Abstract
Enterococcus faecalis bacteria isolated from patients with bacteremia, endocarditis, and urinary tract infections more frequently express the surface protein Esp than do fecal isolates. To assess the role of Esp in colonization and persistence of E. faecalis in an animal model of ascending urinary tract infection, we compared an Esp+ strain of E. faecalis to its isogenic Esp-deficient mutant. Groups of CBA/J mice were challenged transurethrally with 108 CFU of either the parent or mutant strain, and bacteria in the urine, bladder, and kidneys were enumerated 5 days postinfection. Significantly higher numbers of bacteria were recovered from the bladder and urine of mice challenged with the parent strain than from the bladder and urine of mice challenged with the mutant. Colonization of the kidney, however, was not significantly different between the parent and mutant strains. Histopathological evaluations of kidney and bladder tissue done at 5 days postinfection did not show marked histopathological changes consistent with inflammation, mucosal hyperplasia, or apoptosis, and there was no observable difference between the mice challenged with the parent and those challenged with the mutant. We conclude that, while Esp does not influence histopathological changes associated with acute urinary tract infections, it contributes to colonization and persistence of E. faecalis at this site.
The pathogenesis of complicated and uncomplicated urinary tract infection (UTI) is complex and influenced by many host biological and behavioral factors and by properties of the infecting uropathogens. Leading etiological agents of UTIs include Escherichia coli, Candida albicans, Enterococcus faecalis, Pseudomonas aeruginosa, and Proteus mirabilis (27). The incidence of UTIs due to E. faecalis has risen steadily over the years, and infections due to multiple-drug-resistant strains present a significant medical problem (11). Enterococcus spp. rank third among the most common pathogens isolated from intensive care unit patients with UTIs (23) and are a common cause of chronic or recurrent UTIs, especially those associated with structural abnormalities and instrumentation (5, 17). In spite of the role of E. faecalis as a leading cause of nosocomial UTI, little is known about the bacterial factors involved in such infections.
The interaction between enterococci and uroepithelial tissue has been examined previously (16) with the goal of identifying a role for plasmid-encoded aggregation substance in the adhesion of enterococci to renal epithelial cells in vitro. In a study of E. faecalis isolates from patients with UTI and endocarditis, Guzman and coworkers (6) showed that UTI isolates adhered efficiently to urinary tract epithelial cells and less effectively to Girardi heart cells. The adherence of UTI isolates to Girardi heart cells was, however, enhanced eightfold by growth of the bacteria in human serum. The nature of the interaction of enterococci with uroepithelial tissue appears to be quite complex, with a role for bacterial cell surface carbohydrate and protein (6, 26).
About one-third of E. faecalis isolates from patients with bacteremia and UTIs express the Esp protein, compared to its rare occurrence in fecal isolates, suggesting that this surface protein may play an important role during these infections (25). The unique architecture of the Esp protein, with multiple repeat motifs, is characteristic of many bacterial surface protein adhesins involved in binding to host ligands (1, 7, 21). It was hypothesized, therefore, that Esp may play a role similar to that of the fimbriae of E. coli and P. mirabilis in serving as a colonization factor promoting adherence to uroepithelium. To test the role of Esp during UTIs, we constructed an isogenic Esp-deficient mutant by allelic replacement of the esp gene with a chloramphenicol resistance cassette. The wild-type and isogenic mutant strains were then compared in a mouse model of ascending UTI, for their ability to colonize and persist at anatomical sites of the urinary tract.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. faecalis MMH594 is a clinical bacteremia isolate that caused multiple infections in a hospital ward outbreak and is positive for Esp expression (12, 25). The isogenic Esp-deficient mutant (MMH594b) was created by allelic replacement of the esp gene with a chloramphenicol resistance cassette. E. faecalis strains were routinely cultivated in brain heart infusion (Difco Laboratories, Detroit, Mich.), whereas Luria-Bertani broth (24) was used for cultivation of E. coli strains. E. coli strain XL1-Blue was obtained from Stratagene (La Jolla, Calif.), and DH5α was obtained from Life Technologies (Gaithersburg, Md.). Antibiotics (Sigma, St. Louis, Mo.) used for selection of E. faecalis strains included gentamicin (500 μg/ml) for the wild-type strain and gentamicin (500 μg/ml) plus chloramphenicol (20 μg/ml) for the mutant. For maintenance of recombinant constructs in E. coli, ampicillin at 100 μg/ml, chloramphenicol at 20 μg/ml, and tetracycline at 15 μg/ml were used where appropriate. Custom oligonucleotides were obtained from Integrated DNA Technologies (Coralville, Iowa). Restriction and modifying enzymes were purchased from New England Biolabs Inc. (Beverly, Mass.). Plasmids were introduced into electrocompetent E. coli or E. faecalis cells using a Gene Pulser unit (Bio-Rad Laboratories, Hercules, Calif.).
Construction of the isogenic mutant deficient in Esp expression.
A conditionally replicating shuttle-suicide vector (pNS110) was constructed in multiple steps and targeted to the esp gene as follows. In order to generate Esp arms to target the cat cassette to the esp gene, inverse PCR was performed on purified, PstI restricted, and self-ligated MMH594 DNA using the outward-facing primers Esp15B (GAGAgcgcgcGATAGGTCGTGGACTAGCATTAGC) and Esp24N (GAGAgcggccgcCCACGAGTTAGCGGGAACAGGT). Inverse PCR amplification was performed using the Takara LA PCR kit, as suggested by the manufacturer (Panvera Corp., Madison, Wis.). Primers Esp15B and Esp24N corresponded to nucleotide positions 1242 to 1219 and 1551 to 1572 of the esp gene, respectively (25). The ∼4-kb inverse PCR-amplified DNA product was gel purified and restricted with BssHII and NotI to cleave restriction sites built into the primers Esp15B and Esp24N, respectively. An 851-bp chloramphenicol resistance (CAT) determinant was amplified from plasmid pGB354 (30) using the primer pairs GAGAgcgcgcGGCAACGTGAATTTAGGTTTTGA and GAGAgcggccgcGATCACTTACGTGTATAAAATTA, and the amplification product was restricted with BssHII/NotI and subsequently gel purified. The CAT determinant was then ligated to the BssHII/NotI-cut inverse PCR product obtained above from MMH594. Primers Esp58E (GAGAgaattcGGTGTAGGCCTTGTTTTTGGGG; nucleotide positions 187 to 208) and Esp26X (GAGActcgagCGTGCCTACAGAACCATCTTG; nucleotide positions 2280 to 2260 of the esp gene) were then used to amplify from this construct a 2.6-kb DNA segment that consisted of the 851-bp CAT determinant flanked by 1,063-bp and 720-bp regions of the esp structural gene. This 2.6-kb amplified product was restricted with EcoRI and XhoI and cloned into the plasmid vector pBluescript II SK(−) to generate pSK5826, and plasmid DNA was prepared from transformed E. coli XL1-Blue cells.
To facilitate the identification of single-crossover integrants compared with double-crossover integrants, a second antibiotic resistance marker was introduced as follows. The 1.7-kb tetracycline resistance (Tet Kr) determinant from pT181 (3) was PCR amplified using primers T181-L (GAGAggatccCGCCAGTCGATTTAACGGAC) and T181-R (GAGAggatccATACGTGTGCTCTGCGAGGC), restricted with BamHI, and cloned into BamHI-restricted pSK5826. The recombinant plasmid pSKT5826 was purified from transformed E. coli DH5α. For conditional replication in both gram-positive bacteria and E. coli, the temperature-sensitive origin of replication, repA(Ts), of plasmid pTV1OK (4) was PCR amplified using the primers Ts-L (CCACTAATAACTCACAATAGAGAGATGTCACCG) and Ts-R (GAGActcgagGCCTTGAAACATTGGTTTAGTGGG), gel purified, and restricted with XbaI and XhoI. The XbaI/XhoI fragment was cloned into pBluescript SK(−) to generate pSKTs, and recombinant plasmid DNA was purified from XL1-Blue transformants. The Ts replicon region from pSKTs and the 4.5-kb insert from pSKT5826 were gel purified after restriction of the respective plasmids with XbaI/XhoI and ligated together to generate pNS110. The entire construct pNS110 was sequenced using custom Cy5-labeled primers by a standard chain termination method employing the T7 DNA polymerase-based Autoread sequencing kit (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) and determined to be 6,747 bp in size.
An Esp-deficient mutant was generated from parent strain MMH594 by homologous recombination and allele replacement, adopting a protocol reported earlier (18). The single-crossover and double-crossover integrants were analyzed by both PCR and Southern blot hybridization to verify proper integration. The double-crossover integrant (MMH594b) along with the parent strain was used for the studies described in this report.
Antiserum to Esp.
Polyclonal rabbit antiserum to purified Esp was raised by immunization of New Zealand White rabbits, and the reactivity and specificity of the antiserum were determined as previously described (25).
Enzyme-linked immunosorbent assay.
The expression and localization of Esp at the cell surface of parent and mutant strains were verified using whole-cell enzyme-linked immunosorbent assay as follows. Briefly, 105 CFU of the parent and mutant strains suspended in 50 μl of 100 mM carbonate buffer, pH 9.6, was coated in triplicate wells of a 96-well microtiter plate and allowed to bind at 4°C overnight. Loosely adherent cells were washed off by gentle rinsing with phosphate-buffered saline (PBS), and exposed areas of the polystyrene wells were blocked with 2% bovine serum albumin in PBS. One hundred microliters of polyclonal Esp-specific rabbit antiserum, diluted 1:500 in PBS containing 0.05% Tween 20 (PBST), was applied to each well and incubated at 37°C for 4 h. The microtiter wells were rinsed three times with PBST using an automated microtiter plate washer (Bio-Rad Laboratories), followed by the addition of 100 μl of a 1:10,000 dilution of goat anti-rabbit immunoglobulin G (IgG) conjugated to alkaline phosphatase. Incubation was carried out at 37°C for 2 h, the wells were rinsed three times with PBST, and Esp expression was quantified by measuring conversion of the chromogenic substrate p-nitrophenyl phosphate in 10 mM diethanolamine buffer, pH 9.5. The absorbance of each well was read at 405 nm after a 30-min incubation at 37°C.
Immunogold labeling of Esp and high-resolution scanning electron microscopy.
Esp was visualized on the bacterial cell surface using a combination of colloidal gold immunolabeling and low-voltage scanning electron microscopy (LVSEM) by adopting a protocol previously described for enterococcal aggregation substance (22). Overnight or exponential-phase bacterial cultures were washed twice and resuspended to a concentration of 108 cells per ml in 10 mM PBS (pH 7.4). Glass chips (4 by 8 mm) were cleaned with 95% ethanol and coated with 0.1% poly-l-lysine for 10 min. Excess poly-l-lysine was rinsed off, and 30 μl of each bacterial suspension was placed on individual chips for 10 min. Excess bacteria were washed off gently using Hanks' balanced salt solution (HBSS) containing 0.5% bovine serum albumin, and 20 μl of a 1:50 dilution in HBSS of purified IgG (10 mg/ml) from rabbit polyclonal antiserum to Esp was applied for 1 h at 37°C. Bacteria were then gently washed with HBSS containing 0.5% bovine serum albumin, and 20 μl of a 1:5 dilution of goat anti-rabbit IgG conjugated to 12-nm colloidal gold particles (Jackson ImmunoResearch Laboratories, West Grove, Pa.) was applied for 10 min at room temperature. Finally all samples were washed gently with HBSS and placed in fixative (2.5% glutaraldehyde and 0.5% paraformaldehyde in 0.1 M sodium cacodylate buffer containing 7.5% sucrose).
For high-resolution LVSEM, the fixative was washed from the samples twice for 10 min in 0.1 M sodium cacodylate with 7.5% sucrose buffer and postfixed for 30 min in 0.1 M sodium cacodylate containing 1% osmium tetroxide and 7.5% sucrose. The samples were then washed twice with 0.1 M sodium cacodylate, dehydrated with ethanol, critical point dried by the CO2 method, and coated with a 1- to 2-nm discontinuous layer of platinum by using a saddle field ion beam gun (VCR Group, South San Francisco, Calif.). E. faecalis cells were viewed with a Hitachi S-900 field emission scanning electron microscope operated at low accelerating voltages (1.3 to 5 keV), using a scatter electron detector for conventional topographical imaging and a high-resolution yttrium-argon-garnet back scatter electron detector (29) for the visualization of colloidal gold by atomic number contrast.
Phenotypic characterization of the Esp-deficient mutant.
In vitro growth rates in broth cultures were compared for the parent and mutant, in either the presence or the absence of chloramphenicol selection, using standard techniques. The stability of the Cmr determinant in the absence of antibiotic selection was also assessed. A single colony of the allelic replacement mutant was allowed to undergo approximately 96 doublings in broth culture without antibiotic selection. One hundred colonies from this culture were replica plated on agar plates with and without chloramphenicol, to check for loss of chloramphenicol resistance.
CBA mouse model of ascending UTI.
A modified ascending UTI model, as described earlier (13), was used to assess the virulence of parent and mutant E. faecalis strains. Six- to eight-week-old CBA/J mice (Jackson Laboratories, Bar Harbor, Maine) were used. All animal experiments were conducted in accordance with relevant federal guidelines and institutional policies. Prior to bacterial challenge, spontaneously voided urine was collected in a sterile petri dish; bacteriuric mice were not used. Mice (n = 20) were challenged while anesthetized with methoxyflurane (Metofane; Pitman-Moore, Inc., Washington Crossing, N.J.) by inserting a polyethylene catheter (2.5 cm long; outer diameter, 0.61 mm; Clay Adams, Parsippany, N.J.) into the bladder through the urethra and infusing 0.05 ml of a suspension containing 2 × 108 CFU into the bladder over a 30-s period. Mice were challenged with a suspension of either E. faecalis MMH594 or the isogenic Esp-deficient mutant. The urethral catheter was removed immediately after challenge, and mice were cared for by the normal routine. Mice were inspected daily to monitor morbidity and mortality. At 5 days after transurethral challenge, quantitative cultures of the urine, bladder, and kidneys were performed as previously described (13). Segments of bladder and kidneys were preserved in 10% neutral formalin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy. The pathologist examining the tissue sections was blinded to the experimental procedure.
A standard histology scoring system for bladder mucosa and submucosa was followed, and the degree of inflammation was graded as follows: acute, 0, no inflammation; 1+, few neutrophils; 2+, scattered neutrophils not forming microabscesses; 3+, numerous neutrophils in clusters; chronic (based on the degree of lymphocytes and plasma cells in the submucosa), 0, none; 1+, rare; 2+, small aggregates measuring <100 μm; 3+, larger aggregates. The thickness of the epithelium was evaluated, and the degree of hyperplasia was graded as follows: 0, epithelial morphology identical to that of normal controls (two to three layers); 1+, epithelium with three to four cell layers and normal cytoplasmic surface maturation; 2+, epithelium with three to four cell layers and reduced cytoplasmic volume in surface cells; 3+, irregular epithelial crowding with patchy areas showing more than four cell layers and nuclear crowding; 4+, epithelium with diffuse thickening, more than four cell layers, and nuclear crowding-palisading. Increased epithelial cell turnover was graded, based on the presence of apoptotic bodies, as follows: 0, none; 1+, extremely rare; 2+, occasional; 3+, numerous with mitosis.
The histologic criteria used for evaluation of renal lesions included degree and types of inflammatory infiltrates in renal parenchyma and pelvis (epithelium and subepithelial connective tissue), necrosis of transitional and tubular epithelium, purulent casts in collecting ducts, intraparenchymal abscess formation, parenchymal interstitial fibrosis-tubular atrophy, and pelvic fibrosis (13).
Statistics.
Means of quantitative counts of urine, bladder, and kidneys from mice challenged with the parent strain were compared with means from mice challenged with the mutant strain by Student's t test. Differences in the number of mice with urine, bladder, or kidneys colonized with the challenge organisms were compared by chi-square analysis.
RESULTS
Characterization of the Esp-deficient mutant.
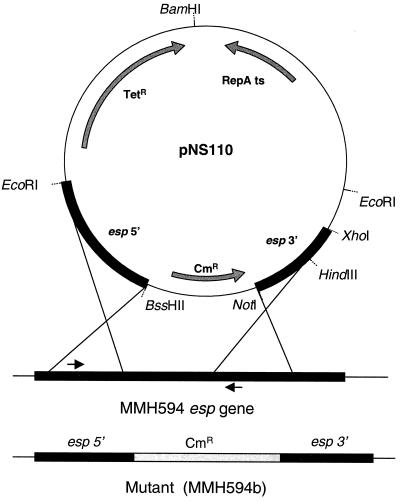
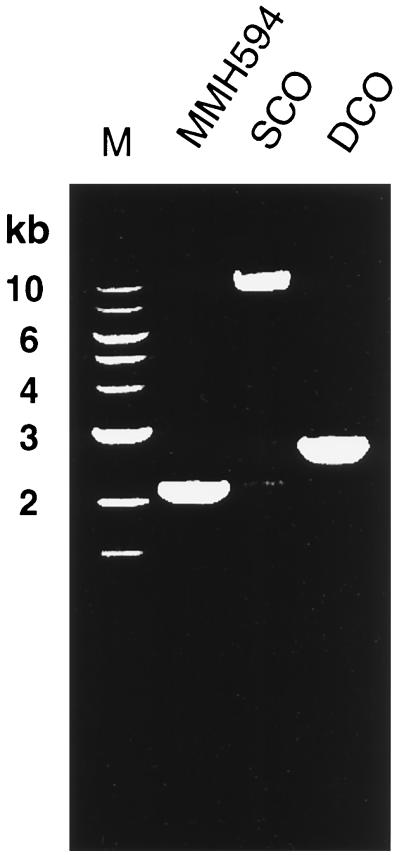
An isogenic mutant of MMH594 that was deficient in Esp expression was constructed by replacement of an intragenic region of the esp gene with a chloramphenicol resistance cassette. As shown in Fig. 1, regions including the 5′ and 3′ ends of the esp gene were cloned into the suicide-shuttle plasmid pNS110, to target the insertion vector to the esp gene on the chromosome of MMH594. Confirmation of the single- and double-crossover mutations was done by PCR, and the amplification products were analyzed by gel electrophoresis (Fig. 2). In separate experiments, genomic DNA from the parent and mutant strains was also analyzed by restriction mapping and Southern blot hybridization, using nucleotide probes to both the esp gene and the chloramphenicol resistance determinant. These experiments confirmed the nature of the mutations (data not shown). To prevent any reversion or possible polar effects stemming from plasmid sequences in the single-crossover integrant, only the double-crossover integrant (MMH594b) was used in further studies.
FIG. 1.
Schematic of the strategy used to create an Esp-deficient mutant. Gray arrows within pNS110 denote the directions of transcription for these determinants. Black arrows adjacent to the esp gene on the MMH594 chromosome indicate the positions of primers used to verify proper integration in the single-crossover and double-crossover mutants.
FIG. 2.
Ethidium bromide-stained agarose gel electrophoresis of PCR-amplified products from parent (MMH594), single-crossover integrant (SCO), and double-crossover integrant (DCO; MMH594b). Molecular size markers are shown in lane M.
Phenotypic characteristics of the mutant.
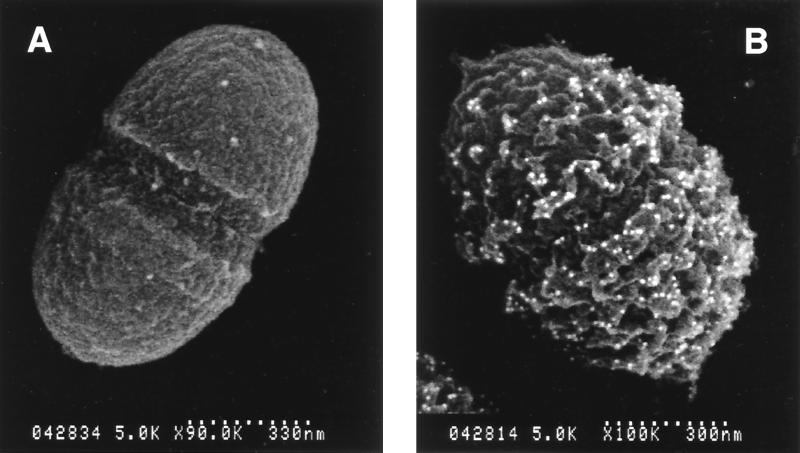
To confirm that the mutant (MMH594b) was indeed deficient in Esp expression as expected, two immunological approaches were employed. In the first instance, specific antiserum to Esp failed to bind to MMH594b cells bound to 96-well polystyrene microtiter plates, as evaluated by enzyme-linked immunosorbent assay (data not shown). Secondly, affinity-purified antibodies to Esp failed to detect any Esp at the cell surface of the mutant strain under conditions where it was readily detected on the surface of the wild-type organism (Fig. 3). These results unambiguously demonstrated that the mutant strain lacked Esp on the cell surface.
FIG. 3.
Back scatter electron imaging of colloidal gold-immunolabeled Esp protein. (A) Representative view of the isogenic mutant strain (MMH594b) which exhibits no binding of colloidal gold to the cell surface. (B) Typical view of the parent strain MMH594.
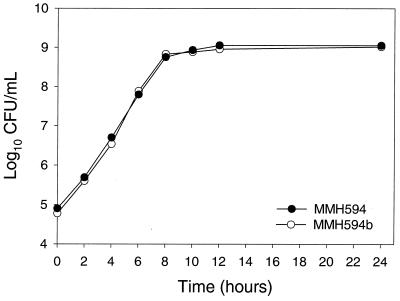
In vitro growth rates in broth cultures were determined to verify that the inactivation of the esp gene did not affect growth and survival of the mutant. As shown in Fig. 4, no difference was observed between parent and mutant during growth in the presence or absence of chloramphenicol selection. Moreover, when a single colony representing the mutant strain was allowed to undergo 96 doublings in the absence of antibiotic selection, 100 of 100 colonies replica plated on agar plates retained the Cmr phenotype, confirming its stability.
FIG. 4.
In vitro growth characteristics of the parent and isogenic mutant strains in brain heart infusion broth. Data points along the growth curve represent the means of three independent measurements from bacteria grown without antibiotic selection. Growth characteristics of parent and mutant strains grown in the presence of antibiotics were identical and superimposable.
Model UTI studies.
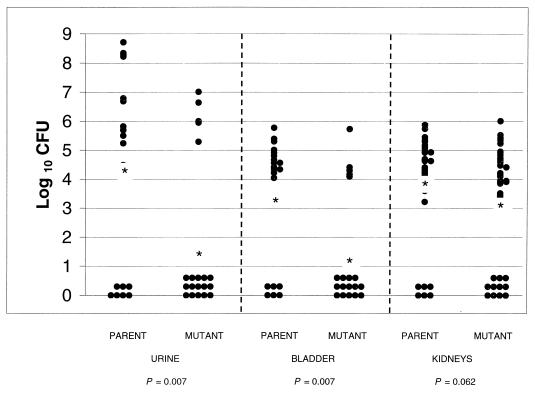
Colonization of both urine and bladder at day 5 after transurethral challenge with 108 CFU of E. faecalis MMH594 parent strain or the Esp-deficient isogenic mutant per mouse was significantly (P < 0.01 for each site) lower in mice challenged with the isogenic mutant (n = 20) than in mice challenged with the parent strain (n = 20). Numbers of CFU (log10 ± standard error of the mean) at each site were as follows: urine, mutant, 1.54 ± 0.62, versus parent, 4.39 ± 0.078; bladder, mutant, 1.34 ± 0.47, versus parent, 3.32 ± 0.50. While levels of colonization of the kidney by the mutant tended to be lower than those of colonization by the parent (3.16 ± 0.33 versus 3.99 ± 0.29), significance at a level of ≤0.05 was not achieved (P = 0.062). Figure 5 shows the distribution data for each animal at each site tested. For urine, 15 of 20 mice challenged with the mutant had counts below 102 CFU/ml versus 7 of 20 mice challenged with the parent strain (P = 0.011, chi-square test). For bladder, 14 of 20 mice challenged with the mutant had counts below 102 CFU/ml versus 6 of 20 mice challenged with the parent strain (P = 0.011), and 11 of 40 kidneys from mice challenged with the mutant had counts below 102 CFU/ml versus 6 of 40 kidneys from mice challenged with the parent strain (P = 0.17).
FIG. 5.
Distribution of CFU quantified from urine, bladder, and kidneys of mice challenged with parent or Esp-deficient E. faecalis. Data are expressed as log10 CFU per milliliter of urine or per gram of bladder or kidney homogenates 5 days after transurethral challenge (n = 20). The mean (*) is indicated for each group.
Although statistically significant differences in quantitative colony counts from urine and bladder were observed, no significant differences in histology scores between mutant and wild-type infections were found. Histology scores for acute inflammation in kidneys were as follows: 14 of 40 kidneys from mice challenged with the mutant versus 13 of 40 kidneys from mice challenged with the parent strain had a histology score of 0, 14 of 40 kidneys from mice challenged with the mutant strain versus 15 of 40 kidneys from mice challenged with the parent strain had a histology score of 1, and 12 of 40 kidneys from mice challenged with the mutant strain versus 12 of 40 kidneys from mice challenged with the parent strain had a histology score of 2. No other histologic changes were observed.
DISCUSSION
Despite the recognition that E. faecalis has emerged as an important uropathogen, much remains to be learned about the pathogenicity of this infection. Numerous studies of the two leading causes of community-acquired UTI, E. coli and P. mirabilis, have identified unique traits that are expressed by specific UTI isolates (2, 9, 10, 14, 19, 20, 27). Uropathogenic strains are highly adapted and possess specific factors that promote bladder colonization, survival in the urinary tract, and often the ability to induce tissue damage, including P fimbriae, hemolysin, serum resistance, and encapsulation. It is well established that adhesion to the bladder epithelium is a key initial step in UTI pathogenesis (20, 32). In E. coli, type 1 fimbriae, P and related fimbriae, and F1C fimbriae mediate the initial adherence preventing washout by urinary flow. Type 1 fimbriae bind to mannose-containing receptors, and the P group fimbriae bind to the Galα(1-4)Gal moiety of the P blood group and related receptors, which are widely distributed on the uroepithelium (31). The high-affinity binding of F1C fimbriae to the GalNAcβ1-4Galβ sequence of glycolipids, asialo-GM1 (GgO4Cer) and asialo-GM2 (GgO3Cer), and low-affinity binding to carbohydrate structures GlcNAcβ1-3Galβ, Galβ1-4Glc, Gal, and Glc of glycolipids have been demonstrated recently (15).
The Esp protein of E. faecalis is displayed on the cell surface. We previously showed a significant association of the Esp protein with E. faecalis isolated from patients with UTI compared to fecal isolates (25; N. Shankar, unpublished data). This localization and enrichment among UTI-derived isolates suggested a possible role for Esp in adherence and colonization. The results of this study support such a role and show that the effect is primarily localized in the bladder. Higher numbers of bacteria recovered from the urine of mice challenged with the parent strain reflect a bacteriuria resulting from colonization of the bladder.
Plasmid-encoded aggregation substance was found to contribute to E. faecalis adhesion to renal epithelial cells in vitro (16). However, to our knowledge, no reports have demonstrated a role for aggregation substance in colonization or persistence in the urinary tract during infection. Guzman et al. (5, 6) have shown that E. faecalis from patients with UTI adhered to urinary tract epithelial cells in vitro and suggested that carbohydrate antigens on the bacterial cell surface were responsible for this adherence. In a recent study (26), it was shown that 5 of 30 E. faecalis isolates from the urine of patients with UTI adhered efficiently to freshly isolated human bladder mucosa and to T-24 bladder carcinoma cells in culture. The adhesiveness of these isolates was inhibited by treatment with fibronectin or trypsin, implying that a specific protein on the bacterial cell surface was responsible for the adhesion. It is apparent from the studies described above that the nature of the interaction between enterococci and uroepithelial tissue can be quite complex, involving surface adhesins of a protein and/or carbohydrate nature.
Preliminary studies in our laboratory have shown no observable differences between the binding of Esp+ parent strains and that of isogenic Esp-deficient mutant strains to the porcine renal tubular cell line LLC-PK1. This observation is not surprising given that the in vivo studies reported here found no significant differences between the number of bacteria recovered from the kidneys of mice challenged with the parent strain and those from kidneys of mice challenged with mutant strains. It is possible that other surface adhesins, such as aggregation substance, contribute to binding to renal epithelial cells, making the pathogenesis of E. faecalis UTI a multistep, multifactorial process. In the present study, both the parent and mutant strains express aggregation substance. We are currently examining strains possessing various combinations of Esp and aggregation substance for differences in localization, as this hypothesis would suggest.
A novel feature of the Esp protein is the presence of identical, large (82- and 84-amino-acid) repeat motifs encoded by nearly identical tandem repeating units within the structural esp gene. Homologous recombination within these repeat units at the genetic level leads to addition or deletion of repeat units, resulting in an alteration in the size of the encoded protein. We have shown previously that E. faecalis isolates do indeed express altered forms of the Esp protein that vary in size depending on the number of repeating units (25). It was postulated that this variation in size of Esp at the cell surface could define an environment-specific function for Esp. Consequently, an extended form of the Esp protein might be involved in adhesion functions during the initial stages of infection, facilitating interaction with host receptors. Subsequent to establishment in the host, an extended form of the surface protein may be detrimental to survival and persistence, favoring expression of a less-extended form of Esp to evade the immune response, analogous to the phase variation observed for uropathogenic E. coli (14).
The ability of E. faecalis to cause pyelonephritis in an experimental mouse model of infection has been reported elsewhere (8). In these experiments, E. faecalis alone when used to infect the bladders of mice at a concentration of 108 CFU caused pyelonephritis in 50% of infected animals after 7 days. These and other studies (28) have also suggested that E. faecalis may enhance the onset and clinical severity of UTIs caused by other uropathogens such as E. coli and P. aeruginosa during mixed infections. The molecular basis for this synergism during mixed infections remains unexplained. Our histopathological data showed no significant differences between bladder and kidney tissue from mice infected with 108 CFU of the parent or mutant E. faecalis strain. One explanation may be that the 5-day postinfection point was suboptimal for pathological changes. Alternatively, E. faecalis may bind and activate bladder epithelial cells, setting the stage for secondary, more symptomatic infection. Identification of the role of Esp in the pathogenesis of enterococcal UTI is an important first step in dissecting this complex process.
ACKNOWLEDGMENTS
We thank Stanley Erlandsen and Gary Dunny, University of Minnesota, for assistance with the LVSEM work.
This work was supported, in part, by Public Health Service grant AI 40651 (N.S.) from the National Institute of Allergy and Infectious Diseases and by the Department of Veterans Affairs (D.E.J.).
REFERENCES
- 1.de Chateau M, Bjorck L. Protein PAB, a mosaic albumin-binding bacterial protein representing the first contemporary example of module shuffling. J Biol Chem. 1994;269:12147–12151. [PubMed] [Google Scholar]
- 2.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H, Warren J, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C.: ASM Press; 1996. pp. 135–174. [Google Scholar]
- 3.Guay G G, Khan S A, Rothstein D M. The tet(K) gene of plasmid pT181 of Staphylococcus aureus encodes an efflux protein that contains 14 transmembrane helices. Plasmid. 1993;30:163–166. doi: 10.1006/plas.1993.1045. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P, Bleiweis A S. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J Bacteriol. 1996;178:4166–4175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman C A, Pruzzo C, LiPira G, Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect Immun. 1989;57:1834–1838. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman C A, Pruzzo C, Plate M, Guardati M C, Calegari L. Serum dependent expression of Enterococcus faecalis adhesins involved in the colonization of heart cells. Microb Pathog. 1991;11:399–409. doi: 10.1016/0882-4010(91)90036-a. [DOI] [PubMed] [Google Scholar]
- 7.Hartford O, McDevitt D, Foster T J. Matrix-binding proteins of Staphylococcus aureus: functional analysis of mutant and hybrid molecules. Microbiology. 1999;145:2497–2505. doi: 10.1099/00221287-145-9-2497. [DOI] [PubMed] [Google Scholar]
- 8.Hirose T, Kumamoto Y, Tanaka N, Yoshioka M, Tsukamoto T. Study on pathogenesis of Enterococcus faecalis in urinary tract. Urol Res. 1989;17:125–129. doi: 10.1007/BF00262034. [DOI] [PubMed] [Google Scholar]
- 9.Hooton T M. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl. A):1–7. [PubMed] [Google Scholar]
- 10.Hull R A, Rudy D C, Wieser I E, Donovan W H. Virulence factors of Escherichia coli isolates from patients with symptomatic and asymptomatic bacteriuria and neuropathic bladders due to spinal cord and brain injuries. J Clin Microbiol. 1998;36:115–117. doi: 10.1128/jcm.36.1.115-117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huycke M M, Sahm D F, Gilmore M S. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huycke M M, Spiegel C A, Gilmore M S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson D E, Russell R G, Lockatell C V, Zulty J C, Warren J W, Mobley H L. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect Immun. 1993;61:2748–2754. doi: 10.1128/iai.61.7.2748-2754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan A S, Kniep B, Oelschlaeger T A, Van Die I, Korhonen T, Hacker J. Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect Immun. 2000;68:3541–3547. doi: 10.1128/iai.68.6.3541-3547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis C M, Zervos M J. Clinical manifestations of enterococcal infection. Eur J Clin Microbiol Infect Dis. 1990;9:111–117. doi: 10.1007/BF01963635. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Kasper D L, Ausubel F M, Rosner B, Michel J L. Inactivation of the alpha C protein antigen gene, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B streptococcus. Proc Natl Acad Sci USA. 1997;94:13251–13256. doi: 10.1073/pnas.94.24.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mobley H L, Island M D, Massad G. Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis. Kidney Int Suppl. 1994;47:S129–S136. [PubMed] [Google Scholar]
- 20.Mobley H L T. Virulence of the two primary uropathogens. ASM News. 2000;66:403–410. [Google Scholar]
- 21.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olmsted S B, Erlandsen S L, Dunny G M, Wells C L. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J Bacteriol. 1993;175:6229–6237. doi: 10.1128/jb.175.19.6229-6237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards M J, Edwards J R, Culver D H, Gaynes R P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Shankar V, Baghdayan A S, Huycke M M, Lindahl G, Gilmore M S. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun. 1999;67:193–200. doi: 10.1128/iai.67.1.193-200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiono A, Ike Y. Isolation of Enterococcus faecalis clinical isolates that efficiently adhere to human bladder carcinoma T24 cells and inhibition of adhesion by fibronectin and trypsin treatment. Infect Immun. 1999;67:1585–1592. doi: 10.1128/iai.67.4.1585-1592.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin N Am. 1997;11:513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchimori N, Hayashi R, Shino A, Yamazaki T, Okonogi K. Enterococcus faecalis aggravates pyelonephritis caused by Pseudomonas aeruginosa in experimental ascending mixed urinary tract infection in mice. Infect Immun. 1994;62:4534–4541. doi: 10.1128/iai.62.10.4534-4541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walther P, Autrata R, Chen Y, Pawley J B. Backscattered electron imaging for high resolution surface scanning electron microscopy with a new type YAG-detector. Scanning Microsc. 1991;5:301–310. [PubMed] [Google Scholar]
- 30.Wirth R, An F Y, Clewell D B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986;165:831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wold A E, Thorssen M, Hull S, Eden C S. Attachment of Escherichia coli via mannose- or Galα1→ 4Galβ-containing receptors to human colonic epithelial cells. Infect Immun. 1988;56:2531–2537. doi: 10.1128/iai.56.10.2531-2537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wullt B, Bergsten G, Connell H, Rollano P, Gebretsadik N, Hull R, Svanborg C. P fimbriae enhance the early establishment of Escherichia coli in the human urinary tract. Mol Microbiol. 2000;38:456–464. doi: 10.1046/j.1365-2958.2000.02165.x. [DOI] [PubMed] [Google Scholar]