Abstract
The impact of dry‐ageing of beef and wet‐ageing of beef, pork and lamb on microbiological hazards and spoilage bacteria was examined and current practices are described. As ‘standard fresh’ and wet‐aged meat use similar processes these were differentiated based on duration. In addition to a description of the different stages, data were collated on key parameters (time, temperature, pH and aw) using a literature survey and questionnaires. The microbiological hazards that may be present in all aged meats included Shiga toxin‐producing Escherichia coli (STEC), Salmonella spp., Staphylococcus aureus, Listeria monocytogenes, enterotoxigenic Yersinia spp., Campylobacter spp. and Clostridium spp. Moulds, such as Aspergillus spp. and Penicillium spp., may produce mycotoxins when conditions are favourable but may be prevented by ensuring a meat surface temperature of −0.5 to 3.0°C, with a relative humidity (RH) of 75–85% and an airflow of 0.2–0.5 m/s for up to 35 days. The main meat spoilage bacteria include Pseudomonas spp., Lactobacillus spp. Enterococcus spp., Weissella spp., Brochothrix spp., Leuconostoc spp., Lactobacillus spp., Shewanella spp. and Clostridium spp. Under current practices, the ageing of meat may have an impact on the load of microbiological hazards and spoilage bacteria as compared to standard fresh meat preparation. Ageing under defined and controlled conditions can achieve the same or lower loads of microbiological hazards and spoilage bacteria than the variable log10 increases predicted during standard fresh meat preparation. An approach was used to establish the conditions of time and temperature that would achieve similar or lower levels of L. monocytogenes and Yersinia enterocolitica (pork only) and lactic acid bacteria (representing spoilage bacteria) as compared to standard fresh meat. Finally, additional control activities were identified that would further assure the microbial safety of dry‐aged beef, based on recommended best practice and the outputs of the equivalence assessment.
Keywords: Meat, wet‐ageing, dry‐ageing, maturation, safety, bacterial growth
Summary
Following a request from the European Commission, the Scientific Panel on Biological Hazards (BIOHAZ) was asked to provide a scientific opinion on the impact of prolonged ageing of meat using the dry‐ageing process for beef and the wet‐ageing process for ungulates on the load of microbiological hazards and spoilage bacteria in comparison with standard fresh meat.
In Term of Reference 1 (ToR1), the European Food Safety Authority (EFSA) was asked to provide an overview of the current practices used by food business operators (FBOs) for dry‐ageing and wet‐ageing of meat (e.g. time, temperature, relative humidity, air flow, type of packaging, etc.).
Historically beef was preserved by removing water in a dry‐ageing process that resulted in tenderisation and enhanced flavour. However, in the 1960s, vacuum packaging was developed and since then most meats, including beef, pork and lamb, are wet‐aged in vacuum packs because it requires less time, incurs less weight loss, has lower investment costs and the resultant product requires less trimming.
There are different combinations of temperature, relative humidity (RH), airflow and time used in academic studies on the dry‐ageing of beef but most used 0–4°C, a RH of 70–80% and an airflow of 0.5–2.5 m/s for 14–35 days. Under these conditions, the surface pH of the beef was usually 5.5–6.0 and the aw 0.95–0.99. Studies on the wet‐ageing of beef, pork and lamb typically used temperatures of −0.6 to 4°C, a RH of 75–85% and an air flow of 0.2–7.0 m/s for 21–35 days (in vacuum packs) resulting in surface pH values of 5.1–6.0, 5.4–6.3 and 5.5–5.9 for beef, pork and lamb, respectively. The aw values for beef were 0.97–0.99 but similar data were not available for pork or lamb.
Information on current commercial practices was obtained using a questionnaire targeting relevant FBOs and their professional associations. Under commercial conditions beef is usually dry‐aged at 1–4°C and a RH of 75–85% for 21–35 days (no data available for air flow). Wet‐ageing of beef is undertaken at 0–4°C for 14–49 days with a resultant surface pH of 5.4–5.8. Wet‐ageing of pork is undertaken at a similar temperature for 2–6 days and the range of pH values is the same as for beef. In contrast, lamb is wet‐aged at −1 to 5°C in a process that requires 7–77 days (pH data not available).
In ToR2, EFSA was requested to identify public health‐relevant microbiological hazards and spoilage bacteria occurring during the dry‐ageing and wet‐ageing of meat, also considering their possible use for the production of minced meat and mechanically separated meat (MSM). The microbiological hazards that may be present in all aged meats included Shiga toxin‐producing Escherichia coli (STEC) (more common in beef), Salmonella spp., Staphylococcus aureus, Listeria monocytogenes, enterotoxigenic Yersinia spp. (usually pork), Campylobacter spp. and Clostridium spp. Moulds such as Aspergillus spp. and Penicillium spp., may produce mycotoxins when conditions are favourable. However, as there is limited information available and that which is available is primarily focused on plant‐based foods, it was unclear as to exactly what temperature conditions prevent mycotoxin production during dry‐ageing of beef. Four studies published in the 1970s report that mycotoxins can be produced at very low concentrations all‐be‐it very slowly by Penicillium spp. at temperatures as low as, for example 0–1°C on fruits, corn and other crops but these do not provide data for meat. Meat and Livestock Australia (MLA) in their 2019 review concluded that Penicillium spp. and Aspergillus spp. are not capable of producing mycotoxins on dry‐aged meat at temperatures between −0.5 and 3°C, a RH of 75–85% and an airflow of 0.2–0.5 m/s.
The main meat spoilage bacteria include Pseudomonas spp., Lactobacillus spp. Enterococcus spp., Weissella spp. and Brochothrix spp., which produce slime on meat, Weissella spp., Leuconostoc spp., Enterococcus spp. and the former Lactobacillus spp., associated with hydrogen peroxide greening, and Shewanella spp. and Clostridium spp., responsible for hydrogen sulfide greening. Meat spoilage is also characterised by off‐odours such as sulfide odour (Clostridium spp. and Hafnia spp.) and cabbage odour (Providencia spp.) and souring caused by lactic acid bacteria (LAB), Enterococcus spp., Micrococcus spp., Bacillus spp. and Clostridium spp. It is generally accepted that Pseudomonas spp. are the main spoilage bacteria under the aerobic conditions encountered during dry‐ageing while facultative anaerobic bacteria such as LAB and strict anaerobes such as Clostridium spp. are the main spoilage bacteria of wet‐aged meat which is vacuum packaged and therefore under anaerobic conditions.
In ToR3, EFSA was requested to assess the impact that dry‐ageing and wet‐ageing of meat, produced according to selected current practices, could have on the load of public health‐relevant microbiological hazards and spoilage bacteria, when compared to standard fresh meat. In ToR4, EFSA was requested to provide those conditions during the production of dry‐aged beef and wet‐aged beef, pork and lamb and possible further storage that would result in a similar or lower load of the relevant microbiological hazards and spoilage bacteria as compared to those obtained during standard fresh meat preparation.
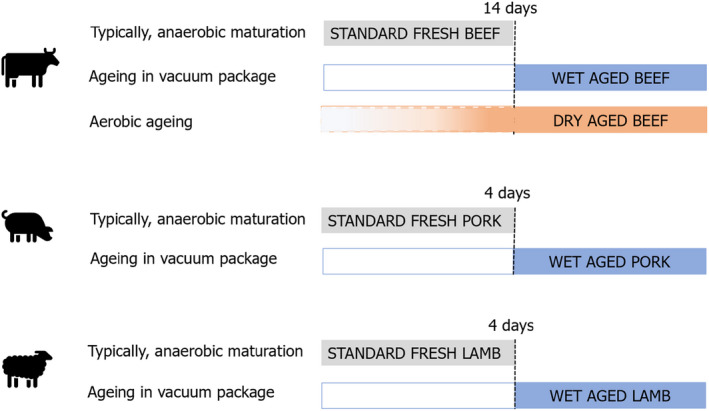
Fresh meat is meat that has not undergone any preserving process other than chilling, freezing or quick‐freezing and the majority of fresh beef, pork and lamb is matured/stored in vacuum packaging under chilled conditions. Wet‐aged meat is meat that has been vacuum packaged and stored under chilled conditions. There is currently no scientific, commercial or legislative basis for differentiating between ‘standard fresh meat’ and ‘wet‐aged meat’. For the purposes of this Opinion, differentiation, as agreed with the European Commission, was based solely on the time in chilled storage. ‘Standard fresh beef’ was considered to be matured in vacuum packs for up to and including 14 days, while for pork and lamb it was typically matured for up to and including 4 days.
Due to the lack of sufficient data on the growth or inactivation of potentially relevant microorganisms during different ageing practices or processes a simulation approach was adopted for ToR3 and ToR4 and compared with the available data. The growth (expressed as log10 increase) of relevant microorganisms during different ageing scenarios was simulated using predictive microbiology models. The relevant bacteria to evaluate were selected from the pathogenic and spoilage bacteria present on the different types of meat, based on their potential for growth and the availability of suitable models. By comparison with experimental data in ComBase and the scientific literature, growth rate predictions of the models were calibrated using bias factors to correct for systematic over‐ or underestimations. To cover differences in practices during standard fresh meat preparation, dry‐ageing of beef and wet‐ageing of beef, pork and lamb in Europe, scenarios of ageing temperature, pH and aw were defined based on the outputs of ToR1.
Under current practices, the ageing of meat may have an impact on the load of microbiological hazards and spoilage bacteria as compared to fresh meat preparation. Based on the ability to grow in the conditions encountered during meat ageing, the most relevant pathogenic bacteria for inclusion in the assessment were L. monocytogenes, and Y. enterocolitica (pork only) and the most relevant spoilage bacteria were LAB and pseudomonads (dry‐aged beef only).
Ageing under defined and controlled conditions can achieve the same or lower loads of microbiological hazards and spoilage bacteria than the variable log10 increases predicted during standard fresh meat preparation. The determination of the actual conditions to achieve equivalence depends on the extent of the variable log10 increase considered typical for that achieved during standard fresh meat preparation, and on the interpretation of the direction and magnitude of the uncertainties of the predictions.
Ageing of meat is a complex process that depends on a multitude of parameters many of which change with time (particularly aw in dry‐ageing), resulting in different bacterial behaviour. This is reflected in a wide range of predicted log10 increases when the minimum and maximum scenarios are considered. For instance, the range of predicted log10 increases of L. monocytogenes after 77 days of dry‐ageing ranged from 0.1 to over 10, and log10 increases of 0.2 to over 10 after 49 days of wet‐ageing. The complexity is also reflected in the occurrence of conflicting studies in some cases reporting growth (log10 increase), survival (no relevant change) or inactivation (log10 decrease), both for wet‐ageing and dry‐ageing. The predictive modelling approach only captured the effects of parameters included in the models and the factors considered in the developed scenarios and uncertainty analysis.
In ToR4, based on the predicted log10 increases during standard fresh meat preparation, it was decided to evaluate conditions of time and temperature during ageing that results in an increase of up to 0.5, 1, 2, 3 or 4 log10 of pathogenic or spoilage bacteria using developed combinations between predicted time and temperature under different scenarios of pH and aw. Different scenarios for aw and pH were analysed since these parameters have an impact on predicted log10 increases, are not easily controlled nor monitored, and vary dynamically during the standard fresh meat preparation and ageing processes. The impact of sources of uncertainty due to, for example the effects of microbial competition between spoilage and pathogenic bacteria (wet and dry‐ageing), inactivation (dry‐ageing), trimming (mainly dry‐aged beef) and storage, and the variable and dynamic (over time) pH and aw were considered and assessed through an expert knowledge elicitation (EKE). Conditions ensuring an equivalent log10 increase of L. monocytogenes as the most relevant pathogen under the scenario of medium pH and aw in the meat were assessed. The outputs included, for example dry‐ageing of beef for 35 days at a temperature of 3°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 2 log10 increase on standard fresh beef with 80–95% certainty.
In ToR5, EFSA was requested to recommend additional good hygiene practices (GHPs) specific to the production and storage of dry‐aged and wet‐aged meat, as compared to those relevant for the production and storage of standard fresh meat.
The food safety of meat is assured through the development and implementation of hazard analysis and critical control point (HACCP) and prerequisite programme (PRP) activities, including GHPs and good manufacturing practices (GMPs). As standard fresh meat preparation and wet‐aged meat differ only in the time applied, the control activities for these processes are similar. The GHP identified from the scientific and grey literature that contribute to the hygienic production of dry‐aged beef included using good quality beef in a dedicated purpose‐built room or chamber; not loading the beef into the chamber until the required temperature and RH have been achieved; hanging from the bone to prevent internal contamination or if using a shelf, ensuring sufficient perforation to facilitate air flow with regular turning using hygienic methods; applying the highest airflow at the start of the ageing process to facilitate early crust development and reduce the surface aw, thereby restricting bacterial growth; regular cleaning and disinfection of the chamber; using air conditioning refrigeration system components that can be effectively cleaned and disinfected; using calibrated thermometers, RH probes and other equipment to accurately monitor and facilitate control of chamber conditions; filtering or UV treating the air in contact with the beef; trimming the crust in a hygienic manner in a dedicated air controlled environment and applying treatments such as heat or high pressure to trimmings to eliminate any pathogens present.
Specific interventions addressed in experimental studies, such as treating the dry‐aged beef trimmings using high pressure processing or washing with hot acidic solutions have been shown to decrease bacterial counts but these may not be suitable for application if, for example the organoleptic characteristics of the product are adversely affected. The outputs of ToR4 may be used to establish time–temperature critical limits for the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb (see Section 4 ‘Conclusions’, ToR4 for examples).
Minced meat must be prepared either within no more than 6 days after the slaughter of the ungulates, or within 15 days in the case of boned, vacuum‐packed beef and veal (Point 2(b) of Chapter III to Section V of Annex III to Regulation (EC) No 853/2004). Before the production of MSM, the maximum storage period of the (chilled) raw material can be no more than 7 days when derived from the on‐site slaughterhouse and 5 days in other cases (Points 3(a) and 4(a)of Chapter III to Section V of Annex III to Regulation (EC) No 853/2004). Thus, meat that is aged for 14 days or more is not currently allowed to be used for minced meat or MSM.
Although it might be expected that longer meat ageing times would facilitate higher bacterial counts, the scientific data to support this is limited and contradictory. Thus, it is currently not possible to conclude on the relative food safety of minced meat and MSM prepared from dry or wet‐aged meat, as compared to standard fresh meat due to the lack of information on the impact of the time between slaughter, chilled storage and minced meat or MSM preparation on bacterial growth and the limited microbiological data on bacterial counts in minced meat and MSM prepared from prolonged (more than 14 days) aged meat. However, if the minced meat (including trimmings from dry‐aged beef) or MSM are thoroughly cooked any vegetative bacterial pathogens such as STEC, Salmonella or L. monocytogenes will be eliminated.
In addition to providing answers to the ToRs, several recommendations are made including research to establish the exact conditions of dry‐ageing of beef in which moulds produce mycotoxins and on the effect of the time between slaughter and minced meat or MSM preparation on the bacterial (pathogenic and spoilage) counts. Finally, research (challenge tests) is also recommended to assess the growth of pathogens such as L. monocytogenes during different conditions of dry‐ageing of beef and wet‐ageing of beef, pork and lamb and during subsequent storage.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Post‐mortem ageing (or ripening or conditioning) is a natural process when meat is subjected to controlled refrigerated storage conditions. It implies cold storage of fresh muscle far beyond the onset of rigor mortis and the enzymatic and physico‐chemical modifications that confers the typical meat characteristics (in particular tenderness and flavour) to the skeletal muscles as in the case of the conventional maturation of carcasses. While meat from any species could be aged, post‐mortem ageing is generally limited to beef, due to the relative youth of pork, lamb, and veal at the time of slaughter. The length of the process is variable, but routinely 14 days are considered the minimum period to obtain the typical characteristics of the aged meat. The aim of meat ageing is to allow and enhance the palatability of meat according to consumers' expectations in terms of meat characteristics (i.e., improving the tenderness, juiciness and flavour).
There are two types of ageing techniques: dry‐ageing and wet‐ageing (Dashdorj et al., 2016; Kim et al., 2018).
Dry‐ageing is the process carried out in aerobic conditions of hanging beef carcasses or sub‐primal or placing primal cuts either unpacked or packed in bags permeable to water vapour in a refrigerated room and left to age for several weeks or even months at controlled environmental conditions of temperature, relative humidity and air flow;
Wet‐ageing is the process carried out in anaerobic conditions of placing the vacuum packaged meat of beef or different ungulates at controlled temperature conditions.
Following post‐mortem inspection, meat of ungulates (other than offal) must be immediately chilled to not more than 7°C throughout the meat (Point 1(a) of Chapter VII to Section I of Annex III to Regulation (EC) No 853/20041). EU requirements do not impose the maximum durability of such meat (left to the food business operator). When chilled (not frozen) meat is used for mincing, the minced meat must be prepared either within no more of six days after the slaughter of the ungulates, or within 15 days in case of boned, vacuum‐packed beef and veal (Point 2(b) of Chapter III to Section V of Annex III to Regulation (EC) No 853/2004). Before the production of mechanically separated meat, the maximum storage period of the (chilled) raw material can be no more than 7 days when derived from the on‐site slaughterhouse and 5 days in other cases (Point 3(a) and 4(a) of Chapter III to Section V of Annex III to Regulation (EC) No 853/2004).
Regulation (EC) No 2073/2005 lays down a number of microbiological criteria that must be applied in any case:
A food safety criterion being no detection of Salmonella in 25 g of minced meat and mechanically separated meat derived from dry‐aged or wet‐aged meat;
Process hygiene criteria on Salmonella, Enterobacteriaceae and aerobic colony counts on carcasses of domestic ungulates;
Process hygiene criteria on E. coli and aerobic colony counts on minced meat and mechanically separated meat.
Meat for ageing must be derived from carcasses that are considered fit for human consumption after the ante‐mortem and post‐mortem inspections. However, such inspection does not routinely detect most public health microbiological hazards. Therefore, microbiological hazards might derive from:
microorganisms already present in the meat but not evidenced by meat inspection,
external contamination during the ageing process, including from e.g., mechanical tenderisation procedures.
Several physical parameters during the dry‐ageing of meat might have an influence on the survival and growth of microorganisms, hence on the safety of aged meat for human consumption.
Additional concerns have been expressed on the safety of such meat since ageing is not only carried out by professional food businesses, but new trends in meat consumption show also the interest of the consumers of meat, with ‘dry‐ageing at home’ advertisement and the publication of videos/tutorials and guidelines (i.e., UmaiDry, BarbecueBible, how to dry‐age steak at home). Furthermore, specific dry‐ager refrigerators are available for domestic and commercial use.
This raises additional concerns about the possible risks related to such processes without professional equipment, to the correct handling/trimming of the meat and to the shelf life of this type of meat. Possible risks that might not be appropriately perceived from consumers.
There is also a demand from certain Member States to allow the use of dry and wet‐aged meat for the production of minced meat and possibly mechanically separated meat. Before considering such authorisation (i.e., the review of the limits for storage period of raw materials for such products), the safety of such an approach should be assessed.
In addition, the European Commission considers that scientific advice is needed as regards the growth of spoilage bacteria during the ageing process. The purpose of this request is to ensure compliance with Article 14(5) of Regulation (EC) No 178/2002 laying down that, ‘in determining whether any food is unfit for human consumption, regard shall be had to whether the food is unacceptable for human consumption according to its intended use, for reasons of contamination, whether by extraneous matter or otherwise, or through putrefaction, deterioration or decay’.
In accordance with Art. 29 of Regulation (EC) No 178/2002 the Commission asks EFSA to deliver a scientific opinion on the impact of prolonged ageing of meat using the dry‐ageing process for beef and the wet‐ageing process for ungulates on the load of microbiological hazards and spoilage bacteria in comparison with standard fresh meat.
More specifically, EFSA is asked:
to provide an overview of the current practices used by food business operators for dry‐ageing and wet‐ageing of meat (e.g., time, temperature, relative humidity, air flow, type of packaging);
to identify public health‐relevant microbiological hazards and spoilage bacteria occurring in the process of prolonged dry‐ageing and wet‐ageing of meat, also considering its possible use for the production of minced meat and mechanically separated meat;
to assess the impact that dry‐ageing and wet‐ageing of meat, produced according to selected current practices, could have on the load of public health‐relevant microbiological hazards and spoilage bacteria, when compared to standard fresh meat;
to provide those conditions during the production of dry‐aged and wet‐aged meat and possible further storage that would result in a similar or lower load of the relevant microbiological hazards and spoilage bacteria as compared to standard fresh meat before consumption (i.e., at the end of the shelf‐life);
to recommend additional good hygiene practices specific to the production and storage of dry‐aged and wet‐aged meat, as compared to those relevant for the production and storage of standard fresh meat.
1.2. Interpretation of the Terms of Reference
When cattle, pigs and sheep are slaughtered, the carcasses are dressed and immediately chilled. This usually occurs at 0–2°C, although the temperature is carefully controlled, especially for beef, to ensure the carcass temperature does not decrease too quickly causing cold shortening and toughening of the meat. Although carcass chilling methods may vary, this process usually requires up to 24 h for pigs and sheep and 24–48 h for beef carcasses (Devine and Dikeman, 2014). Thereafter, the carcasses are either stored chilled for a period of time or are sent to the boning hall and cut into primal and sub‐primal cuts.
Data on the parameters of the meat before ageing is lacking, although the pH is usually 5.5–5.8 for beef (Ahnstrom et al., 2006; Li et al., 2013; Owczarek‐Fendor et al., 2014; Stenstrom et al., 2014; Gudjonsdottir et al., 2015; Hulankova et al., 2018a) and pork (Holmer et al., 2009) while Constantino et al. (2012) reported a pH of 5.6 for lamb. The rate and extent of post‐mortem tenderisation of beef depend on muscle type and processing conditions and there is no standard ageing time (Gagaoua et al., 2021). Moreover, there are no universally agreed ageing times for specific muscles (Stuby‐Souva, 1994; Benech et al., 2021).
Storing meat under chilled conditions for a period of time facilitates tenderisation (Lonergan et al., 2010; Benech et al., 2021). This process is referred to as ageing or maturation. The airflow and RH in the chill room or cabinet are also controlled and the meat may be stored aerobically or anaerobically (in vacuum packs). In Regulation (EC) No 853/2004, ‘fresh meat’ is defined ‘as meat that has not undergone any preserving process other than chilling, freezing or quick‐freezing, including meat that is vacuum packaged or packaged in a controlled atmosphere’. In everyday usage, the term ‘fresh’ is usually associated with food that has been recently harvested and not tinned, frozen or otherwise preserved. Thus, the different meaning of the term ‘fresh’ when applied to meat as compared to other food commodities may lead to confusion. There is no EU definition of ‘standard fresh meat’; nor are there any scientific, commercial or legislative basis for differentiating between ‘standard fresh meat’ and ‘wet‐aged meat’. Therefore, for the purposes of this Opinion, the following applies:
‘Standard fresh beef’ is beef that has been typically matured (usually in vacuum packs) for 14 days or less;
‘Wet‐aged beef’ is beef that has been vacuum packaged and aged for more than 14 days;
‘Dry‐aged beef’ is beef that has been aged aerobically in a dedicated chamber under specific conditions of temperature, RH and airflow for a specific period of time, usually at 1–4°C and a RH of 75–85% for 21–35 days;
‘Standard fresh pork’ is pork that has been matured for 4 days or less;
‘Wet‐aged pork’ is pork that has been vacuum packaged and aged for more than 4 days;
‘Standard fresh lamb’ is lamb that has been matured for 4 days or less; and
‘Wet‐aged lamb’ is lamb that has been vacuum packaged and aged for more than 4 days.
These are referred to as ‘the seven processes’ later in the text and are illustrated in Figure 1.
Figure 1.
Schematic overview of the processes (conditions/types of meat) considered in this opinion
This Scientific Opinion will initially describe the current practices in the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb as required by ToR1. In addition to a description of the different stages and processes used, data on the key parameters such as the surface pH, aw and temperature of the meat, that could influence the survival or growth of pathogenic bacteria and spoilage bacteria as well as moulds and mycotoxin production will be provided. It was agreed with the European Commission that only meat ageing in commercial settings such as meat plants, butcher shops and restaurants was covered while meat ageing performed at home in domestic settings was excluded, and non‐commercial practices such as dry‐ageing in a bag, which has been the subject of academic studies, were also outside of the scope of this mandate.
The microbiological hazards and spoilage bacteria that may be present and grow on the meat during the different ageing processes will be identified as required in ToR2. For dry‐aged beef, mycotoxin production by moulds will also be investigated but other toxic secondary metabolites will be excluded. Furthermore, the European Commission was interested in the behaviour of these bacteria and mycotoxin producing moulds further along the meat chain up to the end of the shelf‐life of minced meat or mechanically separated meat (MSM). This was also clarified with the Commission as Regulation (EC) No 853/2004 requires that minced meat must be prepared within no more than 6 days of slaughter or within no more than 15 days from the slaughter of the animals in the case of boned, vacuum‐packed beef and veal. The same legislation specifies that for the preparation of MSM, the raw material for deboning from an on‐site slaughterhouse must be no more than 7 days old, otherwise raw material for deboning must be no more than 5 days old. Thus, meat that is aged for more than 15 days is not currently allowed to be used for the production of minced meat or MSM. Regardless, for the purposes of this Opinion, specifically to determine if minced meat or MSM from meat that is aged for longer than 15 days has higher bacterial counts and therefore represents a higher risk to consumers, minced meat and MSM were considered to be a part of the meat chain.
ToR3 requires an assessment of the impact that dry‐ageing and wet‐ageing of meat, produced as per the current practices identified in ToR1, have on the load of public health‐relevant microbiological hazards and spoilage bacteria, when compared to standard fresh meat. Since the objective of this ToR was to establish if there was increased growth under the conditions used in dry‐ageing and wet‐ageing of beef as compared to standard fresh beef, only microorganisms capable of growth are considered. Thus, parasites and viruses were excluded and only bacteria and/or mycotoxin‐producing moulds capable of growth under the conditions of pH, aw and temperature on the surface of the meat were relevant for this assessment. The same principle applies to wet‐aged pork vs standard fresh pork and wet‐aged lamb vs standard fresh lamb. Moreover, the European Commission agreed that any differences in the increase of the relevant hazards on the meat surface as a result of the different ageing processes would be expressed in relative terms (e.g. as log10 increase in case of bacterial growth or relative mycotoxin accumulation) rather than a prediction in absolute terms (e.g. bacterial concentration or mycotoxin concentrations) on each type of meat (dry and wet‐aged vs standard fresh). While the growth of bacteria was quantitively assessed through the application of predictive models, the mould growth and mycotoxin production were assessed qualitatively from the evidence reported in the scientific literature.
There is also the possibility that dry or wet‐ageing would reduce bacterial numbers or prevent growth. However, this was not considered in the main assessment thus ensuring the modelling reflects a worst‐case scenario. The impact of this assumption was evaluated in the uncertainty assessment. The European Commission also agreed that the assessment in ToR3 covers the meat ageing stage only and thus started with the carcass, primal or sub‐primal entering the dry‐ageing chamber or, in the case of wet‐aged and standard fresh meat, immediately before vacuum packaging and ended when these ageing processes were completed.
ToR4 will investigate (using the predictive modelling approach developed for ToR3) if changing the conditions (time and temperature) used for dry‐aged and wet‐aged meat could achieve a similar or lower levels of microbial hazards and spoilage bacteria as compared to standard fresh meat. The endpoint for the assessment is the end of ageing. Storage post ageing was not considered due to the lack of information on the impact of trimming and the absence of reliable data on catering/consumer practices and on the surface temperature of the meat during chilled storage in restaurants and domestic refrigeration systems.
Having identified the process parameters that would minimise microbial growth on dry and wet‐aged meat, ToR5 will complement these by identifying control practices during the production and storage of these products, to further assure their microbial safety and quality. This ToR was interpreted to include prerequisite programme (PRP) activities and critical control points (CCPs) that may be part of the hazard analysis and critical control point (HACCP) plan.
The ToRs have been translated into assessment questions (AQs) as follows:
ToR1: AQ1: What are the practices and processes used by meat FBOs and restaurants in the EU for the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb? Specifically, what are the processing conditions (e.g. time, temperature and RH) and the associated intrinsic factors (e.g. pH and aw) of meat surface for each of these processes?
ToR2: AQ2: What are the relevant microbiological hazards and spoilage bacteria that occur and which of these can grow and/or produce toxins during the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb and on the subsequently stored product, including in minced meat or MSM prepared from the aged meat?
ToR3: AQ3: What is the increase in the relevant microorganisms (from AQ2) during the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb (from AQ1) and during subsequent storage, as compared to ‘standard fresh meat’?
ToR4: AQ4: What are the conditions for producing dry‐aged beef and wet‐aged beef, pork and lamb to ensure similar or lower counts/load/concentrations of pathogenic microorganisms, spoilage bacteria and, if relevant, mycotoxins at the end of shelf‐life as compared to standard fresh meat?
ToR5: AQ5: What additional control actions including prerequisite programme (GHP and GMP) and CCPs could be employed to minimise the prevalence and/or concentration of pathogenic and spoilage bacteria and mycotoxin formation (if relevant) on dry and wet‐aged meat?
1.3. Additional information
1.3.1. Approach to answer the ToRs
The approach to answer the ToRs was defined in advance and is described in the protocol (Annex A, available under the Supporting Information section on the online version of the scientific output). This covers both the problem formulation (i.e. what the assessment will address) and the methods that will be used. The problem formulation (‘what’) includes the clarification of the mandate (see further refined in Section 1.2) and consists of the following steps: (1) translation of the mandate into scientifically answerable AQs, (2) definition of the sub‐questions (SQs) for each AQ, and (3) the approach for undertaking the assessment. The planning of the methods for conducting the assessment (‘how’) consisted of: (1) specifying the evidence needs and the methods for answering each SQ, including uncertainty analysis and (2) the methods for integrating evidence across SQs and addressing of the remaining and overall uncertainty. The protocol development followed the draft framework for protocol development for EFSA's scientific assessments (EFSA, 2020).
The SQs related to each AQ are as follows:
SQ1 (for AQ1): What practices and processes are used by meat FBOs and restaurants in the EU to dry‐age beef?
SQ2 (for AQ1): What practices and processes are used by meat FBOs and restaurants in the EU to wet‐age beef, pork and lamb?
SQ3 (for AQ1): What is the shelf‐life of dry‐aged beef and wet‐aged beef, pork and lamb given by FBOs?
SQ4 (for AQ1): What are the resultant characteristics of the meat (surface temperature, pH, aw and concentrations of antimicrobials such as lactic acid)?
SQ4 (for AQ2): What pathogenic and spoilage bacteria occur and which of these can grow on dry‐aged beef and wet‐aged beef, pork and lamb?
SQ5 (for AQ2): Which moulds grow on dry‐aged beef and do these represent a hazard for human health (i.e. do they produce mycotoxins under the conditions used)?
SQ6 (for AQ2): What pathogenic and spoilage bacteria are relevant considering the potential use of dry‐aged beef and wet‐aged beef, pork and lamb for the production of minced meat and mechanically separated meat?
SQ7 (for AQ3): Which are the scenarios that represent current practices of dry‐ageing of beef, wet‐ageing of beef, pork and lamb and standard fresh meat preparation (time/temperature, pH, aw/relative humidity, with/out competition, etc.) including minced meat and MSM preparation from the aged meat?
SQ8 (for AQ3): Which are the relevant pathogenic bacteria, spoilage bacteria and/or mycotoxigenic moulds to evaluate (able to grow and/or produce toxin), relevant for meat type and type of ageing, worst case, available models and data?
SQ9 (for AQ3): What is the log10 change for each scenario and each microorganism and is there toxin produced by moulds?
SQ10 (for AQ4): What are the conditions for producing, handling (including trimming, cutting and packaging) and storing dry‐aged beef to ensure similar or lower counts/load/concentrations of pathogenic microorganisms, spoilage bacteria and, if relevant, mycotoxins at the end of shelf‐life as compared to standard fresh meat?
SQ11 (for AQ4): What are the conditions for producing, handling (including trimming, cutting, packaging) and storing wet‐aged beef, pork and lamb to ensure similar or lower counts/load/concentrations of pathogenic microorganisms, spoilage bacteria and, if relevant, mycotoxins at the end of shelf‐life as compared to standard fresh meat?
SQ12 (for AQ5): What control actions including GHPs and CCPs are currently used in meat plants and restaurants that produce dry‐aged beef and wet‐aged beef, pork and lamb and for the production and storage of ‘standard fresh meat’?
SQ13 (for AQ5): Based on the outcomes from ToR3, are additional control actions including specific GHP required for dry and wet‐aged meat to assure its food safety?
SQ14 (for AQ5): Based on the outcomes from ToR4 and a literature review what changes in current control practices formulated as GHP(s) and/or CCPs could be developed to achieve a similar or lower load of relevant microbiological hazards and spoilage bacteria on dry and wet‐aged meat as compared to standard fresh meat?
2. Data and methodologies
2.1. Data
Information, specifically temperature, RH, airflow and time, was collected about the processes and practices used by meat plants to: (1) dry‐aged beef, (2) wet‐aged beef; (3) wet‐aged pork, and (4) wet‐aged lamb. The same information was collected for dry‐ageing beef in butcher shops and restaurants. This was used to answer ToR1. Information was also collected about the pathogenic and spoilage bacteria that may contaminate dry‐aged beef and wet‐aged beef, pork and lamb. The potential production of mycotoxins by moulds growing on beef during dry‐ageing was also investigated. This information was used to answer ToR2 and to identify the target organisms for the modelling tasks to answer ToR3. Information was also obtained on the current control and hygiene practices to identify additional control actions and GHPs that would minimise the incidence of pathogenic and spoilage bacteria on both dry and wet‐aged meat, as required to answer ToR5.
Data were collected related to the meat surface, specifically pH, aw and temperature of beef at the start, during and at the end of the dry‐ageing process and the pH, aw and temperature of beef, pork and lamb at the start, during and at the end of the wet‐ageing process. The same data were collected for standard fresh meat for beef, pork and lamb. Data were also collected on the duration of each process. All this was used to define the scenarios to be modelled in ToR3 and ToR4.
2.2. Methodologies
2.2.1. ToRs 1 and 2
Information and data, as described in Section 2.1, were obtained by undertaking a comprehensive review of the scientific and grey literature. The search strings used to search the published literature for information and data to answer ToR1 and ToR2 are provided in Annex A (Protocol). This search was limited to scientific papers, book chapters, reviews and reports written in English and published between the years 2000 and 2021. Studies undertaken in laboratory, pilot and industry setting were included from both within and without the EU. Each publication was screened by title, abstract and then full text with publications either included or excluded based on the relevance of the information and data provided. The information gathered was initially assessed by the reviewer extracting the data and later by the members of the WG. As with all scientific studies there were multiple sources of uncertainty as different publications reported different practices and parameters for each of the ageing processes. The sources of uncertainty were identified, listed in Table B.1 (Appendix B) and analysed for their impact on the outcomes using a qualitative approach.
Table B.1.
Qualitative assessment included the ‘sources or location of the uncertainty’, a description of the ‘nature or cause of the uncertainty’ associated with that source and a description of the Impact of the uncertainty on the conclusions
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Defining ‘standard fresh meat’ versus ‘dry‐aged meat’ and ‘wet‐aged meat’ |
By definition, fresh meat is meat that has not undergone any preserving process other than chilling, freezing or quick‐freezing. The majority of fresh beef, pork and lamb is matured in vacuum packaging under chilled conditions. Wet‐aged meat is meat that has been vacuum packaged and stored under chilled conditions. There is no scientific, commercial or legislative basis for differentiating between the ‘standard fresh meat’ and ‘wet‐aged meat’. Thus for the purposes of this Opinion differentiation is based solely on the time in chilled storage. |
A misinterpretation or misunderstanding of what constitutes, ‘standard fresh meat’ versus ‘wet‐aged meat’ could result in an over or underestimation of the predicted growth of pathogenic and/or spoilage bacteria in ToR3, negate the ‘equivalence calculations’ in ToR4 and render any additional GHPs or CCPs in ToR5 redundant. |
|
Inaccurate, incorrect or incomplete information about current practices used by FBOs for dry‐ageing and wet‐ageing beef, pork and/or lamb in the scientific and grey literature. Information for wet‐aged pork and lamb was especially lacking and limited to 3 studies, none of which reported aw values. As none of these were observational studies, the reported values may not be representative. |
The scientific literature reports the time, temperature, RH and/or air flow used in experiments and these are selected based on the objective(s) of that study. Thus, these conditions may or may not reflect those used in commercial operations. Moreover, many of the scientific and technical reports are from countries outside of the EU and the conditions used may not be representative to practices within the EU. |
This lack of information on commercial practices reduces the certainty that the methods and conditions described in the response to ToR1 are an accurate description of current commercial practices in the EU. If parameters such as surface temperature, pH and aw are incorrect there is uncertainty about the outcomes of the predictive modelling in ToRs 3 and 4. |
|
The limited information about current practices provided by commercial FBOs involved in dry‐ageing beef or wet‐ageing beef, pork or lamb in response to the questionnaire. |
As this information was only provided by 8 FBOs and 2 FBO associations data were limited (for example, they do not routinely monitor and record surface temperature, pH and aw) and from a limited number of countries it may not be accurate or a reasonable representative of the entirety of European or regional practices. Moreover, for the dry‐ageing of beef, tradition and personal preference for specific organoleptic qualities in the final product are more important than strictly following a pre‐defined process. Thus, there may be differences in the processes used for different batches. |
An underestimation of the temperatures used for modelling could, for example, result in an underestimation of the growth rate of pathogens/spoilage bacteria. However this has been mitigated to some extent by using a range of temperatures. |
| Information on pathogenic and spoilage bacteria that may be on dry‐aged and wet‐aged meat and how these bacteria will behave in minced meat or MSM prepared the aged meat. | Although several studies have reported and reviewed the prevalence of pathogenic and spoilage bacteria in beef, pork and lamb relatively few have specifically studied dry or wet‐aged meat. | Specific pathogenic or spoilage bacteria may have been erroneously excluded from the answer to ToR2. However, this would have a minimal impact on the conclusions as the range of bacteria including those capable of growth under the conditions encountered were considered. |
| Information on the hygiene status of the carcass and the time between the end of carcass chilling and the preparation of meat cuts for dry and wet‐ageing. |
Few, if any, studies provide this information. Based on the available data, the pre‐ageing time (between slaughter and ageing) may vary considerably and counts at the start of ageing may be highly variable. |
The initial microbial counts at the commencement of dry or wet‐ageing may be higher or lower than reported in the few available studies cited in answering ToR2. However, as the assessment deals with log changes this will not impact on the outputs of the simulations using predictive models in ToRs 3 and 4. |
| The impact of competing microbiota on the predicted growth of L. monocytogenes, Y. enterocolitica and non‐proteolytic Clostridium spp. |
The effect of competing microorganisms was not included in the predictive models used for the main assessment, though the impact has been assessed in the framework of the effect of factors on the predictions focusing on the interaction between LAB and L. monocytogenes |
This uncertainty has been quantified in the uncertainty analysis and in general results in an overestimation (competing microbiota contributes to inhibit and/or stop pathogen growth). A possible exception is Pseudomonas spp. as some articles report the enhancement of L. monocytogenes growth in presence of pseudomonades. However, others have reported a suppression of L. monocytogenes maximum population density by Pseudomonas at low temperature (4°C, Buchanan and Bagi, 1999). |
| Determining if mycotoxins are produced by moulds during the dry‐ageing of beef | The specific conditions under which moulds, specifically Penicillium and Aspergillus spp. will not produce these toxins are based on a limited number (4) of studies, 3 of which focused on plant based foods. These conditions are not defined for meat and/or no specific studies have been undertaken during the dry‐ageing of beef. | It is not possible to definitively state whether or not mycotoxins are produced during the dry‐ageing of beef, even when this process is well described, although our current knowledge would suggest mycotoxin production is inhibited at temperatures below 3°C and at the aw values encountered. |
| Simulation of microbial growth ‐ lag phase | No lag phase was considered for any relevant microorganism assessed for the main assessment. | An overestimation of the potential growth can occur when no lag phase is included (conservative approach). However, if contamination is already present at slaughter, microorganisms can adapt to the environmental conditions during the pre‐ageing time. In this case, the impact of this uncertainty in ToR3 and ToR4 is considered to be limited/low. |
|
Simulation of microbial growth ‐ inactivation |
Only growth rate models were used in the assessment. No inactivation was considered for any relevant microorganism assessed for the main assessment, though the impact of this factor has been assessed in the framework of uncertainty analysis (Section 3.4.4.1). |
An overestimation of the growth can occur when no inactivation is included (conservative approach). This is especially relevant during dry‐ageing and the impact was evaluated and considered in the response to ToR4. |
| Simulation of microbial growth ‐ calibration factors for predictive models |
Observed growth rates from the collected experiments were estimated by fitting the primary growth model to data from challenge test (pathogens) or naturally contaminated means (spoilage). Depending on the design of the experiment and the available data the model fitting will be more or less robust. Predicted growth rates had to be obtained by assuming the input value for aw and for endogenous lactic acid concentration (if considered). For vacuum‐packaged meat, several scientific articles report no growth at all of L. monocytogenes. The growth model will represent the worst‐case scenario For dry‐ageing, the calibration factors have been obtained comparing growth data at high aw. The same behaviour was assumed for the entire range of aw occurring during dry‐ageing. |
Over‐ or underestimation of the Bf can over‐ or underestimate growth predictions in ToR3 and ToR4. |
| The additional actions required in the PRP and HACCP programmes to assure the food safety of dry‐aged beef. | There is a lack of information on the specific GHPs currently used in the meat ageing processes as these are rarely studied and seldom reported. | There may be additional GHPs or CCPs used in FBOs that are not reported and therefore unknown outside of those specific food businesses. Thus the response to ToR5 may be inadequate. |
Data were also sought from relevant stakeholders using questionnaires that were sent to six European associations in the field of meat processing and restaurant establishments for distribution to their members (The Liaison Centre for the Meat Processing Industry in the European Union (CLITRAVI), EuroCommerce Retail &Wholesale, Food Drink Europe, HOTREC Hospitality Europe, International Butchers Confederation (IBC), European Livestock and Meat Trades Union (UECBV)). Replies were received from two associations and eight FBOs. Data were also extracted from a previous Belgian survey (OPTIDRYBEEF) 1 and added to that collated by the WG.
An extended and a shorter version of the questionnaire distributed via different channels (EU survey platform and e‐mail) were used to increase the response rate (see version 1 in Appendix A.1 and version 2 in Appendix A.2). The data sought included data on the meat surface parameters (pH, aw and temperature) as well as bacterial counts including total viable count (TVC), total Enterobacteriaceae count (TEC), Salmonella, Shiga toxin‐producing Escherichia coli (STEC), Listeria monocytogenes, Staphylococcus aureus, Yersinia enterocolitica, and non‐proteolytic Clostridium spp. before, during and after ageing. Participants were asked about testing for mycotoxins in dry‐aged beef, good hygiene practices (GHP) used and storage conditions for each type of aged meat. Questions were also included to gather information about the preparation of minced meat and MSM from the different aged meats.
2.2.2. ToRs 3 and 4
Due to the lack of sufficient data on the growth or inactivation of potentially relevant microorganisms during different ageing practices or processes and the lack of a ‘standardised process’ for dry or wet‐ageing, a simulation approach was adopted for ToR3 and ToR4, and compared with the available data. The predicted growth (expressed as log10 increase) of relevant microorganisms were simulated by applying predictive microbiology models, using different ageing scenarios covering the main factors associated with the meat ageing processes.
Relevant scenarios to evaluate for the seven processes (i.e. standard fresh meat preparation for beef, pork and lamb, dry‐ageing of beef and wet‐ageing of beef, pork and lamb) were developed based on the responses to the questionnaire and literature data, and during discussions with the WG members. In the simulations, the scenarios were defined by the key intrinsic and extrinsic parameters varying over time for each of the processes.
The selection of relevant microorganisms for the different scenarios was based on the magnitude of their growth rate at different values of the main factors associated with meat ageing, i.e. temperature (both for dry and wet‐ageing) and aw (for dry‐ageing).
The predictive models for each relevant microorganism used for evaluating the ageing processes in ToRs 3 and 4 are shown in Table 1. The predictive growth models do not distinguish between microbial growth on different meat types but do take into account the different intrinsic or extrinsic factors characteristic of the meat types. In general, the predictive models were developed using data from experiments made using laboratory media and/or the validation of the predictive performance did not specifically include fresh meat. Furthermore, except for non‐proteolytic C. botulinum (for which anaerobic conditions are assumed), the predictive models do not consider the impact of vacuum‐packaging (wet‐ageing) as compared with aerobic conditions (dry‐ageing). Therefore, growth rate data available in ComBase Browser 2 and the scientific literature dealing with the growth rate of the relevant microorganisms on fresh meat stored under anaerobic (vacuum packaged) and under aerobic conditions were compared with the predictions provided by the models as summarised in Appendix C. The comparison enabled the calculation of the calibration factors that allowed for a correction of the predictions provided by the mathematical models used in the simulations for ToR3 and ToR4 as detailed in Table 1.
Table 1.
Overview of microorganisms and predictive models used to evaluate the log increase of pathogens and spoilage bacteria during different meat ageing processes in ToR3 and ToR4
| Group species | Meat ageing processes | Name | Description secondary model | Source | Calibration factor (b) |
|---|---|---|---|---|---|
| Pathogens | |||||
| Listeria monocytogenes | Wet‐ageing (beef, pork, lamb) | LM | CPM/Gamma (a) | (Mejlholm et al., 2010) | 1 |
| Dry‐ageing (beef) | 0.76 (0.74–0.78) | ||||
| Non‐proteolytic Clostridium botulinum | Wet‐ageing (beef, pork, lamb) | CB | CPM/Gamma (a) | (Koukou et al., 2021) | 1 |
| Yersinia enterocolitica | Wet‐ageing (pork) | YE | Square root model | based on data from (Gill and Reichel, 1989) | 1.10 |
| Non‐pathogens/spoilage bacteria | |||||
| Psychrotolerant LAB |
Wet‐ageing (beef, pork, lamb) Dry‐ageing (beef) |
LAB | CPM/Gamma (a) | (Mejlholm and Dalgaard, 2007) (JFP) or (Mejlholm and Dalgaard, 2013) | 1.89 (2.17–1.30) (c) |
| Pseudomonads | Dry‐ageing (beef) | PS | Square root model (equation for sub‐optimal temperature range) | (Neumeyer et al., 1997) | 1.80 |
LAB: lactic acid bacteria.
Cardinal Parameter Model employing the gamma concept.
Calibration factor equal to 1 means that the original model without correction was implemented. In parentheses the range of calibration factor values considered in the uncertainty analysis.
The same factor applies for aerobic conditions (i.e. when the model is used to simulate microbial interaction between LAB and L. monocytogenes during dry‐ageing). The few data available about LAB growth kinetics on meat under aerobic conditions indicates that the bias factor is within the same order of magnitude as for anaerobic (vacuum packaged) growth.
In ToR3, scenarios were developed and described using distributions for the key intrinsic and extrinsic parameters, i.e. temperature, pH, and aw, to capture the range of variation for each process. Modified Pert distributions were used to describe the parameters and were assumed to reflect variable average conditions in the EU (see Appendix D). To capture the variation of the mean values over time, scenarios were separated into a number of stages, each defined by the intrinsic and extrinsic factors at specified time points during ageing. After having defined and agreed a standard fresh meat preparation time for the different fresh meat species the minimum, maximum, median, 5th and 95th percentiles of the mean growth rates during the standard fresh meat preparation (as sampled at 0 and 14 or 4 days, respectively), were estimated. Between 0 and 14 days (or 4 days), a gradual (linear) change was implemented. To estimate the growth during meat ageing of the most relevant pathogens and spoilage bacteria, the estimated minimum, median, and maximum of the mean growth rates during the standard fresh meat preparation times were then used to predict and illustrate the log increase for different ageing times. Growth models were implemented in the statistical software R, version 4.1.1 (R Core Team, 2021), and in simulations the variable outcomes were estimated by calculating the variable growth rates and log increases over the ageing time by sampling the input temperature, pH and aw distributions at 0 and 14 days in 20,000 iterations.
Besides the overall scenarios covering the entire range of reasonably foreseeable conditions, specific realistic examples were developed for the dry‐ageing of beef. The change in the aw and pH of the meat surface and the temperature (recorded with dataloggers), during the ageing process of beef at different target temperature and RH conditions were collected from the scientific literature. Data were obtained from the authors or extracted from the figures with the WebPlotDigitizer tool (Rohatgi, 2021). The growth model for L. monocytogenes (Table 1) implemented in R was used to simulate growth (as log increase) under dynamic conditions of temperature, pH and aw.
In ToR4, the question of environmental parameters for meat ageing resulting in a similar log increase as compared to standard fresh meat preparation, was evaluated using data reflecting a wider range of conditions than in the scenarios in ToR3, to generate data appropriate for such a scenario analysis. The model was implemented in R. Parameters were kept constant during the standard fresh meat preparation or the meat ageing processes included in the quantitative evaluation. In contrast to ToR3, the duration of the ageing processes was described as variable to allow this factor to be evaluated. The parameters time and temperature were generated by taking a sequence of values between a minimum and maximum value in defined steps, and aw and pH were evaluated in three scenarios using the minimum, median and maximum parameter values (Table 2). This was undertaken to generate defined parameters values rather than evaluating a continuum. The scenario analyses were carried out for L. monocytogenes and LAB in wet‐aged beef, pork and lamb, as well as in dry‐aged beef. This was partly based on the outcome of ToR3 but also for simplicity, LAB were used in dry‐aged beef instead of Pseudomonas thus facilitating a comparison with standard fresh meat preparation, which is considered to be wet‐ageing for up to and including 14 days. Pseudomonas is not expected to grow well if at all, under the anaerobic conditions in vacuum packs during wet‐ageing.
Table 2.
The input parameter values for temperature (T) and duration, and the pH and aw values of the three scenarios for which predictions were generated to address ToR4. The predictions were used for scenario analysis to identify conditions resulting in assumed equivalent log10 increases of relevant pathogens and spoilage bacteria when simulating growth during wet and dry‐ageing of beef, and wet‐agieng of beef, pork and lamb
| Ageing | Stage | Duration (days) (a) | T (b) | Scenarios | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||||||||
| Type | Min | Max | Min | Max | pH | aw | pH | aw | pH | aw | |
| Wet beef | Standard | 14 | 14 | 0 | 5.0 | 5.1 | 0.97 | 5.5 | 0.98 | 5.9 | 0.99 |
| Ageing | 15 | 49 | 0 | 5.0 | 5.1 | 0.97 | 5.5 | 0.98 | 5.9 | 0.99 | |
| Dry beef | Standard | 14 | 14 | 0 | 5.0 | 5.5 | 0.92 | 5.85 | 0.955 | 6.2 | 0.99 |
| Ageing | 15 | 77 | 0 | 5.0 | 5.5 | 0.92 | 5.85 | 0.955 | 6.2 | 0.99 | |
| Wet pork | Standard | 4 | 4 | 0 | 5.0 | 5.4 | 0.95 | 5.85 | 0.97 | 6.3 | 0.99 |
| Ageing | 5 | 28 | 0 | 5.0 | 5.4 | 0.95 | 5.85 | 0.97 | 6.3 | 0.99 | |
| Wet lamb | Standard | 4 | 4 | 0 | 5.0 | 5.5 | 0.95 | 5.7 | 0.97 | 5.9 | 0.99 |
| Ageing | 5 | 21 | 0 | 5.0 | 5.5 | 0.95 | 5.7 | 0.97 | 5.9 | 0.99 | |
In steps of 1 day.
In steps of 1°C.
The parameters, models and scenarios used to answer ToR4 have associated uncertainties. Based on the outcome of the simulations in ToR3, the potential impact of important factors on the predictions, such as not including microbial competition and microbial inactivation, were quantified by estimating their effects on the overall log10 change. In this approach, the ageing processes were divided into two stages, the standard fresh meat preparation stage (up to 14 days) and the ageing stage. The input data, parameters and formulas used in the predictive models were implemented in R (mc2d package, 10,000 iterations) and the conditions of growth, competition and inactivation evaluated are shown in Table 3. The potential impact of competition was quantified using a Jameson effect approach (Jameson, 1962; Giménez and Dalgaard, 2004; Cornu et al., 2011; Leroi et al., 2015; Dalgaard and Mejlholm, 2019), by varying the initial concentrations of L. monocytogenes and LAB, and the maximum total population size (Nmax). The impact of potential inactivation of L. monocytogenes during conditions of ageing of beef that do not support growth was quantified using a Weibull primary model combined with a secondary lambda model (Coroller et al., 2012) or a model developed in this opinion based on decimal reduction times (D values) during dry‐ageing of beef reported by Van Damme et al. (2022). The predicted outcomes were compared with reported inactivation (Van Damme et al., 2022). The impact of drying rate was estimated deterministically, i.e. no iterations, using the data from the case studies (Section 3.6.4.3). The impact of trimming on the concentrations of bacteria was evaluated by using the available data in the scientific literature, although this was limited. The potential impact of neglecting lag phases was not quantified but was evaluated qualitatively together with other factors that could impact on the comparison between standard and ageing processes, based on the literature data.
Table 3.
A summary of input data used in the model for evaluating the effect of inactivation (dry‐aged beef) and competition (wet‐aged beef)
| Process | Parameter | Symbol (a) | Formula | Comment |
|---|---|---|---|---|
| Dry‐ageing | Temperature (°C) |
T1V T2V |
rpert, min = −0.6, mode = 2.0, max = 5.1, shape = 4 | |
| pH |
pH1V pH2V |
rpert, min = 5.5, mode = 5.7, max = 6.2, shape = 4 | ||
| aw | aw1V, aw2V | rpert, min = 0.88, mode = 0.96, max = 0.99, shape = 4 | ||
| Duration (days) |
d1V d2V |
14 rpert, min = 1, mode = 14, max = 42, shape = 4 |
||
| Inactivation rate (1/h) | log_red | (time/delta)p | Parameters depend on T, pH, aw (Coroller et al., 2012) | |
| Inactivation rate (1/h) | log_red_vd | rpert, min = 0.02/24, mode = 0.04/24, max = 0.07/24, shape = 4 | Parameters based on D‐values reported in Van Damme et al. (2022) | |
| Max log increase (log units) | Nmax | rpert, min = 7, mode = 9, max = 11, shape = 4 | ||
| Wet‐ageing | Temperature (°C) |
T1V T2V |
rpert, min = −0.6, mode = 2.0, max = 5.1, shape = 4 | |
| pH |
pH1V pH2V |
rpert, min = 5.1, mode = 5.5, max = 5.9, shape = 4 | ||
| aw | aw1V, aw2V | rpert, min = 0.97, mode = 0.98, max = 0.99, shape = 4 | ||
| Duration (days) |
d1V d2V |
14 rpert, min = 1, mode = 14, max = 35, shape = 4 |
||
|
Max log increase (log units) Max population density (log cfu/g) for competition |
Nmax | rpert, min = 4.0, mode = 7.23, max = 9.2, shape = 4 | ||
| Initial L. monocytogenes concentration (log10 CFU/g) for competition | LM0V | rnorm, mean = −1.40, sd = 0.55, rtrunc = TRUE, linf = −2, lsup = 1 | ||
| Initial concentration (log10 CFU/g) for competition | FF0V | 1, 2, 3 or 4 |
LAB: lactic acid bacteria.
Digit in symbol represents stage 1 is the first 14 days corresponding to standard fresh meat preparation, 2 is the additional ageing time.
2.2.3. ToR5
The control actions (GHPs and CCPs) currently used by FBOs that produce dry‐aged beef and wet‐aged beef, pork and lamb were described based on the information available in the scientific and grey literature. The search strings used to search the published literature (scientific papers, book chapters, reviews and reports written in English and published between the years 2000 and 2021) for information to answer ToR5 are provided in Annex A (Protocol). Each publication was screened as described for ToRs 1 and 2. Additional control activities, that achieve similar or lower bacterial counts for dry‐aged beef and wet‐aged beef, pork and lamb (as compared the standard fresh meat equivalent) were established based on the equivalence assessment in ToR4.
2.2.4. Uncertainty analysis
The uncertainty associated with the outcomes for all of the ToRs were assessed, including the identification and listing of ‘sources or location of the uncertainty’, a description of the ‘nature or cause of the uncertainty’ associated with that source and finally a description of the ‘impact of the uncertainty on the conclusions (e.g. over/underestimation)’. The results of this assessment are provided in Table B.1 in Appendix B.
An informal expert knowledge elicitation (EKE) was also undertaken, with the five members of the Aged Meat WG serving as the experts. To prepare for this EKE, individual judgements were elicited for the correctness of five statements, which were considered sufficient to illustrate the conclusions of the Opinion, one related to ToR2 (mycotoxins) and four related to the outcomes of the modelling undertaken in ToR4. Informed by the evidence collected in the draft opinion, including the uncertainty mentioned above (Table B.1), individual experts were asked to indicate how certain they were that the statements were correct. For expression of the uncertainty, they could use the standard ranges indicated in EFSA's approximate probability scale or any other probability range. Consensus was achieved during discussion in the WG meeting. The outcomes of this exercise are described in Appendix B.
3. Assessment
3.1. ToR1: current dry‐ageing and wet‐ageing practices
3.1.1. Dry‐ageing of beef
3.1.1.1. Introduction
Drying is the process of removing water by evaporation and, before the development of vacuum packaging in the 1960s, was the only method available to age beef (Savell, 2008). Although other meat species may also be dry‐aged, dry‐ageing is almost exclusively used for beef. In the past the whole beef carcasses were dry‐aged but in recent years sub‐primal cuts are more often used (Kim et al., 2018). Dry‐ageing is performed under continuously controlled conditions of temperature, RH and airflow that usually takes several weeks. During this process (shown in Figure 2), water moves to the surface of the carcass or primal and evaporates. This drying process along with enzymatic reactions in the meat results in enhanced flavour and the creation of new flavour compounds (Savell and Gehring, 2018). The increase in the savoury or beefy flavour during ageing is due to the release of compounds such as nucleotides, Maillard reaction‐related sugar fragments (e.g. glucose), lipid oxidation related products as well as volatile compounds such as n‐aldehydes (e.g. hexanal and pentanal) and ketones (Martins et al., 2000; Yaylayan et al., 2000). The complex interaction between sulfur‐containing amino acids, aspartic acid and glutamic acid, nucleotide compounds, and β‐histidyl dipeptides also contribute to the beefy flavour (Dashdorj et al., 2015). Degradation of glycogen releases the substrates responsible for the Maillard reaction while prolonged ageing (over 28 days) increases the volatile compounds responsible for the aroma and enhanced flavour (Martins et al., 2000; Yaylayan et al., 2000; Watanabe et al., 2015).
Figure 2.
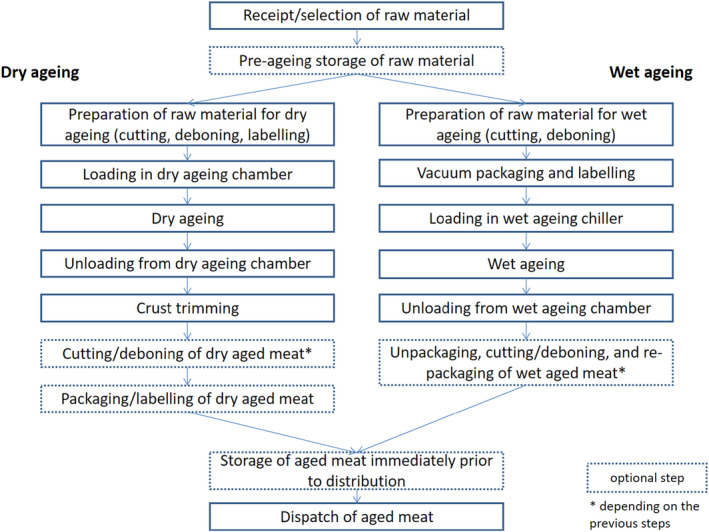
Generic process flow of dry and wet beef ageing
Dry‐aged beef is also more tender (Savell and Gehring, 2018). The tenderisation process occurs due to the post‐rigor degradation of cytoskeletal proteins, myofibrils, muscle fibers and connective tissue (Kristensen and Purslow, 2001; Koohmaraie and Geesink, 2006) by the calpain protease system (Hopkins and Thompson, 2002). The extent of tenderisation depends on the dry‐ageing conditions and the muscle characteristics including the bundle type, size/diameter, composition and extensibility of fibers, the rate of glycolysis and sarcomere length (Ertbjerg and Puolanne, 2017).
Post‐mortem proteolysis is influenced by the pH of the meat. Thus, the initial pH of the meat cuts affects tenderness and flavour formation. During the dry‐ageing process, the pH usually increases although a subsequent decrease has also been reported (Lautenschlaeger, 2012; Stenstrom et al., 2014). Several studies have reported that the final pH of dry‐aged meat is usually slightly higher than that of wet‐aged meat (Dikeman et al., 2013; Li et al., 2014) possibly due to the formation of nitrogenous compounds during the former (Obuz et al., 2014) and the slight acidification due to lactic acid growth under vacuum packaging in the later. However, other studies have reported similar pH values for standard fresh, dry and wet‐aged beef (Ahnstrom et al., 2006, DeGeer et al., 2009; Li et al., 2014; Dashdorj et al., 2016; Dashdorj et al., 2016), while Gudjonsdottir et al. (2015) found there was a correlation between the pH and growth of lactic acid bacteria, regardless of ageing process.
Dry‐ageing of beef (Figures 2 and 3) is considered to be superior, in terms of tenderness and flavour, to other ageing processes although the processes used are not standardised and often achieve inconsistent results (King et al., 1995; Sitz et al., 2006; Laster et al., 2008; Smith et al., 2008). Indeed, dry‐ageing beef is considered to be as much an art as a science‐based activity (Dashdorj et al., 2016).
Figure 3.

Dry‐ageing of beef. © Ana Allende
Conditions such as temperature, RH and airflow determine the characteristics of the final product (Terjung et al., 2021). Sensory trials reported by Kim et al. (Kim et al., 2016) found that dry‐ageing of beef at 3°C, 49% RH and an air flow of 0.2 m/s for 21 days was optimal for organoleptical quality. The same study reported that shear force (a measure of tenderness) is affected by storage temperature and meat dry‐aged at 3°C was more tender than at 1°C. Other authors agree that an RH below 85% achieves a better consumer evaluation (Kang et al., 2017). Lower air velocity may also result in a more tender dry‐aged beef product. Lee et al. (Lee et al., 2019) reported that dry‐ageing for 28 days at air velocities of 2.5 or 5 m/s reduced the shear force by 30 N (N) but at 0 m/s the shear force was reduced by 40 N. At least 2 studies have reported that using the same parameters for dry and wet‐ageing achieves beef products of equal tenderness (Oh et al., 2018; Kim et al., 2020), although it could be argued that the conditions used (85% RH and an airflow of 2–7 m/s) were not suitable for dry‐ageing. Dry‐aged beef has a beefy, buttery, nutty, and sweet flavour and may also have a pleasant savoury (umami) taste due to the high concentrations of glutamate. The dry‐ageing process is comparably costly as it is used on high‐quality cuts, incurs weight losses of 6–15% and trim losses of 3–4%. Moreover, as the beef is not usually packaged, there is a higher risk of open‐air contamination of the meat. This can be reduced by packaging the meat in moisture permeable bags which were first used approximately 15 years ago. Although beef dry‐aged in a bag is reported to have the same flavour as beef aged using unpackaged dry‐ageing this process is not used commercially (Ahnstrom et al., 2006). The different combinations of temperature, RH, airflow and duration used for dry‐ageing of beef in laboratory and/or pilot plant settings are provided in Table E.1 (Appendix E).
Table E.1.
The conditions and corresponding meat parameters reported for dry‐ageing beef in the scientific literature
| Conventionally dry‐aged beef | ||||||||
|---|---|---|---|---|---|---|---|---|
| Conditions | Surface parameters | |||||||
| T (°C)* | RH (%) | Airflow (m/s) | Pre‐ageing time (days) | Ageing time (days) | pH | aw | Surface T (°C) | References |
| 0 | 68–70 | Forced ventilation cell | 5 | 13, 36, 110, 170 and 290 | 5.69–6.00 | 0.965–0.953 | – | Smaldone et al. (2019) |
| −0.6 ± 1.8 | 78 ± 9.3 | – | 9 | 14, 21, 28 and 35 | – | – | – | Laster et al. (2008) |
| 0.5 ± 0.5 | 85 | 0.2–0.5 | 1 | 0, 7, 14, 21 and 28 | 5.6–5.8 | – | – | Kahraman and Gurbuz (2019) |
| 2 ± 1 | 75 ± 10 | 2.5 | – | 0, 7, 14, 21, 28, 35 | 5.7–5.9 | – | – | Oh et al. (2019b) |
| 1 | 85 | 0.5 | 5 | 30 | – | – | – | Kim et al. (2020) |
| 1 | 85 | 2–7 | – | 28 | – | – | – | Kim et al. (2019b) |
| 1 | 80–85 | 0.2–0.3 | 5 | 40 | – | – | – | Kim et al. (2019a) |
| 1 ± 1 | 85 ± 10 | 2–7 | 28 | – | – | – | Oh et al. (2018) | |
| 1 ± 0.5 | 80–85 | 0.5–1.5 | 12 | 20 and 40 | – | – | – | Kim et al. (2017a) |
| 1 | 78 | 1.5 | – | 17 | – | – | – | Kim et al. (2017b) |
| 1 | 75–85 | 5 ± 3 | – | 28 | – | – | – | Lee et al. (2017) |
| 1 | 73–76 | 0.2–0.5 | – | 21 | – | – | – | Kim et al. (2017a,b) |
| 1 | 70 | – | – | 14, 21, 28, 35, 42, and 49 | – | – | – | Lepper‐Blilie et al. (2016) |
| 1 ± 2 | 70–100 | – | – | 14 | – | – | – | Knudsen et al. (2011) |
| 1.6 | – | – | 2 | 13 | 5.6 | – | – | Stenstrom et al. (2014) |
| 1 ± 1 | 85 ± 2 | 0.5 ± 0.2 | – | 12 to 36 | 5.6–5.7 | – | – | Hulankova et al. (2018b) |
| 1 ± 2 | 83 ± 11 | – | 2 | 14, 21, 28 and 35 | – | – | – | Smith et al. (2008) |
| 2 ± 1 | 85 | 2 | – | 0, 20, 24, 40, and 50 | – | – | – | Utama et al. (2020) |
| 0–4 | 75 | 0 | – | 0, 14, 21, 28 | – | – | – | Oh et al. (2019a) |
| 1–3 | 75 | 2.5 | – | 0, 14, 21, 28 | – | – | – | Oh et al. (2019a) |
| 1–3 | 75 | 5 | – | 0, 14, 21, 28 | – | – | – | Oh et al. (2019a) |
| 1–4 | 80–90 | – | – | 3, 25, 40, 50 and 60 | – | – | – | Ryu et al. (2018) |
| 1–4 | 80–90 | – | – | 4, 11, 20, 30, 40, 50 and 60 | – | – | – | Iida et al. (2016) |
| 2 | 50 | 0.8 | – | 42 | 5.5 | 0.99 | – | Ribeiro et al. (2021a) |
| 2 | 85 | 1.5 | 2 | 0, 7 and 14 | 5.5–5.6 | – | – | Shi et al. (2020) |
| 2 ± 1 | 75 ± 2 | 2 ± 0.5 | 3 | 0, 7, 14, 21, 28, 35, or 42 | 5.5 |
0.99 (day 0) 0.97 (day 7) 0.93 (day 42) |
– | da Silva et al. (2019) |
| 2 | 65/75 | 2.5 | – | 20–60 | – | – | – | Ha et al. (2019) |
| 2 ± 1 | 75 ± 10 | 2.5 | – | 0, 7, 14, 21, 28 and − 35 | – | – | – | Oh et al. (2019b) |
| 2 | 78 | ˂ 0.2 | 7 | 28 | – | – | – | Berger et al. (2018) |
| 2 | – | – | 2 | 14 | – | – | – | Jiang et al. (2010) |
| 2.2 | 50 | – | – | 21 and 28 | 5.5 | – | – | DeGeer et al. (2009) |
| 2.5 ± 0.3 | 87 ± 2.6 | “Normal cooler conditions” | 11 | 21 | 5.7 | – | – | Ahnstrom et al. (2006) |
| 2.6 ± 0.4 | – | “Normal cooler conditions” | 11 | 14 | 5.5 | – | – | Ahnstrom et al. (2006) |
| 2.9 | 90 | 1.8–2.5 | – | 35 | 5.6 | – | – | Mikami et al. (2021) |
| 2.9 | 91 | – | 6 | 14 | 5.6 | – | – | Li et al. (2013) |
| 2.9 | – | – | 2 | 8 and 19 |
5.58–5.63 |
– | – | Li et al. (2014) |
| 3 | 49–55 | 0.2–0.5 | – | 21 | – | – | – | Kim et al. (2017a,b) |
| 3 | 80 | 0.25 | – | 28 | – | – | Tittor et al. (2011) | |
| 3.5 ± 1.5 | 75–100 | 0–0.6 | – | 14 | – | – | Knudsen et al. (2011) | |
| 4 | 75 | 2.5 | – | 28 | – | – | – | Kim et al. (2019b, 2020) |
| 4 ± 2 | – | – | – | 0, 7, 14, 21, 28, 42 and 63 | 5.5–6.8 | – | – | Lee et al. (2019) |
| 4 | 75 | 0, 2.5 and 5 | – | 14 and 28 | 5.6–6.0 | – | – | Lee et al. (2019) |
| 4 | 75 | 2.5 | – | 28 | 5.75 | – | – | Lee H.J. et al. (2018) |
| 4 | – | – | – | 7, 14, and 21 | 5.6–5.7 | – | – | Gudjonsdottir et al. (2015) |
| 4.0 ± 1.1 | 98.1 | 2 | 35 | – | – | Smith et al. (2014) | ||
| 8 ± 1 | 75 ± 2 | 2 ± 0.5 | – | 0, 7, 14, 21, 28, 35, and 42 | 5.5 | 0.95–0.88 | – | da Silva et al. (2019) |
The measured surface temperature when provided or when not provided the temperature setting in the chill room/chamber.
3.1.1.2. Temperature
Dry‐ageing must be performed at temperatures high enough to allow the enzymatic processes that are required to achieve tenderisation and flavour development but sufficiently low to inhibit the growth of pathogenic and spoilage bacteria and the development of off‐odours and off‐flavours. Dry‐ageing is therefore usually undertaken at 0–4°C, the same storage temperatures used for other meat products where controlling microbial growth is required. The reported studies have used a range of temperatures, between −0.6°C and 8°C (Table E.1). However, the higher temperatures (> 4°C) are only of academic interest and do not reflect the conditions used in commercial ageing practices. Specific studies of commercial processes are limited in the published literature. Gowda et al. (Gowda et al., 2022) conducted a cross‐sectional study in 15 Belgian companies producing dry‐aged beef and observed that the temperatures of the dry‐ageing chambers were set between –1°C and 3°C, whereas the actual recorded temperatures were as low as 0.0°C and on occasion reached as high as 5.9°C. In Brazil, many dry‐aged beef producers (18/37, 49%) reported using temperature settings during dry‐ageing of between 2 and 4°C (Rezende‐de‐Souza et al., 2021).
3.1.1.3. Relative humidity
Relative humidity (RH) is another important parameter that affects water evaporation rate, yield and microbial growth (Ribeiro et al., 2020). If the RH is too high the meat surface aw will promote the growth of bacteria and if too low it will cause excess product shrinkage and yield loss (Savell, 2008). Different RH values have been reported in experimental studies, varying between 49% and 100% (Table E.1). In the abovementioned cross‐sectional study in Belgium, the RH of the dry‐ageing chambers was set between 40% and 75% while the measured values ranged between 50% and 90% (Gowda et al., 2022). Rezende‐de‐Souza et al. (2021) reported a RH in ageing chambers of between 70% and 80%.
3.1.1.4. Airflow
Airflow is an important parameter as it affects the water evaporation rate from the meat (Lewicki, 2004). Faster air flow results in faster drying and a higher concentration of the flavour compounds after shorter drying times. Uniform drying is essential to minimise spoilage and the development of off‐odours and off‐flavours. Ensuring all surfaces are exposed to the chilled air is achieved using wire racks, perforated shelves, stands and/or hooks. Ultraviolet (UV) light may be used to decontaminate the air that is usually recirculated. Although the majority of studies do not report airflow (Ribeiro et al., 2020), those that have been reported are typically 0.2–2.5 m/s (Kim et al., 2017b; Berger et al., 2018; Hulankova et al., 2018a). Kim et al. (2016) investigated air flow velocities of 0.2 and 0.5 m/s at 1 and 3°C. Although there was no effect on yield or weight losses, the results suggested that dry‐ageing beef loins at 3°C with an air flow of 0.2 m/s and 49% RH was the best treatment for maximising the sensory attributes of the product.
3.1.1.5. Time
The duration of dry‐ageing has a major effect on the flavour, tenderness and juiciness of the end‐product. Determining the number of days of dry‐ageing is based more on personal preference rather than scientific studies, which are often contradictory. Dry‐ageing for 7 days is not sufficient, and it is generally agreed that a minimum of 14 days is required (Campbell et al., 2001). Although the time required to achieve the desired dry‐aged results typically ranges from 14 to 35 days (Ahnstrom et al., 2006; Savell, 2008; Berger et al., 2018; Kim et al., 2018; Hulankova et al., 2018a) there is no standard time and the dry‐ageing period varies considerably in commercial practice and in the scientific studies reported. In Belgian companies, a minimal duration of 3 or 4 weeks is used for dry‐ageing, and the maximum duration varies between 4 and 10 weeks (Gowda et al., 2022). In Brazil, the most common ageing times were between 3 and 8 weeks, though ageing for more than 8 weeks was also reported (15% of responses; (Rezende‐de‐Souza et al., 2021)). Campbell et al. (2001) and Savell (2008) reported that beef starts to show the desirable dry‐aged meat qualities as early as 14 days, while several studies have reported that 21 days are required for noticeable flavour development (Campbell et al., 2001; Smith et al., 2008; DeGeer et al., 2009; Li et al., 2013; Richardson et al., 2018). Thus, the required sensory attributes could be achieved in 3 weeks while minimising weight loss. In contrast, other academic studies have suggested longer periods of at least 40 days (Iida et al., 2016), 50–80 days (Perry, 2012) and 100–240 days (Dashdorj et al., 2016), while the US Meat Exporting Federation recommends 28–55 days. However, Iida et al. (2016) observed no difference in the dry‐aged flavour of beef aged from 30 days to 60 days.
3.1.1.6. Summary
There are different combinations of temperature, RH, airflow and time used in the dry‐ageing of beef reported in experimental studies. Temperatures range from −0.6°C to 8°C but most studies used 0°C to 4°C. RH ranged from 50% to 100% but the majority of studies used 70–85%. Airflow ranged from 0 to 5 m/s but most studies used an airflow of 0.5–2.5 m/s. The duration of dry‐ageing also ranged from 7 to 120 days with most dry‐ageing studies using a period of 14–35 days. The surface pH of the beef usually remained at 5.5–5.9, and the aw at 0.95–0.99 (Table E.1), although it may be as low as 0.81.
3.1.2. Wet‐ageing of beef, pork and lamb
3.1.2.1. Introduction
The vast majority of beef, pork and lamb is vacuum packaged and wet‐aged. The process of wet‐ageing is shown in Figure 2. Despite not achieving the unique and intense flavours associated with dry‐aged meat, wet‐ageing is more commonly practiced in the meat industry because it requires less time, lower investment and results in less loss as the resultant product requires less trimming and water retention is higher (Ahnstrom et al., 2006; Dashdorj et al., 2016). Moreover, wet‐aged meat is less expensive than dry‐aged meat for consumers (Campbell et al., 2001; Sitz et al., 2006; Khan et al., 2016). Carcasses are chilled for 24–48 h and thereafter cut into primals, and sub‐primals in the boning hall. The conditions and corresponding meat parameters reported in scientific studies for wet‐ageing beef, pork and lamb in the scientific literature are shown in Tables E.2 and E.3.
Table E.2.
The conditions and corresponding meat parameters reported for wet‐ageing (WA) beef in the scientific literature
| Surface parameters | Bag properties | Reference | |||||
|---|---|---|---|---|---|---|---|
| T (°C)* | Pre‐ageing time (days) | Ageing time (days) | pH | aw | Surface T (°C) | ||
| −0.6 ± 1.8 | 9 | 14, 21, 28 and 35 | – | – | – | – | Laster et al. (2008) |
| 0.5 ± 0.5 | 1 | 0, 7, 14, 21 and 28 | 5.6–5.7 | – | – | BB3050U vacuum bags | Kahraman and Gurbuz (2019) |
| 0–3 | – | 0 | 5.7 | 0.98–0.99a | – | BB2050U bags, CryoVac | McSharry et al. (2021) |
| 0–3 | – | 37 | 5.5 | 0.98–0.99a | – | BB2050U bags, CryoVac | McSharry et al. (2021) |
| 0–3 | – | 0 | 5.9 | 0.98–0.99a | – | BB2050U bags, CryoVac | McSharry et al. (2021) |
| 0–3 | – | 37 | 5.1 | 0.98–0.99a | – | BB2050U bags, CryoVac | McSharry et al. (2021) |
| 1 | 5 | 7 | – | – | – | – | Kim et al. (2020) |
| 2 ± 1 | 28 | Kim et al. (2019b) | |||||
| 1 | 5 | 21 | – | – | – | Cryovac barrier bags, OTR 3 to 6 cm3/m2 per 24 h | Kim et al. (2019b) |
| 2 ± 1 | – | 28 | – | – | – | oxygen‐impermeable nylon bags | Oh et al. (2018) |
| 1 ± 0.5 | 12 | 20 and 40 | – | – | – | – | Kim et al. (2017a) |
| 1 | – | 21 | – | – | – | Kim et al. (2017a,b) | |
| 1 | – | 37 | – | – | – | – | Sitz et al. (2006) |
| 1 | – | 7 and 21 | – | – | – | Not specified | Liveley et al. (2006) |
| 1.6 | 2 | 13 | 5.6 | – | – | – | Stenstrom et al. (2014) |
| – | – | – | – | ||||
| 1 ± 2 | 2 | 14, 21, 28 and 35 | – | – | – | – | Smith et al. (2008) |
| 2 | 42 | 5.5 | – | – | 3 mil STD barrier, Ultra | Ribeiro et al. (2021a) | |
| 2 | 2 | 0, 7 and 14 | 5.4–5.6 | – | – | DCS00‐FB‐E; PROMAX | Shi et al. (2020) |
| 2 | 20–60 | – | – | Ha et al. (2019) | |||
| 2 | 3 | 7 and 14 | 5.6–5.7 | – | – | – | Owczarek‐Fendor et al. (2014) |
| 2 | 2 | 14 | – | – | – | Jiang et al. (2010) | |
| 2 | 7–35 | 5.5–5.7 | – | – | Not specified | Lee et al. (2008) | |
| 2 | 2, 9, 16, and 23 | 5.4–5.6 | – | – | Combivac (O2 50cm3/m2 per day, CO2 150 cm3/m2 per day) | Smulders et al. (2006) | |
| 2.9 | 35 | 5.6 | – | – | – | Mikami et al. (2021) | |
| 2.9 | 2 | 8 | 5.6 | – | – | CryoVac® 10, BB6050 | Li et al. (2014) |
| 2.9 | 2 | 19 | 5.57 | – | – | CryoVac® 10, BB6050 | Li et al. (2014) |
| 2.9 | 6 | 14 | 5.6 | – | – | CryoVac® 10, BB6050 | Li et al. (2013) |
| 3 | 28 | – | – | – | Cryovac | Tittor et al. (2011) | |
| 3 | – | 1, 5, 10, 15, 23 and 31 | 5.6–5.9 ± 0.05 | – | – | Cryovac | Vásquez et al. (2009) |
| 3.0 ± 0.7 | 2 | 35 | – | – | – | – | Smith et al. (2014) |
| 4 | – | 28 | – | – | – | – | Lee et al. (2021) |
| 4 | – | 28 | – | – | – | HFV‐600 L, Hankook Fujee | Kim et al. (2020) |
| 4 | – | 28 | – | – | – | HFV‐600 L, Hankook Fujee | Lee et al. (2019) |
| 4 | – | 7 | 5.5–5.7 | – | – | – | Gudjonsdottir et al. (2015) |
| 4 ± 2 | 1 | 0, 7 and 14 | 5.7–6.0 | – | – | Polifilm® | Cardoso et al. (2012) |
| 2 or 7 | – | 21, 42 | – | – | – | HFV‐600 L, Sunkyung Co. | da Silva et al. (2021) |
| – | – | 3, 7 and 14 | 5.5 | – | – | – | Beriain et al. (2009) |
| 1 | – | 14, 21, 28, 35, 42, and 49 | – | – | – | – | Lepper‐Blilie et al. (2016) |
| 3.6 | – | 42 | 5.7–6.0 | 0.93–0.99 | −0.65°C after 7 days, ranged from −0.4°C to −0.7°C (14–21 days) and between −0.23°C to −0.53°C (28–42 days) |
BB3055X vacuum bags |
Reid et al. (2017) |
The measured surface temperature when provided or when not provided the temperature setting in the chill room/chamber.
Table E.3.
The conditions and corresponding meat parameters reported for wet‐ageing pork and lamb in the scientific literature
| Conditions | Surface parameters | Bag properties | Reference | ||||
|---|---|---|---|---|---|---|---|
| T (°C)* | Pre‐ageing time (days) | Ageing time (days) | pH | aw | Surface T (°C) | ||
| Wet‐aged pork | |||||||
| −1–2° | Kim et al. (2018) | ||||||
| 2 ± 0.27 | 30 h | 0, 7, 14, 21 and 28 | 5.4–6.3 | – | – | Bush Brothers (OTR 102 cm3/m2 per day at 23°C, 65% RH; moisture transmission 7.9 g/m2 per day at 37.7°C, 90% RH) | Holmer et al. (2009) |
| 4 | 1 | 0–15 | 5.7, (5.4–6.3) | – | – | Not specified | Richardson et al. (2018, USA) |
| Wet‐aged lamb | |||||||
| −1.5 ± 0.5 | – | 21 | 5.9 | – | – | Cryovac® A600 barrier bag | Zhang et al. (2021) |
| 2 ± 2 | – | 14 and 20 | – | – | – | Surlyn (97 cc/m2 per day at 23°C) | McKenna et al. (2003) |
| 5 ± 2 | 1 | 4 and 8 | 5.5–5.6 | – | – | “Oxygen barrier film” | Constantino et al. (2012) |
The measured surface temperature when provided or when not provided the temperature setting in the chill room/chamber.
OTR: O2 transmission rate.
*BB3055X vacuum bags: (17 cm3/m2 O2, 24 h at 23°C, 0% relative humidity; 17 cm3/m2 O2, 24 h at 23°C, 100% relative humidity; 50 cm3/m2 CO2, 24 h at 23°C, 0% relative humidity); Cryovac® BB3050U BAGS: O2 permeability 20 cm3/m2, 24 h, 1 bar (1 kPa) at 23°C and 0% relative humidity; and maximum CO2 permeability of 100 cm3/m2, 24 h, bar at 23 C and 0% relative humidity; HFV‐600 L, low‐density polyethylene/nylon bags: (O2 permeability of 2 mL/m2 per d at 0°C; 0.09 mm thickness; Sunkyung Co., Ltd., Seoul, Korea); Cryovac® A600 barrier bag, oxygen transmission rate 20–50 g/m2 per 24 h at 23°C, Sealed Air®, Auckland, New Zealand at −1.5 ± 0.5°C; HFV‐600L, Hankook Fujee Co., Ltd., Hwaseong, Korea with a low‐density polyethylene/nylon bag (oxygen permeability of 22.5 mL/m2 per 24 h atm at 60% relative humidity (RH)/25°C and water vapour permeability of 4.7 g/m2 per 24 h at 100% RH/25°C).
Meat cuts used include M. longissimus thoracis, M. longissimus lumborum, longissimus dorsi, Gluteus medius, butt, rumps (middle gluteal) tenderloin (boneless) sirloin typically 1–3 but up to 12 days post mortem.
3.1.2.2. Temperature
Wet‐ageing of beef, as reported in the scientific studies, may be as low as −0.6°C (Laster et al., 2008) or 0°C (Kahraman and Gurbuz, 2019; McSharry et al., 2021) but temperatures of up to 4°C have been reported (Table E.3). In reality, the temperature in the chilled storage most likely ranges from 0 to 4°C, depending on the efficiency of the system. Similar temperatures have been reported for pork and lamb (Table E.4) (McKenna et al., 2003; Holmer et al., 2009; Constantino et al., 2012; Zhang et al., 2021).
3.1.2.3. Time
The time reported for wet‐ageing of beef in experimental studies ranged from 7 to 60 days. In this Opinion wet‐ageing for less than 14 days is considered to be ‘standard fresh beef’. These were, for example, as low as 14 days (Laster et al., 2008) and 15 days (Vásquez et al., 2009) to 42 days (Reid et al., 2017) and 60 days (Ha et al., 2019). However, the majority of wet‐ageing times were between 21–35 days (Table E.2). In contrast, pork was wet‐aged for 1–28 days and lamb for 4–21 days (Table E.3).
3.1.2.4. Specific studies
McSharry et al. (2021) investigated the effect of current beef carcass chilling regimes (10°C for 10 h followed by 0°C with a low fan speed) vs four alternatives, ranging from −6°C to 0°C and wind speeds between 1.5 and 6 m/s on the microbiology of beef carcasses in a commercial beef plant. The temperature and RH in the chillers, the carcass core and surface temperature, pH, aw and carcass weight (drip) loss were recorded (McSharry et al., 2021). The initial surface pH of the beef carcasses ranged from 6.6 to 7.4 which decreased to between 5.5 and 5.7, while the initial aw of the carcass surfaces which initially ranged from 0.98 to 0.99, decreased to 0.95 to 0.99 after 48 h regardless of chilling regime applied (McSharry et al., 2021). These values may be considered a good proxy for the parameters for meat cuts at the start of ageing. The same authors published on the microbiology of beef carcass chilling through primal storage to retail steaks in two different plants and reported that the initial pH values of the primals of 5.5–5.7 or 5.1–5.9 were maintained during 16‐ and 37‐days storage, respectively, at 2°C. The aw values were also stable at 0.98–0.99 during primal storage in both plants.
Reid et al. (2017) investigated the microbiology of beef carcasses and primals during chilling and commercial storage. The average core, surface and ambient temperature of the primals during the 6‐week storage were reported. Immediately after vacuum packaging, the average core and surface temperature of the primals was 3.8°C and 3.6°C, respectively. Although set for 0°C, the temperature in the chill room ranged from 1°C to −2.25°C during primal storage. The core temperature decreased to −0.73°C after 1 week which stabilised at −0.6°C in weeks 2 and 3 and fluctuated between −0.23°C to −0.53°C during weeks 4 to 6, inclusive. The surface temperature decreased to −0.65°C after 1 week and fluctuated from −0.4°C to −0.7°C for weeks 2 and 3 and between −0.23°C to −0.53°C in weeks 4–6, inclusive. The mean surface pH values of the beef primals ranged from 5.7 to 6.0 over 6 weeks storage while surface aw values were stable at 0.93–0.99.
3.1.2.5. Summary
The temperatures reported for wet‐ageing beef, pork and lamb in experimental studies ranged from −0.6°C to 4°C. The RH in the chill rooms used for beef ranged from 49% to 91% but the majority of studies used 75–85%. Airflow in beef chillers ranged from 0.2 to 7 m/s and most studies reported ageing for 21–35 days. The surface pH of the beef, pork and lamb ranged from 5.1 to 5.9, 5.4 to 6.3 and 5.5 to 5.9, respectively. The aw values for beef included 0.96, 0.98 and 0.99.
3.1.3. Questionnaire data
The data received in response to the questionnaires was limited and is presented in Appendix A.3 (Table A.1 (dry‐aged beef) and Table A.2 (wet‐aged beef, pork and lamb)). There were 14 respondents and to pseudonymise the data these have been assigned numbers 1–14 with 1 and 2 being FBO Associations and 3–14 (inclusive) being individual FBOs. Respondents 1–9 and 14 provided data on dry‐ageing of beef while 1, 2, 4, 5 and 8–14 provided data on wet‐ageing of beef, pork and/or lamb. Data from a relevant Belgian survey was also added (Herman et al., 2016).
Table A.1.
Conditions reported for dry‐ageing beef in response to the questionnaire, provided by food business operators (FBO) or associations of FBOs
| Respondents | EU/MS | Time between slaughter and ageing (d) | Duration (days) | RH (%) | Specified T (°C) | Actual T (°C) | Specified meat T (°C) |
Min–max meat T (°C) |
Mean meat T (°C) | Start pH (b) | End pH (b) | Start aw | End aw |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EU | – | – | < 85 | 0–4 | – | – | – | – | – | – | – | – |
| EU | 1–5 | Usually between 21–50 but up to 70 days | 80 max | 0–2 | 0–2 | 0–2 | 0–2 |
5.80 max (core) |
– | – | – | ||
| 2 | AT | 3 | 21–35 | 72 (min. 62–76 max) | 1–4 | 1–4 | – | Start: 1–4, End: 1–4 | ‐ | 5.4–5.8 | – | – | – |
| ES | 2 | 21 | 2–3 | – | – | 2–3 | – | 5.8 max | 0.98 | 0.81 | |||
| 3 | ES | 60 | 80 | 2 | – | – | – | 5.4 | |||||
| 4 (a) | DE | 2 | 21–28 | 80 | 0.5–2 | – | – | −0.5 min; 4 max | – | 5.9–5.7 | 6.2 | – | – |
| 5 | BE | 1–2 | 21–28 | 80 (75–85) | 0–2 | – | – | Start: 4° During process: 0–2° | – | – | – | – | – |
| 6 | BE | 3 max | 42 max | 75–85 | 1 | ||||||||
| 7 (a) | DE | 2 to 7 | 21 min | 90, varies depending on temperature | 1–4 | 1–4 | 7 max upon receipt decreasing to 1–4 | – | – | – | – | – | – |
| 8 (a) | DE | 1 to 2 | 21 max | 70 | 2 | – | – | – | 2 ± 0.5 | 5.8 | – | – | – |
| 9 (a) | DE | 4 to 6 |
21, min 30 mean |
75 | 0.5 | – | – | – | < 1 | ‐ | – | – | – |
| 14 | FR | 1–3 | 28 | – | 0–4 | – | – | – | – | 5.7 at 24 h | – | – | – |
| Belgian survey | BE | 1–21 | 14–77 | Set 40–75 Read 46–84 Measured 39–95 | – |
Programmed −1–3 |
– | Read (display) T: 1–3.5 Measured T: −1.2°C‐6.6 | – |
5.4–5.7 (core) |
– | – | – |
Replies to the questionnaire provided by the hearing expert.
Surface unless otherwise stated.
Table A.2.
Conditions reported for wet‐ageing beef, pork and lamb in response to the questionnaire, provided by food business operators (FBO) or associations of FBOs
| Respondents | EU/MS | Time between slaughter and ageing (d) | Duration (d) |
Specified temperature (°C) |
Chill room temperature (°C) |
Minimum – maximum temperature (°C) |
Mean meat temperature (°C) |
Meat pH | Start pH | End pH |
|---|---|---|---|---|---|---|---|---|---|---|
| Beef | ||||||||||
| 1 | EU | – |
30–40 (longer when super chilled) |
– | −2–0 | – | – | Checked in the abattoir | – | – |
| EU | NP | 14, 28 or 42 | 0–5 | – | – | – | Core pH measured 18 & 24 h post‐mortem | – | – | |
| 2 | AT | 3 | 9 | 0–3 | 0–3 |
Start: 1–4 End: 0–3 |
– | Not checked | 5.4–5.8 | |
| 4 | ES | 2 | 40 | < 5, optimum 2 | – | 2–5 | – | Not checked | – | – |
| DE | 2 | – | 2 to 7 | – | 2 to 7 | – | Not answered | – | – | |
| 5 | BE | 1–2 | 28–42 | 0–2 | – | Start: 4, Later: 0–2 | – | Not checked | – | – |
| 8 (a) | DE | 1–2 | 49 max | 2 ± 0.5 | – | – | 1.5–2 | 5.8 | – | – |
| 9 (a) | DE | 4 to 6 | 35–42 | – | 1.5 | – | – | – | – | – |
| 10 | IE | – | Customer specific, typically 14–46 | 0–2 | – | < 4°C start, 2°C within 24 h, thereafter < 2°C | – | Checked pre‐boning | 5.7 | – |
| 11 (a) | DE | 2 | 14–21 | 0–4 | – | 0–4 | – | Not measured | – | – |
| 12 | BE | 2 | 7–14 | 2 | 2 ± 1 | – | – | – | – | – |
| 13 | BE | 2 | – | – | – | – | – | – | – | – |
| 14 | FR | 1–3 | 28 | 4 | ‐ | Not measured | – | Not measured | – | – |
| Belgian study | BE | 1–21 | 5 to 21 but usually 7–14 | – | – | – | – | – | – | – |
| Pork | ||||||||||
| 2 | AT | 3 | min 3 | 0–3 | room: 0–3°C |
Start: 1–4 End: 0–3 |
– | – |
5.4–5.8 |
– |
| 9 | DE | 4–6 | 14–18 | 1.5 | – | – | – | – | – | – |
| 13 | BE | 2 | 18 | 0–4 | – | – | – | Not routinely checked | – | – |
| 14 | FR | 0.5–1 | 6 | 4 | – | Not measured | Not measured | Not measured | – | – |
| Lamb | ||||||||||
| 2 | ES | 2 | 30 |
< 5 optimum 2 |
– | – | 2–5 | – | – | – |
| 10 | IE | – | Customer specific, typically 7–21 | 0–2 | – | – |
Start: 4°C Within 24 h: < 2°C After < 2°C |
– | – | – |
| 12 | BE | – | 70–77 | −1 to 2°C | 2°C ± 1°C | – | – | – | – | – |
– : not provided.
Replies to the questionnaire provided by the hearing expert.
3.1.3.1. Dry‐ageing beef
For dry‐aged beef the time between slaughter and dry‐ageing ranged from 1 to 21 days but mostly between 1 and 5 days when the meat was stored aerobically. The duration for dry‐ageing was 14–77 days but most dry‐ageing required 21–35 days. The RH ranged from 62% to 95% but was usually 75–85%. The specified and actual temperature in the chamber were 1‐4°C with the minimum and maximum meat temperatures being −1.2°C to 6.6°C and the mean temperatures ranging from 1.5°C to 2.5°C. The initial pH of the beef was 5.4–5.9 and the final pH (based on one respondent) was 6.2. The corresponding aw values were 0.98 at the start and 0.81 at the end of the process (based on one respondent only).
3.1.3.2. Wet‐ageing beef
The time between slaughter and wet‐ageing of beef ranged from 1 to 21 days but mostly between 1–2 days. The duration for wet‐ageing was 14–49 days. The specified temperature in the chillers was 0 to 7°C but mostly 0–4°C, while the actual measured temperature was −2 to 2°C. The minimum and maximum meat temperatures were 0–4°C and 0–3°C, respectively, and the mean meat temperatures were 1.5–2°C. The pH of the meat is generally not measured but one respondent reported 5.4–5.8.
3.1.3.3. Wet‐ageing pork
The time between slaughter and wet‐ageing of pork was 2–6 days and the duration of the process was 3–18 days. The specified temperature was 0–4°C, the chiller temperature was 0–3°C with a minimum and maximum meat temperature of 1°C and 4°C, respectively. There was no data available for the mean meat temperature. The pH of the meat at the start of the process was 5.4–5.8.
3.1.3.4. Wet‐ageing lamb
The time between slaughter and wet‐ageing of lamb was 2 days and the process required 7–77 days. The specified temperature was −1 to < 5°C and the actual temperature in the chilling room was 1–3°C. The mean temperature of the meat was 4–5°C at the start of the process and reduced to 2°C.
3.2. Comparison of the dry and wet‐aged beef data from the scientific literature and the questionnaire
The vast majority of experimental studies on dry‐ageing of beef reported in the scientific literature used temperatures of 0–4°C, RH of 75–85% with an airflow 0.2–2.5 m/s for 14–42 days. These resulted in surface pH values of 5.5–5.9 and a surface aw of 0.88–0.99. All 11 respondents to the questionnaire dry‐aged beef at temperatures within the same range (0–4°C) with a majority using a RH 75–85% and ageing was undertaken for 14–35 days. The majority reported initial pH values of 5.4–5.9, final pH 6.2 and one study reported a decrease in the aw from 0.98 to 0.81.
For wet‐aged beef, the temperatures used in the various experimental studies reported in the scientific literature ranged from −0.6 to 4°C for a duration of 14–42 days and resulted in surface pH and aw values of 5.1–5.9 and 0.93–0.99, respectively. These were similar to the values used by commercial meat processors which included temperatures of 0–5°C (although 1 of the respondents suggested they use 7.0°C) for 14–49 days and surface pH values of 5.4–5.8 (3/3, 100%). However, none of the respondents provided aw values.
3.3. Shelf‐life
3.3.1. Dry‐aged beef
Based on a face‐to‐face questionnaire in 15 dry‐aged beef producers in Belgium (Gowda et al., 2022), the median reported shelf life for trimmed steaks was 4 days for unpacked steaks (ranging between 2 and 10 days; 11 FBOs), 18 days for vacuum packed steaks (5–30 days; 11 FBOs) or 5 days for MAP (1 FBO).
The aw of 14 freshly trimmed dry‐aged beef steaks, originating from loins aged for 21–76 days by FBOs varied between 0.98 and 0.99 and had a mean pH of 5.73 ± 0.12 (Gowda et al., 2022). The aw of trimmed aged beef steaks seems to reduce with longer ageing times, but the effect seems only minor as all values remained above 0.98 for meat aged under commercial conditions (Herman et al., 2016). Similarly, da Silva et al. (2019) observed (under experimental conditions) that the aw after trimming remains stable for 21–35 days (0.99 or more) and reduces to 0.98 after 42 days of dry‐ageing at 2°C and 8°C and 75% RH. After storage of trimmed beef steak samples at 4°C and 7°C, aw values were similar, i.e. between 0.98 and 0.99 (aerobic for 3–10 days) and between 0.98 and 0.99 (vacuum packed for 10–21 days) (Herman et al., 2016). The pH of the steaks after storage varied between 5.1 and 6.2.
3.3.2. Wet‐aged beef, pork and lamb
The shelf‐life of wet‐aged beef, pork and lamb depends on the storage temperature, pH, packaging system, etc. (McKenna et al., 2003; Holmer et al., 2009; Clausen et al., 2009). Under aerobic conditions, wet‐aged beef, pork and lamb has a shelf‐life of 3–5 days when stored at 4°C, but this may be extended if the temperature is decreased or using vacuum packs to generate anaerobic conditions. If the storage temperature was above 3°C but less than 8°C, up until recently it was recommended that the shelf‐life of vacuum packaged meat should not exceed 10 days (FSA, 2017) to prevent growth and toxin production by non‐proteolytic C. botulinum. However, this was revised to 13 days by FSA in 2020 based on the recommendation of the Advisory Committee on the Microbiological Safety of Food (ACMSF, 2020). 3
3.4. ToR2: public health‐relevant microbiological hazards and spoilage bacteria in the process of prolonged dry‐ageing and wet‐ageing of meat
The bacteria commonly found on fresh meat belong to a range of different genera, including Achromobacter, Acinetobacter, Aeromonas, Alcaligenes, Alteromonas, Arthrobacter, Bacillus, Campylobacter, Carnobacterium, Citrobacter, Clostridium, Corynebacterium, Enterobacter, Escherichia, Flavobacterium, Hafnia, Klebsiella, Kluyvera, Kocuria, Kurthia, Lactobacillus, Lactococcus, Leuconostoc, Listeria, Microbacterium, Micrococcus, Moraxella, Paenibacillus, Pantoea, Proteus, Providencia, Pseudomonas, Shewanella, Staphylococcus, Streptococcus, Vibrio, Weissella and Yersinia (Nychas et al., 2007). As previously stated, only microbiological hazards and spoilage bacteria capable of growth and/or mycotoxin production are considered relevant for ToR2. The potential microbial hazards therefore include pathogenic bacteria and moulds producing mycotoxins. Bacteria are also primarily responsible for the spoilage of dry and wet‐aged meat. The bacterial counts and spoilage bacteria reported in the scientific literature for dry‐aged beef as well as wet‐aged beef, pork and lamb are provided in Table 5.
Table 5.
The main spoilage defects and causal bacteria (adapted from Nychas et al., 2007)
| Defect | Meat product | Causal bacteria |
|---|---|---|
| Slime | Fresh meat | Pseudomonads, lactic acid bacteria (former Lactobacillus spp.), Enterococci, Weissella spp. and Brochothrix spp. |
| Hydrogen peroxide greening | Aerobically stored fresh meat | Weissella spp., Leuconostoc spp., Enterococci and Lactobacilli |
| Hydrogen sulfide greening | Vacuum‐packed fresh meat | Shewanella spp. and Clostridium spp. |
| Sulfide odour | Vacuum‐packed fresh meat | Clostridium spp. and Hafnia spp. |
| Cheesy or dairy odour | Vacuum‐packed fresh meat | Brochothrix thermosphacta |
| Bone taint | Carcasses | Clostridium spp. and Enterococci |
| Souring | Vacuum‐packed meats | Lactic acid bacteria, Enterococci, Micrococci, Bacilli and Clostridium spp. |
Interesting there are several studies that suggest dry‐ageing may reduce the prevalence and mean concentrations of specific bacteria on the meat. A study at the University of Wisconsin Center for Meat Process Validation, for example, reported that generic E. coli, coliforms and Enterobacteriaceae detected on 69% (3.7 CFU/cm2), 84% (5.8 CFU/cm2) and 93% (7.3 CFU/cm2) respectively, of beef carcasses sampled before dry‐ageing, decreased to 8% (0.17 CFU/cm2), 17% (0.23 CFU/cm2) and 37% (4.9 CFU/cm2) respectively after storage under dry‐ageing conditions for 6 days (Anon, 2006).
3.4.1. Yeasts, moulds and mycotoxins
Several fungal genera have been detected on dry‐aged beef samples, of which D. udenii (yeast), Mucor sp. PG272, Penicillium polonicum, and Penicillium bialowiezens were the most abundant species (Capouya et al., 2020). Thamnidium spp., Pilaira anomala and Debaryomyces hansenii, may have a positive influence on the ageing process by releasing proteases that break down myofibrils and collagenolytic enzymes that produce flavour compounds (Dashdorj et al., 2016; Lee et al., 2019; Oh et al., 2019a,b). In contrast, some moulds may produce mycotoxins, a diverse group of secondary metabolites produced by at least 220 fungal species, with several producing more than one type (Bennett and Klich, 2003; Duraković and Duraković, 2003; Pleadin et al., 2019a,b). These substances are of public health concern and are possible human carcinogens (IARC, 2002; IARC, 2012; Varga et al., 2015; Ostry et al., 2017). The most potent mycotoxins include aflatoxins (AFs), ochratoxins (OTs), fumonisins (FBs), zearalenone (ZEA) or trichothecenes (Asefa et al., 2011; Ismaiel and Papenbrock, 2015; Battilani et al., 2020; Pócsi et al., 2020).
Several studies have investigated the conditions under which moulds grow and produce mycotoxins. Moulds can grow and produce mycotoxins at temperatures between 5–40°C at a pH of 4–4.5 and aw <0.93 (Hamad et al., 2022). However, these are not conditions encountered during the dry‐ageing of beef where the temperature is < 4–5°C and the aw may decrease from approximately 0.99 to as low as 0.80.
A minority of moulds, including Penicillium spp., Aspergillus spp., Cladosporium spp., Thamnidium spp., Rhizopus spp., Mucor spp., Aurobasidium spp., Chrysosporium spp. and Helicostylum spp. are capable of growth at temperatures as low as −5°C (Campano et al., 1985; Gill et al., 1981; Gill and Lowry, 1982; Lowry and Gill, 1984). Of these, Penicillium spp. and Aspergillus spp. may produce mycotoxins such as ochratoxin A (OTA), sterigmatocystin (STC), cyclopiazonic acid (CPA) and citrinin (CIT) on cured and/or dried meat products (e.g. dry‐cured ham, fermented sausages, etc.) during drying/ripening which occurs at temperatures of 10–15°C. (Iacumin et al., 2009; Asefa et al., 2011; Rodriguez et al., 2012; Rodriguez et al., 2015; Zadravec et al., 2019), conditions never encountered during the dry‐ageing of beef.
Mycotoxins can be produced, all‐be‐it at very low concentrations and very slowly by Penicillium spp. at temperatures as low as 0–1°C on fruits, corn and other crops (Table 4) but there are no reports of this occurring in meat.
Table 4.
A summary of mycotoxin production by Penicillium spp. at chill temperatures
| Organism | Mycotoxin | Food | Mycotoxin production at temperature | Reference |
|---|---|---|---|---|
| Penicillium expansum | Patulin | Fruits | 0°C | Buchanan et al. (1974) |
| Penicillium spp. | Penicillic acid | Various agricultural commodities (corn, white rice, barley...) | 1°C | Ciegler and Kurtzman (1970) |
| Penicillium martensii | Penicillic acid | Corn | ≤ 1°C | Kurtzman and Ciegler (1970) |
| Penicillium expansum | Patulin | Apples | 0°C | Sommer et al. (1974) |
The Meat and Livestock Australia (MLA) review published in 2019 concluded that Penicillium spp. and Aspergillus spp. are not capable to produce mycotoxins on dry‐aged meat at temperatures between −0.5°C and 3°C (MLA, 2019). This conclusion was based on 4 key references, 3 of which describe the production of mycotoxins in food (ICMSF, 1996; Hocking and Pitt, 2003; Pitt and Hocking, 2009) and conclude that of 3 genera of concern for human health, specifically, Penicillium, Aspergillus and Fusarium, only Penicillium and Aspergillus spp. are found on meat and neither are capable of producing mycotoxins at temperatures between −0.5°C and 3°C.
The fourth publication was the CSIRO report published in 2018 (Olivier, 2018), which stated that there was no evidence that moulds found on red meat are capable of producing mycotoxins at temperatures between −0.5°C and 3°C and a RH of 75–85%. In addition to the low temperature, mycotoxin production would be further inhibited by the low aw in dry‐aged beef (Olivier, 2018).
PrimeSafe (Victoria, Australia) recommend an air temperature in the dry‐ageing chamber of 1–3°C if the dry‐ageing process takes less than 14 days and −0.5°C to 1.0°C if > 14 days but less than 35 days, with a RH of 75–85% and an air velocity of 0.2–0.5 m/s.
3.4.2. Spoilage bacteria
Fresh meat is highly perishable as the pH, aw and high nutrient content support bacterial growth (Woraprayote et al., 2016). Meat spoilage defects and associated bacteria are shown in Table 5.
The main spoilage bacteria of aerobically stored meat are Pseudomonas spp., Enterobacteriaceae (e.g. Hafnia alvei, Serratia liquefaciens and Pantoea agglomerans), LAB and Brochothrix thermosphacta, all of which are capable of growth at refrigeration temperatures in the presence of oxygen (Nychas et al., 2007; Wang et al., 2017). However, LAB and B. thermosphacta are not major contributors to the spoilage of meat when stored aerobically (Reid et al., 2017). Moreover, LAB may be supressed during the dry‐ageing process (Mikami et al., 2021).
Under anaerobic conditions (e.g. in vacuum packs) Enterobacteriaceae, LAB and B. thermosphacta (all facultative anaerobes) will spoil meat as will psychrophilic and psychrotrophic Clostridium spp. (Ercolini et al., 2006; Hungaro et al., 2016). Yeasts and moulds may also cause meat spoilage, especially at the lower aw conditions found in dry‐aged beef (Capouya et al., 2020). During the wet‐ageing of beef, pork and lamb, when the primals are vacuum packaged and therefore under anaerobic conditions, LAB and to a lesser extent Br. thermosphacta become the dominant spoilage organisms (Russo et al., 2006; Stanborough et al., 2017). It is therefore expected that LAB counts would be higher on wet‐aged as compared to dry‐aged beef and this has been reported by several authors (Parrish et al., 1991; Li et al., 2013; Berger et al., 2018). In contrast, Campbell et al. (2001) obtained higher LAB counts on dry‐ageing beef when compared to wet‐ageing after 14 days.
Compared to other spoilage bacterial groups, psychrotrophic species within the former genus of Lactobacillus (currently named Lactilactobacillus sakei, Lactilactobacillus curvatus, Levilactobacillus brevis etc. Zheng et al., 2020) and other LAB (such as Leuconostoc spp., Weissella spp. and Carnobacterium spp.) are considered strong competitors under reduced oxygen availability and are frequently reported as the predominant spoilage microbiota under vacuum and modified atmosphere packaging (Doulgeraki et al., 2012). The psychrotrophic Br. thermosphacta can be an important proportion of the spoilage microbiota and occasionally become the dominant microorganism (Borch et al., 1996; Doulgeraki et al., 2012). Generally, the growth of Br. thermosphacta is likely to be similar to or faster than LAB in the presence of oxygen (e.g. in O2 containing MAP or in VP that uses a film with low barrier properties) but is more sensitive to carbon dioxide (CO2) as compared to LAB (Devlieghere and Debevere, 2000; Mejlholm and Dalgaard, 2013). In vacuum packaged wet‐aged meat, the composition of the gaseous phase changes during the storage. Residual oxygen decreases while carbon dioxide increases, which gradually becomes a selective factor favouring CO2‐tolerant lactic acid bacteria (Borch et al., 1996). In meat stored under anaerobic chill conditions, it has also been reported that Br. thermosphacta on meat does not compete well against LAB (Sakala et al., 2002; Russo et al., 2006; Doulgeraki et al., 2012; McSharry et al., 2021).
TVCs are often used as indicators to monitor microbial shelf‐life with the end of shelf‐life reached when the counts reach a concentration of 5–7 log10 CFU/g or cm2. Several studies have reported TVC, LAB as well as yeast and mould counts on dry‐aged beef cuts after trimming. The TVC ranged from approximately 2–9 log10 CFU/g or cm2, the LAB counts from approximately 5–7 log10 CFU/g or cm2 while yeasts and moulds ranged from not detected to 7 log10 CFU/g or cm2 (Table 6).
Table 6.
TVC, LAB, and yeasts and mould counts on dry‐aged beef and wet‐aged beef, pork and lamb
|
Temperature (°C) Time (days) in brackets |
Microbial counts (log10 cfu/g or cm2) | Reference | ||
|---|---|---|---|---|
| TVC | LAB | Yeasts (Y) and moulds (M) | ||
| Dry‐aged beef | ||||
| 1–4 (3) | 2–2.5 | 0 | 0 | Ryu et al. (2018) |
| 1–4 (25) | 4–4.2 | 3.6–4.3 | 4.5–5.8 | Ryu et al. (2018) |
| 2 (21) | 3.4 | 2.4 | < 2.0 | da Silva et al. (2021) |
| 2 (42) | 2.8 | 1.4 | – | Ribeiro et al. (2021b) |
| 2.2 (21–28) | 3.5 | 1.3 | ND | DeGeer et al. (2009) |
| 2.2 (21–28) | 2.9 | 1.1 | −0.66 | DeGeer et al. (2009) |
| 2.9 (35) | 4.6 | < 3.0 | – | Mikami et al. (2021) |
| 2.9 (10) |
5.2 (fat) 6.4 (lean) |
0.98 (fat) 2.8 (lean) |
Y: 2.5 (fat); 3.7 (lean) M: 0.16 (fat); 0.01 (lean) |
Li et al. (2014) |
| 2.9 (21) | 6.9 (fat); 8.8 (lean) | 2.3 (fat); 3.2 (lean) |
Y: 4.2 (fat); 5.7 (lean) M: 0.3 (fat); 0.7 (lean) |
Li et al. (2014) |
| 4 (28) | 4.3 | 1.8 | Y: 2.6, M: 2.9 | Lee H.J. et al. (2018) |
| 4 (14) | 5.8 | 3.8 | Y: 5.6, M: 5.4 | Gudjonsdottir et al. (2015) |
| 4 (21) | 6.3 | 3.6 | Y:5.9, M:5.8 | Gudjonsdottir et al. (2015) |
| 2.2 (21–28) | 5.5 | 1.3 | 0.4 | DeGeer et al. (2009) |
| 2.2 (21–28) | 2.6 | 1.4 | ND | DeGeer et al. (2009) |
| 2.9 (10) | 4.7 (fat); 5.3 (lean) | 1.06 (fat); 2.01 (lean) | Y: 2.3 (fat); 2.7 (lean) | Li et al. (2014) |
| 2.9 (21) | 6.2 (fat); 6.6 (lean) | 2.6 (fat); 4.4 (lean) |
Y: 3.9 (fat); 4.9 (lean) M: 0.3 (fat); 0.11 (lean) |
Li et al. (2014) |
| Wet‐aged beef | ||||
| 2 (21) | 6.3 | 6.5 | < 2.0 | da Silva et al. (2021) |
| 2(35) | 2.4–6.7 | 1.8–6.2 | – | Reid et al. (2017) |
| 2 (42) | 3.5 | 2.9 | – | Ribeiro et al. (2021b) |
| 2.9 (10) |
4.44 (fat) 3.28 (lean) |
2.61 (fat) 2.27 (lean) |
Y: 1.11 (fat) 0.37 (lean) M: 0.21 (fat) 0.24 (lean) |
Li et al. (2014) |
| 2.9 (21) | 5.76 (fat); 5.87 (lean) | 4.13 (Fat); 5.34 (lean) |
Y: 0.67 (fat); 0.51 (lean) |
Li et al. (2014) |
| 2.9 (21) | 2.4 | 1.7 |
Y: 0.01, M: 0.01 |
Li et al. (2013) |
| 3 (10) | – | – | – | Vásquez et al. (2009) |
| 3 (15) | – | – | – | Vásquez et al. (2009) |
| 3 (23) | – | – | – | Vásquez et al. (2009) |
| 3 (31) | 6.15 | – | – | Vásquez et al. (2009) |
| (3 or 10) | – | 1.54 | – | Vieira et al. (2009) |
| 4 (14) | 2.65 | 5.26 |
Y: 3.52, M: 2.74 |
Gudjonsdottir et al. (2015) |
| 4 (21) | 2.95 | 6.11 |
Y: 3.48, M: 3.00 |
Gudjonsdottir et al. (2015) |
| 4°C (2–21) | 5.5 ± 0.12 | 5.5 ± 0.12 | – | Bhattacharjee et al. (2011) |
| Wet‐aged pork | ||||
| 2 (7) | 1.1–1.2 | – | – | Holmer et al. (2009) |
| 2 (14) | 1.8–2.7 | – | – | Holmer et al. (2009) |
| 2 (21) | 2.5–4.5 | – | – | Holmer et al. (2009) |
| 2 (28) | 3.3–6.4 | – | – | Holmer et al. (2009) |
| 2 (28) + 4.5 (1–3) | 1–2.5 | – | – | Holmer et al. (2009) |
| Wet‐aged lamb | ||||
| −1.5 ± 0.5 (21) | 5.2 | 2.6 | Y: 2.4, M: < 1 | Zhang et al. (2021) |
| 2 (14) | 1.2 | – | – | McKenna et al. (2003) |
| 2 (20) | 3.5 | – | – | McKenna et al. (2003) |
| 5 ± 2 (5) | 2.42 | – | – | Constantino et al. (2012) |
| 5 ± 2 (9) | 3.01 | – | – | Constantino et al. (2012) |
Gowda et al. (2022) provide TVC data for beef steaks made from dry‐aged meat, which ranged from < 1 to 7.4 log10 CFU/cm2. Significantly lower numbers of psycrotrophic TVC, Pseudomonads and Br. thermosphacta were found on steaks compared to the corresponding loins before trimming. However, they detected Enterobacteriaceae more frequently (40%) with higher maximum counts (7.4 log10 CFU/cm2) on trimmed steaks as compared to the untrimmed loin crust (31%; 4.3 log10 CFU/cm2), suggesting cross‐contamination during trimming had occurred. In addition, Listeria spp. were detected on 10% (3/30) of steaks, but not on any of the 13 loin surfaces sampled. Herman et al. (2016) reported TVC, Pseudomonas spp., Br. thermosphacta and LAB counts on aerobically stored dry‐aged beef of 4.7, 4.8, 3.0, and 3.9 log10 CFU/cm2 after 3 days at 4°C. The corresponding counts on vacuum packaged produce were 7.6, 5.5, 4.9 and 7.0 log10 CFU/cm2 after 10 days at the same temperature.
Even when correctly chilled, wet‐aged beef, pork and lamb may be subject to blown pack spoilage (BPS), resulting in the production of gas (mainly carbon dioxide but also small amounts of hydrogen sulfide), causing severe pack distension/failure, offensive odours and a metallic sheen on the meat (Bolton, 2015). BPS is caused by psychrophilic Clostridium estertheticum and psychrotolerant Clostridium gasigenes. Other Clostridium spp. such as Clostridium algidicarnis, Clostridium frigoris, Clostridium bowmanii, Clostridium frigidicarmis and Clostridium ruminantium may also cause spoilage without gas production (Broda et al., 1999, Broda et al., 2000; Adam et al., 2010; Cavill et al., 2011).
3.4.3. Bacterial pathogens on meat
Beef, pork and lamb carcasses may be contaminated with pathogens from the hide/fleece/skin and digestive tracts/faeces of the animals and from the slaughter and processing environment. These include STEC, Salmonella spp., Campylobacter spp., enteropathogenic Yersinia spp., L. monocytogenes, Clostridium spp. and Staphylococcus aureus. Of these, L. monocytogenes, Yersinia spp. and non‐proteolytic C. botulinum are psychrotrophic pathogens and may grow at the chilled conditions encountered during the ageing of meat.
3.4.3.1. Shiga toxin‐producing E. coli
Phillips et al. (Phillips et al., 2012) reported that 0.1% of beef primals for dry‐ageing were contaminated with STEC. These bacteria were not detected on dry‐aged beef in a study by Ribeiro et al. (2021b) but were present on wet‐aged products. Furthermore, Ryu et al. (2018) failed to detect Escherichia coli O157:H7 during the 3rd, 25th, 40th, 50th, and 60th day of dry‐ageing beef originated from eight carcasses. Tittor et al. (2011) examined the efficacy of dry and wet‐ageing of beef on E. coli O157:H7 on lean and fat beef tissues. After inoculation of the beef samples to approximately 6.0 log10 CFU/cm2, one set was spray chilled with water at 10°C for 15 min and then for 1 min every 17 min for 17 h before vacuum packaging and storage for 28 days. Dry‐ageing was performed at 3°C, RH 80% with an air velocity of 0.25 m/s for a similar time. The final E. coli O157:H7 count on the dry and wet‐aged beef was 1.0 and 3.7 log10 CFU/cm2, respectively. The authors concluded that dry and wet‐ageing could be considered as pathogen control points in the beef chain. Ingham et al. (2010) observed a mean decrease of 0.8 log10 of E. coli O157:H7 after 6 days of dry‐ageing at 4°C and 92% RH. Van Damme et al. (2022) observed a decrease of E. coli O157:H7 of more than 3 log10 after 42 days of dry‐ageing at 2–6°C and 75–85% RH under all conditions, although for certain replicates this 3‐log10 reduction was already observed as early as 14 days.
3.4.3.2. Salmonella spp.
Ribeiro et al. (2021b) investigated differences in microbiological safety and quality between beef (Longissimus dorsi) that was wet‐aged and dry‐aged for 30 days and reported that Salmonella spp. was not isolated from either dry or wet‐aged beef samples. Moreover, Ryu et al. (2018) failed to detect Salmonella serovars during the 3rd, 25th, 40th, 50th, and 60th day of dry‐ageing beef originated from eight carcasses. Knudsen et al. (2011) investigated the survival of different Salmonella strains on beef portions during 14 days of cooling. In a conventional refrigerator (at 1 ± 2°C and RH varying between 70% and 100%), reduction rates varied between −0.216 to −0.113 log10/day, thus requiring 4.6 and 8.9 days to decrease by 1 log10. The authors observed significant differences between strains, including a relatively lower survivability of S. Dublin strains as compared to S. Typhimurium DT104 and S. Enteritidis PT8 and PT4. In the Tittor et al. (2011) study reported above, Salmonella decreased from 6.0 log10 CFU/cm2 on the dry and wet‐aged beef to 1.3 and 2.7 log10 CFU/cm2, respectively. Van Damme et al. (2022) also observed a reduction of at least 3 log10 after 42 days of dry‐ageing.
3.4.3.3. L. monocytogenes
Phillips et al. (2012) reported that 0.1% of beef primals for dry‐ageing were contaminated with L. monocytogenes. However, Ribeiro et al. (2021b) found that these bacteria were not detected after dry‐ageing but were present on wet‐aged beef. These authors suggested that the dehydration of the beef during the dry‐ageing process inhibits bacterial survival thus reducing the occurrence of microbiological hazards. Gowda et al. (2022) also failed to detect L. monocytogenes on dry‐aged beef in 15 companies in Belgium. Furthermore, Hulankova et al. (2018b) failed to detect Listeria spp. in the samples originating from 27 beef carcasses before ageing and in samples that were dry‐aged for up to 36 days. Ryu et al. (2018) similarly failed to detect L. monocytogenes on the 3rd, 25th, 40th, 50th, and 60th day of dry‐ageing beef originated from eight carcasses. da Silva et al. (2019) artificially inoculated L. innocua ATCC 33090 on loin pieces (1.5 kg each; 20 × 13 × 8 cm) from two boneless loins (M. longissimus lumborum). The samples were dry‐aged in refrigeration chambers at 2 ± 1°C and 8 ± 1°C with 75 ± 2% RH and an air velocity of 2 ± 0.5 m/s for up to 42 days. The highest reduction rate occurred in the first seven days of ageing. The time needed to reach the first decimal reduction of L. innocua was 4.7 days at 2°C and 4 days at 8°C. The time required to achieve a 3 log10 reduction was estimated to be 69 and 33 days at 2 and 8°C, respectively. After trimming, 67% of the samples dry‐aged at 2°C and 33% dry‐aged at 8°C were L. innocua positive. The authors concluded that increasing the dry‐ageing time and/or temperature would further decrease L. innocua counts. Van Damme et al. (2022) mostly observed a significant decrease of L. monocytogenes over time when ageing beef loins at 2–6°C and 75–85% RH. The observed reduction varied between 1 and > 3 log10 after 42 days of ageing. Nevertheless, L. monocytogenes numbers significantly increased on lean meat on one of the loins (2°C, 85% RH, pH 6.21), resulting in a 1 log10 higher number at day 42 than the initial inoculation level, suggesting that L. monocytogenes may grow under certain dry‐ageing conditions.
3.4.3.4. Enteropathogenic Yersinia spp.
Pigs are the main reservoirs of pathogenic Y. enterocolitica, and pork and pork products are major sources for human infection (Petsios et al., 2016). Bolton et al. (2013) reported a 0.52% prevalence in Irish pigs, while higher rates of 37% and 93% have been found in Belgium (Van Damme et al., 2010) and Spain (Martínez et al., 2011), respectively. The prevalence on pork carcasses ranges from 0.3% (Gürtler et al., 2005) to 39.7% of carcass surfaces post‐evisceration (Van Damme et al., 2015). Y. enterocolitica can grow at temperatures of between −2°C to 42°C with an optimum of 28–29°C and will multiply faster than L. monocytogenes at chill temperatures (Gill and Reichel, 1989). The minimum pH for growth is between 4.2 and 4.4 while the minimum aw is 0.96 (Stern et al., 1980).
3.4.3.5. Campylobacter spp.
The European Union One Health 2020 Zoonoses Report reported that of the 86 pork and 134 bovine samples tested, 8.5 and 5.1% were positive for Campylobacter, respectively (EFSA and ECDC, 2021). Whyte et al. (2004) detected these bacteria in 7/221 (3.2%) of beef, 10/197 (5.1%) of pork and 31/262 (11.8%) of lamb samples. Thépault et al. (2018) reported a 69% prevalence of thermophilic Campylobacter in French cattle at slaughterhouse level. The prevalence in adult cattle was 39%, while 99.4% of calves were contaminated. C. jejuni was the most prevalent species with prevalence of 37.3% and 98.5%, respectively. The prevalence of C. coli was 3% in adult cattle and 12.5% in calves. Recently, Espunyes et al. (2021) isolated Campylobacter from 23.3% of cattle and 7.7% of sheep, with Campylobacter jejuni being the most frequent species. However, Campylobacter do not grow outside the host and are sensitive to desiccation during the chilling of beef, pork and lamb carcasses and reduced prevalence and counts are obtained after this stage of processing (Narvaez‐Bravo et al., 2017).
3.4.3.6. Clostridium spp.
Bovine, ovine and porcine faeces often contain Clostridium spp. (Russell et al., 2021). These authors used PCR methods to confirm that these were cluster 1 clostridia, which includes C. botulinum, C. sporogenes, C. tetani, C. perfringens, as well as Clostridioides difficile, and spoilage organisms, C. estertheticum and C. gasigenes (Song et al., 2004). Previous research reported C. estertheticum and C. gasigenes in 17.9% and 25.4% of bovine faecal samples, respectively (Moschonas et al., 2009). A more recent study found C. difficile in 7% of bovine faeces as well as in 13% of ovine faeces (Marcos Lázaro et al., 2021). However, the same study failed to detect C. difficile in beef and lamb products at retail. As Clostridium spp. are strict anaerobes, growth and/or toxin production during the dry‐ageing of beef is unexpected but could occur in vacuum packaged beef, pork and lamb during wet‐ageing if chilled conditions were not strictly maintained. Of most concern is C. botulinum with types A, B, E and F associated with human botulism. These belong to two groups that differ mainly with respect to proteolysis. Type A strains are proteolytic, type E are non‐proteolytic while B and F have a mixture. Proteolytic strains do not grow below 10°C, while non‐proteolytic strains may grow at temperatures above approximately 3°C (Lynt et al., 1982; Carter and Peck, 2015; Brunt et al., 2020).
In their report on non‐proteolytic C. botulinum and vacuum and modified atmosphere packaged foods, published in 2020, the Advisory Committee on the Microbiological Safety of Food (ACMSF, 2020) found that there was no evidence of outbreaks having been caused by VP/MAP fresh, chilled meats within the scope of the study: beef, pork and lamb. Indeed, none of the outbreaks associated with any commercial chilled food were caused by correctly stored products. There had been only eight studies on fresh, chilled meat and the experimental approaches used varied markedly, including inoculum size, sample size, methodology for assessing risk (growth or toxin formation), analytical methods and sensitivity of assay. The studies demonstrated variation in the time to toxin formation within and between meat species and it was not possible to draw general conclusions from the studies regarding growth and toxin production of C. botulinum in fresh, chilled meats. The ACMSF subgroup reviewed new evidence to assess the safety of increasing the shelf life of vacuum packaged fresh beef, lamb and pork from 10 days (as stated in Food Safety Agency guidance of 2017 updated on 2020, FSA, 2020a,b) to 13 days. A new series of challenge tests were undertaken with six products representative of the UK market, including meats with both short and long ageing periods and using a temperature profile of < 3°C for 1 day, followed by 5°C for 1 day, 2 h at 22°C (to simulate transport by consumers) and then at 8°C for the remaining period (to simulate domestic storage). According to the study (Peck et al., 2020), toxin was not detected in beef or lamb stored for up to 50 and 35 days, respectively; while in one of the two fresh chilled pork products, toxin was detected at 35 days but not after 25 days. These results provided some evidence about the potential production of non‐proteolytic C. botulinum toxin in vacuum packaged fresh meat when stored at 8°C, though it did not occur within the organoleptic shelf‐life.
3.4.3.7. Staphylococcus aureus
Staphylococcus aureus is a Gram‐positive bacterium that is commonly found on the skin and nasal membranes of production animals, humans and other mammals (Pugazhendhi et al., 2020). It is an opportunistic pathogen, causing a range of illnesses including urinary tract infections, toxic shock syndrome, and food poisoning. However, the main food safety issue is Staphylococcal food poisoning, a food‐borne intoxication caused by staphylococcal enterotoxins. The limits for staphylococcal enterotoxin production are temperatures of between 10°C and 48°C, a pH range of 4–9.6, and NaCl concentrations of 0–10% (Zeaki et al., 2019). Thus, as meat is aged at temperatures below 10°C, enterotoxins are not produced during this process.
Prevalence rates for S. aureus of 64%, 20% and 17% were reported in raw beef, sheep and lamb samples at retail with an overall prevalence of 21% (Şanlıbaba, 2022). Similar studies in the United States of America, Japan and Poland isolated this bacterium from 28%, 33% and 68%, respectively (Hiroi et al., 2012; Krupa et al., 2014; Carrel et al., 2017). Although information on growth on meat products is limited, Phillips et al. (2012) reported that 9% of beef primals for dry‐ageing were contaminated with S. aureus and Yu et al. (2020) predicted these bacteria would grow on vacuum packaged beef achieving 6 log10 CFU/g after 100 h at 25°C. In contrast, Ryu et al. (2018) failed to detect S. aureus on the 3rd, 25th, 40th, 50th and 60th day of dry‐ageing beef, originated from eight carcasses.
3.4.4. Effect of dry and wet‐ageing on bacteria
There are relatively few studies on the effect of dry and wet‐ageing beef on the concentrations of specific pathogens and the data that is available is summarised in Table 7. Interestingly dry‐ageing caused a decrease in E. coli O157:H7, Salmonella spp. and L. innocua (as a surrogate for L. monocytogenes). Van Damme et al. (2022) reported a 1.0 log10 increase or a 3.0 log10 decrease in L. monocytogenes depending on the dry‐ageing conditions and beef muscle used. While a decrease in bacterial pathogens during dry‐ageing may be due to the lowering of the aw, this would not explain the decreases in E. coli O157 and Salmonella spp. on wet‐aged beef reported by Tittor et al. (2011). It should be noted that these findings are specific to the conditions used during ageing and further investigation is required before a more comprehensive conclusion on the fate of specific pathogens during meat ageing can be drawn.
Table 7.
The change in the concentrations of specific pathogenic bacteria under different conditions of dry and wet‐ageing of beef
| Conditions | Pathogen | Log10 | Reference | |
|---|---|---|---|---|
| Increase | Decrease | |||
| Dry‐ageing | ||||
| 3°C, RH 80%, air velocity of 0.25 m/s for 28 days | E. coli O157:H7 | – | 4.8 | Tittor et al. (2011) |
| 4°C, RH 92% for 6 days | E. coli O157:H7 | – | 0.8 | Ingham et al. (2010) |
| 2–6°C, RH 75–85% for 42 days | E. coli O157:H7 | – | 3.5– > 5.0 | Van Damme et al. (2022) |
| 3°C, RH 80%, air velocity of 0.25 m/s for 28 days | Salmonella spp. | – | 4.7 | Tittor et al. (2011) |
| 2–6°C, RH 75–85% for 42 days | Salmonella spp. | – | 2.6–4.0 | Van Damme et al. (2022) |
| 2 ± 1°C, RH 75 ± 2%, air velocity of 2 ± 0.5 m/s for up to 42 days | L. innocua ATCC 33090 (1) | – | 2.4 | da Silva et al. (2019) |
| 8 ± 1°C, RH 75 ± 2%, air velocity of 2 ± 0.5 m/s for up to 35 days | L. innocua ATCC 33090 | – | 3.4 | da Silva et al. (2019) |
| 2–6°C and RH 75–85%, for 42 days | L. monocytogenes | – | 1.0–3.3 | Van Damme et al. (2022) |
| 2°C, RH 85% for 42 days | L. monocytogenes | 1.0 | 1.1–1.8 | Van Damme et al. (2022) |
| Wet‐ageing | ||||
| 3°C, RH 80%, air velocity of 0.25 m/s for 28 days | E. coli O157:H7 | – | 2.2 | Tittor et al. (2011) |
| 3°C, RH 80%, air velocity of 0.25 m/s for 28 days | Salmonella spp. | – | 2.2 | Tittor et al. (2011) |
L. innocua ATCC 33090 was used as a surrogate for L. monocytogenes.
3.5. TOR3: impact of dry‐ageing and wet‐ageing of meat on the load of public health‐relevant microbiological hazards and spoilage bacteria, when compared to standard fresh meat
3.5.1. Selection of relevant microorganisms for the different scenarios
In addition to the ability to grow and/or produce toxin at the chilled temperatures used during meat ageing, the criteria used to select bacteria for the modelling studies included the availability of suitable predictive models to estimate growth under dry and wet‐aged meat conditions, including the relevant aw values (in case of dry‐ageing, Figure 4) and the atmosphere conditions (aerobic for dry‐ageing and anaerobic for vacuum packaged wet‐ageing).
Figure 4.
Growth rate (as square root of μ max ) at 4°C as a function of aw of relevant pathogenic and spoilage bacteria according to predictive models (input value for pH = 6), CB: ComBase; FSSP: Food Spoilage and Safety Predictor. Dotted line represents the square root of the inactivation rate (k max multiplied by 10 to present on the same scale) according to the non‐thermal survival model of ComBase
Moreover, the psychrotrophic pathogenic and spoilage bacteria showing the highest growth rates were considered for the assessment, as representative of the worst‐case scenario. The pathogenic bacteria L. monocytogenes, Y. enterocolitica and, for wet‐ageing only, non‐proteolytic Clostridium spp. were therefore selected. Mesophilic pathogens (e.g. Salmonella and E. coli) were not included in the assessment. Pseudomonas and LAB were the main spoilage bacterial groups (as discussed in Section 3.4.2) that satisfied the criteria for dry and wet‐ageing, respectively.
Figure 4 shows the behaviour of growth rate at 4°C for different aw values for the different bacteria considered according to predictive models available at ComBase portal and FSSP. LAB and L. monocytogenes are the most resistant bacteria to aw reduction, as the reduction of the growth rate due to the decrease of aw was considerably lower as compared to Pseudomonas, Y. enterocolitica and non‐proteolytic C. botulinum. For L. monocytogenes, an overlap for growth and inactivation was noted at aw values below 0.96.
3.5.2. Development of scenarios to evaluate
3.5.2.1. Development of scenarios to evaluate in ToR3 and ToR4
To address ToR3, a range of different scenarios were developed for dry‐aged beef processes as well as wet‐aged and standard fresh meat processes for beef, pork and lamb. These scenarios are based on the scientific literature and information provided in the answers to the questionnaire (see ToR1). The scenarios, distributions and associated parameters used in the predictive modelling are summarised in the Tables D.1, D.2, D.3, D.4–D.1, D.2, D.3, D.4 in Appendix D. Wet‐ageing is assumed to be the standard process used for standard fresh meat preparation, with the times being up to 14 days for beef (Table D.1), 4 days for pork (Table D.3) and 4 days for lamb (Table D.4).
Table D.1.
The min, mode and maximum parameter values for temperature (T), pH and water activity (aw), used in Pert distributions when simulating growth during wet‐ageing of beef
| Stage (days) | T | pH | aw | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Mode | Max | Min | Mode | Max | Min | Mode | Max | |
| 0 | −0.6 | 2.0 | 7.0 | 5.1 | 5.5 | 5.9 | 0.97 | 0.98 | 0.99 |
| 14 | −0.6 | 2.0 | 7.0 | 5.1 | 5.5 | 5.9 | 0.97 | 0.98 | 0.99 |
| 28 | −0.6 | 2.0 | 7.0 | 5.1 | 5.5 | 5.9 | 0.97 | 0.98 | 0.99 |
| 49 | −0.6 | 2.0 | 7.0 | 5.1 | 5.5 | 5.9 | 0.97 | 0.98 | 0.99 |
Table D.2.
The min, mode and maximum parameter values for temperature (T), pH and water activity (aw), used in Pert distributions when simulating growth during dry‐ageing of beef
| Stage (days) | T | pH | aw | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Mode | Max | Min | Mode | Max | Min | Mode | Max | |
| 0 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.95 | 0.97 | 0.99 |
| 14 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.88 | 0.96 | 0.99 |
| 21 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.88 | 0.95 | 0.99 |
| 28 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.88 | 0.95 | 0.99 |
| 35 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.88 | 0.95 | 0.99 |
| 42 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.88 | 0.95 | 0.99 |
| 77 | −0.6 | 2.0 | 5.1 | 5.5 | 5.7 | 6.2 | 0.88 | 0.95 | 0.99 |
Table D.3.
The min, mode and maximum parameter values for temperature (T), pH, and water activity (aw), used in Pert distributions when simulating growth during wet‐ageing of pork
| Stage (Days) | T | pH | aw | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Mode | Max | Min | Mode | Max | Min | Mode | Max | |
| 0 | −1.0 | 2.0 | 4.0 | 5.4 | 5.8 | 6.3 | 0.95 | 0.98 | 0.99 |
| 4 | −1.0 | 2.0 | 4.0 | 5.4 | 5.8 | 6.3 | 0.95 | 0.98 | 0.99 |
| 7 | −1.0 | 2.0 | 4.0 | 5.4 | 5.8 | 6.3 | 0.95 | 0.98 | 0.99 |
| 14 | −1.0 | 2.0 | 4.0 | 5.4 | 5.8 | 6.3 | 0.95 | 0.98 | 0.99 |
| 28 | −1.0 | 2.0 | 4.0 | 5.4 | 5.8 | 6.3 | 0.95 | 0.98 | 0.99 |
Table D.4.
The min, mode and maximum parameter values for temperature (T), pH and water activity (aw), used in Pert distributions when simulating growth during wet‐ageing of lamb
| Stage (days) | T | pH | aw | ||||||
|---|---|---|---|---|---|---|---|---|---|
| min | mode | max | min | mode | max | min | mode | max | |
| 0 | −1.5 | 2.5 | 7.0 | 5.5 | 5.7 | 5.9 | 0.95 | 0.98 | 0.99 |
| 4 | −1.5 | 2.5 | 7.0 | 5.5 | 5.7 | 5.9 | 0.95 | 0.98 | 0.99 |
| 7 | −1.5 | 2.5 | 7.0 | 5.5 | 5.7 | 5.9 | 0.95 | 0.98 | 0.99 |
| 14 | −1.5 | 2.5 | 7.0 | 5.5 | 5.7 | 5.9 | 0.95 | 0.98 | 0.99 |
| 21 | −1.5 | 2.5 | 7.0 | 5.5 | 5.7 | 5.9 | 0.95 | 0.98 | 0.99 |
3.5.2.2. The changing pH, T and aw values during dry‐ageing of beef
Despite the lack of information available in the literature about the drying profile of the meat surface during beef ageing, four references were found reporting the change of aw on the meat surface during the ageing process of beef aged at different target temperature and RH conditions (da Silva et al., 2018; Smaldone et al., 2019; Panella‐Riera et al., 2021; Bover Cid et al., 2022). Five realistic case‐examples of specific combinations of temperature, aw and pH were obtained as shown in Figure 5. These examples are within the wide range of variability covered in the scenarios described in Section 3.5.2.1 (Tables D.1, D.2, D.3, D.4–D.1, D.2, D.3, D.4 in Appendix D)
Figure 5.
Measured, pH and aw values during dry‐ageing of beef for different process conditions (target temperature and RH) reported in the literature used as realistic examples. For case 4 and 5 temperature represent the target value (represented as dotted lines)
3.5.3. Log10 increase of microorganisms during dry‐ageing of beef and prolonged wet‐ageing of beef, pork and lamb compared to standard fresh meat preparation
To assess the potential impact of dry‐ageing of beef and wet‐ageing on beef, pork and lamb under selected current practices, on the load of public health‐relevant microbiological hazards and spoilage bacteria as compared to standard fresh meat and to identify the most relevant bacteria, the potential log10 increases were estimated based on input parameters for the different meat types (Tables D.1, D.2, D.3, D.4–D.1, D.2, D.3, D.4). In this ToR, the effects of inactivation, rate of drying and competition are not included. The potential effects of these factors are shown in Section 3.4.4 when evaluating ToR4.
For wet‐aged beef, based the median of the mean growth rates during standard fresh meat preparation (14 days), the predicted log10 increase of L. monocytogenes after 49 days of wet‐ageing is around 4 log10, and 7 log10 for LAB, whereas practically no growth is predicted for non‐proteolytic C. botulinum. The corresponding minimum log10 increases were approximately 0.5, 0, and 3, for L. monocytogenes, non‐proteolytic C. botulinum and LAB, respectively (Figure 6).
Figure 6.
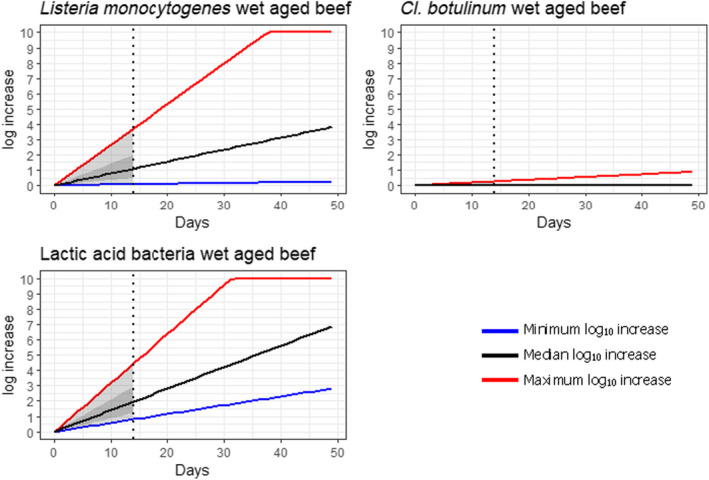
Predicted growth (log10 increase) of selected pathogens and spoilage bacteria during wet‐ageing of beef. The solid lines are the minimum (blue), median (black), and maximum (red) log10 increases during ageing based on constant mean growth rates. These growth rates were estimated from the variable growth rates during the standard fresh meat preparation (dotted vertical line). Light grey area indicates the min and max variable range of log10 increases and dark grey area the 5 and 95 percentile variable range
The predicted log10 increases for the other ageing and meat types are shown in Figures 7, 8, 9–7, 8, 9. The predicted median log10 increases at the end of ageing (i.e. 77 days) in dry‐aged beef were around 5.2, 6.2, and 10 (assumed max log10 increase) log for L. monocytogenes, LAB and Pseudomonas, respectively. In comparison, Van Damme et al. (2022) observed that mean concentrations of Pseudomonas after 42 days of dry‐ageing increased from 2.1 log10 CFU/cm2 to 4.0 log10 CFU/cm2 (i.e. 1.9 log10 increase considering the mean concentrations) on adipose tissue with a maximum concentration of 7.4 log10 CFU/cm2 (i.e. max 5.3 log10 increase). The reported concentrations were smaller on lean tissue. For L. monocytogenes a 1 log10 increase after 42 days was only observed for one replicate of four at 2°C, 85% RH, whereas at other conditions (2°C and 6°C, 75 or 85% RH) varying rates of reductions were observed resulting in 1 to approximately 3 log10 decrease (Van Damme et al., 2022).
Figure 7.
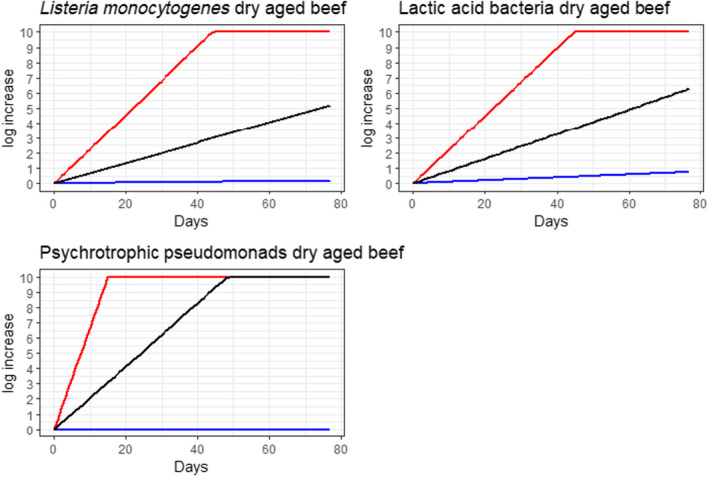
Predicted log10 increases of selected pathogens and spoilage bacteria during dry‐ageing of beef. The solid lines are the minimum (blue), median (black) and maximum (red) log10 increases during ageing based on constant mean growth rates. These growth rates were estimated from the variable growth rates during the first 14 days (standard fresh meat preparation time). The comparison for standard meat is the grey shaded areas in the corresponding graphs in Figure 6
Figure 8.
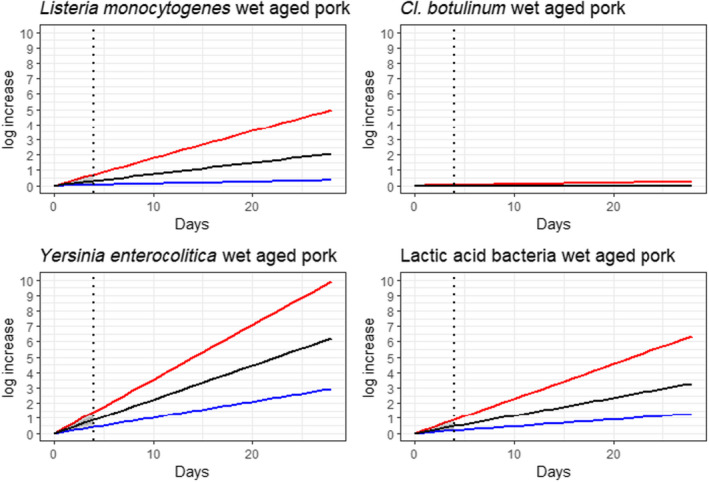
Predicted log10 increases of selected pathogens and spoilage bacteria during wet‐ageing of pork. The solid lines are the minimum (blue), median (black) and maximum (red) log10 increases during prolonged ageing based on constant mean growth rates. These growth rates were estimated from the variable log10 increases during the standard fresh meat preparation time (dotted vertical line). Light grey indicates the min and max variable range of log10 increases and dark grey the 5 and 95 variable percentile range
Figure 9.
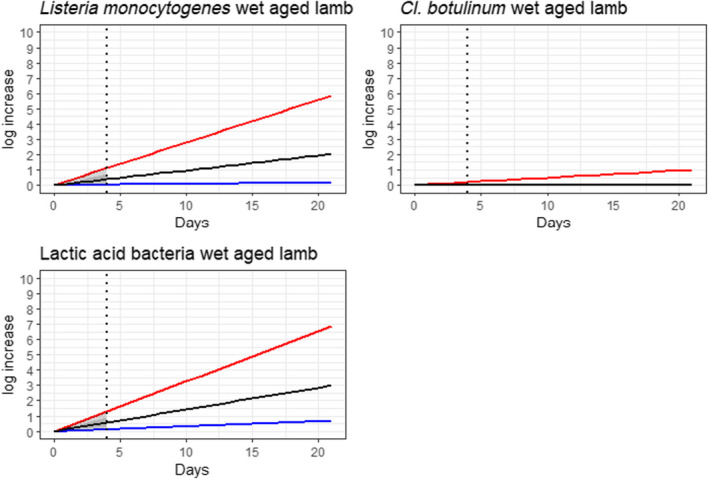
Predicted log10 increases of selected pathogens and spoilage bacteria during wet‐ageing of lamb. The solid lines are the minimum (blue), median (black) and maximum (red) log10 increases during prolonged ageing based on constant mean growth rates. These growth rates were estimated from the variable log10 increases during the standard fresh meat preparation time (dotted vertical line). Light grey indicates the min and max variable range of log10 increases and dark grey the 5 and 95 variable percentile range
In wet‐aged pork, the greatest potential median log10 increase after 28 days was predicted for Y. enterocolitica, at approximately 6.2 log10, whereas the median log predicted increases was around 2 and 3.2 log10 for L. monocytogenes and LAB, respectively (Figure 8). In wet‐aged lamb, the predicted median potential log increases after 21 days of wet‐ageing were approximately 2 and 3 log10 for L. monocytogenes and LAB, respectively (Figure 9).
In comparison, the range of predicted log10 increases of the microbiological hazards and spoilage bacteria during standard fresh meat preparation are indicated by the shaded area in Figures 6, 8 and 9. The median predicted log10 increases during standard fresh meat preparation for L. monocytogenes and LAB varied between 1 and 2 for wet‐aged beef.
Figure 66. The corresponding log10 increases in dry‐aged beef after14 days were similar, except for Pseudomonas with a predicted log10 increase of around 3.0 (Figure 7). In lamb and pork after 4 days, the predicted log10 increases were around 0.5 to 1, with a maximum predicted log10 increase for Yersinia of around 1 (Figures 8 and 9). A summary of the range of predicted log10 increases for pathogens and spoilage bacteria during standard fresh meat preparation and ageing is shown in Appendix G.
Thus, the predictions show that ageing beyond the durations used for standard fresh meat could potentially impact on the load of microbiological hazards and spoilage bacteria under current practices. The estimated minimum predicted log10 increases over time indicate that equivalent loads compared to the range of loads on meat after standard fresh meat preparation times can be achieved, i.e. y‐values of blue lines in figures are lower or equal to the upper grey areas, at least for a part, if not all, of the extended ageing time evaluated. In some cases, e.g. Y. enterolitica in wet‐aged pork, the ageing time appear to be at most around 10–15 days (Figure 8). In ToR4, the conditions resulting in similar log10 increases as standard meat preparation for the relevant pathogens and spoilage bacteria are evaluated. It should be noted that the model predictions in ToR3 are considered overestimations of log10 increases since the effect of competition and inactivation are not included. Additionally, for dry‐ageing the rate of drying is also important. Moreover, the range of variability during standard fresh meat preparation to be used for the determination of equivalence will also affect the outcome of ToR4. The impact of these factors is evaluated in Section 3.4.4.
The predictions discussed above apply to the full range of conditions considered in the scenarios developed in Section 3.5.2.1 and provide the predicted minimum and the maximum log10 increase values, but not the relative frequency or probability that these values could occur within the current practices of meat ageing. Although this type of information could not be collected from the scientific literature or the questionnaire, specific cases of meat ageing process conditions were developed (Section 3.5.2.2) as realistic examples of the dynamic combination of ageing temperature, aw and pH on the meat surface during ageing.
Figure 10 shows the result of the simulation of the growth of L. monocytogenes (as log10 increase) together with the changes of aw and pH of the meat surface and the temperature during the beef dry‐ageing process for each case example. The potential growth of L. monocytogenes varied depending on the specific ageing profiles, from a 1 to 2 log10 increase in cases 1 and 4, that kept temperature at 0°C and case 5 corresponding to the fastest and more intense drying among the assessed examples. The highest log10 increase was predicted for case 2, in which the temperature was 3°C. The magnitude of the simulated growth of these realistic case examples fell within the range of potential log10 increase of L. monocytogenes of the overall scenarios shown above (Figure 7).
Figure 10.
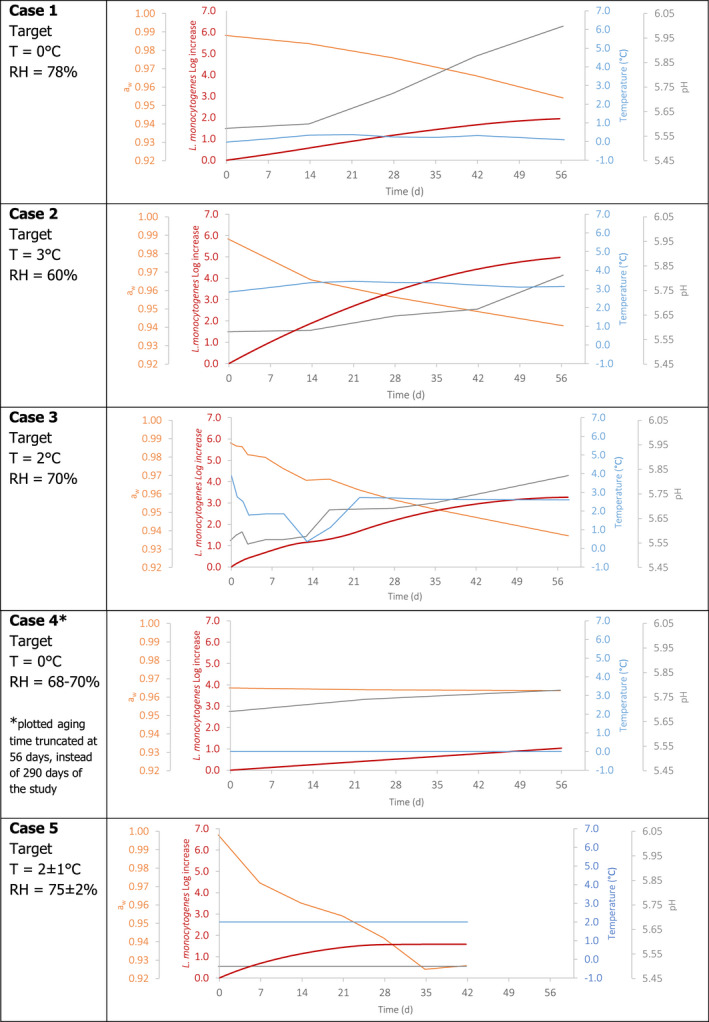
Predicted growth of L. monocytogenes (red) for different realistic cases of dynamic profiles of aw (orange), pH (grey) and temperature (blue) during dry‐ageing of beef under specific target temperature and relative humidity (RH) conditions found in the scientific literature (Bover Cid et al., 2022 for case 1 and 2; Panella‐Riera et al., 2021 for case 3; Smaldone et al., 2019 for case 4; da Silva et al., 2018 for case 5)
3.6. TOR4: conditions during the production of dry‐aged and wet‐aged meat and possible further storage that would result in a similar or lower load of the relevant microbiological hazards and spoilage bacteria as compared to standard fresh meat before consumption (i.e. at the end of the shelf‐life)
3.6.1. Scenario analysis of ageing processes – Approach and scenarios evaluated
In ToR4, the conditions resulting in similar log10 increases as for standard fresh meat preparation are identified. Since temperature and duration are the variables that may be directly controlled, the analysis was focused on the impact of these factors on the potential log10 increase of the fastest growing pathogenic and spoilage bacteria, respectively. Since log10 increase depends on time and temperature equivalent conditions must entail shorter times or lower temperatures, unless maximum bacterial concentrations have already been achieved during the standard fresh meat preparation time However, aw and pH also have an impact on the equivalent time and temperatures. Figures 10 and 11 illustrates this by showing the time and temperature conditions during wet‐ageing of beef that corresponds to predicted log10 increases of L. monocytogenes that are the same or lower than the mean log10 increase during standard fresh beef preparation, i.e. here a 1 log10 increase. For instance, at a pH of 5.1, equivalent conditions are observed at 1 to 4°C for up to 49 days and at 5°C for up to 29 days of ageing (at the range of aw‐values evaluated in the scenario), whereas at a pH of 5.9 the corresponding highest temperature is 1°C and the ageing only at 22 days (presumedly at the lower aw values evaluated, Figure 11a). Thus, at the higher temperatures long duration is only possible at lower values for aw and especially, pH (Figure 11).
Figure 11.
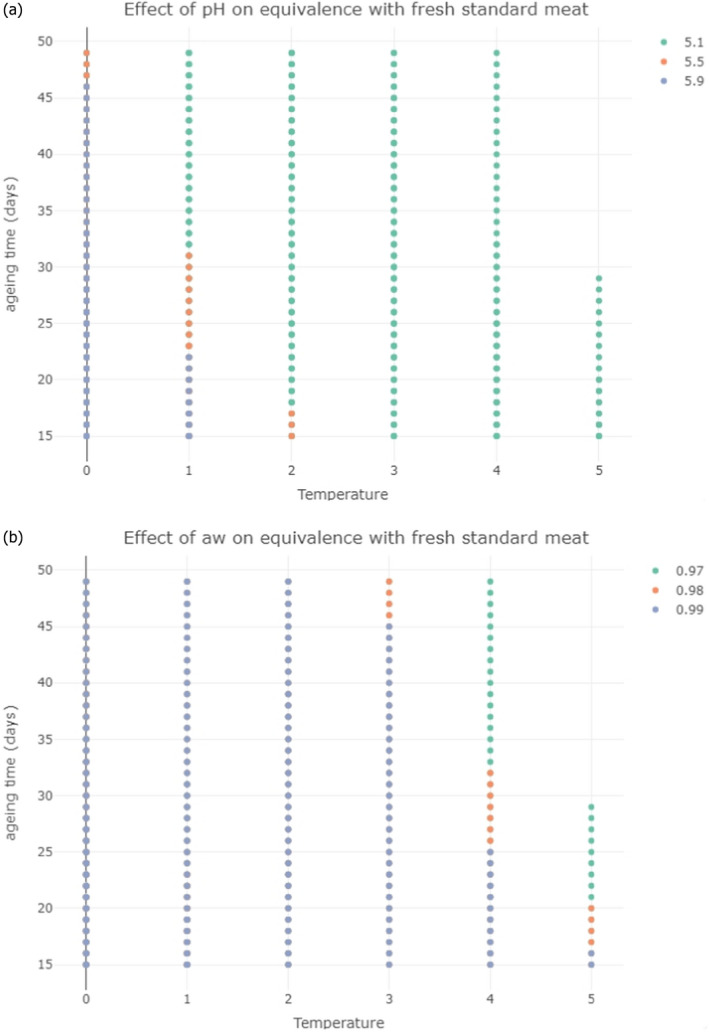
The impact of the range of aw‐values at fixed pH‐values (a) and the range of pH‐values at fixed aw‐values (b) on the equivalent temperature and ageing time conditions. These temperatures and times correspond to predicted log10 increases of L. monocytogenes in wet beef being equal or lower than the mean log10 increase during standard fresh meat preparation
Taking the range of predicted median log10 increases of the microbiological hazards and spoilage bacteria evaluated in ToR3, as well as the potential effect of overestimation arising from not considering competition and the rate of drying, it was decided to evaluate equivalence in terms of 0.5, 1, 2, 3 and 4 predicted log10 increases.
3.6.2. Dry‐ageing – conditions to achieve similar or lower log increase of pathogens or spoilage bacteria before consumption
The relationship between the temperature and the maximum dry‐ageing time that would result in log10 increases equivalent to the log10 increases that represent standard fresh meat preparation, i.e. between 0.5 and 4 log10, are shown in Figures 12 and 13 for L. monocytogenes and LAB. Point estimates of the maximum time or maximum temperature (and the corresponding temperatures and times) conditions that would achieve the different log10 increases considered equivalent to standard fresh beef preparation is shown for different target bacteria in Appendix F, Table F.1. The importance of the pH and aw during ageing is illustrated by the different equivalent conditions resulting using different scenarios for these parameters. The scenarios used for the predictions are based on scenarios with minimum, median, or maximum values of pH and aw during standard fresh meat preparation. For example, assuming that equivalence is represented by a 1 log10 increase of L. monocytogenes, the maximum ageing time would be at least 77 days (at 5°C), the maximum time evaluated, in the minimum scenario, and 20 days (at 0°C) in the maximum scenario (Figure 12). Similarly, if an ageing time of 35 days is required, ageing according to the predictive model need to take place at 0.5°C, 1.25°C, 2.25°C, 3°C or 3.75°C if 0.5, 1, 2, 3, or a 4‐log10 increase, respectively, is considered for equivalence in the medium scenario for pH and aw. The required ageing temperatures results from the predictive model and the small differences between scenarios reflect the great impact of temperature on the predictions. Taking observed temperature variations during ageing into consideration it is difficult to control temperature to this extent under practical conditions. The pH and more especially the aw can be variable during dry‐ageing, and controllable to some extent. The impact of drying rate is evaluated in Section 3.6.4.3.
Figure 12.
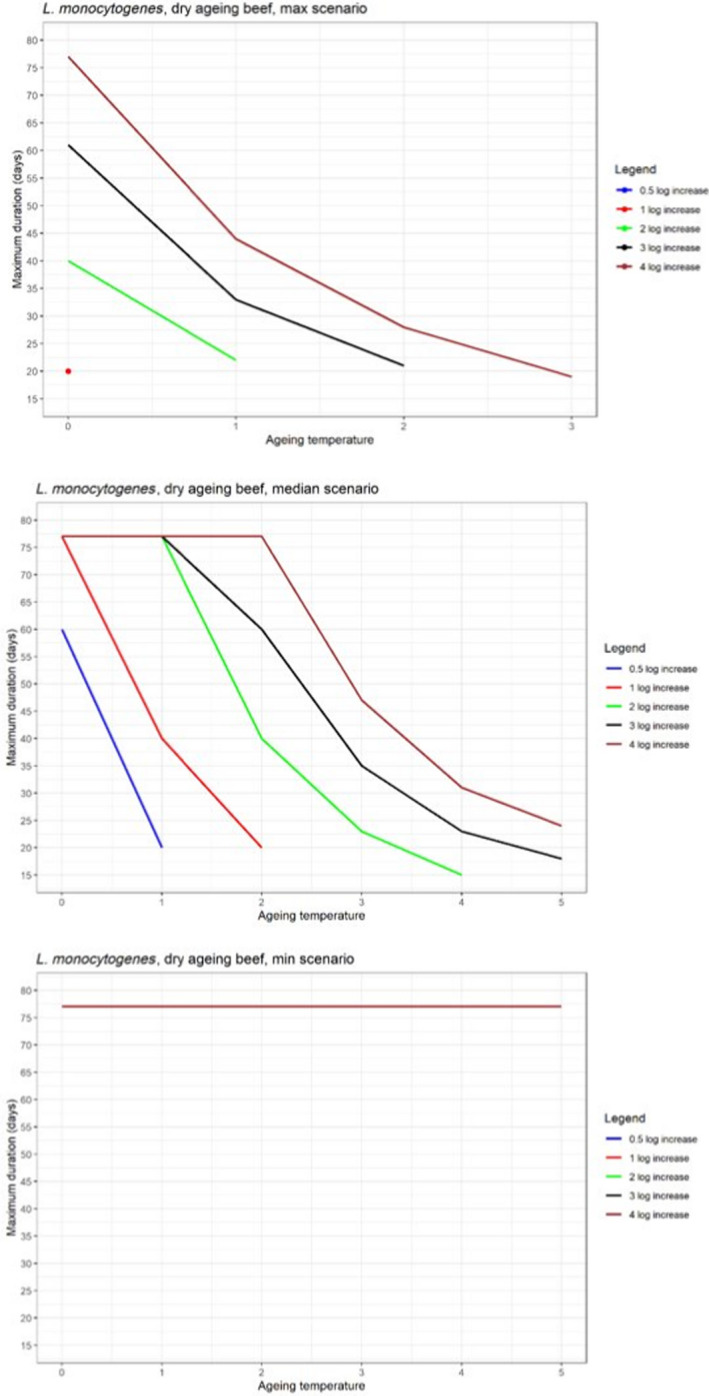
Relationship between ageing temperature and maximum time for dry‐ageing of beef corresponding to different log10 increases of L. monocytogenes considered equivalent to standard beef ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 6.2; aw = 0.99), median (pH = 5.85; aw = 0.955) or minimum scenarios (pH = 5.5; aw = 0.92), and a maximum ageing time of 77 days
Figure 13.
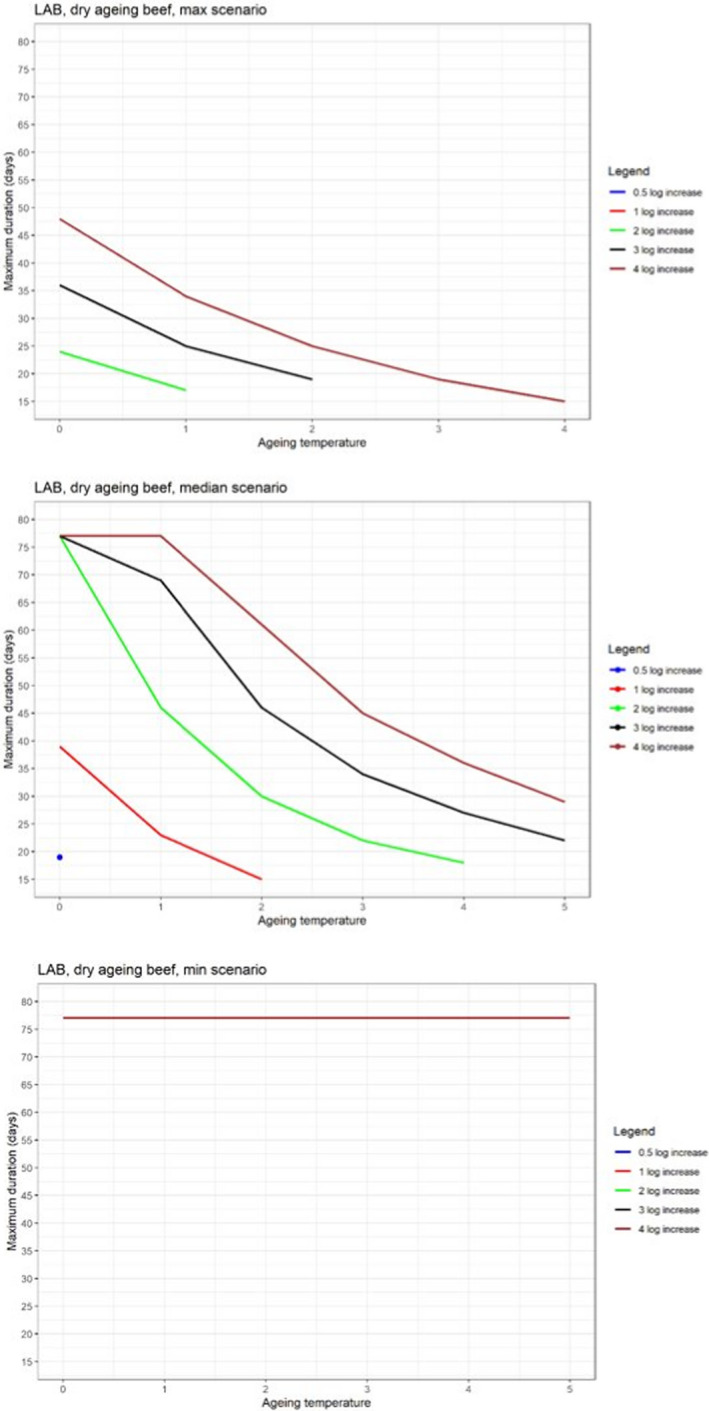
Relationship between ageing temperature and maximum time for dry‐ageing of beef corresponding to different log10 increases of LAB considered equivalent to standard beef ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 6.2; aw = 0.99), median (pH = 5.85; aw = 0.955) or minimum scenarios (pH = 5.5; aw = 0.92), and a maximum ageing time of 77 days
Table F.1.
The estimated maximum time and temperature to stay below different targets corresponding to equivalent log increases as during standard fresh beef preparation for microbiological hazards and spoilage bacteria during dry‐ageing of beef. The scenarios are predicted based on scenarios with minimum, median or maximum values of pH and aw
| Target | Scenario: minimum (a) | Scenario: median (b) | Scenario: maximum (c) | |||
|---|---|---|---|---|---|---|
| Max time (at temperature) | Max temperature (at time) | Max time (at temperature) | Max temperature (at time) | Max time (at temperature) | Max temperature (at time) | |
| Beef | ||||||
| L. monocytogenes | ||||||
| 0.5 | 77 (5) | 5 (77) | 60 (0) | 1 (20) | – (e) | – |
| 1.0 | 77 (5) | 5 (77) | 77 (0) | 2 (20) | 20 (0) | 0 (20) |
| 2.0 | 77 (5) | 5 (77) | 77 (1) | 4 (15) | 40 (0) | 1 (22) |
| 3.0 | 77 (5) | 5 (77) | 77 (1) | 5 (18) | 61 (0) | 2 (21) |
| 4.0 | 77 (5) | 5 (77) | 77 (2) | 5 (24) | 77 (0) | 3 (19) |
| LAB | ||||||
| 0.5 | 77 (5) | 5 (77) | 19 (0) | 0 (19) | – | – |
| 1.0 | 77 (5) | 5 (77) | 39 (0) | 2 (15) | – | – |
| 2.0 | 77 (5) | 5 (77) | 77 (0) | 4 (18) | 24 (0) | 1 (17) |
| 3.0 | 77 (5) | 5 (77) | 77 (0) | 5 (22) | 36 (0) | 2 (19) |
| 4.0 | 77 (5) | 5 (77) | 77 (1) | 5 (29) | 48 (0) | 4 (15) |
| Pseudomonas (d) | ||||||
| 0.5 | 77 (5) | 5 (77) | – | – | – | – |
| 1.0 | 77 (5) | 5 (77) | 15 (0) | 0 (15) | – | – |
| 2.0 | 77 (5) | 5 (77) | 31 (0) | 3 (16) | – | – |
| 3.0 | 77 (5) | 5 (77) | 46 (0) | 5 (17) | – | – |
| 4.0 | 77 (5) | 5 (77) | 62 (0) | 5 (22) | – | – |
Minimum scenario – dry‐ageing: pH = 5.5, aw = 0.92.
Median scenario – dry‐ageing: pH = 5.85, aw = 0.955.
Maximum scenario – dry‐ageing: pH = 6.2, aw = 0.99.
Only temperature and aw (not pH) in a Pseudomonas predictive model.
‘–’ indicates that none of the evaluated conditions result in log increases below the target.
3.6.3. Wet‐ageing – conditions to achieve similar or lower log increase of pathogens or spoilage bacteria before consumption
The relationship between ageing temperature and maximum wet‐ageing time that would result in log10 increases equivalent to the different log10 increases considered to represent standard fresh meat preparation, i.e. between 0.5 and 4 log, are shown in Figures 14, 16, 18 for L. monocytogenes and Figures 15, 17, 19 for LAB. Point estimates of the maximum time or maximum temperature (and the corresponding temperatures and times) conditions that would achieve different log10 increases considered equivalent to standard fresh meat preparation of beef, pork and lamb are shown for different bacteria in Appendix F, Table F.2. The importance of the pH and aw during ageing is illustrated by the different equivalent conditions resulting using different scenarios for these parameters. For instance, assuming that equivalence in pork is represented by a 1 log10 increase of L. monocytogenes, the maximum ageing time would be 28 days (at 3°C) in the minimum scenario, and 26 days (at 0°C) in the maximum scenario (Table F.2). For wet‐aged beef, if an ageing time of 35 days is desirable, ageing according to the predictive model needs to take place at 0.5°C, 1.6°C, 2.6°C or 3.4°C if 1, 2, 3, or a 4‐log10 increase of L. monocytogenes, respectively, is considered for equivalence in the medium scenario for pH and aw (Figure 14). Considering spoilage bacteria and wet‐ageing of beef, assuming that equivalence is represented by a 3‐log10 increase of LAB, the maximum ageing time would be at least 49 days (at 3°C), the maximum time evaluated, in the minimum scenario, and 36 days (at 0°C) in the maximum scenario (Figure 15). Similarly, if an ageing time of 35 days is desirable, the lowest achievable log10 increase according to the predictive model in the medium scenario is a 3‐log10 increase. To allow 35 days ageing time, ageing need to take place at 0.7°C or 1.6°C if a 3‐, or a 4‐log10 increase, respectively, is considered for equivalence in the medium scenario for pH and aw (Figure 15).
Figure 14.
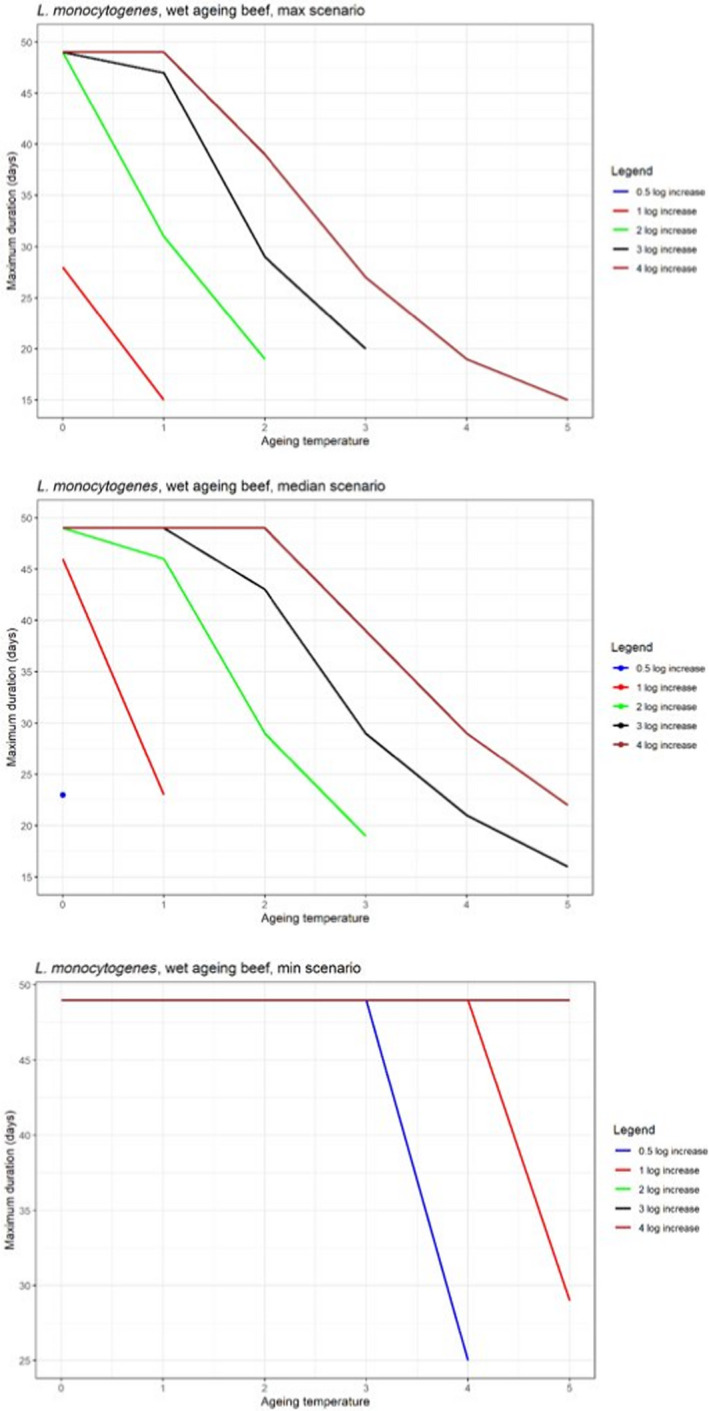
Relationship between ageing temperature and maximum time for wet‐ageing of beef corresponding to different log10 increases of L. monocytogenes considered equivalent to standard beef ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 5.9; aw = 0.99), median (pH = 5.5; aw = 0.98) or minimum scenarios (pH = 5.1; aw = 0.97), and a maximum ageing time of 49 days
Figure 16.
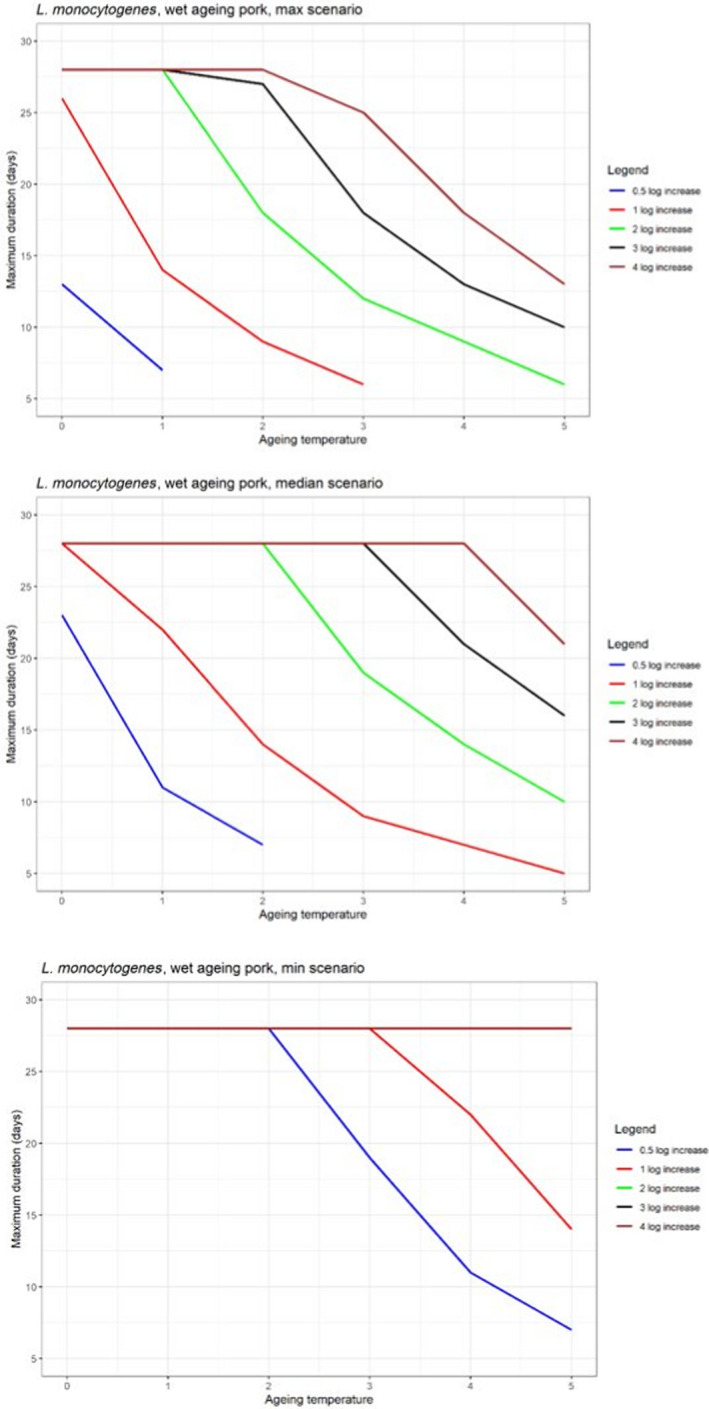
Relationship between ageing temperature and maximum time for wet‐ageing of pork corresponding to different log10 increases of L. monocytogenes considered equivalent to standard pork ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 6.3; aw = 0.99), median (pH = 5.85; aw = 0.97) or minimum scenarios (pH = 5.4; aw = 0.95), and a maximum ageing time of 28 days
Figure 18.
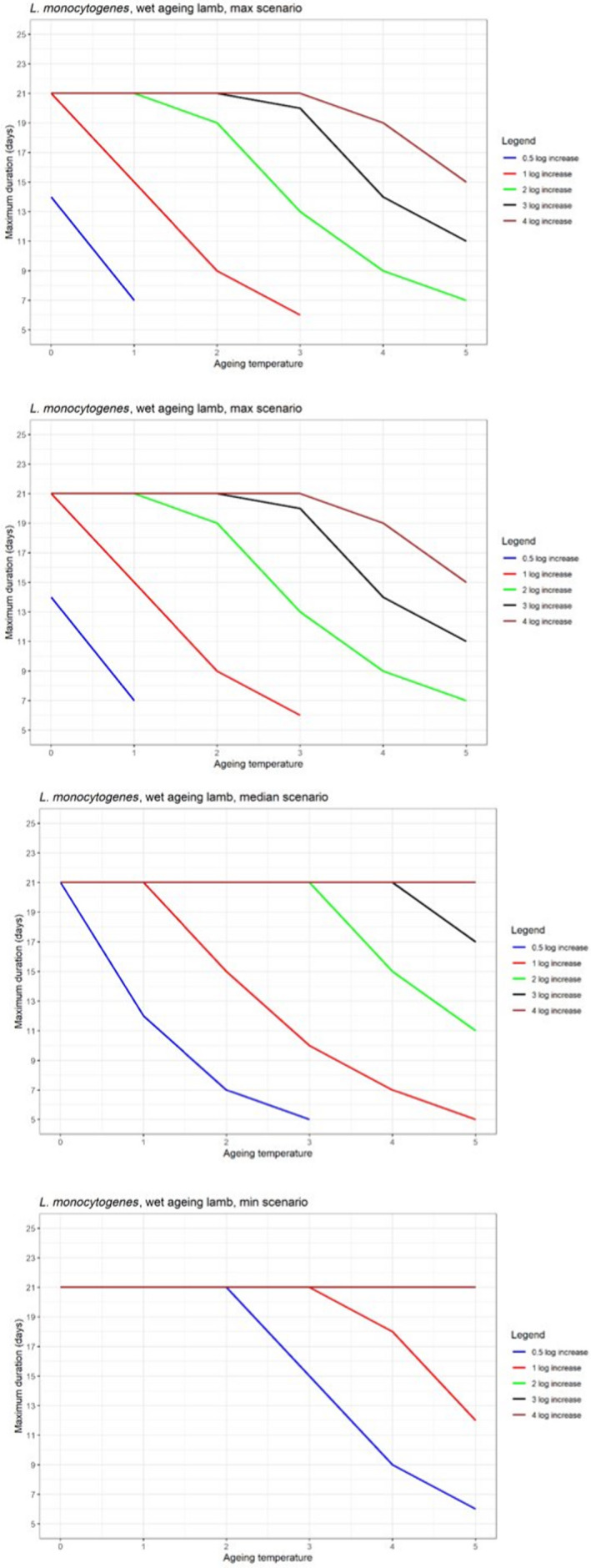
Relationship between ageing temperature and maximum time for wet‐ageing of lamb corresponding to different log10 increases of L. monocytogenes considered equivalent to standard pork ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 5.9; aw = 0.99), median (pH = 5.7; aw = 0.97) or minimum scenarios (pH = 5.5; aw = 0.95), and a maximum ageing time of 21 days
Figure 15.
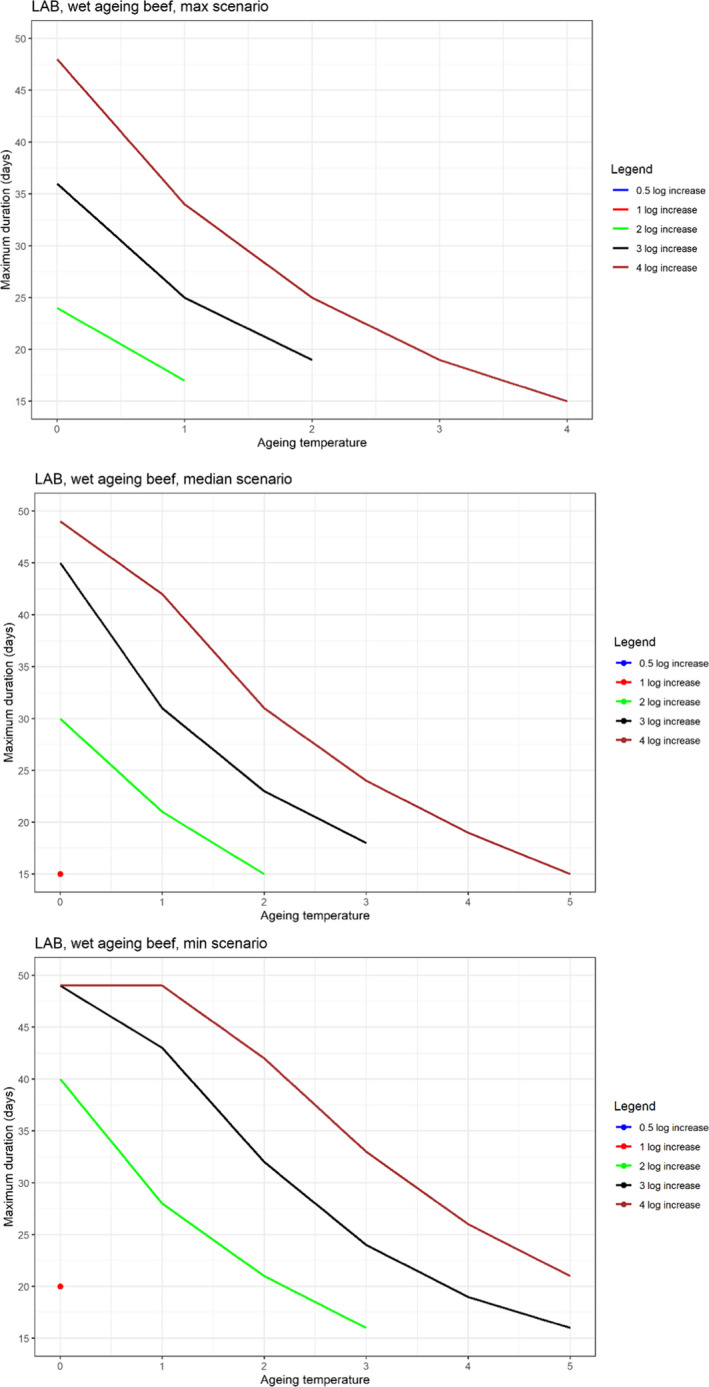
Relationship between ageing temperature and maximum time for wet‐ageing of beef corresponding to different log10 increases of LAB considered equivalent to standard beef ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 5.9; aw = 0.99), median (pH = 5.5; aw = 0.98) or minimum scenarios (pH = 5.1; aw = 0.97), and a maximum ageing time of 49 days
Figure 17.
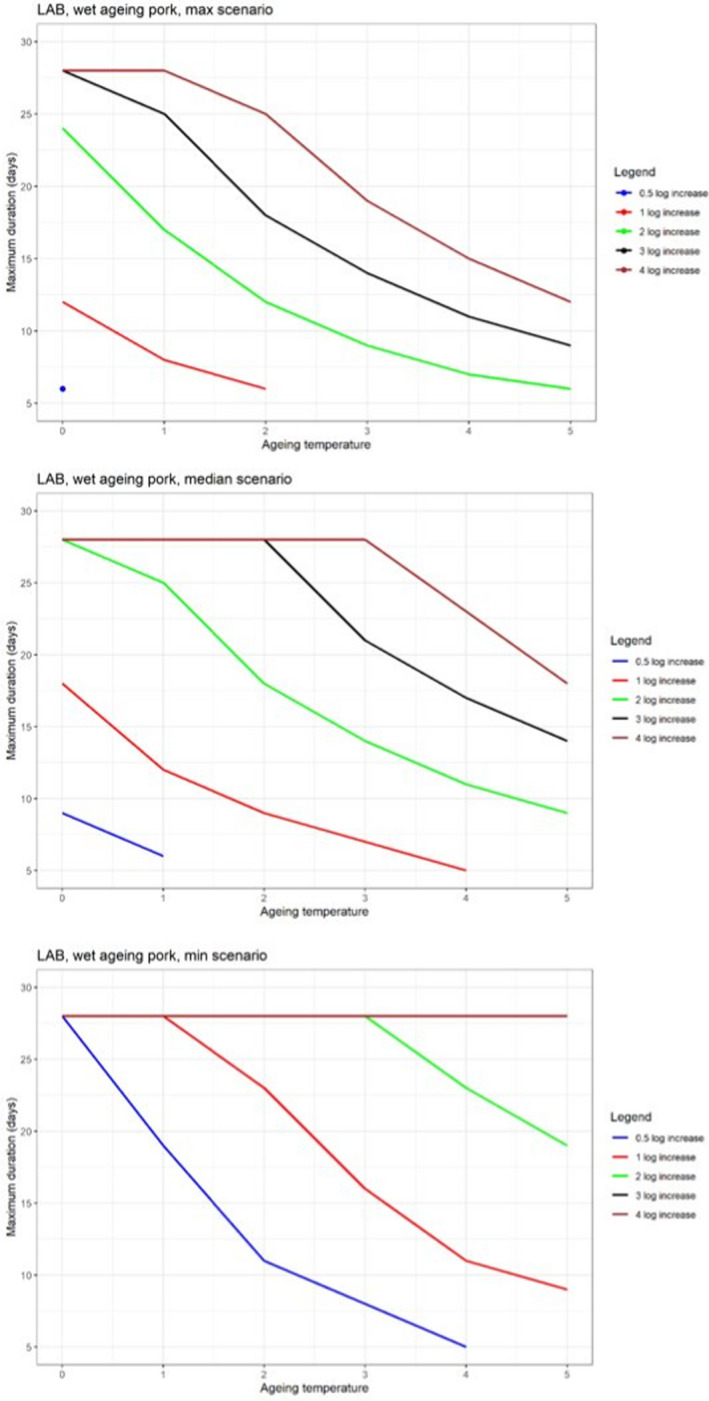
Relationship between ageing temperature and maximum time for wet‐ageing of pork corresponding to different log10 increases of LAB considered equivalent to standard pork ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 6.3; aw = 0.99), median (pH = 5.85; aw = 0.97) or minimum scenarios (pH = 5.4; aw = 0.95), and a maximum ageing time of 28 days
Figure 19.
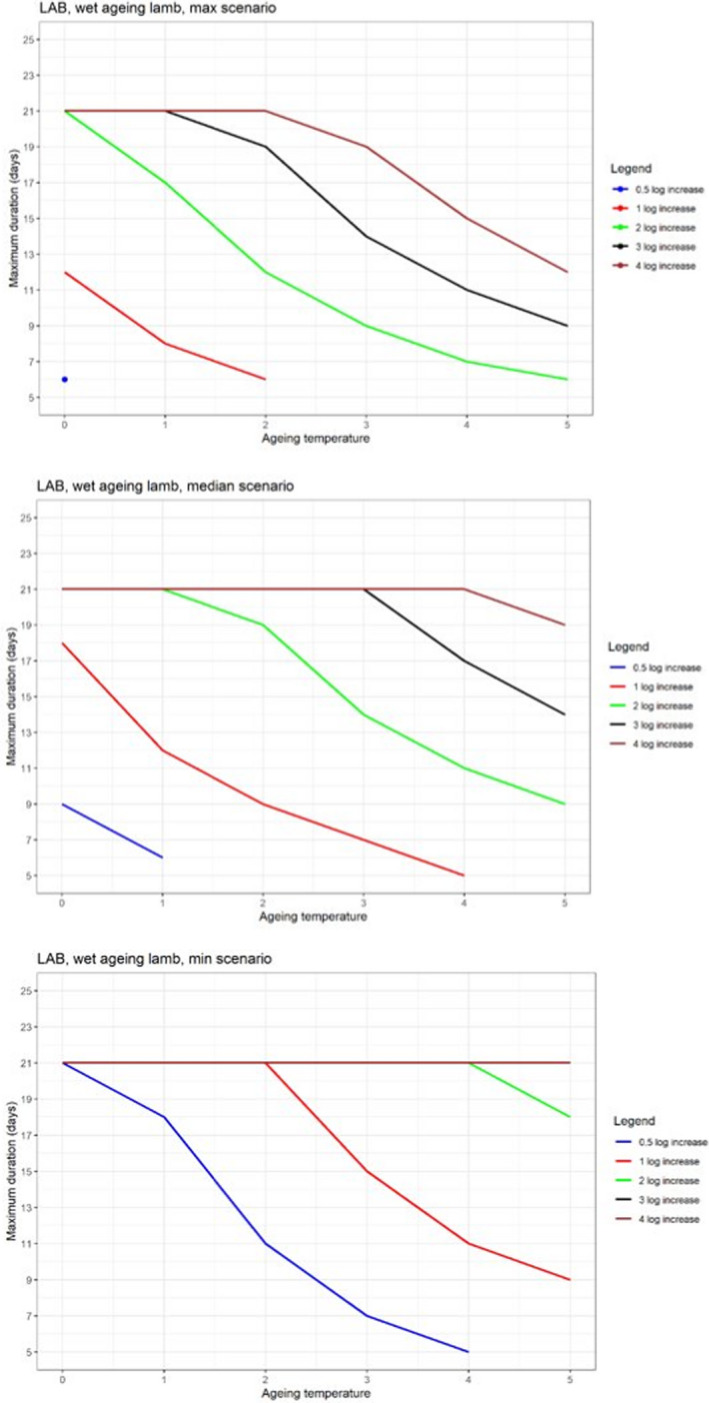
Relationship between ageing temperature and maximum time for wet‐ageing of lamb corresponding to different log10 increases of LAB considered equivalent to standard pork ageing under the assumption of three scenarios of pH and aw, i.e. maximum (pH = 5.9; aw = 0.99), median (pH = 5.7; aw = 0.97) or minimum scenarios (pH = 5.5; aw = 0.95), and a maximum ageing time of 21 days
Table F.2.
The estimated maximum time and temperature to stay below different targets corresponding to equivalent log increases as during standard fresh meat preparation for microbiological hazards and spoilage bacteria during wet‐ageing of beef, pork and lamb. The scenarios are predicted based on the minimum, median or maximum values of pH and aw. Maximum time evaluated was 49 days
| Target | Scenario: minimum (a) | Scenario: median (b) | Scenario: maximum (c) | |||
|---|---|---|---|---|---|---|
| Max time (at temperature) | Max temperature (at time) | Max time (at temperature) | Max temperature (at time) | Max time (at temperature) | Max temperature (at time) | |
| Beef | ||||||
| L. monocytogenes | ||||||
| 0.5 | 49 (3) | 4 (25) | 23 (0) | 0 (23) | – (e) | – |
| 1.0 | 49 (4) | 5 (29) | 46 (0) | 1 (23) | 28 (0) | 1 (15) |
| 2.0 | 49 (5) | 4 (49) | 49 (0) | 3 (19) | 49 (0) | 2 (19) |
| 3.0 | 49 (5) | 5 (49) | 49 (1) | 5 (16) | 49 (0) | 3 (20) |
| 4.0 | 49 (5) | 5 (49) | 49 (2) | 5 (22) | 49 (1) | 5 (15) |
| LAB | ||||||
| 0.5 | – | – | – | – | – | – |
| 1.0 | 20 (0) | 0 (20) | 15 (0) | 0 (15) | – | – |
| 2.0 | 40 (0) | 3 (16) | 30 (0) | 2 (15) | 24 (0) | 1 (17) |
| 3.0 | 49 (0) | 5 (16) | 45 (0) | 3 (18) | 36 (0) | 2 (19) |
| 4.0 | 49 (1) | 5 (21) | 49 (0) | 5 (15) | 48 (0) | 4 (15) |
| Pork | ||||||
| L. monocytogenes | ||||||
| 0.5 | 28 (2) | 5 (7) | 17 (0) | 2 (5) | 13 (0) | 1 (7) |
| 1.0 | 28 (3) | 5 (14) | 28 (0) | 4 (5) | 26 (0) | 3 (6) |
| 2.0 | 28 (5) | 5 (28) | 28 (1) | 5 (9) | 28 (1) | 5 (6) |
| 3.0 | 28 (5) | 5 (28) | 28 (2) | 5 (13) | 28 (1) | 5 (10) |
| 4.0 | 28 (5) | 5 (28) | 28 (3) | 5 (18) | 28 (2) | 5 (13) |
| LAB | ||||||
| 0.5 | 28 (0) | 4 (5) | 9 (0) | 1 (6) | 6 (0) | 0 (6) |
| 1.0 | 28 (1) | 5 (9) | 18 (0) | 4 (5) | 12 (0) | 2 (6) |
| 2.0 | 28 (3) | 5 (19) | 28 (0) | 5 (9) | 24 (0) | 5 (6) |
| 3.0 | 28 (5) | 5 (28) | 28 (2) | 5 (14) | 28 (0) | 5 (9) |
| 4.0 | 28 (5) | 5 (28) | 28 (3) | 5 (18) | 28 (1) | 5 (12) |
| Yersinia (d) | ||||||
| 0.5 | – | – | – | – | – | – |
| 1.0 | 8 (0) | 1 (5) | 8 (0) | 1 (5) | 8 (0) | 1 (5) |
| 2.0 | 16 (0) | 4 (5) | 16 (0) | 4 (5) | 16 (0) | 4 (5) |
| 3.0 | 24 (0) | 5 (6) | 24 (0) | 5 (6) | 24 (0) | 5 (6) |
| 4.0 | 28 (0) | 5 (8) | 28 (0) | 5 (8) | 28 (0) | 5 (8) |
| Lamb | ||||||
| L. monocytogenes | ||||||
| 0.5 | 28 (2) | 5 (6) | 26 (0) | 3 (5) | 14 (0) | 1 (7) |
| 1.0 | 28 (3) | 5 (12) | 28 (0) | 5 (5) | 28 (0) | 3 (6) |
| 2.0 | 28 (4) | 5 (24) | 28 (2) | 5 (11) | 28 (1) | 5 (7) |
| 3.0 | 28 (5) | 5 (28) | 28 (3) | 5 (17) | 28 (2) | 5 (11) |
| 4.0 | 28 (5) | 5 (28) | 28 (4) | 5 (23) | 28 (2) | 5 (15) |
| LAB | ||||||
| 0.5 | 28 (0) | 4 (5) | 9 (0) | 1 (6) | 6 (0) | 0 (6) |
| 1.0 | 28 (1) | 5 (9) | 18 (0) | 4 (5) | 12 (0) | 2 (6) |
| 2.0 | 28 (3) | 5 (18) | 28 (0) | 5 (9) | 24 (0) | 5 (6) |
| 3.0 | 28 (5) | 5 (28) | 28 (2) | 5 (14) | 28 (0) | 5 (9) |
| 4.0 | 28 (5) | 5 (28) | 28 (3) | 5 (19) | 28 (1) | 5 (12) |
Minimum scenario – beef: pH = 5.1, aw = 0.97, Minimum scenario – pork: pH = 5.4, aw = 0.95, Minimum scenario – lamb: pH = 5.5, aw = 0.95.
Median scenario – beef: pH = 5.5, aw = 0.98, Median scenario – pork: pH = 5.85, aw = 0.97, Median scenario – lamb: pH = 5.7, aw = 0.97.
Maximum scenario – beef: pH = 5.9, aw = 0.99, Maximum scenario – pork: pH = 6.3, aw = 0.99, Maximum scenario – lamb: pH = 5.9, aw = 0.99.
Yersinia predictive model is a temperature only model, not pH or aw included in the model.
‘–’ indicates that none of the evaluated conditions result in log increases below the target.
3.6.4. Impact of factors on predictions
3.6.4.1. Competition
The impact of competition from LAB on the potential growth of L. monocytogenes was evaluated for different scenarios of initial concentrations of LAB (Table 3). The initial concentration of L. monocytogenes is also provided to allow calculation of the predicted log10 increase, which was used in other evaluations to quantify the potential growth. There was a clear impact of competition as compared to no competition (No LAB in Table 8) already at a low LAB concentration of 1 log10 CFU/g, and especially for the upper percentiles. The predicted 95th‐percentile was below 100 CFU/g when the LAB concentration was higher than 3 log10 CFU/g but not when it was 2 log10 CFU/g (Table 8).
Table 8.
L. monocytogenes predicted final concentrations (log10 CFU/g) after wet‐ageing depending on initial level of LAB bacteria assuming competition according to the Jameson effect. Conditions for the modelling are shown in Table 3
| Scenario | Stage | L. monocytogenes concentrations (log10 CFU/g) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 75 percentile | 95 percentile | Maximum | ||
| Initial concentration (a) | Start fresh meat preparation/ageing | −1.27 | −1.32 | −0.97 | −0.45 | 0.65 |
| No LAB | End ageing | 0.80 | 0.71 | 1.40 | 2.57 | 5.04 |
| 1 log10 CFU/g | 0.76 | 0.70 | 1.35 | 2.38 | 4.65 | |
| 2 log10 CFU/g | 0.69 | 0.64 | 1.25 | 2.18 | 4.33 | |
| 3 log10 CFU/g | 0.52 | 0.47 | 1.01 | 1.86 | 3.71 | |
| 4 log10 CFU/g | 0.21 | 0.18 | 0.69 | 1.47 | 3.08 | |
LM0V = rnorm, mean = −1.40, sd = 0.55, rtrunc = TRUE, linf = −2, lsup = 1.
3.6.4.2. Inactivation
The effect of including inactivation in the assessment of the microbial behaviour during meat ageing was evaluated for L. monocytogenes on dry‐ageing of beef using different predictive models. The conditions used for the evaluation are shown in Table 3. Inactivation was evaluated under the assumption of no‐growth conditions, or independent of growth conditions (all times). The predicted log10 increase of L. monocytogenes during dry‐ageing of beef based on variable temperature, pH, aw, and duration with or without inactivation considered in the modelling is shown in Table 9. The greatest impact of inactivation was with the model obtained from Van Damme data assuming inactivation occurring in parallel with growth. Under this scenario there was a mean decrease of 0.26 log10, i.e. corresponding to approximately a halving of the L. monocytogenes concentration. The observed log10 reduction after 42 days ranged between approximately 1 to more than 3 logs (Van Damme et al., 2022) contrary to our predictions. However, a log10 increase was also observed in some of the dry‐ageing meat samples in that study. The Coroller et al. (2012) model employs parameters that depend on the environmental conditions so the most reasonable outcome from that model is the ‘all times‐prediction’. The impact of inactivation with this scenario was a decrease around 0.1 log10 except for the maximum log10 increase compared to the prediction with no inactivation (Table 9).
Table 9.
Predicted log10 change of L. monocytogenes during dry‐ageing of beef. Log10 increase is shown for two different inactivation models under the assumption of inactivation occurring all times or only under no‐growth conditions
| Scenario | Model | L. monocytogenes log10 change | |||||
|---|---|---|---|---|---|---|---|
| Mean | Median | 5th percentile | 75th percentile | 95th percentile | Maximum | ||
| No inactivation | – | 1.02 | 0.84 | 0 | 1.49 | 2.68 | 7.68 |
| Inactivation, all times | Van Damme | −0.26 | −0.37 | −1.55 | 0.27 | 1.36 | 4.95 |
| Inactivation, when no growth is predicted | Van Damme | 0.61 | 0.56 | −1.27 | 1.35 | 2.66 | 7.68 |
| Inactivation, all times | Coroller | 0.89 | 0.72 | −0.13 | 1.36 | 2.52 | 7.39 |
| Inactivation, when no growth is predicted | Coroller | 0.97 | 0.81 | −0.12 | 1.47 | 2.68 | 7.68 |
3.6.4.3. Rate of drying
Two studies, cases 2 and 5 of the realistic examples (Section 3.3.2.2), reporting temperature, aw and pH during dry‐ageing of beef were used to evaluate the effect of the rate of drying. These data were used in Section 3.5.3 as input data in the L. monocytogenes growth model to predict the log10 increase during dry‐ageing. To illustrate the effect of the rate of drying, i.e. the decrease of aw, the aw‐data were used here as reported and the temperature and pH were set at 2.5°C and 5.5, respectively. The rate of drying is dependent on temperature, but temperature and pH were similar in these case studies. Using the original aw case study data and the same pH and T, the effect of different rates of aw decrease results in a difference in predicted log10 increase of about 1 log10 after 42 days of ageing (Figure 20).
Figure 20.
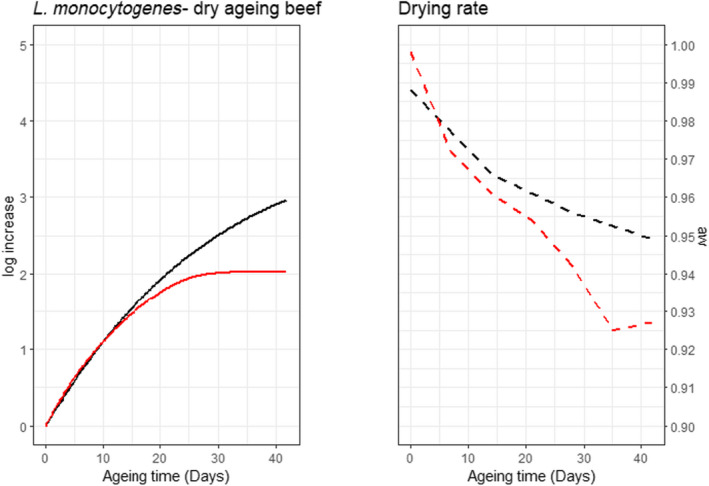
The predicted log10 increase of L. monocytogenes during dry‐ageing of beef depending on the change of aw over time. Temperature was assumed to be 2.5°C and pH 5.5. The black line is case study 2 and the red line case study 5
3.6.4.4. Trimming
One study reported numbers on dry‐aged loin surfaces and freshly trimmed steaks originating from the same loins (Gowda et al., 2022). On average, numbers on the steak surface were 0.8–1.0 log10 lower than on the loin surface before trimming for total psychrotrophic aerobic bacteria, Pseudomonas spp., and Br. thermosphacta. Moulds were detected on 4 out of 13 loins and only 3 out of 30 steaks. However, numbers are very variable after trimming, and occasionally high numbers are found on steaks. Moreover, Enterobacteriaceae were detected on 4 out of 13 loins and 12 out of 30 steaks, in numbers up to 7.4 log10 CFU/cm2. Listeria spp. were found on 3 steaks but not on any of the loins. Although trimming can potentially reduce bacterial/mould contamination, numbers are generally only slightly lower than numbers on the dry‐aged loins, and additional (environmental) contamination may occur during trimming.
Due to the limited data that are available for assessing the effect of trimming, this step in the production of dry‐aged beef was not included in the models. Trimming could result in an increase or decrease of pathogens. When trimming is performed hygienically, it will result in a decrease of pathogens, assuming that there is no internal contamination of the meat.
3.6.4.5. Storage
Due to the lack of data about the storage conditions of dry‐aged, wet‐aged and standard beef, pork and lamb, the growth of pathogens/spoilage bacteria during storage was not predicted.
The aw of trimmed dry‐aged beef steaks is similar to the aw of standard fresh beef. Assuming that the pH is also similar, the growth of Listeria monocytogenes on dry‐aged meat during storage is expected to be equal or lower than that on standard fresh meat. However, differences in storage (time, temperature, packaging) and background microbiota may result in different bacterial counts on standard fresh meat, dry‐aged beef and wet‐aged meat.
3.7. TOR5: to recommend additional good hygiene practices specific to the production and storage of dry‐aged and wet‐aged meat, as compared to those relevant for the production and storage of standard fresh meat
3.7.1. Food safety assurance
The food safety of standard fresh meat, dry‐aged beef and wet‐aged meat is assured through the development and implementation of hazard analysis and critical control point (HACCP) and prerequisite programme (PRP) activities, including good hygiene practice (GHP). HACCP targets specific hazards using CCPs. These are defined as a step at which a control can be applied and is essential to prevent or eliminate a hazard or reduce it to an acceptable level (Codex Alimentarius, 2001 (Joint Fao/Who Codex Alimentarius Commission F and Agriculture Organization of the United Nations WHO, 2001)). Each CCP has critical limits (e.g. temperature) and this parameter(s) is constantly monitored with any breach requiring corrective action. GHP is a standardised way of operating which ensures that foodstuffs are produced safely and hygienically.
The HACCP and PRP should be applied at all stages along the meat chain including slaughter, carcass dressing, chilling, fresh meat preparation, dry or wet‐ageing and during subsequent storage in catering or retail (Ninios et al., 2014). Indeed, Regulation (EC) No 853/2004 lays down specific hygiene requirements that must be implemented by food businesses handling food of animal origin at all stages of the food chain including the specific hygiene requirements for premises and operations such as production, handling, processing, storage and distribution.
A full description of the HACCP and PRP used in meat plants, butcher shops and/or restaurants is beyond the scope of this Opinion. Moreover, as standard fresh meat preparation and wet‐aged meat differ only in the time applied, the control activities for these processes are similar. This section will therefore primarily focus on the current recommendations in the scientific and grey literature for hygienically producing dry‐ageing beef, including international guidance on the conditions required during the dry‐ageing of beef to minimise bacterial growth and/or prevent mycotoxin production. The use of the predictive modelling outputs in ToR4 to develop critical limits (in terms of time and temperature) for dry‐ageing beef and wet‐ageing beef, pork and lamb that ensures the growth of pathogenic and spoilage bacteria is no higher than that obtained on standard fresh meat is also presented. Finally, any additional hazards arising from the preparation of minced meat or MSM from dry or wet‐aged meat are discussed.
3.7.2. Hygienically producing dry‐aged beef
Although limited information is available, the following practices have been reported as contributing to the hygienic production of dry‐aged beef (Asefa et al., 2010; Perry, 2012; López‐Gómez et al., 2013; Park et al., 2018; Rezende‐de‐Souza et al., 2021).
Use fresh meat of good microbial quality. Dark, firm and dry (DFD) meat must not be used as the higher pH would facilitate pathogen growth during the ageing process. Thus, all primals and sub‐primals intended for dry‐ageing should be checked to ensure the pH is in the usual range of 5.5 to 6.2 and inspected for overall condition.
Dry‐ageing should be undertaken in a dedicated purpose‐built room or chamber, thus avoiding cross contamination from other meat products, and the primals/sub‐primals should be aged for the correct period of time. Dry‐aged meat products should be fully traceable.
The beef should not be placed in the chamber until the required temperature and RH have been achieved (to minimise the opportunity for pathogen growth) and the specific conditions of time, temperature, RH and airflow should be strictly applied and continuously monitored.
The meat cuts should be hung from the bone to prevent internal contamination and they should be separated with sufficient space between them to facilitate air flow. If using a shelf, it should be sufficiently perforated to allow for effective air flow and the meat cuts should be turned regularly, using hygienic methods.
The highest airflow should be used at the start of the ageing process to facilitate early crust development and reduce the surface aw, thereby restricting bacterial growth. As a general rule, the ageing time should be as short as required to achieve the desired organoleptic properties as longer storage will allow for growth of pathogenic and spoilage bacteria.
The chamber used for dry‐ageing of the beef must be carefully cleaned and disinfected between batches including racks, shelves, etc. Moreover, the chamber should be empty during cleaning to avoid cross‐contamination with chemical and physical contaminants.
Air conditioning refrigeration system components such as evaporative coolers, evaporative condensers and cooling towers should be designed to facilitate effective cleaning and disinfection.
Thermometers, relative humidity probes and other equipment monitoring the set conditions in the chamber must be routinely calibrated close to the range at which they are used.
The air leaving the evaporator, returning to the evaporator and coming in contact with the beef should be filtered or ultraviolet (UV) treated to eliminate microbial contamination thereby minimising cross contamination.
Trimming of the crust should be carried out in a hygienic manner immediately after removal from the chamber avoiding perforating the meat below the crust. This activity should be performed in an area separate to those used for fresh meat.
The trimming area should be air‐conditioned to avoid cross contamination from other meat areas and have dedicated equipment that is hygienically maintained.
The dry crust should not be used for the preparation of other products as it contains most of the microbial contamination unless subsequently treatments such as heat or high pressure are applied to eliminate any pathogens present.
3.7.3. Preventing mycotoxin production during the dry‐ageing of beef
Mould growth and mycotoxin production during the dry‐ageing of beef have been discussed in Section 3.4.1. Based on the limited available literature, the formation of mycotoxins below 5°C is considered very unlikely (Olivier, 2018). Moreover, due to the reduction in aw on the dry‐aged meat surface, the probability of moulds producing mycotoxins is further reduced. The Meat and Livestock Australia (MLA, 2019) concluded that using dry‐ageing conditions limited to temperatures of −0.5 to 3.0°C with an RH of 75 to 85% for 14–35 days was unlikely to allow for mycotoxin production.
These conditions are also supported by PrimeSafe (Victoria, Australia) who recommend an air temperature in the dry‐ageing chamber of −0.5 to 1.0°C (although > 1 to 3°C could be acceptable where the ageing process lasts 7–14 days), a RH of 75 to 85% and an air velocity of 0.2–0.5 m/s for 14–35 days.
3.7.4. Using the outputs of ToR4 to establish the critical time and temperature limits for dry‐ageing beef and wet‐ageing beef, pork and lamb
The modelling analysis undertaken in ToR4 may be used to estimate equivalence in terms of specific bacterial growth during dry‐ageing (Section 3.4.2) and wet‐ageing (Section 3.4.3). Dry‐ageing of beef for 35 days, for example, at a temperature of 3°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 2‐log10 increase in standard fresh beef. Thus, using these parameters (3°C for no more than 35 days) the FBO can ensure the growth of L. monocytogenes is no higher than would be achieved on standard fresh meat (used as the baseline). In a second example, based on the predictive modelling in ToR4, wet‐ageing of beef for 35 days at a temperature of 2°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 2‐log10 increase in standard fresh beef. Thus, the FBO can use this time–temperature combination to ensure that the wet‐aged beef does not have a higher concentration of L. monocytogenes (and all else being equal, present a higher risk to the consumer) than standard fresh beef.
3.7.5. Bactericidal treatments for dry and wet‐aged meat and consumer information as a PRP
Experimental studies have shown that hot acid solutions (Blagojevic et al., 2015) or high‐pressure processing (Witte et al., 2020) will reduce the bacterial load on the trimmings resulting from dry‐aged meat (Blagojevic et al., 2015; Witte et al., 2020). However, other considerations, such as effect on the organoleptic qualities of the meat and cost under commercial conditions may inhibit application. Both dry and wet‐aged meats are usually cooked before consumption and if cooked thoroughly this final preparation step will eliminate the bacterial non‐spore‐forming pathogens such as Salmonella spp. and STEC. However, aged meats may on occasion be insufficiently cooked or eaten raw (da Silva et al., 2019) to achieve a specific desired flavour (Lee et al., 2021), to increase the tenderness, decrease the cooking loss (Latorre et al., 2019) or simply in error (Suman et al., 2014). Raw meat products, such as, carpaccio or raw kibbeh, are popular in different regions and should include a warning about the risk associated with consumption.
3.7.6. Specific considerations for minced meat of MSM preparation from dry or wet‐aged meat
As previously stated, when chilled (not frozen) meat is used for minced meat it must be prepared either within no more than 6 days after the slaughter of the ungulates, or within 15 days in the case of boned, vacuum‐packed beef and veal (Point 2(b) of Chapter III to Section V of Annex III to Regulation (EC) No 853/2004). Before the production of mechanically separated meat, the maximum storage period of the (chilled) raw material can be no more than 7 days when derived from the on‐site slaughterhouse and 5 days in other cases (Point 3(a) and 4(a)of Chapter III to Section V of Annex III to Regulation (EC) No 853/2004). Thus, meat that is aged for more than 14 days or more is not currently allowed to be used for the production of minced meat or MSM. These regulations are based on the assumption that older meat will have higher concentrations of bacteria due to growth during chilled storage and some of these may be pathogenic to humans.
Minced meat and MSM may be considered to present a higher food safety risk to consumers as bacteria on the meat surface are mixed into the core during mincing and MSM preparation, thereby increasing the probability of survival if the meat product is not thoroughly cooked. Thus, limiting the opportunity for bacterial growth by restricting the time between slaughter and minced meat or MSM preparation would seem a sensible approach. However, there is limited and contradictory data as to whether longer (ageing) times necessarily results in higher bacterial counts. James et al. (2006) reviewed the available scientific evidence and concluded there was no scientific basis for the restrictions on the age of meat used to produce minced meat. A second review by James and James (2012) concluded that total viable, E. coli and Enterobacteriaceae counts from meat after mincing do not increase with the length of storage time prior to mincing. Beef, pork, and lamb (from the UK and New Zealand) were aged for up to 59, 18, 26, 67 days, respectively, after slaughter, before being minced. Total viable counts (TVC) in minced meat varied between 1 and 5.9 log10 CFU/g for beef, between 2.6 and 8.0 log10 CFU/g for pork, and between 2.7 and 8.5 for lamb and there was no correlation between the bacterial concentration in the minced meat and the duration of ageing. In contrast, Garner et al. (2014) reported a mean TVC of 2.9 log10 CFU/g in beef patties prepared from meat matured for 7 days which increased to 3.9–4.7 log10 CFU after 21 days and 6.4–7.2 log10 CFU after 42 days.
Regardless of the bacterial counts, if the minced meat or MSM are thoroughly cooked any bacterial pathogens such as STEC, Salmonella or L. monocytogenes will be killed. Thus, using meat that is aged for longer periods than applied to standard fresh meat and/or trimmings from dry‐aged meat should not present a higher risk to the consumer if proper cooking procedures are consistently applied.
4. Conclusions
ToR1: AQ1: What are the practices and processes used by meat FBOs and restaurants in the EU for the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb? Specifically, what are the processing conditions (e.g. time, temperature and RH) and the associated intrinsic factors (e.g. pH and aw) of meat surface for each of these processes?
In the EU, FBOs and restaurants usually dry‐age beef aerobically in dedicated chambers at 1–4°C and a RH of 75–85% for 21–35 days. Scientific studies are in broad agreement with the majority undertaken at 0–4°C, a RH of 70–80% and an airflow of 0.5–2.5 m/s for 14–35 days.
Wet‐ageing is an anaerobic process, with vacuum packaged primal and sub‐primal cuts vacuum packaged and stored at 0–4°C for 14–49 days (beef), 0–4°C for 4–6 days (pork) or −1 to 5°C for 7–77 days (lamb) in meat plants. The conditions used in scientific studies were similar with most using temperatures of 0.6–4°C, a RH of 75–85% and an air flow of 0.2–7.0 m/s for 21–35 days.
The shelf‐life for unpacked dry‐aged beef steaks was typically 4 days but ranged from 2 to 10 days. If packaged in modified atmosphere the shelf‐life was 5 days and if vacuum packed the shelf‐life increased to 18 days but ranged from 5 to 30 days. The shelf‐life for wet‐aged beef, pork and lamb was 3–5 days for unpacked steaks/chops and 10–14 days for vacuum packaged products. All of the above assume adequate chilled storage conditions at 0–4°C.
Commercial data on the pH and aw is lacking. The data published in the scientific literature (based on samples usually taken at the start and/or end of the ageing process) reported that the surface pH of the beef was usually 5.5–5.9 and the aw 0.95–0.99. The corresponding pH values for wet‐aged beef, pork and lamb were 5.1–5.9, 5.4–6.3 and 5.5–5.9, respectively. The aw values for wet‐aged meat ranged from 0.93 to 0.99, regardless of species.
ToR2: AQ2: What are the relevant microbiological hazards and spoilage bacteria that occur and which of these can grow and/or produce toxins during the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb and on the subsequently stored product, including in minced meat or MSM prepared from the aged meat?
The pathogenic bacteria that may be present on dry‐aged beef and/or wet‐aged beef, pork and lamb include Shiga toxin‐producing E. coli (STEC) (more common in beef), Salmonella spp., Staphylococcus aureus, L. monocytogenes, enterotoxigenic Yersinia spp. (usually pork), Campylobacter spp. and Clostridium spp. However, only L. monocytogenes and Y. enterocolitica are capable of growth on the meat surface under chilled (0–4°C) conditions. All of these pathogenic bacteria are relevant as although some may not grow under the conditions of ageing, they may survive dry‐ageing and wet‐ageing processes and may therefore be present in minced meat or MSM prepared from these meats.
The spoilage bacteria include Pseudomonads, the former Lactobacillus spp., Micrococcus spp., Enterococcus spp., Weissella spp., Brochothrix spp., Leuconostoc spp., Enterococcus spp., Lactococcus spp., Shewanella spp., Bacillus spp. and Clostridium spp., many of which are capable of growth during the ageing process. Pseudomonas spp. are the main spoilage bacteria on dry‐aged beef while Lactobacillus spp. and psychrotrophic/psychrophilic Clostridium spp. are the main spoilage bacteria of wet‐aged beef, pork and lamb.
Thamnidium spp., Pilaira anomala, Debaryomyces hansenii, Aspergillus spp., Cladosporium spp., Trametes gibbosa, Alternaria alternate, Polyporales spp., Nectriaceae spp., Penicillium roquefortii, Pleosporaceae spp., Ascomycota spp., Colletotrichum acutatum, Podospora anserine, Penicillium bialowiezense, Candida spp. and Penicillium polonicum have all been detected on dry‐aged beef. Moulds such as Aspergillus spp. and Penicillium spp. may grow on beef during the dry‐ageing process. These moulds may produce mycotoxins on fruit, grain and other crops at low temperatures, but this has not been reported for meat products. It was judged 60–90% certain that a meat surface temperature of −0.5 to 3.0°C, with a RH of 75–85% and an airflow of 0.2–0.5 m/s will prevent mycotoxin production at least up to 35 days.
ToR3: AQ3: What is the increase in the relevant microorganisms (from AQ2) during the dry‐ageing of beef and the wet‐ageing of beef, pork and lamb (from AQ1) and during subsequent storage, as compared to ‘standard fresh meat’?
-
10
Relevant values for the intrinsic (pH and aw) and extrinsic (temperature) factors, as well as ageing time, were identified and used to develop scenarios covering current practices for dry‐ageing of beef, and wet‐ageing of beef, pork and lamb. Scenario parameters were used as input in predictive models to estimate the potential log10 increases of pathogenic and spoilage bacteria during the preparation of standard fresh meat and ageing of meat.
Standard fresh meat from beef, pork and lamb is not defined in the legislation, and was defined here as typically anaerobic maturation for up to 14 days (beef) or 4 days (pork and lamb).
Other parameters in the standard fresh meat preparation such as temperature, pH and aw vary between producers and thus, the log10 increase during standard fresh meat preparation is variable depending on conditions of the process and the dynamic properties of the meat during the process. In addition, there is uncertainty associated with both the predicted log10 increases during ageing and standard ma fresh meat preparation, due to parameter, model and scenario uncertainties.
Based on the predicted growth, the main microbiological hazard to consider is L. monocytogenes for all meat types, and for pork also Y. enterocolitica. The main spoilage bacteria are LAB for all meat types, and, for dry‐aged beef, also psychrotrophic Pseudomonas.
Considering the scenarios of temperature, pH and aw resulting in minimum, median and maximum growth rate of L. monocytogenes, the predicted ranges of log10 increases of L. monocytogenes were estimated (Appendix G).
Under current practices, ageing of meat has an impact on the load of microbiological hazards and spoilage bacteria compared to standard fresh meat preparation. The extent and direction of the impact depend on the conditions of ageing, the properties of the meat, and the presence of competing microorganisms.
Ageing under defined and controlled conditions can achieve similar or lower loads of microbiological hazards and spoilage bacteria than the variable log10 increases predicted for standard fresh meat preparation.
ToR4: AQ4: What are the conditions for producing, handling (including trimming, cutting, packaging, etc.) and storing dry‐aged beef and wet‐aged beef, pork and lamb to ensure similar or lower counts/load/concentrations of pathogenic microorganisms, spoilage bacteria and, if relevant, mycotoxins at the end of shelf‐life as compared to standard fresh meat?
As the log10 increase in standard fresh meat varies depending on the conditions used, the conditions for meat ageing to achieve equivalent growth are provided for a given log10 increase in standard fresh meat.
Based on the predicted log10 increases during standard fresh meat preparation, conditions of time and temperature during ageing that result in an increase of up to 0.5, 1, 2, 3 or 4 log10 of pathogenic or spoilage bacteria were evaluated using developed relationships between predicted time and temperature under different scenarios of pH and aw.
The impact of sources of uncertainties due to, for instance the effects of microbial competition between spoilage and pathogenic bacteria (wet‐ageing), inactivation (dry‐ageing), trimming and storage, and the variable and dynamic (over time) pH and aw was considered and assessed using informal EKE.
Examples of conditions ensuring an equivalent log10 increase of L. monocytogenes as the most relevant pathogen under the scenario of medium pH and aw in the meat are assessed as:
- Dry‐ageing beef:
-
○It was judged 80–90% certain that dry‐ageing of beef for 35 days at a temperature of 3°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 2‐log10 increase in standard fresh beef.
-
○
- Wet‐ageing beef
-
○It was judged 66–90% certain that wet‐ageing of beef for 35 days at a temperature of 2°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 2‐log10 increase in standard fresh beef.
-
○
- Wet‐ageing pork
-
○It was judged 66–90% certain that wet‐ageing of pork for 10 days at a temperature of 3°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 1‐log10 increase in standard fresh pork.
-
○
- Wet‐ageing lamb
-
○It was judged 66–90% certain that wet‐ageing of lamb for 10 days at a temperature of 3°C will not result in a higher log10 increase in the concentration of L. monocytogenes than an assumed 1‐log10 increase in standard fresh lamb.
-
○
ToR5: AQ5: What additional control actions including prerequisite programme (GHP and GMP) and CCPs could be employed to minimise the prevalence and/or concentration of pathogenic and spoilage bacteria and mycotoxin formation (if relevant) on dry and wet‐aged meat?
Twelve currently used or recommended PRP activities are provided that should be used to prevent contamination and minimise the growth of pathogens and spoilage bacteria.
Conditions for the dry‐ageing of beef that prevent mycotoxin production are provided in the answers to Tor2 (3rd bullet point).
The only difference between standard fresh beef, pork and lamb and wet‐aged beef, pork and lamb is the time used for ageing. The same prerequisite activities and HACCP required for standard fresh meat should therefore be applied for wet‐aged meat. Moulds do not grow on wet‐aged meat and mycotoxin production is therefore not an issue.
The modelling analysis undertaken in ToR4 (Sections 3.4.2 and 3.4.3) provides the time and temperature combinations that could be used for dry‐ageing of beef and wet‐ageing of beef, pork and lamb to ensure bacterial growth is equivalent or less than that which would be achieved during standard fresh meat preparation. This approach could be used by FBOs to further enhance the safety of aged meat products.
It is currently not possible to conclude on the relative food safety of minced meat and MSM prepared from dry or wet‐aged meat, as compared to standard fresh meat due to the lack of information on the impact of the time between slaughter, chilled storage and minced meat or MSM preparation on bacterial growth and the limited microbiological data on bacterial counts in minced meat and MSM prepared from aged meat (more than 14 days). However, if the minced meat (including trimmings from dry‐aged beef) or MSM are thoroughly cooked any vegetative bacterial pathogens such as STEC, Salmonella or L. monocytogenes will be eliminated.
5. Recommendations
Research is required to establish the exact conditions under which moulds such as Aspergillus spp. and Penicillium spp. produce mycotoxins including challenge studies on dry‐aged meat using a range of different combinations of temperature, RH, airflow and time that achieve varying combinations of surface temperature, aw and pH. The information generated should inform future food safety control systems for the dry‐ageing of beef.
Research is also required on the effect of the time between slaughter and minced meat or MSM preparation on the bacterial counts, including psychrotrophic and psychrophilic pathogenic bacteria. This could inform a risk assessment of any additional risks associated with minced meat and MSM prepared from dry‐aged beef or wet‐aged beef, pork or lamb, which would, in turn, facilitate a review of current legislation (Regulation (EC) No 853/2004).
Challenge tests are required to assess the growth of pathogens such as L. monocytogenes during different conditions of dry‐ageing of beef and wet‐ageing of beef, pork and lamb and during subsequent storage to validate the predictions in ToR3 and ToR4.
Abbreviations
- Af
accuracy factor
- AQ
assessment question
- aw
water activity
- Bf
bias factor
- CCP
critical control point
- DFD
dark firm dry
- FBO
food business operator
- FSSP
Food Spoilage and Safety Predictor
- GHP
good hygiene practice
- GMP
good manufacturing practice
- HACCP
Hazard Analysis and Critical Control Point
- LAB
lactic acid bacteria
- MSM
mechanically separated meat
- PRP
prerequisite programme
- RH
relative humidity
- SQ
sub‐question
- STEC
Shiga toxin‐producing Escherichia coli
- TEC
total Enterobacteriaceae count
- ToR
Term of Reference
- TVC
total viable count
Appendix A – Questionnaire
A.1. Version 1 of the questionnaire sent on 27 October 2021
Meat ageing practices in the EU
General Information
The European Food Safety Authority (EFSA) received a request for a ‘scientific opinion on the microbiological safety of aged meat’ with the aim to describe current practices of dry and wet‐ageing of meat and assess its biological safety. You can find further information about the mandate here: EFSA‐Q‐2020‐00527.
The purpose of this survey is to collect data on current practices used by food business operators when preparing ‘standard fresh meat’, dry‐ageing beef and wet‐ageing beef, pork and lamb (e.g. time, temperature, relative humidity, air flow, type of packaging, etc.) to inform the assessment.
For the purposes of this questionnaire ‘standard fresh meat’ is meat that has not undergone dry or wet‐ageing processes. As most beef, pork and lamb is matured in vacuum packs, it is important to distinguish between ‘standard fresh meat’ versus ‘wet‐aged meat’. In this opinion vacuum packaged meat is considered to be ‘standard fresh meat’ if the maturation process takes 14 days or less. More prolonged ageing/maturation in vacuum packs is considered to be ‘wet‐aged’.
Contact information (contact person, e‐mail)
Please select the option that applies to your company:
-
○
Meat plant
-
○
Restaurant
-
○
Other (please specify)
Please select which type of meat ageing practice/s your company applies:
-
○
Dry‐ageing
-
○
Wet‐ageing
-
○
Both
Standard Fresh Meat
The safety assessment of aged meat will be done in comparison to ‘standard fresh meat’. Could you provide data on the minimum maturation time and storage conditions, i.e. temperature and packaging, that is needed to tenderise standard fresh meat when it is not intended to be aged? What temperature, time and bag (e.g. BB2050U bags, CryoVac) are used for ‘standard fresh meat’ including:
| Temperature (°C) | Time (days) | Bag/no bag | |
|---|---|---|---|
| Beef | |||
| Pork | |||
| Lamb |
Dry‐Aged Beef
-
1
What beef cuts are dry‐aged?
-
2
What temperature, relative humidity (RH), airflow, time and bag (e.g. Tumbling® type)/no bag are used? (Please include all of the combinations of meat ageing processes used) (e.g. 2°C, 80%, 0.5 m/s, 35 days, no bag)?
| Meat ageing process(es) | Temperature (°C) | RH (%) | Airflow (m/s) | Duration of ageing (days) | Bag/no bag |
|---|---|---|---|---|---|
-
3
Do you measure the initial and final pH, available water (aw) and/or surface temperature for each combination of meat ageing processes listed above? If so please provide these data in the table below.
| Meat ageing process(es) | Surface pH | Surface aw | Surface T (°C) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
-
4
Do you test the initial and final bacterial load total viable count (TVC) and/or total Enterobacteriaceae count (TEC) and/or other bacteria for each combination?
If so, please provide the most recent data for each meat ageing process.
| Meat ageing process(es) | TVC | TEC | Other specify | Other specify | Other specify | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| 1 | ||||||||||
| 2 | ||||||||||
-
5
Do you test for Salmonella, Shiga toxin‐producing E. coli (STEC), Listeria monocytogenes, Staphylococcus aureus and/or Yersinia enterocolitica (tick as appropriate). If so, what is the average prevalence on the meat before and/or after ageing (e.g. 1% of samples positive)
| Prevalence (average %) | ||
|---|---|---|
| Before ageing | After ageing | |
| Salmonella | ||
| STEC | ||
| Listeria monocytogenes | ||
| Staphylococcus aureus | ||
| Yersinia enterocolitica | ||
|
Other: Please specify |
||
-
6
Do you test dry‐aged beef for moulds? Yes/No
If yes then:
(Sub‐question for yes) Which moulds do you test for and what is the maximum concentration permitted for each mould tested for?
-
7
Do you test for mycotoxins in dry‐aged beef? Yes/No
If yes then:
(Sub‐question for yes) Which mycotoxins and what was the maximum concentration obtained in the last 6 months?
-
8
What hygiene practices do you use when preparing dry‐aged beef? (Please tick as appropriate):
□ Hand washing
□ Knife washing/sterilisation
□ Cleaning & disinfection of the production area
□ Others (please specify all other hygiene measures used):
-
9
What are the recommended storage conditions and the shelf‐life of the dry‐aged beef you produce?
-
10
If you produce minced meat from the dry‐aged beef what are the recommended storage conditions and the shelf‐life of this product?
-
11
If you produce mechanically separated meat (MSM) from the dry‐aged beef (what are the recommended storage conditions and the shelf‐life of this product?
-
12
Other information
| Weight of dry‐aged beef produced in 2020 | Weight of minced meat from dry‐aged beef in 2020 |
Weight of MSM from dry‐aged beef in 2020 |
|
|---|---|---|---|
| Dry‐aged beef: |
-
13
Do you have any additional comments which you consider relevant for the assessment?
Wet/Vacuum Aged Beef, Pork & Lamb
1) What beef, pork and/or lamb cuts are wet/vacuum aged?
Beef: ____________________________________________
Pork: ____________________________________________
Lamb: ___________________________________________
Other: __________________________________________
2) What temperature, time and packaging films (brand & product code) are used? (Please include all of the combinations of meat ageing processes used) (e.g. 2°C, 6 weeks, CryoVac ‐ BB3055x)
| Meat ageing process(es) | Temperature (°C) | Duration of ageing (days) | Packaging (brand & product code) |
|---|---|---|---|
| Beef | |||
| Pork | |||
| Lamb |
3) Do you measure the initial and final pH, available water (aw) and/or surface temperature? If so, please provide these data for your last batch
| Meat ageing condition | pH | aw | Surface T (°C) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| Beef | ||||||
| Pork | ||||||
| Lamb | ||||||
4) Do you test the initial and final bacterial load total viable count (TVC) and/or total Enterobacteriaceae count (TEC) and/or other bacteria (please specify)? If so, please provide the most recent data for each meat ageing process.
| Meat ageing process(es) | TVC | TEC |
Other Specify |
Other Specify |
Other Specify |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| 1 | ||||||||||
| 2 | ||||||||||
5) Do you test for Salmonella, Shiga toxin‐producing E. coli (STEC), Listeria monocytogenes, Staphylococcus aureus and/or Yersinia enterocolitica (tick as appropriate). If so, what is the average prevalence on the meat before and/or after ageing (e.g. 1% of samples positive)
| Prevalence (average %) | ||
|---|---|---|
| Before ageing | After ageing | |
| Salmonella | ||
| STEC | ||
| Listeria monocytogenes | ||
| Staphylococcus aureus | ||
| Yersinia enterocolitica | ||
| Other (please specify) | ||
6) What hygiene practices do you use when preparing dry‐aged beef? (Please tick as appropriate):
□ Hand washing
□ Knife washing/sterilisation
□ Cleaning & disinfection of the production area
□Others (please specify all other hygiene measures used)
7) What are the recommended storage conditions and the shelf‐life of the wet‐aged beef, pork and/or lamb you produce?
8) If you produce minced meat from the wet‐aged beef, pork or lamb, what are the recommended storage conditions and the shelf‐life of this product?
9) If you produce mechanically separated meat (MSM) from wet‐aged beef, pork or lamb, what are the recommended storage conditions and the shelf‐life of this product?
10) Other information
|
Weight of product produced in 2020 |
Weight of minced meat from wet‐aged meat in 2020 |
Weight of MSM from wet‐aged meat in 2020 | |
|---|---|---|---|
| Wet/vacuum aged beef: | |||
| Wet/vacuum aged pork: | |||
| Wet/vacuum aged lamb: |
11) Do you have any additional comments which you consider relevant for the assessment?
A.2. Version 2 of the questionnaire sent on 02 December 2021
General question
What is the usual time between slaughter and start of dry/wet‐ageing?
(e.g. 1 day or 0–3 days)
Questions on dry‐ageing of beef
1) For how long is the beef typically dry‐aged? (e.g. 28 days).
2) What specific temperature do you use for dry‐ageing beef?
(This may be a single numerical value, e.g. 2°C or expressed as a range e.g. 0–3°C).
3) What is the relative humidity in the chamber when dry‐ageing beef? (e.g. 75%).
4) If you check the temperature inside the beef ageing room/chamber and/or on the surface of beef during dry‐ageing, what is the usual oscillation (minimum and maximum) and the actual mean temperature? (e.g. 4°C and the start and 1°C at the end of the dry‐ageing process).
5) If you check the pH of the surface of the beef during dry‐ageing, what are the usual start and finish pH values? If you have additional pH values, please specify.
(e.g. pH 5.9 at the start and 6.5 at the end of the dry‐ageing process).
6) If you check the aw of the surface of the beef during dry‐ageing, what are the usual start and finish aw values? If you have additional aw values, please specify.
(e.g. aw 0.998 and the start and 0.905 at the end of the dry‐ageing process).
Questions on wet‐ageing (maturing in a vacuum pack under chilled conditions) of beef, pork and lamb
1) For how long is the beef, pork or lamb typically wet‐aged (i.e. prolonged ageing)?
(Please specify for each relevant meat species and the time, e.g. beef ‐ 4 weeks, pork ‐ 3 days, lamb ‐ 4 days).
2) What specific temperature do you use for wet‐ageing beef, pork or lamb?
(Please specify for each relevant meat species. This may be a single numerical value, e.g. 2°C or expressed as a range e.g. 0–3°C).
3) If you check the temperature of the chamber or of the surface of the beef, pork or lamb during wet‐ageing, what are the usual oscillation (minimum and maximum)?
(Please specify for each relevant meat species. e.g. 4°C and the start and 1°C at the end of the process).
4) If you check the pH of the surface of the beef, pork or lamb during wet‐ageing, what are the usual start and final pH values?
(e.g. pH 5.9 at the start and 6.5 at the end of the process).
A.3. Summary of the replies of the questionnaire
Appendix B – Uncertainty analysis
An expert knowledge elicitation (EKE) was undertaken on the 8th of November, with the 5 members of the Aged Meat WG serving as experts. To prepare for this EKE, individual judgements were elicited for the correctness of 5 statements. Informed by the evidence collected in the draft opinion, including the uncertainty Table B.1, individual experts were asked to indicate how certain they were that the statements were correct. For expression of the uncertainty, they could use the standard ranges indicated in EFSA's approximate probability scale or any other probability range. Consensus was achieved during discussion in the WG meeting.
The 5 statements and the outcome of the uncertainty assessments were as follows:
ToR2:
‘A meat surface temperature of ‐0.5 to 3.0°C, with a RH of 75 to 85% and an airflow of 0.2–0.5 m/s will prevent mycotoxin production for at least 35 days.’
Certainty: 66–90%.
Comments: The experts mentioned the fact that this statement is based on research published in the 1970s (Ciegler and Kurtzman, 1970; Kurtzman and Ciegler, 1970; Buchanan et al., 1974; Sommer et al., 1974) (which report that mycotoxins can be produced at very low concentrations and very slowly by Penicillium spp. at temperatures as low as 0–1°C on fruits, corn and other crops) and the review published by the Meat and Livestock Australia (MLA) in 2019. The latter relies on 4 references. Three of these describe the production of mycotoxins in different foods (ICMSF, 1996; Hocking and Pitt, 2003; Pitt and Hocking, 2009) and conclude that Penicillium and Aspergillus spp. are incapable of producing mycotoxins at temperatures between −0.5 and 3°C. The fourth (Olivier, 2018) states that there was no evidence that moulds found on red meat are capable of producing mycotoxins at temperatures between −0.5 and 3°C and a RH of 75–85%.
ToR4:
-
2
‘Dry‐ageing of beef for 35 days at 3°C will not result in a higher log increase in the concentration of L. monocytogenes than an assumed 2‐log10 increase in standard fresh beef.’
Certainty: 80–95%.
Comments: The assessment of the pathogen behaviour was based on the application of predictive models for growth rate. Lag time, competition with other bacteria or inactivation were not considered to quantify the log increase. This conservative approach resulted in an overestimation of the predicted log increase. The availability of experimental data showing the behaviour of L. monocytogenes on the surface of beef during dry‐ageing is limited to a single study. This study suggests that L. monocytogenes will decrease during dry‐ageing of beef, probably as a result of the decrease in aw. In comparison, in only one of two replicates in one of the combinations (i.e., at 2°C, 85% RH, pH 6.21) of the factorial design (representing two meat types, temperatures and RH) an increase in L. monocytogenes was observed and this was limited to 1 log at day 42.
-
3
‘Wet‐ageing of beef for 35 days at 2°C will not result a higher log increase in the concentration of L. monocytogenes than an assumed 2‐log10 increase in standard fresh beef.’
Certainty: 66–90%.
Comments: As for dry‐ageing, the assessment of the pathogen behaviour was based on the application of predictive models of growth rate. Lag time, competition with other bacteria or inactivation were not considered to quantify the log increase. This conservative approach resulted in an overestimation of the predicted log increase.
There are several studies showing that L. monocytogenes can grow during the refrigerated storage of vacuum packaged beef. Several studies report no growth of L. monocytogenes but the inactivation extent was not as important as for dry‐ageing (because there is no reduction of the surface aw). It was considered that the degree of overestimation was lower than for dry‐ageing.
-
4
‘Wet‐ageing of pork for 10 days at 3°C will not result a higher log increase in the concentration of L. monocytogenes than an assumed 1‐log10 increase in standard fresh pork.’
Certainty: 66–90%.
Comments: As for statement 3 above.
-
5
‘Wet‐ageing of lamb for 10 days at 3°C will not result a higher log increase in the concentration of L. monocytogenes than an assumed 1‐log10 increase in standard fresh lamb.’
Certainty: 66–90%.
Comments: As for statement 3 above.
Appendix C – Calculation of calibration factor for the correction of predictive models used to simulate microbial growth in raw meat
C.1. Predictive model for Listeria monocytogenes
To simulate the growth of L. monocytogenes in raw meat during wet and dry‐ageing, the growth rate () model of Mejlholm and Dalgaard (Mejlholm and Dalgaard, 2009) was used. The model is part of the user‐friendly tool Food Spoilage and Safety Predictor (FSSP version v4.0, freeware available at http://fssp.food.dtu.dk/). The model was originally developed for processed and ready‐to‐eat seafood including 12 environmental factors and its interaction factor. 4 Its predictive performance was assessed in an international validation study focussed on processed and ready‐to‐eat meat and seafood products, by comparing the growth rate predicted by the model with that observed form 640 growth curves for L. monocytogenes in different types of processed meat, seafood, poultry and dairy products, showing satisfactory results for meat, poultry and non‐fermented dairy products with a bias factor (Bf = 1, indicating that on average there was no systematic over‐ nor underestimation of growth rates 5 ) and accuracy factor (Af = 1.5, as a measure of the average differences between observed and predicted values) (Mejlholm et al., 2010).
However, the validation study was not focussed on raw meat (not processed). Further, the model does not consider anaerobic conditions as an input factor, and the differences between aerobic (dry‐ageing) and anaerobic (vacuum packaged wet‐ageing of vacuum packaged meat).
Therefore, the performance of this predictive model specifically for raw meat stored under aerobic conditions and vacuum‐packaged was evaluated. Growth behaviour and environmental parameters regarding temperature, pH and aw were collected from experimental trials available in the ComBase Browser of the ComBase portal (www.combase.cc) and/or complemented with additional data form scientific literature. When directly reported the log counts were retrieved or digitalised from figures using the WebPlotDigitizer (v4.5) tool (Rohatgi, 2021). Unless provided by the authors, the growth rate was estimated by fitting the Baranyi and Roberts (1994) growth model using the DMFit tool available at the ComBase portal. A total of 66 growth rates values in raw meat under aerobic conditions and 8 growth rates values in vacuum‐packaged raw meat (anaerobic conditions) were obtained, covering different L. monocytogenes strains and experimental conditions (inoculation, meat species/pieces, packaging materials, inoculation procedure, storage temperature, enumeration methods, etc.).
The observed was compared with the predicted by a simplified version of the predictive model quantifying the effect of the temperature, pH, aw and undissociated lactic acid concentration (Eq. 1), thus ignoring the other environmental input factors not relevant for raw meat.
| (1) |
where, = 0.491; T is the reported temperature (°C) of the experiment, pH of the reported initial pH of the meat; aw is the initial water activity of the meat and LACu is the concentration of undissociated lactic acid in the water phase. In most cases, the aw of the meat was not reported by the authors and was assumed to be 0.996. Though, fresh raw meat is known to have a certain amount of lactic acid from endogenous origin, for the baseline predictions this input parameter was set at zero ppm. However, to quantify the possible impact of the uncertainty associated with these assumptions, the predicted was also obtained considering aw = 0.980 and endogenous lactic acid concentration of 4,000 ppm in water phase.
The bias factor (Bf) and accuracy factor (Af) were calculated by comparing the predicted versus observed according to Eq. 2 and Eq. 3, respectively (Baranyi et al., 1999)
| (2) |
| (3) |
The predictive model can be corrected by the matrix effect through the calculated Bf, as the calibration factor dividing the to compensate the systematic bias of the model predictions with respect to the observed in meat.
Figure C1 shows the comparison between the observed for L. monocytogenes in raw meat under aerobic conditions (left, n = 66 data) and vacuum packaging conditions (right, n = 12 data) and the predictions provided by the model. Under aerobic conditions, the predictive model tended to underestimate the with Bf = 0.74, i.e. grey dots were systematically below the equivalence line by 26% on average. After the applying the correction to the the dots (in green) fall just on the equivalence line (no bias on average) and the Af improved to from 1.61 (before correction) to 1.43 (after correction). The calculations for Bf and Af were performed taking into consideration (i) data from different temperature ranges and (ii) using different aw and lactic acid concentration as input factors. When data within temperature range 0–10°C was considered (n = 28) the resulting Bf was 0.78. If a lower aw value (0.98) and an endogenous concentration of lactic acid of 4,000 ppm was used as input for the predictive model, the Bf decreased to 0.44 (Af = 2.56).
Figure C.1.
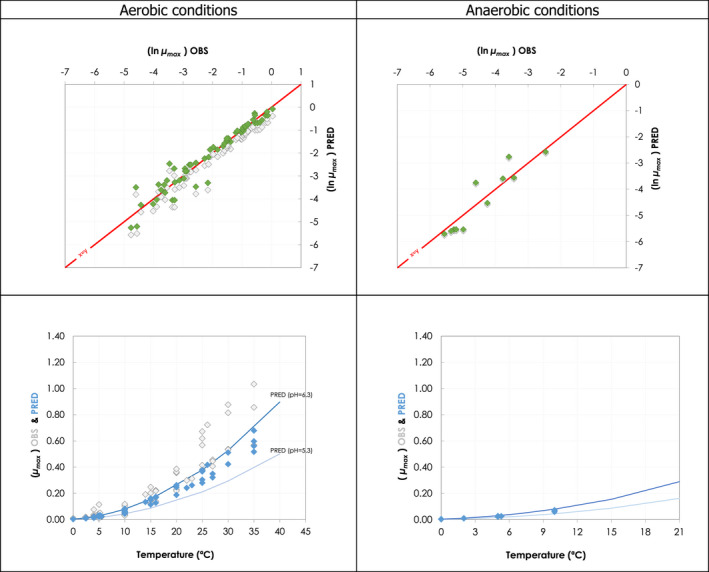
- Data from: (Lee et al., 2014); (Wang et al., 2015); (Solomakos et al., 2008); (Giménez et al., 2021) ComBase records ID: M371_LM; M372_LM; M373_LM; M374_LM; M781_LM; M801_LM; M754_LM; M782_LM; M755_LM; M905_LM; M783_LM; M803_LM; M53_LM; M798_LM; M756_LM; M804_LM; M369_LM; M370_LM; M757_LM; M52_LM; M758_LM; M759_LM; M760_LM; M761_LM; M784_LM; M805_LM; M785_LM; M762_LM; M763_LM; M779_LM; M786_LM; M807_LM; M764_LM; M787_LM; M799_LM; M765_LM; M766_LM; M788_LM; M789_LM; M808_LM; M767_LM; M768_LM; M769_LM; M770_LM; M771_LM; M790_LM; M791_LM; M800_LM; M809_LM; M772_LM; M773_LM; M774_LM; M775_LM; M776_LM; M792_LM; M793_LM; M777_LM; M778_LM; M780_LM; M794_LM; M795_LM; Pawr_1.
For vacuum‐packaged meat, much fewer data were available. Several studies reported no growth or even slight decrease of L. monocytogenes in vacuum packaged meat during the refrigerated storage (Mano et al., 1995; Zhang and Mustapha, 1999; Tsigarida et al., 2000; Dykes and Moorhead, 2002; Saraiva et al., 2016); Combase record ID: M802_LM, M157_3, M157_4, M157_11, M157_12. When growth of the pathogen was observed, on average the predictions matched the observed values (Bf = 0.94), though a poor Af = 1.63 was obtained.
A calibration factor of 0.76 was used to correct the of the predictive model (Eq. 1) to simulate the growth of L. monocytogenes in aerobically stored raw meat. This value corresponds to the average value of Bf obtained for all range of temperatures (0.74) and up to 10°C (0.78). For the uncertainty assessment, a uniform distribution from 0.74 to 0.78 was used.
The simulations for anaerobic conditions (vacuum‐packaged meat during wet‐ageing) were done with the original predictive model without any correction, as the Bf = 0.94 was considered satisfactory.
C.2. Predictive model for lactic acid bacteria
To simulate the growth of LAB in vacuum‐packaged meat during wet‐ageing, the growth rate () model of Mejlholm & Dalgaard (Mejlholm and Dalgaard, 2007, 2013) was used. The model is part of the user‐friendly tool Food Spoilage and Safety Predictor (FSSP version v4.0, freeware available at http://fssp.food.dtu.dk/). The model was originally developed for processed and ready‐to‐eat seafood including 12 environmental factors and its interaction factor. 6 Its predictive performance was assessed by comparing the predicted by the model with that observed form 320 growth curves for lactic acid bacteria in inoculated challenge tests or naturally contaminated seafood (112) and meat products (208), showing satisfactory results with a Bf = 1.1 and Af = 1.3.
However, the validation study was not focussed on raw meat (not processed). Further, the model does not consider anaerobic conditions as an input factor.
Therefore, the performance of this predictive model specifically for vacuum‐packaged meat was evaluated. Growth behaviour and environmental parameters regarding temperature, pH and aw were collected from experimental trials available in the ComBase Browser of the ComBase portal (www.combase.cc) and/or complemented with additional data form scientific literature. A total of 56 growth rates values for of naturally contaminating lactic acid bacteria on vacuum‐packaged raw meat (anaerobic conditions) were obtained, covering different microbial strains and experimental conditions (meat species/pieces, meat source and level of initial contamination, packaging materials, storage temperature, enumeration methods, etc.).
The observed provided by the authors was used or, when not provided, it was estimated by explained in C.1 above. A simplified version of the predictive model of Mejlholm and Dalgaard (2007) quantifying the effect of the temperature, pH and aw (Eq. 4), thus ignoring the other environmental input factors not relevant for raw meat.
| (4) |
where, = 0.583; T is the reported temperature (°C) of the experiment, pH of the reported initial pH of the meat; aw is the initial water activity of the meat and LACu is the concentration of undissociated lactic acid in the water phase. The baseline predictions amount of lactic acid from endogenous origin as input parameter was set at zero ppm. However, to quantify the possible impact of the uncertainty associated with these assumptions, the predicted was also obtained considering aw = 0.980 and endogenous lactic acid concentration of 4,000 ppm in water phase.
Bf and Af were calculated by comparing the predicted versus observed as described in C.1. Figure C.2 shows the comparison between the observed in vacuum packaged raw meat (n = 58 data) and the predictions provided by the model. The predictive model tended to overestimate the by 117% on average, with a Bf = 2.17, i.e., grey dots were systematically above the equivalence line. After the applying the correction to the the dots (in green) fall just on the equivalence line (no bias on average) and the Af improved to from 2.7 (before correction) to 1.85 (after correction). The calculated Bf and Af taking into consideration (i) data from different temperature ranges and (ii) using different aw and lactic acid concentration as input factors ranged from 1.30 to 1.66.
Figure C.2.
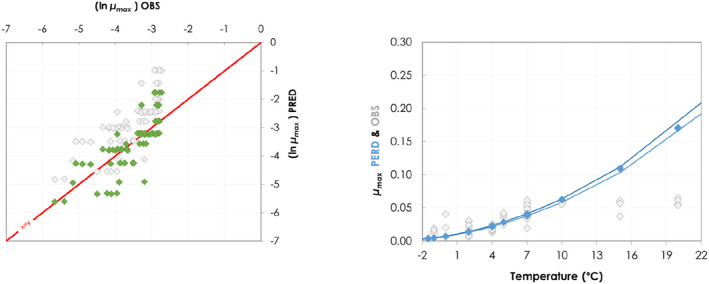
- Data from: (Barrera et al., 2007) Barrera et al. (2007); IRTA unpublished data; ComBase records ID: L‐1MRSA1; L‐1MRSA2; L‐1MRSAn1; L‐1MRSAn2; L2MRSA1; L2MRSA2; L2MRSAn1; L2MRSAn2; L7MRSA1; L7MRSA2; L7MRSAn1; L7MRSAn2; L_LAB0_1; L_LAB0_2; L_LAB‐1.5_1; L_LAB2_1; L_LAB2_2; L_LAB4_1; L_LAB4_2; L_LAB7_1; S_LAB0_1; S_LAB‐1.5_1; S_LAB2_1; S_LAB2_2; S_LAB4_1; S_LAB7_1; S_LAB7_2; CB01k_1_12; CB02k_1_12; CB03k_1_12; CB04k_1_12; CB05k_1_12; CB05k_2_12; CB05k_3_12; CB06k_1_12; CB06k_2_12; CB06k_3_12; CB07k_1_12; CB07k_2_12; CB07k_3_12; CB08k_1_12; CB08k_2_12; CB08k_3_12.
A calibration factor of 1.89 was used to correct the of the predictive model (Eq. 4) to simulate the growth of lactic acid bacteria in vacuum‐packaged meat. This value corresponds to the Bf value obtained for temperatures up to 10°C. For the uncertainty assessment, a uniform distribution from 1.30 to 2.17 was used.
C.3. Predictive model for pseudomonas
To simulate the growth of pseudomonas in meat under aerobic conditions during dry‐ageing, the growth rate () model of Neumeyer et al. (1997) was selected as it accounts for the effect of storage temperature and aw. The model, which is also available in ComBase Premium, was developed from experiments in laboratory growth media and for chilled beef stored between 0 to 20°C, including temperature as the sole environmental factors (Eq. 5).
| (5) |
The performance of this predictive model for aerobically stored meat was evaluated. Growth behaviour and storage temperature were collected from experimental trials available in the ComBase Browser of the ComBase portal (www.combase.cc) and/or complemented with additional data form scientific literature. A total of 80 growth rates values for of naturally contaminating pseudomonas on meat (aerobic conditions) were obtained, covering different microbial strains and experimental conditions (meat species/pieces, meat source and level of initial contamination, packaging materials, storage temperature, enumeration methods, etc.).
Bf and Af were calculated by comparing the predicted versus observed as described in C.1. Figure C.3 shows the comparison between the observed in meat and the predictions provided by the model. The predictive model overestimated (80%) the with a Bf = 1.80, i.e., grey dots were systematically almost above the equivalence line. Moreover, the Af was 1.95 was reduced to 1.37 after the correction, indicating an acceptable precision of the predictive model.
Figure C.3.
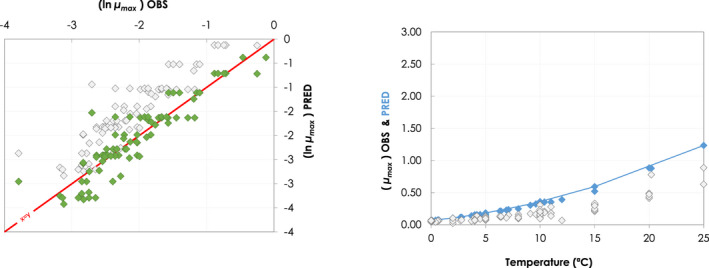
- Data from Skandamis and Nychas (2002a,b); Koutsoumanis et al. (2006); Tsigarida and Nychas (2001); Delaquis and McCurdy (1990); Lasta et al. (1995); Skandamis and Nychas (2002a,b) and ComBase records ID: allP_78; allP_79; allP_80; NP_101; NP_93; NP_97; NP_102; NP_94; NP_98; NP_103; NP_95; NP_99; NP_100; NP_104; NP_96; KTM_13; KTC_17; KTC_21; KTC_25; KTC_29; KMS_11; KML_11; Tas3484; Tas3485; Tas3486; Tas3487; Tas3499; Tas3500; Barrera_Ps1; PA_16; PA_15; PA_14; PA_13; BA_28; BA_27; BA_26; BA_25; PA_40; PA_39; PA_38; PA_37; BA_44; BA_43; BA_42; BA_41; PA_52; PA_51; PA_50.
C.4. Predictive model for Yersinia enterocolitica
Several mathematical models to quantify the growth rate of Y. enterocolitica were found in the literature (Lindberg and Borch, 1994); (Sutherland and Bayliss, 1994); (Adams et al., 1991) (Little et al., 1992) besides those available in ComBase portal and in the Pathogen Modelling Program. However, they were not considered appropriate for the purpose of the opinion because they were based on absorbance or conductance without direct application to quantify the cell concentration as cells or cfu and/or because they were focused on the effect of temperature, pH and acidulant.
A growth rate square root‐based model with temperature as the independent factor was obtained (Eq. 6) by fitting the experimental data extracted from Gill and Reichel (1989). This work dealt with the growth of Y. enterocolitica in high pH beef under vacuum packaging at five different temperatures (from −2°C, to 10°C). The predictions provided by this model were close to the observed (n = 23 data) with a slight overestimation (10% on average) as indicated by a Bf = 1.10 (Figure C.4). However, the accuracy of the predictions was fairly poor as indicated by an Af = 1.68
| (6) |
Figure C.4.
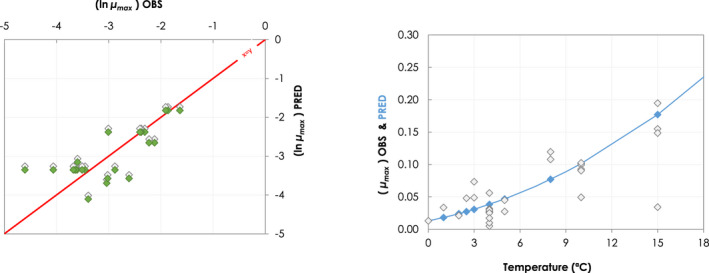
- Data from Gill and Reichel (1989); Bodnaruk and Draughon (1998); Özbaş et al. (1996) and ComBase records ID: Ye03_low_4; Ye03_high_4; Ye09_low_4; Ye09_high_4; Ye03_low_10; Ye03_high_10; Ye09_low_10; Ye09_high_10; Ye03_low_15; Ye03_high_15; Ye09_low_15; Ye09_high_15; M53_Ye; M54_Ye; M52_Ye; YE1_AIR_4; YE2_AIR_4; YE1_VP_4; YE2_VP_4.
Appendix D – Scenarios evaluated in ToR3 and ToR4
The scenarios, distributions and associated parameters used in the predictive modelling are summarised in Tables D.1–D.4.
Appendix E – Conditions and meat parameters reported for meat ageing in the scientific literature
The conditions and corresponding meat parameters reported in scientific studies for ageing beef, pork and lamb in the scientific literature are shown in Tables E.1, E.2, E.3.
The scenarios, distributions and associated parameters used in the predictive modelling are summarised in Tables D.1, D.2, D.3, D.4–D.1, D.2, D.3, D.4.
Appendix F – Tables equivalence conditions hazards and spoilage bacteria
Dry‐ageing
The maximum time or temperature (and the corresponding temperatures and times) conditions that would achieve different log increases considered equivalent to standard fresh beef preparation is shown in Table F.1. The importance of the pH and aw during ageing is illustrated by the different equivalent conditions resulting using different scenarios for these parameters. The scenarios are predicted based on scenarios with minimum, median, or maximum values of pH and aw during standard fresh meat preparation. For instance, assuming that equivalence is represented by a 1 log increase of L. monocytogenes, the maximum ageing time would be 77 days (at 5°C) in the minimum scenario and 20 days (at 0°C) in the maximum scenario (Table F.1). The pH and especially aw can be variable during dry‐ageing, and controllable to some extent. The impact of drying rate is evaluated in Section 3.6.4.3.
Wet‐ageing
The maximum time or temperature (and the corresponding temperatures and times) conditions that would achieve different log increases considered equivalent to standard fresh meat preparation of beef is shown in Table F.2. The importance of the pH and aw during ageing is illustrated by the different equivalent conditions resulting using different scenarios for these parameters. For instance, assuming that equivalence in pork is represented by a 1 log increase of L. monocytogenes, the maximum ageing time would be 28 days (at 3°C) in the minimum scenario and 26 days (at 0°C) in the maximum scenario (Table F.2). The maximum temperature for 1 log increase equivalence is 5°C but then maximum ageing time is only 14 days, whereas under the maximum pork scenario the maximum temperature is 3°C and ageing time only 6 days, i.e. only two more days than standard fresh meat preparation. The predictive model for Yersinia is only including temperature, and not aw or pH so the results are the same for all scenarios. The impact of additional factors that may influence the predicted log increase are evaluated in Section 3.4.4.
Appendix G – Predicted log increases of pathogens and spoilage bacteria during standard fresh meat preparation and ageing (ToR3)
The predicted log10 increase during ageing and standard fresh meat preparation. Numbers represent the range of predicted increases, min, median and max:
-
○Standard fresh meat preparation of beef:
- Lm: After 14 days min = 0, median = 1.1, max = 3.7
- LAB: After 14 days min = 0.8, median = 2.0, max = 4.5
- CB: After 14 days min = 0, median = 0, max = 0.3
-
○Dry‐aged beef:
- Lm: After 77 days min = 0.1, median = 5.1, max > 10
- LAB: After 77 days min = 0.8, median = 6.3, max > 10
- PS: After 77 days min = 0, median > 10, max > 10
-
○Wet‐aged beef
- Lm: After 49 days min = 0.2, median = 3.8, max > 10
- LAB: After 49 days min = 2.8, median = 6.9, max > 10
- CB: After 49 days min = 0, median = 0, max = 0.9
-
○Standard fresh meat preparation of pork
- Lm: After 4 days min = 0.1, median = 0.4, max = 0.6
- Ye: After 4 days min = 0.4, median = 0.9, max = 1.4
- CB: After 4 days min = 0, median = 0, max = 0.1
- LAB: After 4 days min = 0.1, median = 0.5, max = 0.9
-
○Wet‐aged pork
- Lm: After 28 days min = 0.4, median = 2.1, max = 4.9
- Ye: After 28 days min = 2.9, median = 6.1, max = 9.9
- CB: After 28 days min = 0, median = 0, max = 0.3
- LAB: After 28 days min = 1.1, median = 3.2, max = 6.4
-
○Standard fresh meat preparation of lamb
- Lm: After 4 days min = 0.1, median = 0.4, max = 1.1
- CB: After 4 days min = 0, median = 0, max = 0.2
- LAB: After 4 days min = 0.1, median = 0.6, max = 1.3
-
○Wet‐aged lamb
- days min = 0.2, median = 2.0, max = 5.8
- CB: After 21 days min = 0, median = 0, max = 1.0
- LAB: After 21 days min = 0.6, median = 3.0, max = 6.8
Supporting information
Protocol for the Scientific opinion on the microbiological safety of aged meat
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bover‐Cid S, Chemaly M, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Blagojevic B, Van Damme I, Hempen M, Messens W and Bolton D, 2023. Scientific Opinion on the microbiological safety of aged meat. EFSA Journal 2023;21(1):7745, 101 pp. 10.2903/j.efsa.2023.7745
Requestor European Commission
Question number EFSA‐Q‐2020‐00527
Panel members Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies (until 14 October 2022), Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The BIOHAZ Panel wishes to thank the following for the support provided to this scientific output: Maria Francesca Iulietto, Nino Terjung (hearing expert). The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Annex A is available under the Supporting Information section .
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 6 December 2022
Notes
The environmental factors (and the range of applicability) for the full model are: temperature (2–25°C), pH (5.6–7.7), aw (0.996–0.943; equivalent to 0.7–9.0% water phase salt, WPS), lactic acid (0–60,000 ppm in water phase, WP), atmosphere (0–100% CO2), smoke components (phenol: 0–20 ppm), nitrite (0–150 ppm in product), acetic acid (0–11,000 ppm in WP), benzoic acid (0–1800 ppm in WP), citric acid (0–6,500 ppm in WP), diacetate (0–3,000 ppm in WP), and sorbic acid (0–1,300 in WP).
To be considered good or acceptable, growth rates should not be over‐ or underpredicted by more than 43% and 13%, respectively, corresponding to a bias factor of between 1.43 and 0.87 (Ross, 1999; Mejlholm et al., 2010). Accuracy factors higher than 1.5 indicate poor model precision (Mejlholm and Dalgaard, 2013; Koukou et al., 2021).
The environmental factors (and the range of applicability) for the full model are: temperature (2–25°C), pH (5.3–7.7), aw (0.999–0.961; equivalent to 0.1–6.4% water phase salt, WPS), lactic acid (0–67,000 ppm in water phase, WP), atmosphere (0–100% CO2), smoke components (phenol: 0–21.2 ppm), nitrite (0–209 ppm in product), acetic acid (0–12,600 ppm in WP), benzoic acid (0–1800 ppm in WP), citric acid (0–7,300 ppm in WP), diacetate (0–3,000 ppm in WP), and sorbic acid (0–1,300 in WP).
References
- ACMSF , 2020. The safety and shelf‐life of vacuum and modified atmosphere packed chilled foods with respect to non‐proteolytic Clostridium botulinum . Acta Biologica Szegediensis. Available online: http://www2.sci.u-szeged.hu/ABS [Google Scholar]
- Adam KH, Flint SH and Brightwell G, 2010. Psychrophilic and psychrotrophic clostridia: sporulation and germination processes and their role in the spoilage of chilled, vacuum‐packaged beef, lamb and venison. International Journal of Food Science and Technology, 45, 1539–1544. 10.1111/j.1365-2621.2010.02320.x [DOI] [Google Scholar]
- Adams MR, Little CL and Easter MC, 1991. Modelling the effect of pH, acidulant and temperature on the growth rate of Yersinia enterocolitica . Journal of Applied Bacteriology, 71, 65–71. 10.1111/j.1365-2672.1991.tb04664.x [DOI] [PubMed] [Google Scholar]
- Ahnstrom ML, Seyfert M, Hunt MC and Johnson DE, 2006. Dry aging of beef in a bag highly permeable to water vapour. Meat Science, 73, 674–679. 10.1016/j.meatsci.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Anon , 2006. Technology plays a key role. Fleischwirtschaft, 86:43‐44Baranyi J, Pin C and Ross T, 1999. Validating and comparing predictive models. International Journal of Food Microbiology, 48, 159–166. 10.1016/S0168-1605(99)00035-5 [DOI] [PubMed] [Google Scholar]
- Asefa DT, Kure CF, Gjerde RO, Omer MK, Langsrud S, Nesbakken T and Skaar I, 2010. Fungal growth pattern, sources and factors of mould contamination in a dry‐cured meat production facility. International Journal of Food Microbiology, 140, 131–135. 10.1016/j.ijfoodmicro.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Asefa DT, Kure CF, Gjerde RO, Langsrud S, Omer MK, Nesbakken T and Skaar I, 2011. A HACCP plan for mycotoxigenic hazards associated with dry‐cured meat production processes. Food Control, 22, 831–837. 10.1016/j.foodcont.2010.09.014 [DOI] [Google Scholar]
- Baranyi J and Roberts TA, 1994. A dynamic approach to predicting bacterial growth in food. International Journal of Food Microbiology, 23, 277–294. 10.1016/0168-1605(94)90157-0 [DOI] [PubMed] [Google Scholar]
- Baranyi J, Pin C and Ross T, 1999. Validating and comparing predictive models. International Journal of Food Microbiology, 48, 159–166. 10.1016/S0168-1605(99)00035-5 [DOI] [PubMed] [Google Scholar]
- Barrera O, Rodríguez‐Calleja JM, Santos JA, Otero A and García‐López M‐L, 2007. Effect of different storage conditions on E. coli O157:H7 and the indigenous bacterial microflora on lamb meat. International Journal of Food Microbiology, 115, 244–251. 10.1016/j.ijfoodmicro.2006.10.053 [DOI] [PubMed] [Google Scholar]
- Battilani P, Palumbo R, Giorni P, Dall'Asta C, Dellafiora L, Gkrillas A, Toscano P, Crisci A, Brera C, De Santis B, Cammarano R, Seta M, Campbell K, Elliot C, Venâncio A, Gonçalves A, Terciolo C and Oswald I, 2020. Mycotoxin mixtures in food and feed: holistic, innovative, flexible risk assessment modelling approach: MYCHIF. EFSA Supporting Publications 2020:EN‐1757. 10.2903/sp.efsa.2020.EN-1757 [DOI] [Google Scholar]
- Benech N, Aguiar S and Grinspan GA, 2021. Monitoring ageing in beef samples using surface wave elastography: a feasibility study. Journal of Food Engineering, 307, 110647. 10.1016/j.jfoodeng.2021.110647 [DOI] [Google Scholar]
- Bennett JW and Klich M, 2003. Mycotoxins. Microbiology and Molecular Biology Reviews, 16, 497–516. 10.1128/CMR.16.3.497-516.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Kim YHB, Legako JF, Martini S, Lee J, Ebner P and Zuelly SMS, 2018. Dry‐aging improves meat quality attributes of grass‐fed beef loins. Meat Science, 145, 285–291. 10.1016/j.meatsci.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Beriain MJ, Goni MV, Indurain G, Sarries MV and Insausti K, 2009. Predicting Longissimus dorsi myoglobin oxidation in aged beef based on early post‐mortem colour measurements on the carcass as a colour stability index. Meat Science, 81, 439–445. 10.1016/j.meatsci.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee P, Panigrahi S, Lin D, Logue CM, Sherwood JS, Doetkott C and Marchello M, 2011. A comparative qualitative study of the profile of volatile organic compounds associated with Salmonella contamination of packaged aged and fresh beef by HS‐SPME/GC‐MS. Journal of Food Science and Technology‐Mysore, 48, 1–13. 10.1007/s13197-010-0138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagojevic B, Antic D, Adzic B, Tasic T, Ikonic P and Buncic S, 2015. Decontamination of incoming beef trimmings with hot lactic acid solution to improve microbial safety of resulting dry fermented sausages – a pilot study. Food Control, 54, 144–149. 10.1016/j.foodcont.2015.01.047 [DOI] [Google Scholar]
- Bodnaruk PW and Draughon FA, 1998. Effect of packaging atmosphere and pH on the virulence and growth of Yersinia enterocolitica on pork stored at 4°C. Food Microbiology, 15, 129–136. 10.1006/fmic.1997.0150 [DOI] [Google Scholar]
- Bolton DJ, Ivory C and McDowell D, 2013. A small study of Yersinia enterocolitica in pigs from birth to carcass and characterisation of porcine and human strains. Food Control, 33, 521–524. 10.1016/j.foodcont.2013.03.039 [DOI] [Google Scholar]
- Bolton DJ, Carroll J and Walsh D, 2015. A four‐year survey of blown pack spoilage C lostridium estertheticum and C lostridium gasigenes on beef primal cuts. Letters in Applied Microbiology, 61, 153–157. [DOI] [PubMed] [Google Scholar]
- Borch E, Kant‐Muermans ML and Blixt Y, 1996. Bacterial spoilage of meat and cured meat products. International Journal of Food Microbiology, 33, 103–120. 10.1016/0168-1605(96)01135-X [DOI] [PubMed] [Google Scholar]
- Bover‐Cid S, Jofré A, Possas A, Comaposada J, Brun A, Zomeño C, Masferrer G and Panella‐Riera N, 2022. Assessing the microbiological safety of beef aging process: Impact of temperature and relative humidity conditions. Poster presented at the 27th International ICFMH Conference: FoodMicro2022 (ref. P6.7); 28–31 August 2022, Athens Greece.
- Broda DM, Lawson PA, Bell RG and Musgrave DR, 1999. Clostridium frigidicarnis sp. nov., a psychrotolerant bacterium associated with 'blown pack' spoilage of vacuum‐packed meats. International Journal of Systematic Bacteriology, 49 Pt 4, 1539–1550. 10.1099/00207713-49-4-1539 [DOI] [PubMed] [Google Scholar]
- Broda DM, Lawson PA, Bell RG and Musgrave DR, 2000. Clostridium gasigenes sp. nov., a psychrophile causing spoilage of vacuum‐packed meat. International Journal of Systematic and Evolutionary Microbiology, 50 Pt 1, 107–118. 10.1099/00207713-50-1-107 [DOI] [PubMed] [Google Scholar]
- Brunt J, van Vliet AHM, Stringer SC, Carter AT, Lindström M and Peck MW, 2020. Pan‐Genomic Analysis of Clostridium botulinum Group II (Non‐Proteolytic C. botulinum) Associated with Foodborne Botulism and Isolated from the Environment. Toxins, 12, 306. 10.3390/toxins12050306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RL and Bagi LK, 1999. Microbial competition: effect of Pseudomonas fluorescens on the growth of Listeria monocytogenes . Food Microbiology, 16, 523–529. 10.1006/fmic.1998.0264 [DOI] [Google Scholar]
- Buchanan JR, Sommer NF, Fortlage RJ, Maxie EC, Mitchell FG and Hsieh DPH, 1974. Patulin from Penicillium expansum in stone fruits and pears. Journal of the American Society for Horticultural Science, 99, 262–265. [Google Scholar]
- Campano SG, Kotula AW and Kinsman DM, 1985. Antibacterial nature of molds isolated from aged beef. Journal of Food Protection, 48, 699–701. 10.4315/0362-028X-48.8.699 [DOI] [PubMed] [Google Scholar]
- Campbell RE, Hunt MC, Levis P and Chambers E, 2001. Dry‐aging effects on palatability of beef longissimus muscle. Journal of Food Science, 66, 196–199. 10.1111/j.1365-2621.2001.tb11315.x [DOI] [Google Scholar]
- Capouya R, Mitchell T, Clark DI, Clark DL, Bass P, Capouya RD and Bass PD, 2020. A survey of microbial communities on dry‐aged beef in commercial meat processing facilities. Meat and Muscle Biology, 4. [Google Scholar]
- Cardoso TAB, Bridi AM, Fagan EP, Tarsitano MA, Bolfe FC, Perez LM and Prohmann PEF, 2012. Quality of aged meat from Charolais vs Nellore cattle. Ciências Agrárias, Londrina, 33(suplemento 2), 3123–3132. 10.5433/1679-0359.2012v33Supl2p3123 [DOI] [Google Scholar]
- Carrel M, Zhao C, Thapaliya D, Bitterman P, Kates AE, Hanson BM and Smith TC, 2017. Assessing the potential for raw meat to influence human colonization with Staphylococcus aureus . Scientific Reports, 7, 10848. 10.1038/s41598-017-11423-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AT and Peck MW, 2015. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Research in Microbiology, 166, 303–317. 10.1016/j.resmic.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavill L, Renteria‐Monterrubio AL, Helps CR and Corry JEL, 2011. Detection of cold‐tolerant clostridia other than Clostridium estertheticum in raw vacuum‐packed chill‐stored meat. Food Microbiology, 28, 957–963. 10.1016/j.fm.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Ciegler A and Kurtzman CP, 1970. Penicillic acid production by blue‐eye fungi on various agricultural commodities. Applied Microbiology, 20, 761–764. 10.5433/1679-0359.2012v33Supl2p3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen I, Jakobsen M, Ertbjerg P and Madsen NT, 2009. Modified atmosphere packaging affects lipid oxidation, myofibrillar fragmentation index and eating quality of beef. Packaging Technology and Science, 22, 85–96. 10.1002/pts.828 [DOI] [Google Scholar]
- Constantino C, de Azambuja Ribeiro EL, Bridi AM, Tarsitano MA, Koritiaki NA, Peres LM, Mizubuti IY, Pereira ES and Pimentel PG, 2012. Quality of aged ewe meat in vacuum‐packaging system for different storage periods. Semina: Ciencias Agrarias, 33, 3437–3446. 10.5433/1679-0359.2012v33Supl2p3437 [DOI] [Google Scholar]
- Cornu M, Billoir E, Bergis H, Beaufort A and Zuliani V, 2011. Modeling microbial competition in food: Application to the behavior of Listeria monocytogenes and lactic acid flora in pork meat products. Food Microbiology, 28, 639–647. 10.1016/j.fm.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Coroller L, Kan‐King‐Yu D, Leguerinel I, Mafart P and Membré J‐M, 2012. Modelling of growth, growth/no‐growth interface and nonthermal inactivation areas of Listeria in foods. International Journal of Food Microbiology, 152, 139–152. 10.1016/j.ijfoodmicro.2011.09.023 [DOI] [PubMed] [Google Scholar]
- Dalgaard P and Mejlholm O, 2019. In: Bridier A (ed). Modeling Growth of Listeria and Lactic Acid Bacteria in Food Environments. Springer. pp. 247–264. [DOI] [PubMed] [Google Scholar]
- Dashdorj D, Amna T and Hwang I, 2015. Influence of specific taste‐active components on meat flavor as affected by intrinsic and extrinsic factors: an overview. European Food Research and Technology, 241, 157–171. 10.1007/s00217-015-2449-3 [DOI] [Google Scholar]
- Dashdorj D, Tripathi VK, Cho S, Kim Y and Hwang I, 2016. Dry aging of beef; Review. Journal of Animal Science and Technology, 58, 20. 10.1186/s40781-016-0101-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGeer SL, Hunt MC, Bratcher CL, Crozier‐Dodson BA, Johnson DE and Stika JF, 2009. Effects of dry aging of bone‐in and boneless strip loins using two aging processes for two aging times. Meat Science, 83, 768–774. 10.1016/j.meatsci.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Delaquis PJ and McCurdy AR, 1990. Colonization of beef muscle surfaces by Pseudomonas fluorescens and Pseudomonas fragi . Journal of Food Science, 55(4), 898–902. 10.1111/j.1365-2621.1990.tb01560.x [DOI] [Google Scholar]
- Devine C and Dikeman M, 2014. In: Devine C and Dikeman M (eds). Encyclopedia of Meat Sciences. 2nd edn. Academic Press. pp. 1712. [Google Scholar]
- Devlieghere F and Debevere J, 2000. Influence of dissolved carbon dioxide on the growth of spoilage bacteria. LWT ‐ Food Science and Technology, 33(8), 531–537. 10.1006/fstl.2000.0705 [DOI] [Google Scholar]
- Dikeman ME, Obuz E, Gok V, Akkaya L and Stroda S, 2013. Effects of dry, vacuum, and special bag aging; USDA quality grade; and end‐point temperature on yields and eating quality of beef Longissimus lumborum steaks. Meat Science, 94, 228–233. 10.1016/j.meatsci.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Doulgeraki AI, Ercolini D, Villani F and Nychas G‐JE, 2012. Spoilage microbiota associated to the storage of raw meat in different conditions. International Journal of Food Microbiology, 157, 130–141. 10.1016/j.ijfoodmicro.2012.05.020 [DOI] [PubMed] [Google Scholar]
- Duraković, S. and Duraković, L. (2003). Mikologija u biotehnologiji, Zagreb, Croatia: Kugler. pp. 80–94. [Google Scholar]
- Dykes GA and Moorhead SM, 2002. Combined antimicrobial effect of nisin and a listeriophage against Listeria monocytogenes in broth but not in buffer or on raw beef. International Journal of Food Microbiology, 73, 71–81. 10.1016/S0168-1605(01)00710-3 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2021. The European Union One Health 2020 Zoonoses Report. EFSA Journal 2021;19(12):6971, 324 pp. 10.2903/j.efsa.2021.6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Martino, L , Aiassa, E , Halldórsson, TI , Koutsoumanis, PK ; Naegeli, H , Baert, K , Baldinelli, F , Devos, Y , Lodi, F , Lostia, A , Manini, P , Merten, C , Messens, W , Rizzi, V , Tarazona, J , Titz, A and Vos, S , 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA supporting publication 2020;17(4):EN‐1843, 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI] [Google Scholar]
- Ercolini D, Russo F, Torrieri E, Masi P and Villani F, 2006. Changes in the spoilage‐related microbiota of beef during refrigerated storage under different packaging conditions. Applied and Environmental Microbiology, 72, 4663–4671. 10.1128/AEM.00468-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertbjerg P and Puolanne E, 2017. Muscle structure, sarcomere length and influences on meat quality: A review. Meat Science, 132, 139–152. 10.1016/j.meatsci.2017.04.261 [DOI] [PubMed] [Google Scholar]
- Espunyes J, Cabezón O, Dias‐Alves A, Miralles P, Ayats T and Cerdà‐Cuéllar M, 2021. Assessing the role of livestock and sympatric wild ruminants in spreading antimicrobial resistant Campylobacter and Salmonella in alpine ecosystems. BMC Veterinary Research, 17, 79. 10.1186/s12917-021-02784-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L, Van Royen G, Van Damme I and De Zutter L, 2016. FAVV Studieproject SP 2015‐02: Microbiologische risico's van ‘dry aged meat’. Available online: https://www.favv‐afsca.be/professionelen/publicaties/studieprojecten/_documents/FAVVStudieprojectSP2015-02_dryagedmeatsamenvatting_V4_NL.pdf
- FSA (Food Standards Agency) , 2017. The safety and shelf‐life of vacuum and modified atmosphere packed chilled foods with respect to non‐proteolytic Clostridium botulinum . Available online: https://www.food.gov.uk/sites/default/files/media/document/vacpacguide.pdf
- FSA (Food Standards Agency) . 2020a. The safety and shelf‐life of vacuum and modified atmosphere packed chilled foods with respect to non‐proteolytic Clostridium botulinum . Available online: https://www.food.gov.uk/sites/default/files/media/document/the-safety-and-shelf-life-of-vacuum-and-modified-atmosphere-packed-chilled-foods-with-respect-to-non-proteolytic-clostridium-botulinum_1.pdf
- FSA (Food Standards Agency) . 2020b. Subgroup on non‐proteolytic Clostridium botulinum and vacuum and modified atmosphere packaged foods. Final Report. Available online https://acmsf.food.gov.uk/sites/default/files/acmsf-vpmap-subgroup-report_1.pdf
- Gagaoua M, Terlouw EC, Mullen AM, Franco D, Warner RD, Lorenzo JM, Purslow PP, Gerrard D, Hopkins DL, Troy D and Picard B, 2021. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi‐platform proteomics studies. Meat Science, 172, 108311. [DOI] [PubMed] [Google Scholar]
- Garner CM, Unruh FA, Hun MC, Boyle EAE and Houser TA, 2014. Effects of subprimal type, quality grade, and aging time on display color of ground beef patties. Meat Science, 98(2), 301–309. 10.1016/j.meatsci.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Gill CO and Lowry PD, 1982. Growth at sub‐zero temperatures of black spot fungi from meat. Journal of Applied Bacteriology, 52, 245–250. 10.1111/j.1365-2672.1982.tb04846.x [DOI] [PubMed] [Google Scholar]
- Gill CO and Reichel MP, 1989. Growth of the cold‐tolerant pathogens Yersinia enterocolitica, Aeromonas hydrophila and Listeria monocytogenes on high‐pH beef packaged under vacuum or carbon dioxide. Food Microbiology, 6, 223–230. 10.1016/S0740-0020(89)80003-6 [DOI] [Google Scholar]
- Gill CO, Lowry PD and Di Menna ME, 1981. A note on the identities of organisms causing black spot spoilage of meat. Journal of Applied Bacteriology, 51, 1–16. 10.1111/j.1365-2672.1981.tb00922.x 7024231 [DOI] [Google Scholar]
- Giménez B and Dalgaard P, 2004. Modelling and predicting the simultaneous growth of Listeria monocytogenes and spoilage micro‐organisms in cold‐smoked salmon. Journal of Applied Microbiology, 96, 96–109. 10.1046/j.1365-2672.2003.02137.x [DOI] [PubMed] [Google Scholar]
- Giménez B, Graiver N, Giannuzzi L and Zaritzky N, 2021. Treatment of beef with gaseous ozone: Physicochemical aspects and antimicrobial effects on heterotrophic microflora and Listeria monocytogenes . Food Control, 121, 107602. 10.1016/j.foodcont.2020.107602 [DOI] [Google Scholar]
- Gowda TKGM, De Zutter L, Van Royen G and Van Damme I, 2022. Exploring the microbiological quality and safety of dry‐aged beef: a cross‐sectional study of loin surfaces during ripening and dry‐aged beef steaks from commercial meat companies in Belgium. Food Microbiology, 102, 103919. 10.1016/j.fm.2021.103919 [DOI] [PubMed] [Google Scholar]
- Gudjonsdottir M, Gacutan MD, Mendes AC, Chronakis IS, Jespersen L and Karlsson AH, 2015. Effects of electrospun chitosan wrapping for dry‐ageing of beef, as studied by microbiological, physicochemical and low‐field nuclear magnetic resonance analysis. Food Chemistry, 184, 167–175. 10.1016/j.foodchem.2015.03.088 [DOI] [PubMed] [Google Scholar]
- Gürtler M, Alter T, Kasimir S, Linnebur M and Fehlhaber K, 2005. Prevalence of Yersinia enterocolitica in fattening pigs. Journal of Food Protection, 68, 850–854. 10.4315/0362-028X-68.4.850 [DOI] [PubMed] [Google Scholar]
- Ha Y, Hwang I, Van Ba H, Ryu S, Kim Y, Kang SM, Kim J, Kim Y and Cho S, 2019. Effects of dry‐ and wet‐ageing on flavor compounds and eating quality of low fat hanwoo beef muscles. Food Science of Animal Resources, 39, 655–667. 10.5851/kosfa.2019.e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad GM, Mehany T, Simal‐Gandara J, Abou‐Alella S, Esua OJ, Abdel‐Wahhab MA and Hafez EE, 2022. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control, 144, 109350. [Google Scholar]
- Hiroi M, Kawamori F, Harada T, Sano Y, Miwa N, Sugiyama K, Hara‐Kudo Y and Masuda T, 2012. Antibiotic resistance in bacterial pathogens from retail raw meats and food‐producing animals in Japan. Journal of Food Protection, 75, 1774–1782. 10.4315/0362-028X.JFP-11-479 [DOI] [PubMed] [Google Scholar]
- Hocking AD and Pitt JI, 2003. Mycotoxigenic fungi. Foodborne microorganisms of public health significance. 6th edn. pp. 641–673 ref.330. [Google Scholar]
- Holmer SF, McKeith RO, Boler DD, Dilger AC, Eggert JM, Petry DB, McKeith FK, Jones KL and Killefer J, 2009. The effect of pH on shelf‐life of pork during aging and simulated retail display. Meat Science, 82, 86–93. 10.1016/j.meatsci.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Hopkins DL and Thompson JM, 2002. Factors contributing to proteolysis and disruption of myofibrillar proteins and the impact on tenderisation in beef and sheep meat. Australian Journal of Agricultural Research, 53, 149–166. [Google Scholar]
- Hulankova R, Borilova G, Abdullah FAA and Buchtova H, 2018a. Microbiological quality of organic chicken meat during refrigerated storage in air and modified atmospheres. British Poultry Science, 59, 506–513. 10.1080/00071668.2018.1496399 [DOI] [PubMed] [Google Scholar]
- Hulankova R, Kamenik J, Salakova A, Zavodsky D and Borilova G, 2018b. The effect of dry aging on instrumental, chemical and microbiological parameters of organic beef loin muscle. LWT‐ Food Science and Technology, 89, 559–565. 10.1016/j.lwt.2017.11.014 [DOI] [Google Scholar]
- Hungaro H, Caturla MYR, Horita CN, Miranda M and Sant'Ana AS, 2016. Blown pack spoilage in vacuum‐packaged meat: a review on clostridia as causative agents, sources, detection methods, contributing factors and mitigation strategies. Trends in Food Science & Technology, 52(1), 123–138. 10.1016/j.tifs.2016.04.010 [DOI] [Google Scholar]
- Iacumin L, Chiesa L, Boscolo D, Manzano M, Cantoni C, Orlic S and Comi G, 2009. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiology, 26, 65–70. 10.1016/j.fm.2008.07.006 [DOI] [PubMed] [Google Scholar]
- IARC , 2002. iarc monographs on the evaluation of carcinogenic risks to humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 2002(82), 1–556. [PMC free article] [PubMed] [Google Scholar]
- IARC , 2012. IARC monographs on the evaluation of carcinogenic risks to humans. Chemical Agents and Related Occupations, 100F, 1–628. [PMC free article] [PubMed] [Google Scholar]
- ICMSF , 1996. Microorganisms in Foods 5: Microbiological Specifications of Food Pathogens. Chapman & Hall, London. [Google Scholar]
- Iida F, Miyazaki Y, Tsuyuki R, Kato K, Egusa A, Ogoshi H and Nishimura T, 2016. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat Science, 112, 46–51. 10.1016/j.meatsci.2015.10.015 [DOI] [PubMed] [Google Scholar]
- Ingham SC, Algino RJ, Ingham BH and Schell RF, 2010. Identification of Escherichia coli O157:H7 surrogate organisms to evaluate beef carcass intervention treatment efficacy. Journal of Food Protection, 73, 1864–1874. 10.4315/0362-028x-73.10.1864 [DOI] [PubMed] [Google Scholar]
- Ismaiel AA and Papenbrock J, 2015. Mycotoxins: producing fungi and mechanisms of phytotoxicity. Agriculture, 5, 492–537. 10.3390/agriculture5030492 [DOI] [Google Scholar]
- James C and James S, 2012. Quantification of the controls that should be placed on meat prior to mincing. FSA (Food Standards Agency) Project: M01054.
- James C, Vincent C, Lima TIA and James SJ, 2006. The primary chilling of poultry carcasses – a review. International Journal of Refrigeration‐Revue Internationale Du Froid, 29, 847–862. [Google Scholar]
- Jameson J, 1962. A discussion of the dynamics of Salmonella enrichment. Journal of Hygiene, 60, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Busboom JR, Nelson ML, O'Fallon J, Ringkob TP, Rogers‐Klette KR, Joos D and Piper K, 2010. The influence of forage diets and aging on beef palatability. Meat Science, 86, 642–650. 10.1016/j.meatsci.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Joint Fao/Who Codex Alimentarius Commission F and Agriculture Organization of the United Nations WHO , 2001. Codex alimentarius. Rome, Food and Agriculture Organization of the United Nations: World Health Organization.
- Kahraman H and Gurbuz U, 2019. Effects of three aging methods on the Longissimus lumborum muscle from Holstein‐Friesian steers. Medycyna Weterynaryjna, 75, 6182–2019. 10.21521/mw.6182 [DOI] [Google Scholar]
- Kang SM, Chung SG, Seong P‐N, Kim Y, Kim Y, Ba HV, Kim J‐H and Cho S, 2017. Effect of dry‐ageing on sensory properties of loin and tritip muscle from Hanwoo Beef. Proceedings of the 63rd International Congress of Meat Science and Technology. [Google Scholar]
- Khan M, Jung S, Nam K‐C and Jo C, 2016. Postmortem aging of beef with a special reference to the dry aging. Korean Journal for Food Science of Animal Resources, 36, 159–169. 10.5851/kosfa.2016.36.2.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YHB, Kemp R and Samuelsson LM, 2016. Effects of dry‐aging on meat quality attributes and metabolite profiles of beef loins. Meat Science, 111, 168–176. 10.1016/j.meatsci.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Kim J‐H, Kim D‐H, Ji D‐s, Lee H‐J, Yoon D‐K and Lee C‐H, 2017a. Effect of aging process and time on physicochemical and sensory evaluation of raw beef top round and shank muscles using an electronic tongue. Korean Journal for Food Science of Animal Resources, 37, 823–832. 10.5851/kosfa.2017.37.6.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YHB, Meyers B, Kim H‐W, Liceaga AM and Lemenager RP, 2017b. Effects of stepwise dry/wet‐aging and freezing on meat quality of beef loins. Meat Science, 123, 57–63. 10.1016/j.meatsci.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Kim YHB, Ma DY, Setyabrata D, Farouk MM, Lonerg SM, Huff‐Lonergan E and Hunt MC, 2018. Understanding postmortem biochemical processes and post‐harvest aging factors to develop novel smart‐aging strategies. Meat Science, 144, 74–90. 10.1016/j.meatsci.2018.04.031 [DOI] [PubMed] [Google Scholar]
- Kim M, Choe J, Lee HJ, Yoon Y, Yoon S and Jo C, 2019a. Effects of aging and aging method on physicochemical and sensory traits of different beef cuts. Food Science of Animal Resources, 39, 54–64. 10.5851/kosfa.2019.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee HJ, Kim M, Yoon JW, Shin DJ and Jo C, 2019b. Storage stability of vacuum‐packaged dry‐aged beef during refrigeration at 4 degrees C. Food Science of Animal Resources, 39, 266–275. 10.5851/kosfa.2019.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim TK, Shin DM, Kim HW, Kim YB and Choi YS, 2020. Comparative effects of dry‐aging and wet‐aging on physicochemical properties and digestibility of Hanwoo beef. Asian‐Australasian Journal of Animal Sciences, 33, 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M‐F, Matthews MA, Rule DC and Field RA, 1995. Effect of beef packaging method on volatile compounds developed by oven roasting or microwave cooking. Journal of Agricultural and Food Chemistry, 43, 773–778. 10.1021/jf00051a039 [DOI] [Google Scholar]
- Knudsen GM, Sommer HM, Sorensen ND, Olsen JE and Aabo S, 2011. Survival of Salmonella on cuts of beef carcasses subjected to dry aging. Journal of Applied Microbiology, 111, 848–854. 10.1111/j.1365-2672.2011.05094.x [DOI] [PubMed] [Google Scholar]
- Koohmaraie M and Geesink GH, 2006. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science, 74, 34–43. 10.1016/j.meatsci.2006.04.025 [DOI] [PubMed] [Google Scholar]
- Koukou I, Mejlholm O and Dalgaard P, 2021. Cardinal parameter growth and growth boundary model for non‐proteolytic Clostridium botulinum – effect of eight environmental factors. International Journal of Food Microbiology, 346, 109162. 10.1016/j.ijfoodmicro.2021.109162 [DOI] [PubMed] [Google Scholar]
- Koutsoumanis K, Stamatiou A, Skandamis P and Nychas G‐JE, 2006. Development of a microbial model for the combined effect of temperature and pH on spoilage of ground meat, and validation of the model under dynamic temperature conditions. Applied and Environmental Microbiology, 72, 124–134. 10.1128/AEM.72.1.124-134.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen L and Purslow PP, 2001. The effect of ageing on the water‐holding capacity of pork: role of cytoskeletal proteins. Meat Science, 58, 17–23. [DOI] [PubMed] [Google Scholar]
- Krupa P, Bystroń J, Bania J, Podkowik M, Empel J and Mroczkowska A, 2014. Genotypes and oxacillin resistance of Staphylococcus aureus from chicken and chicken meat in Poland. Poultry Science, 93, 3179–3186. 10.3382/ps.2014-04321 [DOI] [PubMed] [Google Scholar]
- Kurtzman CP and Ciegler A, 1970. Mycotoxin from a blue eye mold of corn. Applied Microbiology, 20, 204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasta JA, Pensel N, Masana M, Rodriguez HR and Garcia PT, 1995. Microbial growth and biochemical changes on naturally contaminated chilled‐beef subcutaneous adipose tissue stored aerobically. Meat Science, 39, 149–158. [DOI] [PubMed] [Google Scholar]
- Laster MA, Smith RD, Nicholson KL, Nicholson JDW, Miller RK, Griffin DB, Harris KB and Savell JW, 2008. Dry versus wet aging of beef: retail cutting yields and consumer sensory attribute evaluations of steaks from ribeyes, strip loins, and top sirloins from two quality grade groups. Meat Science, 80, 795–804. 10.1016/j.meatsci.2008.03.024 [DOI] [PubMed] [Google Scholar]
- Latorre ME, Palacio MI, Velázquez DE and Purslow PP, 2019. Specific effects on strength and heat stability of intramuscular connective tissue during long time low temperature cooking. Meat Science, 153, 109–116. 10.1016/j.meatsci.2019.03.016 [DOI] [PubMed] [Google Scholar]
- Lautenschlaeger R, 2012. Latest trends in beef maturation ‐ dry‐aged versus wet‐aged beef. Proceedings of the 58th International Congress of Meat Science and Technology, Montreal, Canada: 12–17th August 2012s. [Google Scholar]
- Lee MS, Apple JK, Yancey JWS, Sawyer JT and Johnson ZB, 2008. Influence of vacuum‐aging period on bloom development of the beef gluteus medius from top sirloin butts. Meat Science, 80, 592–598. 10.1016/j.meatsci.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jung BS, Yoon HJ, Kim K‐T, Paik H‐D and Lee J‐Y, 2014. Predictive model for the growth kinetics of Listeria monocytogenes in raw pork meat as a function of temperature. Food Control, 44, 16–21. 10.1016/j.foodcont.2014.03.024 [DOI] [Google Scholar]
- Lee HJ, Choe J, Kim KT, Oh J, Lee DG, Kwon KM, Choi YI and Jo C, 2017. Analysis of low‐marbled Hanwoo cow meat aged with different dry‐aging methods. Asian‐Australasian Journal of Animal Sciences, 30, 1733–1738. 10.5713/ajas.17.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choe J, Yoon LW, Oh H, Yoon Y and Jo C, 2018. Determination of Salable Shelf‐life for Wrap‐packaged Dry‐aged Beef during Cold Storage. Korean Journal for Food Science of Animal, 38, 251–258. 10.5851/kosfa.2018.38.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Yoon JW, Kim M, Oh H, Yoon Y and Jo C, 2019. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry‐aged beef. Meat Science, 153, 152–158. 10.1016/j.meatsci.2019.03.019 [DOI] [PubMed] [Google Scholar]
- Lee D, Lee HJ, Yoon JW, Kim M and Jo C, 2021. Effect of different aging methods on the formation of aroma volatiles in beef strip loins. Food, 10. 10.3390/foods10010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper‐Blilie AN, Berg EP, Buchanan DS and Berg PT, 2016. Effects of post‐mortem aging time and type of aging on palatability of low marbled beef loins. Meat Science, 112, 63–68. 10.1016/j.meatsci.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Leroi F, Cornet J, Chevalier F, Cardinal M, Coeuret G, Chaillou S and Joffraud J‐J, 2015. Selection of bioprotective cultures for preventing cold‐smoked salmon spoilage. International Journal of Food Microbiology, 213, 79–87. 10.1016/j.ijfoodmicro.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Lewicki PP, 2004. Water as the determinant of food engineering properties. A review. Journal of Food Engineering, 61, 483–495. 10.1016/S0260-8774(03)00219-X [DOI] [Google Scholar]
- Li X, Babol J, Wallby A and Lundstrom K, 2013. Meat quality, microbiological status and consumer preference of beef gluteus medius aged in a dry‐ageing bag or vacuum. Meat Science, 95, 229–234. 10.1016/j.meatsci.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Li X, Babol J, Bredie WLP, Nielsen B, Tomankova J and Lundstrom K, 2014. A comparative study of beef quality after ageing longissimus muscle using a dry‐ageing bag, traditional dry‐ageing or vacuum package ageing. Meat Science, 97, 433–442. 10.1016/j.meatsci.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Lindberg CW and Borch E, 1994. Predicting the aerobic growth of Y. enterocolitica O:3 at different pH‐values, temperatures and L‐lactate concentrations using conductance measurements. International Journal of Food Microbiology, 22, 141–153. [DOI] [PubMed] [Google Scholar]
- Little CL, Adams MR, Anderson WA and Cole MB, 1992. Comparison of a quadratic response surface model and a square root model for predicting the growth rate of Yersinia enterocolitica . Letters in Applied Microbiology, 15, 63–68. 10.1111/j.1472-765X.1992.tb00726.x [DOI] [Google Scholar]
- Lively FO, Keady TWJ, Moss BW, Farmer LG, Gault NFS, Tolland ELC, Patterson DCP and Gordon AG, 2006. The effect of beef genotype, pelvic hanging technique and aging period on the eating quality of some hindquarter muscles. Proceedings of the Britich Society of Animal Science, 2006, 20. 10.1017/S1752756200016975 [DOI] [Google Scholar]
- Lonergan EH, Zhang W and Lonergan SM, 2010. Biochemistry of postmortem muscle ‐ lessons on mechanisms of meat tenderization. Meat Science, 86, 184–195. 10.1016/j.meatsci.2010.05.004 [DOI] [PubMed] [Google Scholar]
- López‐Gómez A, Castaño‐Villar AM, Palop A and Marín‐Iniesta F, 2013. Hygienic design and microbial control of refrigeration and air conditioning systems for food processing and packaging plants. Food Engineering Reviews, 5, 18–35. 10.1007/s12393-012-9060-1 [DOI] [Google Scholar]
- Lowry PD and Gill CO, 1984. Temperature and water activity minima for growth of spoilage moulds from meat. Journal of Applied Bacteriology, 56, 193–199. 10.1111/j.1365-2672.1984.tb01339.x [DOI] [PubMed] [Google Scholar]
- Lynt RK, Kautter DA and Solomon HM, 1982. Heat resistance of proteolytic Clostridium botulinum type F in phosphate buffer and crabmeat. Journal of Food Science, 47, 204–206. 10.1111/j.1365-2621.1982.tb11059.x [DOI] [PubMed] [Google Scholar]
- Mano SB, De Fernando GDG, Lopez‐Galvez D, Selgas MD, Garcia ML, Cambero MI and OrdoÑEz JA, 1995. Growth/survival of natural flora and Listeria monocytogenes on refrigerated uncooked pork and turkey packaged under modified atmospheres. Journal of Food Safety, 15, 305–319. 10.1111/j.1745-4565.1995.tb00142.x [DOI] [Google Scholar]
- Marcos Lázaro P, Whyte P, Rogers T, McElroy M, Fanning S, Frías J and Bolton D, 2021. The prevalence of Clostridioides difficile on farms, in abattoirs and in retail foods in Ireland. Food Microbiology, 98, 103781. 10.1016/j.fm.2021.103781 [DOI] [PubMed] [Google Scholar]
- Martínez PO, Fredriksson‐Ahomaa M, Pallotti A, Rosmini R, Houf K and Korkeala H, 2011. Variation in the prevalence of enteropathogenic Yersinia in slaughter pigs from Belgium, Italy, and Spain. Foodborne Pathogens and Disease, 8, 445–450. 10.1089/fpd.2009.0461 [DOI] [PubMed] [Google Scholar]
- Martins SIFS, Jongen WMF and van Boekel MAJS, 2000. A review of Maillard reaction in food and implications to kinetic modelling. Trends in Food Science & Technology, 11, 364–373. 10.1016/S0924-2244(01)00022-X [DOI] [Google Scholar]
- McKenna DR, Strachan DS, Miller RK, Acuff GR and Savell JW, 2003. Cranberry juice marinade improves sensory and microbiological properties of vacuum‐packaged lamb chops. Journal of Muscle Foods, 14, 207–220. 10.1111/j.1745-4573.2003.tb00701.x [DOI] [Google Scholar]
- McSharry S, Koolman L, Whyte P and Bolton D, 2021. The microbiology of beef from carcass chilling through primal storage to retail steaks. Current Research in Food Science, 4, 150–162. 10.1016/j.crfs.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejlholm OLE and Dalgaard PAW, 2007. Modeling and predicting the growth of lactic acid bacteria in lightly preserved seafood and their inhibiting effect on Listeria monocytogenes . Journal of Food Protection, 70, 2485–2497. 10.4315/0362-028X-70.11.2485 [DOI] [PubMed] [Google Scholar]
- Mejlholm OLE and Dalgaard PAW, 2009. Development and validation of an extensive growth and growth boundary model for Listeria monocytogenes in lightly preserved and ready‐to‐eat shrimp. Journal of Food Protection, 72, 2132–2143. 10.4315/0362-028X-72.10.2132 [DOI] [PubMed] [Google Scholar]
- Mejlholm O and Dalgaard P, 2013. Development and validation of an extensive growth and growth boundary model for psychrotolerant Lactobacillus spp. in seafood and meat products. International Journal of Food Microbiology, 167, 244–260. 10.1016/j.ijfoodmicro.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Mejlholm O, Gunvig A, Borggaard C, Blom‐Hanssen J, Mellefont L, Ross T, Leroi F, Else T, Visser D and Dalgaard P, 2010. Predicting growth rates and growth boundary of Listeria monocytogenes — An international validation study with focus on processed and ready‐to‐eat meat and seafood. International Journal of Food Microbiology, 141, 137–150. 10.1016/j.ijfoodmicro.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Mikami N, Toyotome T, Yamashiro Y, Sugo K, Yoshitomi K, Takaya M, Han K‐H, Fukushima M and Shimada K, 2021. Dry‐aged beef manufactured in Japan: microbiota identification and their effects on product characteristics. Food Research International, 140, 110020. 10.1016/j.foodres.2020.110020 [DOI] [PubMed] [Google Scholar]
- Moschonas G, Bolton DJ, Sheridan JJ and McDowell DA, 2009. Isolation and sources of ‘blown pack’ spoilage clostridia in beef abattoirs. Journal of Applied Microbiology, 107, 616–624. 10.1111/j.1365-2672.2009.04229.x [DOI] [PubMed] [Google Scholar]
- Narvaez‐Bravo C, Taboada NE, Mutschall SK and Aslma M, 2017. Epidemiology of antimicrobial resistant Campylobacter spp. isolated from retail meats in Canada. International Journal of Food Microbiology, 253, 43–47. 10.1016/j.ijfoodmicro.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Neumeyer K, Ross T, Thomson G and McMeekin TA, 1997. Validation of a model describing the effects of temperature and water activity on the growth of psychrotrophic pseudomonads. International Journal of Food Microbiology, 38(1), 55–63. 10.1016/s0168-1605(97)00090-1 [DOI] [PubMed] [Google Scholar]
- Ninios T, Lundén J, Korkeala H and Fredriksson‐Ahomaa M, 2014. Meat Inspection and Control in the Slaughterhouse. Wiley. [Google Scholar]
- Nychas GJE, Marshall D and Sofos J, 2007. Food microbiology: fundamentals and frontiers. Meat Poultry and Seafood. ASM Press, Washington, DC. pp. 105–140. [Google Scholar]
- Obuz E, Akkaya L, Gok V and Dikeman ME, 2014. Effects of blade tenderization, aging method and aging time on meat quality characteristics of Longissimus lumborum steaks from cull Holstein cows. Meat Science, 96, 1227–1232. 10.1016/j.meatsci.2013.11.015 [DOI] [PubMed] [Google Scholar]
- Oh J, Lee HJ, Kim HC, Kim HJ, Yun YG, Kim KT, Choi YI and Jo C, 2018. The effects of dry or wet aging on the quality of the longissimus muscle from 4‐year‐old Hanwoo cows and 28‐month‐old Hanwoo steers. Animal Production Science, 58, 2344–2351. 10.1071/an17104 [DOI] [Google Scholar]
- Oh H, Lee HJ, Lee J, Jo C and Yoon Y, 2019a. Identification of microorganisms associated with the quality improvement of dry‐aged beef through microbiome analysis and DNA sequencing, and evaluation of their effects on beef quality. Journal of Food Science, 84, 2944–2954. 10.1111/1750-3841.14813 [DOI] [PubMed] [Google Scholar]
- Oh J, Lee HJ, Yoon JW, Choe J and Jo C, 2019b. Electrical resistance and mold distribution on beef surface as indicators of dry aging. Journal of Food Process Engineering, 42, e13122. 10.1111/jfpe.13122 [DOI] [Google Scholar]
- Olivier S, 2018. Assessment of the mycological risks associated with the dry‐ageing of meat. MLA project V.MFS.0426. CSIRO, Sydney, NSW. [Google Scholar]
- Ostry V, Malir F, Toman J and Grosse Y, 2017. Mycotoxins as human carcinogens ‐ the IARC Monographs classification. Mycotoxin Research, 33, 65–73. [DOI] [PubMed] [Google Scholar]
- Owczarek‐Fendor A, Vermeulen A, Van Bree I, Eriksson M, Lescouhier S, De Smet S, De Meulenaer B and Devlieghere F, 2014. Effect of muscle, ageing time and modified atmosphere packaging conditions on the colour, oxidative and microbiological stability of packed beef. International Journal of Food Science and Technology, 49, 1090–1098. 10.1111/ijfs.12404 [DOI] [Google Scholar]
- Özbaş ZY, Vural H and Aytaç SA, 1996. Effects of modified atmosphere and vacuum packaging on the growth of spoilage and inoculated pathogenic bacteria on fresh poultry. Zeitschrift für Lebensmittel Untersuchung und Forschung, 203, 326–332. 10.1007/BF01231070 [DOI] [PubMed] [Google Scholar]
- Panella‐Riera N, Possas A, Jofré A, Albano M, Comaposada J and Bover‐Cid S, 2021. Assessment of the microbiological safety associated with the dynamics of the meat surface, aw, pH and temperature during beef dry aging process. Poster presented at the 67th International Congress of Meat Science and Technology, 23 27th August 2021, Kraków, Poland.
- Park B, Yong HI, Choe J and Jo C, 2018. Utilization of the crust from dry‐aged beef to enhance flavor of beef patties. Korean Journal for Food Science of Animal Resources, 38, 1019–1028. 10.5851/kosfa.2018.e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish FC Jr, Boles JA, Rust RE and Olson DG, 1991. Dry and wet aging effects on palatability attributes of beef loin and rib steaks from three quality grades. Journal of Food Science, 56, 601–603. 10.1111/j.1365-2621.1991.tb05338.x [DOI] [Google Scholar]
- Peck MW, Webb MD and Goodburn KE, 2020. Assessment of the risk of botulism from chilled, vacuum/modified atmosphere packed fresh beef, lamb and pork held at 3°C–8°C. Food Microbiol, 91, 103544. 10.1016/j.fm.2020.103544 [DOI] [PubMed] [Google Scholar]
- Perry N, 2012. Dry aging beef. International Journal of Gastronomy and Food Science, 1, 78–80. 10.1016/j.ijgfs.2011.11.005 [DOI] [Google Scholar]
- Petsios S, Fredriksson‐Ahomaa M, Sakkas H and Papadopoulou C, 2016. Conventional and molecular methods used in the detection and subtyping of Yersinia enterocolitica in food. International Journal of Food Microbiology, 237, 55–72. 10.1016/j.ijfoodmicro.2016.08.015 [DOI] [PubMed] [Google Scholar]
- Phillips D, Bridger K, Jenson I and Sumner J, 2012. An Australian National Survey of the microbiological quality of frozen boneless beef and beef primal cuts. Journal of Food Protection, 75, 1862–1866. 10.4315/0362-028X.JFP-12-135 [DOI] [PubMed] [Google Scholar]
- Pitt JI and Hocking AD (Eds), 2009. Aspergillus flavus link. Fungi and Food Spoilage. 3rd edn. Springer, Dordrecht, Netherlands. pp. 305–311. [Google Scholar]
- Pleadin J, Vulic A, Perkovic I, Kudumija N, Lesic T, Kis M, Zadravec M and Mitak M, 2019a. Mycotoxins aflatoxins and ochratoxins ‐ a threat to the safety of traditional meat products. Meso, 21, 186–197. 10.31727/m.21.2.2 [DOI] [Google Scholar]
- Pleadin J, Frece J and Markov K, 2019b. Chapter eight: mycotoxins in food and feed. Advances in Food and Nutrition Research, 89, 297–345. 10.1016/bs.afnr.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Pócsi I, Giacometti F, Ambrus Á and Logrieco AF, 2020. Editorial: Aspergillus‐derived mycotoxins in the feed and food chain. Frontiers in Microbiology, 11 project V.MFS.0426. CSIRO, Sydney, NSW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugazhendhi A, Michael D, Prakash D, Priyadarshini Krishnamaurthy P, Shanmuganathan R, Abdullah Al‐Dhabi N, Duraipandiyan V, Valan Arasu M and Kaliannan T, 2020. Antibiogram and plasmid profiling of beta‐lactamase producing multi drug resistant Staphylococcus aureus isolated from poultry litter. Journal of King Saud University ‐ Science, 32, 2723–2727. 10.1016/j.jksus.2020.06.007 [DOI] [Google Scholar]
- R Core Team , 2021. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/
- Regulation (EC) No 178/2002 , 2002. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002.
- Reid R, Fanning S, Whyte P, Kerry J, Lindqvist R, Yu Z and Bolton D, 2017. The microbiology of beef carcasses and primals during chilling and commercial storage. Food Microbiology, 61, 50–57. 10.1016/j.fm.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Rezende‐de‐Souza JH, Cardello FAB, de Paula APM, Ribeiro FA, Calkins CR and Pflanzer SB, 2021. Profile of producers and production of dry‐aged beef in Brazil. Food, 10. 10.3390/foods10102447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FA, Lau SK, Furbeck RA, Herrera NJ, Henriott ML, Bland NA, Fernando SC, Subbiah J, Sullivan GA and Calkins CR, 2021a. Ultimate pH effects on dry‐aged beef quality. Meat Science, 172, 108365. 10.1016/j.meatsci.2020.108365 [DOI] [PubMed] [Google Scholar]
- Ribeiro JC Jr, Santos IGCD, Dias BP, Augusto WF, Rodrigues EM and Miotto FRC, 2021b. Influence of dry and wet beef maturation on the microbiological quality and safety. Semina: Ciencias Agrarias, 42, 155–166. 10.5433/1679-0359.2021v42n1p155 [DOI] [Google Scholar]
- Ribeiro FA, Lau SK, Henriott ML, Herrera NJ, Bland NA, Subbiah J and Calkins CR, 2020. Effects of relative humidity on meat quality in dry‐aged beef. Nebraska Beef Cattle Reports, 1082. [Google Scholar]
- Richardson EL, Fields B, Dilger AC and Boler DD, 2018. The effects of ultimate pH and color on sensory traits of pork loin chops cooked to a medium‐rare degree of doneness. Journal of Animal Science, 96, 3768–3776. 10.1093/jas/sky258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Rodriguez M, Andrade MJ and Cordoba JJ, 2012. Development of a multiplex real‐time PCR to quantify aflatoxin, ochratoxin A and patulin producing molds in foods. International Journal of Food Microbiology, 155, 10–18. 10.1016/j.ijfoodmicro.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bernaldez V, Rodriguez M, Andrade MJ, Nunez F and Cordoba JJ, 2015. Effect of selected protective cultures on ochratoxin A accumulation in dry‐cured Iberian ham during its ripening process. LWT‐ Food Science and Technology, 60, 923–928. 10.1016/j.lwt.2014.09.059 [DOI] [Google Scholar]
- Rohatgi A, 2021. Webplotdigitizer: Version 4.5. Available online: https://automeris.io/WebPlotDigitizer
- Ross T, 1999. Predictive food microbiology models in the meat industry. Meat and livestock. Australia, Sydney. pp. 196. [Google Scholar]
- Russell L, Galindo CP, Whyte P and Bolton D, 2021. A preliminary study of Salmonella spp., Listeria monocytogenes, Escherichia coli O157, Enterococcus faecalis and Clostridium spp. in Irish cattle. Irish Journal of Agricultural and Food Research, 60. 10.15212/ijafr-2020-0122 [DOI] [Google Scholar]
- Russo F, Ercolini D and Villani F, 2006. Behaviour of Brochothrix thermosphacta in presence of other meat spoilage microbial groups. Food Microbiology, 23(8), 797–802. 10.1016/j.fm.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Ryu S, Park MR, Maburutse BE, Lee WJ, Park DJ, Cho S, Hwang I, Oh S and Kim Y, 2018. Diversity and characteristics of the meat microbiological community on dry‐aged beef. Journal of Microbiology and Biotechnology, 28(1), 105–108. [DOI] [PubMed] [Google Scholar]
- Sakala RM, Hayashidani H, Kato Y, Hirata T, Makino Y, Fukushima A, Yamada T, Kaneuchi C and Ogawa M, 2002. Change in the composition of the microflora on vacuum‐packaged beef during chiller storage. International Journal of Food Microbiology, 74, 87–99. [DOI] [PubMed] [Google Scholar]
- Şanlıbaba P, 2022. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. International Journal of Food Microbiology, 361, 109461. 10.1016/j.ijfoodmicro.2021.109461 [DOI] [PubMed] [Google Scholar]
- Saraiva C, Fontes MC, Patarata L, Martins C, Cadavez V and Gonzales‐Barron U, 2016. Modelling the kinetics of Listeria monocytogenes in refrigerated fresh beef under different packaging atmospheres. LWT ‐ Food Science and Technology, 66, 664–671. 10.1016/j.lwt.2015.11.026 [DOI] [Google Scholar]
- Savell JW, 2008. Dry‐aging of beef: Executive Summary. Place Center for Research and Knowledge Management. National Cattlemen's Beef Association, Center for Research and Knowledge Management. National Cattlemen's Beef Association.
- Savell JW and Gehring K, 2018. Dry‐aged beef revival: new thoughts on an old process. Available online: https://www.meatpoultry.com/articles/19412-dry-aged-beef-revival [Accessed: 17 Jan 2022].
- Shi Y, Zhang W and Zhou G, 2020. Effects of different moisture‐permeable packaging on the quality of aging beef compared with wet aging and dry aging. Food, 9, 647. 10.3390/foods9050649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva ACM, de Oliveira PP, Pflanzer SB and da Silva do Nascimento M, 2019. Effect of different dry aging temperatures on Listeria innocua as surrogate for Listeria monocytogenes . Meat Science, 157, 107884. 10.1016/j.meatsci.2019.107884 [DOI] [PubMed] [Google Scholar]
- da Silva APB, Da Silva ACM, Ferreira FMS, Do Nascimento M and Pflanzer SB, 2020. The effects of time and relative humidity on dry‐aged beef: traditional versus special bag. Food Science and Technology International, 27, 626–634. 10.1177/1082013220976487 [DOI] [PubMed] [Google Scholar]
- Sitz B, Calkins C, Feuz D, Umberger W and Eskridge K, 2006. Consumer sensory acceptance and value of wet‐aged and dry‐aged beef steaks. Journal of Animal Science, 84, 1221–1226. 10.2527/2006.8451221x [DOI] [PubMed] [Google Scholar]
- Skandamis PN and Nychas G‐JE, 2002a. Effect of oregano essential oil on microbiological and physicochemical attributes of minced meat stored in air and modified atmospheres. Journal of Applied Microbiology, 91, 1011–1022. 10.1046/j.1365-2672.2001.01467.x [DOI] [PubMed] [Google Scholar]
- Skandamis PN and Nychas G‐JE, 2002b. Preservation of fresh meat with active and modified atmosphere packaging conditions. International Journal of Food Microbiology, 79(1–2), 35–45. 10.1016/S0168-1605(02)00177-0 [DOI] [PubMed] [Google Scholar]
- Smaldone G, Marrone R, Vollano L, Peruzy MF, Barone CMA, Ambrosio RL and Anastasi A, 2019. Microbiological, rheological and physical‐chemical characteristics of bovine meat subjected to a prolonged ageing period. Italian Journal of Food Safety, 8, 131–136. 10.4081/ijfs.2019.8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Nicholson KL, Nicholson JDW, Harris KB, Miller RK, Griffin DB and Savell JW, 2008. Dry versus wet aging of beef: retail cutting yields and consumer palatability evaluations of steaks from US Choice and US Select short loins. Meat Science, 79, 631–639. 10.1016/j.meatsci.2007.10.028 [DOI] [PubMed] [Google Scholar]
- Smith AM, Harris KB, Griffin DB, Miller RK, Kerth CR and Savell JW, 2014. Retail yields and palatability evaluations of individual muscles from wet‐aged and dry‐aged beef ribeyes and top sirloin butts that were merchandised innovatively. Meat Science, 97, 21–26. 10.1016/j.meatsci.2013.12.013 [DOI] [PubMed] [Google Scholar]
- Smulders FJM, Hiesberger J, Hofbauer P, Dogl B and Dransfield E, 2006. Modified‐atmosphere storage under subatmospheric pressure and beef quality: II. Color, drip, cooking loss, sarcomere length, and tenderness. Journal of Animal Science, 84, 2456–2462. 10.2527/jas.2005-684 [DOI] [PubMed] [Google Scholar]
- Solomakos N, Govaris A, Koidis P and Botsoglou N, 2008. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiology, 25, 120–127. 10.1016/j.fm.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Sommer NF, Buchanan JR and Fortlage RJ, 1974. Production of patulin by Penicillium expansum. Applied Microbiology, 28, 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Liu C and Finegold Sydney M, 2004. Real‐time PCR quantitation of clostridia in feces of autistic children. Applied and Environmental Microbiology, 70, 6459–6465. 10.1128/AEM.70.11.6459-6465.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanborough T, Fegan N, Powell SM, Tamplin M and Chandry PS, 2017. Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Applied and Environmental Microbiology, 83, e02786–e02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstrom H, Li X, Hunt MC and Lundstrom K, 2014. Consumer preference and effect of correct or misleading information after ageing beef longissimus muscle using vacuum, dry‐ageing, or a dry‐ageing bag. Meat Science, 96, 661–666. 10.1016/j.meatsci.2013.10.022 [DOI] [PubMed] [Google Scholar]
- Stern NJ, Pierson MD and Kotula AW, 1980. Effects of pH and sodium chloride on Yersinia enterocolitica growth at room and refrigeration temperatures. Journal of Food Science, 45, 64–67. 10.1111/j.1365-2621.1980.tb03871.x [DOI] [Google Scholar]
- Stuby‐Souva MA, 1994. Tenderness and aging response of beef muscles of different quality grades before and after freezing.
- Suman SP, Hunt MC, Nair MN and Rentfrow G, 2014. Improving beef color stability: practical strategies and underlying mechanisms. Meat Science, 98, 490–504. 10.1016/j.meatsci.2014.06.032 [DOI] [PubMed] [Google Scholar]
- Sutherland JP and Bayliss AJ, 1994. Predictive modelling of growth of Yersinia enterocolitica: the effects of temperature, pH and sodium chloride. International Journal of Food Microbiology, 21, 197–215. 10.1016/0168-1605(94)90028-0 [DOI] [PubMed] [Google Scholar]
- Terjung N, Witte F and Heinz V, 2021. The dry‐aged beef paradox: why dry aging is sometimes not better than wet aging. Meat Science, 172, 108355. 10.1016/j.meatsci.2020.108355 [DOI] [PubMed] [Google Scholar]
- Thépault A, Poezevara T, Quesne S, Rose V, Chemaly M and Rivoal K, 2018. Prevalence of thermophilic Campylobacter in cattle production at slaughterhouse level in France and link between C. jejuni bovine strains and campylobacteriosis. Frontiers in Microbiology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittor AW, Tittor MG, Brashears MM, Brooks JC, Garmyn AJ and Miller MF, 2011. Effects of simulated dry and wet chilling and aging of beef fat and lean tissues on the reduction of Escherichia coli O157:H7 and Salmonella. Journal of Food Protection, 74, 289–293. [DOI] [PubMed] [Google Scholar]
- Tsigarida E and Nychas GJE, 2001. Ecophysiological attributes of Lactobacillus sp. and Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. Journal of Applied Microbiology, 90, 696–705. [DOI] [PubMed] [Google Scholar]
- Tsigarida E, Skandamis P and Nychas GJE, 2000. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5°C. Journal of Applied Microbiology, 89, 901–909. 10.1046/j.1365-2672.2000.01170.x [DOI] [PubMed] [Google Scholar]
- Utama DT, Kim YJ, Jeong HS, Kim J, Barido FH and Lee SK, 2020. Comparison of meat quality, fatty acid composition and aroma volatiles of dry‐aged beef from Hanwoo cows slaughtered at 60 or 80 months old. Asian‐Australasian Journal of Animal Sciences, 33, 157–165. 10.5713/ajas.19.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme I, Habib I and De Zutter L, 2010. Yersinia enterocolitica in slaughter pig tonsils: enumeration and detection by enrichment versus direct plating culture. Food Microbiology, 27, 158–161. 10.1016/j.fm.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Van Damme I, Berkvens D, Vanantwerpen G, Baré J, Houf K, Wauters G and De Zutter L, 2015. Contamination of freshly slaughtered pig carcasses with enteropathogenic Yersinia spp.: distribution, quantification and identification of risk factors. International Journal of Food Microbiology, 204, 33–40. 10.1016/j.ijfoodmicro.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Van Damme I, Varalakshmi S, De Zutter L, Vossen E and De Smet S, 2022. Decrease of Salmonella and Escherichia coli O157:H7 counts during dry‐aging of beef but potential growth of Listeria monocytogenes under certain dry‐aging conditions. Food Microbiology, 104, 104000. 10.1016/j.fm.2022.104000 [DOI] [PubMed] [Google Scholar]
- Varga J, Baranyi N, Chandrasekaran M, Vágvölgyi C and Kocsubé S, 2015. Mycotoxin producers in the Aspergillus genus: an update. Acta Biologica Szegediensis, 59, 151–167. [Google Scholar]
- Vásquez MSM, Héctor SM and Inés MO, 2009. Evaluation of bacteriocines as protective means for the biopreservation of refrigerated meat. Revista Chilena de Nutricion, 36, 228–238. [Google Scholar]
- Vieira C, Diaz MT, Martinez B and Garcia‐Cachan MD, 2009. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Science, 83, 398–404. 10.1016/j.meatsci.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li F, Zhuang H, Chen X, Li L, Qiao W and Zhang J, 2015. Effects of plant polyphenols and alpha‐tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N‐nitrosamines formation during ripening and storage of dry‐cured bacon. LWT‐Food Science and Technology, 60, 199–206. 10.1016/j.lwt.2014.09.022 [DOI] [Google Scholar]
- Wang H, Zhang X, Wang G, Kun J, Xu X and Zhou G, 2017. Bacterial community and spoilage profiles shift in response to packaging in yellow‐feather broiler, a highly popular meat in Asia. Frontiers in Microbiology, 8, 2588. 10.3389/fmicb.2017.02588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kamada G, Imanari M, Shiba N, Yonai M and Muramoto T, 2015. Effect of aging on volatile compounds in cooked beef. Meat Science, 107, 12–19. 10.1016/j.meatsci.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Whyte P, McGill K, Cowley D, Madden RH, Moran L, Scates P, Carroll C, O'Leary A, Fanning S, Collins JD, McNamara E, Moore JE and Cormican M, 2004. Occurrence of Campylobacter in retail foods in Ireland. International Journal of Food Microbiology, 95, 111–118. 10.1016/j.ijfoodmicro.2003.10.018 [DOI] [PubMed] [Google Scholar]
- Witte F, Smetana S, Heinz V and Terjung N, 2020. High‐pressure processing of usually discarded dry‐aged beef trimmings for subsequent processing. Meat Science, 170, 108241. 10.1016/j.meatsci.2020.108241 [DOI] [PubMed] [Google Scholar]
- Woraprayote W, Malila Y, Sorapukdee S, Swetwiwatharna A, Benjakul S and Visessanguan W, 2016. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Science, 120, 118–132. 10.1016/j.meatsci.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Yaylayan VA, Keyhani A and Wnorowski A, 2000. Formation of sugar‐specific reactive intermediates from 13C‐Labeled l‐Serines. Journal of Agricultural and Food Chemistry, 48, 636–641. 10.1021/jf990687a [DOI] [PubMed] [Google Scholar]
- Yu HH, Song YJ, Kim YJ, Lee H, Choi YS, Lee NA‐K and Paik H‐D, 2020. Predictive model of growth kinetics for Staphylococcus aureus in raw beef under various packaging systems. Meat Science, 165, J108108. 10.1016/j.meatsci.2020.108108 [DOI] [PubMed] [Google Scholar]
- Zadravec M, Vahcić N, Brnić D, Markov K, Frece J, Beck R, Lešić T and Pleadin J, 2019. A study of surface moulds and mycotoxins in Croatian traditional dry‐cured meat products. International Journal of Food Microbiology, 317, 108459. 10.1016/j.ijfoodmicro.2019.108459 [DOI] [PubMed] [Google Scholar]
- Zeaki N, Johler S, Skandamis PN and Schelin J, 2019. The role of regulatory mechanisms and environmental parameters in staphylococcal food poisoning and resulting challenges to risk assessment. Frontiers in Microbiology, 10, 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S and Mustapha A, 1999. Reduction of Listeria monocytogenes and Escherichia coli O157:H7 numbers on vacuum‐packaged fresh beef treated with nisin or nisin combined with EDTA†. Journal of Food Protection, 62, 1123–1127. 10.4315/0362-028X-62.10.1123 [DOI] [PubMed] [Google Scholar]
- Zhang R, Yoo MJY, Realini CE, Staincliffe M and Farouk MM, 2021. In‐bag dry‐ vs. wet‐aged lamb: quality, consumer acceptability, oxidative stability and in vitro digestibility. Food, 10, 41. 10.3390/foods10010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P, O'Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Ganzle MG and Lebeer S, 2020. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology, 70, 2782–2858. 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the Scientific opinion on the microbiological safety of aged meat


