Abstract
Onchocerciasis is a debilitating parasitic infection caused by the filarial nematode Onchocerca volvulus. Infections are chronic, and persistence of the parasites for several years argues for highly adapted mechanisms of immune evasion. Due to the restricted host repertoire of O. volvulus, we have used the cattle parasite Onchocerca ochengi to investigate the nature of immunomodulation underpinning these long-term infections. Cattle were infected with a single inoculation of 350 infective-stage larvae under laboratory conditions (n = 6). Intradermal nodules containing immature adult worms were detected from 110 days postinfection, and microfilariae in skin were detected from day 280 postinfection. Parasite-specific responses during early infection were nonpolarized with respect to the major Th cytokines (interleukin-4 [IL-4], IL-2, and gamma interferon [IFN-γ]) produced by antigen-stimulated peripheral blood mononuclear cells (PBMC) or serum antibody isotypes. Antigen-induced proliferation of PBMC peaked shortly after exposure and remained high during the prepatent infection. As the parasites matured and animals developed patent infections, there was a profound down-regulation of lymphoproliferation, accompanied by sharp falls in the expression of both IL-4 and IFN-γ and a gradual decline in IL-2. Levels of immunoglobulin G2 (IgG2) fell, while those of IgG1 remained high. We conclude that neither a classical Th2 response nor a simple Th1-to-Th2 switch is sufficient to explain the immunomodulation associated with patent Onchocerca infections. Instead, there is an initial Th0 response, which matures into a response with some, but not all of the features of a Th2 response. The natural host-parasite relationship of O. ochengi in cattle may be useful as both a descriptive and predictive tool to test more refined models of immunomodulation in onchocerciasis.
Onchocerciasis is a debilitating parasitic infection of sub-Saharan Africa and Latin America caused by the filarial nematode Onchocerca volvulus (2). Characteristically, infections are chronic, and the disease this provokes over the years is associated with a range of dermal and ocular lesions (24). The longevity of O. volvulus adult worms in humans is estimated to be more than a decade (2). This persistence argues for highly adapted mechanisms of immune evasion. An understanding of the processes underlying parasite survival may open the way to new opportunities for curative treatment or the amelioration of disease.
A variety of clinical and experimental observations provide support for the view that O. volvulus modulates the host response to protect the parasite from immune-mediated damage. Based on the study of infected individuals with so-called “generalized disease” (characterized by detectable adult worms and microfilariae, with or without pathology), these observations include depressed cellular responses in skin tests with parasite-specific or ubiquitous recall antigens, hypoplastic and fibrotic draining lymph nodes associated with sites of infection, weak peripheral blood mononuclear cell (PBMC) proliferative responses to parasite antigens in vitro, and reduced levels of type 1 PBMC-derived cytokines (8, 11, 12, 17). This contrasts with the relatively reactive state of patients with “localized” onchocerciasis (or “Sowda”), in which few or no living parasites can be detected, although onchocercal pathology is present. In these cases, delayed hypersensitivity reactions are strong and draining lymph nodes are swollen with active germinal centers (6). Individuals living within areas of endemicity but remaining free of infection (termed “putative immunes”) also exhibit heightened cellular responses. This is manifested by elevated blastogenic responses of PBMC to parasite antigens, accompanied by increased interleukin-2 (IL-2), IL-5, and gamma interferon (IFN-γ) production (8, 27, 36). Experimental infections of chimpanzees with O. volvulus have shown that parasite-specific in vitro proliferative responses and IL-2 production were only observed prior to the onset of patency (28). Cellular proliferative responses of patent animals could be restored by the addition of recombinant IL-4 or IL-6 (19). High levels of IL-10, associated with patent infections in humans, may also be responsible for modulation of type 1 cytokine production and lymphoproliferation in the generalized form of the disease (8, 27).
Many questions in onchocerciasis concerning the interplay between infection status and the balance between immune responsiveness and immune modulation remain to be addressed. To approach this, we have turned to the natural host-parasite relationship of Onchocerca ochengi in cattle. This has many benefits. First, it obviates the need to use chimpanzees, which, while susceptible to O. volvulus, are not natural hosts and come with ethical and logistic constraints on their use. Second, O. ochengi is the parasite most closely related to O. volvulus, based on phylogenetic classification (37), and is biologically very similar. For example, it shares the same vector (Simulium damnosum) and is transmitted sympatrically with O. volvulus across Equatorial Africa (34). Furthermore, O. ochengi is a natural parasite of cattle and, as such, is highly representative of the genus Onchocerca, because this occurs primarily in ungulate hosts. For these reasons, O. ochengi infections in cattle may be one of the best analogs of human infection for experimental investigations.
Here we report our initial results from the O. ochengi-infected cattle system, based on experimental infections undertaken under controlled laboratory conditions. Through the use of single, pulse infections, we find that the response to early infection is nonpolarized with respect to T helper cytokines, follows a profile that accords closely with parasite development, and results in the depression of lymphoproliferation coincident with the maturation of adult worms. Surprisingly, both IL-4 and IFN-γ are down-regulated in cattle with mature O. ochengi infections, at a time when the immunoglobulin G (IgG) response is reaching a peak. These data indicate that the system may be useful as both a descriptive and predictive tool in onchocerciasis.
MATERIALS AND METHODS
Cattle and parasites.
Seven Jersey bull calves (Bos taurus) were obtained locally and maintained off pasture in flyproof accommodations. The animals were treated with a proprietary anthelmintic (Panacur; Hoechst Roussel Vet) prior to entry into the experiment. O. ochengi microfilariae were obtained from the ventral skin of freshly slaughtered cattle from the Adamawa province of northern Cameroon (35). The parasites were extracted from skin and cryopreserved by established procedures (3, 13) and then transported to the United Kingdom in liquid nitrogen. Infective-stage larvae were produced by intrathoracic injection of microfilariae into laboratory-reared blackflies (Simulium ornatum sl.) as described previously (21). A phosphate-buffered saline (PBS) soluble extract of adult parasites was prepared as described previously (16).
Infection of cattle and parasitological examinations.
Three hundred-fifty infective larvae were administered to each of six cattle by subcutaneous injection along the ventral midline in the region of the umbilicus. One animal was retained as an uninfected control. The cattle were bled weekly to 6 weeks postinfection and monthly thereafter. Physical examinations were conducted from 6 months postinfection to detect the first appearance of palpable, intradermal nodules containing O. ochengi adult worms (35). Examinations were repeated at monthly intervals. Nodules were excised and dissected at the end of the experiment to confirm the identity and viability of the parasites they contained. At each examination, three skin biopsies were taken along the ventral midline between the umbilicus and scrotum to detect the presence of microfilariae. The skin was incubated for 24 h at room temperature in RPMI 1640 medium supplemented with 20% fetal calf serum (FCS), 200 U of penicillin per ml, and 200 μg of streptomycin per ml (all from Gibco, Paisley, United Kingdom). Parasites in the medium were counted under low-power microscopy. For greater sensitivity of detection of microfilariae, PCR was employed to amplify the O-150 DNA repeat sequence of Onchocerca (22) from skin biopsies designated negative by parasitological methods. Skin snips were chopped finely in buffer (100 mM NaCl, 10 mM Tris-HCl [pH 8.0], 25 mM EDTA [pH 8.0], 0.5% sodium dodecyl sulfate) containing 100 mg of proteinase K per ml (Boehringer Mannheim GmbH, Mannheim, Germany) and incubated for 12 h at 55°C. Samples were boiled for 30 min in the presence of 20 mM dithiothreitol (Sigma, Poole, United Kingdom) and were subjected to three cycles of freeze-thawing. Genomic DNA was extracted with phenol-chloroform, precipitated in 100% ethanol, and dissolved in 50 μl of water. The PCR was carried out with 1 μl of test sample per reaction, following established procedures (22). Negative (DNA extracted from skin of an uninfected animal) and positive (DNA from plasmid pOVS134 containing the O-150 repeat) control templates were analyzed in parallel.
Lymphocyte proliferation assay.
Heparinized blood was diluted 1:1 in RPMI 1640 medium with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10 U of heparin per ml (Sigma). PBMC were separated by density centrifugation on Histopaque, according to the manufacturer's instructions (Sigma). Cells were washed three times in RPMI 1640 supplemented with 10% FCS, heparin, and antibiotics. The cells were counted and adjusted to a density of 5 × 106 cells/ml in supplemented medium lacking heparin. One-hundred-microliter aliquots of cell suspension were added in triplicate to the wells of 96-well round-bottom microtiter plates (Nunc-Gibco). Cells were stimulated with 100 μl of concanavalin A (final concentration, 10 μg/ml; Sigma), PBS-soluble adult worm antigen (final concentration, 10 or 50 μg/ml), or RPMI 1640 medium alone. Plates were incubated for 72 h at 37°C in 5% CO2 in air, and the cells were pulsed with 0.5 μCi of [3H]thymidine/well (Amersham Life Sciences, Little Chalfont, United Kingdom). After 24 h, the cells were harvested onto filter mats (Skatron Instruments, Ltd., Newmarket, United Kingdom). [3H]thymidine incorporation was measured by liquid scintillation counting and recorded as cpm.
Quantification of bovine cytokines.
Aliquots of 5 × 106 PBMC in 1 ml of supplemented RPMI 1640 medium (10% FCS, 100 U of penicillin per ml, 100 μg of streptomycin per ml) were added in triplicate to the wells of 24-well tissue culture plates (Nunc). Cells were stimulated with PBS-soluble adult parasite antigen at a final concentration of 50 μg/ml. Control cultures received an equivalent volume of PBS alone. Plates were incubated for 72 h at 37°C in 5% CO2 in air. Culture supernatants were obtained by centrifugation of cell suspensions at 400 × g for 5 min at room temperature. Samples were stored at −70°C until read.
Bioassays.
PBMC from a conventionally reared, uninfected Bos taurus heifer were used as a source of B lymphocytes. Cells were adjusted to 3 × 108 cells/ml in PBS containing 0.5% bovine serum albumin and stained with monoclonal antibody IL-A58 (specific for bovine Ig light chain and kindly provided by the International Livestock Research Institute, Nairobi, Kenya). They were incubated with anti-mouse Ig-conjugated superparamagnetic particles (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). Labeled cells were isolated on a MiniMacs column (Miltenyi Biotech) according to the manufacturer's instructions. The purity of the population was assessed by FACscan (Becton Dickinson, Oxford, United Kingdom) and shown to contain greater than 90% B cells. The viability of cell preparations and cultures was assessed by trypan blue exclusion.
IL-2 and IL-4 bioassays.
Culture supernatants were tested for T- and B-cell growth activity on the 4325 long-term IL-2-maintained cell line and purified B cells. For the IL-2 bioassay, 4325 cells were stimulated for 3 days with recombinant bovine IL-2 (rbIL-2) produced in a baculovirus-insect cell system (5). Cells were washed and then made up to 5 × 105 cells/ml in supplemented RPMI 1640 medium, and 100-μl aliquots of cell suspension were added to an equal volume of test sample or rbIL-2, prepared as fivefold serial dilutions. For the IL-4 assay, purified B cells were adjusted to 106 cells/ml, and 100-μl aliquots were dispensed into wells containing test samples diluted as described above. Cultures were incubated for 18 to 24 h before being labeled with 37 kBq of [3H]thymidine (3H-TdR; NEN, Du Pont, Stevenage, United Kingdom). Cells were harvested after 6 h. Incorporated radioactivity was determined by liquid scintillation counting.
IFN-γ ELISA.
Bovine IFN-γ in culture supernatants of PBMC was measured by enzyme-linked immunosorbent assay (ELISA) with a commercial kit (IDEXX Laboratories, Slough, United Kingdom) according to the manufacturer's instructions. Plates were read on a Spectral Max 250 ELISA plate reader (Molecular Devices Corporation, Sunnyvale, Calif.) at A650. Recombinant bovine IFN-γ (Ciba Geigy, Basel, Switzerland) was used as a positive control and to generate a standard curve. Concentrations of IFN-γ in culture supernatants were expressed as nanograms per milliliter.
Quantification of parasite-specific antibodies.
Parasite-specific antibody levels were measured against PBS-soluble adult worm antigen by ELISA as described previously (18), with the following modifications. Plates were coated with antigen at 10 μg/ml, and sera were tested at a dilution of 1:400, as determined by checkerboard titration. Murine monoclonal antibodies against bovine IgG1, IgG2, and IgM were used at a dilution of 1:2,000. The substrate used was 0.3 mg of 2,2′-azino-di-{3-ethylbenzthiazoline sulfonate} peroxidase (ABTS; Sigma) per ml in 1.25 mM citric acid (pH 4.0) (BDH, Poole, United Kingdom) with 0.1% hydrogen peroxide (Merck, Poole, United Kingdom). Plates were read at 405 nm on a Dynatech Microtiter Plate Reader (Dynatech, Billingshurst, United Kingdom).
Statistical analysis.
Data on antigen-specific cytokine production and antibody levels were compared during the prepatent and patent phases of infection. Mean values for each animal over the two observation periods were log transformed, to account for nonnormal distribution, and compared by paired t test analysis.
RESULTS
Parasite development following experimental infection.
Five of six animals developed detectable O. ochengi infections, as determined by palpable intradermal nodules or the detection of microfilariae in the skin (Table 1). The mean time to first detection of nodules was 267 days postinfection (range, 110 to 600 days), with a mean number of three nodules per animal (range, 1 to 7). The mean time to onset of microfilaridermia was 372 days postinfection (range, 279 to 532 days) with a mean peak in microfilarial densities of 4 microfilariae/mg of skin (range, 0.1 to 8.0 microfilariae/mg). Microfilaridermia was detected in four animals (707, 708, 709, and 712) by both microscopy and O-150 PCR. Microfilariae could only be detected by O-150 PCR in animal 706. Microfilaridermia was maintained in animals after the onset of patency to the end of the experiment. Animal 704 remained negative for microfilariae as determined by microscopy and O-150 PCR throughout the period of observation.
TABLE 1.
Cytokine and serum antibody responses to parasite antigen in six calves experimentally infected with O. ochengi, together with data from an uninfected animal
| Animal | IFN-γ concn (ng/ml)a
|
IL-4 (stimulation index)a
|
IL-2 concn (U/ml)a
|
IgG antibody concn (OD405)b
|
Infection intensityc
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prepatentc | Patentd | Prepatent | Patent | Prepatent | Patent | Prepatent | Patent | Mf | Nod | |
| Infected | ||||||||||
| 704 | 614 | 104 | 5 | 3.1 | 2.8 | 1.7 | 0.46 | 0.57 | ND | ND |
| 706 | 121 | 16 | 4.9 | 3.4 | 2 | 2.1 | 0.64 | 0.96 | + | 1 |
| 707 | 568 | 21 | 5 | 1.5 | 2.4 | 0.7 | 0.49 | 0.82 | ++ | 7 |
| 708 | 228 | 9 | 6.3 | 2 | 4.8 | 1.2 | 0.41 | 0.78 | ++ | 4 |
| 709 | 608 | 54 | 4.9 | 3 | 1.8 | 2 | 0.48 | 0.78 | + | ND |
| 712 | 960 | 34 | 8.3 | 3.9 | 5.4 | 3 | 0.22 | 0.43 | + | 1 |
| Geometric mean | 421 | 29d | 5.6 | 2.7d | 2.9 | 1.6d | 0.42 | 0.70d | ||
| Control | ||||||||||
| 710 | 10 | 4 | 1.9 | 1.4 | 1.1 | 0.7 | 0.07 | 0.16 | ||
Cytokines were measured in culture supernatants of PBMC stimulated with antigen in vitro for 72 h. Shown are the mean values for all data points collected within the time interval. Prepatent, days 0 to 279 postinfection; patent, days >280 postinfection.
Mean value for all data points collected within time interval is shown. OD405, optical density at 405 n.m.
Mf, microfilarial density; ND, not detected; +, <1 microfilaria/mg of skin; ++, >1 microfilariae/mg of skin; Nod, number of O. ochengi nodules detected.
Significant difference between prepatent and patent values (P < 0.05).
Parasite-specific lymphoproliferative responses over the course of infection.
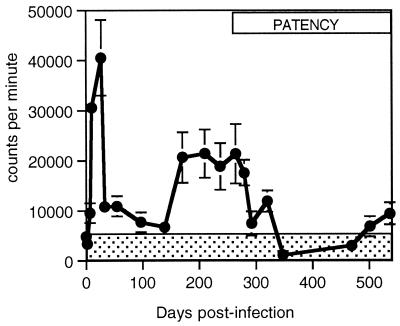
Blastogenesis of PBMC induced by parasite antigen was observed in all six cattle that received O. ochengi infective-stage larvae (Fig. 1). Antigen-specific lymphoproliferation was not observed in PBMC obtained from the uninfected, sentinel animal on 20 successive occasions. Among the infected calves, quantitative differences were observed in lymphoproliferation; however, there was no indication that the level of the response reflected variation in number of nodules, microfilarial density, or prepatent period within the range of values observed (data not shown). Lymphoproliferation rose steeply during the initial period of larval development (Fig. 1). During the prepatent period, the response peaked on day 33 and subsequently oscillated between phases of reduced or elevated responsiveness from days 33 to 139 and 171 to 320, respectively. Proliferation fell to within background levels (represented by the shaded area in Fig. 1 and calculated as the mean + 2 standard errors [SE] for 20 readings taken from the sentinel animal over the period of observation) on day 348. Responses above the background level were again observed on days 500 and 538, although these were relatively low. PBMC responses to concanavalin A were observed in all animals and were maintained over the course of infection, with levels of proliferation always exceeding those of parasite antigen-stimulated cultures (data not shown).
FIG. 1.
Mean levels of proliferation from parasite antigen-stimulated PBMC of cattle infected with O. ochengi (n = 6) as measured by [3H]thymidine incorporation over the course of experimental infection. The shaded area indicates the mean levels of proliferation + 2 SE (n = 20) for an uninfected animal. The period spanning the earliest detection of microfilariae to the termination of the experiment is represented by the box labeled “PATENCY.” Error bars represent SE.
Parasite-specific cytokine dynamics over the course of infection.
Cytokine production following stimulation of PBMC with parasite antigen was observed during recall responses in each of the six infected cattle examined (Table 1). Quantitative and temporal differences were observed in the peaks of cytokine production among individual animals, but the overall kinetics of the responses were relatively constant. For each of the cytokines, a depression in antigen-induced production occurred at the end of the prepatent period (Table 1). No differences in the levels of cytokines that reflected variation in the number of nodules, prepatent period, or density of microfilariae in the skin were seen (data not shown). Background responses measured in the sentinel animal were consistently low (Table 1).
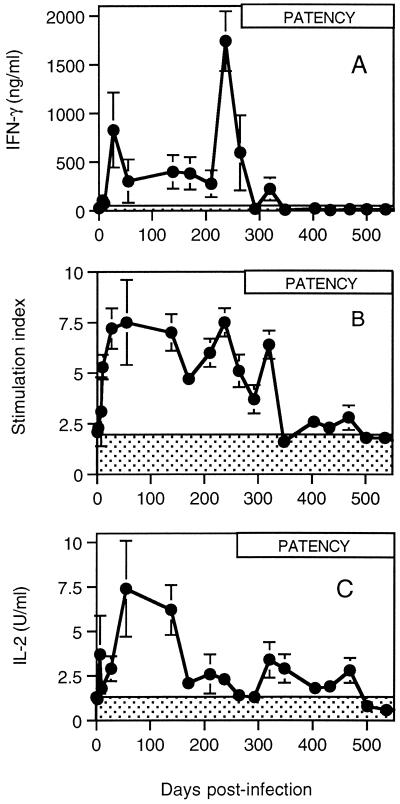
Responses for IFN-γ, IL-4, and IL-2 measured longitudinally over a period of 538 days are shown in Fig. 2. Control values for the sentinel animal are represented by shading and were calculated as the mean + 2 SE for 18 readings taken over the period of observation. IFN-γ production rose sharply after infection and peaked for the first time on day 27 (Fig. 2A). Levels of IFN-γ remained elevated and relatively constant through 210 days after infection. A major peak of production was observed in all animals on day 240, which was followed by a rapid decline. IFN-γ levels from infected cattle were significantly depressed following the onset of patent infections (Table 1, P < 0.05) and had fallen to within control levels by day 290 (Fig. 2A). With the exception of a weak and transient response detected on day 320, no further IFN-γ production above background values was observed.
FIG. 2.
Dynamics of parasite-specific cytokine production. Mean levels of cytokines produced from parasite antigen-stimulated PBMC of cattle infected with O. ochengi (n = 6) as measured by IFN-γ ELISA (A), IL-4 bioassay (B), and IL-2 bioassay (C) over the course of experimental infection. The shaded area indicates the mean levels of cytokines + 2 SE (n = 18) for an uninfected animal. The period spanning the earliest detection of microfilariae to the termination of the experiment is represented by the box labeled “PATENCY.” Error bars represent SE.
The IL-4 response increased rapidly following infection, peaking by day 55 and oscillating at a relatively high level for almost 300 days (Fig. 2B). There was a sharp decline in IL-4 production after day 320, and the level of expression in cells from infected cattle had dropped to control values by day 348. There was no recovery in the production of IL-4 over the remaining period of observation. Changes in IL-4 levels before and after the onset of patent infections affected all infected animals and were significantly different (Table 1, P < 0.05).
IL-2 production was elevated within 7 days of infection, rising to a peak on day 55 (Fig. 2C). Production was maintained at this relatively high level for approximately 84 days and then underwent rapid decline. Low levels of IL-2 production were maintained by PBMC from infected animals that remained above the background level from day 171 to day 469 postinfection. The reduction in responsiveness followed a trend of greatest inhibition with greatest infection intensity (as measured by microfilarial densities in skin) (Table 1). The response in infected cattle fell below the threshold for control values on day 500 and did not recover during the remaining period of observation (Fig. 2C). Again, the changes in levels before and after the onset of patency were significantly different (Table 1, P < 0.05).
Parasite-specific antibody dynamics over the course of infection.
Parasite-specific antibody responses occurred in all six infected cattle and are illustrated in Fig. 3. As with the cellular responses, variations in the responses among individual animals were seen (Table 1), but there was no indication that this reflected variation in number of nodules or prepatent period. Antibody levels did, however, increase as a function of infection intensity as measured by microfilarial densities in skin (data not shown).
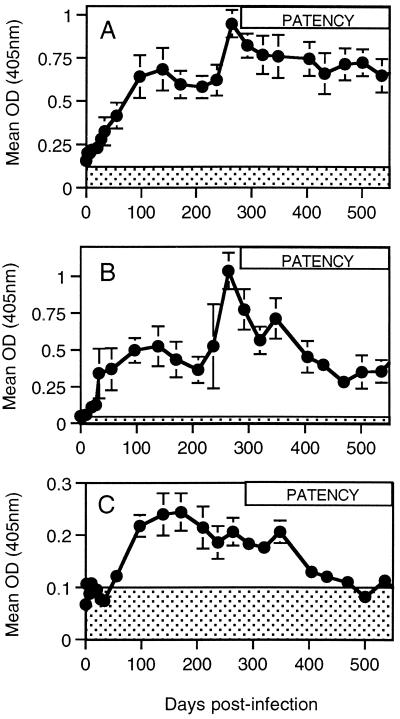
FIG. 3.
Dynamics of parasite-specific antibody responses. Mean parasite-specific total IgG (A), IgG1 (B), and IgG2 (C) antibody levels of cattle infected with O. ochengi (n = 6) as measured by ELISA over the course of experimental infection. The shaded area indicates the mean optical density (OD) reading + 2 SE (n = 24) for an uninfected animal. The period spanning the earliest detection of microfilariae to the termination of the experiment is represented by the box labeled PATENCY. Error bars represent SE.
Levels of parasite-specific total IgG rose above baseline within 7 days after infection (Fig. 3A). Antibody levels increased progressively for the first 100 days, stabilized between days 100 and 220, and rose again sharply to a new level at around 270 days postinfection. Little change was observed subsequently in the level of total IgG production. A significantly elevated antibody response occurred in all infected animals following onset of the patent infection (Table 1, P < 0.05).
Analysis of the response with IgG subclass reagents revealed that the predominant isotype was IgG1 (Fig. 3B). Levels of parasite-specific IgG1 showed a biphasic profile similar to the total IgG response. Parasite-specific IgG2 antibody levels were low, but rose above the threshold for positivity (mean + 2 SE for uninfected cattle serum) from day 50 postinfection (Fig. 3C). Levels peaked between 100 and 200 days postinfection. They fell abruptly between days 350 and 400 and had returned to control values by 500 days postinfection.
DISCUSSION
Cattle infected with O. ochengi under experimental conditions developed intradermal nodules, patent infections, and chronic microfilaridermia in a chronology equivalent to that predicted from natural infections (1, 33). By using a single dose of infective larvae to initiate infection, we were able to interpret the dynamics of the immune response in relation to biological events in the life history of the parasite.
The profile of lymphoproliferation and cytokine production over the course of infection showed significant changes that correlated closely with key stages in parasite development. For example, the sharp rise in proliferation shortly after infection corresponds with the period of development from third-stage larva to the fourth larval moult (2). The subsequent phases of reduced and elevated responsiveness coincide with the periods of adult worm development prior to and after the establishment of intradermal nodules. The final drop in the proliferative response to background levels accords with the end of the prepatent period. Interestingly, this down-regulation occurred in all six infected animals, irrespective of the number of nodules or of our ability to detect microfilariae in the skin. This suggests that very few parasites are needed to achieve immune modulation. That all of the animals were infected and immunologically competent was demonstrated by their antibody responses, which rose even while the markers of cellular responsiveness were being depressed. We assume that the failure to find nodules or microfilariae in one of the six animals is likely to be attributable to technical limitations in detecting small tissue-dwelling nematodes in cattle.
Trends in the expression of cytokines by antigen-stimulated PBMC also varied over time in a similar fashion to lymphoproliferative responses. Levels of IL-4 rose particularly steeply during the development of third- to fourth-stage larvae and remained high, but not beyond the prepatent period. The balance of cytokines observed during the prepatent infection argues for a nonpolarized T helper response during first contact with parasite transmission.
The total IgG parasite-specific antibody response showed a rapid rise in titer following the infection with infective-stage larvae. The levels peaked coincident with the first detection of nodules and presumably following the final larval moult. The response then peaked at the onset of patency, when microfilariae were first appearing in the skin, and remained elevated thereafter. Bovine IgG consists of two isotypes, IgG1 and IgG2 (with allotypes 2a and 2b), neither of which is a homologue of human or murine IgG isotypes. However, regulation of isotype switching in cattle, like that in humans and mice, is reciprocally regulated by IL-4 and IFN-γ, with IL-4 upregulating IgG1 expression and IFN-γ upregulating IgG2 (9). The response was dominated by the IgG1 isotype, which showed an accentuated peak at the onset of patency. This result clearly demonstrates that the animals, despite having impaired cellular responses, and particularly an inability to induce parasite-specific IL-4, were capable of inducing an antibody response. This suggests that the antibody response being generated at the onset of patency may be regulated by an alternative cytokine that is unaffected by the immunomodulatory changes affecting IL-4. IFN-γ has been shown to stimulate IgG2 production from bovine B cells in vitro (9). In infected animals, parasite-specific IgG2 levels decreased to preinfection levels following the ablation of IFN-γ production, which may suggest IFN-γ regulated IgG2 responses in vivo.
The depressed proliferative and elevated antibody responses of chronically infected cattle described here reflect what has been reported for onchocerciasis patients and experimentally infected chimpanzees (12, 19, 28, 36). The down-regulation of parasite-specific IL-2, IL-4, and IFN-γ production observed in this study is particularly informative. If such a down-regulation of cytokine production occurred with O. volvulus infections, it would explain the inability of several studies of onchocerciasis patients to detect parasite-specific IL-2, IL-4, and IFN-γ production (8, 10, 29, 30). A role for IL-4 production in parasite-specific proliferative responses is supported by the observation that the addition of recombinant IL-4 to antigen-stimulated PBMC cultures of O. volvulus-infected chimpanzees partially restored proliferative responses (19). Recent studies of children with onchocerciasis have also shown that IL-4 responses are down-regulated in relation to increasing microfilarial density. This down-regulation also extends to responses induced by Mycobacterium tuberculosis PPD, emphasizing that immunomodulation caused by Onchocerca infection may influence responses to concurrent infections (31).
Several mechanisms have been put forward to explain the underlying mechanisms of T-cell down-regulation in filariasis (20). In studies of lymphatic filariasis, IL-10 has been shown to regulate T-cell responses in both animal models and clinical studies (23, 25). In onchocerciasis, IL-10 has also been shown to play a role, although additional mechanisms are thought to be involved (26). Recent studies have identified a subpopulation of “Th3” cells, isolated from the PMBC of O. volvulus-infected individuals, which mediate cellular hyporesponsiveness through the production of IL-10 and transforming growth factor β (TGF-β) (7).
Most of these studies, including ours, have used crude soluble extracts of parasites that contain a complex mixture of antigens and immunoreactive molecules. A number of defined molecules have been shown to potentially contribute to the induction of T-cell hyporesponsiveness, including two secreted proteins from the rodent filaria Acanthocheilonema viteae: a cysteine protease inhibitor and a phosphorylcholine-containing glycoprotein (14, 15). Additional immunomodulatory molecules that may also influence immune regulation include lipopolysaccharide (LPS)-like molecules derived from endosymbiotic Wolbachia bacteria (32). LPS-like molecules from extracts of O. volvulus and O. ochengi have been shown to induce IL-10 from human monocytes, leading to the down-regulation of major histocompatibility complex and costimulatory molecules (4), both of which are likely to result in impaired antigen presentation.
The ability to investigate the dynamics of the induction of these mechanisms in a natural host-parasite system, as described here, provides a powerful model to define the temporal induction of T-cell activation and subsequent down-regulation. In addition, it will provide the means to address the functional consequences of immune down-regulation for the survival of parasites and/or the regulation of immunopathogenesis.
ACKNOWLEDGMENTS
We thank Mark Bronsvoort and David Ekale for assistance in the collection of parasites in Ngaoundere, Cameroon; Nigel Jones and colleagues at the Veterinary Station of the University of Liverpool for animal husbandry; and Tom Unnasch of the University of Alabama at Birmingham, for the provision of plasmid pOVS134.
This study was supported by a grant (no.05594) from the Edna McConnell Clark Foundation (United States) and was conducted with the approval of the Ministry of Agriculture, Fisheries and Food and Home Office of the United Kingdom. Mark Taylor thanks the Wellcome Trust for fellowship support (no. 047176).
Footnotes
We are sad to report the death of R.A.C. shortly before completion of the manuscript.
REFERENCES
- 1.Achu-Kwi M D, Daiber W H, Renz A, Wahl G, Wanji S. Prepatency period and some aspects of the epizootiology of Onchocerca ochengi infestations in cattle in the Adamawa plateau, Cameroon. Parasite. 1994;1:10. [Google Scholar]
- 2.Bianco A E. Onchocerciasis—river blindness. In: Macpherson C N L, Craig P S, editors. Parasitic helminths, zoonoses, and human health in Africa. London, United Kingdom: Unwin Hayman Press; 1991. pp. 138–189. [Google Scholar]
- 3.Bianco A E, Ham P J, El Sinnary K, Nelson G S. Large-scale recovery of Onchocerca microfilariae from naturally infected cattle and horses. Trans R Soc Trop Med Hyg. 1980;74:109. [Google Scholar]
- 4.Brattig N W, Rathjens U, Ernst M, Geisinger F, Renz A, Tischendorf F W. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filaria Onchocerca volvulus are candidate mediators in the sequence of inflammatory and anti-inflammatory responses of human monocytes. Microbe Infect. 2000;10:1147–1157. doi: 10.1016/s1286-4579(00)01269-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins R A, Tayton H K, Gelder K I, Britton P, Oldham G. Cloning and expression of bovine and porcine interleukin-2 in baculovirus and analysis of species cross-reactivity. Vet Immunol Immunopathol. 1994;40:313–324. doi: 10.1016/0165-2427(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 6.Connor D H, Gibson D W, Neafie R C, Merghi B, Buck A A. Sowda—onchocerciasis in North Yemen: a clinicopathologic study of 18 patients. Am J Trop Med Hyg. 1983;32:123–137. doi: 10.4269/ajtmh.1983.32.123. [DOI] [PubMed] [Google Scholar]
- 7.Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 8.Elson L H, Calvopina M H, Paredes W Y, Araujo E N, Bradley J E, Guderian R H, Nutman T B. Immunity in onchocerciasis: putative immune persons produce a Th1-like response to Onchocerca volvulus. J Infect Dis. 1995;171:652–658. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 9.Estes D M. Differentiation of B cells in the bovine. Role of cytokines in immunoglobulin isotype expression. Vet Immunol Immunopathol. 1996;54:61–67. doi: 10.1016/s0165-2427(96)05684-x. [DOI] [PubMed] [Google Scholar]
- 10.Freedman D O, Lujan-Trangay A, Steel C, Gonzalez-Peralta C, Nutman T B. Immunoregulation in onchocerciasis: functional and phenotypic abnormalities of lymphocyte subsets and changes with therapy. J Clin Investig. 1991;88:231–238. doi: 10.1172/JCI115282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallin M, Edmonds K, Ellner J J, Erttmann K D, White A T, Newland H S, Taylor H S, Greene B M. Cell-mediated immune responses in human infection with Onchocerca volvulus. J Immunol. 1988;140:1999–2007. [PubMed] [Google Scholar]
- 12.Greene B M, Gbakima A A, Albiez E J, Taylor H R. Humoral and cellular immune responses to Onchocerca volvulus infection in man. Rev Infect Dis. 1985;7:789–795. doi: 10.1093/clinids/7.6.789. [DOI] [PubMed] [Google Scholar]
- 13.Ham P J, Townson S, James E R, Bianco A E. An improved technique for the cryopreservation of Onchocerca microfilariae. Parasitology. 1981;83:139–146. doi: 10.1017/s0031182000050113. [DOI] [PubMed] [Google Scholar]
- 14.Harnett W, Deehan M R, Houston K M, Harnett M M. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 1999;21:601–608. doi: 10.1046/j.1365-3024.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253–2260. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 16.Hogarth P J, Bianco A E. IL-5 dominates cytokine responses during expression of protective immunity to Onchocerca lienalis microfilariae in mice. Parasite Immunol. 1998;21:81–88. doi: 10.1046/j.1365-3024.1999.00204.x. [DOI] [PubMed] [Google Scholar]
- 17.Kilian H D, Nielsen G. Cell-mediated and humoral immune responses to BCG and rubella vaccinations and to recall antigens in onchocerciasis patients. Trop Med Parasitol. 1989;40:445–453. [PubMed] [Google Scholar]
- 18.Kuo Y M, Bianco A E. Temporal changes in the humoral immune response of cattle during experimental infections with Onchocerca lienalis. Parasite Immunol. 1995;17:393–404. doi: 10.1111/j.1365-3024.1995.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 19.Luder C G K, Soboslay P T, Prince A M, Greene B M, Lucius R, Schultz-Key H. Experimental onchocerciasis in chimpanzees: cellular responses and antigen recognition after immunization and challenge with Onchocerca volvulus infective third-stage larvae. Parasitology. 1993;107:87–97. doi: 10.1017/s0031182000079440. [DOI] [PubMed] [Google Scholar]
- 20.Maizels R M, Holland M J, Falcone F H, Zang X X, Yazdanbakhsh M. Vaccination against helminth parasites—the ultimate challenge for vaccinologists? Immunol Rev. 1999;171:125–147. doi: 10.1111/j.1600-065x.1999.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 21.McCall P J, Townson H, Trees A J. Morphometric differentiation of the Onchocerca volvulus and Onchocerca ochengi infective larvae. Trans R Soc Trop Med Hyg. 1992;86:63–65. doi: 10.1016/0035-9203(92)90443-g. [DOI] [PubMed] [Google Scholar]
- 22.Meredith S E O, Lando G, Gbakima A A, Zimmerman P A, Unnasch T R. Onchocerca volvulus: application of the polymerase chain reaction to identification and strain differentiation of the parasite. Exp Parasitol. 1991;73:335–344. doi: 10.1016/0014-4894(91)90105-6. [DOI] [PubMed] [Google Scholar]
- 23.Osborne J, Devaney E. Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi. Infect Immun. 1999;67:1599–1605. doi: 10.1128/iai.67.4.1599-1605.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottesen E A. Immune responsiveness and the pathogenesis of human onchocerciasis. J Infect Dis. 1995;171:659–671. doi: 10.1093/infdis/171.3.659. [DOI] [PubMed] [Google Scholar]
- 25.Ravichandran M, Mahanty S, Kumaraswami V, Nutman T B, Jayaraman K. Elevated IL-10 mRNA expression and downregulation of Th1-type cytokines in microfilaraemic individuals with Wurchereria bancrofti infection. Parasite Immunol. 1997;19:69–77. doi: 10.1046/j.1365-3024.1997.d01-185.x. [DOI] [PubMed] [Google Scholar]
- 26.Soboslay P T, Luder C G K, Riesch S, Geiger S M, Banla M, Batchassi E, Stadler A, Schultz-Key H. Regulatory effects of Th1-type (IFN-γ, IL-12) and Th2-type cytokines (IL-10, IL-13) on parasite-specific cellular responsiveness in Onchocerca volvulus-infected humans and exposed endemic controls. Immunology. 1999;97:219–225. doi: 10.1046/j.1365-2567.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soboslay P T, Geiger S M, Weiss N, Banla M, Luder C G K, Drerweck C M, Batchassi E, Boatin B A, Stadler A, Schultz-Key H. The diverse expression of immunity in humans at distinct states of Onchocerca volvulus infection. Immunology. 1997;90:592–599. doi: 10.1046/j.1365-2567.1997.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soboslay P T, Dreweck C M, Taylor H R, Brottman B, Wenk P, Greene B M. Experimental onchocerciasis in chimpanzees. Cell-mediated immune responses, and production and effects of IL-1 and IL-2 with Onchocerca volvulus infection. J Immunol. 1991;147:346–353. [PubMed] [Google Scholar]
- 29.Steel C, Nutman T B. Regulation of IL-5 in onchocerciasis. A critical role for IL-2. J Immunol. 1993;153:5511–5518. [PubMed] [Google Scholar]
- 30.Steel C, Lujan-Trangay A, Gonzalez-Peralta C, Zea-Flores G, Nutman T B. Transient changes in cytokine profiles following ivermectin treatment of onchocerciasis. J Infect Dis. 1994;170:962–970. doi: 10.1093/infdis/170.4.962. [DOI] [PubMed] [Google Scholar]
- 31.Stewart G R, Boussinesq M, Coulson T, Elson L, Nutman T, Bradley J E. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol. 1999;117:517–523. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor M J, Cross H F, Bilo K. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J Exp Med. 2000;191:1429–1436. doi: 10.1084/jem.191.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tchakouté V L, Bronsvoort M, Tanya V, Renz A, Trees A J. Chemoprophylaxis of Onchocerca infections: in a controlled, prospective study ivermectin prevents calves becoming infected with O. ochengi. Parasitology. 1999;118:195–199. doi: 10.1017/s0031182098003680. [DOI] [PubMed] [Google Scholar]
- 34.Trees A J. Onchocerca ochengi: mimic, model or modulator of O. volvulus. Parasitol Today. 1992;8:337–339. doi: 10.1016/0169-4758(92)90068-d. [DOI] [PubMed] [Google Scholar]
- 35.Wahl G, Achu-Kwi M D, Mbah D, Dawa O, Renz A. Bovine onchocerciasis in North Cameroon. Vet Parasitol. 1994;52:297–311. doi: 10.1016/0304-4017(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 36.Ward D J, Nutman T B, Zea-Flores G, Portocarrero C, Lujan A, Ottesen E A. Onchocerciasis and immunity in humans: enhanced T cell responsiveness to parasite antigen in putatively immune individuals. J Infect Dis. 1988;157:536–543. doi: 10.1093/infdis/157.3.536. [DOI] [PubMed] [Google Scholar]
- 37.Xie H, Bain O, Williams S A. Molecular phylogenetic studies on filarial parasites based on 5s ribosomal spacer sequences. Parasite. 1994;1:141–151. doi: 10.1051/parasite/1994012141. [DOI] [PubMed] [Google Scholar]