Abstract
The extract of Tribulus terrestris (TT) has been used as a component of several nutritional supplements for enhancing human vitality. However, its protective effect on ischemic stroke has yet to be fully investigated. In this study, the middle cerebral artery occlusion (MCAO) rat model was established and treated with gross saponin of TT fruit (GSTTF) by gavage to explore its anti-ischemic stroke efficacy. Liquid chromatography–mass spectrometry (LC–MS)-based metabolomics was applied to profile the brain tissue metabolite changes and further obtain the metabolic pathways that were greatly involved in the efficacy of GSTTF. Subsequent molecular biology experiments were applied to validate the findings from metabolomics analysis. The results showed that GSTTF administration remarkably decreased the infarction volume of brain tissue and improved the neurobehavioral scores of MCAO rats. Metabolomics analysis revealed that pathways, including glycerophospholipid metabolism, sphingolipid metabolism, and arachidonic acid metabolism, were considered associated with the protective effect of GSTTF against MCAO, which were greatly involved in the inflammatory responses. The results of the biochemistry analysis showed that GSTTF treatment significantly reduced the levels of TNF-α and IL-6 in brain tissue after MCAO. The anti-inflammatory mechanism of GSTTF was further investigated, which revealed that GSTTF could inhibit the TLR4/MyD88/NF-κB signaling pathway to exert protective effects on MCAO. This study provides the underlying anti-inflammatory mechanism of GSTTF for ischemic stroke protection, which has important implications for the development of GSTTF-related functional foods or food supplements.
Introduction
Stroke has high morbidity and mortality all over the world, which seriously threatens people’s life and health. Nearly 87% of the strokes are ischemic, with the remaining being hemorrhagic. Early intravenous tissue plasminogen activator (t-PA) reperfusion is a well-established standard procedure in the treatment of acute ischemic stroke. However, this treatment has certain limitations due to the narrow therapeutic window, contraindications, and low recanalization rates.1 This factor has contributed to a revolution in endovascular therapy, from thrombolysis to thrombectomy, which uses a specialized endovascular device to remove blocking clots in arteries, thereby restoring blood flow to the brain and reducing brain tissue damage. Mechanical thrombectomy techniques possess higher recanalization rates and are widely used in acute ischemic stroke patients of various ages and severities, providing a safe therapy for individuals who are contraindicated to t-PA due to timing or bleeding risk.2 However, mechanical thrombectomy is a disruptive treatment and requires reorganization of the care system to ensure that patients are treated as soon as possible after stroke onset, which is crucial for optimizing patient outcomes.3
Although the efficacy of candidate neuroprotectants was demonstrated in animal experiments, the majority of these preventative therapies and neuroprotective medicines discovered in preclinical investigations have failed in human trials.4 Therefore, it is of significant importance to develop and establish alternative protective strategies for stroke. The term “nutraceutical” combines contents of “nutrition” and “pharmaceutics”, with the goal of making health-protective products readily available that do not require medical consultation, and is regarded as “more than food but less for than pharmaceuticals”.5 Evidence suggests that certain dietary factors have a greater benefit in preventing ischemic stroke than others. Fruits and vegetables, for example, can help avoid strokes. A daily intake of three to five servings of fruits and vegetables was related to a decreased risk of stroke than a daily intake of fewer than three servings.6 Soy products appeared to protect against ischemic stroke in the Japanese population.7 In addition, the consumption of fatty fish, such as tuna and other grilled fish, was linked to a lower risk of ischemic stroke, with greater protection in women.8
Tribulus terrestris (TT) is a medicinal herb that has been widely used in many countries for thousands of years. Preparations containing TT extract (TTE) are currently marketed as a food supplement in the United States and Europe and are claimed to have a general stimulating effect on athletic activity, muscle tone, and rejuvenation.9 Recent studies have shown that the TT extract, especially the saponins, has cerebrovascular protective effects in vivo and in vitro. Pretreatment of TTE was found to significantly reduce H9c2 cell apoptosis, mitochondrial changes, and other ischemia-induced cell changes.9 Our previous studies also confirmed the protective effects of TTE on ischemic stroke using the middle cerebral artery occlusion (MCAO) rat model with tail vein injection of TT fruit gross saponin (GSTTF).10−13 With the help of omics strategies, we obtained much information about the efficacy of GSTTF against MCAO-induced ischemic stroke, such as the biomarkers, metabolic pathways, key targets, and so on. However, the findings from the omics analysis were not verified by the biochemical analysis. In this study, after analyzing data obtained from metabolomics analysis, we focused on the anti-inflammatory response to perform the validation study. The GSTTF has been widely used as a food supplement in the United States and Europe and is claimed to have a general stimulating effect on athletic activity, muscle tone, and rejuvenation.
The GSTTF has been widely used as a food supplement in the United States and Europe. Our previous studies have confirmed its therapeutic effect on ischemic stroke; thus, we changed the method of treatment from tail vein injection to oral administration to investigate its protective effect on ischemic stroke as a functional food. By combing the metabolomics results and literature reports, we hypothesized that the GSTTF exerted the protective potential on MCAO-induced ischemic stroke via modulating the inflammation-related pathway and then conducted the experiment to validate our hypothesis. In this study, the MCAO rat model was constructed and GSTTF was orally administered to investigate the protective potential against ischemic stroke. The brain tissue was collected and analyzed by high-performance liquid chromatography quadrupole Orbitrap mass spectrometry (HPLC-Q-Orbitrap/MS). LC–MS-based metabolomics combined with histological examination, biochemical analysis, and molecular biology was applied to explore the mechanisms of GSTTF for ischemic stroke protection, which will provide the scientific base for the development of GSTTF as a functional food or food supplement.
Results
GSTTF Reduced Cerebral Ischemia Injury
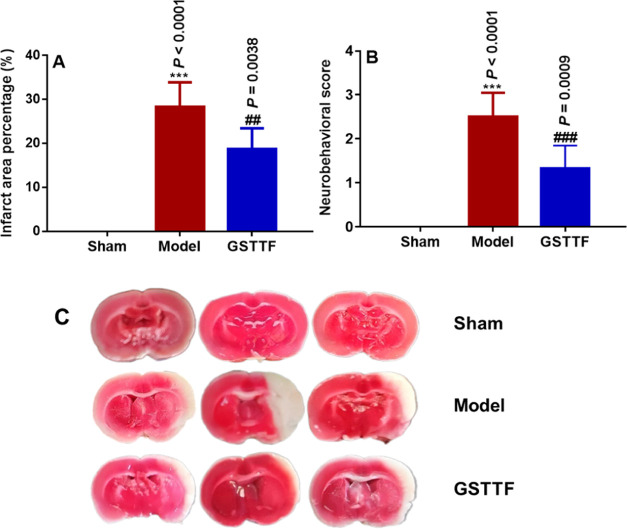
The neuroprotective effects of GSTTF in rats after MCAO surgery were evaluated using the infarction volume and neurobehavioral scores. Rats were treated with GSTTF (200 mg/kg) for 14 consecutive days before MCAO modeling. As shown in Figure 1A,C, there were significant increases in infarct volume in the Model group (28.29 ± 5.64), compared with that in the Sham group. After being treated with GSTTF, the MCAO-induced infarct volume was significantly reduced (18.75 ± 4.7). Neurobehavioral scores (Figure 1B) also showed that the GSTTF administration remarkably alleviated the reduced neurological injury (from 2.50 ± 0.55 to 1.33 ± 0.52).
Figure 1.
Infarction area (A), neurobehavioral score (B), and representative photograph of TTC staining of brain tissue (C) in Sham, Model, and GSTTF-treated rats. ***P < 0.001 vs Sham group; ##P < 0.01, ###P < 0.001 vs Model group.
Metabolic Profile in Brain Tissue
The organic and aqueous extracts of brain tissues were analyzed by UHPLC-Q-Orbitrap/MS in both positive and negative ion modes. The method validation was conducted to ensure the quality of obtained data. As shown in Figure S2, the base peak chromatograms (BPCs) of the QC sample from different brain tissue extracts had a high degree of coincidence, suggesting that the established method had good reproducibility and stability. The representative BPCs of brain tissue samples, including organic and aqueous extracts, are shown in Figures S3 and S4, respectively. Several differences could be observed in the BPCs from different groups, indicating that metabolic changes occurred in the brain during the MCAO modeling and after GSTTF treatment. Since one LC–MS injection could obtain thousands of ion signals, it is hard to handle these pieces of information with a visual inspection. Therefore, the multivariate statistical analysis was introduced to explore the distinction among groups and further find the biomarkers related to the disease and GSTTF efficacy.
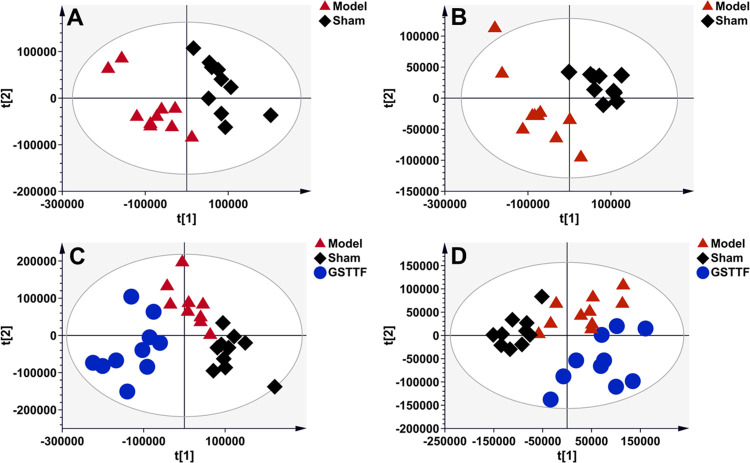
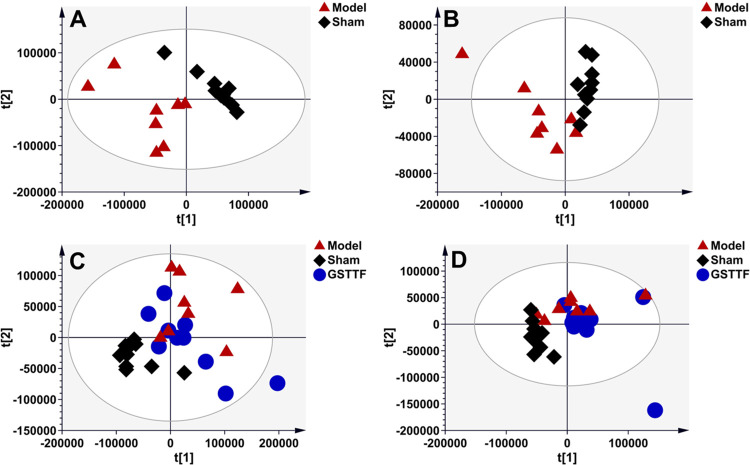
After data preprocessing, the result data set was imported into SIMCA software to construct a partial least-squares discriminant analysis (PLS-DA) model for pattern recognition. As shown in Figure 2A,B, the obvious classifications between the Sham and Model groups were obtained in the PLS-DA score plots established based on the data from the aqueous extract, suggesting that MCAO surgery greatly influenced the composition of brain tissue metabolites. The samples from the GSTTF group showed a clear separation from the Model groups (Figure 2C,D), which indicated that GSTTF treatment could relieve brain injury by regulating certain metabolic pathways. Similarly, the MCAO modeling also led to the disturbance of organic extract of brain tissue, as indicated by the classification between the Sham and Model groups (Figure 3A,B). However, the effect of GSTTF on organic extracts appears to be weaker than its effect on aqueous extracts because although the GSTTF group showed a trend of separation from the Model group, there were still some overlaps (Figure 3C,D).
Figure 2.
PLS-DA score plots of the Sham group (black diamonds), MCAO group (red triangles), and GSTTF group (blue circles) based on the data acquired from aqueous extracts (A, C, ESI+, and B, D, ESI−).
Figure 3.
PLS-DA score plots of the Sham group (black diamonds), MCAO group (red triangles), and GSTTF group (blue circles) based on the data acquired from organic extracts (A, C, ESI+, and B, D, ESI−).
Biomarker Selection and Pathway Analysis
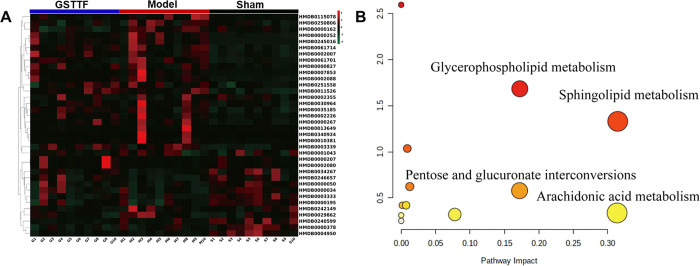
To investigate the metabolites that were related to the MCAO and GSTTF efficacies, the criteria, including importance in the projection (VIP) value from the PLS-DA model, P value, and fold change (FC) value, were used to screen the metabolite that contributed most to the sample classification. As listed in Table 1, 39 metabolites that met the screening criteria (VIP >1, P < 0.05, and FC >2) were identified, which included amino acids, fatty acids, phospholipids, etc. The heat map (Figure 4A) was constructed to visualize the changes in these metabolites from different groups. Most of the intensity changes of metabolite induced by MCAO surgery were reversely regulated after the administration of GSTTF. Then, the metabolic pathway analysis was performed by importing these metabolites into the MetaboAnalyst web tool. The result is shown in Figure 4B; the pathways that were greatly involved in the MCAO operation included glycerophospholipid metabolism, sphingolipid metabolism, arachidonic acid metabolism, pentose and glucuronate interconversions, and so on.
Table 1. Identified Metabolites and Their Changes in Brain Tissues from Different Groups.
| no. | name | HMDB | formula | exact mass | RT (min) | VIP | Model/Sham | GSTTF/Model |
|---|---|---|---|---|---|---|---|---|
| 1 | sphingosine | HMDB0000252 | C18H37NO2 | 299.2823 | 13.51 | 5.67 | ↑b | ↓ |
| 2 | 8-hydroxy-deoxyguanosine | HMDB0003333 | C10 H13N5O5 | 283.0917 | 4.59 | 3.58 | ↓c | ↑ |
| 3 | inosine | HMDB0000195 | C10H12N4O5 | 268.0806 | 4.48 | 5.01 | ↓b | ↑ |
| 4 | adenosine | HMDB0000050 | C10H13N5O4 | 267.0968 | 5.75 | 1.97 | ↓b | ↑ |
| 5 | dl-glutamic acid | HMDB0003339 | C5H9NO4 | 147.0533 | 0.88 | 1.10 | ↑b | ↓ |
| 6 | 2-methylthiazolidine-4-carboxylic acid | HMDB0246657 | C5H9NO2S | 147.0356 | 2.67 | 1.15 | ↓b | ↑d |
| 7 | adenine | HMDB0000034 | C5H5N5 | 135.0546 | 4.09 | 2.59 | ↓a | ↑ |
| 8 | d-valine | HMDB0250806 | C5H11NO2 | 117.0791 | 0.75 | 1.63 | ↑a | ↓d |
| 9 | l-proline | HMDB0000162 | C5H9NO2 | 115.0635 | 0.72 | 1.46 | ↑b | ↓e |
| 10 | docosahexaenoic acid | HMDB0251558 | C22H32O2 | 328.2402 | 13.90 | 6.97 | ↑a | ↓ |
| 11 | 2-aminoethyl oleate | HMDB0245016 | C20H39NO2 | 325.2984 | 13.76 | 1.98 | ↑b | ↓ |
| 12 | oleic acid | HMDB0000207 | C18H34O2 | 282.2559 | 14.88 | 1.07 | ↓a | ↑ |
| 13 | petroselinic acid | HMDB0002080 | C18H34O2 | 282.2559 | 14.88 | 1.07 | ↓a | ↑ |
| 14 | inosine | HMDB0000195 | C10H12N4O5 | 268.0809 | 3.96 | 3.78 | ↓a | ↑ |
| 15 | β-pyrazol-1-ylalanine | HMDB0034267 | C6H9N3O2 | 155.0694 | 0.64 | 2.80 | ↓a | ↑d |
| 16 | l-pyroglutamic acid | HMDB0000267 | C5H7NO3 | 129.0428 | 0.28 | 4.69 | ↓c | ↑ |
| 17 | xylitol | HMDB0242149 | C5H12O5 | 152.0688 | 1.02 | 2.78 | ↑a | ↓ |
| 18 | cyromazine | HMDB0029862 | C6H10N6 | 166.0972 | 0.96 | 2.87 | ↑a | ↓ |
| 19 | 2-methylbutyrylcarnitine | HMDB0000378 | C12H23NO4 | 245.1627 | 1.03 | 2.80 | ↑a | ↓ |
| 20 | 11-hydroxynonadecanoic acid | HMDB0340924 | C19H38O3 | 314.2814 | 15.49 | 2.61 | ↑a | ↓ |
| 21 | oleoylethanolamide | HMDB0002088 | C20H39NO2 | 325.2980 | 13.51 | 3.09 | ↑a | ↓ |
| 22 | eicosapentaenoyl ethanolamide | HMDB0013649 | C22H35NO2 | 345.2668 | 13.77 | 3.16 | ↑a | ↓ |
| 23 | arachidonic acid | HMDB0001043 | C20H32O2 | 304.2402 | 16.15 | 3.32 | ↑b | ↓ |
| 24 | leukotriene E3 | HMDB0002355 | C23H39NO5S | 441.2519 | 6.51 | 2.69 | ↑a | ↓ |
| 25 | LysoPC (15:0) | HMDB0010381 | C23H48NO7P | 481.3167 | 15.11 | 3.10 | ↑a | ↓ |
| 26 | LysoPC (18:1) | HMDB0061701 | C26H52NO7P | 521.3484 | 12.28 | 2.74 | ↑a | ↓ |
| 27 | PA (20:0/20:4) | HMDB0115078 | C43H77O8P | 752.5322 | 21.66 | 2.74 | ↓a | ↑ |
| 28 | l-pyroglutamic acid | HMDB0000267 | C5H7NO3 | 129.0414 | 0.30 | 4.29 | ↓c | ↑ |
| 29 | linolenelaidic acid | HMDB0030964 | C18H30O2 | 278.2247 | 11.98 | 4.28 | ↑c | ↑ |
| 30 | stearic acid | HMDB0000827 | C18H36O2 | 284.2715 | 17.73 | 2.82 | ↓a | ↓ |
| 31 | oleoylethanolamide | HMDB0002088 | C20H39NO2 | 325.2981 | 13.38 | 3.00 | ↑a | ↓ |
| 32 | retinyl acetate | HMDB0035185 | C22H32O2 | 328.2402 | 13.64 | 3.58 | ↑b | ↓ |
| 33 | docosatetraenoic acid | HMDB0002226 | C22H36O2 | 332.2717 | 16.03 | 3.66 | ↑b | ↓ |
| 34 | 13Z,16Z-docosadienoic acid | HMDB0061714 | C22H40O2 | 336.3030 | 18.72 | 2.69 | ↑a | ↓ |
| 35 | tetracosahexaenic acid | HMDB0002007 | C24H36O2 | 356.2718 | 15.81 | 3.52 | ↑b | ↑ |
| 36 | LysoPA (16:0) | HMDB0007853 | C19H39O7P | 410.2433 | 11.85 | 3.40 | ↑b | ↓ |
| 37 | LysoPE (P-18:1(9Z)) | HMDB0240599 | C23H46NO6P | 463.3066 | 14.25 | 2.79 | ↑a | ↓ |
| 38 | LysoPE (22:6) | HMDB0011526 | C27H44NO7P | 525.2848 | 10.61 | 2.82 | ↑a | ↓ |
| 39 | ceramide (d18:1/18:0) | HMDB0004950 | C36H71NO3 | 565.5433 | 21.72 | 2.91 | ↑a | ↓ |
P < 0.05.
P < 0.01.
P < 0.001 vs Sham group.
P < 0.05.
P < 0.01 vs Model group.
Figure 4.
Heat map (A) of the changes in the intensities of biomarkers, and bubble plot (B) of the metabolic pathway analysis.
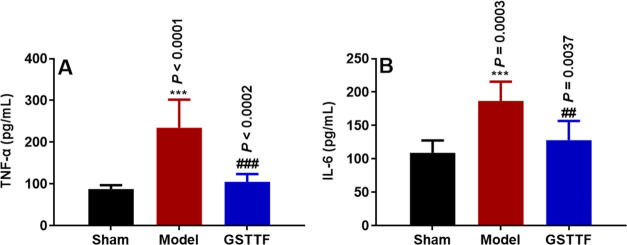
Effect of GSTTF on the Levels of TNF-α and IL-1β in Brain Tissue of Rats after MCAO
The levels of TNF-α and IL-6 in brain tissues after MCAO and GSTTF treatments were determined (Figure 5). Compared with the Sham group, the contents of TNF-α and IL-6 in the brain tissue of the Model group were significantly increased. After treatment with GSTTF, both levels of proinflammatory cytokines were remarkably decreased. These results demonstrated that MCAO could induce the release of inflammatory cytokines, but the GSTTF treatment remarkably attenuated these changes.
Figure 5.
Levels of TNF-α (A) and IL-6 (B) in the brain tissue of rats in each group. ***P < 0.001 vs Sham group; ##P < 0.01, ###P < 0.001 vs Model group.
Effect of GSTTF on TLR4/MyD88/NF-κB Signaling Pathway in Brain Tissue after MCAO
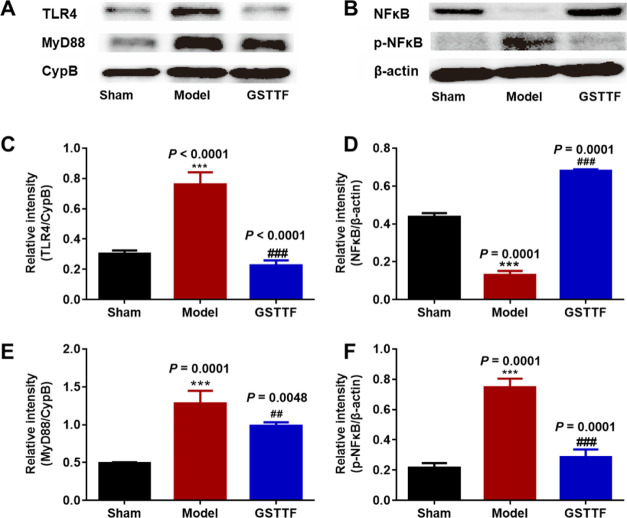
Western blot analysis was applied to detect the protein expressions of TLR4, MyD88, and NF-κB in the signaling pathway. As shown in Figure 6, the expressions of TLR4, MyD88, and p-NF-κB in the brain tissue of the Model group were significantly upregulated, compared with that in the Sham group, indicating that the brain tissue of the MCAO rats was in a state of inflammation and the related signaling pathway was activated. When GSTTF was administered, the expressions of TLR4, MyD88, and p-NF-κB were markedly decreased, suggesting that GSTTF had anti-inflammatory potential on brain tissue of the MCAO rats through the suppression of TLR4/MyD88/NF-κB signaling pathway.
Figure 6.
Protein levels (A, B) and the corresponding statistical graphs (C–F) of TLR4, MyD88, NFκB p65, and p-NFκB p65 in the brain tissue from each group. ***P < 0.001 vs Sham group; ##P < 0.01, ###P < 0.001 vs Model group.
Discussion
Ischemic stroke has become an important factor that seriously threatens the life and health of middle-aged and elderly patients. Investigating the therapy and associated mechanisms of ischemic stroke has far-reaching implications for improving the quality of life of patients. TT is a medicinal herb that has been confirmed to have many health benefits and its extract is used as a food supplement in the United States and Europe. In this study, we investigated the potential of GSTTF as a medicinal food to treat MCAO-induced ischemic stroke using LC–MS-based metabolomics combined with molecular biology analysis.
The efficacy of GSTTF on ischemic stroke was evaluated by brain tissue infarction area and neurobehavioral scores, and the results showed that GSTTF could attenuate MCAO-induced brain injury and improve impaired behavior in rats. Metabolomics is a branch of system biology, which provides a whole picture of metabolites and helps to understand the process of life activities from the perspective of metabolic changes.14 The extracts of brain tissue samples were analyzed using LC–MS-based metabolomics. Consistent with the efficacy assessment, the samples in the Model group were separated from those in the Sham group, and the GSTTF administration could make the samples leave the Model group and approach the Sham group, which suggested that the GSTTF exerted protective effects on MCAO by regulating the changes of metabolites. The statistical analysis highlighted 39 metabolites that were significantly changed during the MCAO modeling and were regarded as biomarkers. Most of them were reversely regulated by GSTTF treatment. The metabolic pathway analysis indicated that these biomarkers were greatly involved in glycerophospholipid metabolism, sphingolipid metabolism, arachidonic acid metabolism, etc.
Arachidonic acid metabolism plays an important role in cardiovascular biology, carcinogenesis, and the development of many inflammatory diseases.15 When the ischemic stroke occurs, cerebral blood flow is stopped, and hypoxic brain tissue triggers the quick cleavage of arachidonic acid from the membrane phospholipid bilayer via the enzymatic hydrolysis of phospholipase A2 (PLA2). Thus, in this study, arachidonic acid was found at a higher intensity in the Model group compared with that in the Sham group, while GSTTF treatment could downregulate its intensity. Free arachidonic acid undergoes enzymatic or nonenzymatic oxidative metabolism to form a number of bioactive molecules that lead to the inflammatory response after stroke onset.16 Besides, glycerophospholipid metabolism can also be mediated by PLA2, which results in the elevation level of LysoPC in blood, cerebrospinal fluid, as well as brain tissue after ischemic and hemorrhagic strokes.17−19 The present study found that the intensities of LysoPCs were significantly increased in the Model group, which was in agreement with the published reports. LysoPCs are potent mediators of poststroke brain inflammation by stimulating IL-1β release and subsequent microglial activation.20−22 The inflammation reaction has been considered a major pathophysiological mechanism, and the drugs that inhibit inflammatory responses and reduce the levels of the related indicators have archived good clinical efficacy in stroke treatment.23 Therefore, we hypothesized that GSTTF exerted protective effects by modulating inflammatory responses.
The proinflammatory cytokines, including TNF-α and IL-6, are released after brain injury to induce inflammation cascades and exert neurotoxicity. The determination of these cytokines in this study revealed that GSTTF treatment greatly reduced their levels in brain tissue after MCAO, suggesting that GSTTF improved cerebral injury through anti-inflammatory effects. NF-κB is a major inflammatory mediator that interacts with a wide range of immunological receptors. Dysregulated NF-κB activation is implicated in various inflammatory diseases; thus, targeting the NF-κB signaling pathway is considered an attractive anti-inflammatory therapeutic approach.24 NF-κB could regulate genes of ischemic stroke-related inflammatory mediators such as TNF-α and IL-6. Our results showed that phosphorylation of NF-κB was markedly activated, suggesting that the NF-κB signaling pathway was associated with the inflammatory response after ischemic stroke. The GSTTF administration significantly downregulated the phosphorylation of NF-κB, which demonstrated the possibly anti-inflammatory mechanism of GSTTF. A previous report also revealed that the therapeutic effect of GSTTF on cerebral ischemic injury was associated with NF-κB signaling.25 However, the effects of GSTTF on the upstream proteins of the NF-κB signaling pathway have not been investigated. TLRs are important components of the innate immune system. When TLRs are activated, they can recruit MyD88 and subsequently facilitate the activation of downstream signaling pathways like the NF-κB and MAPK pathways and then further induce the release of inflammatory cytokines.26,27 Based on these, we further explored the potential protective mechanisms of GSTTF on cerebral ischemic injury. Western blot analysis revealed that GSTTF treatment significantly decreased the higher expression of TLR4 and MyD88 induced by MCAO. These findings suggested that GSTTF provided neuroprotection via anti-inflammatory actions, possibly through the TLR4/MyD88/NF-κB signaling pathway.
Overall, we investigated the metabolic pathways and signaling pathways that were disturbed by MCAO, as well as the effect of GSTTF on these pathways. Metabolomics analysis showed that the protective effects of GSTTF on MCAO were greatly associated with arachidonic acid metabolism and glycerophospholipid metabolism, both of which were closely related to inflammatory responses. Then, the inflammation-related cytokines and proteins were determined, which demonstrated the anti-inflammatory effect of GSTTF and the corresponding mechanism. However, the connection between the metabolic pathway and the TLR4/MyD88/NF-κB signaling pathway is still not fully elucidated. According to the definition of activity metabolomics, the metabolome affects cellular physiology by regulating “omics” levels, including the genome, epigenome, transcriptome, and proteome.28 The metabolites are usually considered the downstream products, whose important regulatory roles are usually ignored. The metabolome could interact with other omics to act directly on biological processes and phenotypes. For example, arachidonic acid, which was highlighted as a biomarker in this study, can be metabolized by three distinct enzymatic systems, including cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes, to produce a series of biologically active fatty acids. These fatty acids produced by arachidonic acid metabolism have received a lot of interest in cardiovascular and cancer biology, particularly in inflammatory processes and diseases. Therefore, further studies are required to explore whether GSTTF exerts its anti-inflammatory effect by directly inhibiting the TLR4/MyD88/NF-κB signaling pathway or by changing the metabolism of active metabolites and then exerting an inhibitory effect.
Besides, lot of information was obtained from the LC–MS-based metabolomics analysis, which gives us many clues to explain the protective effect of GSTTF from different perspectives. It is difficult for us to design experiments to take into account all aspects of metabolic findings; thus, we focused only on the inflammatory response to explain the mechanism by which GSTTF exerts its cerebral protective effects. GSTTF contains multiple components that act synergistically with each other, and this multicomponent, multitarget mode of action could explain the metabolomic finding that multiple metabolic pathways are involved in its protective effects on ischemic stroke, which warrants further investigation.
Conclusions
GSTTF contains multiple compounds and can regulate multiple aspects of our body. The comprehensive regulation effect of GSTTF on MCAO was investigated by LC–MS-based metabolomics, and several inflammation-related metabolites were highlighted as biomarkers. Then, the biochemistry analysis revealed that GSTTF treatment greatly reduced the higher levels of TNF-α and IL-6 induced by MCAO in brain tissue. And this protective efficacy may be archived via suppression of the TLR4/MyD88/NF-κB signaling pathway. This study provides knowledge for understanding the neuroprotective efficacy of GSTTF against ischemic stroke, which expands its medicinal application and also confirms its potential to be developed as a functional food or a food supplement.
Materials and Methods
Materials and Chemicals
The GSTTF with a purity of saponin >60% was manufactured and obtained from Changbaishan Pharmaceutical Co., Ltd. (Jilin, China). The GSTTF was analyzed by HPLC, and the main components were identified by comparison with standard references (Figure S1). HPLC-grade acetonitrile, methanol, and formic acid were purchased from Fisher Scientific Corporation (Loughborough, U.K.). Ultrapure water was prepared using a Milli-Q purification system (Billerica, MA).
Animals and Treatments
Thirty adult male Sprague Dawley rats, weighing 180–220 g, were provided by the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Rats were housed with 12 h of light–dark cycle in a climate-controlled system with 40–65% relative humidity and 19–23 °C. The animals were acclimated for 7 days before the operation. The whole animal treatments were approved by the Animal Ethics Committee, Academy of Traditional Chinese Medicine of Jilin Province (approval no. JLSZKYDWLL2020-002).
All rats were randomly separated into three groups of 10 animals each: (1) Sham group (treated with normal saline), (2) MCAO group (treated with normal saline), and (3) GSTTF group (treated with GSTTF 200 mg/kg). Rats in the GSTTF group were continuously gavaged with GSTTF for 2 weeks prior to MCAO modeling. The MCAO operation was constructed 1 hour after the last treatment according to our previous reports (see the Supporting Material).
LC–MS-Metabolomics Analysis
The aqueous and organic extracts of brain tissue samples were analyzed by a UHPLC-Q-Orbitrap/MS to obtain a comprehensive view of metabolites. The procedures of sample preparation and LC–MS condition are provided in the Supporting Material. After raw data acquisition, the data preprocessing was performed using compound discoverer (CD) software to generate a data set, containing sample groups, accurate m/z value, and peak intensity, for subsequent statistical analysis. Then, the partial least-squares discriminant analysis (PLS-DA) was established by the SIMCA software to exhibit the classification of samples from different groups and screen the biomarkers that contribute most to the sample classification. The features that meet the criteria, including VIP >1, P < 0.05, and FC >2, were selected as a biomarker. The compound annotation was then conducted by matching the MS and MS/MS information with database records.29,30 The metabolic pathway analysis was conducted by importing the annotated metabolites into the MetaboAnalyst web tool.31
Biochemical Determination and Western Blot Analysis
The concentrations of TNF-α and IL-6 in brain tissues were measured using commercial kits (eBioscience, San Diego, CA).
Brain tissue was extracted, and total protein was obtained using RIPA buffer containing PMSF, followed by centrifugation at 12000 rpm for 10 min. Nuclear proteins were extracted and their concentrations were determined by Lowry protein assay. Total proteins were subjected to SDS-PAGE gel electrophoresis and electroblotted onto PVDF membranes, which were blocked in 5% BSA for 1 h and then incubated overnight at 4 °C with the following primary antibodies. The antibodies were as follows: β-actin (loading control; rabbit monoclonal antibody 1:1000), cyclophilin B (CypB; loading control; rabbit monoclonal antibody 1:1000), Toll-like receptor 4 (TLR4; rabbit monoclonal antibody 1:1000), myeloid differentiation factor 88 (MYD88; rabbit monoclonal antibody 1:1000), nuclear factor-kappa B p65 (NF-κB p65; rabbit monoclonal antibody 1:1000), phosphorylated NF-κB p65 (p-NF-κB p65; rabbit monoclonal antibody 1:1000), and then the membranes were incubated with 1:3000 secondary antibodies and visualized with the ECL imaging system. The protein expression was analyzed using the Image-J system.
Data Analysis
The data were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was conducted using GraphPad Prism software. P < 0.05 was considered statistically significant.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (Grant No. 82104366) and the Natural Science Fund Project of Jilin Province, China (Grant No. 20200201502JC).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06625.
HPLC analysis of GSTTF; animals and treatments; HPLC chromatograms of GSTTF (A) and the chromatograms of nine standard references (B) (Figure S1); the base peak chromatograms of the QC sample from aqueous (A, ESI+, and C, ESI−) and organic extracts (B, ESI+, and D, ESI−) (Figure S2); the representative BPC of aqueous brain tissue extract in three groups acquired in positive ion mode (A–C) and negative ion mode (D–F) (Figure S3); and the representative BPC of organic brain tissue extract in three groups acquired in positive ion mode (A–C) and negative ion mode (D–F) (Figure S4) (PDF)
Author Contributions
⊥ H.Z. and W.G. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Furie K. L.; Jayaraman M. V. 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 2018, 49, 509–510. 10.1161/STROKEAHA.118.020176. [DOI] [PubMed] [Google Scholar]
- Samaniego E. A.; Roa J. A.; Limaye K.; Adams H. P. Jr Mechanical thrombectomy: emerging technologies and techniques. J. Stroke Cerebrovasc. Dis. 2018, 27, 2555–2571. 10.1016/j.jstrokecerebrovasdis.2018.05.025. [DOI] [PubMed] [Google Scholar]
- Arora N.; Makino K.; Tilden D.; Lobotesis K.; Mitchell P.; Gillespie J. Cost-effectiveness of mechanical thrombectomy for acute ischemic stroke: an Australian payer perspective. J. Med. Econ. 2018, 21, 799–809. 10.1080/13696998.2018.1474746. [DOI] [PubMed] [Google Scholar]
- O’Collins V. E.; Macleod M. R.; Donnan G. A.; Horky L. L.; van der Worp B. H.; Howells D. W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Watson R. R.; Singh R. B.; Takahashi T.. The role of Functional Food Security in Global Health; Academic Press, 2018. [Google Scholar]
- Gillman M. W. Protective effect of fruits and vegetables on development of stroke in men. JAMA 1995, 273, 1113–1117. 10.1001/jama.1995.03520380049034. [DOI] [PubMed] [Google Scholar]
- Kokubo Y.; Iso H.; Ishihara J.; Okada K.; Inoue M.; Tsugane S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center–based (JPHC) study cohort I. Circulation 2007, 116, 2553–2562. 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- Wennberg M.; Bergdahl I. A.; Stegmayr B.; Hallmans G.; Lundh T.; Skerfving S.; Strömberg U.; Vessby B.; Jansson J.-H. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br. J. Nutr. 2007, 98, 1038–1045. 10.1017/S0007114507756519. [DOI] [PubMed] [Google Scholar]
- Kostova I.; Dinchev D. Saponins in Tribulus terrestris–chemistry and bioactivity. Phytochem. Rev. 2005, 4, 111–137. 10.1007/s11101-005-2833-x. [DOI] [Google Scholar]
- Wang Y.; Zhao H.; Liu Y.; Guo W.; Bao Y.; Zhang M.; Xu T.; Xie S.; Liu X.; Xu Y. GC-MS-based metabolomics to reveal the protective effect of gross saponins of Tribulus terrestris fruit against ischemic stroke in rat. Molecules 2019, 24, 793 10.3390/molecules24040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Guo W.; Liu Y.; Wang J.; Fan M.; Zhao H.; Xie S.; Xu Y. Investigating the protective effect of gross saponins of Tribulus terrestris fruit against ischemic stroke in rat using metabolomics and network pharmacology. Metabolites 2019, 9, 240 10.3390/metabo9100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.; Wang Y.; Fan M.; Xie S.; Zhao H.; Wang J.; Liu Y.; Xu D.; Xu Y. Integrating metabolomics and network pharmacology to explore the protective effect of gross saponins of Tribulus terrestris L. fruit against ischemic stroke in rat. J. Ethnopharmacol. 2020, 263, 113202 10.1016/j.jep.2020.113202. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Guo W.; Xie S.; Liu Y.; Xu D.; Chen G.; Xu Y. Multi-omics analysis of brain tissue metabolome and proteome reveals the protective effect of gross saponins of Tribulus terrestris L. fruit against ischemic stroke in rat. J. Ethnopharmacol. 2021, 278, 114280 10.1016/j.jep.2021.114280. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Liu S.; Hu Y.; Li P.; Wan J.-B. Current state of the art of mass spectrometry-based metabolomics studies–a review focusing on wide coverage, high throughput and easy identification. RSC Adv. 2015, 5, 78728–78737. 10.1039/C5RA14058G. [DOI] [Google Scholar]
- Wang B.; Wu L.; Chen J.; Dong L.; Chen C.; Wen Z.; Hu J.; Fleming I.; Wang D. W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduction Targeted Ther. 2021, 6, 94 10.1038/s41392-020-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W.; Horrocks L. A.; Farooqui A. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Rev. 2006, 52, 201–243. 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Sun G. Y.; Foudin L. L. On the status of lysolecithin in rat cerebral cortex during ischemia. J. Neurochem. 1984, 43, 1081–1086. 10.1111/j.1471-4159.1984.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A.; Yang H. C.; Rosenberger T. A.; Horrocks L. A. Phospholipase A2 and its role in brain tissue. J. Neurochem. 2002, 69, 889–901. 10.1046/j.1471-4159.1997.69030889.x. [DOI] [PubMed] [Google Scholar]
- Hirashima Y.; Nakamura S.; Endo S.; Kuwayama N.; Naruse Y.; Takaku A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem. Res. 1997, 22, 1249–1255. 10.1023/A:1021985030331. [DOI] [PubMed] [Google Scholar]
- Ousman S. S.; David S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 2000, 30, 92–104. . [DOI] [PubMed] [Google Scholar]
- Liu P.; Zhu W.; Chen C.; Yan B.; Zhu L.; Chen X.; Peng C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443 10.1016/j.lfs.2020.117443. [DOI] [PubMed] [Google Scholar]
- Stock C.; Schilling T.; Schwab A.; Eder C. Lysophosphatidylcholine stimulates IL-1β release from microglia via a P2X7 receptor-independent mechanism. J. Immunol. 2006, 177, 8560–8568. 10.4049/jimmunol.177.12.8560. [DOI] [PubMed] [Google Scholar]
- Khoshnam S. E.; Winlow W.; Farzaneh M.; Farbood Y.; Moghaddam H. F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- Liu T.; Zhang L.; Joo D.; Sun S.-C. NF-κB signaling in inflammation. Signal Transduction Targeted Ther. 2017, 2, 17023 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang E.-p.; Li H.; Chen J.-g.; Yang S.-j. Protection by the gross saponins of Tribulus terrestris against cerebral ischemic injury in rats involves the NF-κB pathway. Acta Pharm. Sin. B 2011, 1, 21–26. 10.1016/j.apsb.2011.04.009. [DOI] [Google Scholar]
- Boyd J. H.; Mathur S.; Wang Y.; Bateman R. M.; Walley K. R. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-κB dependent inflammatory response. Cardiovasc. Res. 2006, 72, 384–393. 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Gu J.; Su S.; Guo J.; Zhu Y.; Zhao M.; Duan J.-a. Anti-inflammatory and anti-apoptotic effects of the combination of Ligusticum chuanxiong and Radix Paeoniae against focal cerebral ischaemia via TLR4/MyD88/MAPK/NF-κB signalling pathway in MCAO rats. J. Pharm. Pharmacol. 2018, 70, 268–277. 10.1111/jphp.12841. [DOI] [PubMed] [Google Scholar]
- Rinschen M. M.; Ivanisevic J.; Giera M.; Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. 10.1038/s41580-019-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S.; Feunang Y. D.; Marcu A.; Guo A. C.; Liang K.; Vázquez-Fresno R.; Sajed T.; Johnson D.; Li C.; Karu N.; et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijas C.; Montenegro-Burke J. R.; Domingo-Almenara X.; Palermo A.; Warth B.; Hermann G.; Koellensperger G.; Huan T.; Uritboonthai W.; Aisporna A. E.; et al. METLIN: a technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. 10.1021/acs.analchem.7b04424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J.; Soufan O.; Li C.; Caraus I.; Li S.; Bourque G.; Wishart D. S.; Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.