Abstract
Introduction
Primary biliary cholangitis (PBC) is an autoimmune liver disease involving the small intrahepatic bile ducts; when untreated or undertreated, it may evolve to liver fibrosis and cirrhosis. Ursodeoxycholic Acid (UDCA) is the standard of care treatment, Obeticholic Acid (OCA) has been approved as second-line therapy for those non responder or intolerant to UDCA. However, due to moderate rate of UDCA-non responders and to warnings recently issued against OCA use in patients with cirrhosis, further therapies are needed.
Areas covered. Deep investigations into the pathogenesis of PBC is leading to proposal of new therapeutic agents, among which peroxisome proliferator-activated receptor (PPAR) ligands seem to be highly promising given the preliminary, positive results in Phase 2 and 3 trials. Bezafibrate, the most evaluated, is currently used in clinical practice in combination with UDCA in referral centers. We herein describe completed and ongoing trials involving PPAR agonists use in PBC, analyzing pits and falls.
Expert opinion
Testing new therapeutic opportunities in PBC is challenging due to its low prevalence and slow progression. However, new drugs including PPAR agonists, are currently under investigation and should be considered for at-risk PBC patients.
Keywords: Fibrates, PPAR agonists, Primary biliary cholangitis, Elafibranor, Saroglitazar, Seladelpar, UDCA
Abbreviations: AEs, adverse events; AIH, Autoimmune Hepatitis; ALP, Alkaline Phosphatase; AMA, Antimitochondrial antibodies; BZF, Bezafibrate; CKD, chronic kidney disease; FDA, Food and Drug; FF, Fenofibrate; FXR, Farnesoid X Receptor; GGT, γ-glutamil transferase; HCC, Hepatocellular Carcinoma; HDL, high-density lipoprotein; HR, Hazard Ratio; HSC, Hepatic Stellate Cells; IgM, Immunoglobulin M; IL-1β, Interleukin-1; LDL, low-density- lipoprotein; LT, Liver Transplant; MDR3, multidrug resistance protein 3; NASH, Non Alcoholic Steato-Hepatits; NRS, Numerical Raing Scale; OCA, Obeticholic Acid; OR, Odds Ratio; PBC, Primary Biliary Cholangitis; PC, phosphatidylcholine; PH, Portal Hypertension; PAR, protease-activated receptors; PPAR, peroxisome proliferator-activated receptor; QoL, Quality of Life; RCT, randomized controlled trial; SAE, Severe Adverse Event; TGR, transmembrane G protein-coupled receptor; TLR, Toll Like Receptor; TNF-α, Tumor Necrosis Factor- α; UDCA, ursodeoxycholic acid; ULN, upper limit of normal; UK, United Kingdom; VAS, Visual Analogue Scale; VRS, Verbal Rating Scale
Highlights
-
•
Up to 40% of PBC patients display inadequate response to standard of care with UDCA.
-
•
Among new therapeutic targets under investigation in PBC, PPARs ligands present a very promising profile due to anti-inflammatory and metabolic effects.
-
•
PPARs improve symptoms and quality of life in PBC4. Safety issues on PPAR-agonists use needs further assessment, especially in patients with advanced fibrosis.
1. Introduction
Primary biliary cholangitis (PBC) is an immune-mediated cholestatic liver disease predominantly affecting middle-aged women [1,2] and characterized by progressive non-suppurative inflammation involving small and medium-size intrahepatic bile ducts which are consequently destructed. If left untreated, PBC may lead to liver cirrhosis and its complication including portal hypertension (PH) and Hepatocellular Carcinoma (HCC).
Aim of medical treatment is to improve quality of life (QoL) by management of symptoms, including pruritus and fatigue; and obtain biochemical response, slowing or even avoiding progression to liver fibrosis. Ursodeoxycholic acid (UDCA) at a daily dose of 13–15 mg/kg represents the universal first-line standard of treatment for PBC [3] and, for a long time, it has been the only drug approved by the Food and Drug Administration (FDA). It has anti-apoptotic and anti-inflammatory activities and plays a protective role on cholangiocytes by modification of expression of transport proteins in the canalicular membrane via post-transcriptional and post-translational patterns. Overall, UDCA considerably improves liver biochemistry, reduces rate of disease progression, and is associated with extended transplantation-free survival [[3], [4], [5]]. However, up to 40% of PBC patients do not display complete biochemical response after 1 year of UDCA treatment [4].
From 2016 the FDA has approved Obeticholic Acid (OCA) for use in PBC patients with inadequate response to or intolerance of UDCA; this is a farnesoid X receptor (FXR) agonist involved in regulation of bile acid transport with anti-fibrotic and anti-inflammatory effect [6,7]. Positive results in randomized controlled trial (RCT) POISE and its open-labels extension consisted in reduction of alkaline phosphatase (ALP) and improvement of total bilirubin after 48 months of OCA treatment, both intended as surrogate markers of survival [8,9]. Apart from side effects mainly consisting in itching and increase in low-density- lipoprotein (LDL) levels, use of OCA has recently been restricted by the FDA in advanced cirrhosis due to reported events of liver injury leading either to hepatic decompensation or even liver failure. Prognosis in PBC patients not suitable of available drugs is worst compared to healthy subjects, thus new therapeutic opportunities are strongly needed. Among them, Peroxisome Proliferator-Activated Receptor (PPAR) agonists represent one of the most promising treatments actively investigated. The aim of our review is to provide an update on current research investigating these molecules, including concerns raised so far.
2. Peroxisome proliferator-activated receptors (ppars)
PPARs are nuclear receptors first identified and cloned in 1990, with a key role in regulation of transcription of genes involved in inflammation, carcinogenesis, and metabolic pathways [10]. This consideration makes them crucial molecular targets in cholestatic liver diseases, such as PBC.
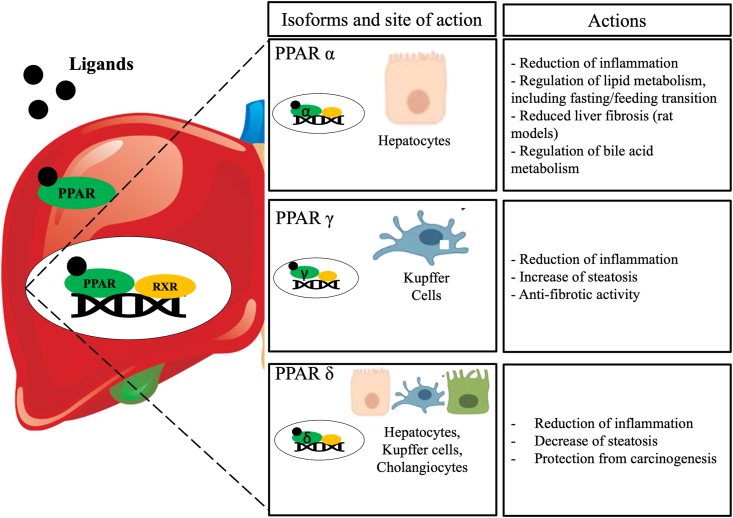
A typical PPARs agonist is made of a carboxylic acid head, a lipophilic tail, and a linker. After binding to their ligands, PPARs form a heterodimer with the retinoid X receptor and interact with specific DNA sequences thus regulating target genes. There are three isotypes of PPARs (PPAR-α, PPAR-γ, PPAR-β/δ), each of them encoded by different genes and characterized by specific tissue allocation and actions [11] (Fig. 1).
Fig. 1.
Mechanisms and effects of peroxisome proliferator-activated receptor (PPAR) activation in the liver.
PPAR-α is predominantly expressed in tissues with high fatty acid oxidation rates including the liver, heart, kidney, brown adipose tissue, and skeletal muscle. In hepatocytes PPAR-α is a transcriptional regulator of genes involved in glucose production, β-oxidation, bile acid homeostasis and lipid transport, including fasting/feeding transition processes [12]. First, hepatic activation of PPAR-α leads to increased fatty-acid oxidation and elimination of triglycerides from plasma, resulting in elevated levels of high-density lipoprotein cholesterol (HDL) [13]. Second, in murine models of atherosclerosis and Nonalcoholic Steatohepatitis (NASH), PPAR-α inhibits expression and duration of action of pro-inflammatory cytokines ad chemokines by trans-repression of AP1 and NF-kB signaling pathway [14], thus reducing both acute and chronic inflammatory processes. Interestingly, anti-fibrotic activity of synthetic PPAR- α agonists in cirrhotic rats has been demonstrated, with effects even on portal hypertension [15,16]. Finally, PPAR- α ligands modulate bile acid metabolisms through four principal ways.
-
a)
inhibition of bile acid synthesis through downregulation of the expression of CYP7A1, the key enzyme of the rate-limiting step of the pathway, and cytochrome sterol 27-hydroxylase (CYP27A1) [17];
-
b)
increased bile acid secretion via up-regulation of BSEP and MRP2 gene expression
-
c)
reduction of bile acid toxicity due to activation and induction of multidrug resistance protein 3 (MDR) gene (ABCB4), this leading to increased insertion of the protein into the canalicular membrane of hepatocytes [18]. MDR3 is mainly responsible of biliary phosphatidylcholine (PC) secretion and mutation and/or polymorphisms of this gene are involved in several kinds of cholestatic diseases such as intrahepatic cholestasis of pregnancy, low-phospholipid cholelithiasis and PFIC-3 [18].
-
d)
detoxification of bile acid thanks to the upregulation of CYP3A4, uridine 5′-diphospho-glucuronosyltransferase (UGT)2B4,1A1, 1A3, 1A4, 1A6, sulfotransferase 2A1 (SULT2A1); and the inhibition of basolateral transporter sodium-taurocholate-cotransporting polypeptide (NTCP) [[19], [20], [21], [22]]. To note, SULT2A1 facilitates elimination of a toxic secondary bile acid named lithocolic acid (LCA), thus improving cholestasis typically observed in PBC.
Given their role in decreasing BA synthesis and bile salt secretion into bile, fibrates, which are PPAR- α agonists, look very promising in the subset of patient with cholestatic liver diseases such as PBC.
PPAR-γ has three splicing variant isoforms (γ1, γ2, and γ3) each of them having different tissue localization but same DNA binding specificity. While isoform γ1 is ubiquitous and isoform γ3 is expressed in the liver at very low levels, isoform γ2 is greatly expressed in adipose tissue where it plays multitude of roles related to adipogenesis, lipid storage and glucose metabolism. (Fig. 2).
Fig. 2.
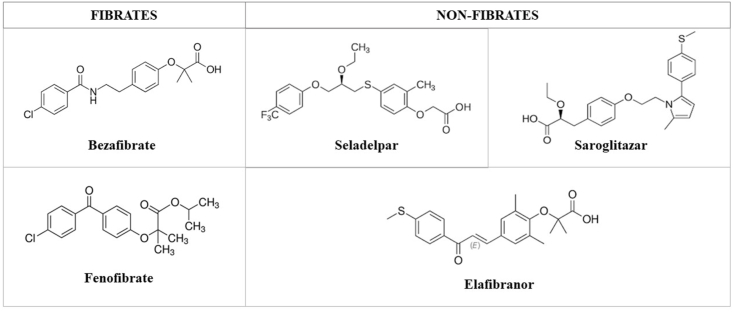
Chemical structure of Peroxisome Proliferator-Activated Receptor (PPAR) agonists.
In hepatic macrophages, PPAR-γ exerts immunomodulator activity with anti-inflammatory properties like PPAR-α, despite mostly limited to Kupffer cells [12,23]; by negatively interfering with NF-κB, it suppresses production of tumor necrosis factor-α (TNF-α) and Interleukin-1β (IL-1β). Moreover, in hepatocytes, activation of PPAR-γ is steatogenic and is typically observed in NASH subjects even when non-obese [24].
Remarkably, PARγ2 is highly expressed in quiescent of Hepatic Stellate Cells (HSC) and involved in the maintenance of this phenotype [12]; after observing that PPAR-γ activation is reduced during HSC activation, new synthetic ligands have been investigated and demonstrated to suppress profibrogenic expression of HSC both in vitro and in vivo [12,24].
Finally, PPAR-β/δ is more ubiquitous and mainly found in skeletal muscle, skin inflammatory cells, heart, adipose tissue and liver, where it is located in hepatocytes, cholangiocytes, HSC, and Kupffer cells [24].
Activation of PPAR-β/δ in Kupffer cells has been observed to improve inflammation and prevent hepatic carcinogenesis; concerning lipid metabolism in hepatocyte, effects are controversial but mainly consisting in decrease of liver steatosis [24]. Furthermore, PPAR-δ does have an anti-inflammatory effect through the BCL6-mediated pathway [25] and plays a role in the regulation of transport and absorption of bile compounds, with consequent therapeutic effect.
Given the effects on metabolism, inflammation, bile acids regulation and liver fibrosis, the creation of selective ligands targeting each isoform of PPAR is an enthusiastic challenge in PBC, with promising results so far; we will analyze them in the following chapter.
3. Peroxisome proliferator-activated receptor agonists
3.1. Fibrates
Fibrates -or fibric acid derivatives-are synthetic PPAR agonists identified as lipid lowering molecules since the 60s and currently licensed in the United States and other countries for the treatment of isolated hypertriglyceridemia and hypercholesterolemia.
Afterwards, next to their newly discovered anti-inflammatory properties, fibrates were noted for their protective effects against bile toxicity, due to inhibition of bile acid synthesis and stimulation of phospholipids excretion via MDR3 gene expression [17,26]. Moreover, fibrates were demonstrated to reduce ALP levels [27], which at present constitute the most accurate surrogate marker of outcome in PBC patients [28].
For all above mentioned, fibrates have been previously investigated in cholestatic liver diseases [29] and are currently suggested for evaluation in patients with inadequate response to UDCA in both American and European guidelines for PBC management [1,30]. However, several safety concerns have been raised about fibrates use in cholestatic diseases due to potential hepatotoxicity. Of note, PBC represents a contraindication in the fenofibrate (FF), label in the US; therefore, the American Association for the Study of the Liver (AASLD) has recently issued an update of the guidelines for PBC management discouraging the use of fibrates in patients with decompensated liver disease [30].
In the next two paragraph we describe clinical studies investigating bezafibrate (BZF) and FF, the two most evaluated fibrates tested in PBC patients (Table 1). Discussion on improvement of pruritus will be taken separately.
Table 1.
Clinical trials and studies evaluating efficacy of Fibrates in PBC patients.
| NCT number or authors | Design | Phase | N. Pts | Duration | Arms of treatment, (n) of pts and treatment | Primary outcome | |
|---|---|---|---|---|---|---|---|
| BEZAFIBRATE |
NCT01654731 (BEZURSO) [34] NCT04751188 NCT02937012 NCT04594694 Smets et al. [36] Tanaka et al. [38] NCT05239468 |
Prosp Prosp Prosp Prosp Prosp case series Retro Prosp |
3 3 3 2 \ \ 2a |
100 11 34 75 11 3908 60 |
24 months 6 months 12 months 12 weeks 1 year 2000–2017 12 weeks |
A. (50): BZF 400 mg qd + UDCA B. (50): placebo + UDCA A. BZF 200 mg bid + UDCA B. Placebo + UDCA A. BZF 200 mg bid + UDCA B. Placebo + UDCA A. BZF 200 mg qd B. BZF 400 mg qd C. OCA 5 mg/10 mg qd + BZF 200 mg qd D. OCA 5 mg/10 mg qd + BZF 400 mg qd BZF 400 mg qd + OCA A. (3162) UDCA B. (746) UDCA + BZF A: BZF 100 mg + placebo B: BZF 400 mg + placebo C: OCA 5 mg + BZF 100 mg D: OCA 5 mg + BZF 400 mg |
Biochemical response a Biochemical response b Biochemical response b Change of ALP levels from baseline to week 12 Biochemical response c Mortality or LT rates Biochemical response |
| FENOFIBRATE |
NCT00575042 Levy et al. [56] Liberopoulos et al. [63] Han et al. [64] Cheung et al. [44] Hegade et al. [41] NCT02965911 NCT02823366 NCT02823353 |
Prosp Prosp Prosp Retro Retro Prosp Prosp Prosp |
2 \ \ \ \ 1–2 3 3 |
20 10 22 121 23 72 104 122 |
12 months 8 weeks >3 months 12 months >12 months \ 24 weeks 48 weeks |
FF 160 mg qd + UDCA A. (4): UDCA B. (6): FF 200 mg qd + UDCA FF 200 mg qd + UDCA A. (46): FF + UDCA B. (74) UDCA FF (median 200 mg qd) + UDCA FF 200 mg/die + UDCA A. FF + UDCA B. UDCA A. FF + UDCA B. UDCA |
Biochemical response and safety Biochemical response Biochemical response HD, death, and LT Biochemical response and prognosis assessment Serum Level of ALP value Biochemical response c Biochemical response c |
| BEZAFIBRATE/FENOFIBRate | Reig A et al. [35] Soret et al. [37] |
Retro Retro |
\ 1 |
277 58 |
12 months 11 months |
A. (65): OCA B. (201) fibrates C. (11) OCA + fibrates A. (19): group UDCA-OCA (2nd line)-fibrates (3rd line) B. (19): group UDCA-fibrates (2nd line)-OCA (3rd line) |
Changes in biochemical tests and AEs with OCA or fibrates Changes in biochemical tests and pruritus with triple therapy |
NCT number: ClinicalTrials.gov identifier; AEs: adverse events; ALP: Alkaline Phosphatase; BZF: bezafibrate; FF: fenofibrate; HD: Hepatic Decompensation; LT: Liver Transplantation; OCA: Obeticholic acid; PBC: Primary Biliary Cholangitis; pts: patients; prosp: prospective; retro: restrospective; UDCA: ursodeoxycholic acid; qd once a day; bid two times a day.
Defined as the normalization of hepatic biochemical tests (aminotransferases, ALP, blood albumin, blood bilirubin and prothrombin index).
Defined as the reduction of ALP lower than 1.5 times the upper limits of normal (ULN), reduction of aminotransferases lower than 1.5 times the ULN and bilirubin lower than 1 mg/DlWithdrawn (No participants enrolled).
Defined as normalization of alkaline phosphatase (ALP) or decrease of ALP by more than 40% compared to the baseline.
3.1.1. Bezafibrate
Different from other fibrates which are specific PPARα ligands, BZF is a weak pan-PPAR activator [31,32]; it enhances biliary excretion of PC by increasing MDR3 expression, thus play a crucial role in detoxification.
BZF was first evaluated in PBC disease in 1999 in a study performed by Iwasaki et al. [33] and soon after introduced as a de facto second line therapy in Japan due to biochemical efficacy emerged in several pilot Japanese studies. After oral administration of BZF 400 mg/day to 11 pre-cirrhotic PBC patients (91% stage of fibrosis I-II) for 12–21 months, Iwasaki et al. observed normalization of ALP in all treated patients and significant decrease of Immunoglobulin M (IgM) levels in almost half of the group.
Additionally, encoraging results came from the BEZURSO (BZF in Combination With Ursodeoxycholic Acid in Primary Biliary Cirrhosis) trial, a double-blind, placebo-controlled, randomized phase III French study (NCT01654731) in which 100 non-cirrhotic PBC patients with inadequate response to UDCA were randomly assigned to receive either BZF 400mg/die or placebo in addiction to UDCA for 24 months [34]. Compared to 0% in the control group, 31% of BZF-treated patients achieved the primary endpoint, consisting in complete biochemical response -that is, normalization of ALP, aminotransferases, serum albumin, total serum bilirubin and normal prothrombin index; moreover, 67% of treated arm showed ALP normalization (secondary endpoint). However, patients with portal hypertension or high ALP levels at baseline reported lower rate of reaching primary endpoint. Interestingly, laboratory improvement was associated with reduction in liver stiffness, a surrogate marker of liver fibrosis evaluated due to insufficient histological data available both at baseline and at the end of the study. Overall, 424 adverse events were reported in the study; rate of serious and non-serious (e.g. myalgias) adverse events did not differ between the two groups, nor did the incidence of liver-related complications. As previously reported34, treatment with BZF was associated with creatinine elevation, noticeable by month 3 of therapy and with not long-term impact on glomerular filtration rate; however, administration in patients at risk for chronic kidney disease (e.g. history of diabetes and arterial hypertension) should be delicately assessed.
At the same time, several studies with retrospective design were performed with the aim to assess biochemical efficacy with either dual (both BZF and FF) or triple combination therapies.
Recently, Reig et al. [35] retrospectively analyzed a Spanish cohort of 277 patients from 30 centers treated with OCA (65 patients, 5 mg qd) or fibrates (201 patients, 84% BZF 400 mg qd; 16% FF 200 -IQR 160–299- mg qd) or both (11 patients) for at least more than 3 months; the authors observed that both treatment decreased significantly ALP, γ-glutamil transferase (GGT) and transaminases levels, with fibrates having significant more effect on ALP reduction and OCA on transaminases decrease. To note, one patient with fibrate presented a severe adverse event.
Concerning triple therapy, after some case series [36] and an interim, retrospective analysis showing higher decrease of ALP levels with combination of UDCA, OCA and fibrates compared to dual therapy, very recently Soret and colleagues [37] published the promising results of their multicentric retrospective study including 58 patients with inadequate response to first- or second-line treatments. Half patients received OCA as second-line and fibrates as third-line therapy (Group OCA-Fibrate), and the other half received the inverse therapeutic sequence (Group Fibrate-OCA), for a mean duration of 11 months. Both arms were not only associated with higher ALP reduction compared to dual regimen, but showed significant decrease even in GGT, aminotransferases and total bilirubin levels, with a 3.4 odds ratio (OR) of reaching normal ALP.
Nevertheless, BZF needs assessment for long-term, clinical outcomes. This is strongly supported by recent, extremely optimistic results of a retrospective, large cohort study by Tanaka et al. [38]; 3908 PBC patients were included, all of them receiving UDCA and 746 receiving BZF as add-on therapy, with an overall average available follow up of 5.5 years from UDCA start. Remarkably, dual therapy was associated with significant reduction in both all-causes and liver-related mortality or need for LT (adjusted Hazard Ratios (HRs) 0.3253, 0.2748 respectively)
3.1.2. Fenofibrate
FF is 10-fold more specific for the α PPAR isoform rather than the γ and, differently from BZF, is currently available in the US where it is approved for the treatment of dyslipidemia.
Mechanisms of action in PBC include reduction of levels of toxic primary and secondary bile acids in serum, and of UDCA; and up-regulation of human MDR3 expression by binding to specific PPREs in the gene's promoter.
Benefits of add-on off-label therapy with FF in PBC early emerged in short-term studies in Japanese cohorts [39,40]. Since then, several but pilot and small-cohort based studies [41] mainly included in two meta-analyses [42,43] confirmed the positive effect of adding FF to UDCA on biochemical markers of PBC, making this class of drugs attractive for cholestatic liver diseases (Table 1).
However, the largest monocentric, retrospective study assessing biochemical response and AEs following FF administration in PBC was published in 2015 by Cheung et al. [44]; 120 PBC patients with inadequate response to UDCA were included, 49 of them receiving FF for a median period of 11 months and 74 continuing UDCA monotherapy. Interestingly, cirrhosis did not constitute an exclusion criterion. FF was associated with improved decompensation-free and transplant-free survival (HR = 0.09) which, together with death, constituted the primary endpoint of the study; also, 41% of the FF group vs 7% in UDCA group met Toronto Criteria for biochemical response. However, three consideration must be outlined: first, biochemical response did not emerge as significant at multivariate analysis, thus raising issues concerning use of ALP as a surrogate of response to therapy in PBC; second, only FF and the absence of cirrhosis or PH were associated with primary outcome after controlling for baseline factors, therefore liver cirrhosis remain a delicate issue in use of fibrates; third, bilirubin increased more rapidly in cirrhotic patients of the FF group, confirming FF must be added-on very cautiously in advanced stage of liver disease [30].
To note, 7 patients (22%) in the FF group early stopped treatment due to side effects (abdominal pain, myalgia, headache, angioedema, and pruritus) and one of them reported severe aminotransferases elevation (5XULN).
Recently, one study assessing efficacy and safety of FF combined with UDCA (NCT02965911) in PBC patients with inadequate response to UDCA was withdrawn due to lack of patients enrolled. Further prospective and large-cohort studies must be performed with the aim to assess long term outcomes.
Of note, a large nationwide observational cohort study conducted in the UK including 457 patients, showed that rates of biochemical response and drug discontinuation appear similar under fibrates and OCA treatment [45].
3.2. Non-fibrates
Beyond fibrates, other PPAR ligands are currently under investigation in PBC patients, as shown in Table 2.
Table 2.
Clinical Trials evaluating non-fibrate PPAR-agonists in PBC patients.
| NCT number/ reference | Design | Phase | Duration | N. Pts | Arms of treatment | Primary outcome | |
|---|---|---|---|---|---|---|---|
|
SELADELPAR (MBX-8025) Seladelpar MBX-8025 |
NCT02609048 [46] NCT02955602 [56] NCT03602560 (ENHANCE) [48] NCT04620733 (RESPONSE) NCT03301506 (ASSURE) NCT04950764 |
Prosp Prosp Prosp Prosp Prosp Prosp |
2 2 2 3 3 1 |
12 weeks 12 months 12 months 12 months 60 months 17 weeks |
41 119 265 180 500 24 |
A. (12) Placebo B. (13) MBX-8025 50 mg qd C. (10) MBX-8025 200 mg qd A. (11) MBX-8025 2 mg qda B. (49) MBX-8025 5 mg qda C. (52) MBX-8025 10 mg qd A. (80) Placebo B. (80) MBX-8025 5–10 mg qd C. (80) MBX-8025 10 mg qd A. Placebo B. MBX-8025 5 mg qd C. MBX-8025 10 mg qd A.MBX-8025 5 mg qd B. MBX-8025 10 mg qd A. MBX-8025 10 mg qd B. MBX-8025 10 mg qd or less |
Effects on ALP levels Effects on ALP levels and safety Composite endpoint of ALP and total bilirubin c Composite endpoint of ALP and total bilirubin c Treatment emergent AEs Safety and tolerability, dose finding |
|
ELAFIBRANOR (GFT-505) Elafibranor |
NCT03124108 [51] NCT04526665 (ELATIVE) |
Prosp Prosp |
2 3 |
12 weeks 52–104 weeks |
45 150 |
A. (15) Placebo B. (15) GFT-5050 80 mg qd C. (15) GFT-5050 120 mg qd A. Placebo B. GFT-5050 80 mg qd |
Relative Change in ALP Levels Effects on cholestasis c |
| SAROGLITAZAR |
NCT03112681 (EPICS) [53,54] NCT05133336 (EPICS-III) |
Prosp Prosp |
2 2b/3 |
16 weeks 52 weeks |
37 192 |
A. (10) Placebo B. (14) Saroglitazar 2 mg qd C. (13) Saroglitazar 4 mg qd A. (10) Placebo B. (14) Saroglitazar 1 mg qd C. (13) Saroglitazar 2 mg qd |
Reduction of ALP Composite endpoint of ALP and total bilirubin |
AEs: Adverse Events; ALP: Alkaline Phosphatase; PBC: Primary Biliary Cholangitis; PPAR: peroxisome proliferator-activated receptor; qd once a day; bid two times a day.
ǂStudy early stopped due to safety concern and need for dose reduction.
Possible up-titration to 10 mg/die after 12 weeks based on biochemical response.
Defined as ALP <1.67 × ULN, ≥15% decrease in AP, and total bilirubin ≤ ULN.
3.2.1. Seladelpar
Seladelpar (MBX-8025) is a PPAR-δ agonist with anti-inflammatory and choleretic properties, the latter due to involvement in downregulation of bile acids synthesis and modulation of their transport and metabolism.
It was first tested in a phase 2 trial including 70 PBC patients non responder to UDCA who were randomly assigned to receive Seladelpar either 50 mg/die or 200 mg/die or placebo [46]. Despite all patients completing 12 weeks of treatment showed complete normalization of ALP levels, the study was prematurely terminated due to grade 3 but fully reversible and asymptomatic aminotransferases increase in 3 patients.
In response to safety issues, further studies were performed with reduced doses. Levy et al. [47] performed a 1 year-phase 2, open-label and uncontrolled dose-finding trial (NCT02955602). In the study, 119 patients were given oral daily doses of 2 mg, 5 mg or 10 mg of Seladelpar, with possible up-titration of doses up to 10 mg after 12 weeks according to biochemical response; after 12 months, no patients remained on 2 mg/die. The composite endpoint consisting of ALP< 1.67x ULN, ALP reduction of 15% and normal total bilirubin was achieved in 53% of 5/10 mg arm and 69% of 10 mg arm. Four patients discontinued the treatment due to AEs, of which two were related to Seladelpar and consisted in grade 2 aminotransferases elevation and grade 1 heartburn; no patients referred pruritus. Over 1 year of study Seladelpar appeared to be safe and well tolerated and well tolerated and 98% of the cohort was enrolled in the long-term follow-up study.
Further, the ENHANCE trial (NCT03602560), a phase III study, randomized 265 patients with inadequate response to UDCA to receive either placebo or Seladelpar 5/10 mg or 10 mg for 52 weeks [48]. To note, only 167 patients underwent final analysis due to premature termination of the study caused by an unexpected -and finally demonstrated unrelated to drug-histologic finding in a clinical trial of MB-8025 for NASH; importantly, the investigators performed safety assessment in all patients included who received at least 1 dose of Seladelpar. The primary endpoint of the study consisted in a composite of ALP and total bilirubin decrease (Table 2) and was reached by almost 78% of the 10 mg group (n = 55) and 57% of the 5 mg group (n = 56) compared to 12.5% of the placebo group (n = 56). The most important AEs observed were pruritus and abdominal pain (13% in placebo, 3% in 5 mg arm and 11% in the 10 mg arm; 3%, 9%, and 7%, respectively).
Several clinical trials assessing either efficacy or safety of Seladelpar are currently ongoing.
From April 2021 the RESPONSE trial (NCT04620733), a 52-week, phase 3 study of Seladelpar 5 mg vs 10 mg vs placebo has started recruitment, with 180 PBC patients non responder to UDCA expected to be included; principal outcome will be biochemical response plus improvement of pruritus will be considered as secondary outcome.
In 2017, a large-cohort, open label, phase 3 study (ASSURE, NCT 03301506) was initiated with the aim to assess Seladelpar safety and tolerability at doses of 5 and 10 mg daily. Five hundred patients are expected to be collected and AEs will be assessed up to 60 months, together with secondary outcome including death, LT, hospitalization for complication of liver cirrhosis and biochemical response. Concerning safety issue, NCT04950764 is currently testing Seladelpar 10 mg in PBC patients with compensated cirrhosis and hepatic impairment with dose-finding tolerability and safety purpose.
3.2.2. Elafibranor
Elafibranor (GFT-505) is a dual PPAR-α/δ agonist, thus not associated with any of PPAR-γ ligands’ typical cardiac adverse effects. It was previously investigated in patients with NASH [49] and in murine models of NAFLD/NASH with liver fibrosis; interestingly, in rodents GFT-505 displayed a protective effect against steatosis, inflammation and fibrosis through mechanisms of inhibition of gene expression of proinflammatory (TNF-α, IL-1 β and F4/80) and profibrotic (tissue inhibitor metalloproteinase 2, transforming growth factor β, collagen type I α1 and collagen type II α2) cytokines [50].
Due to these effects and to observed improvement of hepatic dysfunction markers, Elafibranor was proposed for evaluation in PBC and is currently evaluation in several trials at moderate to high dosage (80–120 mg/die) in order to achieve therapeutic benefits.
In 2021 Schattenberg et al. [51] published the results of a phase 2 trial (NCT03124108) including 45 patients non-responder to UDCA and randomly assigned to receive Elafibranor 80 mg vs 120 mg vs placebo for 12 weeks (1:1:1). The primary endpoint consisted in the relative change (%) in serum ALP levels from baseline to end-of-treatment period at week 12 and was achieved by both the 2 Elafibranor-treated groups with mean changes ±SD equal to-48.3 ± 14.8% in the Elafibranor-80 mg arm, −40.6 ± 17.4% in the Elafibranor-120 mg arm vs +3.2 ± 14.8% in the placebo group; to note, no patient in the placebo group normalized ALP levels at the end of the 12-week treatment period vs 13.3% and 21.4% in the Elafibranor-80 mg and 120 mg arms, respectively.
Moreover, the study reported a consistent reduction in GGT, IgM, and inflammatory markers (C-reactive protein and haptoglobin) in the Elafibranor-treated groups vs placebo; circulating levels of C4, a bile acid intermediate, were significantly reduced by Elafibranor with mechanisms independent from FGF19 levels and similar to those observed for BZF [34] and Seladelpar [46].
Safety assessment represented a one of the numerous secondary outcomes of the study. No death was observed, nor any severe AEs (SAEs) occurred in the placebo or Elafibranor 80 mg groups, while 2 patients in the Elafibranor 120 mg group experienced at least one: the first consisted in a SAE unrelated to drug (ischemic stroke) and ceased the study; the second reported elevation of aminotransferases possibly due to a flare of autoimmune hepatitis (AIH). Non-serious AEs in the treated arms were of mild or moderate intensity and included headache, fatigue, and gastro-intestinal symptoms (nausea, diarrhea), plus a mild and reversible creatinine elevation in the Elafibranor 120 mg group was observed.
The promising results concerning both efficacy and safety of Elafibranor have encouraged further phase III trial (ELATIVE, NCT04526665) initiation at the end of 2020 with the aim to test long-term effect on cholestasis of elafibranor 80 mg daily vs placebo (Table 2).
3.2.3. Saroglitazar
Saroglitazar is a novel PPAR ligand with dual PPAR agonistic activity: it is predominantly a PPAR-α activator plus exerts PPAR-γ agonistic effects. At present, it is approved outside the US for the treatment of hypertriglyceridemia in diabetic patients uncontrolled by statins [52], diabetic dyslipidemia, and NASH. Due to its mechanism of action, Saroglitazar is currently under investigation in PBC.
Promising results have been recently reported in a phase II, proof-of-concept trial (NCT03112681) in which 37 patients were randomly assigned to receive either Saroglitazar 4 mg/die or Saroglitazar 2 mg/die or placebo [53]; to note, the dose of 4 mg daily has been chosen subsequently to ongoing trials in non-cirrhotic NASH (NCT03061721 and NCT03617263). The primary efficacy endpoint (reduction in ALP level at Week 16 of treatment) was observed in the two treated groups, where percentage reduction was almost 50% vs 4% in the placebo arm. However, some safety concerns were raised. Overall, 96 treatment-emergent AEs were reported, of which 7 (53.8%) in the 4 mg group, 4 (28.6%) in the 2 mg group and 3 (30%) in the placebo group were drug-related. In summary, 4 patients discontinued permanently the study (3 in 4 mg arm and 1 in the 2 mg arm), all of them due to asymptomatic elevation of aminotransferases (>5 x ULN in 2 out of 3 patients in 4 mg group) which was not associated to increase of markers of cholestasis; three of the patients reported normalization of liver enzymes within 3 months from withdrawal, while one patients is receiving immunosuppressive therapy for overlap syndrome (PBC with AIH).
Safety concerns emerged in another 16 weeks, open label, phase III study in which 37 patients were screened to enroll 7 patients to be treated with Saroglitazar 4 mg daily for the study period [54]. Despite early termination due to lack of enrollment, six patients reported mean ALP decrease of at least 40% from week 4 of treatments; one patients left the study with no safety concerns, while one participant displayed aminotransferases elevation up to 5-10 x ULN at week 20 but with spontaneous resolution at follow up.
The hepatic safety issues together with promising efficacy results encourage further testing of Saroglitazar with finding-dose aims, reasonably pursuing 1 mg or 2 mg doses at maximum.
4. Pruritus and quality of life
Despite availability of several drugs nowadays, pruritus remains a significant and distinctive symptom in PBC patients, with up to 80% being affected at some point during their clinical course [2]. Even if mainly of mild intensity, itch may sometimes be severe and impair quality of life, due to sleep deprivation and limitations in daily activities [55]. Considering largely prescribed antihistamine drugs do not control symptoms in most of cases, current guidelines suggest a stepwise approach for clinical management (cholestyramine, then rifampicin, naltrexone, and sertraline) but the only licensed therapy is cholestyramine, and together with other therapeutic option has limited efficacy and several side effects. Since refractory pruritus represent a (rare) indication for liver transplantation, there is urgent need for safe and easy therapeutic options for itch control in PBC.
So far, UDCA has not demonstrated any improvement on itch while OCA may be responsible of itch worsening and withdrawal or dose-adjustment of therapy.
On the other hand, PPAR-ligands are receiving much interest due to positive results obtained in clinical trials evaluating as secondary outcome reduction in pruritus, assessed through several scales, such as the visual analogue scale (VAS), the numerical rating scale (NRS) and the verbal rating scale (VRS).
BZF already demonstrated positive effect on pruritus though limited to case series/pilot studies [17,56]; moreover, about one third of BZF-treated patients in the BEZURSO trial reported reduction in (baseline mild) itch assessed by VAS scale [34]. Recently, de Vries et al. [57] presented novel data of their double blind, placebo controlled trial (FITCH) in which 74 patients with cholestatic liver disease (including PBC) and with moderate to severe pruritus (≥5 out of 10 on VAS) were randomized to either receive BZF 400 mg qd or placebo; basically, in the BZF group 45% of patients (55% PBC) reported a reduction of itch of more than 50% vs 11% in the placebo arm (overall) with serum bile acid levels and Autotaxin activity unchanged, suggesting mechanisms of action independent from those of FXR/TGR5 [58] and LPA-ATX [59,60], respectively. Despite being encouraging, some concerns emerged about the study including the very short study period (21 days), lack of safety evaluation and inadequate comparison with standard of therapy (less than 15% of patients were receiving bile acid sequestrants).
Together with BZF, non-fibrate PPAR agonists have been assessed for itch mainly in short-term, pilot studies. Previous reports, though limited by small sample size, of Seladelpar not worsening pruritus [46], were recently confirmed by data emerged after 1 year of treatment [61]. In line with these results, even Elafibranor not only didn't trigger or exacerbate pruritus, but was associated with reduction of VAS score in patients referring pruritus at baseline [51].
Importantly, seladelpar-treated patients also reported improvement in sleep disturbance and fatigue after 1-year of treatment [62]. Although clinical research addressing the benefit of PPAR agonists on the quality of life of PBC patients is still largely missing, these preliminary reports are largely encouraging.
5. Conclusions
Despite UDCA will probably remain the standard of care for PBC, new therapeutic targets are being explored so far. PPAR ligands seem the most promising of them, due to both positive results obtained on improvement of cholestasis and pruritus mitigation. However, clinical trials have mostly evaluated PBC population at pre-cirrhotic stage with brief-term outcomes; besides, safety concerns have been raised especially for most efficacious doses.
Further investigations are strongly needed with both dose-finding and safety purposes; moreover, long-term clinical outcome should be pursued, even including patients with more advanced stage of liver disease who are most characterized by symptoms and worst quality of life, thus deserving alternative, off-label, therapeutic options.
6. Expert opinion
UDCA, at a dose of 13–15 mg/kg/day, is the first-line therapy for PBC and has represented the only available therapy for more than 30 years, with demonstrated efficacy both on biochemical impairment and long-term clinical outcome, including liver transplantation (LT). However, almost 40% of patients are “non responder” to UDCA, and consequently at risk of disease progression and worse outcomes. Considering the poor prognosis of untreated or UDCA-treated patients with inadequate response, further treatments were strongly needed.
OCA was proved by both FDA and EMA in 2016 to be used in combination with UDCA in patients with PBC who have inadequate response, or as monotherapy for those patients who are intolerant to UDCA. Even if recently approved as second-line therapy, several concerns already exist on OCA use: first, frequent development of pruritus often leading to treatment discontinuation; second, reported liver toxicity in patients with decompensated cirrhosis, thus leading to restriction of OCA use in cirrhotic population in the US.
Among new therapeutic targets under investigation, PPAR are the most interesting due to their properties and effects in the liver -that are, anti-inflammatory, anti-fibrotic, bile acid metabolism regulating.
BZF is the most promising candidate so far, due to positive results in terms both of biochemical response and long-term clinical outcomes reached in prospective randomized controlled clinical trial and in retrospective large cohort studies. Moreover, BZF improves symptoms in PBC patients, in particular pruritus, which can be severely debilitating in PBC patients.
However, despite encouraging results, we think that over enthusiasm on BZF should be avoided mainly due to safety concerns; in fact, fibrates should be given with caution in patients at risk for chronic kidney disease, plus administration in cirrhotic patient is critical considering the adverse events observed in clinical study (elevation of bilirubin and impairment of liver function). On the other hand, the large spectrum of actions strongly encourages further investigation and optimization of BZF use instead of alternative agents; also, early diagnosis of the disease, possibly when liver cirrhosis is not established yet would increase the available therapeutic options for patients.
Next to fibrates, several other PPAR ligands are under evaluation such as Seladelpar, Elafibranor, and Saroglitazar; most of them did show ALP improvement, but small sample size, short-term outcome, and selection of pre-cirrhotic PBC population represent a limit for safety evaluation. Thus, the urgent need is to plan dose-finding and safety trials.
In conclusion, we believe that current research support further evaluation of PPAR-agonist. Available data suggest considering highly selected patients for individual dual or triple combination therapies, however, patients with advanced liver diseases should be referred to referral centers.
The PPAR receptor forms a heterodimer with a retinoid X receptor (RXR) consequent to the binding of an activating ligand (i.e. fibrates.). After the recruitment of coactivators and the release of corepressors, the ligand-activated transcriptional complex interacts with DNA and regulates transcription of target genes. Each of the three isotypes of PPAR is characterized by specific cell allocation in the liver and actions, with all of them playing a key anti-cholestatic and anti-inflammatory role in primary biliary cholangitis (PBC).
PPAR agonists, both fibrates and non-fibrate PPARs, are a group of structurally diverse compounds that activate the nueclar receptor PPAR. All their structures bear a certain resemblance to fatty acids, and they are hydrophobic in nature.
Financial support
The authors did not receive any financial support to complete the study or write the manuscript.
Conflict of interest
AL reports receiving consulting fees from Intercept Pharma, AlfaSigma, AbbVie, Gilead and MSD and travel expenses from Intercept Pharma, AlfaSigma and AbbVie. All other authors have nothing to disclose.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Ana Lleo reports a relationship with Gilead Sciences Inc that includes: speaking and lecture fees. Ana Lleo reports a relationship with Intercept Pharmaceuticals that includes: consulting or advisory and speaking and lecture fees. Ana Lleo reports a relationship with Alfasigma SpA that includes: consulting or advisory and speaking and lecture fees. Ana Lleo reports a relationship with Albireo Pharma Inc that includes: consulting or advisory. Ana Lleo reports a relationship with GSK that includes: speaking and lecture fees. Ana Lleo reports a relationship with AstraZeneca Pharmaceuticals LP that includes: consulting or advisory. Ana Lleo reports a relationship with Incyte Corporation that includes: speaking and lecture fees. Ana Lleo reports a relationship with Takeda Pharmaceutical Co Ltd that includes: consulting or advisory. Ana Lleo reports a relationship with Kowa Pharmaceutical Europe Co Ltd that includes: consulting or advisory. Ana Lleo reports a relationship with Merck Sharp & Dohme UK Ltd that includes: speaking and lecture fees. Ana Lleo reports a relationship with AbbVie Inc that includes: speaking and lecture fees. Francesca Colapietro reports a relationship with Intercept Pharmaceuticals Inc that includes: speaking and lecture fees. Research Grants: EU Project D-LIVER, EU COST Action EURO-Cholangio-Net, Italian Ministry of Health, Italian Association for Cancer Research (AIRC) Support for sponsored studies (via Humanitas Research Hospital): Falk, Intercept Pharma.
Handling Editor: Y Renaudineau
Data availability
No data was used for the research described in the article.
References
- 1.European e. e. e. Association for the study of the liver. Electronic address, L. European association for the study of the. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Lleo A., Wang G.Q., Gershwin M.E., Hirschfield G.M. Primary biliary cholangitis. Lancet. 2020;396:1915–1926. doi: 10.1016/S0140-6736(20)31607-X. [DOI] [PubMed] [Google Scholar]
- 3.Corpechot C., Carrat F., Bahr A., Chretien Y., Poupon R.E., Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297–303. doi: 10.1053/j.gastro.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Harms M.H., van Buuren H.R., Corpechot C., Thorburn D., Janssen H.L.A., Lindor K.D., et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J. Hepatol. 2019;71:357–365. doi: 10.1016/j.jhep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Poupon R.E., Lindor K.D., Cauch-Dudek K., Dickson E.R., Poupon R., Heathcote E.J. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 6.Pellicciari R., Costantino G., Camaioni E., Sadeghpour B.M., Entrena A., Willson T.M., et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y.D., Chen W.D., Wang M., Yu D., Forman B.M., Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P., et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 9.Trauner M., Nevens F., Shiffman M.L., Drenth J.P.H., Bowlus C.L., Vargas V., et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–453. doi: 10.1016/S2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 10.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 11.Dubois V., Eeckhoute J., Lefebvre P., Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Invest. 2017;127:1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Hsueh W.A., Law R. The central role of fat and effect of peroxisome proliferator-activated receptor-gamma on progression of insulin resistance and cardiovascular disease. Am. J. Cardiol. 2003;92:3J–9J. doi: 10.1016/s0002-9149(03)00610-6. [DOI] [PubMed] [Google Scholar]
- 14.Wagner M., Zollner G., Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- 15.Toyama T., Nakamura H., Harano Y., Yamauchi N., Morita A., Kirishima T., et al. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem. Biophys. Res. Commun. 2004;324:697–704. doi: 10.1016/j.bbrc.2004.09.110. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Vilarrupla A., Lavina B., Garcia-Caldero H., Russo L., Rosado E., Roglans N., et al. PPARalpha activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J. Hepatol. 2012;56:1033–1039. doi: 10.1016/j.jhep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Post S.M., Duez H., Gervois P.P., Staels B., Kuipers F., Princen H.M. Fibrates suppress bile acid synthesis via peroxisome proliferator-activated receptor-alpha-mediated downregulation of cholesterol 7alpha-hydroxylase and sterol 27-hydroxylase expression. Arterioscler. Thromb. Vasc. Biol. 2001;21:1840–1845. doi: 10.1161/hq1101.098228. [DOI] [PubMed] [Google Scholar]
- 18.Ghonem N.S., Assis D.N., Boyer J.L. Fibrates and cholestasis. Hepatology. 2015;62:635–643. doi: 10.1002/hep.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda A., Ikegami T., Nakamuta M., Miyazaki T., Iwamoto J., Hirayama T., et al. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology. 2013;57:1931–1941. doi: 10.1002/hep.26018. [DOI] [PubMed] [Google Scholar]
- 20.Kok T., Bloks V.W., Wolters H., Havinga R., Jansen P.L., Staels B., et al. Peroxisome proliferator-activated receptor alpha (PPARalpha)-mediated regulation of multidrug resistance 2 (Mdr2) expression and function in mice. Biochem. J. 2003;369:539–547. doi: 10.1042/BJ20020981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoda J., Inada Y., Tsuji A., Kusama H., Ueda T., Ikegami T., et al. Bezafibrate stimulates canalicular localization of NBD-labeled PC in HepG2 cells by PPARalpha-mediated redistribution of ABCB4. J. Lipid Res. 2004;45:1813–1825. doi: 10.1194/jlr.M400132-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Li F., Patterson A.D., Krausz K.W., Tanaka N., Gonzalez F.J. Metabolomics reveals an essential role for peroxisome proliferator-activated receptor alpha in bile acid homeostasis. J. Lipid Res. 2012;53:1625–1635. doi: 10.1194/jlr.M027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada K., Isse K., Kamihira T., Shimoda S., Nakanuma Y. Th1 cytokine-induced downregulation of PPARgamma in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology. 2005;41:1329–1338. doi: 10.1002/hep.20705. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Nakajima T., Gonzalez F.J., Tanaka N. PPARs as metabolic regulators in the liver: lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21062061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C.H., Chawla A., Urbiztondo N., Liao D., Boisvert W.A., Evans R.M., et al. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 26.Chianale J., Vollrath V., Wielandt A.M., Amigo L., Rigotti A., Nervi F., et al. Fibrates induce mdr2 gene expression and biliary phospholipid secretion in the mouse. Biochem. J. 1996;314(Pt 3):781–786. doi: 10.1042/bj3140781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zumoff B. Effect of clofibrate on plasma levels of alkaline phosphatase. N. Engl. J. Med. 1977;297:669. doi: 10.1056/nejm197709222971216. [DOI] [PubMed] [Google Scholar]
- 28.Lammers W.J., van Buuren H.R., Hirschfield G.M., Janssen H.L., Invernizzi P., Mason A.L., et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338–1349. doi: 10.1053/j.gastro.2014.08.029. e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki S., Ohira H., Nishiguchi S., Zeniya M., Kaneko S., Onji M., et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatol. Res. 2008;38:557–564. doi: 10.1111/j.1872-034X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindor K.D., Bowlus C.L., Boyer J., Levy C., Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American association for the study of liver diseases. Hepatology. 2021 doi: 10.1002/hep.32117. [DOI] [PubMed] [Google Scholar]
- 31.Willson T.M., Brown P.J., Sternbach D.D., Henke B.R. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 32.Corpechot C., Poupon R., Chazouilleres O. New treatments/targets for primary biliary cholangitis. JHEP Rep. 2019;1:203–213. doi: 10.1016/j.jhepr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki S., Tsuda K., Ueta H., Aono R., Ono M., Saibara T. Bezafibrate may have a beneficial effect in pre-cirrhotic primary biliary cirrhosis. Hepatol. Res. 1999;16:12–18. doi: 10.1016/S1386-6346(99)00033-9. [DOI] [Google Scholar]
- 34.Corpechot C., Chazouilleres O., Rousseau A., Le Gruyer A., Habersetzer F., Mathurin P., et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N. Engl. J. Med. 2018;378:2171–2181. doi: 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 35.Reig A., Alvarez-Navascues C., Vergara M., Gomez-Dominguez E., Gallego-Moya A., Perez-Medrano I.M., et al. Obeticholic acid and fibrates in primary biliary cholangitis: comparative effects in a multicentric observational study. Am. J. Gastroenterol. 2021;116:2250–2257. doi: 10.14309/ajg.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 36.Smets L., Verbeek J., Korf H., van der Merwe S., Nevens F. Improved markers of cholestatic liver injury in patients with primary biliary cholangitis treated with obeticholic acid and bezafibrate. Hepatology. 2021;73:2598–2600. doi: 10.1002/hep.31613. [DOI] [PubMed] [Google Scholar]
- 37.Soret P.A., Lam L., Carrat F., Smets L., Berg T., Carbone M., et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment. Pharmacol. Ther. 2021;53:1138–1146. doi: 10.1111/apt.16336. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka A., Hirohara J., Nakano T., Matsumoto K., Chazouilleres O., Takikawa H., et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J. Hepatol. 2021;75:565–571. doi: 10.1016/j.jhep.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Dohmen K., Mizuta T., Nakamuta M., Shimohashi N., Ishibashi H., Yamamoto K. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J. Gastroenterol. 2004;10:894–898. doi: 10.3748/wjg.v10.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohira H., Sato Y., Ueno T., Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am. J. Gastroenterol. 2002;97:2147–2149. doi: 10.1111/j.1572-0241.2002.05944.x. [DOI] [PubMed] [Google Scholar]
- 41.Hegade V.S., Khanna A., Walker L.J., Wong L.L., Dyson J.K., Jones D.E.J. Long-term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK-PBC risk score. Dig. Dis. Sci. 2016;61:3037–3044. doi: 10.1007/s10620-016-4250-y. [DOI] [PubMed] [Google Scholar]
- 42.Grigorian A.Y., Mardini H.E., Corpechot C., Poupon R., Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: a meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:296–306. doi: 10.1016/j.clinre.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Li S., He L., Wang F., Chen K., Li J., et al. Combination therapy of fenofibrate and ursodeoxycholic acid in patients with primary biliary cirrhosis who respond incompletely to UDCA monotherapy: a meta-analysis. Drug Des. Dev. Ther. 2015;9:2757–2766. doi: 10.2147/DDDT.S79837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung A.C., Lapointe-Shaw L., Kowgier M., Meza-Cardona J., Hirschfield G.M., Janssen H.L., et al. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment. Pharmacol. Ther. 2016;43:283–293. doi: 10.1111/apt.13465. [DOI] [PubMed] [Google Scholar]
- 45.Abbas N., Culver E.L., Thorburn D., Halliday N., Crothers H., Dyson J.K., et al. UK-wide multicenter evaluation of second-line therapies in primary biliary cholangitis. Clin. Gastroenterol. Hepatol. 2022 doi: 10.1016/j.cgh.2022.07.038. [DOI] [PubMed] [Google Scholar]
- 46.Jones D., Boudes P.F., Swain M.G., Bowlus C.L., Galambos M.R., Bacon B.R., et al. Seladelpar (MBX-8025), a selective PPAR-delta agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 47.Kowdley K.V., Vuppalanchi R., Levy C., Floreani A., Andreone P., LaRusso N.F., et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 2020;73:94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ENHANCE Safety and efficacy of seladelpar in patients with primary biliary cholangitis-A phase 3, international, randomized, placebo-controlled study. Gastroenterol. Hepatol. 2021;Feb;17(2):5–6. [PMC free article] [PubMed] [Google Scholar]
- 49.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–11459 e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 50.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J., et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 51.Schattenberg J.M., Pares A., Kowdley K.V., Heneghan M.A., Caldwell S., Pratt D., et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J. Hepatol. 2021;74:1344–1354. doi: 10.1016/j.jhep.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Jani R.H., Pai V., Jha P., Jariwala G., Mukhopadhyay S., Bhansali A., et al. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI) Diabetes Technol. Therapeut. 2014;16:63–71. doi: 10.1089/dia.2013.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuppalanchi R., Caldwell S.H., Pyrsopoulos N., deLemos A.S., Rossi S., Levy C., et al. Proof-of-concept study to evaluate the safety and efficacy of saroglitazar in patients with primary biliary cholangitis. J. Hepatol. 2022;76(1):75–85. doi: 10.1016/j.jhep.2021.08.025. [DOI] [PubMed] [Google Scholar]
- 54.Vuppalanchi R., Gonzalez-Huezo M.S., Payan-Olivas R., Munoz-Espinosa L.E., Shaikh F., Pio Cruz-Lopez J.L., et al. A multicenter, open-label, single-arm study to evaluate the efficacy and safety of saroglitazar in patients with primary biliary cholangitis. Clin. Transl. Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussain A.B., Samuel R., Hegade V.S., Jones D.E., Reynolds N.J. Pruritus secondary to primary biliary cholangitis: a review of the pathophysiology and management with phototherapy. Br. J. Dermatol. 2019;181:1138–1145. doi: 10.1111/bjd.17933. [DOI] [PubMed] [Google Scholar]
- 56.Levy C., Peter J.A., Nelson D.R., Keach J., Petz J., Cabrera R., et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment. Pharmacol. Ther. 2011;33:235–242. doi: 10.1111/j.1365-2036.2010.04512.x. [DOI] [PubMed] [Google Scholar]
- 57.de Vries E., Bolier R., Goet J., Pares A., Verbeek J., de Vree M., et al. Fibrates for itch (FITCH) in fibrosing cholangiopathies: a double-blind, randomized, placebo-controlled trial. Gastroenterology. 2021;160:734–743 e6. doi: 10.1053/j.gastro.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Green B.G., Shaffer G.S. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain. 1993;53:323–334. doi: 10.1016/0304-3959(93)90228-H. [DOI] [PubMed] [Google Scholar]
- 59.Kremer A.E., Martens J.J., Kulik W., Rueff F., Kuiper E.M., van Buuren H.R., et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018. doi: 10.1053/j.gastro.2010.05.009. 18 e1. [DOI] [PubMed] [Google Scholar]
- 60.Kremer A.E., van Dijk R., Leckie P., Schaap F.G., Kuiper E.M., Mettang T., et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391–1400. doi: 10.1002/hep.25748. [DOI] [PubMed] [Google Scholar]
- 61.Levy C., Bowlus C., Neff G., Swain M., Michael G., Mayo M.J., et al. Durability of treatment response after 1 year of therapy with seladelpar in patients with primary biliary cholangitis (PBC): final results of an international phase 2 study. J. Hepatol. 2020;73:S464. doi: 10.1016/S0168-8278(20)31411-2. –S465 (FRI133) [DOI] [Google Scholar]
- 62.Kremer A.E., Mayo M.J., Hirschfield G., Levy C., Bowlus C.L., Jones D.E., et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2021 doi: 10.1111/liv.15039. [DOI] [PubMed] [Google Scholar]
- 63.Liberopoulos E.N., Florentin M., Elisaf M.S., Mikhailidis D.P., Tsianos E. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc. Med. J. 2010;4:120–126. doi: 10.2174/1874192401004010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han X.F., Wang Q.X., Liu Y., You Z.R., Bian Z.L., Qiu D.K., et al. Efficacy of fenofibrate in Chinese patients with primary biliary cirrhosis partially responding to ursodeoxycholic acid therapy. J Dig Dis. 2012;13:219–224. doi: 10.1111/j.1751-2980.2012.00574.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.