Abstract

A series of chalcone derivatives containing indanone were designed and synthesized by aldehyde-ketone condensation and etherification. The activity test demonstrated that the majority of the compounds had good therapeutic and protective activities against tobacco mosaic virus (TMV) at a concentration of 500 μg/mL when being tested. Among them, the target compounds N2 and N7 showed good therapeutic activities against TMV with EC50 values of 70.7 and 89.9 μg/mL, respectively, which were better than that of ningnanmycin (158.3 μg/mL). N2 and N10 showed better protective activities against TMV with EC50 values of 60.8 and 120.3 μg/mL, which were superior to that of ningnanmycin (175.6 μg/mL). A hydrogen bond interaction was observed between N2 and ARG-341 with a bond length of 3.08 Å and a hydrogen bond was observed between ningnanmycin and ASP-66 with a bond length of 3.72 Å. In contrast, the hydrogen bond length of compound N2 was shorter and its binding was closer. Meanwhile, when the heartleaf tobacco was being treated with N2, its increasing rate of malondialdehyde slowed and its content of defense enzymes significantly increased, again reflecting the good activity of N2 against TMV.
1. Introduction
In the absence of a plant-specific immune system, the parasitic effect of virus on its cells is tremendous, contributing to severe losses, and the prevention and treatment are difficult and complex. Thus, plant viruses are also called “cancer”.1 As an RNA virus, tobacco mosaic virus (TMV) has given rise to infections in 36 crops of the families of Amaranthaceae, Chenopodiaceae, Asteraceae, Cruciferae, and Solanaceae since its discovery, causing huge economic losses.2 Through minor wounds and stomas, TMV destroys the chlorophyll in leaves, weakens photosynthesis, inhibits plant growth, and causes plant growth difficulties, dysplasia, deformity, and even death. At present, the main prevention and treatment have been achieved by means of antiviral agents that induce host defense responses,3 such as ningnanmycin (NNM) and ribavirin. However, the long-term and large-scale use of traditional antiviral agents not only increases the drug resistance of pathogens but also brings a series of environmental issues, such as excessive pesticide residues and eutrophication.4 Therefore, it is of great significance to explore and develop new antiviral agents with high efficiency, low toxicity, and environmental protection for the control of the tobacco mosaic disease.
Chalcone exists widely in licorice,5 safflower,6 oxytropis falcate bunge,7 hops,8 and other plants as an essential natural product and an important intermediate for the synthesis of flavonoids. Chalcone has a wide range of pharmacological activities, such as antibacterial,9 antiviral,10 insecticidal,11 anti-inflammatory,12 antitumor,13 antioxidant,14 and other biological activities,15 due to its flexibility and ability to bind to a variety of receptors. In addition, chalcone contains α,β-unsaturated carbonyl groups in its structure, which can be conjugated with nucleophilic groups (such as sulfhydryl groups in proteins) in bacteria, viruses, and pests, thus causing them to die.16
Indanone also exists widely in the active molecules of natural products, drugs, and pesticides. To name only a few, 1-methoxy-6-methyl-3-oxo-2,3-dihydro-1H-4-aldehyde (I) and pterosin B (II) extracted from marine cyanobacteria and Pteris ensiformis are found to contain indanone structural units,17,18 as shown in Figure 1. Indanones have antibacterial,19 antiviral,20 anti-Alzheimer’s disease,21 antitumor,22 and other biological activities.23 Some of them have been used in clinical practice. For example, donepezil (III),24 an acetylcholinesterase inhibitor, has been approved by the United States Food and Drug Administration for the treatment of Alzheimer’s disease.
Figure 1.

Compounds containing indanone.
In our previous studies,25,26 we found that chalcone derivatives have good anti-TMV activity, so on this basis, we designed and synthesized them in order to obtain effective chalcone antiviral agents. By means of the active splicing principle, a series of chalcone derivatives were synthesized with indanone as the lead compound, and their anti-TMV activities were studied. Activity studies showed that most of the compounds had therapeutic, protective, and passivating activities against TMV, and some of them were better than NNM. The target compound N2 with a higher activity was screened out, and its mechanism of anti-TMV was studied by a preliminary biological activity study, molecular docking, and a microscale thermophoresis test. The changes of malondialdehyde and defense enzymes in vivo were studied so as to provide a theoretical basis for the development of novel pesticides.
2. Results and Discussion
2.1. Antiviral Activity of the Target Compounds N1–N20
The anti-TMV activity of target compounds N1–N20 was tested by the half-leaf dead spot method, and the results are shown in Table 1. When the test concentration was 500 μg/mL, there was a large gap between the therapeutic, protective, and passivation activities of N2 and N7, which had good therapeutic activities, and the inhibition rates were 68.4 and 68.7%, respectively. They were better than those of NNM (61.3%). At the same concentration, the inhibition rates of N1, N2, and N10 on TMV were 72.2, 74.7, and 76.1%, respectively, which were significantly better than that of NNM (66.3%), showing better protective activities. The effect comparison was shown in Figure 2. The inhibition rates of N6 and N16 were 79.4 and 81.2%, respectively, slightly lower than that of NNM (93.2%). The preliminary test results showed that these compounds had good therapeutic and protective activities against TMV.
Table 1. Antiviral Activities (Concentration of 500 μg/mL) of the Target Compounds N1–N20 against TMV In Vivoa.
| compd. | O | R | inhibition ratea (%) |
||
|---|---|---|---|---|---|
| curative | protection | inactivation | |||
| N1 | 4 | Ph | 52.4 ± 6.9 | 72.2 ± 8.8 | 58.5 ± 7.4 |
| N2 | 4 | 3-CH3-Ph | 68.4 ± 2.6 | 74.7 ± 5.7 | 42.0 ± 7.0 |
| N3 | 4 | 4-CH3-Ph | 55.1 ± 8.1 | 61.3 ± 7.8 | 65.2 ± 8.3 |
| N4 | 4 | 4-OCH3-Ph | 46.7 ± 1.8 | 67.2 ± 5.1 | 40.3 ± 6.7 |
| N5 | 4 | 3-Cl-Ph | 47.8 ± 7.4 | 56.0 ± 6.3 | 67.6 ± 5.1 |
| N6 | 4 | 4-Cl-Ph | 42.5 ± 1.8 | 54.8 ± 8.6 | 79.4 ± 8.4 |
| N7 | 4 | 4-F-Ph | 68.7 ± 8.0 | 65.5 ± 5.0 | 29.2 ± 7.7 |
| N8 | 4 | 3,4-di-OCH3-Ph | 27.7 ± 2.5 | 65.5 ± 5.8 | 40.2 ± 5.0 |
| N9 | 5 | Ph | 46.5 ± 8.6 | 65.7 ± 7.2 | 19.8 ± 0.2 |
| N10 | 5 | 3-CH3-Ph | 58.0 ± 6.9 | 76.1 ± 5.6 | 65.7 ± 7.2 |
| N11 | 5 | 4-CH3-Ph | 55.7 ± 8.2 | 49.2 ± 7.7 | 57.3 ± 5.0 |
| N12 | 5 | 3-OCH3-Ph | 60.6 ± 5.3 | 43.1 ± 8.0 | 66.6 ± 4.6 |
| N13 | 5 | 4-OCH3-Ph | 41.5 ± 9.7 | 37.0 ± 3.3 | 51.9 ± 2.4 |
| N14 | 5 | 3-Cl-Ph | 39.0 ± 8.6 | 29.1 ± 3.7 | 56.3 ± 3.2 |
| N15 | 5 | 4-Cl-Ph | 38.7 ± 1.9 | 21.5 ± 1.5 | 55.7 ± 0.8 |
| N16 | 5 | 3-F-Ph | 62.3 ± 8.9 | 45.6 ± 0.7 | 81.2 ± 5.2 |
| N17 | 5 | 4-F-Ph | 60.4 ± 4.9 | 34.2 ± 1.8 | 73.6 ± 8.7 |
| N18 | 5 | 4-Br-Ph | 37.2 ± 6.6 | 17.7 ± 2.9 | 63.1 ± 7.6 |
| N19 | 5 | 3,4-di-OCH3-Ph | 39.4 ± 8.0 | 46.3 ± 5.8 | 70.1 ± 7.5 |
| N20 | 5 | furan-2-Ph | 41.2 ± 1.3 | 41.0 ± 7.7 | 65.7 ± 6.2 |
| NNM | 61.3 ± 1.2 | 66.3 ± 6.6 | 93.2 ± 4.7 | ||
Average of three replicates.
Figure 2.
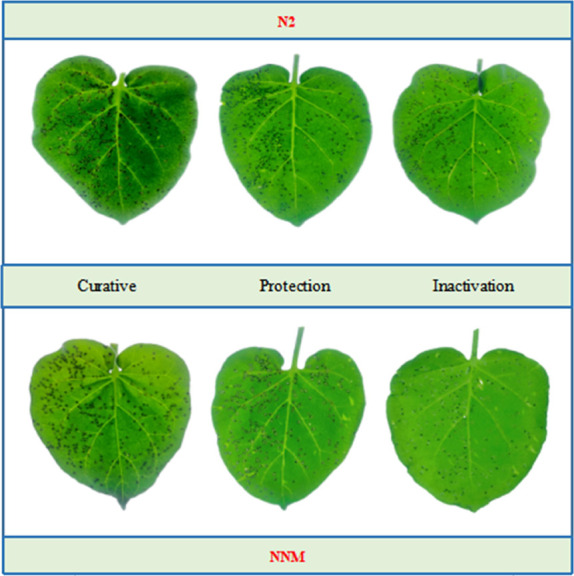
Tobacco leaf morphology effects of N2 and NNM against TMV in vivo. The left side of each leaf was untreated with drug and the right side of each leaf was drug-treated.
The EC50 values of N1, N2, N7, and N10 with high activities were determined to further confirm their good antiviral activities (Table 2). The EC50 values of N2 and N7 against TMV were 70.7 and 89.9 μg/mL, respectively, which were better than that of NNM (158.3 μg/mL). In comparison, the EC50 values of N1, N2, and N10 were 123.6, 60.8, and 120.3 μg/mL, respectively, which were significantly superior to the EC50 value of NNM (175.6 μg/mL). It can be seen that the protective activities of N1, N2, and N10 were better than that of NNM. In particular, the therapeutic and protective activities of N2 were significantly better than those of NNM, and it had good antiviral activity, which was worthy of further research.
Table 2. EC50 Values of Several Target Compounds and NNM against TMV.
| compd. | O | R | toxic regression equation | r2 | EC50/(μg/mL) | |
|---|---|---|---|---|---|---|
| curative activity | N2 | 4 | 3-CH3-Ph | y = 0.9259x + 3.2879 | 0.9873 | 70.7 |
| N7 | 4 | 4-F-Ph | y = 0.6544x + 3.7216 | 0.9679 | 89.9 | |
| NNM | y = 0.6159x + 3.6453 | 0.9426 | 158.3 | |||
| protection activity | N1 | 4 | Ph | y = 1.0654x + 2.7713 | 0.9522 | 123.6 |
| N2 | 4 | 3-CH3-Ph | y = 0.6551x + 3.8315 | 0.9721 | 60.8 | |
| N10 | 5 | 3-CH3-Ph | y = 1.0552x + 2.8049 | 0.9891 | 120.3 | |
| NNM | y = 0.8241x + 3.1503 | 0.9848 | 175.6 | |||
2.2. Structure–Activity Relationship
Most of the target compounds N1–N20 had better antiviral activities than NNM. The therapeutic, protective, and passivating activities vary according to their substituent and positions. As can be seen from Table 1, when R was 3-CH3-Ph (N2) and 4-CH3-Ph (N3), the therapeutic effect on TMV significantly enhanced, and the inhibition rates were 64.8 and 55.1%, respectively, which were better than the inhibition rate when R was Ph (N1) (52.4%), so the therapeutic activity was better when R was an electron donor group. Taking 3-CH3-Ph (N2) and 4-CH3-Ph (N3) as examples, the corresponding target compound N2 had better therapeutic activity than N3. It can be seen that the therapeutic, protective, and passivating activities of substituents in the meta-position were higher than those in the para-position, and the therapeutic, protective, and passivating activities of N1–N20 followed this rule basically.
2.3. Microscale Thermophoresis
Microscale thermophoresis (MST) was used to investigate the binding ability of target compounds N2, N7, and NNM to the TMV coat protein (TMV-CP). The MST test results are shown in Figure 3. The Kd values of N2 and N7 were 0.0427 ± 0.0145 and 0.1890 ± 0.0928 μM, respectively, which were better than that of NNM (2.3471 ± 0.8648 μM). In summary, N2 and N7 were more potent than NNM in binding to TMV-CP, suggesting that N2 and N7 were more potent than the controlled drug.
Figure 3.
MST of compounds N2, N3, N7, and NNM to TMV-CP.
2.4. Molecular Docking
Molecular docking experiments of N2 and NNM with TMV-CP were performed to simulate the interaction between the drug molecules and TMV-CP. As shown in Figure 4, there is a hydrogen bond interaction between N2 and ARG-341 with a bond length of 3.08 Å. There was a hydrogen bond between NNM and SER-138 and ASP-66 with bond lengths of 4.29 and 3.72 Å, respectively. Although the docking of NNM with TMV-CP produced more hydrogen bonds than N2, the hydrogen bond length of N2 was shorter and its binding was closer. Compound N2 was bound to ARG-261 and SER-147 through a C−H bond with bond lengths of 2.95 and 2.33 Å, respectively, and bound to VAL-260, VAL-75, and LYS-253 through a π-alkyl hydrophobic bond with bond lengths of 4.12, 5.28, and 4.92 Å, respectively. NNM binds to GLY-135 via a C−H bond with a bond length of 3.15 Å. In conclusion, N2 binds to TMV-CP through more bonds and their interaction forces are stronger.
Figure 4.
Molecular docking of compounds N2 (A, a) and NNM (B, b) to TMV-CP.
2.5. Malondialdehyde Content
As shown in Figure 5, the content of malondialdehyde (MDA) in leaves was measured after 1, 3, 5, and 7 days of treatment. The accumulation of reactive oxygen species induced membrane lipid peroxidation when plants suffered from various environmental stresses in their life cycle. The main products of membrane lipid peroxidation are MDA, etc. Therefore, the degree of membrane lipid peroxidation, the degree of membrane damage, and the stress resistance of plants can be measured by measuring the MDA content. With the passage of time, the MDA content in leaves treated with CK increased mainly because of environmental adversity and leaf senescence. The content of MDA in the leaves treated with N2 continued to increase, but the content of MDA in the leaves after 5–7 days was relatively lower than that of the untreated leaves because N2 could cause adverse effects in a negative environment. The content of MDA in leaves treated with TMV increased significantly with time, which fully reflected the destruction of leaves caused by TMV. The MDA content of TMV + N2 and TMV + NNM treatment increased, but the rapid damage of the TMV virus to leaves was significantly inhibited. The MDA content of TMV + N2 treatment was lower than that of TMV + NNM treatment from the beginning to end. It could be seen that the activity of the target compound N2 against TMV was significantly better than that of the control drug NNM.
Figure 5.

Changes in the MDA content in tobacco after compound N2 treatment.
2.6. Activities of the Defense Enzyme
2.6.1. Superoxide Dismutase Assay
Figure 6 shows the changes in the superoxide dismutase (SOD) content in leaves after treatment with the target compound N2. The SOD content in leaves with TMV + N2 treatment increased gradually from 1 to 7 days, which was significantly better than that of leaves treated with NNM. Compared with the SOD content of TMV treatment and TMV + N2 treatment, it can be seen that the SOD content of N2 treatment is significantly better than that of TMV treatment, so N2 can induce SOD production in leaves and improve the antivirus ability of leaves.
Figure 6.

Changes in the SOD content in tobacco after compound N2 treatment.
2.6.2. Peroxidase Determination
The changes in the peroxidase (POD) content in leaves treated with N2 and NNM for 1, 3, 5, and 7 days were shown in Figure 7. The POD content in leaves treated with TMV gradually increased over time but the content was less than that before 7 days. The content of POD in TMV + NNM treatment increased significantly, and the difference was more obvious in 1 day. That was to say, NNM treatment induced the production of defense enzymes faster, while the content of POD in TMV + N2 treatment increased within 1, 3, and 5 days, but the increase range was more than that of NNM until 7 days when the POD content exceeded that of NNM. It was concluded that N2 could induce the production of defense enzymes and the induction effect was better in 7 days.
Figure 7.

Changes in the POD content in tobacco after compound N2 treatment.
3. Conclusions
In summary, the derivatives of chalcone containing indanone showed good antiviral activity. The therapeutic and protective activities of N2 and N7 were better than those of the control drug NNM, which was confirmed by the Kd values obtained from MST. The determined MDA content also reflected the activity of N2 against TMV. The results of defense enzyme tests also showed that N2 promoted the production of defense enzymes. That was to say, with the help of N2, tobacco further activated the autoimmune response of tobacco, improving its disease resistance and thus making it more effective against TMV. Therefore, the design and synthesis of chalcone derivatives containing indanone provide value for the development of new antiviral agents.
4. Materials and Methods
4.1. Instruments and Chemicals
The melting point was measured by using an X-4 digital micro-melting point tester (Beijing Tektronix Instruments) without correction. The 1H, 13C, and 19F nuclear magnetic resonance (NMR) spectra of target compounds were characterized by using a JEOL ECX500 500 MHz NMR instrument (JEOL, Japan). High-resolution mass spectrometry (HRMS) was performed using an LC–MS 1100/MSD LC–MS/MS system (Agilent, USA). All chemicals were of analytical grade and purchased from Tianjin Zhiyuan Company. MDA and defense enzyme test kits were purchased from Solarbio.
4.2. Synthesis
4.2.1. General Synthesis Procedure for Intermediates 1 and 2
Hydroxyacetophenone (20 mmol) and aldehydes with different substituents (24 mmol) were added to a three-necked flask followed by (30 mL) ethanol to fully dissolve the reactants and 50 mL of 5% NaOH solution, according to ref (27). Magnetic stirring was carried out at room temperature for 12 h until the reaction was complete. Thin-layer chromatography (TLC) was employed to monitor the raw materials. When the raw material point disappeared, the reaction system was poured into an ice−water mixture. Its pH was adjusted until it was about 5 in the beaker when measured. In intermediate 1, some yellow solids appeared at the bottom of the flask. The intermediate 1 (10 mmol) and potassium carbonate (25 mmol) were dissolved in 30 mL of acetonitrile and heated at 80 °C for 1 h. Then, dibromopropane (50 mmol) was added to the reaction system, and heating was continued until the reaction system was white. It was poured into water for dispersion, and a white solid was precipitated, extracted and evaporated under a reduced pressure, dried, and then obtained intermediate 2.
4.2.2. General Synthesis of Target Compounds N1–N20
Hydroxyindanone (10 mmol), potassium carbonate (25 mmol), and a catalytic dose of iodomethane were added to a round-bottom flask, and 10 mL of N,N-dimethylformamide was added and stirred until it dissolved. After heating to 85 °C, intermediate 2 (12 mmol) was added after 1 h while being monitoring by TLC until the raw materials disappeared. At the end of the reaction, the whole reaction system was poured into water for dispersion, extracted and filtered under a reduced pressure, and dried to obtain the crude products. They were separated and purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1:5). The synthetic route of N1–N20 is shown in Scheme 1.
Scheme 1. Synthetic Route of Target Compounds N1–N20.
4.3. Anti-TMV Bioassay
The therapeutic, protective, and passivating activities of target compounds N1–N20 against TMV were tested at a concentration of 500 μg/mL using the half-leaf dead spot method as described in the literature.28 To determine the antiviral activity of the target compounds, the commercially available 8% NNM was used as a control. The treated tobacco plants were cultured in a greenhouse (28 °C) for 2 days, and the number of spots on the left and right sides was counted when spots appeared on the leaves, and the inhibition rate was calculated. Three parallel experiments were conducted for each drug.
where A1 is the number of spots under a blank control and A2 is the number of spots under treatment control.
4.4. MST Experiment
To further study the affinity between the target compound and the TMV-CP, the NHS-647 dye was added to the purified TMV protein according to ref (29), incubated, and eluted with column B. Sixteen samples with a gradient concentration were prepared, and 10 μL of each sample was introduced into the capillary for the MST experiment. The molecules bound to the dye moved by forcing the heat surge, and the measured Kd value can accurately reflect the interaction among the molecules.
4.5. Molecular Docking
Dock ligands in Discovery Studio were used for molecular docking, which simulated the binding mode and affinity of ligands and receptors through the interaction between them. The coat protein of TMV was coded as 1EI7 according to the reference and retrieved from the official website of Protein Data Bank in Europe.30 After the homology comparison with the CP sequence of TMV, the protein template was imported into Discovery Studio software for clean protein backup. A 3D model of the compound structure was constructed and optimized, and then the molecular docking was performed with the treated protein template to simulate the binding ability between the compound and the protein.
4.6. Determination of the MDA Content
An eight-leaf tobacco with consistent growth and healthy growth was selected. Five treatments were carried out, namely, blank control (CK), target compound N2 (drug control), TMV, TMV + NNM, and TMV + N2, with three plants in each treatment. The samples were picked at intervals of 1, 3, 5, and 7 days. The tobacco leaves were picked and placed in a mortar, placed into liquid nitrogen, ground to powder, divided into 10 mL centrifuge tubes, and stored in a refrigerator at −80 ° C.
The accumulation of reactive oxygen species induces membrane lipid peroxidation when plants suffer from various environmental stresses in their life cycle. The main products of membrane lipid peroxidation are MDA and so on. Therefore, the degree of membrane lipid peroxidation, the degree of membrane damage, and the stress resistance of tobacco leaves treated with TMV + N2 can be measured by the content of MDA. The MDA was determined by the method of thiobarbituric acid.31 The absorbance of each sample at 532 and 600 nm was measured using a microplate reader, and the MDA content was calculated according to the following formula. Origin software was used for processing the data.
where Va is the total volume of the reaction system; ε is the MDA molar absorbance coefficient, 1.55 × 105 L/mol/cm; d is the optical diameter of a 96-well plate; W is the sample quality, g; Vb is the added sample volume; Vc is the added volume of extraction liquid, mL; and F is the dilution factor.
4.7. Determination of the Defense Enzyme Activity
The sample preparation method was the same as that in the MDA content determination.
4.7.1. SOD Assay
SOD is a metalloenzyme that widely exists in animals, plants, and microorganisms. It can catalyze the dismutation reaction of a superoxide radical (O2–) in an organism and it is the natural eliminator of O2– in the body. Thus, scavenging O2– plays an extremely important role in the self-protection system of the organism. It also plays an important role in the immune system. The absorbance value of blue formazan produced by the reduction of nitroblue tetrazolium (NBT) was used to determine the SOD content.32 The absorbance of each tube was measured at 560 nm, and 50% inhibition of the photochemical reduction of NBT was taken as 1 unit of enzyme activity U/g.
4.7.2. POD Determination
The role of POD is mainly to hydrolyze hydrogen peroxide. Hydrogen peroxide (H2O2) is a cytotoxic substance produced in the redox reaction catalyzed by oxidases. Both oxidases and catalase exist in peroxisomes, thereby protecting cells and eliminating the toxicity of hydrogen peroxide and phenol, amines, aldehydes, and benzene. POD was used to oxidize guaiacol in the presence of hydrogen peroxide to produce a brown substance,33 and its absorbance value at 470 nm was measured to calculate the POD content in leaves.
Acknowledgments
The authors gratefully acknowledge the National Nature Science Foundation of China (No. 32072446) and the Science Foundation of Guizhou Province (No. 20192452).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07071.
Characterization data , experimental details, spectrogram data of the target compounds N1–N20, and 1H, 13C, and 19F NMR spectra and HRMS spectra of title compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhou X.; Ye Y. Q.; Liu S. S.; Shao W. B.; Liu L. W.; Yang S.; Wu Z. B. Design, synthesis and anti-TMV activity of novel α-aminophosphonate derivatives containing a chalcone moiety that induce resistance against plant disease and target the TMV coat protein. Pestic. Biochem. Phys. 2021, 172, 104749 10.1016/j.pestbp.2020.104749. [DOI] [PubMed] [Google Scholar]
- Basit A.; Farhan M.; Mo W. D.; Ding H. X.; Ikram M.; Farooq T.; Ahmed S.; Yang Z. F.; Wang Y.; Hashem M.; Alamri S.; Bashir M. A.; El-Zohri M. Enhancement of resistance by poultry manure and plant hormones (salicylic acid & citric acid) against tobacco mosaic virus. Saudi J. Biol. Sci. 2021, 28, 3526–3533. 10.1016/j.sjbs.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. F.; Jiao Y. B.; Shi J.; Xie J. J.; Yin J.; Zhao X. X.; Chen H. M. BLB8, an antiviral protein from Brevibacillus laterosporus strain B8, inhibits Tobacco mosaic virus infection by triggering immune response in tobacco. Pest Manage. Sci. 2021, 77, 4383–4392. 10.1002/ps.6472. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Feng C. H.; Wu K.; Chen W. B.; Chen Y. J.; Hao X. G.; Wu Y. F. Advances and prospects in biogenic substances against plant virus: A review. Pestic. Biochem. Phys. 2017, 135, 15–26. 10.1016/j.pestbp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Wang D. N.; Liang J.; Zhang J.; Wang Y. F.; Chai X. Natural chalcones in chinese materia medica: licorice. Evidence-Based Complementary Altern. Med. 2020, 2020, 14. 10.1155/2020/3821248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C.; Hou J.; Wang C.; Zhang M. Y.; Zheng Y. J.; Yang G.; Hu Y. L. Effects of safflower yellow on cholesterol levels in serum and brain tissue of APP/PS1 mice. Metab. Brain Dis. 2021, 36, 557–569. 10.1007/s11011-021-00680-0. [DOI] [PubMed] [Google Scholar]
- Zhang M. R.; Jiang K.; Yang J. L.; Shi Y. P. Flavonoids as key bioactive components of Oxytropis falcata bunge, a traditional anti-inflammatory and analgesic Tibetan medicine. Nat. Prod. Res. 2020, 34, 3335–3352. 10.1080/14786419.2019.1574786. [DOI] [PubMed] [Google Scholar]
- Nuti E.; Bassani B.; Camodeca C.; Rosalia L.; Cantelmo A. R.; Gallo C.; Baci D.; Bruno A.; Orlandini E.; Nencetti S.; Noonan D. M.; Albini A.; Rossello A. synthesis and antiangiogenic activity study of new hop chalcone Xanthohumol analogues. Eur. J. Med. Chem. 2017, 138, 890–899. 10.1016/j.ejmech.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Mustafa M.; Mostafa Y. A. A facile synthesis, drug-likeness, and in silico molecular docking of certain new azidosulfonamide-chalcones and their in vitro antimicrobial activity. Monatsh. Chem. 2020, 151, 417–427. 10.1007/s00706-020-02568-8. [DOI] [Google Scholar]
- Fu Y.; Liu D.; Zeng H. N.; Ren X. L.; Song B. A.; Hu D. Y.; Gan X. H. New chalcone derivatives: synthesis, antiviral activity and mechanism of action. RSC Adv. 2020, 10, 24483–24490. 10.1039/D0RA03684F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulu A. I.; Oderinlo O. O.; Kruger C.; Isaacs M.; Hoppe H. C.; Smith V. J.; Veale C. G. L.; Khanye S. D. Synthesis, structure and in vitro anti-trypanosomal activity of non-toxic arylpyrrole-based chalcone derivatives. Molecules 2020, 25, 1668. 10.3390/molecules25071668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. L.; Zheng X.; Qi Y.; Pu X. J.; Liu B.; Zhang X.; Li X. S.; Xiao W. L.; Wan C. P.; Mao Z. W. Synthesis and anti-inflammatory evaluation of new chalcone derivatives bearing bispiperazine linker as IL-1β inhibitors. Bioorg. Chem. 2020, 98, 103748 10.1016/j.bioorg.2020.103748. [DOI] [PubMed] [Google Scholar]
- Li K.; Zhao S.; Long J.; Su J.; Wu L. S.; Tao J.; Zhou J. D.; Zhang J. L.; Chen X.; Peng C. A novel chalcone derivative has antitumor activity in melanoma by inducing DNA damage through the upregulation of ROS products. Cancer Cell. Int. 2020, 20, 36. 10.1186/s12935-020-1114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar P. M.; Prabhakar P. K.; Doble M. Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med. Chem. Res. 2011, 20, 482–492. 10.1007/s00044-010-9342-1. [DOI] [Google Scholar]
- Koyii M. M.; Gezegen H.; Taslimi P. Synthesis, characterization, and biological studies of chalcone derivatives containing Schiff bases: Synthetic derivatives for the treatment of epilepsy and Alzheimer’s disease. Arch. Pharmacal Res. 2020, 353, 2000202 10.1002/ardp.202000202. [DOI] [PubMed] [Google Scholar]
- Chen Z. H.; Zheng C. J.; Sun L. P.; Piao H. R. Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. Eur. J. Med. Chem. 2010, 45, 5739–5743. 10.1016/j.ejmech.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Nagle D. G.; Zhou Y. D.; Park P. U.; Paul V. J.; Rajbhandari I.; Duncan C. J. G.; Pasco D. S. A new indanone from the marine cyanobacterium Lyngbya majuscula that inhibits hypoxia-induced activation of the VEGF promoter in Hep3B cells. J. Nat. Prod. 2000, 63, 1431–1433. 10.1021/np000216e. [DOI] [PubMed] [Google Scholar]
- Patil S. A.; Patil R.; Patil S. A. Recent developments in biological activities of indanones. Eur. J. Med. Chem. 2017, 138, 182–198. 10.1016/j.ejmech.2017.06.032. [DOI] [PubMed] [Google Scholar]
- Finkieisztein L. M.; Castro E. F.; Fabian L. E.; Moltrasio G. Y.; Campos R. H.; Cavallaro L. V.; Moglioni A. G. New 1-indanone thiosemicarbazone derivatives active against BVDV. Eur. J. Chem. 2008, 43, 1767–1773. 10.1016/j.ejmech.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Patil S. A.; Patil V.; Patil R.; Beaman K.; Patil S. A. Identification of novel 5,6-dimethoxyindan-1-one derivatives as antiviral agents. Med. Chem. 2017, 13, 787–795. 10.2174/1573406413666170330094822. [DOI] [PubMed] [Google Scholar]
- Costanzo P.; Cariati L.; Desiderio D.; Sgammato R.; Lamberti A.; Arcone R.; Salerno R.; Nardi M.; Masullo M.; Oliverio M. Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. ACS Med. Chem. Lett. 2016, 7, 470–475. 10.1021/acsmedchemlett.5b00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv P. C.; Elsayed M. S.; Agama K.; Marchand C.; Pommier Y.; Cushman M. Design, synthesis, and biological evaluation of potential prodrugs related to the experimental anticancer agent indotecan (LMP400). J. Med. Chem. 2016, 59, 4890–4899. 10.1021/acs.jmedchem.6b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.; Li L.; Feng A. Z.; Su B.; Liang D. M.; Liu Y. X.; Wang Q. M. First total synthesis of Papilistatin. Org. Biomol. Chem. 2011, 9, 2539–2542. 10.1039/c0ob01214a. [DOI] [PubMed] [Google Scholar]
- Van der Zee E. A.; Platt B.; Riedel G. Acetylcholine: future research and perspectives. Behav. Brain Res. 2011, 211, 583–586. 10.1016/j.bbr.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Xia R. J.; Guo T.; He J.; Chen M.; Su S. J.; Jiang S. C.; Tang X.; Chen Y.; Xue W. Antimicrobial evaluation and action mechanism of chalcone derivatives containing quinoxaline moiety. Monatsh. Chem. 2019, 150, 1325–1334. 10.1007/s00706-019-02449-9. [DOI] [Google Scholar]
- Tang X.; Su S. J.; Chen M.; He J.; Xia R. J.; Guo T.; Chen Y.; Zhang C.; Wang J.; Xue W. Novel chalcone derivatives containing a 1,2,4-triazine moiety: design, synthesis, antibacterial and antiviral activities. RSC Adv. 2019, 9, 6011–6020. 10.1039/c9ra00618d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T.; Xia R. J.; Chen M.; He J.; Su S. J.; Liu L. W.; Li X. Y.; Xue W. Biological activity evaluation and action mechanism of chalcone derivatives containing thiophene sulfonate. RSC Adv. 2019, 9, 24942–24950. 10.1039/c9ra05349b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; He F. C.; Chen J. X.; Wang Y. J.; Yang Y. Y.; Hu D. Y.; Song B. A. Purine nucleoside derivatives containing a sulfa ethylamine moiety: design, synthesis, antiviral activity, and mechanism. J. Agric. Food Chem. 2021, 69, 5575–5582. 10.1021/acs.jafc.0c06612. [DOI] [PubMed] [Google Scholar]
- Wang Y. J.; He F. C.; Wu S. K.; Luo Y. Q.; Wu R.; Hu D. Y.; Song B. A. Design, synthesis, anti-TMV activity, and preliminary mechanism of cinnamic acid derivatives containing dithioacetal moiety. Pestic. Biochem. Phys. 2020, 164, 115–121. 10.1016/j.pestbp.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Peng F.; Liu T. T.; Wang Q. F.; Liu F.; Cao X.; Yang J. S.; Liu L. W.; Xie C. W.; Xue W. Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives. J. Agric. Food Chem. 2021, 69, 11085–11094. 10.1021/acs.jafc.1c03755. [DOI] [PubMed] [Google Scholar]
- Soares F. A. C.; Kretzmann Filho V.; Beretta B. F. S.; Linden T. S.; Pöppl A. G.; González F. H. D. Thiobarbituric acid reactive substances in dogs with spontaneous hypercortisolism. Domest. Anim. Endocrinol. 2021, 77, 106634 10.1016/j.domaniend.2021.106634. [DOI] [PubMed] [Google Scholar]
- Bhatia K.; Mal G.; Bhar R.; Jyoti; Attri C.; Seth A. Purification and characterization of thermostable superoxide dismutase from Anoxybacillus gonensis KA 55 MTCC 12684. Int. J. Biol. Macromol. 2018, 117, 1133–1139. 10.1016/j.ijbiomac.2018.06.031. [DOI] [PubMed] [Google Scholar]
- Huyskens-Keil S.; Eichholz-Dündar I.; Hassenberg K.; Herppich W. B. Impact of light quality (white, red, blue light and UV-C irradiation) on changes in anthocyanin content and dynamics of PAL and POD activities in apical and basal spear sections of white asparagus after harvest. Postharvest Biol. Technol. 2020, 161, 111069 10.1016/j.postharvbio.2019.111069. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





