Abstract
Objective
Perimenopause is associated with an increased risk of developing a major depressive (MD) episode. A significant number of women develop their first MD episode during perimenopause, suggesting a unique pathophysiology of perimenopausal (PM) depression. Previous research has shown that depression is associated with decreased gamma-aminobutyric acid (GABA) levels in the medial prefrontal cortex (MPFC) of MD patients. The objective of this study was to compare MPFC GABA+ levels in healthy reproductive-aged (RD) and PM women.
Methods
A total of 18 healthy PM and 20 RD women were included in the study. MPFC GABA+ levels, which include homocarnosine and macromolecules, were measured via magnetic resonance spectroscopy using a 3 Tesla magnet. MPFC GABA+ levels were referenced to creatine + phosphocreatine (Cr+PCr). Absence of current or past psychiatric diagnosis was confirmed via a structured interview. RD participants were scanned during the early follicular phase of the menstrual cycle. PM women were scanned outside of ovulatory cycles.
Results
Mean MPFC GABA+ concentrations (relative to Cr+PCr) were decreased in the PM group compared with the RD group (PM mean = 0.08 ± 0.02, RD mean = 0.09 ± 0.02, t = −2.03, df = 36, P = .05) even after correcting for in percentage in gray matter (GM). Because PM women were inherently older than RD women (aged 48.8 ± 3.55 and 31.5 ± 9.66 years, respectively), the age difference between the 2 groups was statistically significant (P < .001). When age was treated as an independent covariate and included in the model, the difference in GABA+ between PM and RD women was no longer significant (P = .092).
Conclusion
Perimenopause is associated with decreased MPFC GABA+/Cr+PCr levels, which may contribute to the increased risk of experiencing a MD episode during PM.
Keywords: Perimenopause, GABA+, medial prefrontal cortex, depression, magnetic resonance spectroscopy
Significance Statement.
Depression is a debilitating mood disorder that affects millions of people worldwide. Some women with no history of depression present with their first episode during perimenopause, suggesting a unique pathophysiology of perimenopausal (PM) depression. Patients with major depression display dysregulation of glutamate and gamma-aminobutyric acid (GABA) in the medial prefrontal cortex (MPFC). Our laboratory has previously shown that glutamate levels are decreased in the MPFC of PM women, suggesting reproductive status and the associated hormonal fluctuations that occur during perimenopause may increase women’s risks of experiencing depression. Because GABA’s inhibitory activity counterbalances glutamatergic excitatory activity, it was critical to examine GABA levels in the MPFC of PM women. Our findings of decreased MPFC GABA levels in PM women may contribute to our understanding of the brain biological changes that may contribute to the increased risk of MD during perimenopause.
Introduction
Perimenopause is a phase within the normal female reproductive life cycle characterized by menstrual cycle (MC) irregularities. The average age of onset is 46 years, with a duration of approximately 5 years before the transition into menopause (Speroff, 2002). Women with a history of major depression (MD) or a history of mood sensitivity to female hormone fluctuations, that is, postpartum depression or premenstrual dysphoric disorder are at greater risk of developing perimenopausal (PM) depression (Parry, 2008; Freeman, 2015). Some women with no history of MD or mood sensitivity to female hormone fluctuations present with their first episode of MD during perimenopause (Freeman et al., 2004). This suggests a unique pathophysiology of PM depression.
During perimenopause, before the total cessation of production by the ovaries, estrogen concentrations decrease erratically, with successive increases and decreases, while its counterpart, progesterone, declines in a gradual manner (Gordon et al., 2015).
Gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter of the central nervous system, is widespread throughout the brain, and it is estimated that 60%–75% of all synapses are GABAergic (Schwartz, 1988). Several studies have highlighted the importance of GABA in the pathophysiology of MD (Sanacora et al., 1999, 2004; Hasler et al., 2007; Gabbay et al., 2012; Godfrey et al., 2018). Allopregnanolone (ALLO) is a metabolite of progesterone and a potent positive allosteric modulator of the GABAA receptor (Edinoff et al., 2021). Brexanolone and Zuranolone are exogenous formulations of ALLO and have been shown to be effective in treating postpartum depression and MD, respectively (Edinoff et al., 2021; Walkery et al., 2021).
The medial prefrontal cortex (MPFC) is known to play a role in modulating emotional responses due to its reciprocal connections to the amygdala and hippocampus (Wood and Grafman, 2003). It plays a pivotal role in the negative affective and cognitive symptoms experienced by MD patients (Xu et al., 2019). Furthermore, the MPFC is affected by physiological and pharmacologically induced hormone fluctuations (Joffe et al., 2006).
Magnetic resonance spectroscopy (MRS) is the sole noninvasive neuroimaging technique that enables in vivo detection and measurement of brain metabolite concentrations, such as GABA, in localized brain regions (Ramadan et al., 2013). Homocarnosine (Hcar) and macromolecules (MM) are neurometabolites that share similar chemical shifts as GABA in the human brain. MEscher-GArwood Point RESolved Spectroscopy (MEGA-PRESS) is the spectral difference method used to isolate GABA resonance. However, it is difficult to purely obtain GABA resonance without contamination from MM and Hcar; therefore, GABA levels will be referred to as GABA+ levels (Deelchand et al., 2021). MRS studies have shown that GABA+ levels are decreased in the ventromedial prefrontal cortex and anterior cingulate cortex (ACC) of MD patients (Wang et al., 2016; Kantrowitz et al., 2021).
Neuronal networks within the adult vertebrates mainly consist of excitatory (glutamate) and inhibitory (GABA) neurons, and it is thought that these 2 neurotransmitters are in equal flux with one another (E/I balance) to maintain homeostasis. This E/I balance is thought to play a critical role in maintaining neuronal network functions, and dysregulations to this ratio has been associated with MD (Selten et al., 2018).
Previous work from our laboratory has shown that MPFC glutamate (Glu) concentrations referenced to creatine and phosphocreatine (Cr+PCr) are decreased in healthy PM women compared with healthy reproductive-aged (RD) women (Yap et al., 2021). Because GABA’s inhibitory activity counteracts glutamatergic excitatory activity, it is therefore critical to assess MPFC GABA+ levels in PM women.
The objective of the MRS study presented here was to compare MPFC GABA+/Cr+PCr in healthy PM and healthy RD women. Our hypothesis was that MPFC GABA+ levels referenced to Cr+PCr would be decreased in PM women.
Methods
Participants
A total of 18 PM and 20 RD physically and mentally healthy women were recruited for the study. All participants were at least 18 years of age. The study protocol was approved by the Health Research Ethics Board of the University of Alberta and conducted in accordance with the Declaration of Helsinki. A pre-screening telephone interview was first conducted. Written informed consent was collected from all eligible participants. Following collection of informed consent, participants took part in 2 sessions: a screening interview and a scanning visit. An unavoidable methodological difficulty for our comparison study is that PM women are inherently older than RD women.
Inclusion Criteria
Inclusion criteria for both age groups were physically and mentally healthy women who were 18 years of age and older and used a birth control method that did not deliver female hormones. For the RD group, regular occurrence of MC was required for inclusion. For the PM group, inclusion criteria were the following: undergoing perimenopause, with menopausal status being defined as either early PM (menstrual bleeding had occurred in the past 3 months with changes in frequency over the last 12 months) or late PM (no menstrual bleeding within the past 3 months but some menstruation within the last 12 months). This classification is recommended by the World Health Organization and the Stages of Reproductive Aging Workshop (Soules et al., 2001; Bromberger et al., 2010).
Exclusion Criteria
Exclusion criteria for all participants included the following: (1) current or lifetime history of any psychiatric illness (confirmed using the Mini-International Neuropsychiatric Interview based on Diagnostic and Statistical Manual of Mental Disorders-5 criteria) (Pettersson et al., 2018); (2) any contraindications to MRI; (3) pregnancy; (4) use of birth control methods that deliver female hormone; (5) any medical condition that would interfere with the study, for example, an endocrine, chronic, or neurological condition (Riederer et al., 2006); and (6) intake of medications that may impact brain GABA function at any time while participating in the study, that is, benzodiazepines and valerian root (Lane and Phillips-Bute, 1998). Of note, no participants were taking medications for the duration of the study.
Study Protocol
After completing the phone interview, participants who appeared to be eligible for the study, that is, no history of other mental illnesses or use of hormonal contraceptives, were scheduled for a screening interview. During this interview, complete medical and psychiatric history were completed. The Mini-International Neuropsychiatric Interview was used to screen for psychiatric illnesses (Pettersson et al., 2018). Participants who met the inclusion and exclusion criteria were then booked for a scanning visit. The scanning visit was completed between day 2 and 6 of the follicular phase (FP) of the MC for RD women and early PM women (but during an anovulatory MC). Late PM women were scanned after 3 months of anovulatory cycles. Early and late PM women were contacted later to ensure that they did not have any menses during the following month.
All participants underwent an MRS scan and completed Beck’s Depression Inventory (BDI). PM women were additionally administered the Greene Climacteric Scale (GCS) and Menopause Rating Scale (MeRS) to evaluate their PM-related symptomology. A blood sample measuring plasma estradiol and progesterone was collected from all participants. Third-generation Elecsys immunoassay (Roche Diagnostics) was used to measure plasma estradiol, and Access Progesterone assay (Beckman Coulter) was used to measure plasma progesterone. It is important to note that luteinizing hormone and follicle stimulating hormone are useful for the determination of menopause but are not useful for the determination of perimenopause, which is solely a clinical determination. As a result, neither of these hormones was assessed in this study.
MRS and Imaging
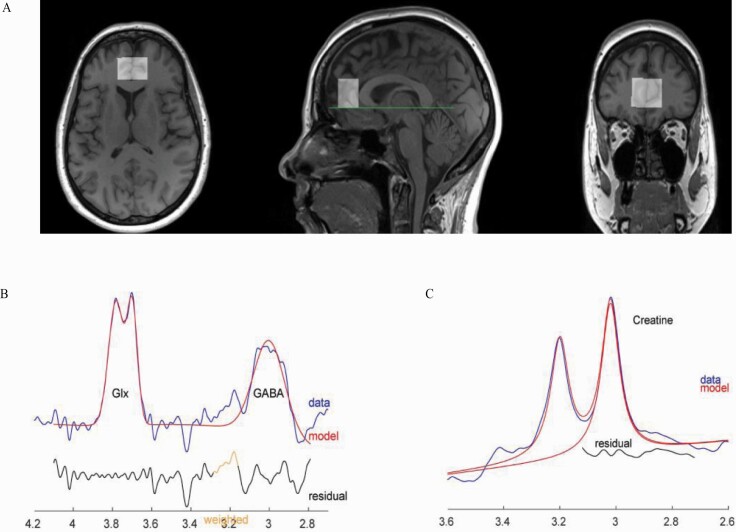
Magnetic resonance data were collected at the Peter S. Allen MR Research Centre, University of Alberta, Edmonton, Canada, using a Siemens Prisma 3 Tesla (T) scanner (Erlangen, Germany) equipped with a 64-channel head-neck coil for signal reception. Anatomical images were acquired using sagittal 3D T1-weighted magnetization-prepared rapid gradient-echo sequence in 3 minutes and 39 seconds (TR: 1800 milliseconds, TE: 2.37 milliseconds, TI: 900 milliseconds, flip angle: 8, field of view: 250 × 250 mm, image matrix: 288 × 288, slice thickness: 0.85 mm, number of slices: 208, resolution: 0.87 × 0.87 × 0.85 mm, parallel acceleration factor: 3). Voxel position and orientation were prescribed so that the voxel was perpendicular to and centered on the midline in transverse and coronal views and parallel to and aligned with the corpus callosum line in a sagittal view (Figure 1A).
Figure 1.
(A) T1 weighted images for a sample participant (55 years old) with overlaid medial prefrontal cortex (MPFC) magnetic resonance spectroscopy (MRS) voxel. Voxel position and orientation were prescribed so that the voxel is perpendicular to and centered on midline in transverse and coronal views and parallel to and aligned with corpus callosum line in sagittal view. (B) Sample gamma-aminobutyric acid including homocarnosine and macromolecules (GABA+) and Glutamix (Glx) spectra (not analyzed in this paper) with fit from Gannet, from the same participant. (C) Sample creatine and phosphocreatine spectrum with fit from Gannet.
These images were used for planning the position of spectroscopy voxels as well as for volumetric and segmentation analysis. MEGA-PRESS, implemented as Siemens “work in progress” WIP859F, was used to measure the spectrum for GABA and Cr+PCr analysis (Harris et al., 2017; Saleh et al., 2019). A sample MEGA-PRESS spectrum is shown in Figure 1B–C.
Adequate signal-to-noise ratio (SNR) was obtained by acquiring data from the voxel positioned in the MPFC (25 × 20 × 30 mm3), summing 320 averages composed of 160 pairs where the editing pulses were either on or off, in 10 minutes and 56 seconds (MEGA-PRESS: TR: 2000 ms, TE: 68 milliseconds, BW: 2000 Hz, editing pulse frequency: 1.9 ppm, delta frequency: 1.7 ppm, 2048 spectral data points). Images in DICOM format and saved raw files (.DAT) for MR spectra were exported from scanner console to Linux Ubuntu 16.04 work station for analysis. Automated metabolite quantification of the proton MR spectra was performed using Gannet software (version 3.0, http://www.gabamrs.com; running in Matlab R2016b), providing relative concentrations of GABA+ to Cr+PCr. No unsuppressed water spectra were acquired.
Manual inspection was conducted on each spectrum fitted by Gannet to assure quality with respect to line shape, line width, and SNR. Data were accepted if SNR > 80 and full width at half maximum (FWHM) < 18 Hz. MRS quality is particularly sensitive to participant motion; the Gannet pipeline automatically excludes samples for which the frequency offset deviates sufficiently to affect the spectrum quality (i.e., it excludes unusable metabolite data because of brief movements during the scanning period). The number of average pairs automatically excluded was similar between the 2 groups (number of excluded samples PM mean = 2.25% ± 2.94%, RD mean = 2.06% ± 2.38%, with maximum excluded samples for 1 participant = 20 out of 320 samples acquired). MRS data for 1 RD and 2 PM participants were severely affected by motion and these research participants were scanned again.
Statistical Parametric Mapping (SPM12) (Penny et al., 2011) were used for T1 image volumetric and segmentation analysis. Included in Gannet package pipeline were steps to generate a mask of the MRS voxel in T1-image space and to utilize the SPM12 “Segmentation” function to calculate relative Grey Matter (GM), white matter (WM), and cerebrospinal fluid (CSF) fractions within MPFC voxel (later referred to as %GM, %WM, and %CSF).
Statistical Analysis
For all statistical tests, the level of significance was defined as P ≤ .05. Statistical analysis was performed using the IBM Statistical Package for Social Sciences software for Windows Version 26.0 (SPSS 26.0) (IBM Corp., Armonk, NY, USA). The data in this study were normally distributed. A 2-tailed t test was used for independent sample analysis of variables (MPFC GABA+/Cr+PCr, %GM, %WM, %CSF, SNR, FitError, and FWHM) between PM and RD women. Additionally, covariate analysis was performed where absolute GM content was treated as a covariate. Metabolite data were analyzed using Cr+PCr as a reference molecule (Figure 1C).
To analyze the impact age had on MPFC GABA+/Cr+PCr levels, simple linear regression was utilized. Multiple regression analysis controlling for age and %GM was conducted to test whether these factors significantly affected MPFC GABA+/Cr+PCr levels. Cross tabulation analysis of Eta coefficient was used to determine the strength of association between participant group and age. A 1-way ANOVA test was used to compare mean MPFC GABA+/Cr+PCr between groups. A 1-way ANCOVA was conducted to determine a statistically significant difference between PM and RD women on MPFC GABA+/Cr+PCr levels, controlling for age, MPFC %GM, and %WM.
The Pearson correlation coefficient was used to analyze the relationship between MPFC GABA+/Cr+PCr levels and age, MPFC GABA+/Cr+PCr levels and female hormone concentrations, and BDI scores and group. Pearson correlation coefficient was also used to analyze the relationship between BDI, GCS, and MeRS scores and mean MPFC GABA+/Cr+PCr levels in the PM group.
Results
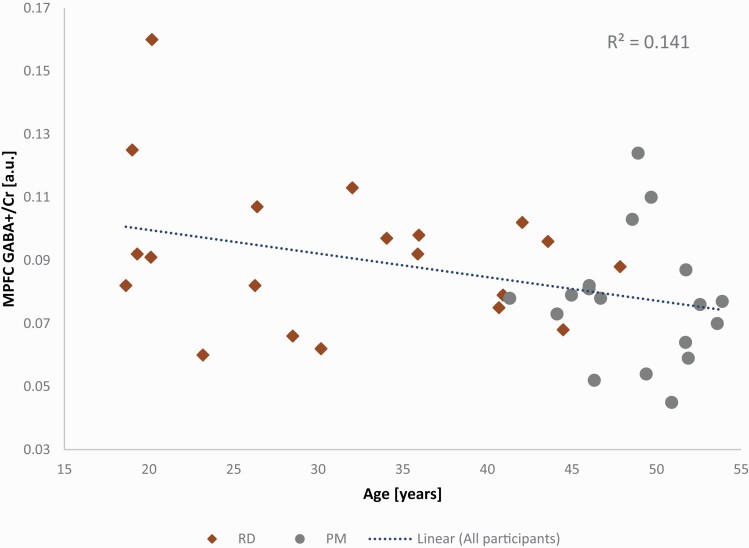
PM women (aged 48.8 ± 3.55 years, range = 41–53 years) were significantly older than RD participants (31.5 ± 9.66 years, range = 18–47 years) (P < .001). Correlational analysis revealed that MPFC GABA+/Cr+PCr levels (Table 3) were significantly related to age with a moderate negative relationship (r = −0.38, P = .02) (Figure 3). Simple linear regression was performed to analyze the impact age had on MPFC GABA+/Cr+PCr levels. Age explained approximately 14% of the observed variance in MPFC GABA+/Cr+PCr [R2 = 0.141, F(1,36) = 5.89, P = .02]. This analysis also showed that age significantly predicted a decrease in MPFC GABA+/Cr+PCr levels (β1 = −0.001, P = .02).
Table 3.
Unadjusted and Covariate Adjusted Descriptive Statistics for MPFC GABA+/Cr+PCr
| Group | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| N | Mean | SE Mean | Mean | SE Mean | |
| Perimenopause | 18 | 0.077 | 0.004 | 0.078 | 0.006 |
| Reproductive | 20 | 0.092 | 0.005 | 0.092 | 0.005 |
Abbreviations: Cr+PCr, creatine + phosphocreatine; GABA+, gamma-aminobutyric acid including homocarnosine and macromolecules; MPFC, medial prefrontal cortex.
Figure 3.
Plot showing negative association between medial prefrontal cortex (MPFC) gamma-aminobutyric acid including homocarnosine and macromolecules (GABA+) levels referenced to creatine+phosphocreatine (Cr+PCr) and age. Both RD and PM groups are shown, with linear regression between MPFC GABA+/Cr+PCr and age shown for all participants.
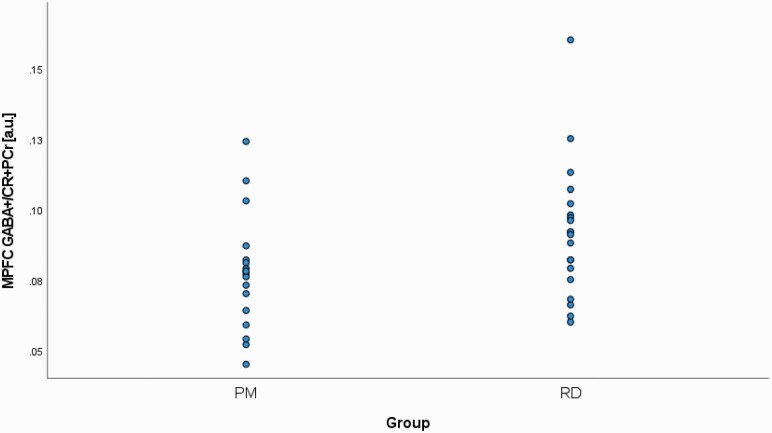
MPFC GABA+/Cr+PCr levels were significantly lower in PM participants (0.08 ± 0.02) compared with RD participants (0.09 ± 0.02) (P = .05) (Figure 2). There was a significant difference in %GM, but not %WM, between PM and RD women (%GM: 54.98 ± 3.51, 58.35 ± 4.62, P = .02; %WM: 27.96 ± 4.33, 27.60 ± 4.89, P = .81), respectively (see Table 1). There was a significant relationship between age and %GM (r = −0.56, P < .001). In addition, there was a significant difference in %CSF between PM and RD women (%CSF: 17.06 ± 3.04, 14.04 ± 2.56, P = .002), respectively (see Table 1).
Figure 2.
Comparison of medial prefrontal cortex (MPFC) creatine and phosphocreatine-referenced (Cr+PCr) gamma-aminobutyric acid including homocarnosine and macromolecules (GABA+) levels in healthy perimenopausal (PM) and reproductive-aged (RD) women. MPFC, medial prefrontal cortex.
Table 1.
Creatine and Phosphocreatine-Referenced GABA+ Concentrations and Brain Tissue Composition Within the MPFC of Healthy PM and RD Women
| PM participants (n = 18) | RD participants (n = 20) | Group | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P |
t
(d.f. = 36) |
|
| Metabolite | ||||||
| GABA+/Cr+PCr | 0.077 | 0.02 | 0.092 | 0.02 | .050* | −2.026 |
| %GM | 54.98 | 3.51 | 58.35 | 4.62 | .017* | −2.510 |
| %WM | 27.96 | 4.33 | 27.60 | 4.89 | .814 | −0.418 |
| %CSF | 17.06 | 3.04 | 14.04 | 2.56 | .002* | 3.318 |
Abbreviations: %CSF, percentage cerebrospinal fluid; GABA+, GABA including homocarnosine and macromolecules; %GM, percentage gray matter; MPFC, medial prefrontal cortex; PM, perimenopausal; RD, reproductive-aged; %WM, percentage white matter.
Brain metabolite measured in institutional units. Metabolite concentration referenced to creatine and phosphocreatine.
*A significant difference between groups.
There was a trend level effect of the combination of age difference and %GM on the observed decrease in MPFC GABA+/Cr+PCr levels [R2 = 0.149, F(1,36) = 3.06, P = .06]. This was shown to be driven by age (β = −0.437, P = .03) and not %GM (β = −0.110, P = .56). Furthermore, when isolating the effects of %GM on MPFC GABA+/Cr+PCr levels, we found that %GM did not significantly explain the decrease in MPFC GABA+/Cr+PCr observed [R2 = 0.018, F(1,36) = 0.669, P = .42]. In addition, %GM on its own did not significantly predict a decrease in MPFC GABA+/Cr+PCr levels (β = .135, P = .42). A multiple regression was conducted to predict MPFC GABA+/Cr+PCr from age and MPFC %GM. These variables did not significantly predict MPFC GABA+/Cr+PCr: F(2,35) = 3.063, P = .059, R2 = .149.
Cross-tabulation analysis of eta coefficient was used to determine the strength of association between participant group and age. This analysis revealed that the participant group was strongly associated with age (η = .77). Knowing that participant group and age were strongly associated, ANOVA analysis without age correction was used to confirm that mean MPFC GABA+/Cr+PCr levels were significantly lower in PM women compared with RD women [F(1,36) = 4.11, P = .05]. In summary, there was a decrease in MPFC GABA+/Cr+PCr levels in PM women compared with RD women, and age differences accounted for approximately 14% for the variances observed.
Based on the ANCOVA analysis, there was no significant effect of reproductive status on MPFC GABA+/Cr+PCr levels after controlling for age (MPFC %GM and %WM, F(1,35) = 3.011, P = .092). On the other hand, a medium effect size was observed (η2 = 0.079) (Table 4).
Table 4.
Analysis of Covariance for MPFC GABA+/Cr+PCr by Group With Brain Tissue Composition as a function of Age (Age, MPFC %GM, and MPFC %WM) as Covariatesa
| Source | SS | df | MS | F | P | η2 |
|---|---|---|---|---|---|---|
| Brain size (covariate) | 6.17E-06 | 1 | 6.17E-06 | 0.013 | 0.912 | 0.00 |
| Group | 0.001 | 1 | 0.001 | 3.011 | 0.092 | 0.079 |
| Error | 0.017 | 35 | 0.00 |
Abbreviations: Cr+PCr, creatine + phosphocreatine; GABA, gamma-aminobutyric acid; GM, gray matter; MPFC, medial prefrontal cortex; WM, white matter.
a R squared = .103 (adjusted R squared = .051).
There were no significant differences in SNR values between PM (122.22 ± 18.51) and RD women (130.94 ± 23.96) (P = .22). Similarly, there were no significant differences in FWHM (P = .74) and FitError (P = .42) values for both groups (see Table 2).
Table 2.
MRS Data Quality Evaluation Between PM and RD Women
| Parameter | PM participants (n = 18) | RD participants (n = 20) | Group | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | t (d.f. = 36) |
|
| SNR | 122.22 | 18.51 | 130.94 | 5.36 | 0.221 | −1.245 |
| FWHM, Hz | 9.96 | 2.03 | 9.77 | 1.56 | 0.739 | 0.336 |
| FitError, % | 11.35 | 2.66 | 10.45 | 3.92 | 0.416 | 0.824 |
Abbreviations: FWHM, full width at half maximum; MRS, magnetic resonance spectroscopy; PM, perimenopausal; RD, reproductive-aged; SNR, signal to noise ratio.
Baseline estradiol (PM: 237.89 ± 280.33; RD: 146.15 ± 53.98; P = .19) and progesterone (PM: 1.74 ± 0.84; RD: 1.79 ± 1.15; P = .89) concentrations did not significantly differ between groups. Correlational analysis revealed that MPFC GABA+/Cr+PCr levels were not significantly correlated to either baseline estradiol (r = −0.12, P = .47) or progesterone (r = −0.01, P = .97) concentrations.
It is important to note that there were instances where hormonal levels were below the detectable range of the assays used. One PM woman (PM group n = 17) had estradiol levels below detectable, and 4 PM (PM group n = 13) and 8 RD women (RD group n = 12) had progesterone levels that were below detectable. Excluding these participants from analysis, we found that estradiol (PM: 250.12 ± 283.97; RD: 146.15 ± 53.98; P = .16) and progesterone (PM: 1.95 ± 0.84; RD: 2.31 ± 1.34; P = .39) concentrations did not significantly differ between groups. Correlational analysis revealed that MPFC GABA+/Cr+PCr levels were not significantly correlated to either baseline estradiol (r = −0.13, P = .44) or progesterone (r = 0.11, P = .58) concentrations.
Although PM women had higher mean BDI scores (3.67 ± 2.89) than RD women (1.45 ± 1.79) (P = .009), scores for both groups were within the range corresponding to scores considered normal mood fluctuations of life. This suggests that participants were not experiencing any clinically significant depressive symptomology. In addition, mean GCS (5.17 ± 4.55) and MeRS (5.22 ± 4.31) scores indicated that PM participants were experiencing little or no PM-related symptomology (RD women were not administered these questionnaires).
Further correlational analyses were conducted between BDI, GCS, and MeRS scores and mean MPFC GABA+/Cr+PCr levels in the PM group. These analyses revealed no significant correlation between BDI (r = 0.41, P = .09) or GCS (r = 0.32, P = .20) scores and mean MPFC GABA+/Cr+PCr levels in the PM group. However, there was a significant correlation between MeRS scores (r = 0.49, P = .04) and mean MPFC GABA+/Cr+PCr levels in PM women.
Discussion
This is the first study, to our knowledge, comparing MPFC GABA+/Cr+PCr levels in healthy PM women and RD women. We showed significantly lower MPFC GABA+ concentrations referenced to Cr+PCr in healthy PM women. This suggests that MPFC GABA+/Cr+PCr levels decrease during the PM period.
Only 1 prior MRS investigation measured GABA levels in the prefrontal cortex of PM women (Wang et al., 2019). Wang et al. (2019) showed that GABA levels in the ACC were decreased in healthy menopausal women compared with healthy PM women. Of note, there was no RD control group in this study.
PM women are inherently older than RD women. The impact of age is therefore difficult to disentangle from PM status. GABA+ is mainly found in GM (Jensen et al., 2005), and full-brain fractional GM declines with age (Ge et al., 2002). Even though we found significant differences in %GM between PM and RD women, multiple regression analysis controlling for age and %GM revealed that these 2 factors did not significantly explain the decrease in MPFC GABA+/Cr+PCr levels observed. It is important to note that when we controlled for age, MPFC %GM, and %WM as covariates, our ANCOVA results suggested that reproductive status no longer had a significant effect on MPFC GABA+/Cr+PCr levels. However, a medium effect size was observed. Despite the loss of statistical significance, our study is still of value because the medium effect size suggests the possibility of finding statistical significance of this comparison with a larger sample size; as some researchers have pointed out, the computation of statistical significance is based on the sample size involved in the analysis (Daniel, 1998; McLean and Ernest, 1998; Cohen, 2013).
As expected from neuroimaging normative studies on the effects of aging on the human brain, the age-associated decrease in GM was associated with an age-associated increase in CSF (Podórski et al., 2021).
Mixed results have also been reported regarding the influence of age on GABA+ levels. For instance, Marenco et al. (2018) found that age was significantly associated with declining levels of GABA+ (referenced to Cr) in the dorsal ACC (mean age = 30 ± 9.2 years). Gao and colleagues (2013) observed a significant negative correlation between age and GABA+ (referenced to Cr) in the frontal region, and women seemed to experience a much faster decline in their GABA+ levels compared with men (men: mean age = 46.1 ± 14.5 years; women: mean age = 45.0 ± 14.7 years). The authors’ voxel of interest was a bit dorsal and caudal compared with ours (Gao et al., 2013). In an older population, Porges and colleagues (2017a) also reported lower GABA concentrations referenced to water, with increased age for both frontal and posterior voxels (a bit dorsal and caudal compared with ours; mean age = 73.12 ± 9.9 years). Aufhaus and colleagues (2013), on the other hand, observed no changes in GABA concentrations (referenced to water) with age in the ACC, using an MM-suppressed MEGA-PRESS sequence (mean age = 34.8 ± 10 years). However, a small age dependency for GABA was observed when MMs were included in the analysis (Aufhaus et al., 2013). Similarly, Porges and colleagues (2017b) observed no significant relationship between age and GABA+ levels (referenced to water) in a frontal voxel when correcting for GM (mean age = 73.2 ± 9.9 years). Interestingly, Pitchaimuthu and co-workers (2017) observed an increase in GABA+ levels (referenced to water) in the visual cortex of older adults (mean = 71; 63–78 years) in contrast to younger adults (mean 28; 20–34 years). This suggests that the relationship between age and GABA+ levels is unclear and might be region specific. In addition, our MPFC voxel partially overlapped with the ACC voxel used in Marenco et al. (2018) and Aufhaus et al. (2013), which makes both studies’ findings relevant to our investigation. The fact that these 2 studies reported different results (one reported a decrease in GABA as a function of age whereas the other reported no change) suggest that age might not have a substantial impact on MPFC GABA+ levels. Additionally, linear regression analysis showed that age contributed to 14% of the variance in MPFC GABA+/Cr+PCr levels, suggesting that other factors, such as PM reproductive status, explain the observed decrease in MPFC GABA+/Cr+PCr levels.
As expected we did not find a significant difference in estrogen and progesterone concentrations between PM and RD women. Indeed, these hormone levels are of little help in clinically determining perimenopause status. This is because female hormones undergo unpredictable variations, especially during perimenopause (Harlow et al., 2012). Accordingly, we saw a greater SD in estradiol levels in PM women compared with RD women. We did not find a significant correlation between concentrations of estradiol and progesterone and MPFC GABA+/Cr+PCr levels in the current study. Female hormones and their metabolites also have a delayed impact on transcription (Bjornstrom and Sjoberg, 2005), which would not be captured by female hormone measurements concomitant to the scanning.
Perimenopause is defined by the physical/physiological consequences of hormonal changes of this unique period of women’s reproductive life (Santoro and Randolph, 2011; Hale et al., 2014). We therefore speculate that brain biological changes such as a decrease in MPFC GABA+/Cr+PCr observed in PM women would be a central physiological consequence of the hormonal changes that indirectly define perimenopause. Epperson and colleagues (2002) observed that healthy menstruating women experienced a significant decrease in occipital GABA levels from the FP to the late luteal phase. This illustrates that fluctuations in female hormonal environments can affect brain GABA levels. Previous research has shown that estrogen has an inhibitory impact on GABA neurotransmission (Murphy et al., 1998), whereas progesterone’s neurometabolite, ALLO, is a positive allosteric modulator of the GABAA receptor (Van Wingen et al., 2008; Deligiannidis et al., 2013). ALLO serum concentrations can fluctuate during perimenopause, with decreased levels being observed (Barbaccia et al., 2000; Bernardi et al., 2003). It is therefore possible that PM women are at an increased risk of developing an MD episode due to their fluctuating ALLO levels. Gordon et al. (2015) posited that as ALLO concentrations fluctuate during perimenopause, GABAA receptors are not able to react as rapidly and adequately compared with their PM stage, which then disrupts the overall GABAergic tone and consequently causes dysfunctions to the hypothalamic-pituitary-adrenal axis, increasing women’s susceptibility to stress and depression. Of note, the exogenous formulation of ALLO, Zuranolone, has been shown to be effective in treating MD (Walkery et al., 2021). The details pertaining to how fluctuations in female hormone levels are associated with GABA+/Cr+PCr levels in perimenopause remain to be elucidated.
It is possible that the design of our study impacted our results. We scanned RD participants during early FP to minimize differences in hormone concentrations, because they are relatively stable at this time compared with other phases of the MC. Different results might have been obtained if scanning had occurred during a different phase of the MC. For instance, Epperson et al. (2002) have shown fluctuations of GABA levels during the MC in healthy reproductive controls.
A limitation of this study was that sample sizes were relatively small for both PM (n = 18) and RD (n = 20) groups. Of the 18 PM participants in total, 12 were identified to be in early perimenopause and the remaining 6 were in late perimenopause. There is a greater risk of experiencing MD in late perimenopause compared with early perimenopause (Colvin et al., 2017). However, due to the small sample size of PM women, we were unable to perform meaningful comparisons of MPFC GABA+/Cr+PCr levels between early PM and late PM women. Future studies with greater numbers of women in late perimenopause should be conducted. Although there is no cutoff for estradiol levels that allows to distinguish early perimenopause from late perimenopause (Su and Freeman, 2009), it has been shown that the greatest decrease in estradiol levels occurs during late perimenopause. A larger number of late PM women in our sample of PM women may have increased the likelihood of finding difference in estradiol levels between PM women and RD women.
Although this study has been conducted on a 3T magnet, allowing for resolving target metabolites, there are also potential limitations to using MRS. First, although MEGA-PRESS is a robust spectral difference method, it is difficult to obtain only GABA resonance without contamination from MM and Hcar, because the latter metabolites share similar chemical shifts as GABA (Deelchand et al., 2021). Thus, our MPFC GABA+/Cr+PCr results could have been confounded by variations in these metabolites. Although Cr+PCr has been used extensively as a reference molecule in previous MRS MD research (Moriguchi et al., 2019), our reported differences in MPFC GABA+/Cr+PCr levels may instead reflect changes in the concentration of Cr+PCr. However, our group recently demonstrated that Cr+PCr was unaffected by the acute female hormone changes experienced during pregnancy and postpartum (McEwen et al., 2021; Ghuman et al., 2022,Ghuman et al., 2022). Age has been shown to affect Cr+PCr in certain brain regions. Because we had an age difference between groups, an age effect on Cr+PCr would be a potential confounding factor in our study. Lind et al. (2020) found that age did not impact Cr+PCr in the ACC when comparing the younger age group (aged 18–26 years) with the middle-age group (aged 39–50 years). Because the younger and middle-age groups were similar in age to our RD and PM groups, respectively, this indicates that age differences were unlikely to have affected the Cr+PCr levels in our study. Together, these findings indicate that Cr+PCr is a viable reference lecule for MRS research in PM women.
Although we postulate that MPFC GABA+/Cr+PCr levels are decreased in PM women due to their reproductive status (and the concomitant hormonal fluctuations), it is still important to acknowledge the possibility of other age-mediated processes responsible for this observation. To elucidate the effect of age on MPFC GABA+/Cr+PCr in PM women, our team will expand the current study moving forward by including a group of menopausal women as controls. These women will be of similar or greater age than the PM women.
We observed a significant correlation between MeRS scores and mean MPFC GABA+/Cr+PCr levels in PM women. However, the mean MeRS scores for PM women (5.22 ± 4.31) correspond to mild symptoms (Heinemann et al., 2004), suggesting a lack of clinical relevance. In addition, there was no significant correlation between GCS scores and mean MPFC GABA+/Cr+PCr levels in PM women (P = .20), which is another tool for assessing PM-related symptomology. Thus, we are very reluctant to discuss this interaction further.
We assume that the decrease of MPFC GABA+/Cr+PCr levels is related to GABAergic neurotransmission. However, Carbon-13 MRS is the only in vivo noninvasive method that allows for determination of both GABAergic neurotransmission and cell-specific energetics with signaling and nonsignaling purposes (Hyder and Rothman, 2017). As a result, it is difficult to determine the source of our GABA+/Cr+PCr measurements in the MPFC because it can be either neurotransmission related, neuronal metabolism related, or both. For the same reasons, Carbon-13 MRS concomitant measurements of both GABA and Glu would also be necessary to assess the balance of GABA and Glu neurotransmission in PM women.
Acknowledgments
We thank the participants for their commitment to this project and Sidney Yap, who assisted with data collection.
Contributor Information
Kim H Tran, University of Alberta, Department of Psychiatry, Edmonton, AB, Canada.
Jessica Luki, University of Alberta, Department of Psychiatry, Edmonton, AB, Canada.
Sarah Hanstock, University of Alberta, Department of Psychiatry, Edmonton, AB, Canada; University of Alberta, Department of Biomedical Engineering, Edmonton, AB, Canada.
Christopher C Hanstock, University of Alberta, Department of Psychiatry, Edmonton, AB, Canada; University of Alberta, Department of Biomedical Engineering, Edmonton, AB, Canada.
Peter Seres, University of Alberta, Department of Biomedical Engineering, Edmonton, AB, Canada.
Katherine Aitchison, University of Alberta, Department of Psychiatry, Edmonton, AB, Canada; University of Alberta, Department of Medical Genetics, Edmonton, AB, Canada; University of Alberta, Neuroscience and Mental Health Institute, Edmonton, AB, Canada; Northern Ontario School of Medicine, Division of Clinical Sciences, Psychiatry Section, Thunder Bay, ON, Canada.
Tami Shandro, Lois Hole Hospital for Women, Royal Alexandra Hospital, Edmonton, AB, Canada.
Jean-Michel Le Melledo, University of Alberta, Department of Psychiatry, Edmonton, AB, Canada.
This research study was funded through the Cranston Family Grant. K.J.A. received a research grants (fellowship grant for trainee) from Janssen, Inc, Canada.
Interest Statement
All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
K.H.T. contributed to investigation, data analysis, methodology, project administration, writing, and revision of the original draft. J.L. contributed to investigation, data collection, project administration, methodology, reviewing, and editing the manuscript. S.H. contributed to investigation, reviewing, and editing the manuscript. C.H. contributed to funding acquisition, data interpretation, reviewing, and editing the manuscript. P.S. contributed to investigation, data analysis, reviewing, and editing the manuscript. T.S. contributed to funding acquisition, investigation, reviewing, and editing the manuscript. K.J.A. contributed to investigation, data interpretation, graduate student funding, reviewing, and editing the manuscript. J.-M.L.M. contributed to writing, editing the manuscript, funding acquisition, conceptualization, and supervision. All authors contributed to the article and approved the submitted version.
Ethical Approval
The study was conducted in accordance with the declaration of Helsinki. Informed consent was obtained from all individual participants. The study protocol was approved by the Health Research Ethics Board of the University of Alberta.
References
- Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, Meyer-Lindenberg A, Boettcher U, Ende G(2013) Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med 69:317–320. doi: 10.1002/mrm.24257. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Lello S, Sidiropoulou T, Cocco T, Sorge RP, Cocchiarale A, Piermarini V, Sabato AF, Trabucchi M, Romanini C(2000) Plasma 5α–androstane–3α, 17βdiol, an endogenous steroid that positively modulates GABAA receptor function, and anxiety: a study in menopausal women. Psychoneuroendocrinology 25:659–675. doi: 10.1016/s0306-4530(00)00017-2. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pieri M, Stomati M, Luisi S, Palumbo M, Pluchino N, Ceccarelli C, Genazzani AR (2003) Effect of different hormonal replacement therapies on circulating allopregnanolone and dehydroepiandrosterone levels in postmenopausal women. Gynecol Endocrinol 17:65–77. doi: 10.1080/gye.17.1.65.77. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M (2005) Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, Randolph JF Jr, Matthews KA (2010) Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Arch Gen Psychiatry 67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (2013) Statistical power analysis for the behavioral sciences. New York: Routledge. [Google Scholar]
- Colvin A, Richardson GA, Cyranowski JM, Youk A, Bromberger JT(2017) The role of family history of depression and the menopausal transition in the development of major depression in midlife women: study of women’s health across the nation mental health study (SWAN MHS). Depress Anxiety 34:826–835. doi: 10.1002/da.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel LG (1998) Statistical significance testing: a historical overview of misuse and misinterpretation with implications for the editorial policies of educational journals. Research in the Schools 25:23–32. [Google Scholar]
- Deelchand DK, Marjańska M, Henry PG, Terpstra M (2021) MEGA-PRESS of GABA+: influences of acquisition parameters. NMR Biomed 34:e4199. doi: 10.1002/nbm.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, Kosma CA, Rothschild AJ, Moore CM (2013) GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res 47:816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinoff AN, Odisho A, Lewis K, Kaskas A, Hunt G, Cornett EM, Kaye AD, Kaye A, Morgan J, Barrilleaux PS, Lewis D, Viswanath O, Urits I (2021) Brexanolone, a GABAA modulator, in the treatment of postpartum depression in adults: a comprehensive review. Front Psychiatry 12:699740. doi: 10.3389/fpsyt.2021.699740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH (2002) Cortical γ-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Freeman EW (2015) Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health 1:1–11. doi: 10.1186/s40695-015-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L (2004) Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry 61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC (2012) Anterior cingulate cortexγ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry 69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB (2013) Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL (2002) Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol 23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Ghuman A, McEwen A, Kim Tran KH, Mitchell N, Hanstock C, Seres P, Jhangri G, Burgess D, Baker G, Le Melledo J-M (2022) Prospective investigation of glutamate levels and percentage of grey matter in the medial prefrontal cortex in females at risk for postpartum depression. Curr Neuropharmacol 20:1988–2000. doi: 10.2174/1570159X20666220302101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KE, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD (2018) Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J Psychiatr Res 105:33–44. doi: 10.1016/j.jpsychires.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, Wisner KL (2015) Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry 172:227–236. doi: 10.1176/appi.ajp.2014.14070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale GE, Robertson DM, Burger HG (2014) The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol 142:121–131. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Harlow SD, et al. (2012) Executive summary of the Stages of Reproductive Aging Workshop+10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97:1159–1168. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Saleh MG, Edden RA (2017) Edited 1H magnetic resonance spectroscopy in vivo: methods and metabolites. Magn Reson Med 77:1377–1389. doi: 10.1002/mrm.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007) Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Heinemann K, Ruebig A, Potthoff P, Schneider HP, Strelow F, Heinemann LA, Do MT (2004) The Menopause Rating Scale (MRS) scale: a methodological review. Health Qual Life Outcomes 2:1–8. doi: 10.1186/1477-7525-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL (2017) Advances in imaging brain metabolism. Annu Rev Biomed Eng 19:485–515. doi: 10.1146/annurev-bioeng-071516-044450. [DOI] [PubMed] [Google Scholar]
- Jensen JE, Frederick B, Renshaw PF (2005) Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed 18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA (2006) Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Dong Z, Milak MS, Rashid R, Kegeles LS, Javitt DC, Lieberman JA, Mann JJ (2021) Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl Psychiatry 11:1–6. doi: 10.1038/s41398-021-01541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Phillips-Bute BG (1998) Caffeine deprivation affects vigilance performance and mood. Physiol Behav 65:171–175. doi: 10.1016/s0031-9384(98)00163-2. [DOI] [PubMed] [Google Scholar]
- Lind A, Boraxbekk CJ, Petersen ET, Paulson OB, Siebner HR, Marsman A (2020) Regional myo-inositol, creatine, and choline levels are higher at older age and scale negatively with visuospatial working memory: a cross-sectional proton MR spectroscopy study at 7 tesla on normal cognitive ageing. J Neurosci 40:8149–8159. doi: 10.1523/JNEUROSCI.2883-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Meyer C, van der Veen JW, Zhang Y, Kelly R, Shen J, Weinberger DR, Dickinson D, Berman KF (2018) Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition. Neuropsyhopharmacology 43:2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen AM, Burgess DTA, Hanstock SEC, Hanstock CC, Seres P, Khalili P, Newman SC, Baker GB, Mitchell ND, Allen PS, Le Melledo J-M (2021). Glutamate levels in the medial prefrontal cortex of healthy pregnant women compared to non-pregnant controls. Psychoneuroendocrinology 133:105382. doi: 10.1016/j.psyneuen.2021.105382. [DOI] [PubMed] [Google Scholar]
- McLean JE, Ernest JM (1998) The role of statistical significance testing in educational research. Research in the schools. 5(2). Neuropsychopharmacology 43:2285–2291. doi: 10.1038/s41386-018-0134-5. [DOI] [Google Scholar]
- Moriguchi S, et al. (2019) Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry 24:952–964. doi: 10.1038/s41380-018-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M (1998) Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL (2008) Perimenopausal depression. Am J Psychiatry 165:23–27. doi: 10.1176/appi.ajp.2007.07071152. [DOI] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, eds. 2011. Statistical parametric mapping: the analysis of functional brain images. UK: Academic Press. doi: 10.1016/B978-0-12-372560-8.X5000-1. [DOI] [Google Scholar]
- Pettersson A, Modin S, Wahlström R, af Winklerfelt Hammarberg S, Krakau I (2018) The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: a mixed-methods study. BMC Fam Pract 19:1–13. doi: 10.1186/s12875-017-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchaimuthu K, Wu QZ, Carter O, Nguyen BN, Ahn S, Egan GF, McKendrick AM (2017) Occipital GABA levels in older adults and their relationship to visual perceptual suppression. Sci Rep 7:1–11. doi: 10.1038/s41598-017-14577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórski P, Bladowska J, Sasiadek M, Zimny A (2021) Novel volumetric and surface-based magnetic resonance indices of the aging brain–does male and female brain age in the same way? Front Neurol 12:645729. doi: 10.3389/fneur.2021.645729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA (2017a) Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging 2:38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Lamb DG, Williamson JB, Cohen RA, Edden RA, Harris AD (2017b) Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage 162:249–256. doi:10.1016%2Fj.neuroimage.2017.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan S, Lin A, Stanwell P (2013) Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed 26:1630–1646. doi: 10.1002/nbm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer F, Bittšanský M, Schmidt C, Mlynárik V, Baumgartner C, Moser E, Serles W (2006) 1H magnetic resonance spectroscopy at 3 T in cryptogenic and mesial temporal lobe epilepsy. NMR Biomed 19:544–553. doi: 10.1002/nbm.1029. [DOI] [PubMed] [Google Scholar]
- Saleh MG, Rimbault D, Mikkelsen M, Oeltzschner G, Wang AM, Jiang D, Alhamud A, Near J, Schär M, Noeske R, Murdoch JB, Ersland L, Craven AR, Dwyer GE, Grüner ER, Pan L, Ahn S, Edden RA (2019) Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. Neuroimage 189:425–431. doi: 10.1016/j.neuroimage.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH (1999) Reduced cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF (2004) Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Santoro N, Randolph JF (2011) Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am 38:455–466. doi: 10.1016/j.ogc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD (1988) The GABAA receptor-gated ion channel: biochemical and pharmacological studies of structure and function. Biochem Pharmacol 37:3369–3375. doi: 10.1016/0006-2952(88)90684-3. [DOI] [PubMed] [Google Scholar]
- Selten M, van Bokhoven H, Kasri NN (2018) Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Res 7:23. doi: 10.12688/f1000research.12155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N (2001) Executive summary: stages of reproductive aging workshop (STRAW). Climacteric 4:267–272. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- Speroff L (2002) The perimenopause: definitions, demography, and physiology. Obstet Gyn Clin N Am 29:397–410. doi: 10.1016/S0889-8545(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Su HI, Freeman EW (2009) Hormone changes associated with the menopausal transition. Minerva Ginecol 61:483–489. [PMC free article] [PubMed] [Google Scholar]
- Van Wingen GA, Van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar J, Fernández G (2008) Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry 13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Walkery A, Leader LD, Cooke E, VandenBerg A (2021) Review of allopregnanolone agonist therapy for the treatment of depressive disorders. Drug Des Devel Ther 15:3017. doi: 10.2147/DDDT.S240856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang A, Zhao B, Gan J, Wang G, Gao F, Liu B, Gong T, Liu W, Edden RAE (2016) GABA+ levels in postmenopausal women with mild-to-moderate depression: a preliminary study. Medicine (Baltim) 95:e4918. doi: 10.1097/MD.0000000000004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang X, Luo MT, Wang H, Li YH (2019) Gamma-aminobutyric acid levels in the anterior cingulate cortex of perimenopausal women with depression: a magnetic resonance spectroscopy study. Front Neurosci 13:785. doi: 10.3389/fnins.2019.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J (2003) Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci 4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Xu P, Chen A, Li Y, Xing X, Lu H (2019) Medial prefrontal cortex in neurological diseases. Physiol Genomics 51:432–442. doi: 10.1152/physiolgenomics.00006.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap S, Luki J, Hanstock CC, Seres P, Shandro T, Hanstock SE, Lirette A, Zhao HH, Aitchison K, Le Melledo J-M (2021) Decreased medial prefrontal cortex glutamate levels in perimenopausal women. Front Psychiatry 12:763562. doi: 10.3389/fpsyt.2021.763562. [DOI] [PMC free article] [PubMed] [Google Scholar]