Abstract
While human regulatory risk assessment (RA) still largely relies on animal studies, new approach methodologies (NAMs) based on in vitro, in silico or non-mammalian alternative models are increasingly used to evaluate chemical hazards. Moreover, human epidemiological studies with biomarkers of effect (BoE) also play an invaluable role in identifying health effects associated with chemical exposures. To move towards the next generation risk assessment (NGRA), it is therefore crucial to establish bridges between NAMs and standard approaches, and to establish processes for increasing mechanistically-based biological plausibility in human studies. The Adverse Outcome Pathway (AOP) framework constitutes an important tool to address these needs but, despite a significant increase in knowledge and awareness, the use of AOPs in chemical RA remains limited. The objective of this paper is to address issues related to using AOPs in a regulatory context from various perspectives as it was discussed in a workshop organized within the European Union partnerships HBM4EU and PARC in spring 2022. The paper presents examples where the AOP framework has been proven useful for the human RA process, particularly in hazard prioritization and characterization, in integrated approaches to testing and assessment (IATA), and in the identification and validation of BoE in epidemiological studies. Nevertheless, several limitations were identified that hinder the optimal usability and acceptance of AOPs by the regulatory community including the lack of quantitative information on response-response relationships and of efficient ways to map chemical data (exposure and toxicity) onto AOPs. The paper summarizes suggestions, ongoing initiatives and third-party tools that may help to overcome these obstacles and thus assure better implementation of AOPs in the NGRA.
Keywords: Adverse outcome pathways, Mechanistic toxicology, Hazard assessment, Regulatory risk assessment, Biomarkers of effect, New approach methodologies
Abbreviations: AEP, aggregated exposure pathways; AO, adverse outcome; AOP, adverse outcome pathway; BDNF, brain-derived neurotrophic factor; BP (A, F or S), bisphenol (A, F or S); BoE, biomarker of effect; CF, Conceptual Framework; DNT, developmental neurotoxicity; EASIS, Endocrine Active Substances Information System; ECHA, European Chemicals Agency; EDC, endocrine disrupting chemical; EFSA, European Food Safety Authority; FB1, fumonisin B1; HBM, human biomonitoring; IATA, integrated approaches to testing and assessment; IVB, in vitro testing battery; JRC, (European commission's) Joint Research Centre; KE, key event; KER, KE relationship; MIE, molecular initiating event; MoA, mode of action; NAMs, new approach methodologies; nFRs, novel flame retardants; NGRA, next generation risk assessment; NTDs, neural tube defects; ODEs, ordinary differential equations; OECD, organization for economic co-operation and development; OHT, OECD harmonized template; PBDE, polybrominated diphenyl ether; PBPK model, Physiologically based pharmacokinetic model; PPR, Plant Protection Products and their Residues; qAOP, quantitative AOP; qIVIVE, quantitative in vitro to in vivo extrapolation; RA, risk assessment; Sa/So, sphinganine/sphingosine ratio; TH, thyroid hormone; US EPA, United States Environmental Protection Agency; WoE, weight of evidence; WPHA, Working Party on Hazard Assessment; WNT, Working Group of National Coordinators of the Test Guidelines program
1. Introduction and objectives
Next generation risk assessment (NGRA) is an approach used for regulatory purposes that has the potential of reducing the use of animal testing that poses several issues related to ethics, relevance to human health, costs and efficacy. The beginning of the 21st century has therefore marked a paradigm shift in toxicity testing from animal-based (in vivo) approaches towards new approach methodologies (NAMs) that mostly rely on molecule- and cell-based (in vitro) and computational (in silico) methods (Andersen et al., 2007; Hartung, 2009). In parallel, epidemiological and biomonitoring studies are crucial to identify hazards potentially associated with chemical exposures in humans. To increase the regulatory acceptance of information from NAMs and epidemiological studies, it is crucial to have a transparent, evidence-based mechanistic knowledge framework linking molecular perturbations to adverse outcomes relevant for human health (Krewski et al., 2020). To help establish these bridges, Adverse Outcome Pathways (AOPs) appear to be an instrumental tool that has become a broadly accepted framework supported by the international OECD Environmental, Health and Safety (EHS) Programme (https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm) (Vinken, 2013). An AOP is a pragmatic evidence-based description of the chain of causally linked biological effects (key events, KEs) and the relationships between them – Key Event Relationships (KERs) - leading from a molecular perturbation (molecular initiating event, MIE) by a stressor to an adverse health effect on the organism or population level (adverse outcome, AO) (Ankley et al., 2010). By synthesizing mechanistic knowledge from different levels of biological organization, the AOP framework should help assessors to relate results obtained from in vitro assays and in silico models (at molecular or cellular levels) to apical endpoints of regulatory relevance. The OECD AOP-Wiki (https://aopwiki.org/) was launched in 2013 and serves as an AOPs open-access repository allowing the contribution to AOP content by international crowdsourcing. However, although the number of AOPs, general awareness of the AOP framework, and training of different stakeholders have substantially increased, the actual application of AOPs in RA processes remains limited, and discussions on how to best use AOPs for regulatory purposes are still ongoing (Carusi et al., 2018; Zuang and Dura, 2022; Hoffmann, 2022; Sauer et al., 2020).
Increasing the communication and understanding between the communities of AOP developers and (potential) AOP users, and having a better overview of concrete examples of the successful application of AOPs in chemical RA, would help to overcome the obstacles in adopting AOPs for regulatory purposes. In that context, various activities have been organized within the European Partnership Human Biomonitoring for Europe, HBM4EU (https://www.hbm4eu.eu/), the Eurion Cluster (https://eurion-cluster.eu/), the United States Environmental Protection Agency (US EPA) (https://www.epa.gov/), the OECD Working Party on Hazard Assessment (WPHA), Working Party on Exposure Assessment (WPEA) project, EU Horizon 2020 projects EuroMix (https://www.euromixproject.eu/) and EU-ToxRisk (https://www.eu-toxrisk.eu/), ASPIS cluster (https://aspis-cluster.eu/) or the Mystery of ROS consortium (Tanabe et al., 2022a, 2022b). These initiatives also organized workshops (Hoffmann, 2022; Paini et al., 2022) or OECD webinars on AOPs (https://www.oecd.org/chemicalsafety/testing/webinars-on-testing-and-assessment-methodologies.htm). To bridge from HBM4EU to a follow-up pan-European Partnership on Risk Assessment of Chemicals (PARC), a workshop was organized in April 2022, which discussed issues related to using AOPs in a regulatory context from various perspectives. The present paper presents the outcomes of the workshop and aims to (1) provide a broad overview of case studies where the AOP framework was successfully applied in the chemical RA process, (2) discuss the needs identified by potential AOP users such as toxicologists or chemical risk assessors, and (3) summarize existing tools and initiatives to further facilitate the application of AOPs for regulatory purposes.
Considering the scope of the HBM4EU project and the expertise of the partners involved, the present paper focuses on human health. It should, however, be highlighted that AOPs were initially proposed as a tool in the environmental ecotoxicological hazard and risk assessment (Ankley et al., 2010), and there are many examples of AOP use in this context (Fay et al., 2017, Legradi et al., 2018, Schmid et al., 2021, Song and Villeneuve, 2021, Toyota et al., 2022, Volz et al., 2011). Likewise, some of the considerations presented in this manuscript are valid for both human and ecological RA.
2. Next generation risk assessment and the use of AOPs
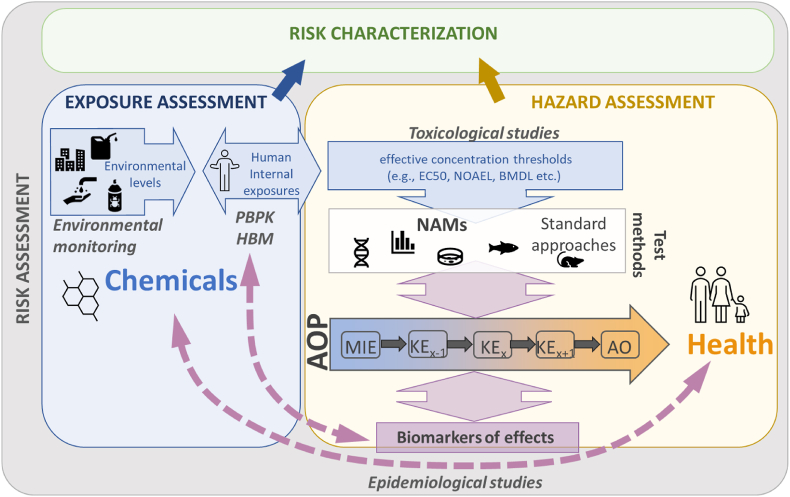
All actors involved in chemical RA and risk management aim to protect environmental and human health from the potential adverse effects of chemicals (sometimes also referred to as “stressors”). For specific compounds such as carcinogenic substances, “generic approach to risk management” (i.e. automatic trigger based on hazardous properties and generic considerations on exposure) has been applied by EU chemical legislation but the risks of most chemicals are typically assessed individually (European Commission, 2020). To date, chemical RA is a standardized process typically conducted through a sequence of steps including exposure assessment, hazard assessment, and risk characterization ((JRC, 2003); ECHA, 2013; UNEP, 1999; WHO, 2021). The exposure assessment estimates the route(s) of exposure, frequency, duration, and levels of exposure to the chemical. The hazard assessment includes hazard identification that evaluates if the substance is capable, in principle, of causing adverse effects and hazard characterization that defines the relationship between the dose and the (markers of) severity or the incidence of anticipated adverse effect(s). It also aims to derive threshold values (e.g., health-based guidance values (HBGVs)); that is, the levels of chemical below which no significant risks to human health are expected. Finally, risk characterization evaluates the risk (probability) of adverse health effects in population groups by integrating the information on exposure and hazard assessments; in particular, addressing if exposure exceeds the threshold value (Fig. 1).
Fig. 1.
The place of the AOP framework in bridging the different components of next generation risk assessment, improving causal inference in exposure-health relationships in epidemiological studies, and identifying and validating biomarkers of effects. Abbreviations: HBM, human biomonitoring; PBPK, physiologically based pharmacokinetic modelling; NAMs, new approach methodologies; AOP, adverse outcome pathway; MIE, molecular initiating event; KE, key event; AO, adverse outcome; EC50, half maximal effective concentration; NOAEL, no observed adverse effect level; BMDL, benchmark dose level.
The long-used, traditional approach for assessing the hazards of chemicals mainly relies on animal tests typically following OECD test guidelines. However, in vivo experiments with animals raise concerns regarding ethics, relevance to human health, costs and efficacy. Human epidemiology and measurements of biomarkers of effects (BoEs) in human biomonitoring (HBM) and epidemiological studies provide invaluable information on hazards associated with chemical exposure in the relevant species. The adoption of alternatives to animal tests and implementation of NAMs - such as in vitro methods (e.g., using human cell-based systems or organoids), utilization of omics (transcriptomics, metabolomics, etc.), epigenetics, or in silico structure-based model predictions - address ethical, financial and efficacy issues (Andersen et al., 2007; Escher et al., 2022; Pistollato et al., 2021; Thomas et al., 2018; Vrijenhoek et al., 2022). Advances in omics technologies and computational approaches bring a major opportunity for a holistic understanding of toxicological mechanisms that should be better captured in AOPs, thus providing substantial advancement to NGRA. In particular, coupling of gene expression-based molecular response pathways (through transcriptomics) with the prevalent pathways identified from bioinformatics analysis of metabolite profiles (through metabolomics) allows to identify the perturbed pathways and their potential links to adverse outcomes and exposures (Barouki et al., 2022; Sarigiannis et al., 2021). These should also be linked to epigenetic changes such as methylation of DNA, histone modifications and noncoding RNAs but linking them to health outcomes (including integration into AOPs) is a major challenge (Angrish et al., 2018). Nevertheless, because of their low cost and high speed, high–throughput and high–content screening are promising approaches for NGRA. The combination of NAMs with computational modelling has fostered the development of a non–animal, NGRA framework to support regulatory decisions relevant to human health (Hernandez, 2021).
NGRA has the advantage of integrating NAMs, that provide information at different levels of biological organization, into the regulatory process. This can be done using, for example, a workflow comprising several levels, tiered approaches, or guidance for reporting omics data (Harrill et al., 2021). However, to our knowledge, there are only a few examples of the acceptance of NAMs in the regulatory RA process, beyond screening, prioritization, and use in IATAs. The same is true for exposure and effect biomarker associations in human biomonitoring and epidemiological studies. Some of the main challenges for adopting NAMs in chemical regulation were presented in a recent Science for Policy report from the European Commission's Joint Research Centre (JRC) based on a survey that aimed at gathering the stakeholders' perceptions. According to this report, stakeholders frequently noted that chemical regulation is insufficiently science-driven and highlighted the importance of establishing bridges between NAMs and standard approaches, and between data and evidence (Carusi et al., 2022).
By providing integrated and curated representation of the mechanistic knowledge connecting data from different levels of biological organization, AOPs have great potential to become a standard tool for NGRA. AOPs are by definition chemical agnostic (i.e. chemical independent), meaning that the biology depicted should hold for any stressor (mostly chemicals) perturbing the biological pathway(s). Information in the AOP-Wiki is therefore limited to “prototypical” stressors (usually those used to provide evidence for AOP development). The AOP-Wiki does not aim at providing a comprehensive database of chemicals perturbing the AOPs, which has the benefit of providing more “universal” mechanistic knowledge but also makes the usability of AOPs for risk assessors more challenging (also discussed later in this paper). The KERs in AOPs can be quantified, thereby offering a formal approach to quantitatively predict an AO from MIEs or KEs, which would greatly support NGRA. The development of quantitative AOPs (qAOPs) will therefore be an important step to consolidating the relationship between toxicokinetics and toxicodynamics within NGRA (Punt et al., 2020). Importantly, a guidance for the weight of evidence (WoE) evaluation based on adapted Bradford-Hill criteria (i.e., biological plausibility, essentiality, and empirical support) has been developed for KEs, KERs and AOPs taking into account the domains of applicability and the levels of uncertainty (OECD, 2022; an online version regularly updated is also available on the AOP-Wiki website). An extensive internal and external standardization and harmonisation of the evaluation and reporting of each AOP is ensured through templates and guidance documents (OECD, 2022, 2017), assignment of dedicated AOP coaches to each AOP, and external review within the OECD AOP development programme (OECD, 2021). Ultimately, endorsement by WPHA and Working Group of National Coordinators of the Test Guidelines program (WNT) ensures that an AOP has undergone the review process and can be disseminated. Finally, if two or more AOPs share some of their KE(s)/MIE/AO, these can be assembled into AOP networks that better represent biological complexity and real-life scenarios, where mixtures of stressors can trigger multiple effects (Knapen et al., 2018). In the past decade, the number of AOPs captured in the AOP-Wiki has increased substantially and are now counting more than 450 at different levels of scientific and review maturity (22 AOPs are endorsed by WPHA/WNT as of August 10th, 2022; see the AOP-Wikifor details).
Several examples exist where AOP knowledge was used to inform chemical hazard and risk assessment, as reviewed in the following section.
3. Existing case studies of AOP application in chemical hazard and risk assessment
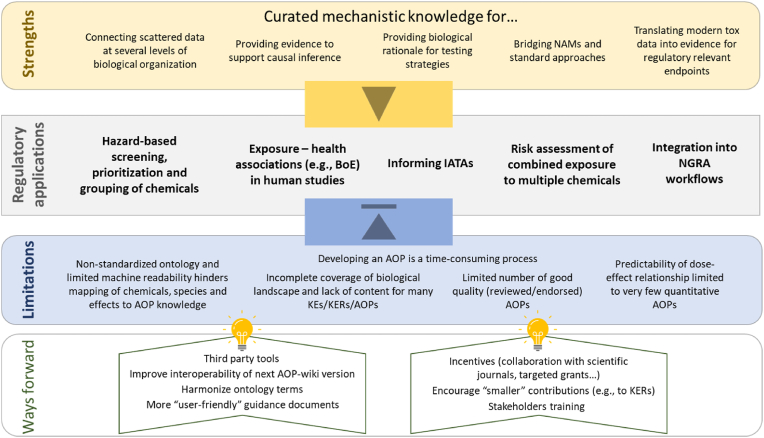
In this section, we identified five main areas in which AOPs can be applied: (1) to support hazard-based screening and prioritization of chemicals, (2) to provide biological plausibility for exposure-health associations (e.g., BoE) in human studies, (3) to inform Integrated Approaches to Testing and Assessment (IATA), (4) to assist risk assessment of combined exposure to multiple chemicals and (5) to become an integral part of NGRA workflows. Fig. 2 summarizes the following paragraphs by highlighting the benefits of AOPs, their main regulatory applications as well as the current drawbacks limiting the use of AOPs, and some possible ways forward.
Fig. 2.
Strengths and limitations of AOPs as a tool to translate scientific data into regulatory relevant knowledge to support risk assessment . Five regulatory applications (light grey box) benefit from the curated and chemical-agnostic AOP-knowledge (light yellow box and yellow arrowhead), but the full adoption of AOPs is currently hindered by several limitations (light blue box and blue arrowhead). Some ongoing or proposed initiatives should help overcome the limitations in future (ways forward). Benefits, applications, limitations and ways forward are all commented in greater details in the manuscript. Abbreviations: AOP, adverse outcome pathway; IATAs, integrated approaches to testing and assessment; BoE, biomarkers of effect; KE, key events; KERs, key event relationships; NGRA, next generation risk assessment. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1. Hazard-based screening and prioritization of chemicals
Humans are exposed to tens of thousands of potentially bioaccumulative and hazardous chemicals (over 26 000 registered in REACH as of May 2022 https://echa.europa.eu/fr/information-on-chemicals/registered-substances), and a proper hazard assessment of all chemicals is not technically or economically feasible through classical approaches. Therefore, hazard-based screening and prioritization of chemicals is an essential step towards pragmatic risk assessment and management. NAMs, assisted by AOP knowledge, can play crucial roles in that process.
A good example is endocrine disrupting chemicals (EDCs). EDCs can adversely interfere with any aspect of hormone action at different levels of hormonal regulation, potentially leading to a wide variety of adverse health outcomes, ranging from infertility to metabolic disorders, developmental neurotoxicity, and other chronic health outcomes (Kucheryavenko et al., 2020; WHO; UNEP, 2012). The Endocrine Disruptor Screening Program (EDSP) of the US EPA focuses on estrogen, androgen, and thyroid hormone signalling pathways, using a variety of NAMs, including in vitro test batteries and computational tools, aimed at identifying and prioritizing EDCs (reviewed in Browne et al., 2017). In this US EPA program, the AOP concept has been used to structure and evaluate mechanistic information, establish connections among pathways leading to different adverse outcomes, and design screening strategies by mapping assays to AOPs and AOP networks. Further, the OECD Conceptual Framework (CF) for Testing and Assessment of Endocrine Disrupters (OECD, 2018a) is a pragmatic example of how the use of non-testing information (CF level 1) can be leveraged with mechanistically-informed in vitro data (CF2), mechanistically-informed in vivo data (CF3), in vivo adverse effects for limited test duration (CF4) and in vivo adverse effects from assays covering more extensive parts of the life cycle of the organism (CF5). The CF is not intended to be a testing strategy nor align directly to AOPs, but it provides a guide to test methods that can populate AOPs related to endocrine disruption. In addition, the European Food Safety Authority (EFSA)/European Chemicals Agency (ECHA) guidance on the identification of EDCs in the EU pesticides regulations requires the identification of an endocrine disrupting-related adversity and mechanism (ECHA, 2018 and EPA, 2018). The key characteristics of EDCs recently described in a consensus paper from leading experts in the field can be used to identify and classify a chemical as EDC (La Merrill et al., 2020). Both the mechanism and the adversity are ideally connected by an AOP. CF could also guide the identification and choice of methods for IATAs. AOP-based IATAs help to combine and establish in vitro methods that are predictive of endocrine-related adversities and may therefore make additional animal testing for some modalities unnecessary (OECD, 2019).
Finally, the JRC has recently published its Endocrine Active Substances Information System (EASIS, https://easis.jrc.ec.europa.eu/), which contains information on the endocrine activity of chemicals as well as adverse effects that may be linked to certain endocrine activities. AOPs were used to identify mechanistic effects that are involved in the endocrine activity. In combination with AOP knowledge, these data help interested parties to get a picture of a substance's potential to be an EDC. EASIS currently contains data on over 600 chemicals collected from around 10 000 study entries covering in vitro and in vivo assays in different species, including some human data. EASIS is a JRC-run installation of IUCLID 6, the software explicitly designed to manage scientific data on chemicals in a regulatory context, for example under the EU Biocides and EU REACH regulations. Parties familiar with the IUCLID software immediately feel comfortable when using EASIS, which improves its usability. Like all IUCLID instances, EASIS uses the OECD Harmonized Templates (OHTs) to facilitate the reuse and exchange of the data. It is actually the first IUCLID installation that makes full use of a special template (OHT 201) dedicated to reporting mechanistic data derived from non-animal methods, mostly from the published scientific literature. This fulfils one of the main requirements of the European Commission's Chemical Strategy for Sustainability, which calls for the increased uptake of non-animal methods and the better use of academic data (European Commission, 2020).
Another application where prioritization is relevant includes hazard identification for substitute chemicals. However, this is particularly challenging because of the typically scarce availability of data for such substitutes. For example, a literature review of 52 novel flame retardants (nFRs) used as substitutes for the restricted brominated flame retardants such as polybrominated diphenyl ethers (PBDEs), showed that hazard data for nFRs are very limited (Bajard et al., 2019). Nine out of 52 nFRs were prioritized based on evidence for hazards, and the biological effects reported in peer-reviewed literature and databases were mapped onto AOP knowledge. Knowledge from the AOP-Wiki provided additional supporting evidence highlighting the health outcomes of highest concern (namely hepatotoxicity, neurotoxicity, and reproductive toxicity) and major data gaps (e.g., insufficient information on MIEs) (Bajard et al., 2019). In a follow-up study, the AOP knowledge was also used to design a testing strategy for screening the effects of nFRs on hepatic steatosis (Negi et al., 2021). The approach refined the prioritization to four nFRs and helped to identify a potential mechanism for this endpoint (Negi et al., 2021). For bisphenol F (BPF) and bisphenol S (BPS), alternatives to the well-known EDC bisphenol A (BPA), an artificial intelligence computational tool, the AOP-helpFinder (http://aop-helpfinder.u-paris-sciences.fr/index.php), was used to automatically decipher connections between data on stressors and the biological events reported in the literature (Carvaillo et al., 2019; Rugard et al., 2019). This approach optimized the identification of dispersed data available and allowed to predict the main health outcomes associated with BPA substitutes in terms of obesity and metabolic disruption (e.g. for BPS) and thyroid cancer (e.g. for BPF) (Carvaillo et al., 2019; Rugard et al., 2019). Associations between exposure to BPS and metabolic disorders were indeed reported in several, but not all, epidemiological studies examining this endpoint (Beausoleil et al., 2022).
These examples illustrate how the stepwise application of AOPs can aid in organizing and simplifying a complicated issue, thereby assisting the regulatory process. The AOP framework has been particularly valuable for the screening, prioritization and hazard identification of chemicals, especially those with limited toxicity data. Knowledge stored in the AOP-Wiki and other data sources, such as the CompTox database (https://comptox.epa.gov/dashboard/), was used to link scattered (toxicological) data at different levels of biological organization. It also facilitated the identification of potential molecular targets and key mechanistic nodes, which could assist in the design of the tiered approaches in the NGRA (Ball et al., 2022).
3.2. Biological plausibility for exposure-health associations in human studies
Epidemiological and HBM studies are invaluable sources of information to evaluate (mostly qualitatively) the potential impact of chemical exposures on human health. Although they offer the great advantage of examining the relevant species (human) in real-world environments, observational epidemiological studies generally provide a lower proof of causality compared to experimental research. Many existing studies also lack a real holistic approach. Although the science and stakeholders call for truly exposome approaches, most of the studies assessed a limited, preselected, small number of compounds that are unlikely to cover the complex exposure situation (Huhn et al., 2021). Tools for evaluating causality in epidemiological studies exist but present several limitations (Shimonovich et al., 2021). Mechanistic evidence from experimental studies (Caporale et al., 2022) and AOPs contribute to provide support to the causal inference of exposure-health associations in human studies.
In epidemiological studies, the associations of health effects with chemical exposures might be observed “directly” as the adverse health outcome itself (e.g., case-control studies or cohort studies' follow-up) or “indirectly” via BoEs. BoEs are measurable indicators of a biological change (e.g., molecular, cellular, physiological, behavioural) in response to a chemical exposure (NRC, 2006). In contrast to overt clinical diseases, molecular BoEs are not apical outcomes but may represent intermediate key events in the causal pathway leading to the adverse outcome, thereby allowing to detect subclinical processes. In HBM and epidemiological studies, analyses of BoEs in parallel with exposure biomarkers (chemicals or their metabolites typically measured in blood or urine) in the same individuals bring a major added value, bridging the exposure and health domains (NRC, 2006). This helps to identify threshold concentrations important for risk management. Advances in epigenetics and omics technologies allowing for simultaneous analyses of responses at different levels (transcriptomics, proteomics, metabolomics, etc.) provide a unique opportunity for the development and validation of novel BoEs. For a BoE to be a reliable tool in HBM studies, it is essential to have strong confidence in the links between BoE and both chemical exposure and health outcomes. In the AOP framework, BoEs tend to coincide with MIEs/KEs between a given exposure and a given adverse outcome (Matos Dos Santos et al., 2020). For BoE identification and/or validation, AOP networks, including feedback loops and modulating factors, are of particular interest, and shared KEs (nodes) are potentially more relevant as they often connect to more MIEs, KEs and/or AOs. These nodes or central KEs can, for example, provide information on whether different chemical families (acting through a single or different MIEs) converge on the same KE or AO and therefore share the same AO. As such, AOPs can support the identification of BoEs that are predictive, translatable, sensitive, specific and robust for regulatory purposes. A challenge for risk assessment will be to acknowledge where subtle and early changes along the toxicodynamic pathway are indicative of an increased chance for downstream adverse outcomes (EFSA, 2017a). Also, considering that real-life exposure often involves multiple chemicals at low doses for prolonged periods with potential fluctuations (Margina et al., 2019), identifying and validating BoEs for low-dose and longer term exposure would be important for the endorsement of BoE within the NGRA framework.
The following examples and case studies illustrate how AOPs can be used to identify and potentially validate BoEs, and/or establish biological causality in epidemiological studies for various groups of hazardous chemicals, supporting thus the science-based assessment of chemical risks.
3.2.1. Reproductive effects associated with phthalate exposure
The AOP framework has been used convincingly by Baken et al. (2019) to provide solid mechanistic support for causal associations between phthalate exposure and reproductive outcomes reported in epidemiology studies. A systematic literature search combined the information on BoEs previously implemented in human observational studies, the mechanisms of action reported in experimental studies as well as knowledge on existing AOPs to which phthalates were listed as stressors and/or that were linked to the identified BoEs (Baken et al., 2019). This approach allowed to (1) show that the majority of the biomarkers of reproductive effects associated with phthalate exposure are supported by mechanistic information described in the AOP-Wiki, and (2) identify novel KEs for the development of BoEs related to phthalate exposure. Readouts of these newly identified KEs are candidates for early or late BoEs, depending on the “position” of the KE in the AOP (upstream or downstream).
3.2.2. BDNF as a neurotoxic biomarker associated with BPA, pesticide and heavy metal exposures
A structured comprehensive literature search was performed on BoEs related to 6 health outcomes associated with BPA exposure. This research identified brain-derived neurotrophic factor (BDNF) as a novel BoE for neurodevelopmental disorders (Mustieles et al., 2020). In a second step, an AOP network containing BDNF as a central KE was constructed, and in vivo toxicological studies linking BPA to BDNF alteration were matched to the AOP network. This approach validated BDNF as a BoE predictive of neurodevelopmental impairments, and demonstrated that BPA interferes through several MIEs (Mustieles et al., 2020). A follow-up pilot study in an existing European cohort “the Childhood and Environment (INMA)-Granada cohort” confirmed that higher childhood urinary BPA concentrations were associated with higher peripheral blood BDNF DNA methylation at adolescence, and that BDNF methylation mediated 34% of the longitudinal association between BPA exposure and behavioural problems (Mustieles et al., 2022). In the same cohort, BDNF has also been associated with exposures to heavy metals and non-persistent pesticides (Rodríguez-Carrillo et al., 2022a, 2022b), suggesting that BDNF could be a BoE for mixtures of neurotoxic chemicals. Altogether, this case study illustrated how AOP data can (1) help to identify, prioritize and/or validate the implementation of BoEs in human studies, synergizing the toxicological and epidemiological approaches, and (2) support the biological plausibility of previously reported associations between stressors and neurodevelopmental outcomes (Mustieles and Fernández, 2020).
3.2.3. Association between fumonisin exposure and neural tube defects
A systematic search for BoE for mycotoxins found that increases in the urinary sphinganine/sphingosine (Sa/So) ratio are associated with fumonisin B1 (FB1) exposure (Al-Jaal et al., 2019; Riley et al., 2015). The Sa/So ratio is often used as a biomarker of fumonisin exposure, and was proposed also as a BoE (HBM4EU, 2020), although it was not fully clear what specific health outcome it might predict. Sphingolipids are known to affect cell membranes, cellular metabolism and basal functioning of cells, and have been assigned a role in the pathogenesis of various metabolic diseases (sphingolipidoses), myocardial infarction, hypertension and diabetes mellitus (Borodzicz et al., 2015; Kolter and Sandhoff, 2006). In addition, one epidemiological study and circumstantial evidence in humans, together with animal studies, suggested that exposure to FB1 might be associated with an increased incidence of neural tube defects (NTDs) (Lumsangkul et al., 2019; Missmer et al., 2006). Recently, the AOP framework has been used to structure and evaluate the available data, and the new AOP (ID 449, https://aopwiki.org/aops/449) describes the chain of events leading from the inhibition of ceramide synthase (MIE) to neural tube defects (AO), through two possible routes (van den Brand et al., 2022). One of these routes, impacts folate uptake, which is associated with NTDs, and the other involves inhibition of histone deacetylases that is linked to NTDs through another existing AOP (ID 275, https://aopwiki.org/aops/275). A dual pathway leading to NTDs is plausible (Gelineau-Van Waes et al., 2005; Sadler et al., 2002), and the proposed AOP provides mechanistic evidence for the fumonisin FB1-NTDs association previously reported in experimental and human studies.
3.2.4. Exposure to pesticides associated with Parkinson's disease
In 2017, the EFSA panel on Plant Protection Products and their Residues (PPR) performed an appraisal of the meta-analyses available at that time and suggested there was sufficient evidence to conclude an association between exposure to pesticides (broad definition) and Parkinson's disease, but a causal relationship with specific pesticides or pesticide classes cannot be established due to several limitations in epidemiological studies (EFSA, 2017b). To acquire evidence for such causality, the Panel recommended, among others, using NAMs and AOPs to establish biological plausibility. An AOP (AOP 3) establishing a link between exposure to pesticides and Parkinson's disease has been developed. The AOP has been endorsed by OECD and provides solid, qualitative, and mechanistic support for linking the inhibition of the mitochondrial complex I of nigrostriatal neurons (MIE) to Parkinsonian motor deficits (AO) (Bal-Price et al., 2018; Terron et al., 2018) (https://aopwiki.org/aops/3). Substantial data link the insecticide rotenone to this AOP, but any stressor perturbing the KEs of this AOP can be potentially connected to Parkinson's disease, as was shown for deguelin (OECD, 2020a). This AOP therefore increases the biological plausibility of human associations and may guide the identification and implementation of BoEs in future studies.
3.2.5. Metabolic perturbations potentially mediating the neurotoxic effects of phthalates and metals
Two recent studies showed that co-exposure to phthalates and metals at real-life exposure levels leads to metabolic perturbations in vitro and in humans (Papaioannou et al., 2021; Sarigiannis et al., 2021), and this could mediate the neurotoxic effects reported in human cohort studies (Sarigiannis et al., 2021). These interdisciplinary studies combining epidemiology with multi-omics analyses benefited from the AOP framework to bring together human exposome analysis and toxicological assays, and helped in identifying and validating BoEs from omics results (Barouki et al., 2022). The urea as well as other BoEs from phosphatidylcholine biosynthesis and phospholipase metabolic pathways were of particular importance since they have been identified as relevant both in experiments and in human samples from two cohorts (Papaioannou et al., 2021; Sarigiannis et al., 2021).
In the examples outlined above, the AOP framework was found to be particularly useful in linking information from different fields. AOPs helped to identify mechanistically based BoEs as (early) indicators of the adverse outcomes demonstrating thus a much-needed approach to strengthen the assessment of causal relationships between chemical exposures and health impacts and the interpretation of human biomonitoring results.
3.3. Inform integrated approaches to testing and assessment (IATA)
IATA are science-based approaches that integrate NAMs and mechanistic knowledge for hazard characterization, in a specific regulatory context (Caloni et al., 2022). The AOP framework can be particularly useful in this case to facilitate the identification of the most suitable assays for measurement of MIE or KEs to predict adverse health effects (Tollefsen et al., 2014; Willett, 2019), as demonstrated by the examples in the following paragraph.
A premium example where the AOP framework has been used to define a panel of suitable tests is the development of IATAs for non-genotoxic carcinogens (Jacobs et al., 2020). Another study also used the AOP-Wiki to identify several modes of action (MoAs) underlying non-genotoxic carcinogenicity for more than 400 agrochemicals (Heusinkveld et al., 2020). Both studies hold promise for using mechanistic-based approaches to reduce the use of standard long-term rodent carcinogenicity studies. In addition, mechanistic knowledge can be used to assess species concordance (particularly human relevance), as proposed by the WHO International Programme on Chemical Safety (Meek et al., 2014b). The OECD IATA Case study project (http://www.oecd.org/chemicalsafety/risk-assessment/iata-integrated-approaches-to-testing-and-assessment.htm#Project) also contains examples wherein AOPs were used, such as the evaluation of approaches for assessing skin sensitizers (Hoffmann et al., 2018; Kleinstreuer et al., 2018; OECD, 2016). In another IATA case study, an AOP network has been developed (based, in part, on 6 AOPs from the AOP-Wiki) to select an in vitro testing battery for chemical-induced liver steatosis (OECD, 2020b). In this effort, 6 MIEs and one converging downstream KE (triglyceride accumulation) were selected for the in vitro evaluation of the potential of 2-Ethylbutyric acid. Another example is the use of the endorsed AOP 3 (Terron et al., 2018) in an OECD IATA case study for the identification and characterization of Parkinsonian hazard liability of rotenone and deguelin, two structurally similar mitochondrial complex I inhibitors. In silico models and in vitro assays were the NAMs selected for a read-across safety assessment (OECD, 2020a). Finally, the EFSA PPR Panel developed two AOP-informed IATA case studies assessing the applicability of the developmental neurotoxicity (DNT) in vitro testing battery (IVB), for hazard identification and characterization of pesticide active substances. The DNT case studies illustrate the usefulness of a postulated AOP network and probabilistic quantification of WoE to improve regulatory decision-making (EFSA, 2021a). They are currently under review in the OECD IATA Case study project. Within this large effort, mapping the assays from the DNT IVB on AOPs and AOP networks has greatly facilitated their use in the IATA case studies and the design of the testing strategies. However, DNT-related AOPs submitted to the AOP-Wiki remain limited. To fill in this gap, new (quantitative) AOPs are being developed (such as the AOP 434) and derived from physiological maps of the developing brain such as the neural tube closure physiological map (Heusinkveld et al., 2021) in the framework of the ongoing European H2020 project ONTOX. The objective is the integration of the qAOP network into an AI-based NAM that includes the DNT IVB and predicts systemic repeated dose toxicity for the purpose of NGRA of chemicals (Vinken et al., 2021).
It should, however, be acknowledged that IATAs also have limitations, and connecting rather simplistic in vitro assays or in silico models with complex regulatory relevant in vivo health outcomes remains a major challenge. For example, predicting the apparent heterogeneity of adverse pregnancy outcomes associated with placental dysfunction (Burton et al., 2019; Dieber-Rotheneder et al., 2012; Jauniaux et al., 2006; Kovo et al., 2013) from the variety of in vitro and ex vivo models is not straightforward (Gundacker and Ellinger, 2020). In general, proper validation and standardization of protocols (e.g., by developing OECD test guidelines) is still lacking for most NAMs, and this important limitation is further discussed below.
3.4. Hazard assessment of chemical mixtures
Current approaches to RA usually involve single chemical assessments, not taking into account potential health risks from combined exposures to multiple chemical mixtures. A framework for RA of combined exposures that includes MoA has been proposed (Meek et al., 2011) and EFSA has recently developed a tiered methodology for grouping chemicals into assessment groups where the AOP/MoA is considered the gold standard (EFSA, 2021b). In the USA and Canada, cumulative effects of different pesticides that have a common mechanism of toxicity are considered in the process of human health risk assessment (Rotter et al., 2018). However, the application of mechanistic knowledge (preferably described in AOPs) for mixture RA is still limited (Kienzler et al., 2016). The need to move from assessments of single substances towards assessment of multiple chemicals has been widely recognized (Rotter et al., 2018). However, the current legal requirements do not fully reflect the regulatory needs in this respect, and the identification of mixtures by grouping chemicals with similar MoAs is a challenging task. Chemicals are often grouped based on shared molecular targets, which may be pertinent in some cases, such as for the effects of combined exposure to estrogenic perfluoroalkyl acids on fetal growth (Bjerregaard-Olesen et al., 2019). However, recent studies highlight the importance of considering adverse outcomes (Kortenkamp, 2022; van der Ven et al., 2022) or the whole AOP, i.e., from molecular target to AO through cellular and tissue effects (Conley et al., 2018; Lichtenstein et al., 2020), when grouping chemicals with a similar toxic action. Similarly, not all chemicals activating the same MIEs may fully trigger adversity, as shown in the case of CAR or PXR transactivation in liver steatosis and thyroid hyperplasia, and it might therefore be necessary to take into account downstream KEs rather than focusing on MIEs alone (Knebel et al., 2019; Kucheryavenko et al., 2020).
AOPs greatly facilitate the identification of mechanisms that are shared by several stressors and thereby highlight and provide supporting evidence for the assessment of mixture effects; AOP networks might be particularly relevant in that context. Along those lines, a methodology for mixture RA in which AOPs play a central role has been developed within the Horizon 2020 EuroMix project, collecting relevant toxicological data, assigning substances into assessment groups, and identifying potential upstream KEs that can be used to calculate relative potency factors (Beronius et al., 2020). In practice, Conley et al. (2018) identified mixtures of anti-androgenic chemicals that trigger the same AOP network. Although the 18 substances included in the mixture targeted five different MIEs, an additive effect was observed. This highlights the importance of considering AOP networks where several AOPs triggered from separate MIEs can converge in downstream KEs and therefore elicit the same adverse outcome. In another study, an AOP network for liver steatosis was used to define a battery of assays for testing the mixture effects of three steatosis-inducing chemicals. The authors demonstrated that the dose addition model was applicable in all different assays, highlighting the relevance of using an AOP-based testing strategy for mixture characterization and, ultimately, mixture hazard assessment (Lichtenstein et al., 2020). In an OECD IATA case study for repeated dose toxicity endpoints (focusing on hepatotoxicity), the MoA/AOP knowledge was used to inform read-across for grouping p-alkylphenols (OECD, 2018b). The study examined the usefulness of the AOP-informed IATA and read-across strategy for substance registration, but the approach might also be relevant for mixture characterization. With regard to mixture risk assessment, the derivation of relative potency factors can help to refine the risk assessment of combinations of stressors, as illustrated by Van der Ven and colleagues (van der Ven et al., 2022). It should also be noted that quantitative hazard characterization is an important and challenging issue in the mixture assessment process. Particularly, effective doses of individual compounds in mixtures are impacted by co-exposures and possible synergistic or antagonistic interactions.
Overall, there is a major potential to use the AOP framework (and the AOP-Wiki) to identify the hazards of chemical mixtures across different chemical groups. Nevertheless, while the current AOP-Wiki can be instrumental in identifying shared mechanisms of toxicity for a defined mixture, it cannot be used efficiently to map chemicals that would interact on a given AOP or AOP network because stressors are not systematically listed in the AOP-Wiki. After all, such listing would require a thorough evaluation of each stressor. This (intentional) disconnect between the AOP-Wiki (biological information) and chemical data is further discussed below.
3.5. Integration within NGRA workflows
Risk characterization integrates the information on hazards and exposure to evaluate whether levels of chemical(s) to which people are exposed may affect their health. It is therefore essential to quantitatively link internal exposures and experimental effective concentrations, using, for example, physiologically based pharmacokinetic (PBPK) and quantitative in vitro to in vivo extrapolation (qIVIVE) models. To derive effect thresholds, or more specially point of departure (POD) values for adverse health effects, it is important to have a quantitative understanding of the dose and time of exposure needed to trigger the entire chain of events from MIE to the downstream AO (Perkins et al., 2019). Activation of an MIE may be sufficient to affect early downstream KEs, but these effects may not be sufficient to reach a threshold to also activate late KEs and AOs. Ideally, AOPs suitable for quantitative RA should have a high level of confidence, meaning that they have gone through a thorough WoE evaluation process being reviewed and endorsed by experts (Coady et al., 2019; Meek et al., 2014a). Nonetheless, considering the precautionary principle (https://www.gdrc.org/u-gov/precaution-3.html) and priorities of the European Chemical strategy for Sustainability (European Commission, 2020), risk managers are encouraged to consider AOP knowledge even before the full formal validation of an AOP by OECD. Although the number of AOPs with quantitative information and a high level of confidence is still limited, some case studies on the integration of AOP within RA workflows are listed below.
A well-described and endorsed AOP for skin sensitization has been developed, along with internationally validated test guidelines for the KEs (OECD, 2016). The qualitative and quantitative evaluation demonstrated that several of the in vitro and in silico approaches used have “equivalent or superior performance to existing animal tests and were successful in predicting human skin sensitization outcomes for both hazard and potency” (Kleinstreuer et al., 2018). Thanks to the high level of confidence in both the AOP and the methods, these alternative approaches may be integrated into regulatory processes (EPA, 2018) and in the next-generation skin allergy risk assessment (Gilmour et al., 2022). Moreover, recently, several AOPs for thyroid disruption have been developed and can be assembled into an AOP network whereby decreased thyroid hormone (TH) levels constitutes a KE shared with diverse MIEs and AOs, including neurodevelopmental defects (Klose et al., 2021; Knapen et al., 2020; Noyes et al., 2019). The evidence for associations between reduced TH levels and neurodevelopmental defects is strong, and some quantitative modelling has been performed, e.g., for polychlorinated biphenyls data (Wise et al., 2012). Therefore, with a quantitative understanding of the mechanisms upstream of maternal TH levels, and based on proper models (Lumen et al., 2015), information about MIE/early KEs may already provide threshold concentrations expected with some probability to trigger an adverse health effect. Additionally, several test guidelines associated with relevant KEs in an AOP network have been identified in fish (Knapen et al., 2020) and further development of cross-species AOPs is ongoing to support the use of the vertebrate species (fish and amphibians) for human hazard assessment. In another example, a pragmatic NGRA workflow (Luijten et al., 2020) was used to validate the use of NAMs for the hazard characterization of three triazole fungicides (Van Der Ven et al., 2020). The authors concluded that the combination of model predictions and in vitro test battery was comparable to in vivo approaches for identifying hazards and may be used in the future within an RA scheme. This NGRA workflow highlighted the usefulness of AOP knowledge for organizing toxicological data and interpreting results from in silico and in vitro tests (Luijten et al., 2020; Van Der Ven et al., 2020).
To further implement qAOPs in regulatory applications or risk assessment, combining information from AOPs with computational models can be a fruitful way forward. Possible pathways were described and illustrated with case examples in published reviews (Perkins et al., 2019; Wittwehr et al., 2017). For example, three case studies demonstrated how Bayesian network modelling can be used to estimate the probability to trigger an AOP network for hepatic steatosis or DNT outcomes, thereby assisting RA for this specific endpoint (EFSA, 2021a; Perkins et al., 2019; Spînu et al., 2022). In another example, Zgheib et al. (2019) compared three different qAOP approaches (dose response modelling, dynamic Bayesian networks and systems biology models in the form of ordinary differential equations (ODEs)) in a renal toxicity case study. This study highlighted that each approach comes with its own advantages and caveats, and the nature of the AOP (network) at hand as well as the data availability jointly set the stage for which qAOP is most suitable. A major advantage of ODE models is that they take into account the dynamic nature of cellular and tissue responses and that ODE models are already available for several of these responses (Kuijper et al., 2017). Nevertheless, due to the likely complicated relation of early KEs with late KEs, it will be challenging to combine and extend ODE models for application in a full qAOP.
In summary, all the examples listed in this section demonstrate that the AOP framework has been used successfully for applications in several aspects of the RA processes. These are mostly related to screening, prioritization, and hazard identification, with some emerging successes in hazard characterization. The benefits of AOPs are further apparent in assisting the identification and validation of BoEs used in epidemiological studies and for improving the inference of causal relationships in exposure-health associations in human studies. Several case studies have also shown that AOP-based chemical grouping can aid the assessment of health risks from combined exposure to chemical mixtures. However, despite the wide recognition of the usefulness of AOPs for hazard assessment, some important limitations hinder a broad adoption of AOPs in chemical regulation. The most prominent issues, as well as suggestions for overcoming these obstacles, are described in the following section.
4. Main limitations in the use of AOPs in the RA process and suggestions for improvement
4.1. Insufficient coverage of the biological landscape by the current AOPs
The information currently available in the AOPs is far from representing all possible mechanisms underlying adverse outcomes relevant for regulatory purposes. Some biological processes and adverse outcomes are generally well covered (such as oxidative stress, TH metabolism, and reproductive toxicity), while others are much less represented (such as immunotoxicity or metabolic disorders). This represents an important limitation when using AOPs for hazard assessment. Incomplete coverage of biological pathways in the AOP-Wiki can be attributable to the fact that the AOP concept is still relatively recent (about 10 years old), elaboration of an AOP is time-consuming, and good incentives to develop AOPs are lacking. Unfortunately, the efforts associated with AOP development are poorly recognized within the general scientific community, and so far underrepresented among the traditional scientists' track records consisting of peer-reviewed papers. In addition, a substantial part of the scientific experts such as academic researchers in biology, pharmacology and medicine may not be well aware of the AOP concept, and the knowledge of this community is thus not fully exploited for the development of new AOPs.
Table 1 provides suggestions and ongoing initiatives to encourage, target and expedite AOP development and broaden the coverage of the biological landscape. We particularly highlight the necessity to raise awareness and upgrade education of early-stage researchers, encouraging the work on smaller and prioritized AOPs and KERs, as well as stimulating the recognition of AOP work within the scientific community.
Table 1.
- Suggested ways forward to expedite and better target AOP development.
| Activity | Description | Notes, examples |
|---|---|---|
| Raising awareness of AOPs among early-stage researchers | Specific courses and trainings, AOPs included in toxicology curricula, offer dedicated workshops, organise theoretical and practical (hands-on) courses at relevant scientific conferences (e.g. SETAC, SOT). | Available resources for AOP training can be found on the AOP forum https://aopwiki.org/forums/showthread.php?tid=18Sections on AOPs have been proposed within summer courses (e.g., organized by HBM4EU or university of Ottawa). |
| International concerted actions for selected AOP projects | Providing guidance and incentives to researchers and regulators for efficient development of priority AOPs and fostering collaborative efforts. | Modelling the COVID-19 pathogenesis with AOPs - CIAO project (https://www.ciao-covid.net/). |
| Development of smaller units (e.g., KERs) | Generating new AOPs and AOP networks through small and easily manageable efforts. Drafting of putative AOPs could also foster continuation by other authors. | Svingen et al. (2021) |
| Prioritize the development of new AOPs that address RA needs and cover gaps | Priority focus on AOPs explaining exposure-health associations from epidemiological studies. Similarly, BoEs from human studies can be used to identify a KE, triggering the development of new AOPs/AOP networks. | van den Brand et al. (2022) |
| Involve risk assessors and risk managers in the selection of AOPs that are most needed | Dedicated discussions of OECD bodies, such as the Working Party for Hazard Assessment (WPHA), the Working Group of the National Coordinators for the Test Guidelines Programme (WNT) and the Working Party on Manufactured Nanomaterial (WPMN). | Stakeholders may recommend focusing on a particular substance/AO and feel integrated into the process of development. The engagement strategy within the field of radiation research and regulation is one example (Chauhan et al., 2022). |
| Foster collaboration with scientific journals to allow the publication of AOP reports alongside creation of an AOP page in the AOP-Wiki | Ongoing initiative promoted by the OECD (e.g. https://youtu.be/Tl1bVpZNYJY). AOP developers prepare a peer-reviewed publication in the format of a citable AOP report (O'Brien and Yauk, 2022). | The first AOP reports were published recently (AOP 296 (Cho et al., 2022), AOP 263 (Song and Villeneuve, 2021), AOP 360 (Schmid et al., 2021)). Development of memorandums of understanding with additional journals are ongoing. |
| Recognize AOPs in the AOP-Wiki as important scientific records themselves | Current discussion (Ritchie, 2022) and implementation of principles of findability, accessibility, interoperability, and reusability (FAIR) suggest that other reporting formats than classical scientific papers may support timely, open access and flexible reporting of new AOPs. | AOP-Wiki is an open living platform that might be better suited for sharing knowledge in modern science. |
| Derive new AOPs from physiological maps | The physiological maps describe underlying mechanisms of human physiology of a relevant organ at the molecular and cellular level. | Vinken et al. (2021) |
4.2. Mistrust by regulators
Mistrust has been highlighted as a major limitation in the acceptance of NAMs and AOPs by the regulatory field stakeholders (Carusi et al., 2022). Although the quality of the information recorded in the AOP-Wiki is ensured via a rigorous review and endorsement process (OECD, 2021), the number of actually reviewed and endorsed AOPs is still limited (22 as of August 10th, 2022). This is because the review and endorsement process is time-consuming and the number of reviewers (working as volunteers) is limited. Also, there is a room for improvement in terms of making the WoE process for AOPs more structured, systematic, harmonized and transparent.
Related to this, there is also a need for consistent and transparent systematic review methodologies to overcome existing inconsistencies in methods across international and national regulatory agencies and organizations (Chartres et al., 2019). Integration of mechanistic evidence in such systematic review frameworks has confronted different challenges like the lack of tools to evaluate the certainty (Rooney et al., 2016). Systematically structuring the mechanistic evidence also represents a challenge, but AOP-inspired frameworks appear as an efficient option to support this process, as shown in a recent evaluation of association between exposure to persistent organic pollutants and endometriosis (Matta et al., 2021). An important additional factor affecting the trust of regulators is the current lack of criteria, and thus lack of consensus, on appropriate methods to be used for measuring MIEs/KEs in AOPs. Many experimental methods are used to generate data for AOPs (“key event readouts”), but they largely lack standardized description and formal validation, which are essential requirements in the regulatory process. Table 2 lists possible ways forward and ongoing initiatives that could help increase the trust in AOPs for risk assessors to encourage their adoption in the NGRA. In addition to raising the awareness among all stakeholders (which is a common theme for most of the improvements needed), mapping of test guidelines to AOPs as well as other standardization and validation efforts related to testing and data reporting are of particular importance.
Table 2.
Suggested ways forward to increase the trust into and adoption of AOPs by risk assessors.
| Activity | Description | Notes, examples |
|---|---|---|
| Raising awareness of AOPs among risk assessors or regulators | Specific education and training programmes. | Training the stakeholders to correctly use AOPs is crucial for their implementation |
| Strengthen the role of test methods in the AOP Framework | Provide a more standardized and reliable description of methods used to measure KEs in the AOP-Wiki (currently described in free text), to better reflect their important role in linking chemicals to AOPs. | An ongoing initiative within OECD EAGMST, especially in its AOP-KB subgroup, is aiming at strengthening the role of test methods in the AOP-Wiki. |
| Mapping test guidelines (TGs) to KEs of AOPs/AOP networks | Putting more emphasis on existing TGs associated with KEs and facilitating their identification. | Including a dedicated section in the KE pages of the AOP-Wiki; Linking information on methods with the TSAR (https://tsar.jrc.ec.europa.eu/). Example case studies - skin sensitization and thyroid hormone regulation (Kleinstreuer et al., 2018; Knapen et al., 2020). |
| Proper method validation and good reporting of data for regulatory risk assessment | Education of (eco)toxicologists, implementation of data reporting standards in (eco)toxicological journals. | The JRC initiative BeAMS (Carusi et al., 2019); SciRAP approach (Beronius et al., 2018; Roth et al., 2021), CRED system (Moermond et al., 2016), ToxTemp (Krebs et al., 2019), FAIR principles (e.g. Mortensen et al., 2022). Efforts to standardize omics data reporting also provide good examples (Bridges et al., 2017; Buesen et al., 2017; Gant et al., 2017; Harrill et al., 2021; Kauffmann et al., 2017). |
| Promote the adoption of systematic literature review methodologies | Improving transparency and reproducibility of the entire process, broadening acceptance of AOPs by regulators through standardized review approaches that are “fit for purpose” and reported in the AOPwiki. | Discussions and elaboration of guidance for implementation of review methodology in AOP development are currently undertaken within an ongoing initiative of OECD EAGMST subgroups. |
| Define criteria for NAMs to be acceptable for regulatory use | Interactions between NAM developers/users and risk assessors to define criteria and accompanying guidance. | One of the objectives of the European Partnership for the Assessment of Risk from Chemicals (PARC). |
| Share the reviewing task through collaboration with scientific journals | The collaboration with scientific journals on the development, scientific review and publishing of AOPs (see also Table 1) will provide a higher level of confidence. | Consider official recognition of the reviewers' contributions (e.g., by issuing certificates or including as co-authors). |
| Make AOP visualisation more intuitive | Rethink the way AOPs are graphically depicted (currently box, arrow, box, arrow, etc.) to meet AOP users' expectations and intuition and better emphasise the crucial role of KERs. . | An ongoing initiative within OECD EAGMST, especially in its AOP-KB subgroup, is examining the role of AOP visualisation and will come up with recommendations to improve them. |
| Provide guidelines for linking chemical data to existing AOPs | Providing criteria, recommendations, tools available and practical advices would enhance the regulatory use of AOPs | An example can be found in the HBM4EU deliverable (HBM4EU, 2021) |
Regulatory confidence in AOP-based NAMs could also be improved by performing their uncertainty analysis where both exposure and hazard are assessed in a probabilistic way. Using the AOP framework to map uncertainties on all its levels would transparently show its weaknesses, but also strengths and advantages (Maertens et al., 2022).
4.3. Missing quantitative information on KERs
Quantitative information is required for several regulatory applications of AOPs to support hazard characterization, (quantitative) risk characterization or associations between chemical exposures and BoE levels. Indeed, sufficient quantitative information describing time-course predictions of exposure and effect, and response–response relationships across KEs (including MIE and AO) is essential for identifying the threshold level of chemical stressors (internal dose) triggering the MIE and leading to an AO (Perkins et al., 2019; Wittwehr et al., 2017). Despite this need, only a limited number of qAOPs have been reported. Partly, this is due to the perception that the quantification of AOPs is highly complex. Indeed, it is a significant effort to calibrate qAOP model parameters in order to properly describe KERs and render the complexity of biological networks. This requires, inter alia, to include feedback loops and knowledge on how factors such as diet, genetic susceptibility/resistance, and disease states modulate the networks. Another limitation associated with AOP quantification is that cell systems may not be fully representative for the tissues in which they reside in vivo. For example, Heldring et al. (2022) recently showed that the effects of cisplatin differ in immortalized vs primary hepatic cell lines for early KEs related to DNA damage signalling. Analogously, there are many other examples documenting the complexity of in vivo toxicokinetics and the development of sufficiently robust PBPK and qIVIVE models. Nevertheless, possible actions to help increase quantitative AOPs are presented in Table 3, which highlights some recent efforts such as the development of a general qAOP modelling framework or concerted crowdsourcing activities focused on smaller units (KERs) within qAOPs.
Table 3.
– Suggested ways forward to increase quantitative information on AOPs.
| Activity | Description | Notes, examples |
|---|---|---|
| Development of a general qAOP modelling framework | A harmonized approach for regulators and scientists that would facilitate the qAOPs modelling. A framework should ideally allow for natural integration with (physiologically-based) pharmacokinetic models. | A framework for qAOP development was proposed and three case studies conducted (Paini et al., 2022). |
| Prioritization of current qualitative AOPs for further qAOP development | Pragmatic prioritization considering (1) the foreseen regulatory application domain (e.g., potency ranking vs quantitative hazard characterization for risk assessment), (2) the existence of established methods for the MIE/KEs, and (3) the expected time lapse between exposure and health effect. | The design of qAOPs may be complicated for endpoints where the adverse outcome only occurs after years of chronic exposure. |
| Concerted action through crowdsourcing and promoting the contribution to smaller units (e.g. quantitative KERs) | Stimulation of concerted activities on smaller parts of quantitative AOPs (quantitative KERs) to facilitate larger interactions and a more rapid generation of quantitative information. | A larger interlaboratory variation could be a limitation (Svingen et al., 2021). |
| Develop in silico extrapolation methods between assays using toxicokinetic models | Account for the toxicokinetic differences between species, assays and level of biological organization. | |
| Establish standardized approach for omics data | Harmonise and standardize the approaches for interpreting and quantitatively connecting omics data (e.g., gene expression and signalling pathways) to a phenotypic outcome. | An example is the Signalling Pathways Project for discovering consensomes, i.e. downstream genomic targets of signalling pathway nodes (receptors, enzymes, transcription factors and co-nodes) and cognate bioactive small molecules (Ochsner et al., 2019). |
| Flexible approach in qAOP development with respect to the available data and modelling tools | Consider other models if dose-response models are not applicable (do not take into account the dynamics of a system). | Hybrid approaches to properly quantify the KERs, i.e., combining different types of equations may be of value. |
4.4. Mapping chemical data to AOP knowledge
Because AOPs are by definition chemically-independent and focused only on toxicodynamic processes, chemical-specific information such as toxicological data, toxicokinetics, or qIVIVE are not emphasized in the current AOP-Wiki. This aims at ensuring that the biology depicted in the AOP should hold for any stressor perturbing the MIE. However, studies are needed to investigate whether the chemically agnostic nature of AOPs generally holds in a quantitative manner. In fact, variability in MIE triggered by different chemicals may translate into a different quantitative relation to the next KE. Such differences could potentially lead to significantly different quantitative conclusions at the AO level despite similar MIEs. Even though AOPs are chemically-agnostic, the use of information from prototypical stressors is encouraged during the development and submission of AOPs to the AOP-Wiki, and can be stored in a dedicated “prototypical stressor” field of the AOP page. However, prototypical stressors are not necessarily representative of chemicals from human exposome or found in the environment, and may thus have limited applicability for realistic exposure scenarios. Assessing chemical structure similarity as a basis for functional grouping (e.g., read-across or quantitative structure-activity relationship) is anticipated to leverage some of these constraints, but is usually missing in the AOP-Wiki as AOPs represent the toxicodynamic part of the toxicity pathways. Another shortcoming is that stressor information currently stored in the AOP-Wiki is of variable quality and some stressors are not supported by sufficiently developed harmonized ontologies, controlled vocabularies or unique identifiers that can facilitate FAIR (Findable, Accessible, Interoperate and Re-useable) compliance. For risk assessment or regulatory use, it is therefore necessary to map chemicals of concern to AOP knowledge. Guidelines/criteria for linking a stressor to an existing AOP may increase the applicability of AOPs in risk assessments, but are not available at the moment.
The following paragraphs and Table 4 provide suggestions and describe examples and tools for establishing links between chemical-specific data from different sources (e.g., peer-reviewed literature, reports from agencies, databases) and the knowledge on AOPs (MIEs/KEs) which is recorded in the AOP-Wiki.
Table 4.
– Tools to assist the mapping of chemical data onto the knowledge organized in AOPs for human RA.
| Activity | Description | Examples, references |
|---|---|---|
| The AOP-helpFinder | A new computational tool based on artificial intelligence, text mining, and graph theory. It screens abstracts from the published scientific literature to identify links between data on stressors and biological information that may be included in AOPs as MIE, KE or AO. Optimized under the HBM4EU and OBERON projects (Audouze et al., 2020). | Freely available as an easy-to-use web interface (http://aop-helpfinder.u-paris-sciences.fr/index.php). Tested in several case studies (Carvaillo et al., 2019; Jornod et al., 2020; Rugard et al., 2019) and developed AOPs (AOP 439, AOP 441) |
| The Abstract Sifter | An Excel-based tool assisting researchers in their PubMed searches (Baker et al., 2017). It allows the researcher to store relevant queries and view quickly the literature landscape linking e.g. stressors with KEs. | Available from the EPA Comptox Chemicals Dashboard download page (https://epa.figshare.com/articles/code/PubMed_Abstract_Sifter/10324379). |
| The Kaptis collaborative project | Develops a tool to improve the visualisation and usability of AOPs in chemical RA, by, for example, highlighting the connection to relevant assays. | https://www.lhasalimited.org/products/kaptis.htm |
| AOP-Wiki content converted into Resource Description Framework (RDF) | AOP-Wiki content converted into RDF and annotated with over twenty ontologies facilitates the connections of AOP-Wiki with external databases, including chemical databases. This allows users to identify AOPs associated with stressors from a specific chemical group. | https://aopwiki.rdf.bigcat-bioinformatics.org/. https://github.com/marvinm2/AOPWikiRDF (Martens et al., 2021) |
Test methods as core elements for connecting chemicals to AOP knowledge.
Information on methods used to measure the KEs of an AOP is essential when connecting toxicological data to AOP content. By bridging the gap between chemical data and AOP knowledge via the introduction of test method information, the picture gets complete. AOP knowledge highlights the necessity to explore certain mechanistic effects (KEs) by describing how these are linked to an adverse outcome. Test method information captures how the mechanistic effects were actually explored. Finally, a chemical tested in a certain method can then be directly linked to the mechanistic effect (KE) in the AOP. As also mentioned above, for regulatory chemical risk assessment, validated methods with test guidelines and properly assigned domains of applicability are preferred. From a practical point of view, in the AOP-Wiki, the information on methods should be in the KE pages “How it is measured or detected” section (see e.g. in the page of the KE 1253 “MLL chromosomal translocation”, https://aopwiki.org/events/1253#measured). (Semi-)automatic connections between the three elements (AOP knowledge, chemical data, and test method description) can be achieved by the introduction of harmonized ontology terms to ensure an efficient match between the assay and KEs. This is currently being implemented at the OECD level in collaboration between the AOP-Wiki development team, the team implementing the OECD Harmonized Template for reporting mechanistic effects (i.e. the template OHT 201) and increasingly also with the test method database developers. The connection between KEs and mechanistic effects reported in OHT 201 is already well under way (Ives et al., 2017), with the ontologies currently being refined and further expanded. Development of systematic ontologies based on AOPs is expected to have direct impacts on the accessibility of highly fragmented mechanistic evidence and its application in risk evaluations using computational methods (Whaley et al., 2020).
Tools to assist AOP users in linking chemical data with existing KEs.
Establishing the connections between the effects of a chemical reported in the literature and databases and the corresponding KE in the AOP-Wiki, can be challenging and time-consuming. In addition, insufficient machine readability of the AOP-Wiki content, and the lack of harmonized ontology terms used to characterize KEs (see above) further complicate the process of linking chemical stressors with AOP-Wiki. Nevertheless, a number of ongoing efforts aim at improving the interoperability of the AOP-Wiki in its future versions, and various tools have been developed. These are outlined in Table 4, which thus clearly indicates the importance of linking chemical-specific data with AOP knowledge assisting potential end-users such as chemical risk assessors.
4.5. Bridging chemical exposures and AOP knowledge
The AOP framework is a powerful tool for organizing biological knowledge, assisting hazard identification. Ultimately, for risk characterization, AOPs also need to be integrated with the outcomes of the exposure assessment. Since the aggregate exposure pathway (AEP) framework includes toxicokinetic processes leading to an internal target site, there is the possibility to integrate AEP, AOP and dose-response data. Connecting chemical external and internal exposure data (such as human biomonitoring data or AEPs) to the AOP knowledge is therefore critical for its final acceptance in the RA process. Even if a chemical is reported to trigger a MIE in toxicological assays, it may not be effective at concentrations relevant for human exposures. Indeed, nominal concentrations traditionally used in toxicological assays can be several orders of magnitude higher than human-relevant concentrations, and the exposure duration in the range of days or weeks rarely corresponds to real-life scenarios in which people may be exposed for years. In addition, continuous exposure may also not realistically represent intermittent or fluctuating exposure scenarios (Geraets et al., 2016; Goeden, 2018). It is therefore essential to (1) document the effective dose required to trigger MIEs (and the subsequent chain of events in AOPs), taking into account that the dose that affects one KE should be typically lower than the dose needed to induce a downstream KE (i.e., dose concordance), (2) translate the external exposure levels into actual internal doses at the target, and (3) relate the actual (measured) exposures with effective doses causing perturbations of MIEs/KEs. In practice, PBPK modelling, coupled with exposure reconstruction algorithms can estimate the internal dose (i.e., the actual exposure metric) needed to activate a MIE (Sarigiannis et al., 2016; Sillé et al., 2020). Similarly to the AOP framework, the AEP framework aims at organizing exposure data from multiple lines of evidence, accounting for sources, fate and transport exposure routes, as well as exposure modifiers such as age, gender, genetic variability, etc. (Tan et al., 2018). Reinforcing and formalizing the connections between these two frameworks is an important way forward.
5. Conclusions
Researchers from different fields such as human biomonitoring and (eco)toxicology support the overarching efforts of risk assessors and risk managers that aim at protecting environmental and public health from chemical exposures. With the growing number of chemicals and the fast increasing data on their hazards, risk assessment processes need to be adapted in order to keep pace. NAMs are the way forward in characterizing chemical hazards, and their use has considerably increased in the past decade(s). RA, however, still lags behind due to relying on old guidelines, lack of trust, and various levels of understanding among stakeholders. On the other hand, NAMs may lack the physiological context, may have poorer predictability of the health outcome and should therefore be combined with information from standard toxicological approaches, epidemiological studies and BoEs. The AOP framework seems to offer an optimal solution for addressing these pressing issues in emerging NGRA. AOPs were shown to be instrumental for integrating heterogeneous (but complementary) sources of information, and for translating modern toxicological and HBM data into evidence relevant to regulators. As illustrated in the present paper, a growing number of examples demonstrates the relevance of AOPs for the screening and prioritization of chemicals, assisting IATAs, supporting quantitative hazard characterization and RA workflows. Various tools, methodologies and initiatives have been developed to assist users, as risk assessors, in the practical implementation of AOPs.
Nonetheless, developments are still needed on both sides. “Traditional” RA undergoes a major transformation into the truly new NGRA, which needs to be open enough to implement NAMs and AOPs. In parallel, the AOP framework needs to mature to become directly applicable in the future NGRA. The main steps forward include (1) overcoming difficulties in mapping toxicological data for environmental chemicals onto the AOP knowledge, (2) increasing the number of quantitative AOPs, (3) establishing criteria and guidance for demonstrating the robustness and reliability of NAMs, and (4) bringing thoroughly evaluated case studies for qIVIVE that can quantitatively link assays at different levels of biological organization to chemical exposures. Given the complexity of the human exposome, linking AEPs to AOPs to characterize scientifically credible “source to outcome pathways” (STOPs) is an additional challenge for the future. Regardless of many challenges, AOPs are likely to evolve into a reliable, robust and specific tool serving future risk assessment and management as outlined for example within the Partnership on Risk Assessment of Chemicals (PARC), a 7-year EU initiative starting in 2022.
Funding and acknowledgments
This research was co-funded from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 733032 (HBM4EU), and grant agreement No 857560 (CETOCOEN Excellence), and from the European Union's Horizon Europe project PARC [grant agreement No 101057014]. This publication reflects only the authors' view and does not necessarily reflect those of the European Union. The European Union is not responsible for any use that may be made of the information it contains. Additional support was provided by the Ministry of Education, Youth and Sports by the RECETOX Research Infrastructure [No. LM2018121] and OP RDE project CETOCOEN Excellence [CZ.02.1.01/0.0/0.0/17_043/0009632]. This research was supported by Japan Agency for Medical Research and Development (AMED) Grant Numbers JP21mk0101216 and JP22mk0101216 , JSPS KAKENHI Grant Number 21K12133.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
This is review paper, all resources used are properly cited.
References
- Al-Jaal B.A., Jaganjac M., Barcaru A., Horvatovich P., Latiff A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: a systematic literature review. Food Chem. Toxicol. 2019;129:211–228. doi: 10.1016/j.fct.2019.04.047. [DOI] [PubMed] [Google Scholar]
- Andersen M., Anderson H., Bailar J.C., Boekelheide K., Brent R., Charnley G., Cheung V.G., Green S., Kelsey K.T., Kerkvliet N.I., Li A. a, McCray L., Meyer O., Patterson R.D., Pennie W., Scala R. a, Solomon G.M., Stephens M., Yager J., Zeise L., Daniel Krewski Daniel Acosta, Jr., Andersen Melvin, Anderson Henry, Bailar John C., III, Kim Boekelheide, Brent Robert, Charnley Gail, Vivian G.Cheung Sidney Green Jr. Kelsey Karl T., Kerkvliet Nancy I., Li Abby A., Lawrence McCray. Toxicity testing in the 21St century: a vision and a strategy. Natl. Acad. Sci. 2007;13:51–138. doi: 10.1080/10937404.2010.483176.TOXICITY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrish M.M., Allard P., McCullough S.D., Druwe I.L., Chadwick L.H., Hines E., Chorley B.N. Epigenetic applications in adverse outcome pathways and environmental risk evaluation. Environ. Health Perspect. 2018;126:1–12. doi: 10.1289/EHP2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley G.T., Bennett R.S., Erickson R.J., Hoff D.J., Hornung M.W., Johnson R.D., Mount D.R., Nichols J.W., Russom C.L., Schmieder P.K., Serrrano J.A., Tietge J.E., Villeneuve D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Audouze K., Sarigiannis D., Alonso-Magdalena P., Brochot C., Casas M., Vrijheid M., Babin P.J., Karakitsios S., Coumoul X., Barouki R. Integrative strategy of testing systems for identification of endocrine disruptors inducing metabolic disorders—an introduction to the oberon project. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajard L., Melymuk L., Blaha L. Prioritization of hazards of novel flame retardants using the mechanistic toxicology information from ToxCast and Adverse Outcome Pathways. Environ. Sci. Eur. 2019;31:14. doi: 10.1186/s12302-019-0195-z. [DOI] [Google Scholar]
- Baken K.A., Lambrechts N., Remy S., Mustieles V., Rodríguez-Carrillo A., Neophytou C.M., Olea N., Schoeters G. A strategy to validate a selection of human effect biomarkers using adverse outcome pathways: proof of concept for phthalates and reproductive effects. Environ. Res. 2019;175:235–256. doi: 10.1016/j.envres.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Baker N., Knudsen T., Williams A. Abstract Sifter: a comprehensive front-end system to PubMed. F1000Research. 2017;6:1–10. doi: 10.12688/f1000research.12865.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A., Leist M., Schildknecht S., Tschudi-Monnet F., Paini A., Terron A. Adverse Outcome Pathway on Inhibition of the mitochondrial complex I of nigro-striatal neurons leading to parkinsonian motor deficits. OECD Series on Adverse Outcome Pathways No. 7. 2018:0–184. [Google Scholar]
- Ball N., Bars R., Botham P.A., Cuciureanu A., Cronin M.T.D., Doe J.E., Dudzina T., Gant T.W., Leist M., van Ravenzwaay B. A framework for chemical safety assessment incorporating new approach methodologies within REACH. Arch. Toxicol. 2022;96:743–766. doi: 10.1007/s00204-021-03215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R., Audouze K., Becker C., Blaha L., Coumoul X., Karakitsios S., Klanova J., Miller G.W., Price E.J., Sarigiannis D. The exposome and toxicology: a win-win collaboration. Toxicol. Sci. 2022;186:1–11. doi: 10.1093/toxsci/kfab149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil C., Le Magueresse-Battistoni B., Viguié C., Babajko S., Canivenc-Lavier M.C., Chevalier N., Emond C., Habert R., Picard-Hagen N., Mhaouty-Kodja S. Regulatory and academic studies to derive reference values for human health: the case of bisphenol S. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112233. [DOI] [PubMed] [Google Scholar]
- Beronius A., Molander L., Zilliacus J., Rudén C., Hanberg A. Testing and refining the Science in Risk Assessment and Policy (SciRAP) web-based platform for evaluating the reliability and relevance of in vivo toxicity studies. J. Appl. Toxicol. 2018;38:1460–1470. doi: 10.1002/jat.3648. [DOI] [PubMed] [Google Scholar]
- Beronius A., Zilliacus J., Hanberg A., Luijten M., van der Voet H., van Klaveren J. Methodology for health risk assessment of combined exposures to multiple chemicals. Food Chem. Toxicol. 2020;143 doi: 10.1016/j.fct.2020.111520. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C., Bach C.C., Long M., Wielsøe M., Bech B.H., Henriksen T.B., Olsen J., Bonefeld-Jørgensen E.C. Associations of fetal growth outcomes with measures of the combined xenoestrogenic activity of maternal serum perfluorinated alkyl acids in Danish pregnant women. Environ. Health Perspect. 2019;127:1–13. doi: 10.1289/EHP1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodzicz S., Czarzasta K., Kuch M., Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14:1–8. doi: 10.1186/s12944-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges J., Sauer U.G., Buesen R., Deferme L., Tollefsen K.E., Tralau T., van Ravenzwaay B., Poole A., Pemberton M. Framework for the quantitative weight-of-evidence analysis of ‘omics data for regulatory purposes. Regul. Toxicol. Pharmacol. 2017;91:S46–S60. doi: 10.1016/j.yrtph.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Browne P., Noyes P.D., Casey W.M., Dix D.J. Application of adverse outcome pathways to U.S. EPA's endocrine disruptor screening program. Environ. Health Perspect. 2017;125 doi: 10.1289/EHP1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesen R., Chorley B.N., da Silva Lima B., Daston G., Deferme L., Ebbels T., Gant T.W., Goetz A., Greally J., Gribaldo L., Hackermüller J., Hubesch B., Jennen D., Johnson K., Kanno J., Kauffmann H.M., Laffont M., McMullen P., Meehan R., Pemberton M., Perdichizzi S., Piersma A.H., Sauer U.G., Schmidt K., Seitz H., Sumida K., Tollefsen K.E., Tong W., Tralau T., van Ravenzwaay B., Weber R.J.M., Worth A., Yauk C., Poole A. Applying ’omics technologies in chemicals risk assessment: report of an ECETOC workshop. Regul. Toxicol. Pharmacol. 2017;91 doi: 10.1016/j.yrtph.2017.09.002. S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G.J., Redman C.W., Roberts J.M., Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:1–15. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- Caloni F., De Angelis I., Hartung T. Replacement of animal testing by integrated approaches to testing and assessment (IATA): a call for in vivitrosi. Arch. Toxicol. 2022;96:1935–1950. doi: 10.1007/s00204-022-03299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N., Leemans M., Birgersson L., Germain P.L., Cheroni C., Borbély G., Engdahl E., Lindh C., Bressan R.B., Cavallo F., Chorev N.E., D'Agostino G.A., Pollard S.M., Rigoli M.T., Tenderini E., Tobon A.L., Trattaro S., Troglio F., Zanella M., Bergman Å., Damdimopoulou P., Jönsson M., Kiess W., Kitraki E., Kiviranta H., Nånberg E., Öberg M., Rantakokko P., Rudén C., Söder O., Bornehag C.G., Demeneix B., Fini J.B., Gennings C., Rüegg J., Sturve J., Testa G. From cohorts to molecules: adverse impacts of endocrine disrupting mixtures. Science. 2022;80:375. doi: 10.1126/science.abe8244. [DOI] [PubMed] [Google Scholar]
- Carusi A., Davies M.R., De Grandis G., Escher B.I., Hodges G., Leung K.M.Y., Whelan M., Willett C., Ankley G.T. Harvesting the promise of AOPs: an assessment and recommendations. Sci. Total Environ. 2018;628–629:1542–1556. doi: 10.1016/j.scitotenv.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi A., Whelan M., Wittwehr C., European Commission. Joint Research Centre. Publications Office of the European Union; 2019. Bridging across Methods in the Biosciences. [DOI] [Google Scholar]
- Carusi A., Wittwehr C., Whelan M. 2022. Addressing Evidence Needs in Chemicals Policy and Regulation. [DOI] [Google Scholar]
- Carvaillo J., Barouki R., Coumoul X., Audouze K. Linking bisphenol S to adverse outcome pathways using a combined text mining and systems biology approach. Environ. Heal. Perpsectives. 2019;127:1–11. doi: 10.1289/EHP4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartres N., Bero L.A., Norris S.L. A review of methods used for hazard identification and risk assessment of environmental hazards. Environ. Int. 2019;123:231–239. doi: 10.1016/j.envint.2018.11.060. [DOI] [PubMed] [Google Scholar]
- Chauhan V., Hamada N., Garnier-Laplace J., Laurier D., Beaton D., Tollefsen K.E., Locke P.A. Establishing a communication and engagement strategy to facilitate the adoption of the adverse outcome pathways in radiation research and regulation. Int. J. Radiat. Biol. 2022;1–8 doi: 10.1080/09553002.2022.2086716. [DOI] [PubMed] [Google Scholar]
- Cho E., Allemang A., Audebert M., Chauhan V., Dertinger S., Hendriks G., Luijten M., Marchetti F., Minocherhomji S., Pfuhler S., Roberts D.J., Trenz K., Yauk C.L. AOP report: development of an adverse outcome pathway for oxidative DNA damage leading to mutations and chromosomal aberrations. Environ. Mol. Mutagen. 2022:1–17. doi: 10.1002/em.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady K., Browne P., Embry M., Hill T., Leinala E., Steeger T., Maślankiewicz L., Hutchinson T. When are adverse outcome pathways and associated assays “fit for purpose” for regulatory decision-making and management of chemicals? Integr. Environ. Assess. Manag. 2019;15:633–647. doi: 10.1002/ieam.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley J.M., Lambright C.S., Evans N., Cardon M., Furr J., Wilson V.S., Gray L.E. Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract malformations in the male rat. Toxicol. Sci. 2018;164:166–178. doi: 10.1093/toxsci/kfy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieber-Rotheneder M., Beganovic S., Desoye G., Lang U., Cervar-Zivkovic M. Complex expression changes of the placental endothelin system in early and late onset preeclampsia, fetal growth restriction and gestational diabetes. Life Sci. 2012;91:710–715. doi: 10.1016/j.lfs.2012.04.040. [DOI] [PubMed] [Google Scholar]
- ECHA . 2013. Guidance for Human Health Risk Assessment Volume III, Part B : Guidance on Regulation (EU) No 528/2012 Concerning the Making Available on the Market and Use of Biocidal Products (BPR) [Google Scholar]
- ECHA, EFSA Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal. 2018;16:1–135. doi: 10.2903/j.efsa.2018.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Opinion of the PPR Panel on the follow‐up of the findings of the External Scientific Report ‘Literature review of epidemiological studies linking exposure to pesticides and health effects. EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Investigation into experimental toxicological properties of plant protection products having a potential link to Parkinson's disease and childhood leukaemia. EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Development of Integrated Approaches to Testing and Assessment (IATA) case studies on developmental neurotoxicity (DNT) risk assessment. EFSA J. 2021;19 doi: 10.2903/j.efsa.2021.6599. [DOI] [Google Scholar]
- EFSA Guidance Document on Scientific criteria for grouping chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals. EFSA J. 2021;19 doi: 10.2903/j.efsa.2021.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . 2018. Interim Science Policy: Use of Alternative Approaches for Skin Sensitization as a Replacement for Laboratory Animal Testing 1–13. [Google Scholar]
- Escher S.E., Aguayo-Orozco A., Benfenati E., Bitsch A., Braunbeck T., Brotzmann K., Bois F., van der Burg B., Castel J., Exner T., Gadaleta D., Gardner I., Goldmann D., Hatley O., Golbamaki N., Graepel R., Jennings P., Limonciel A., Long A., Maclennan R., Mombelli E., Norinder U., Jain S., Capinha L.S., Taboureau O.T., Tolosa L., Vrijenhoek N.G., van Vugt-Lussenburg B.M.A., Walker P., van de Water B., Wehr M., White A., Zdrazil B., Fisher C. Integrate mechanistic evidence from new approach methodologies (NAMs) into a read-across assessment to characterise trends in shared mode of action. Toxicol. Vitro. 2022;79 doi: 10.1016/j.tiv.2021.105269. [DOI] [PubMed] [Google Scholar]
- European Commission . COM; 2020. Chemicals Strategy for Sustainability towards a Toxic-free Environment; p. 667. [Google Scholar]
- Fay K.A., Villeneuve D.L., LaLone C.A., Song Y., Tollefsen K.E., Ankley G.T. Practical approaches to adverse outcome pathway development and weight-of-evidence evaluation as illustrated by ecotoxicological case studies. Environ. Toxicol. Chem. 2017;36:1429–1449. doi: 10.1002/etc.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant T.W., Sauer U.G., Zhang S.D., Chorley B.N., Hackermüller J., Perdichizzi S., Tollefsen K.E., van Ravenzwaay B., Yauk C., Tong W., Poole A. A generic Transcriptomics Reporting Framework (TRF) for ‘omics data processing and analysis. Regul. Toxicol. Pharmacol. 2017;91:S36–S45. doi: 10.1016/j.yrtph.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau-Van Waes J., Starr L., Maddox J., Aleman F., Voss K.A., Wilberding J., Riley R.T. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res. Part A Clin. Mol. Teratol. 2005;73:487–497. doi: 10.1002/bdra.20148. [DOI] [PubMed] [Google Scholar]
- Geraets L., Nijkamp M.M., ter Burg W. Critical elements for human health risk assessment of less than lifetime exposures. Regul. Toxicol. Pharmacol. 2016;81:362–371. doi: 10.1016/j.yrtph.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Gilmour N., Reynolds J., Przybylak K., Aleksic M., Aptula N., Baltazar M.T., Cubberley R., Rajagopal R., Reynolds G., Spriggs S., Thorpe C., Windebank S., Maxwell G. Next generation risk assessment for skin allergy: decision making using new approach methodologies. Regul. Toxicol. Pharmacol. 2022;131 doi: 10.1016/j.yrtph.2022.105159. [DOI] [PubMed] [Google Scholar]
- Goeden H. Focus on chronic exposure for deriving drinking water guidance underestimates potential risk to infants. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundacker C., Ellinger I. The unique applicability of the human placenta to the Adverse Outcome Pathway (AOP) concept: the placenta provides fundamental insights into human organ functions at multiple levels of biological organization. Reprod. Toxicol. 2020;96:273–281. doi: 10.1016/j.reprotox.2020.07.014. [DOI] [PubMed] [Google Scholar]
- Harrill J.A., Viant M.R., Yauk C.L., Sachana M., Gant T.W., Auerbach S.S., Beger R.D., Bouhifd M., O'Brien J., Burgoon L., Caiment F., Carpi D., Chen T., Chorley B.N., Colbourne J., Corvi R., Debrauwer L., O'Donovan C., Ebbels T.M.D., Ekman D.R., Faulhammer F., Gribaldo L., Hilton G.M., Jones S.P., Kende A., Lawson T.N., Leite S.B., Leonards P.E.G., Luijten M., Martin A., Moussa L., Rudaz S., Schmitz O., Sobanski T., Strauss V., Vaccari M., Vijay V., Weber R.J.M., Williams A.J., Williams A., Thomas R.S., Whelan M. Progress towards an OECD reporting framework for transcriptomics and metabolomics in regulatory toxicology. Regul. Toxicol. Pharmacol. 2021;125 doi: 10.1016/j.yrtph.2021.105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T. Toxicology for the twenty-first century. Nature. 2009;460:208–212. doi: 10.1038/460208a. [DOI] [PubMed] [Google Scholar]
- HBM4EU . 2020. Deliverable 14.5 : Selection Criteria and Inventory of Effect Biomarkers for the 2nd Set of Substances. [Google Scholar]
- HBM4EU . 2021. Additional Deliverable 13.7: Draft Guidance and Recommendations for Risk Assessors and Policymakers on the Use of Mechanistic Toxicology Data and AOPs. [Google Scholar]
- Heldring M.M., Wijaya L.S., Niemeijer M., Yang H., Lakhal T., Le Dévédec S.E., van de Water B., Beltman J.B. Model-based translation of DNA damage signaling dynamics across cell types. PLoS Comput. Biol. 2022;18 doi: 10.1371/journal.pcbi.1010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A.F. Toxicological Risk Assessment and Multi-System Health Impacts from Exposure. Elsevier; 2021. In silico toxicology, a robust approach for decision-making in the context of next-generation risk assessment; pp. 31–50. [DOI] [Google Scholar]
- Heusinkveld H., Braakhuis H., Gommans R., Botham P., Corvaro M., van der Laan J.W., Lewis D., Madia F., Manou I., Schorsch F., Wolterink G., Woutersen R., Corvi R., Mehta J., Luijten M. Towards a mechanism-based approach for the prediction of nongenotoxic carcinogenic potential of agrochemicals. Crit. Rev. Toxicol. 2020;50:725–739. doi: 10.1080/10408444.2020.1841732. [DOI] [PubMed] [Google Scholar]
- Heusinkveld H.J., Staal Y.C.M., Baker N.C., Daston G., Knudsen T.B., Piersma A. An ontology for developmental processes and toxicities of neural tube closure. Reprod. Toxicol. 2021;99:160–167. doi: 10.1016/j.reprotox.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. Application of evidence-based methods to construct mechanism-driven chemical assessment frameworks. ALTEX. 2022;39:1–20. doi: 10.14573/altex.2202141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Kleinstreuer N., Alépée N., Allen D., Api A.M., Ashikaga T., Clouet E., Cluzel M., Desprez B., Gellatly N., Goebel C., Kern P.S., Klaric M., Kühnl J., Lalko J.F., Martinozzi-Teissier S., Mewes K., Miyazawa M., Parakhia R., van Vliet E., Zang Q., Petersohn D. Non-animal methods to predict skin sensitization (I): the Cosmetics Europe database. Crit. Rev. Toxicol. 2018;48:344–358. doi: 10.1080/10408444.2018.1429385. [DOI] [PubMed] [Google Scholar]
- Huhn S., Escher B.I., Krauss M., Scholz S., Hackermüller J., Altenburger R. Unravelling the chemical exposome in cohort studies: routes explored and steps to become comprehensive. Environ. Sci. Eur. 2021;33 doi: 10.1186/s12302-020-00444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives C., Campia I., Wang R., Wittwehr C., Edwards S. Creating a structured adverse outcome pathway knowledgebase via ontology-based annotations. Appl. Vitr. Toxicol. 2017;3:298–311. doi: 10.1089/aivt.2017.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M.N., Colacci A., Corvi R., Vaccari M., Aguila M.C., Corvaro M., Delrue N., Desaulniers D., Ertych N., Jacobs A., Luijten M., Madia F., Nishikawa A., Ogawa K., Ohmori K., Paparella M., Sharma A.K., Vasseur P. Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch. Toxicol. 2020;94:2899–2923. doi: 10.1007/s00204-020-02784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E., Poston L., Burton G.J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update. 2006;12:747–755. doi: 10.1093/humupd/dml016.Placental-related. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornod F., Rugard M., Tamisier L., Coumoul X., Andersen H.R., Barouki R., Audouze K. AOP4EUpest: mapping of pesticides in adverse outcome pathways using a text mining tool. Bioinformatics. 2020;36:4379–4381. doi: 10.1093/bioinformatics/btaa545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JRC Technical Guidance Document on Risk Assessment in. European Commission. 2003 [Google Scholar]
- Kauffmann H.M., Kamp H., Fuchs R., Chorley B.N., Deferme L., Ebbels T., Hackermüller J., Perdichizzi S., Poole A., Sauer U.G., Tollefsen K.E., Tralau T., Yauk C., van Ravenzwaay B. Framework for the quality assurance of ’omics technologies considering GLP requirements. Regul. Toxicol. Pharmacol. 2017;91:S27–S35. doi: 10.1016/j.yrtph.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzler A., Bopp S.K., van der Linden S., Berggren E., Worth A. Regulatory assessment of chemical mixtures: requirements, current approaches and future perspectives. Regul. Toxicol. Pharmacol. 2016;80:321–334. doi: 10.1016/j.yrtph.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Kleinstreuer N.C., Hoffmann S., Alépée N., Allen D., Ashikaga T., Casey W., Clouet E., Cluzel M., Desprez B., Gellatly N., Göbel C., Kern P.S., Klaric M., Kühnl J., Martinozzi-Teissier S., Mewes K., Miyazawa M., Strickland J., van Vliet E., Zang Q., Petersohn D. Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit. Rev. Toxicol. 2018;48:359–374. doi: 10.1080/10408444.2018.1429386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose J., Tigges J., Masjosthusmann S., Schmuck K., Bendt F., Hübenthal U., Petzsch P., Köhrer K., Koch K., Fritsche E. TBBPA targets converging key events of human oligodendrocyte development resulting in two novel AOPs. ALTEX. 2021;38:215–234. doi: 10.14573/altex.2007201. [DOI] [PubMed] [Google Scholar]
- Knapen D., Angrish M.M., Fortin M.C., Katsiadaki I., Leonard M., Margiotta-Casaluci L., Munn S., O'Brien J.M., Pollesch N., Smith L.C., Zhang X., Villeneuve D.L. Adverse outcome pathway networks I: development and applications. Environ. Toxicol. Chem. 2018;37:1723–1733. doi: 10.1002/etc.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen D., Stinckens E., Cavallin J.E., Ankley G.T., Holbech H., Villeneuve D.L., Vergauwen L. Toward an AOP network-based tiered testing strategy for the assessment of thyroid hormone disruption. Environ. Sci. Technol. 2020;54:8491–8499. doi: 10.1021/acs.est.9b07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel C., Buhrke T., Süssmuth R., Lampen A., Marx-Stoelting P., Braeuning A. Pregnane X receptor mediates steatotic effects of propiconazole and tebuconazole in human liver cell lines. Arch. Toxicol. 2019;93:1311–1322. doi: 10.1007/s00204-019-02445-2. [DOI] [PubMed] [Google Scholar]
- Kolter T., Sandhoff K. Sphingolipid metabolism diseases. Biochim. Biophys. Acta Biomembr. 2006;1758:2057–2079. doi: 10.1016/j.bbamem.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Invited perspective : how relevant are mode-of-action considerations for the assessment and prediction of mixture. Effects ? 2022;130:10–11. doi: 10.1289/EHP11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovo M., Schreiber L., Bar J. Placental vascular pathology as a mechanism of disease in pregnancy complications. Thromb. Res. 2013;131:S18. doi: 10.1016/S0049-3848(13)70013-6. –S21. [DOI] [PubMed] [Google Scholar]
- Krebs A., Waldmann T., Wilks M.F., van Vugt-Lussenburg B.M.A., van der Burg B., Terron A., Steger-Hartmann T., Ruegg J., Rovida C., Pedersen E., Pallocca G., Luijten M., Leite S.B., Kustermann S., Kamp H., Hoeng J., Hewitt P., Herzler M., Hengstler J.G., Heinonen T., Hartung T., Hardy B., Gantner F., Fritsche E., Fant K., Ezendam J., Exner T., Dunkern T., Dietrich D.R., Coecke S., Busquet F., Braeuning A., Bondarenko O., Bennekou S.H., Beilmann M., Leist M. Template for the description of cell-based toxicological test methods to allow evaluation and regulatory use of the data. ALTEX. 2019;36:682–699. doi: 10.14573/altex.1909271. [DOI] [PubMed] [Google Scholar]
- Krewski D., Andersen M.E., Tyshenko M.G., Krishnan K., Hartung T., Boekelheide K., Wambaugh J.F., Jones D., Whelan M., Thomas R., Yauk C., Barton-Maclaren T., Cote I. Archives of Toxicology. Springer Berlin Heidelberg; 2020. Toxicity testing in the 21st century: progress in the past decade and future perspectives. [DOI] [PubMed] [Google Scholar]
- Kucheryavenko O., Vogl S., Marx-Stoelting P. 2020. Chapter 1. Endocrine Disruptor Effects on Estrogen, Androgen and Thyroid Pathways: Recent Advances on Screening and Assessment; pp. 1–24. [DOI] [Google Scholar]
- Kuijper I.A., Yang H., Van De Water B., Beltman J.B. Unraveling cellular pathways contributing to drug-induced liver injury by dynamical modeling. Expet Opin. Drug Metabol. Toxicol. 2017;13:5–17. doi: 10.1080/17425255.2017.1234607. [DOI] [PubMed] [Google Scholar]
- La Merrill M.A., Vandenberg L.N., Smith M.T., Goodson W., Browne P., Patisaul H.B., Guyton K.Z., Kortenkamp A., Cogliano V.J., Woodruff T.J., Rieswijk L., Sone H., Korach K.S., Gore A.C., Zeise L., Zoeller R.T. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020;16:45–57. doi: 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi J.B., Di Paolo C., Kraak M.H.S., van der Geest H.G., Schymanski E.L., Williams A.J., Dingemans M.M.L., Massei R., Brack W., Cousin X., Begout M.L., van der Oost R., Carion A., Suarez-Ulloa V., Silvestre F., Escher B.I., Engwall M., Nilén G., Keiter S.H., Pollet D., Waldmann P., Kienle C., Werner I., Haigis A.C., Knapen D., Vergauwen L., Spehr M., Schulz W., Busch W., Leuthold D., Scholz S., vom Berg C.M., Basu N., Murphy C.A., Lampert A., Kuckelkorn J., Grummt T., Hollert H. An ecotoxicological view on neurotoxicity assessment. Environ. Sci. Eur. 2018;30:1–34. doi: 10.1186/s12302-018-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein D., Luckert C., Alarcan J., de Sousa G., Gioutlakis M., Katsanou E.S., Konstantinidou P., Machera K., Milani E.S., Peijnenburg A., Rahmani R., Rijkers D., Spyropoulou A., Stamou M., Stoopen G., Sturla S.J., Wollscheid B., Zucchini-Pascal N., Braeuning A., Lampen A. An adverse outcome pathway-based approach to assess steatotic mixture effects of hepatotoxic pesticides in vitro. Food Chem. Toxicol. 2020;139 doi: 10.1016/j.fct.2020.111283. [DOI] [PubMed] [Google Scholar]
- Luijten M., Rorije E., Sprong R.C., Van Der Ven L.T.M. Practical application of next generation risk assessment of chemicals for human health. Chem. Res. Toxicol. 2020;33:693–694. doi: 10.1021/acs.chemrestox.0c00074. [DOI] [PubMed] [Google Scholar]
- Lumen A., McNally K., George N., Fisher J.W., Loizou G.D. Quantitative global sensitivity analysis of a biologically based dose-response pregnancy model for the thyroid endocrine system. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsangkul C., Chiang H.I., Lo N.W., Fan Y.K., Ju J.C. Developmental toxicity of mycotoxin fumonisin B 1 in animal embryogenesis: an overview. Toxins. 2019;11:1–12. doi: 10.3390/toxins11020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens A., Golden E., Luechtefeld T.H., Hoffmann S., Tsaioun K., Hartung T. Probabilistic risk assessment - the keystone for the future of toxicology. ALTEX. 2022;39:3–29. doi: 10.14573/altex.2201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margina D., Nițulescu G., Ungurianu A., Mesnage R., Goumenou M., Sarigiannis D., Aschner M., Spandidos D., Renieri E., Hernandez A., Tsatsakis A. Overview of the effects of chemical mixtures with endocrine disrupting activity in the context of real-life risk simulation: an integrative approach (Review) World Acad. Sci. J. 2019;176:139–148. doi: 10.3892/wasj.2019.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens M., Evelo C.T., Willighagen E.L. Providing adverse outcome pathways from the AOP-Wiki in semantic web format to increase usability and accessibility of the content. Chem. 2021;16 doi: 10.26434/chemrxiv.13524191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos Dos Santos C.E., Miranda R.G., de Oliveira D.P., Dorta D.J. Challenges and opportunities for integrating in silico models and adverse outcomes pathways to set and relate new biomarkers. Water (Switzerland) 2020;12:1–10. doi: 10.3390/w12123549. [DOI] [Google Scholar]
- Matta K., Koual M., Ploteau S., Coumoul X., Audouze K., Bizec B., Le, Antignac, J.P. Cano-Sancho G. Associations between exposure to organochlorine chemicals and endometriosis: a systematic review of experimental studies and integration of epidemiological evidence. Environ. Health Perspect. 2021;129:1–22. doi: 10.1289/EHP8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek M.E.B., Boobis A.R., Crofton K.M., Heinemeyer G., Raaij M., Van, Vickers, C. Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework. Regul. Toxicol. Pharmacol. 2011;60:S1–S14. doi: 10.1016/j.yrtph.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Meek M.E., Boobis A., Cote I., Dellarco V., Fotakis G., Munn S., Seed J., Vickers C. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J. Appl. Toxicol. 2014;34:1–18. doi: 10.1002/jat.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek M.E., Palermo C.M., Bachman A.N., North C.M., Lewis R.J. Mode of action human relevance (species concordance) framework: evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J. Appl. Toxicol. 2014;34:595–606. doi: 10.1002/jat.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer S.A., Suarez L., Felkner M., Wang E., Merrill A.H., Rothman K.J., Hendricks K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006;114:237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moermond C.T.A., Kase R., Korkaric M., Ågerstrand M. CRED: criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem. 2016;35:1297–1309. doi: 10.1002/etc.3259. [DOI] [PubMed] [Google Scholar]
- Mortensen H.M., Martens M., Senn J., Levey T., Evelo C.T., Willighagen E.L., Exner T. The AOP-DB RDF: applying FAIR principles to the semantic integration of AOP data using the research description framework. Front. Toxicol. 2022;4:1–6. doi: 10.3389/ftox.2022.803983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustieles V., Fernández M.F. Bisphenol A shapes children's brain and behavior: towards an integrated neurotoxicity assessment including human data. Environ. Health. 2020;19:66. doi: 10.1186/s12940-020-00620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustieles V., D'Cruz S.C., Couderq S., Rodríguez-Carrillo A., Fini J.B., Hofer T., Steffensen I.L., Dirven H., Barouki R., Olea N., Fernández M.F., David A. Bisphenol A and its analogues: a comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.105811. [DOI] [PubMed] [Google Scholar]
- Mustieles V., Rodríguez-Carrillo A., Vela-Soria F., D'Cruz S.C., David A., Smagulova F., Mundo-López A., Olivas-Martínez A., Reina-Pérez I., Olea N., Freire C., Arrebola J.P., Fernández M.F. BDNF as a potential mediator between childhood BPA exposure and behavioral function in adolescent boys from the INMA-Granada cohort. Sci. Total Environ. 2022;803 doi: 10.1016/j.scitotenv.2021.150014. [DOI] [PubMed] [Google Scholar]
- Negi C.K., Bajard L., Kohoutek J., Blaha L. An adverse outcome pathway based in vitro characterization of novel flame retardants-induced hepatic steatosis. Environ. Pollut. 2021;289 doi: 10.1016/j.envpol.2021.117855. [DOI] [PubMed] [Google Scholar]
- Noyes P.D., Friedman K.P., Browne P., Haselman J.T., Gilbert M.E., Hornung M.W., Barone S., Crofton K.M., Laws S.C., Stoker T.E., Simmons S.O., Tietge J.E., Degitz S.J. Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ. Health Perspect. 2019;127 doi: 10.1289/EHP5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 2006. Human Biomonitoring for Environmental Chemicals, Human Biomonitoring for Environmental Chemicals. [DOI] [Google Scholar]
- Ochsner S.A., Abraham D., Martin K., Ding W., McOwiti A., Kankanamge W., Wang Z., Andreano K., Hamilton R.A., Chen Y., Hamilton A., Gantner M.L., Dehart M., Qu S., Hilsenbeck S.G., Becnel L.B., Bridges D., Ma’ayan A., Huss J.M., Stossi F., Foulds C.E., Kralli A., McDonnell D.P., McKenna N.J. The Signaling Pathways Project, an integrated ‘omics knowledgebase for mammalian cellular signaling pathways. Sci. Data. 2019;6:1–21. doi: 10.1038/s41597-019-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD OECD guidance on the reporting of defined approaches and individual information sources to be used within Integrated Approaches to Testing and Assessment (IATA) for skin sensitization. Toxicol. Lett. 2016;258:S2. doi: 10.1016/j.toxlet.2016.06.027. [DOI] [Google Scholar]
- OECD . 2017. Revised Guidance Document on Developing and Assessing Adverse Outcome Pathways. [Google Scholar]
- OECD . OECD Publishing; 2018. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. [Google Scholar]
- OECD CASE STUDY ON THE USE OF INTEGRATED APPROACHES FOR TESTING AND ASSESSMENT TO INFORM READ-ACROSS OF p_ALKYLPHENOLS. REPEATED-DOSE TOXICITY. 2018;39:1–106. [Google Scholar]
- OECD . vol. 28. ENV/JM/MONO; 2019. (CASE STUDY on the USE of an INTEGRATED APPROACH to TESTING and ASSESSMENT for ESTROGEN RECEPTOR ACTIVE CHEMICALS - Series on Testing and Assessment No. 309). [Google Scholar]
- OECD . 2020. CASE STUDY ON THE USE OF INTEGRATED APPROACHES TO TESTING AND ASSESSMENT FOR IDENTIFICATION AND CHARACTERISATION OF PARKINSONIAN HAZARD LIABILITY OF DEGUELIN BY AN AOP-BASED TESTING AND READ ACROSS 1–57. [Google Scholar]
- OECD . 2020. CASE STUDY ON THE USE OF INTEGRATED APPROACHES TO TESTING AND ASSESSMENT FOR PREDICTION OF A 90 DAY REPEATED DOSE TOXICITY STUDY (OECD 408) FOR 2-ETHYLBUTYRIC ACID USING A READ-ACROSS APPROACH FROM OTHER BRANCHED CARBOXYLIC ACIDS. [Google Scholar]
- OECD Guidance document for the scientific review of adverse outcome pathways. Series on Testing and Assessment No. 344. 2021 [Google Scholar]
- OECD AOP Developers’ Handbook supplement to the Guidance Document for 7 developing and assessing Adverse Outcome Pathways (AOPs) OECD Series on Testing and Assessment. 2022 [Google Scholar]
- O'Brien J.M., Yauk C.L. Introducing AOP reports: collaborative review and publication of adverse outcome pathways. Environ. Mol. Mutagen. 2022;1–2 doi: 10.1002/em.22481. [DOI] [PubMed] [Google Scholar]
- Paini A., Campia I., Cronin M.T.D., Asturiol D., Ceriani L., Exner T.E., Gao W., Gomes C., Kruisselbrink J., Martens M., Meek M.E.B., Pamies D., Pletz J., Scholz S., Schüttler A., Spînu N., Villeneuve D.L., Wittwehr C., Worth A., Luijten M. Towards a qAOP framework for predictive toxicology - linking data to decisions. Comput. Toxicol. 2022;21 doi: 10.1016/j.comtox.2021.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou N., Distel E., de Oliveira E., Gabriel C., Frydas I.S., Anesti O., Attignon E.A., Odena A., Díaz R., Aggerbeck, Μ. Horvat M., Barouki R., Karakitsios S., Sarigiannis D.A. Multi-omics analysis reveals that co-exposure to phthalates and metals disturbs urea cycle and choline metabolism. Environ. Res. 2021;192 doi: 10.1016/j.envres.2020.110041. [DOI] [PubMed] [Google Scholar]
- Perkins E.J., Ashauer R., Burgoon L., Conolly R., Landesmann B., Mackay C., Murphy C.A., Pollesch N., Wheeler J.R., Zupanic A., Scholz S. Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment. Environ. Toxicol. Chem. 2019 doi: 10.1002/etc.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F., Madia F., Corvi R., Munn S., Grignard E., Paini A., Worth A., Bal-Price A., Prieto P., Casati S., Berggren E., Bopp S.K., Zuang V. Archives of Toxicology. Springer Berlin Heidelberg; 2021. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: challenges and opportunities for introducing new approach methodologies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt A., Bouwmeester H., Blaauboer B.J., Coecke S., Hakkert B., Hendriks D.F.G., Jennings P., Kramer N.I., Neuhoff S., Masereeuw R., Paini A., Peijnenburg A.A.C.M., Rooseboom M., Shuler M.L., Sorrell I., Spee B., Strikwold M., van der Meer A.D., van der Zande M., Vinken M., Yang H., Bos P.M.J., Heringa M.B. New approach methodologies (NAMs) for human-relevant biokinetics predictions: meeting the paradigm shift in toxicology towards an animal-free chemical risk assessment. ALTEX. 2020;37:607–622. doi: 10.14573/altex.2003242. [DOI] [PubMed] [Google Scholar]
- Riley R.T., Torres O., Matute J., Gregory S.G., Ashley-Koch A.E., Showker J.L., Mitchell T., Voss K.A., Maddox J.R., Gelineau-van Waes J.B. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize-based foods and living in high exposure communities in Guatemala. Mol. Nutr. Food Res. 2015;59:2209–2224. doi: 10.1002/mnfr.201500499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S. 2022. The Big Idea: Should We Get Rid of the Scientific Paper? Guard. [Google Scholar]
- Rodríguez-Carrillo A., Dćruz S.C., Mustieles V., Suárez B., Smagulova F., David A., Peinado F., Artacho-Cordón F., López L.C., Arrebola J.P., Olea N., Fernández M.F., Freire C. Exposure to non-persistent pesticides, BDNF, and behavioral function in adolescent males: exploring a novel effect biomarker approach. Environ. Res. 2022;211 doi: 10.1016/j.envres.2022.113115. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carrillo A., Mustieles V., D'Cruz S.C., Legoff L., Gil F., Olmedo P., Reina-Pérez I., Mundo A., Molina M., Smagulova F., David A., Freire C., Fernández M.F. Exploring the relationship between metal exposure, BDNF, and behavior in adolescent males. Int. J. Hyg Environ. Health. 2022;239 doi: 10.1016/j.ijheh.2021.113877. [DOI] [PubMed] [Google Scholar]
- Rooney A.A., Cooper G.S., Jahnke G.D., Lam J., Morgan R.L., Boyles A.L., Ratcliffe J.M., Kraft A.D., Schünemann H.J., Schwingl P., Walker T.D., Thayer K.A., Lunn R.M. How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ. Int. 92–. 2016;93:617–629. doi: 10.1016/j.envint.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N., Zilliacus J., Beronius A. Development of the SciRAP approach for evaluating the reliability and relevance of in vitro toxicity data. Front. Toxicol. 2021;3:1–13. doi: 10.3389/ftox.2021.746430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter S., Beronius A., Boobis A.R., Hanberg A., van Klaveren J., Luijten M., Machera K., Nikolopoulou D., van der Voet H., Zilliacus J., Solecki R. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: the potential EuroMix contribution. Crit. Rev. Toxicol. 2018;48:796–814. doi: 10.1080/10408444.2018.1541964. [DOI] [PubMed] [Google Scholar]
- Rugard M., Coumoul X., Carvaillo J.-C., Barouki R., Audouze K. Deciphering adverse outcome pathway network linked to Bisphenol F using text mining and systems toxicology approaches. Toxicol. Sci. 2019;1–9 doi: 10.1093/toxsci/kfz214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler T.W., Merrill A.H., Stevens V.L., Sullards M.C., Wang E., Wang P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology. 2002;66:169–176. doi: 10.1002/tera.10089. [DOI] [PubMed] [Google Scholar]
- Sarigiannis D.A., Karakitsios S.P., Handakas E., Simou K., Solomou E., Gotti A. Integrated exposure and risk characterization of bisphenol-A in Europe. Food Chem. Toxicol. 2016;98:134–147. doi: 10.1016/j.fct.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Sarigiannis D.A., Papaioannou N., Handakas E., Anesti O., Polanska K., Hanke W., Salifoglou A., Gabriel C., Karakitsios S. Neurodevelopmental exposome: the effect of in utero co-exposure to heavy metals and phthalates on child neurodevelopment. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.110949. [DOI] [PubMed] [Google Scholar]
- Sauer U.G., Barter R.A., Becker R.A., Benfenati E., Berggren E., Hubesch B., Hollnagel H.M., Inawaka K., Keene A.M., Mayer P., Plotzke K., Skoglund R., Albert O. 21st century approaches for evaluating exposures, biological activity, and risks of complex substances: workshop highlights. Regul. Toxicol. Pharmacol. 2020;111 doi: 10.1016/j.yrtph.2020.104583. [DOI] [PubMed] [Google Scholar]
- Schmid S., Song Y., Tollefsen K.E. AOP report: inhibition of chitin synthase 1 leading to increased mortality in arthropods. Environ. Toxicol. Chem. 2021;40:2112–2120. doi: 10.1002/etc.5058. [DOI] [PubMed] [Google Scholar]
- Shimonovich M., Pearce A., Thomson H., Keyes K., Katikireddi S.V. Assessing causality in epidemiology: revisiting Bradford Hill to incorporate developments in causal thinking. Eur. J. Epidemiol. 2021;36:873–887. doi: 10.1007/s10654-020-00703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillé F.C.M., Karakitsios S., Kleensang A., Koehler K., Maertens A., Miller G.W., Prasse C., Quiros-Alcala L., Ramachandran G., Rappaport S.M., Rule A.M., Sarigiannis D., Smirnova L., Hartung T. The exposome - a new approach for risk assessment. ALTEX. 2020;37:3–23. doi: 10.14573/altex.2001051. [DOI] [PubMed] [Google Scholar]
- Song Y., Villeneuve D.L. AOP report: uncoupling of oxidative phosphorylation leading to growth inhibition via decreased cell proliferation. Environ. Toxicol. Chem. 2021;40:2959–2967. doi: 10.1002/etc.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spînu N., Cronin M.T.D., Lao J., Bal-Price A., Campia I., Enoch S.J., Madden J.C., Mora Lagares L., Novič M., Pamies D., Scholz S., Villeneuve D.L., Worth A.P. Probabilistic modelling of developmental neurotoxicity based on a simplified adverse outcome pathway network. Comput. Toxicol. 2022;21:3. doi: 10.1016/j.comtox.2021.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingen T., Villeneuve D.L., Knapen D., Panagiotou E.M., Draskau M.K., Damdimopoulou P., O'Brien J.M. A pragmatic approach to adverse outcome pathway development and evaluation. Toxicol. Sci. 2021;184:183–190. doi: 10.1093/toxsci/kfab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.M., Leonard J.A., Edwards S., Teeguarden J., Paini A., Egeghy P. Aggregate exposure pathways in support of risk assessment. Curr. Opin. Toxicol. 2018;9:8–13. doi: 10.1016/j.cotox.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Beaton D., Chauhan V., Choi I., Danielsen P.H., Delrue N., Esterhuizen M., Filipovska J., FitzGerald R., Fritsche E., Gant T., Garcia-Reyero N., Helm J., Huliganga E., Jacobsen N., Kay J.E., Kim Y.J., Klose J., La Rocca C., Luettich K., Mally A., O'Brien J., Poulsen S.S., Rudel R.A., Sovadinova I., Tollefsen K.E., Vogel U., Yepiskoposyan H., Yauk C. Report of the 1st and 2nd Mystery of reactive oxygen species conferences. ALTEX. 2022;39:336–338. doi: 10.14573/altex.2203011. [DOI] [Google Scholar]
- Tanabe S., O’Brien J., Tollefsen K.E., Kim Y., Chauhan V., Yauk C., Huliganga E., Rudel R.A., Kay J.E., Helm J.S., Beaton D., Filipovska J., Sovadinova I., Garcia-Reyero N., Mally A., Poulsen S.S., Delrue N., Fritsche E., Luettich K., La Rocca C., Yepiskoposyan H., Klose J., Danielsen P.H., Esterhuizen M., Jacobsen N.R., Vogel U., Gant T.W., Choi I., FitzGerald R. Reactive Oxygen Species in the Adverse Outcome Pathway Framework: Toward Creation of Harmonized Consensus Key Events. Front. Toxicol. 2022;4:887135. doi: 10.3389/ftox.2022.887135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terron A., Bal-Price A., Paini A., Monnet-Tschudi F., Bennekou S.H., Angeli K., Fritsche E., Mantovani A., Viviani B., Leist M., Schildknecht S. Archives of Toxicology. Springer Berlin Heidelberg; 2018. An adverse outcome pathway for parkinsonian motor deficits associated with mitochondrial complex I inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.S., Paules R.S., Simeonov A., Fitzpatrick S.C., Crofton K.M., Casey W.M., Mendrick D.L. The US Federal Tox 21 Program: a strategic and operational plan for continued leadership. ALTEX. 2018;35:163–168. doi: 10.14573/altex.1803011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen K.E., Scholz S., Cronin M.T., Edwards S.W., de Knecht J., Crofton K., Garcia-Reyero N., Hartung T., Worth A., Patlewicz G. Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA) Regul. Toxicol. Pharmacol. 2014;70:629–640. doi: 10.1016/j.yrtph.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Toyota K., Watanabe H., Hirano M., Abe R., Miyakawa H., Song Y., Sato T., Miyagawa S., Tollefsen K.E., Yamamoto H., Tatarazako N., Iguchi T. Juvenile hormone synthesis and signaling disruption triggering male offspring induction and population decline in cladocerans (water flea): review and adverse outcome pathway development. Aquat. Toxicol. 2022;243 doi: 10.1016/j.aquatox.2021.106058. [DOI] [PubMed] [Google Scholar]
- UNEP . World Heal; Organ: 1999. Chemical Risk Assessment - Human Risk Assessment, Environmental Risk Assessment and Ecological Risk Assessment. [Google Scholar]
- van den Brand A.D., Bajard L., Steffensen I., Brantsæter A.L., Dirven H.A.A.M., Louisse J., Peijnenburg A., Ndaw S., Mantovani A., De Santis B., Mengelers M.J.B. Providing biological plausibility for exposure–health relationships for the mycotoxins deoxynivalenol (DON) and fumonisin B1 (FB1) in humans using the AOP framework. Toxins. 2022;14:279. doi: 10.3390/toxins14040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ven L.T.M., Rorije E., Sprong R.C., Zink D., Derr R., Hendriks G., Loo L.H., Luijten M. A case study with triazole fungicides to explore practical application of next-generation hazard assessment methods for human health. Chem. Res. Toxicol. 2020;33:834–848. doi: 10.1021/acs.chemrestox.9b00484. [DOI] [PubMed] [Google Scholar]
- van der Ven L.T.M., van Ommeren P., Zwart E.P., Gremmer E.R., Hodemaekers H.M., Heusinkveld H.J., van Klaveren J.D., Slob W., Rorije E. Dose addition in the induction of craniofacial malformations in zebrafish embryos exposed to a complex mixture of food relevant chemicals with dissimilar modes of action. Prep. 2022;130:1–15. doi: 10.1289/EHP9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology. 2013;312:158–165. doi: 10.1016/j.tox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Vinken M., Benfenati E., Busquet F., Castell J., Clevert D.A., de Kok T.M., Dirven H., Fritsche E., Geris L., Gozalbes R., Hartung T., Jennen D., Jover R., Kandarova H., Kramer N., Krul C., Luechtefeld T., Masereeuw R., Roggen E., Schaller S., Vanhaecke T., Yang C., Piersma A.H. Safer chemicals using less animals: kick-off of the European ONTOX project. Toxicology. 2021;458:1–7. doi: 10.1016/j.tox.2021.152846. [DOI] [PubMed] [Google Scholar]
- Volz D.C., Belanger S., Embry M., Padilla S., Sanderson H., Schirmer K., Scholz S., Villeneuve D. Adverse outcome pathways during early fish development: a conceptual framework for identification of chemical screening and prioritization strategies. Toxicol. Sci. 2011;123:349–358. doi: 10.1093/toxsci/kfr185. [DOI] [PubMed] [Google Scholar]
- Vrijenhoek N.G., Wehr M.M., Kunnen S.J., Wijaya L.S., Callegaro G., Moné M.J., Escher S.E., Van de Water B. Application of high-throughput transcriptomics for mechanism-based biological read-across of short-chain carboxylic acid analogues of valproic acid. ALTEX. 2022;39 doi: 10.14573/altex.2107261. [DOI] [PubMed] [Google Scholar]
- Whaley P., Edwards S.W., Kraft A., Nyhan K., Shapiro A., Watford S., Wattam S., Wolffe T., Angrish M. Knowledge organization systems for systematic chemical assessments. Environ. Health Perspect. 2020;128:1–11. doi: 10.1289/EHP6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2021. WHO Human Health Risk Assessment Toolkit CHEMICAL HAZARDS. [Google Scholar]
- WHO. UNEP . WHO Press; 2012. State of the Science of Endocrine Disrupting Chemicals. [DOI] [PubMed] [Google Scholar]
- Willett C. Alternatives to Animal Testing. Springer Singapore; Singapore: 2019. The use of adverse outcome pathways (AOPs) to support chemical safety decisions within the context of integrated approaches to testing and assessment (IATA) pp. 83–90. [DOI] [Google Scholar]
- Wise A., Parham F., Axelrad D.A., Guyton K.Z., Portier C., Zeise L., Zoeller R.T., Woodruff T.J. Upstream adverse effects in risk assessment: a model of polychlorinated biphenyls, thyroid hormone disruption and neurological outcomes in humans. Environ. Res. 2012;117:90–99. doi: 10.1016/j.envres.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Wittwehr C., Aladjov H., Ankley G., Byrne H.J., de Knecht J., Heinzle E., Klambauer G., Landesmann B., Luijten M., MacKay C., Maxwell G., Meek M.E.B., Paini A., Perkins E., Sobanski T., Villeneuve D., Waters K.M., Whelan M. How adverse outcome pathways can aid the development and use of computational prediction models for regulatory toxicology. Toxicol. Sci. 2017;155:326–336. doi: 10.1093/toxsci/kfw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgheib E., Gao W., Limonciel A., Aladjov H., Yang H., Tebby C., Gayraud G., Jennings P., Sachana M., Beltman J.B., Bois F.Y. Application of three approaches for quantitative AOP development to renal toxicity. Comput. Toxicol. 2019;11:1–13. doi: 10.1016/j.comtox.2019.02.001. [DOI] [Google Scholar]
- Zuang V., Dura A., et al. Publications Office of the European Union; 2022. Non-animal methods in science and regulation – EURL ECVAM status report 2021, EUR 30960 EN. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is review paper, all resources used are properly cited.