Abstract
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death worldwide. Despite the advent of screening efforts and algorithms to stratify patients into appropriate treatment strategies, recurrence rates remain high. In contrast to first-line treatment for HCC, which relies on several factors, including clinical staging, tumor burden, and liver function, there is no consensus or general treatment recommendations for recurrent HCC (R-HCC). Locoregional therapies include a spectrum of minimally invasive liver-directed treatments which can be used as either curative or neoadjuvant therapy for HCC. Herein, we provide a comprehensive review of recent evidence using salvage loco-regional therapies for R-HCC after failed curative-intent.
Keywords: Recurrent hepatocellular carcinoma, Locoregional therapy, Transarterial chemoembolization, Transarterial embolization, Transarterial radioembolization, Ablation, Salvage therapy
Core Tip: Management of recurrent hepatocellular carcinoma (R-HCC) includes surgical resection, systemic treatment, or locoregional therapies including ablation, transarterial chemoembolization, or radioembolization, and stereotactic body radiation therapy. In the setting of recurrence, locoregional therapies offer unique advantages over surgery for select patients. Recent investigations have also highlighted the potential of combining locoregional therapies or adding systemic retreatments for R-HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 75%-90% of liver malignancies and is the second most common cause of cancer death worldwide[1-3]. While advancements in surveillance efforts have improved prevention and screening, incidence and mortality of HCC in recent decades have gradually increased in the United States[4,5]. Prevalence is increased in East Asia and Africa, and at-risk populations include those with cirrhosis and hepatitis B or C[4,5].
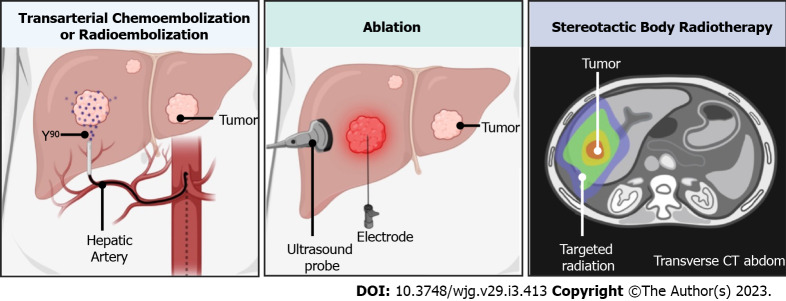
Treatment strategies for patients with HCC are tailored to tumor burden, invasiveness, and liver function, stratified using the Barcelona Clinic Liver Cancer staging (BCLC)[6]. First-line and curative treatment for HCC includes surgical resection or orthotopic liver transplantation with eligibility determined via the Milan criteria[5,7]. In patients with early-stage HCC who are not eligible for liver transplantation, surgical resection may be performed[6]. In patients who do not qualify as surgical candidates, the use of locoregional therapies using image-guided techniques has grown in popularity over the last several decades, providing a minimally invasive treatment approach to HCC[8,9]. Locoregional therapy is comprised of radiofrequency ablation (RFA)/ thermal microwave ablation (MVA), transarterial chemoembolization (TACE), or radioembolization (TARE), which have been commonly used neo-adjunctively to bridge or downstage patients with HCC in order to meet surgical eligibility (Figure 1)[10]. Ablation, in particular, offers a curative-intent option for nonsurgical candidates with early-stage HCC (BCLC 0/A) with a corresponding 5-year survival rate of 50%-80%[11]. Locoregional therapies provide an alternative strategy with the benefit of reduced comorbidity[12], and avoidance of complications that may worsen clinical outcomes associated with traditional surgery[13,14].
Figure 1.
Schematic depiction of locoregional therapies. CT: Computed tomography.
Long-term prognosis for the treatment of HCC remains poor, with a recurrence rate of 41%-70% within 5 years following resection[15-18]. Depending on tumor size, severity, liver function, and clinical indices, repeat hepatectomy may not be suitable for some patients. Therefore, alternative treatment options should be explored after initial curative attempts. No definitive consensus on standard salvage treatment approaches exist for recurrent HCC, but common therapies include repeat resection, liver transplantation, tyrosine kinase inhibitors, locoregional therapies, or a combination of multiple modalities[19]. This manuscript provides a comprehensive review of the current state of the literature for the use of salvage loco-regional therapies for recurrent HCC (R-HCC).
Risk Factors for Recurrent HCC
Prognostic factors associated with the increased risk of recurrence can vary from morphologic and surgical factors to molecular factors[20,21]. Larger tumors, or nodules with diameters ≥ 5 cm, are associated with increased rates of recurrence. Other morphological risk factors include the presence of multiple tumor nodules and satellite lesions[21-23]. The association between tumor size and recurrence is due to its correlation with invasiveness and propensity for portal vein-mediated intrahepatic metastasis and vascular invasion[21,24-26]. Microvascular invasion is a poor prognostic factor for R-HCC[27-29], defined as the histopathological observance of malignant cells within hepatic tissue and vascular cavities of the surrounding portal or hepatic vessels[30]. Other tumor-related factors associated with risk of recurrence after resection or liver transplantation, such as alpha-fetoprotein levels > 400 ug/L[31,32]. Overexpression of other histological and circulating biomarkers are also associated with negative prognostic factors related to recurrence[33].
SALVAGE LOCOREGIONAL THERAPY FOR RECURRENT HCC
Salvage locoregional therapy for R-HCC is frequently used after resection or in the setting of advanced, unresectable disease[8,9,33]. Compared to locoregional therapy or resection, liver transplantation carries a superior survival benefit for R-HCC[34-37]. However, the utility of transplantation is limited due to strict inclusion criteria, donor availability, high treatment costs, and surgical candidacy[9]. In patients who do not meet Milan criteria or not eligible for transplantation, the decision between locoregional therapies such as ablation, or repeated resection remains controversial. While resection is recognized as a primary treatment for HCC[6], portal hypertension, poor functional reserve from the future liver remnant, and technical difficulties (e.g., adhesions, anatomy modifications) can make repeat resection challenging and risky[38,39]. Therefore, the efficacy of alternative methods may be uniquely promising for R-HCC. The following section includes an overview of specific locoregional therapy modalities and their efficacy for R-HCC.
TACE
The liver parenchyma utilizes a dual blood supply with approximately two-thirds of originating from the portal vein and the remaining third from the hepatic artery. Transarterial embolization (TAE) involves selective angiographic occlusion of tumor-supplying vessels from the hepatic artery resulting in tumor ischemia and necrosis[9,40]. Similarly, TACE involves the use of embolizing microparticles combined with regional chemotherapy[9]. Several variations of TACE exist, but embolization is commonly completed using gelatin sponge particles, polyvinyl alcohol particles, or spherical embolic agents[41]. Of note, conventional TACE utilizes a chemotherapeutic agent emulsed with lipiodol, whereas the use of drug-eluting beads carry the added benefit of increased concentration to the target[9,42,43]. Damage to healthy liver parenchyma is spared via arterial supply from the unobstructed portal vein[9,44].
TACE can be used as a bridge to transplantation and is currently a first-line multinodular HCC and intermediate-stage disease (BCLC B)[6,9]. It is also reserved for early-stage disease (BCLC A) who do not meet surgical criteria[9]. TACE after resection is particularly beneficial to patients with poor prognostic factors such as microvascular invasion[45-48]. Similar to primary HCC, TACE for R-HCC is tolerable and an optimal therapeutic modality for patients with poor liver function or multifocal HCC[49-51]. Two recent meta-analyses found adjuvant TACE improved overall survival (hazard ratio: 0.64-0.71)[46,52] and disease free survival (hazard ratio: 0.73)[52]. Overall 1- and 3-year survival rates for TACE for R-HCC are reportedly 28%-82% and 32%-43.9%, respectively[50,53]. Meta-analysis has reported 5-year survival rates for TACE to be 15.5%[54]. Poorer outcomes and prognosis in patients treated with TACE for R-HCC are multiple sessions, tumor size > 5 cm and ≥ 2 lesions[50]. TACE offers a unique benefit in the presence of microvascular invasion or multifocal disease but studies to date have been largely retrospective and a need for randomized control trials is required before clinical considerations are definitive. A prospective investigation of 629 patients found worse outcomes in patients treated with TACE (n = 339), compared to radiofrequency ablation (n = 162), and re-hepatectomy (n = 128)[49]. Yet, a meta-analysis of seven studies including patients with R-HCC reported no overall survival differences between TACE (n = 807) and repeated resection (n = 267). Therefore, TACE appears to be an effective treatment option for R-HCC, with a preferential advantage to patients with morphological factors such as multiple tumors or disease complicated by microvascular invasion[33].
TARE
TARE is a local radiation therapy also referred to as selective internal radiotherapy, whereby Yttrium-90 Labeled microspheres are delivered through the hepatic arteries to the tumor[55,56]. Yttrium-90 is a β-emitter, and has a tumoricidal effect at a sufficient dosage of 400Gy or greater[11]. Similar to TACE, radioembolization is used as a neoadjuvant treatment for downstaging and bridging patients for transplantation or resection[57] and considered a curative approach for early-HCC or BCLC 0/A[58]. TARE has become increasingly popular over the last decade as a safe and tolerable procedure for HCC[59], with shorter hospital length of stay and decreased risk of post-embolization syndrome when compared to TACE[60-62]. Additionally, TARE carries less risk for portal vein tumor thrombosis[63]. Recently, TARE has been adopted within the BCLC algorithm as a second-line treatment for early-stage HCC[11,64]. This change is primarily driven by the LEGACY (Local radioembolization using Glass Microspheres for the Assessment of Tumor Control with Y-90) study, which found radioembolization > 400 Gy to be safe and an effective curative approach for patients with nodules less than 8 cm[65].
For R-HCC, there is a scarcity of investigations determining the utility of TARE after failed curative-intent. Meta-analyses have shown similar outcomes between TACE and TARE for unresectable HCC[66]. It is also important to note that a randomized control trial by Salem et al[65] found better tumor control outcomes in patients with HCC BCLC stages A/B treated with TARE as opposed to TACE (time to progression: > 26 mo; 6.8 mo, respectively). Sangro et al[67] reported no differences in adverse events in patients receiving TARE with prior failed curative-intent treatments (surgical or non-surgical) compared to treatment naïve patients receiving TARE. A retrospective investigation of 41 patients reported a time to progression of 11.3 mo and overall survival of 22.1 mo patients receiving TARE after prior resection[68]. Due to the advantages of TARE listed above, it has been advocated for advanced, unresectable disease[33,69]. More data is needed to determine the efficacy and optimal patient-selection strategies of radioembolization in the context of R-HCC.
Ablation
Ablation involves using a probe placed percutaneously under image guidance into the tumor to induce necrosis via thermal energy[11,70]. Ablation consists of either RFA or MVA. RFA is moderated by the “heat sink effect” which can negatively impact tumor response. Blood flow from nearby tissue can dissipate heat transfer and result in a cooling effect[71]. MVA is less impacted by heat sink due to the use of higher temperatures and larger, homogenous ablation zone, but at a cost of increased risk of injury to adjacent structures[72-75]. For both types of ablation, tumor location efficacy can be impacted by location, where tumors abutting nearby structures like the gallbladder, bowel, and diaphragm can be injured or result in insufficient safety margins that leave residual tumor[76]. Ablation is considered a curative treatment for early-stage HCC (BCLC 0/A)[6,11]. A major advantage of ablation is it can be performed quicker and may be more feasible than surgery with the added benefit of fewer complications and faster recovery[77].
A retrospective review of 211 patients with R-HCC found the 1-year survival rate for locoregional therapy (RFA, TAE, and/or percutaneous ethanol injection; n = 170, 91.6%) to be greater than salvage liver transplantation (n = 41, 90.2%)[37]. However, survival rates became superior in salvage liver transplantations at 3- and 5-years (80.4, and 80.4%, respectively) relative to the locoregional therapy group (71.7, and 51.1%, respectively)[37]. A meta-analysis of retrospective investigations by Chen et al[78] found improved clinical outcomes for 3- and 5-year survival rates in repeated hepatectomy compared to RFA for R-HCC. Therefore, repeated hepatectomy carries improved long-term efficacy, although the authors acknowledge selection bias may confound these results since a higher proportion of patients with improved liver function and limited tumor spread may be candidates for surgery. A meta-analysis of randomized control trials and observational studies by Yuan et al[79] found similar survival rates between ablation (MVI or RFA) compared to re-resection, but lower perioperative morbidity rates were observed in patients undergoing ablation (3.3%) relative to re-resection (17%). The majority of these studies included tumors ≤ 3 cm, and therefore the decision to utilize ablation over surgery for R-HCC may be appropriate for smaller tumors[80]. In tumors ≤ 3 cm, disease-free survival rates are similar to resection, but hospital length of stay and perioperative morbidity is lower in RFA (5 d, 7%, respectively) compared to repeated resection (13 d, 16%, respectively)[81]. Yang et al[82] echoed these findings, illustrating repeat resection for R-HCC has superior overall survival rates, but sub-group analyses of outcomes for smaller tumors diminish survival differences between these two methods. Larger, more homogenous ablation volumes associated with MVA may broaden ablation applicability to larger tumors[83]; however, studies to date evaluating MVA for R-HCC are limited.
Stereotactic body radiotherapy
Stereotactic body radiotherapy (SBRT) is a localized therapy whereby fractionated high-dose radiation is used to ablate liver parenchymal tumors (Figure 1)[84]. Conventionally, SBRT is dedicated to salvage therapy for R-HCC or advanced disease when ablation or embolization has failed or is contraindicated[85]. SBRT is currently not included in the BCLC but is included in the National Comprehensive Cancer Guidelines[84]. Kimura et al[86] reviewed patients with HCC who either failed or were not eligible for resection or other locoregional therapies, reporting safe and satisfactory overall survival rates for first and second SBRT (n = 81, 60.4%, and 61%, respectively). In patients receiving salvage SBRT after TACE, overall survival rates at 3 years were 72.7% (n = 302), with 95.4% tumors reaching complete response[87]. Therefore, in patients who fail TACE and curative modalities are not suitable, salvage SBRT could be offered as a potential subsequent treatment option.
Multimodal Locoregional Therapy Approaches
Approaches that combine locoregional therapies (e.g., TACE and RFA/MVA) have been proposed. Several mechanisms have been suggested to explain the synergistic or additive effects of combining modalities. Multimodality therapies may overcome individual limitations of monotherapy, such as providing adequate control for intermediate to larger tumors[72,88-90]. TACE is suggested to mitigate the heat sink effect and therefore, positively impact the efficacy of RFA[71]. Chemoembolization may also reduce tumor burden, which can aid RFA by extending the safety margin and the resultant coagulation zone[90,91]. A meta-analysis of 8 randomized control trials using RFA-TACE for primary HCC found improved overall survival [hazard ratio (HR) = 0.58, confidence interval (CI) 0.41 - 0.80] and recurrence free survival (HR = 0.65 CI =0.47 - 0.76) compared to RFA alone.
To date, few investigations have sought to determine the efficacy of multimodal therapy as a salvage treatment approach in unresectable disease or instances of R-HCC. For the treatment of larger R-HCC tumors (≤ 7 cm), TACE followed by RFA can reveal additional satellite lesions and have greater 1-, 3-, 4- year survival rates (92.6%, 66.6%, 61.8%) than RFA alone (85.3%, 59%, 45%)[92,93]. Studies comparing the efficacy of TACE-RFA have indicated comparable 1-, 3-, and 5-year survival outcomes between the two salvage treatment approaches for both smaller tumors (≤ 5 cm) [94,95] and larger ones (> 5 cm)[96]. Interestingly, TACE-RFA achieved satisfactory outcomes with a lower rate of complications (e.g., bleeding, liver failure) and shorter hospital stays[94-96]. Yang et al[97] published a retrospective investigation of 103 patients with R-HCC treated with either RFA, TACE, or combination therapy of RFA and TACE. Intrahepatic rates of recurrence were lower in the combination group (20.7%) compared to TACE (57.1%) and the RFA group (43.2%). 1-, 3- and 5-year survival rates were also greater in the combination group (88.5%, 64.6%, 44.3%) compared to the TACE alone group (65.8%, 38.9%, 19.5%). Other multimodal regimens for R-HCC have been explored, including TACE and MVA, of which when combined, improve tumor response and prolong progression-free survival compared to TACE monotherapy for small R-HCC tumors (≤ 3 cm)[98]. Although prospective investigations are required prior to establishing recommendations, in general, current evidence indicates a potential survival benefit to multimodality approaches with some investigators advocating for the adoption of multimodal therapy in future BCLC treatment guidelines[99].
Combining Locoregional Therapy and Systemic Therapy
Sorafenib, an oral tyrosine kinase inhibitor, is reserved for advanced-stage disease (BCLC class C) based on the results of the SHARP trial[6]. Overall, Sorafenib can offer survival benefit for unresectable HCC, but worse tumor response and greater adverse events when compared to locoregional therapies[100,101]. Challenges of using sorafenib are further compounded by heterogenous response rates and acquired resistance[102-104]. However, investigations have explored the utility of combining oral systemic agents with locoregional therapy (Table 1). A retrospective study reviewed 1126 patients with R-HCC in patients who received sorafenib and concurrent TACE or TACE monotherapy. The addition of sorafenib to TACE offered significantly improved survival time compared to TACE alone (20.23 vs 13.87 mo, respectively)[105]. Peng et al[106] retrospectively reviewed patients with advanced R-HCC receiving either sorafenib monotherapy (n = 101), or a combination of sorafenib and TACE-RFA (n = 106). While the toxicity profile was similar between both groups, median overall survival and time to progression in TACE-RFA + sorafenib (14 mo; 7 mo, respectively) was superior to sorafenib monotherapy (9 mo; 4 mo, respectively)[106]. A randomized, multicenter control trial comparing TACE (n = 76 and TACE with sorafenib (n = 80) for unresectable HCC, resection, found median progress-free survival to be greater in the combined treatment group (25.2 vs 13 mo)[107]. Although this trial included treatment naiive patients, a large portion of patients received prior locoregional therapy treatments Multicenter phase III randomized control trials comparing TACE alone and TACE with sorafenib for recurrent, unresectable HCC are currently underway.
Table 1.
Outcomes of multimodal locoregional therapy for recurrent hepatocellular carcinoma
|
Ref.
|
Study design
|
Treatment
|
Number of patients
|
Outcomes
|
| Song et al[95] | Retrospective | Recurrent HCC ≤ 5 cm | 63 TACE; 96 TACE-RFA | TACE-RFA lower disease progression than TACE monotherapy; No difference in overall survival |
| Zhang et al[115] | Retrospective | Treatment Naïve HCC, DEB-TACE-RFA for Recurrent HCC (Group B), and hepatectomy | 40 DEB-TACE as primary treatment; 36 DEB-TACE Recurrent HCC; 40 hepatectomy as primary | DEB-TACE-RFA can prolong survival time for recurrent HCC |
| Zheng et al[96] | Retrospective | TACE-RFA or repeat hepatectomy | 63 TACE-RFA; 38 repeat hepatectomy | Similar overall survival for TACE-RFA (38 months) compared to repeat hepatectomy (42 months); No difference in progression free survival |
| Peng et al[94] | Retrospective | Recurrent HCC ≤ 5 cmTACE-RFA or repeat hepatectomy | 107 TACE-RFA; 79 repeat hepatectomy | No difference in overall survival or disease-free survival; TACE-RFA has lower complications and shorter hospital stays |
| Ji et al[98] | Retrospective | Recurrent HCC with three or fewer tumors < 3 cm | 17 TACE-MWA; 28 TACE | TACE-MWA showed better 1-,3-, 6- month tumor response; TACE-MWA showed prolonged 1-,3-, 5-year progression free survival; No difference in overall survival |
HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; RFA: Radiofrequency ablation; DEB-TACE: Drug-eluting bead transarterial chemoembolization; MWA: Microwave ablation. TACE-RFA: Transarterial chemoembolization and radiofrequency ablation; DEB-TACE-RFA: Drug-eluting bead transarterial chemoembolization and radiofrequency ablation; TACE-MWA: Transarterial chemoembolization and Microwave ablation.
FUTURE DIRECTIONS
Immuno-locoregional combination therapy
Immunological properties associated with HCC have driven a growing use of immune checkpoint modulators such as anti-PD-1 antibodies (e.g., nivolumab, pembrolizumab, camrelizumab) or CTL-A-4 inhibitors (e.g., ipilimumab, tremelimumab)[108-111] over the last decade. Thus far, phase 2 and 3 trials have found promising tumor response rates and safety profiles compared to previous standard systemic therapies[112]. In addition to tumor necrosis, there has been some evidence that locoregional therapy can activate T-cell responses and augment the expression of multiple immune-mediated processes within the tumor microenvironment[113]. Development of treatment strategies for HCC that combine locoregional therapies and immunomodulators have thus emerged. Despite this rise in utilization, Guo et al[109] found no difference in clinical outcomes or tumor response for combined TACE and camrelizumab compared to TACE monotherapy. Studies determining the efficacy of immunotherapy combined with locoregional therapy are scarce, but multiple trials combining immunomodulators and locoregional therapies are currently underway[114]. It should be noted, adverse events with immuno-checkpoint blockers, such as hyperprogressive disease, have been reported and pose a unique challenge influencing clinical judgment to utilize these agents. Hyperprogressive disease is characterized by a rapid increase in tumor burden and subsequent clinical deterioration in patients treated with immunotherapy agents. Other immunotherapies benefits (e.g., vaccines, oncolytic viruses and adoptive cellular therapies) have also been speculated to be therapeutic but remain under clinical investigation[111].
Determining treatment algorithms for recurrent HCC
After the failure of curative-intent or tumor recurrence, the use of locoregional therapies is warranted, especially in patients no longer eligible for surgery. Ablation, however, should be considered as a comparable alternative to repeat-resection in patients with recurrent small solitary tumors, notably ≤ 3 cm. Similar to prior reviews, in patients with early recurrence (< 1 year), multifocal disease (> 2 - 3 nodules) or in the presence of microvascular invasion, TACE should be considered[33]. Moreover, due to lower toxicity and longer time-to-progression for advanced disease[62], the use of radioembolization offers a favorable alternative to TACE. Evidence supports that multimodal therapy provides superior clinical benefit to monotherapy as well as repeat-resection for smaller tumors (Table 1) for R-HCC. To date, it is unclear which additional patient populations (e.g., those not currently suitable for locoregional monotherapy) may benefit from multimodal or strategies that combine locoregional and systemic therapy (Table 2).
Table 2.
Locoregional therapy and oral agents for recurrent hepatocellular carcinoma
|
Ref.
|
Study Design
|
Treatment
|
Number of Patients
|
Outcomes
|
| Wan et al[105] | Retrospective | Recurrent HCC ≤ 5 cm | 127 TACE; 127 Sorafenib + TACE | Sorafenib + TACE increased survival time compared to TACE alone (30.7 vs 18.22 mo); Longer duration of Sorafenib when treated with Sorafenib + TACE associated with survival |
| Peng et al[106] | Retrospective | Recurrent HCC ≤ 7 or five nodules ≤ 3 cm | 106 TACE-RFA + Sorafenib; 101 Sorafenib | Longer median overall survival and time to progression for combination therapy |
| Guo et al[109] | Retrospective | Recurrent HCC | 20 TACE+ camrelizumab; 51 TACE | No difference in tumor response, progression-free survival, or overall survival |
HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; RFA: Radiofrequency ablation; TACE-RFA: Transarterial chemoembolization and radiofrequency ablatio.
CONCLUSION
Treatment strategies for R-HCC remain a challenge, and there is no consensus on how to manage patients who fail curative-intent therapies. The use of targeted locoregional therapies can improve clinical outcomes after recurrence in patients not eligible for or awaiting transplantation, or in cases of advanced disease. The emerging use of multimodal and additive systemic agents exhibit promise as a novel treatment approach in the setting of recurrence; however, prospective studies are necessary before definitive recommendations can be made.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest associated with the contributions to this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 4, 2022
First decision: November 5, 2022
Article in press: January 2, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed MOK, United Kingdom; Yan J, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
Contributor Information
Cody R Criss, Heritage College of Osteopathic Medicine, Ohio University, Athens, Ohio 45701, United States.
Mina S Makary, Department of Radiology, The Ohio State University Wexner Medical Center, Columbus, Ohio 43210, United States. mina.makary@osumc.edu.
References
- 1.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250–261. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812–820.e5. doi: 10.1053/j.gastro.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y) 2014;10:153–161. [PMC free article] [PubMed] [Google Scholar]
- 6.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 8.Makary MS, Ramsell S, Miller E, Beal EW, Dowell JD. Hepatocellular carcinoma locoregional therapies: Outcomes and future horizons. World J Gastroenterol. 2021;27:7462–7479. doi: 10.3748/wjg.v27.i43.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel) 2020;12 doi: 10.3390/cancers12071914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santopaolo F, Lenci I, Milana M, Manzia TM, Baiocchi L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J Gastroenterol. 2019;25:2591–2602. doi: 10.3748/wjg.v25.i21.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zane KE, Nagib PB, Jalil S, Mumtaz K, Makary MS. Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma. World J Hepatol. 2022;14:885–895. doi: 10.4254/wjh.v14.i5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kis B, El-Haddad G, Sheth RA, Parikh NS, Ganguli S, Shyn PB, Choi J, Brown KT. Liver-Directed Therapies for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729244. doi: 10.1177/1073274817729244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Tanaka H, Kubo S, Shuto T, Takemura S, Yamamoto T, Uenishi T, Hai S, Osugi H, Hirohashi K. Bowel injury associated with liver surgery for hepatocellular carcinoma. Hepatogastroenterology. 2006;53:571–575. [PubMed] [Google Scholar]
- 14.Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, Takemura S, Yamamoto T, Uenishi T, Kinoshita H. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484–489. doi: 10.1016/s1072-7515(02)01288-7. [DOI] [PubMed] [Google Scholar]
- 15.Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. doi: 10.1097/00000658-199108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209–217. doi: 10.1001/jamasurg.2018.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Wang K, Bao Q, Sun Y, Xing BC. Hepatic resection provided long-term survival for patients with intermediate and advanced-stage resectable hepatocellular carcinoma. World J Surg Oncol. 2016;14:62. doi: 10.1186/s12957-016-0811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–2047. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 19.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 20.Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328–332. [PubMed] [Google Scholar]
- 21.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healy MA, Choti MA. Hepatocellular Carcinoma Recurrence Risk in the Context of Emerging Therapies. Ann Surg Oncol. 2022 doi: 10.1245/s10434-022-11709-8. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, Xia Y, Li J, Wang K, Yan Z, Wu D, Zhang B, Hüser N, Shen F. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422–427. doi: 10.1111/hpb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka N, Okamoto E, Fujihara S, Kato T, Fujimoto J, Oriyama T, Mitsunobu M, Toyosaka A, Uematsu K, Yamamoto K. Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer. 1992;70:2263–2267. doi: 10.1002/1097-0142(19921101)70:9<2263::aid-cncr2820700909>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- 26.Kaibori M, Ishizaki M, Matsui K, Kwon AH. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2010;102:462–468. doi: 10.1002/jso.21631. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 28.Lauwers GY, Terris B, Balis UJ, Batts KP, Regimbeau JM, Chang Y, Graeme-Cook F, Yamabe H, Ikai I, Cleary KR, Fujita S, Flejou JF, Zukerberg LR, Nagorney DM, Belghiti J, Yamaoka Y, Vauthey JN International Cooperative Study Group on Hepatocellular Carcinoma. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol. 2002;26:25–34. doi: 10.1097/00000478-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Colecchia A, Schiumerini R, Cucchetti A, Cescon M, Taddia M, Marasco G, Festi D. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014;20:5935–5950. doi: 10.3748/wjg.v20.i20.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Guo Y, Zhong J, Wang Q, Wang X, Wei H, Li J, Xiu P. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep. 2021;11:2415. doi: 10.1038/s41598-021-82058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schraiber LDS, de Mattos AA, Zanotelli ML, Cantisani GPC, Brandão ABM, Marroni CA, Kiss G, Ernani L, Marcon PDS. Alpha-fetoprotein Level Predicts Recurrence After Transplantation in Hepatocellular Carcinoma. Medicine (Baltimore) 2016;95:e2478. doi: 10.1097/MD.0000000000002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao LQ, Chen ZL, Feng ZH, Diao YK, Li C, Sun HY, Zhong JH, Chen TH, Gu WM, Zhou YH, Zhang WG, Wang H, Zeng YY, Wu H, Wang MD, Xu XF, Pawlik TM, Lau WY, Shen F, Yang T. Clinical Features of Recurrence After Hepatic Resection for Early-Stage Hepatocellular Carcinoma and Long-Term Survival Outcomes of Patients with Recurrence: A Multi-institutional Analysis. Ann Surg Oncol. 2022 doi: 10.1245/s10434-022-11454-y. [DOI] [PubMed] [Google Scholar]
- 33.Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699–713. doi: 10.1007/s12328-021-01394-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang HL, Mo DC, Zhong JH, Ma L, Wu FX, Xiang BD, Li LQ. Systematic review of treatment strategy for recurrent hepatocellular carcinoma: Salvage liver transplantation or curative locoregional therapy. Medicine (Baltimore) 2019;98:e14498. doi: 10.1097/MD.0000000000014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng J, Cai J, Tao L, Kirih MA, Shen Z, Xu J, Liang X. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis. Int J Surg. 2020;83:196–204. doi: 10.1016/j.ijsu.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, Fan ST, Lo CM. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013;19:411–419. doi: 10.1002/lt.23605. [DOI] [PubMed] [Google Scholar]
- 37.Yong CC, Tsai MC, Lin CC, Wang CC, Lu SN, Hung CH, Hu TH, Chen CL. Comparison of Salvage Living Donor Liver Transplantation and Local Regional Therapy for Recurrent Hepatocellular Carcinoma. World J Surg. 2016;40:2472–2480. doi: 10.1007/s00268-016-3559-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J, Liu Z. Repeat hepatectomy for recurrent hepatocellular carcinoma: a local experience and a systematic review. World J Surg Oncol. 2010;8:55. doi: 10.1186/1477-7819-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagasue N, Kohno H, Hayashi T, Uchida M, Ono T, Yukaya H, Yamanoi A. Repeat hepatectomy for recurrent hepatocellular carcinoma. Br J Surg. 1996;83:127–131. doi: 10.1002/bjs.1800830142. [DOI] [PubMed] [Google Scholar]
- 40.Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Tsochatzis EA, Fatourou E, O'Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3069–3077. doi: 10.3748/wjg.v20.i12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269–280. doi: 10.1007/s00270-007-9226-z. [DOI] [PubMed] [Google Scholar]
- 43.Makary MS, Kapke J, Yildiz V, Pan X, Dowell JD. Conventional vs Drug-Eluting Bead Transarterial Chemoembolization for Neuroendocrine Tumor Liver Metastases. J Vasc Interv Radiol. 2016;27:1298–1304. doi: 10.1016/j.jvir.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425–434. doi: 10.3348/kjr.2009.10.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen ZH, Zhang XP, Zhou TF, Wang K, Wang H, Chai ZT, Shi J, Guo WX, Cheng SQ. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Eur J Surg Oncol. 2019;45:2188–2196. doi: 10.1016/j.ejso.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Liang H, Hu K, Xiong Z, Cao M, Zhong Z, Yao Z, Deng M. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: a systematic review and meta-analysis. Cancer Cell Int. 2021;21:92. doi: 10.1186/s12935-021-01790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Ke Q, Lin N, Zeng Y, Liu J. Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: a meta-analysis. Scand J Gastroenterol. 2019;54:528–537. doi: 10.1080/00365521.2019.1610794. [DOI] [PubMed] [Google Scholar]
- 48.Gao Z, Du G, Pang Y, Fu Z, Liu C, Liu Y, Zhou B, Kong D, Shi B, Jiang Z, Jin B. Adjuvant transarterial chemoembolization after radical resection contributed to the outcomes of hepatocellular carcinoma patients with high-risk factors. Medicine (Baltimore) 2017;96:e7426. doi: 10.1097/MD.0000000000007426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X, Ji Y, Lau WY, Wu M, Shen F. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41:236–242. doi: 10.1016/j.ejso.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Zu QQ, Liu S, Zhou CG, Yang ZQ, Xia JG, Zhao LB, Shi HB. Chemoembolization of recurrent hepatoma after curative resection: prognostic factors. AJR Am J Roentgenol. 2015;204:1322–1328. doi: 10.2214/AJR.14.13343. [DOI] [PubMed] [Google Scholar]
- 51.Shim JH, Kim KM, Lee YJ, Ko GY, Yoon HK, Sung KB, Park KM, Lee SG, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2010;17:869–877. doi: 10.1245/s10434-009-0788-7. [DOI] [PubMed] [Google Scholar]
- 52.Chen W, Ma T, Zhang J, Zhang X, Chen W, Shen Y, Bai X, Liang T. A systematic review and meta-analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB (Oxford) 2020;22:795–808. doi: 10.1016/j.hpb.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Ren Y, Ge S, Xiong B, Zhou G, Feng G, Song S, Zheng C. Transarterial Chemoembolization in Treatment-Naïve and Recurrent Hepatocellular Carcinoma: A Propensity-Matched Outcome and Risk Signature Analysis. Front Oncol. 2021;11:662408. doi: 10.3389/fonc.2021.662408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, Sodergren MH, Jiao LR. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–1442. doi: 10.1002/bjs.10597. [DOI] [PubMed] [Google Scholar]
- 55.Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, Nemcek AA Jr, Omary RA, Madoff DC, Murthy R. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12–29. doi: 10.1053/j.tvir.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Mosconi C, Cappelli A, Pettinato C, Golfieri R. Radioembolization with Yttrium-90 microspheres in hepatocellular carcinoma: Role and perspectives. World J Hepatol. 2015;7:738–752. doi: 10.4254/wjh.v7.i5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed A, Stauffer JA, LeGout JD, Burns J, Croome K, Paz-Fumagalli R, Frey G, Toskich B. The use of neoadjuvant lobar radioembolization prior to major hepatic resection for malignancy results in a low rate of post hepatectomy liver failure. J Gastrointest Oncol. 2021;12:751–761. doi: 10.21037/jgo-20-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, Kulik L, Ganger D, Desai K, Thornburg B, Mouli S, Hickey R, Caicedo JC, Abecassis M, Riaz A, Salem R. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287:1050–1058. doi: 10.1148/radiol.2018171768. [DOI] [PubMed] [Google Scholar]
- 59.Tohme S, Bou Samra P, Kaltenmeier C, Chidi AP, Varley PR, Tsung A. Radioembolization for Hepatocellular Carcinoma: A Nationwide 10-Year Experience. J Vasc Interv Radiol. 2018;29:912–919.e2. doi: 10.1016/j.jvir.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV, Larusso NF, Alberts SR, Gores GJ, Fleming CJ, Slettedahl SW, Harmsen WS, Therneau TM, Wiseman GA, Andrews JC, Roberts LR. Efficacy and safety of transarterial radioembolization vs chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714–723. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goin JE, Roberts CA, Dancey JE, Sickles CJ, Leung DA, Soulen MC. Comparison of post-embolization syndrome in the treatment of patients with unresectable hepatocellular carcinoma: Trans-catheter arterial chemo-embolization vs Yttrium-90 glass microspheres. World J Nucl Med. 2004;3:49–56. [Google Scholar]
- 62.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, Yaghmai V, Ibrahim SM, Senthilnathan S, Baker T, Gates VL, Atassi B, Newman S, Memon K, Chen R, Vogelzang RL, Nemcek AA, Resnick SA, Chrisman HB, Carr J, Omary RA, Abecassis M, Benson AB 3rd, Mulcahy MF. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zane KE, Makary MS. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13215430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guiu B, Garin E, Allimant C, Edeline J, Salem R. TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc Intervent Radiol. 2022;45:1599–1607. doi: 10.1007/s00270-022-03072-8. [DOI] [PubMed] [Google Scholar]
- 65.Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021;74:2342–2352. doi: 10.1002/hep.31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, Sharma R, ElTawil R, Kwon D, Venkat S, Portelance L, Yechieli R. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol. 2016;39:1580–1588. doi: 10.1007/s00270-016-1426-y. [DOI] [PubMed] [Google Scholar]
- 67.Sangro B, Maini CL, Ettorre GM, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Sciuto R, Pizzi G, Lastoria S European Network on Radioembolization with Yttrium-90 resin microspheres (ENRY) Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721–1730. doi: 10.1007/s00259-018-3968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali R, Riaz A, Gabr A, Abouchaleh N, Mora R, Al Asadi A, Caicedo JC, Abecassis M, Katariya N, Maddur H, Kulik L, Lewandowski RJ, Salem R. Clinical outcomes of Y90 radioembolization for recurrent hepatocellular carcinoma following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:2195–2202. doi: 10.1007/s00259-017-3792-3. [DOI] [PubMed] [Google Scholar]
- 69.Rahman SI, Nunez-Herrero L, Berkes JL. Position 2: Transarterial Radioembolization Should Be the Primary Locoregional Therapy for Unresectable Hepatocellular Carcinoma. Clin Liver Dis (Hoboken) 2020;15:74–76. doi: 10.1002/cld.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J Radiol. 2010;2:417–424. doi: 10.4329/wjr.v2.i11.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pillai K, Akhter J, Chua TC, Shehata M, Alzahrani N, Al-Alem I, Morris DL. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015;94:e580. doi: 10.1097/MD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990–e1005. doi: 10.1634/theoncologist.2018-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ward RC, Healey TT, Dupuy DE. Microwave ablation devices for interventional oncology. Expert Rev Med Devices. 2013;10:225–238. doi: 10.1586/erd.12.77. [DOI] [PubMed] [Google Scholar]
- 74.Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation vs radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325. doi: 10.1016/S2468-1253(18)30029-3. [DOI] [PubMed] [Google Scholar]
- 75.Galanakis N, Kehagias E, Matthaiou N, Samonakis D, Tsetis D. Transcatheter arterial chemoembolization combined with radiofrequency or microwave ablation for hepatocellular carcinoma: a review. Hepat Oncol. 2018;5:HEP07. doi: 10.2217/hep-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, Zhang ZY, Wang S, Chen MH. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J Gastroenterol. 2015;21:1554–1566. doi: 10.3748/wjg.v21.i5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lei JY, Wang WT, Yan LN, Wen TF, Li B. Radiofrequency ablation vs surgical resection for small unifocal hepatocellular carcinomas. Medicine (Baltimore) 2014;93:e271. doi: 10.1097/MD.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z, Wang J, Lin Y. Comparison of the efficacy and safety of repeated hepatectomy and radiofrequency ablation in the treatment of primary recurrent liver cancer: a meta-analysis. World J Surg Oncol. 2022;20:182. doi: 10.1186/s12957-022-02649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan BH, Zhu YK, Zou XM, Zhou HD, Li RH, Zhong JH. Repeat hepatic resection vs percutaneous ablation for the treatment of recurrent hepatocellular carcinoma: meta-analysis. BJS Open. 2022;6 doi: 10.1093/bjsopen/zrac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000;1:175–184. doi: 10.3348/kjr.2000.1.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun WC, Chen IS, Liang HL, Tsai CC, Chen YC, Wang BW, Lin HS, Chan HH, Hsu PI, Tsai WL, Cheng JS. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8:104571–104581. doi: 10.18632/oncotarget.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang D, Zhuang B, Wang Y, Xie X. Radiofrequency ablation vs hepatic resection for recurrent hepatocellular carcinoma: an updated meta-analysis. BMC Gastroenterol. 2020;20:402. doi: 10.1186/s12876-020-01544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shampain KL, Hackett CE, Towfighi S, Aslam A, Masch WR, Harris AC, Chang SD, Khanna K, Mendiratta V, Gabr AM, Owen D, Mendiratta-Lala M. SBRT for HCC: Overview of technique and treatment response assessment. Abdom Radiol (NY) 2021;46:3615–3624. doi: 10.1007/s00261-021-03107-7. [DOI] [PubMed] [Google Scholar]
- 85.Kimura T, Doi Y, Takahashi S, Kubo K, Imano N, Takeuchi Y, Takahashi I, Nishibuchi I, Murakami Y, Kenjo M, Nagata Y. An overview of stereotactic body radiation therapy for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2020;14:271–279. doi: 10.1080/17474124.2020.1744434. [DOI] [PubMed] [Google Scholar]
- 86.Kimura T, Takeda A, Tsurugai Y, Kawano R, Doi Y, Oku Y, Hioki K, Miura H, Nagata Y. A Multi-Institutional Retrospective Study of Repeated Stereotactic Body Radiation Therapy for Intrahepatic Recurrent Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2020;108:1265–1275. doi: 10.1016/j.ijrobp.2020.07.034. [DOI] [PubMed] [Google Scholar]
- 87.Lee S, Jung J, Park JH, Kim SY, Choi J, Lee D, Shim JH, Kim KM, Lim YS, Lee HC, Park HH, Kim JH, Yoon SM. Stereotactic body radiation therapy as a salvage treatment for single viable hepatocellular carcinoma at the site of incomplete transarterial chemoembolization: a retrospective analysis of 302 patients. BMC Cancer. 2022;22:175. doi: 10.1186/s12885-022-09263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Chang ZF, Wang Y, Yan JY, Li K. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. World J Gastroenterol. 2013;19:4192–4199. doi: 10.3748/wjg.v19.i26.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan JY, Zhang JL, Wang MQ, Yuan K, Bai YH, Wang Y, Xin HN, Wang ZJ, Liu FY, Duan F, Fu JX. Combined transcatheter arterial chemoembolization and radiofrequency ablation in single-session for solitary hepatocellular carcinoma larger than 7 cm. Asia Pac J Clin Oncol. 2018;14:300–309. doi: 10.1111/ajco.12817. [DOI] [PubMed] [Google Scholar]
- 90.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 91.Zhang YJ, Chen MS, Chen Y, Lau WY, Peng Z. Long-term Outcomes of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation as an Initial Treatment for Early-Stage Hepatocellular Carcinoma. JAMA Netw Open. 2021;4:e2126992. doi: 10.1001/jamanetworkopen.2021.26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation vs RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 93.Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 94.Peng Z, Wei M, Chen S, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Kuang M. Combined transcatheter arterial chemoembolization and radiofrequency ablation vs hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol. 2018;28:3522–3531. doi: 10.1007/s00330-017-5166-4. [DOI] [PubMed] [Google Scholar]
- 95.Song Q, Ren W, Fan L, Zhao M, Mao L, Jiang S, Zhao C, Cui Y. Long-Term Outcomes of Transarterial Chemoembolization Combined with Radiofrequency Ablation Versus Transarterial Chemoembolization Alone for Recurrent Hepatocellular Carcinoma After Surgical Resection. Dig Dis Sci. 2020;65:1266–1275. doi: 10.1007/s10620-019-05733-0. [DOI] [PubMed] [Google Scholar]
- 96.Zheng X, Ren Y, Hu H, Qian K. Transarterial Chemoembolization Combined With Radiofrequency Ablation Versus Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma After Curative Resection: A 10-Year Single-Center Comparative Study. Front Oncol. 2021;11:713432. doi: 10.3389/fonc.2021.713432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang W, Chen MH, Wang MQ, Cui M, Gao W, Wu W, Wu JY, Dai Y, Yan K. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res. 2009;39:231–240. doi: 10.1111/j.1872-034X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 98.Ji J, Yang W, Shi HB, Liu S, Zhou WZ. Transcatheter arterial chemoembolization alone vs combined with microwave ablation for recurrent small hepatocellular carcinoma after resection: a retrospective comparative study. BMC Gastroenterol. 2022;22:321. doi: 10.1186/s12876-022-02387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sparchez Z, Radu P, Bartos A, Nenu I, Craciun R, Mocan T, Horhat A, Spârchez M, Dufour JF. Combined treatments in hepatocellular carcinoma: Time to put them in the guidelines? World J Gastrointest Oncol. 2021;13:1896–1918. doi: 10.4251/wjgo.v13.i12.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 101.Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP, Cheow PC, Chotipanich C, Lim K, Lesmana LA, Manuaba TW, Yoong BK, Raj A, Law CS, Cua IHY, Lobo RR, Teh CSC, Kim YH, Jong YW, Han HS, Bae SH, Yoon HK, Lee RC, Hung CF, Peng CY, Liang PC, Bartlett A, Kok KYY, Thng CH, Low AS, Goh ASW, Tay KH, Lo RHG, Goh BKP, Ng DCE, Lekurwale G, Liew WM, Gebski V, Mak KSW, Soo KC Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36:1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 102.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 103.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 104.Fan G, Wei X, Xu X. Is the era of sorafenib over? Ther Adv Med Oncol. 2020;12:1758835920927602. doi: 10.1177/1758835920927602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wan X, Zhai X, Yan Z, Yang P, Li J, Wu D, Wang K, Xia Y, Shen F. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget. 2016;7:83806–83816. doi: 10.18632/oncotarget.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Chen M, Qian G, Kuang M. Advanced Recurrent Hepatocellular Carcinoma: Treatment with Sorafenib Alone or in Combination with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;287:705–714. doi: 10.1148/radiol.2018171541. [DOI] [PubMed] [Google Scholar]
- 107.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo Y, Ren Y, Chen L, Sun T, Zhang W, Sun B, Zhu L, Xiong F, Zheng C. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer. 2022;22:270. doi: 10.1186/s12885-022-09325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X, Wang Y, Ye X, Liang P. Locoregional Combined With Systemic Therapies for Advanced Hepatocellular Carcinoma: An Inevitable Trend of Rapid Development. Front Mol Biosci. 2021;8:635243. doi: 10.3389/fmolb.2021.635243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng H, Sun G, Chen H, Li Y, Han Z, Zhang P, Yang L. Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res. 2019;9:1536–1545. [PMC free article] [PubMed] [Google Scholar]
- 112.van Doorn DJ, Takkenberg RB, Klümpen HJ. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: An Overview. Pharmaceuticals (Basel) 2020;14 doi: 10.3390/ph14010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh P, Toom S, Avula A, Kumar V, Rahma OE. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2020;7:11–17. doi: 10.2147/JHC.S187121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999–1007. doi: 10.1016/j.jhep.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y, Zhang MW, Fan XX, Mao DF, Ding QH, Zhuang LH, Lv SY. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg. 2020;12:355–368. doi: 10.4240/wjgs.v12.i8.355. [DOI] [PMC free article] [PubMed] [Google Scholar]