Abstract
Purpose
To synthesize the body of knowledge on the factors influencing the quality of life (QoL) after ischemic stroke (IS) in young adults.
Methods
Guidelines regarding the scoping review methodology developed by the Joanna Briggs Institute, and the PRISMA-ScR checklist for a scoping review was used in this paper. A total of 1197 studies were identified through a bibliographic search in Web of Science, MEDLINE, PsycInfo, ScienceDirect, Scopus, and ProQuest Science Database. Articles published between the years 2000–2021 were included.
Results
A total of nine papers were finally selected to respond to the research question. Three studies were prospective longitudinal studies compared QoL between young stroke and age-matched controls from the general population. Across all the analysed studies, 14 variables potentially associated with QoL were identified. QoL in young patients is mainly affected by clinical outcomes after IS (scored by the modified Rankin scale and the Barthel index—favourable initial functional status and higher independence in ADL leads to higher QoL) and psychological factors (post-stroke fatigue and depression—higher levels of fatigue and depression lead to lower QoL). The reviewed studies emphasized the importance of functional outcomes, post-stroke depression, fatigue and anxiety and early return to work.
Conclusion
Further longitudinal studies are needed to identify the trajectory of post-stroke psychosocial symptoms over time and other potential predictors of unfavourable long-term QoL, thus specific young stroke rehabilitation and stroke self-management support programmes should be developed (address physical, psychological factors which influence the psychosocial adaptation post-stroke and the perception of the QoL).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12955-023-02090-5.
Keywords: Young ischemic stroke, Quality of life, Health-related quality of life, Functional outcome, Post-stroke depression, Fatigue
Introduction
Ischaemic stroke (IS) is traditionally considered a disease of middle-aged and elderly patients. In the literature focused on IS in the young population, there is no uniform age cut-off to define “young adults” [1–5]. Lower age limits range from 15 and 18 years [2]. The most pronounced inconsistencies relate to the upper age limit for IS in young adults [2, 3, 5]. The recent reviews used a limit of 45 years [5] or 50 years [1, 3]. On the other hand, some previous studies used the upper age limit of 55 years [3] and the World Health Organisation (WHO) used even a cut-off of 65 years for the upper age limit in the Global Burden of Disease analyses [6–8]. Epidemiological evidence published in the recent narrative reviews highlighted the increasing incidence of IS in young adults and subsequently the increasing number of young patients who live with physical and psychosocial sequels after IS [1–5].
The incidence of IS in young patients has a global increasing trend with higher mortality and morbidity and risk of recurrence [1]. The prevalence of IS in young adults in the US accounts for approximately 10–15% of all strokes [2, 5, 9]. Incidence in Europe ranges between 3.4–21.7/100,000 and the lowest one is in Northern Europe (10.8–11.4/100,000) [4, 10]. Higher IS incidence in young patients was reported previously in the Hispanic population (26/100,000) [11] and in African Americans in the USA (96/100,000) [12]. A sex difference in IS incidence in young patients was also observed [13].
Stroke outcomes in young patients are generally favourable with a high rate of a good 3-month functional outcome and with lower short-term mortality compared with older stroke patients [2, 14, 15]. Nevertheless, young IS patients may face psychological tasks and concerns about their future and the risk of IS recurrence during the period of “active” life (society, family, and work) [7, 14, 15]. Several quantitative studies with an observational design focused on the long-term issues relating to young stroke emphasized a complexity of problems or “invisible dysfunctions” perceived by young individuals, including fatigue [16], cognitive impairment [17–19], fear from stroke recurrence [20], anxiety, depression [21–23], sexual dysfunctions [24], loss of employment [25–28], family conflicts, social isolation, lack of specialist support, reduction in mobility and life roles, negative body image, and impaired self-efficacy and self-esteem [25, 26].
IS at a young age may have a long-term impact on a patient’s multidimensional health-related quality of life (HRQoL), with an accompanying socioeconomic burden [2–8, 14, 29]. However, there is a paucity of comprehensive information regarding post-stroke HRQoL trajectories in young adult stroke survivors and therefore much of the variance of HRQoL after IS remains unexplained in this population. Existing literature reviews [1–5] and prospective cohort studies [30–34] provide information predominantly about the aetiology, risk factors and prognosis of IS in young adults. Moreover, most of the current understanding of the long-term consequences of IS comes from older populations [5]. A considerable number of systematic reviews [35–38] focused mainly on post-stroke trajectories and predictors of the HRQoL in the older population. In addition, the previous studies and their syntheses evaluating HRQoL after a stroke at a young age were heterogeneous regarding the type of stroke included or the age limits definitions for young adults [39–41]. The only available review [41] describing the determinants of the HRQoL did not specifically address the issue of HRQoL after IS but it comprised studies with other subtypes of juvenile stroke and focused on HRQoL and resilience. This review also did not provide evidence to address the impact of physical consequences of IS on the HRQoL in this specific age group.
HRQoL has been widely recognised as one of the key indicators to measure post-stroke outcomes [42]. Further investigation of predictors of post-stroke HRQoL in young-onset patients is needed for the creation, implementation and evaluation of specific young stroke rehabilitation and stroke self-management programs. Furthermore, the evaluation of currently used HRQoL measures should be performed to identify those measures that accurately reflect the complexity of problems in this specific age group and that could be used in stroke self-management and rehabilitation of young adults with IS [43].
In this review, we aimed to focus comprehensively on differences between younger and older individuals regarding HRQoL and the main factors contributing to HRQoL in young adults with IS.
Methods
Guidelines for the scoping review (ScR) methodology developed by the Joanna Briggs Institute (JBI) [44], and the PRISMA-ScR checklist [45, 46] for a scoping review were used.
Review questions
The review addressed the primary (overarching) question: What is known about the HRQoL in young adult stroke survivors?
The specific research questions that guided the scoping review were:
What are the differences in the HRQoL between younger and older stroke survivors and between young adult stroke survivors and age-matched controls?
How has the HRQoL been assessed, and which instruments have been used to measure HRQoL in young adult stroke survivors?
What are the predictors of quality of life in young adult stroke survivors?
A bibliographic search of the literature was conducted from May to June 2021. The relevant studies were identified based on a search of publications between 2000 and May 2021. The ‘‘PCC’’ mnemonic (population, concept, and context [44]) was used to identify meaningful criteria for this scoping review.
Inclusion criteria
(a) Participants: This review has mentioned include the adult population only, and the age range between 18 and 65 years was applied for “young stroke” age definition in this review. Studies with individuals over 65 years at the time of the index IS were excluded, but relevant studies comparing the young and the old stroke patients were included. Studies involving participants with a clinical diagnosis of transient ischemic attack (TIA) or other stroke types than IS were excluded. However, relevant heterogeneous studies comprising a sample of individuals with IS as well as other stroke types were considered if a substantial part of the sample (more than 80%) consisted of young adults with IS.
(b) Concepts: HRQoL was the central concept investigated in this scoping review [37]. Thus, we included studies focused on the health aspect of QoL, long-term functional disabilities and physical and psychosocial consequences of IS to cover comprehensively multidimensionality or all relevant domains of HRQoL in young stroke. Studies using stroke specific or generic HRQoL measures were considered. Therefore, studies focused on functional or health status only and did not explore QoL as the main outcome were excluded.
(c) Context: The studies were included if conducted in long-term period after stroke or in chronic phase of stroke.
(d) Type of studies: After the search was done, quantitative studies with an observational design were included in the analysis. Studies focused on HRQoL measured by standardised questionnaires only were considered. Therefore, qualitative studies did not clearly respond to the inclusion criteria and were excluded from this review. Qualitative studies have explored particular lived experiences of stroke from the perspective of young adults (e.g., the coping experiences [47], the perceived needs and priorities [48], the experience of parenting [49], the lived experiences during the transition period from hospital discharge through the first weeks at home [50], the experiences of QoL in the recovery process across countries [51], the roles of service provision and return to work [52] etc.). Published protocols of studies, discussion papers, reviews, editorials, conference abstracts, books, reports, and dissertations were excluded. Grey literature was also excluded.
Search strategy
The following search terms and their combinations were identified to cover all long-term consequences of IS according to previously published reviews [1–5]:
Participant: (‘stroke’ OR ‘cerebrovascular stroke’ OR ‘cerebrovascular accident’ OR ‘ischemic stroke’ OR ‘ischaemic stroke’) AND (‘young’ OR ‘adult, young’ OR ‘young adult’ OR ‘middle-aged adults’ OR ‘working age’ OR ‘stroke patient’ OR ‘post-stroke’ OR ‘stroke survivor’).
Concept: (‘quality of life’ OR ‘health-related quality of life’) AND (‘health status’) AND (‘functional status’ OR ‘activities of daily living’ OR ‘functional disability’).
Context: ‘long-term’ OR ‘stroke care’ OR ‘post stroke care’ OR ‘prognosis’
The electronic databases Web of Science, MEDLINE (Ovid), PsycInfo (EBSCO), ScienceDirect (Elsevier), Scopus (Elsevier), and ProQuest Science Database were used to gather data for a review of quantitative studies. Selection terms were modified for use in each database (Additional file 1).
Study selection
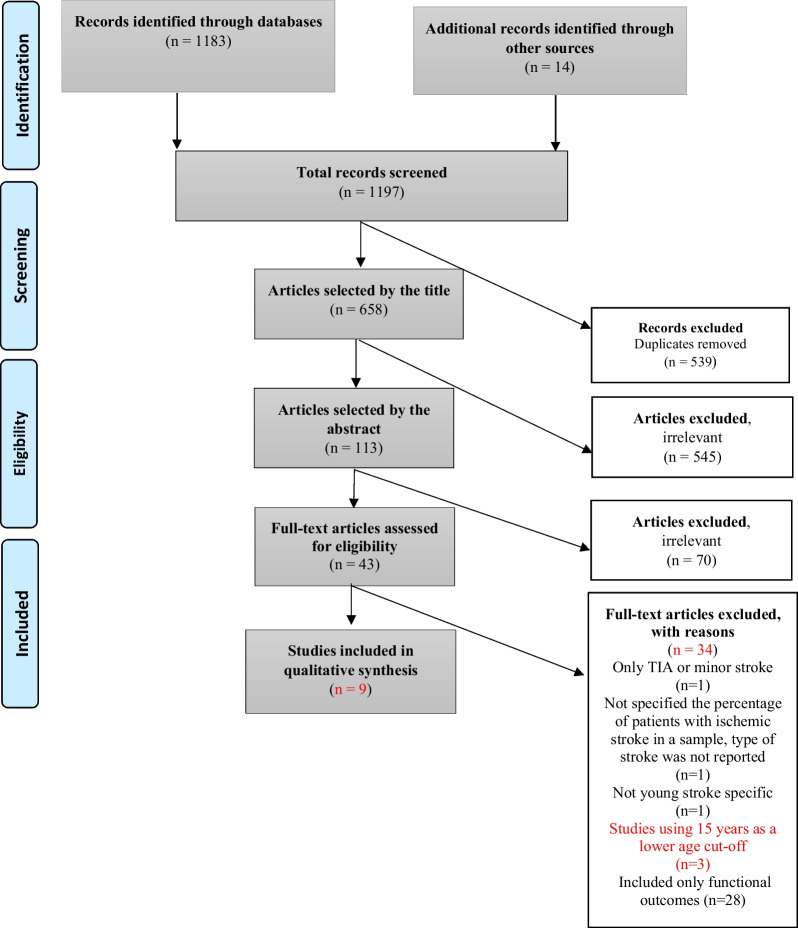
All citations of the identified records were uploaded into a web-based reference manager, and duplicates were eliminated. Two researchers (E.G., L.Š.) screened studies using the titles, and selected studies were analysed using the abstracts based on the pre-specified inclusion/exclusion criteria. Eligibility and data extraction were conducted by two independent researchers (E.G., L.Š.). The full texts of selected sources were then studied by two researchers (E.G., L.Š.) for the final inclusion in the scoping review. A third researcher (D.Š.) evaluated the studies without the previous agreement about the inclusion (Fig. 1). A disagreement about individual study inclusion was solved by the joint discussion until a consensus was reached.
Fig. 1.
Flow diagram of the study selection process
Data extraction and analysis
The extracted data included the following information: the author, year, country of origin, study aim, study design, participants and sampling, age mean, assessment tool, measure time (follow-up) and main findings. The data extraction was performed by three researchers; two researchers extracted data (E.G., L.S.) and one researcher (P.M.) cross-checked the extraction. Data were subsequently analysed and reported thematically with the stated research questions [53].
Results
In total, the search produced 1197 sources, 658 studies were screened using the titles, and a total of 113 studies were analysed using the abstracts based on the pre-specified inclusion/exclusion criteria. The full texts of 43 sources were studied and 9 studies were finally included in the scoping review (Fig. 1).
Characteristics of included studies
Studies included in this review were conducted across multiple countries and most were carried out in European countries. Four studies included patients only with IS [55, 57, 59, 60]. Five studies included patients with IS and haemorrhagic stroke [20, 26, 54, 56, 58], and the proportion of patients with IS in such studies ranges from 77.7% [20] to 92.4% [26]. Eight studies recruited participants from stroke hospital registries, and one study recruited patients from a national stroke clinical registry [54]. The number of participants in the follow-up (FUP) assessment ranged from 63 [56] to 5154 [54]. Three studies assessed the HRQoL within the time of FUP ≤ 12 months [54, 55, 60]. The other studies evaluated the HRQoL within the time of FUP between one to 6 years (Tables 1, 2, 4).
Table 1.
Differences in QoL between young stroke patients and age-matched controls from the general population
| Author/Year/Country | Study design | Participants and sampling | Age mean (SD), range or median (years) | HRQoL measure | Measure time (Time of assessment after stroke) | Main findings and conclusion |
|---|---|---|---|---|---|---|
|
de Bruijn et al. (2015) [57] The Netherlands |
Prospective study |
Young ISs (18–49 years): 170 Controls: 61 |
Age at a stroke: 41.4 (7) Age at follow-up: 46.3 (7.1) Controls: 48 (6.6) |
WHOQOL-BREF 26 | A mean time to FUP: 4.9 years |
There were no statistical differences in QoL between young adults with IS and controls after long-term follow-up Fatigue, depression, anxiety, and unemployment affect the QoL in young adults with IS of mild severity The stroke-specific factors were not confirmed as significant contributing factors of QoL in young adults with IS |
|
Palmcrantz et al. (2014) [58] Sweden |
Prospective study |
Young adult (< 65 years):150 (80% ISs) Controls: 2661 |
Young adult Mean: 57 (6) Median: 59 IQR: 54–62 Controls Median: 46, IQR: 38–55 |
MYS questionnaire (body functions, limitations, restrictions, personal and environmental factors) EQ-5D |
< 6 years after stroke | The negative effects of stroke, on self-rated global health among young individuals living in the community, appear to be substantial, multifactorial and long-standing. The young individuals with IS reported significantly lower health status regarding mobility, self-care, usual activities, anxiety, and depression than the matched general population. Limitations and restrictions regarding previous leisure activities and returning to work were the main predictors of the HRQoL in young individuals with IS |
|
Schneider et al. (2021) [59] Estonia |
Prospective study |
Young ISs (18–54 years): 352 Controls (18-64 years): 2304 |
Age at stroke Median: 48.8 Range: 19.2–54.9 Age at follow-up Median: 54 Range: 27–65 Controls: Median: 47 Range: 27–64 |
EQ-5D-3L | A mean time to FUP: 5.7 years | ISs reported significantly lower health status regarding mobility, self-care, usual activities, anxiety, and depression than controls. The most significant differences between young stroke patients and their non-stroke counterparts were in the physical domain. Lower QoL of young stroke patients was predicted by stroke-specific (coronary heart disease during the index event, longer follow-up duration, recurrent stroke, functional disability) and psychosocial factors (depression, unemployment). Young patients with excellent recovery after IS reported higher QoL than the controls. Young ISs have long-term decreased HRQOL, except for those with excellent functional recovery |
EQ-5D-3L EuroQoL-5 dimension-3 level, EQ-5D EuroQoL-5 dimension, FUP follow-up, ISs Ischemic stroke survivors, IQR Inter quartile range, mRS Modified Rankin Scale, MYS Mapping Young persons with Stroke, WHOQOL-BREF 26 shortened World Health Organization Quality of Life scale
Table 2.
HRQoL and related outcomes
| Author/Year/Country | Aim | Study design | Participants and sampling | Age mean (SD), range or median | HRQoL measure | Measure time (Time of assessment post-stroke) | Main findings and conclusion |
|---|---|---|---|---|---|---|---|
|
Rhudy et al. (2020) [60] USA |
To investigate QoL in young adult stroke survivors at baseline and 6 months post-discharge | Prospective study | ISs (18–65 years):18 |
55.56 (9.45) Range: 34–64 |
PROMIS and NeuroQoL | 6-month | The statistically significant improvement from baseline to 6-month follow-up was found only in independence in ADL and cognitive function |
|
Westerlind et al. (2017) [26] Sweden |
To explore factors affecting the return to work after stroke | Prospective study |
Young adult (18–63 years): 211 (77.7% ISs) |
Median: 53 ≤ 50 years: 41.2% ≥ 51 years: 58.8% |
FUP assessment EQ5D |
A mean time to FUP: 6 years | Young ISs who did return to work reported higher VAS scores. There were no significant differences regarding the index value calculated from EQ-5D |
|
Yoon et al. (2021) [20] South Korea |
To test a predictive model of QoL in young adults with stroke | Cross-sectional study |
Young adult (18–49 years): 237 (92.4% ISs) |
46.7 (4.70) Range: 20–49 |
SS-QoL | A mean time since the stroke: 19.3 (± 13.1) months | QoL was mainly influenced by stroke severity, social support, depression, functional disability, fear of stroke recurrence and perception of health status |
ADL activities of daily living, EQ-5D-3L EuroQoL-5 dimension-3 level, EQ-5D EuroQoL-5 dimension, FUP follow-up, ISs Ischemic stroke survivors, IQR Inter quartile range, NeuroQoL Quality of Life in Neurological Disorders, SS-QoL Stroke Specific Quality of Life scale, VAS Visual analogue scale
Table 4.
Differences in QoL between younger and older individuals
| Author/Year/Country | Study design | Participants and sampling | Age mean (SD), range or median (years) | HRQoL measure | Measure time (Time of assessment after stroke) | Main findings and conclusion |
|---|---|---|---|---|---|---|
|
Lannin et al. (2017) [54] Australia |
Retrospective study |
Working age adults (18–64 years): 5154 ISs: 79% Older group (≥ 65 years):15,317 |
Not reported | EQ-5D-3L | 90 and 180 days after hospital admission | The younger group reported problems with self-care (only 21%), performing usual activities (53%), and pain/discomfort (45%) less often compared with the older one. There were no statistical differences in mobility and emotional domain |
|
Lisabeth et al. (2018) [55] USA |
Retrospective study |
The midlife ISs (45–64): 1618 Older ISs (≥ 65 years): 3240 |
The midlife ISs: 56.8 Median: 52.2 Older ISs: 78.3, Median: 72.4 |
SS-QoL-12 | 90 Days after IS | Despite more favourable 90-day neurologic, functional, and cognitive outcomes, the midlife stroke survivors’ group did not report better QoL than the old one |
|
Palmcrantz et al. (2012) [56] Sweden |
Prospective study |
Young adult (< 65 years):63 (84% ISs) Older group (≥ 65 years):129 |
Young: 53 (11), 25–64 Old: 78 (8), 65–94 |
SIS | 12 Months after IS | No statistical differences in global recovery between the groups. Young adults reported greater use of care and rehabilitation, higher levels of strength, self-care/domestic life, and mobility. Self-perceived global recovery in the younger group was mainly affected by hand function and depression. The initial sense of coherence, stroke severity and follow-up independence in ADL were associated with self-perceived global recovery in young adults at 12 months |
ADL activities of daily living, EQ-5D-3L EuroQoL-5 dimension-3 level, EQ-5D EuroQoL-5 dimension, ISs Ischemic stroke survivors, IQR Inter quartile range, mRS Modified Rankin Scale, SS-QoL-12 short-form Stroke Specific Quality of Life scale, SIS Stroke Impact scale, VAS Visual analogue scale
The studies included in the review used different age limits to define young patients. The mean age of the participants ranged from 47 years [57] to 57 years [58] with the age range between 18 and 65 years. The upper limit of 50 years was used in two studies [20, 57], and the upper limit of 65 years was used in six studies [26, 54–56, 58, 60]. The upper limit of 55 years was used in one study [59].
HRQoL between young adults with IS and age-matched controls
Differences in HRQoL between young adults with IS and age-matched controls from the general population were reported in four studies, in which generic measures (WHOQOL-BREF 26, EQ-5D) were applied. Young stroke patients rated significantly lower global health [59], physical functioning [58] and all domains of the EQ-5D, except pain/discomfort (Table 1).
Instruments used for assessing the HRQoL
Seven outcome measures (Tables 1, 2, 4) were applied in the nine quantitative studies; five were generic (EQ-5D; EQ-5D-3L; WHOQOL-BREF 26) and four stroke-specific QoL measures (SS-QoL; SS-QoL-12; SIS; NeuroQoL). The most used measures were the EuroQoL instruments, which had been applied in four studies. Stroke-specific QoL measures had been applied in four studies [20, 55, 56, 60].
Predictors of the HRQoL in young adults
Of all analysed studies, 14 variables (Table 3) potentially associated with the HRQoL were identified, and in most studies, several factors were examined in parallel. In most studies, multiple regression analyses were used (Table 3). Clinically related physical factors were more frequently addressed, followed by those centred on the psychosocial stroke sequels and the individual or sociodemographic factors. The severity of the stroke, functional status, or disability, independence in ADL, motor dysfunction, hand function, fatigue, and coronary heart disease during the index event were clinically related factors. Cognitive status, depression/anxiety, fear of stroke recurrence, return to work/unemployment, restrictions and limitations in leisure activities, self-perceived health status, and sense of coherence were psychosocial factors.
Table 3.
Predictors of HRQoL in young adults with IS
| Category | Factors | Measure | Statistical analyses | Main findings |
|---|---|---|---|---|
| Clinically related factors | Neurological outcomes, the severity of stroke | NIHSS | (SEM) | The severity of the stroke at the time of follow-up had significant direct, indirect and total effects on HRQoL [20] |
| Functional outcomes, independence in ADL | mRS, BI | Regression model | Favourable initial functional status and higher independence in ADL: higher HRQOL [56] | |
| SEM | The functional status and higher independence in ADL at the time of follow-up had significant direct, and total effects on HRQoL [20] | |||
| Motor impairment | MRC | Regression model | Lower motor dysfunction: higher HRQOL [56] | |
| SIS | ||||
| Hand function | Upper extremity function of the SIS | Regression model | HRQoL at 12 months after stroke was predicted by hand function explaining a total of 32% of variance [56] | |
| Fatigue | FSS, FAS | Regression model | Higher levels of fatigue: lower HRQOL [57] | |
| Coronary heart disease during the index event | Regression model | No coronary heart disease during the index event: higher HRQOL [59] | ||
| Psychosocial factors | Depression | BDI, HADS, MADRS | Regression model | Higher levels of depression: lower HRQOL [57, 59] |
| SEM | Depression at the time of follow-up had significant indirect, and total effects on HRQoL [20] | |||
| Anxiety | HADS | Regression model | Higher levels of anxiety: lower HRQOL [57] | |
| Fear of stroke recurrence | FSRS | SEM | Fear of stroke recurrence at the time of follow-up had significant direct, indirect, and total effects on HRQoL [20] | |
| Restrictions and limitations in leisure activities | MYS questionnaire | Regression model | Higher levels of restrictions and limitations in leisure activities: lower HRQOL [58] | |
| Self-perceived health status | HPQ | SEM | General health perception had a significant direct effect on the HRQoL [20] | |
| Sense of coherence | SCS | Regression model | The higher initial sense of coherence: higher HRQOL [56] | |
| Return to work/unemployment | Regression model | Return to work: higher HRQoL | ||
| Unemployment: lower HRQoL [20, 57–59] | ||||
| Social support | ENRICHD SSI | SEM | Higher social support: higher HRQOL | |
| Social support had significant direct, and total effects on HRQoL [20] |
BI Barthel Index, BDI Beck Depression Inventory, ENRICHD SSI Coronary Heart Disease Patients Social Support Inventory, FAS Fatigue Assessment Scale, FSS Fatigue Severity Scale,– FSRS Fear of Stroke Recurrence Scale, HPQ Health Perception Questionnaire, HADS Hospital Anxiety and Depression Scale, ADL Activities of Daily Living, MRC Medical Research Council motor scale, MMSE Mini-Mental State Examination, MMMS Modified Mini-Mental State Examination, mRS Modified Rankin Scale, MADRS Montgomery-Åsberg Depression Rating Scale, NIHSS National Institutes of Health Stroke Scale, SCS Sense of Coherence scale, SIS Stroke Impact scale, SEM Structural equation modelling
Consistent results were reported for functional outcomes, independence in ADL, fatigue, depression, and return to work were demographic factors (Table 3). Poor functional outcomes, dysarthria, motor impairment and impaired hand function, depression/anxiety, fatigue, fear of stroke recurrence, coronary heart disease during the index event, and restrictions and limitations in leisure activities had a negative impact on the HRQoL in young adults with IS. Independence in ADL, social support, return to work, and higher general health perception had a positive influence on the HRQoL.
Three studies [55, 56, 60] confirmed a predictive value of clinical outcomes assessed using the modified Rankin Scale (mRS) and the Barthel index (BI) for the HRQoL. HRQoL had no significant association with mRS [57] in a long-term FUP. The impact of mRS on FUP was explained mostly by its association with physical functioning. However, in the study from the Netherlands, mRS in FUP did not significantly contribute to any domains of the HRQoL [57]. The proportion of independence in ADL based on BI ranged from 78% [60] to 84% [56] after a mean FUP of 6 to 12 months. Inconclusive results were found in the stroke severity. Stroke severity (scored by the National Institutes of Health Stroke Scale, NIHSS) was often reported at baseline (during the hospital admission or discharge) and two studies only provided the neurological deficits within months after discharge [20, 55]. The severity of persisting neurological deficit over time had negative effects on the HRQoL in the Korean study [20]. In the US study, the median of NIHSS score was significantly lower in the midlife stroke survivors’ group (45–64 years, median NIHSS: 2) than in the older group (≥ 65 years, median NIHSS: 3) [49]. The admission NIHSS had no significant impact on the HRQoL in stroke patients in the Netherlands study [57]. Three of the included studies assessed depression and/or anxiety [20, 57, 59], however, only in one of them, fatigue and depression were measured simultaneously (Table 3) [57].
Discussion
The aim of this review was to investigate and map conceptually the current evidence in HRQoL in young adults. In comparison with the previous review [41], this scoping review contributes to the existing evidence with a deeper insight into what is known about the differences in the HRQoL poststroke between younger and older patients and about the relationship between functional outcomes and HRQoL in young adults.
Studies comparing the HRQoL between the younger and older stroke patients (Table 4) have produced inconsistent findings; the methods of the assessments of variables varied among studies and resulted in inconclusive data. Aspects of the HRQoL after stroke differed between younger and older individuals, most significantly in the functional outcomes and in the self-care [55, 56]. No significant difference was found in global recovery or global HRQoL [55, 56] and in the emotional aspects of the HRQoL [54, 56]. Between younger and older stroke survivors. Based on the available literature, it appears that the time of FUP and the type of HRQoL assessment may explain the inconsistent results regarding the differences in the HRQoL between younger and older individuals. Studies examining differences in the disease specific HRQoL between young and old patients provide consistent evidence that overall HRQoL assessed more than 12 months after IS was significantly higher in the younger patients (< 65 years) compared to older those [54, 56]. It was also well documented that young adults reported a higher level of self-care, mobility, functional outcome, and strength/energy 12 months after IS than old ones. However, inconsistent findings were found within the time of FUP ≤ 12 months [54, 55].
Aspects of HRQoL in the long-term FUP differ most significantly among young adults and their age-matched controls in physical functioning and in almost all domains of the EQ-5D. However, the studies comparing HRQoL between young stroke patients and their non-stroke counterparts (Table 2) predominantly addressed the perceived global health measured by generic instruments (the EQ-5D) and not QoL per se.
Instruments used for assessing the HRQoL
However, only one instrument (the MYS questionnaire) was developed specifically to assess relevant aspects of the functioning and disability of young individuals with IS [58]. Conceptual and psychometric limitations of this instrument are the most important, mainly the lack of explicit, a priori models of the construct. For the measurement of post-stroke HRQoL, generic instruments were predominantly used. However, these measures do not comprehensively cover all relevant domains to HRQoL in young strokes. Therefore, there is a continuing need for examining the feasibility of methods to assess all domains of long-term consequences in patients with young stroke.
Factors associated with the HRQoL in young adults
A wide spectrum of functional, and psychosocial factors related to the HRQoL has been investigated in many studies. In line with previous reviews [35, 61], various predictors of the HRQoL were identified in young adults with IS in this scoping review.
The incidence of poor functional outcomes in young adults at the time of hospital discharge is generally low [62]. Favourable functional status and low severity of the stroke were found to be the significant factors contributing to better long-term outcomes [21, 27, 63] and return to work [20, 63]. Several prospective and retrospective cohort studies on the long-term prognosis of young adults after IS have revealed that a substantial number of patients achieved independence in activities of daily living [21, 27, 63–65] and young patients achieved better clinical outcomes after IS in comparison with old ones [55, 56]. There are only a few studies that examined the associations between long-term functional outcomes and the HRQoL. Stroke severity (measured by the NIHSS) was not consistently associated with the HRQoL, but the NIHSS score was confirmed as the independent predictor of the HRQoL in several prospective studies. The predictability of the NIHSS score for the HRQoL was also observed in previous longitudinal studies measuring the HRQoL in the older populations at three [66], 12 months [66] or 2 years after IS [62]. Although NIHSS was identified as a predictor of good clinical outcomes in young, no prospective study assessed the impact of the baseline NIHSS on the HRQoL after IS in them. Thus, further studies related to changes in the severity status and functional outcomes over time (years after discharge) or their influence on the HRQoL in young adults are needed. In addition, the size effect of different functional outcome measures on the HRQoL was not also investigated. To our knowledge, some prospective studies in stroke survivors found that mRS is a better predictor of the HRQoL in long-term FUP than other scales (e.g. BI) [49, 66–68].
Getting sufficient evidence about long-term functional outcomes and remaining disability is strongly needed in young stroke survivors because of their long-life expectancy. Younger adults live with disabilities for a longer time, and their HRQoL is strongly influenced not only by independence in ADL. Young adults require and expect to achieve a higher level of functioning or independence in more complex roles because of their parenting and challenging family or work responsibilities [49, 56, 59]. Return to work (RTW) or the ability to stay at work significantly contributed to better HRQoL [20]. Post-stroke unemployment was assessed in six of the included studies and mainly using self-reported data. The Swedish study used data from the Swedish Health Insurance Office [26, 27]. In the Dutch study, young adults had a higher risk of post-stroke unemployment eight years after IS than their peers did [68]. Several predictors of RTW after IS (e.g., functional outcomes, stroke severity, fatigue, depression) reported in recent studies were like predictors of HRQoL identified in this review [25–27, 68, 69]. We could suggest a reciprocal relationship between the HRQoL and RTW after IS, which means that better HRQoL facilitates RTW and vice versa [25].
Psychological characteristics, namely post-stroke fatigue (PSF), depression (PSD) and anxiety (PSA) have proved the highest relevance as independent factors contributing to low HRQoL at a young age. Post-stroke depressive symptoms together with PSF have been recognised as common and persistent complaints jeopardizing the HRQoL after stroke [70–72]. Moreover, PSF [57] and PSD [57] among young adults correlated with the domains of the HRQoL stronger than functional outcomes (mRS). However, all the included studies used self-reported measures for assessing the level of fatigue, depression, and anxiety. The findings of this review emphasize the need to monitor PSF, PSD, and PSA also many years after IS and to improve awareness among healthcare professionals about their prevalence and impact on functional outcomes, the HRQoL or on return to pre-stroke activities among young stroke patients. The influence of PSD and PSF was observed even after a mean FUP of 5 to 6 years after IS [57]. Furthermore, PSD, PSA, and PSF had a negative influence on functional outcomes in young adults a decade after IS [16, 22]. Nevertheless, temporal relationships between PSD and PSF have not yet been reported. Pre-stroke depression significantly contributed to PSD [73].
In addition, the relationship between cognitive dysfunction or post-stroke pain and the HRQoL was not investigated in any study included in this review. However, the prevalence and course of cognitive dysfunction [6, 17–19] or post-stoke pain [74] in young IS patients have been examined in several studies.
The HRQoL was not measured repeatedly over time in most studies and only one pilot study compared the HRQoL in young patients between two-time points (baseline and 6 months after discharge) [60]. A significant improvement was found only in the independence in ADL and cognitive function, whereas the HRQoL did not improve over time [60]. Other studies examined the HRQoL only in FUP assessments (Table 1, 2, 3). Future research investigating the trajectories of the HRQoL domains and post-stroke symptoms at a young age is needed. In the Dutch quantitative study of 351 old IS patients (mean age of 67 ± 13 years), the trajectories of the HRQoL were investigated in the first 12 months after stroke [75]. The authors identified a four-trajectory model (high, low, recovery, and decline) for physical and psychosocial HRQoL and predictors of these diverse trajectories. Psychological factors (personality, coping competencies, illness cognitions, and self-efficacy) were the most significant factors in identifying individuals at risk of unfavourable post-stroke HRQoL outcomes [75]. In this review, we identified the following three psychosocial factors affecting the HRQoL: the sense of coherence [56], fear of stroke recurrence [20] and perceived social support [20]. Although the role and importance of psychological factors (such as personality, coping, locus of control, illness cognitions, self-efficacy, or self-worth) for the post-stroke HRQoL have been systematically studied, these factors have not been specifically examined in young stroke patients [29]. Indeed, psychological factors may influence the psychosocial adaptation post-stroke and therefore the perception of the HRQoL. The most examined predictors of post-stroke HRQoL in young adults were stroke-related factors (functional outcomes and PSD), and RTW. Further longitudinal studies are needed to identify the trajectory of post-stroke psychosocial symptoms over time and other potential predictors of unfavourable long-term HRQoL so that specific young stroke rehabilitation and stroke self-management support programmes could be developed.
Most of the studies included in this review were from Western countries, and only two studies were conducted outside of this area (South Korea). A paucity of studies conducted in other parts of World (especially in developing countries) remains, however, epidemiological evidence published in the recent narrative reviews [1, 5] has highlighted that data on incidence and prevalence are still scarce for many African and Asian countries.
Study limitations
Cohen’s kappa was not used to calculate concurrence between authors. Disagreement was solved only by the joint discussion until a consensus was reached. The search was limited to the electronic scientific databases accessible to the authors’ institution. The comparative analysis of included studies was limited, mainly due to the different study designs across studies and inherent heterogeneity in the subtypes of stroke. For measuring the HRQoL, different tools were used, mainly generic instruments. In addition, the HRQoL was measured at a different time of FUP, and a varying number of variables were used in the included studies. These factors could contribute to inconclusive findings. This scoping review suggested that the HRQoL in young adults with IS can be affected mainly by commonly used stroke clinical outcomes (measured by mRS and BI) and psychological factors (post-stroke fatigue and depression).
Conclusion
There is still a gap in the evidence for the HRQoL outcomes after IS and in the role of psychosocial variables for the HRQoL in young IS patients. The reviewed studies emphasized the importance of functional outcomes, post-stroke depression, fatigue and anxiety and early return to work. The findings of our review can provide deeper insight and a better understanding of the various factors contributing to the long-term HRQoL after IS and may support the development of specific interventions for stroke self-management programmes. Further large prospective studies focusing on the factors affecting the HRQoL in young patients after IS are warranted.
Supplementary Information
Additional file 1. Detailed search strategy in databases.
Acknowledgements
Not applicable.
Author contributions
Made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data: EG, DS, LS, PM. Involved in drafting the manuscript, revising it critically for important intellectual content: EG, DS, PM. Given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content: EG, DS, PM. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.: EG, DS, LS, PM. All authors read and approved the final manuscript.
Funding
This study was supported by the Ministry of Health of the Czech Republic (Grant number NU22-09-00021) and by the Internal Grant Agency of Palacký University Olomouc (Grant number IGA_LF UP_013_2022).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elena Gurková, Email: elena.gurkova@upol.cz.
Lenka Štureková, Email: lenka.sturekova@upol.cz.
Petra Mandysová, Email: petra.mandysova@upol.cz.
Daniel Šaňák, Email: daniel.sanak@fnol.cz.
References
- 1.Boot E, Ekker MS, Putaala J, Kittner S, De Leeuw FE, Tuladhar AM. Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry. 2020;91(4):411–417. doi: 10.1136/jnnp-2019-322424. [DOI] [PubMed] [Google Scholar]
- 2.Ekker MS, Boot EM, Singhal AB, Tan KS, Debette S, Tuladhar AM, de Leeuw FE. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018;17(9):790–801. doi: 10.1016/S1474-4422(18)30233-3. [DOI] [PubMed] [Google Scholar]
- 3.Ekker MS, Jacob MA, van Dongen MME, Aarnio K, Annamalai AK, Arauz A, Arnold M, Barboza MA, Bolognese M, Brouns R, Chuluun B, Chuluunbaatar E, Dagvajantsan B, Debette S, Don A, Enzinger C, Ekizoglu E, Fandler-Höfler S, Fazekas F, Fromm A, Gattringer T, Gulli G, Hoffmann M, Hora TF, Jern C, Jood K, Kamouchi M, Kim YS, Kitazono T, Kittner SJ, Kleinig TJ, Klijn CJM, Korv J, Lee TH, Leys D, Maaijwee NAM, Martinez-Majander N, Marto JP, Mehndiratta MM, Mifsud V, Montanaro VV, Owolabi MO, Patel VB, Phillips MC, Piechowski-Jozwiak B, Pikula A, Ruiz-Sandoval JL, Sarnowski B, Schreuder FHBM, Swartz RH, Tan KS, Tanne D, Tatlisumak T, Thijs V, Tuladhar AM, Viana-Baptista M, Vibo R, Wu TY, Yesilot N, Waje-Andreassen U, Pezzini A, Putaala J, de Leeuw FE. Global Outcome Assessment Life-long after stroke in young adults initiative-the GOAL initiative: study protocol and rationale of a multicentre retrospective individual patient data meta-analysis. BMJ Open. 2019;9(11):e031144. doi: 10.1136/bmjopen-2019-031144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putaala J. Ischemic stroke in the young: current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur Stroke J. 2016;1(1):28–40. doi: 10.1177/2396987316629860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yahya T, Jilani MH, Khan SU, Mszar R, Hassan SZ, Blaha MJ, Blankstein R, Virani SS, Johansen MC, Vahidy F, Cainzos-Achirica M, Nasir K. Stroke in young adults: current trends, opportunities for prevention and pathways forward. Am J Prevent Cardiol. 2020;3:100085. doi: 10.1016/j.ajpc.2020.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 7.Defining Young Stroke. Young Stroke. 2017. https://youngstroke.org/wpcontent/uploads/2017/04/DefiningYoungStroke.pdf
- 8.Siriratnam P, Godfrey A, O'Connor E, Pearce D, Hu CC, Low A, Hair C, Oqueli E, Sharma A, Kraemer T, Sahathevan R. Prevalence and risk factors of ischaemic stroke in the young: a regional Australian perspective. Intern Med J. 2020;50(6):698–704. doi: 10.1111/imj.14407. [DOI] [PubMed] [Google Scholar]
- 9.Tibæk M, Dehlendorff C, Jørgensen HS, Forchhammer HB, Johnsen SP, Kammersgaard LP. Increasing incidence of hospitalization for stroke and transient ischemic attack in young adults: a registry-based study. J Am Heart Assoc. 2016;5(5):e003158. doi: 10.1161/JAHA.115.003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naess H, Nyland HI, Thomassen L, Aarseth J, Nyland G, Myhr KM. Incidence and short-term outcome of cerebral infarction in young adults in western Norway. Stroke. 2002;33(8):2105–2108. doi: 10.1161/01.str.0000023888.43488.10. [DOI] [PubMed] [Google Scholar]
- 11.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R, Broderick J. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35(2):426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 12.Kittner SJ, Stern BJ, Wozniak M, Buchholz DW, Earley CJ, Feeser BR, Johnson CJ, Macko RF, McCarter RJ, Price TR, Sherwin R, Sloan MA, Wityk RJ. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;50(4):890–894. doi: 10.1212/wnl.50.4.890. [DOI] [PubMed] [Google Scholar]
- 13.Putaala J, Yesilot N, Waje-Andreassen U, Pitkäniemi J, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, Kloss M, Ferrari J, Lemmens R, Coban O, Haapaniemi E, Maaijwee N, Rutten-Jacobs L, Bersano A, Cereda C, Baron P, Borellini L, Valcarenghi C, Thomassen L, Grau AJ, Palm F, Urbanek C, Tuncay R, Durukan-Tolvanen A, Van Dijk EJ, De Leeuw FE, Thijs V, Greisenegger S, Vemmos K, Lichy C, Bereczki D, Csiba L, Michel P, Leys D, Spengos K, Naess H, Bahar SZ, Tatlisumak T. Demographic and geographic vascular risk factor differences in European young adults with ischemic stroke: the 15 cities young stroke study. Stroke. 2012;43(10):2624–2630. doi: 10.1161/STROKEAHA.112.662866. [DOI] [PubMed] [Google Scholar]
- 14.Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, van Dijk EJ, de Leeuw FE. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol. 2014;10(6):315–325. doi: 10.1038/nrneurol.2014.72. [DOI] [PubMed] [Google Scholar]
- 15.Rutten-Jacobs LC, Maaijwee NA, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, van der Vlugt MJ, van Dijk EJ, de Leeuw FE. Long-term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74(4):592–601. doi: 10.1002/ana.23953. [DOI] [PubMed] [Google Scholar]
- 16.Maaijwee NA, Arntz RM, Rutten-Jacobs LC, Schaapsmeerders P, Schoonderwaldt HC, van Dijk EJ, de Leeuw FE. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J Neurol Neurosurg Psychiatry. 2015;86(10):1120–1126. doi: 10.1136/jnnp-2014-308784. [DOI] [PubMed] [Google Scholar]
- 17.Pinter D, Enzinger C, Gattringer T, Eppinger S, Niederkorn K, Horner S, Fandler S, Kneihsl M, Krenn K, Bachmaier G, Fazekas F. Prevalence and short-term changes of cognitive dysfunction in young ischaemic stroke patients. Eur J Neurol. 2019;26(5):727–732. doi: 10.1111/ene.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaapsmeerders P, Maaijwee NA, van Dijk EJ, Rutten-Jacobs LC, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, Kessels RP, de Leeuw FE. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke. 2013;44(6):1621–1628. doi: 10.1161/STROKEAHA.111.000792. [DOI] [PubMed] [Google Scholar]
- 19.Synhaeve NE, Schaapsmeerders P, Arntz RM, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, Dorresteijn LD, de Kort PL, van Dijk EJ, Kessels RP, de Leeuw FE. Cognitive performance and poor long-term functional outcome after young stroke. Neurology. 2015;85(9):776–782. doi: 10.1212/WNL.0000000000001882. [DOI] [PubMed] [Google Scholar]
- 20.Yoon S, Kim HY, Kim SR. A prediction model of health-related quality of life in young adult patients with stroke. J Clin Nurs. 2021;30(13–14):2023–2035. doi: 10.1111/jocn.15755. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor A, Si K, Yu A, Lanctot KL, Herrmann N, Murray BJ, Swartz RH. Younger age and depressive symptoms predict high risk of generalized anxiety after stroke and transient ischemic attack. Stroke. 2019;50(9):2359–2363. doi: 10.1161/STROKEAHA.119.025464. [DOI] [PubMed] [Google Scholar]
- 22.Maaijwee NA, Tendolkar I, Rutten-Jacobs LC, Arntz RM, Schaapsmeerders P, Dorresteijn LD, Schoonderwaldt HC, van Dijk EJ, de Leeuw FE. Long-term depressive symptoms and anxiety after transient ischaemic attack or ischaemic stroke in young adults. Eur J Neurol. 2016;23(8):1262–1268. doi: 10.1111/ene.13009. [DOI] [PubMed] [Google Scholar]
- 23.Tanislav C, Kropp P, Grittner U, Holzhausen M, Fazekas F, Jungehülsing GJ, Tatlisumak T, von Sarnowski B, Putaala J, Huber R, Thijs V, Schmidt R, Kaps M, Enzinger C, Dichgans M, Norrving B, Rolfs A. Clinically relevant depressive symptoms in young stroke patients—results of the sifap1 study. Neuroepidemiology. 2015;44(1):30–38. doi: 10.1159/000371389. [DOI] [PubMed] [Google Scholar]
- 24.Bugnicourt JM, Hamy O, Canaple S, Lamy C, Legrand C. Impaired sexual activity in young ischaemic stroke patients: an observational study. Eur J Neurol. 2014;21(1):140–146. doi: 10.1111/ene.12277. [DOI] [PubMed] [Google Scholar]
- 25.Aarnio K, Rodríguez-Pardo J, Siegerink B, Hardt J, Broman J, Tulkki L, Haapaniemi E, Kaste M, Tatlisumak T, Putaala J. Return to work after ischemic stroke in young adults: a registry-based follow-up study. Neurology. 2018;91(20):e1909–e1917. doi: 10.1212/WNL.0000000000006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerlind E, Persson HC, Sunnerhagen KS. Return to work after a stroke in working age persons. A six-year follow up. PLoS ONE. 2017;12(1):e0169759. doi: 10.1371/journal.pone.0169759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerlind E, Persson HC, Eriksson M, Norrving B, Sunnerhagen KS. Return to work after stroke: a Swedish nationwide registry-based study. Acta Neurol Scand. 2020;141(1):56–64. doi: 10.1111/ane.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris R. The psychology of stroke in young adults: the roles of service provision and return to work. Stroke Res Treat. 2011;2011:534812. doi: 10.4061/2011/534812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasikumar S, Pikula A. Psychosocial needs and occupational functioning of younger adults after stroke. EC Neurology. 2018;10:34–47. [Google Scholar]
- 30.Arntz RM, van Alebeek ME, Synhaeve NE, Brouwers PJ, van Dijk GW, Gons RA, den Heijer T, de Kort PL, de Laat KF, van Norden AG, Vermeer SE, van der Vlugt MJ, Kessels RP, van Dijk EJ, de Leeuw FE. Observational Dutch Young Symptomatic StrokE studY (ODYSSEY): study rationale and protocol of a multicentre prospective cohort study. BMC Neurol. 2014;14(1):55. doi: 10.1186/1471-2377-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douven E, Schievink SHJ, Verhey FRJ, van Oostrenbrugge RJ, Aalten P, Staals J, Köhler S. The Cognition and Affect after Stroke—a Prospective Evaluation of Risks (CASPER) study: rationale and design. BMC Neurol. 2016;16:65. doi: 10.1186/s12883-016-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40(4):1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 33.Rutten-Jacobs LC, Maaijwee NA, Arntz RM, Van Alebeek ME, Schaapsmeerders P, Schoonderwaldt HC, Dorresteijn LD, Overeem S, Drost G, Janssen MC, van Heerde WL, Kessels RP, Zwiers MP, Norris DG, van der Vlugt MJ, van Dijk EJ, de Leeuw FE. Risk factors and prognosis of young stroke. The FUTURE study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:109. doi: 10.1186/1471-2377-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yesilot Barlas N, Putaala J, Waje-Andreassen U, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, Kloss M, Ferrari J, Lemmens R, Coban O, Haapaniemi E, Maaijwee N, Rutten-Jacobs L, Bersano A, Cereda C, Baron P, Borellini L, Valcarenghi C, Thomassen L, Grau AJ, Palm F, Urbanek C, Tuncay R, Durukan Tolvanen A, van Dijk EJ, de Leeuw F-E, Thijs V, Greisenegger S, Vemmos K, Lichy C, Bereczki D, Csiba L, Michel P, Leys D, Spengos K, Naess H, Tatlisumak T, Baha SZ. Etiology of first-ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol. 2013;20(11):1431–1439. doi: 10.1111/ene.12228. [DOI] [PubMed] [Google Scholar]
- 35.Bello UM, Chutiyami M, Salihu D, Abdu SI, Tafida BA, Jabbo AA, Gamawa A, Umar L, Lawan A, Miller T, Winser SJ. Quality of life of stroke survivors in Africa: a systematic review and meta-analysis. Quality Life Res: Int J Quality Life Aspects Treat Care Rehabil. 2021;30(1):1–19. doi: 10.1007/s11136-020-02591-6. [DOI] [PubMed] [Google Scholar]
- 36.Kruithof WJ, van Mierlo ML, Visser-Meily JM, van Heugten CM, Post MW. Associations between social support and stroke survivors' health-related quality of life—a systematic review. Patient Educ Couns. 2013;93(2):169–176. doi: 10.1016/j.pec.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 37.van Mierlo ML, Schröder C, van Heugten CM, Post MW, de Kort PL, Visser-Meily JM. The influence of psychological factors on health-related quality of life after stroke: a systematic review. Int J Stroke: Off J Int Stroke Soc. 2014;9(3):341–348. doi: 10.1111/ijs.12149. [DOI] [PubMed] [Google Scholar]
- 38.Wang R, Langhammer B. Predictors of quality of life for chronic stroke survivors in relation to cultural differences: a literature review. Scand J Caring Sci. 2018;32(2):502–514. doi: 10.1111/scs.12533. [DOI] [PubMed] [Google Scholar]
- 39.Goeggel Simonetti B, Cavelti A, Arnold M, Bigi S, Regényi M, Mattle HP, Gralla J, Fluss J, Weber P, Hackenberg A, Steinlin M, Fischer U. Long-term outcome after arterial ischemic stroke in children and young adults. Neurology. 2015;84(19):1941–1947. doi: 10.1212/WNL.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor RJ, Cassidy EM, Delargy MA. Late multidisciplinary rehabilitation in young people after stroke. Disabil Rehabil. 2005;27(3):111–116. doi: 10.1080/09638280400007414. [DOI] [PubMed] [Google Scholar]
- 41.Bartholomé L, Winter Y. Quality of life and resilience of patients with juvenile stroke: a systematic review. J Stroke Cerebrovasc Dis. 2020;29(10):105129. doi: 10.1016/j.jstrokecerebrovasdis.2020.105129. [DOI] [PubMed] [Google Scholar]
- 42.Hamza AM, Al-Sadat N, Loh SY, Jahan NK. Predictors of poststroke health-related quality of life in Nigerian stroke survivors: a 1-year follow-up study. Biomed Res Int. 2014;2014:350281. doi: 10.1155/2014/350281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norrving B, Barrick J, Davalos A, Dichgans M, Cordonnier C, Guekht A, Kutluk K, Mikulik R, Wardlaw J, Richard E, Nabavi D, Molina C, Bath PM, Stibrant Sunnerhagen K, Rudd A, Drummond A, Planas A, Caso V. Action plan for stroke in Europe 2018–2030. Eur Stroke J. 2018;3(4):309–336. doi: 10.1177/2396987318808719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters M, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–2126. doi: 10.11124/JBIES-20-00167. [DOI] [PubMed] [Google Scholar]
- 45.Tricco A, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, Levac D, Ng C, Sharpe JP, Wilson K, Kenny M, Warren R, Wilson Ch, Stelfox HT, Straus SE. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15. doi: 10.1186/s12874-016-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 47.Opoku S, Eliason C, Akpalu A. Why me?: A qualitative study on the experiences of young stroke survivors in the Accra Metropolis of Ghana, West Africa. J Patient Exp. 2020;7(6):1788–1796. doi: 10.1177/2374373520967505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence M. Young adults' experience of stroke: a qualitative review of the literature. Br J Nurs (Mark Allen Publ) 2010;19(4):241–248. doi: 10.12968/bjon.2010.19.4.46787374373520967505. [DOI] [PubMed] [Google Scholar]
- 49.Harris Walker G, Oyesanya TO, Hurley A, Sandhu S, Liu C, Mulla M, Prvu Bettger J. Recovery experiences of younger stroke survivors who are parents: a qualitative content analysis. J Clin Nurs. 2021;30(1–2):126–135. doi: 10.1111/jocn.15529. [DOI] [PubMed] [Google Scholar]
- 50.Connolly T, Mahoney E. Stroke survivors' experiences transitioning from hospital to home. J Clin Nurs. 2018;27(21–22):3979–3987. doi: 10.1111/jocn.14563. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen SG, Anke A, Aadal L, Pallesen H, Moe S, Arntzen C. Experiences of quality of life the first year after stroke in Denmark and Norway. A qualitative analysis. Int J Qual Stud Health Well Being. 2019;14(1):1659540. doi: 10.1080/17482631.2019.1659540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindgren I, Brogårdh C, Pessah-Rasmussen H, Jonasson SB, Gard G. Work conditions, support, and changing personal priorities are perceived important for return to work and for stay at work after stroke—a qualitative study. Disabil Rehabil. 2020 doi: 10.1080/09638288.2020.1836522. [DOI] [PubMed] [Google Scholar]
- 53.Gustafsson N, Leino-Kilpi H, Prga I, Suhonen R, Stolt M, RANCARE consortium COST Action – CA15208 Missed care from the patient's perspective—a scoping review. Patient Prefer Adherence. 2020;14:383–400. doi: 10.2147/PPA.S238024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lannin NA, Anderson CS, Kim J, Kilkenny M, Bernhardt J, Levi C, Dewey HM, Bladin C, Hand P, Castley H, Hill K, Faux S, Grimley R, Grabsch B, Middleton S, Donnan G, Cadilhac DA. Treatment and outcomes of working aged adults with stroke: results from a national prospective registry. Neuroepidemiology. 2017;49(3–4):113–120. doi: 10.1159/000484141. [DOI] [PubMed] [Google Scholar]
- 55.Lisabeth LD, Baek J, Morgenstern LB, Zahuranec DB, Case E, Skolarus LE. Prognosis of midlife stroke. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc. 2018;27(5):1153–1159. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmcrantz S, Holmqvist LW, Sommerfeld DK, Tistad M, Ytterberg C, von Koch L. Differences between younger and older individuals in their use of care and rehabilitation but not in self-perceived global recovery 1year after stroke. J Neurol Sci. 2012;321(1–2):29–34. doi: 10.1016/j.jns.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 57.de Bruijn MA, Synhaeve NE, van Rijsbergen MW, de Leeuw FE, Mark RE, Jansen BP, de Kort PL. Quality of life after young ischemic stroke of mild severity is mainly influenced by psychological factors. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc. 2015;24(10):2183–2188. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 58.Palmcrantz S, Widén Holmqvist L, Sommerfeld DK. Young individuals with stroke: a cross sectional study of long-term disability associated with self-rated global health. BMC Neurol. 2014;14:20. doi: 10.1186/1471-2377-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider S, Taba N, Saapar M, Vibo R, Kõrv J. Determinants of long-term health-related quality of life in young ischemic stroke patients. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc. 2021;30(2):105499. doi: 10.1016/j.jstrokecerebrovasdis.2020.105499. [DOI] [PubMed] [Google Scholar]
- 60.Rhudy LM, Wells-Pittman J, Flemming KD. Psychosocial sequelae of stroke in working-age adults: a pilot study. J Neurosci Nurs: J Am Assoc Neurosci Nurses. 2020;52(4):192–199. doi: 10.1097/JNN.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearce G, Pinnock H, Epiphaniou E, Parke HL, Heavey E, Griffiths CJ, Greenhalgh T, Sheikh A, Taylor SJ. Experiences of self-management support following a stroke: a meta-review of qualitative systematic reviews. PLoS ONE. 2015;10(12):e0141803. doi: 10.1371/journal.pone.0141803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kono Y, Terasawa Y, Sakai K, Iguchi Y, Nishiyama Y, Nito C, Suda S, Kimura K, Kanzawa T, Imafuku I, Nakayama T, Ueda M, Iwanaga T, Kono T, Yamashiro K, Tanaka R, Okubo S, Nakajima M, Nakajima N, Mishina M, Yaguchi H, Oka H, Suzuki M, Osaki M, Kaneko N, Kitagawa K, Okamoto S, Nomura K, Yamazaki M, Nagao T, Murakami Y. Risk factors, etiology, and outcome of ischemic stroke in young adults: a Japanese multicenter prospective study. J Neurol Sci. 2020;417:117068. doi: 10.1016/j.jns.2020.117068. [DOI] [PubMed] [Google Scholar]
- 63.Varona JF, Bermejo F, Guerra JM, Molina JA. Long-term prognosis of ischemic stroke in young adults. Study of 272 cases. J Neurol. 2004;251(12):1507–1514. doi: 10.1007/s00415-004-0583-0. [DOI] [PubMed] [Google Scholar]
- 64.Synhaeve NE, Arntz RM, Maaijwee NA, Rutten-Jacobs LC, Schoonderwaldt HC, Dorresteijn LD, de Kort PL, van Dijk EJ, de Leeuw FE. Poor long-term functional outcome after stroke among adults aged 18 to 50 years: follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation (FUTURE) study. Stroke. 2014;45(4):1157–1160. doi: 10.1161/STROKEAHA.113.004411. [DOI] [PubMed] [Google Scholar]
- 65.Leys D, Bandu L, Hénon H, Lucas C, Mounier-Vehier F, Rondepierre P, Godefroy O. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;59(1):26–33. doi: 10.1212/wnl.59.1.26. [DOI] [PubMed] [Google Scholar]
- 66.Yeoh YS, Koh GC, Tan CS, Lee KE, Tu TM, Singh R, Chang HM, De Silva DA, Ng YS, Ang YH, Yap P, Chew E, Merchant RA, Yeo TT, Chou N, Venketasubramanian N, Young SH, Hoenig H, Matchar DB, Luo N. Can acute clinical outcomes predict health-related quality of life after stroke: a one-year prospective study of stroke survivors. Health Qual Life Outcomes. 2018;16(1):221. doi: 10.1186/s12955-018-1043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, Thrift AG. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2004;35(10):2340–2345. doi: 10.1161/01.STR.0000141977.18520.3b. [DOI] [PubMed] [Google Scholar]
- 68.Maaijwee NA, Rutten-Jacobs LC, Arntz RM, Schaapsmeerders P, Schoonderwaldt HC, van Dijk EJ, de Leeuw FE. Long-term increased risk of unemployment after young stroke: a long-term follow-up study. Neurology. 2014;83(13):1132–1138. doi: 10.1212/WNL.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 69.Edwards JD, Kapoor A, Linkewich E, Swartz RH. Return to work after young stroke: a systematic review. Int J Stroke: Off J Int Stroke Soc. 2018;13(3):243–256. doi: 10.1177/1747493017743059. [DOI] [PubMed] [Google Scholar]
- 70.Duncan F, Wu S, Mead GE. Frequency and natural history of fatigue after stroke: a systematic review of longitudinal studies. J Psychosom Res. 2012;73(1):18–27. doi: 10.1016/j.jpsychores.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Wright F, Wu S, Chun HY, Mead G. Factors associated with poststroke anxiety: a systematic review and meta-analysis. Stroke Res Treat. 2017;2017:2124743. doi: 10.1155/2017/2124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarkar A, Sarmah D, Datta A, Kaur H, Jagtap P, Raut S, Shah B, Singh U, Baidya F, Bohra M, Kalia K, Borah A, Wang X, Dave KR, Yavagal DR, Bhattacharya P. Post-stroke depression: chaos to exposition. Brain Res Bull. 2021;168:74–88. doi: 10.1016/j.brainresbull.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Taylor-Rowan M, Momoh O, Ayerbe L, Evans JJ, Stott DJ, Quinn TJ. Prevalence of pre-stroke depression and its association with post-stroke depression: a systematic review and meta-analysis. Psychol Med. 2019;49(4):685–696. doi: 10.1017/S0033291718002003. [DOI] [PubMed] [Google Scholar]
- 74.Harno H, Haapaniemi E, Putaala J, Haanpää M, Mäkelä JP, Kalso E, Tatlisumak T. Central poststroke pain in young ischemic stroke survivors in the Helsinki Young Stroke Registry. Neurology. 2014;83(13):1147–1154. doi: 10.1212/WNL.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 75.van Mierlo M, van Heugten C, Post M, Hoekstra T, Visser-Meily A. Trajectories of health-related quality of life after stroke: results from a one-year prospective cohort study. Disabil Rehabil. 2018;40(9):997–1006. doi: 10.1080/09638288.2017.1292320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed search strategy in databases.
Data Availability Statement
Not applicable.