Abstract
As an important treatment for acute myeloid leukemia, allogeneic hematopoietic stem cell transplantation (allo-HSCT) plays an important role in reducing relapse and improving long-term survival. With rapid advancements in basic research in molecular biology and immunology and with deepening understanding of the biological characteristics of hematopoietic stem cells, allo-HSCT has been widely applied in clinical practice. During allo-HSCT, preconditioning, the donor, and the source of stem cells can be tailored to the patient’s conditions, greatly broadening the indications for HSCT, with clear survival benefits. However, the risks associated with allo-HSCT remain high, i.e. hematopoietic reconstitution failure, delayed immune reconstitution, graft-versus-host disease, and post-transplant relapse, which are bottlenecks for further improvements in allo-HSCT efficacy and have become hot topics in the field of HSCT. Other bottlenecks recognized in the current treatment of individuals diagnosed with acute myeloid leukemia and subjected to allo-HSCT include the selection of the most appropriate conditioning regimen and post-transplantation management. In this paper, we reviewed the progress of relevant research regarding these aspects.
Keywords: Hematopoietic stem cell, Transplantation, Allogeneic hematopoietic stem cell transplantation, Leukemia, Treatment
Core Tip: Allogeneic stem cell transplantation remains an important player in the therapeutic armamentarium of acute myeloid leukemia. However, this procedure has its advantages and disadvantages. In this narrative review, we explore the obstacles and opportunities of allogeneic stem cell transplantation in acute myeloid leukemia as well as the recent advances in the field.
INTRODUCTION
Leukemia is a malignant disease caused by the abnormal proliferation and differentiation of hematopoietic stem cells (HSCs). Chemotherapy is still one of the main treatments for leukemia, with most patients achieving complete remission (CR) after induction and consolidation chemotherapy. However, some patients relapse after months or years despite CR followed by maintenance chemotherapy. To improve the prognosis of leukemia subjects, some researchers have tried to increase the dose of induction and consolidation chemotherapy to kill as many leukemia cells as possible before they become resistant to certain antineoplastic drugs. The results, however, are unsatisfactory. Certain malignant cells, such as leukemic stem cells (LSCs), hide in the bone marrow (BM) niche, resulting in minimal residual disease (MRD), which is difficult to clear and is an important cause of resistance and relapse[1]. In addition, high-dose chemotherapy drugs can easily damage HSCs and cause BM suppression[2-5]. Therefore, an appropriate post-CR treatment plan is important for improving the disease-free survival of leukemia patients.
Hematopoietic stem cell transplantation (HSCT) has been one of the most important breakthroughs in the therapy of malignant tumors over the last five decades. In 1957, Professor Thomas, a renowned hematologist, first used allogeneic BM transplantation to successfully treat hematological malignancies. Since then, allogeneic (allo)-HSCT technology has been improving and has been implemented worldwide. Allo-HSCT has completely transformed the treatment of hematological malignancies, with substantial survival benefits via the graft-versus-leukemia (GVL) effect. However, the risks of allo-HSCT include hematopoietic reconstitution failure, delayed immune reconstitution, graft-versus-host disease (GVHD), and post-transplant relapse, which are past and current challenges and research topics in the field of HSCT[1-5].
According to the most recent guidelines of the National Comprehensive Cancer Network, allo-HSCT can be considered in patients diagnosed with acute myeloid leukemia (AML) in the following clinical contexts[6]: Subjects aged less than 60 years who display induction failure after induction with high-dose cytarabine, i.e. after at least two courses of intensive induction therapy, and the patient does not achieve complete response or complete response with incomplete hematological recovery; in the setting of post-induction therapy in subjects aged 60 years or more who achieve complete response after induction with standard dose cytarabine and are fit to be subjected to conventional consolidation or in those individuals who display induction failure in whom allo-HSCT preferably should be performed in the setting of a clinical trial; in the setting of post-induction therapy in subjects aged 60 years or more who achieve response after being subjected to lower-intensity regimens; and in patients with relapsed AML after the use of targeted therapy or chemotherapy, depending on the genomic profile of the malignancy.
HEMATOPOIETIC RECONSTITUTION AFTER BONE MARROW TRANSPLANTATION
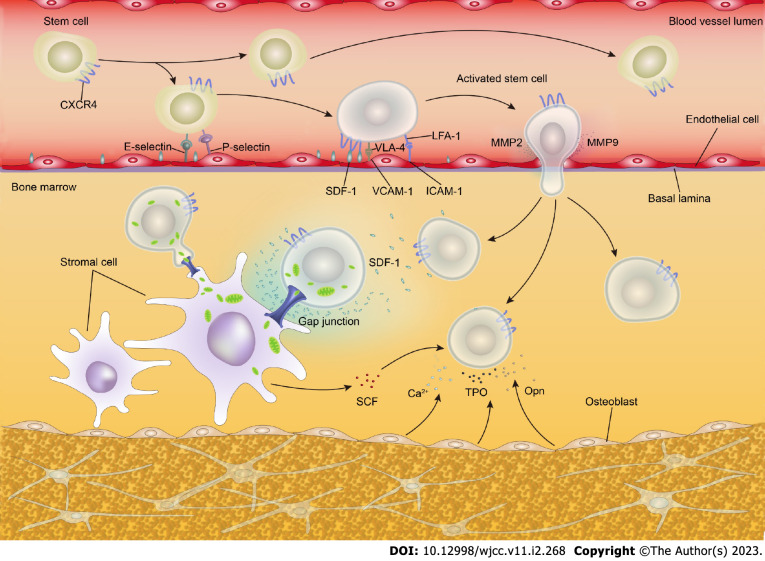
During allo-HSCT, whether the transplanted donor hematopoietic stem and progenitor cells (HSPCs) can successfully home to the BM niche, with successful hematopoietic reconstitution in an appropriate hematopoietic microenvironment, is key for the success of allo-HSCT. HSPC homing and engraftment is a complex multistep process that involves complex interactions between HSPCs and a range of stromal cells in the hematopoietic microenvironment as well as various molecules, e.g., adhesion molecules and chemokines[7,8].
Primitive CD34+ HSPCs express a wide range of cell adhesion molecules, some of which are closely related to HSPC homing, e.g., P-selectin glycoprotein ligand 1, integrins such as very late antigen-4, lymphocyte Peyer’s patch adhesion molecule-1, lymphocyte function-associated antigen-1, specific antigens such as CD44, and cadherins[7,9]. Most of the adhesion molecules on HSPCs have corresponding ligands on the BM mesenchymal stromal cells (MSCs) and the extracellular matrix. The adhesion molecules and their ligands recognize each other and mediate the adhesion of HSPCs (Table 1).
Table 1.
Molecules that mediate hematopoietic stem and progenitor cell adhesion and chemotaxis during transplantation
|
HSPC receptor
|
Bone marrow ligands
|
Effect
|
Ref.
|
| PSGL-1/CD162 | Selectins (P and E) | Promote HSPC homing | [10] |
| β1 integrin | Opn | Contribute to HSC trans-marrow migration toward the endosteal region | [17,18] |
| VLA-4/α4 β1 | VCAM-1, fibronectin | Promote HSPC homing | [7,11] |
| VLA-5/α5 β1 | Fibronectin | Promote HSPC homing and proliferation | [19,20] |
| LFA-1/αL β2 | ICAM-1 | Promote HSPC homing | [7,11] |
| LPAM-1/α4 β7 | MAdCAM-1 | Promote HSPC homing and engraftment | [21] |
| Cx43 | Participate in the formation of intercellular transmembrane channels, facilitate the transportation of mitochondria or other substances, and promote bone marrow regeneration and HSPC engraftment | [22] | |
| CXCR4 | SDF-1 | Promote HSPC homing and engraftment and participate in the regulation of HSPC survival and proliferation | [7] |
| c-kit | SCF | The transmembrane isoform of SCF is critical in the lodgment and detainment of HSCs within the bone marrow niche | [23] |
| c-MPL | TPO | TPO promotes the survival and proliferation of HSPCs and upregulates SDF-1 in the bone marrow niche, thereby contributing to HSPC homing and engraftment | [24,25] |
| CD44/Pgp-1 | Selectins (P, E and L), HA | CD44 and HA play a key role in SDF-1-dependent transendothelial migration of HSPCs and their final anchorage within the bone marrow niche | [26] |
| CD82/KAI1 | CD82 modulates HSPC bone marrow maintenance, homing, and engraftment | [27] | |
| Anxa2r | Annexin II/Anxa2 | Regulate stem cell adhesion, homing, and engraftment | [28] |
| CaR | Ca2+ | Enhance HSC lodgment and engraftment in the bone marrow niche | [29] |
| N-cadherin | N-cadherin | N-cadherin-mediated cell adhesion is functionally required for the establishment of hematopoiesis in the bone marrow niche after bone marrow transplantation | [30] |
Anxa2r: Annexin II receptor; c-kit: Tyrosine-protein kinase kit; c-MPL: Thrombopoietin receptor; Ca2+: Calcium; CaR: Calcium-sensing receptor; CD162: cluster of differentiation 162; CD44: Cluster of differentiation 44; CD82: Cluster of differentiation 82; Cx43: Connexin 43; CXCR4: C-X-C chemokine receptor 4; HA: Hyaluronic acid; HSC: Hematopoietic stem cell; HSPC: Hematopoietic stem and progenitor cells; ICAM-1: Intercellular adhesion molecule 1; KAI1: Kangai 1; LFA-1: Lymphocyte function-associated antigen-1; LPAM-1: Lymphocyte Peyer's patch adhesion molecule-1; MAdCAM-1: Mucosal addressin cell adhesion molecule-1; MPL: Myeloproliferative leukemia gene; Opn: Osteopontin; Pgp-1: Phagocytic glycoprotein-1; PSGL-1: P-selectin glycoprotein ligand-1; Ref.: Reference; SCF: Stem cell factor; SDF-1: Stromal cell-derived factor-1; TPO: Thrombopoietin; VCAM-1: Vascular cellular adhesion molecule-1; VLA-4: Very late antigen 4; VLA-5: Very late antigen 5.
Upon entering the BM cavity from the blood circulation, the initial adherence of HSPCs to the BM sinusoidal endothelial cells requires P-selectin glycoprotein ligand 1, P-selectin, and E-selectin[10]. After this, the adhesion between HSPCs and BM sinusoidal endothelial cells becomes tighter, and the HSPCs enter the BM hematopoietic microenvironment by passing through BM sinusoidal endothelial cells. The process requires integrins and immunoglobulin (Ig) superfamily members, especially the very late antigen-4/vascular cell adhesion molecule-1 and lymphocyte function-associated antigen-1/ intercellular adhesion molecule-1 pathways[7,11]. In the BM hematopoietic microenvironment, HSPCs adhere and interact with stromal cells and the extracellular matrix and stimulate BM stromal cells to secrete hematopoietic cytokines to regulate the quiescence, self-renewal, proliferation, and differentiation of HSPCs[12-30].
A large body of evidence indicates that the axis composed of stromal derived factor-1 (SDF-1/CXCL12) secreted by osteoblasts and endothelial cells and the HSC surface receptor CXCR4 plays a critical role in HSC homing and subsequent engraftment. The SDF-1/CXCR4 signal induces HSPCs to pass through the endothelial layer to adhere to the BM matrix, promoting HSPC homing and engraftment via chemotaxis and participating in the regulation of HSPC survival and proliferation[7,31]. Given the important role of SDF-1/CXCR4 in HSPC homing and engraftment, the regulation of this signal axis is also important for promoting post-transplant hematopoietic reconstitution. Studies have shown that mild heat treatment, prostaglandin E2, histone deacetylase inhibitors, and hypoxia inducible factor-1α enhance the SDF-1/CXCR4 signal and promote HSPC homing and engraftment[11]. In addition to the SDF-1/CXCR4 axis, other chemokine axes and numerous molecules, e.g., receptor tyrosine kinase, thrombopoietin, and matrix metalloproteinases, are involved in HSPC maintenance, homing, and engraftment[23,25,32,33] (Table 1).
Recent research has delineated that the adhesion molecule connexin-43 plays an important role in BM regeneration and HSPC engraftment after irradiation preconditioning. With connexin-43-mediated cell-to-cell contact, donor HSPCs transfer mitochondria to postradiation recipient MSCs, promoting the metabolic recovery of radiation-damaged MSCs and improving the BM hematopoietic compartment reconstitution and donor HSPC engraftment[22]. The mechanism of HSPC homing and engraftment is depicted in Figure 1.
Figure 1.
Mechanism underlying hematopoietic stem and progenitor cells homing and engraftment. Numerous adhesion molecules and chemokines are involved in the regulation of hematopoietic stem and progenitor cells (HSPCs) homing and engraftment. The interaction between recipient bone marrow stromal cells and donor HSPCs contributes to HSPC homing and engraftment. In addition, donor HSPCs improve the metabolism of recipient bone marrow stromal cells via mitochondrial transfer, accelerating the recovery of damaged bone marrow stromal cells. Ca2+: Calcium; CXCR4: C-X-C chemokine receptor 4; ICAM-1: Intercellular adhesion molecule-1; LFA-1: Lymphocyte function-associated antigen-1; MMP-2: Matrix metalloproteinase 2; MMP-9: Matrix metalloproteinase 9; Opn: Osteopontin; SCF: Stem cell factor; SDF-1: Stromal derived factor-1; TPO: Thrombopoietin; VCAM-1: Vascular cell adhesion molecule-1; VLA-4: Very late antigen 4.
MSCs are the main components of the BM hematopoietic microenvironment and play important roles in supporting, regulating, and protecting HSPCs[34,35]. During HSCT preconditioning, chemotherapy/ radiotherapy causes damage to MSCs, resulting in severely low numbers of MSCs, impaired cytokine production and adhesion molecule expression, and impaired function in supporting and regulating hematopoiesis[36].
Histocompatibility is another consideration for allogeneic transplantation, as transplantation failure may also occur not only due to immune rejection but also to major histocompatibility complex (MHC) restriction between donor HSCs and recipient stromal cells and because recipient stromal cells do not support the proliferation and differentiation of donor HSCs. There is a complex interplay between MSCs and HSCs in HSCT, as MSCs are known to support HSCs and enhance their engraftment. Due to their properties, i.e. adherence to plastic and ability to be expanded ex vivo as well as the lack of reported side effects after their administration, MSCs have been employed in clinical patient research, and MSC infusion has been co-administered with HSCs to enhance the engraftment of the latter, particularly in the setting of haploidentical allo-HSCT with/without T cell depletion. In addition, MSCs secrete soluble molecules (e.g., interferon (IFN)-γ, cytokines, chemokines, etc.) and exhibit immunomodulatory actions, having already been employed successfully in the prevention and treatment of GVHD in individuals who had been subjected to allo-HSCT.
Several of the processes in which MSCs are involved include a decrease in inflammation and in the proliferation of B cells and T cells as well as an increase in tissue repair[34,37,38]. In vitro cell culture studies have demonstrated that when primed with nitric oxide MSCs can significantly boost the engraftment potential of HSCs via the intercellular transfer of microvesicles harboring mRNAs encoding HSC-supportive genes[39]. In vitro research has revealed that, under mild hypoxia (5% oxygen), MSCs promote CXCR4 expression in CD34+ CD38− cells, thereby enhancing HSPC homing[40].
MSCs were investigated in phase I/II clinical trials of HSCT to promote HSC engraftment. In clinical applications, MSCs have been used to expand HSCs in vitro[34]. Previous assessments have reported that the engraftment success rate is related to several factors, e.g., the number of stem cells, the stem cell source, donor-specific anti-human leukocyte antigen (HLA) antibodies (DSAs), and the pretreatment protocol. In most cases, increasing the HSPC infusion dose contributes to successful HSPC engraftment and hematopoietic reconstitution. In addition, the quantity and quality of the grafts, as well as the age of donor, can also affect immune reconstruction after allo-HSCT. For example, allo-HSCT from donors aged > 50 years has been linked with lower CD8+CD45RA+ naïve T cell and CD19+ B cell counts, reduced serum IgM and IgA concentrations, and higher Epstein-Barr virus reactivation rates[12,41-45].
However, it should be noted that for allogeneic peripheral blood stem cell transplantation (allo-PBSCT), a high dose of CD34+ cells increases the risk of extensive chronic graft-versus-host disease (cGVHD)[46]. The higher incidence of extensive cGVHD leads to adverse effects on the patient’s prognosis and increases transplant-related mortality, particularly among subjects receiving T cell depleted allogeneic transplantation with myeloablative conditioning. In contrast, individuals who are subjected to low-intensity preconditioning rather than myeloablative regimens may benefit from a higher dose of CD34+ cells, as it has been shown that relapse and/or progression rates were significantly lower (9% vs 36%) in subjects who had received an elevated number of CD34+ cells[46]. Most clinical studies have indicated that in HLA-identical sibling donor transplantation, the application of peripheral blood-derived stem cells accelerates platelet and neutrophil engraftment, which is related to the use of G-CSF during mobilization of peripheral blood-derived stem cells[47-49]. In addition to successfully mobilizing CD34+ stem cells from the BM of healthy donors, G-CSF can induce changes in immune cell function, redirect T cell polarization, and change the expression of adhesive molecules, resulting in rapid and long-lasting engraftment[50].
With the widespread development of HLA haploidentical stem cell transplantation in recent years, engraftment failure and poor engraftment are still an urgent problem to be solved in HLA haploidentical transplantation. DSAs are the most important factors causing engraftment failures of HLA haploidentical transplantation. Chang et al[51] suggested that high DSA levels were associated with primary engraftment failure and poor primary engraftment in HLA haploidentical transplantation. Ciurea et al[52] used plasma exchange, rituximab, intravenous immunoglobulins, and irradiated donor buffy coat to intervene in patients with high DSA levels and found that the engraftment success rate was higher in patients with decreased DSAs and negative complement component 1q. Pretreatment protocols can also affect the engraftment success rate. The Beijing Protocol and the post-transplant cyclophosphamide protocol are some of the most commonly used pretreatment protocols for HLA haploidentical transplantation worldwide. Recently, Tang et al[53] conducted a retrospective study and found that the Beijing Protocol exhibited some advantages vs single-cell source + the post-transplant cyclophosphamide transplant regimens in terms of 30-d neutrophil engraftment rate, 90-d platelet engraftment rate, median neutrophil engraftment time, and platelet engraftment time. Other pretreatment protocols have been previously summarized by Baumeister et al[54] elsewhere.
In recent years, researchers have paid more attention to alternative routes of stem cell administration to reduce the ineffective homing of donor cells[55,56]. Animal model studies have highlighted that compared with intravenous infusion, intra-bone marrow injections for HSPC transplantation are more effective in promoting hematopoietic reconstitution and reducing the incidence of GVHD[57-59]. At present, the clinical application of intra-bone marrow injections is still in its infancy and is mostly used for umbilical cord blood cell transplantation.
POST-TRANSPLANT IMMUNE RECONSTITUTION
HSCs have the capacity for self-renewal, to proliferate, and to differentiate into hematopoietic cells and immune cells. Therefore, HSCT is essentially a dual transplantation of hematopoietic cells and immune cells. After allo-HSCT, the recipient’s hematopoietic system and immune system are reconstituted simultaneously. The restoration of immune function helps patients fight pathogens and ensures successful HSCT. For allo-HSCT recipients, immune reconstitution is a highly dynamic process, including the innate immune system reconstitution and adaptive immune system reconstitution. Post-transplant immune reconstitution takes time, and different immune cells follow different reconstitution patterns, having important implications for the outcome of allo-HSCT.
The innate immune system is mainly composed of natural killer (NK) cells, neutrophils, monocytes, macrophages, and antigen presenting cells (APCs)[60], of which NK cells are the first group of lymphocytes to recover after transplantation, taking only 1-4 mo to return to normal levels, independent of the stem cells source[61-64]. The function of NK cells is regulated by the interaction between killer immunoglobulin-like receptors and the ligand HLA[65]. For haploidentical transplantation, HSCTs with alloreactive donor NK cells (killer immunoglobulin-like receptor-HLA mismatched HCT) are shown to be associated with less relapse and better overall survival[66-69]. Such alloreactive NK cells may also have a beneficial effect on alleviating GVHD because they can eliminate host APCs that prime alloreactive T cells that cause GVHD[67,70,71]. As with NK cells, neutrophils and monocytes also recover in a short period of time after transplantation. Dendritic cells (DCs), shown to be the most potent APC, take longer to recover. In adults, while donor DCs can be detected in the peripheral blood in the first few weeks after stem cell transplantation, the total number may not return to normal even after a year[60,72,73]. Furthermore, previous investigations have pinpointed that while peripheral blood DCs are mainly derived from donors (> 80% by day 14), up to 70% of tissue DCs may still come from the host[60,74-76]. These tissue DCs of host origin may persist for up to a year following HSCT[75]. Researchers have confirmed that host APCs, rather than donor APCs, play an important role in the post-allo-HSCT GVL effect and GVHD[77-79]. Therefore, proper regulation of host APCs may alleviate GVHD and enhance the GVL effect.
Adaptive immune reconstitution mainly includes the restoration of the number and function of B cells and T cells. Reconstitution of the B cell compartment after HSCT occurs primarily through de novo regeneration from BM progenitors[80]. Generally, the proportion of total B cells in most patients reaches normal levels by 3 mo, but the absolute number may not return to normal for 6-12 mo[81-83]. During the 1st year of HSCT, most reconstituted B cells are mainly composed of transitional and naive subsets. However, the restoration of memory B cells takes much longer[83]. Consistent with this, IgM levels recover in 2 to 6 mo after transplantation, and then IgG levels return to close to normal in 3 to 18 mo after transplantation, whereas IgA reconstitution may be delayed for up to 3 years[60].
T cell immune reconstitution is markedly different from B cell immune reconstitution. T cells mainly include two subgroups, CD4+ T cells and CD8+ T cells, which are reconstituted through thymus-independent and thymus-dependent pathways. The early increase in blood T lymphocyte numbers is related to the thymus-independent peripheral expansion of mature donor T cells. The recovery of a broader T cell repertoire depends on the de novo generation of naïve T cells through the thymus after the engraftment and differentiation of hematopoietic stem cells in the BM[80,84]. Preconditioning or GVHD impairs thymus function, resulting in decreased CD4+ T cells after transplantation. Memory or effector CD8+ T cells can rapidly expand through a thymus-independent pathway and return to normal in 12 mo. Therefore, an inverted CD4:CD8 ratio after transplantation is one of the earliest signs of T cell reconstitution and may last for several years, depending on preconditioning and GVHD prevention regimens[83].
CD4+ CD25+ regulatory T cells (Tregs), a subgroup of CD4+ T-cells, play an important role in HSCs maintenance. Cytotoxic T-= cell activation and decreased Treg counts are believed to be the etiology of idiopathic severe aplastic anemia[85]. Tregs reconstitute faster than effector T cells after HSCT. They suppress the activation and proliferation of effector T cells and downregulate the body’s response to foreign antigens or autoantigens, thereby maintaining immune tolerance[86]. Numerous recent studies show that an imbalance between Tregs and effector T cells may be an important link in the occurrence of GVHD[87,88].
Post-transplant immune reconstitution is affected by many factors, such as the intensity of preconditioning, recipient thymus function, recipient age, graft source, and GVHD (Table 2). Delayed immune reconstitution makes HSCT recipients susceptible to various infections. In fact, despite the use of routine peritransplant prophylactic antibiotics, approximately 80%-85% of HSCT recipients contract infections, which is one of the leading causes of nonrelapse death after allo-HSCT[89]. At present, there is no “standard-of-care” approach to enhance post-transplant immune reconstitution. However, several strategies such as protecting the thymic epithelium, stimulating thymopoiesis, or increasing the number of T lymphoid precursors, are being investigated in preclinical models as well as early clinical trials[83]. The effectiveness of these measures remains to be further verified and improved in practice.
Table 2.
Main factors for post-transplant immune reconstitution
|
Factors
|
Effect
|
Ref.
|
| Recipient age | Several studies show that immune reconstitution, especially the reconstitution of CD4+ T cells, is inversely related to age. However, some studies report that age has no effect on the reconstitution of any subgroup of lymphocytes | [63,90,91] |
| Graft source | Immune reconstitution occurs faster after PBSCT than after BMT. This may be because PBSCT grafts are rich in mature lymphocytes. Delayed immune reconstitution after UCBT is related to low lymphocyte count and immature immune cells in umbilical cord blood | [61,92-95] |
| HLA matching between donor and recipient | HLA mismatch causes delayed reconstitution of neutrophils and T cells | |
| Intensity of preconditioning | Several studies show that compared with MA-SCT, RICSCT reduces thymus damage and promotes immune reconstitution. However, some studies show no significant difference in recipient immune reconstitution between these two transplantation methods | [60,96-98] |
| GVHD | GVHD damages thymus structure and function and interferes with T cell differentiation at all stages, thereby affecting T cell reconstitution. GVHD also affects the recovery of B cell number and function | [84,99] |
| GVHD prevention | Donor TCD reduces the risk of GVHD; however, the lack of T cells increases the risk of infection and delayed immune reconstitution | [100] |
| The use of ATG or alemtuzumab has a negative effect on the reconstitution of T cells and B cells | [101-103] |
ATG: Antithymocyte globulin; BMT: Bone marrow transplantation; CD4: Cluster of differentiation 4; GVHD: Graft-versus-host disease; HLA: Human leukocyte antigen; MA-SCT: Myeloablative stem cell transplantation; PBSCT: Peripheral blood stem cell transplantation; Ref.: Reference; RICSCT: Reduced-intensity conditioning stem cell transplantation; TCD: T cell depletion; UCBT: Umbilical cord blood stem cell transplantation.
Several studies show that immune reconstitution, especially the reconstitution of CD4+ T cells, is inversely related to age. However, some studies report that age has no effect on the reconstitution of any subgroup of lymphocytes[63,90,91].
Graft source
Immune reconstitution occurs faster after PBSCT than after BMT. This may be because PBSCT grafts are rich in mature lymphocytes. Delayed immune reconstitution after umbilical cord blood cell transplantation is related to low lymphocyte count and immature immune cells in umbilical cord blood[61,92-95].
HLA matching between donor and recipient
HLA mismatch causes delayed reconstitution of neutrophils and T cells.
Intensity of preconditioning
Several studies show that compared with myeloablative stem cell transplantation, reduced-intensity conditioning stem cell transplantation reduces thymus damage and promotes immune reconstitution. However, some studies show no significant difference in recipient immune reconstitution between these two transplantation methods[60,96-98].
GVHD
GVHD damages thymus structure and function and interferes with T cell differentiation at all stages, thereby affecting T cell reconstitution. GVHD also affects the recovery of B cell number and function[84,99].
GVHD prevention
Donor T cell depletion reduces the risk of GVHD; however, the lack of T cells increases the risk of infection and delayed immune reconstitution[100]. The use of antithymocyte globulin (ATG) or alemtuzumab has a negative effect on the reconstitution of T cells and B cells[101-103].
REGULATION OF GVL AND GVHD
For allo-HSCT, an important mechanism for the treatment of leukemia is that donor immune cells recognize the surface antigens of recipient leukemia cells and trigger an immune response to attack and clear any residual leukemia cells, which is known as the GVL effect. GVL effect is closely related to GVHD, as both have similar pathways, effector cells, and cytokines. Therefore, during immune reconstitution after allo-HSCT, the precise regulation of GVL and GVHD (i.e. suppressing GVHD while preserving GVL) plays an important role in the final outcome of allo-HSCT[104,105].
The mechanism of action of GVHD and GVL is very complex and not entirely clear. The interactions between many donor and recipient cells and cytokines make the mechanism even more challenging to understand. It is believed that donor T cells play an important role in the occurrence of GVHD and GVL. Acute GVHD (aGVHD) has three pathophysiological stages: (1) Activation of APCs by the underlying disease and the HCT conditioning regimen. The damaged host tissue produces a large amount of proinflammatory cytokines, e.g., tumor necrosis factor alpha (TNF-α) and chemokines, with elevated expression of adhesion molecules, MHC antigens, and costimulators on host APCs; (2) Donor T cell activation. Donor T cells proliferate and differentiate in response to host APCs. Activated donor T cells secrete a large amount of Th1 cytokines, such as IFN-γ, interleukin (IL)-2, and TNF-α, which trigger aGVHD; and (3) Cellular and inflammatory effector phase. The complex cascade of cytotoxic T lymphocytes, NK cells, and soluble inflammatory mediators (e.g., TNF-α, IFN-γ, and IL-1) produces synergistic effects and causes further local tissue injury, inflammation, and target tissue damage[106].
The pathophysiology of cGVHD differs from that of aGVHD and is believed to be related to the following factors: (1) Thymus damage and defective negative selection of T-cells, promoting the production of autoreactive T cells; (2) Decreased CD4+ CD25+ Tregs, affecting the suppressive effect of Tregs on effector T cells; (3) Abnormal activation of B cells, promoting the production of autoantibodies and subsequently an autoimmune response; and (4) The formation of profibrotic lesions[107].
GVHD-related tissue damage, as well as GVL-linked tumor elimination, seem to share common immunological mechanisms[108]. Based on this understanding, mitigating the risk of GVHD while maximizing the GVL effect seems to be unrealistic. Clinically, clearing donor T cells effectively reduces the occurrence of and damage by GVHD; however, this approach also weakens the GVL effect, which results in a much higher risk of recurrent leukemia, especially chronic myeloid leukemia. For recurrent cases, donor lymphocyte infusion (DLI) (containing primarily T cells) enables long-term remission[109,110]. These data indicate that GVHD and the GVL effect are interdependent and that both are T cell dependent. However, recent studies show that GVHD and the GVL effect may be mediated by different subgroups of T cells. In the peripheral blood, the αβ T cell receptor is expressed by 95% of T cells, whereas the γδ T cell receptor is expressed by the remaining T cells. Since the primary mediators in GVHD are alloreactive αβ T cells, their depletion from the graft is expected to decrease the chances of GVHD development. In contrast, γδ T cell receptor-expressing lymphocytes exert anti-infectious and anti-leukemia actions, are not marked by alloreactivity, and are not involved in GVHD occurrence. Notwithstanding, the interest towards the use of γδ T cells in allo-HSCT has increased and are currently under investigation by the international scientific community[111-115].
Allo-HSCT studies in mice show that naïve T cells consistently cause severe GVHD, whereas memory T cells cause milder or no GVHD and have critical graft-versus-tumor functions[116-118]. Subsequent clinical trials have confirmed that the removal of donor naïve T cells effectively reduces the incidence of GVHD and opportunistic infections, without any significant increase in relapse[118-120]. In addition, GVL and GVHD effector T cells have different target antigens. For GVHD effector T cells, the target antigens are MHC antigens and minor histocompatibility antigens (MiHAs); for GVL effector T cells, the target antigens are mainly MiHAs on recipient leukemia cells. Therefore, hematopoietic system-specific MiHAs expressed on leukemic cells are considered important targets for leukemia-specific cellular immunotherapy with a low risk of GVHD[121].
Cytokines are critical drivers of both GVHD and GVL, and current evidence indicates that different cytokines may play different roles in GVHD vs the GVL effect[122-133]. In a recent study, Tugues et al[108] used an MHC-mismatched HSCT mouse model and found that donor T cell-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) can drive GVHD pathology by licensing donor-derived phagocytes to produce inflammatory mediators such as IL-1 and reactive oxygen species (ROS). Moreover, anti-GM-CSF treatment improved the survival of recipient mice without affecting the GVL effect of alloreactive T cells, suggesting that GM-CSF may be an important target for GVHD-GVL uncoupling. These data indicate that GVHD and the GVL effect are somewhat independent of each other and are not completely parallel, which makes it possible to target GVHD and the GVL effect separately in allo-HSCT recipients. In addition, alloreactive NK cells seem to play a role in the GVL effect especially in the early period that follows the execution of allo-HSCT (via interaction in the BM of the recipient between the donor HLA environment and the reconstitution of NK cells) without being involved in the development of GVHD[68].
To improve allo-HSCT efficacy and safety, researchers are making great progress in separating GVHD and the GVL effect, including the early prediction of GVHD risk, the modification of donor graft cells, and drug interventions (Table 3). However, the outcomes in clinical practice are still unsatisfactory. An important reason is an inadequate understanding about the mechanism of action of GVHD and the GVL effect. While it is known that GVHD and the GVL effect may involve different subgroups of T cells, it is challenging to identify these T cells. With the advent and application of new detection methods such as sequencing, a solution may be developed to address this issue. For example, T cell receptor high-throughput sequencing can be used to analyze and identify the entire T cell library involved in GVHD and the GVL effect, thus helping researchers learn more about relevant T cells, clarify the targets and mechanisms of different effector cells, and better separate GVHD and GVL.
Table 3.
Strategies to separate graft-versus-host disease and graft-versus-leukemia
|
Separation strategies
|
Approaches
|
Brief description
|
Ref.
|
| GVHD risk prediction | GVHD biomarker testing | Contributes to GVHD diagnosis and provides evidence for the early use of anti-GVHD drugs | [123] |
| Cytokine gene polymorphism testing | Helps to identify patients with a high risk of severe GVHD and take preventive measures | [124] | |
| Modification of donor graft cells | Donor T cell depletion | Donor T cell depletion reduces GVHD while increasing the risk of infections, graft rejection, and disease relapse | [109] |
| Graft-specific cell population depletion | Removing specific cell populations such as naïve T cells in the graft that consistently cause severe GVHD | [118] | |
| DLI to treat relapse | DLI is very effective in the treatment of relapsed slow-growing hematopoietic malignancies such as CML; however, the mechanism is unknown | [122] | |
| Application of CAR T cell | The combination of scFv that identifies leukemia-specific antigens and the activating domain of T cells enhances specific identification and killing of leukemia cells | [125,126] | |
| Suicide gene transduced donor lymphocyte infusion | A genetically modified suicide gene is introduced. Donor lymphocytes expressing this gene are sensitive to prodrugs, a feature that can be used when needed to regulate GVHD through the drug clearance of transduced cells | [127] | |
| Selecting memory T cells | Memory T cells cause mild or no GVHD and have critical graft-versus-tumor functions | [118] | |
| Enhancing activated γδ T cells | γδ T cells have the ability to kill leukemic blasts, and allogeneic TCR γδ T cells are not alloreactive and do not cause GVHD | [113] | |
| Selecting Tregs | Tregs suppress the activation and proliferation of effector T cells and downregulate the body’s response to foreign antigens or autoantigens | [86] | |
| Modifying/selecting other cells in the grafts | Selecting mesenchymal cells, NK cells, and manipulating dendritic cells and dendritic cell subsets | [79,122,129] | |
| Drug intervention | Application of immunosuppressants | Various immunosuppressants suppress T cells and reduce GVHD via different mechanisms | [130] |
| Application of HDACis | HDACis, such as vorinostat, downregulate inflammatory cytokines and increase the number of Tregs, thereby reducing the occurrence of GVHD, without effecting the GVL effect of donor CTLs | [131,132] | |
| Suppression of cytokines related to the occurrence of GVHD | Th1 cytokines such as TNF-α, IFN-γ, and IL-6 are related to aGVHD; Th2 cytokines such as IL-4, IL-5, and IL-10 are related to cGVHD. Appropriate regulation of these cytokines facilitates GVHD management | [122] | |
| Enhancing cytokines that suppress GVHD | Various cytokines such as IL-11 and keratinocyte growth factor reduce GVHD while preserving the GVL effect | [122] | |
| Targeting MiHAs on hematopoietic cells | CTLs targeting MiHAs such as HA-1 and HA-2 (expressed on hematopoietic cells only) promote the GVL effect | [121] | |
| Development and application of tumor vaccines | Vaccines targeting MiHAs on hematopoietic cells and leukemia-specific antigens improve GVL specificity | [133] |
aGVHD: Acute GVHD; CAR: Chimeric antigen receptor; cGVHD: chronic GVHD; CML: Chronic myeloid leukemia; CTLs: Cytotoxic T lymphocytes; DLI: Donor lymphocyte infusion; GVHD: Graft-versus-host disease; GVL: Graft versus leukemia; HA-1: Histocompatibility antigens 1; HA-2: Histocompatibility antigens 2; HDACis: Histone deacetylase inhibitors; IFN-γ: Interferon-γ; IL-10: Interleukin 10; IL-4: Interleukin 4; IL-5: Interleukin 5; IL-6: Interleukin 6; MiHAs: Minor histocompatibility antigens; NK: Natural killer; Ref.: Reference; scFv: Single-chain variable fragment; TCR: T cell receptor; Th2: T-helper 2; TNF-α: Tumor necrosis factor α; Tregs: Regulatory T cells.
MECHANISM OF POST-TRANSPLANT LEUKEMIA RELAPSE
Over the past decades, the transplant-related mortality due to post-transplant complications such as GVHD and infections has decreased due to continuous improvements in stem cell transplantation technology. Post-allo-HSCT relapse has become the major cause of treatment failure and is associated with a dismal prognosis[134]. Post-allo-HSCT relapse may come from normal donor cells, known as donor cell leukemia (DCL; rare, 0.12% to 5.0%), or recipient cells (most cases)[135,136,137]. Despite the remarkable advancement in allo-HSCT technology in recent years, there has been little progress on how to reduce post-allo-HSCT relapse or improve the survival of relapsed patients. The main reason is a lack of information about the mechanism of post-allo-HSCT relapse.
DCL was first recognized in 1971. Since then, few DCL cases have been reported. The molecular mechanisms involved in DCL occurrence seem to involve cytogenetic abnormalities (chromosome 7 monosomy has been depicted in more than one-fifth of DCL cases) or genetic aberrations that arise in RUNX1, ASXL1, DNMT3A, IDH1/2, EZH2, JAK2, CEBPA, GATA2, and other genes. In addition, it has been hypothesized that leukemia cells could have been transferred from the donor during the allo-HSCT procedure. Moreover, several theories support the fact that DCL can arise due to reduced immune surveillance following allo-HSCT, the genomic instability of the donor cells, or an aberrant stromal niche that exhibits a pro-leukemia potential[138,139].
For leukemia relapse derived from recipient cells, researchers had believed that MRD was the root cause of the relapse. However, a growing body of evidence indicates that this theory cannot fully explain the mechanism of leukemia relapse. With advancements in human whole genome sequencing technology, several studies have demonstrated the presence of clonal evolution in leukemia relapse[140-142]. Mullighan et al[140] analyzed the genome-wide DNA copy number in the diagnosis and relapse samples of 61 children with acute lymphoblastic leukemia (ALL) and found concordance between the postchemotherapy relapse leukemia clone and the diagnosis clone in only 8% of the patients; in most cases, the relapse leukemia clone evolved from the diagnosis clone or normal ancestral clones. In an analysis of 92 cases of relapsed pediatric ALL, Waanders et al[141] found that relapsed leukemic cells propagate primarily from clones already expanded at diagnosis and rarely from unexpanded dormant ancestral clones, suggesting that the information gleaned through subclonal mutation analysis at diagnosis may help to predict relapse risk and select rational therapeutic measures with minimal relapse potential.
In recent years, minor diagnosis subclones that initiate an evolutionary trajectory toward relapse (termed diagnosis relapse initiating clones, dRI) had been identified in both ALL and AML[143,144]. Compared with other diagnosis subclones, dRIs are drug tolerant with distinct engraftment and metabolic properties[143]. Genomic analysis of matched diagnosis and relapse samples showed that relapse often arose from dRIs[143], suggesting that the isolation and identification of dRIs and the elimination of dRIs by targeting the unique metabolic and transcription pathways may be novel approaches to prevent leukemia relapse.
Another important factor for post-allo-HSCT relapse is the immune escape of leukemia cells. With immune escape, some leukemia cells avoid a potent GVL effect after transplantation and hide in the BM niche to form MRD and eventually lead to leukemia relapse. Several studies showed that the loss of HLA on the surface of leukemia cells prevented T cells from recognizing leukemia cells, an important mechanism of immune escape[145,146]. In addition, the changes in the number and function of T cell subsets after allo-HSCT, as well as the high expression of the T cell immune coinhibitory receptors programmed cell death protein 1, cytotoxic T lymphocyte-associated antigen-4, and T cell immunoreceptor with Ig and ITIM domains (TIGIT), are closely related to the immune escape of leukemia cells[147,148]. The mechanism of post-allo-HSCT relapse is very complex and multifactorial, and more extensive and in-depth research is needed to clarify the mechanism.
INTERVENTION AND TREATMENT STRATEGIES FOR POST-ALLO-HSCT RELAPSE
Post-allo-HSCT relapse is a challenging issue for the treatment of leukemia. The overall incidence of post-allo-HSCT relapse is 20% to 30%. For refractory and high-risk leukemia, the relapse rate is 50% or higher[149,150]. Post-transplant relapse has severe impacts on allo-HSCT outcomes because it affects long-term survival and is a major cause of death in leukemia patients after transplantation. Therefore, the identification of the risk factors for post-allo-HSCT relapse and post-transplant indicator monitoring are useful for preventing post-transplant relapse and for the timely identification of early relapse. Furthermore, optimizing treatment strategies with a personalized treatment plan will help to reduce post-transplant relapse and improve survival.
Many factors are related to post-transplant relapse, including disease type, pretransplant disease status, risk stratification, donor source, stem cell source, preconditioning, and GVHD (Table 4). Pretransplant disease status is the most important factor. The risk of relapse is high in nonremission patients and patients with a high level of residual leukemia cells before transplantation[151]. Studies have proven that pre-HSCT MRD may be an independent prognostic factor for relapse in AML patients after receiving myeloablative HSCT. The 2-year overall relapse rate is significantly higher for patients with MRD than for patients without MRD before transplantation (58% vs 14%). The 5-year overall survival rate is 26% and 79%, respectively, suggesting that the presence of pretransplant MRD is positively correlated with post-transplant relapse and mortality[151].
Table 4.
Main factors for post-hematopoietic stem cell transplantation relapse
|
Factors
|
Brief description
|
Ref.
|
| Disease type | The relapse rate is highest in ALL patients, followed by AML patients and CML patients | [161] |
| Pretransplant disease status | The risk of relapse is significantly higher in nonremission patients and patients with a high level of residual leukemia cells before transplantation | [151] |
| Risk stratification | The level of risk is positively correlated with the relapse rate and negatively correlated with the disease-free survival rate | [162] |
| Stem cell source | Peripheral blood stem cells contain more lymphocytes with a more potent GVL effect; as a result, the relapse rate of BMT is higher than that of PBSCT | [163,164] |
| Preconditioning | Myeloablative preconditioning is more effective in reducing post-transplant relapse than reduced intensity conditioning and nonmyeloablative preconditioning; T cell depletion is associated with increased relapse rates in CML and AML | [164,165] |
| GVHD | Post-transplant GVHD, especially cGVHD, is associated with a significantly lower relapse rate and a higher survival rate | [166,167] |
ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; BMT: Bone marrow transplantation; cGVHD: Chronic GVHD; CML: Chronic myeloid leukemia; GVHD: Graft versus host disease; GVL: Graft versus leukemia; PBSCT: Peripheral blood stem cell transplantation; Ref.: Reference.
Kebriaei et al[151] retrospectively analyzed the data of 68 adult patients with AML/myelodysplastic syndrome and found that the transplantation outcome was inversely related to the pretransplant tumor load. The mortality rate due to post-transplant relapse increased 1.21 times for every 10% increase in the percentage of leukemia blasts in the BM before transplantation. These findings suggest that reducing the pretransplant tumor burden and achieving stable disease or remission before transplantation are critical for reducing post-transplant relapse. This requires preparatory regimens that maximize leukemia cell removal without increasing side effects. Clinical experience shows that the low selectivity of traditional chemotherapy drugs for leukemia cells is an important factor for pretransplant preconditioning. Therefore, improving selectivity with targeted drugs, such as inhibitors of BCR-ABL or FLT3, as well as targeting LSCs, may offer treatment breakthroughs[152,153].
Over the last decade, the tumor-specific killing prodrug strategy based on the high level of ROS in tumor cells has provided a novel method for improving chemotherapy selectivity, enhancing efficacy, and reducing side effects[154,155]. Recently, several studies confirmed through in vivo and in vitro experiments that ROS-responsive anticancer prodrugs with ROS-sensitive linkers have precise killing effects on various types of leukemia cells and do not damage normal cells[156-160]. However, ROS-responsive anticancer prodrugs are ineffective in clearing MRD because the level of intracellular ROS in quiescent LSCs may be too low to activate the prodrug system, thus sparing these LSCs[161-167].
Another mechanism involved in post-allo-HSCT relapse is HLA loss, which has been reported in HSCT from both unrelated donors as well as sibling donors. Loss of HLA antigens reduces the efficacy of the GVL effect and favors the immune escape of AML cells. In haploidentical HSCT, as there is no incompatible target to stimulate alloreactivity, the GVL effect remains low[168,169]. Wu et al[169] analyzed nearly 800 cases of AML and ALL that were subjected, following an ATG T cell-replete conditioning regimen, to haploidentical HSCT and delineated that relapse occurred faster in AML patients who experienced loss of HLA antigens vs those who did not (223 d vs 321 d, P = 0.03). The factors linked with HLA loss in AML were aGVHD (odds ratio = 4.84) and body mass index < 18.5 kg/m2 (odds ratio = 0.10). Similarly, Jan et al[170] evaluated HLA loss in the setting of haploidentical HSCT and concluded that minor HLA antigens might be involved in the process of immune recognition.
Prevention and pre-emptive treatment of post-allo-HSCT relapse remain major challenges for hematologists who manage individuals diagnosed with AML. The choice of therapy is dictated by measurable residual disease levels. If MRD is undetectable, subjects should undergo maintenance therapy, whereas detectable MRD requires pre-emptive management strategies, e.g., DLIs[171]. A recently published meta-analysis highlighted that FLT3 inhibitors are a safe and tolerable therapy option for individuals who undergo allo-HSCT for FLT3-mutated AML. The use of these pharmacological agents as maintenance therapy post-allo-HSCT was associated with prolonged overall and relapse-free survival, with no significant differences between the treatment and control groups in terms of non-relapse mortality, GVHD, or adverse events[172]. Moreover, sorafenib maintenance therapy following allo-HSCT for FLT3-mutated AML was linked with increased overall survival and reduced cumulative incidence of relapse in AML patients who were subjected to allo-HSCT in the first complete remission[173].
Similarly, Fathi et al[174] explored, in the setting of a clinical trial, the benefits of 100 mg/d enasidenib maintenance post-allo-HSCT for IDH2-mutated AML. In their investigation, 2-year progression-free survival was 69%, overall survival was 74%, and the cumulative incidence of moderate/severe GVHD and of relapse were 42% and 16%, respectively, with only 1 patient experiencing AML relapse while on enasidenib maintenance. Another attractive option for post-allo-HSCT maintenance in the management of AML is represented by hypomethylating agents, namely azacitidine and decitabine. A meta-analysis of 14 studies delineated that the use of hypomethylating agents in this setting was correlated with reduced rates of cumulative incidence of relapse and GVHD, as well as higher rates of overall and relapse-free survival vs observation only[175]. Similarly, the combination of low-dose decitabine and venetoclax, i.e. a BCL-2 inhibitor, was associated with lower lates of relapse in high-risk AML patients who received this combination as maintenance therapy post-allo-HSCT[171,175].
Many strategies have been developed for post-allo-HSCT relapse, including withdrawal of immunosuppressants, immunotherapy, DLI, radiotherapy and chemotherapy, molecular targeted drugs, and second transplantation. At present, DLI is the most used and most effective in clinical practice. However, the efficacy of DLI varies greatly for different types of hematological malignancies. Clinical data show that DLI enables most patients with relapsed chronic myeloid leukemia to achieve CR in the early stage of relapse, while the remission rate is lower than 30% for patients with relapsed acute leukemia[169]. The main side effects of DLI are GVHD and pancytopenia. Data have indicated that, after DLI, aGVHD and/or cGVHD will be diagnosed in approximately one-third of the subjects. Moreover, 5%-20% of these individuals will experience treatment-related mortality following DLI[176,177]. To reduce DLI-related side effects, transplant specialists are modifying traditional DLI. Clinical experience shows that several modification measures, such as selective deletion of CD8+ cells and escalating cell dosage regimens, have decreased GVHD-related morbidity without any impact on the DLI-mediated effect of GVL[178]. However, these methods cannot completely eliminate the risk of GVHD. Currently, researchers are developing conditional suicide protocols utilizing the HSV-tk or fas receptor-derived genes to achieve selective killing at will of the transduced cells if uncontrollable GVHD develops[178].
Apart from DLIs, other cell-based therapies, such as a second allo-HSCT, as well as chimeric antigen receptor (CAR)-T and CAR-NK cell-based treatments, have been developed. A second allo-HSCT can be attempted in younger patients, in whom relapse occurs at least 6 mo after the first allo-HSCT and who already have a matched related donor following the first allo-HSCT. However, there is a current need to conduct prospective studies to assess the benefits and risk of a second allo-HSCT, as most data have been derived from retrospective investigations. Impressive overviews of a second allo-HSCT in the setting of relapsed AML post-allo-HSCT has been published elsewhere[171,179].
Hypomethylating agents, i.e. azacitidine and decitabine, IDH1/2 inhibitors, and venetoclax have been recognized as members of the therapeutic armamentarium in the setting of post-allo-HSCT AML relapse as well. In addition, immune checkpoint inhibitors (e.g., ipililumab and magrolimab), monoclonal antibodies (gemtuzumab ozogamicin and the anti-IL3 agent CLS360) and vaccines are displaying promising results. In addition, several novel targeted agents are currently being developed and/or investigated[171,179]: Small-molecule inhibitors (apart from FLT3 inhibitors and the BCL-2 inhibitor venetoclax), trametinib (anti-MEK agent), glasdegib (a molecule that interacts with the Hedgehog pathway), and uproleselan (anti-E-selectin agent); histone-deacetylase inhibitors, panobinostat; and IDH1/2 inhibitors, ivosidenib and enasidenib.
In addition, the survival of individuals who have undergone allo-HSCT is also affected by the compatibility of specific HLA loci of the donor and recipient. A recent publication pointed out that HLA matching and the age of the recipient are simple factors that can accurately stratify subjects into prognostic groups as well as predict overall survival and non-relapse mortality in allo-HSCT[180]. However, a meta-analysis of 19 investigations with a patient sample of 3336 individuals concluded that mismatched allo-HSCT from unrelated donors remains a safe procedure that is linked with favorable outcomes[181].
Furthermore, several predictors of relapse in haploidentical HSCT have been identified. For example, as compared to intermediate cytogenetic risk, higher relapse rates and shorter overall survival were noted in patients diagnosed with AML with adverse risk cytogenetic abnormalities who were subjected to haploidentical HSCT without T cell depletion[182]. Pre-allo-HSCT MRD levels are also implicated in the outcome of haploidentical HSCT. Zhang et al[183] demonstrated that AML subjects with undetectable MRD pre-allo-HSCT registered elevated overall survival and disease-free survival vs MRD-positive cases. In addition, cumulative incidence of relapse was similar between MRD-positive and MRD-negative cases in the setting of haploidentical allo-HSCT, which was also linked with a better prognosis vs HLA-matched allo-HSCT for individuals who remained MRD-positive pre-allo-HSCT.
Similarly, Al Hamed et al[184] identified predictors of relapse in NMP1-mutated AML individuals who were subjected to haploidentical HSCT. Detectable MRD pre-allo-HSCT, presence of FLT3 mutations, and allo-HSCT in the second complete remission negatively impacted leukemia-free survival and were linked with elevated percentages of relapsed cases. Overall survival was shorter in cases with concomitant detectable MRD pre-allo-HSCT, presence of FLT3 mutations, and older age, whereas haploidentical HSCT was correlated with elevated overall survival rates. Similarly, Canaani et al[185] confirmed that MRD status pre-allo-HSCT was a predictor of relapse following haploidentical HSCT. Undetectable MRD was correlated with elevated percentages of leukemia-free survival and reduced relapse rates, whereas haploidentical HSCT in MRD-positive AML cases was linked with better outcomes when the donor had anti-cytomegalovirus antibodies.
In recent years, with continuous advancements in immunology, novel cellular immunotherapies such as CAR-T cells have emerged and are being investigated in clinical trials, generating certain effects, such as significantly enhancing the capacity of immune cells to specifically recognize and kill leukemia cells. However, there are some obstacles for the clinical application of CAR-T cell therapy. For example, CAR-T cells target only cover certain types of leukemia, with a risk of attacking normal tissues and cells due to off-target effects and an inflammatory storm. Moreover, more research is needed to validate the long-term effects of CAR-T cell therapy[186-189]. The implementation of CAR-T cell therapy in AML is extremely challenging as the targeted antigen needs to be primarily expressed by AML blast cells and not by hematopoietic cells, activated T cells, or other cells in the body. In addition, the targeted antigen should be involved in or be a driver of the proliferation of AML blast cells as well as be present solely on AML blast cells and LSCs.
Currently, the following antigens have been studied as potential targets of CAR-T cell therapy in AML: CD33, CD123, CD38, FLT3, Lewis Y, NKG2D ligand, CD116, CD117, CD70, CD93, CD44v6, CD276, CLL1, ILT3, TIM-3, Siglec-6, FRβ, h8F4, and the PR1/HLA-2 complex. Moreover, antigen pairs have also been studied by molecular biology techniques, with several research teams identifying CD33+ADGRE2, CLEC12A+CCR1, CD33+CD70, CD33+TIM3, CLL1+TIM3, CLL1+CD123 and CLL1+CD33 as potential candidates for the “ideal antigen” for CAR-T cell therapy. Furthermore, as the manufacture of autologous CAR-T cells can require several weeks, the development of allogeneic CAR-T and allogeneic CAR-NK cell therapies has also been taken into consideration but has failed to produce satisfactory results in the management of AML due to toxicity.
Another potential future strategy is to target AML-associated, e.g., WT1 or PR1, rather than AML-specific antigens using peptide vaccines[137,190,191]. However, at present, allo-HSCT remains the standard of care for individuals diagnosed with AML and who display evidence of intermediate or unfavorable risk, and the potential benefits of CAR-T cell therapy in conjunction with pharmacological agents and/or allo-HSCT in the management of AML remains to be decided in future studies[192].
In addition, other cell-based therapies, such as CAR-NK cell therapies, have emerged from the drug pipeline landscape. Ureña-Bailén et al[193] reported that NK-92 cells transduced with CD276-CAR constructs with a triple knock-out of CBLB, NKG2A, and TIGIT (inhibitory checkpoints of NK cells), CD276-CAR-NK-92 with CBLB knock-out as well as CD276-CAR-NK-92 with TIGIT knock-out exerted significant cytotoxicity against cellular models of AML. Similarly, CD123-CAR-NK constructs exhibited antileukemic potential and a satisfactory safety profile in a cellular model of CD123+ AML[194]. Similarly, NPM1-mutation-specific T cell receptor-like CAR cytokine-induced memory-like NK cell constructs displayed significant antileukemic potential against a cellular model and patient-derived NMP1-mutated AML samples[195]. Thus, we hypothesize that NK-CAR constructs might emerge as future therapies of AML.
Monitoring is critical for the prevention and treatment of post-allo-HSCT relapse. It also plays an important role in the long-term survival of leukemia patients. Flow cytometry is useful for identifying leukemia-related abnormal phenotypes, real-time quantitative PCR can be used to detect leukemia-specific fusion genes, and fluorescence in situ hybridization can detect specific chromosomal translocations or deletions. These methods can be used to monitor MRD to facilitate the early detection of post-transplant relapse[196,197].
In recent years, next-generation sequencing technology has been widely used in the clinic. Because next-generation sequencing has the advantages of high throughput, accurate quantification and high sensitivity, it is of great significance for evaluating the curative effect, guiding treatment and predicting relapse[198]. In addition, graft mosaicism is a highly sensitive measure for predicting relapse and guiding immune intervention[199]. MRD monitoring may be combined with graft mosaicism monitoring. In cases of elevated MRD or decreased donor mosaicism, immunosuppressants may be reduced or stopped, and targeted drugs or DLI may be used for timely intervention[200-202]. Currently, no consensus has been established for the frequency of and cutoff values for MRD and graft mosaicism monitoring, and the technologies and methods must be further standardized. Moreover, further research is needed to investigate the timing of monitoring, how fast to reduce or stop immunosuppressants, the timing of DLI, and the number of cells infused.
OTHER COMPLICATIONS OF ALLO-HSCT
Although only briefly discussed in this narrative review, allo-HSCT can also be associated with various complications. A serious complication of allo-HSCT is graft failure. Graft failure can be either primary, i.e. HSCs from the donor fail to engraft at all, or secondary, i.e. HSCs from the donor engraft successfully but a loss of donor cells occurs at some time point[203,204]. In addition, poor graft function has also been identified as a complication of allo-HSCT, yet it must be differentiated from graft failure. In both graft failure and poor graft function, cytopenias are present, the bone marrow is hypocellular, and there is no evidence of relapse. In terms of chimerism, poor graft function is associated with full-donor chimerism, whereas in graft failure it is either full-recipient or mixed. Initial donor engraftment is noted in both primary and secondary poor graft function, and also in secondary graft failure but not in primary graft failure. However, initial hematological recovery only occurs in secondary graft failure and secondary poor graft function, whereas it is absent in both primary graft failure and primary poor graft function.
Risk factors for graft failure include major ABO incompatibility, HLA mismatch, pretransplantation MRD and disease type, stem cell source and dose, conditioning regimen, and others, whereas poor graft function seems to be influenced more by the presence of BM fibrosis, damage to HSCs or stromal cells caused by the selected conditioning regimen or other pharmacological agents, infections, or GVHD as well as a low infusion dose of HSCs. Graft failure, poor graft function, and their management have been reviewed elsewhere[203,204].
In addition, apart from cGVHD, allo-HSCT poses the threat and several late onset complications that can develop in the context of GVHD or accompany it. Late-onset complications of allo-HSCT can affect the skin and mucosa, eyes, gastrointestinal tract, lungs (e.g., bronchiolitis obliterans syndrome), muscles and connective tissue, endocrine system and the metabolism (hypogonadism, thyroid dysfunction, osteoporosis, diabetes), kidneys, nervous system, and/or the heart. In addition, infections (e.g., with viruses such as varicella zoster virus, Epstein-Barr virus, or cytomegalovirus reactivation, fungi, or encapsulated bacteria) and the development of secondary malignancies in allo-HSCT recipients have emerged as “swords of Damocles” in the survival of AML patients in the post-allo-HSCT setting. These complications have been discussed in detail elsewhere[205,206].
A recent investigation of over 40000 leukemia patients who were subjected to allo-HSCT revealed that the most frequent late-onset complications of this therapeutic procedure were azoospermia (approximately 71.0%), cGVHD (5-year post-allo-HSCT prevalence at approximately 43.0%), secondary malignancies (20-year post-allo-HSCT prevalence at approximately 21.0%), depression (post-allo-HSCT prevalence at approximately 18.0%), hypothyroidism (15-year post-allo-HSCT prevalence at approximately 11.0%), bronchiolitis obliterans syndrome (4-mo post-allo-HSCT prevalence at approximately 10.0%), cardiovascular disease (15-year post-allo-HSCT prevalence at approximately 7.5%), and avascular necrosis (10-year post-allo-HSCT prevalence at approximately 5.0%)[207]. However, future prospective studies are needed to clarify the exact epidemiology of late complications of allo-HSCT.
Future directions of research in the field of allo-HSCT should also focus on potential opportunities in this expanding field, such as the combination of allo-HSCT with CAR-T cell based therapies, the application of novel drugs in conditioning regimens, the use of ATG in combination with post-transplant cyclophosphamide, and others.
CONCLUSION
With continuous developments in immunology, molecular biology, and related disciplines, allo-HSCT is advancing rapidly with proven results and has emerged as a key factor in the management of AML. In recent years, with improved preconditioning regimens, optimized donor selection strategies, novel targeted drugs, and monoclonal antibodies, the incidence and severity of transplant-related complications have been greatly reduced, and the long-term survival of leukemia patients after allo-HSCT has significantly improved. In-depth research on the molecular mechanisms that drive AML will ensure the development of better treatments to further improve remission, prevent relapse, manage early and late onset complications of allo-HSCT, and improve patient survival.
Footnotes
Conflict-of-interest statement: All authors declare having no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 10, 2022
First decision: November 16, 2022
Article in press: January 5, 2023
Specialty type: Hematology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Serafi I, Sweden; Feng Y, China; Goebel WS, United States; Zhang R, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Yong-Feng Chen, Department of Basic Medical Sciences, School of Medicine of Taizhou University, Taizhou University, Taizhou 318000, Zhejiang Province, China.
Jing Li, Department of Histology and Embryology, North Sichuan Medical College, Nanchong 637000, Sichuan Province, China.
Ling-Long Xu, Department of Hematology, Taizhou Central Hospital, Taizhou 318000, Zhejiang Province, China.
Mihnea-Alexandru Găman, Faculty of Medicine, “Carol Davila” University of Medicine and Pharmacy, Bucharest 050474, Romania. mihneagaman@yahoo.com.
Zhen-You Zou, Department of Scientific Research,Brain Hospital of Guangxi Zhuang Autonomous Region, Liuzhou 545005, Guangxi Zhuang Autonomous Region, China.
References
- 1.Moses BS, Slone WL, Thomas P, Evans R, Piktel D, Angel PM, Walsh CM, Cantrell PS, Rellick SL, Martin KH, Simpkins JW, Gibson LF. Bone marrow microenvironment modulation of acute lymphoblastic leukemia phenotype. Exp Hematol . 2016;44:50–9.e1. doi: 10.1016/j.exphem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adair JE, Kubek SP, Kiem HP. Hematopoietic Stem Cell Approaches to Cancer. Hematol Oncol Clin North Am . 2017;31:897–912. doi: 10.1016/j.hoc.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Dalle IA, Paranal R, Zarka J, Paul S, Sasaki K, Li W, Ning J, Short NJ, Ohanian M, Cortes JE, Jabbour EJ, Issa GC. Impact of luteinizing hormone suppression on hematopoietic recovery after intensive chemotherapy in patients with leukemia. Haematologica . 2021;106:1097–1105. doi: 10.3324/haematol.2020.256453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloos RQH, Pieters R, van den Bos C, van Eijkelenburg NKA, de Jonge R, van der Sluis IM. The effect of asparaginase therapy on methotrexate toxicity and efficacy in children with acute lymphoblastic leukemia. Leuk Lymphoma . 2019;60:3002–3010. doi: 10.1080/10428194.2019.1613537. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Liang Y, Luo X, Hu Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis . 2020;11:291. doi: 10.1038/s41419-020-2488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, de Lima M, Fathi AT, Foran JM, Gojo I, Hall AC, Jacoby M, Lancet J, Mannis G, Marcucci G, Martin MG, Mims A, Neff J, Nejati R, Olin R, Percival ME, Prebet T, Przespolewski A, Rao D, Ravandi-Kashani F, Shami PJ, Stone RM, Strickland SA, Sweet K, Vachhani P, Wieduwilt M, Gregory KM, Ogba N, Tallman MS. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J Natl Compr Canc Netw . 2021;19:16–27. doi: 10.6004/jnccn.2021.0002. [DOI] [PubMed] [Google Scholar]
- 7.Marquez-Curtis LA, Turner AR, Sridharan S, Ratajczak MZ, Janowska-Wieczorek A. The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev Rep. 2011;7:590–607. doi: 10.1007/s12015-010-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Găman MA, Cozma MA, Dobrică EC, Crețoiu SM, Găman AM, Diaconu CC. Liquid Biopsy and Potential Liquid Biopsy-Based Biomarkers in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Life (Basel) 2021;11:677.. doi: 10.3390/life11070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Broxmeyer HE. Progress towards improving homing and engraftment of hematopoietic stem cells for clinical transplantation. Curr Opin Hematol . 2019;26:266–272. doi: 10.1097/MOH.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 10.Ratajczak MZ, Suszynska M. Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells. Stem Cell Rev Rep . 2016;12:121–128. doi: 10.1007/s12015-015-9625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: From physiology to therapeutics. Stem Cells . 2020;38:1241–1253. doi: 10.1002/stem.3242. [DOI] [PubMed] [Google Scholar]
- 12.Jiang P, Cai Y, Zhou X, Yang J, Tong Y, Huang C, Qiu H, Zhou K, Xu X, Zhang Y, Niu J, Shen C, Xia X, Wei Y, Song X, Wan L. Immune reconstitution and survival of patients after allogeneic hematopoietic stem cell transplantation from older donors. Clin Transplant . 2022:e14844. doi: 10.1111/ctr.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JS, Harley BA. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci Adv . 2017;3:e1600455. doi: 10.1126/sciadv.1600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastelaro de Rezende M, Zenker Justo G, Julian Paredes-Gamero E, Gosens R. Wnt-5A/B Signaling in Hematopoiesis throughout Life. Cells . 2020;9:1801. doi: 10.3390/cells9081801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wielockx B, Grinenko T, Mirtschink P, Chavakis T. Hypoxia Pathway Proteins in Normal and Malignant Hematopoiesis. Cells . 2019;8:155. doi: 10.3390/cells8020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirth F, Lubosch A, Hamelmann S, Nakchbandi IA. Fibronectin and Its Receptors in Hematopoiesis. Cells . 2020;9:2717. doi: 10.3390/cells9122717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanetti C, Krause DS. "Caught in the net": the extracellular matrix of the bone marrow in normal hematopoiesis and leukemia. Exp Hematol . 2020;89:13–25. doi: 10.1016/j.exphem.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Cao H, Cao B, Heazlewood CK, Domingues M, Sun X, Debele E, McGregor NE, Sims NA, Heazlewood SY, Nilsson SK. Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche. Cells . 2019;8:985. doi: 10.3390/cells8090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Windisch R, Pirschtat N, Kellner C, Chen-Wichmann L, Lausen J, Humpe A, Krause DS, Wichmann C. Oncogenic Deregulation of Cell Adhesion Molecules in Leukemia. Cancers (Basel) . 2019;11:311. doi: 10.3390/cancers11030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caocci G, Greco M, La Nasa G. Bone Marrow Homing and Engraftment Defects of Human Hematopoietic Stem and Progenitor Cells. Mediterr J Hematol Infect Dis . 2017;9:e2017032. doi: 10.4084/MJHID.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami JL, Xu B, Franco CB, Hu X, Galli SJ, Weissman IL, Chen CC. Evidence that β7 Integrin Regulates Hematopoietic Stem Cell Homing and Engraftment Through Interaction with MAdCAM-1. Stem Cells Dev . 2016;25:18–26. doi: 10.1089/scd.2014.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golan K, Singh AK, Kollet O, Bertagna M, Althoff MJ, Khatib-Massalha E, Petrovich-Kopitman E, Wellendorf AM, Massalha H, Levin-Zaidman S, Dadosh T, Bohan B, V Gawali M, Dasgupta B, Lapidot T, Cancelas JA. Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood . 2020;136:2607–2619. doi: 10.1182/blood.2020005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol . 2003;31:1284–1291. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Zhang B, Wang S, Zhang J, Liu Y, Wang J, Fan Z, Lv Y, Zhang X, He L, Chen L, Xia H, Li Y, Pei X. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci Rep . 2015;5:12993. doi: 10.1038/srep12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Ding L, Zhang B, Deng Z, Han Y, Wang S, Yang S, Fan Z, Zhang J, Yan H, Han D, He L, Yue W, Wang H, Li Y, Pei X. Thrombopoietin enhances hematopoietic stem and progenitor cell homing by impeding matrix metalloproteinase 9 expression. Stem Cells Transl Med . 2020;9:661–673. doi: 10.1002/sctm.19-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao H, Heazlewood SY, Williams B, Cardozo D, Nigro J, Oteiza A, Nilsson SK. The role of CD44 in fetal and adult hematopoietic stem cell regulation. Haematologica . 2016;101:26–37. doi: 10.3324/haematol.2015.135921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito-Reis CA, Marjon KD, Pascetti EM, Floren M, Gillette JM. The tetraspanin CD82 regulates bone marrow homing and engraftment of hematopoietic stem and progenitor cells. Mol Biol Cell . 2018;29:2946–2958. doi: 10.1091/mbc.E18-05-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood . 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam BS, Cunningham C, Adams GB. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood . 2011;117:1167–1175. doi: 10.1182/blood-2010-05-286294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood . 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi N, Zhang TT, Nakanishi T. Involvement of CXCR4 in Normal and Abnormal Development. Cells . 2019;8:185. doi: 10.3390/cells8020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirvaikar N, Marquez-Curtis LA, Janowska-Wieczorek A. Hematopoietic Stem Cell Mobilization and Homing after Transplantation: The Role of MMP-2, MMP-9, and MT1-MMP. Biochem Res Int . 2012;2012:685267. doi: 10.1155/2012/685267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alomari M, Almohazey D, Almofty SA, Khan FA, Al Hamad M, Ababneh D. Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation. Cells . 2019;8:630. doi: 10.3390/cells8060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crippa S, Bernardo ME. Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation. Hemasphere . 2018;2:e151. doi: 10.1097/HS9.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Luo X, Zou Z, Liang Y. The Role of Reactive Oxygen Species in Tumor Treatment and its Impact on Bone Marrow Hematopoiesis. Curr Drug Targets . 2020;21:477–498. doi: 10.2174/1389450120666191021110208. [DOI] [PubMed] [Google Scholar]
- 36.Shipounova IN, Petinati NA, Bigildeev AE, Drize NJ, Sorokina TV, Kuzmina LA, Parovichnikova EN, Savchenko VG. Alterations of the bone marrow stromal microenvironment in adult patients with acute myeloid and lymphoblastic leukemias before and after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma . 2017;58:408–417. doi: 10.1080/10428194.2016.1187277. [DOI] [PubMed] [Google Scholar]
- 37.Ciciarello M, Corradi G, Loscocco F, Visani G, Monaco F, Cavo M, Curti A, Isidori A. The Yin and Yang of the Bone Marrow Microenvironment: Pros and Cons of Mesenchymal Stromal Cells in Acute Myeloid Leukemia. Front Oncol . 2019;9:1135. doi: 10.3389/fonc.2019.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv . 2020;4:5877–5887. doi: 10.1182/bloodadvances.2020002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalnapurkar S, Moirangthem RD, Singh S, Limaye L, Kale V. Microvesicles Secreted by Nitric Oxide-Primed Mesenchymal Stromal Cells Boost the Engraftment Potential of Hematopoietic Stem Cells. Stem Cells . 2019;37:128–138. doi: 10.1002/stem.2912. [DOI] [PubMed] [Google Scholar]
- 40.Mohammadali F, Abroun S, Atashi A. Mild hypoxia and human bone marrow mesenchymal stem cells synergistically enhance expansion and homing capacity of human cord blood CD34+ stem cells. Iran J Basic Med Sci . 2018;21:709–716. doi: 10.22038/IJBMS.2018.26820.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elmariah H, Naqvi SMH, Kim J, Nishihori T, Mishra A, Perez L, Faramand R, Lazaryan A, Liu HD, Khimani F, Nieder M, Anasetti C, Pidala J, Bejanyan N. Impact of infused CD34+ stem cell dosing for allogeneic peripheral blood stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant . 2021;56:1683–1690. doi: 10.1038/s41409-021-01219-8. [DOI] [PubMed] [Google Scholar]
- 42.Patel SS, Rybicki LA, Corrigan D, Dumont C, Bolwell B, Dean R, Figueroa P, Hanna R, Liu H, Gerds AT, Hill B, Jagadeesh D, Kalaycio M, Pohlman B, Ricci K, Sobecks R, Lu W, Hamilton BK, Majhail NS. Effect of bone marrow CD34+cells and T-cell subsets on clinical outcomes after myeloablative allogeneic hematopoietic cell transplantation. Bone Marrow Transplant . 2019;54:775–781. doi: 10.1038/s41409-018-0380-5. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Xu LP, Liu KY, Chen H, Chen YH, Zhang XH, Wang Y, Wang FR, Han W, Wang JZ, Yan CH, Huang XJ. Higher dose of CD34+ peripheral blood stem cells is associated with better survival after haploidentical stem cell transplantation in pediatric patients. Clin Transplant . 2017;31:e12880. doi: 10.1111/ctr.12880. [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Almaguer D, Gómez-Peña Á, Jaime-Pérez JC, Gómez-Guijosa MÁ, Cantú-Rodríguez O, Gutiérrez-Aguirre H, Martínez-Cabriales SA, García-Rodríguez F, Olguín-Ramírez LA, Salazar-Riojas R, Méndez-Ramírez N. Higher doses of CD34+ progenitors are associated with improved overall survival without increasing GVHD in reduced intensity conditioning allogeneic transplant recipients with clinically advanced disease. J Clin Apher . 2013;28:349–355. doi: 10.1002/jca.21278. [DOI] [PubMed] [Google Scholar]
- 45.Martin PS, Li S, Nikiforow S, Alyea EP 3rd, Antin JH, Armand P, Cutler CS, Ho VT, Kekre N, Koreth J, Luckey CJ, Ritz J, Soiffer RJ. Infused total nucleated cell dose is a better predictor of transplant outcomes than CD34+ cell number in reduced-intensity mobilized peripheral blood allogeneic hematopoietic cell transplantation. Haematologica . 2016;101:499–505. doi: 10.3324/haematol.2015.134841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Díez-Campelo M, Pérez-Simón JA, Ocio EM, Castilla C, González-Porras JR, Sánchez-Guijo FM, Vázquez L, Caballero MD, Cañizo MC, San Miguel JF. CD34 + cell dose and outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Leuk Lymphoma . 2005;46:177–183. doi: 10.1080/10428190400014900. [DOI] [PubMed] [Google Scholar]
- 47.Fan Q, Liu H, Liang X, Yang T, Fan Z, Huang F, Ling Y, Liao X, Xuan L, Xu N, Xu X, Ye J, Liu Q. Superior GVHD-free, relapse-free survival for G-BM to G-PBSC grafts is associated with higher MDSCs content in allografting for patients with acute leukemia. J Hematol Oncol . 2017;10:135. doi: 10.1186/s13045-017-0503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao XS, Chen Y, Zhao XY, Liu DH, Xu LP, Wang Y, Han W, Chen YH, Chen H, Zhang XH, Liu KY, Huang XJ. Improved outcomes using G-CSF-mobilized blood and bone marrow grafts as the source of stem cells compared with G-PB after HLA-identical sibling transplantation in patients with acute leukemia. Clin Transplant . 2013;27:844–851. doi: 10.1111/ctr.12225. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Jiang M, Xu C, Chen J, Li B, Wang J, Hu J, Ning H, Chen H, Chen S, Hu L. Granulocyte colony-stimulating factor-primed bone marrow: an excellent stem-cell source for transplantation in acute myelocytic leukemia and chronic myelocytic leukemia. Chin Med J (Engl) . 2015;128:20–24. doi: 10.4103/0366-6999.147790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang YJ, Huang XJ. Use of G-CSF-stimulated marrow in allogeneic hematopoietic stem cell transplantation settings: a comprehensive review. Clin Transplant . 2011;25:13–23. doi: 10.1111/j.1399-0012.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- 51.Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, Chen H, Wang FR, Mo XD, Zhang YY, Huo MR, Zhao XS, Y K, Liu KY, Huang XJ. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol . 2015;8:84. doi: 10.1186/s13045-015-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciurea SO, Al Malki MM, Kongtim P, Zou J, Aung FM, Rondon G, Chen J, Taniguchi M, Otoukesh S, Nademanee A, Forman SJ, Champlin R, Gendzekhadze K, Cao K. Treatment of allosensitized patients receiving allogeneic transplantation. Blood Adv . 2021;5:4031–4043. doi: 10.1182/bloodadvances.2021004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang F, Xu Y, Chen H, Xu L, Zhang X, Wang Y, Liu Q, Wu D, Huang X. Comparison of the clinical outcomes of hematologic malignancies after myeloablative haploidentical transplantation with G-CSF/ATG and posttransplant cyclophosphamide: results from the Chinese Bone Marrow Transplantation Registry Group (CBMTRG) Sci China Life Sci . 2020;63:571–581. doi: 10.1007/s11427-019-9594-7. [DOI] [PubMed] [Google Scholar]
- 54.Baumeister SHC, Rambaldi B, Shapiro RM, Romee R. Key Aspects of the Immunobiology of Haploidentical Hematopoietic Cell Transplantation. Front Immunol . 2020;11:191. doi: 10.3389/fimmu.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He XC, Li Z, Sugimura R, Ross J, Zhao M, Li L. Homing and migration assays of hematopoietic stem/progenitor cells. Methods Mol Biol . 2014;1185:279–284. doi: 10.1007/978-1-4939-1133-2_19. [DOI] [PubMed] [Google Scholar]
- 56.Baron F, Nagler A. Novel strategies for improving hematopoietic reconstruction after allogeneic hematopoietic stem cell transplantation or intensive chemotherapy. Expert Opin Biol Ther . 2017;17:163–174. doi: 10.1080/14712598.2017.1269167. [DOI] [PubMed] [Google Scholar]
- 57.Goto T, Murata M, Nishida T, Terakura S, Kamoshita S, Ishikawa Y, Ushijima Y, Adachi Y, Suzuki S, Kato K, Hirakawa A, Nishiwaki S, Nishio N, Takahashi Y, Kodera Y, Matsushita T, Kiyoi H. Phase I clinical trial of intra-bone marrow cotransplantation of mesenchymal stem cells in cord blood transplantation. Stem Cells Transl Med . 2021;10:542–553. doi: 10.1002/sctm.20-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura K, Inaba M, Sugiura K, Yoshimura T, Kwon AH, Kamiyama Y, Ikehara S. Enhancement of allogeneic hematopoietic stem cell engraftment and prevention of GVHD by intra-bone marrow bone marrow transplantation plus donor lymphocyte infusion. Stem Cells . 2004;22:125–134. doi: 10.1634/stemcells.22-2-125. [DOI] [PubMed] [Google Scholar]
- 59.Chen C, Su Y, Chen J, Song Y, Zhuang R, Xiao B, Guo S. Specially modified stromal and immune microenvironment in injected bone marrow following intrabone transplantation facilitates allogeneic hematopoietic stem cell engraftment. Exp Hematol . 2016;44:614–623.e3. doi: 10.1016/j.exphem.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol . 2008;21:579–596. doi: 10.1016/j.beha.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hattori N, Saito B, Sasaki Y, Shimada S, Murai S, Abe M, Baba Y, Watanuki M, Fujiwara S, Kawaguchi Y, Arai N, Kabasawa N, Tsukamoto H, Uto Y, Ariizumi H, Yanagisawa K, Harada H, Nakamaki T. Status of Natural Killer Cell Recovery in Day 21 Bone Marrow after Allogeneic Hematopoietic Stem Cell Transplantation Predicts Clinical Outcome. Biol Blood Marrow Transplant . 2018;24:1841–1847. doi: 10.1016/j.bbmt.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 62.de Koning C, Plantinga M, Besseling P, Boelens JJ, Nierkens S. Immune Reconstitution after Allogeneic Hematopoietic Cell Transplantation in Children. Biol Blood Marrow Transplant . 2016;22:195–206. doi: 10.1016/j.bbmt.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Bae KW, Kim BE, Koh KN, Im HJ, Seo JJ. Factors influencing lymphocyte reconstitution after allogeneic hematopoietic stem cell transplantation in children. Korean J Hematol . 2012;47:44–52. doi: 10.5045/kjh.2012.47.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charrier E, Cordeiro P, Brito RM, Mezziani S, Herblot S, Le Deist F, Duval M. Reconstitution of maturating and regulatory lymphocyte subsets after cord blood and BMT in children. Bone Marrow Transplant . 2013;48:376–382. doi: 10.1038/bmt.2012.176. [DOI] [PubMed] [Google Scholar]
- 65.Dębska-Zielkowska J, Moszkowska G, Zieliński M, Zielińska H, Dukat-Mazurek A, Trzonkowski P, Stefańska K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells . 2021;10:1777. doi: 10.3390/cells10071777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol . 2012;19:324–335. doi: 10.1097/MOH.0b013e328353bc7d. [DOI] [PubMed] [Google Scholar]
- 67.Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L. NK Cells Mediate a Crucial Graft-versus-Leukemia Effect in Haploidentical-HSCT to Cure High-Risk Acute Leukemia. Trends Immunol . 2018;39:577–590. doi: 10.1016/j.it.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Gao F, Ye Y, Gao Y, Huang H, Zhao Y. Influence of KIR and NK Cell Reconstitution in the Outcomes of Hematopoietic Stem Cell Transplantation. Front Immunol . 2020;11:2022. doi: 10.3389/fimmu.2020.02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SY, Lee H, Han MS, Shim H, Eom HS, Park B, Kong SY. Post-Transplantation Natural Killer Cell Count: A Predictor of Acute Graft-Versus-Host Disease and Survival Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation. Clin Lymphoma Myeloma Leuk . 2016;16:527–535.e2. doi: 10.1016/j.clml.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka J, Miller JS. Recent progress in and challenges in cellular therapy using NK cells for hematological malignancies. Blood Rev . 2020;44:100678. doi: 10.1016/j.blre.2020.100678. [DOI] [PubMed] [Google Scholar]
- 71.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood . 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klangsinsirikul P, Carter GI, Byrne JL, Hale G, Russell NH. Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood . 2002;99:2586–2591. doi: 10.1182/blood.v99.7.2586. [DOI] [PubMed] [Google Scholar]
- 73.Chklovskaia E, Nowbakht P, Nissen C, Gratwohl A, Bargetzi M, Wodnar-Filipowicz A. Reconstitution of dendritic and natural killer-cell subsets after allogeneic stem cell transplantation: effects of endogenous flt3 ligand. Blood . 2004;103:3860–3868. doi: 10.1182/blood-2003-04-1200. [DOI] [PubMed] [Google Scholar]
- 74.Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, Najfeld V, Phelps RG, Grosskreutz C, Scigliano E, Frenette PS, Merad M. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med . 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Auffermann-Gretzinger S, Eger L, Bornhäuser M, Schäkel K, Oelschlaegel U, Schaich M, Illmer T, Thiede C, Ehninger G. Fast appearance of donor dendritic cells in human skin: dynamics of skin and blood dendritic cells after allogeneic hematopoietic cell transplantation. Transplantation . 2006;81:866–873. doi: 10.1097/01.tp.0000203318.16224.57. [DOI] [PubMed] [Google Scholar]
- 76.Auffermann-Gretzinger S, Lossos IS, Vayntrub TA, Leong W, Grumet FC, Blume KG, Stockerl-Goldstein KE, Levy R, Shizuru JA. Rapid establishment of dendritic cell chimerism in allogeneic hematopoietic cell transplant recipients. Blood . 2002;99:1442–1448. doi: 10.1182/blood.v99.4.1442. [DOI] [PubMed] [Google Scholar]
- 77.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med . 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 78.Yu H, Tian Y, Wang Y, Mineishi S, Zhang Y. Dendritic Cell Regulation of Graft-Vs.-Host Disease: Immunostimulation and Tolerance. Front Immunol . 2019;10:93. doi: 10.3389/fimmu.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant . 2003;9:170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 80.Stern L, McGuire H, Avdic S, Rizzetto S, Fazekas de St Groth B, Luciani F, Slobedman B, Blyth E. Mass Cytometry for the Assessment of Immune Reconstitution After Hematopoietic Stem Cell Transplantation. Front Immunol . 2018;9:1672. doi: 10.3389/fimmu.2018.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA, Witherspoon RP, Bensinger W, Flowers ME, Martin P, Storb R, Appelbaum FR, Boeckh M. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood . 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 82.Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant . 1993;12:387–398. [PubMed] [Google Scholar]
- 83.Mehta RS, Rezvani K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence . 2016;7:901–916. doi: 10.1080/21505594.2016.1208866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krenger W, Blazar BR, Holländer GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood . 2011;117:6768–6776. doi: 10.1182/blood-2011-02-334623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han H, Yan H, King KY. Broad-Spectrum Antibiotics Deplete Bone Marrow Regulatory T Cells. Cells . 2021;10:277. doi: 10.3390/cells10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang S, Tsang J, Tam P. Regulatory T cell immunotherapy for transplantation tolerance: step into clinic. Int Immunopharmacol . 2010;10:1486–1490. doi: 10.1016/j.intimp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Elias S, Rudensky AY. Therapeutic use of regulatory T cells for graft-versus-host disease. Br J Haematol . 2019;187:25–38. doi: 10.1111/bjh.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whangbo JS, Antin JH, Koreth J. The role of regulatory T cells in graft-versus-host disease management. Expert Rev Hematol . 2020;13:141–154. doi: 10.1080/17474086.2020.1709436. [DOI] [PubMed] [Google Scholar]
- 89.Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, Herrera MI, Reynolds CG, Alyea EP, Ho VT, Koreth J, Armand P, Chen YB, Ballen K, Soiffer RJ, Antin JH, Cutler CS, Ritz J. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant . 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalwak K, Gorczyńska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, Latos-Grazyńska E, Król M, Boguslawska-Jaworska J, Chybicka A. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol . 2002;118:74–89. doi: 10.1046/j.1365-2141.2002.03560.x. [DOI] [PubMed] [Google Scholar]
- 91.Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation . 2002;73:1154–1158. doi: 10.1097/00007890-200204150-00026. [DOI] [PubMed] [Google Scholar]
- 92.Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Luo X, Curtsinger J, Mehta RS, Warlick E, Cooley SA, Blazar BR, Miller JS, Weisdorf D, Wagner JE, Verneris MR. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv . 2018;2:909–922. doi: 10.1182/bloodadvances.2017014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood . 2014;124:3201–3211. doi: 10.1182/blood-2014-07-589176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH, Champlin RE, Confer DL, DiPersio JF, Fernandez-Viña M, Hartzman RJ, Horowitz MM, Hurley CK, Karanes C, Maiers M, Mueller CR, Perales MA, Setterholm M, Woolfrey AE, Yu N, Eapen M. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood . 2016;127:260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens . 2012;79:83–89. doi: 10.1111/j.1399-0039.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- 96.Jiménez M, Martínez C, Ercilla G, Carreras E, Urbano-Ispízua A, Aymerich M, Villamor N, Amézaga N, Rovira M, Fernández-Avilés F, Gaya A, Martino R, Sierra J, Montserrat E. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol . 2005;33:1240–1248. doi: 10.1016/j.exphem.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 97.Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, Sandmaier B, Maloney D, Storb R, Storek J. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol . 2003;31:941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Perez L, van Roon L, Schilham MW, Lankester AC, Pike-Overzet K, Staal FJT. Combining Mobilizing Agents with Busulfan to Reduce Chemotherapy-Based Conditioning for Hematopoietic Stem Cell Transplantation. Cells . 2021;10:1077. doi: 10.3390/cells10051077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Maas NG, Berghuis D, van der Burg M, Lankester AC. B Cell Reconstitution and Influencing Factors After Hematopoietic Stem Cell Transplantation in Children. Front Immunol . 2019;10:782. doi: 10.3389/fimmu.2019.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hobbs GS, Perales MA. Effects of T-Cell Depletion on Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in AML Patients. J Clin Med . 2015;4:488–503. doi: 10.3390/jcm4030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J, Xu LP, Bian Z, Chang YJ, Wang Y, Zhang XH, Huang XJ. Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. J Transl Med . 2015;13:391. doi: 10.1186/s12967-015-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdel-Azim H, Elshoury A, Mahadeo KM, Parkman R, Kapoor N. Humoral Immune Reconstitution Kinetics after Allogeneic Hematopoietic Stem Cell Transplantation in Children: A Maturation Block of IgM Memory B Cells May Lead to Impaired Antibody Immune Reconstitution. Biol Blood Marrow Transplant . 2017;23:1437–1446. doi: 10.1016/j.bbmt.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Willemsen L, Jol-van der Zijde CM, Admiraal R, Putter H, Jansen-Hoogendijk AM, Ostaijen-Ten Dam MM, Wijnen JT, van Kesteren C, Waaijer JL, Lankester AC, Bredius RG, van Tol MJ. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant . 2015;21:473–482. doi: 10.1016/j.bbmt.2014.11.674. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, Chen HM, Ma G, Zhou Z, Raulet D, Rivera AL, Chen SH, Pan PY. The mechanistic study behind suppression of GVHD while retaining GVL activities by myeloid-derived suppressor cells. Leukemia . 2019;33:2078–2089. doi: 10.1038/s41375-019-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang YJ, Zhao XY, Huang XJ. Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease. Front Immunol . 2018;9:3041. doi: 10.3389/fimmu.2018.03041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet . 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Min CK. The pathophysiology of chronic graft-versus-host disease: the unveiling of an enigma. Korean J Hematol . 2011;46:80–87. doi: 10.5045/kjh.2011.46.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tugues S, Amorim A, Spath S, Martin-Blondel G, Schreiner B, De Feo D, Lutz M, Guscetti F, Apostolova P, Haftmann C, Hasselblatt P, Núñez NG, Hottiger MO, van den Broek M, Manz MG, Zeiser R, Becher B. Graft-versus-host disease, but not graft-versus-leukemia immunity, is mediated by GM-CSF-licensed myeloid cells. Sci Transl Med . 2018;10:eaat8410. doi: 10.1126/scitranslmed.aat8410. [DOI] [PubMed] [Google Scholar]
- 109.Booth C, Veys P. T cell depletion in paediatric stem cell transplantation. Clin Exp Immunol . 2013;172:139–147. doi: 10.1111/cei.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho C, Perales MA. Expanding Therapeutic Opportunities for Hematopoietic Stem Cell Transplantation: T Cell Depletion as a Model for the Targeted Allograft. Annu Rev Med . 2019;70:381–393. doi: 10.1146/annurev-med-120617-041210. [DOI] [PubMed] [Google Scholar]
- 111.Rådestad E, Sundin M, Törlén J, Thunberg S, Önfelt B, Ljungman P, Watz E, Mattsson J, Uhlin M. Individualization of Hematopoietic Stem Cell Transplantation Using Alpha/Beta T-Cell Depletion. Front Immunol . 2019;10:189. doi: 10.3389/fimmu.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rådestad E, Wikell H, Engström M, Watz E, Sundberg B, Thunberg S, Uzunel M, Mattsson J, Uhlin M. Alpha/beta T-cell depleted grafts as an immunological booster to treat graft failure after hematopoietic stem cell transplantation with HLA-matched related and unrelated donors. J Immunol Res . 2014;2014:578741. doi: 10.1155/2014/578741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Handgretinger R, Schilbach K. The potential role of γδ T cells after allogeneic HCT for leukemia. Blood . 2018;131:1063–1072. doi: 10.1182/blood-2017-08-752162. [DOI] [PubMed] [Google Scholar]
- 114.Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, Lamb LS. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant . 2007;39:751–757. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]
- 115.Li Pira G, Malaspina D, Girolami E, Biagini S, Cicchetti E, Conflitti G, Broglia M, Ceccarelli S, Lazzaro S, Pagliara D, Meschini A, Bertaina A, Montanari M, Locatelli F. Selective Depletion of αβ T Cells and B Cells for Human Leukocyte Antigen-Haploidentical Hematopoietic Stem Cell Transplantation. A Three-Year Follow-Up of Procedure Efficiency. Biol Blood Marrow Transplant . 2016;22:2056–2064. doi: 10.1016/j.bbmt.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 116.Bertaina A, Roncarolo MG. Graft Engineering and Adoptive Immunotherapy: New Approaches to Promote Immune Tolerance After Hematopoietic Stem Cell Transplantation. Front Immunol . 2019;10:1342. doi: 10.3389/fimmu.2019.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dutt S, Tseng D, Ermann J, George TI, Liu YP, Davis CR, Fathman CG, Strober S. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol . 2007;179:6547–6554. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 118.Bleakley M. Naive T-cell depletion in stem cell transplantation. Blood Adv . 2020;4:4980. doi: 10.1182/bloodadvances.2020001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, Gooley TA, Sommermeyer F, Riddell SR, Shlomchik WD. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest . 2015;125:2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teschner D, Distler E, Wehler D, Frey M, Marandiuc D, Langeveld K, Theobald M, Thomas S, Herr W. Depletion of naive T cells using clinical grade magnetic CD45RA beads: a new approach for GVHD prophylaxis. Bone Marrow Transplant . 2014;49:138–144. doi: 10.1038/bmt.2013.114. [DOI] [PubMed] [Google Scholar]
- 121.Mutis T. Targeting alloreactive donor T-cells to hematopoietic system-restricted minor histocompatibility antigens to dissect graft-versus-leukemia effects from graft-versus-host disease after allogeneic stem cell transplantation. Int J Hematol . 2003;78:208–212. doi: 10.1007/BF02983796. [DOI] [PubMed] [Google Scholar]
- 122.Li JM, Giver CR, Lu Y, Hossain MS, Akhtari M, Waller EK. Separating graft-versus-leukemia from graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Immunotherapy . 2009;1:599–621. doi: 10.2217/imt.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Budde H, Papert S, Maas JH, Reichardt HM, Wulf G, Hasenkamp J, Riggert J, Legler TJ. Prediction of graft-versus-host disease: a biomarker panel based on lymphocytes and cytokines. Ann Hematol . 2017;96:1127–1133. doi: 10.1007/s00277-017-2999-5. [DOI] [PubMed] [Google Scholar]
- 124.Martínez-Laperche C, Buces E, Aguilera-Morillo MC, Picornell A, González-Rivera M, Lillo R, Santos N, Martín-Antonio B, Guillem V, Nieto JB, González M, de la Cámara R, Brunet S, Jiménez-Velasco A, Espigado I, Vallejo C, Sampol A, Bellón JM, Serrano D, Kwon M, Gayoso J, Balsalobre P, Urbano-Izpizua Á, Solano C, Gallardo D, Díez-Martín JL, Romo J, Buño I GVHD/Immunotherapy Committee of the Spanish Group for Hematopoietic Transplantation. A novel predictive approach for GVHD after allogeneic SCT based on clinical variables and cytokine gene polymorphisms. Blood Adv . 2018;2:1719–1737. doi: 10.1182/bloodadvances.2017011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin Z, Xu L, Li Y. Approaches for generation of anti-leukemia specific T cells. Cell Regen . 2018;7:40–44. doi: 10.1016/j.cr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klobuch S, Hammon K, Vatter-Leising S, Neidlinger E, Zwerger M, Wandel A, Neuber LM, Heilmeier B, Fichtner R, Mirbeth C, Herr W, Thomas S. HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation. Cells . 2020;9:1264. doi: 10.3390/cells9051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, Traversari C, Sanvito F, Toma S, Radrizzani M, La Seta-Catamancio S, Ciceri F, Bordignon C, Bonini C. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood . 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- 128.Zeiser R. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol. 2019;187:563–572. doi: 10.1111/bjh.16190. [DOI] [PubMed] [Google Scholar]
- 129.Zamai L, Del Zotto G, Buccella F, Gabrielli S, Canonico B, Artico M, Ortolani C, Papa S. Understanding the Synergy of NKp46 and Co-Activating Signals in Various NK Cell Subpopulations: Paving the Way for More Successful NK-Cell-Based Immunotherapy. Cells . 2020;9:753. doi: 10.3390/cells9030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Negrin RS. Graft-versus-host disease versus graft-versus-leukemia. Hematology Am Soc Hematol Educ Program . 2015;2015:225–230. doi: 10.1182/asheducation-2015.1.225. [DOI] [PubMed] [Google Scholar]
- 131.Thangavelu G, Blazar BR. Achievement of Tolerance Induction to Prevent Acute Graft-vs.-Host Disease. Front Immunol . 2019;10:309. doi: 10.3389/fimmu.2019.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim S, Santhanam S, Lim S, Choi J. Targeting Histone Deacetylases to Modulate Graft-Versus-Host Disease and Graft-Versus-Leukemia. Int J Mol Sci . 2020;21:4281. doi: 10.3390/ijms21124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rezvani K, Barrett AJ. Characterizing and optimizing immune responses to leukaemia antigens after allogeneic stem cell transplantation. Best Pract Res Clin Haematol . 2008;21:437–453. doi: 10.1016/j.beha.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol . 2021;14:4. doi: 10.1186/s13045-020-01017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ruiz-Delgado GJ, León Peña AA, Gómez-de-León A, Ruiz-Argüelles GJ. Clearance of donor cell leukemia by means of graft versus leukemia effect: A case report. Hematology . 2016;21:470–473. doi: 10.1080/10245332.2015.1117741. [DOI] [PubMed] [Google Scholar]
- 136.Sala-Torra O, Hanna C, Loken MR, Flowers ME, Maris M, Ladne PA, Mason JR, Senitzer D, Rodriguez R, Forman SJ, Deeg HJ, Radich JP. Evidence of donor-derived hematologic malignancies after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant . 2006;12:511–517. doi: 10.1016/j.bbmt.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 137.Pasvolsky O, Daher M, Alatrash G, Marin D, Daver N, Ravandi F, Rezvani K, Shpall E, Kebriaei P. CARving the Path to Allogeneic CAR T Cell Therapy in Acute Myeloid Leukemia. Front Oncol . 2021;11:800110. doi: 10.3389/fonc.2021.800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Williams L, Doucette K, Karp JE, Lai C. Genetics of donor cell leukemia in acute myelogenous leukemia and myelodysplastic syndrome. Bone Marrow Transplant . 2021;56:1535–1549. doi: 10.1038/s41409-021-01214-z. [DOI] [PubMed] [Google Scholar]
- 139.Deshmukh KG, Kelemen K. Lessons Learned from Donor Cell-Derived Myeloid Neoplasms: Report of Three Cases and Review of the Literature. Life (Basel) . 2022;12:559. doi: 10.3390/life12040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aldoss I, Clark M, Marcucci G, Forman SJ. Donor derived leukemia in allogeneic transplantation. Leuk Lymphoma. 2021;62:2823–2830. doi: 10.1080/10428194.2021.1929966. [DOI] [PubMed] [Google Scholar]
- 141.Waanders E, Gu Z, Dobson SM, Antić Ž, Crawford JC, Ma X, Edmonson MN, Payne-Turner D, van de Vorst M, Jongmans MCJ, McGuire I, Zhou X, Wang J, Shi L, Pounds S, Pei D, Cheng C, Song G, Fan Y, Shao Y, Rusch M, McCastlain K, Yu J, van Boxtel R, Blokzijl F, Iacobucci I, Roberts KG, Wen J, Wu G, Ma J, Easton J, Neale G, Olsen SR, Nichols KE, Pui CH, Zhang J, Evans WE, Relling MV, Yang JJ, Thomas PG, Dick JE, Kuiper RP, Mullighan CG. Mutational landscape and patterns of clonal evolution in relapsed pediatric acute lymphoblastic leukemia. Blood Cancer Discov . 2020;1:96–111. doi: 10.1158/0008-5472.BCD-19-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mat Yusoff Y, Ahid F, Abu Seman Z, Abdullah J, Kamaluddin NR, Esa E, Zakaria Z. Comprehensive analysis of mutations and clonal evolution patterns in a cohort of patients with cytogenetically normal acute myeloid leukemia. Mol Cytogenet . 2021;14:45. doi: 10.1186/s13039-021-00561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dobson SM, García-Prat L, Vanner RJ, Wintersinger J, Waanders E, Gu Z, McLeod J, Gan OI, Grandal I, Payne-Turner D, Edmonson MN, Ma X, Fan Y, Voisin V, Chan-Seng-Yue M, Xie SZ, Hosseini M, Abelson S, Gupta P, Rusch M, Shao Y, Olsen SR, Neale G, Chan SM, Bader G, Easton J, Guidos CJ, Danska JS, Zhang J, Minden MD, Morris Q, Mullighan CG, Dick JE. Relapse-Fated Latent Diagnosis Subclones in Acute B Lineage Leukemia Are Drug Tolerant and Possess Distinct Metabolic Programs. Cancer Discov . 2020;10:568–587. doi: 10.1158/2159-8290.CD-19-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, Medeiros JJF, Rao-Bhatia A, Jaciw-Zurakowsky I, Marke R, McLeod JL, Doedens M, Bader G, Voisin V, Xu C, McPherson JD, Hudson TJ, Wang JCY, Minden MD, Dick JE. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature . 2017;547:104–108. doi: 10.1038/nature22993. [DOI] [PubMed] [Google Scholar]
- 145.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, Perrelli NF, Cosentino C, Torri F, Angius A, Forno B, Casucci M, Bernardi M, Peccatori J, Corti C, Bondanza A, Ferrari M, Rossini S, Roncarolo MG, Bordignon C, Bonini C, Ciceri F, Fleischhauer K. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med . 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 146.Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, Barcella M, Spinelli O, Greco R, Crucitti L, Cieri N, Noviello M, Manfredi F, Montaldo E, Ostuni R, Naldini MM, Gentner B, Waterhouse M, Zeiser R, Finke J, Hanoun M, Beelen DW, Gojo I, Luznik L, Onozawa M, Teshima T, Devillier R, Blaise D, Halkes CJM, Griffioen M, Carrabba MG, Bernardi M, Peccatori J, Barlassina C, Stupka E, Lazarevic D, Tonon G, Rambaldi A, Cittaro D, Bonini C, Fleischhauer K, Ciceri F, Vago L. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med . 2019;25:603–611. doi: 10.1038/s41591-019-0400-z. [DOI] [PubMed] [Google Scholar]
- 147.Hutten TJA, Norde WJ, Woestenenk R, Wang RC, Maas F, Kester M, Falkenburg JHF, Berglund S, Luznik L, Jansen JH, Schaap N, Dolstra H, Hobo W. Increased Coexpression of PD-1, TIGIT, and KLRG-1 on Tumor-Reactive CD8(+) T Cells During Relapse after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant . 2018;24:666–677. doi: 10.1016/j.bbmt.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 148.Hammrich J, Wittig S, Ernst T, Gruhn B. CTLA-4 polymorphism rs231775: Influence on relapse and survival after allogeneic hematopoietic stem cell transplantation in childhood. Eur J Haematol . 2019;102:251–255. doi: 10.1111/ejh.13200. [DOI] [PubMed] [Google Scholar]
- 149.Leung AY, Tse E, Hwang YY, Chan TS, Gill H, Chim CS, Lie AK, Kwong YL. Primary treatment of leukemia relapses after allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning second transplantation from the original donor. Am J Hematol . 2013;88:485–491. doi: 10.1002/ajh.23439. [DOI] [PubMed] [Google Scholar]
- 150.Xuan L, Fan Z, Zhang Y, Zhou H, Huang F, Dai M, Nie D, Lin D, Xu N, Guo X, Jiang Q, Sun J, Xiao Y, Liu Q. Sequential intensified conditioning followed by prophylactic DLI could reduce relapse of refractory acute leukemia after allo-HSCT. Oncotarget . 2016;7:32579–32591. doi: 10.18632/oncotarget.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kebriaei P, Kline J, Stock W, Kasza K, Le Beau MM, Larson RA, van Besien K. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant . 2005;35:965–970. doi: 10.1038/sj.bmt.1704938. [DOI] [PubMed] [Google Scholar]
- 152.Chen Y, Li J, Xu L, Găman MA, Zou Z. The genesis and evolution of acute myeloid leukemia stem cells in the microenvironment: From biology to therapeutic targeting. Cell Death Discov . 2022;8:397. doi: 10.1038/s41420-022-01193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sen R, Natarajan K, Bhullar J, Shukla S, Fang HB, Cai L, Chen ZS, Ambudkar SV, Baer MR. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol Cancer Ther . 2012;11:2033–2044. doi: 10.1158/1535-7163.MCT-12-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen Y, Li J, Zhao Z. Redox Control in Acute Lymphoblastic Leukemia: From Physiology to Pathology and Therapeutic Opportunities. Cells . 2021;10:1218. doi: 10.3390/cells10051218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen YF, Liu H, Luo XJ, Zhao Z, Zou ZY, Li J, Lin XJ, Liang Y. The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol Hematol . 2017;112:21–30. doi: 10.1016/j.critrevonc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 156.Chen W, Fan H, Balakrishnan K, Wang Y, Sun H, Fan Y, Gandhi V, Arnold LA, Peng X. Discovery and Optimization of Novel Hydrogen Peroxide Activated Aromatic Nitrogen Mustard Derivatives as Highly Potent Anticancer Agents. J Med Chem . 2018;61:9132–9145. doi: 10.1021/acs.jmedchem.8b00559. [DOI] [PubMed] [Google Scholar]
- 157.Liao Y, Xu L, Ou S, Edwards H, Luedtke D, Ge Y, Qin Z. H(2)O(2)/Peroxynitrite-Activated Hydroxamic Acid HDAC Inhibitor Prodrugs Show Antileukemic Activities against AML Cells. ACS Med Chem Lett . 2018;9:635–640. doi: 10.1021/acsmedchemlett.8b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hagen H, Marzenell P, Jentzsch E, Wenz F, Veldwijk MR, Mokhir A. Aminoferrocene-based prodrugs activated by reactive oxygen species. J Med Chem . 2012;55:924–934. doi: 10.1021/jm2014937. [DOI] [PubMed] [Google Scholar]
- 159.Marzenell P, Hagen H, Sellner L, Zenz T, Grinyte R, Pavlov V, Daum S, Mokhir A. Aminoferrocene-based prodrugs and their effects on human normal and cancer cells as well as bacterial cells. J Med Chem . 2013;56:6935–6944. doi: 10.1021/jm400754c. [DOI] [PubMed] [Google Scholar]
- 160.Daum S, Chekhun VF, Todor IN, Lukianova NY, Shvets YV, Sellner L, Putzker K, Lewis J, Zenz T, de Graaf IA, Groothuis GM, Casini A, Zozulia O, Hampel F, Mokhir A. Improved synthesis of N-benzylaminoferrocene-based prodrugs and evaluation of their toxicity and antileukemic activity. J Med Chem . 2015;58:2015–2024. doi: 10.1021/jm5019548. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, Chen H, Chen YH, Wang FR, Wang JZ, Sun YQ, Huang XJ. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer . 2013;119:978–985. doi: 10.1002/cncr.27761. [DOI] [PubMed] [Google Scholar]
- 162.Armand P, Kim HT, Zhang MJ, Perez WS, Dal Cin PS, Klumpp TR, Waller EK, Litzow MR, Liesveld JL, Lazarus HM, Artz AS, Gupta V, Savani BN, McCarthy PL, Cahn JY, Schouten HC, Finke J, Ball ED, Aljurf MD, Cutler CS, Rowe JM, Antin JH, Isola LM, Di Bartolomeo P, Camitta BM, Miller AM, Cairo MS, Stockerl-Goldstein K, Sierra J, Savoie ML, Halter J, Stiff PJ, Nabhan C, Jakubowski AA, Bunjes DW, Petersdorf EW, Devine SM, Maziarz RT, Bornhauser M, Lewis VA, Marks DI, Bredeson CN, Soiffer RJ, Weisdorf DJ. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant . 2012;18:280–288. doi: 10.1016/j.bbmt.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Urbano-Ispizua A. Risk assessment in haematopoietic stem cell transplantation: stem cell source. Best Pract Res Clin Haematol . 2007;20:265–280. doi: 10.1016/j.beha.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 164.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol . 2008;142:877–888. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 165.Atilla E, Ataca Atilla P, Demirer T. A Review of Myeloablative vs Reduced Intensity/Non-Myeloablative Regimens in Allogeneic Hematopoietic Stem Cell Transplantations. Balkan Med J . 2017;34:1–9. doi: 10.4274/balkanmedj.2017.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Z, Yin C, Zhang W, Tang W, Song X, Hu X, Ni X, Qiu H, Yang J, Hu J, Wang J. The benefit of chronic graft-versus-host disease in patients with acute myeloid leukemia relapsed after allogeneic stem cell transplantation. Ann Hematol . 2019;98:1765–1773. doi: 10.1007/s00277-019-03682-2. [DOI] [PubMed] [Google Scholar]
- 167.Shokouhi S, Bray S, Bakhtiyari S, Sayehmiri K, Alimoghadam K, Ghavamzadeh A. Effects of aGVHD and cGVHD on Survival Rate in Patients with Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation. Int J Hematol Oncol Stem Cell Res . 2015;9:112–121. [PMC free article] [PubMed] [Google Scholar]
- 168.Arnold PY. Review: HLA loss and detection in the setting of relapse from HLA-mismatched hematopoietic cell transplant. Hum Immunol . 2022;83:712–720. doi: 10.1016/j.humimm.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 169.Wu H, Shi J, Luo Y, Yu J, Lai X, Liu L, Fu H, Ouyang G, Xu X, Xiao H, Huang H, Zhao Y. Assessment of Patient-Specific Human Leukocyte Antigen Genomic Loss at Relapse After Antithymocyte Globulin-Based T-Cell-Replete Haploidentical Hematopoietic Stem Cell Transplant. JAMA Netw Open . 2022;5:e226114. doi: 10.1001/jamanetworkopen.2022.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, Wollison BM, Ducar MD, Thorner AR, Leppanen S, Baronas J, Stevens J, Lane WJ, Kekre N, Ho VT, Koreth J, Cutler CS, Nikiforow S, Alyea EP 3rd, Antin JH, Soiffer RJ, Ritz J, Lindsley RC, Ebert BL. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation. Blood Adv . 2019;3:2199–2204. doi: 10.1182/bloodadvances.2019000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kreidieh F, Abou Dalle I, Moukalled N, El-Cheikh J, Brissot E, Mohty M, Bazarbachi A. Relapse after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia: an overview of prevention and treatment. Int J Hematol . 2022;116:330–340. doi: 10.1007/s12185-022-03416-7. [DOI] [PubMed] [Google Scholar]
- 172.Fei X, Zhang S, Gu J, Wang J. FLT3 inhibitors as maintenance therapy post allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia patients with FLT3 mutations: A meta-analysis. Cancer Med . 2022 doi: 10.1002/cam4.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aydin S, Passera R, Scaldaferri M, Dellacasa CM, Poggiu M, Cattel F, Zallio F, Brunello L, Giaccone L, Dogliotti I, Busca A. Sorafenib maintenance after hematopoietic stem cell transplantation improves outcome of FLT3-ITD-mutated acute myeloid leukemia. Int J Hematol . 2022;116:883–891. doi: 10.1007/s12185-022-03427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fathi AT, Kim HT, Soiffer RJ, Levis MJ, Li S, Kim AS, Mims AS, DeFilipp Z, El-Jawahri A, McAfee SL, Brunner AM, Narayan R, Knight LW, Kelley D, Bottoms AS, Perry LH, Wahl JL, Brock J, Breton E, Ho VT, Chen YB. Enasidenib as maintenance following allogeneic hematopoietic cell transplantation for IDH2-mutated myeloid malignancies. Blood Adv . 2022;6:5857–5865. doi: 10.1182/bloodadvances.2022008632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kungwankiattichai S, Ponvilawan B, Roy C, Tunsing P, Kuchenbauer F, Owattanapanich W. Maintenance With Hypomethylating Agents After Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Systematic Review and Meta-Analysis. Front Med (Lausanne) . 2022;9:801632. doi: 10.3389/fmed.2022.801632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cirillo M, Tan P, Sturm M, Cole C. Cellular Immunotherapy for Hematologic Malignancies: Beyond Bone Marrow Transplantation. Biol Blood Marrow Transplant . 2018;24:433–442. doi: 10.1016/j.bbmt.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 177.Tomblyn M, Lazarus HM. Donor lymphocyte infusions: the long and winding road: how should it be traveled? Bone Marrow Transplant . 2008;42:569–579. doi: 10.1038/bmt.2008.259. [DOI] [PubMed] [Google Scholar]
- 178.Stamouli M, Gkirkas K, Tsirigotis P. Strategies for improving the efficacy of donor lymphocyte infusion following stem cell transplantation. Immunotherapy . 2016;8:57–68. doi: 10.2217/imt.15.100. [DOI] [PubMed] [Google Scholar]
- 179.Leotta S, Condorelli A, Sciortino R, Milone GA, Bellofiore C, Garibaldi B, Schininà G, Spadaro A, Cupri A, Milone G. Prevention and Treatment of Acute Myeloid Leukemia Relapse after Hematopoietic Stem Cell Transplantation: The State of the Art and Future Perspectives. J Clin Med . 2022;11:253. doi: 10.3390/jcm11010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mushtaq MU, Shahzad M, Tariq E, Iqbal Q, Chaudhary SG, Zafar MU, Anwar I, Ahmed N, Bansal R, Singh AK, Abhyankar SH, Callander NS, Hematti P, McGuirk JP. Outcomes with mismatched unrelated donor allogeneic hematopoietic stem cell transplantation in adults: A systematic review and meta-analysis. Front Oncol . 2022;12:1005042. doi: 10.3389/fonc.2022.1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Garcia-Horton A, Cyriac SL, Gedde-Dahl T, Floisand Y, Remberger M, Mattsson J, Michelis FV. Patient age and donor HLA matching can stratify allogeneic hematopoietic cell transplantation patients into prognostic groups. Eur J Haematol . 2022;109:672–679. doi: 10.1111/ejh.13850. [DOI] [PubMed] [Google Scholar]
- 182.Nagler A, Labopin M, Dholaria B, Ciceri F, Fraccaroli A, Blaise D, Fanin R, Bruno B, Forcade E, Vydra J, Chevallier P, Bulabois CE, Jindra P, Bornhäuser M, Canaani J, Sanz J, Savani BN, Spyridonidis A, Giebel S, Brissot E, Bazarbachi A, Esteve J, Mohty M. Impact of Cytogenetic Risk on Outcomes of Non-T-Cell-Depleted Haploidentical Hematopoietic Cell Transplantation in Patients with Relapsed or Refractory Acute Myeloid Leukemia. Transplant Cell Ther . 2022;28:773.e1–773.e8. doi: 10.1016/j.jtct.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Y, Wang P, Cassady K, Zou Z, Li Y, Deng X, Yang W, Peng X, Zhang X, Feng Y. Pretransplantation minimal residual disease monitoring by multiparameter flow cytometry predicts outcomes of AML patients receiving allogeneic hematopoietic stem cell transplantation. Transpl Immunol . 2022;72:101596. doi: 10.1016/j.trim.2022.101596. [DOI] [PubMed] [Google Scholar]
- 184.Al Hamed R, Labopin M, Daguindau E, Niittyvuopio R, Huynh A, Socié G, Srour M, Henri Bourhis J, Kröger N, Tholouli E, Choi G, Poiré X, Martin H, Rubio MT, Jindra P, Blaise D, Beelen D, Labussière-Wallet H, Nagler A, Bazarbachi A, Mohty M. Measurable residual disease, FLT3-ITD mutation, and disease status have independent prognostic influence on outcome of allogeneic stem cell transplantation in NPM1-mutated acute myeloid leukemia. Cancer Med . 2022;11:1068–1080. doi: 10.1002/cam4.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Canaani J, Labopin M, Huang XJ, Ciceri F, Van Lint MT, Bruno B, Santarone S, Diez-Martin JL, Blaise D, Chiusolo P, Wu D, Mohty M, Nagler A. Minimal residual disease status predicts outcome of acute myeloid leukaemia patients undergoing T-cell replete haploidentical transplantation. An analysis from the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) Br J Haematol . 2018;183:411–420. doi: 10.1111/bjh.15540. [DOI] [PubMed] [Google Scholar]
- 186.Miazek-Zapala N, Slusarczyk A, Kusowska A, Zapala P, Kubacz M, Winiarska M, Bobrowicz M. The "Magic Bullet" Is Here? Cells . 2021;10:1511. doi: 10.3390/cells10061511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Britten O, Ragusa D, Tosi S, Kamel YM. MLL-Rearranged Acute Leukemia with t(4;11)(q21;q23)-Current Treatment Options. Is There a Role for CAR-T Cell Therapy? Cells . 2019;8:1341. doi: 10.3390/cells8111341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pellegrino M, Del Bufalo F, De Angelis B, Quintarelli C, Caruana I, de Billy E. Manipulating the Metabolism to Improve the Efficacy of CAR T-Cell Immunotherapy. Cells . 2020;10:14. doi: 10.3390/cells10010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Magnani CF, Tettamanti S, Alberti G, Pisani I, Biondi A, Serafini M, Gaipa G. Transposon-Based CAR T Cells in Acute Leukemias: Where are We Going? Cells . 2020;9:1337. doi: 10.3390/cells9061337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vishwasrao P, Li G, Boucher JC, Smith DL, Hui SK. Emerging CAR T Cell Strategies for the Treatment of AML. Cancers (Basel) . 2022;14:1241. doi: 10.3390/cancers14051241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Marvin-Peek J, Savani BN, Olalekan OO, Dholaria B. Challenges and Advances in Chimeric Antigen Receptor Therapy for Acute Myeloid Leukemia. Cancers (Basel) . 2022;14:497. doi: 10.3390/cancers14030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dholaria B, Savani BN, Hamilton BK, Oran B, Liu HD, Tallman MS, Ciurea SO, Holtzman NG, Ii GLP, Devine SM, Mannis G, Grunwald MR, Appelbaum F, Rodriguez C, El Chaer F, Shah N, Hashmi SK, Kharfan-Dabaja MA, DeFilipp Z, Aljurf M, AlShaibani A, Inamoto Y, Jain T, Majhail N, Perales MA, Mohty M, Hamadani M, Carpenter PA, Nagler A. Hematopoietic Cell Transplantation in the Treatment of Newly Diagnosed Adult Acute Myeloid Leukemia: An Evidence-Based Review from the American Society of Transplantation and Cellular Therapy. Transplant Cell Ther . 2021;27:6–20. doi: 10.1016/j.bbmt.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 193.Ureña-Bailén G, Dobrowolski JM, Hou Y, Dirlam A, Roig-Merino A, Schleicher S, Atar D, Seitz C, Feucht J, Antony JS, Mohammadian Gol T, Handgretinger R, Mezger M. Preclinical Evaluation of CRISPR-Edited CAR-NK-92 Cells for Off-the-Shelf Treatment of AML and B-ALL. Int J Mol Sci . 2022;23:12828. doi: 10.3390/ijms232112828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Caruso S, De Angelis B, Del Bufalo F, Ciccone R, Donsante S, Volpe G, Manni S, Guercio M, Pezzella M, Iaffaldano L, Silvestris DA, Sinibaldi M, Di Cecca S, Pitisci A, Velardi E, Merli P, Algeri M, Lodi M, Paganelli V, Serafini M, Riminucci M, Locatelli F, Quintarelli C. Safe and effective off-the-shelf immunotherapy based on CAR.CD123-NK cells for the treatment of acute myeloid leukaemia. J Hematol Oncol . 2022;15:163. doi: 10.1186/s13045-022-01376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dong H, Ham JD, Hu G, Xie G, Vergara J, Liang Y, Ali A, Tarannum M, Donner H, Baginska J, Abdulhamid Y, Dinh K, Soiffer RJ, Ritz J, Glimcher LH, Chen J, Romee R. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc Natl Acad Sci U S A . 2022;119:e2122379119. doi: 10.1073/pnas.2122379119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guan Y, Zhang M, Zhang W, Wang J, Shen K, Zhang K, Yang L, Huang L, Wang N, Xiao M, Zhou J. Clinical Utility of Droplet Digital PCR to Monitor BCR-ABL1 Transcripts of Patients With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Post-chimeric Antigen Receptor19/22 T-Cell Cocktail Therapy. Front Oncol . 2021;11:646499. doi: 10.3389/fonc.2021.646499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen X, Wood BL. Monitoring minimal residual disease in acute leukemia: Technical challenges and interpretive complexities. Blood Rev . 2017;31:63–75. doi: 10.1016/j.blre.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 198.Press RD, Eickelberg G, Froman A, Yang F, Stentz A, Flatley EM, Fan G, Lim JY, Meyers G, Maziarz RT, Cook RJ. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol . 2019;94:902–912. doi: 10.1002/ajh.25514. [DOI] [PubMed] [Google Scholar]
- 199.Jacque N, Nguyen S, Golmard JL, Uzunov M, Garnier A, Leblond V, Vernant JP, Bories D, Dhédin N. Chimerism analysis in peripheral blood using indel quantitative real-time PCR is a useful tool to predict post-transplant relapse in acute leukemia. Bone Marrow Transplant . 2015;50:259–265. doi: 10.1038/bmt.2014.254. [DOI] [PubMed] [Google Scholar]
- 200.Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, Gorin NC, Giebel S, Mohty M, Savani BN, Nagler A. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant . 2016;51:1431–1438. doi: 10.1038/bmt.2016.167. [DOI] [PubMed] [Google Scholar]
- 201.Mo XD, Lv M, Huang XJ. Preventing relapse after haematopoietic stem cell transplantation for acute leukaemia: the role of post-transplantation minimal residual disease (MRD) monitoring and MRD-directed intervention. Br J Haematol . 2017;179:184–197. doi: 10.1111/bjh.14778. [DOI] [PubMed] [Google Scholar]
- 202.Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH, Wang Y, Huang H, Bai H, Huang F, Ma X, Huang XJ. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft-versus-Host Disease-Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia. Biol Blood Marrow Transplant . 2017;23:1311–1319. doi: 10.1016/j.bbmt.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 203.Ozdemir ZN, Civriz Bozdağ S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci . 2018;57:163–167. doi: 10.1016/j.transci.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 204.Huang XJ. Overcoming graft failure after haploidentical transplantation: Is this a possibility? Best Pract Res Clin Haematol . 2021;34:101255. doi: 10.1016/j.beha.2021.101255. [DOI] [PubMed] [Google Scholar]
- 205.Atilla E, Atilla PA, Toprak SK, Demirer T. A review of late complications of allogeneic hematopoietic stem cell transplantations. Clin Transplant . 2017;31:e13062. doi: 10.1111/ctr.13062. [DOI] [PubMed] [Google Scholar]
- 206.Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother . 2016;22:505–514. doi: 10.1016/j.jiac.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 207.Kanagasundram S, Amini F. Late Complications of Allogenic Stem Cells Transplantation in Leukaemia. Tissue Eng Regen Med . 2019;16:1–9. doi: 10.1007/s13770-018-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]