Abstract
Meningococcal group A+C capsular polysaccharide (PS) conjugate vaccines may prime for serum immunoglobulin G (IgG) memory responses to meningococcal capsular PS. It is not known whether these vaccines induce immunological memory at the mucosal level, which may be important in reducing nasopharyngeal carriage. Mucosal immune responses to meningococcal conjugate and PS vaccines in young adults were investigated. Healthy university students were randomized to receive either a groups A+C meningococcal conjugate vaccine (MACconj, n = 100) or a group A+C meningococcal PS vaccine (MACPS, n = 95). One year after the primary immunization, both groups were randomized again to receive a MACconj or a MACPS booster vaccination. Saliva samples were collected before and 1 month after the primary and booster vaccinations. Anti-meningococcal A (MenA) and C (MenC) PS IgA and IgG antibody levels were measured by a standard enzyme-linked immunosorbent assay. After the primary vaccination, salivary MenA and MenC IgG and MenA IgA concentrations were significantly increased after immunization with both MACconj and MACPS vaccines, but the salivary Men C IgA level was increased only after MACPS vaccine (P < 0.01). IgA responses to both serogroups were greater for MACPS than MACconj vaccine (P < 0.05), whereas no significant differences were seen for IgG responses. MenA IgG titers were higher after the MACPS booster in MACconj-primed subjects than after the MACPS primary vaccination, suggesting the presence of IgG memory. Antibody responses to a dose of either MACPS or MACconj were not significantly reduced in those previously given MACPS compared to the primary responses to those vaccines. Meningococcal A+C conjugate and PS vaccines induce significant mucosal responses in young adults. MACconj priming may induce IgG memory at the mucosal level, which is likely to be a reflection of an anamnestic serum IgG response. No evidence of mucosal hyporesponsiveness was observed after MACPS priming in this study.
Parenteral immunisation with meningococcus group C polysaccharide (PS) vaccine may induce local immunity in the nasopharynx against meningococci, since a significantly lower percentage of vaccinated military recruits became carriers of group C meningococci than did unvaccinated controls (6). The local immunity induced was specific for meningococcus group C. We and others have shown that upper respiratory tract mucosal antibodies are produced following meningococcus PS vaccination (20, 26), which could play an important role in preventing immunized individuals becoming carriers. We also observed that the levels of these antibodies declined rapidly to near-prevaccination levels after 6 to 12 months, raising the possibility that protection could be short-term (26). However, if vaccines induce immunological memory at the mucosal level, long-term protection could be achieved. The widespread use of such vaccines would not only protect immunized individuals against invasive disease but also potentially interrupt the chain of transmission, thereby protecting the unimmunized population through “herd immunity” or “population immunity.” Meningococcal group A and C PS conjugate vaccines have recently been developed and shown to prime for anamnestic serum immunoglobulin G (IgG) responses to meningococcus serogroup C PS vaccine (13, 14, 16, 22). However, little information is available about whether these vaccines induce immunological memory at the mucosal level.
There has been an increase in the proportion of group C strains in England and Wales, and older children and young adults have been at particular risk (8). Outbreaks of Neisseria meningitidis group C disease have occurred recently in British universities, prompting the widespread use of meningococcal PS vaccines for first-year students in 1999. There have been recent reports suggesting that meningococcus group C PS vaccine may induce hyporesponsiveness or tolerance to subsequent doses of PS vaccine (7, 15, 23), raising the question whether use of meningococcus group C PS vaccine as a primary immunization has potential disadvantages.
We have studied the primary and booster mucosal immune responses to an A+C meningococcal conjugate vaccine and an A+C meningococcal PS vaccine in young adults to assess whether these vaccines induce memory mucosal responses and whether primary immunization with a PS vaccine induces hyporesponsiveness to subsequent PS or conjugate vaccine doses.
MATERIALS AND METHODS
Study subjects and vaccines.
Healthy University of Sheffield students aged 17 to 30 years were randomized to receive a single dose (0.5 ml) of either a group A+C meningococcus conjugate vaccine (MACconj [Aventis Pasteur]) (group 1, n = 100) or a group A+C meningococcus PS vaccine (MACPS [Aventis Pasteur]) (group 2, n = 95). One year after the first (primary) immunization, both groups were randomized again to receive a single dose of MACconj or MACPS vaccination (booster); the groups were as follows: group 1A (n = 41), MACconj (primary) and MACconj (booster); group 1B (n = 43), MACconj and MACPS; group 2A (n = 32), MACPS and MACconj; group 2B (n = 41), MACPS and MACPS. This was a randomized, controlled study, and the primary phase was observer blinded. Study participants were immunized in a separate area of the study clinic by study nurses not otherwise involved in the study. The subjects were not informed which vaccine they received, although the appearance of the vaccines was not identical. The study was performed before general immunization against meningococcus was introduced in the United Kingdom, and none of the subjects had previously been immunized.
For the MACconj vaccine, a 0.5-ml dose containing 4 μg each of meningococcus group A and C PS and 48 μg of diphtheria toxoid (protein carrier) was injected intramuscularly into the left deltoid muscle. For the MACPS vaccine, a 0.5-ml dose containing 50 μg of each PS was similarly injected intramuscularly. The same batches of MACconj or MACPS vaccines were used for the primary and booster immunizations.
The study was approved by the South Sheffield local research ethics committee, and written informed consent was obtained from all subjects before enrolment.
Sample collection.
Before and 1 month after the primary vaccination and before and 1 month after the booster (day 0, day 30, day 360, and day 390), unstimulated saliva was collected by inserting a sponge swab in the mouth until the swab was saturated with saliva. Samples were transported at 4°C to the laboratory within 3 h and stored at −70°C until assayed.
Immunoassay for salivary anti-meningococcal A and C PS-specific IgG and IgA antibodies.
Specific salivary antibodies against serogroup A (MenA) and C (MenC) meningococcal PS were determined using an enzyme-linked immunosorbent assay as described previously (26). In brief, Immulon 1 microtiter plates (Dynex, Chantilly, Va.) were coated overnight at 4°C with 5 μg of meningococcal A or C PS (the gift of George Carlone, Centers for Disease Control and Prevention) (CDC) per ml diluted in phosphate-buffered saline (PBS), containing 5 μg of methylated human serum albumin (the gift of Mike Bybel, Aventis Pasteur) per ml. After the plates were washed, 10% fetal bovine serum in PBS was added to block nonspecific binding. Diluted saliva and standard serum (CDC 1992 reference, the gift of George Carlone) samples were added to each plate, and the plates were incubated. Subsequently, alkaline phosphatase-conjugated anti-human IgG (Sigma) was added for the IgG assay. Substrate (p-nitrophenyl phosphate) was added, and the plates were incubated. The optical density (OD) at 405 nm was measured using a plate reader (Dynex), and concentrations of IgG were calculated by interpolation on the standard curve derived from serial dilutions of the reference serum (CDC 1992). For MenA and MenC PS-specific IgA measurements, the plates were incubated at room temperature (RT) on a horizontal rotator for 2 h after addition of samples. Murine monoclonal antibodies to human IgA (1:5,000) were then added to the plates, and the plates were incubated at RT for 2 h. Alkaline phosphatase-conjugated goat anti-mouse antibodies (Stratech) were added, and the plates were incubated overnight at RT. Subsequent procedures were as for the IgG assay.
Measurement of total salivary IgA and IgG levels.
Total salivary IgA and IgG levels were also measured by immunoassay as described previously (3, 26), so that the ratio of MenA and MenC PS-specific IgA or IgG could be calculated for each subject as a method of compensating for dilution of saliva.
Statistical analysis.
Antibody concentrations were logarithmically transformed (base 10), and geometric mean concentrations (GMC) with 95% confidence intervals (CI) were calculated for each study group. Antibody titers below the limit of detection were arbitrarily assigned to half the lower limit of detection for each assay. The lower limit of detection was determined from calculation of the lowest concentration in the standard curve derived from the reference standard, whose OD was at least 2 standard deviations above the mean OD of 10 PBS blank controls (10 PBS solutions in triplicate were assayed three times instead of test samples) for each assay. Comparisons between vaccine groups were made using Student's t test. Comparisons between pre- and postvaccination GMCs or between post-primary and post-booster immunization GMCs within groups were made using paired t tests. Analysis of the age and gender distribution among groups was done using one-way analysis of variance and χ2 tests, respectively. Statistical analysis was done using SPSS for Windows (version 9.0; SPSS Inc., Chicago, Ill.). P < 0.05 was considered to indicate statistical significance.
RESULTS
A total of 195 young adults entered the primary phase of the study. The number of adults completing each phase of the study and for whom saliva samples were analyzed for each antibody type are shown in Tables 1 and 2. There were no significant differences between the two primary phase groups and four booster phase groups with respect to age and gender (data not shown).
TABLE 1.
Salivary antibody responses to MenA and MenC PS after primary immunization with MACconj or MACPSa
| Antibody | Time (days) after primary vaccination | Group 1 (MACconj)
|
Group 2 (MACPS)
|
Pb | ||
|---|---|---|---|---|---|---|
| Antibody level in saliva (ng/ml) (95% CI) | No. of subjects | Antibody level in saliva (ng/ml) (95% CI) | No. of subjects | |||
| MenA IgG | 0 | 9.92 (6.96, 14.08) | 88 | 15.60 (11.40, 21.40) | 86 | NSf |
| 30 | 31.24c (20.50, 47.50) | 88 | 46.26c (32.39, 66.10) | 86 | NS | |
| MenA IgA | 0 | 40.81 (27.18, 61.28) | 87 | 49.26 (32.92, 73.71) | 85 | NS |
| 30 | 67.75d (46.30, 99.15) | 87 | 126.91c (87.42, 183.70) | 85 | <0.05 | |
| MenC IgG | 0 | 6.88 (5.10, 9.30) | 88 | 8.17 (6.00, 11.12) | 86 | NS |
| 30 | 15.69c (10.73, 23.0) | 88 | 22.44c (15.64, 32.20) | 86 | NS | |
| MenC IgA | 0 | 9.44 (6.95, 12.82) | 87 | 7.25 (5.33, 9.85) | 85 | NS |
| 30 | 8.90e (6.56, 12.05) | 87 | 33.81c (24.55, 46.57) | 85 | <0.001 | |
Data on the subjects who completed the primary phase of the study and for whom saliva samples were analyzed for each antibody type are presented.
P values of comparisons of antibody concentrations between two vaccines at each time point.
P < 0.001 (between the day 0 and day 30 results).
P < 0.01 (between the day 0 and day 30 results).
P = NS (between the day 0 and day 30 results).
NS, not significantly different.
TABLE 2.
Comparison of salivary antibody responses to MenA and MenC PS between primary and booster immunizationsa
| Antibody | Time (days) after primary vaccination | Group 1A (MACconj-MACconj)
|
Group 1B (MACconj-MACPS)
|
Group 2A (MACPS-MACconj)
|
Group 2B (MACPS-MACPS)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Antibody level in saliva (ng/ml) (95% CI) | No. of subjects | Antibody level in saliva (ng/ml) (95% CI) | No. of subjects | Antibody level in saliva (ng/ml) (95% CI) | No. of subjects | Antibody level in saliva (ng/ml) (95% CI) | No. of subjects | ||
| MenA IgG | 30 | 35.79 (19.20, 66.90) | 39 | 25.49 (13.00, 50.10) | 38 | 36.53 (20.30, 65.80) | 27 | 63.60 (34.90, 116.00) | 33 |
| 390 | 63.27 (33.60, 119.00) | 39 | 147.00b (78.80, 274.00) | 38 | 46.01 (22.40, 94.50) | 27 | 139.20c (84.00, 231.00) | 33 | |
| MenC IgG | 30 | 17.73 (9.77, 32.17) | 39 | 16.03 (8.78, 29.25) | 38 | 20.85 (10.48, 41.45) | 27 | 26.69 (15.40, 46.30) | 33 |
| 390 | 24.97 (14.64, 42.60) | 39 | 39.35b (24.20, 64.00) | 38 | 18.54 (8.78, 39.14) | 27 | 38.87 (23.44, 64.46) | 33 | |
| MenA IgA | 30 | 51.02 (29.51, 88.10) | 38 | 75.45 (38.37, 148.25) | 36 | 79.51 (36.73, 172.19) | 26 | 146.73 (78.52, 274.16) | 31 |
| 390 | 81.05 (46.34, 141.58) | 38 | 201.56b (134.59, 302.00) | 36 | 115.53 (61.94, 215.28) | 26 | 269.35d (189.67, 382.82) | 31 | |
| MenC IgA | 30 | 8.22 (5.28, 12.79) | 38 | 9.00 (5.30, 15.31) | 36 | 24.93 (12.33, 50.47) | 26 | 50.99 (30.06, 86.50) | 31 |
| 390 | 21.96b (14.62, 32.96) | 38 | 47.92b (33.57, 68.39) | 36 | 30.30 (16.07, 57.15) | 26 | 57.44 (35.08, 94.19) | 31 | |
Data on the subjects who completed the booster phase of the study and for whom saliva samples were analyzed for each antibody type are presented.
P < 0.01 (between the day 30 and day 390 results).
P = 0.024 (between the day 30 and day 390 results).
P = 0.028 (between the day 30 and day 390 results).
Primary anti-meningococcal A and C PS-specific IgA and IgG responses.
MenA and MenC PS-specific IgA and IgG responses after the primary immunization are shown in Table 1. Both salivary MenA and MenC IgG antibody concentrations were significantly increased after immunization with MACconj and MACPS vaccines (P < 0.001). There was no significant difference between the IgG responses to the two vaccines. There were significant increases in salivary MenA and MenC IgA concentrations after MACPS vaccination, whereas only the MenA IgA level rose significantly for the MACconj vaccine (P < 0.01). Compared to the MACconj vaccine, the MACPS vaccine induced higher concentrations of both MenA IgA (P < 0.05) and MenC IgA (P < 0.001).
Booster MenA and MenC PS-specific IgA and IgG responses.
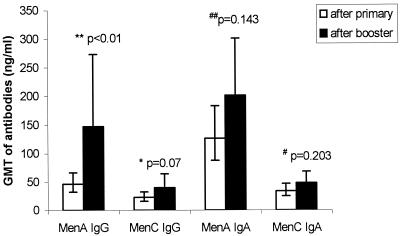
Booster responses after the second dose of vaccine were compared with primary responses (in nanograms per milliliter (Fig. 1; table 2). MenA salivary IgG responses after MACPS booster in MACconj-primed subjects (group 1B) were significantly higher than MACPS primary responses (group 2) (P < 0.01) (Fig. 1). The equivalent comparison for MenC IgG responses did not show a statistically significant difference (P = 0.07) (Fig. 1). There were also no significant differences in IgA titers between such MACPS booster and MACPS primary responses for MenA (P = 0.143) and MenC (P = 0.203) (Fig. 1).
FIG. 1.
Comparison of salivary IgG and IgA responses to MenA and MenC PS between booster MACPS responses in MACconj vaccine-primed subjects (group 1B) and primary MACPS responses (group 2).
In group 1A, whose members were primed with MACconj and boosted with the same MACconj vaccine, the MenA IgA, MenA IgG, and MenC IgG antibody mean titers after the booster immunization were not significantly different from the primary responses. However, the MenC IgA titers after the booster were significantly higher than after primary immunization (the response to which was very poor) (P < 0.01) (Table 2).
In group 2B, whose members received primary MACPS and booster MACPS vaccines, both MenA PS IgG and IgA titers were significantly higher after the booster (day 390) than after the primary (day 30) vaccination (P = 0.024 and 0.028 respectively) (Table 2). MenC PS IgG and IgA titers after the booster were not significantly different from those after the primary vaccination.
When antibody responses after MACconj booster in MACPS-primed individuals (group 2A, day 390) were compared with primary MACconj responses (groups 1A and 1B, day 30), no significant differences were found for the MenA PS IgA and IgG and MenC PS IgG titers but a significant difference was found for the MenC IgA titers (P < 0.05) (Table 2).
When anti-MenA and MenC PS-specific IgA and IgG responses were expressed as the ratio of the specific antibody concentrations to total IgA or IgG in saliva (data not shown), the results of the statistical analysis of antibody concentrations comparing vaccine groups, pre- and postimmunisation values, or post-primary and post-booster immunization values were closely similar to the results expressed as absolute concentrations (nanograms per milliliter), and thus all conclusions drawn are the same using both approaches to analysis.
DISCUSSION
Mucosal immunity is important in protecting the host against mucosal pathogens such as N. meningitides, since the human nasopharyngeal mucosa is the only natural reservoir of this organism, which is transferred from person to person through direct contact or via respiratory secretions. Meningococcal PS-specific antibodies may promote the clearance of nasopharyngeal carriage. Haemophilus influenzae type b (Hib) conjugate vaccines induce anti-PS mucosal antibody responses (10) and are associated with reduced carriage rates when widely used (2, 9–11, 19, 24, 25).
We have previously reported anti-MenC mucosal immune responses to a different MenC conjugate vaccine in adolescents compared to the same A and C PS vaccine used in this study (26). In that study, the IgG responses to the conjugate vaccine were greater and the IgA responses were similar. The fact that IgG and IgA responses to the conjugate vaccine in this study were poorer may simply reflect the PS doses in the two conjugate vaccines (4 μg in this study and 10 μg in the previous study) and in the MACPS vaccine (50 μg each of A and C PS).
To investigate whether these meningococcal PS vaccines induce immunological memory or tolerance, booster immunizations were given 1 year after the primary vaccinations. Our comparison of mucosal responses to MACPS in naive subjects and those previously primed with MACconj suggests a priming effect for MenA IgG responses. We and others have previously presented evidence suggesting that the salivary IgG response to MenC capsular PS is serum derived (3, 20, 26), and there is evidence that MenC conjugate vaccines prime for memory-type IgG responses to PS boosters in serum (13, 22). These mucosal data may reflect IgG memory responses to MenA in serum.
We have previously presented data suggesting that salivary MenC PS IgA antibody is largely secretory IgA and thus is likely to be locally produced rather than serum derived (26). We have also presented evidence supporting the existence of IgA mucosal memory responses to pneumococcal PS vaccine in young children primed with conjugate vaccine (4). The comparison of responses to MACPS in naive and MACconj-primed subjects in this study does not suggest the presence of IgA mucosal memory. Whether this is affected by the antigens used, their doses, the timing of sample collection, or the ages of the subjects remains to be studied. However, our observation of approximately mean 2.5-fold increases in MenC IgA titers on administration of a second dose of MACconj (group 1A) compared to the poor response to a first dose (groups 1) offers some evidence of priming for mucosal IgA responses by the conjugate vaccine if the same vaccine is used to boost.
Detectable titers of both MenA and MenC IgA and IgG antibody were observed in the saliva of many subjects prior to immunization (table 1). This may be due to previous exposure and colonization with these or antigenically related bacteria. The carrier state is an immunizing process, which gives rise to antibodies to several meningococcal antigens including group-specific polysaccharides (5). It is not known whether this “natural immunization” induces immunological memory. If it does, it might explain why some individuals produced very high antibody titers (IgA and/or IgG) after just one MACPS vaccination, which could be acting as a booster for these individuals. In this situation, subsequent vaccinations may not necessarily boost their immune responses further. Such effects could confound observations made in a study of this design and explain why the results are less straightforward than those obtained in studies of younger subjects. Future studies designed to include more subjects and to stratify the study population according to the levels of preexisting antibody will help define whether and how preexisting antibody may influence the immunogenicity of “primary” and/or “booster” vaccination in young adults.
Classically PS antigens are not thought to induce immunological memory or to induce a T-cell-dependent antibody response (12, 18). Recent studies have reported a degree of immunological tolerance induced by MenC PS given in certain dose regimens (7, 15, 23) and first described in the 1970s (1). Our data do not show evidence of such hyporesponsiveness in either IgA or IgG responses at the mucosal level to MenC PS. In contrast, we demonstrate augmented IgA and IgG mucosal responses to MenA after a second dose of MACPS, suggesting that the PS antigen primes for memory responses. Like the MenC tolerance phenomenon, anamnestic responses caused by MenA PS have been observed previously in the serum of immunised children (17, 21). It is clear that the nature of the antibody responses to PS vaccine antigens varies in important and fundamental ways among antigens. Further studies with humans are needed to optimize the potential of vaccines to interrupt nasopharyngeal carriage and transmission of N. meningitidis.
ACKNOWLEDGMENTS
We thank the students of University of Sheffield who took part in the study. We thank Helena Käyhty and Maija Horkeila (KTL, Helsinki, Finland) and Ray Borrow (PHLS, Manchester, United Kingdom) for advice on salivary immunoassays. We also thank Lynn Seymour, Karen McMurtrie, Annie Wright, Lorna Ward, Lynne Shaw, and Gillian Race (this group) and Karen Crowther, Laurence Pollissard, Beatrice Buffin, and Chantal Ethevenaux (Aventis Pasteur) for their help throughout the study, and we thank Nicolas Rouyrre (Aventis Pasteur) for help in data analysis.
We acknowledge Aventis Pasteur and the Children's Appeal, Sheffield, United Kingdom, for financial support.
REFERENCES
- 1.Artenstein M S, Brandt B L. Immunologic hyporesponsiveness in man to group C meningococcal polysaccharide. J Immunol. 1975;115:5–7. [PubMed] [Google Scholar]
- 2.Barbour M L, Booy R, Crook D W, Griffiths H, Chapel H M, Moxon E R, Mayon-White D. Haemophilus influenzae type b carriage and immunity four years after receiving the Haemophilus influenzae oligosaccharide-CRM197 (HbOC) conjugate vaccine. Pediatr Infect Dis J. 1993;12:478–484. doi: 10.1097/00006454-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Borrow R, Fox A J, Cartwright K, Begg N T, Jones D M. Salivary antibodies following parenteral immunization of infants with a meningococcal serogroup A and C conjugated vaccine. Epidemiol Infect. 1999;123:201–208. doi: 10.1017/s0950268899002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo S, Zhang Q, Seymour L, Akhtar S, Finn A. Primary and booster salivary antibody responses to a 7-valent pneumococcal conjugate vaccine in infants. J Infect Dis. 2000;182:1260–1263. doi: 10.1086/315834. [DOI] [PubMed] [Google Scholar]
- 5.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotschlich E C, Goldschneider I, Artenstein M S. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med. 1969;129:1385–1395. doi: 10.1084/jem.129.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granoff D M, Gupta R K, Belshe R B, Anderson E L. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis. 1998;178:870–874. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- 8.Kaczmarski E B. Meningococcal disease in England and Wales: 1995. Commun Dis Rep CDR Rev. 1997;4:R55–R59. [PubMed] [Google Scholar]
- 9.Kauppi-Korkeila M, van Alphen L, Madore D, Saarinen L, Kayhty H. Mechanism of antibody-mediated reduction of nasopharyngeal colonization by Haemophilus influenzae type b studied in an infant rat model. J Infect Dis. 1996;174:1337–1340. doi: 10.1093/infdis/174.6.1337. [DOI] [PubMed] [Google Scholar]
- 10.Kauppi M, Eskola J, Kayhty H. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J. 1995;14:286–294. doi: 10.1097/00006454-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kauppi M, Saarinen L, Kayhty H. Anti-capsular polysaccharide antibodies reduce nasopharyngeal colonization by Haemophilus influenzae type b in infant rats. J Infect Dis. 1993;167:365–371. doi: 10.1093/infdis/167.2.365. [DOI] [PubMed] [Google Scholar]
- 12.Kayhty H, Karanko V, Peltola H, Makela P H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984;74:857–865. [PubMed] [Google Scholar]
- 13.Leach A, Twumasi P A, Kumah S, Banya W S, Jaffar S, Forrest B D, Granoff D M, LiButti D E, Carlone G M, Pais L B, Broome C V, Greenwood B M. Induction of immunological memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J Infect Dis. 1997;175:200–204. doi: 10.1093/infdis/175.1.200. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald N E, Halperin S A, Law B J, Forrest B, Danzig L E, Granoff D M. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers. JAMA. 1998;280:1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan J, Obaro S, Deeks J, Williams D, Pais L, Carlone G, Moxon R, Greenwood B. Immune response to revaccination with meningococcal A and C polysaccharides in Gambian children following repeated immunisation during early childhood. Vaccine. 1999;17:3086–3093. doi: 10.1016/s0264-410x(99)00139-5. [DOI] [PubMed] [Google Scholar]
- 16.MacLennan J M, Shackley F, Heath P T, Deeks J J, Flamank C, Herbert M, Griffiths H, Hatzmann E, Goilav C, Moxon E R. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. JAMA. 2000;283:2795–2801. doi: 10.1001/jama.283.21.2795. [DOI] [PubMed] [Google Scholar]
- 17.Makela P H, Peltola H, Kayhty H, Jousimies H, Pettay O, Ruoslahti E, Sivonen A, Renkonen O V. Polysaccharide vaccines of group A Neisseria meningitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977;136(Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- 18.Mosier D E, Subarrao B. Thymus-independent antigens: complexity of B-lymphocyte activation revealed. Immunol Today. 1982;3:217–222. doi: 10.1016/0167-5699(82)90095-0. [DOI] [PubMed] [Google Scholar]
- 19.Murphy T V, Pastor P, Medley F, Osterholm M T, Granoff D M. Decreased Haemophilus colonization in children vaccinated with Haemophilus influenzae type b conjugate vaccine. J Pediatr. 1993;122:517–523. doi: 10.1016/s0022-3476(05)83529-2. [DOI] [PubMed] [Google Scholar]
- 20.Nurkka A, MacLennan J, Jantti V, Obaro S, Greenwood B, Kayhty H. Salivary antibody response to vaccination with meningococcal A/C polysaccharide vaccine in previously vaccinated and unvaccinated Gambian children. Vaccine. 2000;19:547–556. doi: 10.1016/s0264-410x(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 21.Peltola H, Safary A, Kayhty H, Karanko V, Andre F E. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatrics. 1985;76:91–96. [PubMed] [Google Scholar]
- 22.Richmond P, Borrow R, Miller E, Clark S, Sadler F, Fox A, Begg N, Morris R, Cartwright K. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis. 1999;179:1569–1572. doi: 10.1086/314753. [DOI] [PubMed] [Google Scholar]
- 23.Richmond P, Kaczmarski E, Borrow R, Findlow J, Clark S, McCann R, Hill J, Barker M, Miller E. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis. 2000;181:761–764. doi: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- 24.Takala A K, Eskola J, Leinonen M, Kayhty H, Nissinen A, Pekkanen E, Makela P H. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982–986. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 25.Takala A K, Santosham M, Almeido-Hill J, Wolff M, Newcomer W, Reid R, Kayhty H, Esko E, Makela P H. Vaccination with Haemophilus influenzae type b meningococcal protein conjugate vaccine reduces oropharyngeal carriage of Haemophilus influenzae type b among American Indian children. Pediatr Infect Dis J. 1993;12:593–599. doi: 10.1097/00006454-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Choo S, Everard J, Jennings R, Finn A. Mucosal immune responses to meningococcal group C conjugate and group A and C polysaccharide vaccines in adolescents. Infect Immun. 2000;68:2692–2697. doi: 10.1128/iai.68.5.2692-2697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]