Abstract
People across the world are affected by the "coronavirus disease 2019 (COVID-19)", brought on by the "SARS-CoV type-2 coronavirus". Due to its high incidence in individuals with diabetes, metabolic syndrome, and metabolic-associated fatty liver disease (MAFLD), COVID-19 has gained much attention. The metabolic syndrome's hepatic manifestation, MAFLD, carries a significant risk of type-2-diabetes. The link between the above two conditions has also drawn increasing consideration since MAFLD is intricately linked to the obesity epidemic. Independent of the metabolic syndrome, MAFLD may impact the severity of the viral infections, including COVID-19 or may even be a risk factor. An important question is whether the present COVID-19 pandemic has been fueled by the obesity and MAFLD epidemics. Many liver markers are seen elevated in COVID-19. MAFLD patients with associated comorbid conditions like obesity, cardiovascular disease, renal disease, malignancy, hypertension, and old age are prone to develop severe disease. There is an urgent need for more studies to determine the link between the two conditions and whether it might account for racial differences in the mortality and morbidity rates linked to COVID-19. The role of innate and adaptive immunity alterations in MAFLD patients may influence the severity of COVID-19. This review investigates the implications of COVID-19 on liver injury and disease severity and vice-versa. We also addressed the severity of COVID-19 in patients with prior MAFLD and its potential implications and therapeutic administration in the clinical setting.
Keywords: Metabolic-associated fatty liver disease, COVID-19, Metabolic syndrome, Non-alcoholic steatohepatitis, Angiotensin converting enzyme 2
Core Tip: The severity of coronavirus disease 2019 (COVID-19) symptoms and sequelae positively correlate with high rates of hepatic decompensation and elevated transaminases in patients with chronic liver disease and cirrhosis. Implicated mechanisms linking cirrhosis with severe COVID-19 symptoms include cirrhosis-related immune dysregulation, systemic inflammation, coagulopathy, and metabolic derangements. Metabolic-associated fatty liver disease (MAFLD) is characterized as the hepatic manifestation of the metabolic syndrome and therefore is highly associated with other comorbidities such as obesity, diabetes, and hyperlipidemia. Those comorbidities are also risk factors for severe COVID-19. The hepatic distribution of the angiotensin-converting enzyme 2 receptor, the main viral entry receptor for SARS-CoV-2, may determine the severity of hepatic involvement. In addition, moderate hepatic dysfunction could alter the severity of COVID-19, as well as the safety profile, and the therapeutic efficacy of antiviral drugs metabolized in the liver. Therefore, it is of high clinical priority to enhance our understanding of COVID-19 infection-associated liver injury in MAFLD patients to treat both of these conditions effectively.
INTRODUCTION
A substantial hazard to public health has suddenly emerged from the "severe acute respiratory syndrome (SARS)" global pandemic caused by the coronavirus SARS-CoV-2 “[coronavirus disease 2019 (COVID-19), Sarbecovirus subgenus, Betacoronavirus genus, Coronaviridae family]"[1-5]. Up until the September 2, 2022, the total infected cases were 607013841; total deaths were 6508326 and total vaccinated were 12185442365[2,6]. The most common and important clinical manifestation of COVID-19, alternating from moderate respiratory symptoms to severe pneumonia, is respiratory involvement, even though many people still show no symptoms. The severe Corona virus infection, however, is a systemic illness that can cause myocardial injury, heart failure, vascular inflammation, myocarditis, cardiac arrhythmias, hypoxic encephalopathy, multi-organ failure, and eventually death[1-5,7-11].
Though severe liver damage is rare, the liver remains a potential target for Coronavirus. This infection poses a novel challenge for hepatologists because it may harm the liver by direct (viral translocation from the gastrointestinal tract to the liver) or indirect pathways (systemic inflammation, hepatic ischemia and hypoxia, effects on pre-existing liver illnesses, and drug-related liver injury)[12-17].
Notably, nonalcoholic fatty liver disease (NAFLD), a chronic dysmetabolic pandemic with a prevalence rate of > 30% in the global population, has become the most widespread liver disease in the world. Furthermore, NAFLD is a "fellow traveler" with a number of risk factors, metabolic syndrome, and diseases rather than a stand-alone disorder. Along with this viewpoint, the term "metabolic-associated fatty liver disease" (also known as "MAFLD") has recently given the acronym NAFLD a second look[18,19]. Therefore, NAFLD/MAFLD may impact how COVID-19-infected “patients” fare. Additionally, in situations of chronic injury, the liver itself is more vulnerable to medicines.
In this setting, individuals with NAFLD/MAFLD and COVID-19 infections exhibit inflammatory response pathways, particularly those involving cytokines that may aggravate the clinical result by causing an increase in liver inflammation or by serving as a marker of metabolic risk factors. A precise understanding of the behavior of the virus and the risk factors contributing to the initiation and progression of COVID-19 will be crucial in the near future to predict virus-related events around the globe due to the pandemic characteristics and high mortality rate of SARS-CoV-2 infection.
According to Wang et al[20], analysis of COVID-19-infected patients revealed independent risk factors for hypertension, diabetes, chronic obstructive pulmonary disease, cardiovascular disease, and cerebrovascular disease [odds ratio (OR): 2.29-5.97]. A recent study on COVID-19-infected individuals who were hospitalized in New York reported that 48.7% of the patients had a BMI > 40 kg/m2, suggesting that BMI is one of the strongest predictors of hospitalization (OR: 6.2), only being surpassed by ages ≥ 75 years (OR: 66.8) and age 65-74 years (OR: 10.9)[21]. Finally, MAFLD was found to be independently linked with COVID-19 progression in a study of 202 consecutive individuals with confirmed COVID-19[22]. Acute COVID-19 epidemic and chronic MAFLD, which is a member of a larger group of metabolic illnesses, are the two pandemic conditions that are the subject of this article's discussion. The underlying MAFLD may contribute to more severe hepatic and metabolic consequences during COVID-19 infection and may develop into another prognostic indicator of the viral illness[21-26].
COVID-19 AND MAFLD
In contrast to the hepatocytes which are the predominant liver cells and of which only 3% express angiotensin I converting enzyme 2 (ACE2) receptors, about 60% of the cholangiocytes expresses ACE2 receptors even though they occupy only 3% to 5% of the liver cell population[27]. Acute liver injury was common in 15.4% of Chinese patients with COVID-19 illness[1]. However, it has been noted that the liver is involved in roughly 60% of cases, and the likelihood of liver malfunction appears to rise with age. A report by Ji et al[22] on 202 COVID-19-positives showed that 50% of the patients had some form of liver abnormalities upon admission, and 75% of patients developed liver dysfunction during the course of their stay in the hospital. Most of the liver injury was mild, and only 3% of the patients had ductular or mixed patterns of liver abnormalities. Male gender, older age > 60 years, a high BMI, underlying comorbidities, and MAFLD were all linked to COVID-19 development[28]. MAFLD was identified as having an OR of 6.4 with a 95% confidence interval (CI) of 1.5 to 31.2 in this study by multivariate logistic regression analysis[29]. However, this survey has limitations due to the small number of cases that were available, various severity criteria, underlying comorbidities, and unclear liver disorders[22,30-32]. In contrast, the presence of intermediate or high fibrosis-4 (FIB-4) scores significantly and independently enhanced the probability of severe COVID-19 illness in a sample of 310 individuals with COVID-19 and MAFLD[33]. Due to their increased metabolic risk, patients with MAFLD exhibit a distinct risk[23-26,34].
ACE2 receptors are required for the spike viral proteins to attach to the target cells in order for the COVID-19 disease to progress to its first stage[12]. These receptors are mainly expressed on alveolar epithelial cells (type II) and ciliated cells in the human lung, as well as on the epithelia of the upper respiratory tract (nasopharynx), which is a key site of replication[12]. The vascular endothelium, the brush border of intestinal enterocytes, and cholangiocytes express the ACE2 receptor[14]. Therefore, COVID-19 may cause symptoms to appear in the gastrointestinal tract[15].
According to a recent United States survey, 61% of people who tested positive for COVID-19 had clinically obvious gastrointestinal symptoms[16]. Because ACE2 receptors are found in the glandular cells of the stomach, duodenum, and distal enterocytes, their presence may cause malabsorption, imbalanced intestinal secretion, and enteric nervous system activation, all of which can result in gastrointestinal symptoms[12].
COVID-19 AND LIVER BIOCHEMISTRY PATTERNS AND FREQUENCY
Although the specific impact of COVID-19 on the liver is yet unknown, patients with COVID-19 frequently experience liver biochemistry abnormalities, affecting 15%-65% of SARS-CoV-2-infected people[35-42]. The large variation in these reported frequencies may be due to various interpretations of what constitutes the upper limit of normal, variable lab results regarded as liver enzymes and regional variations in the prevalence and nature of the underlying chronic liver disease (CLD). An estimated 29%-39% and 38%-63% of patients, respectively, have been reported to have mild (1-2 times the upper limit of normal) elevations of their serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, which characterize liver biochemistry abnormalities in COVID-19[36,38,43]. Although severe liver damage, increased blood bilirubin levels, and hepatic synthetic malfunction are all uncommon in SARS-CoV-2 patients, hypoalbuminemia, a non-specific index of illness severity, has been linked to worse COVID-19 outcomes. Non-specific findings from liver biopsies in SARS-CoV-2 patients have included steatosis, moderate lobular and/or portal inflammation, and vascular pathology[10,44,45].
The majority of the time, abnormal biochemistries are likely multifactorial, with direct infection of hepatocytes as well as immune-mediated inflammatory response, drug-induced liver injury, hepatic congestion, and extrahepatic release of transaminases all having a potential role. Elevations in serum AST levels among COVID-19 hospitalized patients positively correlate with levels of ALT but not with markers of systemic inflammation [such as C-reactive protein (CRP) and ferritin] or muscle breakdown (such as creatinine kinase)[21]. Despite the rarity of reports of rhabdomyolysis (muscle breakdown) related to COVID-19[46], these findings suggest that high liver enzymes in COVID-19 are the result of direct hepatic injury. Finally, during COVID-19, AST is frequently higher than ALT, which is unusual for a classic hepatocellular pattern of liver injury outside of specific situations like alcohol-related liver disease, some drug-induced liver injuries (like those caused by lamotrigine), ischemic hepatitis, and cirrhosis. The causes of an AST-predominant aminotransferase increase are not fully understood, although they may include mitochondrial failure linked to COVID-19, hepatic steatosis brought on by SARS-CoV-2, and altered hepatic perfusion brought on by microthrombotic disease[34,47,48].
EFFECTS OF COVID-19 ON MAFLD DISEASE PROGRESSION
Comorbidities associated with MAFLD
MAFLD is a serious public health issue and a leading cause of CLD globally. MAFLD has been identified as a hepatic manifestation of an insulin resistance-related metabolic syndrome. A growing body of research evidence suggests that systemic disorders like type 2 diabetes, obesity, metabolic syndrome, chronic kidney disease, and cardiovascular disease are all linked to MAFLD. The primary cause of death in MAFLD patients is cardiovascular disease. Rather than just being steatosis, these findings are intricately linked to nonalcoholic steatohepatitis (NASH). MAFLD should be seen as an early mediator of systemic disease in addition to being a liver-specific condition. In relation to other medical illnesses, the pathophysiology, and underlying processes of MAFLD are still poorly understood. Future therapeutic approaches for MAFLD require more research[49]. The various risk factors associated with severe COVID-19 in patients with MAFLD are enumerated in Table 1.
Table 1.
risk factors associated with severe coronavirus disease 2019 in metabolic-associated fatty liver disease patients
|
Common risk factors for severe COVID-19 infections
| |
| Obesity | High serum IL-6 at admission |
| Advanced age > 65 yr | Male gender |
| Black race | High ferritin level at admission |
| Liver fibrosis | High EWS at admission |
| Dyslipidemia | Type 2 diabetes mellitus |
EWS: Early warning score; IL-6: Interleukin-6; COVID-19: Coronavirus disease 2019.
Systemic inflammation and hypoxia
Patients with COVID-19 infection who have chronic liver disorders may express more ACE2 receptors and hypoxia-inducible factors (HIFs), a class of transcription factors triggered by hypoxia[50]. The progression of metabolic illnesses like MAFLD may be accelerated by such changes[31,35-37,39,51-55]. Clinically, biliary ductal abnormalities are uncommon in COVID-19-infected patients; as a result, the ACE2-mediated liver injury may primarily result from the localization of these receptors in endothelial cells. Additionally, the progression of MAFLD involves increased production of reactive oxygen species and nitric oxide derivatives, inflammatory pathways that result in cellular communication with Kupffer cells, and upregulation of HIF through suppression of fatty acid oxidation. This theory is somewhat corroborated by liver histology from patients who died from severe COVID-19, which showed minor lobular and portal activity as well as moderate microvesicular steatosis, presumably as a direct result of SARS-CoV-2 infection or drug-induced liver injury[51,55,56].
Altered liver response
Lipids, which are part of the cell membrane, exosomes, and energy storage components, are strongly associated with the viral life cycle. Infected cells typically have changes in their metabolism of circulating lipids[41,43]. In order to facilitate their replication, viruses alter lipid metabolism, including the expression and activity of crucial enzymes involved in lipid biosynthesis. Changes in lipid metabolism may also be linked to the host's reaction to an infection. SARS-CoV-2 is not an exception and causes significant modifications in lipid metabolism after infection[42,43,57]. SARS-CoV-2 infection specifically causes a general down-regulation of approximately 100 serum lipids, including fatty acids, sphingolipids, and glycerophospholipids. Lipids are not only altered in COVID-19, but they are also linked to pathophysiology and the development of the illness. Changes in bilirubin and bile acids provide evidence that the observed down-regulation of lipids during SARS-CoV-2 infection is related to liver damage. Many of the COVID-19 lipid and lipoprotein changes that have been reported are connected to hepatic activities. The investigation of plasma lipidomic analysis was conducted during COVID-19. Sphingomyelin and monosialodihexosyl ganglioside levels were upregulated, and diacylglycerol levels were downregulated, accounting for the majority of the significantly altered lipids. The severity of the condition was positively linked with higher monosialodihexosyl ganglioside levels. Again, disruption of the normal circulating lipid profiles may be caused by inflammation and infection. Unsaturated fatty acids may be released as a defense mechanism in response to a cytokine storm. When COVID-19 illness is present, proinflammatory lipids and lipid mediators may modify the immunological response[43,57,58]. In addition to the lipid metabolism, liver detoxification and protein synthesis are significantly impaired in the COVID-19 patient. In an autopsy study, looking at the transcriptome of the severe COVID-19 patient with non-covid patient, the cytochrome P450 gene - ACAD11, CIDEB, GNMT and GPAM were significantly down regulated[59]. This consequently affects the detoxification of drugs and metabolites through the CYP 450 system. The liver is the powerhouse of protein synthesis. It does not only synthesize the anabolic proteins but it also synthesizes proteins involved in both innate and acquired immune responses. This is very much reduced in the MAFLD patient who are in a state of immune dysregulation but the exact role of each component of hepatic immune dysregulation to COVID-19 severity is difficult to delineate[3].
Liver steatotic state/ lipid derangement
The development of steatosis and liver fibrosis in MAFLD patients is facilitated by active innate immunity in the infectious state, which not only directly causes and intensifies liver inflammation but also interferes with the control of lipid metabolism. In COVID-19 patients, proteomic and metabolomic analysis identified dyslipidemia, including lipid build-up and downregulation of apolipoproteins[57]. In turn, it was discovered that SARS-CoV-2 infection can alter lipid synthesis and absorption pathways, increasing the accumulation of lipid droplets (LD) in human cells[43]. SARS-CoV-2 can also highjack LDs to increase its ability to replicate. Recent research has shown that ACE2 is crucial for maintaining metabolic homeostasis from a mechanistic perspective. A SARS-CoV-2 infection reduces ACE2 expression, which leads to aberrant metabolic processes. Patients with COVID-19 may experience MAFLD development as a result of the metabolic imbalance brought on by ACE2 deficiency[27,42,43,57,58,60].
ELEVATED FIB-4 AND POOR COVID-19 OUTCOMES
A straightforward, thoroughly tested point-of-care measure called the FIB-4 index is used to categorize individuals with suspected MAFLD according to their likelihood of developing liver fibrosis. It uses a combination of patient's age, ALT, AST, and platelet count, all of which may be quickly determined by front-line healthcare professionals[33]. FIB-4 is helpful in identifying liver disease patients who are more likely to experience a negative clinical outcome connected to the liver. FIB-4 has also been demonstrated to predict non-liver clinical outcomes in MAFLD patients, such as cardiovascular mortality or risk of atrial fibrillation. Similarly, FIB-4 has been used to predict mortality in the general population as well as clinical outcomes in clinical situations unrelated to the liver. In the study of Ibáñez-Samaniego et al[33], increased FIB-4 Levels were linked to a poor outcome in COVID 19 patients.
The chance of developing an enhanced inflammatory response, a feature of severe COVID-19, may be increased by advanced hepatic fibrosis. Advanced liver disease is actually characterized by a persistent stimulation of immune cells by pathogen-associated molecular patterns and damage-associated molecular patterns[58]. This stimulation causes immune cells to become activated and increases the production of cytokines, chemokines, and growth factors. These growth factors are then released to attract and activate additional inflammatory cells, maintaining a state of chronic low-grade systemic inflammation[33]. Patients with obesity and insulin resistance have been noted to experience a similar level of low-grade inflammation. In fact, the degree of obesity and the likelihood of developing type-2 diabetes mellitus have been linked to increased serum levels of Interleukin-6 (IL-6)[32]. Activated macrophages release IL-6 during an acute infection, which is a significant inducer of the creation of acute phase reactant proteins in hepatocytes (CRP, ferritin, complement, clotting factors). The hepatocytes' acute phase proteins have a direct effector role on innate immunity, facilitating pathogen clearance[21,33,46,61-65].
Increased hepatic decompensation rates in cirrhotic patients
Data on decompensated cirrhosis and COVID-19 is limited. In the first 152 cases of clinically and laboratory-confirmed COVID-19 infections with CLD in two international reporting registries (n = 103 with cirrhosis and n = 40 with chronic liver disease) (COVID-Hep.net and COVIDCirrhosis.org)[66], the probability of death after hepatic decompensation during COVID-19 was significantly higher in those with new decompensation: 63.2% died compared to 26.2% in those without new decompensation. Notably, 24.3% of people with new hepatic decompensation at the time of diagnosis had no pulmonary symptoms of COVID-19. Therefore, decompensated liver disease is a significant risk factor for mortality in COVID-19 patients. As a result, all patients with decompensated liver disease should be hospitalized, and any recent decompensation in a cirrhotic patient should be tested for COVID-19 at this time[13,66]. In a metanalysis of observational studies of COVID-19 infection with cirrhosis, the patient with cirrhosis not only had higher rate of decompensation but the odds for mortality has been 2.48 (CI 2.2-3.04) when compared to the non-cirrhotic patients[67].
EFFECTS OF PRE-EXISTING MAFLD ON COVID-19 DISEASE SEVERITY
Coagulopathy
Proinflammatory cells may produce cytokines, which can increase the synthesis of procoagulant molecules like tissue factor and von Willebrand factor. This can result in a hypercoagulable condition, which can lead to widespread micro-/macrovascular thrombosis[63]. In addition to elevated levels of tissue factor and von Willebrand factor in the bloodstream, MAFLD patients also have increased levels of platelet activation and plasminogen activator inhibitor type 1 concentration. Patients with COVID-19 who have MAFLD have greater levels of circulating D-dimer than patients without MAFLD, indicating that the pro-coagulant condition associated with MAFLD may be a factor in the severity of COVID-19[65]. According to findings from a retrospective investigation on a group of COVID-19 patients, people who presented with deep vein thrombosis, confirmed by Doppler ultrasound, had a greater prevalence of MAFLD[46,62]. In addition, COVID-19 patients with MAFLD had a higher mean admission and peak serum D-dimer concentrations than those without MAFLD[46]. In MAFLD individuals, COVID-19 may potentially further boost the production of proinflammatory cytokines, resulting in the activation of the coagulation cascade and thrombosis. In fact, a pathologic analysis of the pulmonary arteries in COVID-19 patients revealed extensive thrombosis with microangiopathy in addition to hepatic steatosis affecting 50%-60% of the liver parenchyma[68]. Hepatic steatosis and pulmonary thrombi were discovered in 55% and 73%, respectively, of COVID-19 patients, according to an Italian post-mortem examination, which corroborated this report[32]. These findings strongly imply a connection between these disorders, with the proinflammatory hypercoagulable state acting as a common pathogenic pathway to severe COVID-19, which promotes thrombosis and the spread of the disease[62-64].
Cytokine production
Prolonged and significant lymphopenia, an abnormal inflammatory response related to aberrant and uncontrolled cytokine activation, and lung mononuclear cell infiltration are all associated with COVID-19[52]. The prognosis of an illness depends on the degree of involvement of additional organs. In fact, observational studies showed that increased levels of inflammatory markers in the blood (CRP, ferritin, and D-dimer), a higher neutrophil-to-lymphocyte ratio, as well as elevated levels of inflammatory cytokines and chemokines were linked to the disease severity and a poor prognosis[21]. One element of liver damage in COVID-19 may be dysregulation of the innate immune response. Inflammatory indicators, such as abnormally high levels of CRP, lymphocytes, neutrophils, and cytokines, are usual in COVID-19 patients[3]. Due to the loss of control over cytokine regulation, pulmonary and extrapulmonary damage occurs. During the early stages, this control could help to slow the evolution of the disease[62]. Hypercytokinemia, that is deadly or fulminant, may set off a series of events that damage or fail many organs, including the liver. Jaundice, hepatic encephalopathy, hepatomegaly, and increased blood transaminase levels could be brought on by the inflammatory response[62]. Since COVID-19 is associated with cytokine storm there is overlap of cytokines involved in both the disorders, however, it will be difficult to point out that these are sole causative agents for hepatic decompensation in MAFLD as there are more factors in play than the cytokines alone. It is also interesting to note that MAFLD patients had a distinct cytokine profile with higher concentrations of IL-6, IL-8, IL-10, and IFN-β when compared to the patients without MAFLD[69]. Higher levels of IL-8 and IL-10 are associated with the worst prognosis and delayed time to recovery[69].
Obesity
Apart from diabetes, the presence of an "overfat" condition (excess body fat that harms health) has become a global pandemic and can occur in obese, overweight, and even normal weight subjects with excess fat involving the liver in the form of steatosis[70]. Several abnormalities can cluster together with overfat, including obesity, overweight, chronic "metabolic" inflammation, and insulin resistance, ultimately configuring the metabolic syndrome[18,20,30,32,44,45]. As seen by the higher prevalence of both autoimmune and immunological illnesses, excess body fat may impede the immunity[41]. Adiposity underlies a compromised immune response (mostly mediated by T cells and macrophages) that increases the risk of infections and chronic respiratory illnesses. Notably, being overweight appears to increase the risk of contracting contagious viral infections[47,48,71,72]. In particular, being overfat may have a negative impact on the immune system performance and host defense mechanism, while being overfat causes hosts to respond improperly to viral and bacterial attacks[48].
The association between obesity and COVID-19 severity persisted after adjusting for age, sex, smoking habits, diabetes, hypertension, and dyslipidemia. In MAFLD patients, obesity was associated with a 6-fold increased risk of severe infection[72]. Patients with MAFLD, especially those who are obese, have been found to have higher levels of IL-6, which has been linked to an aggravation of the COVID-19 infection[72-74].
Pre-existing CLD
Investigations on COVID-19 rarely include patients with pre-existing liver illness, and these patients' features according to their Child's status or model for end-stage liver disease (MELD) score have not been independently assessed in these studies. The innate immune response against the virus likely caused important changes in the liver enzymes and coagulation profile in 63 patients with severe COVID-19 disease, and CLD was not proven to affect the severity of COVID-19[3]. Cirrhosis patients are now recognized as an independent predictor of COVID-19 severity and a higher hospitalization risk[61]. According to the Child Turcotte Pugh score or MELD score, patients with cirrhosis have a greater mortality rate, and this rate increased as the severity of the liver disease grew[66]. Patients with cirrhosis had a significant 30-d death rate and a 20%-30% chance of decompensation manifesting as acute on chronic liver failure[66]. Data on patients with COVID-19-associated autoimmune hepatitis, chronic viral hepatitis, and alcoholic liver disease are few. Immunosuppressive medications should not be reduced for immunosuppressed patients, whether for autoimmune hepatitis or post-transplantation, out of concern for COVID-19[75]. Data on post-transplant patients are scarce, although there is no evidence of significant COVID-19-related mortality during the peri-transplant period[76]. Routine endoscopy and liver biopsy should be avoided, but urgent procedures for variceal bleed and cholangitis should be carried out according to the correct protocols designed for COVID-19 patients. Various drug combinations are being used with varying degrees of success in treating COVID-19 in patients with cirrhosis. According to the combined findings of the preliminary COVID-19 data, CLD had a negligible impact on patient progression to the severe stage of the disease. However, further research conclusively demonstrated that underlying CLD was associated with worse outcomes and more severe COVID-19 illness[48].
HEPATIC IMMUNE MODULATION
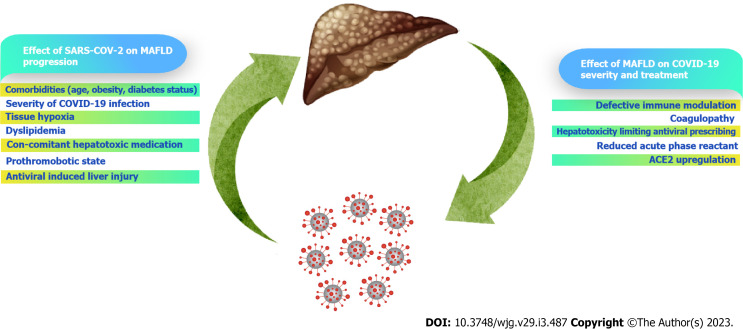
Disconcerting, the inherent chronic activation of inflammatory pathways in MAFLD appears to increase liver damage in patients with COVID-19, perhaps worsening outcomes in those with prior comorbid metabolic disorders. The likelihood of developing more severe types of COVID-19 infection has also been reported to be increased in people with pre-existing chronic liver disorders[23]. Patients with severe COVID-19 infection had high levels of inflammatory markers like CRP, serum ferritin, lactate dehydrogenase, D-dimer, and interleukins (IL-6, IL-2)[36]. Individuals with MAFLD have been found to have elevated IL-6 Levels. When individuals with COVID-19 infection experience a "cytokine storm," IL-6 is a key player. Particularly, IL-6 seems crucial in the beginning and development of the "cytokine storm" seen in COVID-19-infected patients[52]. Elevated IL-6 Levels are linked to MAFLD, which may be a marker or mediator of the comorbidities and related atherosclerosis that are typically observed in COVID-19-infected patients[23]. The cytokine, monocyte chemoattractant protein (MCP-1), is commonly raised in patients with COVID-19 infection, which exacerbates steatohepatitis[23,24,77]. The interplay between COVID-19 and MAFLD in modulating the pathophysiology and outcomes of either disease is shown in Figure 1.
Figure 1.
Interplay between coronavirus disease 2019 and metabolic-associated fatty liver disease. COVID-19: Coronavirus disease 2019; MAFLD: Metabolic-associated fatty liver disease; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
DIAGNOSTIC CHALLENGES
An accurate clinical history, radiographic and laboratory tests, and histologic data are all necessary to diagnose MAFLD. In the absence of significant alcohol intake, other hepatic steatosis-causing factors, and the presence of other liver illnesses, hepatic steatosis must be present in order to diagnose the disease. MAFLD, diagnosed with abdominal imaging, reduces the need for invasive tests like liver biopsies[38]. A liver biopsy may be helpful when deciding between basic steatosis (NAFL) and NASH. Also, a liver biopsy can help in assessing the likelihood of other conditions that will worsen the MAFLD. Patients with COVID-19: Liver biopsy should be postponed in most patients because: (1) Liver biopsy may pose a risk for viral transmission (although the virus has not yet been detected in the liver tissue), the expression of its receptor on cholangiocytes suggests that the virus might be present[49]; (2) COVID-19 treatment/care outweighs diagnosis of concurrent liver disease; and (3) Systemic inflammation associated with COVID-19 will obscure etiology-specific histologic characteristics.
The patient's clinical history must be considered while interpreting test results (Table 2). The "World Gastroenterology Organization" suggests the following guidelines for treating people with liver disease generally in the COVID-19 era[49]: (1) Routine outpatient testing of liver biochemistry is not advised in the COVID-19 era; (2) Discard viral hepatitis in patients with increased ALT or AST levels. Due to the possibility that patients in developing nations have never undergone testing, this may be very crucial; (3) Local context and availability should be considered throughout routine investigation to rule out other etiologies; and (4) Regular imaging should only be done if it will change management.
Table 2.
Analysis of liver test results in coronavirus disease 2019 patients
|
Test
|
Comments
|
| Prolonged INR or thrombocytopenia | In one-third of sick patients |
| Spontaneous coagulopathy/DIC may be present | |
| Thromboembolic incidents are probably frequent | |
| There may be a chance of ACLF | |
| Imaging | Where chest-CT is frequently performed: Assessing liver/biliary tract disease might be helpful |
| Do US, if necessary, but refrain from using US for superfluous imaging (not formally investigated) | |
| Hypoalbuminemia | Common in people with systemic inflammatory response |
| May also be a sign of acute hepatic decompensation or acute liver failure in people with pre-existing liver cirrhosis | |
| High transaminases or bilirubin (> 3 × ULN) | Although not typical for COVID-19, ACLF may be present in patients with cirrhosis who already have liver disease |
| Dyselectrolytemia | Diarrhea and other GI problems might result in numerous electrolyte abnormalities |
| Anemia | Consider bleeding due to variceal hemorrhage in the context of MAFLD cirrhosis, portal hypertensive gastropathy or stress mucosal GI ulcer |
ACLF: Acute on chronic liver failure; CT: Computed tomography; DIC: Disseminated intravascular coagulation; GI: Gastrointestinal; ULN: Upper limit of normal; US: Ultrasonography; COVID-19: Coronavirus disease 2019; MAFLD: Metabolic-associated fatty liver disease.
NONINVASIVE MARKERS FOR FIBROSIS DETECTION
The increasing prevalence of MAFLD, the limits of liver biopsy, and the lack of consensus regarding clinical predictors of NASH have generated a market for next-generation noninvasive biomarkers and imaging modalities to aid in the distinction between MAFLD and NASH. Aminotransferases, cytokeratin-18, and numerous scoring systems that incorporate laboratory indicators such as the AST/platelet ratio index, NAFLD fibrosis score, FIB-4 index, and Fibrotest are some examples of indirect markers. The extracellular matrix contains direct fibrosis indicators such as fibronectin, elastin, laminin, and hyaluronic acid which develop in the presence of prolonged hepatocyte damage and have also been included in certain ratings[33,78].
ENDOSCOPY FOR PATIENTS WITH COVID-19
Patients who are at risk of variceal bleeding, such as those with a history of variceal bleeding or symptoms of significant portal hypertension (ascites, low thrombocyte count, ALT > 5 × ULN) of unknown etiology, should be considered for esophago-gastro-duodenoscopic variceal screening (in case of suspected autoimmune liver disease, treatment without a histological diagnosis can be considered based on individual risk-benefit considerations)[49,75].
DETECTION OF ACE2 POLYMORPHISM
Studies on whether ACE1/ACE2 genetic variability influences the clinical course of COVID-19 in diverse ethnic communities remain elusive[14]. Between Asians and Caucasians, ACE2 demonstrated significant minor allele frequency differences due to four missense mutations[71]. 64 K26R and I468W, two of these variations, may influence how the S protein of SARS-CoV-2 binds to the hACE2 receptor[55]. A difference in male and female individuals was found in ACE2 expression between Asians and others[79]. The ACE2 variant rs2285666 was not connected to the course of the disease when ACE2 genetic variation was examined in the COVID-19 progression[71]. Nevertheless, numerous studies have shown a substantial correlation between COVID-19 and ACE1-insertion/deletion (I/D)[71,77,80]. When compared to ACE1-II people, ACE1-DD carriers had higher blood levels of ACE-I that are roughly twice as high and have been linked to hypertension, ARDS, and in-hospital mortality[81]. As a result, although the ACE1-II genotype negatively correlates with infection rate and mortality, the deletion allele positively corresponds with COVID-19 progression and SARS-CoV-2 infection rate and mortality. However, ACE1-I/D allele frequency ratio was substantially linked to the rise in recovery rate but not to mortality in a meta-analysis of 48758 healthy adults from 30 different nations[82]. Additionally, ACE1-I/D polymorphisms may help to explain how COVID-19 manifests in different ethnic populations. African Americans (29%, 60%, and 11%, respectively), Indians (19%, 50%, and 31%, respectively), and Whites (29%, 40%, and 31%, respectively) all had statistically different distributions of the D/D, I/D, and I/I genotype frequency ratios[82]. Additionally, there was a statistically significant difference in the frequency of the deletion allele among African Americans, Indians, and whites (0.59, 0.49, and 0.44, respectively)[79,81]. More research is necessary to determine whether these indications could explain COVID-19 progression in various populations. Overall, more in vitro and functional research are needed to fully understand the importance of the ACE and ACE2 allele frequency ratio findings and how they relate to the COVID-19 studies[71]. These investigations ought to look into the morbidity and mortality hazards linked to COVID-19 and MAFLD in these racially varied genetic variants.
CLINICAL INTERVENTIONS AND MANAGEMENT
Antivirals and monoclonal antibodies
There is a paucity of information on the safety and effectiveness of new and existing COVID-19 treatments in patients with MAFLD, CLD, and cirrhosis. Clinical professionals' key worries are around adverse immune-related events, long-term effects, and drug interactions. On the basis of the presumption that dysregulated immune responses need to be suppressed; a number of medicines have been evaluated in COVID-19. Steroids, such as dexamethasone, prednisolone, methylprednisolone, or intravenous hydrocortisone, which act through the glucocorticoid receptor and effector genes, are one of the principal treatments. According to the World Health Organization recommendations; systemic corticosteroid medication is not recommended for everyday usage[5,83]. Only patients who have cytokine storm, ARDS, severe cardiac failure, acute kidney injury, and high serum D-dimer levels should receive it. Janus Kinase JAK inhibitors, IL-1 and IL-6 inhibitors, anti-tumor necrosis factor-alpha (often referred to as anti-TNF-alpha) medications, corticosteroids, colchicine, and intravenous immunoglobulin are other immunomodulators studied in COVID-19 infection[83]. Chloroquine and hydroxychloroquine have been shown to lessen COVID-19-mediated damage by stopping the cytokine storm, activating CD8+ cells, or blocking the virus from being taken up by endocytosis[84]. By building up in lysosomes and raising the pH of the endosome, chloroquine and hydroxychloroquine block the entry and departure of viruses from cells. Additionally, these medications block the ACE2 receptor, inhibiting SARS-CoV-2 entrance. Chloroquine and hydroxychloroquine may lessen the ACE2 receptor's glycosylation, preventing the virus from attaching to and infecting new cells. Chloroquine and hydroxychloroquine have been known to cause QT prolongation due to a delay in the cardiomyocyte depolarization rate[85]. There have been reports of patients developing torsades de pointes with the use of chloroquine[86]. Major studies, however, failed to demonstrate any alleged COVID-19 prophylactic and therapeutic benefit, and these medications have subsequently fallen out of favor due to their serious cardiovascular complication risk[87]. Other direct antivirals, like remdesivir and favipiravir, similarly did not demonstrate any appreciable efficacy or survival advantage[83]. In COVID-19, tocilizumab, a humanized IgG1 monoclonal antibody to the IL-6 receptor, has shown only patchy success. However, the adverse effects can include hepatotoxicity, diverticulitis, hypertriglyceridemia, and increased susceptibility to infection[88]. Cytokine dialysis, utilizing blood ultrafiltration, diffusion, and adsorption circuits in dialysis machines, has also been tested as an alternative to medications that directly decrease the immune response[44]. Theoretically, restoring immunological IL-6/IL-1 levels and other proinflammatory molecules protect against organ failures, but the clinical effectiveness of this protection is still unknown, and immune dysregulation is just one issue among many[89]. It has been suggested to use immunomodulators based on mesenchymal stem cells to prevent and control the cytokine storm. Mesenchymal stem cell transplantation intravenously was proven successful in COVID-19 patients in a study[90].
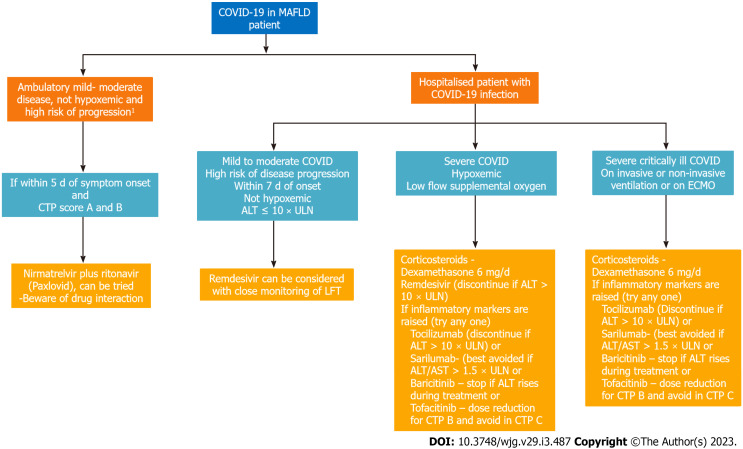
Of the drugs listed in Table 3, the commonly used agents and their hepatoxicity profile are shown in Table 4. The appropriate selection of drugs in MAFLD patients depends on the severity of COVID-19 infection, duration of the disease, ALT level, and potential drug interactions with other medications. Our approach is in line with the Infectious Disease Society of America 2022 guidelines and is shown in Figure 2[91]. Hence when these drugs are used, liver function tests should be routinely monitored, and manufacturers’ advice regarding dose adjustment should be followed until more studies are available in MAFLD patients. A very rare case of acute severe hepatitis with the use of Tocilizumab was noted in a patient who had previous lopinavir and ritonavir exposures[88]. In general, the management of these drug induced liver injury is usually symptomatic and in severe cases, when the ALT is more than six times the upper limit of normal, the medication may need to be temporarily stopped[44].
Table 3.
Drugs and vaccines used in the management and prevention of coronavirus disease 19
|
Classification
|
Drugs
|
|
| Antiviral agents | Favipiravir, molnupiravir, paxlovid, remdesivir | |
| Immunomodulatory agents | ||
| JAK inhibitors | Baricitinib, ruxolitinib, tofacitinib | |
| Monoclonal antibodies to IL-6 | Sarilumab, tocilizumab | |
| Corticosteroids | Cortisol, dexamethasone, methylprednisolone | |
| Monoclonal antibodies to SARS-CoV-2 | Bamlanivimab, casirivimab, etesevimab, imdevimab, sotrovimab | |
| COVID-19 vaccines | ||
| mRNA | BNT162b2 [Pfizer-BioNTech], mRNA-1273 [Moderna] | |
| Adenovirus vector | ChAdOx1-S [AstraZeneca, Oxford]; Ad26.COV2.S [Johnson and Johnson, Janssen], Sputnik-V-Gam - COVID Vac Ad5+Ad26 [Gamleaya] | |
| Recombinant nanoparticles | NVX-CoV2373 [Novavax] | |
| Miscellaneous agents | Azithromycin, chloroquine, dexamethasone, fluvoxamine, hydroxychloroquine, ivermectin | |
COVID-19: Coronavirus disease 19; IL-6: Interleukin-6; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Table 4.
Hepatoxicity profile of the commonly used drugs to treat coronavirus disease 2019 infection
|
Medication
|
Hepatotoxicity pattern
|
| Dexamethasone | None reported at the dose given for COVID |
| Protease inhibitors (e.g., lopinavir, ritonavir) | Mostly hepatitis pattern with ALT raise up to 6 times the normal, but rarely cholestatic pattern reported[43] |
| Nucleoside analogue: Remdesivir | Hepatitis pattern with mild to moderate ALT raise (up to 6 times the normal)[77] |
| Monoclonal antibodies to IL-6: Tocilizumab | Rarely can cause acute severe hepatitis in patients on concomitant or previous hepatotoxic drug usage[83] |
ALT: Alanine transaminase; COVID: Coronavirus disease; IL-6: Interleukin-6.
Figure 2.
Selection of coronavirus disease 2019 therapy in metabolic-associated fatty liver disease patient. 1High risk of progression - advanced age ≥ 65-yr-old, immunocompromised state or multiple medical co-morbidities. Hypoxemia: SpO2 ≤ 94% on room air; ECMO: Extracorporeal membrane oxygenation; Mild disease: Cough, upper respiratory tract symptom and absence of dyspnea; Severe: Hypoxemia or need for supplemental oxygen; Moderate: Dyspneic patient and absence of severe disease features. ALT: Alanine transaminase; AST: Aspartate transaminase; COVID: Coronavirus disease; CTP: Child Pugh score; LFT: Liver function test; ULN: Upper limit of normal.
Vaccines
At least 85 vaccine proposals were being researched in clinical trial phases, and 184 vaccines were being evaluated in pre-clinical stages, according to the most recent version of the WHO report from April 2, 2021[28]. Other vaccines, including the plant-derived vaccine and the Bacillus Calmette-Guérin vaccine, have also been proven in tests to potentially aid in the management of the COVID-19 pandemic[92].
The currently commercially available vaccines include Oxford-AstraZeneca, Pfizer-BioNTech, Moderna, Sinopharm-Beijing, Gamaleya (Sputnik V), Sinovac, Sinopharm-Wuhan, Johnson & Johnson, Bharat Biotech (Covaxin), CanSino and Vector Institute (EpiVacCorona). In the multicenter study conducted by Wang et al[93], in patients with MAFLD who had two doses of inactivated vaccine against SARS-CoV2 without a history of SARS-CoV-2 infection, these vaccines were found to be safe with good immunogenicity. In a multicentric study from China, the inactivated vaccine induced adequate antibody titer against SARS-CoV-2 in 95% of the patients with MAFLD. The adverse event profile was similar to the individuals without MAFLD and hence the vaccine is safe and equally immunogenic as in the normal population[93].
CONCLUSION
SARS-CoV-2 infection's pandemic traits and high mortality rate have sparked worries about the processes causing harm to vulnerable patients. The people most susceptible to COVID-19 had pre-existing illnesses. As a result of metabolic irregularities, the accumulation of metabolically active fat (also known as the "overfat state") coexists with chronic inflammatory alterations, the emergence of insulin resistance, the buildup of fat in the liver, and perhaps even hepatic fibrosis in the long run. This interplay between the numerous inflammatory pathways constantly present in MAFLD can dramatically increase the risk for COVID-19 infection and intensify liver damage. MAFLD should therefore be considered as a prognostic indication during COVID-19, while on the other hand, close long-term monitoring of individuals with MAFLD who experienced COVID-19 may be required. Finally, reducing the vulnerability to non-communicable diseases and boosting personal resistance to future epidemics are additional challenges in diagnosing and treating individuals with MAFLD.
Footnotes
Conflict-of-interest statement: All authors report having no relevant conflict of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 12, 2022
First decision: November 15, 2022
Article in press: December 27, 2022
Specialty type: Infectious diseases
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gambuzza ME, Italy; Li Z, China; Wang TJ, China; Zhang LL, China; Zhu YY, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
Contributor Information
Mohammad Sadiq Jeeyavudeen, Department of Endocrinology and Metabolism, University Hospitals of Edinburgh, Edinburgh EH4 2XU, United Kingdom.
Rahul Chaudhari, Department of Gastroenterology, Hepatology and Nutrition, Virginia Commonwealth University, Richmond, VA 23298, United States.
Joseph M Pappachan, Department of Endocrinology and Metabolism, Lancashire Teaching Hospitals NHS Trust, Preston PR2 9HT, United Kingdom; Faculty of Science, Manchester Metropolitan University, Manchester M15 6BH, United Kingdom; Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PL, United Kingdom. drpappachan@yahoo.co.in.
Sherouk Fouda, School of Health and Biomedical Sciences, RMIT University, Melbourne VIC, Australia.
References
- 1.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez MA, Franco S. Impact of COVID-19 in Liver Disease Progression. Hepatol Commun. 2021;5:1138–1150. doi: 10.1002/hep4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Sessa A, Lanzaro F, Zarrilli S, Picone V, Guarino S, Miraglia Del Giudice E, Marzuillo P. COVID-19 and pediatric fatty liver disease: Is there interplay? World J Gastroenterol. 2021;27:3064–3072. doi: 10.3748/wjg.v27.i22.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jebril N. World Health Organization Declared a Pandemic Public Health Menace: A Systematic Review of the Coronavirus Disease 2019 “COVID-19”. 2020. [Google Scholar]
- 6.Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2022 [Accessed September 2, 2022] Available from: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 .
- 7.Eurosurveillance editorial team. Latest assessment on COVID-19 from the European Centre for Disease Prevention and Control (ECDC) Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.8.2002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verelst F, Kuylen E, Beutels P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID-19) cases, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.13.2000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albano D, Bertagna F, Bertoli M, Bosio G, Lucchini S, Motta F, Panarotto MB, Peli A, Camoni L, Bengel FM, Giubbini R. Incidental Findings Suggestive of COVID-19 in Asymptomatic Patients Undergoing Nuclear Medicine Procedures in a High-Prevalence Region. J Nucl Med. 2020;61:632–636. doi: 10.2967/jnumed.120.246256. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 13.Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1295. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 [Google Scholar]
- 15.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology. 2020;159:373–375.e2. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Méndez-Sánchez N, Valencia-Rodríguez A, Qi X, Yoshida EM, Romero-Gómez M, George J, Eslam M, Abenavoli L, Xie W, Teschke R, Carrion AF, Keaveny AP. What Has the COVID-19 Pandemic Taught Us so Far? J Clin Transl Hepatol. 2020;8:0024. doi: 10.14218/JCTH.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 24.Biquard L, Valla D, Rautou PE. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic-associated fatty liver disease. J Hepatol. 2020;73:717–718. doi: 10.1016/j.jhep.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai L, Li H. Innate immune regulatory networks in hepatic lipid metabolism. J Mol Med (Berl) 2019;97:593–604. doi: 10.1007/s00109-019-01765-1. [DOI] [PubMed] [Google Scholar]
- 26.Prins GH, Olinga P. Potential implications of COVID-19 in non-alcoholic fatty liver disease. Liver Int. 2020;40:2568. doi: 10.1111/liv.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asemota J, Aduli F. The Impact of Nonalcoholic Fatty Liver Disease on the Outcomes of Coronavirus Disease 2019 Infection. Clin Liver Dis (Hoboken) 2022;19:29–31. doi: 10.1002/cld.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motamedi H, Ari MM, Dashtbin S, Fathollahi M, Hossainpour H, Alvandi A, Moradi J, Abiri R. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int Immunopharmacol. 2021;96:107763. doi: 10.1016/j.intimp.2021.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, Erőss B, Szakács Z, Hegyi P, Pár G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front Med (Lausanne) 2021;8:626425. doi: 10.3389/fmed.2021.626425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji D, Cheng G, Lau G. Reply to: "NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues". J Hepatol. 2021;74:484–485. doi: 10.1016/j.jhep.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji D, Xu J, Qin E, Zhang D, Cheng G, Wang Y, Lau G. Reply to: 'No evidence for an increased liver uptake of SARS-CoV-2 in metabolic-associated fatty liver disease'. J Hepatol. 2020;73:718–719. doi: 10.1016/j.jhep.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji D, Zhang M, Qin E, Zhang L, Xu J, Wang Y, Cheng G, Wang F, Lau G. Letter to the Editor: Obesity, diabetes, non-alcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease are proinflammatory hypercoagulable states associated with severe disease and thrombosis in Covid-19. Metabolism. 2021;115:154437. doi: 10.1016/j.metabol.2020.154437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibáñez-Samaniego L, Bighelli F, Usón C, Caravaca C, Fernández Carrillo C, Romero M, Barreales M, Perelló C, Madejón A, Marcos AC, Albillos A, Fernández I, García-Samaniego J, Calleja JL, Bañares R. Elevation of Liver Fibrosis Index FIB-4 Is Associated With Poor Clinical Outcomes in Patients With COVID-19. J Infect Dis. 2020;222:726–733. doi: 10.1093/infdis/jiaa355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747–749. doi: 10.1016/j.jhep.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Chen Q. COVID-19 Pandemic: Insights into Interactions between SARS-CoV-2 Infection and MAFLD. Int J Biol Sci. 2022;18:4756–4767. doi: 10.7150/ijbs.72461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Yang X, Bian H, Xia M. Metabolic dysfunction associated fatty liver disease and coronavirus disease 2019: clinical relationship and current management. Lipids Health Dis. 2021;20:126. doi: 10.1186/s12944-021-01564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamadrid P, Alonso-Peña M, San Segundo D, Arias-Loste M, Crespo J, Lopez-Hoyos M. Innate and Adaptive Immunity Alterations in Metabolic Associated Fatty Liver Disease and Its Implication in COVID-19 Severity. Front Immunol. 2021;12:651728. doi: 10.3389/fimmu.2021.651728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruzzone C, Bizkarguenaga M, Gil-Redondo R, Diercks T, Arana E, García de Vicuña A, Seco M, Bosch A, Palazón A, San Juan I, Laín A, Gil-Martínez J, Bernardo-Seisdedos G, Fernández-Ramos D, Lopitz-Otsoa F, Embade N, Lu S, Mato JM, Millet O. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience. 2020;23:101645. doi: 10.1016/j.isci.2020.101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dias SSG, Soares VC, Ferreira AC, Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR, Teixeira L, Nunes da Silva MA, Barreto E, Mattos M, de Freitas CS, Azevedo-Quintanilha IG, Manso PPA, Miranda MD, Siqueira MM, Hottz ED, Pão CRR, Bou-Habib DC, Barreto-Vieira DF, Bozza FA, Souza TML, Bozza PT. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16:e1009127. doi: 10.1371/journal.ppat.1009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferron PJ, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266–274. doi: 10.1016/j.biochi.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dongiovanni P, Meroni M, Longo M, Fracanzani AL. MAFLD in COVID-19 patients: an insidious enemy. Expert Rev Gastroenterol Hepatol. 2020;14:867–872. doi: 10.1080/17474124.2020.1801417. [DOI] [PubMed] [Google Scholar]
- 46.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P, Kumar A, Anikhindi S, Bansal N, Singla V, Shivam K, Arora A. Effect of COVID-19 on Pre-existing Liver disease: What Hepatologist Should Know? J Clin Exp Hepatol. 2021;11:484–493. doi: 10.1016/j.jceh.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamid S, Alvares da Silva MR, Burak KW, Chen T, Drenth JPH, Esmat G, Gaspar R, LaBrecque D, Lee A, Macedo G, McMahon B, Ning Q, Reau N, Sonderup M, van Leeuwen DJ, Armstrong D, Yurdaydin C. WGO Guidance for the Care of Patients With COVID-19 and Liver Disease. J Clin Gastroenterol. 2021;55:1–11. doi: 10.1097/MCG.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15:21–32. doi: 10.1038/s41574-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor: Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology. 2022;75:757–759. doi: 10.1002/hep.32269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L, Bronsert P, Schwabenland M, Prinz M, Mogler C, Neumann-Haefelin C, Thimme R, Hofmann M, Bengsch B. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77:653–659. doi: 10.1016/j.jhep.2022.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai J, Zhang XJ, Li H. The Role of Innate Immune Cells in Nonalcoholic Steatohepatitis. Hepatology. 2019;70:1026–1037. doi: 10.1002/hep.30506. [DOI] [PubMed] [Google Scholar]
- 57.Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, Quan S, Zhang F, Sun R, Qian L, Ge W, Liu W, Liang S, Chen H, Zhang Y, Li J, Xu J, He Z, Chen B, Wang J, Yan H, Zheng Y, Wang D, Zhu J, Kong Z, Kang Z, Liang X, Ding X, Ruan G, Xiang N, Cai X, Gao H, Li L, Li S, Xiao Q, Lu T, Zhu Y, Liu H, Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammoudeh SM, Hammoudeh AM, Bhamidimarri PM, Mahboub B, Halwani R, Hamid Q, Rahmani M, Hamoudi R. Insight into molecular mechanisms underlying hepatic dysfunction in severe COVID-19 patients using systems biology. World J Gastroenterol. 2021;27:2850–2870. doi: 10.3748/wjg.v27.i21.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, Peng M, Chen P, Liu C, Hu A, Zhang Y, Peng J, Liu J, Li Y, Li W, Zhu W, Guan D, Chen H, Li J, Fan D, Huang K, Lin F, Zhang Z, Guo Z, Luo H, He X, Zhu Y, Li L, Huang B, Cai W, Gu L, Lu Y, Deng K, Yan L, Chen S. Imatinib and methazolamide ameliorate COVID-19-induced metabolic complications via elevating ACE2 enzymatic activity and inhibiting viral entry. Cell Metab. 2022;34:424–440.e7. doi: 10.1016/j.cmet.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao Y, Pan H, She Q, Wang F, Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5:528–529. doi: 10.1016/S2468-1253(20)30080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgos-Blasco B, Güemes-Villahoz N, Santiago JL, Fernandez-Vigo JI, Espino-Paisán L, Sarriá B, García-Feijoo J, Martinez-de-la-Casa JM. Hypercytokinemia in COVID-19: Tear cytokine profile in hospitalized COVID-19 patients. Exp Eye Res. 2020;200:108253. doi: 10.1016/j.exer.2020.108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, Peyvandi F, Bertelli C, Valenti L, Fargion S. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61:148–154. doi: 10.1016/j.jhep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, Hubens G, Van Marck E, Staels B, Michielsen P, Van Gaal L. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59:121–129. doi: 10.1002/hep.26510. [DOI] [PubMed] [Google Scholar]
- 65.Virović-Jukić L, Stojsavljević-Shapeski S, Forgač J, Kukla M, Mikolašević I. Non-alcoholic fatty liver disease - a procoagulant condition? Croat Med J. 2021;62:25–33. doi: 10.3325/cmj.2021.62.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Middleton P, Hsu C, Lythgoe MP. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2021-000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papic N, Samadan L, Vrsaljko N, Radmanic L, Jelicic K, Simicic P, Svoboda P, Lepej SZ, Vince A. Distinct Cytokine Profiles in Severe COVID-19 and Non-Alcoholic Fatty Liver Disease. Life (Basel) 2022;12 doi: 10.3390/life12060795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verdelho Machado M, Cortez-Pinto H. Fatty liver in lean patients: is it a different disease? Ann Gastroenterol. 2012;25:1–2. [PMC free article] [PubMed] [Google Scholar]
- 71.Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nseir WB, Mograbi JM, Amara AE, Abu Elheja OH, Mahamid MN. Non-alcoholic fatty liver disease and 30-day all-cause mortality in adult patients with community-acquired pneumonia. QJM. 2019;112:95–99. doi: 10.1093/qjmed/hcy227. [DOI] [PubMed] [Google Scholar]
- 74.Nseir W, Taha H, Khateeb J, Grosovski M, Assy N. Fatty liver is associated with recurrent bacterial infections independent of metabolic syndrome. Dig Dis Sci. 2011;56:3328–3334. doi: 10.1007/s10620-011-1736-5. [DOI] [PubMed] [Google Scholar]
- 75.Madhu D, Sharma S, Agarwal A, Saraya A. Special Considerations in the Management of Autoimmune Hepatitis in COVID-19 Hotspots: A Review. J Clin Transl Hepatol. 2021;9:568–575. doi: 10.14218/JCTH.2021.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, Kumar P, Sharma M, Rao PN, Reddy DN. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv. 2020 [Google Scholar]
- 78.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, Gong W, Han JJ. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19 doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faridzadeh A, Mahmoudi M, Ghaffarpour S, Zamani MS, Hoseinzadeh A, Naghizadeh MM, Ghazanfari T. The role of ACE1 I/D and ACE2 polymorphism in the outcome of Iranian COVID-19 patients: A case-control study. Front Genet. 2022;13:955965. doi: 10.3389/fgene.2022.955965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hatami N, Ahi S, Sadeghinikoo A, Foroughian M, Javdani F, Kalani N, Fereydoni M, Keshavarz P, Hosseini A. Worldwide ACE (I/D) polymorphism may affect COVID-19 recovery rate: an ecological meta-regression. Endocrine. 2020;68:479–484. doi: 10.1007/s12020-020-02381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sinha N, Balayla G. Hydroxychloroquine and COVID-19. Postgrad Med J. 2020;96:550–555. doi: 10.1136/postgradmedj-2020-137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiu MN, Bhardwaj M, Sah SP. Safety profile of COVID-19 drugs in a real clinical setting. Eur J Clin Pharmacol. 2022;78:733–753. doi: 10.1007/s00228-021-03270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szekely Y, Lichter Y, Shrkihe BA, Bruck H, Oster HS, Viskin S. Chloroquine-induced torsades de pointes in a patient with coronavirus disease 2019. Heart Rhythm. 2020;17:1452–1455. doi: 10.1016/j.hrthm.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gasmi A, Peana M, Noor S, Lysiuk R, Menzel A, Gasmi Benahmed A, Bjørklund G. Chloroquine and hydroxychloroquine in the treatment of COVID-19: the never-ending story. Appl Microbiol Biotechnol. 2021;105:1333–1343. doi: 10.1007/s00253-021-11094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Premkumar M, Kedarisetty CK. Cytokine Storm of COVID-19 and Its Impact on Patients with and without Chronic Liver Disease. J Clin Transl Hepatol. 2021;9:256–264. doi: 10.14218/JCTH.2021.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma A, Kulkarni R, Sane H, Awad N, Bopardikar A, Joshi A, Baweja S, Joshi M, Vishwanathan C, Gokulchandran N, Badhe P, Khan M, Paranjape A, Kulkarni P, Methal AK. Phase 1 clinical trial for intravenous administration of mesenchymal stem cells derived from umbilical cord and placenta in patients with moderate COVID-19 virus pneumonia: results of stage 1 of the study. Am J Stem Cells. 2022;11:37–55. [PMC free article] [PubMed] [Google Scholar]
- 91.Bhimraj A, Morgan RL, Shumaker AH, Baden L, Cheng VCC, Edwards KM, Gallagher JC, Gandhi RT, Muller WJ, Nakamura MM, O'Horo JC, Shafer RW, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borges KCM, da Costa AC, de Souza Barbosa LC, Ribeiro KM, Dos Anjos LRB, Kipnis A, Junqueira-Kipnis AP. Tuberculosis, BCG Vaccination, and COVID-19: Are They Connected? Mini Rev Med Chem. 2022;22:1631–1647. doi: 10.2174/1389557522666220104152634. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, Zhang A, Zhang N, Wang F, Li X, Wang Y, Liu X, Lv C, Chen S, Liu D, Ji X, Liu C, Ren T, Sun J, Zhao Z, Wu F, Li F, Wang R, Yan Y, Zhang S, Ge G, Shao J, Yang S, Huang Y, Xu D, Ai J, He Q, Zheng MH, Zhang L, Xie Q, Rockey DC, Fallowfield JA, Zhang W, Qi X. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol. 2021;75:439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]