Abstract
Background
In many countries, including Italy, there are few national data on pre-pregnancy Body Mass Index (BMI) and gestational weight gain (GWG), despite these being important predictors of maternal and neonatal health outcomes. This dearth of information makes it difficult to develop and monitor intervention policies to reduce the burden of disease linked to inadequate BMI status and/or GWG in pregnant women. This study describes the setting up and initial implementation of a regional surveillance system on pre-pregnancy BMI and GWG.
Methods
Between 1 January 2017 and 31 December 2018, anthropometric data were collected from all pregnant women accessing public health services in the Friuli Venezia Giulia region (Italy) for first ultrasound check (T1) and at delivery (T2). Anthropometric data collected at T1 (self-reported pre-pregnancy weight and measured weight and height) and T2 (measured weight and self-reported pre-pregnancy weight and height) were compared.
Results
The system was able to reach 43.8% of all the women who gave birth in the region, and provided complete data for 6400 women of the 7188 who accessed the services at T1. At the beginning of pregnancy 447 (7.0%) women were underweight, 4297 (67.1%) had normal weight, 1131 (17.7%) were overweight and 525 (8.2%) had obesity. At delivery, 2306 (36.0%) women were within the appropriate weight gain range, while for 2021 (31.6%) weight gain was insufficient and for 2073 (32.4%) excessive. Only minor differences were observed between measured and self-reported anthropometric data.
Conclusions
The surveillance system offers an overview of the weight status of women during pregnancy. About 1/3 of women entered pregnancy with unsatisfactory BMI and 2/3 did not achieve the recommended weight gain. This surveillance system can be an effective tool to guide public health interventions.
Keywords: Pregnancy, BMI, Gestational weight gain, Surveillance system, Nutritional status
Introduction
Pre-pregnancy Body Mass Index (BMI) and gestational weight gain (GWG) are important predictors of maternal and neonatal health outcomes. Both excessive BMI at the beginning of the pregnancy and excessive GWG can affect the course of gestation and the outcomes at childbirth [1–10]. Maternal obesity seems to negatively affect fetal development [11–13] as well as long term child health, increasing the offspring’s vulnerability to obesity [14, 15]. Similarly to obesity, maternal pre-pregnancy underweight is also associated with negative health effects with short and long-term consequences for the mother and the fetus [16–21].
Both excessive and insufficient GWG affect fetal growth and weight at birth [22, 23]. Excessive body mass gained during pregnancy can become persistent even in women with normal pre-gestational BMI [2, 24–27]. This condition is a major predictor of long-term obesity, and becoming pregnant again might drive the cycle towards obesity further [2, 27]. Thus, the public health burden of inadequate pre-pregnancy and pregnancy weight status is significant, extending well beyond delivery, and should be adequately addressed. The 2009 guidelines of the Institute of Medicine (IOM, now the National Academy of Medicine, NAM) provide a range for adequate weight gain for each BMI class, indicating 0.5–2 kg as normal GWG at the end of the first trimester, regardless of pre-pregnancy BMI [28].
Unfortunately, in many countries, including Italy, data on pre-pregnancy BMI and on GWG are scarce. National statistics show a constant increase of weight excess in the total adult female population (age range: 18–69) and suggest that around 22% of women in the reproductive years (18–44 years of age) [29, 30] are likely to have a BMI above the normal range (18.5–24.9 kg/m2) [31]. However, no specific information is available for the pregnant population subgroup. This makes it difficult to develop and monitor intervention policies aimed at reducing the burden of disease due to inadequate BMI and/or weight gain of pregnant women. The recommendations developed by IOM, addressing health workers and women of childbearing age with regards to pregnancy, include the need to implement surveillance systems to monitor weight gain during pregnancy and postpartum weight retention, collecting data on pre-conception BMI class, age and socio-economic status [28]. Particular attention, should be paid to population subgroups that are at greater risk of having high BMI, such as women with low income, low level of schooling or belonging to historically marginalized communities [32, 33]. These recommendations are also supported by the objectives identified in the latest WHO European Food and Nutrition Action Plan 2015–2020 [34].
Based on all the above, we designed and set up in one Italian region, a surveillance system that provides: 1) annual estimates and trends of the prevalence of inadequate BMI before and during pregnancy and 2) data on GWG. In addition to this, we also sought to assess the reliability of self-reported versus measured anthropometric data. This paper describes the process of setting up the surveillance system, the difficulties encountered in its implementation, and the reliability of the data collection method, also with regards to the sustainability of the system.
Materials and methods
This surveillance system on the weight status of women in pregnancy (in a gender inclusive perspective, in this paper, the term “women” is meant as including any person capable of pregnancy), was designed and developed through a collaboration between the Regional Health Authority of the Friuli Venezia Giulia (FVG) region and the Institute for Maternal and Child Health – IRCCS ‘Burlo Garofolo’ of Trieste, a third level university and research hospital. FVG is a region in the north-east of Italy with about 1.2 million inhabitants and 8,000 deliveries per year, about 99% of which take place in public hospitals. Eleven centers, including all the public maternity units and private birth clinics of the region, plus one private antenatal clinic affiliated with the regional health system, were involved in the surveillance. Data collection began in pilot form on 1 January 2015 and the system was fully implemented by the beginning of 2016. This paper presents the results of two years of monitoring, from 1 January 2017 to 31 December 2018. Data were collected following Italian rules and regulations: women signed a standard privacy form to give consent to the routine acquisition and storage of health data. Approval to conduct the survey was granted by the FVG Regional Health Authority.
Set up
The surveillance system was set up in collaboration with the healthcare staff of the centers involved in the project, taking into account their needs and routines. The accuracy of the reported data was assessed through periodic data extraction.
Two data collection times were established:
First ultrasound check (T1), between 11 and 14 weeks of gestation: women were asked to self-report their pre-pregnancy weight (PPW) and a direct measurement of weight and height was carried out by the health staff routinely in charge of providing pre-natal care. This time-point was selected because it’s when the greatest proportion of pregnant women in the Region access the public hospital system. The indications were: to measure women in light clothing and without shoes, to record weight to the nearest 0.1 kg using a SECA 877 scale, and height to the nearest 0.1 cm using a SECA 217 stadiometer. An ad hoc two-hour training was provided to the personnel involved, before starting the surveillance. Collected data were entered into the Regional hospital’s electronic clinical records system, known as G2, and extracted periodically to assess the accuracy of the measurements (i.e. number of decimals). The results of this periodic assessment were reported back to the health professionals for discussion.
Hospital admission for delivery (T2): women’s weight was measured before delivery using the same equipment and standardized protocol. On this occasion, women were also asked to provide self-reported pre-pregnancy weight and height for comparison with the information recorded at T1. The collected information was registered in the Certificate of Delivery Care (CeDAP), a national administrative form routinely filled in electronically by trained health care personnel after delivery, that includes sociodemographic information, data on pregnancy and delivery, and on selected maternal and neonatal outcomes. In FVG, for the specific purpose of this survey, since 2015, weight measured at delivery and self-reported pre-pregnancy weight and height are included among the information collected through the CeDAP system.
CeDAP data feed into the Regional Repository of MicroData (RRMD), an automated centralized record system that stores administrative and clinical data from the Italian National Health Service, using unique anonymous regional identification codes.
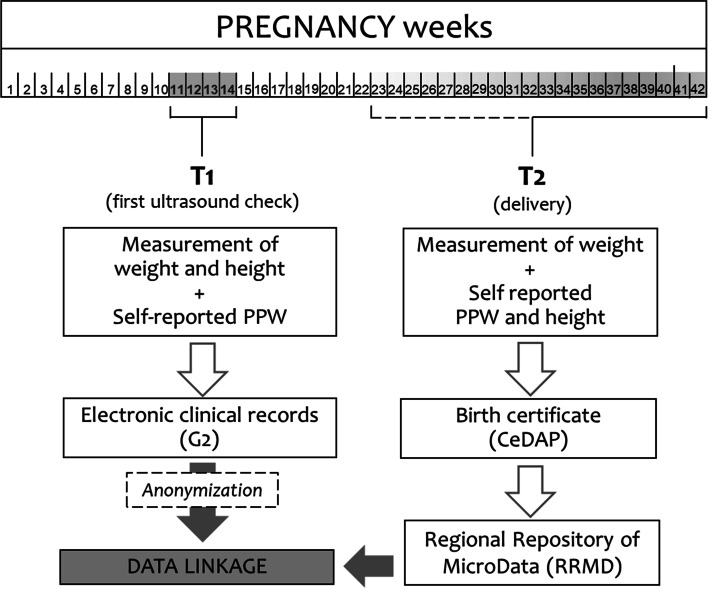
Anthropometric data recorded, for the purposes of this study, in the G2 system at T1, were integrated with information deriving from the CeDAP through deterministic record linkage with the databases of the Regional Repository of MicroData (RRMD) of FVG, using an anonymous identifier. A synthetic flow-chart of the study design is shown in Fig. 1.
Fig. 1.
Design of the study
Population
The study population comprised all pregnant women who accessed the public health services of FVG for first ultrasound check (T1), and subsequently gave birth in the Region (T2). Only women for whom information at T1 was available, were included in the survey.
Outcomes
The main outcome of the study was the evaluation of the weight status of the women based on pre-pregnancy BMI and GWG. Pre-pregnancy BMI was calculated as self-reported PPW in kilograms divided by measured height in meters squared, and categorized, according to WHO definitions [30], as underweight (BMI < 18.5 kg/m2), normal weight (BMI ≥ 18.5 kg/m2 to ≤ 24.9 kg/m2), overweight (BMI ≥ 25.0 kg/m2 to ≤ 29.9 kg/m2) and obese (BMI ≥ 30.0 kg/m2). The GWG was calculated by subtracting the PPW from the measured weight at delivery adjusted for gestational age, and categorized as insufficient, appropriate or excessive, based on current IOM guidelines which provide a range of adequate weight gain for each pre-pregnancy BMI class [28].
Statistical analysis
Categorical variables are presented as absolute frequency and percentage; continuous variables as mean and standard deviation. Data on the weight status of pregnant women (pre-pregnancy BMI and GWG), based on current IOM recommendations, are presented descriptively. Differences between years were evaluated with the Chi-square test or Fisher’s exact test, when appropriate.
To evaluate the regional representativeness of the surveyed women, their socio-economic status, smoking habits during the 5 years before pregnancy, access to medical services during pregnancy, parity and pre-pregnancy BMI, were compared with those of women who had not been surveyed (women who gave birth in the Region but lacked information at T1). For continuous variables differences were evaluated with the Student t-test, for categorical variables with the Chi-square test or Fisher’s exact test, when appropriate.
To evaluate the accuracy of self-reported data and their influence on BMI classification, comparisons were carried out between: a) self-reported PPW and measured weight at T1; b) PPW self-reported at delivery and at T1; c) measured height at T1 and self-reported height at delivery; d) GWG calculated using self-reported PPW or using weight measured at T1. Differences were evaluated with the paired t-test for continuous variables, and with the weighted Cohen Kappa test for categorical variables. All analyses were performed using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
During the two-year data collection period (01.01.17 to 31.12.18), a total of 16,428 women gave birth in FVG, 7188 (43.8%) accessed the services at the first ultrasound check (T1) and were included in the assessment of the surveillance system’s accuracy and representativeness. However, only 6400 records (89.0%) had complete data and could be used to evaluate the BMI status of pregnant women. The remaining 788 records were excluded from the analysis because of missing data or because weight was ≤ 40 kg or ≥ 140 kg, and/or height was ≤ 141 cm or ≥ 198 cm.
BMI status of pregnant women
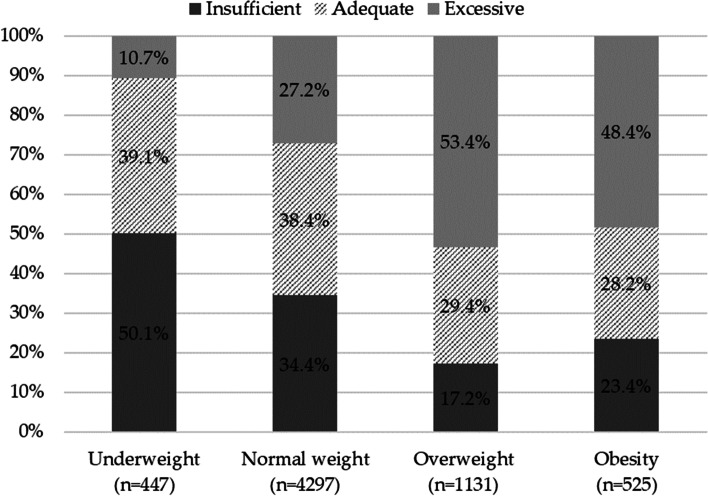
At the beginning of the pregnancy, 7.0% (n = 447) of women were underweight, 67.1% (n = 4297) were normal weight, 17.7% (n = 1131) were overweight and 8.2% (n = 525) had obesity, and these percentages remained unchanged over the study period (2017 vs 2018) (p = 0.99). At the end of the pregnancy, 36.0% (n = 2306) of women had achieved adequate GWG, 31.6% (n = 2021) had grown insufficiently, and 32.4% (n = 2073) excessively. A statistically significant difference in GWG categories was observed between 2017 and 2018 (p = 0.04). In 2018, the percentage of women with insufficient GWG was greater than in 2017 (33.0% vs 30.2%) and the percentage of those with adequate GWG was lower (34.9% vs 37.1%), while the percentage of women with excessive GWG remained virtually unchanged (32.1% vs 32.6%). As expected, insufficient GWG was particularly marked in underweight women (Fig. 2), and this did not change between 2017 and 2018 (49.1% vs 51.2%).
Fig. 2.
Comparison of GWG by BMI category in 2017 and 2018
Excessive GWG mostly occurred in women with a BMI in the overweight and obese range, accounting for more than 50% of the percentage distribution in both categories (Fig. 2), with a significant increase between 2017 and 2018 among women with obesity (44.9% vs 51.9%).
Regional representativeness of women surveyed
Table 1 presents the main socio-economic characteristics of women who accessed the services at T1 (n = 7188), compared to those who didn’t (n = 9240). The differences between the two groups are more evident for the variables “Employed” and “Born in Italy”, which are higher in the survey group.
Table 1.
Characteristics of women involved and not involved in the survey
|
Women involved n (%), N = 7188 |
Women not involved n (%), N = 9240 |
|
|---|---|---|
| Age at delivery | ||
| < 25 years | 564 (7.9) | 874 (9.5) |
| 25–34 years | 4177 (58.1) | 5114 (55.3) |
| ≥ 35 years | 2447 (34.0) | 3252 (35.2) |
| Level of education | ||
| None/completed primary school | 64 (0.9) | 185 (2.0) |
| Completed secondary school | 1101 (15.3) | 1473 (15.9) |
| Completed high school or equivalent | 3381 (47.0) | 4360 (47.2) |
| Bachelor degree or higher | 2642 (36.8) | 3222 (34.9) |
| Born in Italy | ||
| Yes | 5705 (79.4) | 6477 (70.1) |
| Employed | ||
| Yes | 4733 (65.8) | 5470 (59.2) |
| Parity | ||
| Nulliparous | 3705 (51.5) | 4629 (50.1) |
| Smoking during the five years before pregnancy | ||
| Yes | 1709 (23.8) | 2218 (24.0) |
| Medical service mainly used during pregnancy | ||
| Public Local Family Health Unit | 1151(16.0) | 1578 (17.1) |
| Public hospital services | 2605 (36.2) | 3180 (34.4) |
| Private gynaecologist/obstetrician | 2807 (39.1) | 3721 (40.3) |
| Private Local Family Health Unit | 68 (1.0) | 181 (2.0) |
| None | 3 (0.0) | 22 (0.2) |
Accuracy of self-reported versus measured anthropometric data
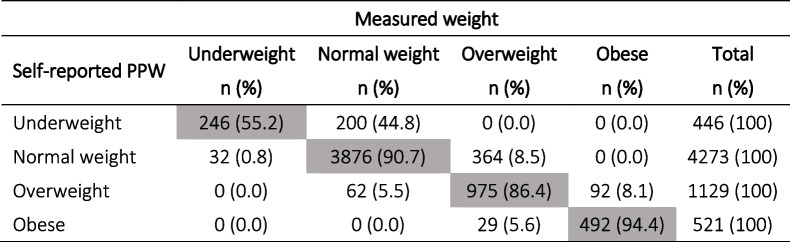
The mean difference between self-reported PPW and measured weight at T1 was 1.45 kg (SD 2.48; p < 0.0001; N = 6393; 95% CI: 1.39–1.52) which significantly affected pre-pregnancy BMI class distribution. Despite the level of agreement being strong (Kappa = 0.82; 95% CI: 0.81–0.83), using weight measured at T1 instead of self-reported pre-pregnancy PPW to calculate the BMI, 44.8% of underweight women shifted to the normal weight category, 8.5% of normal weight women shifted to the overweight category, 5.5% of women in the overweight category downshifted to normal weight and 8.1% shifted to the obese BMI category, while 94.4% of women with obesity maintained their BMI class, as reported in Table 2.
Table 2.
Distribution of BMI classes calculated using self-reported PPW vs weight measured at T1 and measured height at T1 for both (N = 6369)
Grey boxes indicate the number and % of women who remained in the same BMI class regardless of how the weight data was collected
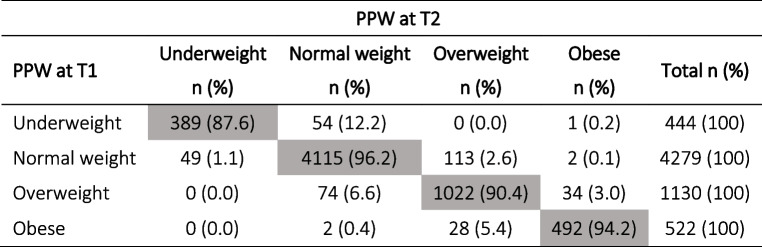
The comparison between PPW self-reported at T1 and at delivery (T2) shows a mean difference of 0.1 kg (SD 2.10, p < 0.0001; N = 6400). As can be inferred from Table 3, this difference mostly concerned underweight women, whose pre-pregnancy BMI class shifted to normal weight in 12.2% of cases, with a strong level of agreement (Kappa = 0.91; 95% CI: 0.90–0.92).
Table 3.
Distribution of BMI classes calculated using PPW self-reported at T1 vs PPW self-reported at T2 and measured height at T1 for both (N = 6375)
Grey boxes indicate the number and % of women who remained in the same BMI class regardless of when PPW data was collected
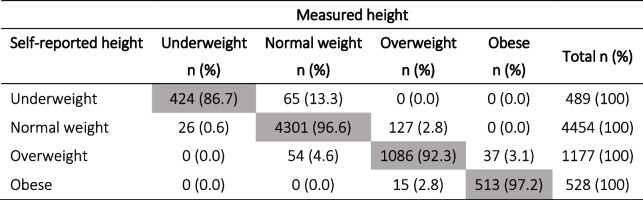
The mean difference between height self-reported at T2 and measured at T1 was 0.4 cm (SD 2.15; p < 0.001; N = 6649). Using height measured at T1 instead of self- reported height to calculate the BMI, 96.6% of normal weight women, 92.3% of overweight women and 97.2% of women with obesity maintained their BMI class, while 13.3% of underweight women shifted to normal weight (Table 4), with a very strong level of agreement (Kappa = 0.93; 95% CI: 0.92–0.94).
Table 4.
Distribution of BMI classes calculated using self-reported height vs height measured at T1 and self-reported PPW for both (N = 6648)
Grey boxes indicate the number and % of women who remained in the same BMI class regardless of how the height data was collected
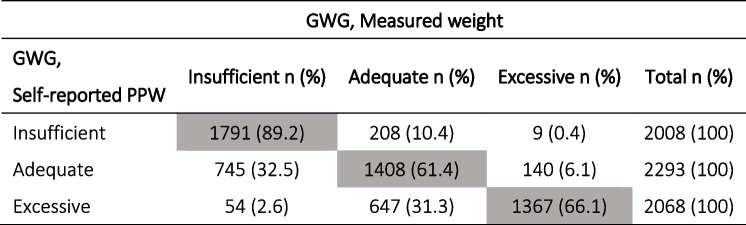
In terms of overall GWG, using the weight measured at T1 instead of the self-reported pre-pregnancy PPW, 31.3% of women classified as having excessive GWG, shifted to adequate, 32.5% of women with adequate GWG shifted to insufficient and 10.4% of women with insufficient GWG shifted to adequate (Table 5). These differences are reflected in a weak level of agreement (Kappa = 0.53; 95% CI: 0.51–0.55).
Table 5.
Distribution of GWG classes using self-reported PPW vs measured weights (N = 6369)
Grey boxes indicate the number and % of women who remained in the same GWG class regardless of how the weight data was collected
Discussion
The results show that our surveillance system, run by health professionals routinely involved in pregnancy care and based on two different data collection times, was able to assess and monitor the anthropometric profile of pregnant women in FVG and to identify groups of women to whom integrative interventions should be targeted. To our knowledge this is the first experience in Italy of setting up a system to monitor the BMI and GWG status of pregnant women using measured data.
In FVG, 25.9% of women are overweight or with obesity at the beginning of pregnancy. Furthermore, only 36.0% of women have GWG appropriate for their pre-pregnancy BMI category, while 32.4% experience excessive weight gain. Additional relevant findings concern underweight before pregnancy and insufficient GWG, which affect 7.0% and 31.6% of women, respectively. These issues should be adequately addressed, given their possible consequences on neonatal health.
It is difficult to compare our findings with other national and international data, because this information is not available for most European countries. In the European region, the proportion of women with overweight and obesity ranges from around 30% to 50% of all women, of which between 8 and 26% have BMI ≥ 30.0 kg/m2 [35]. For Italy, the only available national data derive from the WHO 2009 database that includes all women of childbearing age (20 years or above) and reports an obesity rate of 15% [36]. At national level, our data can be compared to those obtained from self-reported weight and height at delivery in the CeDAP of the Emilia Romagna region. In 2017 and 2018, the overweight and obesity rate in Emilia Romagna was slightly higher than the one in FVG over the same period (27.5% vs 25.9%) [37, 38]. In Emilia Romagna, 53% of women had not achieved the recommended GWG at the end of pregnancy (22% excessive and 31% insufficient GWG), versus 64% (32.4% excessive and 31.6% insufficient) in FVG. These findings may not only be due to population differences, but also to the use of different data collection methods (self-reported only vs a mix of self-reported and actual measurements). At the international level, the overall rate of inadequate GWG observed in FVG (64%) is comparable to those described in a 2018 systematic review by Goldstein et al. for western Europe (69%), and Asia (67%), but lower than that described for the USA (72%) [39]. However, when the data are compared by GWG class, our rate of insufficient GWG is higher (32% vs 21% USA, 18% Europe, 16% Asia), while excessive GWG is lower (32% vs. 51% for all three regions).
One of the aims of our survey was to assess the accuracy of anthropometric data (both weight and height) self-reported by women at the beginning and at the end of pregnancy, and to investigate to what extent self-reported data affect the categorization of pre-pregnancy BMI. Our results show that self-reported PPW was lower than the weight measured at the first prenatal check, as would be expected from the physiological weight gain during the first trimester. In our population, the mean difference between self-reported PPW and measured weight at T1 (1.5 kg) was similar to that reported in other studies [40, 41]. The 2009 IOM recommendations indicate 0.5–2 kg as the normal weight gain for all women in the first 13 weeks of gestation [28]. Thus, our self-reported PPW at T1 appears to be congruent with both weight measured at T1 and data from the literature, thereby providing a reliable approximation of the actual PPW for the purpose of calculating pre-pregnancy BMI and GWG. Since the differences in BMI and GWG we observed are in line with the physiological GWG during the first trimester, they may not be related to PPW misreporting.
Our data also show that self-reported PPW recorded at T2 does not differ from the one collected at T1, and can thus be used as proxy for the latter. It is worth noting that also IOM recommendations on GWG [28] are based on self-reported anthropometric data, especially pre-pregnancy weight, since these values are rarely available as measured data [40, 42].
With regard to height, literature shows that it is often misreported, mostly overestimated, by women, resulting in a shift to a lower BMI class [43]. In our study, the difference between measured and self-reported height was modest (0.4 cm), affecting BMI classification only slightly, and mainly for underweight women. This finding might be explained by the fact that self-reported height at delivery could be influenced by the measurement taken at T1, which in turn can be affected by the difficulty to harmonize data collection procedures and data entering systems. Thus, while in some centers height was measured and entered correctly at T1, in others it was misregistered and rounded to the nearest integer.
A surveillance system such as the one we propose, that links anthropometric data to the socio-demographic information included in the CeDAP, can allow for the individuation of possible predictors that may have an association with maternal BMI status and GWG, as already described in other studies [33, 44]. The identification of segments of the population most at risk for under- or overweight, can usefully support policy makers and Health Authorities in planning targeted strategies of intervention.
The main limitation of our surveillance system is the unexpectedly small number of women who access public health facilities at the beginning of their pregnancy in our region, preferring private services (gynecologist/obstetrician) for antenatal care. As a result of this, the survey was able to reach only 43.8% of the population of pregnant women. The surveyed women were, however, representative of the total population of the region and their characteristics differed only modestly from those of the women who were not included in the survey: for almost all the variables considered, the differences between the two groups were of small entity.
Another limitation of this surveillance system is the long-term sustainability of the data collection effort. The healthcare staff involved in the surveillance reported experiencing difficulties with the routine acquisition and entry of the women's anthropometric data during the first ultrasound check (T1). This could explain the misregistration of measurements and missing data of the 788 women who were excluded from the analysis.
Despite its current limitations, a surveillance system such as the one implemented in FVG, is able to fulfil its purpose as an epidemiological tool to systematically monitor the weight status of women during pregnancy in the larger public context of a region, but is also flexible enough to be adapted to different settings and scales. For example, the surveillance could be simplified by collecting self-reported information on PPW at delivery, rather than during the first ultrasound check, and ensuring that height is measured during maternity stay after childbirth. This would allow the surveillance system to reach the whole of the pregnant population, regardless of whether women in pregnancy are cared for in public or private healthcare services, thereby addressing both study limitations. The system is bound to be relevant also for clinicians, who have a key role in supporting and empowering pregnant women to achieve and maintain a healthy body weight during and after pregnancy.
Conclusions
In Italy, as in other countries, there is no systematic collection of data on maternal overweight and obesity. The only available data refer to the whole female population of childbearing age. Considering the growing obesity epidemic, and in order to pursue the objectives of the latest WHO Food and Nutrition Action Plan (2015–2020) [34], it is crucial to set up surveillance systems that can provide high quality health information to support decision-making on health practices and policies for pregnant women and new born infants.
The present experience in conducting a survey on maternal BMI during pregnancy is in line with these objectives and offers an overview of the weight status of women during the gestational period, even if only at regional level. Despite some limitations, the system allows for continuous standardized collection of anthropometric data, potentially comparable at national and international level, and can be an effective tool to guide public health interventions on maternal and child health. Further studies are needed to assess the effectiveness of our surveillance system and its reproducibility in different contexts.
Acknowledgements
Gestational Weight Survey group (GWS group): Caterina Businelli, Adriano Cattaneo, Claudia Carletti, Maura De Grassi, Enrica Dovier, Manuela Giangreco, Alessandra Glavina, Alessandra Knowles, Valentina Lazzari, Paola Pani, Luca Ronfani, Cristina Tomasi, Giuseppa Verardi (Institute for Maternal and Child Health—IRCCS “Burlo Garofolo”—Trieste); Elena Clagnan, Stefania Del Zotto, Michele Gobbato (ARCS – Azienda Regionale di Coordinamento per la Salute FVG, Udine); Elisa Michelesio (Insiel SPA, Udine); Valentina Capodicasa, Alessandra Citossi, Lorenza Driul; Jessica Fasan, Chiara Mattiussi, Emanuela Vogrig, Serena Xodo (Presidio Ospedaliero Universitario Santa Maria della Misericordia ASUFC, Udine); Anna Gianesini, Diletta Lorenzon, Stefania Maccor, Ilaria Pecile (Policlinico San Giorgio, Pordenone), Marzia Pignat (Ospedale Santa Maria degli Angeli—AS FO, Pordenone); Rubina Banco, Giulia De Zuane, Silvia Raccanelli, (AIED, Pordenone); Carmen Zampis (Dipartimento di Prevenzione—AS FO, Pordenone); Fiorenza Basaldella, Giulia Boscarol, Diletta Degenhardt, Noemi Filipaz, Diandra Gaetani, Roberta Giornelli, Gloria Godeas, Rosa Valentina Zippo (ASUGI); Alessandra Biffi, Paola Cescutti, Annalisa Ianni, Caterina Stefanutti (Presidio Ospedaliero di Tolmezzo, ASU FC); Cristina Alloi, Francesca Magrini, Lucia Pecci, (Presidio Ospedaliero di San Daniele, ASU FC). The authors also wish to thank Dr. Loris Zanier for his contribution to the design and the coordination of the overall study, Dr. Silvana Widmann and Dr. Roberta Rescazzi for helping set up the survey and implementing data collection in their respective study sites, and the healthcare staff of the maternity units that took part in the study for assisting with the collection of anthropometric data.
Abbreviations
- BMI
Body Mass Index
- CeDAP
Certificato di Assistenza al Parto (Certificate of Delivery Care)
- CI
Confidence Interval
- FVG
Friuli Venezia Giulia
- GWG
Gestational Weight Gain
- IOM
Institute of Medicine of the National Academies of Washington
- PPW
Pre-pregnancy weight
- RRMD
Regional Repository of MicroData
- SD
Standard Deviation
- WHO
World Health Organization
Authors’ contributions
PP and CC, contributing equally to this paper, designed, planned and coordinated the study, participated in data analysis and interpretation, and drafted and revised the paper. AK planned and coordinated the study, participated in data analysis and interpretation, and drafted and revised the paper. AC, LR conceived the study, contributed to data analysis and interpretation, critically revised and helped edit the manuscript. MGi defined the statistical analysis plan, performed the analysis and revised the drafts of the paper. EC, MGo and SD helped to design and coordinated the study, and revised the paper. All other members of the GWS group helped to set up the survey, and were in charge of organizing and implementing data collection in the different study sites, with the exception of EM who provided technical support for G2-RRMD data linkage. All authors read and approved the final version of the paper.
Funding
This survey study was supported by a fund allocated by the Regional Health Authority of FVG through the Regional Prevention Plan 2014–2018/ DGR 1243/2015 and by the Ministry of Health, Rome—Italy, in collaboration with the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste—Italy.
Availability of data and materials
Restrictions apply to the availability of these data. Data were obtained from the FVG Regional Health Authority and are available from the authors with the permission of the FVG Regional Health Authority.
Declarations
Ethics approval and consent to participate
The Health Authority (Direzione Centrale Salute) of the Friuli Venezia Giulia Region approved the project (Regional Deliberation n.42 dated 08.04.2008). The same Health Authority gave us consent to use the data routinely collected by the Regional Information System and fully anonymized in the Regional Repository of Microdata, for this epidemiological study of regional relevance, thereby waiving the need for informed consent.
Methods
All methods were performed in accordance with the relevant Italian rules and regulations with participants signing a standard privacy form giving informed consent to the routine acquisition and storage of health data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alessandra Knowles, Email: alessandra.knowles@burlo.trieste.it.
on behalf of the Gestational Weight Survey Group (GWS group):
Caterina Businelli, Maura De Grassi, Enrica Dovier, Alessandra Glavina, Valentina Lazzari, Cristina Tomasi, Giuseppa Verardi, Elisa Michelesio, Valentina Capodicasa, Alessandra Citossi, Lorenza Driul, Jessica Fasan, Chiara Mattiussi, Emanuela Vogrig, Serena Xodo, Anna Gianesini, Diletta Lorenzon, Stefania Maccor, Ilaria Pecile, Marzia Pignat, Rubina Banco, Giulia De Zuane, Silvia Raccanelli, Carmen Zampis, Fiorenza Basaldella, Giulia Boscarol, Diletta Degenhardt, Noemi Filipaz, Diandra Gaetani, Roberta Giornelli, Gloria Godeas, Rosa Valentina Zippo, Paola Cescutti, Annalisa Ianni, Caterina Stefanutti, Cristina Alloi, Francesca Magrini, and Lucia Pecci
References
- 1.Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. 2008;9:635–683. doi: 10.1111/j.1467-789X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- 2.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 3.Patro Golab B, Santos S, Voerman E, Lawlor DA, Jaddoe VW, Gaillard R, et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta-analysis. Lancet Child Adolesc Health. 2018;2:812–821. doi: 10.1016/S2352-4642(18)30273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voerman E, Santos S, Inskip H, Amiano P, Barros H, LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321:1702–15. doi: 10.1001/jama.2019.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachenderan J, Bradford J, McLean M. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol. 2008;48:228–235. doi: 10.1111/j.1479-828X.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- 6.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287 213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 7.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 8.Bogaerts A, Witters I, van den Bergh BRH, Jans G, Devlieger R. Obesity in pregnancy: altered onset and progression of labour. Midwifery. 2013;29:1303–1313. doi: 10.1016/j.midw.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368–374. doi: 10.1097/01.EDE.0000059921.71494.D1. [DOI] [PubMed] [Google Scholar]
- 10.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 13.Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197:223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019;16(6):e1002817. [DOI] [PMC free article] [PubMed]
- 15.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 16.Roseboom TJ, van der Meulen JHP, Ravelli ACJ, Osmond C, Barker DJP, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. doi: 10.1016/S0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 17.Salihu HM, Mbah AK, Alio AP, Clayton HB, Lynch O. Low pre-pregnancy body mass index and risk of medically indicated versus spontaneous preterm singleton birth. Eur J Obstet Gynecol Reprod Biol. 2009;144:119–123. doi: 10.1016/j.ejogrb.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Han Z, Mulla S, Beyene J, Liao G, McDonald SD. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2011;40:65–101. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 19.Lynch AM, Hart JE, Agwu OC, Fisher BM, West NA, Gibbs RS. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am J Obstet Gynecol. 2014;210:428.e1–428.e9. doi: 10.1016/j.ajog.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Girsen AI, Mayo JA, Carmichael SL, Phibbs CS, Shachar BZ, Stevenson DK, et al. Women’s prepregnancy underweight as a risk factor for preterm birth: a retrospective study. BJOG. 2016;123:2008. doi: 10.1111/1471-0528.14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balsells M, García-Patterson A, Corcoy R. Systematic review and meta-analysis on the association of prepregnancy underweight and miscarriage. Eur J Obstet Gynecol Reprod Biol. 2016;207:73–79. doi: 10.1016/j.ejogrb.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Siega-Riz AM, Deierlein A, Stuebe A. Implementation of the new institute of medicine gestational weight gain guidelines. J Midwifery Womens Health. 2010;55:512–519. doi: 10.1016/j.jmwh.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melzer K, Schutz Y. Pre-pregnancy and pregnancy predictors of obesity. Int J Obes (Lond) 2010;34:S44–52. doi: 10.1038/ijo.2010.239. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity - Results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes (Lond) 2007;31:1642–1651. doi: 10.1038/sj.ijo.0803655. [DOI] [PubMed] [Google Scholar]
- 26.Rong K, Yu K, Han X, Szeto IMY, Qin X, Wang J, et al. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr. 2015;18:2172–2182. doi: 10.1017/S1368980014002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooney BL, Schauberger CW, Mathiason MA. Impact of Perinatal Weight Change on Long-Term Obesity and Obesity-Related Illnesses LEVEL OF EVIDENCE II-2. Obstet Gynecol. 2005;106:1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen KM, Yaktine AL, Institute of Medicine (US), National Research Council (US) Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, D.C.: National Academies Press; 2009. [PubMed] [Google Scholar]
- 29.ISTAT. Istituto Nazionale di Statistica. I.Stat, the complete data warehouse for experts. Multipurpose survey on households: aspects of daily life. Health statistics- Body mass index and weight control. Years 2018–2019. Available online at: http://dati.istat.it/# .
- 30.Istituto Superiore di Sanità. Epidemiology for public health. PASSI surveillance system. Data available online at: https://www.epicentro.iss.it/passi/dati/sovrappeso?tab-container-1=tab1#dati.
- 31.World Health Organization. Regional Office for Europe. Body Mass Index - BMI Available online at: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on June 2022).
- 32.Shrewsbury VA, Robb KA, Power C, Wardle J. Socioeconomic differences in weight retention, weight-related attitudes and practices in postpartum women. Matern Child Health J. 2009;13:231–240. doi: 10.1007/s10995-008-0342-4. [DOI] [PubMed] [Google Scholar]
- 33.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36 821 women over a 15-year period. BJOG. 2007;114:187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Regional Office for Europe. European food and nutrition action plan 2015–2020. World Health Organization, Regional Office for Europe (Copenhagen). 2015.
- 35.Euro-Peristat Project. EUROPEAN PERINATAL HEALTH REPORT. Core indicators of the health and care of pregnant women and babies in Europe in 2015. Available online at: https://www.europeristat.com/images/EPHR2015_Euro-Peristat.pdf
- 36.Devlieger R, Benhalima K, Damm P, van Assche A, Mathieu C, Mahmood T, et al. Maternal obesity in Europe: Where do we stand and how to move forward?: A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG) Eur J Obstet Gynecol Reprod Biol. 2016;201:203–208. doi: 10.1016/j.ejogrb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Perrone E, Lupi C, Basevi V, Battaglia S, Gargano G. LA NASCITA IN EMILIA ROMAGNA 15° Rapporto sui dati del Certificato di Assistenza al Parto (CedAP) – Anno 2017. 2018. [Google Scholar]
- 38.Perrone E, Formisano D, Gargano G, Battaglia S, di Mario S, Fieni S, et al. LA NASCITA IN EMILIA-ROMAGNA 16° Rapporto sui dati del Certificato di Assistenza al Parto (CedAP)-Anno 2018. 2019. [Google Scholar]
- 39.Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16(1):153. [DOI] [PMC free article] [PubMed]
- 40.Holland E, Simas TAM, Curiale DKD, Liao X, Waring ME. Self-reported pre-pregnancy weight versus weight measured at first prenatal visit: Effects on categorization of pre-pregnancy body mass index. Matern Child Health J. 2013;17:1872–1878. doi: 10.1007/s10995-012-1210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell A, Gillespie S, Satya S, Gaudet LM. Assessing the Accuracy of Pregnant Women in Recalling Pre-Pregnancy Weight and Gestational Weight Gain. J Obstet Gynaecol Can. 2013;35:802–809. doi: 10.1016/S1701-2163(15)30836-7. [DOI] [PubMed] [Google Scholar]
- 42.Shin D, Chung H, Weatherspoon L, Song WO. Validity of Prepregnancy Weight Status Estimated from Self-reported Height and Weight. Matern Child Health J. 2014;18:1667–1674. doi: 10.1007/s10995-013-1407-6. [DOI] [PubMed] [Google Scholar]
- 43.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 44.Bogaerts A, van den Bergh B, Nuyts E, Martens E, Witters I, Devlieger R. Socio-demographic and obstetrical correlates of pre-pregnancy body mass index and gestational weight gain. Clin Obes. 2012;2:150–159. doi: 10.1111/cob.12004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from the FVG Regional Health Authority and are available from the authors with the permission of the FVG Regional Health Authority.