Abstract
Objective:
To test associations between parent-reported confidence to avoid hospitalization and caregiving strain, activation, and health-related quality of life (HRQOL).
Study Design:
In this prospective cohort study, enrolled parents of CMC (n=75) from three complex care programs received text messages (random times, every 2 weeks for 3 months) to rate confidence on avoiding hospitalization in the next month. Low confidence, measured on a 10-point Likert scale (1=not confident; 10=fully confident), was defined as mean rating <5. Caregiving measures included caregiver strain questionnaire (CGSQ7), family caregiver activation in transition (FCAT), and caregiver HRQOL (SF12). Relationships between caregiving and confidence were assessed with hierarchical logistic regression and classification and regression trees (CART).
Results:
Parents were mostly mothers (77%), linguistically diverse (20% spoke Spanish as primary language), and 18% had low confidence on average. Demographic and clinical variables had weaker associations with confidence. In regression models, low confidence was associated with higher caregiver strain, aOR (95% CI), 3.52 (1.45–8.54). Better mental HRQOL was associated with lower likelihood of low confidence, 0.89 (0.80–0.97). In the CART model, higher strain similarly identified parents having lower confidence. In all models, low confidence was not associated with caregiver activation (FCAT) or physical HRQOL (SF12) scores.
Conclusion:
Parents of CMC with high strain and low mental HRQOL had low confidence in the range where intervention to avoid hospitalization would be warranted. Future work should determine how adaptive interventions to improve confidence and prevent hospitalizations should account for strain and low mental HRQOL.
Keywords: medical complexity, mHealth, confidence, caregiving, mixed-methods
INTRODUCTION
Parents of children with medical complexity (CMC) often put tremendous effort into keeping their children healthy and thereby preventing the need to be hospitalized.1 By delivering substantial hours of sophisticated healthcare to their children, parents of CMC develop unique caregiving expertise about managing health crises.2,3 Recent studies have supported the notion that parents can predict when hospitalization may be imminent.4–6
Although clinical programs are responsive to parent concerns, responses can be reactive since systems for families to effortlessly express real-time needs are limited. To proactively take advantage of parents’ expertise about their child’s health trajectory, and support more rapid and precise interventions at times of concern, we developed a text-messaging platform to repeatedly collect ratings of parent confidence to avoid hospitalization. In our first of two planned studies, we observed that lower confidence did predict hospitalization.6 Importantly, a construct like confidence varies over time and can be both high and low in families who are capable of providing quality care for their child. Within an adaptive digital intervention framework, low confidence represents a key window of opportunity for intervention,7 i.e., when parents feel that a hospitalization may be imminent, this is a red flag that an increase in clinical care is needed. In this manner confidence is a valuable tailoring variable – a participant-specific determinant of which/when elements of an intervention should be delivered.7
The purpose of this study was to explore patterns and predictors of parent confidence to avoid hospitalization, based on theorized relationships from our conceptual model (Figure 1, online only). Our model was adapted from the capability-opportunity-motivation-behavior (COM-B) model,8 integrating findings from our prior research on preventing hospitalizations.1,6,9–11 In the present study, we test the specific hypothesis that low confidence is associated with greater caregiving strain, poorer parent heath, and greater challenges with caregiving tasks. Better understanding determinants of confidence will both refine our conceptual model and identify unique drivers of hospitalization for CMC as well as opportunities to further optimize an adaptive intervention to reduce hospitalizations at times of low confidence.12
Figure 1. Conceptual Model Linking CMC Parent Confidence to Hospitalization in the Context of a Future Adaptive Digital Intervention.
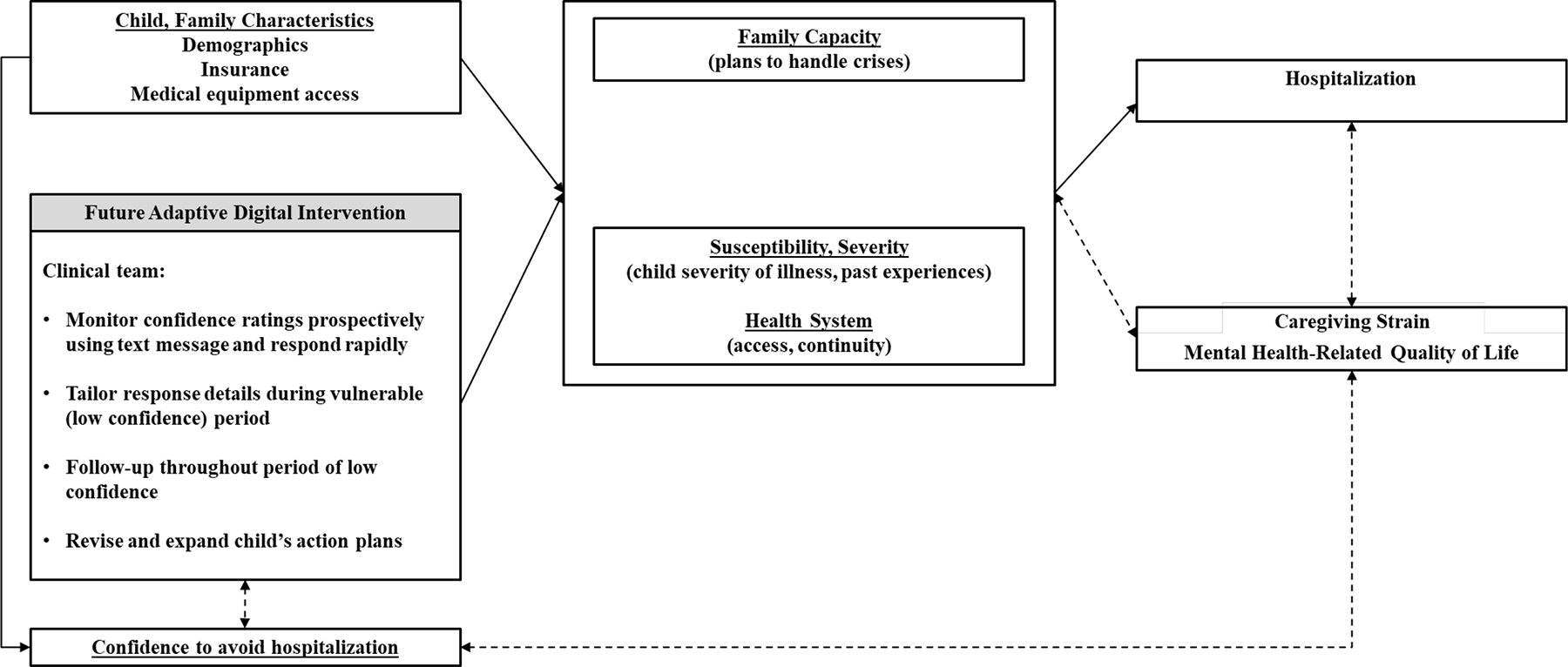
Prior research on preventing hospitalizations,1,6,9–11 combined with behavioral intervention theory8 suggests that decisions to seek hospital care are influenced by family capacity, susceptibility, health system, and confidence. Solid lines represent hypothesized causal assumptions to be tested in future studies. Dashed lines represent potential confounding association.
METHODS
Study Design
This multisite prospective cohort study was the second phase of a 2-part study evaluating repeated text messaging with parents of CMC to rate their confidence to avoid hospitalization.6 In the first study, we confirmed confidence was associated with hospitalization, while in this second study we identify variables associated with low confidence.
Participants and Setting
Primary caregivers, typically parents, of children enrolled in outpatient pediatric complex care programs at the University of Wisconsin American Family Children’s Hospital, UCLA Mattel Children’s Hospital and Boston Children’s Hospital were recruited between August 2018 and May 2019. We enrolled n=75 parent caregivers (n=25/site), purposively sampled to facilitate diversity in age, gender, race/ethnicity and English/Spanish primary language. All Spanish-speaking caregivers were recruited at UCLA by native Spanish-speaking team members, and their study activities were all conducted in Spanish. Inclusion criteria for caregivers were being ≥18 years of age, having a child < 18 years enrolled in the site’s complex care program, and having a personal phone capable of sending/receiving text messages.
Our complex care programs similarly aim to set and meet family-identified goals, coordinate primary, specialty, home- and community-based services, reduce unmet needs, and reduce health services use. UCLA’s program differs from the other two sites by also delivering primary care. Enrollment criteria are similar at each program and include numbers of affected organ systems, subspecialists involvement, and past health services use. Children in each program are frequently, though not exclusively, assisted by medical technology (e.g., enteral tubes, implanted devices). None of the programs focus on complexity related primarily to behavioral health needs. Each program has been described in detail previously.9,13,14 This study was approved by the Institutional Review Boards at each site.
Confidence
Modeled after our earlier observational studies4,5,15 and guidance for creating scales to measure self-efficacy,6,16 our text message prompt was, “How confident are you that your child can avoid an unplanned hospitalization over the next month? Please respond on a scale from 1 (‘not confident’) to 10 (‘fully confident’).” Text messages were sent at random days and times every two weeks over a period of three months. We defined low confidence as ratings <5, which was based on our a priori assumption and supported in our previous study through parent focus group data, and confirmed with received operative characteristic curves analyses most optimally predicting future hospitalization when confidence was rated <5. We defined parents having mean confidence <5 over the study period as having low confidence.
Parent Caregiving Measures
Each caregiving measure was evaluated at study enrollment.
Strain.
The caregiver strain questionnaire (CGSQ7)17 evaluated consequences of caregiving. This 7-item survey quantifies the extent to which caregivers report disruptions in personal time, family relationships, work, feeling tired, worried, sad, or financially strained, as a result of caregiving. Responses to the CGSQ7 range from 1 (not a problem at all) to 5 (very much a problem). For this study, we used global strain, which represents a sum of the means of the two subscale, and therefore ranges from 2–10, with lower scores indicating less strain.
Activation.
To measure activation, i.e., confidence, skills and attitudes to manage health,18,19 we used the Family Caregiver Activation in Transition (FCAT) scale.19 The 10-item FCAT asks caregivers to self-evaluate performance of common caregiving tasks. This includes understanding the care plan, effectively communicating with healthcare professionals, knowing what things to watch for that mean the condition is getting worse, getting to appointments, and knowing medications, doses, and how to ensure consistent access and delivery, among others. Responses range from 1–5, with higher scores indicating higher activation. We calculated the summary FCAT score as the average of all 10 items.
HRQOL.
We used the Medical Outcomes Study Short Form 12 (SF12v1) to measure caregiver physical and mental health-related quality of life.20,21 The SF12 includes 12 questions about health experiences over the previous 4 weeks. Two different 0–100 component scores (physical, PCS, and mental, MCS) are created. Mental and physical component SF12 scores of US adults are normalized to mean 50 and SD 10.
Data Collection and Covariates
During a study enrollment telephone call, a trained research team member collected baseline caregiving data, i.e., CGSQ7, FCAT, SF12, using a structured questionnaire. Additional covariate data included in this study were caregiver age, primary language for study activities (English or Spanish), household gross income (<$40,000, $40,000–79,999, >$80,000), household structure (married/domestic partnership, single/never-married, divorced/separated). Since we expected important differences might exist by race/ethnicity due to known differences in experience navigating health system or access to care, we included race/ethnicity. CMC covariates included duration of complex care program enrollment, and numbers affected organ systems, specialists, medications, hospitalizations and ED visits in the 12 months prior to study enrollment and during the study period, as well as technology assistance. Study data from all sites were entered into a REDCap database (Vanderbilt, https://www.project-redcap.org/) at University of Wisconsin.
Statistical Analysis
Hierarchical logistic regression, with random effects to account for clustering of data by study site, identified associations between measures of CMC caregiving (i.e., strain, activation and HRQOL) and low confidence. Multivariable models used pre-selected variables consistent with our initial analysis, and included caregiver age, study language, child technology assistance and duration of complex care clinic enrollment. To further explore variable relationships with confidence, we conducted classification and regression tree (CART) analyses using mean confidence as a continuous outcome. CART models use statistical testing with all included variables to recursively split the cohort into subgroups that have statistically significant differences from one another in the outcome. A branch is identified when specific variables are associated with the outcome and create the most separation in the outcome among the resulting groups.22 This analysis allows investigators to identify higher-order interactions with an outcome that might otherwise be difficult to observe.
We also conducted two secondary analyses. First, to present more interpretable relationships between low confidence and caregiving measures, we calculated the difference in scale scores for those with low compared to not low confidence. We estimated marginal differences of each caregiving scale score with random effects negative binomial regression models. Second, because we observed significant relationships with large effect sizes between caregiving strain and confidence, we also calculated marginal differences in strain scores by caregiver or child variables using random effects negative binomial regression. Two-sided p<.05 was considered statistically significant and analyses were conducted in STATA (version 16.0, College Station, TX).
RESULTS
We enrolled n=75 caregivers (25 caregivers per site), representing 93% of those approached. Caregivers were mostly mothers (77%), median (IQR) 40 (33–52) years old, and demographically and socioeconomically diverse, as previously reported (Table 1).6 About half (55%) lived in 2-parent households, 20% were single, never married, and 20% were divorced or separated. Educational experience varied, with 40% reporting general educational diploma (GED) or less, and 36% reporting college or graduate degrees. Children in the cohort had median (IQR) 7 (3–11) medications, 6 (4–8) subspecialists, and 1 (0–2) hospitalization in the 12 months prior to study enrollment, and over two-thirds (69%) were assisted by medical technology.
Table 1.
Demographic and Clinical Characteristics of the Study Participants
| Caregiver Characteristics | n (%) |
|---|---|
| Age, years, median (IQR) | 39.5 (33–52) |
| Gender | |
| Female | 65 (89.0) |
| Study Language | |
| English | 60 (80.0) |
| Spanish | 15 (20.0) |
| Race/Ethnicity | |
| White, non-Hispanic | 29 (38.7) |
| Black, non-Hispanic | 7 (9.3) |
| Hispanic | 34 (45.3) |
| Other | 5 (6.7) |
| Household Income (pre-tax) | |
| >$80,000 | 14 (18.7) |
| $40,000–79,999 | 17 (22.7) |
| < $40,000 | 35 (46.7) |
| Did not answer | 9 (12.0) |
| Household Status | |
| Married or Domestic Partnership | 41 (54.7) |
| Single, Never-Married | 15 (20.0) |
| Divorced or separated | 15 (20.0) |
| Widowed | 4 (5.3) |
| Child Characteristics | |
| Complex Care Clinic Enrollment, months, median (IQR) | 32.5 (12–58.5) |
| Affected Organ Systems, mean (SD) | 5.4 (1.9) |
| Medications upon enrollment, median (IQR) | 7 (3–11) |
| Subspecialists 12 months prior to enrollment, median (IQR) | 6 (4–8) |
| Presence of Technology Assistance | 51 (68.9) |
| Hospitalizations 12 months prior to enrollment, median (IQR) | 1 (0–2) |
Table 2 summarizes the cohort’s caregiving measure results. Mean (SD) confidence rating by text message during the study period was 7.7 (2.1) out of 10, with 18% of the cohort having low confidence (i.e., mean rating <5) during the study period. Mean (SD) CGSQ7 score, which ranges from 2 (least) to 10 (most) strain was 5.2 (2.2), suggesting that caregivers tended to feel that caregiving created at least some degree of disruption, though there was substantial variability across items and individuals. The FCAT score, which ranges from 1 (least) to 5 (most) activated, was 4.5 (0.6), suggesting that caregivers tended to ‘agree’ or ‘agree strongly’ when self-evaluating items reflecting their caregiving knowledge, skill, and motivation. Physical and mental component SF12 HRQOL scores were 51.8 (7.9) and 48.5 (11.2), respectively. These numbers can be interpreted in reference to the SF12 HRQOL’s score for US adults being normalized to mean 50 (10).
Table 2.
Confidence, Strain, Activation and Health-Related Quality of Life among Study Participants
| Scale Range | Cohort Mean (SD) | |
|---|---|---|
| Mean text response, confidence to avoid hospitalization | 1–10 | 7.7 (2.1) |
| Caregiver Reported Measures | ||
| Caregiver Strain, Global (CGSQ7) | 2–10a | 5.2 (2.2) |
| Family Caregiver Activation in Transition (FCAT) | 1–5b | 4.5 (0.6) |
| Health Related Quality of Life (SF12) Physical | 0–100 | 51.8 (7.9) |
| Health Related Quality of Life (SF12) Mental | 0–100 | 48.5 (11.2) |
Lower values indicate less strain
Higher values indicate more activation
Demographic and Clinical Associations with Confidence
Most demographic and clinical characteristics included in the study were not associated with confidence (Table 3, online only). Female caregivers and parents of children with a higher number of hospitalizations in the 12 months prior to study entry were significantly more likely to have low confidence during the study.
Table 3.
Demographic and Clinical Associations with Confidence to Avoid Hospitalization
| Low Confidencea | ||
|---|---|---|
| OR (95% CI) | p | |
| Caregiver Characteristics | ||
| Age, years | 0.92 (0.82–1.04) | 0.20 |
| Gender, Ref: Male | ||
| Female | 8.85 (1.53–51.10) | 0.015 |
| Language, Ref: English | ||
| Spanish | 0.62 (0.25–1.52) | 0.67 |
| Race/Ethnicity, Ref: non-Hispanic White | ||
| Black, non-Hispanic | 0.77 (0.08–7.86) | 0.82 |
| Hispanic | 0.14 (0.02–1.31) | 0.09 |
| Household Income (pre-tax), Ref: >$80,000 | ||
| $40,000–79,999 | 0.24 (0.02–2.68) | 0.25 |
| < $40,000 | 0.23 (0.03–1.56) | 0.13 |
| Did not answer | 0.46 (0.04–5.26) | 0.53 |
| Household Status, Ref: Married / Domestic Partnership | ||
| Single, Never-Married | 0.64 (0.07–6.26) | 0.70 |
| Divorced or separated | 1.50 (0.24–9.25) | 0.24 |
| Child Characteristics | ||
| Complex Care Clinic Enrollment Duration | 0.98 (0.96–1.01) | 0.28 |
| Affected Organ Systems | 1.46 (0.96–2.21) | 0.07 |
| Medications upon enrollment | 1.03 (0.93–1.14) | 0.60 |
| Subspecialists 12 months prior to enrollment | 1.25 (0.91–1.71) | 0.18 |
| Technology Assistance | 0.55 (0.11–2.70) | 0.46 |
| Hospitalizations 12 months prior to enrollment | 2.03 (1.22–3.36) | 0.006 |
| ED visits 12 months prior to enrollment | 1.22 (0.77–1.94) | 0.40 |
Low confidence defined as having mean confidence responses <5.
Caregiving Associations with Confidence
Among the caregiving measures of strain, activation, and HRQOL, low confidence was significantly associated with CGSQ7 strain scores and the SF12 mental component in bivariable and multivariable models (Table 4). Each additional point on the CGSQ7 was associated with 3.52 times higher adjusted odds of having low confidence (95% CI 1.45–8.54). For each additional point on the SF12 mental component (i.e., better mental HRQOL), the odds of having low confidence was 11% lower, aOR 0.89 (95% CI 0.80–0.97). Confidence was not associated with FCAT or physical SF12 scores.
Table 4.
Associations between Low Confidence and Caregiver Strain, Activation and Health-Related Quality of Life
| Low Confidencea | ||
|---|---|---|
| OR (95% CI) | aOR (95% CI)b | |
| Caregiver Strain, Global (CGSQ7) | 2.97 (1.37–6.47) | 3.52 (1.45–8.54) |
| Family Caregiver Activation (FCAT) | 0.72 (0.23–2.23) | 0.41 (0.01–1.74) |
| Health Related Quality of Life (SF12) Physical | 1.09 (0.94–1.26) | 1.14 (0.93–1.40) |
| Health Related Quality of Life (SF12) Mental | 0.91 (0.85–0.98) | 0.89 (0.80–0.97) |
Low confidence defined as having mean confidence responses <5.
Models adjusted for caregiver age, study language, child technology assistance and duration of complex care clinic enrollment.
CART modeling similarly differentiated caregiver higher strain associations with lower confidence (Figure 2). Baseline CGSQ7 strain scores ≥7.7 identified the group with the lowest confidence whose mean confidence rating was 4.4. We observed that 30% of this group’s children were hospitalized during the study period. Within this lower caregiver strain group, the CART model identified two subgroups, those with lower confidence at study entry (baseline confidence ≤7), who continued to have lower confidence (mean confidence 6.1); and those with higher confidence at study entry (baseline confidence >7), who continued to have higher confidence (mean confidence 8.9). Among both subgroups having lower strain, we observed the hospitalization rates of approximately 18%.
Figure 2. CART model depicting relationships with mean confidence to avoid hospitalization among parents of children with medical complexity.
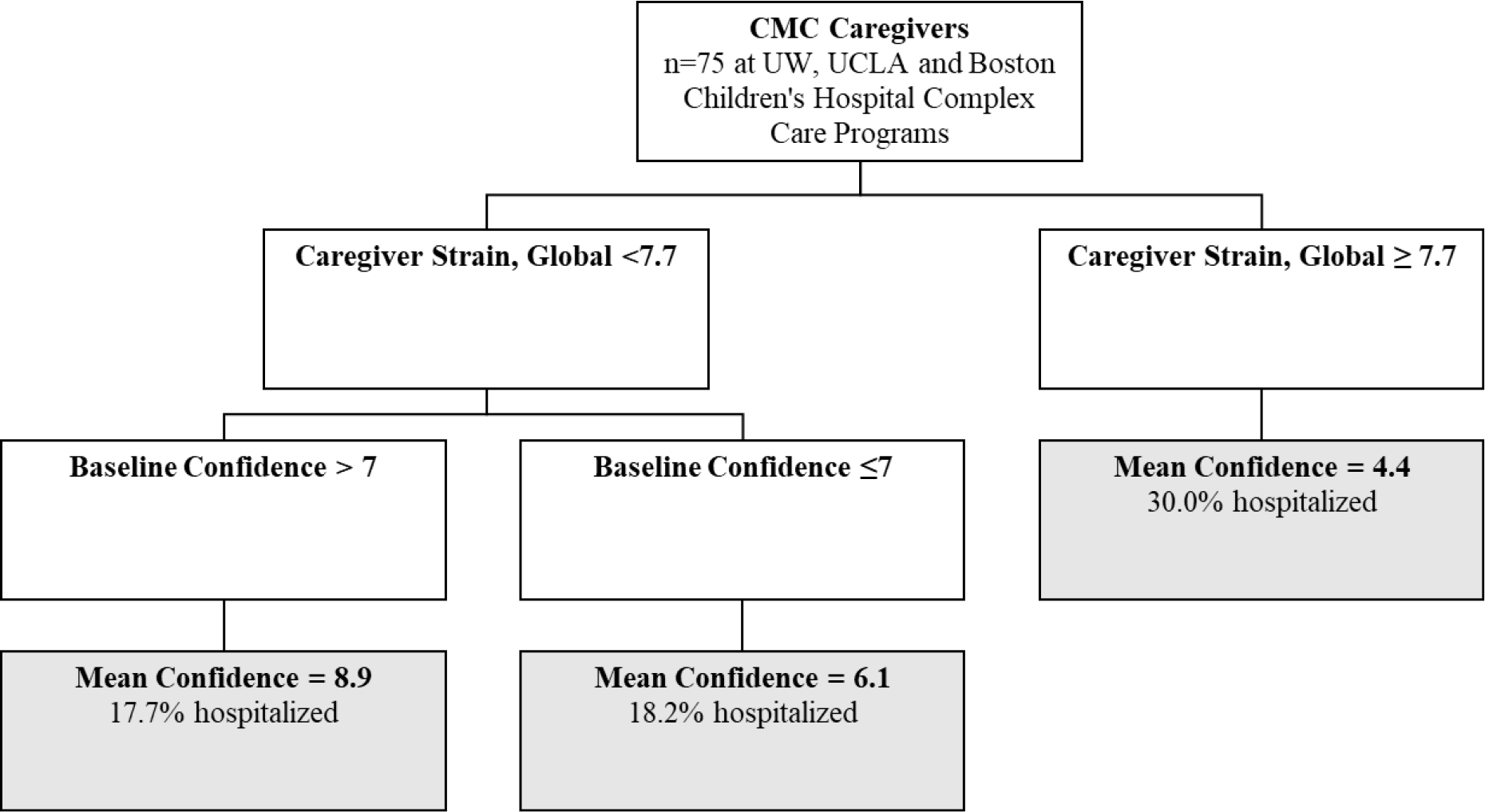
Confidence ranges from 1 (lowest) to 10 (highest), and strain ranges from 2 (lowest) to 10 (highest). Splits were significant at P < .05. CMC, children with medical complexity; UW, University of Wisconsin.
Secondary Analyses
In the first secondary analysis, differences in caregiving scale scores for those with low (compared to not low) confidence were estimated (Table 5, online only). Estimated CGSQ7 scale scores (95% CI) were 3.5 (1.2–5.9) points lower in those with low confidence. SF12 mental health component scores were 12.8 (4.9–20.6) points lower for those with low confidence. In the second analysis (Table 6, online only), children with more affected organ systems and more medications also had caregivers with more strain. The lowest estimated strain scores, CGSQ7 around 4, were observed with caregivers over 40 years or children with only 2 affected organ systems or 1 medication. No differences in strain scores were observed by caregiver gender, race/ethnicity, income, household status, or complex care program enrollment duration.
Table 5.
Differences in Strain, Activation and Quality of Life for Caregivers with Low Confidence to Avoid Hospitalization.
| Score Difference for Parents with Low Confidencea (95% CI)a | |
|---|---|
| Mean text response, confidence to avoid hospitalization | −4.5 (−5.8, −3.2) |
| Caregiver Reported Measures | |
| Caregiver Strain, Global (CGSQ7)b | +3.5 (1.2, 5.9) |
| Family Caregiver Activation in Transition (FCAT)c | −0.1 (−1.8, 1.5) |
| Health Related Quality of Life (SF12) Physical | +3.9 (−2.6, 10.3) |
| Health Related Quality of Life (SF12) Mental | −12.8 (−20.6, −4.9) |
Estimated difference in scale scores for those with low (vs not low) confidence.
Lower indicates less strain
Higher indicates more activation
Table 6.
Demographic and Clinical Variable Associations with Caregiver Strain
| Global Strain (higher=more strain) | p | |
|---|---|---|
| Estimated Score (95% CI)a | ||
| Caregiver Characteristics | ||
| Age | ||
| 20 years | 6.6 (4.5–8.6) | 0.037 |
| 40 years | 5.2 (4.0–6.3) | |
| 60 years | 4.0 (2.7–5.3) | |
| Gender | ||
| Male | 5.6 (3.7–7.6) | 0.63 |
| Female | 5.3 (4.1–6.3) | |
| Race/Ethnicity | ||
| White, non-Hispanic | 5.8 (4.5–7.0) | (ref) |
| Black, non-Hispanic | 6.0 (4.1–7.9) | 0.84 |
| Hispanic | 4.4 (3.4–5.4) | 0.11 |
| Household Income | ||
| >$80,000 | 5.9 (4.1–7.6) | (ref) |
| $40,000–79,999 | 4.5 (3.2–5.8) | 0.10 |
| < $40,000 | 4.8 (3.6–6.1) | 0.20 |
| Did not answer | 6.8 (4.6–9.0) | 0.38 |
| Household Status | ||
| Married or Domestic Partnership | 5.1 (3.9–6.3) | (ref) |
| Single, Never-Married | 5.2 (3.7–6.8) | 0.84 |
| Divorced or separated | 5.6 (4.1–7.2) | 0.44 |
| Child Characteristics | ||
| Complex Care Clinic Enrollment | ||
| 1 year | 5.2 (4.1–6.3) | 0.60 |
| 3 years | 5.1 (4.1–6.2) | |
| 5 years | 5.1 (4.0–6.1) | |
| Affected Organ Systems | ||
| 2 organ systems | 4.0 (2.9–5.1) | 0.01 |
| 5 organ systems | 4.9 (4.0–5.8) | |
| 8 organ systems | 6.2 (4.8–7.6) | |
| Medications upon enrollment | ||
| 1 medication | 4.2 (3.3–5.0) | 0.002 |
| 10 medications | 5.4 (4.5–6.2) | |
| 20 medications | 7.1 (5.3–9.0) | |
| Subspecialists 12 months prior to enrollment | ||
| 2 subspecialists | 4.1 (2.6–5.5) | 0.18 |
| 5 subspecialists | 4.8 (4.1–5.5) | |
| 8 subspecialists | 5.7 (4.6–6.8) | |
| Technology Assistance | ||
| No | 4.9 (3.6–6.2) | (ref) |
| Yes | 5.2 (4.1–6.4) | 0.61 |
| Hospitalizations 12 months prior to enrollment | ||
| 0 | 4.8 (3.8–5.8) | 0.12 |
| 2 | 5.3 (4.3–6.3) | |
| 4 | 5.9 (4.4–7.3) |
To make the relationships with caregiver strain more interpretable, random effects regression models estimated average strain scores for each demographic and clinical variable. Specific predictor values (e.g., caregiver age 20, 40, or 60 years) were selected by the authors to provide an illustrative range of values across the cohort.
DISCUSSION
This multisite study extends prior results linking low parent confidence to avoid hospitalization with hospitalization for CMC6 by identifying baseline predictors of low confidence for inclusion within our adaptive digital intervention. The results confirmed our hypothesis that CMC caregivers with high strain and low mental HRQOL had low confidence in the range associated with hospitalization; however, we observed no associations between activation or physical HRQOL and confidence. These results advance our understanding of CMC parent caregiving, including our conceptualization of confidence as a unique hospitalization risk indicator and intervention tailoring variable when fewer hospitalizations is a target outcome (Figure 2).
The study’s observation that parents of CMC had generally high activation levels despite having discernible variation in confidence to avoid hospitalization has valuable implications. Activation is typically defined as the motivation, knowledge, skills, and confidence to make effective decisions to manage health.23 The lack of association between activation and confidence in our study suggests that, despite having generally high motivation and skill, parents can still experience vulnerable periods of low confidence presumably influenced by different forces, such as demands exceeding knowledge or capacity. This fits with models of digital interventions that seek to identify tailoring variables that are associated with high value outcomes (i.e., hospitalization) but wax and wane throughout an intervention period.7,24
Our study suggests that confidence to make effective decisions generally and confidence to avoid hospitalization may be different constructs. For example, health status changes not previously experienced by the caregiver, or unexpected changes in family resources and supports, might lead to low confidence that is missed when only measuring activation. The focus of interventions triggered during periods of low confidence likely need to adapt to what is causing low confidence, and may even need to shift to focus on support services for parents. Importantly, the data suggest that general interventions to raise activation levels might have less impact on hospitalization risk than more tailored or just-in-time adaptive interventions seeking to intervene at moments of low confidence.7,24
As hypothesized, caregiving strain and mental HRQOL were tightly correlated with low confidence. The temporality of our measurement scheme was designed to evaluate how strain measured at baseline may affect confidence measured subsequently, but it is important to acknowledge that the direction of these relationships is unknown, and low confidence may actually drive strain experienced by caregivers. Although CGSQ7 has not be used in studies involving CMC, the mean and variance of global strain scores of our families were comparable to those reported by parents of adolescents with mental health illness.17 Since strain was strongly associated with confidence, we also identified variables associated with strain. Previous research on strain from related pediatric populations was consistent with our study. For example, high levels of strain have been associated with more complex or severe child functional impairment,25,26 similar to our result that strain was higher when children had more affected organ systems or daily medications. Our observation linking caregiving strain and mental HRQOL is also consistent with prior studies.27–29 For example, among caregivers of children with autism spectrum disorders, strain has been the most important predictor of caregiver mental HRQOL.30 These newly articulated associations give us opportunity to add even more precision to our future digital intervention algorithm. For instance, when families have low mental HRQOL at the outset of the study, it may be appropriate to deliver a brief series of sessions of supportive parental counseling in addition to our intervention targeting the child’s physical health.
Since prior studies have also underscored links between unmet needs, poorer social support, family functioning, and maladaptive coping as predictors of strain,25,31,32 it was notable that our results did not observe significant caregiver or family associations with strain. For example, strain was not associated with income, household structure, activation, or other caregiver characteristics, though this study may have been underpowered to detect these relationships. While we did not identify racial/ethnic disparities in strain, this should continue to be studied as inequities in strain have been described.33,34
Better understanding the longitudinal and dynamic patterns of confidence would advance our understanding about how confidence is influenced by, or influences key health outcomes related to CMC caregiving. The variance in our confidence data highlights not only that mean confidence differs across the sample, but also that meaningful variation in confidence may occur within the same individual over time. The design of our study limited more sophisticated analyses of confidence trajectories; however, prior research has shown that not only an individual’s average levels of self-efficacy but also the variability in those levels predict psychological strain and coping in the face of demands.35 We speculate that variation in an individual’s confidence to avoid hospitalization may reflect differences in the demands placed on the family due to changes with the child’s health or the supports at the family’s disposal.36 For example, families who reported stable high confidence might experience the strain associated with the changing demands of their child’s fluctuating health differently than those whose confidence is less stable, even if their average confidence is similar. Better understanding patterns in confidence, and the longitudinal relationships between confidence, caregiving strain, and health services outcomes may help us understand mechanisms underlying adaptive coping in CMC caregiving and how to optimize outcomes of care delivered by CMC families.
This study’s findings must be interpreted with several limitations in mind. The observational design prohibits us from determining causality or the directions of identified relationships. The study duration and power was limited, which precluded us from evaluating these constructs over longer time horizons or exploring unique patterns. The measures used in our study have unknown psychometric properties in our study population. We also note that high activation in our study could have occurred by recruiting families enrolled in complex care programs, potentially selecting for more activated families or because the programs themselves increased activation.
It would be informative for future research to confirm our findings with activation measures developed specifically for this population and applied amongst CMC caregivers in different circumstances, such as less experience caring for a CMC. Although we recruited from three geographically diverse complex care programs, the findings may not generalize to CMC and families in different regions. Additionally, resource constraints only supported enrollment of Spanish-speaking families at one site, and findings may not generalize to other Spanish-speaking communities. In our study, confidence appears to be dynamic for many individuals, and future studies which measure stress and stress triggers more frequently are needed to characterize whether these constructs are similarly dynamic.
Despite these limitations, this multisite study provides valuable insights about caregiving for CMC. The findings underscore that many caregivers of CMC self-perceive high levels of motivation and skill, despite important subsets experiencing substantial strain associated with low confidence for their child to avoid hospitalization. Although complex care programs typically aim to overcome challenges in daily caregiving, and appear to reduce hospitalizations,37,38 it is not yet known whether enrollment into these programs improves parent confidence, strain, activation, or HRQOL, or what services could be provided that would achieve such gains. The associations between strain, mental HRQOL, and confidence to avoid hospitalization in this population suggests that these are potentially high-yield targets for future adaptive intervention design and testing. For example, our team is now conducting a pilot randomized clinical trial pairing action planning9 and a just-in-time clinical response when families report low confidence by text message. Such intervention activities could foreseeably be used in both general pediatric and complex care settings. Measuring the constructs from this study through this trial, and continuing to iterate intervention designs12 based on what we learn from subsequent studies, will help uncover effective pathways to prevent CMC hospitalization.
Supplementary Material
ACKNOWLDGEMENTS:
The authors would like to thank Drs. Ed Schor and Chris Stille for their thoughtful comments on an earlier draft of this manuscript.
Funding Source:
This project is supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under UA6MC31101 Children with Special Health Care Needs Research Network. Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R34HL153570. The project was also supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government.
Footnotes
Financial Disclosure: All authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: All authors have no conflicts of interest to disclose.
Clinical Trial Registration: None
REFERENCES
- 1.Nelson BB, Coller RJ, Saenz AA, et al. How Avoidable are Hospitalizations for Children With Medical Complexity? Understanding Parent Perspectives. Academic pediatrics. 2016;16(6):579–586. [DOI] [PubMed] [Google Scholar]
- 2.Romley JA, Shah AK, Chung PJ, Elliott MN, Vestal KD, Schuster MA. Family-provided health care for children with special health care needs. Pediatrics. 2017;139(1). [DOI] [PubMed] [Google Scholar]
- 3.Schall TE, Foster CC, Feudtner C. Safe Work-Hour Standards for Parents of Children With Medical Complexity. JAMA Pediatr. 2020;174(1):7–8. [DOI] [PubMed] [Google Scholar]
- 4.Coller RJ, Klitzner TS, Saenz AA, Lerner CF, Nelson BB, Chung PJ. The Medical Home and Hospital Readmissions. Pediatrics. 2015;136(6):e1550–1560. [DOI] [PubMed] [Google Scholar]
- 5.Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coller RJ, Lerner CF, Berry JG, et al. Linking Parent Confidence and Hospitalization through Mobile Health: A Multisite Pilot Study. J Pediatr. 2021;230:207–214 e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine. 2018;52(6):446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michie S, Van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation science. 2011;6(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care Hospital Use and Postdischarge Coaching: A Randomized Controlled Trial. Pediatrics. 2018;142(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coller RJ, Nelson BB, Klitzner TS, et al. Strategies to Reduce Hospitalizations of Children with Medical Complexity through Complex Care: Expert Perspectives. Acad Pediatr. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing Hospitalizations in Children With Medical Complexity: A Systematic Review. Pediatrics. 2014;134(6):e1628–e1647. [DOI] [PubMed] [Google Scholar]
- 12.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2015;34(10):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nackers A, Ehlenbach M, Kelly MM, Werner N, Warner G, Coller RJ. Encounters From Device Complications Among Children With Medical Complexity. Hosp Pediatr. 2019;9(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry JG, Agrawal R, Kuo DZ, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159(2):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coller RJ, Klitzner TS, Saenz AA, et al. Discharge Handoff Communication and Pediatric Readmissions. Journal of hospital medicine. 2017;12(1):29–35. [DOI] [PubMed] [Google Scholar]
- 16.Bandura A Guide for Constructing Self-Efficacy Scales. In: Pajares F, Urdan T, eds. Self-Efficacy Beliefs of Adolescents. Greenwich, CT: Information Age Publishing; 2006. [Google Scholar]
- 17.Brannan AM, Athay MM, de Andrade AR. Measurement Quality of the Caregiver Strain Questionnaire-Short Form 7 (CGSQ-SF7). Adm Policy Ment Health. 2012;39(1–2):51–59. [DOI] [PubMed] [Google Scholar]
- 18.Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27(5):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman EA, Ground KL, Maul A. The Family Caregiver Activation in Transitions (FCAT) Tool: A New Measure of Family Caregiver Self-Efficacy. Jt Comm J Qual Patient Saf. 2015;41(11):502–507. [DOI] [PubMed] [Google Scholar]
- 20.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 21.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. Journal of clinical epidemiology. 1998;51(11):1171–1178. [DOI] [PubMed] [Google Scholar]
- 22.Wray CM, Byers AL. Methodological Progress Note: Classification and Regression Tree Analysis. Journal of hospital medicine. 2020;15(9):549–551. [DOI] [PubMed] [Google Scholar]
- 23.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health services research. 2004;39(4p1):1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spruijt-Metz D, Nilsen W. Dynamic models of behavior for just-in-time adaptive interventions. IEEE Pervasive Computing. 2014;13(3):13–17. [Google Scholar]
- 25.Green AL, Kutash K, Duchnowski AJ. Strain in caregivers of students with emotional and behavioral disorders receiving school-based services. School Mental Health. 2016;8(4):441–451. [Google Scholar]
- 26.Bradshaw J, Gillespie S, McCracken C, et al. Predictors of Caregiver Strain for Parents of Children with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litzelman K, Skinner HG, Gangnon RE, Nieto FJ, Malecki K, Witt WP. The relationship among caregiving characteristics, caregiver strain, and health-related quality of life: Evidence from the Survey of the Health of Wisconsin. Quality of Life Research. 2015;24(6):1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litzelman K, Skinner HG, Gangnon RE, Nieto FJ, Malecki K, Witt WP. Role of global stress in the health-related quality of life of caregivers: evidence from the Survey of the Health of Wisconsin. Quality of Life Research. 2014;23(5):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raina P, O’Donnell M, Rosenbaum P, et al. The health and well-being of caregivers of children with cerebral palsy. Pediatrics. 2005;115(6):E626–E636. [DOI] [PubMed] [Google Scholar]
- 30.Khanna R, Madhavan SS, Smith MJ, Patrick JH, Tworek C, Becker-Cottrill B. Assessment of health-related quality of life among primary caregivers of children with autism spectrum disorders. Journal of autism and developmental disorders. 2011;41(9):1214–1227. [DOI] [PubMed] [Google Scholar]
- 31.Khanna R, Madhavan SS, Smith MJ, Tworek C, Patrick JH, Becker-Cottrill B. Psychometric properties of the Caregiver Strain Questionnaire (CGSQ) among caregivers of children with autism. Autism : the international journal of research and practice. 2012;16(2):179–199. [DOI] [PubMed] [Google Scholar]
- 32.Stuart M, McGrew JH. Caregiver burden after receiving a diagnosis of an autism spectrum disorder. Research in autism spectrum disorders. 2009;3(1):86–97. [Google Scholar]
- 33.Shin SH, Brown TA. Racial and ethnic disparities in caregiver strain and the use of child mental health services: a structural equation model. Psychiatric services (Washington, DC). 2009;60(8):1039–1045. [DOI] [PubMed] [Google Scholar]
- 34.McCabe KM, Yeh M, Lau A, Garland A, Hough R. Racial/ethnic differences in caregiver strain and perceived social support among parents of youth with emotional and behavioral problems. Mental health services research. 2003;5(3):137–147. [DOI] [PubMed] [Google Scholar]
- 35.Peng AC, Schaubroeck JM, Xie JL. When confidence comes and goes: How variation in self-efficacy moderates stressor–strain relationships. Journal of occupational health psychology. 2015;20(3):359. [DOI] [PubMed] [Google Scholar]
- 36.Lang FR, Featherman DL, Nesselroade JR. Social self-efficacy and short-term variability in social relationships: The MacArthur Successful Aging Studies. Psychology and Aging. 1997;12(4):657. [DOI] [PubMed] [Google Scholar]
- 37.Bergman DA, Keller D, Kuo DZ, et al. Costs and Use for Children With Medical Complexity in a Care Management Program. Pediatrics. 2020;145(4). [DOI] [PubMed] [Google Scholar]
- 38.Mosquera RA, Avritscher EB, Samuels CL, et al. Effect of an enhanced medical home on serious illness and cost of care among high-risk children with chronic illness: a randomized clinical trial. Jama. 2014;312(24):2640–2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


