Abstract
On a global scale, the incidence and mortality rates of lung cancer are gradually increasing year by year. A number of bad habits and environmental factors are associated with lung cancer, including smoking, second-hand smoke exposure, occupational exposure, respiratory diseases and genetics. At present, low-dose spiral computed tomography is routinely the first choice in the diagnosis of lung cancer. However, pathological examination is still the gold standard for the diagnosis of lung cancer. Based on the classification and stage of the cancer, treatment options such as surgery, radiotherapy, chemotherapy, targeted therapy and immunotherapy are available. The activation of the EGFR pathway can promote the survival and proliferation of tumor cells, and the VEGF pathway can promote the formation of blood vessels, thereby promoting tumor growth. In non-small cell lung cancer (NSCLC) with EGFR mutation, EGFR activation can promote tumor growth by promoting VEGF upregulation through a hypoxia-independent mechanism. The upregulation of VEGF can make tumor cells resistant to EGFR inhibitors. In addition, the expression of the VEGF signal is also affected by other factors. Therefore, the use of a single EGFR inhibitor cannot completely inhibit the expression of the VEGF signal. In order to overcome this problem, the combination of VEGF inhibitors and EGFR inhibitors has become the method of choice. Dual inhibition can not only overcome the resistance of tumor cells to EGFR inhibitors, but also significantly increase the progression-free survival time of patients with NSCLC. The present review discusses the associations between the EGFR and VEGF pathways, and the characteristics of dual inhibition of the EGFR-VEGF pathway.
Keywords: dual inhibition, EGFR, VEGF, non-small cell lung cancer, combination therapy, targeted therapy
1. Introduction
Worldwide, in the past few decades, lung cancer has become the most prolific malignant tumor endangering human health, and the number of cases and associated deaths from lung cancer has been on the rise (1,2). Lung cancer accounts for 21% of all tumors and 27% of all cancer-associated deaths. In 2018, an estimated 2.1 million people were newly found to have lung cancer, including ~1.38 million cases of lung cancer in men, with the highest incidence in Eastern Asia (>40/100,000 in China, Korea and Japan), Micronesia/Polynesia and much of Europe (>50/100,000), especially in Eastern Europe (49.3/100,000). At the same time, lung cancer rates in men in Africa remain generally low. Overall, in women, lung cancer rates are lower than those in men, with the highest incidence rates occurring in Western Europe (25.7/100,000), Northern Europe (26.9/100,000) and North America (30.7/100,000) (2). Furthermore, there is a geographical difference in the incidence of smoking among women compared with men, which may be ascribed to differences in smoking history among different regions (1,2). It should be noted that the incidence rate among Chinese women (22.8/100,000) is no different from that of western European countries such as France (22.5/100,000), despite the epidemiology of smoking being different in the two countries (lower smoking rate among Chinese women) (2). However, the high incidence of this type of lung cancer reflects the possible increased exposure to charcoal burning or smoke production (3). There are two histological types of lung cancer: NSCLC (80-85%) and SCLC. Although the etiology of lung cancer is not completely clear, it is mainly related to the following risk factors: Smoking, occupational exposure (such as asbestos and radon), ionizing radiation, exposure to second-hand smoke, genetic factors and infectious or non-infectious respiratory diseases (2) (Fig. 1). One current treatment for NSCLC is the dual inhibition of the EGFR and VEGF pathways, as EGFR and VEGF play an important role in the development of NSCLC. According to a previous study, the EGFR and VEGF pathways are correlated, and double inhibition has a good therapeutic effect compared with the simple inhibition of the EGFR pathway (4). The present study reviews the causes of lung cancer, effective therapeutic measures, and the advantages of EGFR and VEGF dual inhibition in the treatment of NSCLC.
Figure 1.
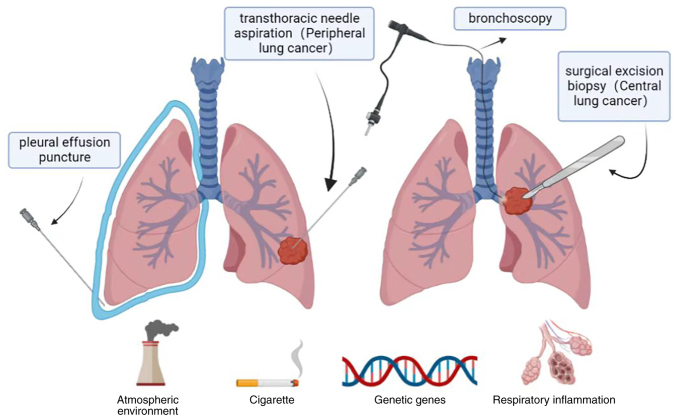
Causes of lung cancer include smoking, second-hand smoke exposure, infectious or non-infectious respiratory disease, occupational exposure and genetic factors. For central lung cancer, biopsies are performed by bronchoscopy, while for peripheral lung cancer, biopsies are performed by pleural lung puncture.
2. Risk factors of lung cancer
The pathogenesis of lung cancer is very complex, involving multiple gene mutations, such as EGFR/KEAS/TP53/P13KCAA mutations, ALK/RET/ROS1 rearrangements and EGFR/MET/FGFR1 amplifications (2,5). Some cascade reactions in lung cancer have been confirmed in broncho-lung cancer studies: Acinar/BA-lepidic/micro-papillary patterns express TTF1 and mutated EGFR; bronchial-pulmonary adenocarcinomas in non-smoking females exhibit mutated EGFR and ERCC1 expression; vimentin/RB/ERCC1 was expressed in micropapillary tumors; EGFR and HER2 multibody and CK7/vimentin are expressed in epidermoid carcinoma, and this expression can be used for epithelial-mesenchymal transformation into non-pure epidermoid carcinoma (5,6). Lung cancer is also connected with smoking, exposure to second-hand smoke, occupational exposure, ionizing radiation, infectious or non-infectious respiratory diseases, and genetic factors (5).
Tobacco smoking
The most important risk factor for lung cancer is tobacco smoking (7). According to statistics, the risk of lung cancer in smokers is 20 times that of lifelong non-smokers, and this risk is related to the number of years of smoking and the number of cigarettes smoked per day (8-10). Tobacco smoke contains thousands of compounds in the gas and particle phases. The 'Hoffman List' (11) highlights >60 carcinogens in cigarette smoke that are known as major carcinogens and includes nitrosamines, polycyclic aromatic hydrocarbons, volatile organic compounds, aromatic amines, aldehydes and metals, among others (11). Seven tobacco-specific nitrosamines have been found in tobacco products, but two of these, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N'-nitroso or nicotine, are the most important due to their carcinogenic activity (11). NNK requires metabolic activation by cytochrome P450-catalyzed α-hydroxylation to exert its carcinogenic properties. This process is the key to its carcinogenicity. NNK and its main metabolite [4-(methylnitrosa mino)-1-(3-pyridyl)-1-butanol] are formed by the reaction of methanediazosynthesis with DNA to form well-known DNA adducts, which lead to mutations and lung tumors (12,13).
Tobacco exposure can lead to mutations in KRAS and TP53 genes, which in turn are closely related to the occurrence of lung cancer. Tobacco smoke components or its metabolic activation products directly damage the TP53 tumor suppressor gene, leading to mutation and loss of the control mechanism of normal cell growth, and the change in p53 plays an important role in malignant transformation, invasion and metastasis (14,15). A study has shown that these mutations are caused by DNA adducts of tobacco carcinogens (14).
Exposure to second-hand smoke
Second-hand smoke is a form of indirect carcinogenic exposure from burning tobacco. Carcinogens related to lung cancer can be detected in second-hand smoke, including nitrosamines, polycyclic aromatic hydrocarbons and aromatic amines (2). Studies have shown that the risk of lung cancer from second-hand smoke is strongly related to the duration of exposure. Long-term exposure to secondhand smoke is estimated to increase the risk by 18-23% (16). A study evaluating the connection between second-hand smoke exposure and lung cancer among non-smokers in Japan reported a 28% increased risk compared with the risk in unexposed non-smokers (17). Therefore, for the prevention of lung cancer in non-smokers, it is very important to reduce their exposure to second-hand smoke. At the same time, the implementation of relevant measures to reduce the exposure to second-hand smoke can also reduce the opportunities for smoking overall, thus achieving a win-win effect of tobacco control and second-hand smoke exposure control (16,18).
Occupational exposure and lung cancer
Occupational exposure is also an important cause of lung cancer, for which asbestos is the most common exposure factor. Asbestos includes amphibole and chrysotile, which are widely used in building materials, car brakes, asbestos panels and insulation (2,19-21). The mechanism of asbestos-induced lung cancer is very complex and is currently considered to be related to changes in apoptosis regulation, oxidative stress response, chronic and persistent inflammation, genetic and epigenetic changes, cytotoxicity and fibrosis (2,19). At the same time, research has shown that asbestos exposure and smoking have a synergistic effect to increase the risk of lung cancer (1,20,22). However, the current synergy between tobacco smoke and asbestos exposure, and the specific mechanism for its induction of lung cancer is not entirely clear. This mechanism may be related to improving chemical carcinogens, promoting the absorption of carcinogens in smoke, chronic inflammation and promoting tumor proliferation (19,20). In addition to asbestos exposure, there is now good evidence that lung cancer is linked to the exposure to arsenic, chromium, and paint, diesel and welding fumes (2,23).
Infectious or non-infectious respiratory disease
Some diseases of the respiratory tract, including chronic obstructive pulmonary disease (COPD) (chronic bronchitis, emphysema), pneumonia, tuberculosis and asthma, and the occurrence of lung cancer also have a certain relationship. Both COPD and asthma are chronic airway inflammatory diseases. The former is an irreversible change, while the latter is mainly manifested by airway hyper-responsiveness (2). Published meta-analyses have shown that patients with COPD and asthma have an increased risk of lung cancer, and that patients with a history of COPD have a 2-3 times higher risk of the cancer (2,24,25). The mechanism behind this may be related to long-term inflammation in the respiratory tract. At present, several mechanisms have been suggested: i) Ciliary dysfunction caused by COPD may lead to longer exposure to carcinogens in the airway; ii) inflammatory responses can damage DNA and lead to mutations; and iii) oxidative stress responses can also damage DNA and lead to mutations (26,27). Pneumonia, tuberculosis and other infectious lung diseases can also increase the risk of lung cancer (25,28). The mechanism of pneumonia-induced lung cancer may be related to substances in chronic local inflammatory mediators, which can lead to DNA damage mutations, signal transduction and neovascularization. Tuberculosis, on the other hand, can induce inflammation and fibrosis, changes that can lead to a higher mutation rate (28), and a pooled analysis found that a history of tuberculosis increased the risk of lung cancer by 48% (29).
Inherited genetics
Among the causes of lung cancer, environmental factors (such as smoking) are the most important (3), but genetic variation accounts for 12-21% of the risk of lung cancer (30,31). Over the past few decades, genome-wide association studies (GWASs) have certified a variety of genetic risk factors connected with lung cancer susceptibility (32,33). GWASs have also identified 45 lung cancer risk loci in different populations (34). Based on a large-scale GWAS of lung cancer, 6 new loci of variation were identified and 13 previously reported mutations associated with the development of NSCLC were validated (34). It is of great importance to understand the relationship between genetics and lung cancer, and to predict the risk of lung cancer for individual prevention and the screening of lung cancer.
3. Diagnosis of NSCLC
The diagnostic methods of NSCLC could be roughly divided into imaging examination, lung biopsy and biomarker examination. Each test has its own advantages and disadvantages. The pathological biopsy of lung tissue is the gold standard for the diagnosis of NSCLC.
Imaging examination
Computed tomography (CT) is more effective than plain radiographs in detecting peripheral lung lesions, but is less sensitive to centrally located tumors (35,36). In patients with suspected metastatic lung cancer, positron emission tomography is a non-invasive examination that can be used in addition to a routine chest CT scan. The two together are more effective than either approach alone. As a relatively inexpensive and non-invasive test, a CT scan has become the preferred method of lung cancer screening (37,38).
Lung biopsy
Biopsies are the gold standard for the diagnosis of malignant tumors, as they have a very high accuracy and can be used to determine the subtype of lung cancer, which is of great significance for diagnosis and treatment (39). There are several techniques available through which physicians can obtain tissue specimens, including bronchoscopy, transthoracic needle aspiration, surgical excision biopsy and pleural effusion puncture (Fig. 1). Experiments show that for patients with peripheral lung cancer, pleural aspiration is more sensitive than bronchoscopy (40). However, these methods are expensive and prone to complications (39).
Biomarkers
Sputum cytology is an adjunctive examination that is highly sensitive to tumors in the larger bronchi and less sensitive to peripheral tumors. The sensitivity of sputum cytology to early lung cancer is 20-30% (41). The role of plasma microRNA in the early detection of lung cancer has been investigated. Studies have shown differences in the miRNAs in the plasma of patients with lung cancer compared with healthy individuals (42). The role of microRNA biomarkers in sputum samples has also been explored and found to be helpful in the diagnosis of lung cancer (43).
Bronchoalveolar lavage (BAL) is another method of using molecular markers to diagnose lung cancer by pathological analysis of the cells obtained. Some studies have compared tumor and BAL cells with lung cancer molecular markers and found that they have high sensitivity. The exact genes related to tumorigenesis can be found in BAL samples. Therefore, it is of positive significance to study these molecular markers for the early diagnosis of tumors (44).
Peripheral blood and urine specimens are readily available and are non-invasive. With advances in technology, it has been found that the amount of DNA in the blood and urine of patients with cancer is approximately four times higher than the amount of free DNA in healthy individuals. Urine also has some potential with regard to the detection of biomarkers for lung cancer, such as volatile organic compounds (VOCs). While VOCs are promising, more clinical studies are needed to prove their usefulness (45).
Biomarkers are easy to obtain, so it is currently the direction of early lung cancer diagnosis research. Biomarkers are not limited to the aforementioned sputum cytology, BAL and peripheral blood and urine specimens, so more clinical studies to verify the role of biomarkers in the early diagnosis of lung cancer are required.
For patients diagnosed with advanced NSCLC, biomarkers derived from blood or other tissues are also needed to guide tumor treatment. The detection of biomarkers is of great help to precision treatment and to the improvement of the therapeutic effect (46). The European Society for Medical Oncology (ESMO) Clinical Practice Guidelines recommend routine testing for ALK, ROS1and EGFR. Meanwhile, in 2018, the ESMO and the Chinese Society of Clinical Oncology jointly issued Pan-Asian guidelines recommending routine testing for ALK rearrangement, EGFR mutation, BRAF mutation, ROS1 rearrangement and programmed cell death-ligand 1 (PD-L1) immunohistochemistry for patients with advanced NSCLC (47,48). In addition to conventional biomarkers activating HER2 mutations (49), MET exon 14 skipping mutations (50) and RET rearrangements (51) may also be present in NSCLC. Detection of NTRK gene rearrangements and KRAS mutations is also of some value (52,53). In general, all patients with advanced NSCLS should be routinely tested for at least the ROS1 rearrangements, and ALK and EGFR mutations. PD-L1, RET, MET, HER2 and KRAS and BRAF mutations and immunohistochemistry should also be evaluated in a broader search for affected genes.
4. Treatment of NSCLC
Lung cancer can be divided into SCLC and NSCLC. The main treatment methods include surgery, drug therapy (chemotherapy, targeted therapy and immunotherapy), radiotherapy, and a combination of several treatments (e.g., chemotherapy combined with immunotherapy or surgery, radiotherapy or targeted therapy) (54-57). The choice of specific treatment is related to the type and stage of cancer, and although there are numerous treatment methods, except for that in a few local cancer types, the treatment effect of other therapies is mostly poor.
Treatment of stage I and stage II NSCLC
The main and best treatment for early lung cancer is surgery (58). Although platinum-assisted chemotherapy is recommended for stage II NSCLC and increases the survival rate by 5 years, recurrence rates and toxicity are high (59). Postoperative adjuvant chemotherapy has not been proven to be beneficial for stage I patients. Molecular targeted therapy for early lung cancer also has no obvious therapeutic effect; it may also lead to early entry into the ranks of those with advanced cancer (59).
Treatment of stage III NSCLC
In total, >70% of patients with NSCLC are diagnosed with advanced stage disease (60), and treatment for these patients often depends on the location of the tumor and whether it can be resected. Some patients in stage IIIA have operable disease, for which the standard treatment is surgery and chemotherapy. Studies have shown that adjuvant chemotherapy can prolong overall survival (OS) rates, and that neoadjuvant chemotherapy can increase the 5-year survival rate by 5-6%. For patients with inoperable stage IIIA and IIIB disease, the standard of care consists of sequential or concurrent chemotherapy and radiation therapy (54,55,61,62).
Treatment of stage IV NSCLC
Stage IV NSCLC accounts for 40% of cases. The choice of treatment is influenced by a variety of factors (such as physical condition, whether the tumor is metastatic and whether it is sensitive to radiotherapy and chemotherapy) and the specific standard regimen includes palliative external radiotherapy, combination chemotherapy, combination chemotherapy with targeted therapy, and other treatments that may alleviate the patient's symptoms. Surgical treatment may be used in some cases to relieve symptoms, but is not the preferred treatment (56).
Targeted therapy
Targeted therapy has a positive effect on improving the prognosis of patients with advanced NSCLC. Inhibitors targeting alterations in certain genes (EGFR, ALK, ROS1, RET, BRAF V600E, MET14 exons and NTRK) are currently certified for the treatment of patients with NSCLC (63). These drugs can improve the median OS and progression-free survival (PFS) times of patients (64). Immunotherapy has also been shown to benefit survival in patients with advanced NSCLC by improving OS time (65,66).
Immunotherapy
Immunotherapy alone is generally better than first-line chemotherapy for tumors with high expression of PD-L1. No matter how PD-L1 is expressed in the tumor, combination therapies of chemotherapy and immunotherapy have also been proved to be superior to chemotherapy (57). For patients with advanced squamous cell carcinoma or non-squamous cell carcinoma without contraindications to programmed death 1/PD-L1 inhibitors, monotherapy or combination therapy is now the standard first-line treatment (63). In KEYNOTE-024, a phase III randomized trial comparing the efficacy of pembrolizumab monotherapy with platinum chemotherapy in patients with untreated stage IV NSCLC, pembrolizumab monotherapy was found to be superior to chemotherapy in terms of response rate (44.8 vs. 27.8%) and OS time [median OS, 30.0 months (95% CI, 18.3-not reached) vs. 14.2 months (95% CI, 9.8 vs. 19.0) (57).
5. EGFR and inhibition of the EGFR pathway in NSCLC
EGFR, also referred to as human EGF receptor-1 (HER-1), is a member of the HER/erbB family of receptor tyrosine kinases (67,68); it is a multi-domain glycoprotein consisting of an extracellular ligand binding domain, a hydrophobic transmembrane domain and a cytoplasmic domain containing a tyrosine kinase domain (67,69). Currently, the possible mechanisms behind EGFR leading to malignant phenotypes include EGFR overexpression and increased EGFR signaling pathway activity. Based on the fact that EGFR is upregulated in numerous tumors, we can speculate that increased expression of EGFR-mediated signaling pathways may promote the initiation of unregulated cell proliferation, leading to the emergence of a malignant phenotype (70). EGFR pathway activation is mainly due to endogenous ligands, such as EGF, TGF-α, epiregulin or heparin-binding EGF, which bind to the extracellular domain of EGFR and form dimers (67-69,71,72). His dimerization activates the cytoplasmic EGFR tyrosine kinase, leading to autophosphorylation, and the phosphorylated EGFR tyrosine kinase regulates cell proliferation and apoptosis by stimulating downstream intracellular signal transduction cascades through several pathways (73). EGFR is associated with the formation and development of a number of tumors. Solid tumors such as breast, colon and non-small cell carcinoma express EGFR, and EGFR is also involved in the occurrence and development of tumors (68,71). The activation of the EGFR pathway is associated with the metastasis, proliferation, differentiation and migration of tumor cells (68). EGFR mutations occur in 7-37% of Caucasian patients and 40-60% of Asian patients with NSCLC (74).
Cetuximab and panitumumab
Cetuximab and panitumumab are antibodies that bind to the extracellular domain of EGFR, inhibit receptor activation and block signal transduction (75,76). A study of patients treated with locally advanced squamous cell carcinoma of the head and neck showed that patients receiving cetuximab plus radiotherapy experienced an improvement in median survival time by nearly 20 months compared with patients treated with radiotherapy alone (77). In addition, cetuximab combined with gemcitabine has shown a significant clinical response in the treatment of pancreatic cancer (78). Cetuximab combined with docetaxel also has certain beneficial clinical activity in the second-line treatment of NSCLC. Based on this phenomenon, a further study is evaluating the use of cetuximab in combination with chemotherapeutic agents in first-line treatment of NSCLC (79).
EGFR tyrosine kinase inhibitors (TKIs)
EGFR TKIs are small molecules that bind to the cytoplasmic domain of EGFR containing the tyrosine kinase domain to inhibit EGFR autophosphorylation, thus inhibiting receptor activation and signal transduction. EGFR TKIs include erlotinib and gefitinib (first generation), afatinib and dacomitinib (second generation), and osimertinib and vandetanib (third generation) (4).
6. VEGF and inhibition of the VEGF pathway in NSCLC
Angiogenesis, the formation of new blood vessels, plays an important role in human life activities. Angiogenesis is regulated by both antiangiogenic factors and pro-angiogenic factors (71,80). VEGF belongs to the platelet-derived growth factor family, including VEGF-A, B, C and D (81). VEGF-A is a main stimulator in angiogenesis, and its expression is of great significance to the tissue angiogenesis system. Other family members are involved in lymphatic angiogenesis and embryo angiogenesis (82-84). In patients with cancer, tumor cells secrete VEGF, which forms new blood vessels by acting on endothelial cells in existing blood vessels and promoting their migration (71). The genesis of this pathological blood vessel provides a pathway for tumor growth and metastasis (85). VEGF mainly binds to VEGF receptor 1 and VEGF receptor 2, and the receptor is made up of extracellular, transmembrane and intracellular domains. Studies have shown that the binding ability of VEGF with VEGF receptor 2 is lower than that of VEGF1, but VEGF receptor 2 has been shown to be the primary receptor for VEGF signaling in endothelial cells and to play a key role in angiogenesis. After the binding of VEGF and VEGF receptor 2, a dimer is formed, resulting in a phosphorylated activation pathway that promotes endothelial cell proliferation and angiogenesis (82,86,87).
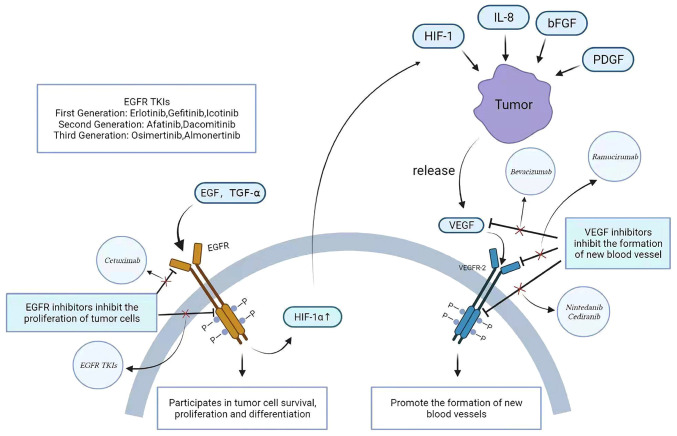
The expression of VEGF is affected by a number of factors, EGF receptor 2, methylation, transforming growth factor (TGF)-α and β, and TNF-α, among which oxygen tension is the most important for pathological angiogenesis (88,89). Hypoxia can rapidly induce VEGF mRNA expression through hypoxia-inducible factor-1 (HIF-1), thus promoting downstream signal transduction (90). In a normal oxygen tension environment, HIF-1 is degraded and downregulated (82), while in a hypoxic environment, the HIF-1 degradation pathway is blocked (90) and HIF-1 is phosphorylated through an oncogenic signal, thus binding to the promoter of the VEGF gene, promoting the transcription of VEGF genes and participating in angiogenesis. Hypoxia may be the most important regulator of VEGF mRNA expression (91,92). The activation of the VEGF pathway can act on existing vascular endothelial cells to promote their migration, thus forming new blood vessels and promoting tumor growth (71). There are several targeted drugs that inhibit VEGF pathway expression, including some that bind to the intracellular domain of VEGFR-2, such as vandetanib, nintedanib, axitinib, cediranib and ramucirumab, a monoclonal antibody that binds to the extracellular domain of VEGFR-2 and a monoclonal antibody (bevacizumab) that inhibits VEGF-A protein. The targeted therapies inhibit angiogenesis and tumor growth by binding to VEGF and inhibiting its activity (93).
In the treatment of NSCLC, EGFR inhibition is an important therapeutic strategy, and the inhibition of the VEGF pathway is an important potential complementary target (81). EGF and VEGF share a common downstream signaling pathway and may play an independent role in tumorigenesis. Various ligands bind to EGFR and VEGF-2 to trigger activation of both PI3K/AKT and RAS/RAF/ERK pathways. At the same time, EGFR activation can promote the expression of the VEGF gene, which is involved in HIF-1 upregulation (4).
7. Dual-inhibition of EGFR-VEGF
EGFR- or VEGF-targeted drugs have been shown to be effective in the treatment of NSCLC, but have limited therapeutic effects when used alone. As aforementioned, cetuximab in combination with chemotherapeutic agents is more effective than the chemotherapeutic agents alone. However, the combination of different targeted agents may be more useful in improving the efficacy and may avoid the safety and tolerability problems associated with the use of chemotherapeutic agents compared with the combination of chemotherapy agents (94). At the same time, there are also drug resistance, escape of certain targets and other problems. The activation of the EGFR pathway can promote HIF-1 production, thus upregulating VEGF expression. However, an EGFR inhibitor alone cannot completely block the production of VEGF, as the expression of VEGF is affected by numerous factors, and it is impossible to completely inhibit the release of VEGF in the interstitium. Therefore, the effect of an EGFR inhibitor alone is not sufficient (4,94). Several studies have also shown that tumor cell resistance to EGFR inhibitors is related to increased VEGF levels, and that this overexpression increases tumor cell resistance (95,96). VEGF expression can promote angiogenesis, and as the disease progresses, the pro-angiogenesis pathway increases, and the dependence of tumor cells on VEGF may decrease (97). Some tumors drive pericyte activation through PDGF receptors, showing VEGF-resistance through an escape mechanism, as this process is not VEGF-dependent in endothelial cells (97-99). To address these concerns, the combined use of EGFR inhibitors and VEGF inhibitors may help (Fig. 2).
Figure 2.
Association and inhibition of EGFR and VEGF pathways. EGFR is activated when EGF and TGF-α bind to it and is involved in the growth, proliferation and differentiation of tumor cells through the conduction of downstream pathways. After EGFR activation, HIF-1 can be upregulated, resulting in VEGF gene expression. HIF-1, IL-8, bFGF and PDGF can promote the release of VEGF by tumors, thereby activating the VEGF pathway and promoting the formation of new blood vessels. Drugs targeting the EGFR and VEGF pathways are also used clinically, including EGFR inhibitors and VEGF-2 inhibitors. EGFR binds to the intracellular domain of EGFR cell TKIs (erlotinib, gefitinib, icotinib, dacomitinib, afatinib, almonertinib and osimertinib). Mechanisms of VEGF inhibitors include interference with VEGF (bevacizumab), binding to VEGFR-2 intracellular domains (nintedanib and cediranib) and interfering with VEGFR-2 extracellular domains (ramucirumab). The use of EGFR inhibitors can downregulate HIF-1 expression, but the activation of the VEGF pathway is affected by a variety of factors, so EGFR inhibitors alone are not effective in the treatment of advanced non-small cell lung cancer. TKI, tyrosine kinase inhibitor; HIF-1, hypoxia-inducible factor.
8. Advantages of dual inhibition of EGFR-VEGF
Dual inhibition resists acquired resistance of tumor cells to EGFR inhibitors
As aforementioned, simple EGFR inhibitors cannot completely prevent VEGF from promoting angiogenesis, and the overexpression of VEGF can also cause the drug resistance of tumor cells to EGFR inhibitors. Several studies have also demonstrated this phenomenon. Long-term administration of EGFR inhibitors in mice with colon cancer showed that mice could produce drug-resistant colon cancer cell lines with VEGF overexpression, which could make tumor cells resistant to EGFR inhibitors. As VEGF levels increased, tumor angiogenesis potential also increased (96). If a treatment requires EGFR inhibitors, a combination of VEGF inhibitors is needed to inhibit angiogenesis. Combination therapy is more effective than blocking a single pathway, and may also help overcome tumor resistance mechanisms.
Dual inhibition increases PFS times
The JO25567 trial of 154 Japanese patients, showed that erlotinib combined with bevacizumab compared with erlotinib alone exhibited a significantly increased PFS time (median PFS time, 16.0 vs. 9.7 months; HR, 0.54; P=0.0015) (100). The phase 3 NEJ026 trial also evaluated erlotinib alone and erlotinib with bevacizumab in patients with complete NSCLC with EGFR mutation. The results showed that the combination of erlotinib and bevacizumab increased the PFS time (16.9 vs. 13.3 months; HR, 0.605; P=0.016) (101). Erlotinib + ramucirumab vs. erlotinib + placebo was evaluated in the phase 3 RELAY trial, which showed a significant increase in PFS time for the erlotinib + ramucirumab group (median PFS, 19.4 vs. 12.4 months; HR, 0.59; P<0.0001) (64). In a randomized controlled trial meta-analysis of 1,918 patients with advanced NSCLC, the dual inhibition of EGFR and VEGF pathways significantly improved the PFS time (HR, 0.71; 95% CI, 0.58-0.86; P<0.001) (102). A meta-analysis comparing the efficacy of targeted combination therapy vs. erlotinib alone in advanced NSCLC showed that the combination therapy significantly improved the OS time (HR, 0.90; 95% CI, 0.82-0.99; P=0.024), PFS time (HR, 0.83; 95% CI, 0.72-0.97; P=0.018) and overall response rate (OR, 1.35; 95% CI, 1.01-1.80; P=0.04) in 2,417 patients (103) (Table I). Based on these data, the combination of EGFR and VEGF inhibitors can significantly increase PFS time. The National Comprehensive Cancer Network Clinical Practice Guidelines for Oncology has identified erlotinib plus ramucirumab and erlotinib plus bevacizumab as first-line therapies for EGFR-mutated NSCLC (101).
Table I.
Clinical results after double inhibition of EGFR-VEGF and related meta-analysis results.
| First author, year | Study | Test area | Sample size, n | EGFR inhibitor | Dual inhibition of EGFR-VEGF | Median PFS (alone vs. combined), months | PFS (hazard ratio) | Added PFS time, months | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Seto et al | JO25567 trial, phase 2 | Japan | 154 | Erlotinib | Erlotinib + bevacizumab | 9.7 vs. 16.0 | 0.540 | 6.3 | (100) |
| Saito et al | NEJ026 trial, phase 3 | Japan | 228 | Erlotinib | Erlotinib + bevacizumab | 13.3 vs. 16.9 | 0.605 | 3.5 | (101) |
| Nakagawa et al | RELAY trial, phase 3 | Global | 449 | Erlotinib | Erlotinib + ramucirumab | 12.4 vs. 19.4 | 0.590 | 7.0 | (64) |
| Qi et al | Meta-analysis of 5 randomized controlled trials | / | 1736 | Erlotinib | Erlotinib + ramucirumab | / | 0.63 | / | (103) |
| Zhanget al | Meta-analysis of 4 randomized controlled trials | / | 1918 | EGFR-TKIs | EGFR-TKIs + VEGF inhibitors | / | 0.71 | / | (102) |
PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
9. Conclusions
EGF and VEGF have the same downstream signaling pathway and play a very important role in tumor growth. EGFR inhibitors alone cannot completely inhibit the angiogenesis-promoting effect of VEGF, and the overexpression of VEGF will also increase the resistance of tumor cells to EGFR inhibitors, so the combination of drugs becomes a good choice. A large body of data has shown that dual inhibition of EGFR and VEGF pathways significantly reduces tumor cell resistance to EGFR inhibitors and improves PFS time compared with EGFR inhibitors alone. Under the support and guidance of a large number of clinical trials, the pathways and targets that are not yet understood will be solved one by one. The dual inhibition of EGFR-VEGF in the treatment of advanced NSCLC applied to a greater extent, which will be of great help to improve the survival of patients. Of course, the double inhibition of the EGFR-VEGF pathway has also been applied in other malignant tumors (such as colorectal, head and neck, and breast cancer), but the use of EGFR-VEGF dual inhibition in the treatment of tumors such as breast cancer, gastrointestinal cancer, renal cancer, and head and neck tumors, is limited by the lack of clinical trials. The field of combination therapy for NSCLC with EGFR mutation is still being studied, and solutions to adverse reactions (such as renal dysfunction) and cost increases after double inhibition are also being actively sought. It is believed that in the future, double inhibition to treat EGFR mutations in NSCLC and other tumors associated with the EGFR-VEGF pathway will be more widely used in clinical practice.
Acknowledgments
Not applicable.
Funding Statement
This study was supported by the Foundation of the Xinglin Project of Chengdu University of Traditional Chinese Medicine (grant no. MPRC2021012).
Availability of data and materials
Not applicable.
Authors' contributions
LS and MZ designed the research and were responsible for the project conception. QW and AZ drafted the manuscript. LS and MZ revised the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Schabath MB, Cote ML. Cancer progress and priorities: Lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1563–1579. doi: 10.1158/1055-9965.EPI-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, Ares LP, Frimodt-Moller B, Wolff K, Visseren-Grul C, et al. Dual EGFR-VEGF pathway inhibition: A promising strategy for patients with EGFR-Mutant NSCLC. J Thorac Oncol. 2021;16:205–215. doi: 10.1016/j.jtho.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 5.de Sousa VML, Carvalho L. Heterogeneity in lung cancer. Pathobiology. 2018;85:96–107. doi: 10.1159/000487440. [DOI] [PubMed] [Google Scholar]
- 6.Sousa V, Espírito Santo J, Silva M, Cabral T, Alarcão AM, Gomes A, Couceiro P, Carvalho L. EGFR/erB-1, HER2/erB-2, CK7, LP34, Ki67 and P53 expression in preneoplastic lesions of bronchial epithelium: An immunohistochemical and genetic study. Virchows Arch. 2011;458:571–581. doi: 10.1007/s00428-011-1062-5. [DOI] [PubMed] [Google Scholar]
- 7.Thun MJ. Early landmark studies of smoking and lung cancer. Lancet Oncol. 2010;11:1200. doi: 10.1016/S1470-2045(09)70401-2. [DOI] [PubMed] [Google Scholar]
- 8.Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 surgeon general's report: 'The health consequences of smoking-50 years of progress ': A paradigm shift in cancer care. Cancer. 2014;120:1914–1916. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto J. That the effects of smoking should be measured in pack-years: Misconceptions 4. Br J Cancer. 2012;107:406–407. doi: 10.1038/bjc.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy SE, Palomino A, Hecht SS, Hoffmann D. Dose-response study of DNA and hemoglobin adduct formation by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res. 1990;50:5446–5452. [PubMed] [Google Scholar]
- 13.Jalas JR, McIntee EJ, Kenney PM, Upadhyaya P, Peterson LA, Hecht SS. Stereospecific deuterium substitution attenuates the tumorigenicity and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Dose-response study of DNA and hemoglobin adduct formation by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Chem Res Toxicol. 2003;16:794–806. doi: 10.1021/tx034022l. [DOI] [PubMed] [Google Scholar]
- 14.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12:3–13. doi: 10.1158/1541-7786.MCR-13-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan P, Buffler PA, Reynolds P, Wu AH, Wichmann HE, Agudo A, Pershagen G, Jöckel KH, Benhamou S, Greenberg RS, et al. Secondhand smoke exposure in adulthood and risk of lung cancer among never smokers: A pooled analysis of two large studies. Int J Cancer. 2004;109:125–131. doi: 10.1002/ijc.11682. [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K. Secondhand smoke exposure and risk of lung cancer in Japan: A systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol. 2016;46:942–951. doi: 10.1093/jjco/hyw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heloma A, Jaakkola MS, Kähkönen E, Reijula K. The short-term impact of national smoke-free workplace legislation on passive smoking and tobacco use. Am J Public Health. 2001;91:1416–1418. doi: 10.2105/AJPH.91.9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebe S, Leigh J, Henderson DW, Nurminen M. Asbestos, smoking and lung cancer: An update. Int J Environ Res Public Health. 2019;17:258. doi: 10.3390/ijerph17010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngamwong Y, Tangamornsuksan W, Lohitnavy O, Chaiyakunapruk N, Scholfield CN, Reisfeld B, Lohitnavy M. Additive synergism between asbestos and smoking in lung cancer risk: A systematic review and meta-analysis. PLoS One. 2015;10:e0135798. doi: 10.1371/journal.pone.0135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villeneuve PJ, Parent MÉ, Harris SA, Johnson KC, Canadian Cancer Registries Epidemiology Research Group Occupational exposure to asbestos and lung cancer in men: Evidence from a population-based case-control study in eight Canadian provinces. BMC Cancer. 2012;12:595. doi: 10.1186/1471-2407-12-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topinka JB, Loli P, Dusinská M, Hurbánková M, Kováciková Z, Volkovová K, Kazimírová A, Barancoková M, Tatrai E, Wolff T, et al. Mutagenesis by man-made mineral fibres in the lung of rats. Mutat Res. 2006;595:174–183. doi: 10.1016/j.mrfmmm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Ketfi A, Zanoun N, Laouedj I, Gharnaout M, Fraga S. Primary lung cancer and occupational exposure in a North African population. Pan Afr Med J. 2020;37:120. doi: 10.11604/pamj.2020.37.120.21755. In French. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Jiang N, Wang L, Liu H, He R. Chronic obstructive pulmonary disease and risk of lung cancer: A meta-analysis of prospective cohort studies. Oncotarget. 2017;8:78044–78056. doi: 10.18632/oncotarget.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu YL, Liu J, Zhang LX, Wu CM, Chu AJ, Wen BL, Ma C, Yan XY, Zhang X, Wang DM, et al. Asthma and the risk of lung cancer: A meta-analysis. Oncotarget. 2017;8:11614–11620. doi: 10.18632/oncotarget.14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozinovski S, Vlahos R, Anthony D, McQualter J, Anderson G, Irving L, Steinfort D. COPD and squamous cell lung cancer: Aberrant inflammation and immunity is the common link. Br J Pharmacol. 2016;173:635–648. doi: 10.1111/bph.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh A, Boucher RC, Tarran R. Airway hydration and COPD. Cell Mol Life Sci. 2015;72:3637–3652. doi: 10.1007/s00018-015-1946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keikha M, Esfahani BN. The relationship between tuberculosis and lung cancer. Adv Biomed Res. 2018;7:58. doi: 10.4103/abr.abr_182_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PLoS One. 2011;6:e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Zhu M, Zhou W, Du J, Xiang Y, Shu XO, Hu Z, Zhou W, Chen K, Xu J, et al. Estimation of heritability for nine common cancers using data from genome-wide association studies in Chinese population. Int J Cancer. 2017;140:329–336. doi: 10.1002/ijc.30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson JN, Wheeler WA, Yeager M, Panagiotou O, Wang Z, Berndt SI, Lan Q, Abnet CC, Amundadottir LT, Figueroa JD, et al. Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J Natl Cancer Inst. 2015;107:djv279. doi: 10.1093/jnci/djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay JD, Hung RJ, Han Y, Zong X, Carreras-Torres R, Christiani DC, Caporaso NE, Johansson M, Xiao X, Li Y, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet 4. 2017;9:1126–1132. doi: 10.1038/ng.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bossé Y, Amos CI. A decade of GWAS results in lung cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:363–379. doi: 10.1158/1055-9965.EPI-16-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai J, Lv J, Zhu M, Wang Y, Qin N, Ma H, He YQ, Zhang R, Tan W, Fan J, et al. Identification of risk loci and a polygenic risk score for lung cancer: A large-scale prospective cohort study in Chinese populations. Lancet Respir Med. 2019;7:881–891. doi: 10.1016/S2213-2600(19)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobue T, Moriyama N, Kaneko M, Kusumoto M, Kobayashi T, Tsuchiya R, Kakinuma R, Ohmatsu H, Nagai K, Nishiyama H, et al. Screening for lung cancer with low-dose helical computed tomography: Anti-lung cancer association project. J Clin Oncol. 2002;20:911–920. doi: 10.1200/JCO.2002.20.4.911. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda Y, Nakayama T, Kusunoki Y, Iso H, Suzuki T. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br J Cancer. 2008;98:1602–1607. doi: 10.1038/sj.bjc.6604351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, von Schulthess GK, Steinert HC. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 38.Guhlmann A, Storck M, Kotzerke J, Moog F, Sunder-Plassmann L, Reske SN. Lymph node staging in non-small cell lung cancer: Evaluation by [18F]FDG positron emission tomography (PET) Thorax. 1997;52:438–441. doi: 10.1136/thx.52.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch FR, Prindiville SA, Miller YE, Franklin WA, Dempsey EC, Murphy JR, Bunn PA, Jr, Kennedy TC. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: A randomized study. J Natl Cancer Inst. 2001;93:1385–1391. doi: 10.1093/jnci/93.18.1385. [DOI] [PubMed] [Google Scholar]
- 40.Arroliga AC, Matthay RA. The role of bronchoscopy in lung cancer. Clin Chest Med. 1993;14:87–98. doi: 10.1016/S0272-5231(21)01150-3. [DOI] [PubMed] [Google Scholar]
- 41.Risse EK, Vooijs GP, van't Hof MA. Relationship between the cellular composition of sputum and the cytologic diagnosis of lung cancer. Acta Cytol. 1987;31:170–176. [PubMed] [Google Scholar]
- 42.Tsoulos N, Papadopoulou E, Metaxa-Mariatou V, Tsaousis G, Efstathiadou C, Tounta G, Scapeti A, Bourkoula E, Zarogoulidis P, Pentheroudakis G, et al. Tumor molecular profiling of NSCLC patients using next generation sequencing. Oncol Rep. 2017;38:3419–3429. doi: 10.3892/or.2017.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Y, Todd NW, Liu Z, Zhan M, Fang H, Peng H, Alattar M, Deepak J, Stass SA, Jiang F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer 6. 2010;7:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13:361–366. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]
- 45.Rotunno M, Hu N, Su H, Wang C, Goldstein AM, Bergen AW, Consonni D, Pesatori AC, Bertazzi PA, Wacholder S, et al. A gene expression signature from peripheral whole blood for stage I lung adenocarcinoma. Cancer Prev Res (Phila) 2011;4:1599–1608. doi: 10.1158/1940-6207.CAPR-10-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non-small cell lung cancer: Real-world issues and tough choices. Am Soc Clin Oncol Educ Book. 2019;39:531–542. doi: 10.1200/EDBK_237863. [DOI] [PubMed] [Google Scholar]
- 47.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 48.Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 49.Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, Kris MG, Varella-Garcia M, Arcila ME. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11:414–419. doi: 10.1016/j.jtho.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reungwetwattana T, Liang Y, Zhu V, Ou SI. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The why, the how, the who, the unknown, and the inevitable. Lung Cancer. 2017;103:27–37. doi: 10.1016/j.lungcan.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, Wirth LJ, Stock S, Smith S, Lauriault V, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrer I, Zugazagoitia J, Herbertz S, John W, Paz-Ares L, Schmid-Bindert G. KRAS-mutant non-small cell lung cancer: From biology to therapy. Lung Cancer. 2018;124:53–64. doi: 10.1016/j.lungcan.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Edell ES, Cortese DA. Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest. 1992;102:1319–1322. doi: 10.1378/chest.102.5.1319. [DOI] [PubMed] [Google Scholar]
- 55.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: Reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol. 2004;22:3860–3867. doi: 10.1200/JCO.2004.01.153. [DOI] [PubMed] [Google Scholar]
- 56.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 2015;1856:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 58.Sangha R, Price J, Butts CA. Adjuvant therapy in non-small cell lung cancer: Current and future directions. Oncologist. 2010;15:862–872. doi: 10.1634/theoncologist.2009-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 60.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 61.Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, Martelli M, Clerici M, Cognetti F, Tonato M, Adjuvant Lung Project Italy/European Organisation for Research Treatment of Cancer-Lung Cancer Cooperative Group Investigators Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 62.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, et al. Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 63.Alexander M, Kim SY, Cheng H. Update 2020: Management of non-small cell lung cancer. Lung. 2020;198:897–907. doi: 10.1007/s00408-020-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 65.Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15:288–293. doi: 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 67.Arteaga CL. The epidermal growth factor receptor: From mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 2001;19(Suppl 18):32S–40S. [PubMed] [Google Scholar]
- 68.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 69.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(Suppl 2):S21–S26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 70.Laskin JJ, Sandler AB. Epidermal growth factor receptor inhibitors in lung cancer therapy. Semin Respir Crit Care Med. 2004;25(Suppl 1):S17–S27. doi: 10.1055/s-2004-829641. [DOI] [PubMed] [Google Scholar]
- 71.Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–1021. viii. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-I. [DOI] [PubMed] [Google Scholar]
- 73.Hirsch FR, Scagliotti GV, Langer CJ, Varella-Garcia M, Franklin WA. Epidermal growth factor family of receptors in preneoplasia and lung cancer: Perspectives for targeted therapies. Lung Cancer. 2003;41(Suppl 1):S29–S42. doi: 10.1016/S0169-5002(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 74.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 75.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 76.Prewett M, Rothman M, Waksal H, Feldman M, Bander NH, Hicklin DJ. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinoma xenografts in nude mice. Clin Cancer Res. 1998;4:2957–2966. [PubMed] [Google Scholar]
- 77.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 78.Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II Trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 79.Kim ES. Cetuximab as a single agent or in combination with chemotherapy in lung cancer. Clin Lung Cancer. 2004;6(Suppl 2):S80–S84. doi: 10.3816/CLC.2004.s.019. [DOI] [PubMed] [Google Scholar]
- 80.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 81.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 82.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 83.Karkkainen MJ, Mäkinen T, Alitalo K. Lymphatic endothelium: A new frontier of metastasis research. Nat Cell Biol. 2002;4:E2–E5. doi: 10.1038/ncb0102-e2. [DOI] [PubMed] [Google Scholar]
- 84.Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59:455–467. [PubMed] [Google Scholar]
- 85.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 86.Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 87.Muñoz-Chápuli R, Quesada AR, Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224–2243. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: Focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev. 2013;39:839–850. doi: 10.1016/j.ctrv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Li N, Zeng A, Wang Q, Chen M, Zhu S, Song L. Regulatory function of DNA methylation mediated lncRNAs in gastric cancer. Cancer Cell Int. 2022;22:227. doi: 10.1186/s12935-022-02648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin Oncol. 2002;29(Suppl 6):S10–S14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 92.Longo R, Sarmiento R, Fanelli M, Capaccetti B, Gattuso D, Gasparini G. Anti-angiogenic therapy: Rationale, challenges and clinical studies. Angiogenesis. 2002;5:237–256. doi: 10.1023/A:1024532022166. [DOI] [PubMed] [Google Scholar]
- 93.Manzo A, Montanino A, Carillio G, Costanzo R, Sandomenico C, Normanno N, Piccirillo MC, Daniele G, Perrone F, Rocco G, Morabito A. Angiogenesis inhibitors in NSCLC. Int J Mol Sci. 2017;18:2021. doi: 10.3390/ijms18102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tabernero J. The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203–220. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- 95.Vallböhmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA, Rhodes KE, Sherrod AE, Iqbal S, Danenberg KD, et al. Molecular determinants of cetuximab efficacy. J Clin Oncol. 2005;23:3536–3544. doi: 10.1200/JCO.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 96.Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: A role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 97.Rak J, Yu JL, Kerbel RS, Coomber BL. What do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors? Cancer Res. 2002;62:1931–1934. [PubMed] [Google Scholar]
- 98.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI200317929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viloria-Petit AM, Kerbel RS. Acquired resistance to EGFR inhibitors: Mechanisms and prevention strategies. Int J Radiat Oncol Biol Phys. 2004;58:914–926. doi: 10.1016/j.ijrobp.2003.09.091. [DOI] [PubMed] [Google Scholar]
- 100.Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 101.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 102.Zhang TT, Wang RM, Yang Z, Chen GB. Dual inhibiting EGFR and VEGF pathways versus EGFR-TKIs alone in the treatment of advanced non-small-cell lung cancer: A meta-analysis of randomized controlled trials. Clin Transl Oncol. 2016;18:576–581. doi: 10.1007/s12094-015-1402-z. [DOI] [PubMed] [Google Scholar]
- 103.Qi WX, Wang Q, Jiang YL, Sun YJ, Tang LN, He AN, Min DL, Lin F, Shen Z, Yao Y. Overall survival benefits for combining targeted therapy as second-line treatment for advanced non-small-cell-lung cancer: A meta-analysis of published data. PLoS One. 2013;8:e55637. doi: 10.1371/journal.pone.0055637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.