Abstract
Background
We aimed to determine the prevalence of hepatitis B virus (HBV) infection among people with human immunodeficiency virus (PWH) in rural and periurban communities in Botswana.
Methods
PWH from a previous population-based study, the Botswana Prevention Combination Project, which enrolled adults in 30 communities across Botswana (2013–2018), were screened for HBV surface antigen (HBsAg) and HBV core antibody (anti-HBc). HBsAg-positive (HBsAg+) samples were further screened for HBV core immunoglobulin M antibodies (anti-HBc immunoglobulin M [IgM]) and HBV e antigen (HBeAg). We quantified HBV viral load on participants who tested positive (n = 148) and negative for HBsAg (n = 381).
Results
Of 3304 participants tested, 271 (8% [95% confidence interval {CI}, 7%–9%]) were HBsAg+ while 1788 (56% [95% CI, 54%–57%]) of 3218 PWH whom we tested had positive anti-HBc. Approximately 88% of HBsAg+ participants were on antiretroviral therapy (ART), 40% and 56% of whom were receiving lamivudine- and tenofovir-containing ART, respectively. Male sex (relative risk ratio [RRR], 1.8 [95% CI, 1.2–2.7]) and the northern geographic region (RRR, 2.5 [95% CI, 1.4–4.7]) were independent predictors of HBV infection (HBsAg+). Of 381 persons with negative HBsAg who were tested for occult HBV, 126 (33% [95% CI, 29%–38%]) had positive HBV DNA. Eleven participants were highly viremic with high HBV viral load while on a lamivudine- or tenofovir-containing regimen. Ten (91%) of these participants also had positive HBeAg serology, while 4 (36%) had positive anti-HBc IgM serology.
Conclusions
The prevalence of HBV was high among PWH in Botswana while on ART regimens with activity against HBV.
Keywords: Africa, Botswana, hepatitis B virus, human immunodeficiency virus, occult HBV
In a near-nationwide, predominantly rural population–based random sample of >3300 adults with HIV, HBsAg and occult HBV prevalence was 8% and 33%, respectively. Male sex and the northern region were independent predictors of HBV infection in Botswana.
Hepatitis B virus (HBV) infection is one of the leading causes of liver disease worldwide, with sub-Saharan Africa having the highest HBV-associated mortality [1]. In 2019 the global prevalence of chronic HBV was 3.8%, affecting 296 million people and leading to 820 000 deaths annually [2].
In Botswana, universal childhood HBV vaccination was introduced in 2000 with 4 HBV vaccine doses being given to infants [3]. The reported coverage of the HBV vaccine is 94% while coverage of timely hepatitis B birth dose was shown to be 74% [4]. The prevalence of individuals with hepatitis B surface antigen–positive (HBsAg+) serology in Botswana has been reported to range between 1.1% and 10.6%, with studies conducted primarily in the Gaborone (capital) region [5–7]. Studies from other African countries have reported HBV prevalence ranging from 0.4% to 22.9% [8–13], but few of these studies are from HBV population-based studies in sub-Saharan Africa [14, 15]. National and regional HBV prevalence data are important for implementing programs for eliminating HBV by 2030, as per the Sustainable Development goals.
Some studies have shown that HBV/human immunodeficiency virus (HIV) coinfection leads to worse clinical outcomes than either infection alone [16]. This may, however, not always be the case as HIV/HBV-coinfected patients may be better linked to care and be better managed than HBV-monoinfected patients. In one study, for example, a higher proportion of coinfected individuals were on ART and were less likely to have undetectable HBV viremia and features of liver disease compared to HBV-monoinfected individuals [17]. HIV prevalence in Botswana is estimated to be 20.8% [18]. HBV screening among people with HIV (PWH) in Botswana is not routinely done; however, people found to have concomitant HBV and HIV are initiated on Truvada (emtricitabine/tenofovir disoproxil fumarate [TDF]) [19]. Botswana has a robust National Antiretroviral Treatment Programme that provides treatment to all PWH since the year 2016 [19], and the success of this program is shown in Botswana reaching the Joint United Nations Programme on HIV/AIDS (UNAIDS) 95–95–95 targets at 95–98–98 [18]. Currently, the first-line regimen is Truvada and dolutegravir, while second-line regimens are based on resistance test results and consultations with an HIV specialist. Prior to the treat-all era, the first-line regimen was efavirenz/emtricitabine/TDF with the second-line regimen being zidovudine/lamivudine (3TC)/lopinavir/ritonavir.
Occult HBV (OBI), defined as the presence of replication-competent HBV DNA in the absence of HBsAg [20], often goes unreported in national HBV prevalence data. The clinical relevance of OBI infections includes vertical transmission [21], reactivation (particularly in immunocompromised individuals) [22], drug resistance [23], and diagnostic failure when using only serological assays to screen for HBV. Mutations identified in various HBV open reading frames may explain OBI phenotype characteristics such as reduced replication leading to low viral loads and reduced HBsAg secretion [24–26].
We aimed to determine the prevalence of HBV infection, including OBI, among a nationally representative cohort of PWH in rural and periurban communities in Botswana.
METHODS
Study Population
For this study, we used existing data and stored plasma samples that were collected from PWH who participated in a population-based household survey of the Botswana Combination Prevention Project (BCPP) in 30 geographically dispersed villages throughout Botswana [27]. BCPP was a cluster-randomized trial conducted in 15 paired communities matched by size, preexisting health services, population age structure, and geographic location. In brief, a random sample of approximately 20% of adult residents (16–64 years) of these 30 communities were enrolled from 2013–2018 into a survey; a total of 12 610 participants enrolled, of whom 3596 were HIV positive [28]. We tested stored leftover plasma from PWH who enrolled into this survey for various HBV serological markers following the manufacturer's protocols.
Patient Consent Statement
In the parent BCPP study, written informed consent was obtained from participants. Only samples from participants who consented for their samples to be used for future research were used. Our current study was approved by the Human Research Development Committee at the Botswana Ministry of Health (HPDME 13/18/1) in which we received a waiver of consent.
Laboratory Investigations
Plasma samples were initially screened for HBsAg (Murex Version 2, Diasorin, Dartford, United Kingdom) and total core antibodies (anti-HBc) using the Monolisa anti-HBc PLUS enzyme-linked immunosorbent assay kit (Bio-Rad, Marnes-la-Coquette, France). HBsAg+ samples were further screened for hepatitis B e antigen (HBeAg) and anti-HBc immunoglobulin M (IgM) using the Monolisa HBe Ag/Ab and Monolisa anti-HBc Plus 1 Plaque (Bio-Rad, Marnes-la-Coquette, France), respectively. HBV DNA was quantified in 148 HBsAg+ and 381 HBsAg-negative (HBsAg–) participants (screening for OBI) using the COBAS AmpliPrep/COBAS TaqMan HBV Test version 2.0 (Roche Diagnostics, Mannheim, Germany) following the manufacturer's instructions with a broad linear range from 20 to 1.7 × 108 IU/mL. Viral load quantification for OBI samples was done regardless of anti-HBc results. The assay has an analytical sensitivity of ≥95% and specificity of 100% with a confidence limit of 99.54% as reported by the manufacturer [29]. This kit also comes with 1 high-positive control, 1 low-positive control, and a negative control; results were considered valid if the controls passed validation. Using a prevalence estimate of 26.5% [30] at 80% power and 15% nonresponse, we estimated that the required sample size to be used for OBI estimation would be 306 samples. The sample size was enriched for downstream processes. The sample selection was mostly impacted by sample volume and sample availability.
Data Analysis
We calculated the Wilson 95% confidence interval (CI) around prevalence estimates. Factors associated with HBsAg positivity were determined using a multivariable multinomial logistic regression adjusting for clustering by community through the sandwich variance estimator model. Covariates were chosen a priori. Kruskal-Wallis test was used to compare continuous variables between OBI-infected and OBI-uninfected participants while χ2 test was used to compare categorical variables. P values <.05 were considered statistically significant. All statistical analyses were performed on Stata version 14.1 (StataCorp LLC, College Station, Texas).
RESULTS
Participant Characteristics and HBV Risk Factors
Characteristics of participants are summarized in Table 1 in 3 categories; HBsAg+ (infected group), HBsAg–/anti-HBc+ (exposed group), and HBsAg–/anti-HBc− (uninfected group). Approximately 73% of the participants were female, median age was 41 years, and 84% were on antiretroviral therapy (ART) (Table 1, Supplementary Table 1). In a multivariate multinomial logistic regression model, male sex, and the northern geographic region were independent predictors of positive HBsAg (relative risk ratio [RRR], 1.8 [95% CI, 1.2–2.7] and 2.5 [95% CI, 1.4–4.7], respectively; Table 2). HBsAg prevalence was significantly higher in men than women aged >55 years (P < .01), whereas this significance was not observed in younger participants (Supplementary Figure 1).
Table 1.
Participant Characteristics
| Characteristic | HBsAg+ (n = 271) | HBsAg–/Anti-HBc+ (n = 1561) | HBsAg–/Anti-HBc– (n = 1343) |
|---|---|---|---|
| Sex | |||
| Female | 173 (64) | 1135 (73) | 1016 (76) |
| Male | 98 (36) | 426 (27) | 327 (24) |
| Age category, y | |||
| ≤35 | 77 (28) | 320 (20) | 511 (38) |
| >35 | 194 (72) | 1241 (80) | 832 (62) |
| Region | |||
| South | 49 (18) | 407 (26) | 374 (28) |
| Central | 127 (47) | 779 (50) | 706 (53) |
| North | 95 (35) | 375 (24) | 263 (20) |
| Nadir CD4, cells/µL (n = 1295) | |||
| <100 | 15 (13) | 79 (13) | 47 (8) |
| 100–199 | 28 (24) | 114 (19) | 100 (18) |
| 200–499 | 55 (47) | 305 (50) | 288 (51) |
| ≥500 | 18 (16) | 112 (18) | 134 (24) |
| HIV viral load suppression (n = 3170) | |||
| Undetectable | 217 (80) | 1276 (82) | 1046 (78) |
| Detectable | 53 (20) | 283 (18) | 295 (22) |
| ART status (n = 3151) | |||
| ART-naive | 33 (12) | 227 (15) | 233 (17) |
| On ART | 235 (88) | 1322 (85) | 1101 (83) |
| Type of ART (n = 1799) | |||
| Non-TDF, non-3TC | 7 (4) | 41 (4) | 35 (5) |
| 3TC-containing, non-TDF | 67 (40) | 400 (43) | 272 (39) |
| TDF-containing | 93 (56) | 490 (53) | 394 (56) |
| Duration on ART, mo (n = 2241), median (IQR) | 6.6 (4.5–9.5) | 7.4 (3.9–10.7) | 6.4 (2.8–10.1) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: 3TC, lamivudine; anti-HBc, hepatitis B core antibody; ART, antiretroviral therapy; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; IQR, interquartile range; TDF, tenofovir disoproxil fumarate.
Table 2.
Multivariate Multinomial Logistic Regression of Factors Associated With Exposure to Hepatitis B Virus and Hepatitis B Surface Antigen Positivity
| Characteristic | HBsAg–/Anti-HBc+ (n = 1561), RRR (95% CI) | P Value | HBsAg+ (271), RRR (95% CI) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 1 (Ref) | 1 (Ref) | ||
| Male | 1.32 (1.02–1.71) | 0.03 | 1.82 (1.21–2.73) | <.01 |
| Age category, y | ||||
| ≤35 | 1 (Ref) | 1 (Ref) | ||
| >35 | 2.11 (1.60–2.80) | <.001 | 1.27 (.83–1.95) | .27 |
| Region | ||||
| South | 1 (Ref) | 1 (Ref) | ||
| Central | .99 (.74–1.33) | .74 | 1.37 (.88–2.13) | .16 |
| North | 1.41 (.93–2.16) | .93 | 2.54 (1.37–4.70) | <.01 |
| HIV viral load suppression | ||||
| Undetectable | Ref | Ref | ||
| Detectable | .99 (.70–1.41) | .98 | 1.21 (.65–2.28) | .55 |
| Type of ART | ||||
| Non-TDF, non-3TC | 1 (Ref) | 1 (Ref) | ||
| 3TC-containing, non-TDF | 1.12 (.66–1.91) | .68 | 1.10 (.41–2.97) | .84 |
| TDF-containing | 1.03 (.66–1.62) | .89 | 1.06 (.41–2.72) | .91 |
Abbreviations: 3TC, lamivudine; anti-HBc, hepatitis B core antibody; ART, antiretroviral therapy; CI, confidence interval; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; RRR, relative risk ratio; TDF, tenofovir disoproxil fumarate.
HBV Markers
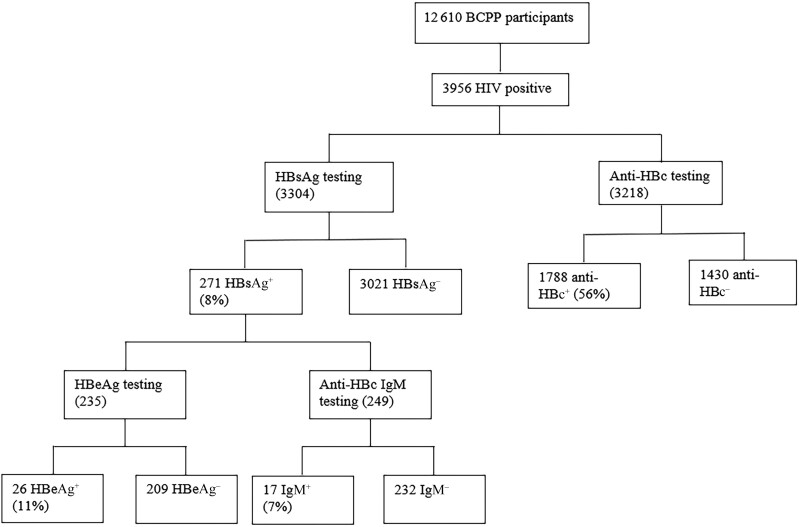
Of 3218 PWH tested for anti-HBc, we found evidence of HBV exposure (positive anti-HBc) in 1788 or 56% (95% CI, 54%–57%) (Figure 1). Of 3304 participants screened for HBsAg, 271 (8% [95% CI, 7%–9%]) had positive HBsAg. Of 249 HBsAg+ participants tested for anti-HBc IgM, 17 (7% [95% CI, 4%–11%]) tested IgM+. HBeAg was positive in 26 of 235 HBsAg+ participants tested (11% [95% CI, 8%–16%]) (Figure 1).
Figure 1.
Hepatitis B virus marker screening algorithm. Abbreviations: +, positive; –, negative; anti-HBc, hepatitis B core antibody; BCPP, Botswana Prevention Combination Project; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; IgM, immunoglobulin M.
HBsAg Prevalence by Community
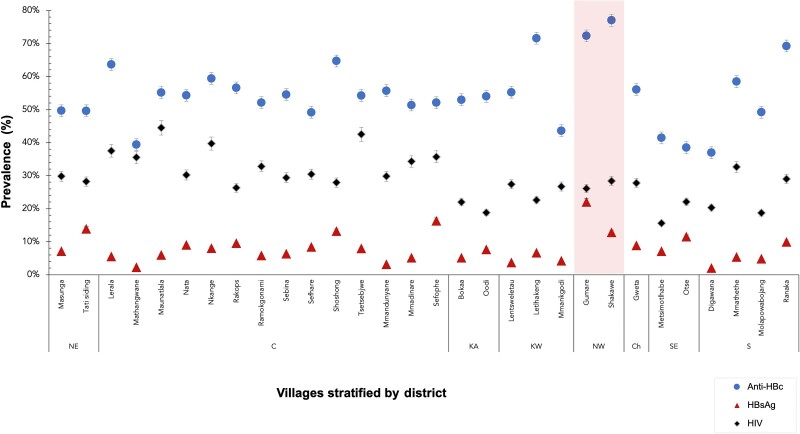
HBsAg prevalence varied substantially between communities in Botswana; the lowest prevalence of positive HBsAg was found in Digawana (2% [95% CI, .4%–11%]) while the highest prevalence was in Gumare (22% [95% CI, 16%–30%]). The Northwest district of Botswana (Gumare and Shakawe) showed high HBV exposure (positive anti-HBc) at 77% and 72%, respectively; however, HBsAg prevalence in these 2 communities varied greatly (22% and 12%, respectively; Figure 2).
Figure 2.
Hepatitis B surface antigen, hepatitis B core antibody, and human immunodeficiency virus prevalence in Botswana rural and periurban communities. Abbreviations: anti-HBc, hepatitis B core antibody; C, Central District; Ch, Chobe District; HBsAg, hepatitis B surface antigen; HIV, human immunodeficiency virus; KA, Kgatleng District; KW, Kweneng District; NE, North-East District; S, Southern District; SE, South-East District.
High HBV Viral Loads in Participants on Anti-HBV Therapy
Of participants who were positive for HBsAg, 39 (26%) had undetectable HBV viral loads, 80 (54%) had viral loads <2000 IU/mL, 13 (9%) had viral loads between 2000 and 19 999 IU/mL, 5 (3%) between 20 000 and 199 999 IU/mL, and 11 (7%) >200 000 IU/mL (Supplementary Table 2). The median HBV viral load was 409 IU/mL (95% CI, 104–1905 IU/mL) among participants with viral loads within the broad linear range. Table 3 shows HBsAg+ participants who had viral loads >200 000 IU/mL while on TDF- and 3TC-containing regimens. Participants on the 3TC-containing regimen made up 73% of these participants while those on the TDF-based regimen made up 27% of the participants. All participants on an ART regimen that included 3TC but not TDF had viral loads >170 000 000 IU/mL. All but 2 participants had suppressed HIV viral loads despite having HBV viral loads >200 000 IU/mL. Table 3 also shows participants with positive anti-HBc IgM and participants with actively replicating virus (HBeAg+) while on ART. For participants that had both HBV viral load and HBeAg serology results, 67% (12/18) of participants with positive HBeAg serology had HBV viral loads >20 000 IU/mL while 3% (4/121) with negative HBeAg serology had HBV viral loads >20 000 IU/mL (Supplementary Table 3)
Table 3.
Participants With Hepatitis B Virus Viral Loads ≥200 000 IU/mL While on Antiretroviral Therapy Containing Either Lamivudine or Tenofovir Disoproxil Fumarate
| Participant | HBV VL, IU/mL |
Regimen | Sex | Age, y | HIV VL, Copies/mL |
ART Duration, y | Anti-HBc | IgM | HBeAg |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 325 429 | EFV + FTC + TDF | F | 44 | 40 | NA | POS | NEG | POS |
| 2 | 377 860 | FTC + NVP + TDF | M | 52 | 40 | NA | POS | NEG | NEG |
| 3 | 157 534 884 | EFV + FTC + TDF | F | 27 | 40 | 6.9 | POS | POS | POS |
| 4 | >170 000 000 | 3TC + NVP + ZDV | M | 40 | 40 | 7.2 | NEG | NEG | POS |
| 5 | >170 000 000 | 3TC + NVP + ZDV | F | 49 | 40 | 13.8 | NEG | POS | POS |
| 6 | >170 000 000 | 3TC + NVP + ZDV | M | 45 | 40 | 16.2 | POS | NEG | POS |
| 7 | >170 000 000 | 3TC + NVP + ZDV | M | 47 | 40 | NA | POS | NEG | POS |
| 8 | >170 000 000 | 3TC + NVP + ZDV | F | 31 | 40 | 9.5 | POS | NEG | POS |
| 9 | >170 000 000 | 3TC + NVP + ZDV | F | 41 | 40 | 11.3 | POS | POS | POS |
| 10 | >170 000 000 | 3TC + NVP + ZDV | F | 35 | 21 574 | 6.8 | POS | NEG | POS |
| 11 | >170 000 000 | 3TC + NVP + ZDV | F | 30 | 80 680 | NA | POS | POS | POS |
Abbreviations: 3TC, lamivudine; anti-HBc, hepatitis B core antibody; ART, antiretroviral therapy; EFV, efavirenz; F, female; FTC, emtricitabine; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IgM, immunoglobulin M; M, male; NA, data not available; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; VL, viral load; ZDV, zidovudine.
Occult HBV Infection
Samples from 381 participants who had negative HBsAg were screened for HBV DNA, 126 (33.1% [95% CI, 28.5%–37.9%]) of whom had detectable DNA and hence OBI. Of persons with detectable HBV viral load, 85 of 126 (67.5%) had viral loads <20 IU/mL while the rest had viral loads ≥20 IU/mL. One participant had a viral load of 7 874 196 IU/mL. The participant was an ART-naive female aged 53 years with an HIV viral load of 1 505 646 copies/mL. A total of 67 of 118 (56.8%) participants with detectable viral load tested positive for anti-HBc while the remaining 43.2% were negative for anti-HBc. There was no statistically significant difference between OBI-infected individuals and OBI-uninfected participants in sex, age, nadir CD4+ T-cell count, log HIV viral load, ART status, ART type, or duration on ART (Supplementary Table 4).
DISCUSSION
In a near-nationwide, predominantly rural population–based random sample of >3300 adults with HIV, more than half had evidence of HBV exposure (positive anti-HBc), nearly 10% had positive HBsAg, and a high proportion of HBV-exposed, HBsAg– individuals had occult HBV by HIV DNA testing.
The HBV prevalence that was observed was comparable to that described by previous studies in Botswana [31] and in neighboring South Africa where a prevalence of 6.4% was observed in a similar cohort [14]. It is important to note that the majority of participants in our study were already on HIV ART with at least 1 drug active against HBV. As some patients clear HBsAg after being on ART [32], our results may be an underestimation of the true HBsAg prevalence in this population.
We also observed participants with positive anti-HBc IgM, HBeAg, and high HBV viral load who were already on TDF- and 3TC-containing regimens, which has been observed elsewhere [33, 34]. Since all participants had been on treatment for >6 years, the lack of HBV suppression could have not been due to duration on ART, but several reasons may be likely. We postulate that these participants may have acquired antiviral drug-resistant HBV as has been previously reported [35]. Persistent HBV viremia while on ART with anti-HBV activity is also attributed to drug resistance mutations in the HBV polymerase region [36] with resistance mutation rtM204I/V being reported to be the most prevalent in Africa [37]. Drug resistance may pose a challenge in the elimination of hepatitis B by 2030 [38]. It is worth noting that we could not ascertain whether these anti-HBc IgM+ cases are acute or reactivations as has been shown to be the likely case [39]. Evidence of tenofovir resistance has been discussed in chronic HBV patients [40] and therefore would be the likely situation in our anti-HBc IgM+ cases who could possibly be chronic carriers experiencing HBV reactivation.
OBI prevalence was estimated to be 33% in our population, which is slightly higher than the reported prevalence in a previous Botswana study among ART-naive individuals at 26.5% [30]. A much lower OBI prevalence of 5.6% was observed among treatment-naive PWH in Kenya [41]. Recently, it was revealed that there is regional variation of OBI in the African continent, with the southern region having the highest OBI prevalence compared to other regions [42]. Despite the different cohorts studied, OBI prevalence rates may vary due to the sensitivity of nucleic acid testing platforms used. Furthermore, the varying OBI prevalence in different African regions may be due to HBV genetic variability where high OBI prevalence were shown in genotype D [43]. The deletion present in genotype D was postulated to result in less HBsAg secretion than genotype A [44].
Being male was an independent risk factor for HBV infection in our study as has been observed elsewhere [45]. Exposure events may be attributed to this high prevalence in males than females [46] with high anti-HBc being observed in males in our study (data not shown) and elsewhere [45]. A combination of factors including behavioral factors may also be attributed to the difference in HBV prevalence between males and females [46].
HBV prevalence also varied by region in Botswana and was significantly higher in communities in the northern region of the country. HIV, anti-HBV, and HBsAg prevalence did not always follow the same trends (by community). For example, the Shakawe community had the highest HBV exposure (77% had positive anti-HBc) but did not have the highest HBsAg or HIV prevalence. We hypothesize that the varying HBsAg prevalence rates could be due to differences in host factors and genotype-specific seroclearance among different communities. Genotypes A and D are predominant in Botswana [5, 47]; however, their distribution across the country may differ. HBsAg seroclearance has been shown to be higher in genotype A than in genotype D [48, 49]. Furthermore, host-genetic factors, such as specific single-nucleotide polymorphisms like rs9277535, have also been associated with spontaneous seroclearance [50]. Transmission rates within each community may also vary, resulting in varying HBV prevalences. A combination of unique cultural practices, behaviors, uptake of HBV prevention strategies, and access to healthcare at birth, which could impact age at HBV exposure, may also contribute to the varying HBV prevalences by region.
Among persons with positive HBsAg, 93% had positive anti-HBc IgM serology. With the median age of HBsAg-positive participants being 42 years, we can assume that the majority of these individuals were not previously vaccinated, as universal childhood HBV vaccination in Botswana was introduced in 2000 [3].
Our study has several strengths. The primary strengths are the large representative random population-based sample of individuals from 30 communities across Botswana during an era of high ART coverage, and the high rate of completion of testing. Our study also has several limitations. We did not have results of liver function tests or other HBV-related clinical assessments. We did not screen for HBV surface antibody, which would have ascertained if the OBI cases identified were seropositive or seronegative cases. The study is also only in PWH; hence, results are not necessarily generalizable to people without HIV. Finally, we do not have longitudinal assessment of HBV test results.
CONCLUSIONS
This is the largest HBV study in Botswana and one of the largest in the region, and the first to report on HBV in understudied rural and periurban communities. HBV exposure and prevalence are still high among PWH in Botswana, a country that is showing success in achieving HIV epidemic control [18]. We also note an even higher OBI prevalence than previously reported, which could represent a population reservoir that may get missed during routine screening and which has implications for blood donations (particularly in resource-limited settings where nucleic acid testing may not be available). Our study has revealed that there is regional variation in HBV prevalence in Botswana and has identified areas with high prevalence rates that may require specific interventions from the Botswana Ministry of Health to curb these infections. HBV viral loads were found in some tenofovir- and lamivudine-experienced participants. The next steps involve sequencing HBV from these participants to look for drug resistance mutations. Screening of PWH for HBV infection prior to treatment initiation should be scaled up in the country. We recommend HBV surveillance studies in Botswana to shed more light into the dynamics of the infection in the population, particularly in areas that were not included in our study.
Supplementary Material
Contributor Information
Bonolo B Phinius, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Motswedi Anderson, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Irene Gobe, School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Margaret Mokomane, School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Wonderful T Choga, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Sharon R Mutenga, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Applied Biological Sciences and Biotechnology, Faculty of Science and Technology, Midlands State University, Gweru, Zimbabwe.
Gorata Mpebe, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Biological Sciences, Faculty of Sciences, University of Botswana, Gaborone, Botswana.
Molly Pretorius-Holme, Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Rosemary Musonda, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Tendani Gaolathe, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Mompati Mmalane, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Roger Shapiro, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Joseph Makhema, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Shahin Lockman, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Vlad Novitsky, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Max Essex, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Sikhulile Moyo, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Simani Gaseitsiwe, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Conceptualization: S. G., M. A., B. B. P. Methodology: B. B. P. Resources: S. G., M. A., M. P., T. G., M. Mm, R. S., J. M., S. L., V. N., M. E., S. M., S. G., R. M. Formal analysis; B. B. P., W. T. C., S. R. M. Investigation: B. B. P. Validation: B. B. P., G. M., S. R. M., W. T. C. Visualization; B. B. P., W. T. C. Writing–original draft preparation: B. B. P. Writing–review and editing: M. A., I. G., M. Mo, W. T. C., S. R. M., G. M., M. P., R. M., T. G., M. Mm, R. S., J. M., S. L., V. N., M. E., S. M., S. G. Supervision: S. G., M. A., I. G., M. Mo. Funding acquisition: S. G., M. A., R. M. All authors have read and agreed to the published version of the manuscript.
Acknowledgments. The authors thank the Botswana Prevention Combination Project study participants, Dikgosi and other community leaders, the clinic staff, District Health Management Teams, and Community Health Facilities at study sites; the Ya Tsie Study Team at the Botswana Harvard AIDS Institute Partnership, the Harvard T. H. Chan School of Public Health, the Centers for Disease Control and Prevention (CDC) Botswana, CDC Atlanta, and the Botswana Ministry of Health. The authors also thank those who served on the Ya Tsie Community Advisory Board, Laboratory Staff, and Management of Botswana Harvard HIV Reference Laboratory.
Financial support. This work was supported by the Wellcome Trust (grant number 218770/Z/19/Z). B. B. P. and S. G. are supported by the National Institutes of Health (NIH) Common Fund, award number U41HG006941 (H3ABioNet). H3ABioNet is an initiative of the Human Health and Heredity in Africa Consortium (H3Africa) program of the African Academy of Science. B. B. P., R. M., and S. M. are also supported by Trials of Excellence in Southern Africa (TESAIII), which is part of the EDCTP2 program supported by the European Union (grant number CSA2020NoE-3104 TESAIII). S. L. and R. S. received support from the NIH (award numbers K24 AI131928 and K24 AI131924, respectively). W. T. C., S. M., and S. G. are partly supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE 2.0) from the Bill & Melinda Gates Foundation (INV-033558). S. G. is supported by the Fogarty International Center at the US National Institutes of Health (D43 TW009610).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stanaway JD, Flaxman AD, Naghavi M, et al. . The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016; 388:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Interim guidance for country validation of viral hepatitis elimination. Geneva, Switzerland: WHO, 2021. [Google Scholar]
- 3. Tolle M, Anabwani G, Davis S, et al. . Prevalence of hepatitis B and hepatitis C coinfections in an adult HIV centre population in Gaborone, Botswana. Am J Trop Med Hyg 2011; 85:390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moturi E, Tevi-Benissan C, Hagan J, et al. . Implementing a birth dose of hepatitis B vaccine in Africa: findings from assessments in 5 countries. J Immunol Sci 2018; 5(Suppl):31–40. [PMC free article] [PubMed] [Google Scholar]
- 5. Choga WT, Anderson M, Zumbika E, et al. . Molecular characterization of hepatitis B virus in blood donors in Botswana. Virus Genes 2018; 55:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthews PC, Beloukas A, Malik A, et al. . Prevalence and characteristics of hepatitis B virus (HBV) coinfection among HIV-positive women in South Africa and Botswana. PLoS One 2015; 10:e0134037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wester CW, Bussmann H, Moyo S, et al. . Serological evidence of HIV-associated infection among HIV-1-infected adults in Botswana. Clin Infect Dis 2006; 43:1612–5. [DOI] [PubMed] [Google Scholar]
- 8. Makuwa M, Mintsa-Ndong A, Souquière S, et al. . Prevalence and molecular diversity of hepatitis B virus and hepatitis delta virus in urban and rural populations in northern Gabon in Central Africa. J Clin Microbiol 2009; 47:2265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ducancelle A, Abgueguen P, Birguel J, et al. . High endemicity and low molecular diversity of hepatitis B virus infections in pregnant women in a rural district of north Cameroon. PLoS One 2013; 8:e80346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molu JP, Essome MCN, Monamele CG, et al. . Sero-prevalence of HBsAg in naive HIV-infected patients in a rural locality of Cameroon. BMC Res Notes 2018; 11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forbi JC, Vaughan G, Purdy MA, et al. . Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria. PLoS One 2010; 5:e11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suesstrunk J, Djongali FB. Hepatitis B virus prevalence in rural areas in south-west Chad. Trop Doct 2017; 47:374–7. [DOI] [PubMed] [Google Scholar]
- 13. Barth RE, Huijgen Q, Tempelman HA, et al. . Presence of occult HBV, but near absence of active HBV and HCV infections in people infected with HIV in rural South Africa. J Med Virol 2011; 83:929–34. [DOI] [PubMed] [Google Scholar]
- 14. Samsunder N, Ngcapu S, Lewis L, et al. . Seroprevalence of hepatitis B virus: findings from a population-based household survey in KwaZulu-Natal, South Africa. Int J Infect Dis 2019; 85:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimakawa Y, Lemoine M, Mendy M, et al. . Population-based interventions to reduce the public health burden related with hepatitis B virus infection in The Gambia, West Africa. Trop Med Health 2014; 42(2 Suppl):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thio CL, Seaberg EC, Skolasky R, et al. . HIV-1, hepatitis B virus, and risk of liver-related mortality in the multicenter cohort study (MACS). Lancet 2002; 360:1921–6. [DOI] [PubMed] [Google Scholar]
- 17. Maponga TG, McNaughton AL, van Schalkwyk M, et al. . Treatment advantage in HBV/HIV coinfection compared to HBV monoinfection in a South African cohort. J Infect 2020; 81:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mine M, et al. Botswana Achieved the Joint United Nations Programme on HIV/AIDS (UNAIDS) 95-95-95 targets: results from the Fifth Botswana HIV/AIDS Impact Survey (BAIS V), 2021 [231]. In: 24th international AIDS conference, Montreal, Canada, 29 July–2 August2022. Poster exhibition abstract (PELBC01) presented at AIDS2022. [Google Scholar]
- 19. Ministry of Health of Botswana, Masa, and Treat All . Handbook of the Botswana 2016 integrated HIV clinical care guidelines. Gaborone, Botswana:Ministry of Health;2016. [Google Scholar]
- 20. Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol 2007; 46:160–70. [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann CJ, Mashabela F, Cohn S, et al. . Maternal hepatitis B and infant infection among pregnant women living with HIV in South Africa. J Int AIDS Soc 2014; 17:18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coppola N, Tonziello G, Pisaturo M, et al. . Reactivation of overt and occult hepatitis B infection in various immunosuppressive settings. J Med Virol 2011; 83:1909–16. [DOI] [PubMed] [Google Scholar]
- 23. Selabe SG, Lukhwareni A, Song E, Leeuw YGM, Burnett RJ, Mphahlele MJ. Mutations associated with lamivudine-resistance in therapy-naïve hepatitis B virus (HBV) infected patients with and without HIV co-infection: implications for antiretroviral therapy in HBV and HIV co-infected South African patients. J Med Virol 2007; 79:1650–4. [DOI] [PubMed] [Google Scholar]
- 24. Fang Y, et al. . Molecular characterization and functional analysis of occult hepatitis B virus infection in Chinese patients infected with genotype C. J Med Virol 2009; 81:826–35. [DOI] [PubMed] [Google Scholar]
- 25. Kim H, Lee SA, Kim BJ. X region mutations of hepatitis B virus related to clinical severity. World J Gastroenterol 2016; 22:5467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology 2008; 48:88–98. [DOI] [PubMed] [Google Scholar]
- 27. Makhema J, Wirth KE, Pretorius Holme M, et al. . Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019; 381:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaolathe T, Wirth KE, Holme MP, et al. . Botswana’s progress toward achieving the 2020 UNAIDS 90–90–90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3:e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roche . COBAS AmpliPrep/COBAS TaqMan HBV Test, v2.0. 2022.https://diagnostics.roche.com/global/en/products/params/cobas-ampliprep-cobas-taqman-hbv-test-v2-0.html. Accessed 12 August 2022.
- 30. Ryan K, Anderson M, Gyurova I, et al. . High rates of occult hepatitis B virus infection in HIV-positive individuals initiating antiretroviral therapy in Botswana. Open Forum Infect Dis 2017; 4:ofx195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson M, et al. . Slow CD4+ T cell recovery in human immunodeficiency virus/hepatitis B virus–coinfected patients initiating Truvada-based combination antiretroviral therapy in Botswana. Open Forum Infect Dis 2016; 3:ofw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chihota BV, Wandeler G, Chilengi R, et al. . High rates of hepatitis B virus (HBV) functional cure among human immunodeficiency virus-HBV coinfected patients on antiretroviral therapy in Zambia. J Infect Dis 2020; 221:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su F, Dai J, Yang S, et al. . Prevalence and types of drug-resistant variants in Chinese patients with acute hepatitis B. J Med Virol 2015; 87:1527–31. [DOI] [PubMed] [Google Scholar]
- 34. Baxa DM, Thekdi AD, Golembieski A, et al. . Evaluation of anti-HBV drug resistant mutations among patients with acute symptomatic hepatitis B in the United States. J Hepatol 2013; 58:212–6. [DOI] [PubMed] [Google Scholar]
- 35. Locarnini S. Transmission of antiviral drug resistant hepatitis B virus: implications for public health and patient management. J Gastroenterol Hepatol 2010; 25:649–51. [DOI] [PubMed] [Google Scholar]
- 36. Msomi N, Parboosing R, Wilkinson E, et al. . Persistent hepatitis B viraemia with polymerase mutations among HIV/HBV co-infected patients on HBV-active ART in KwaZulu-Natal, South Africa. Viruses 2022; 14:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mokaya J, McNaughton AL, Hadley MJ, et al. . A systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa: a call for urgent action. PLoS Negl Trop Dis 2018; 12:e0006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization (WHO) . Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 39. Downs LO, McNaughton AL, de Cesare M, et al. . Case report: application of hepatitis B virus (HBV) deep sequencing to distinguish between acute and chronic infection. Wellcome Open Res 2020; 5:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mokaya J, Maponga TG, McNaughton AL, et al. . Evidence of tenofovir resistance in chronic hepatitis B virus (HBV) infection: an observational case series of South African adults. J Clin Virol 2020; 129:104548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salyani A, Shah J, Adam R, et al. . Occult hepatitis B virus infection in a Kenyan cohort of HIV infected anti-retroviral therapy naive adults. PLoS One 2021; 16:e0244947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kajogoo VD, Swai SS, Gurung S. Prevalence of occult hepatitis B among HIV-positive individuals in Africa: a systematic review and meta-analysis. SAGE Open Med 2022; 10:20503121211072748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel NH, Meier-Stephenson V, Genetu M, et al. . Prevalence and genetic variability of occult hepatitis B virus in a human immunodeficiency virus positive patient cohort in Gondar, Ethiopia. PLoS One 2020; 15:e0242577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sengupta S, Panda SK, Acharya SK, Durgapal H. Role of hepatitis B virus genotype D and its mutants in occult hepatitis B infection. Indian J Med Res 2013; 138:329–39. [PMC free article] [PubMed] [Google Scholar]
- 45. Hamilton E, Yang L, Mentzer A, et al. . Conventional and genetic risk factors for chronic hepatitis B virus infection in a community-based study of 0.5 million Chinese adults. Sci Rep 2022; 12:12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown R, Goulder P, Matthews PC. Sexual dimorphism in chronic hepatitis B virus (HBV) infection: evidence to inform elimination efforts. Wellcome Open Res 2022; 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anderson M, Gaseitsiwe S, Moyo S, et al. . Molecular characterisation of hepatitis B virus in HIV-1 subtype C infected patients in Botswana. BMC Infect Dis 2015; 15:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marcellin P, Bonino F, Lau GKK, et al. . Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology 2009; 136:2169–79.e1–4. [DOI] [PubMed] [Google Scholar]
- 49. Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med 2015; 5:a021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng HR, Liu C-J, Tseng T-C, et al. . Host genetic factors affecting spontaneous HBsAg seroclearance in chronic hepatitis B patients. PLoS One 2013; 8:e53008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.