Abstract
The evolution and spread of antibiotic‐resistant bacteria have renewed interest in phage therapy, the use of bacterial viruses (phages) to combat bacterial infections. The delivery of phages in cocktails where constituent phages target different modalities (e.g., receptors) may improve treatment outcomes by making it more difficult for bacteria to evolve resistance. However, the multipartite nature of cocktails may lead to unintended evolutionary and ecological outcomes. Here, we compare a 2‐phage cocktail with a largely unconsidered group of phages: generalists that can infect through multiple, independent receptors. We find that λ phage generalists and cocktails that target the same receptors (LamB and OmpF) suppress Escherichia coli similarly for ~2 days. Yet, a “trained” generalist phage, which previously adapted to its host via 28 days of coevolution, demonstrated superior suppression. To understand why the trained generalist was more effective, we measured the resistance of bacteria against each of our phages. We find that, when bacteria were assailed by two phages in the cocktail, they evolved mutations in manXYZ, a host inner‐membrane transporter that λ uses to move its DNA across the periplasmic space and into the cell for infection. This provided cross‐resistance against the cocktail and untrained generalist. However, these mutations were ineffective at blocking the trained generalist because, through coevolutionary training, it evolved to bypass manXYZ resistance. The trained generalist's past experiences in training make it exceedingly difficult for bacteria to evolve resistance, further demonstrating the utility of coevolutionary phage training for improving the therapeutic properties of phages.
Keywords: coevolution, generalist, phage cocktail, phage therapy, phage training, resistance
1. INTRODUCTION
The evolution of antibiotic‐resistant bacteria is a major threat to human health. Recent estimates suggest that >1 million deaths were attributable to antibiotic‐resistant infections in 2019, with mortality expected to climb to >10 million deaths in the next 30 years (Murray et al., 2022; World Health Organization, 2019). To combat the evolution and spread of resistance, many are developing evolutionarily informed strategies of antibiotic use, such as deploying drugs sequentially, in fluctuation, or at concentrations less likely to select for high levels of resistance (Andersson & Hughes, 2014; Imamovic & Sommer, 2013; Kim et al., 2014; Oz et al., 2014). In the case of life‐threatening infections, clinicians often administer combinations (i.e., cocktails) of antibiotic drugs in unison, hoping to reduce bacteria's ability to evolve resistance by forcing them into the difficult challenge of evolving resistance against multiple drugs simultaneously (Joshi, 2011; Mouton, 1999; Tamma et al., 2012). However, as bacteria have and continue to evolve resistance to the panacea of available drugs and the pipeline of drug development slows, there is growing interest in alternative ways to treat bacterial infections (Cooper & Shlaes, 2011; Tommasi et al., 2015).
Phage therapy, the use of bacterial viruses (phages) to treat bacterial infections, is a promising alternative to antibiotic drugs (Chan et al., 2018; Dedrick et al., 2022; Schooley et al., 2017). Often, phages are administered to patients in cocktails, comprised of multiple, distinct phage strains (Dedrick et al., 2022; Schooley et al., 2017). Akin to antibiotic, antiviral, and cancer combination therapies, the goal is to target multiple, distinct modalities to improve bacterial killing and prevent or delay the evolution of resistance (Gordillo Altamirano & Barr, 2021). In the context of phages, modalities constitute different infection mechanisms (e.g., the use of distinct receptors). Multiple studies of phage therapy have found that cocktails are more suppressive than individual constituent phages, especially in cases where they contain phages known to target different receptors (Gu et al., 2012; Tanji et al., 2004; Wright et al., 2019, 2021; Yang et al., 2020). However, increasing the complexity of multipartite phage cocktails is expected to reduce the predictability of evolutionary, ecological, and pharmacokinetic dynamics resulting from their use (Chan et al., 2013; Gordillo Altamirano & Barr, 2021). In the complex environment inside a patient, some of the phages may not be maintained at all sites of the infection, allowing bacteria to sequentially gain resistance to each constituent phage.
One unique solution to the evolution of resistance may be the deployment of “generalist” phages that infect cells through multiple, alternative receptors. Generalist phages are distinct from phages that use co‐receptors to assist in adsorption, as well as from all other antimicrobials because a single genotype can kill cells via different, independent modalities, like an all‐in‐one cocktail. Because they are comprised of a single genotype, generalist phages may be less susceptible to the complex dynamics and stochastic changes that affect the composition of multi‐phage cocktails. This may allow generalists to provide a more consistent pressure on bacteria at all sites of infection, reducing their susceptibility to the evolution of resistance.
By reciprocally adapting to changes in their hosts (coevolution), phages can evolve from single‐receptor specialists into multi‐receptor generalists (Meyer et al., 2012). This evolutionary capacity can be harnessed to produce generalist phages that have improved therapeutic efficacy. Previously, we demonstrated that by preemptively adapting a specialist phage to target bacterial hosts in a process called coevolutionary phage training, we could evolve generalist phages that showed 1000‐fold improved bacterial suppression and delayed the evolution of resistance 14+ days compared to their phage ancestor (Borin et al., 2021). These trained generalists were much more suppressive than trained specialists, suggesting that the dominant driver of improved efficacy was the evolution to use two receptors.
These results suggest that generalist phages could be advantageous for treating bacterial infections. Yet, their use in phage therapy has not been described and no direct comparison between generalists and cocktails has been made. This is likely due to two factors: (1) practitioners often do not know the receptor(s) that their phages use (Gordillo Altamirano & Barr, 2021) and (2) generalists seem to be rare in nature. In a meta‐analysis of 17 gram‐positive and 64 gram‐negative phages, Bertozzi Silva et al. (2016) show that the majority of phages either use a single receptor or a primary co‐receptor that improves adsorption before irreversibly binding a secondary receptor. Phage T2 is an exception because it can infect using either receptor OmpF or FadL (Black, 1988; Hantke, 1978; Kortright et al., 2020). We also determined the receptors of 17 lambdoid coliphages in our collection and found that all specialize on a single receptor (Table S1).
In this study, we evaluate the efficacy of dual‐receptor phage generalists and a phage cocktail comprised of two specialists to suppress bacteria in vitro. To make direct, controlled comparisons, we consider highly related λ phage genotypes. These include two different generalists that both exploit host outer membrane protein receptors LamB and OmpF, as well as two specialist phages that exploit either LamB or OmpF. When we compared our cocktail with an early generalist phage (that recently evolved the ability to use two receptors), we find similar dynamics of bacterial suppression, suggesting treatments are equivalent. However, phage generalists that have been “trained” via coevolution with their hosts for a prolonged period were significantly more suppressive than the cocktail for 15 days. By characterizing the resistance of coevolved bacterial isolates, we find that the trained generalist is more effective because, in coevolutionary training, it evolved mutations that allow it to bypass intracellular forms of resistance.
2. MATERIALS AND METHODS
2.1. Bacterial and phage strains
To make useful comparisons between phage cocktails and phage generalists, we leveraged a collection of highly related λ phage genotypes (Table 1, Figure 1). All phages are derived from the strictly lytic λ strain cI26 (λanc). When λanc coevolves with Escherichia coli B strain REL606, it repeatably evolves the ability to use a novel outer membrane protein receptor (OmpF) in addition to its native receptor LamB (Daegelen et al., 2009; Meyer et al., 2012). The early generalist phage (λegen) was isolated on day 8 of a coevolution experiment, immediately after gaining the ability to use a new receptor, OmpF, in addition to its native receptor, LamB (EvoC in Meyer et al., 2012). For our cocktail, we use one LamB specialist and one OmpF specialist (λLspec and λOspec, evolved as in Meyer et al., 2016). Because coevolutionary dynamics between λ and E. coli lead to dual‐receptor generalists (Gupta, Zaman, et al., 2022), our specialists were obtained by evolving λegen on hosts possessing either LamB or OmpF for 35 days. Hosts were replenished daily and therefore did not coevolve alongside the phages. A handful of mutations separate λanc, λegen, λLspec, and λOspec, with most mutations in the phages' host recognition protein J (Table S2). We also compared a trained generalist phage (λtgen), isolated after 20 more days of coevolution from the same population as λegen (λtrn in Borin et al., 2021). We consider this phage “trained” because it has coevolved with its host for a prolonged period of time. During this period, λtrn evolved point mutations in genes H, lom, Orf‐401, and Orf‐64, as well as a recombination in J, and therefore differs from the other phages at more sites in the genome (Table S2).
TABLE 1.
Phage strains used in the study and their receptor preferences.
| Phage | Abbreviation | Receptor used |
|---|---|---|
| λ ancestor | λanc | LamB |
| λ early generalist | λegen | LamB & OmpF |
| λ trained generalist | λtgen | LamB & OmpF |
| λ LamB specialist | λLspec | LamB |
| λ OmpF specialist | λOspec | OmpF |
FIGURE 1.
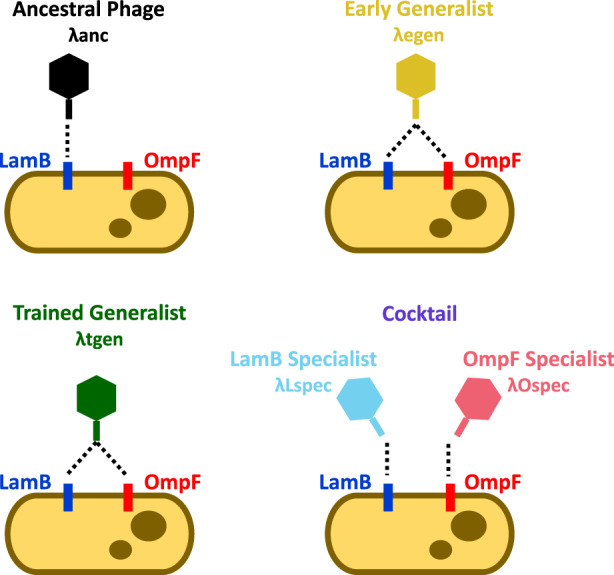
Schematic of phage strains in the study. The ancestral phage (λanc) is cI26, a strictly lytic strain of phage λ. The early generalist (λegen) is a descendant of λanc isolated after 8 days of coevolution with E. coli. The trained generalist (λtgen) was isolated from the same population as λegen after 20 more days of coevolution. The cocktail is comprised of a LamB specialist (λLspec) and an OmpF specialist (λOspec) which are descendants of λegen. λanc uses LamB, λegen and λtgen can use LamB and OmpF, and λLspec and λOspec use LamB or OmpF, respectively. Colors indicate phages/treatments throughout the study. Dotted lines indicate which receptor(s) the phages can use.
2.2. Bacterial suppression experiments
To evaluate the suppressive ability of phages, we inoculated REL606 host and respective phages into 50‐ml flasks with 10 ml modified M9‐G (recipe in Meyer et al., 2012). Cultures were incubated at 37°C for 24 h and every day 100 μl from each population was propagated into new flasks with 10 ml of fresh media. Aliquots were removed each day to estimate bacterial and phage densities, as well as to preserve communities for later analyses. To estimate bacterial and phage densities, aliquots were serially diluted in M9‐G media. Bacteria were plated on Luria‐Bertani (LB) agar plates that were incubated at 37°C to count colony‐forming units (CFU). Phages were aliquoted in 2‐μl droplets onto a soft agar overlay (LB agar except with 0.8% w/w agar) infused with ~108 cells of REL606 and incubated at 37°C to enumerate plaque forming units (PFU). To preserve communities, aliquots were preserved by freezing at −70°C in 15% v/v glycerol. Experiments were initiated with the following bacterial and phage inoculums: To compare λanc, λegen, and the cocktail (comprised of λLspec and λOspec) in the first suppression experiment, ~107 phages and ~105 cells were inoculated into flasks. Effort was made to balance the ratio of λLspec to λOspec in the cocktail but it was inoculated at ~9:1. To compare λegen and the cocktail in the second suppression experiment, ~106 phages and ~105 cells were inoculated into flasks and the ratio of λLspec to λOspec in the cocktail was ~3:1. Bacterial densities were similar in experiments 1 and 2 but phage inoculums differed ~10‐fold; however, we find no evidence that differences in inoculums affected the coevolutionary dynamics or outcomes of the study; statistical comparisons of suppression and receptor preference are similar between experiments (see Results). To compare λtgen and the cocktail in the third suppression experiment, ~106 λtgen or ~107 cocktail phages (λLspec λOspec ratio of ~3:1) were inoculated into flasks with ~106 cells. More cells were used in experiment 3 to reduce bacterial extinction caused by λtgen.
2.3. Measuring receptor preference
We measured the receptor preference of phage populations by enumerating those that could use the LamB and OmpF receptors. We did this by aliquoting phages onto soft agar infused with E. coli K‐12 Keio gene knockout collection strains (Baba et al., 2006) that were either ΔompF (O−, strain JW0912) or ΔlamB (L−, strain JW3996). To quantify the phages' proclivity for LamB and OmpF receptors, phage titers on LamB (O− hosts) and OmpF (L− hosts) were used to calculate the Specialization Index (SI), where SI = (titerLamB – titerOmpF)/(titerLamB + titerOmpF). SI can range from −1 to +1, indicating complete specialization on OmpF or LamB, respectively.
2.4. Bacterial isolation and measuring phage resistance
We isolated bacteria by streaking ~2 μl of preserved, frozen communities onto LB agar plates. Plates were incubated overnight at 37°C and then colonies were isolated and streaked twice more to purify them of phage and obtain isogenic strains. Lastly, bacterial isolates were grown overnight at 37°C in LB Lennox broth and preserved by freezing. To determine when phage resistance evolved, we isolated 12 coevolved bacteria from each community across various days of the suppression experiment (~600 strains). We measured resistance by aliquoting phages onto soft agar plates infused with different isolates. By dividing the number of plaques produced on different bacterial isolates by the number of plaques formed on the ancestor (REL606), we calculated the efficiency of plaquing (EOP, a metric often used to indicate how well phages are adapted to different hosts) for each phage–host pair (~2400 pairwise EOPs).
2.5. Survey and test of manXYZ mutations
To determine which bacterial populations and timepoints had evolved mutations in the manXYZ operon, we streaked one representative bacterial isolate from each population across days 1–5 on tetrazolium‐mannose indicator plates (10 g tryptone, 1 g yeast extract, 5 g sodium chloride, 16 g agar, 10 g mannose per liter of water, and supplemented to a final concentration of 0.005% triphenyl tetrazolium chloride [TTC] indicator dye). Colonies formed by bacteria with mutations that disrupt mannose metabolism appear dark red and colonies without manXYZ mutations are pink (Burmeister et al., 2021). We then tested whether manXYZ mutations recapitulate the resistance we observed in coevolved bacteria from the suppression experiment by measuring the EOP of phages on ΔmanY and ΔmanZ Keio knockout collection strains (JW1807 and JW1808, respectively) with respect to K‐12 wildtype (Baba et al., 2006).
3. RESULTS
3.1. Suppression by the phage cocktail and early generalist
Initially, we compared the suppressive efficacy of our phage cocktail, early generalist (λegen), and their wild‐type phage ancestor (λanc) (Expt. 1, Figure 2a). Consistent with previous studies (Borin et al., 2021), we found that the ancestral phage lost the ability to suppress bacteria in ~1 day. Both early generalist and cocktail treatments appeared more suppressive than λanc for the first 2 days since all three replicates for λegen and all six replicates for the cocktail had less dense bacterial populations than all three λanc replicates. The cocktail was significantly more suppressive (p = 0.028 and p = 0.024 for days 1 and 2, respectively, Mann–Whitney U‐test); however we did not find statistical significance between λanc and λegen (p = 0.1) because of the reduced number of replicates for λegen. Given the nonparametric test we used (Mann–Whitney U‐test) and the low number of replicates, it would be impossible to compute a p‐value lower ≤0.05. In comparisons between the cocktail and early generalist, bacterial suppression was similar; both λegen and the cocktail eliminated bacteria from 1 of 3 and 2 of 6 flasks, respectively. However, the cocktail was more suppressive on day 2 (p = 0.048). Because bacterial titers were highly variable and sample sizes were small, we repeated the experiment with more replicates. (Expt. 2, n = 10 λegen flasks and 11 cocktail flasks). On average, both λegen and the cocktail suppressed bacteria ~100‐fold for the first 4 days of the experiment (Expt. 2, Figure 2b). There were no significant differences between treatments, suggesting that the cocktail and early generalist are equally effective at suppressing bacteria.
FIGURE 2.

Bacterial suppression due to phage treatments. Panel (a): Experiment 1, comparison of λanc, λegen, and cocktail treatments. Lines and shapes represent replicates (3, 3, and 6 flasks for λanc, λegen, and cocktail, respectively). Panel (b): Experiment 2, comparison of λegen and cocktail treatments. Median bacterial titer is emboldened and trajectories of replicate flasks are translucent (10 and 11 flasks for λegen and cocktail, respectively). Mann–Whitney U‐tests were used to compare bacterial titer between treatments for each day. No significant differences were found (α = 0.05, “ns” indicated above days). Panels (a) and (b): λanc, λegen, and cocktail are colored black, gold, and purple, respectively. Dashed line and gray shading indicate the limit of detection (10 CFU/ml). Lines that drop below the limit of detection and do not reemerge indicate that bacterial populations were driven extinct.
Combination therapies rely on the concurrent exploitation of multiple modalities to improve efficacy and delay resistance. Loss of, or asymmetries in targeted modalities can reduce the effectiveness of treatment. Because the cocktail is a multipartite treatment comprised of two distinct specialist phages, we investigated how its composition (and therefore preference for different receptors) differed from the early generalist treatment, which is comprised of a single genotype. In addition to estimating the overall phage titer each day, we measured the number of phages that could infect using LamB or OmpF receptors by aliquoting the community phage lysate on hosts lacking either OmpF (O−) or LamB (L−), respectively. We then calculated the Specialization Index (SI), which quantifies phages' preference on a scale from 1 (LamB specialization) to −1 (OmpF specialization). We hypothesized that over time the SI of the cocktail would either (H1) fix at −1 or 1, coinciding with fixation of a specialist genotype, (H2) occupy intermediate values suggesting a balance between the specialist phages or (H3) fluctuate between −1 and 1 in a negative frequency‐dependent manner. Support for H1 or H3 associated with a loss of bacterial suppression could suggest that efficacy is lost due to a loss of, or asymmetry in receptor use.
We find that SI values of the cocktail fluctuated between 1 and −1, indicating that the composition of the cocktail alternated between λLspec and λOspec phages (Expt. 2, Figure 3a). These oscillations, which initially favor LamB specialization (SI ~ 1), give way to OmpF specialization (SI ~ −1) as the cocktail maintains suppression, and then return to SI ~ 1. In contrast, the SI of the early generalist maintained intermediate values (Figure 3b). When we repeated the experiment with more replicate populations (Expt. 2, Figure 2b), we found similar dynamics, albeit with more variable periods of oscillation (Expt. 2, Figure S1). We did not observe any relationship between the predominance of λLspec or λOspec and the cocktail's ability to maintain suppression. For example, in cocktail population 5, the rapid shift to LamB specialization correlates with lower suppression, whereas in population 4, the shift to LamB specialization coincides with increased suppression (Figure S1). Altogether, these results show that fluctuations in the composition and receptor preference of the cocktail helped maintain phages throughout the experiment and possibly prolonged the cocktail's effectiveness.
FIGURE 3.

Preference of λegen and cocktail treatment for LamB and OmpF receptors over days of the first suppression experiment (Expt. 1, lines and shapes represent replicates and correspond with those in Figure 2a). The cocktail (a) is colored purple and λegen (b) is gold. Receptor preference was quantified using a specialization index (SI) where SI = (titerLamB − titerOmpF)/(titerLamB + titerOmpF). SI ranges from 1 (LamB specialization) to −1 (OmpF specialization).
3.2. Suppression by the phage cocktail and trained generalist
In previous work, we demonstrated that the ability to infect through 2 distinct receptors substantially improves bacterial suppression (Borin et al., 2021). We did this by comparing phages that were “trained” via coevolution with their host for 20 days. Half of the phages we compared had evolved the ability to use an additional receptor during training and were far more suppressive (~1000‐fold) than the phage ancestor. However, the other half of the phages in our comparison did not evolve to use a new receptor and showed only meager improvements in suppression. This led us to conclude that the ability to infect through two receptors (which evolved as a result of phage training) drastically improves suppressive efficacy. However, when we compared λegen and the cocktail (Expt. 1 and 2, above), which both exploit two receptors, neither seemed as suppressive as the trained dual‐receptor phages from the previous study.
To investigate this discrepancy, we compared a trained generalist phage (λtgen) against our cocktail in another suppression experiment (Expt. 3). λtgen was significantly more suppressive for 15 days, and bacteria were driven extinct in 3 of 5 λtgen flasks (Expt. 3, Figure 4), clearly showing that the trained generalist was more suppressive than the early generalist and phage cocktail. Again, we found that the cocktail fluctuated between LamB and OmpF specialization, whereas the trained generalist maintained intermediate SI values like the early generalist (Expt. 3, Figure S2). Because λegen, λtgen, and the cocktail all use the same two receptors, these results suggested that λtgen has some other property that makes it more effective at suppressing bacteria.
FIGURE 4.

Experiment 3, bacterial suppression in λtgen (green) and cocktail (purple) treatments. Lines represent replicate flasks (n = 5 with λtgen and 6 with the cocktail). Bacteria were driven extinct in 3 λtgen flasks; for λtgen flasks where bacteria survived, dashed and dotted lines indicate populations 1 and 2, respectively. The dashed line and gray shading denote the limit of detection. Significant differences are indicated above days, calculated via Mann–Whitney U‐test (not significant [ns] p > 0.05, * p < 0.05, ** p < 0.005).
3.3. Evolution of resistance
To investigate why the trained generalist was able to suppress bacteria for so much longer than the cocktail, we first measured when bacteria evolved resistance in each treatment. We isolated 12 coevolved bacteria from various timepoints of each population in the suppression experiment (Expt. 3). Then, we measured how resistant each coevolved bacterial isolate was to each of our phages using efficiency of plaquing (EOP, a metric of how well phages infect different hosts). By aliquoting all of our phages (λtgen, λLspec, λOspec, λegen) on each host, we measured how resistant bacteria were to phages within their treatment, as well as to phages outside of their treatment. For example, we measured the resistance of bacteria in the λtgen treatment to λtgen (within treatment), as well as to λegen, λLspec, and λOspec (outside of treatment).
Consistent with previous work, we found that λtgen maintained suppression because it delayed the evolution of phage resistance (Figure 5a). Of the two surviving λtgen bacterial populations, high levels of resistance did not evolve for >10 days and, in population 2, high levels of resistance to λtgen never evolved. Because the pathway by which REL606 evolves resistance to λtgen has previously been characterized (Borin et al., 2021), we focused our investigation on how bacteria evolved resistance in the cocktail treatment.
FIGURE 5.

Median resistance of n = 12 coevolved bacterial isolates from λtgen Panel (a) and cocktail Panel (b) populations across various days of the suppression experiment against our panel of four phages (Expt. 3). Resistance of bacteria to λegen, λLspec, λOspec, and λtgen is indicated by gold, blue, red, and green lines, respectively. Resistance was measured by calculating the efficiency of plaquing (EOP = plaques on coevolved bacteria/plaques on bacterial ancestor). EOP is presented on a log10 scale where the dashed line indicates EOP = 1 (where bacterial isolates are as sensitive as the ancestor). Points along the x‐axis indicate where phages were completely unable to form plaques on bacteria from that timepoint (EOP = 0, log10EOP = −infinity).
Bacteria in all cocktail populations evolved high levels of resistance (EOP <10−4) to both λLspec and λOspec within 1 day (Figure 5b), explaining why the cocktail treatment lost efficacy much earlier than λtgen. We also noticed that in all populations, resistance to λLspec and λOspec coincided with high levels of resistance to λegen. Yet, the bacteria in four of six cocktail populations did not rapidly evolve concomitant levels of λtgen resistance, indicating that resistance mutations that evolved early against the cocktail also conferred high levels of resistance to λegen, but not to λtgen. Of these four cocktail populations, three eventually evolved high levels of λtgen resistance, concomitant with even greater levels of λLspec, λOspec, and λegen resistance. These results suggest that λtgen may be more suppressive because it is less susceptible to easily acquired mutations that confer cocktail (λLspec, λOspec) and λegen resistance.
Next, we investigated how bacteria evolved resistance. Because all of our treatments, including λtgen, exploit the same two receptors, it seemed unlikely that initial cocktail resistance would be explained by mutations in LamB and OmpF. Therefore, we considered other mechanisms that might provide resistance against the cocktail and early generalist phages but not to λtgen. A large body of work has shown that the E. coli mannose transporter, encoded by the manXYZ operon (formerly ptsM, a phosphoenolpyruvate‐dependent phosphotransferase system), which is embedded in the inner, cytoplasmic membrane, is used by phage λ to move its DNA across the periplasmic space and into the cell for infection (Casjens & Hendrix, 2015; Scandella & Arber, 1976). Additionally, the evolution of manYZ mutations have been found in previous coevolution experiments between λ and E. coli (Burmeister et al., 2021; Gupta, Peng, et al., 2022; Meyer et al., 2012). Therefore, we used tetrazolium‐mannose indicator plates to survey bacterial isolates from days 1–5 of all λtgen and cocktail populations for manXYZ mutations. We found manXYZ mutants in every isolate from the cocktail treatment and none in λtgen populations. The ubiquity of manXYZ mutations in cocktail populations by day 1 suggests that it is the primary cause of phage resistance and loss of bacterial suppression. Moreover, the lack of manXYZ mutants in λtgen populations suggest that they did not arise because these mutations may not be effective for λtgen‐resistance.
To directly test whether mutations in manXYZ provide protection from infection, we measured the resistance of ΔmanY and ΔmanZ strains (that do not have any other resistance mutations) against each of our phages. We find that the resistance profiles of ΔmanY and ΔmanZ strains closely resemble the resistance of coevolved bacteria from the cocktail treatment (Figure 6); ΔmanY and ΔmanZ hosts are highly resistant to λLspec, λOspec, and λegen (EOP = 0.05–3 × 10−5) and less resistant to λtgen (EOP ~ 0.25). The relative resistance of ΔmanY and ΔmanZ strains also matches patterns from the cocktail treatment; they are most resistant (lowest EOP) to λegen, followed by λOspec, λLspec, and then λtgen. Altogether, these results further support that, when treated with the cocktail, bacteria rapidly evolved resistance via mutations in manXYZ and also that these mutations do not grant high levels of resistance to λtgen.
FIGURE 6.

Resistance of E. coli K‐12 ΔManY and ΔManZ knockout bacteria to our panel of four phages. Resistance to λegen, λLspec, λOspec, and λtgen is indicated in gold, blue, red, and green, respectively. Resistance was measured by calculating the efficiency of plaquing (EOP = plaques on knockout bacteria/plaques on bacterial ancestor). EOP is presented on a log10 scale where the dashed line indicates EOP = 1 (focal bacteria is as sensitive as the ancestor).
Previous studies of phage λ offer insight into how λtgen is able to bypass manXYZ resistance mutations. Researchers have found that adaptive mutations in λ genes V (major tail subunit) or H (tape measure protein and tail component) can allow it to infect resistant manXYZ mutants (Esquinas‐Rychen & Erni, 2001; Scandella & Arber, 1976). In the case of our phages, λOspec and λtgen each have a unique H mutation (Table S2). λOspec's H mutation clearly does not allow it to overcome manXYZ resistance. However, for λtgen, the ability to infect manXYZ mutants that are resistant to λegen and the cocktail may be due to its H mutation, due to a different mutation (λtgen also has mutations in lom, Orf‐401, and Orf‐64, for which functions are not fully understood), or due to a combination of mutations. Yet, these results explain why manXYZ mutants did not evolve in the λtgen treatment; manXYZ mutations do not confer high levels of resistance to λtgen because λtgen has already evolved mutations for counter‐resistance. These counter‐measures allowed λtgen to remain effective while the phage cocktail lost suppression because of easily acquired manXYZ resistance mutations.
The persistence of cocktail phages, despite rapid resistance evolution suggests that the specialist phages were able to counter manXYZ‐based resistance over time. To test this, we isolated coevolved phages from cocktail populations that survived to the end of the experiment (T = 16) by plating from frozen communities onto either ΔLamB or ΔOmpF cells (for Population 2 we only recovered phages on ΔOmpF). Next, we measured each isolates ability to bypass manXYZ resistance as described above. We found that all surviving specialist phages drastically improved at infecting manXYZ mutants (EOP from ~10−3 to EOP ~0, Figure S3). Therefore, by the end of the coevolution experiment, surviving specialist phages had evolved the ability to overcome manXYZ‐based cross‐resistance mutations, similar to λtgen. As a check to make sure that these isolates were indeed specialists and not λtgen contamination, we measured the Specialization Index for each and confirmed that they were still specialists (Figure S4). Beyond confirming the identity of these phages, this measurement indicated that the phage in the cocktail treatment maintained high levels of specialization, showing that fluctuations in receptor use measured at the population level (Figure 3a, Figure S1) were due to oscillations in the phage specialists and not the de novo evolution of phage preferences.
4. DISCUSSION
In phage therapy, multi‐phage cocktails are often used to improve treatment outcomes. Ideally, cocktails are comprised of phages that work additively or synergistically to kill target bacterial pathogens. For example, phages that target different host receptors can make it more difficult for bacteria to evolve resistance (Gu et al., 2012; Tanji et al., 2004; Yang et al., 2020). Previous work has also demonstrated that generalist phages, which infect using multiple, distinct receptors, can also improve bacterial killing and stem the evolution of resistance (Borin et al., 2021). Here, we leveraged a collection of highly related λ phage strains to make novel comparisons between cocktails and generalists.
We conducted initial comparisons between a cocktail, comprised of two specialist phages (λLspec and λOspec) and an early generalist phage (λegen) that recently evolved the ability to use two receptors. We found that the cocktail and λegen treatments were both more suppressive than their phage ancestor, supporting previous studies demonstrating that phage therapeutics that target multiple receptors improve efficacy (Gu et al., 2012; Tanji et al., 2004; Wright et al., 2019, 2021; Yang et al., 2020). We also found that the cocktail and λegen showed similar levels of suppression. The composition of the cocktail fluctuated throughout the experiment. However, we found no link between fluctuations and a loss or gain in suppressive efficacy. Our results suggest that similar outcomes in therapy may be achieved, regardless of whether the targeted host receptors are exploited by a single generalist or multiple specialists. However, caution should be used when extrapolating cocktail treatment efficacy in vitro to animal host infections: On the one hand, kill‐the‐winner dynamics that promote negative frequency‐dependence and maintain constituent phages at the site of infection could improve therapeutic outcomes (Maslov & Sneppen, 2017). However, the fact that fluctuations arose in a simplified flask experiment may be concerning for in vivo applications if spatial heterogeneity and environmental complexity exacerbate asymmetries in the treatment and one or more constituent phages are lost from the infection site. Future studies should evaluate whether the inherent compositional differences between phage cocktails and phage generalists lead to diverging outcomes when administered for in vivo phage therapy.
We also compared our cocktail against a trained generalist (λtgen) that previously demonstrated strong suppressive capabilities (Borin et al., 2021). Although both of our treatments use the same two receptors, λtgen was significantly more suppressive. It took bacteria >10 days to evolve high levels of resistance to λtgen (resistance to λtgen is described in Borin et al., 2021), whereas bacteria evolved resistance to the cocktail in 1 day. By measuring how bacteria evolved resistance in each treatment, we discovered why the cocktail and λegen performed similarly, as well as why the trained generalist was superior.
Bacteria evolved resistance to both phages in the cocktail by acquiring mutations in manXYZ, a mannose transporter that λ uses to traffic its DNA across the periplasm and into the host cell (Esquinas‐Rychen & Erni, 2001). These manXYZ mutants were also resistant to the early generalist, explaining why the efficacy of our cocktail and λegen treatments were similar. When besieged by phages targeting two different receptors, bacteria evolved cross‐resistance by altering an intracellular pathway instead of mutating each receptor separately (see also Wright et al., 2018). This blocked infection, regardless of the receptor(s) used by the phage.
The trained generalist was superior to all other treatments because manXYZ mutations were not effective to block λtgen infection. Previously, we concluded that coevolutionary training improved phages by selecting for those that had evolved to use two receptors (Borin et al., 2021). It is now clear that training also selects for phages that experience and evolve to bypass other forms of resistance, like manXYZ, thereby producing phages that were far less susceptible to the evolution of resistance. Because mutations to counter manXYZ resistance evolved after the appearance of dual‐receptor generalists (λegen lacks manXYZ counter resistance), our results suggest that it is advantageous to continue training phages after they have acquired novel functions, in order for them to experience and counter new forms of resistance in their hosts.
We believe that our results strongly recommend the consideration of multi‐receptor phages for therapy. Yet, generalist phages appear to be rare in nature. This perception could be due to poor surveillance of phage receptors preferences; however, there may also be costs associated with generalization. Previous work demonstrates that λ can rapidly and repeatably evolve to use new receptors, suggesting that researchers may be able to generate multi‐receptor phages in the lab (Meyer et al., 2012). However, studies also show that the evolution of tail fiber mutations that enable the innovation to use a new receptor also destabilize the protein (Petrie et al., 2018; Strobel et al., 2022). This could be problematic for therapy if it reduces phage shelf life or increases clearance from the body (Bull et al., 2019). Therefore, more study is needed to determine whether destabilization is a general phenomenon in the evolution of receptor innovations. Moreover, we recommend that researchers test whether naturally isolated multi‐receptor phages, like T2, are unstable and whether generalist phages of interest can be restabilized through evolution or genetic engineering (Favor et al., 2020).
In this study, we used highly related λ strains to control for factors apart from receptor use. This could have made it easier for bacteria to evolve cross‐resistance. Cocktails comprised of more distantly related phages may reduce the likelihood of cross‐resistance; however, these results highlight the importance of determining whether cross‐resistance mutations are easily acquired, even when phages target different receptors (Abedon et al., 2021; Wright et al., 2018).
Altogether our results demonstrate strong support for the use of multi‐receptor targeting phage cocktails and generalists for therapy. We believe that practitioners should first determine whether target bacteria can readily evolve cross‐resistance, as this may rapidly cause treatments to fail. We also find more support for coevolutionary phage training, as it produces phages that bypass mechanisms of cross‐resistance and drastically improves efficacy. We find that coevolutionary training is a particularly powerful approach because it employs the natural algorithm of evolution. Through coevolution with their hosts, phages like λtgen “solve” evolutionary challenges that were neither known nor anticipated by their trainers.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank the labs of Ry Young, Sherwood Casjens, and Carolin Wendling for sending us lambdoid phages. This study benefitted from useful discussion with all members of Justin R. Meyer and Sergey Kryazhimskiy's laboratories. The work herein was supported by the National Science Foundation (award 1934515) and the National Institutes of Health Cell and Molecular Genetic Training Program (T32GM007240).
Borin, J. M. , Lee, J. J. , Gerbino, K. R. , & Meyer, J. R. (2023). Comparison of bacterial suppression by phage cocktails, dual‐receptor generalists, and coevolutionarily trained phages. Evolutionary Applications, 16, 152–162. 10.1111/eva.13518
DATA AVAILABILITY STATEMENT
Data and code related to the study have been made available at the following GitHub repository: https://github.com/joshborin/CocktailGeneralist.
REFERENCES
- Abedon, S. T. , Danis‐Wlodarczyk, K. M. , & Wozniak, D. J. (2021). Phage cocktail development for bacteriophage therapy: Toward improving spectrum of activity breadth and depth. Pharmaceuticals, 14(10), 1019. 10.3390/ph14101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, D. I. , & Hughes, D. (2014). Microbiological effects of sublethal levels of antibiotics. Nature Reviews Microbiology, 12(7), 465–478. 10.1038/nrmicro3270 [DOI] [PubMed] [Google Scholar]
- Baba, T. , Ara, T. , Hasegawa, M. , Takai, Y. , Okumura, Y. , Baba, M. , Datsenko, K. A. , Tomita, M. , Wanner, B. L. , & Mori, H. (2006). Construction of Escherichia coli K‐12 in‐frame, single‐gene knockout mutants: The Keio collection. Molecular Systems Biology, 2(1), 2006.0008. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi Silva, J. , Storms, Z. , & Sauvageau, D. (2016). Host receptors for bacteriophage adsorption. FEMS Microbiology Letters, 363(4), fnw002. 10.1093/femsle/fnw002 [DOI] [PubMed] [Google Scholar]
- Black, P. N. (1988). The fadL gene product of Escherichia coli is an outer membrane protein required for uptake of long‐chain fatty acids and involved in sensitivity to bacteriophage T2. Journal of Bacteriology, 170(6), 2850–2854. 10.1128/jb.170.6.2850-2854.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin, J. M. , Avrani, S. , Barrick, J. E. , Petrie, K. L. , & Meyer, J. R. (2021). Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proceedings of the National Academy of Sciences of the United States of America, 118(23), e2104592118. 10.1073/pnas.2104592118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull, J. J. , Levin, B. R. , & Molineux, I. J. (2019). Promises and pitfalls of in vivo evolution to improve phage therapy. Viruses, 11(12), 1083. 10.3390/v11121083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister, A. R. , Sullivan, R. M. , Gallie, J. , & Lenski, R. E. (2021). Sustained coevolution of phage lambda and Escherichia coli involves inner‐ as well as outer‐membrane defences and counter‐defences. Microbiology, 167(5), 001063. 10.1099/mic.0.001063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens, S. R. , & Hendrix, R. W. (2015). Bacteriophage lambda: Early pioneer and still relevant. Virology, 479–480, 310–330. 10.1016/j.virol.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, B. K. , Abedon, S. T. , & Loc‐Carrillo, C. (2013). Phage cocktails and the future of phage therapy. Future Microbiology, 8(6), 769–783. 10.2217/fmb.13.47 [DOI] [PubMed] [Google Scholar]
- Chan, B. K. , Turner, P. E. , Kim, S. , Mojibian, H. R. , Elefteriades, J. A. , & Narayan, D. (2018). Phage treatment of an aortic graft infected with Pseudomonas aeruginosa . Evolution, Medicine, and Public Health, 2018(1), 60–66. 10.1093/emph/eoy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, M. A. , & Shlaes, D. (2011). Fix the antibiotics pipeline. Nature, 472(7341), 32. 10.1038/472032a [DOI] [PubMed] [Google Scholar]
- Daegelen, P. , Studier, F. W. , Lenski, R. E. , Cure, S. , & Kim, J. F. (2009). Tracing the ancestors and relatives of Escherichia coli B, and the derivation of B strains REL606 and BL21(DE3). Journal of Molecular Biology, 394(4), 634–643. 10.1016/j.jmb.2009.09.022 [DOI] [PubMed] [Google Scholar]
- Dedrick, R. M. , Smith, B. E. , Cristinziano, M. , Freeman, K. G. , Jacobs‐Sera, D. , Belessis, Y. , Whitney Brown, A. , Cohen, K. A. , Davidson, R. M. , van Duin, D. , Gainey, A. , Garcia, C. B. , Robert George, C. R. , Haidar, G. , Ip, W. , Iredell, J. , Khatami, A. , Little, J. S. , Malmivaara, K. , … Hatfull, G. F. (2022). Phage therapy of mycobacterium infections: Compassionate‐use of phages in twenty patients with drug‐resistant mycobacterial disease. Clinical Infectious Diseases. 10.1093/cid/ciac453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquinas‐Rychen, M. , & Erni, B. (2001). Facilitation of bacteriophage lambda DNA injection by inner membrane proteins of the bacterial phosphoenol‐pyruvate: Carbohydrate phosphotransferase system (PTS). Journal of Molecular Microbiology and Biotechnology, 3(3), 361–370. [PubMed] [Google Scholar]
- Favor, A. H. , Llanos, C. D. , Youngblut, M. D. , & Bardales, J. A. (2020). Optimizing bacteriophage engineering through an accelerated evolution platform. Scientific Reports, 10(1), 1–10. 10.1038/s41598-020-70841-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo Altamirano, F. L. , & Barr, J. J. (2021). Unlocking the next generation of phage therapy: The key is in the receptors. Current Opinion in Biotechnology, 68, 115–123. 10.1016/j.copbio.2020.10.002 [DOI] [PubMed] [Google Scholar]
- Gu, J. , Liu, X. , Li, Y. , Han, W. , Lei, L. , Yang, Y. , Zhao, H. , Gao, Y. , Song, J. , Lu, R. , Sun, C. , & Feng, X. (2012). A method for generation phage cocktail with great therapeutic potential. PLoS One, 7(3), e31698. 10.1371/journal.pone.0031698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Peng, S. , Leung, C. Y. , Borin, J. M. , Medina, S. J. , Weitz, J. S. , & Meyer, J. R. (2022). Leapfrog dynamics in phage‐bacteria coevolution revealed by joint analysis of cross‐infection phenotypes and whole genome sequencing. Ecology Letters, 25(4), 876–888. 10.1111/ele.13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Zaman, L. , Strobel, H. M. , Gallie, J. , Burmeister, A. R. , Kerr, B. , Tamar, E. S. , Kishony, R. , & Meyer, J. R. (2022). Host–parasite coevolution promotes innovation through deformations in fitness landscapes. eLife, 11, e76162. 10.7554/eLife.76162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke, K. (1978). Major outer membrane proteins of E. coli K12 serve as receptors for the phages T2 (protein Ia) and 434 (protein Ib). Molecular and General Genetics MGG, 164(2), 131–135. 10.1007/BF00267377 [DOI] [PubMed] [Google Scholar]
- Imamovic, L. , & Sommer, M. O. A. (2013). Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Science Translational Medicine, 5(204), 204ra132. 10.1126/scitranslmed.3006609 [DOI] [PubMed] [Google Scholar]
- Joshi, J. M. (2011). Tuberculosis chemotherapy in the 21st century: Back to the basics. Lung India: Official Organ of Indian Chest Society, 28(3), 193–200. 10.4103/0970-2113.83977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Lieberman, T. D. , & Kishony, R. (2014). Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance. Proceedings of the National Academy of Sciences of the United States of America, 111(40), 14494–14499. 10.1073/pnas.1409800111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortright, K. E. , Chan, B. K. , & Turner, P. E. (2020). High‐throughput discovery of phage receptors using transposon insertion sequencing of bacteria. Proceedings of the National Academy of Sciences of the United States of America, 1–10, 18670–18679. 10.1073/pnas.2001888117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov, S. , & Sneppen, K. (2017). Population cycles and species diversity in dynamic kill‐the‐winner model of microbial ecosystems. Scientific Reports, 7(1), 39642. 10.1038/srep39642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, J. R. , Dobias, D. T. , Medina, S. J. , Servilio, L. , Gupta, A. , & Lenski, R. E. (2016). Ecological speciation of bacteriophage lambda in allopatry and sympatry. Science, 1–5, 1301–1304. 10.1126/science.aai8446 [DOI] [PubMed] [Google Scholar]
- Meyer, J. R. , Dobias, D. T. , Weitz, J. S. , Barrick, J. E. , Quick, R. T. , & Lenski, R. E. (2012). Repeatability and contingency in the evolution of a key innovation in phage lambda. Science, 335(6067), 428–432. 10.1126/science.1214449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton, J. W. (1999). Combination therapy as a tool to prevent emergence of bacterial resistance. Infection, 27(2), S24–S28. 10.1007/BF02561666 [DOI] [PubMed] [Google Scholar]
- Murray, C. J. , Ikuta, K. S. , Sharara, F. , Swetschinski, L. , Robles Aguilar, G. , Gray, A. , Han, C. , Bisignano, C. , Rao, P. , Wool, E. , Johnson, S. C. , Browne, A. J. , Chipeta, M. G. , Fell, F. , Hackett, S. , Haines‐Woodhouse, G. , Kashef Hamadani, B. H. , Kumaran, E. A. P. , McManigal, B. , … Naghavi, M. (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet, 399(10,325), 629–655. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz, T. , Guvenek, A. , Yildiz, S. , Karaboga, E. , Tamer, Y. T. , Mumcuyan, N. , Ozan, V. B. , Senturk, G. H. , Cokol, M. , Yeh, P. , & Toprak, E. (2014). Strength of selection pressure is an important parameter contributing to the complexity of antibiotic resistance evolution. Molecular Biology and Evolution, 31(9), 2387–2401. 10.1093/molbev/msu191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie, K. L. , Palmer, N. D. , Johnson, D. T. , Medina, S. J. , Yan, S. J. , Li, V. , Burmeister, A. R. , & Meyer, J. R. (2018). Destabilizing mutations encode nongenetic variation that drives evolutionary innovation. Science, 359(6383), 1542–1545. 10.1126/science.aar1954 [DOI] [PubMed] [Google Scholar]
- Scandella, D. , & Arber, W. (1976). Phage λ DNA injection into Escherichia coli pel‐ mutants is restored by mutations in phage genes V or H. Virology, 69(1), 206–215. 10.1016/0042-6822(76)90207-5 [DOI] [PubMed] [Google Scholar]
- Schooley, R. T. , Biswas, B. , Gill, J. J. , Hernandez‐Morales, A. , Lancaster, J. , Lessor, L. , Barr, J. J. , Reed, S. L. , Rohwer, F. , Benler, S. , Segall, A. M. , Taplitz, R. , Smith, D. M. , Kerr, K. , Kumaraswamy, M. , Nizet, V. , Lin, L. , McCauley, M. D. , Strathdee, S. A. , … Hamilton, T. (2017). Development and use of personalized bacteriophage‐based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrobial Agents and Chemotherapy, 61(10), 1–14. 10.1128/aac.00954-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel, H. M. , Horwitz, E. K. , & Meyer, J. R. (2022). Viral protein instability enhances host‐range evolvability. PLoS Genetics, 18(2), e1010030. 10.1371/journal.pgen.1010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma, P. D. , Cosgrove, S. E. , & Maragakis, L. L. (2012). Combination therapy for treatment of infections with gram‐negative bacteria. Clinical Microbiology Reviews, 25(3), 450–470. 10.1128/CMR.05041-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji, Y. , Shimada, T. , Yoichi, M. , Miyanaga, K. , Hori, K. , & Unno, H. (2004). Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Applied Microbiology and Biotechnology, 64(2), 270–274. 10.1007/s00253-003-1438-9 [DOI] [PubMed] [Google Scholar]
- Tommasi, R. , Brown, D. G. , Walkup, G. K. , Manchester, J. I. , & Miller, A. A. (2015). ESKAPEing the labyrinth of antibacterial discovery. Nature Reviews Drug Discovery, 14(8), 529–542. 10.1038/nrd4572 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2019). Interagency coordination group on antimicrobial resistance, “No time to wait: Securing the future from drug‐resistant infections. Report to the secretary‐general of the United Nations”. World Health Organization. [Google Scholar]
- Wright, R. C. , Friman, V. P. , Smith, M. C. , & Brockhurst, M. A. (2018). Cross‐resistance is modular in bacteria‐phage interactions. PLoS Biology, 16(10), e2006057. 10.1371/journal.pbio.2006057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, R. C. , Friman, V. P. , Smith, M. C. , & Brockhurst, M. A. (2019). Resistance evolution against phage combinations depends on the timing and order of exposure. MBio, 10(5), e01652‐19. 10.1128/mBio.01652-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, R. C. , Friman, V. P. , Smith, M. C. , & Brockhurst, M. A. (2021). Functional diversity increases the efficacy of phage combinations. Microbiology, 167(12), 001110. 10.1099/mic.0.001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Shen, W. , Zhong, Q. , Chen, Q. , He, X. , Baker, J. L. , Xiong, K. , Jin, X. , Wang, J. , Hu, F. , & Le, S. (2020). Development of a bacteriophage cocktail to constrain the emergence of phage‐resistant Pseudomonas aeruginosa . Frontiers in Microbiology, 11, 327. 10.3389/fmicb.2020.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data and code related to the study have been made available at the following GitHub repository: https://github.com/joshborin/CocktailGeneralist.


