Abstract
Background
Debate exists about whether extra protection of elderly and other vulnerable individuals is feasible in COVID-19. We aimed to assess the relative infection rates in the elderly vs the non-elderly and, secondarily, in children vs adults.
Methods
We performed a systematic review and meta-analysis of seroprevalence studies conducted in the pre-vaccination era. We identified representative national studies without high risk of bias through SeroTracker and PubMed searches (last updated May 17, 2022). We noted seroprevalence estimates for children, non-elderly adults, and elderly adults, using cut-offs of 20 and 60 years (or as close to these ages, if they were unavailable) and compared them between different age groups.
Results
We included 38 national seroprevalence studies from 36 different countries comprising 826 963 participants. Twenty-six of these studies also included pediatric populations and twenty-five were from high-income countries. The median ratio of seroprevalence in elderly vs non-elderly adults (or non-elderly in general, if pediatric and adult population data were not offered separately) was 0.90-0.95 in different analyses, with large variability across studies. In five studies (all in high-income countries), we observed significant protection of the elderly with a ratio of <0.40, with a median of 0.83 in high-income countries and 1.02 elsewhere. The median ratio of seroprevalence in children vs adults was 0.89 and only one study showed a significant ratio of <0.40. The main limitation of our study is the inaccuracies and biases in seroprevalence studies.
Conclusions
Precision shielding of elderly community-dwelling populations before the availability of vaccines was indicated in some high-income countries, but most countries failed to achieve any substantial focused protection.
Registration
Open Science Framework (available at: https://osf.io/xvupr)
Coronavirus disease 2019 (COVID-19) is characterized by a steep age-gradient in risk of serious disease and death [1-3]. Death risk after infection increases approximately 3-fold per 10-year increments, thus differing more than a thousand-fold between pediatric and geriatric populations. The total fatalities footprint of a pandemic with such strong risk stratification is expected to depend on how effectively high-risk, vulnerable individuals are protected from infection [4]. This is particularly important for the pre-vaccination period, but remains relevant even as effective interventions such as vaccines are carried out.
The ability to more aggressively protect the elderly and other vulnerable individuals (ie, precision shield) has been contested [5,6]. For a widely circulating virus, it may be difficult to effectively shield only high-risk individuals. Nursing home residents, a particularly high-risk group of elderly people, were even disproportionately more frequently infected early in the pandemic [7-11]. Infections were massively spread in such facilities, as testified by high death tolls and high seroprevalence rates in their populations [9-13]. However, the question of age-stratified precision shielding remains open for community-dwelling populations. It is possible that infection rates vary in different age groups. Perhaps community-dwelling elderly might have been less mobile and less exposed than other adults. Conversely, children and adolescents may have had lower infection rates, given the widely implemented school closures.
Insights on the relative infection rates across age strata can be obtained from seroprevalence studies. Hundreds of such studies have been conducted to date [14]. However, such surveys are also susceptible to numerous biases [15]. To answer the question of whether age-specific precision shielding was achieved in the pre-vaccination period, we performed a systematic review and meta-analysis of national seroprevalence studies without high risk of bias. Through the meta-analysis, we aimed to estimate the relative infection rates in the elderly vs the non-elderly and, secondarily, in children vs adults.
METHODS
We pre-registered this meta-analysis as part of a broader ongoing project on COVID-19 seroprevalence and infection fatality (protocol: https://osf.io/xvupr). Protocol amendments and clarifications are available in the Supplementary Table 1 in the Online Supplementary Document.
Search strategy and eligibility criteria
We identified seroprevalence studies in the live systematic review SeroTracker [14-16]. We also performed PubMed searches using the string “seroprevalence AND (national OR stratified) AND COVID-19”. The initial search performed on February 8, 2022 was updated on May 17, 2022.
We included studies on SARS-CoV-2 seroprevalence had a national, representative sample, completed the sampling by February 28, 2021, included adults (with or without children and/or adolescents), and provided seroprevalence for non-elderly people (preferably for <70 years and/or <60 years, but any cut-off between 60 and 70 years was acceptable). We excluded studies focusing on patient cohorts, blood donors, workers, and insurance applicants and any other study where the examined population might have had lower or higher risk than the general population. In SeroTracker, only studies in the categories of “Household and community samples” and “Multiple general populations” without high risk of bias (reported by the SeroTracker team using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for Prevalence Studies) were considered for further scrutiny. Similar criteria were applied to any additional PubMed-retrieved studies. Following previous work [17], for studies done in the USA, we retained only those that have adjusted the seroprevalence estimates for race/ethnicity [18].
For studies with several sampled (sub)regions of a country, we accepted those where the sampling locations might reasonably represent the entire country. Conversely, we excluded studies when only urban populations or when only rural populations were sampled, or when locations were selected only when hard hit or only when lightly hit. One reviewer selected the studies for the title/abstract review and two reviewers independently for full text eligibility.
We excluded seroprevalence studies where crude overall seroprevalence in the population was less than one-test specificity and/or the 95% confidence interval (CI) of the seroprevalence went to 0%, since the uncertainty on seroprevalence for them is very large.
To avoid any substantive impact of vaccination, we only considered seroprevalence studies where the sampling had been completed by the end of February 2021 and at least 90% of the samples had been collected before end of January 2021.
Extracted information
Two authors independently performed the data extraction for eligible articles in duplicate, discussing any disagreements between themselves and consulting a third author (JPAI) if consensus was not achieved.
We extracted information on country, dates of sample collection, overall sample size (number tested) and sample size in pediatric, non-elderly adults, and elderly populations, and types of antibodies measured (immunoglobulin G (IgG), immunoglobulin G (IgM), immunoglobulin A (IgA)) from all eligible seroprevalence studies. We also extracted the estimated unadjusted seroprevalence (positive samples divided by all samples tested) and the most fully adjusted seroprevalence in children, non-elderly adults, and the elderly. We also noted the factors that the authors considered for adjustment in the most fully adjusted calculations. If multiple different time points when seroprevalence was assessed existed in a study, we selected the time point that gives the highest overall seroprevalence estimate; when there was a tie, we chose the earliest time point.
We defined groups of children (including adolescents), non-elderly adults, and elderly according to preferred age cut-offs of 20 years and 60 years; therefore, these groups ideally referred to 0-20 years, 21-60 years, and >60 years, respectively. For separating pediatric and non-elderly adult populations, we accepted cut-offs in the range 14-20, preferring the one available that was closest to 20. For separating non-elderly adults from elderly, we accepted cut-offs in the range of 54-70, preferring the one available that was closest to 60. Available seroprevalence data on more granular age strata were merged within the three main age groups.
Data synthesis
For each eligible study, we calculated ratios of seroprevalence across children/adolescents, non-elderly adults, and elderly adults. For the main analysis, we focused on the ratio of seroprevalence in the elderly vs non-elderly (non-elderly adults or any non-elderly, if pediatric and adult population data were not offered separately). In the sensitivity analyses, we examined the ratios of seroprevalence in the elderly vs any non-elderly, and elderly vs strictly non-elderly adults. These ratios are “shielding ratios” [4] and allow for the evaluation of whether elderly individuals (a high-risk group) were more protected (and if so, by how much) and if there were consistent patterns across different countries. The observed ratios may thus provide estimates of the extent of precision shielding achieved in different countries [4] under the assumption that selection biases in sampling, test performance, and seroreversion are not substantially different in different age strata. We performed calculations using the crude numbers (tested positive/total tested) in each age stratum; when these were not available, we used the adjusted seroprevalence estimates and converted the adjusted proportion to an equivalent number of seropositives. When both crude numbers and adjusted estimates were available, we examined whether the latter changed the results.
In the secondary analysis, we examined the ratio of seroprevalence in children/adolescents vs non-elderly adults to evaluate whether there was preferential shielding of pediatric populations.
We had anticipated that substantial heterogeneity may exist across countries to preclude formal data synthesis by meta-analysis. Therefore, we expressed the results by using medians and by describing studies with extreme values. We also formally estimated the between-study heterogeneity of these ratios using the I2 statistic [19]. In the exploratory analyses, we evaluated whether results differed in high-income countries vs other countries (assuming that perhaps focused protection might be more feasible in the former). Analyses were conducted in STATA (StataCorp, USA).
RESULTS
Eligible studies
After in-depth screening (Figure 1 and Supplementary Table 2 in the Online Supplementary Document), we included 38 eligible studies in the analyses: 36 had separate seroprevalence data on an elderly age stratum, while two (Afghanistan, Oman) could only separate pediatric vs adult population seroprevalence.
Figure 1.
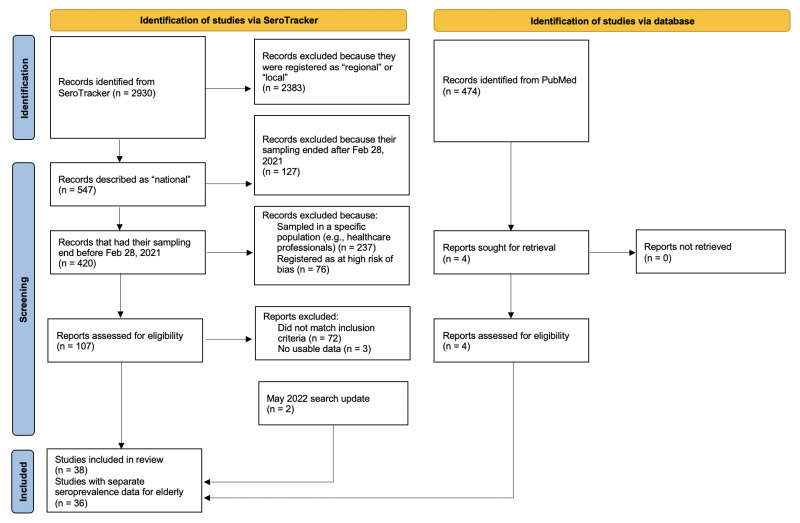
Flowchart for screening and selection of eligible studies. May 2022 search update: Among 3412 identified SeroTracker records, were manually assessed nine for eligibility (after applying relevant SeroTracker filters according to first search) and excluded seven. Details on the totally 79 reports excluded among 116 reports manually assessed for eligibility are available in Supplementary Table 2 in the Online Supplementary Document.
Study characteristics
Table 1 presents the 38 eligible studies. According to JBI risk of bias criteria, 18 studies [20-37] were deemed to be at low risk of bias and 19 [38-56] were deemed to be at moderate risk of bias. One study [57] was deemed at unclear risk of bias (no studies at high risk of bias were eligible upfront). The eligible studies came from 36 different countries (France and USA had two eligible studies each). More than half of the studies were performed in Europe (n = 20), 13 were performed in Asia, four in the Americas, and one in Africa. Twenty-five of the 38 studies came from high-income countries. Sample sizes varied substantially, but tended to be higher in high-income countries. Twenty-four studies had a total sample exceeding 5000, but this applied to only six out of 13 studies from non-high-income countries. Twenty-six studies provided separate data for a pediatric population with cut-off ages varying between 14 and 19 years, and 36/38 provided separate data for an elderly population with cut-offs varying between 54 and 70 years. Eleven studies assessed all antibodies, seven assessed IgG and IgM, and 20 assessed IgG only. Twenty studies performed all their sampling before or up to October 2020.
Table 1.
Eligible studies – population and sampling details
| Country | Children | Non-elderly adults | Elderly | Non-elderly | Age cut-off, pediatric | Age cut-off, elderly | Antibodies | Sampling time |
|---|---|---|---|---|---|---|---|---|
| Afghanistan |
4346 |
5168 |
|
9514 |
17 |
NA |
IgG, IgM |
June 2020 |
| Andorra |
10 590 |
38 765 |
10 331 |
49 355 |
19 |
59 |
IgG, IgM |
May 2020 |
| Canada |
|
|
1029 |
5789 |
NA |
59 |
IgG only |
May to September 2020 |
| Czech Republic |
|
|
1215 |
5665 |
NA |
59 |
IgG only |
December 2020 to January 2021 |
| Denmark* |
1126 |
13 500 |
3540 |
14 626 |
17 |
64 |
All |
September to December 2020 |
| England |
|
|
21 953 |
77 955 |
NA |
64 |
IgG only |
June to July 2020 |
| Faroe Islands |
14 616 |
25 851 |
12 387 |
40 467 |
19 |
59 |
All |
November 2020 |
| France (Warszawski) |
1438 |
47 555 |
14 531 |
48 993 |
17 |
64 |
IgG only |
November 2020 |
| France (Carrat) |
|
|
41 933 |
40 193 |
NA |
59 |
IgG only |
May to September 2020 |
| Germany |
|
|
3819 |
11 302 |
NA |
64 |
IgG only |
October 2020 to February 2021 |
| Hungary |
|
|
2386 |
8088 |
NA |
64 |
IgG only |
May 2020 |
| Iceland* |
|
|
3400 |
27 176 |
NA |
70 |
All |
April to June 2020 |
| India |
2290 |
23 271 |
3037 |
25 561 |
17 |
60 |
IgG only |
December 2020 to January 2021 |
| Iran |
2302 |
7596 |
1358 |
9898 |
17 |
59 |
IgG only |
August to October 2020 |
| Ireland |
198 |
1224 |
311 |
1422 |
19 |
59 |
IgG only |
June to July 2020 |
| Israel |
5864 |
32 809 |
15 687 |
38 673 |
19 |
59 |
IgG only |
June to September 2020 |
| Italy* |
2788 |
21 434 |
12 176 |
24 222 |
17 |
59 |
IgG only |
May to July 2020 |
| Japan |
|
|
2794 |
5156 |
NA |
59 |
All |
June 2020 |
| Jersey |
|
|
298 |
1077 |
NA |
64 |
IgG, IgM |
June 2020 |
| Jordan |
1486 |
3027 |
470 |
5513 |
19 |
59 |
IgG, IgM |
December 2020 to January 2021 |
| Lao PDR |
233 |
1849 |
351 |
2082 |
18 |
60 |
IgG, IgM |
August to September 2020 |
| Lebanon |
293 |
1449 |
200 |
1742 |
19 |
59 |
IgG only |
December 2020 to January 2021 |
| Lithuania |
|
|
2218 |
868 |
NA |
64 |
IgG, IgM |
October 2020 |
| Maldives |
410 |
1396 |
83 |
1806 |
17 |
59 |
IgG only |
October to November 2020 |
| Mexico* |
1891 |
5785 |
1787 |
7676 |
19 |
59 |
All |
August to November 2020 |
| Mongolia |
1898 |
2784 |
317 |
4682 |
19 |
59 |
IgG only |
October to December 2020 |
| Nepal |
455 |
2275 |
310 |
2730 |
14 |
64 |
All |
October 2020 |
| Netherlands |
1128 |
3472 |
2213 |
4600 |
19 |
59 |
IgG only |
June to August 2020 |
| Norway |
868 |
21 396 |
5436 |
22 264 |
19 |
66 |
IgG only |
November 2020 to February 2021 |
| Oman |
57 |
4007 |
|
4064 |
14 |
NA |
IgG only |
November 2020 |
| Pakistan |
1995 |
2030 |
975 |
4022 |
19 |
59 |
IgG, IgM |
October to November 2020 |
| Portugal |
2108 |
6495 |
4795 |
8603 |
17 |
54 |
All |
September to October 2020 |
| Russia |
9705 |
44 921 |
19 432 |
54 626 |
17 |
59 |
IgG only |
June to July 2020 |
| Senegal |
462 |
867 |
117 |
1329 |
15 |
60 |
All |
October to November 2020 |
| Slovenia* |
174 |
673 |
364 |
847 |
20 |
60 |
All |
October to November 2020 |
| Spain |
8636 |
27 453 |
15 320 |
36 089 |
19 |
59 |
IgG only |
November 2020 |
| USA (Sullivan) |
|
3481 |
1173 |
3481 |
NA |
64 |
All |
August to December 2020 |
| USA (Kalish) | 6785 | 1273 | 6785 | NA | 69 | All | April to August 2020 |
NA – not available, IgG – immunoglobin G, IgM – immunoglobin M
*Denmark – numbers are approximated based on number of invited persons and proportion participating. Iceland – numbers are approximated based on estimated age-stratified national seroprevalence, number of people tested and the population pyramid. Italy – numbers are approximated based on the proportion positive and the 95% CI in each age group. Mexico – numbers are approximated from adjusted seroprevalence. Slovenia – October 3 estimates used according to predefined eligibility criteria.
Seroprevalence in the different age groups
Table 2 shows the seroprevalence estimates for the pre-specified groups of children, non-elderly adults, non-elderly, and elderly. There was a wide range of values from 0% in Faroe Islands to over 40% in the Czech Republic. Whenever available, adjusted seroprevalence estimates tended to be similar to unadjusted estimates, with few exceptions (Table 2). Parameters used for adjustments are shown in Supplementary Table 3 in the Online Supplementary Document.
Table 2.
Seroprevalence estimates (%) for age groups – unadjusted (and adjusted in parenthesis)
| Country | Seroprevalence in children | Seroprevalence in non-elderly adults | Seroprevalence in non-elderly | Seroprevalence in elderly |
|---|---|---|---|---|
| Afghanistan |
25.3 |
35.2 |
30.7 |
|
| Andorra |
12.6 |
11.0 |
11.3 |
13.1 |
| Canada |
|
|
2.1 |
0.7 |
| Czech Republic |
|
|
43.7 |
41.6 |
| Denmark |
6.5 (6.5) |
4.2 (4.3) |
4.4 (4.5) |
2.3 (2.3) |
| England |
|
|
6.1 (6.8) |
3.6 (3.2) |
| Faroe Islands |
0.0 |
0.0 |
0.0 |
0.0 |
| France (Warszawski) |
8.9 (9.8) |
6.7 (6.4) |
6.8 (6.5) |
4.2 (4.2) |
| France (Carrat) |
|
|
6.8 |
2.3 |
| Germany |
|
|
1.3 (1.9) |
1.1 (0.6) |
| Hungary |
|
|
0.6 (0.7) |
0.8 (0.8) |
| Iceland |
|
|
1.0 |
0.5 |
| India |
27.7 (27.0) |
25.6 (24.0) |
25.7 (24.5) |
28.2 (26.0) |
| Iran |
(11.5) |
(12.6) |
(12.4) |
(19.4) |
| Ireland |
1.5 (1.4) |
2.0 (1.7) |
1.9 (1.7) |
1.9 (1.7) |
| Israel |
(7.2) |
(4.0) |
(4.5) |
(2.2) |
| Italy |
2.2 (2.2) |
2.5 (2.5) |
2.5 (2.5) |
2.6 (2.6) |
| Japan |
|
|
0.1 |
0.1 |
| Jersey |
|
|
3.8 |
5.4 |
| Jordan |
36.2 |
33.8 |
34.6 |
34.5 |
| Lao PDR |
3.9 (4.2) |
4.9 (5.1) |
4.8 (5.0) |
8.8 (9.3) |
| Lebanon |
15.4 (17.8) |
15.9 (18.3) |
15.8 (18.2) |
18.5 (21.4) |
| Lithuania |
|
|
1.4 |
2.1 |
| Maldives |
4.9 |
15.2 |
12.8 |
31.3 |
| Mexico |
22.5 (22.5) |
27.8 (27.9) |
26.5 (25.7) |
18.6 (18.6) |
| Mongolia |
1.1 (0.8) |
1.7 (1.3) |
1.5 (1.1) |
1.3 (1.2) |
| Nepal |
(8.8) |
(15.7) |
(14.1) |
(10.7) |
| Netherlands |
2.4 (3.7) |
5.2 (4.9) |
4.5 (4.2) |
5.0 (4.9) |
| Norway |
1.8 (1.9) |
0.9 (0.8) |
0.9 (0.9) |
0.6 (0.5) |
| Oman |
(23.5) |
(21.5) |
(21.5) |
|
| Pakistan |
4.2 |
8.9 |
6.6 |
8.7 |
| Portugal |
(2.4) |
(2.3) |
(2.3) |
(1.9) |
| Russia* |
21.6 |
15.6 |
16.6 |
17.4 |
| Senegal |
19.3 (19.3) |
31.7 (32.1) |
27.4 (27.4) |
24.8 (25.1) |
| Slovenia* |
4.9 |
5.6 |
5.5 |
2.1 |
| Spain |
7.8 (7.6) |
10.4 (10.5) |
9.8 (9.3) |
10.4 (10.3) |
| USA (Sullivan) |
|
5.6 (5.5) |
5.6 (5.5) |
2.9 (1.9) |
| USA (Kalish) | 3.8 (4.1) | 3.8 (4.1) | 3.6 (3.5) |
*Russia – median percentage across tested regions. Slovenia – October 3 estimates used according to predefined eligibility criteria. Inverse-variance fixed effects meta-analysis used to combine age sub-strata.
Ratio of seroprevalence in different age groups
Figure 2 shows the ratio of seroprevalence in the elderly vs the seroprevalence in non-elderly (non-elderly adults or non-elderly in general, if pediatric and adult population data were not offered separately). We found large between-study variability, with I2 = 98%.
Figure 2.
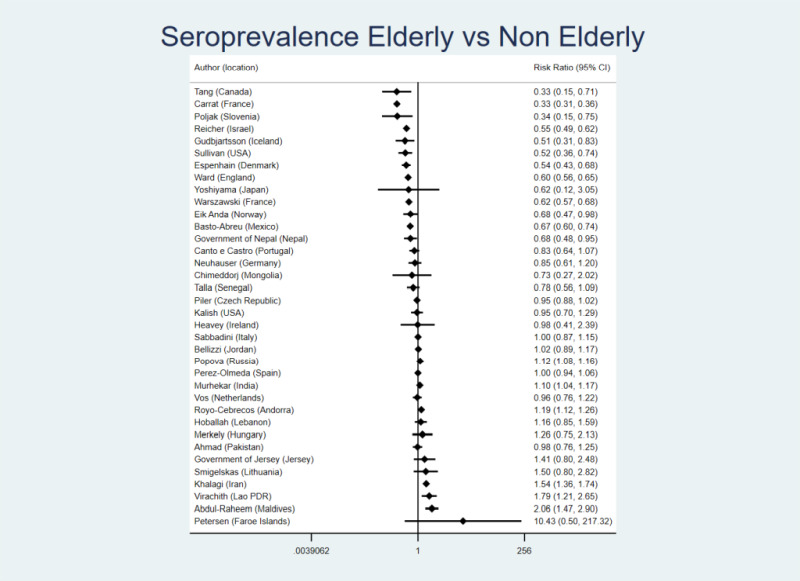
Seroprevalence ratio for the elderly vs non-elderly (non-elderly adults or non-elderly in general, if pediatric and adult population data were not offered separately). The definitions of age groups are detailed in the “Methods” section. We estimated the presented 95% CIs using crude counts in a two by two table for each study ((number elderly positive/number elderly tested)/(number non-elderly positive/number non-elderly tested)). When only adjusted seroprevalence estimates were available without crude data, we converted them to equivalent of number positive (number positive = adjusted seroprevalence × number tested in the specific age group).
The median ratio was 0.95 (0.90 if adjusted seroprevalence estimates were given priority in the calculations), suggesting a slightly lower seroprevalence in elderly populations; 23/36 studies had point estimates in this direction. For the two countries with two studies each, the point estimates were in the same direction, but the magnitude of the estimated protection of the elderly varied. Twelve studies with suggested protection of elderly and six studies with suggested inverse protection (higher seroprevalence in the elderly) had 95% CIs excluding a ratio of 1.00. Canada, Slovenia, one of the two studies in France and (in adjusted analyses only) Germany, and one of the USA studies suggested protection over 2.5-fold (ratio <0.40) with 95% CIs excluding 1.00. We did not observe inverse protection of such magnitude (ratio >2.5) with 95% CIs excluding 1.00 in any study.
The sensitivity analyses gave similar results: the median ratio of seroprevalence in the elderly vs any non-elderly was 0.95, with 20/36 studies offering point estimates in the direction of some protection of the elderly; the median ratio of seroprevalence in the elderly vs strictly non-elderly adults was 0.98 with 14/24 studies offering point estimates in the direction of some protection in the elderly.
We also observed large between-study heterogeneity (I2 = 96%) in the comparison of pediatric populations vs non-elderly adults (Figure 3). The median ratio of seroprevalence was 0.89 and 15/26 studies presented point estimates in the direction of greater protection of children/adolescents than non-elderly adults. Fifteen studies had 95% CIs excluding a ratio of 1.00 (with lower seroprevalence in the pediatric populations in eight and higher in seven). Only one study (Maldives) showed a ratio of <0.40 with 95% CIs excluding 1.00 and none had a ratio >2.5 with 95% CIs excluding 1.00.
Figure 3.
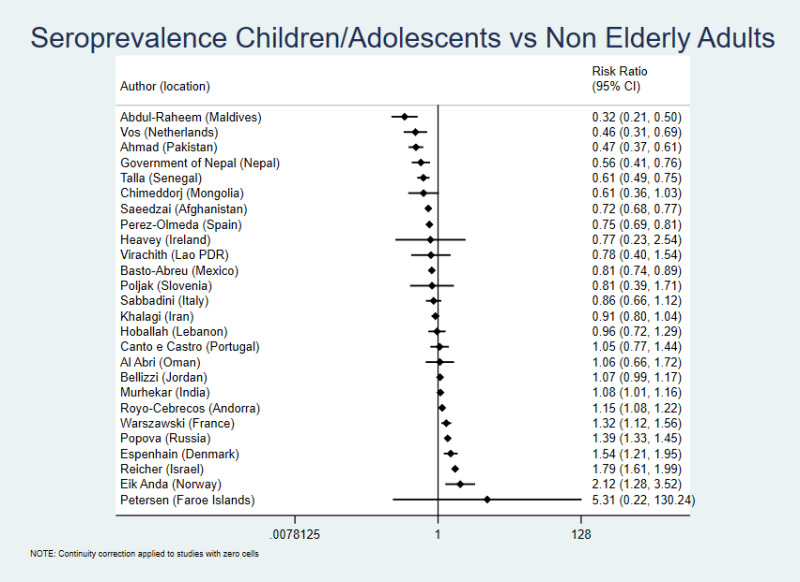
Seroprevalence ratio for pediatric populations vs non-elderly adults. The definitions of age groups are detailed in the “Methods” section. We estimated the presented 95% CIs using crude counts in a two by two table for each study ((number of pediatric population positive/number of pediatric population tested)/(number of non-elderly adults positive/number of non-elderly adults tested)). When only adjusted seroprevalence estimates were available without crude data, these were converted to equivalent of number positive (number positive = adjusted seroprevalence × number tested in the specific age group).
High income vs non-high-income countries
For the main analysis of elderly vs non-elderly (the latter group comprising of non-elderly adults or non-elderly in general, if pediatric and adult population data were not offered separately), the median ratio was 0.85 in 25 studies done in high-income countries (0.83 if priority were given to adjusted estimates) and 1.02 in 11 studies done in non-high-income countries. All five statistically significant estimates of >2.5-fold protection of the elderly were in high-income countries. For a more modest protection threshold, all nine estimates of >1.5-fold protection of the elderly (ratio <0.67) with 95% CIs excluding 1.00 were in high income countries, while both estimates of >1.5-fold inverse protection (higher seroprevalence in the elderly) with 95% CIs excluding 1.00 were in non-high-income countries.
DISCUSSION
Our analysis of data from 38 national seroprevalence studies for COVID-19 showed that, before the advent of massive vaccination, there was large heterogeneity across countries on the extent to which elderly people in the community were protected or not from infection compared with younger populations. On average, there was very little extra shielding of the elderly. However, several countries apparently did achieve substantial precision shielding of this vulnerable group. Conversely, in a few countries, the elderly were apparently infected slightly more frequently than non-elderly adults. Conclusive evidence for substantial preferential protection of the elderly in the community was seen only in some high-income countries. In non-high-income countries, the average ratio of seroprevalence between age groups suggested no preferential protection by age. There was also little difference in seroprevalence in children vs non-elderly adults overall, but the pattern differed across countries. On average, children were slightly less frequently infected than non-elderly adults.
These data suggest that precision shielding of vulnerable elderly populations is feasible, but strong shielding of the community-dwelling elderly populations was uncommon during the COVID-19 pandemic. It is unclear if this failure reflects practical difficulties of achieving major precision shielding, especially in disadvantaged settings [58,59], or the fact that pandemic response policies may not have focused much on this aspect, instead aiming for more horizontal measures. Country-level responses may have differed in this regard. Even within countries, heterogeneity may have existed across states and local communities on the timing and duration of specific measures that may protect some parts of the population more than others. For example, some measures may be more stringent on reducing the exposure of the elderly population (eg, avoidance of visits), while others (eg, school closures) might attempt to reduce exposure among children. While some may argue that the most draconian lockdown should be associated with equal protection of all age groups, this may not be so in practice, as (for example) essential workers who continue to be substantially exposed are non-elderly adults.
Given that a large share of COVID-19 deaths among community-dwelling people happen in the elderly, protection shielding exceeding 2.5-fold, as documented for five countries in our analysis, reflects roughly halving the total COVID-19 deaths among community-dwelling populations, showing that the benefit can be very significant. Unfortunately, however, the countries that apparently did achieve some substantial shielding of their community-dwelling elderly, failed in protecting resident of long-term care facilities [7-13], where infection fatality rates can be much higher (even 10-fold higher) than in community-dwelling elderly [17]. This resulted in numerous deaths of elderly residents in countries like USA, Canada, France, Germany, and Slovenia. Seroprevalence studies have documented extremely high rates of infection in nursing homes, much higher than in the community, in diverse countries, especially during 2020 [9-13]. Nursing home residents and other institutionalized people are routinely excluded from sampling in national seroprevalence studies and are, in any case, a small portion of the total population of any country.
Some limitations of our work should be discussed. First, we tried to select studies with maximal representativeness, but some samples may not have ended being fully representative, as some subpopulations are more difficult to recruit that may have different rates of infection than those recruited (e.g. the homeless and other marginalized groups). Second, both overall seropositivity and in specific age groups may vary temporally; for example, in some settings, an age group may have been protected or over-exposed compare to others at some point, depending on the measures taken. Third, screening schemes and approaches differed across countries, possibly creating heterogeneity in the results. Fourth, we focused on studies that were rated as having low or moderate risk of bias according to SeroTracker, but this does not offer absolute protection from many biases that may have affected the results. Fifth, given the unavoidable between-country heterogeneity, conclusions from the results of the meta-analysis need to be drawn carefully.
Additionally, the probability of seroconversion after infection and the rapidity of seroreversion may vary depending on age [60,61]. Elderly people may have initially stronger immune responses linked to more severe disease or weaker responses due to immune system senescence. If anything, old age and more symptomatic disease tend to be associated with longer persistence of antibodies [60]. If so, the precision shielding of elderly may have been slightly larger than what we calculated. Different antibody assays may also exhibit different rates of seroreversion and this may also vary per age group. However, given that most studies evaluated here were done early in the pandemic, seroreversion was probably not large.
For some studies, seroprevalence rates were very low and 95% CIs very wide. Depending on what adjustments are made, seroprevalence ratios might also differ in such cases, although in most studies we observed similar results for adjusted and unadjusted calculations.
The counterfactual seroprevalence ratios in the absence of any restrictive measures are unknown. Evidence from influenza seroprevalence assessments suggests that often children and/or young adults may be infected more frequently than elderly individuals, perhaps due to greater mobility and exposures, but this is not absolute and may vary per year and location [62-65]. Extrapolations to SARS-CoV-2 are tenuous.
Finally, focused protection may have varied in subsequent phases of the pandemic, with different infection rate ratios across age groups. Different waves due to different SARS-CoV-2 variants may have exhibited different age patterns and their underlying age-related variation in susceptibility and/or infectiousness is not well understood. Vaccine availability in 2021 was typically prioritized for the elderly leading to shifts in the age distribution of COVID-19 impact [66]. Vaccination also allowed more mobility and higher population exposure. After the Delta and Omicron waves, most people were infected at least once in most countries [67]. Even if precision shielding of the elderly can be achieved (as our data suggest), it is unknown whether it can be maintained effectively for pandemic-long circles lasting two or more years. Moreover, adverse consequences of trying to diminish exposures of vulnerable elderly may be substantial for their social well-being and their mental health [68,69]. Adverse consequences are likely for all age groups, including for children after school closures [70].
CONCLUSIONS
Despite these limitations, we can conclude that precision shielding was feasible in several high-income countries in the first year of the pandemic, but most countries had no major differences in infection rates across age groups. Precision shielding remains an attractive concept given the extreme variability of fatality risk of SARS-CoV-2 infection across age groups [17,71], but when it is not achieved (or worse, when vulnerable individuals, such as elderly and nursing home residents are even more frequently infected than the non-vulnerable) the death toll and excess deaths become high [72]. These observations may be useful for future pandemic preparedness, especially for pathogens exhibiting large fatality rate variability across different population groups.
Additional material
Acknowledgments
Data availability: All data are in the manuscript and supplementary files.
Footnotes
Funding: The work of John Ioannidis is supported by an unrestricted gift by Sue and Bob O’Donnell. The work of Angelo Maria Pezzullo in this research has been supported by the European Network Staff Exchange for Integrating Precision Health in the Healthcare Systems project (Marie Skłodowska-Curie Research and Innovation Staff Exchange No. 823995). Cathrine Axfors has received funding outside this work from the Knut and Alice Wallenberg Foundation’s Postdoctoral Fellowship (KAW 2019.0561) and postdoctoral grants from Uppsala University (E o R Börjesons stiftelse; Medicinska fakultetens i Uppsala stiftelse för psykiatrisk och neurologisk forskning), The Sweden-America Foundation, Foundation Blanceflor, Swedish Society of Medicine, and Märta och Nicke Nasvells fond. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Authorship contributions: J.P.A.I. had the original idea. J.P.A.I. and C.A. designed the study with contributions from A.M.P. and D.G.C-I. All authors edited the protocol. All authors collected data. J.P.A.I., A.M.P. and D.G.C.-I. performed analyses and all authors interpreted the results. J.P.A.I. wrote the first draft and all authors edited it for content. All authors approved the final version.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
REFERENCES
- 1.Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG.Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890. 10.1016/j.envres.2020.109890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Driscoll M, Dos Santos GR, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140-5. 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis JPA.Precision shielding for COVID-19: metrics of assessment and feasibility of deployment. BMJ Glob Health. 2021;6:e004614. 10.1136/bmjgh-2020-004614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenzer J.Covid-19: Experts debate merits of lockdowns versus “focused protection”. BMJ. 2020;371:m4263. 10.1136/bmj.m4263 [DOI] [PubMed] [Google Scholar]
- 6.Lenzer J.Covid-19: Group of UK and US experts argues for “focused protection” instead of lockdowns. BMJ. 2020;371:m3908. 10.1136/bmj.m3908 [DOI] [PubMed] [Google Scholar]
- 7.Levin AT, Jylhävä J, Religa D, Shallcross L.COVID-19 prevalence and mortality in longer-term care facilities. Eur J Epidemiol. 2022;37:227-34. 10.1007/s10654-022-00861-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comas-Herrera A, Zalakaín J, Lemmon E, Henderson D, Litwin C, Hsu A, et al. Mortality associated with COVID-19 in care homes: international evidence. Article in LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE, 14 October 2020. 2020.
- 9.Verschoor CP, Bowdish DME.Estimating SARS-CoV-2 seroprevalence in long-term care: a window of opportunity. Lancet Healthy Longev. 2022;3:e2-3. 10.1016/S2666-7568(21)00304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman SM, Davidow AL, Gurumurthy M, Peymani R, Webb J, Desai K, et al. Antibody Seroprevalence, Infection and Surveillance for SARS-CoV-2 in Residents and Staff of New Jersey Long-Term Care Facilities. J Community Health. 2022;47:774-782. 10.1007/s10900-022-01104-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krutikov M, Stirrup O, Nacer-Laidi H, Azmi B, Fuller C, Tut G, et al. COVID-19 Genomics UK consortium Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study. Lancet Healthy Longev. 2022;3:e347-55. 10.1016/S2666-7568(22)00093-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candel FJ, Barreiro P, San Román J, Del Mar Carretero M, Sanz JC, Pérez-Abeledo M, et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the Community of Madrid: the SeroSOS study. Age Ageing. 2021;50:1038-47. 10.1093/ageing/afab096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chudasama DY, Milbourn H, Nsonwu O, Senyah F, Florence I, Cook B, et al. Penetration and impact of COVID-19 in long term care facilities in England: population surveillance study. Int J Epidemiol. 2022;50:1804-1813. 10.1093/ije/dyab176 [DOI] [PubMed] [Google Scholar]
- 14.Arora RK, Joseph A, Van Wyk J, Rocco S, Atmaja A, May E, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2021;21:e75-e76. 10.1016/S1473-3099(20)30631-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannidis JPA.Reconciling estimates of global spread and infection fatality rates of COVID-19: An overview of systematic evaluations. Eur J Clin Invest. 2021;51:e13554. 10.1111/eci.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SeroTracker, in: https://serotracker.com/en/Explore, last accessed May 17, 2022.
- 17.Axfors C, Ioannidis JPA.Infection fatality rate of COVID-19 in community-dwelling elderly populations. Eur J Epidemiol. 2022;37:235-49. 10.1007/s10654-022-00853-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holden TM, Simon MA, Arnold DT, Halloway V, Gerardin J.Structural racism and COVID-19 response: higher risk of exposure drives disparate COVID-19 deaths among Black and Hispanic/Latinx residents of Illinois, USA. BMC Public Health. 2022;22:312. 10.1186/s12889-022-12698-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Patsopoulos NA, Evangelou E.Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914-916. 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royo-Cebrecos C, Vilanova D, López J, Arroyo V, Pons M, Francisco G, et al. Mass SARS-CoV-2 serological screening, a population-based study in the Principality of Andorra. Lancet Reg Health Eur. 2021;5:100119. 10.1016/j.lanepe.2021.100119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward H, Cooke GS, Atchison C, Whitaker M, Elliott J, Moshe M, et al. Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: Serial cross-sectional studies of 365,000 adults. Lancet Reg Health Eur. 2021;4:100098. 10.1016/j.lanepe.2021.100098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen MS, Strøm M, Fjallsbak JP, Hansen JL, Larsen S, Eliasen EH, et al. Low Seroprevalence among Undetected COVID-19 Cases, Faroe Islands, November 2020. Emerg Infect Dis. 2022;28:242-4. 10.3201/eid2801.210917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warszawski J, Meyer L, Franck JE, Rahib D, Lydié N, Gosselin A, et al. Trends in social exposure to SARS-Cov-2 in France. Evidence from the national socio-epidemiological cohort-EPICOV. PLoS One. 2022;17:e0267725. 10.1371/journal.pone.0267725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhauser H, Rosario AS, Butschalowsky H, Haller S, Hoebel J, Michel J, et al. Germany’s low SARS-CoV-2 seroprevalence confirms effective containment in 2020: Results of the nationwide RKI-SOEP study. medRxiv. 2021 Jan 1.
- 25.Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Kumar MS, Selvaraju S, et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020-January 2021. Int J Infect Dis. 2021;108:145-55. 10.1016/j.ijid.2021.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalagi K, Gharibzadeh S, Khalili D, Mansournia MA, Mirab Samiee S, Aghamohamadi S, et al. Prevalence of COVID-19 in Iran: results of the first survey of the Iranian COVID-19 Serological Surveillance programme. Clin Microbiol Infect. 2021;27:1666-71. 10.1016/j.cmi.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshiyama T, Saito Y, Masuda K, Nakanishi Y, Kido Y, Uchimura K, et al. Prevalence of SARS-CoV-2-Specific Antibodies, Japan, June 2020. Emerg Infect Dis. 2021;27(2):628-631. 10.3201/eid2702.204088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Government of Jersey. SARS-CoV-2: Prevalence of antibodies in Jersey. St Helier, Statistics Jersey. 2020 May 5. Available: https://www.gov.je/SiteCollectionDocuments/Government%20and%20administration/R%20Prevalence%20of%20antibodies%202020508%20SJ.pdf. Accessed: 7 January 2023.
- 29.Hoballah A, El Haidari R, Siblany G, Abdel Sater F, Mansour S, Hassan H, et al. SARS-CoV-2 antibody seroprevalence in Lebanon: findings from the first nationwide serosurvey. BMC Infect Dis. 2022;22:42. 10.1186/s12879-022-07031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chimeddorj B, Mandakh U, Le LV, Bayartsogt B, Deleg Z, Enebish O, et al. SARS-CoV-2 seroprevalence in Mongolia: Results from a national population survey. Lancet Reg Health West Pac. 2021;17:100317. 10.1016/j.lanwpc.2021.100317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Government of Nepal. Enhanced surveillance on sero-prevalence of SARS-CoV-2 in general population. Kathmandu, Government of Nepal Ministry of Health and Population. 2021 Apr 4. Available: https://mohp.gov.np/attachments/article/708/First%20Sero-prevalence_final_report_04-04-2021.pdf. Accessed: 7 January 2023.
- 32.Vos ERA, van Boven M, den Hartog G, Backer JA, Klinkenberg D, van Hagen CCE, et al. Associations Between Measures of Social Distancing and Severe Acute Respiratory Syndrome Coronavirus 2 Seropositivity: A Nationwide Population-based Study in the Netherlands. Clin Infect Dis. 2021;73:2318-21. 10.1093/cid/ciab264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anda EE, Braaten T, Borch KB, Nøst TH, Chen SLF, Lukic M, et al. Seroprevalence of antibodies against SARS-CoV-2 in the adult population during the pre-vaccination period, Norway, winter 2020/21. Euro Surveill. 2022;27:2100376. 10.2807/1560-7917.ES.2022.27.13.2100376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Abri SS, Al-Wahaibi A, Al-Kindi H, Kurup PJ, Al-Maqbali A, Al-Mayahi Z, et al. Seroprevalence of SARS-CoV-2 antibodies in the general population of Oman: results from four successive nationwide sero-epidemiological surveys. Int J Infect Dis. 2021;112:269-77. 10.1016/j.ijid.2021.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talla C, Loucoubar C, Roka JL, Barry MA, Ndiaye S, Diarra M, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Senegal: a national population-based cross-sectional survey, between October and November 2020. IJID Regions. 2022;3:117-25. 10.1016/j.ijregi.2022.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan PS, Siegler AJ, Shioda K, Hall EW, Bradley H, Sanchez T, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Cumulative Incidence, United States, August 2020-December 2020. Clin Infect Dis. 2022;74:1141-50. 10.1093/cid/ciab626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalish H, Klumpp-Thomas C, Hunsberger S, Baus HA, Fay MP, Siripong N, et al. Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Sci Transl Med. 2021;13:eabh3826. 10.1126/scitranslmed.abh3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeedzai SA, Osmani A, Noormal B. Prevalence of COVID-19 and its related deaths in Afghanistan: a Nationwide, Population-Based Seroepidemiological Study. Islamic Republic of Afghanistan, Ministry of Public Health, Kabul, Afghanistan; 2020.
- 39.Tang X, Sharma A, Pasic M, Brown P, Colwill K, Gelband H, et al. Assessment of SARS-CoV-2 Seropositivity During the First and Second Viral Waves in 2020 and 2021 Among Canadian Adults. JAMA Netw Open. 2022;5:e2146798. 10.1001/jamanetworkopen.2021.46798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piler P, Thon V, Andrýsková L, Doležel K, Kostka D, Pavlík T, et al. Nationwide increases in anti-SARS-CoV-2 IgG antibodies between October 2020 and March 2021 in the unvaccinated Czech population. Commun Med (Lond). 2022;2:19. 10.1038/s43856-022-00080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espenhain L, Tribler S, Sværke Jørgensen C, Holm Hansen C, Wolff Sönksen U, Ethelberg S.Prevalence of SARS-CoV-2 antibodies in Denmark: nationwide, population-based seroepidemiological study. Eur J Epidemiol. 2021;36:715-25. 10.1007/s10654-021-00796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrat F, Lapidus N, Ninove L, Blanché H, Rahib D, Saba Villarroel PM, et al. Age, COVID-19-like symptoms and SARS-CoV-2 seropositivity profiles after the first wave of the pandemic in France. Infection. 2022;50:257-62. 10.1007/s15010-021-01731-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merkely B, Szabó AJ, Kosztin A, Berényi E, Sebestyén A, Lengyel C, et al. Novel coronavirus epidemic in the Hungarian population, a cross-sectional nationwide survey to support the exit policy in Hungary. Geroscience. 2020;42:1063-74. 10.1007/s11357-020-00226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724-34. 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heavey L, Garvey P, Colgan AM, Thornton L, Connell J, Roux T, et al. The Study to Investigate COVID-19 Infection in People Living in Ireland (SCOPI): A seroprevalence study, June to July 2020. Euro Surveill. 2021;26:2001741. 10.2807/1560-7917.ES.2021.26.48.2001741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reicher S, Ratzon R, Ben-Sahar S, Hermoni-Alon S, Mossinson D, Shenhar Y, et al. Nationwide seroprevalence of antibodies against SARS-CoV-2 in Israel. Eur J Epidemiol. 2021;36:727-34. 10.1007/s10654-021-00749-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabbadini LL. Primi risultati dell’indagine di sieroprevalenza SARS-CoV-2. Roma, Istituto Nazionale di Statistica. 2020. Available: https://www.istat.it/it/files//2020/08/ReportPrimiRisultatiIndagineSiero.pdf. Accessed: 7 January 2023.
- 48.Bellizzi S, Alsawalha L, Sheikh Ali S, Sharkas G, Muthu N, Ghazo M, et al. A three-phase population based sero-epidemiological study: Assessing the trend in prevalence of SARS-CoV-2 during COVID-19 pandemic in Jordan. One Health. 2021;13:100292. 10.1016/j.onehlt.2021.100292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virachith S, Pommelet V, Calvez E, Khounvisith V, Sayasone S, Kounnavong S, et al. Low seroprevalence of COVID-19 in Lao PDR, late 2020. Lancet Reg Health West Pac. 2021;13:100197. 10.1016/j.lanwpc.2021.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Šmigelskas K, Petrikonis K, Kasiulevičius V, Kalėdienė R, Jakaitienė A, Kaselienė S, et al. SARS-CoV-2 Seroprevalence in Lithuania: Results of National Population Survey. Acta Med Litu. 2021;28:48-58. 10.15388/Amed.2020.28.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdul-Raheem R, Moosa S, Waheed F, Aboobakuru M, Ahmed IN, Rafeeg FN, et al. A sero-epidemiological study after two waves of the COVID-19 epidemic. Asian Pac J Allergy Immunol. 2021. [DOI] [PubMed] [Google Scholar]
- 52.Basto-Abreu A, Carnalla M, Torres-Ibarra L, Romero-Martínez M, Martínez-Barnetche J, López-Martínez I, et al. Nationally representative SARS-CoV-2 antibody prevalence estimates after the first epidemic wave in Mexico. Nat Commun. 2022;13:589. 10.1038/s41467-022-28232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmad AM, Shahzad K, Masood M, Umar M, Abbasi F, Hafeez A.COVID-19 seroprevalence in Pakistan: a cross-sectional study. BMJ Open. 2022;12:e055381. 10.1136/bmjopen-2021-055381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canto E Castro L, Gomes A, Serrano M, Pereira AHG, Ribeiro R, et al. Longitudinal SARS-CoV-2 seroprevalence in Portugal and antibody maintenance 12 months after infection. Eur J Immunol. 2022;52:149-60. 10.1002/eji.202149619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poljak M, Oštrbenk Valenčak A, Štrumbelj E, Maver Vodičar P, Vehovar V, Resman Rus K, et al. Seroprevalence of severe acute respiratory syndrome coronavirus 2 in Slovenia: results of two rounds of a nationwide population study on a probability-based sample, challenges and lessons learned. Clin Microbiol Infect. 2021;27:1039.e1-7. 10.1016/j.cmi.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gobierno de España. Estudio ENE-COVID: Cuarta ronda: Estudio nacional de sero-epidemiología de la infección por SARS-CoV-2 en España. Madrid, Gobierno de España Ministerio de Ciencia y Innovación. 2020. Available: https://www.sanidad.gob.es/gabinetePrensa/notaPrensa/pdf/15.12151220163348113.pdf. Accessed: 7 January 2023.
- 57.Popova AY, Smirnov VS, Andreeva EE, Babura EA, Balakhonov SV, Bashketova NS, et al. SARS-CoV-2 Seroprevalence Structure of the Russian Population during the COVID-19 Pandemic. Viruses. 2021;13:1648. 10.3390/v13081648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rocha R, Atun R, Massuda A, Rache B, Spinola P, Nunes L, et al. Effect of socioeconomic inequalities and vulnerabilities on health-system preparedness and response to COVID-19 in Brazil: a comprehensive analysis. Lancet Glob Health. 2021;9:e782-92. 10.1016/S2214-109X(21)00081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barron GC, Laryea-Adjei G, Vike-Freiberga V, Abubakar I, Dakkak H, Devakumar D, et al. Safeguarding people living in vulnerable conditions in the COVID-19 era through universal health coverage and social protection. Lancet Public Health. 2022;7:e86-92. 10.1016/S2468-2667(21)00235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peghin M, De Martino M, Fabris M, Palese A, Visintini E, Graziano E, et al. The fall in antibody response to SARS-CoV-2: a longitudinal study of asymptomatic to critically ill patients up to 10 months after Recovery. J Clin Microbiol. 2021;59:e0113821. 10.1128/JCM.01138-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailie CR, Tseng YY, Carolan L, Kirk MD, Nicholson S, Fox A, et al. Trend in sensitivity of SARS-CoV-2 serology one year after mild and asymptomatic COVID-19: unpacking potential bias in seroprevalence studies. Clin Infect Dis. 2022;75:e357-e360. 10.1093/cid/ciac020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomaa MR, Badra R, El Rifay AS, Kandeil A, Kamel MN, Abo Shama NM, et al. Incidence and seroprevalence of seasonal influenza a viruses in Egypt: Results of a community-based cohort study. Influenza Other Respir Viruses. 2022;16:749-55. 10.1111/irv.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vinh DN, Nhat NTD, de Bruin E, Vy NHT, Thao TTN, Phuong HT, et al. Koopmans M, Boni MF. Age-seroprevalence curves for the multi-strain structure of influenza A virus. Nat Commun. 2021;12:6680. 10.1038/s41467-021-26948-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu JT, Leung K, Perera RA, Chu DK, Lee CK, Hung IF, et al. Inferring influenza infection attack rate from seroprevalence data. PLoS Pathog. 2014;10:e1004054. 10.1371/journal.ppat.1004054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hopkins RS, Kite-Powell A, Goodin K, Hamilton JJ.The ratio of emergency department visits for ILI to seroprevalence of 2009 pandemic influenza A (H1N1) virus infection, Florida, 2009. PLoS Curr. 2014;6. 10.1371/currents.outbreaks.44157f8d90cf9f8fafa04570e3a00cab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pastorino R, Pezzullo AM, Villani L, Causio FA, Axfors C, Contopoulos-Ioannidis DG, et al. Change in age distribution of COVID-19 deaths with the introduction of COVID-19 vaccination. Environ Res. 2022;204:112342. 10.1016/j.envres.2021.112342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ioannidis JPA.The end of the COVID-19 pandemic. Eur J Clin Invest. 2022;52:e13782. 10.1111/eci.13782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dove A, Guo J, Calderón-Larrañaga A, Vetrano DL, Fratiglioni L, Xu W.Association between social isolation and reduced mental well-being in Swedish older adults during the first wave of the COVID-19 pandemic: the role of cardiometabolic diseases. Aging (Albany NY). 2022;14:2462-74. 10.18632/aging.203956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bankole A.Impact of Coronavirus Disease 2019 on geriatric psychiatry. Psychiatr Clin North Am. 2022;45:147-59. 10.1016/j.psc.2021.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viner R, Russell S, Saulle R, Croker H, Stansfield C, Packer J, et al. School Closures During Social Lockdown and Mental Health, Health Behaviors, and Well-being Among Children and Adolescents During the First COVID-19 Wave: A Systematic Review. JAMA Pediatr. 2022;176:400-9. 10.1001/jamapediatrics.2021.5840 [DOI] [PubMed] [Google Scholar]
- 71.Levitt M, Zonta F, Ioannidis JP.Comparison of pandemic excess mortality in 2020-2021 across different empirical calculations. Environ Res. 2022;213:113754. 10.1016/j.envres.2022.113754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pezzullo AM, Axfors C, Contopoulos-Ioannidis DG, Apostolatos A, Ioannidis JPA.Age-stratified infection fatality rate of COVID-19 in the non-elderly population. Environ Res. 2023;216:114655. 10.1016/j.envres.2022.114655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


