Abstract
Various genotypes of Borrelia burgdorferi sensu stricto have been previously identified among a large collection of isolates cultured from patients with Lyme disease in the United States. Furthermore, association of specific genotypes with hematogenous dissemination early in the disease course has been observed. The present study assessed kinetics of spirochete dissemination and disease severity in C3H/HeJ mice infected with two different genotypes of B. burgdorferi. Spirochete load in plasma and ear and other tissue samples of infected mice was measured by quantitative PCR, and these data were compared to those obtained by culture and histopathologic analysis. In mice infected with isolate BL206 (a type 1 strain), the peak number of spirochetes was observed in plasma between day 4 and 7, in heart and ear tissue on day 14, and in joints on day 28 postinoculation. There was a correlation between the peak number of spirochetes in plasma on day 4 or 7 and that in ear biopsy and joint specimens on day 14. By contrast, spirochete burdens in plasma of mice infected with isolate B356 (a type 3 strain) were 16- and 5-fold lower than those of BL206-infected mice on days 7 and 14 of infection, respectively. Similarly, approximately 6- and 13-fold fewer spirochetes were detected in the heart tissues of B356-infected mice compared to BL206-infected mice. Histopathologically, severe arthritis and aortitis were noted only in mice infected with isolate BL206. Spirochete dissemination and disease severity vary significantly in mice infected with distinct genotypes of B. burgdorferi, suggesting that genotypic differences in the infecting spirochetes play a key role in the pathogenesis and development of clinical disease.
The clinical features of Lyme disease in patients infected with Borrelia burgdorferi sensu lato range from transient cutaneous infection at the site of tick bite (erythema migrans [EM]) to more serious or prolonged manifestations such as carditis, neuroborreliosis, arthritis, or acrodermatitis chronica atrophicans (28, 37, 40). Disease severity in humans may depend on a number of factors, including characteristics of the infecting strain of B. burgdorferi sensu lato, coinfection with other tick-borne pathogens and inherent differences in individual host responses to infection (4, 9, 16, 27, 31, 44). Studies employing murine models of infection by B. burgdorferi or other pathogenic species of Borrelia support the notion that strain differences in the spirochete may be one of the critical determinants of disease severity (9, 12, 23, 31, 34). In an experimental study with the relapsing fever agent Borrelia turicatae, Pennington et al. reported that arthritis severity and spirochete burden in C3H and CB-17 SCID mice were determined mainly by the serotype of the infecting strain (31).
The population of B. burgdorferi is genetically heterogeneous. Ten different Borrelia species have been delineated within the B. burgdorferi sensu lato complex (40). Three of these species, B. burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii, are responsible for causing human Lyme disease in Europe (3, 7, 40). Moreover, an association between the clinical manifestations of Lyme disease and the infecting B. burgdorferi sensu lato species has been suggested (2, 38, 40, 44). Three B. burgdorferi sensu lato species (B. burgdorferi sensu stricto, Borrelia andersonii, and Borrelia bissettii) have been described in the United States (14, 22, 32). However, unlike the situation in Europe, to date only a single species, B. burgdorferi sensu stricto, has been cultured from patients with Lyme disease in North America (40). Previously, we characterized B. burgdorferi sensu stricto isolates cultured from patients with Lyme disease in Westchester County, N.Y., by restriction fragment length polymorphism (RFLP) analysis of the 16S-23S rRNA spacer. All of the clinical isolates were classified into one of three RFLP types (17–19). Interestingly, for Lyme disease patients with skin infected with type 1 strains a significantly higher percentage of (43%) blood cultures for B. burgdorferi yielded positive results compared to cultures of blood from those infected in skin with type 2 (20%) or type 3 (3%) isolates (44). The frequency of multiple EM lesions was also significantly greater among patients infected with type 1 strains, suggesting an association of specific B. burgdorferi RFLP types with hematogenous dissemination in patients with early Lyme disease (44). This finding was supported by a study which demonstrated that only 4 of 21 different B. burgdorferi OspC genotypes were associated with invasive infection in humans (36). In Europe, of seven genetically distinct OspA genotypes, serotype 4 B. garinii isolates were cultured from the cerebrospinal fluid samples of patients with neuroborreliosis significantly more frequently than the other genotypes (21, 41, 43).
To elucidate further whether infections with different genotypes of B. burgdorferi sensu stricto could potentially affect disease pathogenesis, the kinetics of spirochete dissemination and the severity of the resultant disease were evaluated in a murine model of Lyme disease. Two B. burgdorferi clinical isolates representing two different RFLP types were inoculated into genetically susceptible C3H/HeJ mice. Dissemination of spirochetes to blood and tissues was evaluated by in vitro cultivation and quantitative PCR (qPCR), and disease severity was assessed by caliper measurement of ankle joint swelling and histopathologic assessment of inflammatory cell infiltrates in heart and ankle joint tissues.
MATERIALS AND METHODS
B. burgdorferi isolates.
Two clinical isolates of B. burgdorferi sensu stricto, BL206 at passage 3 and B356 at passage 4, were used in this study. Isolate BL206 was cultured from the blood of a patient with EM, and isolate B356 was cultured from the skin biopsy of an EM lesion of a different patient. These two isolates were classified as RFLP type 1 and 3, respectively, based on ribosomal DNA spacer RFLP analysis (17, 18).
Mice.
Virus antibody-free, male and female 3-week-old C3H/HeJ mice (n = 30) were obtained from Jackson Laboratory (Bar Harbor, Maine). Mice were maintained in separate cages in the Department of Comparative Medicine at New York Medical College, according to the guidelines of the National Institutes of Health for care and use of laboratory animals.
Infection of mice with B. burgdorferi.
Thirty mice were randomly divided into three groups of 10 mice each. Spirochetes were cultured in BSK-H medium supplemented with 6% rabbit serum (Sigma Chemical Co., St. Louis, Mo.) at 33°C for 5 to 7 days. Spirochete concentrations were determined by fluorescence microscopy by mixing 10-μl aliquots of the diluted culture material with 10 μl of an acridine orange solution (100 μg/ml). The motility of the spirochetes was also examined by dark-field microscopy. Cultures were adjusted to a final concentration of 105 spirochetes/ml in sterilized phosphate-buffered saline (PBS) (pH 7.4), and each mouse in the experimental groups was inoculated intradermally with 0.1 ml of the culture material (104 spirochetes) in the shaven back. Mice from the control group were injected at the same site with an equal volume of sterile PBS. In addition, six mice were inoculated with 106 (n = 2) and 107 (n = 4) B356 organisms, respectively.
Blood samples from each mouse were collected at 4, 7, 14, and 28 days after inoculation. Tibiotarsal joints were measured at the same time points with a digital metric caliper (SPI Digmax; Ralmike's Tool-A-Rama, South Plainfield, N.J.) through the thickest antero-posterior diameter of the ankle. Five mice from each of the three groups were sacrificed by exposure to carbon dioxide at days 14 and 28 postinoculation. Samples of blood, ear, and various internal tissues (joint, heart, spleen, urinary bladder, and brain) were collected aseptically from each mouse for culture, qPCR, and histopathologic analysis.
Culture of B. burgdorferi from mouse samples.
Three to five drops of whole blood (approximately 120 μl) were added directly into 5 ml of BSK-H medium. For culture of spirochetes from ear and other tissues, a 2-mm-diameter ear punch and samples of internal organs were immersed separately in 70% ethanol for several minutes, washed twice in PBS (pH 8.0) in 24-well culture plates, and incubated in 5 ml of BSK-H medium containing rifampin (50 μg/ml; Sigma). All cultures were maintained at 33°C for up to 6 weeks and examined for the presence of spirochetes every 7 to 10 days by dark-field microscopy.
Preparation of DNA from mouse samples.
Five to thirty milligrams (up to 80 mg for joints) of each tissue was digested with 20× (vol/wt) of 0.1% type I collagenase A (Sigma) in PBS (pH 7.4) at 37°C for at least 4 h and then mixed with an equal volume of 0.2-mg/ml proteinase K in 200 mM NaCl, 20 mM Tris-HCl (pH 8.0), 50 mM EDTA, and 1% sodium dodecyl sulfate. Brain tissues were digested with only 40× (vol/wt) 0.1% proteinase K in the above buffer. After an overnight incubation at 55°C, aliquots of 100 to 200 μl of the above-mentioned digestion mixtures or 100 μl of plasma specimens were subjected to DNA isolation by means of a commercial nucleic acid extraction kit (Isoquick; Orca Research, Bothell, Wash.). The purified DNA was resuspended in a total volume of 50 to 100 μl of sterile water, such that 2 μl equaled the amount of DNA extracted from 0.1 mg of tissue.
Conventional PCR amplification of the mouse nidogen gene.
For monitoring the quality of DNA extraction, a 150-bp fragment of the mouse nidogen gene was amplified by conventional PCR with primers nido.F and nido.R, which are described below. PCR was performed in a 25-μl reaction mixture containing 1× PCR buffer (Roche Molecular Biochemicals, Indianapolis, Ind.), a 200 μM concentration of each deoxynucleoside triphosphate, 1 U of Taq DNA polymerase (Roche), a 0.5 μM concentration of each primer, and 2 μl of DNA template in thermocycler (Eppendorf Scientific Inc., Westbury, N.Y.). The amplification profiles consisted of preheating at 94°C for 5 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 45 s; and a final extension at 72°C for 5 min.
Quantification of B. burgdorferi DNA in mouse tissues by qPCR.
The Lightcycler PCR system (Roche Diagnostics, Mannheim, Germany) was used for amplification and online quantification of B. burgdorferi-specific recA and mouse-specific nidogen. PCR was performed in glass capillaries in a final volume of 10 μl containing 1× Lightcycler master mix (Roche), 3 mM MgCl2, a 1 μM concentration of each set of primers, and 2 μl of mouse DNA template (1 μl for ear biopsy specimens) or external standard template. The primers used to amplify B. burgdorferi-specific recA were nTM17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and nTM17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′) (26). The primers used to detect mouse nidogen were nido.F (5′-CCA GCC ACA GAA TAC CAT CC-3′) and nido.R (GGA CAT ACT CTG CTG CCA TC-3′) (26). The amplification program for recA and nidogen consisted of heating at 95°C for 30 s followed by 35 cycles (for nidogen) or 45 cycles (for recA) of heating at 20°C/s to 95°C with a 1-s hold, cooling at 20°C/s to 60°C with a 1-s hold (4 s for skin and plasma samples), and heating at 20°C/s to 72°C with a 10-s hold (8 s for nidogen). The fluorescent product was collected at 80°C for recA and 82°C for nidogen at the last step of each cycle to minimize signal from nonspecific products. A melting curve was acquired by heating the product at 20°C/s to 95°C, cooling it at 20°C/s to 60°C, and slowly heating it at 0.2°C/s to 95°C, with fluorescence collection at 0.2°C intervals.
Quantification data were analyzed with the Lightcycler software provided by the manufacturer. Only the log-linear portion of the amplification was chosen for analysis. The background fluorescence was removed by setting a noise band, and a standard curve was prepared by plotting the crossing point (Ct) versus the log of copy number based on standards included in each run. Copy numbers for the experimental samples were calculated by comparing the crossing points of the samples with those of the standards. Melting curves were used to determine the specificity of the PCR products.
External standards for qPCR.
Two external standard sets were developed to quantify B. burgdorferi-specific recA and mouse-specific nidogen. For recA, genomic DNA was prepared from B. burgdorferi isolate B356. The recA concentration in the purified B. burgdorferi DNA (copies per microliter) was estimated based on DNA concentration as determined by the optical density at 260 nm, assuming a genome size of 1.5 Mbp. This was further confirmed by a PCR-based limiting-dilution assay. DNAs containing 10 to 105 copies of recA were mixed with 200 ng of control mouse DNA and used as standards for qPCR. Alternatively, 100 to 107 B. burgdorferi isolate BL206 organisms were spiked into 100 μl of mouse tissue digestion mixtures, followed by DNA extraction as described above. The purified DNA that contains both mouse and spirochete DNA was used subsequently as template for qPCR, assuming a single copy of recA per spirochete (10).
Mouse nidogen was amplified by conventional PCR as described above, cloned into the pGEM-T vector (Promega, Madison, Wis.), and transformed into Escherichia coli DH5α. The plasmid DNA containing mouse nidogen was purified using the Mini Plasmid Extraction Kit (Qiagen), and the concentration of nidogen in the purified plasmid DNA was estimated as described above for recA. Tenfold serial dilutions of the purified plasmid DNA containing 100 to 106 copies of nidogen in 2 μl were prepared and used as standards to calculate nidogen copy number in mouse tissues.
Semiquantitative assessment of tissue histopathology.
One hindlimb (ankle joint) and half of the heart from each mouse sacrificed at 2 or 4 weeks after infection were immersion fixed in 10% neutral buffered formalin. For hindlimb tissues, bone and joint were decalcified by 5% formic acid treatment overnight prior to processing. All tissues were processed to slides by dehydration in graded ethanol, clearing in xylene, embedding in paraffin, sectioning at thickness of 5 μm, and staining with hematoxylin and eosin, and then coverslips were placed on the slides. All histopathologic sections were labeled in code, and observers were blinded to the study conditions until after the histopathologic features were assessed.
Each histopathologic parameter was assessed and scored separately using a semiquantitative criterion-based scoring methodology. Scores of 0 (absent), 10 (mild), 20 (moderate), or 30 (severe) were assigned to each parameter, and the average scores of all these parameters were used to reflect the overall severity of carditis and arthritis for each group. The scoring criteria were as follows.
Heart tissues were scored for myocarditis (score of 10, one collection of three or more lymphocytes among myofibers; score of 20, two or three such collections; score of 30, more than three collections), valvulitis (score of 10, a valve with 3 to 5 lymphocytes; score of 20, a valve with 6 to 10 lymphocytes; score of 30, valve with >10 lymphocytes), and aortitis (score of 10, great vessel with an infiltrate of 1 to 3 lymphocytes, typically in adventitia; score of 20, 4 to 10 lymphocytes; score of 30, >10 lymphocytes).
Limb tissues were scored for synovitis (score of 10, infiltrate of 3 to 10 lymphocytes; score of 20, more than 10 lymphocytes without synovial proliferation; score of 30, >10 lymphocytes with synovial proliferation), joint capsule inflammation (score of 10, joint capsule infiltrates of three to six lymphocytes; score of 20, more than six lymphocytes without fibrocyte (fibroblast) proliferation; score of 30, more than six lymphocytes with fibroblast proliferation), and myositis (score of 10, skeletal muscle with one collection of three to six lymphocytes; score of 20, two or more such collections without myofiber atrophy; score of 30, more than two such collections with myofiber atrophy).
Serology.
Mouse serum and/or plasma samples were collected and stored at −20°C until use. The presence of anti-B. burgdorferi specific antibodies was determined by Western blotting with a commercial kit (MarDx, Carlsbad, Calif.). Serum and plasma samples were diluted to 1:160 or 1:320 with Tris-buffered saline. A 1:3,000 dilution of alkaline phosphatase-labeled goat anti-mouse IgM-IgG-IgA antibody (Kirkegaard & Perry Lab, Gaithersburg, Md.) was used to develop the blots following the procedure recommended by the manufacturer.
Statistical analysis.
Statistical analysis was performed using programs included in the SPSS software package (version 10; SPSS Inc., Chicago, Ill.).
RESULTS
Clinical signs and measurements of ankle joints.
Groups of 10 C3H/HeJ mice were inoculated with either B. burgdorferi isolate BL206 (type 1) or B356 (type 3) or sterile PBS (control group). All mice inoculated with BL206 developed clinically apparent arthritis. This was heralded by the appearance of clinical swelling and redness of the rear ankles which appeared between day 10 and 12 after inoculation and lasted until the mice were sacrificed. In one mouse the joint swelling resolved by day 25. No joint swelling or redness was apparent among any mice inoculated with isolate B356 or any mice in the control group.
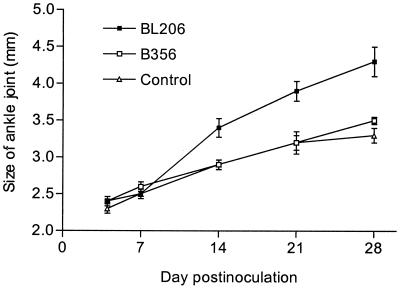
Ankle diameters of each animal were measured with a digital caliper on days 4, 7, 14, 21, and 28 (Fig. 1). No significant difference in the average values was observed among the three groups of mice at day 4 or 7. However, the average ankle diameter was significantly greater in BL206-infected mice than those in B356-infected and control animals at days 14, 21, and 28 after inoculation (P < 0.01). No significant difference in rear ankle diameter was noted between B356-infected and control mice (P > 0.05).
FIG. 1.
Ankle swelling of C3H/HeJ mice infected with different B. burgdorferi isolates. Data represent the averages and standard deviations (error bars) of values from each group of 10 mice on days 4, 7, and 14 and from 5 mice on days 21 and 28. Significant differences between BL206- and B356-infected mice were observed on days 14, 21, and 28 (P = 0.007, 0.004, and 0.009, respectively).
Culture of B. burgdorferi from blood, ear biopsy specimens, and tissues.
Blood and ear biopsy samples were collected for culture of B. burgdorferi from all mice on days 4, 7, and 14 after inoculation and on day 28 from the remaining five mice in each group. In addition, at sacrifice the heart, spleen, urinary bladder, and brain of each mouse were collected aseptically and portions of each tissue were subjected to culture. The results are summerized in Table 1. All blood samples collected on days 4 and 7 from mice inoculated with isolate BL206 were positive. By day 28, only two of five blood samples grew spirochetes. In total, 30 of 35 (85.7%) blood samples from BL206-infected mice were positive by culture. In contrast, all the ear punch biopsy specimens collected on days 4 and 7 from BL206-infected mice were negative by culture, but 9 of 10 ear biopsy samples taken on day 14 and all ear biopsy specimens (5 of 5) taken at day 28 were positive by culture. The recovery rate of B. burgdorferi from internal organs of BL206-infected mice was relatively higher in tissues harvested on day 14 (14 of 18 tissues [77.8%]) than those collected on day 28 (10 of 18 tissues [55.6%]), although this difference is not significant (P > 0.05). In contrast, all blood, ear biopsy, and tissue samples collected at each time point from mice infected with 104 to 107 B356 organisms were negative by culture.
TABLE 1.
Culture of B. burgdorferi from blood, ear biopsy specimens, and selected tissues of C3H/HeJ mice inoculated with isolates BL206 and B356a
| Strain | RFLP typeb | Inoculumc | Day postinoculation | No. positive/no. testedd
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Blood | Ear | Heart | Spleen | Bladder | Brain | ||||
| BL206 | 1 | 104(n = 10) | 4 | 10/10 | 0/10 | ||||
| 7 | 10/10 | 0/9 | |||||||
| 14 | 8/10 | 9/10 | 4/4 | 3/5 | 5/5 | 2/4 | |||
| 28 | 2/5 | 5/5 | 4/5 | 1/4 | 3/4 | 2/5 | |||
| B356 | 3 | 104(n = 10) | 4 | 0/10 | 0/10 | ||||
| 7 | 0/9 | 0/9 | |||||||
| 14 | 0/9 | 0/9 | 0/4 | 0/4 | 0/4 | 0/4 | |||
| 28 | 0/4 | 0/4 | 0/5 | 0/5 | 0/4 | 0/5 | |||
| 106(n = 2e) | 14 | 0/2 | 0/2 | 0/2 | NT | 0/2 | NT | ||
| 107(n = 4e) | 14 | 0/4 | 0/4 | 0/3 | NT | 0/3 | NT | ||
| 28 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
All cultures of specimens from control group mice (n = 10) were negative.
RFLP types were based on ribosomal DNA spacer RFLP analysis as described previously (17).
The numbers of mice inoculated with each inoculum are shown in parentheses.
Five mice from each group were sacrificed at days 14 and 28, respectively. Contaminated samples were not included. NT, not tested.
For these animals, cultures were also performed on skin biopsy specimens taken from inoculation sites on days 4 and 14 postinfection, and all cultures were negative.
Sensitivity, specificity, and reproducibility of qPCR.
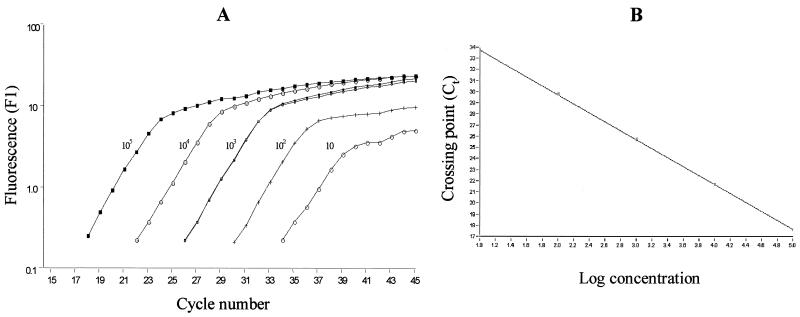
Genome sequence analysis and experimental evidence indicates that there is only one copy of recA in a single chromosome of B. burgdorferi (10, 26). Previously, this chromosome-encoded B. burgdorferi-specific gene was successfully used to quantify spirochete loads in infected mice by Lightcycler PCR (26). In this study, 10-fold serial dilutions containing approximately 1 to 107 copies of the recA gene per 2 μl were prepared from purified genomic DNA of B. burgdorferi isolate B356. The copy numbers of recA in these dilutions were further confirmed by a PCR-based limiting-dilution assay. Although single-copy sensitivity was achieved with this recA primer set in the Lightcycler assay (data not shown), very low copy numbers (<10 spirochetes) in 10 μl of PCR mixture resulted in a high degree of variability. Therefore, serial dilutions which contained 10 to 105 copies of recA per 2 μl were employed in the Lightcycler to yield a standard curve for quantifying B. burgdorferi in blood, ear biopsy, and tissue specimens from infected mice. As shown in Fig. 2, plotting the Ct values relative to the number of spirochetes always resulted in a linear correlation with a coefficient (r) value of >0.98. The specificity of this assay was evaluated by using DNAs from control mice and DNAs from several, unrelated species of bacteria (e.g., E. coli and Salmonella enterica serovar Typhimurium). No amplification of recA was observed with these samples (data not shown).
FIG. 2.
Standard curve used in qPCR for estimation of B. burgdorferi-specific recA in mouse specimens. PCR was performed in 10 μl of reaction mixture containing 200 ng of control mouse DNA and B. burgdorferi DNAs extracted from 10 to 105 spirochetes. (A) Data obtained in a typical run as presented by the Lightcycler software. A duplicate sample containing 103 spirochetes was included. (B) Standard curve of Ct versus log concentration of B. burgdorferi.
Reproducibility of the qPCR assay was assessed by comparison of the copy numbers obtained from at least two different PCR runs for a total of 50 randomly selected specimens. The interassay variations of the PCR ranged from 7 to 26.2% in different types of mouse samples (7% for ear biopsy specimens [258 ± 17; n = 10], 12.7% for plasma [279 ± 36; n = 20], 14.2% for heart tissues [964 ± 137; = 10], and 26.2% for joint [229 ± 60; n = 10] [means ± standard deviations]). In addition, 10 to 105 spirochetes were spiked into mouse heart tissues to test the efficiency of DNA extraction. A high correlation was found between the expected numbers of spirochetes and those determined by qPCR in the spiked samples (r = 0.99; 95% confidence interval [CI], 0.99 to 1.00).
Quantification of B. burgdorferi DNA in plasma and tissue specimens.
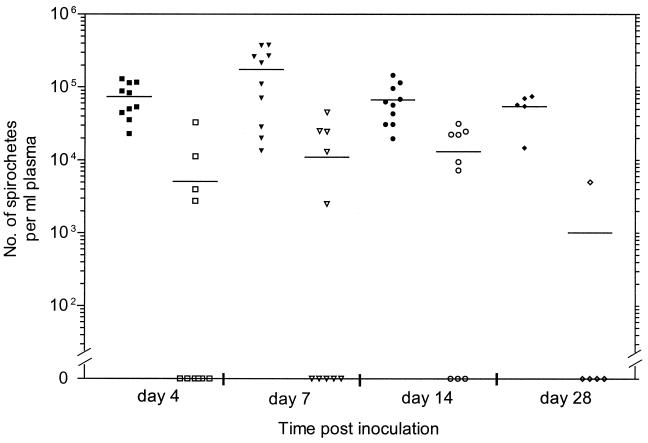
To analyze the kinetics of dissemination of B. burgdorferi, spirochete burdens in plasma of infected mice were monitored by qPCR over the course of infection. As depicted in Fig. 3, the overall spirochete burdens in plasma were 16- and 5-fold higher in mice infected with isolate BL206 than those infected with isolate B356 at day 7 and 14 postinfection, respectively. For mice infected with BL206, spirochetes were detected at day 4, with a peak load (1.8 × 105/ml plasma) occurring on day 7 and then gradually subsiding by days 14 and 28. In contrast, spirochete DNA was detectable in the blood of only 4 of 10 mice infected with B356 at day 4 after infection. The numbers of spirochetes in the plasma of these mice increased on day 7, reached a peak (1.3 × 104/ml plasma) on day 14, and were undetectable by day 28 in four of the five mice.
FIG. 3.
Spirochete burden in the plasma of C3H/HeJ mice infected with B. burgdorferi. BL206 (solid symbols) and B356 (open symbols) DNA was measured by qPCR as described in Materials and Methods. The samples in which no spirochetes were detected are plotted as zero on the x axis. The average number of spirochetes for each group of mice on different days is indicated by the horizontal line.
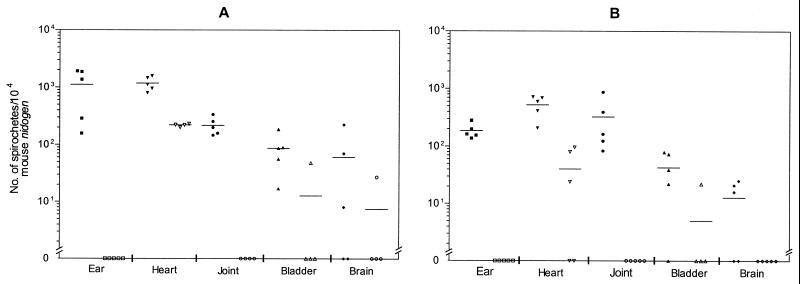
Spirochete burdens in the ear biopsy, heart, joint, bladder, and brain tissues collected from mice infected with isolates BL206 and B356 on days 14 and 28 postinoculation are shown in Fig. 4. Spirochete numbers are normalized to 104 nidogen copies to account for variation in mouse cell density in different tissue specimens. For mice infected with BL206, the highest spirochete loads were detected in the ear biopsy (1.12 × 103 spirochetes/104 nidogen genes) and heart (1.18 × 103 spirochetes/104 nidogen genes) specimens on day 14 of infection. Assuming that each mouse has at least 100 mg of heart tissue and that there are approximately 104 copies of mouse nidogen in DNA extracted from 0.1 mg of this tissue, it is estimated that, on average, at least 1.2 × 106 spirochetes are harbored in the heart of an infected mouse. Relatively lower numbers of spirochetes were detected in urinary bladder (87 spirochetes/104 nidogen genes) and brain (60 spirochetes/104 nidogen genes) specimens. At four weeks after infection, the numbers of B. burgdorferi organisms decreased in most of the examined specimens (ear biopsy, heart, bladder, and brain) of BL206-infected mice. However, peak spirochete loads in the ankle joints (mean ± standard error, [3.3 × 102 ± 1.4 × 102]/104 nidogen genes) were observed at 4 weeks. This was in accordance with the increased severity of arthritis on day 28 as shown by histopathology (see below) and is in agreement with previous reports (6, 26, 33).
FIG. 4.
Number of spirochetes in tissue of C3H/HeJ mice infected with B. burgdorferi. Five mice from each group were sacrificed on days 14 and 28. DNA was extracted from the indicated tissues and subjected to qPCR. Numbers of spirochetes in the tissues were normalized to 104 copies of mouse nidogen. Data from BL206- and B356-infected mice are shown as solid and open symbols, respectively. The average number of spirochetes for each group of mice is indicated by the horizontal line. (A) 2 weeks postinfection; (B) 4 weeks postinfection.
In contrast to the findings in BL206-infected mice, no B. burgdorferi DNA was detected in ear or joint tissues of B356-infected mice at either 14 or 28 days. Spirochetal DNA was found in only two urinary bladder samples and one brain specimen. However, B. burgdorferi-specific DNA was consistently detected in heart tissues of these mice, with the number of spirochetes approximately 6- and 13-fold lower compared to those of BL206-infected mice at day 14 and 28 after infection, respectively. A greater difference in average bacterial burdens was noted in the heart tissues of B356-infected mice between day 14 and 28 (from 220 spirochetes/104 nidogen genes to 40/104 nidogen genes) relative to that observed in BL206-infected animals (from 1,183 spirochetes/104 nidogen genes to 526/104 nidogen genes).
Correlation of spirochete burden in plasma and tissues of the infected mice.
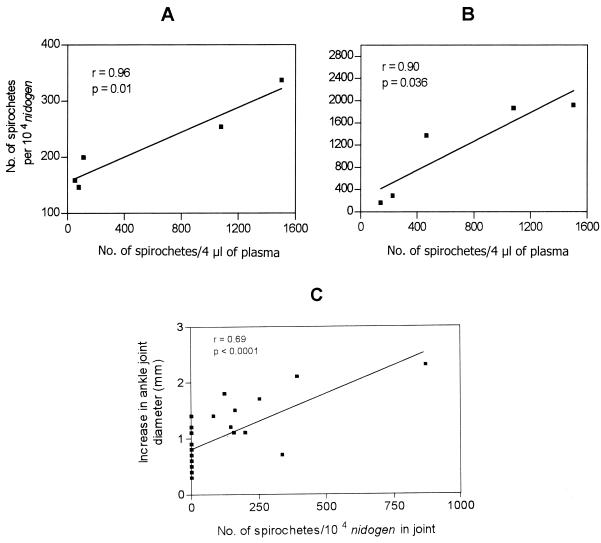
To evaluate the potential effect of bacteremia on the dissemination of B. burgdorferi to various tissues and on the development of clinical disease, the peak level of spirochetemia in blood on day 4 or 7 and the number of spirochetes in the ear biopsy, heart, and joint tissues of BL206-infected mice sacrificed at day 14 were investigated. A strong correlation was found between the spirochete load in day 7 plasma and the number of spirochetes in joints on day 14 (r = 0.96; 95% CI, 0.49 to 0.99; P = 0.01) (Fig. 5A). Similarly, the number of spirochetes in ear biopsy specimens was significantly correlated with peak spirochetemia (r = 0.90; 95% CI, 0.10 to 0.99; P = 0.036) (Fig. 5B). No significant correlation was noted between the peak spirochete load in blood and the number of spirochetes in heart tissues on day 14 (r = 0.45; 95% CI, −0.30 to 0.86; P > 0.05).
FIG. 5.
Correlation between the spirochete load in plasma on day 4 or 7 and spirochete numbers in joint (A) and ear biopsy (B) specimen on day 14 of C3H/HeJ mice infected with B. burgdorferi isolate BL206. (C) Correlation between ankle joint swelling and spirochete burden in the joints of mice infected with B. burgdorferi and controls. The increased ankle joint diameter obtained on day 14 or 28 for each mouse is plotted versus the spirochete numbers in the joints at the same time points as measured by qPCR.
Association of joint disease and spirochete burden in the joints.
Caliper measurements of ankle swelling in mice appear to reflect the degree of edema associated with the regions directly proximal to the ankle and provide one indicator of the inflammatory response and severity of disease (6, 31, 45). Therefore, the difference between the ankle diameter on the day of sacrifice and that measured on day 4 and the spirochete load in the joint tissue were assessed for 28 mice for which such data were available. There was a significant correlation between the spirochete load in the joints and the severity of joint swelling (r = 0.69, 95% CI, 0.43 to 0.85; P < 0.0001) (Fig. 5C).
Comparison between culture and qPCR.
A total of 72 blood, ear biopsy, and tissue specimens from BL206-infected mice were subjected simultaneously to culture and qPCR for detection of B. burgdorferi. Sixty (83.3%) of these were positive by both culture and qPCR, and B. burgdorferi-specific DNA was detected in 8 of the 12 culture-negative specimens. This results in an overall PCR positivity rate of 94.4%. None of the four tissues that were negative by PCR was positive by culture. The comparison of culture and qPCR is limited to BL206-infected animals since no positive cultures were obtained from B356-infected mice.
Histopathology.
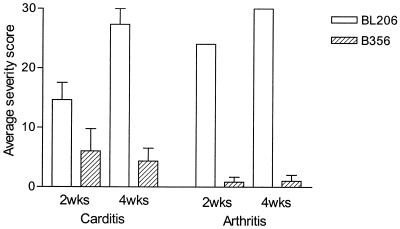
Histopathologic findings are summarized in Fig. 6. Analysis of BL206-infected mice at 2 weeks revealed that four of five had aortitis (average score, 20), two of five had moderate valvulitis (average score, 10), and all had varying degrees of myocarditis (average score, 14). Three of five mice had severe synovitis, with the other two mice having moderate or mild synovitis (average score, 24). At 4 weeks, all animals had severe aortitis (average score, 30) and valvulitis (average score, 30) and three of the infected animals had developed a severe myocarditis (average score, 22); all of the animals had severe synovitis, joint capsule inflammation, and myositis (average score, 30).
FIG. 6.
Histopathology of Lyme carditis and arthritis in mice infected with B. burgdorferi isolates BL206 and B356. Five mice in each group were sacrificed at 2 and 4 weeks after inoculation. The joint and heart tissues from each mouse were subjected to histopathologic analysis as described in Materials and Methods. The severity scores of carditis were averages of the valvulitis, myocarditis, and base-vessel inflammation scores of the heart tissues; the severity scores of arthritis were averages of the synovitis, capsule inflammation, and myositis scores of the joints. All differences in disease severity between BL206- and B356-infected mice were significant at both 2 and 4 weeks (P < 0.001). Error bars, standard deviations.
In contrast, analysis of B356-infected mice at 2 and 4 weeks showed less-severe histopathology. At 2 weeks, two of four mice had mild valvulitis (average score, 5), three of four had mild or moderate myocarditis (average score, 13), and none had aortitis. One animal had a mild synovitis, without joint capsule thickening or myositis (average score, 2). At 4 weeks, none of the mice had valvulitis, two of three had mild myocarditis (average score, 7), and one of five had severe aortitis (average score 6). Only one animal had mild joint inflammation at 4 weeks.
Serology.
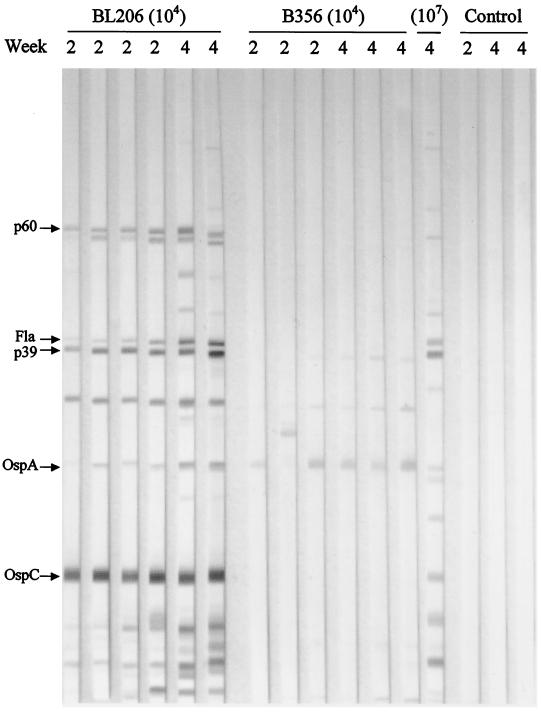
B. burgdorferi-specific antibodies present in the serum of infected mice were assessed by Western blotting. Representative blots using sera collected on days 14 and 28 from mice of each group are shown in Fig. 7. The production of B. burgdorferi-specific antibodies in BL206-infected mice was strikingly different from those in B356-infected mice. Notably, all BL206-infected mice had antibody responses against several B. burgdorferi proteins, including OspC, p39, and flagellin, and to a lesser extent against p18, OspA and p60. The antibody profiles were very similar for sera on both days 14 and 28 with only a few additional bands appearing in the blots with day 28 sera. Antibody response to OspA was stronger in day 28 sera than in day 14 sera. In contrast to the findings in BL206-infected animals, sera from B356-infected mice showed only weak reactivity to a few B. burgdorferi proteins. However, mice inoculated with 107 B356 organisms (as opposed to 104 organisms) showed serologic responses similar to those of BL206-inoculated mice.
FIG. 7.
Western blot of B. burgdorferi-specific antibodies in C3H/HeJ mice infected with different clinical isolates. Serum or plasma specimens were collected at 2 or 4 weeks after infection. The major bands on the blot are indicated by arrows on the left.
DISCUSSION
In previous studies, three RFLP types of B. burgdorferi sensu stricto were identified among a large collection of clinical isolates from Westchester County, N.Y. (17–19). Moreover, an association between the frequency of hematogenous dissemination and subtype of the infecting B. burgdorferi sensu stricto was observed (44). Specifically, Lyme disease patients with RFLP type 1 infection in skin were 15 times more likely to yield a positive blood culture for B. burgdorferi than those with RFLP type 3 infection. The present study was aimed at assessing the pathogenicity of different subtypes of B. burgdorferi sensu stricto in a murine model of Lyme disease.
Evidence from culture, qPCR, and histopathology indicated that isolate BL206 (type 1) disseminated more readily to distant tissues than isolate B356 (type 3). Plasma spirochete burdens in mice infected with BL206 were 16-fold higher (day 7) and 5-fold higher (day 14) than those in mice infected with B356. Similarly, the number of spirochetes in heart tissues of mice infected with BL206 was approximately 6- and 13-fold higher than those of B356-infected mice in samples collected 14 and 28 days after infection, respectively. In contrast to BL206-infected mice, a greater reduction in bacterial burdens was noted in plasma and tissues of B356-infected mice by day 28. In addition, severe carditis and arthritis were only observed in mice infected with isolate BL206. The peak spirochete burden in these mice was seen in plasma between days 4 and 7, in heart and ear biopsy specimens on day 14, and in joints on day 28. Although the spirochete load in plasma diminished gradually after the peak, organisms were detectable through day 28. All blood samples collected at days 4 and 7 were positive by culture, but the recovery rate decreased to 80 and 40% on days 14 and 28, respectively. This reduction in culture yield coincides with detection of anti-B. burgdorferi-specific antibodies in serum after day 14. In BL206-infected mice, a significant correlation between the peak spirochete load in plasma on day 4 or 7 and the number of spirochetes in ear biopsy specimens and joints on day 14 was noted. This is similar to the previously reported finding in C3H and CB-17 SCID mice infected with the relapsing fever spirochete, B. turicatae, in which the level of spirochetemia on day 9 was predictive of the degree of swelling and spirochete infiltration of joints on day 15 (31). Interestingly, while the numbers of spirochetes in the ear biopsy and heart tissues were reduced, the spirochete load increased in the joints from day 14 to day 28. This corresponded with increased joint swelling and a greater degree of joint inflammation observed by histopathology on day 28 relative to that observed on day 14.
No spirochetes could be cultured from any of the mice inoculated with isolate B356 at any time after inoculation. Despite this, findings from qPCR, serology, and histopathology suggest that these mice were indeed infected. First, spirochetes were detected in the plasma of B356-infected mice on days 7 and 14 postinoculation, and the estimated total numbers of spirochetes in these mice exceeded the number (104) of spirochetes in the inoculum. This increase in spirochete number above the initial inoculum indicates that the organisms were multiplying in the infected mice. Second, immunoblot of serum samples from mice inoculated with B356 clearly showed an immune response, including antibodies against OspA, p35, and p39. The weak and limited serologic response relative to that observed in BL206-infected mice may be due to the lower numbers of circulating spirochetes in B356-infected mice, since inoculation with 107 B356 organisms yielded an antibody response very similar to that of BL206-infected mice by day 28. In an additional experiment with three C3H/HeJ mice, B. burgdorferi could not be recovered in culture from skin biopsy samples taken at the site of inoculation with B356 on days 1, 2, 4, 7, 14, and 28 after infection (data not shown). This behavior is not peculiar to isolate B356, since a second clinical isolate with the same RFLP type also demonstrated the same pathogenic characteristics and was not cultivable from inoculated mice (unpublished data).
Since isolate B356 was cultured from the skin biopsy specimen of a patient with EM, it is assumed to be infectious and pathogenic in humans. It is not clear why viable B. burgdorferi B356 cannot be recovered from infected mice. Based on qPCR data, it appears that spirochetes did disseminate and were present in the plasma and some tissues for a short time, after which they were rapidly cleared. The possibility that a large inoculum of B356 organisms is required to establish disseminated infection was considered. However, cultivation of tissues from mice inoculated with 107 B356 organisms (versus 104 BL206 organisms) did not yield any positive culture. It is possible that the C3H/HeJ mice are not susceptible to disseminated infection by B. burgdorferi isolates with a B356-like genotype, since variable host susceptibility to different B. burgdorferi isolates and complement sensitivity among different Borrelia species has been reported (11, 15, 39). Some hosts could act as filters to predispose and transmit only particular species or isolates (15). If so, how are such type 3 isolates maintained in nature, acquired by ticks, and transmitted to patients? One possibility is that other mouse strains, in particular Peromyscus leucopus, the primary reservoir for B. burgdorferi in nature (1, 24), are more susceptible to disseminated infection with B356-like isolates. Alternatively, other small mammals (e.g., raccoon or opposum) may be the preferred reservoir hosts for this B. burgdorferi genotype. It is also possible that coinfection with a mixture of B. burgdorferi genotype (18) facilitates the survival and dissemination of B356-like isolates in patients. Further studies on infection of other mouse strains with B356-like isolates, on the distribution of B. burgdorferi genotypes in reservoir hosts and ticks, on the complement sensitivity of type 1 and type 3 isolates to C3H/HeJ mice serum, and on the pathogenicity of mixtures of B356-like and other genotypes should be helpful in elucidating the mechanisms involved in resistance to infection with B356-like isolates.
The nature of infection with B. burgdorferi and the manner in which it is manifested is determined by both host- and pathogen-related factors. Numerous studies have examined host contributions to this process (4, 5, 8, 13, 20, 25, 45). For example, different mouse strains display various susceptibilities to infection by B. burgdorferi (4, 35, 42). Previous experimental evidence has shown that severity of B. burgdorferi-induced arthritis is determined by the genetic background of the mice and is associated with persistence of large numbers of spirochetes in tissues (30, 31, 42, 45). The present study revealed that pathogen genotype also plays an important role in this process.
In summary, distinct kinetics of dissemination and severity of disease were observed in genetically susceptible C3H/HeJ mice infected with different genotypes of B. burgdorferi sensu stricto clinical isolates. High spirochete burdens in plasma and tissues and severe carditis and arthritis were detected only in BL206-infected mice. This study, together with other previous reports (9, 12, 29, 31, 36), suggests that dissemination of B. burgdorferi and development of clinical disease in C3H/HeJ mice is determined in part by the genotype of the infecting spirochetes. A drawback of the present study is that only two clinical isolates, each representing one RFLP type, were examined. Further studies on the kinetics of dissemination and severity of disease with additional clinical isolates are under way in order to clarify further the potential role of genotypic variation of B. burgdorferi in the pathogenesis of Lyme disease.
ACKNOWLEDGMENTS
We thank M. Aguero-Rosenfeld for assistance with the serologic testing, D. Liveris for genotypic analysis of the tested B. burgdorferi isolates, and H. Wu and J. Xia for technical assistance with the Lightcycler PCR.
This work was supported by Public Health Service grant AR41511 from the National Institutes of Health.
REFERENCES
- 1.Anderson J F. Epizootiology of Lyme borreliosis. Scand J Infect Dis Suppl. 1991;77:23–34. [PubMed] [Google Scholar]
- 2.Balmelli T, Piffaretti J C. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–340. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Brown C R, Reiner S L. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect Immun. 1999;67:1967–1973. doi: 10.1128/iai.67.4.1967-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Field J A, Glickstein L, Molloy P J, Huber B T, Steere A C. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum. 1999;42:1813–1822. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Escudero R, Barral M, Perez A, Vitutia M M, Garcia-Perez A L, Jimenez S, Sellek R E, Anda P. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J Clin Microbiol. 2000;38:4026–4033. doi: 10.1128/jcm.38.11.4026-4033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 11.Gern L, Estrada-Pena A, Frandsen F, Gray J S, Jaenson T G, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall P A. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentbl Bakteriol. 1998;287:196–204. doi: 10.1016/s0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- 12.Golde W T, Dolan M C. Variation in antigenicity and infectivity of derivatives of Borrelia burgdorferi, strain B31, maintained in the natural, zoonotic cycle compared with maintenance in culture. Infect Immun. 1995;63:4795–4801. doi: 10.1128/iai.63.12.4795-4801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R C. Borrelia burgdorferi sp. nov.: etiological agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 15.Kurtenbach K, Sewell H S, Ogden N H, Randolph S E, Nuttall P A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66:1248–1251. doi: 10.1128/iai.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin M L, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. 2000;68:2183–2186. doi: 10.1128/iai.68.4.2183-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liveris D, Gazumyan A, Schwartz I. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1995;33:589–595. doi: 10.1128/jcm.33.3.589-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman R B, Wormser G P, Schwartz I. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–569. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liveris D, Wormser G P, Nowakowski J, Nadelman R, Bittker S, Cooper D, Varde S, Moy F H, Forseter G, Pavia C S, Schwartz I. Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1996;34:1306–1309. doi: 10.1128/jcm.34.5.1306-1309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconi R T, Hohenberger S, Jauris-Heipke S, Schulte-Spechtel U, Lavoie C P, Rossler D, Wilske B. Genetic analysis of Borrelia garinii OspA serotype 4 strains associated with neuroborreliosis: evidence for extensive genetic homogeneity. J Clin Microbiol. 1999;37:3965–3970. doi: 10.1128/jcm.37.12.3965-3970.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuzawa T, Kurita T, Kawabata H, Yanagihara Y. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol Lett. 1994;123:319–324. doi: 10.1111/j.1574-6968.1994.tb07242.x. [DOI] [PubMed] [Google Scholar]
- 24.Mather T N, Wilson M L, Moore S I, Ribeiro J M, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 25.Moody K D, Barthold S W, Terwilliger G A. Lyme borreliosis in laboratory animals: effect of host species and in vitro passage of Borrelia burgdorferi. Am J Trop Med Hyg. 1990;43:87–92. doi: 10.4269/ajtmh.1990.43.87. [DOI] [PubMed] [Google Scholar]
- 26.Morrison T B, Ma Y, Weis J H, Weis J J. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadelman R B, Horowitz H W, Hsieh T C, Wu J M, Aguero-Rosenfeld M E, Schwartz I, Nowakowski J, Varde S, Wormser G P. Simultaneous human granulocytic chrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- 28.Nadelman R B, Wormser G P. Lyme borreliosis. Lancet. 1998;352:557–565. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 29.Norris S J, Howell J K, Garza S A, Ferdows M S, Barbour A G. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun. 1995;63:2206–2212. doi: 10.1128/iai.63.6.2206-2212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahl A, Kuhlbrandt U, Brune K, Rollinghoff M, Gessner A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–1963. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postic D, Ras N M, Lane R S, Hendson M, Baranton G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potter M R, Noben-Trauth N, Weis J H, Teuscher C, Weis J J. Interleukin-4 (IL-4) and IL-13 signaling pathways do not regulate Borrelia burgdorferi-induced arthritis in mice: IgG1 is not required for host control of tissue spirochetes. Infect Immun. 2000;68:5603–5609. doi: 10.1128/iai.68.10.5603-5609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadziene A, Barbour A G, Rosa P A, Thomas D D. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993;61:3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaible U E, Kramer M D, Wallich R, Tran T, Simon M M. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur J Immunol. 1991;21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 36.Seinost G, Dykhuizen D E, Dattwyler R J, Golde W T, Dunn J J, Wang I N, Wormser G P, Schriefer M E, Luft B J. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanek G, O'Connell S, Cimmino M, Aberer E, Kristoferitsch W, Granstrom M, Guy E, Gray J. European Union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–747. [PubMed] [Google Scholar]
- 38.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 39.van Dam A P, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G, van Dam A P, Schwartz I, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev. 1999;12:633–653. doi: 10.1128/cmr.12.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, van Dam A P, Spanjaard L, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J Clin Microbiol. 1998;36:768–776. doi: 10.1128/jcm.36.3.768-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weis J J, McCracken B A, Ma Y, Fairbairn D, Roper R J, Morrison T B, Weis J H, Zachary J F, Doerge R W, Teuscher C. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- 43.Wilske B, Busch U, Fingerle V, Jauris-Heipke S, Preac Mursic V, Rossler D, Will G. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection. 1996;24:208–212. doi: 10.1007/BF01713341. [DOI] [PubMed] [Google Scholar]
- 44.Wormser G P, Liveris D, Nowakowski J, Nadelman R B, Cavaliere L F, McKenna D, Holmgren D, Schwartz I. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–725. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]