Abstract
The pandemic of COVID-19 in worldwide causes recent millions of morbidity and mortality in all countries and is the most important challenge in the world in recent years. Coronavirus is a single-stranded RNA virus and infection with COVID-19 leads to acute respiratory distress syndrome, lung inflammation, cytokine storm, and death. The other complications include endothelial dysfunction, activation of coagulation, thromboembolic events, and vascular disease. Cardiovascular complications such as myocardial and stroke ischemia, pulmonary thromboembolism, systemic arterial, and deep vein thrombosis were reported. In this review, we presented immuno-pathological mechanisms and the effects of COVID-19 on the cardiovascular system, heart, vessels, coagulation system, and molecular glance of immuno-inflammation to the COVID-19’s pathology on the cardiovascular system.
Keywords: Corona virus, infection, immune system, heart, blood
The COVID-19 has strong effect on cardiovascular system.
Endothelial dysfunction, myocardial ischemia, and arterial and vein thromboembolism are the side effects of SARS-CoV-2.
In COVID-19, the inflammation dysregulates cardiac ion channels which results in arrhythmia.
These downstream effects of COVID-19 result in cardiac valvular disease.
Introduction
COVID-19, a recently discovered disease, is circulated rapidly worldwide and this pandemic involved more than several hundred million cases that were diagnosed and confirmed; also, these numbers have continued to increase. Clinically, the COVID-19 is markedly heterogeneous and has range from asymptomatic form to severe viral pneumonia and may progress to cytokine storm (CS), acute respiratory distress syndrome (ARDS), and death.1,2
SARS-CoV-2 is an enveloped novel strain in the coronavirus family, singled-stranded positive-sense RNA viruses. Additional other members of this family are SARS-CoV-1 and MERS-CoV. The rapid spread of COVID-19, caused by SARS-CoV-2, leads to ARDS in patients. Recent studies indicate that in SARS-CoV-2, CS and hyper-inflammation contribute to disease severity and COVID-19 mortality.1,3,4 The rapid transmission and divergent presentation of the SARS-CoV-2 strengthened the COVID-19’s status as a major public health threat.5,6 The hyper-inflammatory syndrome in COVID-19 results from a dysregulated innate immune response of the patients. The potential therapeutic approaches to the immune system modulation and abrogate lung injury in COVID-19 are necessary.1,7
Although the COVID-19 in the world has been substantial, concerns have arisen about the direct and indirect effects of the pandemic on higher-risk patients with cardiovascular disease (CVD). During the COVID-19 pandemic, hospitalizations for acute CVD, including heart failure, myocardial infarction (MI), and stroke, have declined precipitously. Population deaths due to cardiovascular causes were increased over this period and may also inform public health responses and policy efforts in states. CVD is associated with increasing mortality among hospitalized COVID-19 patients. Identifying the significant risk factors is urgent to reduce the risk of hospitalized patients. Therefore, in this study, we aimed to overview the immune-inflammatory responses of COVID-19 and its effect on the CVD such as heart failure, congenital heart disease, coronary heart disease, ischemic heart disease, hypertensive problems, and other diseases of circulatory system. Also, the COVID-19’s impact on the heart, CVD, summarizing the pathophysiological basis and the clinical management of these patients, and ultimately reduce mortality.8–10
Pathology and pathogenesis
SARS-CoV-2 uses the angiotensin-converting enzyme-2 (ACE2) receptor for cell entry in humans. The predominant lung injury pattern in COVID-19 was identified as diffuse alveolar hemorrhage and damage accompanied by platelet–fibrin–microthrombi in the pulmonary vessels. On the postmortem analysis, congested and diffusely edematous of the lung parenchyma, and clinical diagnosis of ARDS were observed. Pathologic features accompanying the interstitial and intra-alveolar exudate have dilated alveolar and collapsed alveoli, capillary congestion, formation of hyaline membrane, and pneumocytes desquamation. Viral particles within pneumocytes type 1 and 2, and localized SARS-CoV-2 antigen to the ACE2 bronchiolar epithelium are dominant. The microthrombosis was present in the small- and medium-pulmonary arterial vessels and CD61 megakaryocytes were increased in alveolar capillaries. In COVID-19, direct viral invasion via the ACE2 receptor may trigger endotheliitis and contribute to endothelial injury.1,11,12
Patients with COVID-19 have infiltration of the mononuclear inflammatory cells (lymphocytes and macrophages) in the lung parenchyma. The infiltrated lymphocytes are T cells (CD4 and CD8) in the bronchioles and alveoli, and CD4 T-cells are predominance, which aggregate around small vessels that often contained microthrombi. The CD68 macrophages were also localized in the alveolar lumen.1,13,14
Immune-inflammatory response
Innate immunity, as the first line of defense, has a key role in inhibiting in the COVID-19. Pathogen-associated molecular patterns (PAMPs) of the SARS-CoV-2 such as viral genomic single-stranded ribonucleic acid (ssRNA), replication of double-stranded ribonucleic acid, and proteins are recognized by pattern-recognition receptors (PRRs). Coronavirus ssRNA is sensed by endosomal toll-like receptors (TLR)3 and TLR7. Likewise, RIG-I and MDA5, as cytoplasmic RNA sensors, mediate signaling in the induction of antiviral interferon responses and respond to coronavirus RNAs. Stimulator of interferon genes (SING), as another PRR, is a cytosolic DNA sensor, and SING-mediated signaling was reported following coronavirus infections. The PAMP-PRR interactions result in the transcription factor activation of inflammation promoting, which contribute to the expression of interferon-I (IFN-I) and other proinflammatory cytokines.15–17 Viral replication also stimulates the production of chemokines such as IL8, IP10, and MCP1, leading to the accumulation of new sources for proinflammatory mediators. SARS-CoV Viroporin 3a stimulates the NLR-P3 inflammasome and the secretion of IL-1β, which contributes to pyroptosis. Adaptive immune response against COVID-19 is categorized into humoral via neutralizing antibodies (IgM and/or IgG) and cellular responses via CD8+ cytotoxic T cells against intracellular viruses. Both of the elements are regulated by antigen-specific CD4+ T cells.15,18–20
Cytokine storm
In COVID-19 patients, enhanced clinical inflammatory markers were prognostic of mortality and disease severity. ARDS is higher in the patients with elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, PGF, VEGF, FGF, G-CSF, GM-CSF, IP-10, MCP-1, MIP-1a and b, IFN-γ, TNF-α, IL-1b, IL-2, IL-6, IL-7, and IL-8 levels.1,21,22
SARS-CoV-2, as a cytopathic virus, induces cell death during replication. Viral replication in cells causes high levels of pyroptosis (inflammatory form of programed cell death), that is hyper-inflammatory response to SARS-CoV-2 infection and releases IL-1b from cells. Resident alveolar macrophages and lung epithelial cells detect the PAMP such as viral RNA, using several PRR. The PRR and IL-1R activation stimulate the secretion of pro-inflammatory cytokines, which recruit activated T cells and macrophages to the infection site, whereby these immune effector cells potentiate inflammatory reaction with additional cytokine secretion and lung parenchyma destruction.1,23–25 The cytokine profile in COVID-19 patients is Th1 and classic M1 macrophage is predominant. Also, ACE2-infected pneumocytes express high levels of pro-inflammatory cytokines (i.e., IL-1b, MCP-1, IL-6, and TNF-α). On the other hand, severe COVID-19 patients have increased TLR, IL-1R, and downstream signaling molecules (i.e., TRAF6, IRAK1, IRAK4, MYD88, and p65) expression that have strong effect on inflammation. Therefore, identifying the exact drivers of the pathologic inflammation and immune markers that predict a hyperinflammatory response to SARS-CoV-2 infection is important.1,24,26,27
CVD and COVID-19
The CVD and hypertension are the most common comorbidities in patients with COVID-19, which are associated with the severity of COVID-19. The several cardiovascular biomarkers are elevated in severe COVID-19 cases. Angiotensin I is converted to angiotensin II by ACE1 and angiotensin II is converted to angiotensin 1–7 (Ang 1–7) by ACE2 that induces vasodilatory response. However, ACE inhibitor/angiotensin-receptor blocker therapy in patients increase the risk of SARS-CoV-2 infection and severity. In addition, in patients with CVD, the cardiotoxicity of antiviral therapies with cardiovascular drugs should be paid attention in COVID-19.28–31
These involvements of the cardiovascular with COVID-19 can be explained by the several mechanisms. First, the systemic oxidative stress in SARS that is induced by hypoxemia directly damages cardiomyocytes, via mitochondrial damage and intracellular acidosis. Second, ACE2 receptors on the cardiovascular system can dysregulate the renin–angiotensin–aldosterone system (RAAS), leading to ventricular remodeling that alters myocardial demand and further induces cardiomyocyte damage. Third, in the CS of COVID-19 infection, mononuclear inflammatory infiltrate cells in cardiac interstitial are dominant. Finally, local and systemic effects of immune system induce cardiac microvasculature damage resulting in perfusion defects.28,32–35
The cumulative cardiomyocyte damage elucidated may result in increased levels of hscTn1, CK, troponin, furthermore, D-dimer, and prothrombin levels in hypercoagulable state which are associated with poor outcomes in COVID-19 patients. Elevation of the troponin is associated with the increased risk of mortality and adverse outcomes in COVID-19 patients. Therefore, in COVID-19 patients, coronary angiography and primary percutaneous coronary intervention are necessary.28,36,37
Cardiovascular cellular pathogenesis
The SARS-CoV-2 enters into the cells via binding to the ACE2. SARS-CoV-2 internalizes and downregulates the ACE2 expression on the cell surface. Since ACE2 converts angiotensin I and II to angiotensin 1–9 and 1–7 cardioprotective peptides, its loss on cell surface potentiates cardiac damage. In addition, the ACE2 loss on endothelium exacerbates endothelial dysfunction, inflammation, and thrombosis. Also, the reduced ACE2 induces cytokine release through dysregulating RAAS, depresses ACE2/Mas receptor axis, and activates ACE2/bradykinin B1R/DABK axis. The cardiac complications in COVID-19 patients can be divided into electrical and mechanical dysfunction. In arrhythmias, the electrical aberrance is seen, whereas vascular, pericardial, myocardial, and valvular complications arise due to mechanical dysfunction.38–42
Arrhythmia in COVID-19 patients can be secondary activated by pulmonary disease, drug side effects, electrolyte imbalance, protein kinase C (PKC), and direct oxidized Ca2+/CAMKII (calmodulin-dependent protein kinase II). PKC is activated by angiotensin II and can initiate arrhythmias. Hypo Ca2+ and hypo Mg2+ are commonly seen in COVID-19 patients due to angiotensin II-mediated urinary excretion. The hypoxia in ARDS increases the arrhythmias risk. The upregulated angiotensin II activates NADPH oxidase (increases the reactive oxygen species (ROS)) that oxidizes the CaMKII into ox-CaMKII and the ox-CaMKII in turn phosphorylates RyR2, and increases diastolic sarcoplasmic Ca2+ leak that induces atrial and ventricular arrhythmias. In COVID-19, the inflammation dysregulates the post-translational modification of cardiac ion channels resulting in arrhythmia.38,43–45
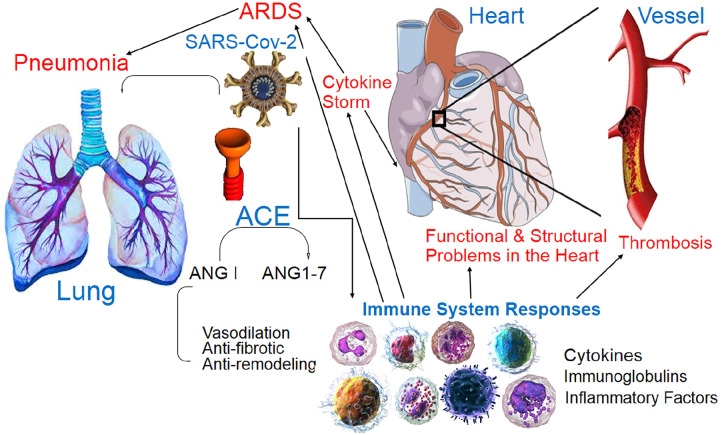
Elevated angiotensin II facilitates inflammatory cascade, elevated IL-6, and VEGF. These pro-inflammatory cytokines promote fibrosis and inflammation that present as pericarditis. On the other side, SARS-CoV-2 can cause pericarditis which progresses to pericardial effusion and can further complicate Takotsubo cardiomyopathy. In COVID-19, myocardial dysfunction presents as acute coronary syndrome, myocarditis, cardiomyopathy, heart failure, and shock. SARS-CoV-2 can induce systemic inflammatory response syndrome through a combination of CS and dysregulated immune activity which leads to myocardial dysfunction. In the CS of the SARS-CoV-2 infection, cytokines contribute to the damage of the myocard by facilitating the release of ROS, endogenous NO, and superoxide anion.38,46–48 Finally, damage-associated molecular proteins (DAMPs) like heat-shock proteins, HMGB1, oxidized lipoproteins, and histones are released from the damaged myocardial cells and further activate the inflammation and also myocytes damage. These excessive DAMPs create an ongoing defective inflammatory cycle resulting in septic/COVID-19 cardiomyopathy. The ACE2 is broadly expressed on the stromal fibroblasts of the cardiac valves, especially aortic valves and in stenotic valves, the ACE2 expression is downregulated. The depression of ACE2/angiotensin-(1–7)/Mas receptor axis potentiates inflammation, fibrosis, and valvular sclerosis (Figure 1). These downstream effects of COVID-19 result in the damage of the valves, which may present chronically due to the slow progressive nature of cardiac valvular disease.38,49,50
Figure 1.
The SARS-Cov-2 enters via respiration and is localized in pneumocytes via ACE2- that is dominant in bronchiolar epithelium and can active immune response (innate and adaptive) against COVID-19. Pneumonia and ARDS are main problems in COVID-19. Inflammation, pro-inflammatory cytokines release, and CS lead to the structural and functional (mechanical and electrical) CVD (cardiomyopathy, endomyocardial, pericarditis, arrhythmia, etc.) and coagulopathy (microthrombi, thromboembolism, ischemia, DIC, etc.).
ANG, angiotensin; ACE, angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; CS, cytokine storm; CVD, cardiovascular disease; DIC, disseminated intravascular coagulation.
Myocardial injury
COVID-19 patients with myocardial injury have elevated CRP, procalcitonin, and NT-proBNP that are associated with increased risk of in-hospital mortality. The mechanism of myocardial injury in COVID-19 may involve a direct myocyte injury with viral infection, and significant inflammation provoked by cytokine dysregulation.5,51–54
Endomyocardial biopsies from COVID-19 patients demonstrated the presence of lymphocytic infiltrates in the right ventricular myocardium, cardiomyocyte hypertrophy, and necrosis, and also macrophage infiltration in the myocardium. Potential etiologies with the COVID-19 include myocarditis, cardiac microvascular and coronary disease, ischemic injury, septic cardiomyopathy, Takotsubo’s cardiomyopathy, and secondary hypoxic injury.5,55,56
Non-invasive diagnostic tools such as electrocardiography (ECG) and cardiac imaging (including echocardiography, magnetic resonance imaging, and computed tomography imaging) determine the extent of cardiac structure and function in the setting of COVID-19 infection. ECG abnormalities, such as PR depression and ST elevation, may be observed in myocarditis, but these are not conclusive.5,57,58
Coagulopathy
Overproduction of pro-inflammatory cytokines in the immune response to SARS-CoV-2 activates the coagulation cascade. Some cytokines facilitate the adhesion of platelets by increasing the production of Von Willebrand factor. Moreover, high levels of IL-1, IL-6, TNF-α, and thrombin promote clot formation in COVID-19 patients by activating platelets. Also, thrombin activates the PAR-1, and endothelial cells (ECs) upregulate the expression of tissue factor (TF) at mucosal surfaces. Tissue factor activates the extrinsic pathway of the coagulation, downregulates the activated protein C that is an inhibitor of clotting, and inhibits the fibrinolytic processes. Moreover, plasminogen activator inhibitor-1 upregulation blocks the plasminogen activation, thus decreases the breakdown of fibrin clots.5,59–62
An imbalance between coagulants and anti-coagulants promotes the pro-coagulation state. This can explain the presence of pulmonary microthrombi and disseminated intravascular coagulation (DIC) diagnosed in critically COVID-19 patients. The formation of microthrombi increases venous thromboembolism incidence and its related problems.5,63–65
Thrombotic complications
There are substantial evidences for a significant risk of thrombosis in COVID-19 patients. Complications may include pulmonary embolism (PE), deep-vein thrombosis, systemic arterial embolism, ischemia, and MI. Usually, these patients have abnormal coagulation parameters, such as D-dimer and activated partial thromboplastin time. Important risk factors of the venous thrombosis involve endothelial injury, stasis, and a hypercoagulable state, together known as Virchow’s triad.66–71
Inflammation has a main role in thrombosis through perpetuating a hypercoagulable state and endothelial injury that can be done via fibrinolysis reduction, stimulation of the TF pathway, and NETosis. Also, complement activation is involved in thrombosis. The C3a and membrane attack complex (C5b-9) are involved in platelet activation and C5a enhances plasma and cellular TF expression. These intravascular pulmonary microthrombi are linked to the development of hypoxemia in the early stages of ARDS in COVID-19. Primary pulmonary thrombosis could be underpinned by ACE2-mediated CS, endothelial injury, and the development of a hypercoagulable state in patients with COVID-19.66,72–74
Myocardial and microvascular injuries by SARS-CoV-2 cause sub-endothelium and collagen exposure, which lead to platelet activation and possible contact pathway activation follows polyphosphate release in platelet degranulation. Endothelial trauma actives the TF pathway via the cleavage of FVII to FVIIa and also, ACE2-SARS-CoV-2 interactions dysregulate the kallikrein/kinin system. Inflammation due to SARS-CoV-2 generates inflammatory mediators such as CRP and pro-inflammatory cytokines. IL-6 is a key regulator of fibrinogen transcription and increases the level of plasma fibrinogen. Interestingly, SARS-CoV-2’ RNAs have interaction with platelets via TLR7 and 9 in a similar way to stimulate cytokine releasing and leukocytes activation.66,75–77
The balance between thrombolysis and thrombus is maintained by tissue-type plasminogen activator (tPA), urinary-type plasminogen activator (uPA), and their inhibitor PAI-1. In COVID-19 patients, impaired tPA and uPA fibrinolytic function further perpetuates a pro-thrombotic state. Hyperactivity of the fibrinolytic is supported by significant rises in D-dimer. On the other side, pro-inflammatory cytokines trigger EC activation and the release of PAI-1 and tPA. DIC is a potentially lethal mechanism that leads to multi-organ dysfunction and fibrinolysis derangement in COVID-19. The DIC pathophysiology in SARS-CoV-2 includes EC, leukocyte, and platelet activation, fibrin deposition, resulting in diffuse inflammation and coagulopathy.66,78–80
It was proposed that the COVID-19 microthrombus phenotype aligns more closely with complement-mediated thrombotic microangiopathy that is supported by the presence of anemia, elevated LDH, and renal dysfunction in severe SARS-CoV-2 cases. Microvascular dysfunction in SARS-CoV-2 via ACE2 receptor interactions and mannan-binding lectin serine protease (MASP) MASP-2 in the lectin pathway of complement activation is done. MASP-1 and -2 can cleave prothrombin to form thrombin and activate FXIII and fibrinogen, but future studies are needed to address the role of MASPs in COVID-19.66,81–83
Immuno-inflammatory biomarkers in CVD
Several immune-inflammatory biomarkers were explored in COVID-19 patients for possible monitoring of patients with CVD and treatment, which include full blood cells count, D-dimer, APTT and prothrombin time, cytokines, CRP, and other inflammatory markers such as CRP, fibrinogen, ferritin, IL-2, 6, 7, and TNF-α.66,84–86
D-dimer is an important prognostic marker for COVID-19 mortality. Thrombocytopenia in SARS-CoV-2 is observed due to intravascular pulmonary microthrombus formation. However, thrombocytopenia in COVID-19 rarely results in a bleeding phenotype. Elevation of the inflammatory markers is observed 7–14 days after initial onset. IL-6, as a main inflammatory cytokine, decreased during intensive care unit (ICU) admission. CRP is also elevated in the hospitalized COVID-19 patients. Furthermore, CRP levels are linked with elevated cardiac troponins and also myocardial injury. Serum ferritin, as a potential prognostic inflammatory marker in COVID-19, is a cellular damage marker.66,87–90 Elevated ferritin is associated with ICU admission and in-hospital mortality. Troponins T and I as cardiac-specific markers are highly sensitive to myocardial damage and released into the blood following heart trauma or infection. Elevated troponin is associated with myocarditis, MI, and PE resulting in myocardial ischemia. Troponin is consistently elevated in SARS-Cov-2 infection and considered a marker of poor prognosis. However, it should be confirmed in ICU admission patients, in-hospital mortality, and comorbidity.36,66,91–95
Lung vascular immunopathology
Pathology of the lung in COVID-19 patients shows marked hemorrhage and microvascular thrombosis linked to interstitial and alveolar inflammation. Lung-restricted vascular immunopathology, as diffuse pulmonary intravascular coagulopathy in COVID-19, is an early stage of DIC. The immune mechanism underlying pulmonary interstitial and alveolar inflammation triggers extensive immunothrombosis.96–100
IL-1, IL-6, and TNF-α trigger EC activation and dysfunction, determine vessel wall permeability, vasculopathy, thromboinflammatory, ventilation perfusion mismatch, and a clinical phenotype of refractory ARDS. Innate immune response, dysregulation of ACE2 expression, and adaptive antiviral immune responses in COVID-19 contribute to extensive pulmonary immunovascular coagulopathy, pulmonary immunothrombosis, and diffuse pulmonary intravascular coagulopathy.96,101–110
Pulmonary artery problems in COVID-19
One of the serious COVID-19 complication is thrombosis, and prophylactic anticoagulation to prevent thrombosis and reduce mortality is necessary.111,112 In-hospital, the acute PE rate is higher in ICU patients than in hospitalized general wards that may reflect a more severe pro-coagulant state. Therefore, the higher rate in COVID-19 patients makes it urgent to establish the antithrombotic treatment to minimize the risk in patients. Indeed, the immunothrombosis scenario is triggered by the SARS-CoV-2 infection, and EC dysfunction in the pulmonary microvasculature plays an important role in the thromboinflammatory processes. In this regard, CS and macrophage activation syndrome could trigger activating the coagulation cascade. Immune responses in local lymph nodes against pathogens (such as corona virus) can have strong effect on target tissue. At least, immunothrombosis prevention by standard thromboprophylaxis and available anticoagulant regiments is necessary.113–115
One of the potential pulmonary morbidities in COVID-19 patients is the tendency to thromboembolic phenomena. Also, chronic thromboembolic pulmonary hypertension (CTEPH) was reported in a series of patients with acute PE after COVID-19 pneumonia. It is possible that recovered patients from COVID-19 may develop the chronic thromboembolic disease, CTEPH, and PH secondary to lung disease.116,117 In COVID-19 patients (even in non-advanced disease), pulmonary parenchymal damage and altered hemodynamics determine pulmonary hypertension and right ventricular involvement.118 Post-PE clinical syndrome results from the mix of heterogeneous factors, from deconditioning to hemodynamic derangements. And also, the combination of viral pneumonia and ARDS is a possible precursor to pulmonary fibrosis.119 Therefore, immunomodulatory and anti-complement treatments to reduce the inflammatory cascade in COVID-19 are necessary and in routine, post-COVID-19 follow-up should be done in all COVID-19 patients.
Concluding remarks
COVID-19 infection is linked to myocardial injury and leads to severe disease progression. The main mechanism of myocardial injury is immune-inflammatory responses against SARS-Cov-2. SARS-CoV-2 with its receptor, the ACE2 involve in CVD and by inflammation and cytokines, involve immune system with cardiovascular system. ARDS is the main issue in COVID-19 patients that is initiated by cytokine hyper secretion and can have dangerous effect on cardiovascular system, and coagulopathy is another problem in COVID-19 patients. The interplay between the inflammation, immune response, coagulation, and CVD in COVID-19 requires further research, to establish their pathophysiology mechanism of the disease in further detail. In addition, the relationship between CVD pathogenesis and immune response in SARS-Cov-2 infection needs deep survey to have applicable prophylactic and therapeutic protocols for control and management of CVD in COVID-19 patients. Therefore, more attention to cardiovascular injury and CVD in patients with COVID-19 infection is necessary for prevention and limitation of the morbidity and mortality.
Footnotes
Author contributions: EMN, HA, RHM, and SSA participated in the study design, and drafting the manuscript. SSA supervised the study. All authors approved the submitted version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Seyyed Shamsadin Athari  https://orcid.org/0000-0002-6355-6378
https://orcid.org/0000-0002-6355-6378
References
- 1.Gustine JN, Jones D.Immunopathology of hyperinflammation in COVID-19. Am J Pathol 2021; 191(1): 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athari SS.COVID-19 pandemic is not apocalypse, is strong warning. Ann Immunol Immunother 2020; 2(2): 000119. [Google Scholar]
- 3.Huang C, Wang Y, Li Xet al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Chen X, Cai Yet al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamouche W, Bisserier M, Brojakowska Aet al. Pathophysiology and pharmacological management of pulmonary and cardiovascular features of COVID-19. J Mol Cell Cardiol 2021; 153: 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Yang K, Wang Wet al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Valle DM, Kim-schulze S, Hsin-hui Het al. An inflammatory cytokine signature helps predict COVID-19 severity and survival. Nat Med 2020; 26: 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadhera RK, Shen C, Gondi Set al. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol 2021; 77(2): 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox SE, Akmatbekov A, Harbert JLet al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020; 8: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Wu D, Guo Wet al. Clinical and immunological features of severe and moderate Coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay MZ, Poh CM, Rénia Let al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink SL, Cookson BT.Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 2005; 73: 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L, Ding Y, Zhang Qet al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2 cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 2006; 210: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco-Melo D, Nilsson-Payant BE, Liu W-Cet al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036.e9–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khosroshahi LM, Rokni M, Mokhtari Tet al. Immunology, immunopathogenesis and immunotherapeutics of COVID-19: an overview. Int Immunopharmacol 2021; 93: 107364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubernatorova EO, Gorshkova EA, Polinova AIet al. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev 2020; 53: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satterfield BA, Bhatt DL, Gersh BJ.Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol 2022; 19(5): 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surazakov A, Klassen A, Gizinger O.The bioenergetics of COVID-19 immunopathology and the therapeutic potential of biophysical radiances. J Photochem Photobiol, B: Biol 2020; 213: 112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang S, Hillyer C, Du L.Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 2020; 41(5): 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju B, Zhang Q, Ge Jet al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020; 584(7819): 115–119. [DOI] [PubMed] [Google Scholar]
- 21.St John AL, Rathore APS. Early insights into immune responses during COVID-19. J Immunol 2020; 205: 555–564. [DOI] [PubMed] [Google Scholar]
- 22.Liao M, Liu Y, Yuan Jet al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26: 842–844. [DOI] [PubMed] [Google Scholar]
- 23.Hadjadj J, Yatim N, Barnabei Let al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369: 718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Yang X, Zheng Yet al. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell 2014; 5: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Fu B, Zheng Xet al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 2020; 7: 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills CD, Kincaid K, Alt JMet al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164: 6166–6173. [DOI] [PubMed] [Google Scholar]
- 27.Yap JKY, Moriyama M, Iwasaki A.Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J Immunol 2020; 205: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levett JY, Raparelli V, Mardigyan Vet al. Cardiovascular pathophysiology, epidemiology, and treatment considerations of coronavirus disease 2019 (COVID-19): a review. CJC Open 2021; 3: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tikellis C, Thomas MC.Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012; 2012: 256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng YY, Ma YT, Zhang JYet al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang L, Karakiulakis G, Roth M.Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers LC, Parodi SM, Escobar GJet al. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 2020; 323: 2195–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz JH.Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020; 27(3): taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esler M, Esler D.Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens 2020; 38: 781–782. [DOI] [PubMed] [Google Scholar]
- 35.Castiglione V, Chiriacò M, Emdin Met al. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother 2020; 6: 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippi G, Lavie CJ, Sanchis-Gomar F.Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a metaanalysis. Prog Cardiovasc Dis 2020; 63: 390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onder G, Rezza G, Brusaferro S.Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020; 323: 1775–1776. [DOI] [PubMed] [Google Scholar]
- 38.Sattar Y, Ullah W, Rauf Het al. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. IJC Heart Vasc 2020; 29: 100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Napoli C, Tritto I, Benincasa Get al. Cardiovascular involvement during COVID-19 and clinical implications in elderly patients. A review. Ann Med Surg 2020; 57: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.South AM, Diz DI, Chappell MC.COVID-19, ACE2, and the cardiovascular consequences, Am J Physiol Heart Circ Physiol 2020; 318: H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasab ME, Athari SS.Chest pain in COVID-19 patients may not refer to cardiac ischemia. J Med Microbiol Infect Dis 2020; 8(3): 125–126. [Google Scholar]
- 42.Sanders JM, Monogue ML, Jodlowski TZet al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 323: 1824–1836. [DOI] [PubMed] [Google Scholar]
- 43.Lippi G, South AM, Henry BM.Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem 2020; 57: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal M.Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020; 14: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirano T, Murakami M.COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020; 52: 731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Li M, Zhou Zet al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun 2020; 111: 102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo P, Vieceli Dalla Sega F, Fortini Fet al. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol 2020; 115: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quiros Roldan E, Biasiotto G, Magro Pet al. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol Res 2020; 158: 104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X, Li L, Yang Q.Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: implications from clinical features to pathologic findings. Circulation 2020; 141: 1736–1738. [DOI] [PubMed] [Google Scholar]
- 50.Vaduganathan M, Vardeny O, Michel Tet al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kariyanna PT, Sutarjono B, Grewal Eet al. A systematic review of COVID-19 and myocarditis. Am J Med Case Rep 2020; 8(9): 299–305.32747875 [Google Scholar]
- 52.Chen G, Wu D, Guo Wet al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130(5): 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh MN, Sorgente A, Fischman DLet al. The COVID-19 pandemic and cardiovascular complications: what have we learned so far? JACC Case Rep 2020; 2(9): 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ortega JT, Serrano ML, Pujol FHet al. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: an in silico analysis. EXCLI J 2020; 19: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giustino G, Croft LB, Oates CPet al. Takotsubo Cardiomyopathy in COVID-19. J Am Coll Cardiol 2020; 76(5): 628–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao XH, Li TY, He ZCet al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua bing li xue za zhi = Chin J Pathol 2020; 49(5): 411–417. [DOI] [PubMed] [Google Scholar]
- 57.Venugopal VK, Mahajan V, Rajan Set al. A systematic meta-analysis of CT features of COVID-19: lessons from radiology. medRxiv: the preprint server for health sciences. 2020; DOI: 2020.04.04.20052241. [Google Scholar]
- 58.Li X, Ma X.Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care 2020; 24(1): 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leonard-Lorant I, Delabranche X, Severac Fet al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020; 296: E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fauvel C, Weizman O, Trimaille Aet al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020; 41(32): 3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nasab EM, Athari SS.COVID-19 cytokine storm complications in asthmatic patients. Open Public Health J 2020; 13: 625–626. [Google Scholar]
- 62.Tang N, Li D, Wang Xet al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18(4): 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varga Z, Flammer AJ, Steiger Pet al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395(10234): 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poyiadji N, Cormier P, Patel PYet al. Acute pulmonary embolism and COVID-19. Radiology 2020; 297(3): E335–E338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poissy J, Goutay J, Caplan Met al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation 2020; 142(2): 184–186. [DOI] [PubMed] [Google Scholar]
- 66.Page ED, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res 2021; 200: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava K.Association between COVID-19 and cardiovascular disease. IJC Heart Vasc 2020; 29: 100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roifman I, Arora RC, Bewick Det al. Cardiovascular care delivery during the second wave of COVID-19 in Canada. Can J Cardiol 2021; 37: 790e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leong DP, Biostat M, Banerjee Aet al. COVID-19 vaccination prioritization on the basis of cardiovascular risk factors and number needed to vaccinate to prevent death. Can J Cardiol 2021; 37(7): 1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hantoushzadeh S, Nabavian SM, Soleimani Zet al. COVID-19 disease during pregnancy and peripartum period: a cardiovascular review. Curr Probl Cardiol 2022; 47(1): 100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berry C, Mangion K.Cardiovascular complications are very uncommon in healthcare workers with mild or asymptomatic COVID-19 infection. JACC: Cardiovasc Imaging 2021; 14(11): 2167–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao X.COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 2020; 20: 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schurink B, Roos E, Radonic Tet al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 2020; 1: e290–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunal S, Sharma SM, Sharma SKet al. Cardiovascular complications and its impact on outcomes in COVID-19. Indian Heart J 2020; 72: 593e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boechat JL, Chora I, Morais Aet al. The immune response to SARS-CoV-2 and COVID-19 immunopathology-current perspectives. Pulmonology 2021; 27(5): 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhalete AEI, Rhazi I, Bensaid Aet al. Cardiovascular injuries during COVID-19 infection: a PROCESS-compliant case series from the Eastern Morocco. Ann Med Surg 2021; 65: 102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minhas A, Shade JK, Cho SMet al. The role of sex and inflammation in cardiovascular outcomes and mortality in COVID-19. Int J Cardiol 2021; 337: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guijarro C.COVID-19 and cardiovascular disease. Clin Investig Arterioscler 2020; 32(6): 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dai X, Ostrikov KK.ROS-Driven selection pressure on COVID-19 patients with cardiovascular comorbidities. Innovation. Epub ahead of print 27April2021. DOI: 10.1016/j.xinn.2021.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonnie KY, Mann DL.COVID-19 clinical trials: a primer for the cardiovascular and cardio-oncology communities. JACC: Cardio Oncol 2020; 2(2): 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yiangou L, Davis RP, Mummery CL.Using cardiovascular cells from human pluripotent stem cells for COVID-19 research: why the heart fails. Stem Cell Rep J 2021; 16: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maines M, Zorzi A, Benetollo PPet al. Short-term outcome associated with remote evaluation (telecardiology) of patients with cardiovascular diseases during the COVID-19 pandemic. IJC Heart Vasc 2020; 30: 100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Egeli B, Sparks JA, Kim AHJet al. Hydroxychloroquine for the treatment of COVID-19 and its potential cardiovascular toxicity: hero or villain? Best Pract Res Clin Rheumatol 2021; 35: 101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zuo Y, Kanthi Y, Knight JSet al. The interplay between neutrophils, complement, and microthrombi in COVID-19. Best Pract Res Clin Rheumatol 2021; 35: 101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.García-Guimaraes M, Mojón D, Calvo Aet al. Influence of cardiovascular disease and cardiovascular risk factors in COVID-19 patients. Data from a large prospective Spanish cohort. RECCardio Clin 2021; 56(2): 108–117. [Google Scholar]
- 86.Aldien AS, Ganesan GS, Wahbeh Fet al. Systemic inflammation may induce cardiac injury in COVID-19 patients including children and adolescents without underlying cardiovascular diseases: a systematic review. Cardiovasc Revasc Med 2022; 35: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bérard E, Kai SHY, Coley Net al. Lockdown-related factors associated with the worsening of cardiovascular risk and anxiety or depression during the COVID-19 pandemic. Prev Med Rep 2021; 21: 101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naeini MB, Sahebi M, Nikbakht Fet al. A meta-meta-analysis: evaluation of meta-analyses published in the effectiveness of cardiovascular comorbidities on the severity of COVID-19. Obes Med 2021; 22: 100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medzikovic L, Cunningham CM, Li Met al. Sex differences underlying preexisting cardiovascular disease and cardiovascular injury in COVID-19. J Mol Cell Cardiol 2020; 148: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang SC, Wang YF.Cardiovascular protective properties of oxytocin against COVID-19. Life Sci 2021; 270: 119130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lazaridis C, Vlachogiannis NI, Bakogiannis Cet al. Involvement of cardiovascular system as the critical point in coronavirus disease 2019 (COVID-19) prognosis and recovery. Hell J Cardiol 2020; 61: 381e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long J, Luo Y, Wei Yet al. The effect of cardiovascular disease and acute cardiac injury on fatal COVID-19: a meta-analysis. Am J Emerg Med 2021; 48: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Polito MV, Silverio A, Bellino Met al. Cardiovascular involvement in COVID-19: what sequelae should we expect? Cardiol Ther Actions 2021; 10(2): 377–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nishiga M, Wang DW, Han Yet al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tersalvi G, Vicenzi M, Calabretta Det al. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail 2020; 26: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGonagle D, O’Donnell JS, Sharif Ket al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020; 2: e437–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang N, Li D, Wang Xet al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian S, Hu W, Niu Let al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020; 15(5): 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang N, Bai H, Chen Xet al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18(5): 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fox SE, Akmatbekov A, Harbert JLet al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. Lancet Respir Med 2020; 8(7): 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGonagle D, Sharif K, O’Regan Aet al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020; 19(6): 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ji HL, Zhao R, Matalon Set al. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 2020; 100: 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shoenfeld Y.Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev 2020; 19: 102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nasab EM, Heidarzadeh S, Yavari Bet al. Acute upper limb ischemia in a patient with COVID-19: a case report. Ann Vasc Surg 2021; 77: 83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nasab EM, Heidarzadeh S, Athari SS.Right atrial clot and pulmonary embolism in a patient with COVID-19: a case report. Radiol Case Rep 2021; 16: 3392–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nasab EM, Athari SS.Post-coronavirus era: should we expect a surge in allergic diseases and asthma? Open Public Health J 2021; 14: 291–293. [Google Scholar]
- 107.Rivellese F, Prediletto E.ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev 2020; 19(6): 102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Athari SS.Targeting cell signaling in allergic asthma. Signal Transduct Target Ther 2019; 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Athari SS, Athari SM.The importance of eosinophil, platelet and dendritic cell in asthma. Asian Pac J Trop Dis 2014; 4(Sup 1): S41–S47. [Google Scholar]
- 110.Bagheri Lankarani K, Honarvar B, Athari SS. The mechanisms underlying helicobacter pylori-mediated protection against allergic asthma. Tanaffos 2017; 16(4): 251–259. [PMC free article] [PubMed] [Google Scholar]
- 111.Sasaki K, Murata M, Nakamura Ket al. A case of severe COVID-19 with pulmonary thromboembolism related to heparin-induced thrombocytopenia during prophylactic anticoagulation therapy. Pulmonary artery problems in COVID-19. J Infect Chemother 2022; 28(8): 1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Puk O, Nowacka A, Smulewicz Ket al. Pulmonary artery targeted therapy in treatment of COVID-19 related ARDS. Literature review. Biomed Pharmacother 2022; 146: 112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haddadzadeh HR, Athari SS, Abedini Ret al. One-Humped camel (Camelus dromedarius) infestation with linguatula serrata in Tabriz, Iran. Iran J Arthropod Borne Dis 2010; 4(1): 54–59. [PMC free article] [PubMed] [Google Scholar]
- 114.Roncon L, Zuin M, Barco Set al. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med 2020; 82: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hami M, Naddaf SR, Mobedi Iet al. Prevalence of Linguatula serrata infection in domestic bovids slaughtered in Tabriz Abattoir, Iran. Iranian J Parasitol 2009; 4(3): 25–31. [Google Scholar]
- 116.Cueto-Robledo G, Roldan-Valadez E, Graniel-Palafox LEet al. Chronic thromboembolic pulmonary hypertension (CTEPH): a review of another sequel of severe post-covid-19 pneumonia. Curr Probl Cardiol. Epub ahead of print 25March2022. DOI: 10.1016/j.cpcardiol.2022.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zotzmann V, Mueller-Peltzer K, Bode Cet al. Clinical implication of pulmonary artery thrombi in COVID-19. Respir Med 2021; 176: 106247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pagnesi M, Baldetti L, Beneduce Aet al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart 2020; 106: 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhawan RT, Gopalan D, Howard Let al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med 2021; 9: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]