Abstract
The slightest change in the extra/intracellular concentration of metal ions results in amplified effects by signaling cascades that regulate both cell fate within the tumor microenvironment and immune status, which influences the network of antitumor immunity through various pathways. Based on the fact that metal ions influence the fate of cancer cells and participate in both innate and adaptive immunity, they are widely applied in antitumor therapy as immune modulators. Moreover, nanomedicine possesses the advantage of precise delivery and responsive release, which can perfectly remedy the drawbacks of metal ions, such as low target selectivity and systematic toxicity, thus providing an ideal platform for metal ion application in cancer treatment. Emerging evidence has shown that immunotherapy applied with nanometallic materials may significantly enhance therapeutic efficacy. Here, we focus on the physiopathology of metal ions in tumorigenesis and discuss several breakthroughs regarding the use of nanometallic materials in antitumor immunotherapeutics. These findings demonstrate the prominence of metal ion-based nanomedicine in cancer therapy and prophylaxis, providing many new ideas for basic immunity research and clinical application. Consequently, we provide innovative insights into the comprehensive understanding of the application of metal ions combined with nanomedicine in cancer immunotherapy in the past few years.
Graphical Abstract
Keywords: Metal ions, Nanometallic materials, Cancer immunotherapy, Nanotechnology, Tumor microenvironment
Introduction
Because of its high mortality rate and limited treatment modalities, cancer has become a public health threat. As a result, some novel cancer therapies, including immunotherapy, photodynamic therapy (PDT), and photothermal therapy (PTT), are emerging in clinical practice and trials [1]. Cancer immunotherapy has attracted increasing attention due to its ability to induce powerful immune activation and persistent immune memory [2]. As many immune modulators or activators have been investigated, metal ions have demonstrated their superiority with high efficiency and ideal biocompatibility [3].
Metal ions can serve as elements that improve anticancer immunity and exert the function of cancer clearance. With the deepening understanding of the function of metal ions in cancer immunotherapy, a new term, “metalloimmunology”, was proposed in 2020 by Jiang et al. [4], and “cancer metalloimmunotherapy” was subsequently described by James J. et al. in 2021 [5]. The fewer side effects, feasible accumulation process and relative sensitivity to cancer cells indicate the advantages of metal ions for cancer immunotherapy.
In recent years, the development of nanotechnology has provided novel opportunities for the clinical application of tumor immunotherapy [6]. Multifunctional nanocarriers for tumor immunotherapy show various advantages, such as targeted delivery of immune activators to immune cells, thermal sensitivity systems of PTT/PDT, and reduction of side effects [7].
Nanometallic materials have become a promising delivery system because of their nanocrystalline strengthening effect, high photoabsorptivity, high surface energy, and single magnetic region performance [8]. Simultaneously, nanoparticulate delivery systems (NDs) are widely applied in tumor treatment because of their excellent efficiency [9].
Since the systemic application of metal ions may have toxic side effects [10], functionalized nanoparticles are needed to carry various metal ions directly into target cells. Some metal nanoprobes inherently reflect local and systemic information, which allows the integration of nanodelivery and nanobioimaging technologies in cancer metalloimmunotherapy [6, 11]. This combination of “nanometalloimmunotherapy” shows significant potential for facilitating precise drug delivery and synergistic effects [11, 12]. Moreover, nanometalloimmunotherapy overcomes the inherent limitations of traditional immunotherapy [13] and the drawbacks of metal ion-based antitumor therapeutics, including the short circulation time, low target selectivity, and systematic toxicity [14], exhibiting extraordinary practical potential by eliminating barriers to immunology and other fields [15].
The general function of metal ions in tumorigenesis and antitumor immunity
Metal ions are essential for cellular life because they participate in many fundamental biological processes, such as membrane excitability, signal transduction, metalloprotein catalysis, and cell death [16]. A slight change in their concentration or cellular compartments may elicit metabolic dysfunction and disrupt the homeostasis of metal ions [17, 18]. Since the late 1980s, the application of metal ions has become especially prominent in cancer treatment because they participate in many cancer hallmarks, such as unrestricted proliferation, evasion of apoptosis, tissue invasion, and metastasis [19–22].
In addition to directly influencing cancer cell metabolism, metal ions contribute to cancer therapy by regulating hypoxia in the tumor microenvironment (TME) [7, 23–25]. The TME is characterized by a lower pH and higher levels of glutathione (GSH) and hydrogen peroxide (H2O2), which leads to the accumulation of immunosuppressive cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [26, 27]. Metal ions can reverse the low response of conventional cancer immunotherapy by inducing redox reactions serving as reducing agents with simultaneous oxygen production [14, 28, 29].
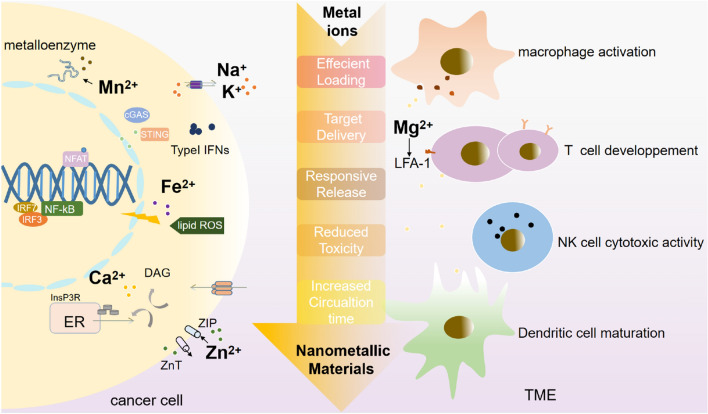
At the same time, metal ions are essential for the activation and proliferation of immune cells in the TME [30]. On the one hand, metal ions can promote innate immunity by enhancing the presentation capacity of dendritic cells (DCs) and macrophages and the cytotoxicity of natural killer (NK) cells [31–33]. On the other hand, metal ions can stimulate the activation and proliferation of adaptive immune cells, including CD8+ T cells (Fig. 1) [34], which exert a dominant function in antitumor immunity [35].
Fig. 1.
Metabolism of metal ions in cancer cells related to tumorigenesis
In the TME, the metabolism of metal ions in cancer cells and immune cells plays a major role in the development and metastasis of cancer. For instance, colon cancer cells display enhanced store-operated Ca2+ entry (SOCE), whose molecular players include ORAI1 and TRPC1 channels and stromal interacting molecules (STIMs) 1 and 2. In addition to abnormal growth, cancer cells resist cell death, such as ferroptosis, in a process involving metal ions. The disturbed signaling network of metal ions determines the features of cancer cells and their surroundings and supports the formation of tumor-associated macrophages (TAMs) and the dysfunction of lymphocytes.
Metal ions participate in innate and adaptive immune activation in cancer
Metal ions with innate immune activation ability in the TME
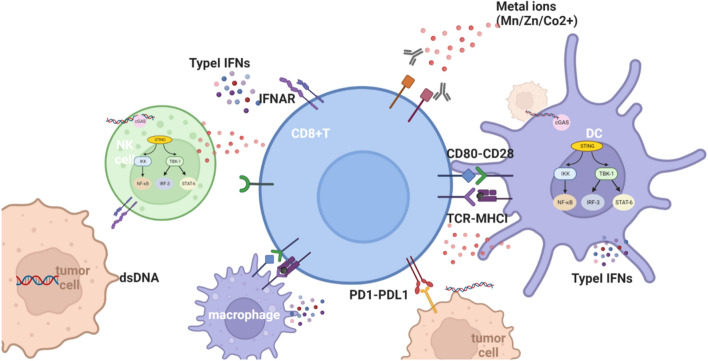
Innate immunity involves a wide range of immune cells, such as DCs, macrophages, and NK cells [36]. Although innate immune cells can detect tumors and induce and amplify adaptive immune responses, the immunosuppressive microenvironment at the tumor bed represses their functions [37]. Metal ions have been revealed to promote the pathogen‒host interaction and activate the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway and NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasomes, which can serve as innate immunity activators and boost anticancer immunity.
Fe2+/Zn2+/Cu2+ modulates the pathogen‒host interaction
Dysbiosis is considered a contributing factor in the origin and development of colon cancer because disturbed host-microbe interactions may lead to chronic inflammation. Metal ions have been confirmed to be essential for intestinal inflammation recovery [38, 39].
Mouse experiments have revealed that intestinal stimulation by microbes can induce type 2 conventional DCs (cDC2s) to release hepcidin, which is a key regulator of systemic Fe2+ homeostasis. At the same time, cDC2s play a dominant role in human intestinal inflammation and local mucosal repair by promoting ferroportin-mediated Fe2+ sequestration from intestinal macrophages that have phagocytosed erythrocytes [40]. Colorectal cancers (CRCs) often result in intestinal bleeding and hence anemia, and limited Fe2+ levels in the intestinal lumen can reduce tissue gut infiltration and therefore promote intestinal repair [41, 42].
Zn2+ is an essential but toxic microelement in high concentrations and can serve as an antimicrobial strategy for Mycobacterium [43, 44], which can provoke chronic inflammation related to cancer pathogenesis [45]. Meanwhile, the P1B-type ATPase CtpG (Rv3270) was recently identified as a Zn2+ efflux transporter via the CmtR-CtpG-Zn2+ regulatory pathway that enhances mycobacterial resistance to Zn2+ toxicity because the accumulation of Zn2+ can contribute to ROS detoxification in mycobacterial cells [46].
The intestinal microbiota can also affect the therapeutic effects of antineoplastic agents, such as disulfiram, whose anticancer effect is enhanced by combining with antibiotics and Cu2+ by significantly reducing the expression of phosphorylated protein kinase B (p-AKT)/protein kinase B (AKT), Toll-like receptor 4 (TLR-4), and phosphorylated nuclear factor kappa-B (p-NFκB)/NFκB in tumors [39].
Zn2+/Mn2+/Co2+ activates the cGAS-STING signaling pathway
The stimulator of interferon genes (STING) pathway has been proven to play critical roles in the initiation of antitumor immunity. In particular, the STING pathway can ameliorate the immunosuppressive network in “cold” tumors [37, 38]. In brief, cyclic GMP-AMP synthase (cGAS) detects damage-associated double-stranded DNA (dsDNA) in the cytosol and catalyzes the generation of cyclic GAMP (cGAMP), which serves as the second messenger to activate STING and induce type-I interferons (IFNs) [47, 48]. As a result of chromosomal instability, cytosolic dsDNA in cancer cells elicits cancer immunogenicity via cGAS-STING pathway activation, which originates primarily from the vulnerable membrane of micronuclei [49]. To escape from the suppressive signaling axis, cancer cells inhibit the cGAS-STING pathway by reducing the expression of cGAS and STING and co-opting STING-dependent DNA sensing [50].
In addition to abnormal dsDNA, some metal ions, such as Mn2, Zn2+, and Co2+, have been recently shown to be activators of the cGAS-STING pathway [51, 52]. In 2018, J. Chen et al. proved that Zn2+, Mn2+ and Co2+ can significantly promote the combination activity of cGAS in vitro, which could induce DNA-induced cGAS phase separation even at low concentrations [53]. Moreover, Zn2+ can largely enhance cGAS activity by binding to cGAS and stabilizing the cGAS-DNA complex [47].
Wang et al. revealed that Mn2+ can replace Mg2+ as a cofactor and markedly enhance activation of the cGAS-STING signaling axis not only by sensitizing cGAS and its adaptor STING but also by largely increasing the STING-cGAMP binding affinity [53]. In addition, an increasing number of studies have shown that Mn2+ can potentiate the STING pathway by amplifying cGAS-STING recognition in immune cells via a direct interaction with tumor cells mediated by Mn2+ [53]. Additionally, Mn2+ promotes cross-presentation between DCs and CD8+ T cells and the cytotoxic function of NK cells and cytotoxic T lymphocytes (CTLs). Among cancer patients with multidrug resistance or advanced metastatic solid tumors, Mn2+ rescues the clinical efficiency of some cancer treatments such as PD-1/PD-L1 therapy and greatly improves their prognosis [51, 54] (Fig. 2).
Fig. 2.
Mn2+/Zn2+/Co2+ is indispensable for host defense triggered by cytosolic dsDNA, which activates the cGAS-STING signaling axis and produces type-I IFNs
Mn2+, Zn2+ and Co2+ can contribute to cGAS binding to STING at the endoplasmic reticulum (ER), and this complex translocates from the ER to Golgi compartments. STING serves as a signaling platform for TANK-binding kinase 1 (TBK1) and IkappaB kinase (IKK). TBK1 phosphorylates STING, which in turn recruits interferon regulatory factor 3 (IRF3) for TBK1-mediated phosphorylation, which stimulates the transcriptional expression of IFNs and other immune-stimulatory genes.
Ca2+/K+/Na+ activates the NLRP3 inflammasome
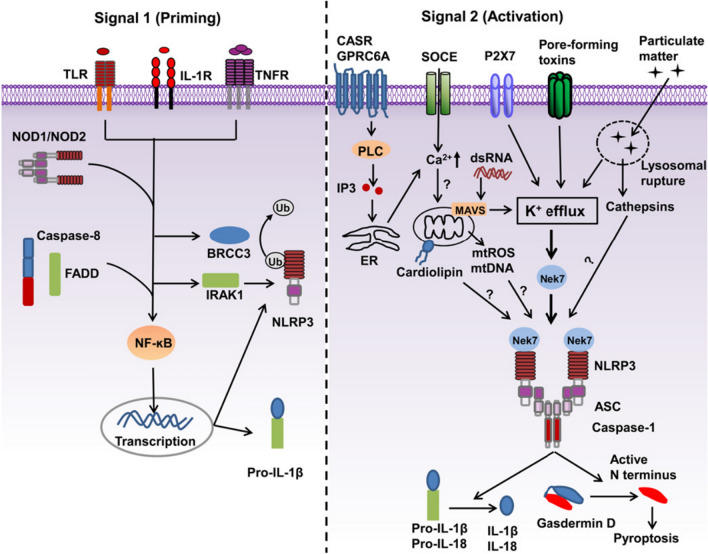
Chronic inflammation and persistent infection contribute to different malignancies, such as melanoma, since some inflammasomes, including NLRP3 inflammasomes, have a pathogenic role in triggering a broad range of cellular perturbations, such as damage-associated molecular patterns (DAMPs) [55]. NLRP3 inflammasomes provoke the release of the pro-inflammatory cytokines IL-1β and IL-18 in the foundation of caspase-1, which is considered as a promoting factor for cancer development, invasion, metastasis and chemoradioresistance [56, 57]. Metal ions such as Ca2+/K+/Na+ can activate the NLRP3 inflammasome and caspase-1, thereby inducing high levels of bioactive IL-1β and tumorigenesis [58, 59].
The increase in [Ca2+]e detected by monocytes can activate the phosphatidyl–inositol/Ca2+ pathway, which in turn leads to NLRP3 activation [60, 61]. At the same time, as Ca2+ uptake through the mitochondrial Ca2+ uniporter (MCU) can enhance the phagocytosis-dependent NLRP3 inflammatory response, the amplified release of IL-1β aggravates tissue damage via inefficient inflammatory pathways induced by mitochondrial DAMPs, which promotes neoplastic disorders [62, 63].
NLRP3 activation is also determined by membrane permeability to K+ and Na+. In particular, the reduction in [K+]i is necessary and sufficient for caspase-1 activation [57]. K+ efflux is widely accepted as a conjoint point in the activation of the NLRP3 inflammasome since internalization by phagocytosis can induce lysosomal membrane damage and trigger the opening of membrane pores permeable to K+ [59, 63]. In addition, Na+ influx can modulate NLRP3 activation independent of K+ efflux but is not an absolute requirement. Recently, the correlation of enhanced epithelial sodium channel (ENaC)-dependent Na+ influx with exacerbated NLRP3 inflammasome activation was observed in a monogenic disease accompanied by increased K+ efflux [58] (Fig. 3).
Fig. 3.
A two-signal model for NLRP3 inflammasome activation [55] @Copyright 2022, Elsevier Ltd
Metal ions with adaptive immune activation ability in the TME
Adaptive immune responses are crucial for the recognition and elimination of infective and neoplastic cells [37]. Homeostasis of metal ion metabolism is essential for the differentiation and function of immune cells, especially for the main adaptive immune effector CD8+ T cell [64]. Considering that CD8+ T-cell exhaustion within the TME usually causes a low response to cancer immunotherapy [26], the regulation of metal ions contributes to the infiltration of CD8+ T cells, thus reversing local immunosuppression [65].
K+ is crucial for CD8+ T-cell function and stemness
Studies have shown that the increase in extracellular K+ impairs Akt-mTOR phosphorylation driven via TCR and facilitates subsequent effector processes, which are dependent on the activity of the serine/threonine phosphatase PP2A [65, 66]. Overexpression of the K+ channel Kv1.3 in melanoma-bearing mice can increase K+ efflux in tumor-specific T cells and enhance effector functions, thus promoting tumor clearance. The elimination of K+ from T cells restores their ability to attack cancer [67]; as a result, K+ can act as an ionic checkpoint blocking T-cell function in cancer immunotherapy.
Recently, an increasing concentration of K+ in the TME has been proven to promote the persistence and stemness of tumor-infiltrating lymphocytes (TILs) through functional caloric restriction, which promotes autophagy and metabolic reprogramming [68]. Increased [K+]e can selectively induce mitochondrial acetyl-CoA (AcCoA) synthetase 1, which drives metabolically abundant oxygen utilization for antitumor T cells [69]. As a result, K+ efflux improves T-cell persistence in tumor-bearing mice, which enhances tumor clearance and mouse survival time [70]. The increase in [K+] in the TME can reversibly and durably cultivate the expansion of T cells in vitro, indicating its application potential as an adoptive immunotherapy modulator.
Mg2+ regulates CD8+ T-cell effector function via LFA-1
Leukocyte function-associated antigen 1 (LFA-1) is an integrin that participates in T cell activation via immune synapse formation as well as in leukocyte trafficking and extravasation [71]. LFA-1 conformational changes are mediated by the T cell antigen receptor (TCR) signal stimulation, which is regulated by the binding of Mg2+ to metal-ion-dependent adhesion sites (MIDAS) [72]. This demonstrates that Mg2+ can affect T cell function by modulating proximal and distal signaling activity, such as focal adhesion kinase (FAK) and extracellular signal-regulated protein kinase 1/2 (ERK1/2) phosphorylation, respectively [73].
Mechanically, the extension and conformational opening of LFA-1 is regulated by Mg2+ on activated T cells, which is essential for FAK phosphorylation, calcium flux, and downstream effector functions in T-cell blasts [74]. As Mg2+ is an essential cofactor for DNA damage repair enzymes, deficiency of Mg2+ results in the accumulation of DNA damage and contributes to cancer progression. A low concentration of Mg2+ is related to TCR signal suppression, which inhibits T-cell proliferation and contributes to T-cell exhaustion [65].
Ca2+ promotes CD8+ T-cell activation by CD3 phosphorylation
Ca2+ can bind directly to anionic phospholipids, serving as a modulator for membrane protein function. The ionic interactions between positively charged CD3 cytoplasmic domains and negatively charged phospholipids in the plasma membrane regulate the activation of TCR. Since increasing the [Ca2+]i concentration can induce the dissociation of CD3 from the membrane and the solvent exposure of tyrosine residues, CD3 tyrosine phosphorylation can be significantly enhanced by Ca2+ influx. Instead of initiating CD3 phosphorylation, this Ca2+ regulatory pathway can amplify and sustain CD3 phosphorylation, which enhances T-cell sensitivity to some antigens, such as major histocompatibility complex (MHC) [75].
Ca2+ influx can increase the accessibility of the immunoreceptor tyrosine-based activation motifs (ITAMs) in the CD3 cytoplasmic domains to lymphocyte-specific protein tyrosine kinase (Lck) phosphorylation and can thus trigger the signaling cascade to activate T cells. The robust Ca2+ influx can compete with phospholipids to help CD3 ITAMs release from the inner leaflet of the plasma membrane. TCR signal transduction is crucial to T-cell activation in immune responses; therefore, the participation of Ca2+ in T-cell-engaging therapies deserves to be further explored [76, 77].
The application of metal ion-based antitumor therapeutics and the perspective of nanometallic materials in cancer immunotherapy
Metal ions exert pivotal functions in cancer immunology by modulating cellular metabolism and the TME, which enhance the efficacy of cancer immunotherapy and demonstrate their important clinical potential through delicate mechanisms (Table 1).
Table 1.
The important roles of metal ions in antitumor immunology
| Metal ion | Location | ↑↓ | Functions | Mechanisms | Applications | Refs. |
|---|---|---|---|---|---|---|
| K+ | TME | ↑ | T-cell effector dysfunction; CD8+ T-cell stemness preservation | Functional caloric restriction; autophagyautophage↑; effector programs↓ | Adoptive cell transfer therapy | [68] |
| Mn2+ | TME | ↑ | NK cell activation; DC function↑; CD8+ T proliferation↑ differentiation↑; M1 polarization | Sensitizes cGAS and its adaptor STING; STING-cGAMP binding affinity; type-I IFN ↑ | Innate immunity activator | [5, 51] |
| Mg2+ | TME | ↑ | T-cell activation; T-cell effector function↑; T-cell cytotoxicity↑; Mg2 + influx acts as second messenger in TCR signaling | Increases LFA-1 outside-in signaling; directly interacts with IL-2-inducible T-cell kinase (ITK) promoting its activation | Combined with PD-1 blockade; Target Mg2+ transporters | [73, 96] |
| Fe2+/3+ | Cancer cells | ↑ | Immunotherapy-activated CD8+ T cells enhance ferroptosis-specific lipid peroxidation in cancer cells | CD8+ T cells secrete IFNγ; downregulates the expression of SLC3A2 and SLC7A11 | T-cell-promoted tumor ferroptosis + checkpoint blockade | [97] |
| Ca2+ | CD8+ T cells | ↓ | Activation of CD8+ T cells and NLR3 inflammasomes; NK cells function↑ | CD3 phosphorylation↑; TCR signal transduction; T-cell sensitivity↑ | Store-operated Ca2+ entry (SOCE) block | [98, 99] |
| Zn2+ | T lymphocytes | ↑ | Boosts immune functions; targets T-cell metabolism; immune surveillance | Increases in T -cell receptor-derived excision circles (TRECs) and CD4+ naïve lymphocytes | Zinc supplementation | [100] |
| Cu2+ | Cancer cells | ↓ | Modulates PD-L1 expression; cancer immune evasion; CD8+ T, NK cells↑ | Inhibits phosphorylation of STAT3 and epidermal growth factor receptor (EGFR); promotes ubiquitin-mediated degradation of PD-L1 | Copper chelators | [101] |
| Pt2+ | TME | ↑ | Induces increases in antigen processing machinery (APM) component expression; upregulation of immune checkpoints or impairment of T-cell function | Enhances antigen presentation and T-cell killing; increases tumor cell expression of PD-L1; impair T-cell function | Cisplatin; combination with immune checkpoint antibody | [102, 103] |
| Au+/3+ | Immune cells | ↑↓ | Stimulates the activation and proliferation of T as well as B cells; promotes the T-cell-based anticancer immunity cycle via DC | Activates TLR3 signaling; stimulates immune cells to secrete key inflammatory cytokines; inhibits the DNA binding activity of NF-κB | Aurothioglucose; Auranofin; sodium aurothiomalate; HAuCl4 [Au(III)]; gold sodium thiomalate | [94] |
↑: upregulation; ↓: downregulation
Traditional therapy: Metallodrugs participate in antitumor immunity
Cis-diamminedichloroplatinum(II), known as cisplatin (Pt(NH3)2Cl2), is a first-line medicine for a broad range of cancers, such as lung, head, and neck cancer [78], and, which induces tumor-specific cytotoxicity based on structural lesions [79]. In addition to the induction of tumor cell apoptosis, cisplatin has been proven to optimize immune checkpoint blockade by increasing PD-L1 expression in non-small cell lung cancer [80], which indicates that cisplatin-based neoadjuvant chemotherapy could improve the clinical effectiveness of PD-1/PD-L1 treatment.
Sustained exposure to MnCl2 enhances humoral immunity. Nearly 30 years ago, intraperitoneal injection of MnCl2 was shown to enhance the activity of murine NK cells, which is probably mediated by the production of type I IFNs [81]. In addition, intratumor injection of MgCl2 was recently proven to enhance cellular immunity by regulating CD8+ T-cell activation and cytotoxicity [73]. In addition, magnesium supplementation has the potential to combat cisplatin resistance [82] and to modulate inflammatory factors, such as tumor necrosis factor (TNF-α) [83].
It was considered that sustained exposure to MnCl2 enhances humoral immunity. Nearly 30 years ago, intraperitoneal injection of MnCl2 was shown to enhance the activity of murine NK cells, which is probably mediated by the production of type I IFNs. In the meanwhile, intratumor injection of MgCl2 is recently proved to enhance the cellular immunity by shaping CD8+ T cells activation and cytotoxicity, meanwhile, magnesium supplementation has the potential of cisplatin resistance repair as well as inflammation factors modulation, like TNF-α.
Targeted therapy: Metal ion channels and transporters regulate the TME
“Oncochannelopathy” is a term that was first proposed in 2018 by Prevarskaya et al. and refers to cancer hallmarks viewed as pathological states that are mainly caused by abnormal expression and/or dysfunction of certain ion channels [19]. Metal ion homeostasis and metabolism are highly regulated by distinct ion channels. Owing to the significant functions of metal ion channels in tumorigenesis and metastasis, many novel anticancer therapies have emerged that target metal ion transporters as agonists or inhibitors [84].
For Zn2+, the SLC39A (zrt/irt-like proteins; ZIP) family and SLC30A (cation diffusion; ZnT) Zn2+ family are widely investigated in cancer immunotherapy. Zinc transporters play an important role in B-cell development at different stages; for instance, SLC39A10 deficiency leads to a reduced population of both pro- and pre-B cells, and, SLC39A7 deficiency is a negative regulator of phosphatases with impaired BCR-dependent signaling in pre- and immature B cells [85, 86]. In addition, SLC39A6 in DCs and T cells can indirectly activate the TCR activation pathway, which leads to cell proliferation and cytokine production [87].Ladiratuzumab vedotin is a new drug of antibody drug conjugates (ADCs) targets for of SLC39A6, which is under evaluation in an ongoing open-label phase Ib/II trial.
SLC41A1 and SLC41A2 are two key transporters expressed in lymphoid cell lines and various immune cells, respectively [87]. SLC41A functions as a Mg2+/Na+ exchanger whose overexpression could partially repair the deletion of other Mg2+-permeable ion channels, such as TRPM7 (transient receptor potential cation channel subfamily M member 7), thus rescuing the reduction in cell growth and maintaining the normal growth of lymphocytes [88].
Pyroptosis/ferroptosis/cuproptosis: Metal ions induce nonapoptotic cell death
Pyroptosis is a special type of inflammatory cell death that evokes a proinflammatory signal and stimulates tumor immunogenicity via gasdermin D (GSDMD). The first metal complex-based pyroptosis inducer was recently reported to be carbonic anhydrase IX (CAIX)-anchored ruthenium(I) photosensitizer CA-Re, which promoted the maturation and antigen-presenting ability of DCs and activated the T-cell-dependent adaptive immune response [89].
Ferroptosis is a type of Fe2+-dependent cell death that damages polyunsaturated fatty acid-containing phospholipids in cellular membranes mediated by lipid peroxidation (LPO) [90]. In recent years, increasing numbers of natural and synthetic drugs related to ferroptosis have been identified [91]. Conventionally, inhibition of GSH and glutathione peroxidase 4 (GPX4) expression leads to the accumulation of lipid peroxides, inducing ferroptosis. Innovatively, vaccination with early ferroptotic cancer cells is reported as an inducer of efficient antitumor immunity, which promotes the phenotypic maturation of bone marrow-derived dendritic cells (BMDCs) and elicits adaptive immunity activation [92].
A recent study revealed that Cu2+-dependent death is another type of cell death that relies on mitochondria by means of direct binding of copper to lipoylated components of the tricarboxylic acid (TCA) cycle. The subsequent lipoylated protein aggregation and iron-sulfur cluster protein loss leads to proteotoxic stress and cell death [93]. Many studies have noted that the cuproptosis-related gene (CRG) signature can predict immune characteristics in various cancers, which provides guidance for prognosis, clinicopathological features, immune characteristics, and treatment preference in precise and individual cancer strategies.
Drawbacks for metal ion-based antitumor therapeutics and possible solutions
Metal ion-based complexes such as cisplatin are only effective against limited types of tumors and have a variety of serious side effects, such as gastrointestinal and nervous system toxicity and bone marrow suppression. Additionally, intrinsic and acquired drug resistance attenuate the effectiveness of these agents. [94] More importantly, systemic toxicity, short circulation time, and low target selectivity have hampered their clinical applications to a great extent. While the application of metal ions alone has many deficiencies, the clinical application of nanometallic materials showed predominant advantages, such as efficient loading, selective delivery and responsive release with longer circulation retention time. Cisplatin and copper ionophores represent an example.
A multifunctional nanogel (designated Valproate-D-Nanogel) was capable of reactivating cisplatin and enhancing early apoptosis. This nanogel can effectively inhibit cisplatin-resistant cancer through combined pathways and provides an effective approach for overcoming cisplatin resistance in cancer treatment [95]. With the development of nanotechnology, many copper ionophores, such as dithiocar-bamates (DTCs) and thiosemicarbazones (TSCs), have been developed and combined with cuproptosis inducers to increase the intracellular copper content and elicit efficient cancer cell damage, which can greatly improve the efficiency of cuproptosis induction and is regarded as a promising cancer strategy.
Several breakthroughs of nanometallic materials in cancer immunotherapy
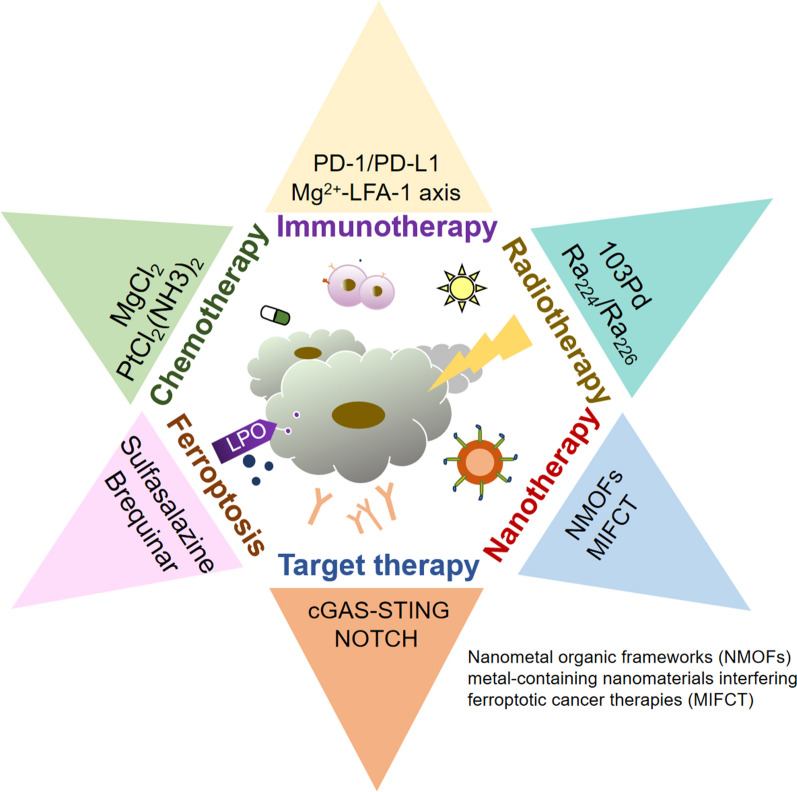
As we have mentioned above, many metal ions exert critical functions in antitumor immunity, and the synergistic strategy can enhance the effectiveness of both therapies and overcome their inherent limitations (Fig. 4) [11]. Considering that the combination of metal ions and nanotechnology possesses predominant advantages, including more stability, better efficiency at lower doses, and more importantly, fewer side effects than metal ions alone, we will introduce several essential metal ion nanoparticles applied in cancer immunotherapy in the last few years.
Fig. 4.
Various recent therapies for the treatment of cancer patients and the main techniques applied with metal ions of each treatment.
Cancer therapies can be combined with others, resulting in a better therapeutic effect and prognosis. Prospectively, the combination of different antitumor modalities will provide a new concept for cancer treatment and prevention.
Manganese and MnO2-based nanoimmune activators
Manganese catalyzes the Fenton reaction but is also an activator of the cGAS-STING pathway in antitumor immunity. Amorphous porous manganese phosphate (APMP) NPs have been designed with a high responsive ability to TME, which could be loaded with doxorubicin (DOX) and phospholipid (PL)-coated hybrid nanoparticles to maintain stability in systemic circulation and activate the cGAS-STING signal pathway [104].
A metal-phenolic network (MPN)-based immune-active nanoparticle is newly synthesized by coordinating tannic acid with Mn2+ by electroporation and subsequent coating with CpG-oligodeoxynucleotides (CpG-ODNs) via hydrogen bonding. CpG-ODN-coated Mn-phenolic network nanoparticles can effectively internalize into macrophages and stimulate M1 polarization to promote the release of proinflammatory cytokines as an effective immune activator [28].
Manganese oxide (MnO2) nanomaterials are biodegradable with stable structures, excellent physiochemical features and biosecurity. MnO2 can catalyze H2O2 into dissolved O2 and consume GSH, which can react with ROS within the TME, serving as an enhancer for PDT-induced immunotherapy and photothermal agents (PTAs) [105]. In addition, Mn@CaCO3/ICG nanoparticles loaded with PD-L1-targeting small interfering RNA (siRNA), a type of TME-sensitive O2-generating nanosystem MnO2@Chitosan-CyI (MCC) and cGAS-STING activating MnIIIPC@DTX@PLGA@Mn2+@HA (MDPMH) nanoparticles have also been designed for precise individualized diagnosis and treatment of various tumors [106].
Iron and iron oxide derivative-based nanoimmune activators
Iron oxide nanoparticles (IONPs) are ideal magnetic drug carriers with reasonable biodegradability and biocompatibility. IONPs have been applied as magnetic resonance imaging (MRI) contrast agents in cancer diagnosis, which could be guided to a specific area of interest [107, 108]. The magnetic hyperthermia triggered by IONPs can not only generate effective PTT/PDT but also enhance antigen presentation and DC maturation; at the same time, T lymphocytes can be recruited into lymph nodes, and immunosuppressive Tregs are depleted by the enhanced immune response. Thus, magnetic-responsive immunostimulatory nanoagents (MINPs) possessing magnet-guided and immunostimulatory properties have been designed and specifically eliminate primary and metastatic tumors [109].
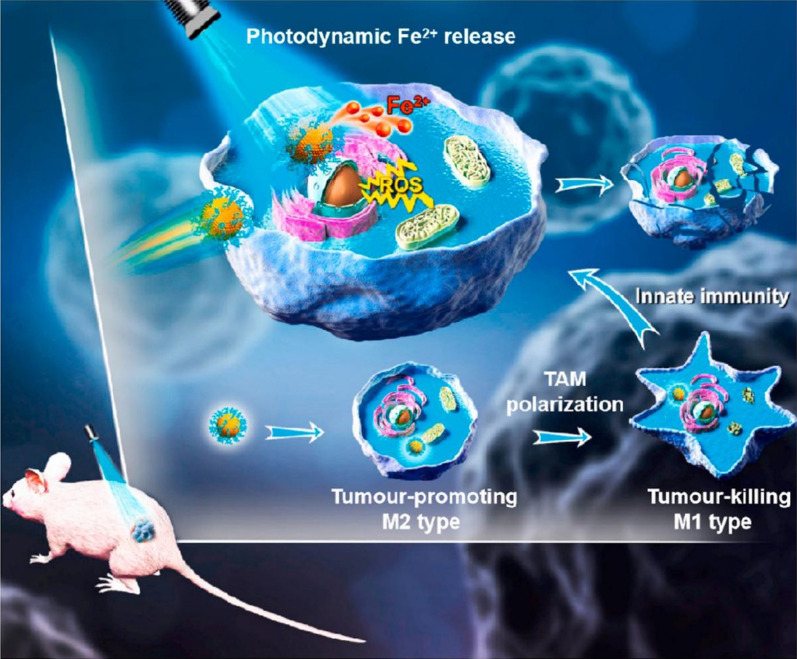
Biocompatible PEG-coated ferrihydrite nanoparticles (PEG-Fns) show the therapeutic potential of ferrihydrite to combine ROS-based CDT, PDT, and M1-activating immunotherapy with Fe2+ as the connecting point (Fig. 5) [110]. Triggered by visible blue light instead of UV light, the photoresponsive system generates Fe2+ and ROS and inhibits GPX4, which leads to apoptosis- and ferroptosis-dependent cancer cell proliferation inhibition [111]. TAM polarization from the tumor-promoting M2 type to the tumor-killing M1 type is simultaneously activated by PEG-Fns, which concomitantly inhibits tumor growth and prevents pulmonary metastasis in vivo [112].
Fig. 5.
Reproduced with permission from [111] @Copyright 2022, Elsevier Ltd
Gold nanoparticle (AuNP)-based nanoimmune activators
AuNPs are applied in light-triggered thermal therapy in the form of gold–gold sulfide nanoparticles, hollow gold nanoshells, and gold–silica nanoshells because of their high absorption property in the second near-infrared (NIR) light window, which can lead to hyperthermia in tumor sites for PTT/PDT and facilitate drug release [113, 114].
Intravenous injection of CpG immunostimulants by AuNP delivery systems can lead to better accumulation of nanoparticles in the reticuloendothelial system (RES) [115]. Moreover, AuNPs can induce macrophage and DC infiltration, which can inhibit cancer development in the context of B16-ovalbumin (OVA) [116, 117]. This provides novel insight and an ideal platform for cancer vaccine design; in this context, phagocytosis by macrophages and DCs is favorable for antigen transportation to increase the overall amount of the vaccine delivered to APCs [117–119].
Mangiferin-functionalized gold nanoparticulate agents (MGF-AuNPs) were designed to treat prostate cancer by elevating the levels of certain antitumor cytokines, such as IL-12 and TNF-α, and simultaneously reducing the levels of protumor cytokines, such as IL-10 and IL-6, which target macrophages in the spleen via NF-kB signaling pathway activation [120]. Immunomodulatory metal nanoparticles transform protumor M2 macrophages into antitumor M1 macrophages, substantially improving patients’ therapeutic outcomes [121].
An innovative immunological AuNP (AuNP@B16F10) has been developed; melanoma B16F10 cells were first employed to generate AuNPs, and then these cells shed nanoparticles with retained tumor antigen-trapped vesicles into the extracellular environment. AuNP@DCB16F10 particles were further constructed by introducing the nanoparticles into DCs, which can induce hyperthermia and provoke antitumor immune responses for combinatorial PTT and immunotherapy [122].
Ag2S-based and sliver nanoparticle (AgNP) nanoimmune activators
Ag2S with an appropriate size can elicit a photothermal-based tumor-killing effect. At the same time, Ag2S is applied in imaging due to its ideal tissue penetration ability to provide more detailed cancer diagnosis information [123, 124]. An NIR light-responsive NO delivery system was developed for the controlled and precise release of NO to hypoxic tumors during radiotherapy [125].
Ag2S quantum dots (QDs) coupled with an efficient NO source, tert-butyl nitrite, are able to generate NO under NIR irradiation induced by the thermal effect [126]. These Ag2S@BSA-SNO NPs can ameliorate the immunosuppressive TME by significantly enhancing anti-PD-L1 immune checkpoint blockade therapy. Multifunctional cancer radioimmunotherapy based on Ag2S NO delivery platforms showed a 100% survival rate, which remarkably maximized radiotherapy effects to inhibit tumor growth in a CT26 tumor model [127].
Among the gut microbiota, Fusobacterium nucleatum (Fn) selectively increases the proportion of MDSCs in CRC. M13@Ag was designed by assembling AgNPs electrostatically on the surface capsid protein M13 to specifically clear Fn and remodel the suppressive TME by activating APCs to further strengthen the host immune system. Combined with immune checkpoint inhibitors (α-PD1) or chemotherapeutics (FOLFIRI), M13@Ag prolonged overall mouse survival in the orthotopic CRC model to a greater extent [128].
Copper and CuS-based nanoimmune activators
Intratumoral copper levels influence PD-L1 expression in cancer cells and contribute to cancer immune evasion. The expression levels of the major copper influx transporter copper transporter 1 (CTR-1) and PD-L1 are closely related across many cancers but not in corresponding normal tissues [101].
The treatment prognosis, sensitivity to chemotherapy based on immunophenotype, and immunotherapy response can be predicted based on cuproptosis-related genes in bladder cancer as the infiltrating landscape of immune cells (especially T cells and DCs) induce a nonapoptotic type of programmed cell death caused by excess copper [129]. Further application remains to be reported in triple-negative breast cancer (TNBC) [130].
Featuring the high photothermal conversion efficiency of copper sulfide (CuS) nanoparticles, abundant deposition inside the large pores of dendritic large-pore mesoporous silica nanoparticles (DLMSNs), and the immune adjuvant resiquimod (R848), AM@DLMSN@CuS/R848 has been incorporated to treat TNBC by combining photothermal ablation and immune remodeling through tumor vaccination and T lymphocyte activation [131].
In addition, cancer cell-macrophage hybrid membrane-coated, NIR-responsive, hollow CuS nanoparticles can encapsulate sorafenib and be surface-modified with anti-VEGFR (CuS-SF@CMV NPs); the other ataxia telangiectasia mutated (ATM) inhibitor-loaded hollow-structured CuS NPs with surface modification with anti-TGF-β have the function of target specificity and immune activation (CuS-ATMi@TGF-βNPs); both these structures show application potential in hepatocellular carcinoma (HCC) as they synergize with PTT, chemotherapy and immunotherapy [132].
Zinc and ZnS-based nanoimmune activators
Zn2+ is essential for innate and adaptive immune activation and proper function of innate and adaptive immune cells, including the cytotoxic activity of NK cells and T cells [133]. Taking advantage of the excellent bioavailability of encapsulated ionotropic drugs, hesperidin-loaded Zn2+@ sodium alginate/pectin (SA/PCT) nanocomposites were designed to inhibit the proliferation of colon carcinoma cells and induce apoptosis under in vitro conditions [134].
In addition, the innovative delivery system FEGCG/Zn is integrated with fluorinated-coordinative-epigallocatechin gallate (EGCG) and Zn2+. The robust therapeutic effects of FEGCG/Zn depend on excellent delivery of small interfering RNA of PD-L1 (siPD-L1) and further siPD-L1 accumulation in tumors, which enhances antitumor immunotherapy through alleviation of T-cell exhaustion by regulating PD-L1 expression in tumor cells [135].
Another new ZnNP is ZnPP@MSN-RGDyK: zinc protoporphyrin (ZnPP)-loaded mesoporous silicon nanoparticles are combined with a new PD-L1 inhibitor RGDyK, which was reported recently with high photodynamic therapy efficiency, excellent immunotherapeutic effects and precise integrin β3 (β3-int) targeting in an NSCLC-SM mouse model [136].
ZnS@BSA (bovine serum albumin) nanoclusters have been synthesized to activate cGAS-STING signals in mice, promoting the infiltration of CD8+ T cells at the tumor site and cross-presentation of DCs, which can improve immunotherapy efficacy against HCC [137]. Systemic evaluation of in vitro cytotoxicity demonstrated the good biocompatibility of the proposed BSA-conjugated ZnS nanoparticles (Table 2). These studies suggest that the prepared BSA-conjugated ZnS nanoparticles are promising for future applications in antitumor immunity and biomedical engineering.
Table 2.
Several metallic nanoparticles have been applied in cancer therapy for immune modulation
| Nanoparticle | Metallic material | Delivery technology | Additional agents | Cancer type | Immune modulation | Effectiveness | Refs. |
|---|---|---|---|---|---|---|---|
| PL/APMP-DOX | Mn3 (PO4)2(APMP) |
Lipid bilayer coating (PL) |
Doxorubicin (DOX)Generate DNA damage |
Breast cancer (4T1 cells) |
Augments cGAS/STING activity |
Tumor targeting; DC maturation; T cell infiltration; NK recruitment; Type I-IFNs,TNF-a↑ |
[80] |
| H-MnO2-PEG/C&D | Hollow MnO2 | Polyethylene-glycol (PEG) | Photodynamic agent chlorine e6 (Ce6); DOX | Breast cancer (4T1 cells) | Enhances macrophages infiltration; facilitates M2/M1 polarization |
T1WI MRI imaging; H2O2 decomposition; metastasis inhibition; combined chemo-PDT |
[81] |
| Mn@CaCO3/ICG@siRNA | Walnut-like MnO2 NPs | PH-response cover layer of CaCO3 | PD-L1-targeting siRNA; ICG | Lung cancer (Lewis cells) |
Improves CTL-mediated antitumor immunities |
Enhances PDT effect; relieves tumor hypoxia; silences PD-L1 gene; DC infiltration, effector↑ |
[82] |
|
CpG@PLGA-PLLmPEG/SPIO (MINPs) |
Superparamagnetic iron oxide (SPIO) |
mPEG-PLGA-PLL triblock copolymer |
Immunoadjuvant CpG-ODNs Activate TLR9 |
Breast cancer (4T1 cells) | Activates DCs; induces TNF-α, IL-6 and IFN-γ secretion |
Magnetic targeting; synergistic PTT; precise MR/PA image; photo-immunotherapy |
[85] |
| PEG-coated ferrihydrite nanoparticles (PEG-Fns) | Fe(NO3)3·9H2O |
Biocompatible PEG-coating |
/ | Lung cancer (SCC-7 cells) |
Induces M1 macrophage polarization |
Promotes ROS-based CDT; increased oxidative stress; apoptosis/ferroptosis↑ metastasis prevention |
[87] |
| Fe2+/siRNA/PDA nanoparticles (FesiRNAPNPs) | FeCl2 | Polydopamine (PDA) |
Fe2+ chelator -GMP; GAPDH siRNA |
Colon cancer (CT26 cells) |
ROS generation & GSH consumption in the TME leads to tumor killing |
Accumulation of LPO; Fe2+-induced ferroptosis Transformation of H2O2 into ·OH Suppression of tumor growth |
[88] |
| PolyA-CpG-AuNPs | Nano-gold (AuNPs) | Poly-adenine (polyA) tail | Immunoadjuvant CpG-ODNs |
Leukemia (RAW264.7) |
Immune- stimulatory activity via TLR9 |
Efficient delivery; induces expression of proinflammatory cytokines |
[91] |
| AuNP4 |
HAuCl4 · 3H2O |
Monolayer Mercaptosuc-cinic acid (MSA) |
Radiation |
Breast cancer (MCF7) |
Tumor neoantigen presentation, cytokine secretion; priming of host antitumor T cells |
Enhances RT induced ICD tumor growth delay; TME immunogenicity↑ Radiosensitization |
[92] |
| Mangiferin functionalized (MGF)-AuNPs | NaAuCl4 | Mangiferin (polyphenol:Dglucoside) | / |
prostate cancer (PC-3 cells) |
Antitumor cytokines (IL-12 and TNF-α)↑; IL-10, IL-6↓ |
Targets macrophages via the NF-kB pathway; anti-angiogenesis agent; reprograms M2 macrophages to M1 macrophages |
[96] |
| AuNP@B16F10 | HAuCl4 |
Exocytosis of B16F10/ DC 2.4 |
Coincubation with DC2.4; exposure to UV irradiation; NIR |
Melanoma (B16F10 cells) | Provides a pro-inflammatory immune environment |
Promoted DC maturation Tumor-infiltrating lymphocytes↑; induces hyperthermia; anti-metastasis effect |
[98] |
| Silver/silver sulfide Janus nanoparticle (Ag/Ag2S JNP) | AgNO3 | PEGylated | H2O2; NIR-II | Breast cancer (MCF7) | PTT; noninvasive location and diagnosis in vivo |
H2O2-activated imaging; NIR-II fluorescence switching specifically activated by H2O2 |
[101] |
|
AM@DLMSN@ CuS/R848 |
Copper sulfide (CuS) | Dendritic large-pore mesoporous silica nanoparticles (DLMSNs) | Anti-PD-1 peptide AUNP-12; Resiquimod (R848)(TLR7/8 agonist); NIR | Breast cancer (4T1 cells) | Photothermal ablation induced ICD | High photothermal conversion efficiency; PTT agent suppressing TNBC growth and metastasis; block the PD-1/PD-L1 pathway | [108] |
|
CuS-sorafenib-anti-VEGFR antibodies (CuS-SF@CMV) |
Hollow-CuS | Cancer cell-macrophage hybrid membrane-coating |
Sorafenib; anti-VEGFR antibodies |
Hepatocell-ular carcinoma (HepG2 cells) | Localizes to the homotypic cells and escapes immune cell-mediated elimination | Inhibits tumor cell proliferation and angiogenesis via the Ras/Raf/MEK/ERK and PI3K/AKT pathways | [109] |
| ZnS@BSA | ZnS | BSA (bovine serum albumin) | Generated H2S | Hepatoma | Activates cGAS/STING pathway |
ROS production; infiltration of CD8+ T cells, cross-presentation of DCs↑ |
[114] |
| Zn2+@ SA/PCT | ZnCl2 | Hesperidin | Sodium alginate (SA), pectin(PCT) | Colon cancer (HCT116) | Induces apoptosis via excessive generation of ROS | Inhibited proliferation of colon carcinoma cell and induced apoptosis in in vitro condition | [111] |
Synergistic application of multiple metal ions
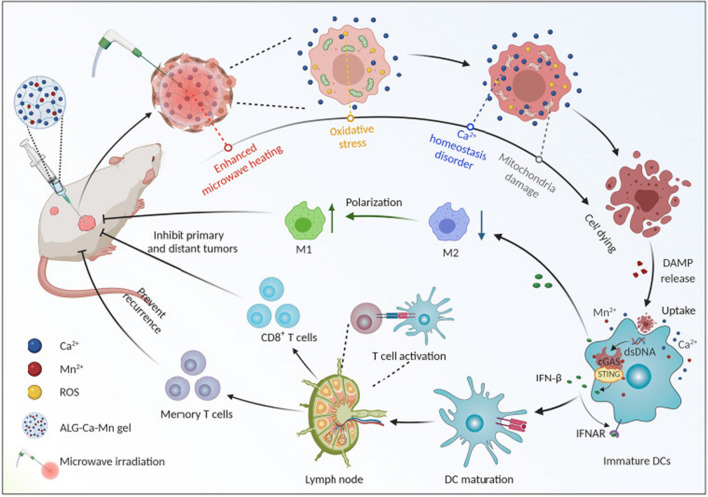
Sodium alginate (ALG)-Ca-Mn gel is an innovative microwave ablation (MWA) nanomaterial developed for local thermal ablation of tumors using the heat generated by the intense oscillation of metal ions under microwave exposure. The combination of increased [Ca2+]e (e.g., 4 mM) with mild hyperthermia (43 °C) induces the immunogenic cell death (ICD) of cancer cells by eliciting intracellular Ca2+ overloading as well as mitochondrial dysfunction, which leads to effective cancer cell killing. Moreover, Mn2+ can elicit potent innate and adaptive antitumor immunity via cGAS-STING activation (Fig. 6). This immune nanoactivator based on metal ions shows great capacity to improve MWA treatment effectiveness [138]. Recently, mPEG-b-PHEP incorporating IR780 dye and manganese zinc sulfide nanoparticles (ZMS) (PPIR780-ZMS) was established. This thermally responsive biopolymer micelle with Zn0.43Mn0.57S2 nanoparticles can readily induce antitumor immunity. With NIR induced by IR780 dye, the precise release of ZMS nanoparticles produces DAMPs and then boosts ICD. In particular, Mn2+ can not only generate ROS but can also enhance immune filtration in neoplastic foci, which reverses the suppressive phenotype of the TME to allow effects against the primary tumor and pulmonary metastases with safe systemic cytokine expression via the synergism of PDT/CDT and immunotherapy [139].
Fig. 6.
Reproduced with permission from [138] @Copyright 2022, American Association for the Advancement of Science
GNRs@SiO2@MnO2@MDSCs (GSMM) is obtained by combining gold nanorods (GNRs) with MnO2, which further disguises the MDSC membrane on its surface. Mn2+ catalyzes H2O2 into ·OH for CDT, leading to the activation of cGAS-STING but also directly acts on STING, inducing the secretion of type-I interferons, proinflammatory cytokines and chemokines. Additionally, PTT and CDT-mediated ICD of tumor cells can further enhance antitumor immunity via exposure to calreticulin (CRT), high mobility group protein B1 (HMGB1) and adenosine triphosphate (ATP) [140].
Discussion
As we have generally described, the metabolism of several metal ions in the TME, especially concerning antitumor immunity, has been well established. The slightest change in their extra/intracellular concentration results in amplified effects by signaling cascades that determine cell fate and immunity status [141, 142]. Moreover, the self-regulation of metal ion metabolism and application of nanometallic materials influence the network of antitumor immunity through various pathways. Because metal ions influence the fate of cancer cells and participate in both innate and adaptive immunity, they are widely applied in antitumor treatments [143]. However, there are some issues that remain to be resolved.
First, the delivery system and administration route should be taken into account. For example, some administration routes, including intranasal, intravenous, and intratumoral routes, induce systemic antitumor responses, which might be applicable to cases of widely metastatic cancers, whereas local injection might be more suitable for early-stage cancers or certain types, such as retinoblastoma [144].
Secondly, unwanted damage to normal tissues should be avoided by designing selective metal ion-based immune activators in cancer cells. In addition, in consideration of tumor diversity and heterogeneity, ultrasensitive nanomaterials should be designed to distinguish the tumor margin from normal tissues based on enzymatic activity and acidity [145].
More importantly, specific drug delivery vehicles are essential to realize precise delivery of biomimetic drugs in diverse tumor microenvironments with various biological barriers. The design of a loading platform depends on the application of nanotechnology in the domain of physiopathology concerned with metal and tumor physio-biochemical characteristics.
Despite the fact that metal ion application is considered to be a promising cancer therapy without the introduction of exogenous substances, we should focus on evaluation before treatment and monitoring after treatment because metal ions can directly affect cell excitability and manifest cytotoxicity beyond a certain dosage [139]. As potential agonists of the immune system, we should discover a balance between immune surveillance and homeostasis to avoid hyperinflammatory reactions that will be a double-edged sword for cancer therapy.
Acknowledgements
This work was supported by General Program of National Natural Science Foundation of China (No.81972667 to J.S.), National Key R&D Program of China (2021YFC2701103 to J.S.), The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No.TP2018046 to J.S.), Shanghai Municipal Education Commission-Two Hundred Talent (No.20191817 to J.S.).
Author contributions
J.S. and X.F. provided direction and guidance throughout the preparation of this manuscript. F.S. and Y.F. contributed equally to this manuscript. They collected and interpreted the study and were major contributors to the writing and editing of the manuscript, Y.W and M.Z. made some constructive revisions to the manuscript. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feiyang Shen and Yan Fang have contributed equally to this work
Contributor Information
Feiyang Shen, Email: sophiesfy@163.com.
Yan Fang, Email: yanyan2021fang@sjtu.edu.com.
Yijia Wu, Email: wyj_med@sjtu.edu.com.
Jianfeng Shen, Email: jfshen@shsmu.edu.cn.
Xianqun Fan, Email: fanxq@sjtu.edu.cn.
References
- 1.Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019. doi: 10.1016/j.ctrv.2020.102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 3.Ni K, Luo T, Nash GT, Lin W. Nanoscale metal-organic frameworks for cancer immunotherapy. Acc Chem Res. 2020;53:1739–1748. doi: 10.1021/acs.accounts.0c00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wulff H, Christophersen P, Colussi P, Chandy KG, Yarov-Yarovoy V. Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019;18:339–357. doi: 10.1038/s41573-019-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X, et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat Nanotechnol. 2021;16:1260–1270. doi: 10.1038/s41565-021-00962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MX, Yang YW. Metal-Organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater. 2017 doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Ma X, Hu H. Application of nano-drug delivery system based on cascade technology in cancer treatment. Int J Mol Sci. 2021 doi: 10.3390/ijms22115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Li J, et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev. 2018;47:2322–2356. doi: 10.1039/c7cs00543a. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Tumor-microenvironment-triggered ion exchange of a metal-organic framework hybrid for multimodal imaging and synergistic therapy of tumors. Adv Mater. 2020;32:e2001452. doi: 10.1002/adma.202001452. [DOI] [PubMed] [Google Scholar]
- 12.Qian X, Xu Z. Fluorescence imaging of metal ions implicated in diseases. Chem Soc Rev. 2015;44:4487–4493. doi: 10.1039/c4cs00292j. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Synthesis and characterization of Mn:ZnSe/ZnS/ZnMnS Sandwiched QDs for multimodal imaging and theranostic applications. Small. 2016;12:534–546. doi: 10.1002/smll.201503352. [DOI] [PubMed] [Google Scholar]
- 14.Fei W, et al. Engineering of bioactive metal sulfide nanomaterials for cancer therapy. J Nanobiotechnol. 2021;19:93. doi: 10.1186/s12951-021-00839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H, et al. New anti-cancer explorations based on metal ions. J Nanobiotechnol. 2022;20:457. doi: 10.1186/s12951-022-01661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Zhang R, Wei X, Lv M, Jiang Z. Metalloimmunology: the metal ion-controlled immunity. Adv Immunol. 2020;145:187–241. doi: 10.1016/bs.ai.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Bird AJ. Cellular sensing and transport of metal ions: implications in micronutrient homeostasis. J Nutr Biochem. 2015;26:1103–1115. doi: 10.1016/j.jnutbio.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie TK, et al. Sodium homeostasis in the tumour microenvironment. Biochim Biophys Acta Rev Cancer. 2019;1872:188304. doi: 10.1016/j.bbcan.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevarskaya N, Skryma R, Shuba Y. Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol Rev. 2018;98:559–621. doi: 10.1152/physrev.00044.2016. [DOI] [PubMed] [Google Scholar]
- 20.Stelling MP, et al. Metal ions and the extracellular matrix in tumor migration. Febs J. 2019;286:2950–2964. doi: 10.1111/febs.14986. [DOI] [PubMed] [Google Scholar]
- 21.Boonstra J, Mummery CL, Tertoolen LG, Van Der Saag PT, De Laat SW. Cation transport and growth regulation in neuroblastoma cells. Modulations of K+ transport and electrical membrane properties during the cell cycle. J Cell Physiol. 1981;107:75–83. doi: 10.1002/jcp.1041070110. [DOI] [PubMed] [Google Scholar]
- 22.Martial S. Involvement of ion channels and transporters in carcinoma angiogenesis and metastasis. Am J Physiol Cell Physiol. 2016;310:C710–727. doi: 10.1152/ajpcell.00218.2015. [DOI] [PubMed] [Google Scholar]
- 23.Panyi G, Beeton C, Felipe A. Ion channels and anti-cancer immunity. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130106. doi: 10.1098/rstb.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, et al. DNA-based MXFs to enhance radiotherapy and stimulate robust antitumor immune responses. Nano Lett. 2022;22:2826–2834. doi: 10.1021/acs.nanolett.1c04888. [DOI] [PubMed] [Google Scholar]
- 26.Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019–1033. doi: 10.1016/j.molcel.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater. 2021;6:1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han JH, et al. Combination of metal-phenolic network-based immunoactive nanoparticles and bipolar irreversible electroporation for effective cancer immunotherapy. Small. 2022;18:e2200316. doi: 10.1002/smll.202200316. [DOI] [PubMed] [Google Scholar]
- 29.Zhao DH, et al. tumor microenvironment-activated theranostics nanozymes for fluorescence imaging and enhanced chemo-chemodynamic therapy of tumors. ACS Appl Mater Interfaces. 2021;13:55780–55789. doi: 10.1021/acsami.1c12611. [DOI] [PubMed] [Google Scholar]
- 30.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, et al. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13:355. doi: 10.1038/s41419-022-04775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab A, Loeck T, Najder-Nalepa K. STIM2: Redox-sensor and effector of the (tumor) microenvironment. Cell Calcium. 2021;94:102335. doi: 10.1016/j.ceca.2020.102335. [DOI] [PubMed] [Google Scholar]
- 33.Sang Y, et al. Remodeling macrophages by an iron nanotrap for tumor growth suppression. ACS Nano. 2021;15:19298–19309. doi: 10.1021/acsnano.1c05392. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Q, et al. Target reprogramming lysosomes of CD8+ T cells by a mineralized metal-organic framework for cancer immunotherapy. Adv Mater. 2021;33:e2100616. doi: 10.1002/adma.202100616. [DOI] [PubMed] [Google Scholar]
- 35.St Paul M, Ohashi PS. The Roles of CD8(+) T Cell subsets in antitumor immunity. Trends Cell Biol. 2020;30:695–704. doi: 10.1016/j.tcb.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Demaria O, et al. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 37.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 38.Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol Aspects Med. 2019;69:93–106. doi: 10.1016/j.mam.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu H, et al. Intestinal microbiota regulates anti-tumor effect of disulfiram combined with Cu(2+) in a mice model. Cancer Med. 2020;9:6791–6801. doi: 10.1002/cam4.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bessman NJ, et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science. 2020;368:186–189. doi: 10.1126/science.aau6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minton K. Ironing out the details of intestinal repair. Nat Rev Immunol. 2020;20:350–351. doi: 10.1038/s41577-020-0310-9. [DOI] [PubMed] [Google Scholar]
- 42.Rescigno M. The “iron will” of the gut. Science. 2020;368:129–130. doi: 10.1126/science.abb2915. [DOI] [PubMed] [Google Scholar]
- 43.Lonergan ZR, Skaar EP. Nutrient zinc at the host-pathogen interface. Trends Biochem Sci. 2019;44:1041–1056. doi: 10.1016/j.tibs.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souffriau J, et al. Zinc inhibits lethal inflammatory shock by preventing microbe-induced interferon signature in intestinal epithelium. EMBO Mol Med. 2020;12:e11917. doi: 10.15252/emmm.201911917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fol M, Koziński P, Kulesza J, Białecki P, Druszczyńska M. Dual nature of relationship between mycobacteria and cancer. Int J Mol Sci. 2021 doi: 10.3390/ijms22158332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, et al. A novel stress-inducible CmtR-ESX3-Zn(2+) regulatory pathway essential for survival of Mycobacterium bovis under oxidative stress. J Biol Chem. 2020;295:17083–17099. doi: 10.1074/jbc.RA120.013017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon J, Bakhoum SF. The cytosolic DNA-Sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10:26–39. doi: 10.1158/2159-8290.Cd-19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakhoum SF, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reisländer T, Groelly FJ, Tarsounas M. DNA damage and cancer immunotherapy: a STING in the tale. Mol Cell. 2020;80:21–28. doi: 10.1016/j.molcel.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Lv M, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–979. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozenberg JM, et al. The role of the metabolism of Zinc and Manganese Ions in human cancerogenesis. Biomedicines. 2022 doi: 10.3390/biomedicines10051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48:675–687.e677. doi: 10.1016/j.immuni.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, et al. A peritumorally injected immunomodulating adjuvant Elicits robust and safe metalloimmunotherapy against solid tumors. Adv Mater. 2022 doi: 10.1002/adma.202206915. [DOI] [PubMed] [Google Scholar]
- 55.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–559. doi: 10.1038/s41590-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scambler T, et al. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. Elife. 2019 doi: 10.7554/eLife.49248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muñoz-Planillo R, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong H, et al. Mitochondrial calcium uniporter promotes phagocytosis-dependent activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2022;119:e2123247119. doi: 10.1073/pnas.2123247119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18:1141–1160. doi: 10.1038/s41423-021-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman NM, Chi H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity. 2022;55:14–30. doi: 10.1016/j.immuni.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eil R, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537:539–543. doi: 10.1038/nature19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conforti L. Potassium channels of T lymphocytes take center stage in the fight against cancer. J Immunother Cancer. 2017 doi: 10.1186/s40425-016-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandy KG, Norton RS. Immunology: channelling potassium to fight cancer. Nature. 2016;537:497–499. doi: 10.1038/nature19467. [DOI] [PubMed] [Google Scholar]
- 68.Vodnala SK, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019 doi: 10.1126/science.aau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baixauli F, Villa M, Pearce EL. Potassium shapes antitumor immunity. Science. 2019;363:1395–1396. doi: 10.1126/science.aaw8800. [DOI] [PubMed] [Google Scholar]
- 70.Lien EC, Lau AN, Vander Heiden MG. Putting the K(+) in K(+)aloric Restriction. Immunity. 2019;50:1129–1131. doi: 10.1016/j.immuni.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Vardhana S, Dustin ML. Magnesium for T cells: strong to the finish! Trends Immunol. 2022;43:277–279. doi: 10.1016/j.it.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Al-Aghbar MA, Jainarayanan AK, Dustin ML, Roffler SR. The interplay between membrane topology and mechanical forces in regulating T cell receptor activity. Commun Biol. 2022 doi: 10.1038/s42003-021-02995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bird L. Magnesium: essential for T cells. Nat Rev Immunol. 2022;22:144–145. doi: 10.1038/s41577-022-00688-2. [DOI] [PubMed] [Google Scholar]
- 74.Lötscher J, et al. Magnesium sensing via LFA-1 regulates CD8(+) T cell effector function. Cell. 2022;185:585–602.e529. doi: 10.1016/j.cell.2021.12.039. [DOI] [PubMed] [Google Scholar]
- 75.Shi X, et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493:111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- 76.Leitenberg D, Falahati R, Lu DD, Takeda A. CD45-associated protein promotes the response of primary CD4 T cells to low-potency T-cell receptor (TCR) stimulation and facilitates CD45 association with CD3/TCR and lck. Immunology. 2007;121:545–554. doi: 10.1111/j.1365-2567.2007.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma Y, et al. Clustering of the ζ-Chain Can Initiate T Cell Receptor Signaling. Int J Mol Sci. 2020 doi: 10.3390/ijms21103498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925. doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 79.Farooq MA, et al. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: an overview. Artif Cells Nanomed Biotechnol. 2019;47:1674–1692. doi: 10.1080/21691401.2019.1604535. [DOI] [PubMed] [Google Scholar]
- 80.Fournel L, et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019;464:5–14. doi: 10.1016/j.canlet.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Smialowicz RJ, et al. Manganese chloride enhances murine cell-mediated cytotoxicity: effects on natural killer cells. J Immunopharmacol. 1984 doi: 10.3109/08923978409026455. [DOI] [PubMed] [Google Scholar]
- 82.Li T, et al. Magnesium-assisted cisplatin inhibits bladder cancer cell survival by modulating Wnt/β-catenin signaling pathway. Front Pharmacol. 2021;12:804615. doi: 10.3389/fphar.2021.804615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Draghi PF, et al. Magnesium supplementation: effect on the expression of inflammation genes in Erlich’s tumor. J Diet Suppl. 2022;19:483–498. doi: 10.1080/19390211.2021.1897056. [DOI] [PubMed] [Google Scholar]
- 84.Pedersen SF, Stock C. Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658–1661. doi: 10.1158/0008-5472.Can-12-4188. [DOI] [PubMed] [Google Scholar]
- 85.Miyai T, et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc Natl Acad Sci USA. 2014;111:11780–11785. doi: 10.1073/pnas.1323549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shrivastava P, Katagiri T, Ogimoto M, Mizuno K, Yakura H. Dynamic regulation of Src-family kinases by CD45 in B cells. J Blood. 2004;103:1425–1432. doi: 10.1182/blood-2003-03-0716. [DOI] [PubMed] [Google Scholar]
- 87.Lee K, Sung C, Kim BG, Hahn JS. Activation of Aro80 transcription factor by heat-induced aromatic amino acid influx in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2013;438:43–47. doi: 10.1016/j.bbrc.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Song W, Li D, Tao L, Luo Q, Chen L. Solute carrier transporters: the metabolic gatekeepers of immune cells. Acta Pharm Sin B. 2020;10:61–78. doi: 10.1016/j.apsb.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su X, et al. A carbonic anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer evokes Pyroptosis for enhanced anti-tumor immunity. Angew Chem Int Ed Engl. 2022;61:e202115800. doi: 10.1002/anie.202115800. [DOI] [PubMed] [Google Scholar]
- 90.Xu J, et al. Regulation mechanism of ferroptosis and its research progress in tumor immunotherapy. Front Mol Biosci. 2022;9:1045548. doi: 10.3389/fmolb.2022.1045548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang R, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Efimova I, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsvetkov P, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yue S, Luo M, Liu H, Wei S. Recent advances of gold compounds in anticancer immunity. Front Chem. 2020;8:543. doi: 10.3389/fchem.2020.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun M, He L, Fan Z, Tang R, Du J. Effective treatment of drug-resistant lung cancer via a nanogel capable of reactivating cisplatin and enhancing early apoptosis. Biomaterials. 2020;257:120252. doi: 10.1016/j.biomaterials.2020.120252. [DOI] [PubMed] [Google Scholar]
- 96.Li D, Molldrem JJ, Ma Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J Biol Chem. 2009;284:21001–21010. doi: 10.1074/jbc.M109.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang W, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao H, et al. STIM1 is a metabolic checkpoint regulating the invasion and metastasis of hepatocellular carcinoma. Theranostics. 2020;10:6483–6499. doi: 10.7150/thno.44025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sergi CM. The role of Zinc in the T-cell metabolism in infection requires further investigation—an opinion. Front Immunol. 2022;13:865504. doi: 10.3389/fimmu.2022.865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voli F, et al. Intratumoral copper modulates PD-L1 expression and influences tumor immune evasion. Cancer Res. 2020;80:4129–4144. doi: 10.1158/0008-5472.Can-20-0471. [DOI] [PubMed] [Google Scholar]
- 102.Tran L, et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 2017;5:1141–1151. doi: 10.1158/2326-6066.Cir-17-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Glorieux C, et al. Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer. J Adv Res. 2022;40:109–124. doi: 10.1016/j.jare.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou L, et al. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano. 2020;14:3927–3940. doi: 10.1021/acsnano.9b06111. [DOI] [PubMed] [Google Scholar]
- 105.Yang G, et al. Hollow MnO(2) as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, et al. A tumor microenvironment responsive biodegradable CaCO(3)/MnO(2)—based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019;9:6867–6884. doi: 10.7150/thno.37586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chung S, Revia RA, Zhang M. Iron oxide nanoparticles for immune cell labeling and cancer immunotherapy. Nanoscale Horiz. 2021;6:696–717. doi: 10.1039/d1nh00179e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv. 2019;16:69–78. doi: 10.1080/17425247.2019.1554647. [DOI] [PubMed] [Google Scholar]
- 109.Guo Y, et al. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials. 2019;219:119370. doi: 10.1016/j.biomaterials.2019.119370. [DOI] [PubMed] [Google Scholar]
- 110.Matsumura S, Aoki I, Saga T, Shiba K. A tumor-environment-responsive nanocarrier that evolves its surface properties upon sensing matrix metalloproteinase-2 and initiates agglomeration to enhance T2 relaxivity for magnetic resonance imaging. Mol Pharm. 2011;8:1970–1974. doi: 10.1021/mp2001999. [DOI] [PubMed] [Google Scholar]
- 111.Yang Y, et al. Blue light-triggered Fe(2+)-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials. 2021;271:120739. doi: 10.1016/j.biomaterials.2021.120739. [DOI] [PubMed] [Google Scholar]
- 112.Wang Y, et al. Metal ions/nucleotide coordinated nanoparticles comprehensively suppress tumor by synergizing ferroptosis with energy metabolism interference. J Nanobiotechnology. 2022;20:199. doi: 10.1186/s12951-022-01405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta N, Malviya R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188532. doi: 10.1016/j.bbcan.2021.188532. [DOI] [PubMed] [Google Scholar]
- 114.Lin Y, Ren J, Qu X. Nano-gold as artificial enzymes: hidden talents. Adv Mater. 2014;26:4200–4217. doi: 10.1002/adma.201400238. [DOI] [PubMed] [Google Scholar]
- 115.Chen N, et al. Self-assembly of poly-adenine-tailed CpG oligonucleotide-gold nanoparticle nanoconjugates with immunostimulatory activity. Small. 2014;10:368–375. doi: 10.1002/smll.201300903. [DOI] [PubMed] [Google Scholar]
- 116.Janic B, et al. Therapeutic enhancement of radiation and immunomodulation by gold nanoparticles in triple negative breast cancer. Cancer Biol Ther. 2021;22:124–135. doi: 10.1080/15384047.2020.1861923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin AY, et al. Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS ONE. 2013;8:e63550. doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rai A, Ferreira L. Biomedical applications of the peptide decorated gold nanoparticles. Crit Rev Biotechnol. 2021;41:186–215. doi: 10.1080/07388551.2020.1853031. [DOI] [PubMed] [Google Scholar]
- 119.Almeida JPM, Lin AY, Figueroa ER, Foster AE, Drezek RA. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;11:1453–1459. doi: 10.1002/smll.201402179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khoobchandani M, et al. Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci Rep. 2021;11:16797. doi: 10.1038/s41598-021-96224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patra N, Dehury N, Pal A, Behera A, Patra S. Preparation and mechanistic aspect of natural xanthone functionalized gold nanoparticle. Mater Sci Eng C Mater Biol Appl. 2018;90:439–445. doi: 10.1016/j.msec.2018.04.091. [DOI] [PubMed] [Google Scholar]
- 122.Zhang D, et al. Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 2019;19:6635–6646. doi: 10.1021/acs.nanolett.9b02903. [DOI] [PubMed] [Google Scholar]
- 123.Chen H, et al. Characterization of tumor-targeting Ag2S quantum dots for cancer imaging and therapy in vivo. Nanoscale. 2014;6:12580–12590. doi: 10.1039/c4nr03613a. [DOI] [PubMed] [Google Scholar]
- 124.Shen Y, et al. Perspectives for Ag(2)S NIR-II nanoparticles in biomedicine: from imaging to multifunctionality. Nanoscale. 2019;11:19251–19264. doi: 10.1039/c9nr05733a. [DOI] [PubMed] [Google Scholar]
- 125.Zhang X, et al. Plasmonic-fluorescent janus Ag/Ag(2)S nanoparticles for In Situ H(2)O(2)-activated NIR-II fluorescence imaging. Nano Lett. 2021;21:2625–2633. doi: 10.1021/acs.nanolett.1c00197. [DOI] [PubMed] [Google Scholar]
- 126.Zhao J, Zhang Q, Liu W, Shan G, Wang X. Biocompatible BSA-Ag(2)S nanoparticles for photothermal therapy of cancer. Colloids Surf B Biointerfaces. 2022;211:112295. doi: 10.1016/j.colsurfb.2021.112295. [DOI] [PubMed] [Google Scholar]
- 127.Zhou X, et al. Near-infrared light-responsive nitric oxide delivery platform for enhanced radioimmunotherapy. Nanomicro Lett. 2020;12:100. doi: 10.1007/s40820-020-00431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong X, et al. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020 doi: 10.1126/sciadv.aba1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song Q, Zhou R, Shu F, Fu W. Cuproptosis scoring system to predict the clinical outcome and immune response in bladder cancer. Front Immunol. 2022;13:958368. doi: 10.3389/fimmu.2022.958368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sha S, et al. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front Immunol. 2022;13:922780. doi: 10.3389/fimmu.2022.922780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng Y, et al. An intelligent biomimetic nanoplatform for holistic treatment of metastatic triple-negative breast cancer via photothermal ablation and immune remodeling. ACS Nano. 2020;14:15161–15181. doi: 10.1021/acsnano.0c05392. [DOI] [PubMed] [Google Scholar]
- 132.Ji B, et al. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020;111:363–372. doi: 10.1016/j.actbio.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 133.Wessels I, Fischer HJ, Rink L. Dietary and physiological effects of zinc on the immune system. Annu Rev Nutr. 2021;41:133–175. doi: 10.1146/annurev-nutr-122019-120635. [DOI] [PubMed] [Google Scholar]
- 134.Yang Z, Yang H, Dong X, Pu M, Ji F. Hesperidin loaded Zn(2+)@ SA/PCT nanocomposites inhibit the proliferation and induces the apoptosis in colon cancer cells (HCT116) through the enhancement of pro-apoptotic protein expressions. J Photochem Photobiol B. 2020 doi: 10.1016/j.jphotobiol.2019.111767. [DOI] [PubMed] [Google Scholar]
- 135.Wu P, et al. Engineered EGCG-containing biomimetic nanoassemblies as effective delivery platform for enhanced cancer therapy. Adv Sci. 2022;9:e2105894. doi: 10.1002/advs.202105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou L, et al. Precisely targeted nano-controller of PD-L1 level for non-small cell lung cancer spinal metastasis immunotherapy. Adv Healthc Mater. 2022 doi: 10.1002/adhm.202200938. [DOI] [PubMed] [Google Scholar]
- 137.Cen D, et al. ZnS@BSA Nanoclusters Potentiate Efficacy of Cancer Immunotherapy. Adv Mater. 2021;33:e2104037. doi: 10.1002/adma.202104037. [DOI] [PubMed] [Google Scholar]
- 138.Zhu Y, et al. Metallo-alginate hydrogel can potentiate microwave tumor ablation for synergistic cancer treatment. Sci Adv. 2022 doi: 10.1126/sciadv.abo5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z, et al. Immunogenic cell death augmented by manganese zinc sulfide nanoparticles for metastatic melanoma immunotherapy. ACS Nano. 2022 doi: 10.1021/acsnano.2c08013. [DOI] [PubMed] [Google Scholar]
- 140.Zhao Y, et al. Biomimetic manganese-based theranostic nanoplatform for cancer multimodal imaging and twofold immunotherapy. Bioact Mater. 2023;19:237–250. doi: 10.1016/j.bioactmat.2022.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aguilera-Juárez A, et al. LptD-antigen system on gold nanoparticles: an innovative strategy in the nanovaccine development. Nanotechnology. 2022 doi: 10.1088/1361-6528/ac659b. [DOI] [PubMed] [Google Scholar]
- 142.Chen C, et al. Cytosolic delivery of Thiolated Mn-cGAMP nanovaccine to enhance the antitumor immune responses. Small. 2021;17:e2006970. doi: 10.1002/smll.202006970. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y, Xu Y, Zheng L. Disease ionomics: understanding the role of ions in complex disease. Int J Mol Sci. 2020 doi: 10.3390/ijms21228646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2014;98:292–297. doi: 10.1136/bjophthalmol-2013-303885. [DOI] [PubMed] [Google Scholar]
- 145.Wu D, Wang S, Yu G, Chen X. Cell death mediated by the pyroptosis pathway with the aid of nanotechnology prospects for cancer therapy. Angew Chem Int Ed Engl. 2021;60:8018–8034. doi: 10.1002/anie.202010281. [DOI] [PubMed] [Google Scholar]