Abstract
Amebic liver abscesses (ALAs) are the most commonly encountered extraintestinal manifestation of human invasive amebiasis, which results from Entamoeba histolytica (E. histolytica) spreading extraintestinally. Amebiasis can be complicated by liver abscess in 9% of cases, and ALAs led to almost 50000 fatalities worldwide in 2010. Although there have been fewer and fewer cases in the past several years, ALAs remain an important public health problem in endemic areas. E. histolytica causes both amebic colitis and liver abscess by breaching the host’s innate defenses and invading the intestinal mucosa. Trophozoites often enter the circulatory system, where they are filtered in the liver and produce abscesses, and develop into severe invasive diseases such as ALAs. The clinical presentation can appear to be colitis, including upper-right abdominal pain accompanied by a fever in ALA cases. Proper diagnosis requires nonspecific liver imaging as well as detecting anti-E. histolytica antibodies; however, these antibodies cannot be used to distinguish between a previous infection and an acute infection. Therefore, diagnostics primarily aim to use PCR or enzyme-linked immunosorbent assay to detect E. histolytica. ALAs can be treated medically, and percutaneous catheter drainage is only necessary in approximately 15% of cases. The indicated treatment is to administer an amebicidal drug (such as tinidazole or metronidazole) and paromomycin or other luminal cysticidal agent for clinical disease. Prognosis is good with almost universal recovery. Establishing which diagnostic methods are most efficacious will necessitate further analysis of similar clinical cases.
Keywords: Amebic liver abscess, Entamoeba histolytica, Polymerase chain reaction, Enzyme-linked immunosorbent assay, Percutaneous catheter drainage, Amebicidal drug
Core Tip: Amebic liver abscesses are the most commonly encountered extraintestinal manifestation of human invasive amebiasis, which results from Entamoeba histolytica. It breaches the host’s innate defenses and invades the intestinal mucosa. Trophozoites enter the circulatory system and are filtered in the liver and produce abscesses. The diagnostics primarily aim to use PCR or enzyme-linked immunosorbent assay to detect Entamoeba histolytica. Medical treatment of amebic liver abscesses is possible using an amebicidal drug and a luminal cysticidal agent. Prognoses are generally good. Elucidating the detailed pathogenesis and establishing which diagnostic methods are most efficacious will necessitate further analyses of similar clinical cases.
INTRODUCTION
General information on amebic liver abscess
Worldwide the most commonly encountered manifestation of invasive extraintestinal amebiasis in humans is amebic liver abscesses (ALAs), which occur when Entamoeba histolytica (E. histolytica) spreads extraintestinally[1-5]. In 9% of cases, liver abscesses develop as a complication of amebiasis, and in 2010, ALAs led to a total of nearly 50000 fatalities[6,7]. Though cases have declined in number in recent years, ALAs are still a major public health issue within endemic areas[8]. ALA patients may present with what appears to be colitis, with pain in the upper right abdomen and sometimes accompanied by a fever; however, asymptomatic infections may also occur[1,9]. Hepatitis E virus infection and amebiasis are endemic in India and coexisting acute hepatitis E and ALA has also been reported[10]. The aim of this review was to share the general information of ALA and its pathogenesis, examinations, diagnosis, treatment, complications, prognosis, and prevention.
General information on amebiasis
E. histolytica is an anaerobic parasitic invasive enteric protozoan, and infections of E. histolytica correlate to high mortality and morbidity rates[11,12]. Each year, this protozoan causes 40000-100000 deaths, ranking only behind malaria in patient mortality[13-15]. According to a previous report, invasive amebiasis develops in fewer than one-tenth of patient infections[11]. The geographic distribution of amebiasis has worldwide amplitude and a high rate of incidence, and it remains a public health concern in low- and middle-income developing countries in the tropics, particularly in environments that are crowded and lacking in adequate sanitation and clean water due to the oral-fecal route of pathogen transmission (including ingestion of food or water that contains cysts from this protozoan)[6,16-19]. On the other hand, this pathogen is only rarely seen in wealthier countries but is epidemiologically growing; in particular, recent immigrants from endemic regions (or travelers returning from a long-term stay in an endemic region) have a greater risk of developing amebiasis[6,20-22].
Maintaining a high index of suspicion is recommended for amebiasis regarding other groups that are at greater risk, such as men who have sex with men, people with acquired immunodeficiency syndrome or HIV, immunocompromised hosts such as patients with cirrhosis, or people who reside in group homes or mental health facilities[6,23]. In particular, relatively large numbers of cases have been reported in Japan in individuals infected with HIV-1, and it was found that these individuals commonly suffered from subclinical amebiasis[24]. In addition, asymptomatic individuals infected with HIV-1 who have a high anti-E. histolytica titer run a risk of invasive amebiasis, most likely as a result of subclinical amebiasis exacerbation[24].
In the Western world, the low overall prevalence, as well as the fact that the latency period between infection by the underlying pathogen and clinical symptom onset may be lengthy, creates a risk of delaying diagnosis of amebiasis and thus inadequate treatment[20]. Additionally, pregnancy has also been found to be an invasive amebiasis risk factor; management of pregnant patients becomes especially complex[20]. Mortality due to amebiasis is primarily the result of extraintestinal infections, with the most common of these being ALAs[25].
PATHOGENESIS
The route of transmission of E. histolytica that leads to ALA has yet to be thoroughly elucidated; broadly, after E. histolytica breaches the host’s innate defenses, it causes liver abscess and amebic colitis by invading the intestinal mucosa[8,26]. Often, trophozoites enter the circulatory system. They are then filtered in the liver and produce abscesses and can develop further into severe invasive diseases, such as ALAs[27]. On the other hand, conditions of immune-compromised individuals and/or momentaneous immune modulation in humans have been reported to increase both bacterial and viral activities/ infections and related diseases[28-31]. ALA may arise following an impairment of the anti-E. histolytica immune system, and the immune evasion is a typical mode of action of pathogens in humans.
Regarding its molecular mechanism, E. histolytica uses the virulence factor Gal/GalNAc lectin in order to invade the host tissue; this molecule not only protects against ALAs but also induces an adherence-inhibitory antibody response[3]. In addition, E. histolytica has a pair of low-molecular-weight protein tyrosine phosphatase (LMW-PTP) genes, EhLMW-PTP1 and EhLMW-PTP2, which are expressed through cysts, cultured trophozoites, and clinical isolates[32]. There is a single amino acid sequence difference, at position A85V, between the proteins EhLMW-PTP1 and EhLMW-PTP2[32]. Both of these genes are expressed in cultured trophozoites, particularly EhLMW-PTP2; trophozoites that are recovered from ALAs show downregulated EhLMW-PTP1 expression[32].
In an in vitro study, the compound linearolactone, as isolated from Salvia polystachya, demonstrated antiparasitic activity against E. histolytica through the production of reactive oxygen species and was able to induce apoptosis-like effects in trophozoites of E. histolytica through intracellular reactive oxygen species production, which affected the structure of the actin cytoskeleton[33]. Therefore, linearolactone served to more actively reduce ALA development[33]. Furthermore, calreticulin is a highly conserved protein in the endoplasmic reticulum and serves in an important capacity in regulating vital cellular functions[34]. In patients with acute phase ALAs, interleukin levels (interleukin-6, interleukin-10, granulocyte colony stimulating factor, and transforming growth factor β1) were higher, while resolution phase ALA patients had higher levels of interferon gamma detected[34].
Entamoeba dispar is a separate amoeba species that annually infects 12% of the global population, and it has been classified as “noninvasive” in the past[17]. However, this amoeba has been isolated from patients suffering from symptomatic non-dysenteric colitis, and DNA sequences from this species have been both detected and genotyped in samples from dysenteric colitis patients as well as samples from ALA patients, suggesting that this amoeba may play some role in human large intestine and liver lesion development[17].
EXAMINATIONS
It is difficult to distinguish ALA from pyogenic liver abscesses using only clinical, laboratory, and radiological findings[35,36]. In order to diagnose the various ALA-related complications, computed tomography (CT) scans serve as an ideal tool[37]. As a result, serologic E. histolytica tests are a necessary part of accurate evaluations of liver abscesses within high-risk groups[35,36]. On the other hand, in a study to use CT findings to determine different morphological types of ALA and to determine any differences in their clinical features, ALAs were found to have three distinct CT morphological types, each varying in terms of its laboratory and clinical features[38].
Type I abscesses (representing 66% of the total) have walls that were either absent or incomplete as well as peripheral septa and edges that are ragged and exhibit enhancement that is both irregular and interrupted[38]. Here, we show a CT from our institution of a 44-year-old woman with a type I abscess (Figure 1). Clinically, these abscesses had an acute presentation alongside severe disease. Laboratory parameters were significantly deranged, and they had higher incidences of rupture with higher rates of admission to inpatient care and/or intensive care[38]. In a large majority of type I abscesses (81%), disease severity prompted percutaneous drainage to be carried out immediately[38].
Figure 1.
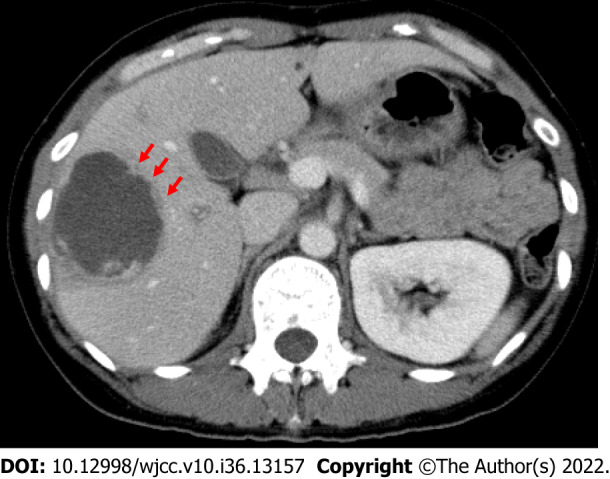
Computed tomography of a 44-year-old woman with a type I abscess. The axial computed tomography image illustrates the non-enhancing and ragged edge of the abscess in the absence of a definite wall, peripheral septa, and ragged edges; these edges exhibited both irregular and interrupted enhancement (arrows).
Type II abscesses (representing 28% of the total) have complete walls with both peripheral hypodense halo and rim enhancement. Type III abscesses (representing 6% of the total) demonstrate walls but without enhancement[38]. The type II and III abscesses feature delayed presentations, with near-normal laboratory findings and mild to moderate disease[38]. On the other hand, whether ALA patients are infected with HIV cannot be determined through the clinical characteristics alone. Even in the absence of HIV symptoms, it is advisable to routinely test ALA patients for HIV[39].
DIAGNOSIS
Despite the rarity of ALAs, a high index of suspicion should be maintained by physicians working with patients who have presented with synchronous lesions of the colon and liver, particularly because in recent years travel has increased to regions where they are endemic[40]. Crucial predictors of ALAs include habitual alcohol consumption and low socioeconomic status[25]. A number of diagnostic tools are available for diagnosis; if there is a suspicion of amebiasis, testing yield can be maximized through a combination of stool testing and serology[6]. Diagnosis relies on nonspecific liver imaging and on detecting anti-E. histolytica antibodies, which cannot be used to distinguish between acute and previous infections[5,21]. Therefore, diagnostics must focus primarily on detecting E. histolytica using PCR or enzyme-linked immunosorbent assay[1]. Among these options, a parallel analysis using indirect enzyme-linked immunosorbent assay with crude soluble antigen together with excretory-secretory antigen for ALA serodiagnosis improved the overall amebic serology efficacy compared to either assay on its own[41].
Recently, the XEh Rapid® IgG4-based rapid dipstick test for rapid detection of ALAs (based on detecting the anti-E. histolytica pyruvate phosphate dikinase IgG4 antibody) demonstrated high diagnostic specificity in infected patients (97%-100%), with diagnostic sensitivity varying between 38% and 94%[42]. The various evaluation process-related difficulties have been discussed elsewhere; nonetheless, it has demonstrated promise for development into a point-of-care test, especially for settings that have relatively restricted resources, and consequently further investigation to confirm its sensitivity as a diagnostic is warranted[42]. On the other hand, one valuable antigen for amebiasis serodiagnosis is the C-terminal region of the intermediate subunit of E. histolytica galactose- and N-acetyl-D-galactosamine-inhibitable lectin[43]. The newly developed immunochromatographic kit, which uses fluorescent silica nanoparticles coated with the C-terminal region of the intermediate subunit of E. histolytica galactose- and N-acetyl-D-galactosamine-inhibitable lectin prepared in Escherichia coli, has proven beneficial for rapid amebiasis serodiagnosis[43].
Ultrasound is currently the criterion standard for liver abscess diagnoses[14]. Acute abdominal pain can be the result of a variety of diseases, but even in non-endemic Western countries parasitic abscess should not be overlooked as a potential diagnosis[14]. Contrast-enhanced ultrasound is a promising new technique, with the potential for greater accuracy in recognizing liver abnormalities, including abscesses; however, definition of differential diagnoses will require retrospective population-wide studies[14].
It is difficult to definitively diagnose ALAs because sensitive point-of-care molecular tests are not readily commercially available[44]. A diagnostic study was performed in order to compare the available methods for E. histolytica laboratory diagnoses in pus samples, stool samples, and blood samples taken from patients who had radiological and/or clinical diagnoses of ALA with loop-mediated isothermal amplification. The results found that loop-mediated isothermal amplification had significantly greater sensitivity (88%) then reverse transcriptase PCR (64%) as well as outstanding specificity (100%)[44]. On the other hand, in ALAs, cell-free circulating E. histolytica DNA can be detected in serum in ALAs, which could prove beneficial for not only positive diagnosis but also the efficacy of follow-up treatments[21]. Additional innovative detecting methods have been developed for E. histolytica, and stool samples were analyzed using PCR-denaturing gradient gel electrophoresis in order to distinguish between pathogenic E. histolytica (pathogenic) and non-pathogenic Entamoeba dispar[45]. The PCR amplification target was a relatively small region (228 bp) of the adh112 gene, which was selected for greater test sensitivity[45]. These results, validated by nested PCR-restriction fragment length polymorphism, would imply that PCR-denaturing gradient gel electrophoresis may have promise as a tool to distinguish between Entamoeba infections and contribute to the determination of a specific course of treatment for E. histolytica patients, thus obviating unnecessary treatment of patients who have been infected with Entamoeba dispar, which is non-pathogenic[45].
Additionally, diagnosis is possible through abdominal ultrasound and echography-guided liver puncture[46]. If liver abscess fluid bacterial cultures remain negative, amebic abscess should be considered as a possibility, even if the patient has no personal history of tropical or subtropical travel[1]. In culture-negative cases, 16S rRNA abscess fluid analysis plays a part in improved microbiological diagnoses[35].
TREATMENT
ALAs can be treated medically. Percutaneous catheter drainage (PCD) is required in only 15% of cases[5,47]. They generally respond well to treatment using metronidazole, alongside drainage if indicated[2,4,48]. In uncomplicated cases, it is advisable to avoid surgical drainage[48].
Safe, effective complex abscess decompression has been enabled through surgical drainage with preoperative CT and intraoperative ultrasonography[48]. In particular, liver abscesses in the caudate lobe can be accessed without major complications via different percutaneous drainage routes, despite its deep location and the fact that it is surrounded by large blood vessels[4]. Thus, PCD or percutaneous needle aspiration (PNA) could be regarded as a first-line therapy for caudate lobe amebic abscess management, in adjunct to medical therapy[4]. Following substantial reduction or cessation of PCD output along with clinical recovery, treating physicians may be concerned with residual collections on radiological evaluations[49]. However, both the significance and prevalence of such collections remain unknown, and it is subsequently unclear what approach should be taken in order to tackle them. On the other hand, PCD removal can be expedited successfully in ALAs, even when residual collections are present[49]. In pediatric patients, PNA and drain placement were both found to be effective as ALA treatments, though PNA had greater efficacy[50].
On the other hand, ultrasound-guided PCD has been found to be both safe and effective as a treatment method for ruptured ALAs, including free ruptures with diffuse intraperitoneal fluid collections. For ruptured ALAs, PCD is also recommended as the first line of therapy[51]. At present, metronidazole on its own as well as PNA and PCD play unclear roles in treating uncomplicated ALAs[52]. Compared to metronidazole on its own, PNA results in earlier resolution of both pain and tenderness in patients suffering from medium to large ALAs[52]. On the other hand, PCD is preferable for larger ALAs[52]. However, further efforts to generate more accurate and reliable data are needed due to therapeutic dilemmas caused by discrepancies in randomized controlled trials[52]. In addition, the literature seems to be conflicting on the topic with proponents of both percutaneous methods and laparoscopic drainage[4,53]. Given the rarity of amebiasis, the rarity of the complication itself, and the possibility that PCD may prove ineffective due to viscosity of the abscess content, catheter dislocation etc, a step-up approach would be advisable in that case.
The indicated treatment is to use an amebicidal drug such as metronidazole or tinidazole as well as paromomycin or another luminal cysticidal agent for clinical disease[1,6,54]. Treatment involves oral administration of 500-750 mg of metronidazole (or another nitroimidazole if necessary), three times daily, for 7-10 d[55]. As an alternative option, 2000 mg of tinidazole can be administered orally on a daily basis for 3 d[55]. However, in 40%-60% of patients, the parasites persist within the intestine. Therefore, nitroimidazole treatment should always be followed with a luminal agent such as a 7-d regimen of 500 mg of paromomycin three times a day or a 20-d regimen of 650 mg of iodoquinol three times a day[55].
The drug of choice for treating ALAs is often metronidazole, a common antibacterial and antiprotozoal drug; though it has long been preferred, it is also associated with a number of different adverse effects in some clinical situations, including intolerance[54,56,57]. The mechanisms of resistance to metronidazole, as well as mutagenic potential, have previously been described[23]. Though ordinarily safe, under rare circumstances this drug is capable of causing serious central nervous system disturbances. In particular, metronidazole neurotoxicity as well as characteristic bilateral symmetrical cerebellar dentate hyperintensities have been shown on brain magnetic resonance imaging[58]. However, neurotoxicity is not dependent on dose, and with discontinuation of the drug it can be fully reversed[57,58]. Additionally, it is still unknown what effects, if any, the drug has when used by pregnant or lactating patients (and consequently in breastfeeding infants)[54].
The efficacy of nitazoxanide has been demonstrated in invasive intestinal amebiasis treatment; however, a study has shown that in uncomplicated ALAs nitazoxanide has efficacy comparable to metronidazole and enjoys the advantages of both superior tolerability and simultaneous luminal clearance, leading to a lower likelihood of recurrence[54].
In comparison to metronidazole, tinidazole has an earlier clinical response, a shorter course of treatment, a more favorable rate of recovery, and a higher tolerability; consequently, for ALAs tinidazole can be considered preferable to metronidazole[56]. The recommended treatment for asymptomatic infections is a luminal cysticidal agent, in order to reduce the chances of either invasive disease or transmission[6].
For surgical treatment, a laparoscopic approach imposes the least physical burden resulting from the laparotomy[46]. According to the latest research results, ubiquitin Ehub antibodies are induced solely in patients with ALA or other invasive amoebiasis, and the antibody response is mainly to the glycoprotein, indicating that the glycans are immunodominant[59]. Therefore, Ehub glycan inhibitors hold potential as an amoebiasis treatment through selective damage to trophozoites[59].
COMPLICATIONS
In rare cases, abscesses can rupture into the peritoneum, pericardium, or pleura, or into the hilum of the bile duct; they may also lead to septic emboli[2]. Thromboses of the hepatic vein and the inferior vena cava are uncommon ALA complications (though well documented) and are generally attributed to the inflammation and mechanical compression that accompany larger abscesses[60]. With ALAs, the combination of portal vein thrombosis and hepatic vein thrombosis is a common occurrence, frequently manifesting as segmental hypoperfusion in the portal venous phase and indicating ischemia[61]. When events such as these are detected using CT, they may indicate a more severe disease that demands more aggressive management, including percutaneous drainage[61]. However, there has been one report of a left hepatic ALA in a patient who had no clear source of infection, initially presenting with a left portal vein thrombosis[22].
In rare cases, hepatic artery aneurysms can complicate amebiasis in hepatic abscess patients[13]. In addition to the significant harm caused by the disease, particularly in developing countries, there is only sporadic case report data available, which suggests that there may be an underreporting bias[13]. Further studies are necessary in order to further elucidate vascular involvement in this setting of parasitological interest[13]. Furthermore, intrahepatic pseudoaneurysms due to ALAs are exceptionally uncommon. There are only a handful of published reports[62]. In every known symptomatic case, the treatment was embolization of the hepatic artery; consequently, the natural course of the disease remains poorly understood, as do the effects of abscess drainage on outcomes[62]. On the other hand, according to one report regarding symptomatic intracavitary intrahepatic pseudoaneurysms as a result of an ALA, an ultrasound-guided abscess PCD caused the intrahepatic pseudoaneurysms to spontaneously resolve[62]. Recently, there has been a case reported of ALA copresenting with coronavirus disease 2019. Based on pathophysiological similarities, coinfection with both of these could affect the clinical course of the patient[18].
PROGNOSIS
There are highly varied infection outcomes for amebiasis due to the protozoan parasite E. histolytica[63]. Prognosis is favorable, and there is near-universal recovery[5]. A study of the relationship between the genotypes of parasites and amebic infection outcomes found a significant association with disease outcomes related to single nucleotide polymorphisms (both non-synonymous and synonymous) within the protein 2 (kerp2) locus, which is rich in both lysine and glutamic acid[63]. An incomplete linkage disequilibrium value has also been found to exist at the kerp2 locus, with potential recombination events and significant values for population differentiation[63]. At the kerp2 locus, disease-specific single nucleotide polymorphisms, potential recombination events, and significant values for population differentiation are present, indicating that the host continuously exerts selection pressure on the parasite on the kerp2 gene and its gene products; this could potentially serve as a way to determine the outcome of disease caused by E. histolytica infections[63].
Additionally, in isolation from asymptomatic carriers, E. histolytica is closer, phylogenetically, to species that cause human liver abscesses, and they exhibit potential interpopulation recombination[63]. Individuals who experience persistent asymptomatic infections of E. histolytica could have a greater likelihood of future ALA development, and asymptomatic people who live in areas where it is endemic should always be mandated to undergo close investigations[63]. On the other hand, potentially valuable predictors of recurrent ALA include the presence of resistance genes (nim) and Prevotella in the abscess fluid, accompanied by elevated levels of matrix metalloproteinase-9 and large abscess size (11 cm × 10.8 cm); recurrence rates were 8.9%[64].
PREVENTION
Despite the knowledge that has been gained and the scientific advances that have been made, there are still no effective treatments to prevent this infection[65]. The extended duration of subclinical E. histolytica infection makes it difficult to control this disease not only in individual amebiasis patients but also epidemiologically[24]. Anti-E. histolytica testing targeting individuals who are at greater risk could prove beneficial in early subclinical amebiasis diagnosis, and earlier treatment of infected patients could halt invasive amebiasis from developing, thus preventing community transmission[24].
The compound curcumin can demonstrate anti-amebic effects within the liver, which would suggest that administering curcumin daily could help to significantly decrease infection incidence rates[65]. Immunization using a chimeric vaccine (using the recombinant protein PEΔIII-LC3-KDEL3) was successful in preventing invasive amebiasis, avoiding acute proinflammatory response, and rapidly activating a protective response. Ultimately, this recombinant protein induced increased serum levels of IgG[3]. Additionally, in order to proactively eliminate the disease, it would be beneficial to have greater awareness among at-risk members of the public[8].
CONCLUSION
The aim of this minireview was to highlight the pathogenesis of and difficulty of diagnosing ALAs. Methods of pathogenesis and accurate diagnosis have yet to be determined. However, accurate diagnoses can be achieved through newer molecular biological techniques, and these can lead to appropriate management of infections due to this organism. Future studies should ideally aim to elucidate pathogenesis and determine more effective diagnoses for effective ALA management.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 7, 2022
First decision: October 17, 2022
Article in press: December 8, 2022
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hakimi T, Afghanistan; Pantelis AG, Greece; Priyadarshi RN, India S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
Contributor Information
Daisuke Usuda, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan. d.usuda.qa@juntendo.ac.jp.
Shiho Tsuge, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Riki Sakurai, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Kenji Kawai, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Shun Matsubara, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Risa Tanaka, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Makoto Suzuki, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Hayabusa Takano, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Shintaro Shimozawa, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Yuta Hotchi, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Shungo Tokunaga, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Ippei Osugi, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Risa Katou, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Sakurako Ito, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Kentaro Mishima, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Akihiko Kondo, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Keiko Mizuno, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Hiroki Takami, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Takayuki Komatsu, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan; Department of Sports Medicine, Faculty of Medicine, Juntendo University, Bunkyo 113-8421, Tokyo, Japan.
Jiro Oba, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Tomohisa Nomura, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
Manabu Sugita, Department of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Nerima 177-8521, Tokyo, Japan.
References
- 1.Heil TC, Dercksen MW, Blank SN. Infection or metastases? Ned Tijdschr Geneeskd. 2018;162 [PubMed] [Google Scholar]
- 2.Goel R, Roy A, Ray D, Chaluvashetty SB, De A. A case of amoebic liver abscess complicated by bilhaemia and venous thrombosis. Trop Doct. 2021;51:249–250. doi: 10.1177/0049475520975948. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Hernández SL, Becerra-González VM, Muñoz-Ortega MH, Loera-Muro VM, Ávila-Blanco ME, Medina-Rosales MN, Ventura-Juárez J. Evaluation of the PEΔIII-LC3-KDEL3 Chimeric Protein of Entamoeba histolytica-Lectin as a Vaccine Candidate against Amebic Liver Abscess. J Immunol Res. 2021;2021:6697900. doi: 10.1155/2021/6697900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav T, Patel RK, Bansal A, Chatterjee N, Patidar Y, Mukund A. Caudate lobe amebic abscesses: percutaneous image-guided aspiration or drainage. Abdom Radiol (NY) 2022;47:1157–1166. doi: 10.1007/s00261-021-03395-z. [DOI] [PubMed] [Google Scholar]
- 5.Roediger R, Lisker-Melman M. Pyogenic and Amebic Infections of the Liver. Gastroenterol Clin North Am. 2020;49:361–377. doi: 10.1016/j.gtc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Smith L, Diakiw A. Amebiasis and Amebic Liver Abscess in Children. Pediatr Clin North Am. 2022;69:79–97. doi: 10.1016/j.pcl.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca Z, Díaz-Godínez C, Mora N, Alemán OR, Uribe-Querol E, Carrero JC, Rosales C. Entamoeba histolytica Induce Signaling via Raf/MEK/ERK for Neutrophil Extracellular Trap (NET) Formation. Front Cell Infect Microbiol. 2018;8:226. doi: 10.3389/fcimb.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannathasan S, Murugananthan A, Kumanan T, de Silva NR, Rajeshkannan N, Haque R, Iddawela D. Epidemiology and factors associated with amoebic liver abscess in northern Sri Lanka. BMC Public Health. 2018;18:118. doi: 10.1186/s12889-018-5036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Den Broucke S, Verschueren J, Van Esbroeck M, Bottieau E, Van den Ende J. Clinical and microscopic predictors of Entamoeba histolytica intestinal infection in travelers and migrants diagnosed with Entamoeba histolytica/dispar infection. PLoS Negl Trop Dis. 2018;12:e0006892. doi: 10.1371/journal.pntd.0006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Kar P. HBsAg carrier with simultaneous amebic liver abscess and acute hepatitis E. Indian J Gastroenterol. 1999;18:124. [PubMed] [Google Scholar]
- 11.Medina-Rosales MN, Muñoz-Ortega MH, García-Hernández MH, Talamás-Rohana P, Medina-Ramírez IE, Salas-Morón LG, Martínez-Hernández SL, Ávila-Blanco ME, Medina-Rosales B, Ventura-Juárez J. Acetylcholine Upregulates Entamoeba histolytica Virulence Factors, Enhancing Parasite Pathogenicity in Experimental Liver Amebiasis. Front Cell Infect Microbiol. 2020;10:586354. doi: 10.3389/fcimb.2020.586354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulido-Ortega J, Talamás-Rohana P, Muñoz-Ortega MH, Aldaba-Muruato LR, Martínez-Hernández SL, Campos-Esparza MDR, Cervantes-García D, Leon-Coria A, Moreau F, Chadee K, Ventura-Juárez J. Functional Characterization of an Interferon Gamma Receptor-Like Protein on Entamoeba histolytica. Infect Immun. 2019;87 doi: 10.1128/IAI.00540-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvestri V, Ngasala B. Hepatic aneurysm in patients with amoebic liver abscess. A review of cases in literature. Travel Med Infect Dis. 2022;46:102274. doi: 10.1016/j.tmaid.2022.102274. [DOI] [PubMed] [Google Scholar]
- 14.Marenga G, Traficante S, Ragonici S, Vincenzi C, Rocchetti M, De Rito G, Fonsi GB, Messineo D. Successful Diagnosis of a Longstanding Giant Amoebic Liver Abscess Using Contrast-Enhanced Ultrasonography (CEUS): A Case Report in a Western Country. Am J Case Rep. 2019;20:493–498. doi: 10.12659/AJCR.914378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivos-García A, Nequiz M, Liceaga S, Mendoza E, Zúñiga P, Cortes A, López-Velázquez G, Enríquez-Flores S, Saavedra E, Pérez-Tamayo R. Complement is a rat natural resistance factor to amoebic liver infection. Biosci Rep. 2018;38 doi: 10.1042/BSR20180713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez Zamora P, Gallotti AC, Ramos R, Ligero López J, González Y, Mejía RA, Orozco Vinasco AC, Fuentes I, Merino FJ. An Unexpected Case of Disseminated Amebiasis with Cerebral Involvement and Successful Recovery in a Non-Endemic Context. Am J Case Rep. 2021;22:e934188. doi: 10.12659/AJCR.934188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva CAV, de Oliveira IMC, Cruz RE, Silva Prado GK, Santos FV, Neves NCV, Gomes MA, Silva Oliveira FM, Caliari MV. South American Entamoeba dispar strains produce amoebic liver abscesses with different pathogenicities and evolutionary kinetics. Acta Trop. 2021;224:106114. doi: 10.1016/j.actatropica.2021.106114. [DOI] [PubMed] [Google Scholar]
- 18.Maricuto AL, Velásquez VL, Pineda J, Flora-Noda DM, Rodríguez I, Rodríguez-Inés CA, Noya-González ÓO, Contreras R, Omaña-Ávila ÓD, Escalante-Pérez IA, Camejo-Ávila NA, Kuffaty-Akkou NA, Carrión-Nessi FS, Carballo M, Landaeta ME, Forero-Peña DA. Amoebic liver abscess in a COVID-19 patient: a case report. BMC Infect Dis. 2021;21:1134. doi: 10.1186/s12879-021-06819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin MJ, Leslie JL, Petri WA Jr. Host Protective Mechanisms to Intestinal Amebiasis. Trends Parasitol. 2021;37:165–175. doi: 10.1016/j.pt.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser RWJ, Allgeier J, Philipp AB, Mayerle J, Rothe C, Wallrauch C, Op den Winkel M. Development of amoebic liver abscess in early pregnancy years after initial amoebic exposure: a case report. BMC Gastroenterol. 2020;20:424. doi: 10.1186/s12876-020-01567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghelfenstein-Ferreira T, Gits-Muselli M, Dellière S, Denis B, Guigue N, Hamane S, Alanio A, Bretagne S. Entamoeba histolytica DNA Detection in Serum from Patients with Suspected Amoebic Liver Abscess. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01153-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borro M, Murdaca G, Greco M, Negrini S, Setti M. A rare hepatic mass in an Italian resident. BMC Gastroenterol. 2020;20:295. doi: 10.1186/s12876-020-01440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iritani S, Kawamura Y, Yamashige D, Muraishi N, Kajiwara A, Fujiyama S, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F, Arase Y, Ikeda K, Suzuki Y, Kumada H. An encapsulated bulky abdominal abscess due to amoeba. Clin J Gastroenterol. 2021;14:555–559. doi: 10.1007/s12328-020-01331-0. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Aoki T, Nagata N, Tanuma J, Kikuchi Y, Oka S, Gatanaga H. Clinical significance of high anti-entamoeba histolytica antibody titer in asymptomatic HIV-1-infected individuals. J Infect Dis. 2014;209:1801–1807. doi: 10.1093/infdis/jit815. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Banerjee T, Kumar R, Shukla SK. Prevalence of cases of amebic liver abscess in a tertiary care centre in India: A study on risk factors, associated microflora and strain variation of Entamoeba histolytica. PLoS One. 2019;14:e0214880. doi: 10.1371/journal.pone.0214880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon-Coria A, Kumar M, Chadee K. The delicate balance between Entamoeba histolytica, mucus and microbiota. Gut Microbes. 2020;11:118–125. doi: 10.1080/19490976.2019.1614363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nushijima Y, Ishida H, Watanabe Y, Nakaguchi K, Nakanishi K, Hoshida Y, Kabuto T. Amebic liver abscess rupturing into the lesser omentum space. J Hepatobiliary Pancreat Surg. 2006;13:252–255. doi: 10.1007/s00534-005-1044-6. [DOI] [PubMed] [Google Scholar]
- 28.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazziotta C, Pellielo G, Tognon M, Martini F, Rotondo JC. Significantly Low Levels of IgG Antibodies Against Oncogenic Merkel Cell Polyomavirus in Sera From Females Affected by Spontaneous Abortion. Front Microbiol. 2021;12:789991. doi: 10.3389/fmicb.2021.789991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su S, Hua D, Li JP, Zhang XN, Bai L, Cao LB, Guo Y, Zhang M, Dong JZ, Liang XW, Lan K, Hu MM, Shu HB. Modulation of innate immune response to viruses including SARS-CoV-2 by progesterone. Signal Transduct Target Ther. 2022;7:137. doi: 10.1038/s41392-022-00981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sierra-López F, Baylón-Pacheco L, Vanegas-Villa SC, Rosales-Encina JL. Characterization of low molecular weight protein tyrosine phosphatases of Entamoeba histolytica. Biochimie. 2021;180:43–53. doi: 10.1016/j.biochi.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Velázquez-Domínguez JA, Hernández-Ramírez VI, Calzada F, Varela-Rodríguez L, Pichardo-Hernández DL, Bautista E, Herrera-Martínez M, Castellanos-Mijangos RD, Matus-Meza AS, Chávez-Munguía B, Talamás-Rohana P. Linearolactone and Kaempferol Disrupt the Actin Cytoskeleton in Entamoeba histolytica: Inhibition of Amoebic Liver Abscess Development. J Nat Prod. 2020;83:3671–3680. doi: 10.1021/acs.jnatprod.0c00892. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez Rivas E, Ximenez C, Nieves-Ramirez ME, Moran Silva P, Partida-Rodríguez O, Hernandez EH, Rojas Velázquez L, Serrano Vázquez A, Magaña Nuñez U. Entamoeba histolytica Calreticulin Induces the Expression of Cytokines in Peripheral Blood Mononuclear Cells Isolated From Patients With Amebic Liver Abscess. Front Cell Infect Microbiol. 2018;8:358. doi: 10.3389/fcimb.2018.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neill L, Edwards F, Collin SM, Harrington D, Wakerley D, Rao GG, McGregor AC. Clinical characteristics and treatment outcomes in a cohort of patients with pyogenic and amoebic liver abscess. BMC Infect Dis. 2019;19:490. doi: 10.1186/s12879-019-4127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim E, Park DH, Kim KJ, Kim TO, Park SH, Park J, Choi JH, Lee J, Park YE, Oh EH, Hwang JS, Heo NY. Current Status of Amebic Liver Abscess in Korea Comparing with Pyogenic Liver Abscess. Korean J Gastroenterol. 2020;76:28–36. doi: 10.4166/kjg.2020.76.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sodhi KS, Ojili V, Sakhuja V, Khandelwal N. Hepatic and inferior vena caval thrombosis: vascular complication of amebic liver abscess. J Emerg Med. 2008;34:155–157. doi: 10.1016/j.jemermed.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 38.Priyadarshi RN, Sherin L, Kumar R, Anand U, Kumar P. CT of amebic liver abscess: different morphological types with different clinical features. Abdom Radiol (NY) 2021;46:4148–4158. doi: 10.1007/s00261-021-03093-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu MS, Hsieh SM, Chen MY, Hung CC, Chang SC. Association between amebic liver abscess and human immunodeficiency virus infection in Taiwanese subjects. BMC Infect Dis. 2008;8:48. doi: 10.1186/1471-2334-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bronswijk M, Van Gool S. A case of amoebic colitis with amoeboma and simultaneous liver abscesses. A diagnosis by colonoscopy. Acta Gastroenterol Belg. 2019;82:539–541. [PubMed] [Google Scholar]
- 41.Wong WK, Foo PC, Olivos-Garcia A, Noordin R, Mohamed Z, Othman N, Few LL, Lim BH. Parallel ELISAs using crude soluble antigen and excretory-secretory antigen for improved serodiagnosis of amoebic liver abscess. Acta Trop. 2017;172:208–212. doi: 10.1016/j.actatropica.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Noordin R, Yunus MH, Saidin S, Mohamed Z, Fuentes Corripio I, Rubio JM, Golkar M, Hisam S, Lee R, Mahmud R. Multi-Laboratory Evaluation of a Lateral Flow Rapid Test for Detection of Amebic Liver Abscess. Am J Trop Med Hyg. 2020;103:2233–2238. doi: 10.4269/ajtmh.20-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tachibana H, Kakino A, Kazama M, Feng M, Asai S, Umezawa K, Nozaki T, Makiuchi T, Kamada T, Watanabe H, Horiki N, Cheng X, Masuda G. Development of a sensitive immunochromatographic kit using fluorescent silica nanoparticles for rapid serodiagnosis of amebiasis. Parasitology. 2018;145:1890–1895. doi: 10.1017/S0031182018000690. [DOI] [PubMed] [Google Scholar]
- 44.Handa D, Gupta M, Lehl SS, Gupta A, Singh R. Utility of loop-mediated isothermal amplification as a point-of-care test in diagnosis of amoebic liver abscess. Trop Doct. 2021;51:488–491. doi: 10.1177/00494755211018050. [DOI] [PubMed] [Google Scholar]
- 45.López-López P, Martínez-López MC, Boldo-León XM, Hernández-Díaz Y, González-Castro TB, Tovilla-Zárate CA, Luna-Arias JP. Detection and differentiation of Entamoeba histolytica and Entamoeba dispar in clinical samples through PCR-denaturing gradient gel electrophoresis. Braz J Med Biol Res. 2017;50:e5997. doi: 10.1590/1414-431X20175997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koumaré S, Koné T, Keita S, Soumaré L, Sissoko MS, Camara M, Sacko O, Camara A, Koïta A, Togo S, Ouattara MA, Dicko H, Konaté M, Coulibaly Y, Diallo M, Sanogo ZZ, Sangaré D. Diagnosis and therapeutic aspects of the amoebic liver abscesses in the surgery at point "G" Hospital. Mali Med. 2018;33:1–5. [PubMed] [Google Scholar]
- 47.Khim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132:45–52. doi: 10.1093/bmb/ldz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kouzu K, Einama T, Nishikawa M, Fukumura M, Nagata H, Iwasaki T, Miyata Y, Obuchi Y, Hase K, Ueno H, Kishi Y, Yamamoto J. Successful surgical drainage with intraoperative ultrasonography for amebic liver abscess refractory to metronidazole and percutaneous drainage: a case report. BMC Surg. 2020;20:112. doi: 10.1186/s12893-020-00776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyal A, Dhaliwal HS, Nampoothiri RV, Singh R, Abraham J, Sharma R, Soloman R, Lahan S, Kaur P, Bansal P, Gill CS. Percutaneous catheter drainage of uncomplicated amoebic liver abscess: prospective evaluation of a clinical protocol for catheter removal and the significance of residual collections. Abdom Radiol (NY) 2021;46:2855–2864. doi: 10.1007/s00261-021-02949-5. [DOI] [PubMed] [Google Scholar]
- 50.Salim A, Jeelani SM, Qazi SH, Mirza W. Amoebic liver abscess: Outcomes of percutaneous needle aspiration vs drain placement in paediatric population. J Pak Med Assoc. 2019;69(Suppl 1):S29–S32. [PubMed] [Google Scholar]
- 51.Priyadarshi RN, Prakash V, Anand U, Kumar P, Jha AK, Kumar R. Ultrasound-guided percutaneous catheter drainage of various types of ruptured amebic liver abscess: a report of 117 cases from a highly endemic zone of India. Abdom Radiol (NY) 2019;44:877–885. doi: 10.1007/s00261-018-1810-y. [DOI] [PubMed] [Google Scholar]
- 52.Kumar R, Ranjan A, Narayan R, Priyadarshi RN, Anand U, Shalimar Evidence-based therapeutic dilemma in the management of uncomplicated amebic liver abscess: A systematic review and meta-analysis. Indian J Gastroenterol. 2019;38:498–508. doi: 10.1007/s12664-019-01004-y. [DOI] [PubMed] [Google Scholar]
- 53.Dhir U, Ghuman S, Singhvi S, Rawat S. Caudate Lobe Liver Abscess: Laparoscopic Drainage the best approach. HPB. 2019;21(Suppl 2):S385. [Google Scholar]
- 54.Goel V, Jain A, Sharma G, Jhajharia A, Agarwal VK, Ashdhir P, Pokharna R, Chauhan V. Evaluating the efficacy of nitazoxanide in uncomplicated amebic liver abscess. Indian J Gastroenterol. 2021;40:272–280. doi: 10.1007/s12664-020-01132-w. [DOI] [PubMed] [Google Scholar]
- 55.Jackson-Akers JY, Prakash V, Oliver TI. Amebic Liver Abscess. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 56.Pandey S, Gupta GK, Wanjari SJ, Nijhawan S. Comparative study of tinidazole vs metronidazole in treatment of amebic liver abscess: A randomized control trial. Indian J Gastroenterol. 2018;37:196–201. doi: 10.1007/s12664-018-0848-7. [DOI] [PubMed] [Google Scholar]
- 57.Kaur U, Kumar I, Singh A, Kumar M, Chakrabarti SS. Cerebellar Dysfunction in an Elderly Male After a Brief Course of Metronidazole. Curr Drug Saf. 2019;14:163–166. doi: 10.2174/1574886314666190206154735. [DOI] [PubMed] [Google Scholar]
- 58.Arora N, Wasti KP, Babbar N, Saroch A, Pannu AK, Sharma N. Neurological complications during treatment of liver abscess: think of metronidazole toxicity. Trop Doct. 2020;50:165–166. doi: 10.1177/0049475520903651. [DOI] [PubMed] [Google Scholar]
- 59.Flores MS, Tamez E, Rangel R, Monjardin J, Bosques F, Obregón A, Trejo-Avila L, Quintero I, Gandarilla F, Arevalo K, Alemán E, Galán L. Ubiquitin of Entamoeba histolytica induces antibody response in patients with invasive amoebiasis. Parasite Immunol. 2022;44:e12919. doi: 10.1111/pim.12919. [DOI] [PubMed] [Google Scholar]
- 60.Martin L, Burute N, Haider E, Serrano PE, O'Shea T, Siegal D. Occult Amebic Liver Abscess as Cause of Extensive Inferior Vena Cava and Hepatic Vein Thrombosis. Am J Trop Med Hyg. 2017;97:1214–1217. doi: 10.4269/ajtmh.17-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Priyadarshi RN, Kumar P, Kumar R, Anand U, Shyama Venous thrombosis and segmental hypoperfusion in amebic liver abscess: MDCT demonstration and its implications. Abdom Radiol (NY) 2020;45:652–660. doi: 10.1007/s00261-020-02409-6. [DOI] [PubMed] [Google Scholar]
- 62.Priyadarshi RN, Kumar R, Anand U. Case Report: Spontaneous Resolution of Intracavitary Hepatic Artery Pseudoaneurysm Caused by Amebic Liver Abscess following Percutaneous Drainage. Am J Trop Med Hyg. 2019;101:157–159. doi: 10.4269/ajtmh.19-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das K, Sardar SK, Ghosal A, Saito-Nakano Y, Dutta S, Nozaki T, Ganguly S. Multilocus sequence typing (MLST) of Entamoeba histolytica identifies kerp2 as a genetic marker associated with disease outcomes. Parasitol Int. 2021;83:102370. doi: 10.1016/j.parint.2021.102370. [DOI] [PubMed] [Google Scholar]
- 64.Singh A, Banerjee T, Shukla SK. Factors Associated with High Rates of Recurrence of Amebic Liver Abscess (ALA) in North India. Am J Trop Med Hyg. 2021;104:1383–1387. doi: 10.4269/ajtmh.20-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macías-Pérez JR, Aldaba-Muruato LR, Martínez-Hernández SL, Muñoz-Ortega MH, Pulido-Ortega J, Ventura-Juárez J. Curcumin Provides Hepatoprotection against Amoebic Liver Abscess Induced by Entamoeba histolytica in Hamster: Involvement of Nrf2/HO-1 and NF-κB/IL-1β Signaling Pathways. J Immunol Res. 2019;2019:7431652. doi: 10.1155/2019/7431652. [DOI] [PMC free article] [PubMed] [Google Scholar]


