and B). Cystic fibrosis was suspected based on the history of pancreatitis and chest CT findings, and a sweat test was performed; the Cl− concentration in the sweat was higher than normal. Genetic analysis revealed mutations in dele16-17b and R347H, confirming the diagnosis of cystic fibrosis. The aforementioned chest CT scan coincidentally also showed some nodular lesions in the left superior and both inferior pulmonary lobes (Figs. 1C-E). These lesions were suspected to be AVMs as the patient's hypoxemia (PaO2 = 57 mmHg) could not be explained entirely by the bronchiectasis from cystic fibrosis. A dynamic CT confirmed the diagnosis of multiple pulmonary AVMs in 3 locations (Figs. 2A-E). Coil embolization was first performed on the AVMs in the left lung. Since hypoxemia was not completely resolved, coil embolization was also performed on the AVM in the inferior lobe of the right lung, and hypoxemia resolved. At present, at the age of 16 years, she is doing well with no worsening of hypoxemia and no new AVMs.
Fig. 1.
Axial CT scans of the chest. (A) Bronchiectasis is present predominantly in the superior right pulmonary lobe (white arrow). (B) Bronchiectasis, mucoid impaction in the bronchi (white arrow), and tree-in-bud sign (yellow arrow) are visible in the right superior pulmonary lobe. (C-E) Nodular shadows in the left superior and both the inferior lobes (white arrows).
Fig. 2.
Coronal maximum intensity projection (MIP) CT images and 3-dimensional (3D) reconstructions. (A-C) Coronal MIP images clearly show the multiple contiguous vascular structures (white arrows). (D,E) 3D reconstructions visualizing the inflow arteries, sac, and outflow veins of each lesion (white arrows).
Discussion
Cystic fibrosis is caused by a dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-dependent Cl− channel, which maintains the pH and water content of the luminal fluid at appropriate levels; when CFTR function is lost, the lumen is obstructed by viscous secretions with a low water content that tends toward acidity, resulting in a variety of clinical findings including repeated and intractable airway infections, pancreatic exocrine insufficiency, biliary stasis liver injury, ileus gravidarum, and electrolyte abnormalities. The diagnosis of cystic fibrosis is made by clinical symptoms, sweat testing, and CFTR genetic testing, and even in this patient, the final diagnosis was also made based on a comprehensive evaluation all of these factors.
On high-resolution CT, cystic fibrosis may present with diffuse central and peripheral bronchiectasis initially with a predominant distribution to the superior lobes, particularly on the right side in some patients [3]. Cylindrical bronchiectasis is the most common type in patients with cystic fibrosis, followed by the cystic form [4]. The bronchial wall and peribronchial interstitium are often thickened [5]. Furthermore, early findings reflecting peripheral bronchial obstruction include mucoid impaction in the bronchi, granular lesions, tree-in-bud sign, mosaic perfusion, and air trapping [5], [6], [7], [8]. This patient had imaging findings suggestive of cystic fibrosis, associated with pulmonary lesions. This genetic disease may cause several pulmonary complications, such as pneumonia, pneumothorax, and hemorrhage; however, to the best of our knowledge, an association with pulmonary AVMs has not been previously reported in the literature. This case may be the first report of this association.
Pulmonary AVMs are pathological anastomoses between arterial and venous vessels in the lungs; they are classified as simple, with a single inflow artery and outflow vein, or complex, with 2 or more inflow arteries and outflow veins [9]. Dynamic CT in this patient clearly showed 2 or more inflow arteries, a sac, and 2 or more outflow veins in all lesions; thus, classified as complex-type pulmonary AVMs. These malformations may manifest with hypoxemia due to the reduced gas exchange caused by intrapulmonary shunts. In this case, the multiple AVMs were the likely cause of hypoxemia.
Pulmonary AVMs are secondary to hereditary hemorrhagic telangiectasia (HHT) in 47%-80% of cases [10], and 15%-30% of HHT patients have multiple AVMs [11]. Therefore, HHT was also suspected in this case; however, a detailed systemic examination and family history showed no clinical features consistent with HHT, and a genetic search for this disease was not performed.
Chronic inflammation associated with bronchiectasis and liver cirrhosis is also a known cause of secondary pulmonary AVMs [10,12]. In this case, the bronchiectasis was most prominent in the right superior lobe, whereas the AVMs were located in the left superior and both inferior lobes. Therefore, bronchiectasis was unlikely to be the cause of the AVMs. Only one case of cystic fibrosis with AVM has been previously reported [13], and the AVM was located in the pelvis. It was speculated that in that case the liver disease associated with cystic fibrosis may have caused high estrogen levels and promoted the AVM formation. However, it is difficult to consider estrogen involvement in this case because the liver function was preserved.
An association between Chiari I type malformation and cystic fibrosis was found in the literature [14]; however, no association has ever been reported between this malformation and pulmonary AVMs.
In conclusion, this report describes a rare case of cystic fibrosis associated with multiple pulmonary AVMs. In cases of severe hypoxemia in cystic fibrosis that cannot be explained by bronchiectasis, it is necessary to consider the possibility of a pulmonary AVM with careful CT examination. Perhaps, similar cases occurred in the past and they were not recognized due to imaging limitations. The pathological origin of the pulmonary AVMs, in this case, is unclear; however, the possible association of cystic fibrosis and pulmonary AVMs should be investigated in the future.
Patient consent
The patient and her parents provided written informed consent for the publication of this report.
Acknowledgments
The authors would like to thank Keisuke Dasai for image editing and Michale G. Piscitello for English language assistance in writing the paper.
Footnotes
Competing Interests: None.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Yamashiro Y, Shimizu T, Oguchi S, Shioya T, Nagata S, Ohtsuka Y. The estimated incidence of cystic fibrosis in Japan. J Pediatr Gastroenterol Nutr. 1997;24:544–547. doi: 10.1097/00005176-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, et al. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175:943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179:783–788. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 5.Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Götz M, Wojnarowski C, et al. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology. 1999;213:537–544. doi: 10.1148/radiology.213.2.r99nv04537. [DOI] [PubMed] [Google Scholar]
- 6.Webb WR. Thin-section CT of the secondary pulmonary lobule: anatomy and the image-the 2004 Fleischner lecture. Radiology. 2006;239:322–338. doi: 10.1148/radiol.2392041968. [DOI] [PubMed] [Google Scholar]
- 7.Gruden JF, Webb WR, Warnock M. Centrilobular opacities in the lung on high-resolution CT: diagnostic considerations and pathologic correlation. AJR Am J Roentgenol. 1994;162:569–574. doi: 10.2214/ajr.162.3.8109498. [DOI] [PubMed] [Google Scholar]
- 8.Goris ML, Zhu HJ, Blankenberg F, Chan F, Robinson TE. An automated approach to quantitative air trapping measurements in mild cystic fibrosis. Chest. 2003;123:1655–1663. doi: 10.1378/chest.123.5.1655. [DOI] [PubMed] [Google Scholar]
- 9.White RI, Mitchell SE, Barth KH, Kaufman SL, Kadir S, Chang R, et al. Angioarchitecture of pulmonary arteriovenous malformations: an important consideration before embolotherapy. AJR Am J Roentgenol. 1983;140:681–686. doi: 10.2214/ajr.140.4.681. [DOI] [PubMed] [Google Scholar]
- 10.Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J. 2002;78:191–197. doi: 10.1136/pmj.78.918.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colon N, Schlegel C, Pietsch J, Chung DH, Jackson GP. Congenital lung anomalies: can we postpone resection? J Pediatr Surg. 2012;47:87–92. doi: 10.1016/j.jpedsurg.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki Y, Nojiri M, Oikawa T, Nishiki K, Nakase K, Takahara Y, et al. Acquired pulmonary arteriovenous malformation associated with bronchiectasis: a case report. J Med Case Rep. 2022;16:24. doi: 10.1186/s13256-021-03233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledson MJ, Wahbi Z, Harris P, Walshaw MJ. A large pelvic arteriovenous malformation in an adult patient with cystic fibrosis. Postgrad Med J. 1999;75:353–354. doi: 10.1136/pgmj.75.884.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AJ, Raol VH, Jea A. Rare association between cystic fibrosis, Chiari Ⅰ malformation, and hydrocephalus in a baby: a case report and review of the literature. J Med Case Rep. 2011;5:366. doi: 10.1186/1752-1947-5-366. [DOI] [PMC free article] [PubMed] [Google Scholar]