Abstract
Campylobacter jejuni encodes a cytolethal distending toxin (CDT) that causes cells to arrest in the G2/M transition phase of the cell cycle. Highly related toxins are also produced by other important bacterial pathogens. CDT activity requires the function of three genes: cdtA, cdtB, and cdtC. Recent studies have established that CdtB is the active subunit of CDT, exerting its effect as a nuclease that damages the DNA and triggers cell cycle arrest. Microinjection of CdtB into target cells led to G2/M arrest and cytoplasmic distention, in a manner indistinguishable from that caused by CDT treatment. Despite this progress, nothing is known about the composition of the CDT holotoxin or the function of CdtA and CdtC. We show here that, when applied individually, purified CdtA, CdtB, or CdtC does not exhibit toxic activity. In contrast, CdtA, CdtB, and CdtC when combined, interact with one another to form an active tripartite holotoxin that exhibits full cellular toxicity. CdtA has a domain that shares similarity with the B chain of ricin-related toxins. We therefore proposed that CDT is a tripartite toxin composed of CdtB as the enzymatically active subunit and of CdtA and CdtC as the heterodimeric B subunit required for the delivery of CdtB.
Campylobacter jejuni is the leading cause of bacterial food-borne illness in the United States and Europe (21). Despite its great importance in human health, relatively little is known about its pathogenesis (34). This paucity of knowledge is probably due to the relatively recent recognition of C. jejuni as an important human pathogen (6, 30), the lack of simple animal models to facilitate its study (9, 31), the deficiency of the available genetic tools to generate random mutants (10), or a combination of these and other factors. A very significant development in Campylobacter research has been the recent completion of the determination of the entire nucleotide sequence of its genome (24). Despite this major advancement, there are only a few C. jejuni genes that are known or predicted to be directly involved in virulence. One of these factors is the cytolethal distending toxin (CDT), which causes eukaryotic cells to arrest in the G2/M transition phase of the cell cycle (25, 33). In addition to C. jejuni, CDTs have also been described in other important bacterial pathogens, such as Campylobacter coli (15), Campylobacter fetus (15), Shigella spp. (23), Haemophilus ducreyi (3), Actinobacillus actinomycetemcomitans (32), Helicobacter hepaticus (35), and certain pathogenic strains of Escherichia coli (16, 28). Cells intoxicated with CDT exhibit a characteristic distention of their cytoplasm, an increase in their DNA content, and the accumulation of the inactive, tyrosine phosphorylated form of Cdc2, a key regulator of cell cycle progression (2, 5, 29, 33).
CDT is encoded by three genes, cdtA, cdtB, and cdtC, which are required for cytotoxicity (26). Recent studies provided major insight into the mechanism of action of this toxin. Elwell and Dreyfus (7) and Lara-Tejero and Galan (19) reported that CdtB exhibits a striking amino acid sequence similarity to members of the DNase I protein family. The amino acid sequence similarity is limited to a number of residues that mutagenesis and structural analysis have shown to be essential for nuclease activity (8, 18, 20). Mutations in these residues completely abolished CDT activity (7, 19). These results, coupled to the observation that purified CdtB exhibited in vitro nuclease activity, led to the hypothesis that CDT exerts its effect by damaging DNA, thereby triggering a cell cycle control checkpoint that leads to G2/M arrest. Indeed, it was shown that transient expression of CdtB in host cells resulted in catastrophic changes in the chromatin (19). More importantly, CdtB, when microinjected into host cells, by itself was capable of recapitulating all the toxic effects of the CDT holotoxin, including G2/M arrest and cytoplasm distention (19).
Although these recent studies demonstrated that CdtB is the active subunit of CDT, nothing is known about the composition of the CDT holotoxin and the function of CdtA and CdtC (26). For example, it is not known whether CdtA and/or CdtC are components of a putative CDT holotoxin or whether these proteins play a role in the secretion and/or translocation into target cells of the active subunit. Studies on CDT have been hampered by the difficulties in its isolation (26). These technical difficulties, coupled to the apparent high specific activity of the toxin, have led to several contradictory reports that cannot be reconciled with one another. For example, some reports have indicated that CdtB (29) or CdtC (27) by themselves could induce toxic effects. However, none of these reports could demonstrate that the preparations used in the studies were devoid of the other components of the toxin.
We report here that purified CdtA, CdtB, or CdtC do not exhibit toxic activity when applied to cells individually. However, CdtA, CdtB, and CdtC when combined, interact with one another to form an active tripartite holotoxin.
MATERIALS AND METHODS
Bacterial strains, media, growth conditions, and plasmid constructions.
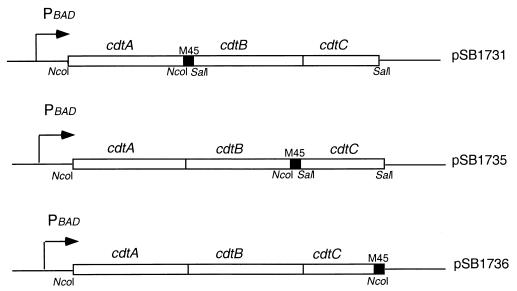
E. coli DH5α was the host strain for recombinant CDT expression. E. coli M15(pREP4) was the host strain for recombinant CdtA-His6 and CdtC-His6 expression. Expression of glutathione S-transferase (GST)–CdtA and GST-CdtB was carried out in E. coli BL21(DE3). Plasmids alternatively expressing the cdt operon with one of the three Cdt proteins tagged at their C terminus with an epitope derived from the E4-6/7 protein of adenovirus and recognized by the M45 monoclonal antibody (22) were constructed using standard molecular biology techniques as follows. The vector backbone for all the three constructs was the C-terminal M45 epitope-tagging vector pSB616 (1), which allows expression of the cloned operon from the tightly regulated PBAD promoter that can be induced upon the addition of arabinose (11). A plasmid encoding the cdt operon with an M45 epitope-tagged version of CdtA was constructed by cloning the PCR-amplified cdtA gene upstream of and in frame with the M45 tag using the unique NcoI site of pSB616. A PCR-amplified DNA segment encoding cdtBC was cloned immediately downstream of the epitope-tagged cdtA into a unique SalI site, yielding the plasmid pSB1731. A plasmid encoding the cdt operon with an M45 epitope-tagged version of cdtB was constructed by cloning a PCR-amplified DNA fragment encoding cdtAB in frame with the M45 tag of pSB616 using the unique NcoI site. A DNA segment encoding cdtC was then cloned in the SalI site downstream of the epitope tag, resulting in plasmid pSB1735. A plasmid encoding a cdt operon with an M45 version of cdtC was constructed by cloning in frame with the M45 tag a PCR-amplified DNA fragment encoding cdtABC into the unique NcoI site of pSB616 (Fig. 1 shows the organization of these M45 epitope-tagged operons). All the genes were amplified from C. jejuni 81-176 chromosomal DNA. All plasmid constructs led to expression of the different Cdt subunits upon induction with arabinose and were as toxic as the wild-type operon, indicating that the presence of the epitope tags did not affect the function of the different Cdt proteins. All epitope-tagged proteins were readily detectable by Western blot analysis using an anti-M45 monoclonal antibody. Plasmids encoding the individual Cdt epitope-tagged subunits were constructed by PCR amplifying the different DNA segments from C. jejuni 81-176 chromosomal DNA and subsequent cloning into the epitope tagging vector pSB616. This procedure yielded the plasmids pSB1723, pSB1382, and pSB1738, which encode M45 epitope-tagged versions of CdtA, CdtB, and CdtC, respectively. Plasmids encoding His6-tagged CdtA or CdtC lacking their secretion signal sequence were constructed by PCR amplification of the appropriate DNA fragments from C. jejuni 81-176 chromosomal DNA and their subsequent cloning into the tagging vector pQE60 (Qiagen), resulting in the plasmids pSB1958 and pSB1961, respectively. Plasmids encoding GST-tagged CdtA and GST-tagged CdtB without their signal sequence were constructed by PCR amplifying the relevant DNA segments from C. jejuni 81-176 chromosomal DNA and subsequently cloning them into the tagging vector pGEX-KG (Pharmacia) to yield plasmids pSB1727 and pSB1953, respectively.
FIG. 1.
Diagram of plasmid constructs expressing M45 epitope-tagged versions of the different Cdt proteins.
Tissue culture.
Henle-407 intestinal epithelial cells were grown as described previously (19) in Dulbecco modified Eagle medium (DMEM) supplemented with 10% bovine calf serum (BCS) under a 5% CO2 atmosphere.
Toxicity assay.
Henle-407 intestinal epithelial cells seeded at ∼20% confluency were treated for 2 h with 30 μl of each column fraction or a series of twofold dilutions of these preparations. After the treatment, cells were washed with phosphate-buffered saline (PBS), and fresh complete DMEM medium was added. Cells were observed for up to 72 h. A given dilution was considered toxic when at least 50% of the cells on a dish showed clear signs of intoxication, as determined by the characteristic cytoplasmic distention and enlargement of the nucleus. A toxic titer was assigned as the reciprocal of the higher dilution that was considered toxic.
Protein purification for toxin reconstitution assay.
C-terminal His6 tagged CdtA and CdtC recombinant proteins were purified as follows from inclusion bodies obtained from E. coli M15(pREP4, pSB1958) and M15(pREP4, pSB1961), respectively. E. coli M15(pREP4, pSB1958) and M15(pREP4, pSB1961) encoding CdtA-His6 and CdtC-His6, respectively, were grown on 1 liter of 2×TY supplemented with 25 μg of kanamycin and 100 μg of ampicillin per ml to an optical density at 600nm (OD600) of 0.6 to 0.7. Expression of CdtA-His6 and CdtC-His6 was then induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and cultures were incubated for 5 h to allow expression of the recombinant proteins. Bacterial cells were then harvested by centrifugation, washed in cold (PBS) at pH 7.4, and resuspended in 10 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole; pH 8). Cells were lysed by two passages through a French pressure cell at 100,000 kPa. The cell lysate was clarified by centrifugation at 16,000 rpm for 30 min to recover the pellet containing the cellular debris and inclusion bodies. Inclusion bodies were solubilized by resuspension in 10 ml of solubilization buffer (8 M urea, 100 mM NaH2PO4, 10 mM Tris; pH 8). The suspension was stirred at room temperature for at least 1 h. After incubation, the solubilized material was clarified by centrifugation at 16,000 rpm for 30 min. The supernatant containing the solubilized His6-tagged protein was transferred to a 14-ml conical tube containing 1 ml of Ni-nitrilotriacetic acid agarose beads (Qiagen), and the mixture was incubated with gentle rocking for 1 h at 4°C. After binding, the beads were pelleted by centrifugation at 1,500 rpm and washed five times with 10 ml of ice-cold wash solution (8 M urea, 100 mM NaH2PO4, 10 mM Tris; pH 6.5). Protein elution was carried out by incubation for 10 min in 5 ml of elution buffer (8 M urea, 100 mM NaH2PO4, 100 mM EDTA, 10mM Tris; pH 4.5). The elution procedure was repeated five times, and all of the eluents were pooled. The eluted protein was diluted to 0.1 mg/ml in the solubilization buffer and refolded by dialysis against a renaturalization buffer (25 mM Tris, pH 8; 200 mM NaCl; 10% glycerol; 2 mM EDTA; 5 mM dithiothreitol [DTT]). Once refolded, the protein was concentrated using Centriprep-10 concentrator device (Amicon).
N-terminal GST-tagged CdtB recombinant protein was purified as follows. E. coli BL21(DE3)(pSB1953) was grown at 37°C in 1 liter of 2×TY supplemented with 100 μg of ampicillin per ml to an OD600 of 1.0. At that point, expression of GST-CdtB was induced by the addition of 1 mM IPTG, and cells were incubated for an additional 5 h at 30°C. After expression of the recombinant protein, cells were harvested by centrifugation, washed in ice-cold PBS, and resuspended in 10 ml of lysis buffer (50 mM Tris-HCl, pH 8; 200 mM NaCl; 10 mM DTT). Cells were lysed by two passages through a French pressure cell, and the lysate was clarified as described above. Then, 2 ml of 50% slurry of glutathione-Sepharose beads (Amersham Pharmacia) was added to the clarified lysate, and the mixture was incubated for 1 h at 4°C with gentle rocking. Beads were washed five times with 10 ml of lysis solution containing 500 mM NaCl. To release the CdtB moiety, the beads were incubated at room temperature for 1 h in 1 ml of thrombin cleavage buffer (50 mM Tris-HCl, pH 8; 150 mM NaCl; 2.5 mM CaCl2) containing 2.5 U of thrombin. The supernatant containing the purified CdtB protein was transferred to a fresh tube, and the thrombin was inactivated by the addition of 1 mM phenylmethylsulfonyl fluoride (PMSF). Quantification of the purified recombinant proteins was carried out by Coomassie blue-stained sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) or with the Bio-Rad Protein Assay kit, using known quantities of bovine serum albumin as standards.
Cell cycle analysis.
Henle-407 intestinal epithelial cells were lightly seeded on 60-mm tissue culture dishes. After 24 h the cells were treated with the different samples for 2 h. Cells were then washed with PBS, and fresh complete DMEM medium was added. At 56 h after intoxication, the cells were processed for flow cytometry as follows. Cells were dislodged from dishes with trypsin, which was subsequently neutralized with serum-containing medium. The cell suspensions were centrifuged for 5 min at 1,500 rpm at room temperature. The supernatants were removed, and the cell pellets were resuspended in 500 μl of PBS at room temperature. The cell suspensions were added dropwise to a tube containing 4 ml of −20°C ethanol as a fixative with continuous vortexing. Cells were kept in fixative for at least 2 h on ice. The ethanol-suspended cells were collected by centrifugation, and the ethanol was decanted thoroughly. Pellets were resuspended in 5 ml of PBS and incubated for 5 min, and the cells were centrifuged again as described above. The cell pellets were resuspended in 1 ml of a solution containing 0.1% Triton X-100, 20 mg of DNase-free RNase A, and 20 μg of propidium iodide (Molecular Probes) per ml in PBS. The stained cells were analyzed by flow cytometry with a FACStar Plus flow cytometer (Beckton Dickinson).
GST pulldown assays.
N-terminal GST-tagged CdtA and CdtB proteins used in GST pulldowns were purified as described above except that the fusion proteins were eluted from the beads by incubation for 10 min in 1 ml of elution buffer (50 mM Tris-HCl, pH 8.0; 200 mM NaCl; 10 mM DTT; 5 mM reduced glutathione). The elution procedure was repeated five times; the elutes were pooled and concentrated using a Centricon-10 concentrator device (Amicon), which also removed the glutathione from the purified protein. Quantification of the recombinant purified proteins was carried out by Coomassie blue-stained SDS-PAGE, using known quantities of bovine serum albumin as standards.
Overnight cultures of E. coli strains harboring each of the plasmids encoding M45 epitope-tagged versions of the different Cdt proteins in the context of the cdt operons were diluted 1:50 in 25 ml of Luria broth containing 100 μg of ampicillin per ml. Cultures were grown at 37°C with shaking to an OD600 of 0.5 to 0.6. Expression of the operons was induced by the addition of 0.02% arabinose, and cultures were incubated for an additional 4 h. Cells were then collected by centrifugation at 10,000 rpm for 30 min. The supernatants were discarded, and the cell pellets were resuspended in 250 μl of PBS containing 0.1% NP-40 and 1 mM PMSF. Cells were lysed by sonication, the lysates were clarified by centrifugation at 14,000 rpm for 30 min, and the supernatants were transferred to a fresh tube. Binding reactions were set up by adding 20 μg of the appropriate GST fusion protein to a 1:10 dilution of bacterial lysates in PBS containing 0.1% NP-40 in a final volume of 750 μl. The mixtures were incubated for 3 h at 4°C under gentle rocking conditions. Next, 50 μl of a 50% slurry of glutathione-Sepharose beads was added to each of the mixtures, and the samples were incubated for 1 h under the same conditions. Beads were pelleted by centrifugation at 7,500 rpm for 1 min and washed four times in 1 ml of PBS containing 0.1% NP-40. Beads were loaded onto a 10% SDS-PAGE gel, along with pre- and postincubation samples of the lysate. Bound proteins were detected by anti-M45 immunoblotting.
Coimmunoprecipitation assays.
Lysates of E. coli strains harboring each of the plasmids encoding M45 epitope-tagged versions of the different Cdt proteins in the context of the cdt operons were prepared as described above. The appropriate rabbit antisera directed to the different Cdt proteins were added to the different samples at a final dilution of 1:100, and the mixtures were kept on ice for 90 min with occasional mixing. Then, 50 μl of protein A-Sepharose slurry was added to the binding reactions, and the samples were incubated for an additional 90 min at 4°C with gentle rocking. Beads were washed four times with 1 ml of PBS containing 0.1% NP-40, resuspended in Laemmli loading buffer, boiled for 5 min, and loaded onto a 10% SDS-polyacrylamide gel along with pre- and postincubation samples of the lysate. Bound proteins were detected by Western immunoblotting with an anti-M45 monoclonal antibody.
Analyis of complex formation by gel filtration.
Each of the purified Cdt proteins were individually loaded onto a Superdex 200 (SD200) gel filtration column (Amershan Pharmacia) previously equilibrated in 25 mM Tris (pH 8)–200 mM NaCl. Fractions (1 ml) were collected, and 25 μl of each fraction was analyzed by SDS-PAGE and Coomassie blue staining. The fractions containing each of the purified Cdt proteins were mixed in equimolar concentrations and incubated for 90 min at room temperature to allow complex formation. After incubation the complex was loaded onto the SD200 column, and 1-ml fractions were collected as described above. Monolayers of Henle-407 intestinal epithelial cells were then treated with 50 μl of a series of twofold dilutions of the relevant fractions (13 to 18) to quantitate the CDT activity. In addition, 200 μl of the same fractions was analyzed by SDS-PAGE and Coomassie blue staining.
Amino acid sequence comparison.
Amino acid sequence threading was conducted with the program THREADER2 (17). When CdtA was compared against a library of more than 1,900 folds, the B chain of ricin ranked first with a highly significant Z-score (4.24 e-3) that clearly distinguished it from all other proteins.
RESULTS
Reconstitution of the CDT holotoxin with purified Cdt components.
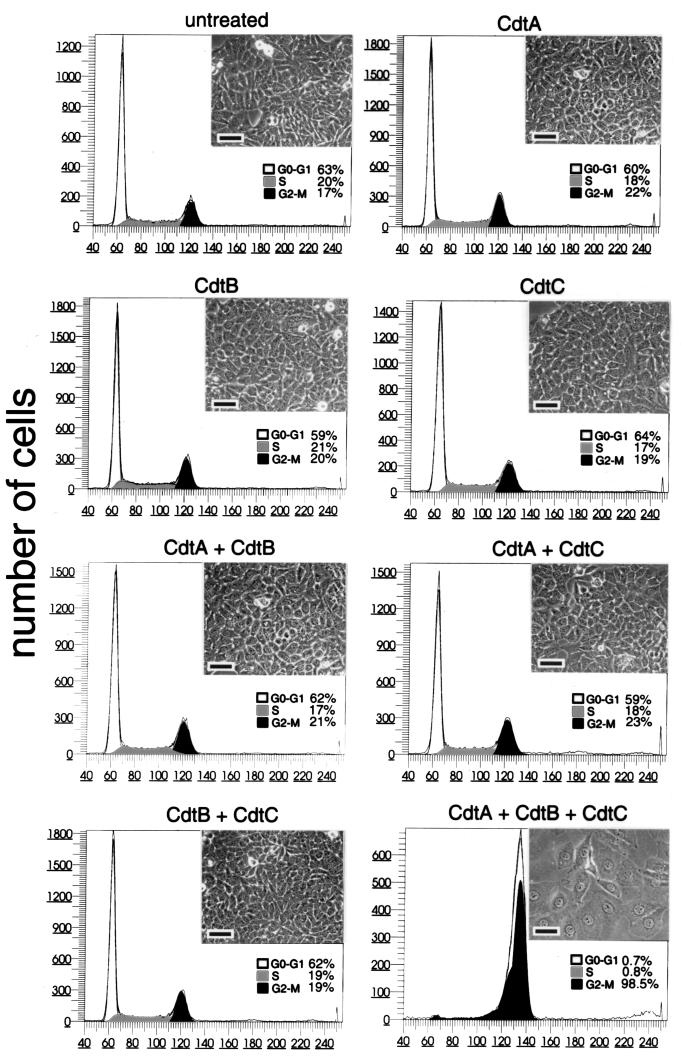
CDT activity requires the function of CdtA, CdtB, and CdtC. We have recently shown that microinjection of small amounts (a solution of 1 μg/ml) of purified CdtB into the cytoplasm of cultured cells can recapitulate all the cytotoxic effects of CDT such as cell cycle arrest and cytoplasm distention (19). These results indicated that CdtB is the enzymatically active component of the holotoxin. The contribution of CdtA and CdtC to CDT activity, however, is not known. Either one or both of these proteins may be involved in the translocation of CdtB into the eukaryotic cell and/or may play an exclusive role in the bacterial cell to process, modify, assemble, or secrete the active holotoxin. To gain insight into some of these issues, we investigated the possibility that the CDT activity could be reconstituted from purified components. Purification of the individual Cdt proteins has proven to be very challenging since the bulk of these proteins remain tightly associated to the bacterial envelope (13). Furthermore, the amount of toxin present in culture supernatants is not enough for meaningful biochemical experiments. These features, coupled to the extremely high specific activity of CDT, have been largely responsible for the often contradictory reports in the literature ascribing functions to the individual components of the toxin. To circumvent these problems, we optimized the expression and purification of the individual recombinant Cdt proteins using different E. coli expression systems. CdtA and CdtC were purified as C-terminal His6-tagged proteins lacking the signal sequence. CdtB was purified as an N-terminal fusion to GST also without its signal sequence. All preparations were soluble and >95% pure (Fig. 2). The expression and purification of each Cdt protein individually ensure the absence of cross-contamination. The purified Cdt proteins were then used in experiments to determine whether toxicity could be reconstituted from purified components. The treatment of Henle-407 intestinal epithelial cells with CdtA, CdtB, or CdtC resulted in no toxicity (Fig. 3). These results are in sharp contrast to previous reports indicating that purified CdtC (27) or CdtB (29) retain toxic activity. Since the microinjection of purified CdtB can recapitulate all of the CDT effects (19), these results suggest that the delivery of CdtB into target cells may require the function of other Cdt proteins. To investigate this possibility, we treated cells with CdtB in conjunction with either CdtA or CdtC. As shown in Fig. 3, neither combination resulted in detectable toxicity. However, treatment of Henle-407 cells with a combination of the three individually purified components resulted in a full display of toxicity, such as overt cytoplasmic distention and G2/M cell cycle arrest (Fig. 3). These results indicate that CdtA and CdtC are required for the delivery of the active subunit CdtB into the target cell.
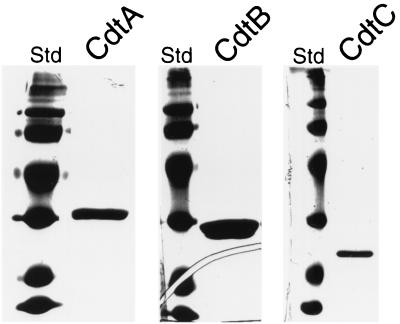
FIG. 2.
Purified Cdt proteins. Recombinant Cdt proteins were purified as indicated in Materials and Methods, run on a polyacrylamide gel, and stained with Coomassie blue.
FIG. 3.
Effect of purified Cdt proteins on cultured intestinal epithelial cells. Purified Cdt proteins alone or in combination (as indicated) were added at a concentration of 10 μg/ml to culture intestinal Henle-407 cells. At 72 h after addition of the proteins, the cells were examined under a phase microscope or processed to measure DNA content by flow cytometry as indicated in Materials and Methods. The scale bar in the microphotographs of cultured intestinal cells treated with the different samples represents 50 μm.
The Cdt proteins interact with one another.
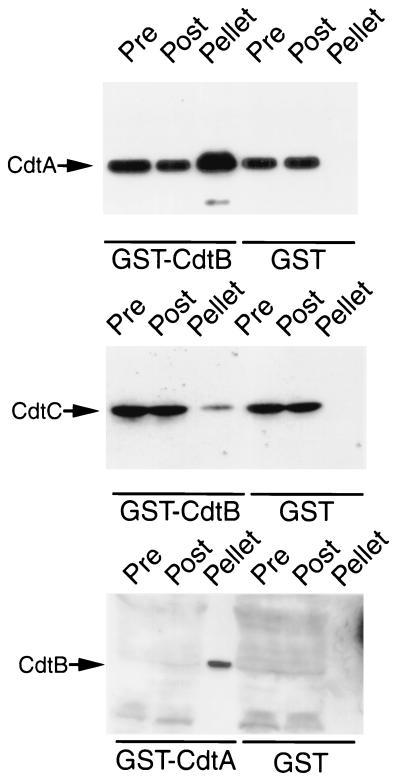
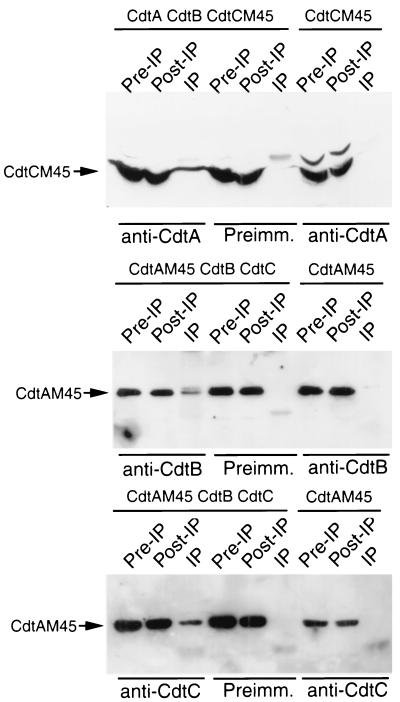
The three Cdt proteins are required to reconstitute CDT toxicity, indicating that CdtA and CdtC must play a role in the translocation of CdtB into the target cell. It is therefore possible that these proteins or a subset of them may interact with one another to form a complex prior to delivery of the active subunit into the cell. To address this possibility, we investigated the potential interaction of the different Cdt proteins with several in vitro binding experiments. We first investigated the interaction of CdtB, the active subunit, with CdtA and CdtC by GST pulldown assays. CdtA-M45 and CdtC-M45 readily bound to GST-CdtB but not to GST (Fig. 4). Conversely, GST-CdtA, but not GST, was able to interact with CdtB-M45. These interactions were further confirmed by coimmunoprecipitation assays using specific antibodies and lysates of E. coli expressing the three Cdt proteins (Fig. 5). An antibody directed to CdtA immunoprecipitated CdtC and, conversely, an antibody directed to CdtC brought down CdtA (Fig. 5). Furthermore, an anti-CdtB antibody was able to bring down CdtA (Fig. 5). In all cases, preimmune antisera did not immunoprecipitate any of these proteins (Fig. 5). Taken together, these results indicate that the three Cdt proteins are able to interact with one another and therefore may form a complex.
FIG. 4.
GST pulldown assays for Cdt protein interactions. Whole-cell lysates of E. coli expressing cdt operons encoding M45 epitope-tagged CdtA, CdtB, or CdtC proteins (as indicated to the left of each panel) were probed with purified GST-CdtB, GST-CdtA, or GST (as a negative control) as indicated below each panel. Cdt proteins in the samples before (Pre) and after (Post) the pulldowns or bound to the glutathione-agarose beads (pellet) were detected by Western blotting with a monoclonal antibody directed to the M45 epitope tag. The levels of CdtB in E. coli lysates were not sufficient for its detection in the pre- or postpulldown samples without prior concentration. However, precipitation with GST-CdtA resulted in the concentration of CdtB, which allowed its detection in the sample.
FIG. 5.
Coimmunoprecipitation assays for Cdt protein interactions. Whole-cell lysates of E. coli expressing different M45 epitope-tagged Cdt proteins in combination with other untagged Cdt proteins (as indicated at the top of each panel) were subjected to coimmunoprecipitation assays with rabbit polyclonal antibodies specific to different Cdt proteins (as indicated below each panel). M45 epitope-tagged Cdt proteins in samples before (Pre-IP) and after (Post-IP) the immunoprecipitation or bound to the protein A-Sepharose beads (pellet) were detected by Western blotting with a monoclonal antibody directed to the M45 epitope. As controls for the specificity of the antibodies, lysates expressing different epitope-tagged Cdt proteins alone (as indicated on the top of the last three lanes of each panel) were treated with the anti-Cdt polyclonal antibodies (as indicated below the last three lanes of each panel), and the immunoprecipitates were analyzed by Western blotting with the M45 monoclonal antibody. Preimm., preimmune serum.
CdtA, CdtB, and CdtC form a tripartite complex.
The previous set of experiments suggested that the three Cdt proteins might be organized in a tripartite complex since they are all required to reconstitute CDT toxicity and they are able to interact with one another in GST pulldown and coimmunoprecipitation assays. We therefore investigated this possibility directly by using gel filtration chromatography. We first determined the elution peaks of the individually purified recombinant Cdt proteins loaded onto an SD200 gel filtration column. CdtA and CdtB eluted in fractions 16 and 17, which correspond to elution profiles of proteins of their predicted molecular masses (29,919.36 kDa for CdtA and 28,972.97 kDa for CdtB). CdtC, which has a predicted molecular mass of 21,157 kDa, eluted in fractions 14 through 16, which correspond to the elution profile of a larger size protein, indicating that at least a proportion of CdtC may multimerize in solution. The gel-filtrated purified proteins were then mixed in equimolar ratios and incubated at room temperature for 90 min to allow the formation of a putative complex. The mixture was then loaded onto the same SD200 column, and the eluted fractions were monitored by SDS-PAGE and Coomassie blue staining. The Cdt proteins eluted in fractions corresponding to an approximate molecular mass of ∼80 kDa, which is consistent with the formation of a tripartite complex composed of CdtA, CdtB, and CdtC (Fig. 6). More importantly, the elution profiles of CdtA and CdtB were shifted from their original monomeric position in fractions 16 and 17 to fractions 14 and 15, consistent with the formation of such a complex (Fig. 6). The toxicity of the different fractions was tested in Henle-407 intestinal epithelial cells. Consistent with the formation of an active toxin complex, the highest specific activity was associated with fraction 14, decreasing sharply after that, and almost no toxic activity was detected in fraction 17 and beyond. Furthermore, toxicity was correlated with the presence of the three Cdt protein components. Taken together with the protein-protein interaction experiments, these results indicate that CdtA, CdtB, and CdtC form a tripartite complex that is competent for toxicity.
FIG. 6.
Gel filtration chromatography of Cdt proteins. Purified Cdt proteins, individually or in combination, were loaded onto a Superdex 200 gel filtration column as indicated in Materials and Methods. Then, 1-ml fractions were collected and examined by SDS-PAGE and Coomassie blue staining. Superimposed over the protein bands are the chromatography absorption profiles. Where indicated, the toxicity was examined as described in Materials and Methods using 50 μl of each fraction. The toxicity index represents the inverse of the dilution of each sample that caused visible morphological changes in culture intestinal Henle-407 cells.
DISCUSSION
It has been well established that the CDT activity of C. jejuni and other gram-negative pathogens requires the function of three proteins: CdtA, CdtB, and CdtC (25, 28). However, the individual contribution of each of these proteins to CDT toxicity has been the subject of contradictory reports (27, 29). Furthermore, it had remained unclear whether the different Cdt proteins form part of CDT holotoxin or play an unknown role in the secretion and/or processing of a putative active subunit(s). Some of these issues were recently clarified when it was shown that microinjection of purified CdtB into target cells can reproduce all of the effects observed in cells treated with CDT (19). It was also reported that CdtB exhibits amino acid sequence similarity to the DNase I-type proteins and has nuclease activity in vitro (7, 19). These observations indicate that CdtB's nuclease activity damages the target cell's DNA, triggering a cell cycle control check point that causes the G2/M arrest characteristic of CDT intoxication (7, 19). These results therefore indicate that CdtB is in effect the active or “A” subunit of CDT. We have expanded these studies here and show that CdtA, CdtB, and CdtC form a tripartite active complex that composes the CDT holotoxin.
There have been several conflicting reports regarding the individual contribution of each Cdt protein to CDT toxicity (27, 29). Most likely, these conflicting results are a consequence of the high specific activity of the CDT toxin coupled to the inherent difficulty in the purification of the Cdt proteins due to their tendency to associate to the bacterial outer membrane (13). These obstacles have made it virtually impossible to draw conclusions from experiments conducted with individual Cdt proteins isolated from bacteria expressing all the cdt genes since minor contamination can lead to artifactual results. To avoid these pitfalls, we purified each recombinant Cdt protein from E. coli expressing each individual cdt gene, thereby avoiding the potential problems that can arise from subunit cross-contamination. The treatment of cells with purified CdtB resulted in no detectable toxicity. Since microinjection of the same preparation of CdtB resulted in toxicity (19), these results indicated that this subunit by itself is unable to reach the intracellular compartment to exert its toxic effect. Therefore, these results also suggested that CdtA, CdtC, or both may be required for the cellular translocation of CdtB. The treatment of cells with a combination of CdtB and either CdtC or CdtA did not result in toxicity. However, the treatment of cells with a mixture of individually purified CdtA, CdtB, and CdtC caused identical changes to those caused by extracts from bacteria expressing the wild-type cdt operon. These changes included the typical cytoplasmic distention, nuclear enlargement, and G2/M arrest. These results indicate that the three proteins are required for toxicity and that an active toxin can be reconstituted from individually purified components. These results also suggest that CdtA and CdtC are most likely not involved in the secretion of CdtB (the active subunit) but rather in its delivery into the host cell.
The reconstitution of CDT toxicity with a combination of the three Cdt proteins suggested the possibility that these three proteins were indeed subunits of a tripartite holotoxin. In addition, Purven et al. have shown that CDTs purified from bacterial extracts using a monoclonal antibody directed against CdtC display full toxicity (27). Since CdtB is essential for CDT toxicity, these results are also consistent with the notion that the Cdt proteins form an active complex. Our results showed this to be the case. CdtA, CdtB, and CdtC were shown to interact with one another by a variety of protein-protein interaction assays, and a fully toxic tripartite complex made of the three Cdt proteins was isolated by gel filtration chromatography. These results suggest that CDT is an AB2 toxin composed of CdtB, as the enzymatically active (A) subunit, and CdtA and CdtC, as the heterodimeric B subunit required for the delivery CdtB into the target cell. Also in support of this hypothesis, we have detected in CdtA, using amino acid sequence threading, a region encompassing amino acids 160 through 220 that exhibits similarity to a lectin fold present in the B chain of ricin and abrin, two well-characterized AB toxins (12). A smilar observation was made by Hofmann et al. using a different approach (14). The B subunit of ricin binds to galactose residues of receptor glycoproteins, facilitating the entry of the A subunit into the cytosol of the target cell by receptor-mediated endocytosis. CDT has been recently reported to enter cells via clathrin-coated pits, which is consistent with a receptor-mediated process (4). It is therefore possible that CdtA, perhaps in association with CdtC, may direct the translocation of CdtB by engaging a cellular receptor. Experiments are under way to investigate this possibility.
In summary, these studies indicate that CDT is a tripartite toxin composed of CdtB as the enzymatically active subunit, and CdtA and CdtC, required for the delivery of CdtB. Such a composition suggests that CDT is an AB2 heterodimeric toxin.
ACKNOWLEDGMENTS
We thank M. Blaser for bacterial strains and plasmids and Erec Stebbins for useful discussions and help with the Cdt purification.
REFERENCES
- 1.Collazo C M, Galan J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comayras C, Tasca C, Peres S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. Cellular Internalization of cytolethal distending toxin from Haemophilus ducreyi. Infect Immun. 2000;68:6903–6911. doi: 10.1128/iai.68.12.6903-6911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Investig. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekeyser P, Gossuin-Detrain M, Butzler J P, Sternon J. Acute enteritis due to related vibrio: first positive stool cultures. J Infect Dis. 1972;125:390–392. doi: 10.1093/infdis/125.4.390. [DOI] [PubMed] [Google Scholar]
- 7.Elwell C A, Dreyfus L A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 8.Evans S J, Shipstone E J, Maughan W N, Connolly B A. Site-directed mutagenesis of phosphate-contacting amino acids of bovine pancreatic deoxyribonuclease I. Biochemistry. 1999;38:3902–3909. doi: 10.1021/bi9824893. [DOI] [PubMed] [Google Scholar]
- 9.Fox J G, Ackerman J I, Taylor N, Claps M, Murphy J C. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. Am J Vet Res. 1987;48:85–90. [PubMed] [Google Scholar]
- 10.Golden N J, Camilli A, Acheson D W. Random transposon mutagenesis of Campylobacter jejuni. Infect Immun. 2000;68:5450–5453. doi: 10.1128/iai.68.9.5450-5453.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazes B. The (Q×W)3 domain: a flexible lectin scaffold. Protein Sci. 1996;5:1490–1501. doi: 10.1002/pro.5560050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey T E, McVeigh A L, Scott D A, Michielutti R E, Bixby A, Carroll S A, Bourgeois A L, Guerry P. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun. 2000;68:6535–6541. doi: 10.1128/iai.68.12.6535-6541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci USA. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 16.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 17.Jones D T, Taylor W R, Thornton J M. A new approach to protein fold recognition. Nature. 1992;358:86–89. doi: 10.1038/358086a0. [DOI] [PubMed] [Google Scholar]
- 18.Jones S J, Worrall A F, Connolly B A. Site-directed mutagenesis of the catalytic residues of bovine pancreatic deoxyribonuclease I. J Mol Biol. 1996;264:1154–1163. doi: 10.1006/jmbi.1996.0703. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Tejero M, Galan J E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q Y, Ribecco M, Pandey S, Walker P R, Sikorska M. Apoptosis-related functional features of the DNase I-like family of nucleases. Ann N Y Acad Sci. 1999;887:60–76. doi: 10.1111/j.1749-6632.1999.tb07922.x. [DOI] [PubMed] [Google Scholar]
- 21.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obert S, O'Connor R J, Schmid S, Hearing P. The adenovirus E4–6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 24.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 25.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickett C L, Whitehouse C A. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 27.Purven M, Frisk A, Lonnroth I, Lagergard T. Purification and identification of Haemophilus ducreyi cytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenker B J, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Antinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 30.Skirrow M B. Campylobacter enteritis: a “new” disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanfield J T, McCardell B A, Madden J M. Campylobacter diarrhea in an adult mouse model. Microb Pathog. 1987;3:155–165. doi: 10.1016/0882-4010(87)90092-1. [DOI] [PubMed] [Google Scholar]
- 32.Sugai M, Kawamoto T, Peres S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wooldridge K G, Ketley J M. Campylobacter-host cell interactions. Trends Microbiol. 1997;5:96–102. doi: 10.1016/S0966-842X(97)01004-4. [DOI] [PubMed] [Google Scholar]
- 35.Young V B, Knox K A, Schauer D B. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]