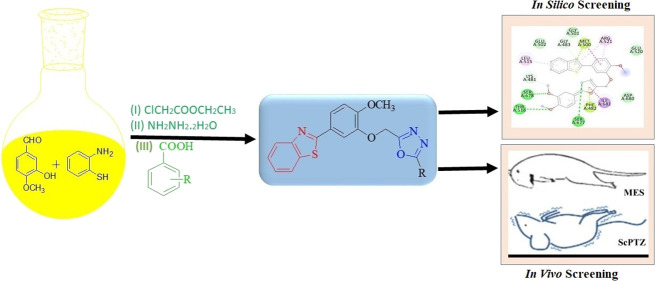
Abstract
In the presented manuscript, a new series of 2-[4-methoxy-3-(5-substituted phenyl-[1,3,4]oxadiazol-2-ylmethoxy)-phenyl]-benzothiazoles (6a–n) have been synthesized and studied in vivo and in silico for their anticonvulsant potential. Maximum electroshocks (MES) and subcutaneous pentylenetetrazol (scPTZ) models have been used for in vivo anticonvulsant activity. Auto Dock 4.2 software was used for in silico studies, and the targeted protein was 5IOV.sThe antidepressant activity of selected compounds (most active) was determined as a reduction in locomotor activity through an actophotometer. In vivo and In silico studies proved that among all the synthesized compounds, 6f, 6h, 6j, and 6l were the most potent with no neurotoxicity as compared to conventional drugs (phenytoin and phenobarbital). The in silico studies also indicated about different binding interactions of synthetic compounds to localize the binding receptors. The most likely mode of action for these drugs, according to the docking analysis of active compounds with various targets, is their binding to the VGCC and NMDA receptors.
1. Introduction
Epilepsy is a serious neurologic condition associated with stigma, psychiatric comorbidity, and high economic costs.1−3 By using the meta-analytic technique in population-based studies of the prevalence and incidence of epilepsy, it is established that the point prevalence of active epilepsy was 6.38 per 1000 persons while the lifetime prevalence was 7.60 per 1000 persons. The same study also established that the annual cumulative incidence of epilepsy was 67.77 per 100,000 persons while the incidence rate was 61.44 per 100,000 person-years.4 Epilepsy is also more common among high-income countries, lower socioeconomic groups, and persons of different ethnic origins within the same community.5 According to the International League Against Epilepsy (ILAE), epilepsy can be characterized by the following: (1) one unprovoked (or reflex) seizure and a risk of further seizures similar to the general recurrence risk (at least 60%) after 2 unprovoked seizures occurring within the next 10 years; (2) at least 2 unprovoked (or reflex) seizures occurring >24 h apart; and (3) diagnosis of an epilepsy syndrome.6 In the middle of the 19th century, the first anticonvulsant drug named potassium bromide was reported.7 Since then, several antiepileptic drugs (AEDs) have been approved by scientists and the medical community and are available as primary treatment for people suffering from epilepsy.8,9 Currently, barbiturates and benzodiazepines are widely used as antiepileptic drugs; however, these are inefficient in controlling seizures in more than 30% of the patient and have a low therapeutic window, drug–drug interaction, and various types of adverse effects.10 Other marketed AEDs like gabapentin, pregabalin, vigabatrin, lacosamide, lamotrigine, levetiracetam, etc. also are associated with several adverse effects such as gastrointestinal disturbances, nausea, hirsutism, hepatotoxicity, weight gain, resistance, and neurotoxicity.11,12 The efficacy of an AED for a specific type of seizure and also its tolerability and safety are factors taken into consideration in the selection of antiepileptic drugs for treatment.12−14 Recently, the U.S. Food and Drug Administration (FDA) permitted three new AEDs—brivaracetam, cannabidiol, and stiripentol.15,16 However, there is always a need to search for new chemical entities with more efficacy and fewer side effects as AEDs.
Medicinal chemists are continuously working to explore the pharmacological potential of heterocyclic compounds in which benzothiazoles are interesting prospects. It has been established that the endocyclic sulfur and nitrogen activities in this heterocyclic nucleus are essential for anticonvulsant activity.17 Benzothiazole contains benzene fused with a five-membered thiazole ring.18,19 Benzothiazole is an affluent endocyclic ring system with multiple biological activities including anticancer,20 antidiabetic,21 antimicrobial,22 anti-inflammatory,23 antiviral,24 antituberculosis,25 etc. Further, researchers have found that the presence of an oxadiazole moiety in the heterocyclic ring shows potent anticonvulsant properties. 1,3,4-Oxadiazole is a five-membered ring containing one oxygen and two nitrogen atoms.26 Oxadiazole containing heterocyclic compounds also have a wide range of biological activities such as antimicrobial,27 anti-inflammatory,28 antituberculosis,29 antibacterial,30 antioxidant,31 etc. To maximize the pharmacological potential of both moieties, we created some novel hybrid compounds of benzothiazole and hydrazones while taking into account the structural requirements and wide range of biological activities of both moieties. Utilizing the MES (maximal electroshock seizure method) and scPTZ standard protocols, the synthesized compounds were assessed for their anticonvulsant activity (subcutaneous pentylenetetrazole).
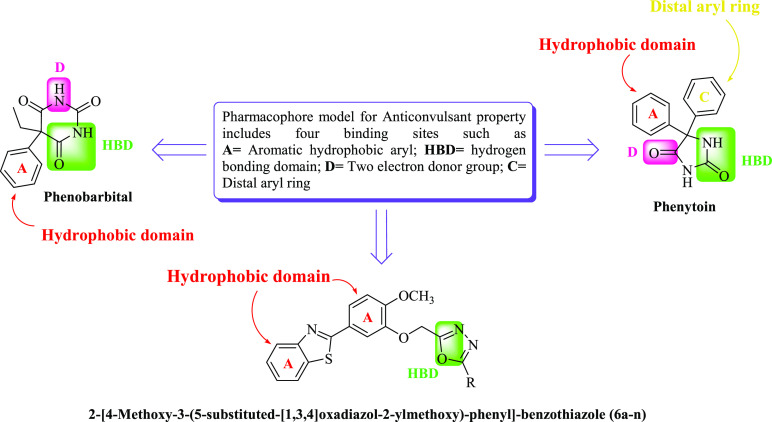
The pharmacophore model for anticonvulsant drugs consists of four binding sites by way of illustration; an aryl hydrophobic binding site (A), a hydrogen bonding domain (HBD), an electron donor-acceptor system (D), and a hydrophobic aryl ring (C).32 The standard drug phenytoin and phenobarbital are considered as references for designing the pharmacophore model of newly synthesized compounds (Figure 1).33,34
Figure 1.
Structural requirements (pharmacophore model) of anticonvulsant activity.
2. Results and Discussion
2.1. Chemistry
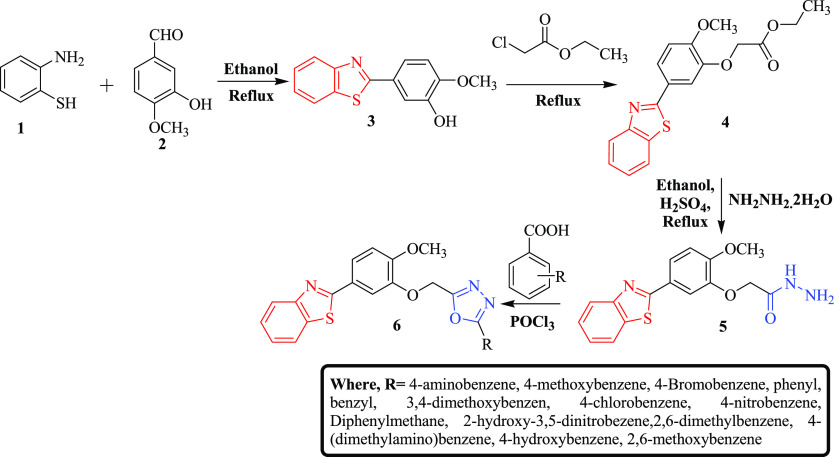
Targeted compounds (6a–n) were synthesized by following the reaction scheme as illustrated in Scheme 1. In the initial step, 5-benzothiazol-2-yl-2-methoxy-phenol (3) was synthesized via cyclization of 2-amino thiophenol (1) with 3-hydroxy-4-methoxy-benzaldehyde (2). The presence of peaks (cm–1) at 3244 (O–H str), 3065 (C–H str, Ar), 2932–2845 (C–H str, alkane), 1586 (C=N str), 1530–1431(C=C, Ar), 1174 (C–O), 1127(C–N), and 731 (C–S–C) in the IR spectra confirmed the synthesis of compound 3. The synthesis of compound 3 was also confirmed by the presence of signals at 4.821 and 3.827 for OH and OCH3 protons, respectively, in IH NMR. In the next step, (5-benzothiazol-2yl-2-methoxy-phenoxy)-acetic acid ethyl ester (4) was prepared by esterification with chloroethyl acetate.35 The peaks (cm–1) at 3064 (C–H, Ar), 2935–2849 (C–H, alkane), 1763 (C=O), 1586 (C=N), 1523–1432 (C=C, Ar), 1193 (C–O), 1136 (C–N), and 727 (C–S–C) in the IR spectra confirm the synthesis of compound 4. The signals at 4.912, 4.128, 3.831, and 1.298 due to the presence of OCH3, CH3, and CH2 groups in the 1H NMR again bolstered the confirmation of intermediate 4 synthesis. In the third step, the reaction of intermediate 4 with hydrazine hydrate yielded (5-benzothiazol-2-yl-2-methoxy-phenoxy)-acetic acid hydrazide (5),35 which was confirmed by the presence of peaks (cm–1) at 3648 (N–H), 3065 (C–H, Ar), 2932–2849 (C–H, alkane), 1757 (C=O), 1558 (C=N), 1524–1435 (C=C, Ar), 1173 (C–O), 1146 (C–N), and 729 (C–S–C), respectively, in the IR spectra. The structure of compound 5 was again bolstered by the presence of signals of NH2, NH, OCH3, and CH2 protons at 8.001, 4.809, 3.830, and 2.321, respectively, in the 1H NMR. In the final step, the targeted compounds (6a–n) were obtained by the reaction of (5-benzothiazol-2-yl-2-methoxy-phenoxy)-acetic acid hydrazide (5) with substituted aromatic carboxylic acids via the Vilsmeier–Haack reaction followed by cyclization.36,37 The structures of targeted compounds were established by characterization using FT-IR, 1H NMR, 13C NMR, and mass spectral data followed by elemental analysis. The IR spectra of the synthesized compounds showed C–H stretching (aromatic and aliphatic) in the range of 3090–2800 cm–1. The C=N and C=C stretching peaks appeared in the range of 1650–1590 and 1600–1400 cm–1, respectively, whereas C–N and C–O bending peaks were found in the range of 1300–1100 cm–1. Characteristic absorption bands at 780–650 cm–1 corresponded to the C–S, C–Cl, and C–Br bending functions of the structures. The substituted functional groups such as N–H and O–H were observed in the region of 3500–3300 cm–1. In 1H-NMR spectra, a doublet and triplet of aromatic protons of the benzothiazoyl and benzene rings are found resonating in the range of 8.034–7.330 ppm. The characteristic peaks of the −OH, −CH2, −NH, and −OCH3 groups appeared at 10.414, 4.816, 4.292, and 3.964 ppm, respectively.
Scheme 1. Synthesis of Target Compounds (6a–n).
2.2. Biological Evaluation Studies
2.2.1. Acute Toxicity (LD50) Study
The median lethal dose (LD50) of the derived compounds (6a–n) was determined by utilizing data obtained through acute toxicity studies, which were conducted as per OECD 423 guidelines.38 All the prepared compounds were administered to different groups of either sex of albino mice. Each animal was observed for 24 h after the administration of definite doses. The data obtained are shown in Table 1, which suggests that animals with a dose concentration of 300 mg/kg body weight of compounds, 6c, 6d, 6e, 6g, 6i, and 6k show mortality with involuntary movements like unwanted body stiffening and jerky movements. No other signs and mortality have been reported in any groups at 100 mg/kg dose concentration except compounds 6c and 6e as they showed slight spasmodic behavior after a few minutes of administration. At lower dose concentrations of 30 and 5 mg/kg body weight, all animals survived and stayed healthy.
Table 1. Acute Toxicity Studies of Standards and Synthesized Compounds (6a–n).
| number
of animals dead/tot number of animals tested,
dosage (mg/kg) |
||||
|---|---|---|---|---|
| compounds | 5 | 30 | 100 | 300 |
| 6a | 0/3 | 0/3 | 0/3 | 0/3 |
| 6b | 0/3 | 0/3 | 0/3 | 0/3 |
| 6c | 0/3 | 0/3 | 1/3 | 2/3 |
| 6d | 0/3 | 0/3 | 0/3 | 2/3 |
| 6e | 0/3 | 0/3 | 1/3 | 1/3 |
| 6f | 0/3 | 0/3 | 0/3 | 0/3 |
| 6g | 0/3 | 0/3 | 0/3 | 1/3 |
| 6h | 0/3 | 0/3 | 0/3 | 0/3 |
| 6i | 0/3 | 0/3 | 0/3 | 1/3 |
| 6j | 0/3 | 0/3 | 0/3 | 0/3 |
| 6k | 0/3 | 0/3 | 0/3 | 1/3 |
| 6l | 0/3 | 0/3 | 0/3 | 0/3 |
| 6m | 0/3 | 0/3 | 0/3 | 0/3 |
| 6n | 0/3 | 0/3 | 0/3 | 0/3 |
| phenytoin | 0/3 | 0/3 | 0/3 | 0/3 |
| phenobarbital | 0/3 | 0/3 | 0/3 | 0/3 |
2.2.2. In Vivo Anticonvulsant Activity
The maximal electroshock (MES) and subcutaneous pentylenetetrazole (scPTZ) induced convulsion animal models were used for investigating the antiepileptic potential of the synthesized compounds (6a–n) in albino mice. The purpose of these studies was to find compounds that might prevent both generalized absence (petit mal) and generalized tonic–clonic (grand mal) seizures. The chosen groups of mice were given all the synthetic compounds intraperitoneally (i.p.) at doses of 30, 100, and 300 mg/kg of body weight, and observations were made at two distinct times (0.5 and 4 h).
As indicated in Table 2, the majority of the produced compounds demonstrated protection in both antiepileptic screening methods. Compounds 6f, 6h, 6j, and 6l in the MES and scPTZ model showed protection against seizures spread across both 0.5 and 4.0 h periods at 30 mg/kg of body weight, indicating a potential short onset and prolonged duration of action. Compounds 6a and 6d showed protection at the same dose after 0.5 h but needed 100 mg/kg after 4.0 h, indicating a rapid onset but brief duration of action. By needing a greater dosage of 100 mg/kg of body weight and 300 mg/kg of body weight for 0.5 and 4 h interval protection, respectively, compounds 6e and 6k displayed intermediate activity in MES screening, whereas in scPTZ screening, compound 6g is also added into the same category of potential along with 6e and 6k. Further, in MES screening, compounds 6g, 6i, and 6n also showed signs of antiepileptic activity since they offered protection at 100 mg/kg of body weight at intervals of 0.5 and 4 h, but compounds 6b, 6c, and 6m were shown to have antiepileptic potential because it showed action at 300 and 100 mg/kg of body weight at the 0.5 h, which failed to exhibit activity after the time of 4.0 h, indicating that their effects are limited to the dosages utilized (100 and 300 mg/kg). In scPTZ screening, compound 6n showed signs of antiepileptic activity since they offered protection at 100 mg/kg of body weight at intervals of 0.5 h, but compounds 6c and 6m were shown to have antiepileptic potential because they showed action at 300 mg/kg of body weight at the same period. Compounds 6i, 6c, 6m, and 6n failed to exhibit activity after the times of 0.5 and 4.0 h, indicating that their effects are limited to the dosages utilized (100 and 300 mg/kg).
Table 2. Anticonvulsant Activity and Neurotoxicity of Synthesized Compounds (6a–n).
| intraperitoneal injection in micea |
||||||
|---|---|---|---|---|---|---|
| HLTE | scPTZ | neurotoxicity | ||||
| compound no | 0.5 h | 4.0 h | 0.5 h | 4.0 h | 0.5 h | 4.0 h |
| 6a | 30 | 100 | 30 | 100 | ||
| 6b | 300 | 300 | 300 | |||
| 6c | 300 | 300 | 300 | 300 | ||
| 6d | 30 | 100 | 30 | 100 | ||
| 6e | 100 | 300 | 100 | 100 | ||
| 6f | 30 | 30 | 30 | 30 | ||
| 6g | 100 | 100 | 100 | 100 | ||
| 6h | 30 | 30 | 30 | 30 | ||
| 6i | 100 | 100 | 300 | |||
| 6j | 30 | 30 | 30 | 30 | ||
| 6k | 100 | 300 | 100 | 300 | ||
| 6l | 30 | 30 | 30 | 30 | ||
| 6m | 100 | 300 | ||||
| 6n | 100 | 100 | 100 | |||
| phenobarbital | 30 | 100 | 30 | 100 | 100 | 300 |
| phenytoin | 30 | 30 | 30 | 30 | 100 | 100 |
Administered doses were taken as 30, 100, and 300 mg/kg, and the above data indicates the minimum dose for antiepileptic potential at 0.5 and 4 h intervals. (−) indicates an absence of activity at the maximum dose administered. n = 5; time span 5 min; the percent inhibition for each group was calculated by comparison with the control group. All values are expressed as mean ± SEM (n = 5). *P ≤ 0.05, **P ≤ 0.01 as compared with control. Data were analyzed by one-way ANOVA followed by Dunnett’s test.
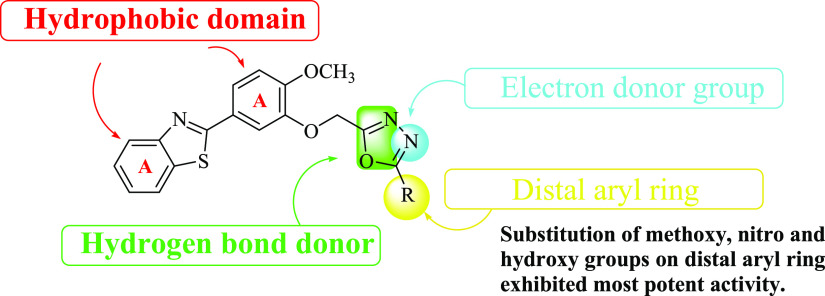
The structure–activity relationship of the synthesized compound showed that the substitution of methoxy, nitro, and hydroxyl groups on the distal aryl ring exhibited the most potent activity as shown in Figure 2. The presence of an oxadiazole ring in the structure exerts an electron donor ring, which is essential for antiepileptic activity. The substitution of the phenyl ring at R with nitro, methoxy, and hydroxy exerts potent activity due to its electron-donating potency forms a hydrogen bond with targeted protein and also the lipophilicity of the distal aryl ring increased, which is required for activity. The presence of other aryl rings and benzothiazoyl rings is also involved in the formation of hydrophobic bonds.
Figure 2.
Structure–activity relationship of synthesized compounds.
2.2.3. Neurotoxicity Screening
Using rotarod equipment, the prepared compounds’ in vivo neurotoxicity in albino mice of either sex was evaluated. A normal mouse can stay balanced on a revolving rod for at least 1 min without any neurological damage.38 Failure to keep balance on a revolving rod on each attempt is a sign of neurological impairment. In contrast to standard (diazepam), all of the synthesized compounds were assessed at dosages of 30, 100, and 300 mg/kg of body weight (diazepam). As illustrated in Table 3, compound 6c demonstrated neurotoxicity at 300 mg/kg of body weight, although neither of the tested compounds showed any evidence of neurotoxicity since neither of them was unable to maintain balance on the revolving rod of the rotarod device.
Table 3. Antidepressant Activity of Most Active Anticonvulsant Compoundsa.
| compound no. | basal means ± SEM | after treatment means ± SEMa | % reduction |
|---|---|---|---|
| 6f | 379.4 ± 2.83 | 369.2 ± 3.08ns | 2.68 |
| 6h | 374.8 ± 3.21 | 373.4 ± 3.85ns | 0.37 |
| 6j | 370.6 ± 1.63 | 368.8 ± 2.63ns | 0.48 |
| 6l | 378.6 ± 3.26 | 375.8 ± 3.65ns | 0.73 |
| PEG-200 | 384.8 ± 2.67 | 379 ± 3.64ns | 1.50 |
| diazepam | 382.2 ± 4.70 | 74.6 ± 2.65** | 80.4 |
n = 5; time = 5 min; the percent inhibition for each group was calculated by comparison with the control group. Dose = 100 mg/kg (p.o.); all values expressed as mean ± SEM (n = 5). *P ≤ 0.05, **P ≤ 0.01 as compared with control. Data were analyzed by one-way ANOVA followed by Dunnett’s test. 4 mg/kg (intraperitoneally).
2.2.4. Antidepressant Activity
The most effective anticonvulsant compounds were chosen, and their antidepressant efficacy was measured as a fall in locomotor activity using a conventional actophotometer.39,40 The test substances were injected intraperitoneally into either sex of mice at a dose of 100 mg/kg body weight. Based on these findings, a % decrease in locomotor activity was determined and is shown in Table 3 along with the activity score. When compared to diazepam, the most effective compounds, 6f, 6h, 6j, and 6l, showed a negligible decrease in the range of 0.37 to 2.68. (80.4). Hence, it was established that compounds 6f, 6h, 6j, and 6l were not depressants, according to the study’s findings.
2.2.5. In Silico Studies
2.2.5.1. In Silico ADME Properties
All of the synthesized compounds (6a–n) were subjected to in silico analyses to assess their physiochemical characteristics (based on Lipinski’s rule). According to the Lipinski rule, there is a direct correlation between an epileptic drug’s absorption and its physicochemical characteristics, such as its molecular weight, log P, number of hydrogen bond donors, and acceptors. According to Lipinski’s criteria, every one of the functional variants has 6–8 hydrogen bond acceptor and 1–3 hydrogen bond donor domains. The protocol of the pkCSM descriptors method was used to evaluate the preliminary ADME profiles of the synthesized derivatives, as indicated in Table S4. Since the synthesized compounds are less water soluble and have similar intestinal absorptivity to standard drugs (phenytoin and phenobarbital), it is clear from the tabulated data that they are more lipophilic. The produced compounds’ excellent lipophilicity causes an improvement in bioavailability in the conclusion. The volume of distribution of synthetic derivatives, which suggests that the volume of distribution at a steady state (VDss) is close to that of standard drugs, was studied to corroborate the aforementioned remark. These values indicate the capacity of substances to permeate the BBB and CNS, which are located in the ranges of −0.048 to −1.832 and −1.852 to −3.548, respectively. Total clearance values indicated that all compounds had similar projected maximum total clearance values. When compared to the typical anticonvulsant medications phenobarbital and phenytoin, the value of total clearance showed that all compounds had equivalent anticipated maximum total clearance values (0.536 to 0.009, respectively, compared to 0.209 and 0.277). The lowest overall clearance value among them is for compound 6a (0.009). As a result, these substances may be excreted more quickly, necessitating shorter dose intervals. Compound 6a is projected to have slower clearance rates than the reference drug phenobarbital, which might favor longer dosage intervals for the latter two compounds.
2.2.5.2. In Silico Docking Studies
To determine the different binding interactions of synthetic compounds within the pocket of well-known antiepileptic medicines target such as VGCCs and NMDA receptors, molecular docking research was conducted. The comparative analysis of interactions with various targets also contributed to the development of the tested drugs’ anticonvulsant action. All the synthetic compounds that have shown antiepileptic activity in vivo have been chosen for in silico molecular docking studies with various targets. Table S5 lists the binding energies of the active substances (6a, 6d–h, 6j–l, and 6n) and standard drugs (phenytoin and phenobarbital) with chosen targets. All active compounds have binding energy to 5IOV that is between −7.96 and −6.19 kcal/mol, which is quite comparable to the standard drugs phenytoin (−5.48 kcal/mol) and phenobarbital (−5.36 kcal/mol). However, interactions of the investigated compounds with other targets are not as substantial. The 2D interaction pictures of the most active substances (6f, 6h, 6j, and 6l) and reference drugs (phenytoin and phenobarbital) with 5IOV clearly show a resemblance with one another (Figures 3–8). Figures 3–6 show that the carbonyl group of acetamide makes hydrogen bonds with SER A:677, SER A:678, THR A:516, GLU A:502, GLU A:526, LYS A:754, SER A:678, GLY A:483, GLN A:676, GLN A:485, and LEU A:503 residues. The methoxy group in compound 6f forms a conventional hydrogen bond with SER A:678 and THR A:516 residues. The nitro group in compound 6h forms a conventional hydrogen bond with LYS A:754 and GLU A:526 residues. The nitro group in compound 6j forms a conventional hydrogen bond with SER A:678. The hydroxyl group in compound 6l forms a conventional hydrogen bond with GLN A:485. All the above information indicates good interaction between tested compounds and targets. All the above-stated information established that the synthesized compounds have good antiepileptic potential and they exerted their action primarily through NMDAs (5IOV) as the standard drugs (phenytoin and phenobarbital) do. Also, it has seemed that the synthesized compounds on docking with 2COJ, i.e., voltage-gated calcium channel receptor, have significant activity against convulsion. The compounds that are most active against NMDA also inhibit VGCC except, compound 6l. So, it concludes that both are possible mechanisms for anticonvulsant activity.
Figure 3.
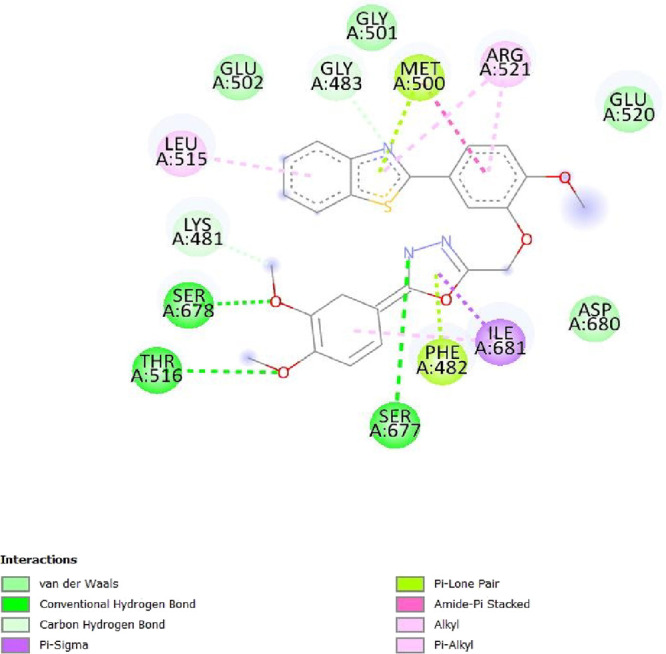
2D interaction of compound 6f on the site of 5IOV.
Figure 8.
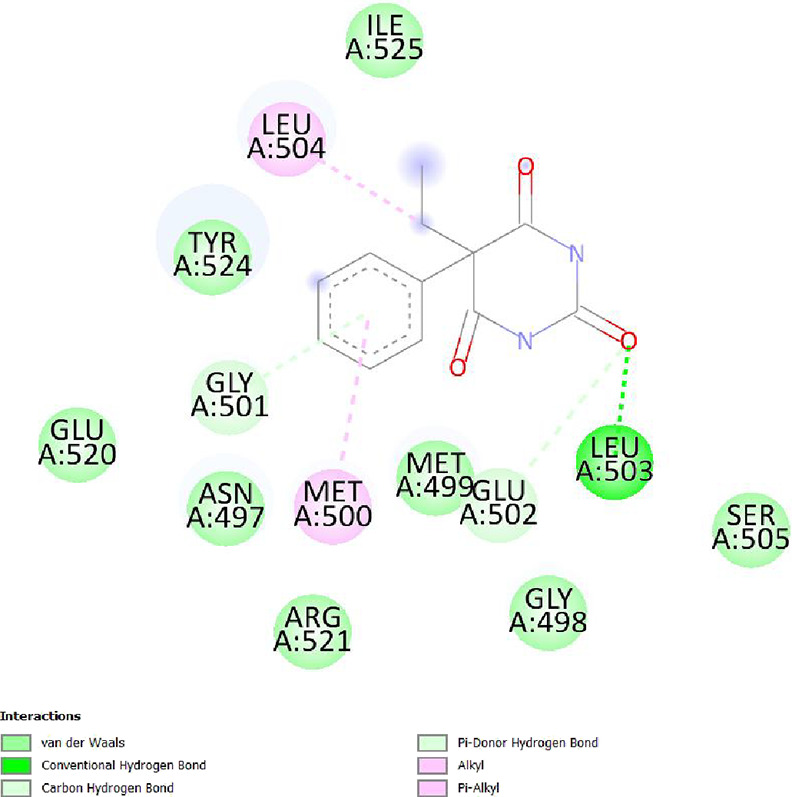
2D interaction of compound phenobarbital on the site of 5IOV.
Figure 6.
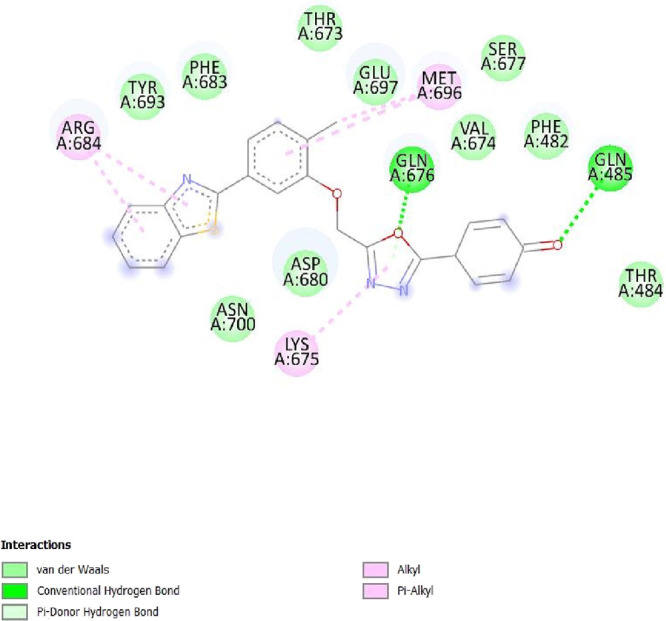
2D interaction of compound 6l on the site of 5IOV.
Figure 4.
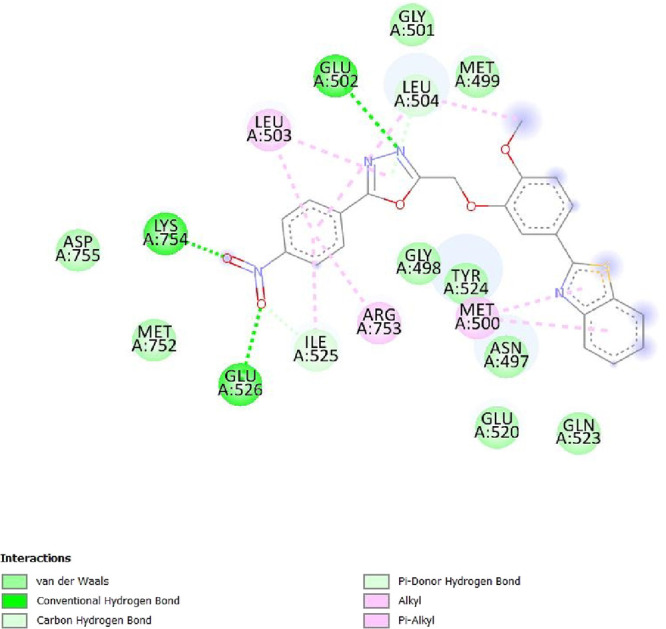
2D interaction of compound 6h on the site of 5IOV.
Figure 5.
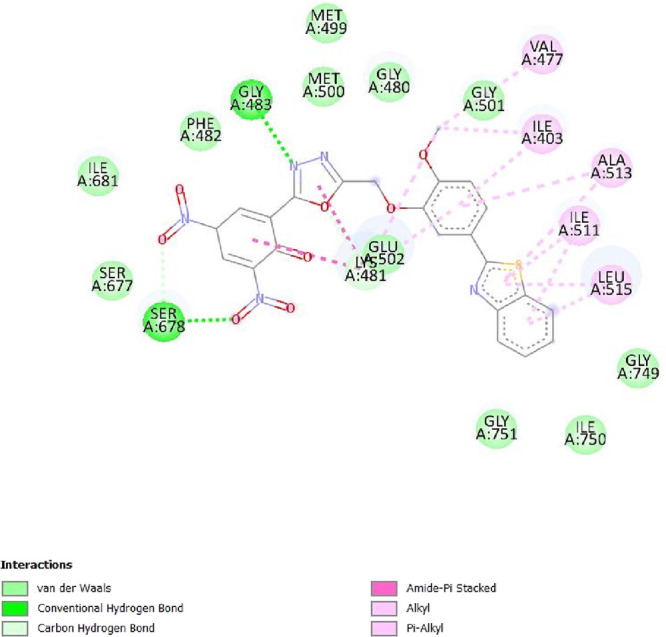
2D interaction of compound 6j on the site of 5IOV.
Figure 7.
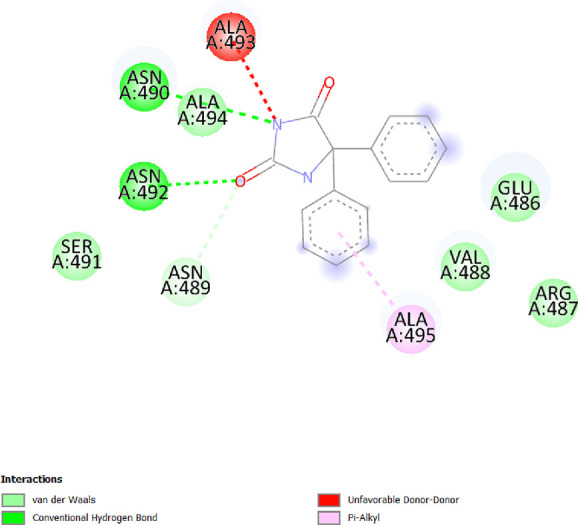
2D interaction of compound phenytoin on the site of 5IOV.
3. Experimental Section
3.1. Chemistry
3.1.1. General
The chemical and reagents are collected from S. D. Fine Chemicals Ltd. (Mumbai, India), Sigma-Aldrich (Missouri, USA), CDH (Central Drug House), and E. Merck (Darmstadt, Germany). For confirming the purity of compounds, thin-layer chromatography (TLC) was performed using silica gel-G as the stationary phase and toluene:ethyl acetate:formic acid (5:4:1) and benzene:acetone (8:2) as solvent systems. The iodine was used as the visualizing agent. The melting points were detected by the Thiele tube apparatus. A Bruker FTIR (Model-Alpha) spectrometer was used to determine Fourier transform infrared (FT-IR) spectra using KBr pellets. 1H NMR and 13C NMR spectra in CDCl3 solution were recorded at CDRI, Lucknow on an NMR spectrometer (300 MHz, Bruker-400 Ultra shield TM) using TMS [(CH3)4Si] as the internal standard. Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; m, multiplet. Mass spectra were recorded at CDRI, Lucknow on a mass spectrometer (Waters Synapt). The physicochemical and pharmacokinetic parameters of the synthesized compounds were determined by the free online version of Molinspiration and pkCSM software. AutoDock 4.2 and Biovia drug discovery studios were used for visualizing the molecular docking and interaction complexes, respectively.
3.1.2. 5-Benzothiazol-2-yl-2-methoxy-phenol (3)
5-Benzothiazol-2-yl-2-methoxy-phenol (3) was synthesized according to the literature procedure. Yield: 70%; m.p. 160–170 °C.10
3.1.3. (5-Benzothiazol-2-yl-2-methoxy-phenoxy)-acetic Acid Ethyl Ester (4)
(5-Benzothiazol-2-yl-2-methoxy-phenoxy)-acetic acid ethyl ester (4) was synthesized according to the literature procedure. Yield: 69%; m.p. 160–165 °C.10
3.1.4. (5-Benzothiazol-2-yl-2-methoxy-phenoxy)-acetic Acid Hydrazide (5)
(5-Benzothiazol-2-yl-2-methoxy-phenoxy)-acetic acid hydrazide (5) was synthesized according to the literature procedure. Yield: 72%; m.p. 170–175 °C.10
3.1.5. Synthesis of 2-[4-Methoxy-3-(5-substituted-phenyl-[1,3,4]oxadiazol-2-ylmethoxy)-phenyl]-benzothiazoles (6a–n)
Compound 5 (0.01 M) was dissolved into 10 mL of phosphorous oxychloride followed by the addition of different substituted carboxylic acids (0.01 M). The mixture is then refluxed for 4–5 h at 120 °C. The mixture was cooled, then poured onto crushed ice to turn basic with NaHCO3 then filtered, dried, and recrystallized with ethanol. The completion of the reaction and purity optimization of the derived compounds can be found through thin-layer chromatography.
3.1.6. Synthesis of 4-[5-(5-Benzothiazol-2-yl-2-methoxy-phenoxy methyl)-[1,3,4]oxadiazol-2-yl]-phenylamine (6a)
It was obtained as brownish powder in color, yield 66.6%, m.p. 136–138 °C, Rf 0.88; IR (KBr), Vmax (cm–1): 3460 (NH2, str), 3090–2933.8 (str, C–H, Ar), 2835 (C–H, str, alkane), 1528–1438 (C=C, Ar), 1210 (C–O), 1149 (C–N), 756 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 10.414 (s, 2H, NH2), 8.035–8.008 (J = 8.1, d, 1H, Ar–H), 7.888–7.861 (J = 8.1, d, 1H, Ar–H), 7.693–7.659 (t, 1H, Ar–H), 7.502–7.448 (m, 4H, Ar–H), 7.386–7.332 (t, 1H, Ar–H), 7.265 (s, 1H, Ar–H), 6.998–6.970 (J = 8.1, d, 1H, Ar–H), 4.816 (s, 2H, −CH2−), 3.965 (s, 3H, −OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.82 (1C, N=C–S), 160.30 (1C, C–NH2), 152.30 (2C, =C–O), 147.71 (2C, −C=), 126.73–66.62 (15C, Ar–C), 61.67 (1C, CH2), 56.33 (1C, OCH3); EI-MS (m/z): 431.21 [M + 1], HR-MS (m/z): 430.2195 (M+); Anal. calcd. for C23H18N4O3S: C, 64.17; H, 4.21; N, 13.01; O, 11.15; S, 7.45. Found: C, 64.24; H, 4.28; N, 13.17; O, 11.25; S, 7.32.
3.1.7. Synthesis of 2-{4-Methoxy-3-[5-(4-methoxy-phenyl)-[1,3,4]oxadiazol-2-ylmethoxy]-phenyl}-benzothiazole (6b)
It was obtained as a beige powder in color, yield 62.6%, m.p. 140–142 °C, Rf 0.84; IR (KBr), Vmax (cm–1): 2970–2929 (str, C–H, Ar), 2835 (str, C–H, alkane), 1613 (str, C=N), 1603–1433 (C=C, Ar), 1246 (C–O), 1170 (C–N), 754 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.035–8.008 (J = 8.1, d, 1H, Ar–H), 7.888–7.861 (J = 8.1, d, 1H, Ar–H), 7.693–7.659 (t, 1H, Ar–H), 7.502–7.447 (m, 4H, Ar–H), 7.386–7.332 (t, 1H, Ar–H), 7.264 (s, 1H, Ar–H), 6.997–6.970 (J = 8.1, d, 2H, Ar–H), 4.816 (s, 2H, -CH2–), 3.964 (s, 6H, -OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.81 (1C, N=C–S), 154.31 (1C, =C–S), 152.29 (2C, =C–O), 147.70 (2C, −C=), 126.74–66.62 (16 C, Ar–C), 61.66 (1C, CH2), 56.32 (2C, OCH3); EI-MS (m/z): 445.11, HR-MS (m/z): 445.1182; Anal. calcd. for C24H19N3O4S: C, 64.71; H, 4.30; N, 9.43; O, 14.37; S, 7.20. Found: C, 64.78; H, 4.22; N, 9.34; O, 14.45; S, 7.12.
3.1.8. Synthesis of 2-{3-[5-(4-Bromo-phenyl)-[1,3,4]oxadiazol-2-ylmethoxy]-4-methoxy-phenyl}-benzothiazole (6c)
It was obtained as a creamy color powder, yield 61.33%, m.p. 144–146 °C, Rf 0.80; IR (KBr), Vmax (cm–1): 2933 (str, C–H, Ar), 2830 (str, C–H, alkane), 1630 (str, C=N), 1600–1434 (C=C, Ar), 1246 (C–O), 1170 (C–N), 754 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.039–7.978 (J = 18.3, d, 2H, Ar–H), 7.889–7.862 (s, 1H, Ar–H), 7.695–7.661 (t, 1H, Ar–H), 7.503–7.448 (m, 4H, Ar–H), 7.388–7.334 (t, 1H, Ar–H), 7.264 (s, 1H, Ar–H), 6.999–6.972 (J = 8.1, d, 1H, Ar–H), 6.999–6.972 (J = 8.1, d, 1H, Ar–H), 4.817 (s, 2H, −CH2−), 3.965 (s, 3H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.82 (1C, N=C–S), 152.31 (2C, =C–O), 147.73 (2C, −C=), 126.73–66.63 (15 C, Ar–C), 61.64 (1C, CH2), 56.32 (1C, OCH3); EI-MS (m/z): 493.04, HR-MS (m/z): 494.0514 (M + 1); Anal. calcd. for C23H16BrN3O3S: C, 55.88; H, 3.26; Br, 16.16; N, 8.50; O, 9.71; S, 6.49. Found: C, 55.96; H, 3.34; Br, 16.24; N, 8.58; O, 9.79; S, 6.38.
3.1.9. Synthesis of 2-[4-Methoxy-3-(5-phenyl-[1,3,4]oxadiazol-2-ylmethoxy)-phenyl]-benzothiazole (6d)
It was obtained as a white powder, yield 68.6%, m.p. 132–134 °C, Rf 0.82; IR (KBr), Vmax (cm–1): 2933 (str, C–H, Ar), 2839 (str, C–H, alkane), 1590 (C=N), 1526–1430 (C=C, Ar), 1246 (C–O), 1148 (C–N), 764 (C–S).; 1H NMR (300 MHz, CDCl3) δ (ppm): 8.035–8.008 (J = 8.1, d, 1H, Ar–H), 7.889–7.862 (J = 8.1, d, 1H, Ar–H), 7.695–7.659 (t, 1H, Ar–H), 7.502–7.451 (t, 3H, Ar–H), 7.387–7.336 (t, 3H, Ar–H), 7.263 (s, 1H, Ar–H), 6.999–6.972 (J = 8.1, d, 1H, Ar–H), 4.817 (s, 2H, −CH2−), 3.965 (s, 3H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.82 (1C, N=C–S), 160.30 (1C, =C−), 152.30 (2C, =C–O), 147.69 (2C, −C=), 126.73–66.63 (15 C, Ar–C), 61.67 (1C, CH2), 56.38 (1C, OCH3); EI-MS (m/z): 415.10, HR-MS (m/z): 415.1164 (M+); Anal calcd. for C23H17N3O3S: C, 66.49; H, 4.12; N, 10.11; O, 11.55; S, 7.72. Found: C, 66.35; H, 4.22; N, 10.19; O, 11.62; S, 7.80.
3.1.10. Synthesis of 2-[3-(5-Benzyl-[1,3,4]oxadiazol-2-ylmethoxy)-4-methoxy-phenyl]-benzothiazole (6e)
It was obtained as a brown solid, yield 73.5%, m.p. 130–132 °C, Rf 0.86; IR (KBr), Vmax (cm–1): 2972–2935 (str, C–H, Ar), 2836 (str, C–H, alkane), 1635 (str, C=N), 1608–1435 (C=C, Ar), 1246 (C–O), 1138 (C–N), 754 (C–S);1H NMR (300 MHz, CDCl3) δ (ppm): 8.036–8.007 (J = 8.7, d, 1H, Ar–H), 7.887–7.861 (J = 7.8, d, Ar, H), 7.693–7.658 (t, 1H, Ar–H), 7.501–7.448 (m, 5H, Ar–H), 7.386–7.333 (t, 1H, Ar–H), 7.264 (s, 1H, Ar–H), 6.998–6.971 (J = 8.1, d, 2H, Ar–H), 4.816 (s, 2H, −CH2−), 3.965 (s, 3H, OCH3), 1.790 (s, 2H, −CH2). 13C NMR (300 MHz, CDCl3) δ (ppm): 168.81 (1C, N=C–S), 152.30 (2C, =C–O), 145.03 (2C, −C=), 126.76–66.64 (16 C, Ar–C), 61.68 (2C, CH2), 56.33 (1C, OCH3); EI-MS (m/z):429.11, HR-MS (m/z): 429.1158 (M+); Anal. calcd. for C24H19N3O3S: C, 67.12; H, 4.46; N, 9.78; O, 11.18; S, 7.47. Found: C, 67.18; H, 4.51; N, 9.84; O, 11.12; S, 7.32.
3.1.11. Synthesis of (E)-N-[1-(1H-Benzimidazol-2-yl)-2-phenylethyl]-1-(2-chloroquinolin-3-yl)-methenamine (6f)
It was obtained as a white powder, yield 66.1%, m.p. 146–148 °C, Rf 0.87; IR (KBr), Vmax (cm–1): 2966 (str, C–H, Ar), 2843 (str, C–H, alkane), 1610 (C=N), 1526–1434 (C=C, Ar), 1247 (C–O), 1148 (C–N), 756 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.034–8.006 (J = 8.4, d, 1H, Ar–H), 7.888–7.860 (J = 8.4, d, 1H, Ar–H), 7.693–7.658 (t, 1H, Ar–H), 7.501–7.448 (m, 3H, Ar–H), 7.386–7.331 (t, 1H, Ar–H), 7.263 (s, 2H, Ar–H), 6.998–6.971 (J = 8.1, d, 1H, Ar–H), 6.998–6.971 (J = 8.1, d, 1H, Ar–H), 4.816 (s, 2H, −CH2−), 3.964 (s, 9H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 174.83 (O–C=N), 168.83 (1C, N=C–S), 154.30 (1C, =C−), 152.29 (2C, =C–O), 147.71 (2C, −C=), 126.74–66.62 (15 C, Ar–C), 61.66 (1C, CH2), 56.32 (2C, OCH3); EI-MS (m/z):475.10, HR-MS (m/z): 475.1076 (M+); Anal. calcd. for C25H21N3O5S: C, 63.15; H, 4.45; N, 8.84; O, 16.82; S, 6.74. Found: C, 63.15; H, 4.45; N, 8.84; O, 16.76; S, 6.68.
3.1.12. Synthesis of 2-{3-[5-(4-Chloro-phenyl)-[1,3,4]oxadiazol-2-ylmethoxy]-4-methoxy-phenyl}-benzothiazole (6g)
It was obtained as a beige powder, yield 71.4%, m.p. 142–144 °C, Rf 0.81; IR (KBr), Vmax (cm–1): 2970–2925 (str, C–H, Ar), 2839 (str, C–H, alkane), 1540 (C=N), 1520–1434 (C=C, Ar), 1246 (C–O), 1138 (C–N), 765 (C-Cl), 754 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.038–7.978 (J = 18, d, 2H, Ar–H), 7.888–7.861 (J = 8.1, d, 1H, Ar–H), 7.695–7.661 (t, 1H, Ar–H), 7.502–7.448 (m, 4H, Ar–H), 7.388–7.334 (t, 1H, Ar–H), 7.265 (s, 1H, Ar–H), 6.999–6.971 (J = 8.1, d, 1H, Ar–H), 4.817 (s, 2H, −CH2−), 3.965 (s, 3H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.82 (1C, N=C–S), 152.31 (2C, =C–O), 147.71 (2C, −C=), 126.73–66.63 (15 C, Ar–C), 61.67 (1C, CH2), 56.32 (1C, OCH3); EI-MS (m/z):449.06, 450.06 (M + 1), HR-MS (m/z): 450.0716 (M + 1); Anal. calcd. for C23H16ClN3O3S: C, 61.40; H, 3.58; Cl, 7.88; N, 9.34; O, 10.67; S, 7.13. Found: C, 61.32; H, 3.66; Cl, 7.80; N, 9.24; O, 10.75; S, 7.20.
3.1.13. Synthesis of 2-{4-Methoxy-3-[5-(4-nitro-phenyl)-[1,3,4]oxadiazol-2-ylmethoxy]-phenyl}-benzothiazole (6h)
It was obtained as a grayish powder, yield 70.1%, m.p. 182–186 °C, Rf 0.79; IR (KBr), Vmax (cm–1): 3085–2983 (str, C–H, Ar), 2843 (str, C–H, alkane), 1635 (C=N), 1603–1431 (C=C, Ar), 1270 (N-O), 1246 (C–O), 1149 (C–N), 754 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.038–7.979 (J = 17.7, d, 2H, Ar–H), 7.889–7.862 (J = 8.1, d, 1H, Ar, H), 7.695–7.662 (t, 1H, Ar–H), 7.502–7.448 (m, 4H, Ar–H), 7.389–7.335 (t, 1H, Ar–H), 7.265 (s, 1H, Ar–H), 6.998–6.973 (J = 7.5, d, 1H, Ar–H), 4.816 (s, 2H, −CH2−), 3.964 (s, 3H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.82 (1C, N=C–S), 152.30 (2C, =C–O), 147.70 (2C, −C=), 126.73–66.65 (15 C, Ar–C), 61.64(1C, CH2), 56.33 (1C, OCH3); EI-MS (m/z):460.09, HR-MS (m/z): 460.0671 (M+); Anal. calcd. for C23H16N4O5S: C, 59.99; H, 3.50; N, 12.17; O, 17.37; S, 6.96. Found: C, 59.92; H, 3.56; N, 12.24; O, 17.43; S, 6.84.
3.1.14. Synthesis of 2-[3-(5-Benzhydryl-[1,3,4]oxadiazol-2-ylmethoxy)-4-methoxy-phenyl]-benzothiazole (6i)
It was obtained as a dull brown powder, yield 61.9%, m.p. 160–162 °C, Rf 0.89; IR (KBr), Vmax (cm–1): 3059–2929 (str, C–H, Ar), 2839 (str, C–H, alkane), 1613 (C=N), 1529–1421 (C=C, Ar), 1246 (C–O), 1148 (C–N), 764 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.036–8.008 (J = 8.4, d, 1H, Ar–H), 7.888–7.862 (J = 7.8, d, 1H Ar–H), 7.692–7.659 (t, 2H, Ar–H), 7.502–7.448 (m, 6H, Ar–H), 7.388–7.332 (t, 2H, Ar–H), 7.265 (s, 1H, Ar–H), 6.998–6.971 (J = 8.1, d, 3H, Ar–H), 4.817 (s, 2H, −CH2−), 3.965 (s, 3H, OCH3), 1.790 (s, 2H, -CH-); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.80 (1C, N=C–S), 152.30 (2C, =C–O), 145.03 (2C, −C=), 140.02–66.63 (22C, Ar–C), 61.67 (2C, CH2), 56.33 (1C, OCH3), 14.44; EI-MS (m/z): 505.15, HR-MS (m/z): 505.1506 (M+); Anal. calcd. for C30H23N3O3S: C, 71.27; H, 4.59; N, 8.31; O, 9.49; S, 6.34. Found: C, 71.34; H, 4.54; N, 8.37; O, 9.42; S, 6.38.
3.1.15. Synthesis of 2-[5-(5-Benzothiazol-2-yl-2-methoxy-phenoxymethyl)-[1,3,4]oxadiazol-2-yl]-4,6-dinitro-phenol (6j)
It was obtained as a grayish powder, yield 63.49%, m.p. 138–141 °C, Rf 0.84; IR (KBr), Vmax (cm–1): 3465 (O–H str), 3090–2933 (str, C–H, Ar), 2835 (str, C–H, alkane), 1528–1438 (C=C, Ar), 1266 (N–O), 1210 (C–O), 1150 (C–N), 757 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.036–8.007 (J = 8.7, d, 1H, Ar–H), 7.889–7.863 (J = 7.8, d, 1H, Ar–H), 7.692–7.657 (t, 1H, Ar–H), 7.388–7.332 (t, 1H, Ar–H), 7.264 (s, 3H, Ar–H), 6.998–6.973 (J = 7.5, d, 2H, Ar–H), 4.815 (s, 2H, −CH2−), 4.292 (s, 1H, OH), 3.964 (s, 3H, −OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.80 (1C, N=C–S), 152.30 (2C, =C–O), 147.72 (2C, −C=), 126.70–66.65 (15C, Ar–C), 61.67 (1C, CH2), 56.32 (1C, OCH3); EI-MS (m/z):521.05, HR-MS (m/z): 521.0576 (M+); Anal. calcd. for C23H15N5O8S: C, 52.98; H, 2.90; N, 13.43; O, 24.55; S, 6.15. Found: C, 52.93; H, 2.98; N, 13.36; O, 24.42; S, 6.23.
3.1.16. Synthesis of 2-{3-[5-(2,6-Dimethylphenyl)-[1,3,4]oxadiazol-2-ylmethoxy]-4-methoxy-phenyl}-benzothiazole (6k)
It was obtained as a creamy color powder, yield 70.9%, m.p. 135–137 °C, Rf 0.83; IR (KBr), Vmax (cm–1): 2967–2930 (str, C–H, Ar), 2836 (str, C–H, alkane), 1610 (C=N), 1526–1431 (C=C, Ar), 1211 (C–O), 1149 (C–N), 764 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.036–8.008 (J = 8.4, d, 1H, Ar–H), 7.888–7.862 (J = 7.8, d, 1H, Ar–H), 7.695–7.658 (t, 1H, Ar–H), 7.502–7.452 (t, 3H, Ar–H), 7.387–7.337 (t, 4H, Ar–H), 7.264 (s, 1H, Ar–H), 6.998–6.972 (J = 7.8, d, 1H, Ar–H), 4.817 (s, 2H, −CH2−), 3.964 (s, 3H, OCH3), 1.754 (s, 6H, -CH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.81 (1C, N=C–S), 152.28 (2C, =C–O), 147.71 (2C, −C=), 126.76–66.64 (15C, Ar–C), 61.68 (1C, CH2), 56.33 (1C, OCH3), 14.43 (CH3), 10.41–10.20 (CH3). EI-MS (m/z):443.13, HR-MS (m/z): 443.1386 (M+); Anal. calcd. for C25H21N3O3S: C, 67.70; H, 4.77; N, 9.47; O, 10.82; S, 7.23. Found: C, 67.74; H, 4.71; N, 9.42; O, 10.79; S, 7.28.
3.1.17. Synthesis of 4-[5-(5-Benzothiazol-2-yl-2-methoxy-phenoxy methyl)-[1,3,4]oxadiazol-2-yl]-phenol (6l)
It was obtained as a beige color powder, yield 70.17%, m.p. 158–162 °C, Rf 0.85; IR (KBr), Vmax (cm–1): 3466 (O-H, str), 3090–2934 (str, C–H, Ar), 2835 (str, C–H, alkane), 1527–1438 (C=C, Ar), 1210 (C–O), 1149 (C–N), 757 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.036–8.007 (J = 8.7, d, 1H, Ar–H), 7.888–7.862 (J = 7.8, d, 1H, Ar–H), 7.692–7.658 (t, 1H, Ar–H), 7.502–7.448 (m, 4H, Ar–H), 7.387–7.333 (t, 1H, Ar–H), 7.264 (s, 1H, Ar–H), 6.998–6.972 (J = 7.8, d, 2H, Ar–H), 4.816 (s, 2H, −CH2−), 4.292 (S, 1H, OH), 3.964 (s, 3H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.81 (1C, N=C–S), 160.31 (1C, C–OH), 152.30 (2C, =C–O), 147.72 (2C, −C=), 126.72–66.63 (15C, Ar–C), 61.67 (1C, CH2), 56.32 (1C, OCH3); EI-MS (m/z):431.08, HR-MS (m/z): 431.0776 (M+); Anal. calcd. for C23H17N3O4S: C, 64.03; H, 3.97; N, 9.74; O, 14.83; S, 7.43. Found: C, 64.12; H, 3.91; N, 9.68; O, 14.72; S, 7.37.
3.1.18. Synthesis of {4-[5-(5-Benzothiazol-2-yl-2-methoxy-phenoxymethyl)-[1,3,4]oxadiazol-2-yl]-phenyl}-dimethylamine (6m)
It was obtained as a creamy color powder, yield 69.8%, m.p. 146–148 °C, Rf 0.78; IR (KBr), Vmax (cm–1): 2967–2930 (str, C–H, Ar), 2836 (C–H, str, alkane), 1611 (C=N), 1526–1431 (C=C, Ar), 1210 (C–O), 1150 (C–N), 759 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm): 8.039–7.978 (J = 18.3, d, 2H, Ar–H), 7.889–7.861 (J = 8.4, d, 1H, Ar–H), 7.695–7.662 (t, 1H, Ar–H), 7.502–7.448 (m, 4H, Ar–H), 7.387–7.334 (t, 1H, Ar–H), 7.263 (s, 1H, Ar–H), 6.998–6.972 (J = 7.8, d, 1H, Ar–H), 4.818 (s, 2H, −CH2−), 3.964 (s, 3H, OCH3), 3.564 (s, 6H, -CH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 168.82 (1C, N=C–S), 160.30 (1C, =C–N), 152.30 (2C, =C–O), 147.71 (2C, −C=), 126.73–66.62 (15C, Ar–C), 61.65 (1C, CH2), 56.33 (1C, OCH3), 46.30–40.33 (2C, N–CH3). EI-MS (m/z): 458.14, HR-MS (m/z): 458.1452 (M+). Anal. calcd. for C25H22N4O3S: C, 65.48; H, 4.84; N, 12.22; O, 10.47; S, 6.99. Found: C, 65.41; H, 4.78; N, 12.19; O, 10.42; S, 6.91.
3.1.19. Synthesis of 2-{3-[5-(2,6-Dimethoxyphenyl)-[1,3,4]oxadiazol-2-ylmethoxy]-4-methoxy-phenyl}benzothiazole (6n)
It was obtained as a grayish powder, yield 64.2%, m.p. 160–164 °C, Rf 0.88; IR (KBr), Vmax (cm–1): 2965–2930 (C–H, str, Ar), 2835 (C–H, str, alkane), 1630 (C=N), 1603–1430 (C=C, Ar), 1211 (C–O), 1149 (C–N), 754 (C–S); 1H NMR (300 MHz, CDCl3) δ (ppm) = 8.034–8.008 (d, 1H, Ar–H), 7.692–7.658 (t, 1H, Ar–H), 7.502–7.448 (m, 3H, Ar–H), 7.386–7.330 (t, 1H, Ar–H), 6.998–6.970 (d, 1H, Ar–H), 4.817 (s, 2H, −CH2−), 3.965 (s, 9H, OCH3); 13C NMR (300 MHz, CDCl3) δ (ppm): 174.82 (1C, =C–O), 168.83 (1C, N=C–S), 154.30 (1C, =C–O), 152.29 (2C, =C–O), 147.71 (2C, −C=), 126.74–66.63 (14C, Ar–C), 61.66 (1C, CH2), 56.32 (3C, OCH3); EI-MS (m/z):475.10, HR-MS (m/z): 475.1056 (M+); Anal. calcd. for C25H21N3O5S: C, 63.15; H, 4.45; N, 8.84; O, 16.82; S, 6.74. Found: C, 63.19; H, 4.48; N, 8.88; O, 16.74; S, 6.65.
3.2. Pharmacological Activities
For evaluation of the anticonvulsant potential of synthesized compounds, the standard protocols established by the epilepsy branch of the National Institute of Neurological Disorders and Stroke (NINDS), and Antiepileptic Drug Development (ADD) program have been followed. These include screening of all the newly synthesized compounds by Maximal electroshock test (MES) and subcutaneous Pentylenetetrazole (scPTZ) test. In the laboratory environment, five animals are kept in separate plastic cages with 12 h of light/dark cycles. Before the experiment, they fasted for a night. The compounds were given intraperitoneally a solution of polyethyleneglycol (PEG-200) and tested in three different doses of 30, 100, and 300 mg/kg at two different time intervals (0.5 and 4 h). The actophotometer apparatus test was used for the antidepressant activity.
3.2.1. Acute Toxicity (LD50) Study
Acute toxicity studies (LD50) were conducted on albino mice to observe the median lethal dose and assess the chance of the toxic effect of the prepared derivatives. All the prepared compounds were injected intraperitoneally in different dose concentrations (5, 30, 100, and 300 mg/kg) into Swiss albino mice.38 Each animal was observed for 24 h after the drug administration.
3.2.2. Maximum Electroshock (MES)-Induced Seizure Test
The anticonvulsant activity in an MES-induced seizure model was indicated by the lower dose that protected the hind limb tonic extension in more than half of the animals (n = 5). Each animal received an i.p. injection of the test compounds (30, 100, and 200 mg/kg) followed by electroshock with 60 cycles of alternating current of 50 mA for 0.25 s through an ear clip electrode using an electro convulsometer as per the reported procedure and activity was assessed at 0.5 and 4 h after administration.41
3.2.3. Subcutaneous Pentylenetetrazole (scPTZ)-Induced Seizure Screening
For anticonvulsant activity test, compounds were administered 0.5 h before the scPTZ treatment and protection was detected in terms of failure to observe an episode of clonic spasms for 5 s duration. The control group received subcutaneous PTZ solution (in PEG-200) in the posterior midline of the mice, and the onset and severity of convulsion were noted.42
3.2.4. Rotarod Neurotoxicity Test
The neurotoxicity of all the test compounds was detected by using the rotarod apparatus. Mice were trained to balance on the rotating rod (3.2 cm diameter) that rotates at 6 rpm. Trained animals were treated with test compounds at a dose of 30, 100, and 300 mg/kg of body weight intraperitoneally. Neurotoxicity was determined by the inability of the animal to sustain steadiness on the rotarod for at least 1 min in each of three successive trials.43
3.2.5. Antidepressant Activity
The antidepressant activity of selected compounds (most active) was determined as a reduction in locomotor activity, which was calculated with an actophotometer through a standard protocol. The test compounds (100 mg/kg body weight) and standard drug (diazepam) have been administered intraperitoneally into animals. The activity scores (basal and after the administration of tested compounds) were noted, and the percentage reduction in locomotor activity was calculated.39
3.3. In Silico Studies
3.3.1. In Silico ADME Prediction
A computational study of the title compounds was performed for the prediction of physicochemical and ADME properties. Log P value, number of rotatable bonds, molecular volume, number of hydrogen donors, number of hydrogen bond acceptor atoms, violations of Lipinski’s rule of five, intestinal absorption, total clearance, etc. were calculated using the Molinspiration (https://www.molinspiration.com/) and pkCSM (http://biosig.unimelb.edu.au/pkcsm/prediction) online property calculation tool kits. The physicochemical properties and ADME data are given in Table S5.
3.3.2. Molecular Docking Study
Molecular docking studies have been used to find out the interaction between ligands and proteins concerning standard drugs. The molecular docking between ligands and two different proteins such as reduced human cytosolic branched-chain aminotransferase (VGSCs, PDB ID: 2COJ) and GluN1/GluN2B ligand binding complex (NMDA, PDB ID: 5IOV) was performed using Auto Dock 4.2 software. The protein was retrieved from the RCSB protein data bank (https://www.rcsb.org/) in PDB format, and heteroatoms and water molecules are removed from the Swiss PDB viewer and Biovia drug discovery studios. The ligands are prepared in the Marvin Sketch structure drawing tool in Tripos.mol2 format. The 2D interaction complex of the target protein and ligand is obtained from visualizing in Biovia drug discovery studio software.
4. Conclusions
In search of better antiepileptic agents, a new series of benzothiazoles bearing a 1,3,4-oxadiazole moiety (6a–n) have been synthesized. The synthesized compounds have drug-like characteristics and also fulfilled the structural requirements (pharmacophore model) for antiepileptic drugs. The data obtained after the characterization, in silico, and in vivo studies established the anticonvulsant potential of synthesized compounds. Among all, compounds 6f, 6h, 6j, and 6l were found to have the most significant antiepileptic potential at the dose of 30 mg/kg after 0.5 and 4 h with no neurotoxicity. The antidepressant screening also showed that their effect on locomotor activity was minimum in comparison with standard drugs. The in silico studies were also performed to determine the different binding interactions of synthetic compounds to localize the binding receptors. The most likely mode of action for these drugs, according to the docking analysis of active compounds with various targets, is their binding to the VGCC and NMDA receptors.
Acknowledgments
The authors are thankful to Dr. O.P. Aggarwal, Managing Director, Noida Institute of Engineering and Technology (Pharmacy Institute), Greater Noida, India, for his continuous support and encouragement.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06967.
Spectral data of the compounds (FT-IR, 1H NMR, 13C NMR, mass spectra, HRMS spectra), molecular properties, ADME profile, and binding energies of synthesized compounds (6a–n) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fiest K.-M.; Birbeck G.-L.; Jacoby A.; Jette N. Stigma in epilepsy. Curr. Neurol. Neurosci. Rep. 2014, 14, 444. 10.1007/s11910-014-0444-x. [DOI] [PubMed] [Google Scholar]
- Begley C.-E.; Beghi E. The economic cost of epilepsy: a review of the literature. Epilepsia 2002, 43, 3–9. 10.1046/j.1528-1157.43.s.4.2.x. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno J.-F.; Patten S.-B.; Jetté N.; Williams J.; Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia 2007, 48, 2336–2344. 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- Fiest K.-M.; Sauro K.-M.; Wiebe S.; Patten S.-B.; Kwon C.-S.; Dykeman J.; Pringsheim T.; Lorenzetti D.-L.; Jetté N. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E.; Hesdorffer D. Prevalence of epilepsy—an unknown quantity. Epilepsia 2014, 55, 963–967. 10.1111/epi.12579. [DOI] [PubMed] [Google Scholar]
- Fisher R.-S.; Acevedo C.; Arzimanoglou A.; Bogacz A.; Cross J.-H.; Elger C.-E.; Engel J.-J.; Forsgren L.; French J.-A.; Glynn M.; Hesdorffer D.-C.; Lee B. I.; Mathern G. W.; Moshé S. L.; Perucca E.; Scheffer I. E.; Tomson T.; Watanabe M.; Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Chackalamannil S.; Rotella D.; Ward S.. Comprehensive medicinal chemistry III. Elsevier. 2017. [Google Scholar]
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212839s000lbl.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212839Orig1s000ClinPharmR.pdf
- Singh H.; Kumar R.; Mazumder A.; Yadav R.-K.; Chauhan B.; Datt V.; Shabana K.; Abdullah M. Design, synthesis, in vivo and in silico evaluation of novel benzothiazole-hydrazone derivatives as new antiepileptic agents. Med. Chem. Res. 2022, 31, 1431–1447. 10.1007/s00044-022-02923-w. [DOI] [Google Scholar]
- Quintas R.; Raggi A.; Giovannetti A.-M.; Pagani M.; Sabariego C.; Cieza A.; Leonardi M. Psychosocial difficulties in people with epilepsy: a systematic review of literature from 2005 until 2010. Epilepsy Behav. 2012, 25, 60–67. 10.1016/j.yebeh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Regesta G.; Tanganelli P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 1999, 34, 109–122. 10.1016/S0920-1211(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Kwan P.; Brodie M.-J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Cosford N.-D.-P.; McDonald I. A.; Schweiger E. J. Recent progress in antiepileptic drug research. Annu. Rep. Med. Chem. 1998, 33, 61–70. 10.1016/S0065-7743(08)61072-6. [DOI] [Google Scholar]
- Abou-Khalil B.-W. Update on antiepileptic drugs 2019. CONTINUUM: Lifelong Learning in Neurology 2019, 25, 508–536. 10.1212/CON.0000000000000715. [DOI] [PubMed] [Google Scholar]
- Baulac M.; Rosenow F.; Toledo M.; Terada K.; Li T.; de Backer M.; Werhahn K. J.; Brock M. Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled-release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2017, 16, 43–54. 10.1016/S1474-4422(16)30292-7. [DOI] [PubMed] [Google Scholar]
- Nath R.; Yar M.-S.; Pathania S.; Grover G.; Debnath B.; Akhtar M.-J. Synthesis and anticonvulsant evaluation of indoline derivatives of functionalized aryloxadiazole amine and benzothiazole acetamide. J. Mol. Struct. 2021, 1228, 129742 10.1016/j.molstruc.2020.129742. [DOI] [Google Scholar]
- Sharma P.-C.; Sinhmar A.; Sharma A.; Rajak H.; Pathak D.-P. Medicinal significance of benzothiazole scaffold: an insight view. J. Enzyme Inhib. Med. Chem. 2013, 28, 240–266. 10.3109/14756366.2012.720572. [DOI] [PubMed] [Google Scholar]
- Keri R.-S.; Patil M.-R.; Patil S.-A.; Budagupi S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. 10.1016/j.ejmech.2014.10.059. [DOI] [PubMed] [Google Scholar]
- Irfan A.; Batool F.; Zahra Naqvi S.-A.; Islam A.; Osman S.-M.; Nocentini A.; Alissa S.-A.; Supuran C.-T. Benzothiazole derivatives as anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 265–279. 10.1080/14756366.2019.1698036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan G.; Prabhat P.; Sutharson L.; Banerjee J.; Patangia U.; Nath S. Synthesis and antidiabetic evaluation of benzothiazole derivatives. J. Korean Chem. Soc. 2012, 56, 251–256. 10.5012/jkcs.2012.56.2.251. [DOI] [Google Scholar]
- Soni B.; Ranawat M.-S.; Sharma R.; Bhandari A.; Sharma S. Synthesis and evaluation of some new benzothiazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2938–2942. 10.1016/j.ejmech.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Kharbanda C.; Alam M.-S.; Hamid H.; Javed K.; Bano S.; Dhulap A.; Ali Y.; Nazreen S.; Haider S. Synthesis and evaluation of pyrazolines bearing benzothiazole as anti-inflammatory agents. Bioorg. Med. Chem. 2014, 22, 5804–5812. 10.1016/j.bmc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Asiri Y.-I.; Alsayari A.; Muhsinah A.-B.; Mabkhot Y.-N.; Hassan M.-Z. Benzothiazoles as potential antiviral agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. 10.1111/jphp.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopala K.-N.; Chandrashekharappa S.; Pillay M.; Bhandary S.; Kandeel M.; Mahomoodally F.-M.; Morsy M.-A.; Chopra D.; Aldhubiab B.-E.; Attimarad M.; Alwassil O.-I.; Harsha S.; Mlisana K.; Odhav B. Synthesis and structural elucidation of novel benzothiazole derivatives as anti-tubercular agents: In-silico screening for possible target identification. Med. Chem. 2019, 15, 311–326. 10.2174/1573406414666180703121815. [DOI] [PubMed] [Google Scholar]
- Nazar S.; Siddiqui N.; Alam O. Recent progress of 1,3,4-oxadiazoles as anticonvulsants: Future horizons. Arch. Pharm. 2020, 1900342. 10.1002/ardp.201900342. [DOI] [PubMed] [Google Scholar]
- Glomb T.; Świątek P. Antimicrobial activity of 1, 3, 4-oxadiazole derivatives. Int. J. Mol. Sci. 2021, 22, 6979. 10.3390/ijms22136979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari S.-V.; Bothara K.-G.; Raut M.-K.; Patil A.-A.; Sarkate A.-P.; Mokale V.-J. Design, synthesis and evaluation of anti-inflammatory analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1, 3, 4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg. Med. Chem. 2008, 16, 1822–1831. 10.1016/j.bmc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ahsan M.-J.; Samy J.-G.; Khalilullah H.; Nomani M.-S.; Saraswat P.; Gaur R.; Singh A. Molecular properties prediction and synthesis of novel 1, 3, 4-oxadiazole analogues as potent antimicrobial and antitubercular agents. Bioorg. Med. Chem. Lett. 2011, 21, 7246–7250. 10.1016/j.bmcl.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Othman A.-A.; Kihel M.; Amara S. 1, 3, 4-Oxadiazole, 1, 3, 4-thiadiazole and 1, 2, 4-triazole derivatives as potential antibacterial agents. Arab. J. Chem. 2019, 12, 1660–1675. 10.1016/j.arabjc.2014.09.003. [DOI] [Google Scholar]
- Kotaiah Y.; Harikrishna N.; Nagaraju K.; Rao C.-V. Synthesis and antioxidant activity of 1, 3, 4-oxadiazole tagged thieno [2, 3-d] pyrimidine derivatives. Eur. J. Med. Chem. 2012, 58, 340–345. 10.1016/j.ejmech.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Kamal M.; Jawaid T.; Dar U. A.; Shah S. A. Amide as a Potential Pharmacophore for Drug Designing of Novel Anticonvulsant Compounds. Chem. Biol. Potent Nat. Prod. Synth. Compd. 2021, 319–342. 10.1002/9781119640929.ch11. [DOI] [Google Scholar]
- Sahu M.; Siddiqui N.; Naim M.-J.; Alam O.; Yar M.-S.; Sharma V.; Wakode S. Design, synthesis, and docking study of pyrimidine–triazine hybrids for GABA estimation in animal epilepsy models. Arch. Pharm. 2017, 350, 1700146. 10.1002/ardp.201700146. [DOI] [PubMed] [Google Scholar]
- Khatoona Y.; Shaquiquzzamanb M.; Singha V.; Sarafrozc M. Synthesis, characterization and anticonvulsant activity of some novel 4, 5-disubstituted-1, 2, 4-triazole derivatives. J. App. Pharmaceut. Sci. 2017, 7, 158–167. 10.7324/JAPS.2017.70724. [DOI] [Google Scholar]
- Ilgın S.; Osmaniye D.; Levent S.; Sağlık B.-N.; AcarÇevik U.; Çavuşoğlu B.-K.; Özkay Y.; Kaplancıklı Z.-A. Design and synthesis of new benzothiazole compounds as selective h MAO-B inhibitors. Molecules 2017, 22, 2187. 10.3390/molecules22122187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagthara P.-R.; Shah N.-S.; Doshi R.-K.; Parekh H.-H.. Synthesis of 2, 5-disubstituted 1, 3, 4-oxadiazoles as biologically active heterocycles, 1999.
- Farshori N.-N.; Rauf A.; Siddiqui M.-A.; Al-Sheddi E.-S.; Al-Oqail M.-M. A facile one-pot synthesis of novel 2, 5-disubstituted-1, 3, 4-oxadiazoles under conventional and microwave conditions and evaluation of their in vitro antimicrobial activities. Arab. J. Chem. 2017, 10, S2853–S2861. 10.1016/j.arabjc.2013.11.010. [DOI] [Google Scholar]
- OECD423: OECD guideline for testing of chemicals, Section 4, Adopted: 17th December 2001
- Verma R.; Bhatia R.; Singh G.; Kumar B.; Mehan S.; Monga V. Design, synthesis and neuropharmacological evaluation of new 2,4-disbsituted-1,5-benzodiazepines as cns active agents. Bioorg. Chem. 2020, 101, 104010 10.1016/j.bioorg.2020.104010. [DOI] [PubMed] [Google Scholar]
- Pratima A.; Nikalje G.; Shaikh S.-I.; Khan F.-A.-K.; Shaikh S.; Sangshetti J.-N. Molecular sieves promoted, ultrasound-mediated synthesis, niological evaluation and docking study of 3-(5-substituted-1,3,4*thiazol-2-ylimino) indolin-2-ones as a potential anticonvulsant agents. Med. Chem. Res. 2015, 24, 4058–4069. 10.1007/s00044-015-1458-x. [DOI] [Google Scholar]
- Krall R.-L.; Penry J.-K.; White B.-G.; Kupferberg H.-J.; Swinyard E.-A. Antiepileptic drug development: ii. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. 10.1111/j.1528-1157.1978.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Abulkhair H. S.; Elmeligie S.; Ghiaty A.; El-Morsy A.; Bayoumi A. H.; Ahmed H. E.; El-Adl K.; Zayed M. F.; Hassan M. H.; Akl E. N.; El-Zoghbi M. S. In vivo - and in silico - driven identification of novel synthetic quinoxalines as anticonvulsants and ampa inhibitors. Arch. Pharm. 2021, 354, 2000449. 10.1002/ardp.202000449. [DOI] [PubMed] [Google Scholar]
- Dehestani L.; Ahangar N.; Mahdieh S.; Irannejad H.; Honarchian P.; Shakiba A.; Emami S. Bioorganic chemistry design, synthesis, in vivo and in silico evaluation of phenacyltriazolehydrazones as new anticonvulsant agents. Bioorg. Chem. 2018, 78, 119–129. 10.1016/j.bioorg.2018.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.