Abstract
Rodent models are useful for understanding the mechanisms that underlie opioid addiction, but most preclinical studies have focused on rewarding and consummatory aspects of opioids without components of dependence-induced escalation of drug taking or seeking. We characterized several opioid-related behaviors in mice using a model of vaporized fentanyl self-administration. Male and female C57BL/6J mice were assigned to short-access (ShA; 1 h, nondependent) or long-access (LgA; 6 h, dependent) fentanyl vapor self-administration and subsequently tested in a battery of behavioral tests, followed by blood collection during withdrawal. Compared with mice in the ShA group, mice in the LgA group escalated their fentanyl intake, were more motivated to work to obtain the drug, exhibited greater hyperalgesia, and exhibited greater signs of naloxone-precipitated withdrawal. Principal component analysis indicated the emergence of two independent behavioral constructs: “intake/motivation” and “hyperalgesia/punished seeking.” In mice in the LgA condition only, “hyperalgesia/punished seeking” was associated with plasma levels of proinflammatory interleukin-17 (IL-17), chemokine (C-C motif) ligand 4 (CCL-4), and tumor necrosis factor α (TNF-α). Overall, the results suggest that extended access to opioids leads to addiction-like behavior, and some constructs that are associated with addiction-like behavior may be associated with levels of the proinflammatory cytokines/chemokines IL-17, TNF-α, and CCL-4 in blood.
Keywords: Substance use disorder (SUD), Opioid use disorder (OUD), Hyperalgesia, Extended access, Addiction-like behavior, Operant self-administration, Punishment
1. Introduction
Opioid use disorder (OUD) is a chronic relapsing disorder that is characterized by a pattern of opioid use that leads to clinically significant impairment and distress [1]. In 2019, 1.2% of the population worldwide used opioids, and opioids were present in over 70% of overdose deaths [2]. The nonmedical use of opioids is a major problem in North America but has remained stable since 2010. Opioid overdose deaths doubled in the same period, suggesting an increase in the nonmedical use of potent opioids, such as fentanyl and its analogs [2]. During the coronavirus disease 2019 (COVID-19) pandemic, the United States Centers for Disease Control and Prevention reported a 35% increase in opioid overdose deaths (https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html ).
Opioid use disorder involves a compulsion to seek and take opioids, the loss of control over intake, and the emergence of a negative affect state during withdrawal, also known as hyperkatifeia, which are symptoms that reflect multiple sources of motivation for opioid seeking [3]. There are also physical symptoms, such as body aches, diarrhea, greater pain sensitivity, and lower pain tolerance (hyperalgesia) during opioid withdrawal [4, 5]. The treatment of OUD is largely limited to drugs that act on the opioid system (e.g., μ-opioid receptor agonist methadone, μ-opioid receptor partial agonist buprenorphine, and preferential μ-opioid receptor antagonist naltrexone). The preferential μ-opioid receptor antagonist naloxone (Narcan®) is used to reverse opioid overdose. Treatment with the α2-adrenergic receptor agonists lofexidine and clonidine is restricted as an adjuvant during medically supervised withdrawal [6]. Adherence and retention to the medication-assisted treatment of OUD faces several barriers, such as poor accessibility, high cost, and stigma [6, 7]. Additionally, chronic treatment with buprenorphine and methadone may not improve hyperalgesia [8], which may contribute to the maintenance of drug taking and relapse. Therefore, understanding the biological mechanisms that underlie motivational withdrawal and contribute to drug seeking and taking may shed light on OUD etiology and assist with medication development.
Animal models are a key tool for understanding the neurocircuitry that underlies OUD [9, 10] and testing novel pharmacological targets [11, 12]. One of these models involves extended access to intravenous opioid self-administration, which recapitulates several characteristics of OUD. Rats [13, 14] and mice [15] escalate their heroin intake, exhibit greater signs of naloxone-induced withdrawal, and are more motivated for the drug when given extended access (e.g., 6–24 h self-administration sessions) to the drug compared with limited access (e.g., 1–3 h self-administration sessions). Although intravenous self-administration models are considered the gold-standard in addiction research, they pose several technical challenges, such as high rates of catheter failure, especially in mice [16, 17]. Considering the importance of fentanyl and its analogs in the current opioid crisis, we developed vaporized fentanyl and sufentanil self-administration models in both rats [18, 19] and mice [20] that produce motivational and physical (somatic) signs of opioid withdrawal without the need for a catheter implant. Additionally, most research has focused on reward and consummatory behaviors without components of dependence-induced increases in drug taking and seeking, and drug taking and seeking despite punishment (i.e., addiction-like behaviors, [21]). Whether extended access to fentanyl vapor self-administration leads to addiction-like behaviors in mice remains to be determined.
Addiction-like behaviors are characterized by persistent, repetitive drug seeking and taking that can prevent or provide relief from distress, anxiety, or stress [1, 22, 23]. For the purposes of the present study, elements of addiction-like drug seeking were divided into the following phenotypic components: (i) escalation of drug intake, (ii) increase in drug seeking under progressive-ratio (PR) and progressive-delay conditions, and (iii) drug seeking and taking despite aversive consequences [24].
Neuroimmune alterations in the brain have been shown to significantly alter key neurotransmitters involved in substance use disorders [25]. As such, the overproduction of cytokines and other immune signaling molecules can decrease dopamine and increase glutamate signaling and release (for review, see [26–28]). Conversely, prolonged exposure to an addictive drug affects the neuroimmune system by eliciting microglia and astrocyte activation, leading to the release of cytokines and chemokines [29]. Opioids induce many neuroinflammatory alterations in the brain that may contribute to the development of addiction through myriad mechanisms [29]. Opioids activate microglia (i.e., resident immune cells in the brain) and astrocytes (i.e., neuronal regulatory cells; [30]) leading to the release of proinflammatory cytokines [31]. Recent studies reported an upregulated expression of cytokines such as TNF-α, IL-1β, IL-6 and IL-17 [32]. Opioids can also increase the production of chemokines like CCL2 and CCL4 in human neurons [33] and astrocytes [34]. However, the role of peripheral cytokines and chemokines as biomarkers of addiction-like opioid intake remains to be investigated. Therefore, we chose to explore the impact of prolonged opioid exposure on those specific pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-17) and chemokines (CCL2, CCL4) in blood.
Using a battery of behavioral tests, the present study investigated opioid dependence-related behaviors, including drug seeking and taking despite punishment, in the vaporized fentanyl self-administration model in male and female mice. The secondary goal was to assess blood levels of cytokines and chemokines as potential biomarkers of opioid dependence. Our hypothesis was that extended access to fentanyl vapor self-administration will result in qualitative and quantitative changes in opioid-related behaviors and that blood cytokine and chemokine levels serve as biomarkers for some of these behaviors.
2. Methods
2.1. Animals
C57BL/6J mice (41 females and 41 males) were purchased from Jackson Laboratories (Bar Harbor, ME, USA) at 7 weeks of age and were 8–10 weeks old at the beginning of the experiment. The mice were kept in groups of two to four per cage and housed under a reverse light cycle (lights on at 7 PM) with controlled temperature (22 °C ± 2 °C) and humidity (50–60%). The mice had free access to water and food except during testing. Tests were performed during the dark cycle. Body weight was recorded at least weekly. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were previously approved by the National Institute on Drug Abuse Animal Care and Use Committee.
2.2. Drugs
Fentanyl citrate (Mallinckrodt, St. Louis, MO, USA) was dispensed by the National Institute on Drug Abuse Intramural Research Program pharmacy. The stock solution was prepared by adding 6 mL of sterile water to 1 g fentanyl and 44 mL of vehicle (80% vegetal glycerin [VG]/20% propylene glycol [PG]). The stock solution (20 mg/mL) was diluted with vehicle to a concentration of 5 mg/mL for the self-administration experiments [20]. Capsaicin-adulterated fentanyl was used for drug seeking and taking despite punishment. Capsaicin (catalog no. N735–5 g, AK Scientific, Union City, CA, USA) was dissolved in 0.5 mL of 200-proof ethanol (catalog no. 111,000,200, Pharmco-Aaper, Brookfield, CT, USA). This solution was then added to the vehicle (VG/PG) or 5 mg/mL fentanyl in VG/PG. The mixture was sonicated at 40 °C for 1 h.
2.2.1. Fentanyl and norfentanyl measurement in blood
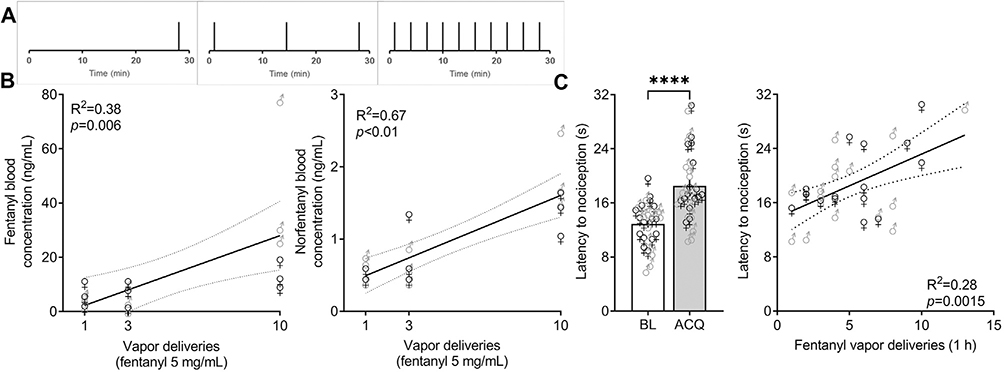
All mice received 1, 3, or 10 noncontingent vapor deliveries (1.5 s, 60 W) of fentanyl, evenly spaced (for three and 10 deliveries) in 30 min (Fig. 1A). Immediately after the end of the session (2 min after the last fentanyl delivery), the mice were deeply anesthetized in a chamber that was saturated with isoflurane and euthanized by decapitation. Trunk blood was collected in microtubes that contained 45 μL of 4% ethylene-diaminetetraacetic acid (EDTA). Blood samples were analyzed by high-performance liquid chromatography/tandem mass spectrometry at NMS Labs (Horsham, PA, USA).
Fig. 1. Fentanyl vapor leads to detectable blood fentanyl and norfentanyl levels and antinociception.
(A) Temporal distribution of noncontingent fentanyl vapor deliveries. (B) Concentration of fentanyl and norfentanyl in blood in male and female mice. Eighteen mice were exposed to 1, 3, or 10 fentanyl vapor deliveries (1.5 s, 60 W) and euthanized 2 min after the last vapor exposure for blood collection. Fentanyl and norfentanyl levels were analyzed by liquid chromatography/tandem mass spectroscopy. The data are expressed as individual points and were analyzed by linear regression (n = 6/number of deliveries). (C) Antinociceptive response to fentanyl vapor self-administration. Male and female C57BL/6J mice were tested in the hot plate test (52.5 °C, 30 s cutoff) immediately before and after the fifth 1-h fentanyl vapor self-administration acquisition session. Fentanyl vapor self-administration increased the latency to a nociceptive response. The data are expressed as the mean ± SEM and were analyzed using paired Student’s t -test. ∗ ∗ ∗ ∗ p < 0.0001. Antinociceptive responses positively correlated with the number of fentanyl vapor deliveries in the self-administration session. The data are expressed as individual points and were analyzed by linear regression. BL, baseline; ACQ, acquisition (n = 16 mice/sex).
2.3. Nociception tests
To measure antinociception, we used a hot plate (Ugo Basile, Gemonio, Italy) at 52.5 °C. The latency to the first nociceptive behavior was recorded at baseline immediately before an acquisition self-administration session (BL) and immediately after an acquisition self-administration session (ACQ). Nociceptive behaviors included jumps and rear paw licking or vigorous flicking. A cutoff time of 30 s was imposed to prevent tissue damage. To measure mechanical hyperalgesia during withdrawal, we used an electronic von Frey device (Ugo Basile, Gemonio, Italy). All mice were tested before acquisition session 1 for their baseline responding and immediately before escalation session 10 (i.e., 36 h after the end of their last self-administration session). The maximum force was set to 50 gf, and the paw withdrawal threshold was determined as the force necessary to elicit a response (i.e., paw removal). Two measures were taken for each rear paw with an interstimulus interval of at least 30 s. We calculated the average of these measures and used this as a measure of mechanical sensitivity. For principal component analysis (PCA), this value was multiplied by −1, so higher values represented more hyperalgesia.
2.4. Fentanyl vapor self-administration
The fentanyl vapor self-administration operates in a closed system. The vaporizer only works with the chamber closed in an airtight environment. The pump produces negative pressure to pull fentanyl vapor inside the chambers. The outlet tubes connect to a HEPA filter before the vapor is released to the building exhaust system. All procedures were performed as previously described [35]. Briefly, mice were trained to lever press for vaporized fentanyl (1.5 s, 60 W, 1 min timeout) on a fixed-ratio 1 (FR1) schedule of reinforcement, in which each lever press on the active (left) lever resulted in vapor delivery. The concentration of fentanyl used was determined in our previous work [20]. Drug delivery was accompanied by activation of a cue light. The cue light remained on during the timeout period to signal that the drug was not available. Presses on the inactive (right) lever and during the timeout period were recorded but had no consequence. After acquisition (six 1-h sessions) of fentanyl vapor self-administration, the mice were split into two groups. One group was allowed 1 h access (short access [ShA]), and the other group was allowed 6 h access (long access [LgA]) to fentanyl self-administration in 10 FR1 sessions (escalation phase). The mice were then tested for motivation to obtain fentanyl under PR and delay schedules (described below), nociception, and capsaicin-punished drug seeking. After 3 weeks of abstinence, all mice underwent 10 re-escalation sessions, after which they were tested for naloxone-precipitated withdrawal and capsaicin-punished drug taking (described below). An FR1 session following the same parameters described above was performed between tests to maintain stable levels of lever pressing in the ShA group and opioid dependence in the LgA group.
2.5. Progressive-ratio test
We tested the mice in a PR task, in which the number of lever presses that was required for vapor delivery was sequentially increased by six (PR 6; i.e., 1, 7, 13, 19, 25, 31, etc.). A 30 min period without vapor delivery or a total of 6 h ended the session. The breakpoint was determined as the last ratio (“effort”) that was completed in the session.
2.6. Delay
We also tested the mice in a delayed-reward task, in which the interval between lever pressing and vapor delivery was sequentially increased by 6 s (i.e., 1, 7, 13, 19, 25, 31, etc.) for each subsequent drug delivery. A 30 min period without vapor delivery or a total of 6 h ended the session. The breakpoint was determined as the last ratio (in seconds;”time”) that was completed in the session.
2.7. Precipitated withdrawal test
Immediately after the end of the FR1 self-administration session, each mouse received an intraperitoneal (IP) injection of naloxone (1 mg/kg, 10 mL/kg) and were placed in a transparent box in front of a mirror at a 45° angle for the improved visibility of behavior. The observation and recording period lasted 20 min. The videos were analyzed by an experienced scorer who was blind to group assignment. The number of paw tremors (“clapping”), jumps, and wet-dog shakes were recorded, and each occurrence was attributed one point. The presence of other”physical” signs, such as diarrhea, genital grooming, abnormal posture, ptosis, salivation, and teeth chattering, were also recorded, and attributed one point each, regardless of frequency. A precipitated global withdrawal score was calculated as the sum of points [36, 15].
2.8. Drug seeking and self-administration in the presence of an aversive stimulus
To model punished drug seeking, we tested the mice for the self-administration of capsaicin alone (i.e., vehicle without fentanyl). The mice were exposed to four concentrations of capsaicin (0, 0.01, 0.03, and 0.1%, w/v) in VG/PG in 1-h sessions for both ShA and LgA groups. The presentation of capsaicin followed a Latin-square design, 24 h apart, to avoid the confound of extinction-like behavior. To model punished drug taking, the fentanyl solution (5 mg/mL) was adulterated with increasing concentrations of capsaicin. The concentrations of capsaicin followed a log scale, starting at 0.01% (capsaicin in fentanyl [w/v]) up to 3%. The sessions with capsaicin-adulterated fentanyl lasted 1 h for both ShA and LgA and were performed 24 h apart in an ascending order of concentration.
2.9. Plasma collection
After the end of the behavioral test battery, all mice underwent one final FR1 self-administration session (1 h for ShA and 6 h for LgA). After 36 h, the mice were deeply anesthetized with isoflurane and decapitated. Trunk blood was collected in microtubes that contained EDTA. Blood samples were centrifuged at 10,000 × g at 4 °C for 10 min. Plasma was collected and stored at −80 °C until use.
2.10. Corticosterone measurement
Plasma samples were diluted 1:100 with assay buffer, and the enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s instructions (catalog no. ab108821, Abcam, Cambridge, UK).
2.11. Cytokine and chemokine measurement
Plasma samples were diluted by a factor of 1.5 and analyzed using an Ella microfluid multiplex cartridge according to the manufacturer’s instructions (ProteinSimple, San Jose, CA, USA).
2.12. Data analysis
After conducting analyses of variance (ANOVAs) for possible sex interactions and finding none (see below), we reanalyzed the male and female data combined using two-way repeated-measures ANOVA, followed by Duncan’s post hoc test, or paired or unpaired Student’s t-test as appropriate. All data separated by sex are presented in the Supplemental Material. The data are expressed as the mean ± standard error of the mean (SEM). Linear regression was used to determine correlations between nociception and fentanyl vapor deliveries. Values of p < 0.05 were considered statistically significant. To assess relationships among variables, we used Varimax-normalized principal components analysis (PCA) with Eigenvalues greater than 1. Loading factors ≥ 0.6 were considered. We arbitrarily selected loading factors higher than 0.6 to be conservative with the representation of variables in each factor. The larger a loading’s relative magnitude, the more the variables are positively correlated with each other (positive values) or negatively correlated with each other (negative values). We then extracted the individual loading values of each mouse and used Pearson’s test to determine correlations between PCA values and blood chemokines/cytokines and corticosterone levels (two-tailed comparison, α= 1%). One female ShA mouse was not included in the cytokine and corticosterone analysis because of technical issues during plasma sample processing.
3. Results
3.1. Sex differences
We analyzed all data for sex effects (Supplemental Tables S1–4). We employed three-way ANOVAs and found no sex × group interactions for any of the variables analyzed. An overall effect of sex on punished seeking was found (F 1,28 = 17.026, p = 0.0003; F > M), with no interaction between sex and any other variable (Supplemental Tables S1, S3). Because no sex × group interactions were found, we collapsed the male and female data for subsequent analyses.
3.2. Fentanyl vapor leads to detectable blood fentanyl levels and antinociception
We previously reported that vaporized fentanyl and sufentanil leads to concentration-dependent levels of fentanyl and sufentanil in blood [18, 20]. Here, we confirmed that cumulative exposure to fentanyl vapor led to higher blood levels of fentanyl (F1,16 = 9.888, p = 0.006) and its metabolite, norfentanyl (F1,16 = 32.79, p < 0.001), in male and female mice (Fig. 1B). One vapor delivery of fentanyl 5 mg/mL led to a mean plasma level of 4.55 ng/mL, whereas 10 vapor deliveries resulted in an average plasma concentration of 28.63 ng/mL.
Next, we trained 32 mice (16 male and 16 female) to lever press for fentanyl vapor delivery. Upon stable active lever pressing (~5–6 sessions), we tested their antinociceptive response immediately after the end of the session in the hot plate test (Fig. 1C). Fentanyl vapor self-administration caused an antinociceptive response (t 31 = 6.210, p < 0.0001) that positively correlated with the number of lever presses (R 2 = 0.28, F 1,30 = 12.13, p = 0.0015). This indicates that mice will self-administer fentanyl to levels of biological effect (i.e., antinociception).
3.3. Escalation of fentanyl vapor self-administration in dependent mice
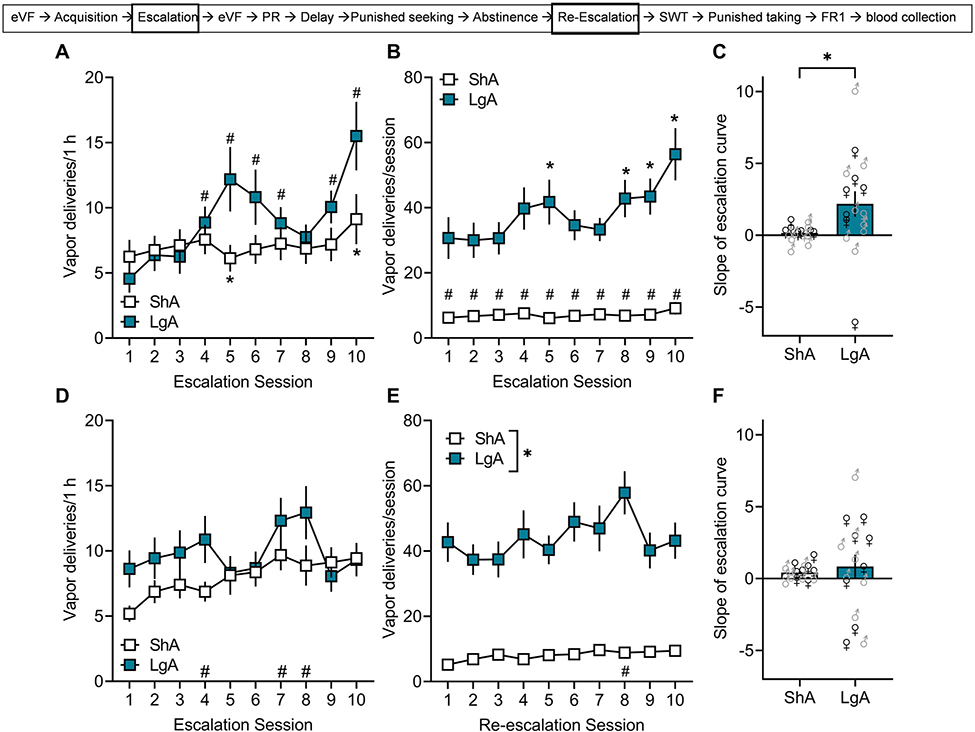
For the first hour of the session, the two-way ANOVA showed a significant group × session interaction (F 9,270 = 3.880, p = 0.00012; Fig. 2A). The Duncan post hoc test showed that mice in the LgA group had more vapor deliveries in session 4 (p = 0.01), session 5 (p < 0.0001), session 6 (p < 0.0001), session 7 (p = 0.01), session 9 (p = 0.0007), and session 10 (p < 0.0001) compared with session 1 and more vapor deliveries in session 5 (p = 0.02) and session 10 (p = 0.006) compared with mice in the ShA group. For the total 6 h session, the two-way repeated-measures ANOVA showed a significant group × session interaction for the number of vapor deliveries (F9,270 = 2.917, p = 0.0026; Fig. 2B). The Duncan post hoc test showed that mice in the LgA group had more vapor deliveries in session 5 (p = 0.03), session 8 (p = 0.02), session 9 (p = 0.01), and session 10 (p < 0.0001) compared with session 1. There were no differences in vapor deliveries across sessions in the ShA group (Fig. 2A, B). Using linear regression, we calculated the slope of the escalation curve for each mouse. Mice in the LgA group had significantly higher slopes than mice in the ShA group, further confirming their escalation of intake (t 30 = 2.259, p = 0.03; Fig. 2C). For the first hour of re-escalation, the two-way repeated-measures ANOVA did not show a significant group × session interaction (F 9,270 = 1.880, p = 0.055; Fig. 2D) nor main effect of group (F1,30 = 1.657, p = 0.21) but showed a main effect of session (F9,270 = 3.473, p = 0.0005). Duncan’s post hoc showed that the overall number of vapor deliveries was higher on sessions 7 (p = 0.00008), 8 (p = 0.0001) and 10 (p = 0.02) compared with session 1. The two-way repeated-measures ANOVA did not show a significant group × session interaction for the number of vapor deliveries for the total 6 h during re-escalation (F9,270 = 1.567, p = 0.12; Fig. 2E) but showed main effects of group (F1,30 = 77.029, p < 0.0001; ShA < LgA) and session (F9,270 = 2.115, p = 0.03). Duncan’s post hoc test showed that the overall number of vapor deliveries was higher in session 8 than in session 1 (p = 0.012). The calculated slopes for the re-escalation did not differ between ShA and LgA conditions (t30 = 0.48, p = 0.63; Fig. 2F). Mice allowed LgA escalated their fentanyl intake during the escalation phase and maintained higher levels of fentanyl intake but did not escalate further on the re-escalation phase.
Fig. 2. Escalation and re-escalation of fentanyl vapor self-administration.
Male and female C57BL/6J mice were trained to lever press for fentanyl vapor deliveries on an FR1 schedule of reinforcement. Upon stable lever pressing, the mice were split in short-access (ShA; 1 h) and long-access (LgA; 6 h) conditions. (A) Mice in the LgA condition escalated their intake in the first hour of the session across days. The data are expressed as the mean ± SEM and were analyzed using two-way repeated-measures ANOVA. #p < 0.05, compared with session 1; *p < 0.05, compared with LgA. (B) Mice in the LgA condition escalated their fentanyl intake across the 6 h sessions. The data are expressed as the mean ± SEM and were analyzed using two-way repeated-measures ANOVA. #p < 0.05, compared with session 1; *p < 0.05, compared with LgA. (C) Mice in the LgA condition had higher calculated slopes of the escalation curve compared with mice in the ShA condition. The data are expressed as the mean ± SEM and were analyzed using unpaired Student’s t-test. *p < 0.05, compared with ShA. (D) Fentanyl intake on the first re-escalation self-administration sessions. The data are expressed as the mean ± SEM and were analyzed using two-way repeated-measures ANOVA. #p < 0.05, compared with session 1. (E) Mice in the LgA condition maintained higher fentanyl intake across sessions in the re-escalation phase. The data are expressed as the mean ± SEM and were analyzed using two-way repeated-measures ANOVA. # p < 0.05, compared with session 1; *p < 0.05, compared with LgA. (F) The calculated re-escalation slope did not differ between ShA and LgA conditions. The data are expressed as the mean ± SEM and were analyzed using unpaired Student’s t-test. (n = 8/sex/group).
3.4. Increases in naloxone-precipitated withdrawal signs, hyperalgesia during spontaneous withdrawal, and motivation for fentanyl
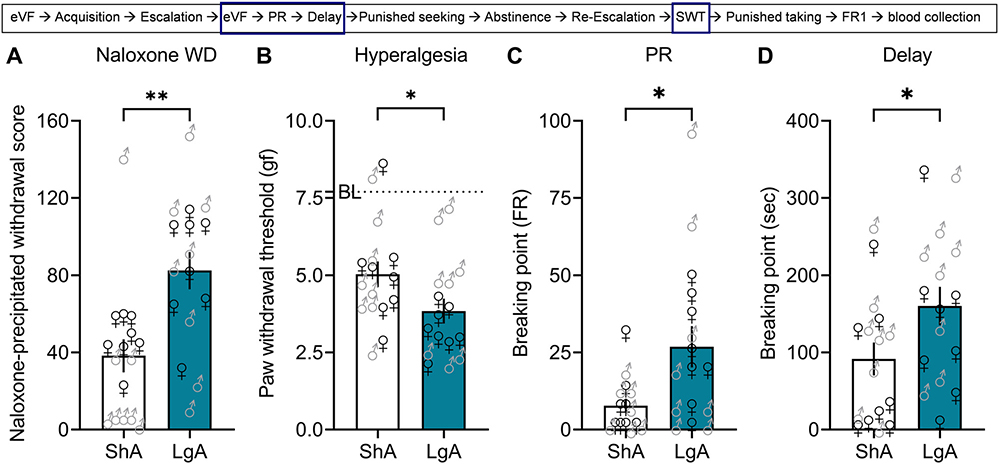
Unpaired Student’s t-test showed that mice in the LgA group exhibited more signs of naloxone-precipitated withdrawal compared with mice in the ShA group (t30 = 3.424, p = 0.002; Fig. 3A). Unpaired Student’s t-test showed that mice in the LgA group had lower thresholds than mice in the ShA group in response to mechanic stimulation with an electronic von Frey device, indicating the development of hyperalgesia (t30 = 2.409, p = 0.049; Fig. 3B). Unpaired Student’s t-test showed that mice in the LgA group had a higher breakpoint than mice in the ShA group in the PR test (t30 = 2.299, p = 0.03; Fig. 3C). Unpaired Student’s t-test showed that mice in the LgA group had a higher time breakpoint than mice in the ShA group in the delay task (t30 = 2.090, p = 0.04; Fig. 3D). In summary, mice allowed LgA to fentanyl self-administration showed more signs of somatic withdrawal, more hyperalgesia and were more motivated to obtain fentanyl than the ShA group.
Fig. 3. Naloxone-induced signs of withdrawal, hyperalgesia during spontaneous withdrawal, and motivation for fentanyl.
After escalation, male and female C57BL/6J mice were tested in a battery of tests to assess somatic and motivational signs of withdrawal. (A) Naloxone-precipitated withdrawal. Immediately after fentanyl vapor self-administration session 10, all mice received naloxone (1 mg/kg, IP) and were observed for 20 min for signs of withdrawal. The data are expressed as the mean ± SEM and were analyzed using unpaired Student t-test. **p < 0.01, compared with ShA. (B) Mechanical hyperalgesia. Between 36 to 40 h after a self-administration session (i.e., during spontaneous withdrawal), the mice were tested for mechanical hyperalgesia using an electronic von Frey device. The data are expressed as the mean ± SEM and were analyzed using unpaired Student t-test. *p < 0.05, compared with ShA. The dotted line represents the average baseline measure (i.e., before fentanyl exposure) for all mice; both groups developed hyperalgesia compared with the baseline (BL) measure. (C) Progressive ratio test (motivation or”effort”). After escalation, all mice were tested in a progressive-ratio task, in which the number of lever presses that were required for the next fentanyl delivery increased by 6. The data are expressed as the mean ± SEM and were analyzed using unpaired Student’s t-test. *p < 0.05, compared with ShA. (D) Time delay task (motivation). After escalation, all mice were tested in the delayed-reward task, in which the time between lever presses and vapor delivery increased by 6 s. The data are expressed as the mean ± SEM and were analyzed using unpaired Student’s t-test. *p < 0.05, compared with ShA (n = 8/sex/group).
3.5. Self-administration despite punishment
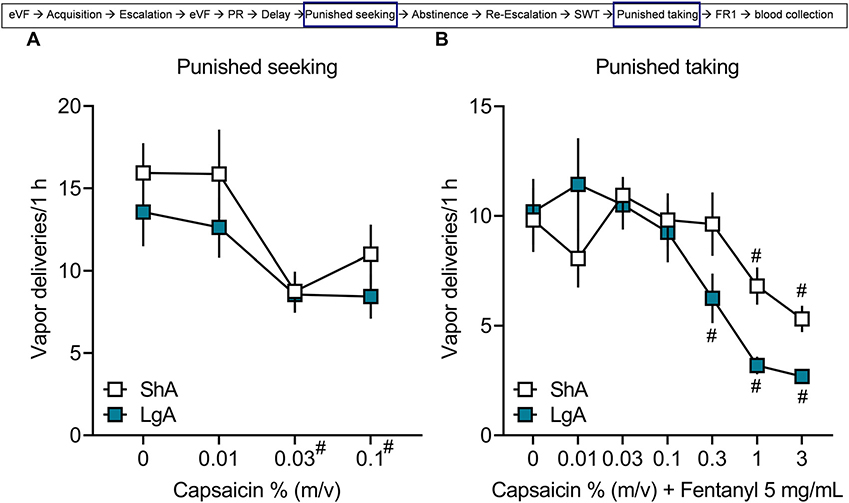
For punished fentanyl seeking, the two-way repeated-measures ANOVA showed no difference between groups (p > 0.05) and no group × capsaicin concentration interaction (p > 0.05) but a significant effect of capsaicin concentration (F3,90 = 12.60, p < 0.0001; Fig. 4A), in which the self-administration of 0.03% and 0.1% capsaicin was lower than vehicle without capsaicin (i.e., 0%). For punished taking, the two-way repeated-measures ANOVA showed a significant group × capsaicin concentration interaction (F6,180 = 3.797, p = 0.0014; Fig. 4B). The post hoc comparisons did not indicate a difference between ShA and LgA groups at any concentration of capsaicin-adulterated fentanyl. However, in the ShA group, responding for 1% capsaicin (p = 0.03) and 3% capsaicin (p = 0.001) was significantly lower than 0% capsaicin. In the LgA group, responding for 0.3% capsaicin (p = 0.006), 1% capsaicin (p < 0.0001), and 3% capsaicin (p < 0.0001) was significantly lower than vehicle. These data indicate that mice in both the ShA and LgA groups reduced their drug seeking and taking in the presence of capsaicin.
Fig. 4. Self-administration despite punishment.
Male and female C57BL/6J mice were tested for vapor self-administration in 1 h-sessions with vehicle or fentanyl that was adulterated with increasing concentrations of capsaicin. (A) Vapor deliveries for each concentration of capsaicin alone in vehicle without fentanyl. The data are expressed as the mean ± SEM and were analyzed using two-way repeated-measures ANOVA. #p < 0.05, compared with 0% regardless of group. (B) Vapor deliveries for each concentration of capsaicin in fentanyl. The data are expressed as the mean ± SEM and were analyzed using two-way repeated-measures ANOVA. #p < 0.05, compared with 0% (n = 8/sex/group).
3.6. Principal component analysis
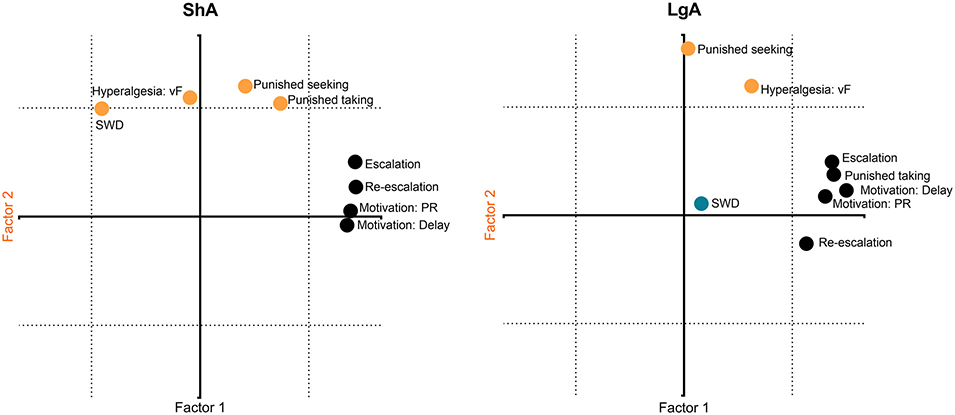
To elaborate multidimensional phenotypes that are associated with LgA to fentanyl compared with ShA, we performed a PCA with all behavioral variables, combining males and females. We used the average of the last three escalation sessions (i.e., sessions that were different from session 1), the average of the 10 re-escalation sessions, the average of 0.03% and 0.1% capsaicin in vehicle without fentanyl, and the average of fentanyl that was adulterated with 0.3%, 1%, and 3% capsaicin. We analyzed the ShA and LgA groups separately. We did not restrict the Varimax-normalized PCA to a pre-specified number of factors. Factor loadings ≥ 0.6 were considered (Fig. 5). For ShA, factor 1 represented 42.26% of total variance of the data and comprised positive associations between fentanyl intake (escalation and re-escalation) and motivation (PR and delay). Factor 2 comprised 22.73% of the data variance and included positive associations between hyperalgesia, capsaicin-punished seeking and taking, and naloxone-precipitated withdrawal. For the LgA group, factor 1 (42.42% of the variance) showed positive associations between fentanyl intake (escalation and re-escalation), motivation (PR and delay), and capsaicin-punished taking. Factor 2 (19.56% of the variance) showed a positive association between hyperalgesia and capsaicin-punished seeking. Factor 3 (14.84% of the variance) comprised naloxone-precipitated withdrawal signs. These data suggest that there are qualitative differences on the behavioral phenotype of mice allowed ShA or LgA to fentanyl. Although PR and delay measures (motivation) were associated with FR1 (intake) in both groups, drug taking despite punishment was associated with FR1 in the LgA group only. Only in the ShA group hyperalgesia and naloxone-induced withdrawal were part of the same behavioral construct.
Fig. 5. Principal component analysis.
The behavioral measures were analyzed separately for each group (ShA and LgA) by a Varimax-normalized PCA. Factor loadings ≥ 0.6 were considered. Black symbols represent all measures that loaded on factor 1 (x-axis). Orange circles represent measures that loaded on factor 2 (y-axis). The blue circle represents the only measure that loaded on factor 3. SWD, precipitated (somatic) withdrawal; PR, progressive ratio; vF, von Frey mechanical nociception (n = 8/sex/group).
3.7. Plasma levels of cytokines and corticosterone
There were no significant group differences in plasma corticosterone levels (p > 0.05), or cytokine and chemokine levels (p > 0.05), including iIL-1β, IL-6, IL-17, CCL-2, CCL-4, and TNF-α (Table 1).
Table 1.
Plasma levels of corticosterone and cytokines.
| ShA | LgA | |
|---|---|---|
|
| ||
| Corticosterone (ng/mL) | 135.7 ± 19.85 | 152.3 ± 21.79 |
| CCL-2 (pg/mL) | 26.67 ± 3.15 | 23.17 ± 1.76 |
| CCL-4 (pg/mL) | 21.49 ± 1.35 | 23.15 ± 1.89 |
| IL-1β (pg/mL) | 1.87 ± 0.73 | 0.83 ± 0.15 |
| IL-6 (pg/mL) | 2.02 ± 0.84 | 1.03 ± 0.25 |
| IL-17 (pg/mL) | 4.14 ± 0.45 | 2.85 ± 0.39 |
| TNF- α (pg/mL) | 1.75 ± 0.10 | 1.49 ± 0.13 |
After the behavioral test battery, the mice underwent an FR1 self-administration session and were euthanized 36 h later (i.e., the time point at which they would have undergone the next self-administration session during spontaneous withdrawal). Trunk blood was collected, and plasma was separated for corticosterone and cytokine analysis. The data are expressed as the mean ± SEM and were analyzed using unpaired Student’s t-test (n = 7–8/sex/group).
3.8. Correlation analysis
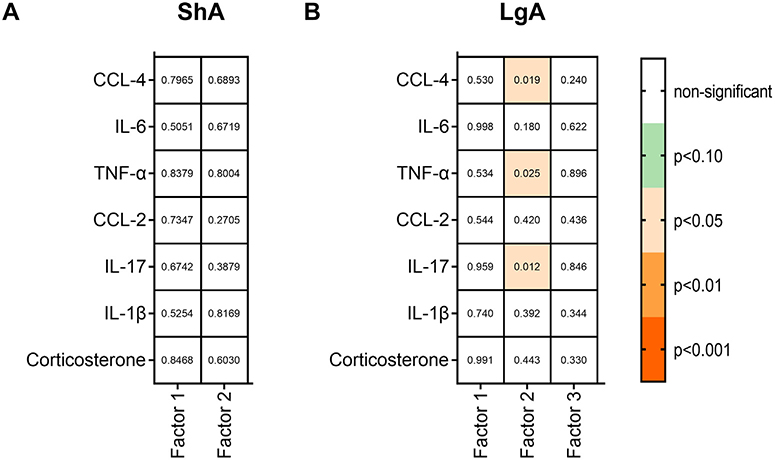
We extracted values of the principal components for each mouse and correlated these values with plasma levels of cytokines/chemokines and corticosterone using Pearson’s correlation (Fig. 6 and Supplemental Table S5). We did not observe any correlations for the ShA group (Fig. 6A). For the LgA group, factor 2 from the PCA showed negative correlations with plasma levels of CCL-4 (r = −0.576, p = 0.019) and TNF-α (r = −0.556, p = 0.025) and a positive correlation with plasma levels of IL-17 (r = 0.611, p = 0.012; Fig. 6B). We then correlated the behavior comprised in factor 2 for the LgA group, hyperalgesia, and capsaicin-punished seeking, with IL-17, CCL-4 and TNF-α. Punished seeking showed an inverse relationship with CCL-4 (r = −0.658, p = 0.006) and TNF-α (r = −0.566, p = 0.022), and positive correlations with hyperalgesia (r = 0.476, p = 0.06) and IL-17 (r = 0.586, p = 0.017).
Fig. 6. Correlation analysis.
Individual factor loadings from the PCA were correlated with plasma levels of cytokines and corticosterone in ShA (A) and LgA (B) groups. Data were analyzed by Pearson’s correlations using two-tailed p values with α= 1%. The p values are represented inside the boxes. Orange squares denote p < 0.05–0.001. Green squares denote p < 0.10. (n = 7–8/sex/group).
4. Discussion
The present study found that (i) the number of fentanyl vapor deliveries positively correlated with blood levels of fentanyl and its metabolite, norfentanyl. (ii) Fentanyl vapor self-administration positively correlated with antinociception. (iii) Mice that were given LgA to fentanyl escalated their intake, exhibited more signs of naloxone-precipitated withdrawal, developed more robust hyperalgesia during spontaneous withdrawal, and were more motivated to obtain fentanyl than mice given ShA to fentanyl (iv) Fentanyl self-administration under the FR1 and PR (both effort and time) schedules comprised the same behavioral construct (“intake/motivation”) in both the ShA and LgA groups. In mice in the LgA group, fentanyl self-administration despite punishment (i.e., punished taking) was associated with the drug”intake/motivation” construct. However, punished seeking and hyperalgesia in mice in the LgA group comprised an independent construct (“hyperalgesia/punished seeking”). Precipitated signs of withdrawal constituted a third, independent factor in the LgA group. (v) Overall, plasma levels of corticosterone, chemokines, and cytokines were not different between the ShA and LgA groups. However, in mice in the LgA group, more responding during punished seeking positively correlated with IL-17 and negatively correlated with CCL-4 and TNF-α.
We found that increasing the number of passive fentanyl vapor deliveries resulted in higher blood levels of fentanyl and norfentanyl. With this noncontingent drug delivery, no conditioned responses were expected to form, and the mice inhaled fentanyl vapor that dispersed throughout the chamber. It is difficult to determine the extent to which this drug concentration relates to drug use in humans, due to, for example, inconsistent purity and cross-contamination with different drugs. Kilmer et al. [37] reported an estimated use of fentanyl at 3–7 mg/day. Applying allometric scaling [38] to the blood fentanyl levels in the mice, the estimated concentrations would range from 0.37 to 2.33 ng/mL, which is within the concentration used to induce analgesia in humans [39]. Although we did not measure drug levels following operant self-administration, we would argue that fentanyl levels are higher during self-administration because the mice likely improve their navigation in the operant chambers and quickly move close to the vapor delivery port for greater inhalation [20]. Fentanyl vapor self-administration caused analgesia, and analgesia positively correlated with the number of fentanyl vapor deliveries. These findings replicate previous studies with vapor-delivered opioids [20,40,41] and validate our experimental conditions, indicating that mice achieved sufficient fentanyl levels during self-administration that caused behavioral effects. We did not investigate the development of tolerance to antinociceptive responses because the LgA group escalated their intake in the first hour of the session which would be a confounding factor for the analysis.
We [18,19,20,35] and others [40–42] have developed opioid vapor self-administration models in rodents. Mice and rats that are allowed extended access to opioids escalate their intake over time and exhibit greater motivation [13,43,14]. In our previous work, LgA mice were given 12 h access to fentanyl [20]. Here, in male and female mice, 6 h access to vaporized fentanyl was sufficient to produce an escalation of intake over time, and these mice exhibited an increase in dependence as defined by naloxone-precipitated opioid withdrawal. We did not observe major sex differences in opioid intake or the rate of escalation (Supplemental Tables S1, S2), which contrasts with findings in mice that self-administered heroin intravenously or oxycodone orally, in which females had higher overall intake [15,44]. One potential explanation for this difference is drug-specific sex differences (e.g., [45]).
Both somatic and motivational withdrawal symptoms are caused by the prolonged use of opioids. The somatic symptoms are generally observed only during acute withdrawal, and are short lasting [46]. In rodent models, both somatic and motivational signs of withdrawal can be precipitated by the administration of μ-opioid receptor antagonists, such as naloxone. Consistent with previous reports [14,18,15,20], we found that mice in the LgA group exhibited more signs of precipitated withdrawal compared with the ShA group (Fig. 3A).
The motivational component of addiction-like behaviors (e.g., increases in drug taking and seeking) can be modeled in rodents with such tasks as progressive-ratio tests [47,45]. We chose two tasks to assess motivation: progressive-ratio schedules [47] in which the number of operant responses (i.e., lever presses) and the time delay between an operant response and reinforcer delivery that were necessary to obtain the next reinforcer increased progressively. Mice in the LgA group exhibited greater motivation to work (Fig. 3C) and wait (Fig. 3D) to obtain the subsequent reinforcer compared with mice in the ShA group.
Another element that contributes to motivational drive in opioid dependence is hyperalgesia (i.e., increase in pain sensitivity/decrease in pain tolerance) during spontaneous withdrawal, an effect that is longlasting and hypothesized to contribute to addiction from the perspective of drug seeking to relieve a negative emotional state [48, 46]. Opioid withdrawal-induced hyperalgesia is observed in humans [49, 4,5] and rodents [50–54]. Here, we observed that both ShA and LgA groups exhibited a reduction of mechanical sensitivity thresholds compared with baseline (i.e., before any opioid exposure), confirming that even low doses of opioids can cause hyperalgesia, likely through a sensitization process [50], yet hyperalgesia was more pronounced in the LgA group.
Another construct that is associated with addiction-like behavior in rodent models is drug seeking and taking despite adverse consequences [55–57]. Commonly used punishers with opioid self-administration include taste adulterants, such as quinine, for oral self-administration [58]; irritants, such as histamine, for intravenous self-administration [59]; and mechanical punishers, such as air puff [60] and foot shock [61,59]. Here, we used the respiratory irritant capsaicin as a punisher for vaporized drug seeking and taking. Capsaicin activates nonselective cation transient receptor potential vanilloid type 1 (TRPV1) channels [62]. Heat and protons can activate TRPV1 channels on their own. Furthermore, TRPV1 channels are upregulated in the sciatic nerve, dorsal root ganglia, spinal cord [63], sensory cortex, and thalamus [64] following chronic morphine treatment. The administration of TRPV1 channel antagonists in the nucleus accumbens significantly reduces morphine self-administration in rats [65]. In the hyperalgesic state, there is a higher extracellular proton concentration and because protons can activate TRPV1, this state potentiates responses to capsaicin, [62], which may have contributed to the apparent higher sensitivity of LgA mice to punished fentanyl taking (see 0.3% on Fig. 4B).
In the punished seeking test, capsaicin at different concentrations was mixed with vapor vehicle (VG/PG), with no fentanyl in the solution. Thus, responding during this test was motivated by conditioned positive and negative reinforcement responses [66]. Both groups equally reduced their drug seeking, measured by vapor deliveries, when the vehicle was adulterated with 0.03% and 0.1% capsaicin (Supplemental Table S3). To model punished taking, we adulterated the fentanyl solution with increasing concentrations of capsaicin so that the motivation to lever press for drug in this test results from both conditioned effects and drug effects. Both groups exhibited a reduction of drug taking at the three highest capsaicin concentrations (0.3%, 1%, and 3%). The strong analgesia and consequent hyperalgesic effects of opioids make it notoriously difficult to employ punishment in opioid self-administration. As such, we found fewer studies in PubMed that employed punishment for opioids (28 studies) vs. cocaine (69 studies) and alcohol (66 studies).
We conducted a PCA of data from the ShA and LgA groups separately to gain a better understanding of interactions among multiple dimensions that are associated with these two drug exposure conditions. Quite similar patterns of behavioral constructs emerged for the two groups. Escalation, re-escalation, and motivation (both effort and delay) loaded onto one factor (“intake/motivation”). Punished taking also loaded on this factor in the LgA group. Hyperalgesia and punished seeking loaded on an independent factor (“hyperalgesia/punished seeking”). Punished taking and naloxone-precipitated withdrawal loaded on this factor in the ShA group. Although punished seeking was not higher in the LgA group compared with the ShA group (Fig. 4, Supplemental Table S4), the data suggest that higher hyperalgesia is associated with higher aversion-resistant drug seeking in both groups. Heightened mechanical sensitivity was associated with higher opioid preference in mice with a history of stress but not in controls [67]. Naloxone-precipitated withdrawal in the LgA group loaded on a third independent factor, suggesting different brain circuitries compared with hyperalgesia in LgA mice [68]. These findings highlight qualitative differences between behavioral phenotypes of mice with different levels of drug access. In animals with ShA to fentanyl, sensitivity to punishment, hyperalgesia and somatic signs of dependence comprised the same behavioral construct, and these variables may engage similar biological mechanisms. In mice allowed LgA to fentanyl, a different behavioral phenotype was observed. Somatic signs of dependence were independent from all behavioral measures. Additionally, in mice in the LgA group, the mechanisms that underlie resistance to punished drug taking, motivation for fentanyl and fentanyl intake likely shared biological mechanisms as well as the mechanism underlying sensitivity to punished drug seeking and hyperalgesia. It is important to note that these are correlative results, and studies will be needed to test their functional relationship.
Given that PCA factors are considered independent from each other, the biological mechanisms that underlie these behavioral constructs are also likely different. Consistent with this hypothesis, punishment insensitivity may constitute a unique phenotype that is independent from reward seeking and Pavlovian fear [69] and separate from motivation for alternative rewards and the drug [70]. Aoun et al. found that”intake/motivation” and “punished drinking/anxiety” emerged as independent constructs in alcohol-dependent and nondependent rats [71]. Rats that were identified to be shock-resistant alcohol drinkers were also more resistant to quinine adulteration, but they were less motivated for alcohol in a progressive-ratio test [72]. These findings suggest that conflict is a component of “aversion resistance.” Aoun et al. [71] used the elevated plus-maze conflict model of anxiety; herein, we presented a conflict between receiving drug/drug-associated cue and punishment. The inhibition of synaptotagmin-2 in the prefrontal cortex increased aversion-resistant alcohol (adulterated with quinine) drinking without changing the FR1 self-administration of non-adulterated alcohol [73], providing support for different molecular mechanisms between intake and aversion-resistant intake. However, motivation, resistance to punishment, and drug seeking were correlated in cocaine self-administering rats [55]. Thus, LgA to drugs to the point of dependence (i.e., the manifestation of withdrawal) in preclinical models can increase various behaviors, such as escalation, motivation, hyperalgesia, and punished responding that comprise different behavioral constructs [13,74,18,20,54,75] that contribute to the overall phenotype of addiction-like behavior and provide insights into biological mechanisms.
Lastly, we searched for correlations between behavioral constructs and blood chemokine/cytokine levels. Overall, we did not observe differences between the ShA and LgA groups in plasma levels of corticosterone and chemokines/cytokines (IL-1β, IL-6, CCL-2, CCL-4, and TNF-α). We are unaware of studies that investigated blood chemokine/cytokine levels in opioid dependence. Mahajan et al. [34] reported downregulated mRNA expression of CCL2 and CCL4 chemokines in human astrocytes following chronic morphine exposure [34], whereas other studies revealed that prolonged administration of oxycodone and withdrawal raised protein levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-17) [32]. These findings corroborate other studies that associated opioid exposure with changes in neuroimmune markers [31,51,76–79] in rodent brain tissue. Opioids may also cause the reactivity of microglia and astrocytes in rodent brain tissue [30] and human brain tissue (e.g., central nucleus of the amygdala; [45]). In the ShA group, we did not find correlations between principal component factor 1 (escalation, re-escalation, PR, delay) and factor 2 (, hyperalgesia, precipitaded withdrawal, punished seeking and punished taking) with corticosterone or cytokines. However, in the LgA group, the “hyperalgesia/punished seeking” factor was associated with CCL-4, TNF-α, and IL-17, all of which are proinflammatory cytokines. This association was driven more by the punished seeking behavior than hyperalgesia. Further analysis confirmed that punished seeking positively correlated with IL-17 and negatively correlated with CCL-4 and TNF-α in the LgA group. These preliminary findings encourage further investigations of chemokines/cytokines as potential biomarkers and/or mediators of opioid dependence in blood and other tissues.
5. Conclusion
Our results suggest that extended fentanyl vapor self-administration leads to the development of addiction-like behaviors. The measurement of several opioid-related behaviors allowed us to identify independent behavioral constructs that may capture different aspects of opioid seeking with extended access. We also provided preliminary evidence of the potential of blood proinflammatory chemokine/cytokine levels to serve as biomarkers of opioid-related behaviors in opioid dependence.
Supplementary Material
Acknowledgements
We thank Dr. Shelley Jackson and Sara Deschaine from the Translational Analytical Core at the National Institute on Drug Abuse Intramural Program for performing the ELISA and ELLA analysis. We thank Michael A. Arends and Dr. Peter Manza for proofreading the manuscript. This study was supported by the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism Intramural Research Programs.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Renata C.N. Marchette: Conceptualization, Methodology, Formal analysis, Data curation, Writing – review & editing. Erika R. Carlson: Methodology, Writing – review & editing. Nadia Said: Writing – review & editing. George F. Koob: Conceptualization, Methodology, Writing – review & editing. Leandro F. Vendruscolo: Conceptualization, Methodology, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.addicn.2022.100057.
Data availability
Data will be made available on request.
References
- [1].American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association Publishing, 2022. [Internet]. DSM-5-TR[cited 2022 Aug 9]. Available from https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425787. [Google Scholar]
- [2].Labor Unooda. World drug report 2021 (SET OF 5 BOOKLETS). S.l.: UNITED NA-TIONS; 2022.
- [3].Koob GF, Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement, Biol. Psychiatry 87 (1) (2020) 44–53 [Internet]Jan [cited 2021 Sep 30]Available from https://linkinghub.elsevier.com/retrieve/pii/S0006322319314350. [DOI] [PubMed] [Google Scholar]
- [4].Carcoba LM, Contreras AE, Cepeda-Benito A, Meagher MW, Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals, J. Addict. Dis 30 (3) (2011) 258–270 [Internet]Jul [cited 2020 Mar 16]Available from http://www.tandfonline.com/doi/abs/10.1080/10550887.2011.581985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Higgins C, Smith BH, Matthews K, Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis, Br. J. Anaesth. 122 (6) (2019) e114–e126 [Internet]Jun [cited 2020 Jun 17]Available from https://linkinghub.elsevier.com/retrieve/pii/S0007091218307633. [DOI] [PubMed] [Google Scholar]
- [6].Blanco C, Volkow ND, Management of opioid use disorder in the USA: present status and future directions, Lancet 393 (10182) (2019) 1760–1772 [Internet]Apr [cited 2022 Apr 13]Available from https://linkinghub.elsevier.com/retrieve/pii/S0140673618330782. [DOI] [PubMed] [Google Scholar]
- [7].Morgan JR, Quinn EK, Chaisson CE, Ciemins E, Stempniewicz N, White LF, et al. , Variation in initiation, engagement, and retention on medications for opioid use disorder based on health insurance plan design, Med. Care 60 (3) (2022) 256–263 [Internet]Mar [cited 2022 Apr 13]Available from https://journals.lww.com/10.1097/MLR.0000000000001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Compton P, Canamar CP, Hillhouse M, Ling W, Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy, J. Pain 13 (4) (2012) 401–409 [Internet]Apr [cited 2022 Aug 9]Available from https://linkinghub.elsevier.com/retrieve/pii/S152659001200017X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmed SH, The science of making drug-addicted animals, Neuroscience 211 (2012) 107–125 [Internet]Jun [cited 2022 Aug 10]Available from https://linkinghub.elsevier.com/retrieve/pii/S0306452211009559. [DOI] [PubMed] [Google Scholar]
- [10].Belin-Rauscent A, Fouyssac M, Bonci A, Belin D, How preclinical models evolved to resemble the diagnostic criteria of drug addiction, Biol Psychiatry 79 (1) (2016) 39–46 [Internet]Jan [cited 2022 Apr 13]Available from https://linkinghub.elsevier.com/retrieve/pii/S0006322315000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grenald SA, Largent-Milnes TM, Vanderah TW, Animal models for opioid addiction drug discovery, Expert Opin. Drug Discov 9 (11) (2014) 1345–1354 [Internet]Nov [cited 2022 Apr 11]Available from http://www.tandfonline.com/doi/full/10.1517/17460441.2014.966076. [DOI] [PubMed] [Google Scholar]
- [12].Kreek MJ, Reed B, Butelman ER, Current status of opioid addiction treatment and related preclinical research, Sci. Adv. 5 (10) (2019) eaax9140 [Internet]Oct 4 [cited 2022 Apr 13]Available from https://www.science.org/doi/10.1126/sciadv.aax9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ahmed S, Persistent increase in the motivation to take heroin in rats with a history of drug escalation, Neuropsychopharmacology 22 (4) (2000) 413–421 [Internet]Apr [cited 2022 Aug 8]Available from http://www.nature.com/doifinder/10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- [14].Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF, Escalation patterns of varying periods of heroin access, Pharmacol. Biochem. Behav. 98 (4) (2011) 570–574 [Internet]Jun [cited 2022 Jul 7]Available from https://linkinghub.elsevier.com/retrieve/pii/S009130571100075X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Towers EB, Tunstall BJ, McCracken ML, Vendruscolo LF, Koob GF, Male and female mice develop escalation of heroin intake and dependence following extended access, Neuropharmacology 151 (2019) 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arena DT, Covington HE, DeBold JF, Miczek KA, Persistent increase of IV cocaine self-administration in a subgroup of C57BL/6J male mice after social defeat stress, Psychopharmacology 236 (7) (2019) 2027–2037 (Berl) [Internet]Jul [cited 2022 Apr 13]Available from http://link.springer.com/10.1007/s00213-019-05191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Y, Svenningsson P, Picetti R, Schlussman SD, Nairn AC, Ho A, et al. , Cocaine self-administration in mice is inversely related to phosphorylation at Thr34 (protein kinase A site) and Ser130 (kinase CK1 site) of DARPP-32, J. Neurosci. Off. J. Soc. Neurosci. 26 (10) (2006) 2645–2651 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vendruscolo JCM, Tunstall BJ, Carmack SA, Schmeichel BE, Lowery-Gionta EG, Cole M, et al. , Compulsive-like sufentanil vapor self-administration in rats, Neuropsychopharmacology 43 (4) (2018) 801–809 [Internet]Mar [cited 2020 Jun 30]Available from http://www.nature.com/articles/npp2017172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McConnell SA, Brandner AJ, Blank BA, Kearns DN, Koob GF, Vendruscolo LF, et al. , Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration, Neuropharmacology 182 (2021) 108355 [Internet]Jan [cited 2022 Apr 13]Available from https://linkinghub.elsevier.com/retrieve/pii/S0028390820304238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moussawi K, Ortiz MM, Gantz SC, Tunstall BJ, Marchette RCN, Bonci A, et al. , Fentanyl vapor self-administration model in mice to study opioid addiction, Sci. Adv. 6 (32) (2020) eabc0413 [Internet]Aug [cited 2021 Sep 30]Available from https://advances.sciencemag.org/lookup/doi/10.1126/sciadv.abc0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McLellan AT, Koob GF, Volkow ND, Preaddiction-a missing concept for treating substance use disorders, JAMA Psychiatry 79 (8) (2022) 749 [Internet]Aug 1 [cited 2022 Oct 27]Available from https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2793694. [DOI] [PubMed] [Google Scholar]
- [22].el-Guebaly N, Mudry T, Zohar J, Tavares H, Potenza MN, Compulsive features in behavioural addictions: the case of pathological gambling: compulsivity in addictions, Addiction 107 (10) (2012) 1726–1734 [Internet]Oct [cited 2022 Aug 17]Available from https://onlinelibrary.wiley.com/doi/10.1111/j.1360-0443.2011.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Robbins T, Curran H, de Wit H, Special issue on impulsivity and compulsivity, Psychopharmacology 219 (2) (2012) 251–252 (Berl) [Internet]Jan [cited 2022 Aug 17]Available from http://link.springer.com/10.1007/s00213-011-2584-x. [DOI] [PubMed] [Google Scholar]
- [24].Moore CF, Sabino V, Koob GF, Cottone P, Dissecting compulsive eating behavior into three elements, in: Compulsive Eating Behavior and Food Addiction, Elsevier, 2019, pp. 41–81. https://linkinghub.elsevier.com/retrieve/pii/B9780128162071000032. [Internet][cited 2022 Aug 17]Available from. [Google Scholar]
- [25].Hofford RS, Russo SJ, Kiraly DD, Neuroimmune mechanisms of psychostimulant and opioid use disorders, Eur. J. Neurosci. 50 (3) (2019) 2562–2573 [Internet]Aug [cited 2022 Aug 18]Available from https://onlinelibrary.wiley.com/doi/10.1111/ejn.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Felger JC, Miller AH, Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise, Front. Neuroendocrinol. 33 (3) (2012) 315–327 [Internet]Aug [cited 2022 Aug 18]Available from https://linkinghub.elsevier.com/retrieve/pii/S0091302212000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. , Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression, Mol. Psychiatry 21 (10) (2016) 1358–1365 [Internet]Oct [cited 2022 Aug 18]Available from http://www.nature.com/articles/mp2015168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Felger JC, Treadway MT, Inflammation effects on motivation and motor activity: role of dopamine, Neuropsychopharmacology 42 (1) (2017) 216–241 [Internet]Jan [cited 2022 Aug 18]Available from https://www.nature.com/articles/npp2016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cui C, Shurtleff D, Harris RA, Neuroimmune mechanisms of alcohol and drug addiction, Int. Rev. Neurobiol (2014) 1–12 [Internet]. Elsevier; [cited 2022 May 4]Available from https://linkinghub.elsevier.com/retrieve/pii/B9780128012840000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR, Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia, Pharmacol. Rev 63 (3) (2011) 772–810 [Internet]Sep [cited 2022 Aug 9]Available from http://pharmrev.aspetjournals.org/lookup/doi/10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roeckel LA, Le Coz GM, Gavériaux-Ruff C, F Simonin, Opioid-induced hyperalgesia: cellular and molecular mechanisms, Neuroscience 338 (2016) 160–182 [Internet]Dec [cited 2022 Aug 9]Available from https://linkinghub.elsevier.com/retrieve/pii/S0306452216302597. [DOI] [PubMed] [Google Scholar]
- [32].Kumar M, Rainville JR, Williams K, Lile JA, Hodes GE, Vassoler FM, et al. , Sexually dimorphic neuroimmune response to chronic opioid treatment and withdrawal, Neuropharmacology 186 (2021) 108469 [Internet]Mar [cited 2022 Oct 12]Available from https://linkinghub.elsevier.com/retrieve/pii/S002839082100023X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rock RB, Hu S, Sheng WS, Peterson PK, Morphine stimulates CCL2 production by human neurons, J. Neuroinflamm 3 (1) (2006) 32 [Internet][cited 2022 Oct 12]Available from http://jneuroinflammation.biomedcentral.com/articles/10.1186/1742-2094-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mahajan S, Schwartz S, Aalinkeel R, Chawda R, Sykes D, Nair M, Morphine modulates chemokine gene regulation in normal human astrocytes, Clin. Immunol. 115 (3) (2005) 323–332 [Internet]Jun [cited 2022 Oct 12]Available from https://linkinghub.elsevier.com/retrieve/pii/S1521661605000380. [DOI] [PubMed] [Google Scholar]
- [35].Marchette R, Tunstall B, Vendruscolo L, Moussawi K, Operant vapor self-administration in mice, Bio Protoc 11 (10) (2021) [Internet][cited 2021 Sep 30]Available from https://bio-protocol.org/e4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maldonado R, Negus S, Koob GF, Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists, Neuropharmacology 31 (12) (1992) 1231–1241 [Internet]Dec [cited 2020 May 22]Available from https://linkinghub.elsevier.com/retrieve/pii/002839089290051P. [DOI] [PubMed] [Google Scholar]
- [37].Kilmer B, Pardo B, Caulkins JP, Reuter P, How much illegally manufactured fentanyl could the U.S. be consuming? Am. J. Drug Alcohol Abuse 48 (4) (2022) 397–402 Jul 4. [DOI] [PubMed] [Google Scholar]
- [38].Nair A, Jacob S, A simple practice guide for dose conversion between animals and human, J. Basic Clin. Pharm 7 (2) (2016) 27 [Internet][cited 2022 Oct 16]Available from http://www.jbclinpharm.org/text.asp?2016/7/2/27/177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Peng PWH, Sandler AN, A review of the use of fentanyl analgesia in the management of acute pain in adults, Anesthesiology 90 (2) (1999) 576–599 [Internet]Feb 1 [cited 2022 Oct 6]Available from, doi: 10.1097/00000542-199902000-00034. [DOI] [PubMed] [Google Scholar]
- [40].Gutierrez A, Nguyen JD, Creehan KM, Taffe MA, Female rats self-administer heroin by vapor inhalation, Pharmacol. Biochem. Behav. 199 (2020) 173061 [Internet]Dec [cited 2022 Apr 27]Available from https://linkinghub.elsevier.com/retrieve/pii/S0091305720305943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gutierrez A, Creehan KM, Taffe MA, A vapor exposure method for delivering heroin alters nociception, body temperature and spontaneous activity in female and male rats, J. Neurosci. Methods 348 (2021) 108993 [Internet]Jan [cited 2022 Apr 27]Available from https://linkinghub.elsevier.com/retrieve/pii/S0165027020304167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shelton KL, Nicholson KL, Reinforcing effects of fentanyl and sufentanil aerosol puffs in rats, Psychopharmacology (2022) (Berl) [Internet]Apr 15 [cited 2022 Apr 27]; Available from https://link.springer.com/10.1007/s00213-022-06129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, Compulsive-like responding for opioid analgesics in rats with extended access, Neuropsychopharmacology 40 (2) (2015) 421–428 [Internet]Jan [cited 2022 Jul 7]Available from http://www.nature.com/articles/npp2014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, et al. , Oral prescription opioid-seeking behavior in male and female mice, Addict. Biol 25 (6) (2020) e12828 Nov. [DOI] [PubMed] [Google Scholar]
- [45].Carmack SA, Vendruscolo JCM, Adrienne McGinn M, Miranda-Barrientos J, Repunte-Canonigo V, Bosse GD, et al. , Corticosteroid sensitization drives opioid addiction, Mol. Psychiatry 27 (5) (2022) 2492–2501 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koob GF, Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development, Pharmacol. Rev 73 (1) (2021) 163–201 [Internet]Jan [cited 2022 Aug 9]Available from http://pharmrev.aspetjournals.org/lookup/doi/10.1124/pharmrev.120.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hodos W, Progressive ratio as a measure of reward strength, Science 134 (3483) (1961) 943–944 Sep 29. [DOI] [PubMed] [Google Scholar]
- [48].Shurman J, Koob GF, Gutstein HB, Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain, Pain Med. 11 (7) (2010) 1092–1098 [Internet]Jul [cited 2022 Aug 1]Available from https://academic.oup.com/painmedicine/article-lookup/doi/10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ren ZY, Shi J, Epstein DH, Wang J, Lu L, Abnormal pain response in painsensitive opiate addicts after prolonged abstinence predicts increased drug craving, Psychopharmacology 204 (3) (2009) 423–429 (Berl) [Internet]Jun [cited 2020 Mar 16]Available from http://link.springer.com/10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Célèrier E, Laulin JP, Corcuff JB, Le Moal M, G Simonnet, Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process, J. Neurosci. Off. J. Soc. Neurosci. 21 (11) (2001) 4074–4080 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. , Evidence that opioids may have toll-like receptor 4 and MD-2 effects, Brain Behav. Immun 24 (1) (2010) 83–95 [Internet]Jan 1 [cited 2020 Mar 16]Available from http://www.sciencedirect.com/science/article/pii/S0889159109003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF, Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia, Addict. Biol 20 (2) (2015) 275–284 [Internet]Mar 1 [cited 2020 Apr 15]Available from https://onlinelibrary.wiley.com/doi/full/10.1111/adb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Roeckel LA, Utard V, Reiss D, Mouheiche J, Maurin H, Robé A, et al. , Morphineinduced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide, Sci. Rep. 7 (1) (2017) 1–15 [Internet]Sep 4 [cited 2020 Mar 16]Available from https://www.nature.com/articles/s41598-017-11120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Marchette RCN, Gregory-Flores A, Tunstall BJ, Carlson ER, Jackson SN, Sulima A, et al. , κ-opioid receptor antagonism reverses heroin withdrawal-induced hyperalgesia in male and female rats, Neurobiol. Stress 14 (2021) 100325 [Internet]May [cited 2022 Aug 9]Available from https://linkinghub.elsevier.com/retrieve/pii/S2352289521000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Deroche-Gamonet V, Belin D, Piazza PV, Evidence for addiction-like behavior in the rat, Science 305 (5686) (2004) 1014–1017 [Internet]Aug 13 [cited 2022 Aug 9]Available from https://www.science.org/doi/10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- [56].Vanderschuren LJMJ, Everitt BJ, Drug seeking becomes compulsive after prolonged cocaine self-administration, Science 305 (5686) (2004) 1017–1019 [Internet]Aug 13 [cited 2022 Aug 8]Available from https://www.science.org/doi/10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- [57].Hopf FW, Lesscher HMB, Rodent models for compulsive alcohol intake, Alcohol 48 (3) (2014) 253–264 Fayettev NMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Monroe SC, Radke AK, Aversion-resistant fentanyl self-administration in mice, Psychopharmacology 238 (3) (2021) 699–710 (Berl) [Internet]Mar [cited 2022 Jun 16]Available from http://link.springer.com/10.1007/s00213-020-05722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Honeycutt SC, Paladino MS, Camadine RD, Mukherjee A, Loney GC, Acute nicotine treatment enhances compulsive-like remifentanil self-administration that persists despite contextual punishment, Addict. Biol 27 (3) (2022) [Internet]May [cited 2022 Jun 16]Available from https://onlinelibrary.wiley.com/doi/10.1111/adb.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Skupio U, Sikora M, Korostynski M, Wawrzczak-Bargiela A, Piechota M, Ficek J, et al. , Behavioral and transcriptional patterns of protracted opioid self-administration in mice: patterns of opioid addiction, Addict. Biol 22 (6) (2017) 1802–1816 [Internet]Nov [cited 2022 Jun 16]Available from https://onlinelibrary.wiley.com/doi/10.1111/adb.12449. [DOI] [PubMed] [Google Scholar]
- [61].Blackwood CA, McCoy MT, Ladenheim B, Cadet JL, Escalated oxycodone self-administration and punishment: differential expression of opioid receptors and immediate early genes in the rat dorsal striatum and prefrontal cortex, Front. Neurosci. 13 (2020) 1392 [Internet]Jan 9 [cited 2022 Jun 16]Available from https://www.frontiersin.org/article/10.3389/fnins.2019.01392/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. , The cloned capsaicin receptor integrates multiple pain-producing stimuli, Neuron 21 (3) (1998) 531–543 [Internet]Sep [cited 2022 Jun 16]Available from https://linkinghub.elsevier.com/retrieve/pii/S0896627300805644. [DOI] [PubMed] [Google Scholar]
- [63].Chen Y, Geis C, Sommer C, Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway, J. Neurosci. 28 (22) (2008) 5836–5845 [Internet]May 28 [cited 2022 Jun 16]Available from https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nguyen TL, Nam YS, Lee SY, Jang CG, Repeated morphine administration increases TRPV1 mRNA expression and autoradiographic binding at supraspinal sites in the pain pathway, Biomol. Ther (2022) [Internet]May 26 [cited 2022 Jun 16]; Available from http://www.biomolther.org/journal/view.html?doi=10.4062/biomolther.2022.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ma SX, Kim HC, Lee SY, Jang CG, TRPV1 modulates morphine self-administration via activation of the CaMKII-CREB pathway in the nucleus accumbens, Neurochem. Int. 121 (2018) 1–7 [Internet]Dec [cited 2022 Jun 16]Available from https://linkinghub.elsevier.com/retrieve/pii/S0197018618303772. [DOI] [PubMed] [Google Scholar]
- [66].Pantazis CB, Gonzalez LA, Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF, Cues conditioned to withdrawal and negative reinforcement: neglected but key motivational elements driving opioid addiction, Sci. Adv. 7 (15) (2021) eabf0364 [Internet]Apr 9 [cited 2022 Aug 5]Available from https://www.science.org/doi/10.1126/sciadv.abf0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].O’Brien C, Vemireddy R, Mohammed U, Barker DJ, Stress reveals a specific behavioral phenotype for opioid abuse susceptibility, J. Exp. Anal. Behav 117 (3) (2022) 518–531 [Internet]May [cited 2022 Aug 8]Available from https://onlinelibrary.wiley.com/doi/10.1002/jeab.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alvarez-Bagnarol Y, Marchette RCN, Francis C, Morales M, Vendruscolo LF, Neuronal correlates of hyperalgesia and somatic signs of heroin withdrawal in male and female mice, eNeuro 9 (4) (2022) [Internet]Jul [cited 2022 Jul 7]ENEURO.0106-22.2022. Available from https://www.eneuro.org/lookup/doi/10.1523/ENEURO.0106-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jean-Richard-dit-Bressel P, Ma C, Bradfield LA, Killcross S, McNally GP, Punishment insensitivity emerges from impaired contingency detection, not aversion insensitivity or reward dominance, eLife 8 (2019) e52765 [Internet]Nov 26 [cited 2022 Jul 20]Available from https://elifesciences.org/articles/52765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, et al. , Chronic EtOH effects on putative measures of compulsive behavior in mice: etOH and compulsive behavior, Addict. Biol 22 (2) (2017) 423–434 [Internet]Mar [cited 2022 Aug 10]Available from https://onlinelibrary.wiley.com/doi/10.1111/adb.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Aoun EG, Jimenez VA, Vendruscolo LF, Walter NAR, Barbier E, Ferrulli A, et al. , A relationship between the aldosterone–mineralocorticoid receptor pathway and alcohol drinking: preliminary translational findings across rats, monkeys and humans, Mol. Psychiatry 23 (6) (2018) 1466–1473 [Internet]Jun [cited 2022 Aug 9]Available from http://www.nature.com/articles/mp201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Domi E, Xu L, Toivainen S, Nordeman A, Gobbo F, Venniro M, et al. , A neural substrate of compulsive alcohol use, Sci. Adv. 7 (34) (2021) eabg9045 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, et al. , DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity, J. Neurosci. 35 (15) (2015) 6153–6164 [Internet]Apr 15 [cited 2022 Aug 9]Available from https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.4571-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, et al. , Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats, J. Neurosci. 32 (22) (2012) 7563–7571 [Internet]May 30 [cited 2022 Aug 9]Available from https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].George O, Ahmed SH, Gilpin NW, Are we compulsively chasing rainbows? Neuropsychopharmacology (2022) [Internet]Aug 18 [cited 2022 Aug 22]; Available from https://www.nature.com/articles/s41386-022-01419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Carranza-Aguilar CJ, Hernández-Mendoza A, Mejias-Aponte C, Rice KC, Morales M, González-Espinosa C, et al. , Morphine and fentanyl repeated administration induces different levels of NLRP3-dependent pyroptosis in the dorsal raphe nucleus of male rats via cell-specific activation of TLR4 and opioid receptors, Cell. Mol. Neurobiol. 42 (3) (2022) 677–694 [Internet]Apr [cited 2022 Aug 9]Available from https://link.springer.com/10.1007/s10571-020-00957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT, et al. , Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats, Brain Behav. Immun 87 (2020) 725–738 [Internet]Jul [cited 2022 Aug 9]Available from https://linkinghub.elsevier.com/retrieve/pii/S0889159119314333. [DOI] [PubMed] [Google Scholar]
- [78].Cisneros IE, Cunningham KA, Self-administered fentanyl profoundly impacts rat brain innate immune targets, Neuropsychopharmacology 46 (1) (2021) 247 [Internet]Jan [cited 2022 Aug 18];247Available from https://www.nature.com/articles/s41386-020-00853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ahearn OC, Watson MN, Rawls SM, Chemokines, cytokines and substance use disorders, Drug Alcohol Depend. 220 (2021) 108511 [Internet]Mar [cited 2022 Aug 18]Available from https://linkinghub.elsevier.com/retrieve/pii/S0376871621000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.