Abstract
Engineering studies of Candida (Pseudozyma) antarctica lipase A (CalA) have demonstrated the potential of this enzyme in the selective hydrolysis of fatty acid esters of different chain lengths. CalA has been shown to bind substrates preferentially through an acyl-chain binding tunnel accessed via the hydrolytic active site; it has also been shown that selectivity for substrates of longer or shorter chain length can be tuned, for instance by modulating steric hindrance within the tunnel. Here we demonstrate that, whereas the tunnel region is certainly of paramount importance for substrate recognition, residues in distal regions of the enzyme can also modulate substrate selectivity. To this end, we investigate variants that carry one or more substitutions within the substrate tunnel as well as in distal regions. Combining experimental determination of the substrate selectivity using natural and synthetic substrates with computational characterization of protein dynamics and of tunnels, we deconvolute the effect of key substitutions and demonstrate that epistatic interactions contribute to procuring selectivity toward either long-chain or short/medium-chain fatty acid esters. We demonstrate that various mechanisms contribute to the diverse selectivity profiles, ranging from reshaping tunnel morphology and tunnel stabilization to obstructing the main substrate-binding tunnel, highlighting the dynamic nature of the substrate-binding region. This work provides important insights into the versatility of this robust lipase toward diverse applications.
Enzyme engineering has become a powerful tool to endow enzymes with desired catalytic features while shedding light on the properties and mode of action of the enzyme itself.1−3 In particular, it can help to uncover regions or residues that procure the greatest impact on enzymatic function, providing knowledge to direct further engineering experiments in an iterative loop.4−6 Indeed, integral properties such as protein dynamics, stability, allostery, or epistasis, each of which underlies catalytic efficacy, are often revealed through the process of in vitro evolution.5,7−14 In-depth characterization of those properties in engineered enzyme variants and application of that knowledge to guide further cycles of improvement is the key to effective modulation or creation of catalytic features.15−21
Lipases (EC 3.1.1.3) are among the most heavily used enzymes for industrial biocatalysis, particularly in the detergents, food and beverage, agrochemical, flavor, and pharmaceutical industries.22−26 In addition to hydrolyzing glycerides to fatty acids and glycerol, they can be applied to achieve esterification and transesterification, aminolysis, alcoholysis, and acidolysis, depending on the reaction conditions. Contrary to the highly engineered and most widely used hydrolase in biocatalysis, Candida (Pseudozyma) antarctica lipase B (CalB, UniProtKB P41365),27,28Candida (Pseudozyma) antarctica lipase A (CalA, UniProtKB W3VKA4) is surprisingly understudied.29−31 CalA shows great potential for industrial applications in the food, energy, and pharmaceutical sectors as a result of its high solvent and thermal (up to 100 °C) tolerance,29 substrate and activity promiscuity, preference for hydrolysis of triglycerides at the sn2 position and trans fatty acid selectivity.30−32
CalA shows a preference in the hydrolysis of short-to-medium-chain fatty acid esters (where we refer to as short < C6, medium C6–C10, and long > C10).32,33 This is not intuitive, since it harbors an acyl-chain binding tunnel that can accommodate up to 25 methylene units.34 Its crystal structure was resolved in 2008,34 yet few directed evolution experiments have been performed on CalA to date.30,31,35−37 Moreover, its low sequence identity to other known lipases prevents making inferences about its conformational dynamics, activation, or substrate binding, justifying deeper investigation into the effects of mutations.38
Chain-length selectivity is highly sought in the food industry to enhance the composition of specific fatty acids that offer health benefits, flavor, or texture.39−41 Selectivity for fatty acid esters shorter than C8 has been achieved in several lipases including CalA by steric blockage of the acyl-chain binding tunnel, thereby precluding binding of long chains.42−44 Achieving selectivity toward fatty acid esters greater than six carbons in length has proven to be more challenging, since activity for shorter chain lengths tends to be preserved.37,45 Nevertheless, it has been proposed that selectivity toward long-chain fatty acid esters can be increased in certain lipases by widening the entrance to the acyl-chain binding tunnel.45,46 Further mechanisms of relevance reported in other lipases to achieve short or long chain-length selectivity include conformational changes and lid modifications.47−50 Distal amino acid substitutions can lead to nonadditive (epistatic) interactions that are not easily predictable, whether beneficial or deleterious to the overall enzymatic activity.51−53 To our knowledge, there is a single report of an epistatic interaction in CalA between two substitutions at the extremity of the acyl-chain binding tunnel, conferring pNO2-phenyl-C22:1/pNO2-phenyl C18:1 selectivity.13 This highlights the potential for engineering substrate selectivity in the CalA enzyme.
In this work, we investigate the properties of CalA variants exhibiting widely differing chain-length selectivity. Some are derived from a thorough enzyme engineering campaign previously led on CalA. In that study, we generated region-focused libraries of CalA to identify variants with enhanced chain-length selectivity.36,37 This confirmed the importance of the acyl-chain binding tunnel in substrate recognition54 and uncovered CalA variants showing significantly improved short- or long-chain selectivity.36,37
Here we study chain-length selective variants of CalA to identify factors contributing to the observed phenotypes. In most cases, substitutions in the acyl-chain binding tunnel were accompanied by distal substitutions.37 To shed light on whether these substitutions contributed to the observed phenotype, a deconvolution campaign was carried forward. Epistatic interactions were identified by means of deconvolution of multiply substituted variants. Chain-length selectivity was determined in vivo and in vitro in crude lysate of E. coli using natural triglyceride substrates and synthetic, activated pNO2-phenyl esters of varying chain lengths. Although this approach does not address factors such as variant stability, expression level, selectivity for the sn2 position of triglycerides, and kinetic parameters (which can all be affected by the introduction of substitutions) it provides an effective means to compare hydrolysis of various substrates. Simulations of the substrate-binding tunnel identified potential alternatives for binding, revealing morphological features that appear to be common for the short- and long-chain variants analyzed, thus expanding our knowledge of the factors affecting chain-length selectivity in CalA.
Materials and Methods
Deconvolution of the Multiply Substituted CalA Variants
The WT CalA construct used as a template for mutagenesis was previously reported.36 Briefly, it was codon-optimized for expression in E. coli. The pelB signal peptide was N-terminally fused for periplasmic export, and a poly hexahistidine tag was C-terminally fused. The construct was cloned into plasmid pD441. CalA deconvoluted variants were created through whole-plasmid site-directed mutagenesis55 using primers (Alpha DNA) designed according to the QuikChange Lightning kit (Table S1). Mutations were introduced either by using the QuikChange Lightning kit (Agilent) or Phusion polymerase (Thermo Fisher) according to the manufacturer’s guidelines using ∼100 ng of pD441-pelB-CalA template in a total volume of 50 μL. Adding a final concentration of 3% DMSO improved the reaction efficiency for mutations introduced in high GC-rich regions. After completion, the products were digested with DpnI (NEB) for 1 h at 37 °C, and 5 μL was transformed into chemically competent E. coli DH5α prepared following the CaCl2 protocol.56 The transformed cells were plated onto Luria–Bertani agar plates containing 50 μg/mL kanamycin as a selective marker. Plasmid DNA from colonies obtained after overnight incubation at 37 °C was isolated using the Monarch Plasmid miniprep kit (NEB) and sequenced at the Genome Quebec platform (Sainte-Justine Hospital, Montreal) using primers pFWD (5′-TTACGAGCTTCATGCACAG-3′) and pRVS (5′-TGGTAGTGTGGGGACTC-3′). Confirmed sequences were then transformed into electrocompetent E. coli BL21 (DE3), and the sequence was verified once more.
Protein Expression and Cell Lysis
E. coli BL21(DE3) cell cultures transformed with the relevant CalA genes were grown to saturation at 37 °C in Luria–Bertani media supplemented with 50 μg/mL kanamycin. For quantitative activity assays using pNO2-phenyl esters, 150 μL of saturated cultures was used to inoculate 15 mL of ZYP-5052 of autoinducing medium57 with kanamycin and grown at 30 °C. After 24 h, cultures were pelleted and resuspended in 1 mL of 50 mM Tris-HCl pH 8.0 as previously described.37 Following 3 × 30 s sonication pulses until clear, the lysate was centrifuged, and the supernatant assayed. Protein quantification of protein lysates was done using the Bradford Protein Assay Kit (Bio-Rad).
For all variants related to LS_66, expression and lysis have been performed as described above, except that pellets were resuspended with 5 mL of 50 mM Tris-HCl pH 8.0 and that only 1 mL of the resuspended cells was kept for sonication. This is due to the fact that some deconvoluted variants were highly active; the lysate needed to be diluted further to measure activity in the desired range.
Lipase Activity Assays
A. Semiquantitative Halo-Formation Assay for Hydrolysis of Triglycerides on Agar Plates
The semiquantitative plate assays containing emulsions of glyceryl tributyrate (ACROS organics), glyceryl trioctanoate (Sigma), or olive oil (Filippo Berio; C18, one unsaturation:1) were as previously described with a few modifications.37 Briefly, the appropriate triglyceride was emulsified with autoinducing medium containing 3% agar and 50 μg/mL kanamycin57 to obtain a final concentration of 1% v/v triglyceride and cast into rectangular Nunc OmniTray dishes (Thermo Fisher). Glyceryl tributyrate and octanoate-containing plates are opaque, and lipase activity is detected as a halo of clearance around the colony. Rhodamine B (0.001% w/v, Sigma) was included in olive oil plates to detect lipase activity as a halo of fluorescence around the colony. E. coli precultures with a final concentration of 50 μg/mL kanamycin were prepared for all variants. Precultures were spotted in triplicates onto the plates. E. coli expressing cTEM-19m ß-lactamase and type B dihydrofolate reductase DfrB1 (UniProtKB P00383) were used as negative controls for lipase activity. Following 16–18 h incubation at 30 °C and 48 h incubation at 4 °C, images of the plates were obtained with an iBright FL1500 device for subsequent analysis with the image processing software Fiji. Either the area of the halo of clearance (glyceryl tributyrate/trioctanoate) or the fluorescence signal (olive oil) were measured using ImageJ.58 In both cases, colony size was assessed to normalize the halo of clearance or the fluorescence to colony growth. For each CalA variant, the halo area or the fluorescence signal was normalized to values obtained with WT CalA on the same plate. Subsequently, normalized values were divided by calculated colony size to obtain the ratio of activity to colony size. Ratios are given as the average of at least triplicates.
B. Quantitative Assay for Hydrolysis of Single-Chain Esters in Solution
The quantitative assay using pNO2-phenyl fatty acid esters with chain lengths C4:0 to C16:0 was done as previously described.37 Briefly, 20 μL of E. coli lysate was assayed in a total reaction volume of 200 μL containing 0.556 mM pNO2-phenyl fatty acid ester, 9% isopropanol, and 0.9% Triton in 50 mM Tris-HCl pH 8.0. Lipase activity was monitored at 45°C as the increase in absorbance of pNO2-phenolate at 405 nm (e405 = 18.1 mM–1 cm–1) upon its release from the corresponding ester (4-nitrophenyl butyrate, octanote, dodecanoate, and palmitate from Sigma, 4-nitrophenyl decanoate from Biosynth). The assay was performed at least in triplicate in 96-well plates using a BMG FLUOstar 96-well plate spectrophotometer.
The specific activity (S.A.) was calculated according to the following formula:
where Δ450 is the linear increase in absorption at 405 nm in min–1, l is the length of the optical path (cm), εpNO2phenolate corresponds to 18.1 mM–1 cm–1, Vt is the total volume in each well in mL, Vl is the volume of lysate in each well (mL), and Cl is the concentration of lysate (mg/mL).
The S.A. (U/mg) for WT CalA ranges from 0.49 to 0.56 for pNO2-phenylbutyrate, 0.66 to 0.74 for pNO2-phenyloctanoate, 0.59 to 0.78 for pNO2-phenyldecanoate, 0.51 to 0.63 for pNO2-phenyldodecanoate, and 0.40 to 0.52 for pNO2-phenylpalmitate. The WT CalA used in Figure 8 was diluted along with all variants related to LS_66 due to high activity of some of them. Its S.A. was of 0.31 for pNO2-phenylbutyrate, 0.30–0.31 for pNO2-phenyloctanoate, 0.39–0.41 for pNO2-phenyldecanoate, 0.35–0.40 for pNO2-phenyldodecanoate, and 0.31–0.36 for pNO2-phenylpalmitate.
Figure 8.

Hydrolytic activity of CalA variant LS_66 and its deconvoluted variants. Top panel: In vitro assays using pNO2-phenyl esters. Bottom panel: In vivo assays with triglycerides. Assays were performed in triplicate with clarified E. coli lysates. Activity for each variant is reported relative to hydrolysis of the same substrate with WT CalA. Specific activities for this series of assays are reported in Figure S13.
Dynamic Protein Tunnel Calculations
The conformational space of CalA (PDB ID: 2VEO) and its chain-length selective variants was sampled using the Protein Local Motion script on the Protein Energy Landscape Exploration (PELE) server (https://pele.bsc.es/)59 at the Barcelona Supercomputing Center. This script performs backbone perturbations in several Monte Carlo steps coupled to large normal-mode analysis predicted by an anisotropic network model of backbone perturbations, side-chain optimizations, and a final constrained minimization. This approach allows to sample large conformational motions that occur at the time scale of ligand binding with a lower computational cost than MD methods.60,61
CalA variants were generated in silico using PyMOL (Schrodinger, LLC. 2010. The PyMOL Molecular Graphics System, Version 2.5) from the WT CalA crystal structure (PDB ID: 2VEO). Prior to conformational sampling, all structures were prepared in Maestro (Schrödinger, LLC, 2021, Maestro, Schrödinger release 2019-4) using Schrödinger’s Protein Preparation Wizard (version 12.2.012), eliminating solvent molecules and the cocrystallized PEG-4 molecule. The resulting protein motions were analyzed with VMD 1.4.62 RMSF calculations were performed using a script on the Tk console of VMD 1.4. The first 150 frames of all simulations were eliminated to account for equilibration of the system.
The PELE Protein Local Motion script output was used as input for dynamic tunnel calculations using CAVER 3.0 command-line version.63 The catalytic nucleophile residue Ser184 was defined as the starting point residue for tunnel calculations, with a probe radius of 0.6 Å. Results were analyzed in PyMOL (Schrödinger, LLC. 2010. The PyMOL Molecular Graphics System, Version 2.5).
Docking
The different variants subjected to docking were generated in silico in PyMOL using the crystal structure 2VEO corresponding to wild-type CalA. The structures were subsequently prepared in Maestro as previously described. The prepared molecules were subjected to accurate docking using the online serve SwissDock (http://www.swissdock.ch/docking). The coordinates chosen for the accurate docking correspond to Ser184. Flexibility of the side-chains was allowed within 5 Å of any atom of the ligand. Once a docking pose was identified, the protein with the ligand was subjected to binding refinement to obtain docking poses where the ester bond was <3.5 Å from the catalytic Ser184. Binding refinement was done using the online server for the Protein Energy 152 Landscape Exploration algorithm (PELE) (https://pele.bsc.es/). PELE allows for sampling of the conformational space by allowing protein and ligand perturbations.59 Analysis of the obtained poses was done using PyMOL and Maestro.
Results and Discussion
Chain-length selectivity can be determined in vivo or in vitro using lipase substrates of different chain lengths such as natural triglycerides or synthetic substrates bearing an activated ester group. Triglyceride substrates are bulkier and can be used for a semiquantitative, rapid, in vivo determination of natural activity by observing halo formation on solid medium containing the natural substrates.36,37 Alternatively, change in pH resulting from hydrolysis of the triglyceride can be determined in lysates using a phenol red assay or a pH stat.64−67 In contrast, the spectrophotometric quantification of hydrolysis of single-chain, pNO2-phenyl esters allows for more accurate screening; pNO2-phenyl esters are routinely used as standards in industrial settings for the determination of lipase activity, despite those non-natural esters being activated and less bulky than natural triglycerides.68 It is important to note that with both assays (in vivo and with lysates in vitro), one cannot distinguish whether the absolute change in the level of enzymatic activity observed is due to higher activity of the variant or a different expression level. Nonetheless, because the same lysate is used to determine the variant’s selectivity on different substrates, the chain-length profile is expected to be independent of the expression level.
Although it has a long acyl-chain binding tunnel,34 the WT CalA lipase shows similar, albeit slightly higher, activity toward short- to medium-chain fatty acid esters.32 In one study, CalA displayed approximately 70% lower activity toward olive oil (C18:1) than glyceryl tributyrate (C4:0).33 Here, we verified the selectivity of WT CalA with respect to acyl-chain length by determining its specific activity for in vitro hydrolysis of C4–C16 pNO2-phenyl esters. Under these assay conditions, reactivity for the C16 ester was approximately 80% of that of the C4 ester; highest activity was with C8 and C10 esters, at approximately 130% of the C4 ester and 160% of the C16 ester (Figure S1).
Panel of CalA Variants That Procure Chain-Length Selectivity
In a prior study, we generated and recombined region-focused libraries of CalA to identify variants with enhanced chain-length selectivity while allowing for potential epistasis between multiple substitutions.36,37 This semirational approach to directed evolution uncovered several CalA variants showing improved selectivity for either short- or long-chain substrates.36,37 Our results confirmed the existence of a key region for substrate recognition within the acyl-chain binding tunnel54 while uncovering further residues that may contribute to defining chain-length selectivity. To gain insight into the mechanisms that determine chain-length selectivity in CalA, we focus on variants that confer either of the two distinct, selective phenotypes for further investigation (Figure 1). We name short/medium-chain selective variants “SS” (short-chain selective) for simplicity, and long chain-selective variants are named “LS”.
Figure 1.
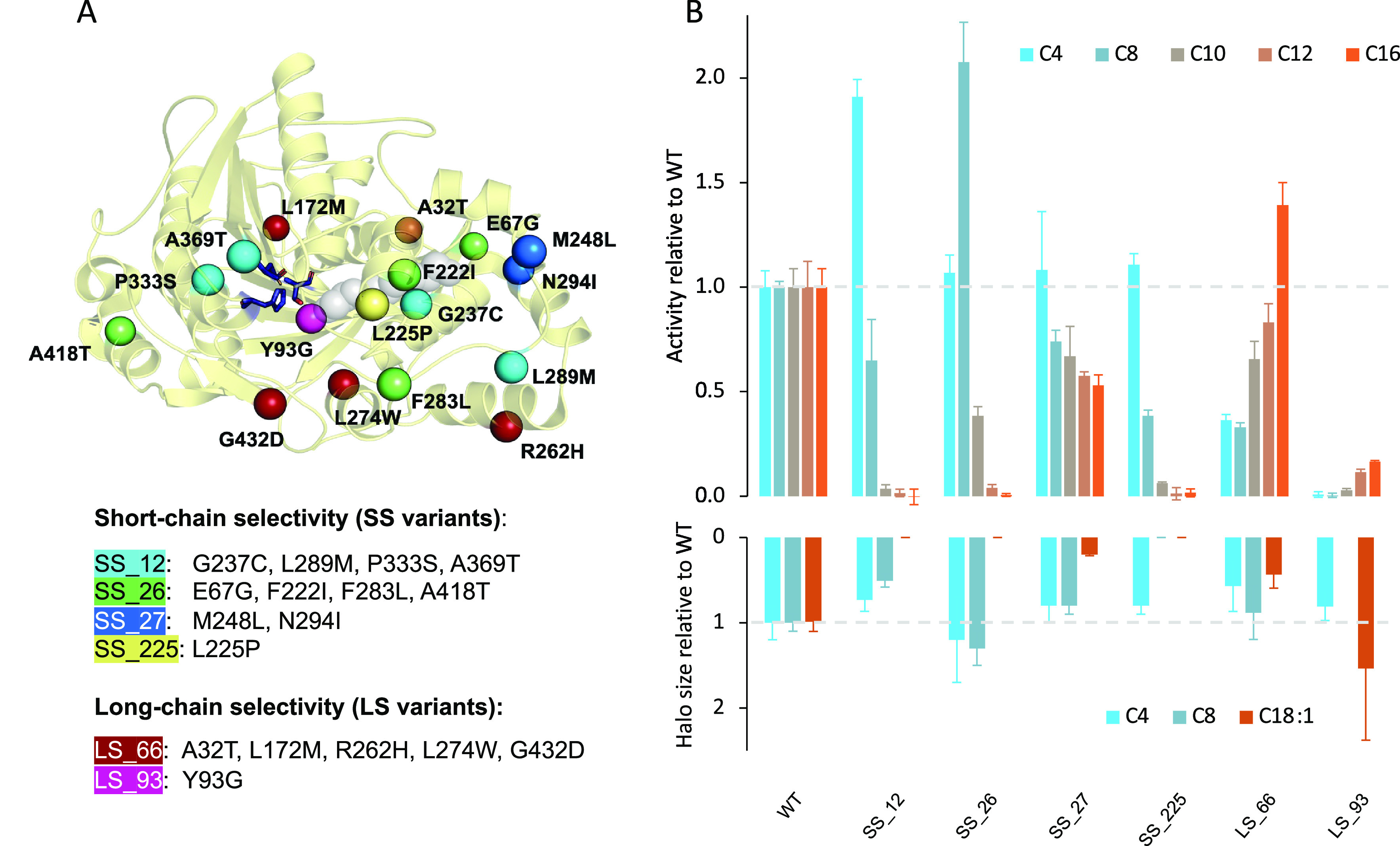
Short-chain selective and long-chain selective variants of CalA. (A) CalA structure (PDB ID: 2VEO) with the catalytic triad in purple sticks and key substitutions represented as spheres: SS_12 (cyan), SS_26 (green), SS_27 (blue), SS_225 (yellow), LS_66 (red), and LS_93 (magenta). A PEG4 molecule cocrystallized inside the acyl-chain binding tunnel is represented in light gray spheres. (B) Hydrolytic activity of chain-length selective variants. Top panel: In vitro assays using pNO2-phenyl esters. Bottom panel: In vivo assays with triglycerides. Assays were performed in triplicate. Activity for each variant is reported relative to hydrolysis of the same substrate with WT CalA. Hydrolysis of the C8 triglyceride is not available for LS_93. Specific activities for this series of assays are reported in Figure S2.
To establish a robust point of comparison for the panel of chain-length selective CalA variants, activity was quantified both in vitro on activated pNO2-phenyl esters of varying length (C4 to C16, saturated) and in vivo with the triglycerides tributyrin (C4), trioctanoin (C8), and olive oil (C18:1). Despite the different nature of single-chain pNO2-phenyl esters and triglycerides, we had previously observed a generally good correspondence in the selectivity of CalA variants determined with the two types of esters.36,37
Short-chain selectivity had previously been achieved in the literature by individual or combined substitutions at residues T221, G237, V238, and V290 that prevented binding of long fatty acid esters in the acyl-chain binding tunnel by hindering insertion into said tunnel.13,44,69Variant SS_12 contains a substitution in one among those previously described positions (G237C) in combination with further substitutions (Figure 1A).44 Here we verified that this variant exhibits high short-chain selectivity for single-chain pNO2-phenyl esters with a nearly 2-fold increase in activity for pNO2-phenyl butyrate relative to WT (Figure 1B, top panel; specific activity is reported in Figure S2), reflected also in its preference for tributyrin (Figure 1B, bottom panel), whereas hydrolysis with pNO2-phenyl dodecanoate and palmitate (C12 and C16) and olive oil (C18:1) was essentially undetectable.
The short-chain selective SS_225 variant carries a single substitution, L225P, in the middle portion of the acyl-chain binding tunnel (Figure 1A). This single substitution suffices to confer excellent selectivity, with a pattern similar to SS_12 though without the increase in activity toward the single-chain pNO2-phenyl butyrate. Variant L225V, at the same position, has been shown to provide enantioselectivity adjacent to the ester carbonyl of bulky esters, confirming the importance of this position in substrate selectivity.35 Interestingly, the short-chain selectivity of variant SS_26 differs from SS_12 and SS_225 in that hydrolysis of pNO2-phenyl octanoate was improved 2-fold relative to WT, with retention of WT-like activity for both tributyrin and trioctanoin (Figure 1B); here hydrolysis of pNO2-phenyl palmitate and of olive oil was essentially undetectable. Though none of the four modified residues of SS_26 has previously been reported, we note that the substitution T221F and T221W, previously reported in the context of short-chain selectivity,69 is the immediate neighbor of the F222I substitution of variant SS_26, in the middle portion of the acyl-chain binding tunnel.
In contrast, the final short-chain selective variant we retained for study, SS_27, displayed modest selectivity (Figure 1B). The selectivity may result from a different mechanism than the three former variants, as its two substitutions are at the bottom of the acyl-chain binding tunnel instead of in the middle portion (Figure 1A). Two further variants, the point-substituent G240C and double-substituted V321A/S377R, identified in the past as being weakly selective for short-chain triglyceride substrates,37 were also assayed but showed negligible or no activity with pNO2-phenyl ester substrates under the conditions used here (not shown); they were not further investigated.
The mechanism for long-chain selectivity is more elusive, since long-chain selective variants tend to retain some reactivity toward tributyrin and pNO2-phenylbutyrate.37 In previous work, we reported for the first time CalA variant LS_93, which carries the single substitution Y93G, for its long-chain selectivity.36,37 Here, we see that hydrolysis of pNO2-phenyl esters shorter than C12 was essentially abolished, leaving a modest reactivity for pNO2-phenyl dodecanoate and palmitate (Figure 1B, upper panel). However, the selectivity for triglycerides was less pronounced (Figure 1B, lower panel). Y93G occupies a key position at the entrance of the acyl-chain binding tunnel just below the catalytic triad (Figure 1A) and forms part of the oxyanion hole.38Variant LS_66 is equally remarkable: although it retained weak activity for short-chain pNO2-phenyl esters, it displayed increased activity toward substrates longer than 12 carbon atoms (140% activity relative to WT toward pNO2-phenylpalmitate; Figure 1B). Once again, the selectivity for triglycerides was less pronounced. LS_66 carries substitution L274W near the active site as well as G432D (Figure 1A). Residue 432 belongs to CalA “flap”, a short C-terminal segment composed of residues Gly426 to Gly436 that regulates access to CalA active-site cavity;34 its substitution modifies the flap and could modulate substrate recognition.
Tunnel Morphology Analysis
Individual substitution of several tunnel-lining residues has been shown to sterically block the main tunnel, modulating chain-length selectivity.44,70 Here, in silico tunnel prediction was performed with all the variants to gain insight into the short- and long-chain selectivity that was observed experimentally with respect to hydrolysis of single-chain pNO2-phenyl esters and of triglycerides. This assumes that relevant conformational changes occur prior to ligand binding. Indeed, conformational selection is the most widely accepted binding model for several enzymes, including lipases.4,71,72 Conformational sampling of the variants by normal-mode simulations coupled to side-chain optimization was generated with the Protein Local Motion script using the Protein Energy Landscape Exploration (PELE) approach.59 The PELE algorithm performs the local perturbation of the protein structure to describe the global motion of the protein, optimization of side-chains using a library of rotamers, and energy minimization of the structure.59 The three steps constitute one move, which is either accepted or rejected by the method; this process is repeated iteratively. Contrary to MD simulations, this method can, at low computational cost, capture processes that occur on the millisecond time-scale,60,61 describing conformational sampling at a time-scale relevant to ligand binding and catalysis.73−75
Snapshots from the simulations served as input for tunnel prediction with CAVER 3.0, a software that uses a spherical probe to identify tunnels in proteins.63 This revealed common features as well as specificities that distinguish short-chain and long-chain selective variants. CAVER predicted a variety of tunnels which differed to various degrees in all variants both for shape and priority (Table 1 and Figure S3). Unless otherwise specified, we focus on the area where tunnel prediction by CAVER 3.0 has the highest priority in the WT CalA, which corresponds to the region of the protein where a PEG4 molecule was cocrystallized in previous work by others (Figure S4A).34 We refer to this as the “the tunnel”, which in WT is constituted by a “main portion”, and three branches (Figure 2).
Table 1. Properties of the Most Probable Substrate-Binding Tunnel Predicted in WT CalA and CalA Variants.
| Variant | Snapshots/totala (%) | Lengthb (Å) | Priorityc | Curvatured | Average bottleneck radiuse (Å) |
|---|---|---|---|---|---|
| WT | 88 | 25.6 ± 3.6 | 0.15 | 1.6 ± 0.2 | 0.7 ± 0.07 |
| SS_12 | 97 | 32.8 ± 1.7 | 0.14 | 1.7 ± 0.1 | 0.8 ± 0.08 |
| SS_27 | 100 | 28.3 ± 2.8 | 0.15 | 1.6 ± 0.2 | 0.7 ± 0.05 |
| SS_225 | 14 | 25.1 ± 0.8 | 0.014 | 1.8 ± 0.1 | 0.66 ± 0.04 |
| LS_15f | 97 | 24.8 ± 4.6 | 0.18 | 1.6 ± 0.3 | 0.7 ± 0.1 |
| LS_93 | 94 | 24.1 ± 4.4 | 0.23 | 1.6 ± 0.3 | 0.8 ± 0.1 |
Snapshots that include the main substrate-binding tunnel defined for WT CalA, divided by the total number of snapshots analyzed.
Average of the shortest distance between the start and end point of each tunnel in the cluster, as per the CAVER3.0 manual.
Tunnel probability ranking for a given tunnel cluster calculated by averaging the sum of tunnels predicted in that cluster throughout all snapshots for that variant, as per the CAVER3.0 manual. The higher the priority, the higher the probability of observing that tunnel.
Ratio between tunnel length and distance between the start and end point of the tunnel.
The maximal probe size that can fit in the narrowest segment of the tunnel, averaged over the snapshots predicting that tunnel.
LS_15 stands in for LS_66; it has substitutions R262H/L274W/G432D which have the greatest impact on phenotype (vide infra).
Figure 2.
Predicted substrate-binding tunnels in WT CalA and chain-length selective variants. Top panel: Tunnels were predicted using CAVER 3.0 on poses generated by a normal-mode simulation. The acyl-chain binding tunnel of WT CalA and its three predominant branches (numbered 1–3 for WT CalA) are represented in gray and serve as a reference in all panels. Each line represents a tunnel predicted in one of the poses, and branches are numbered according to similarity to those in the WT CalA; the sum of all predicted tunnels in this region defines the cluster, the properties of which are displayed in Table 1. The mouth and main portion of the tunnel (left-hand portion in all panels, with the catalytic Ser184 as a reference) and branches (right-hand portion of the panels) are colored as Figure 1 (SS_12: cyan; SS_27: blue; SS_225: yellow; LS_15: red; LS_93: magenta). No tunnel was predicted for SS_26. LS_15 stands in for LS_66, as it has substitutions R262H/L274W/G432D which have the greatest impact on phenotype (vide infra).
In WT CalA and in all variants but one (for SS_26), the presence of the tunnel was predicted, as expected (Figure 2) and with similar priority to the WT CalA, except for SS_225 (Table 1); the main portion of the tunnel, which is readily identifiable by visual inspection, holds the ∼14 Å long PEG4 entirely buried inside it (Figure S4A).34 Its mouth is near the catalytic triad such that the ester bond of the fatty acid ester substrate is correctly positioned for hydrolysis, while the aliphatic portion slips into the tunnel (Figure S4). In the variants, this main portion of the tunnel is shifted to various degrees and does not completely overlap with that of the WT. Docking pNO2-phenyloctanoate in WT CalA allows to estimate that an acyl chain of 12 carbons or less can be accommodated in said portion (Figure S4B).
In WT CalA, the main portion of the tunnel then splits into the three distal branches (Figure 2) by bifurcating at ∼12 Å from the tunnel mouth (Figure S4B). The region of the protein where the distal branches were simulated constitutes the largest of two highly dynamic regions of CalA (Figures S5 and S6). Interestingly, the variants are predicted to differ somewhat in branch bifurcation (Figure 2). This suggests that alteration in the tunnel-branching region is modulated by the amino acid substitutions of the variants, coherent with its highly dynamic nature providing plasticity to the region. Visual inspection of the tunnel morphology reveals variations in branch bifurcation and likelihood of each predicted branch, where a compact overlay of a greater number of predicted paths corresponds to a higher likelihood of a branch being populated.
Short-chain selective variant SS_26 is readily distinguished from all others as no tunnel is present: the F222I substitution occludes tunnel formation. Even upon repeating the tunnel simulation using a smaller probe diameter (a sphere of 0.55 Å instead of 0.6 Å), no tunnel was observed (hence, no tunnel for this variant shown in Figure 2).
Though all clearly forming the main portion of the tunnel, visual inspection shows that none among the three remaining short-chain selective variants (SS_12, SS_27, and SS_225) explored all three tunnel branches predicted in the WT CalA. This observation corroborates the finding that a change in amino acid can result in a change in tunnel formation and shape. The new branching may contribute to explain the short-chain selective phenotypes of these variants. Namely, it appears that the substitutions in SS_12 and SS_225 alter the local protein morphology, creating alternate, less favored branches that disfavor binding of acyl chains longer than C8. In fact, variants SS_12 and SS_225 maintain only one of the three branches (2a and 1 respectively), illustrating the magnitude of perturbation in the branching region (Figure 2). It should be noted that this has no direct implication in the different rates of hydrolysis of variants SS_12 and SS_225 toward C4 or C8 substrates, as these substrates are too short to access said branches (Figure S4). Nonetheless, changes in the branching region might contribute to the phenotype of variant SS_27, where substrates longer than C8, while disfavored relative to WT, can still bind. Consistent with this hypothesis, the branch exploration of SS_27 is most similar to WT CalA with SS_27 displaying only weak short-chain selectivity.
The substitutions in the short-chain selective variants are, thus, predicted here to have modified the favored path of fatty acid ester accommodation. Shortening the tunnel cavity of CalA has been reported to increase short-chain selectivity.44,70 Here, the main portion of the tunnel was occluded in short-chain selective variant SS_26, but for SS_12, SS_225, and SS_27, there is otherwise no visible correlation between the calculated average length of the newly predicted tunnel (including main portion and new branches) and chain-length selectivity (Table 1). Variant SS_225 is the only short-chain selective variant showing a substantial decrease in priority (10-fold lower, Table 1 and Figure S3). This correlates with a lower probability of finding said tunnel, with alternative tunnels more favored in this case (Figure S3). Short-chain selectivity could be also linked to changes in the morphology of other tunnels that acquired increased priority due to the substitutions (Figure S3). Indeed, “alternative” binding tunnels have been reported in CalA.44 Although the CAVER results provide insightful information, to attempt to conclude on the relevance of the many potential binding tunnels toward selectivity would be speculative.
The long-chain selective variants analyzed in this study showed instead a significantly higher tunnel priority than WT CalA (0.18 and 0.23 respectively compared to 0.15 for the WT) (Table 1). For those variants, the branching region is always observed, providing a favorable environment for the binding of long-chained substrates. The long-chain selective variants explored ramifications that correspond to branches 1 and 2 of WT CalA, yet did not significantly explore branch 3 or the neighboring region (Figure 2). This suggests that maintenance of integrity of tunnel-branches 1 and 2 favors maintenance of long-chain binding. Interestingly, SS_12 displayed branch 2a, which is visibly shifted relative to branch 2 in WT CalA and the long-chain selective variants, suggesting that 2a is not favorable to long-chain binding. We note that these observations do not address the relative reduction in reactivity toward short-chain substrates. Here, variant LS_15 was analyzed as a simplified proxy for LS_66: both include substitutions R262H/L274W/G432D, which have the greatest impact on phenotype (described below); the additional A32T/L172 M substitutions of LS_66 affected activity but not selectivity.
The enlargement of the tunnel cavity has been proposed to increase long-chain selectivity in other lipases.45,46 Consistent with those findings, an increase in the bottleneck radius of the highest priority tunnel in variant LS_93 (0.8 Å) was predicted, relative to WT CalA (0.7 Å) (Table 1), such that this may be a contributing factor to its long-chain selectivity. However, no such increased bottleneck was seen in LS_15. This should be interpreted with caution since bottleneck radii of other variants are similar, including the increased radius in short-chain selective variant SS_12 (0.8 Å). This suggests that a combination of factors contributes to increasing the tunnel priority and selectively favoring hydrolysis of long-chain substrates in these variants.
Analysis of the crystal structure of CalA reported that the acyl-chain binding tunnel of WT CalA curves toward the bottom.34 Average curvatures of the predicted substrate-binding tunnels did not significantly differ in WT CalA and the selective variants, suggesting that tunnel curvature is probably not a major determinant of chain-length selectivity (Table 1).
It should be highlighted that, for these variants, the sole analysis of tunnel parameters such as length or bottleneck radius could have led to erroneous interpretation of phenotype.
Overall, this analysis performed in the absence of substrate shows evidence of a causal relationship between amino acid substitution and alteration of tunnel morphology. Furthermore, the combined analysis of tunnel parameters and shape shows a plausible correlation between tunnel-branch exploration, tunnel priority, and chain-length phenotypes, not necessarily linked only to steric hindrance generated by amino acid substitutions. These results point to a variety of complex mechanisms determining chain-length selectivity in the tunnel region of the CalA lipase.
Deconvolution of Multisubstituted Chain-Length Selective CalA Variants
As the majority of the chain-length selective variants includes several substitutions (Figure 1A), deconvolution of the substitutions was undertaken to reveal the key determinants of selectivity, including any epistatic effects. The activity of all deconvoluted variants was quantified both in vitro on pNO2-phenyl esters of varying length (C4 to C16, saturated) and in vivo with triglycerides against tributyrin (C4), trioctanoin (C8), and olive oil (C18, one unsaturation). Despite the chemical and steric differences between those two classes of substrates, a remarkable concordance between the two assays was found, unless otherwise specified.
Short-Chain Selective Variant SS_12: Steric Hindrance in the Main Tunnel Is Not the Only Factor Influencing Selectivity
Variant SS_12 contains substitutions G237C (in the main tunnel region), L289 M (on an external loop belonging to the dynamic tunnel-branching region), P333S, and A369T (flanking the active site) (Figure 1A). Displaying a remarkable ability to hydrolyze exclusively substrates with a chain length ≤ C8, the concordance between hydrolysis of single-chain pNO2-esters and triglycerides was clear (Figure 1B). It was previously observed that substitution G237C alone confers the short-chain selectivity.37,44 Here, the addition of substitutions L289M, P333S, and A369T maintained selectivity and nearly doubled activity toward C4 substrates (191%) relative to the WT CalA.
Deconvolution of variant SS_12 clearly demonstrates that the G237C substitution is its unique source of discriminative capacity: short-chain discrimination always accompanied inclusion of G237C and was not seen in its absence (Figure 3). Importantly, each of the three further point substitutions of SS_12 significantly reduced catalytic activity, particularly with respect to triglyceride substrates. Each of those three residues is too far from any atom of the substrate to exert direct influence on catalysis. Even substitutions P333S and A369T that are in the active-site region are at least 10 Å away from the substrate (Figure S7). The addition of G237C to any of the three substitutions partly recovered enzymatic activity. Finally, only by the combination of all four substitutions did variant SS_12 acquire higher activity for hydrolysis of pNO2-phenylbutyrate. These results clearly indicate that epistatic effects define the highly active and selective phenotype of variant SS_12. We note that the trait of increased absolute activity toward pNO2-phenylbutyrate can result from a variety of factors that were not investigated here, including stabilization of the variant, increased expression, and/or effects on kcat and/or KM.
Figure 3.
Hydrolytic activity of CalA variant SS_12 and its deconvoluted variants. The position of the substitutions is shown in cyan spheres with the catalytic triad in sticks and the PEG4 cocrystallized inside the acyl-chain binding tunnel in light gray spheres (PDB ID: 2VEO). Top panel: In vitro assays using pNO2-phenyl esters. Bottom panel: In vivo assays with triglycerides. Assays were performed in triplicate with clarified E. coli lysates. Activity for each variant is reported relative to hydrolysis of the same substrate with WT CalA. Specific activities for this series of assays are reported in Figure S8C.
The impact of position 237 on selectivity was previously studied by Bornscheuer and colleagues and further investigated by our group.37,44 Interestingly, saturation mutagenesis demonstrated that any amino acid substitution of G237, while maintaining the enzyme active, conferred short-chain selectivity in all cases.37 The activity toward pNO2-phenylbutyrate was at least 80% of the WT, except for Arg (<10%), Glu, Cys, and Trp (approximately 50%), and essentially inactive for pNO2-phenyldecanoate or longer substrates.37 Considering that G237 is located in the middle of the main substrate tunnel, it was inferred that substituting the glycine causes steric hindrance of the tunnel, preventing entrance of acyl chains longer than C8 (Figure S4B).
To gain more insight into this hypothesis, we modeled tunnel conformations for a diverse panel of G237 variants: Ala, Cys, Asp, Glu, Gln, Arg, Ser, and Trp (Figure 4). For variants G237W and G237E, tunnel simulation predicts clear and complete steric hindrance and tunnel collapse (not illustrated, since no tunnel was modeled). The remaining variants showed a predicted substrate-binding tunnel. Visual inspection shows that the branching region differs from that of WT CalA. Furthermore, the path of the main tunnel has deviated from that seen in WT CalA and is accompanied by significantly lower tunnel priority, except for G237S (Figure 4; Table S2); this suggests that the main tunnel path in those G237X variants might be disfavored and that the predicted path, in turn, disfavors fatty acid binding greater than C8. Decreased branching is also observed for most variants, which is likely to affect the binding of long-chain substrates: however, it should not directly affect binding of C8 substrates or shorter, which occurs before tunnel branching (Figure S4B). Variant G237S differs: its tunnel is predicted with high priority but is the shortest predicted and shows a wider bottleneck (Table S2). Nonetheless, it results in a similar phenotype.37 These results illustrate that while steric hindrance can play a role in disfavoring long-chain fatty acid ester binding when G237 is substituted, it is not the root cause for the determination of all short-chain selective phenotypes, with alteration of the priority and path of the main tunnel appearing to be contributors to short-chain selectivity. Again, it is plausible that increased priority of alternative tunnels might be linked with the acquired short-chain selective phenotype (Figure S3); for instance, accommodation of the substrate in variants G237W and G237E, which completely lost the main tunnel, requires an alternative binding site.
Figure 4.
Predicted substrate-binding tunnels in selected G237 variants. The acyl-chain binding tunnels of WT CalA are represented in black and serve as a reference in all panels. Each line represents a tunnel predicted in one of the poses. Position 237 is represented as a green sphere. The three main tunnel branches of WT CalA are numbered in the top left panel.
Short- To Medium-Chain Selective Variant SS_26: Epistasis between a Tunnel Residue and Distal Substitutions
Variant SS_26 displayed a clear preference for hydrolysis of C4 and C8 substrates, both when tested with pNO2-phenyl esters and with triglycerides. Interestingly, the hydrolysis of pNO2-phenylbutyrate was comparable to WT yet the hydrolysis of pNO2-phenyloctanoate was increased nearly 2-fold (specific activity of SS_26 = 1.2 U/mg compared to 0.66 U/mg for WT; Figure S8A). This variant contains substitution F222I in the middle region of the main tunnel (positioned ∼4 Å from the last carbon of the docked pNO2-phenyloctanoate, Figure S4B). Deconvolution showed that F222I is the main contributor to this selectivity (Figure 5). In addition to our previous observation of a link to short-chain selectivity,37 substitutions at position F222 were previously reported to provide selectivity for trans fatty acids over cis fatty acids.54
Figure 5.
Hydrolytic activity of CalA variant SS_26 and its deconvoluted variants. The position of the substitutions is shown in green spheres with the catalytic triad in sticks and the PEG4 cocrystallized inside the acyl-chain binding tunnel in light gray spheres (PDB ID: 2VEO). Top panel: In vitro assays using pNO2-phenyl esters. Bottom panel: In vivo assays with triglycerides. Assays were performed in triplicate with clarified E. coli lysates. Activity for each variant is reported relative to hydrolysis of the same substrate with WT CalA. Specific activities for this series of assays are reported in Figure S8A.
The three remaining substitutions of variant SS_26 are E67G, F283L, and A418T. The three are distal to the active site and to the tunnel region, each in a different area of the protein. Residue 67 is the farthest from the catalytic Ser184 (Cα distance = 27.4 Å) and is located in a domain that does not participate in the tunnel region. Substitution E67G produced a moderate reduction of activity with all substrates and offered slight selectivity only toward the shortest triglyceride (tributyrin). E67G provided only an increase of activity toward pNO2-phenyl octanoate when combined with the other substitutions in SS_26 (Figure 5); we therefore focused on the remaining substitutions.
In contrast, the individual substitutions F283L (Cα distance from S184 = 20.4 Å; below the tunnel, to the right of the active site, Figure 1A) and A418T (Cα distance from S184 = 21.7 Å; to the left of the active site, distal from the tunnel) had a stronger effect, reducing hydrolysis of all pNO2-phenyl ester substrates and abolishing hydrolysis of triglycerides; their combination had a similar effect (Figure 5). However, the addition of substitution F222I to F283L and/or A418T maintained the selectivity profile produced by F222I as well as a similar level of activity, where the F222I/A418T combination most favored the hydrolysis of pNO2-phenyloctanoate.
Interestingly, the F222I/F283L combination produced a successful double variant for selective hydrolysis of short- to medium-chain triglycerides, with essentially no activity toward olive oil (Figure 5). Yet, only by combining all four substitutions, forming SS_26, was the large increase in hydrolysis of pNO2-phenyloctanoate observed (Figures 5 and S8); in that case, the preference for C8 was observed for the single-chain ester substrates but not for triglycerides.
Intriguingly, tunnel simulations of variant F222I with CAVER show that substitution of the aromatic, bulky Phe with the smaller Ile resulted in the closure, or “collapse”, of the tunnel (not shown, as no tunnel is detected). Instead of abolishing the enzyme activity, this substitution unlocks short-chain selectivity, as in previous variants G237W/E, probably by favoring the formation of alternative tunnels with higher priority (Figure S3). We hypothesized that F222 contributes to tunnel formation by π-stacking with a nearby aromatic residue to maintain an open tunnel; its substitution could collapse the midsection of the tunnel eventually preventing tunnel formation. Among the three Phe residues identified within a 7 Å radius of F222, F287 is the best oriented for π-stacking with F222 (Figure 6A). We verified whether substituting F287 to Leu would disrupt the hypothetical π-stacking with F222, thereby collapsing the tunnel and unlocking a short-chain selective phenotype; instead, the F287L variant was nearly inactive, even toward short-chain substrates (Figure 6B). However, through CAVER analysis, we did not predict a tunnel collapse for mutation F287L (Figure S9), as had been predicted for F222I.
Figure 6.
Hydrolytic activity of CalA variants with potential for π-stacking with F222. (A) Aromatic residues within 7 Å of tunnel residue F222. (B) Top panel: In vitro assays using pNO2-phenyl esters. Bottom panel: In vivo assays with triglycerides. Assays were performed in triplicate with clarified E. coli lysates. Activity for each variant is reported relative to hydrolysis of the same substrate with WT CalA. Specific activities for this series of assays are reported in Figure S11.
Interestingly, variant F233I/F287L had been identified as showing weak short-chain selectivity with the triglyceride assay in our previous work,37 as confirmed here (Figure 6B, lower panel). Here, we produced and assayed the singly substituted F233I: it was nearly inactive toward all substrates tested (Figure 5). Therefore, the combination of the two essentially inactivating substitutions F233I and F287L displays clear epistasis, “rescuing” both and resulting in a moderately active phenotype for pNO2-phenyl substrates of all chain lengths (without discrimination) and selectively hydrolyzing tributyrin among the three triglycerides tested. In WT CalA, the aromatic ring of F233 is not positioned near F222 or F287; therefore, its effect in modulating activity and selectivity upon substitution does not involve its π-stacking with these residues. Its substitution to the smaller Ile is likely to alter the backbone of the α-helix where it is located, which lies proximal to F222 and F287, modifying local interactions.
Overall, there is no clear evidence that F222 contributes to tunnel formation by means of π-stacking, yet its role is intricately held in balance by neighboring residues. We note that variant F233I/F287L provides a clear example of different patterns of chain-length preference for bulky triglycerides and single-chain, activated pNO2-phenyl esters.
Short-Chain Selective Variant SS_27: The Distal Contribution of Previously Unreported Tunnel Substitutions
Although variant SS_27 showed only moderate selectivity for short-chain substrates, its particular interest lies in the fact that its phenotype results from two substitutions that lie deep in the tunnel-branching region (Figures 1 and S10) and have not previously reported for chain-length selectivity. As opposed to short-chain selective variants SS_12 and SS_26 where long-range epistasis was observed implicating residues located outside of the tunnel region, here two neighboring residues buried deep in the dynamic, tunnel-branching region modulate binding to disfavor long-chain substrates.
The deconvolution of SS_27 again revealed clear epistatic effects. Variant M248L had virtually no effect on activity toward any substrate, whereas N294I reduced activity to roughly 50% for all substrates; a slight tendency toward short-chain selectivity was suggested with both substrate classes (Figures 7 and S8). Their combination produced a WT-like activity toward pNO2-phenylbutyrate and 2-fold decrease toward pNO2-phenylpalmitate; a difference of about 4-fold was observed in relative rates of hydrolysis of tributyrin and olive oil. These two substitutions disrupt binding of longer chain substrates. Interestingly, they are in the neighboring region of two previously reported substitutions at residues V290 and V286 at the bottom of the tunnel (Figure S10), which also decrease hydrolysis of fatty acids longer than C18.13 This reinforces the impact of that region in modifying fatty ester binding, It should be noted that Mg2+ has been proposed to bind near residue N294, which could have indirect effects on the reaction rate when mutating this residue.76
Figure 7.
Hydrolytic activity of CalA variant SS_27 and its deconvoluted variants. Top panel: In vitro assays using pNO2-phenyl esters. Bottom panel: In vivo assays with triglycerides. Assays were performed in triplicate with clarified E. coli lysates. Activity for each variant is reported relative to hydrolysis of the same substrate with WT CalA. Specific activities for this series of assays are reported in Figure S8B.
Long-Chain Selective Variant LS_66: Epistasis between Two Gatekeeper Residues Disfavors the Hydrolysis of Short-Chain Substrates
Selectivity for long-chain over short-chain substrates has been more difficult to engineer in CalA than the inverse. The preference of CalA variants for long-chain substrates is best illustrated in the point-substituted LS_93 variant, where an increase in the bottleneck radius of the highest priority tunnel may contribute to its long-chain selectivity, as discussed above. Here we deconvoluted variant LS_66 to gain insight into its long-chain selective phenotype.
Variant LS_66 includes substitutions A32T (positioned just above the tunnel), L172 M (above the active site), R262H (in a distal part of the protein), and L274W and G432D (gatekeepers at the entrance of the tunnel, Figure S12). While some hydrolysis of short-chain pNO2-phenyl ester substrates was maintained, LS_66 demonstrated a propensity to hydrolyze long-chain substrates at a faster rate (140% relative to WT; Figure 8). Importantly, this phenotype was achieved for every combination tested that contains both L274W and G432D (the gatekeeper residues), yet not when these substitutions are tested alone. Substitutions L274W and G432D lie close to each other (Cα 11 Å apart; nearest side-chain distance 7.3 Å; Figure S12) near the entrance to the active-site area, serving as gatekeepers; G432D is in the moderately dynamic flap at the surface of CalA (Figure S12), suggesting the potential for dynamic interaction with L274W that is near the substrate (6.7 Å with docked pNO2-phenyloctanoate; Figure S12).
The best selectivity was attained only when the five mutations were present, with an activity for pNO2-phenylpalmitate 5-fold higher than for pNO2-phenylbutyrate. Variant L274W was essentially inactive toward all substrates tested, whereas G432D showed the opposite phenotype: an increase of 3.5- to 6-fold in activity for pNO2-phenyl ester substrates, the greatest increases being seen for short-chain substrates. A similar moderate short-chain selectivity was also observed for the triglyceride substrates, with slight loss of activity relative to WT.
This interaction between substitutions L274W and G432D therefore concerns both magnitude of activity, where G432D “rescued” the inactive L274W, as well as L274W inverting the moderate short-chain selectivity of G432D to procure moderate long-chain selectivity. The effect of these combined substitutions cannot be justified by the additive effect of the single mutants. Furthermore, no single substitution or multiple substituent that does not contain L274W and G432D displayed the pattern of pNO2-phenyl-ester substrate hydrolysis of LS_66. Variants A32T, L172M, and L274W appear to be destabilizing, and R262H displayed similar activity to the WT with a slight preference for substrates with shorter chains (up to C12). Finally, the combination G432D with R262H behaved similarly to the single G432D mutant.
For both LS variants (LS_66 and LS_93, where Y93G sits just below the active-site triad), higher hydrolysis activity of longer substrates may come from their favorable binding in the tunnel (tunnel priority is higher than WT for both variants) and the modulation of the tunnel entrance and prevalence of the branching region (Figures 2 and S3 and Table 1). It is important to note that these features cannot account for the decreased ability of these variants to hydrolyze short-chain fatty acids, which remains unexplained.
As with the short-chain variant F233I/F287L, this series of deconvoluted variants vividly exemplifies how assays carried out with bulky triglycerides and with single-chain, activated pNO2-phenyl esters do not always produce corresponding results. Because hydrolysis of either type of substrate is of interest for specific applications, this highlights the value of assaying both substrate classes as well as the risk of assuming that results obtained using one type of substrate adequately predict effects toward the other.
Conclusions
CalA is an excellent candidate for industrial use in the enrichment of specific fatty acid esters of determined chain length. While selectivity for fatty acid esters shorter than C6 has been achieved in the past with several lipases, selecting for longer carbon chains is harder because of residual hydrolytic activity on short-chain esters.
The main binding tunnel and its branches naturally evolved to procure efficient binding of triglycerides of differing lengths. Our normal-mode simulations clearly identify the tunnel domain as the dominantly dynamic region of the protein. The tunnel morphology simulations demonstrate the impact of substitutions within the tunnel as well as in distal regions. Specifically, the location of the predicted path, its length and branching pattern, and/or the tunnel priority were significantly modified in short-chain selective variants. For long-chain selective variants, there is an evident conservation or enhancement of the WT-like tunnel. It appears that the tunnels in short-chain selective variants were altered upon insertion of single or combined substitutions, disfavoring binding of substrates carrying longer carbon chains. These results indicate that a variety of mechanisms can modulate chain-length selectivity in CalA, including but not limited to steric hindrance.
Our analysis provides new insights into the tunnel engineering for altered substrate selectivity. It increases our understanding of the binding region while highlighting the importance of epistatic effects between close and distal mutations, paving the way for further engineering of CalA.
Acknowledgments
The authors gratefully acknowledge Manuel Ferrer Martinez and Cristina Coscolín for proofreading and guidance in protein motion simulations using PELE and thank Samy Cecioni for access to the iBright FL1500 imaging system and BioTek Synergy Neo2 microplate reader.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.2c00513.
List of primers (Table S1), properties of G237 variants from CAVER analysis (Table S2), specific activity of CalA WT (Figure S1) and of chain-length selective variants (Figure S2), predicted tunnels in CalA (Figure S3), substrate-binding tunnel (Figure S4), highly flexible regions mapped on structure (Figure S5), RMSF plots (Figure S6), relative positions of substitutions in variant SS_12 (Figure S7), specific activity of deconvoluted short-chain selective variants (Figure S8), predicted substrate-binding tunnels for variants within π-stacking distance of F222I (Figure 9), key distances for substitutions in variant SS_27 (Figure S10), specific activity of variants within π-stacking distance of F222I (Figure S11), positions of substitutions in variant LS_66 (Figure S12), and specific activity of deconvoluted long-chain selective variant LS_66 (Figure S13) (PDF)
Author Contributions
∞ L.A. and C.L.-S.-D. contributed equally to this paper. L.A. undertook all computations and subsequent data analysis. L.A. and C.L.-S.-D. performed the molecular biology. L.A., C.L.-S.-D., and D.Q. performed and analyzed in vitro and in vivo activity assays. L.A. wrote the first draft, and all authors edited subsequent versions. L.A. and C.L.-S.-D. produced figures. L.A. and D.Q. directed the storyline. D.Q. and J.N.P. supervised the project and provided guidelines. J.N.P. provided funding. All authors have given approval to the final version of the manuscript.
This work was supported by operating grant RGPIN-2018-04686 from the Natural Science and Engineering Research Council of Canada (NSERC) and the Canada Research Chairs Program (to J.N.P). C.L.-S.-D. was supported by PhD graduate scholarships from NSERC, CREATE-APRENTICE, Hydro-Québec, and Université de Montréal, and LA by PhD graduate scholarships from FQRNT, PROTEO, and Université de Montréal.
The authors declare no competing financial interest.
Supplementary Material
References
- Jackel C.; Hilvert D. Biocatalysts by Evolution. Curr. Opin Biotechnol 2010, 21 (6), 753–759. 10.1016/j.copbio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Mazurenko S.; Prokop Z.; Damborsky J. Machine Learning in Enzyme Engineering. ACS Catal. 2020, 10 (2), 1210–1223. 10.1021/acscatal.9b04321. [DOI] [Google Scholar]
- Bornscheuer U. T.; Huisman G. W.; Kazlauskas R. J.; Lutz S.; Moore J. C.; Robins K. Engineering the Third Wave of Biocatalysis. Nature 2012, 485 (7397), 185–194. 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- Maria-Solano M. A.; Serrano-Hervás E.; Romero-Rivera A.; Iglesias-Fernández J.; Osuna S. Role of Conformational Dynamics in the Evolution of Novel Enzyme Function. Chem. Commun. 2018, 54 (50), 6622–6634. 10.1039/C8CC02426J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E.; Kaltenbach M.; Correy G. J.; Carr P. D.; Porebski B. T.; Livingstone E. K.; Afriat-Jurnou L.; Buckle A. M.; Weik M.; Hollfelder F.; Tokuriki N.; Jackson C. J. The Role of Protein Dynamics in the Evolution of New Enzyme Function. Nat. Chem. Biol. 2016, 12 (11), 944–950. 10.1038/nchembio.2175. [DOI] [PubMed] [Google Scholar]
- Wilding M.; Hong N.; Spence M.; Buckle A. M.; Jackson C. J. Protein Engineering: The Potential of Remote Mutations. Biochem. Soc. Trans. 2019, 47 (2), 701–711. 10.1042/BST20180614. [DOI] [PubMed] [Google Scholar]
- Bershtein S.; Segal M.; Bekerman R.; Tokuriki N.; Tawfik D. S. Robustness-Epistasis Link Shapes the Fitness Landscape of a Randomly Drifting Protein. Nature 2006, 444 (7121), 929–932. 10.1038/nature05385. [DOI] [PubMed] [Google Scholar]
- Bloom J. D.; Labthavikul S. T.; Otey C. R.; Arnold F. H. Protein Stability Promotes Evolvability. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (15), 5869–5874. 10.1073/pnas.0510098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatis P. E.; Fabiane S. M.; Simona F.; Carloni P.; Sutton B. J.; Vila A. J. Adaptive Protein Evolution Grants Organismal Fitness by Improving Catalysis and Flexibility. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (52), 20605–20610. 10.1073/pnas.0807989106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz M. T. The Importance of Additive and Non-Additive Mutational Effects in Protein Engineering. Angew. Chem., Int. Ed. Engl. 2013, 52 (10), 2658–2666. 10.1002/anie.201207842. [DOI] [PubMed] [Google Scholar]
- Gobeil S. M. C.; Clouthier C. M.; Park J.; Gagné D.; Berghuis A. M.; Doucet N.; Pelletier J. N. Maintenance of Native-like Protein Dynamics May Not Be Required for Engineering Functional Proteins. Chemistry & Biology 2014, 21 (10), 1330–1340. 10.1016/j.chembiol.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Sato T. K.; Tremaine M.; Parreiras L. S.; Hebert A. S.; Myers K. S.; Higbee A. J.; Sardi M.; McIlwain S. J.; Ong I. M.; Breuer R. J.; Avanasi Narasimhan R.; McGee M. A.; Dickinson Q.; La Reau A.; Xie D.; Tian M.; Reed J. L.; Zhang Y.; Coon J. J.; Hittinger C. T.; Gasch A. P.; Landick R. Directed Evolution Reveals Unexpected Epistatic Interactions That Alter Metabolic Regulation and Enable Anaerobic Xylose Use by Saccharomyces Cerevisiae. PLoS Genet 2016, 12 (10), e1006372. 10.1371/journal.pgen.1006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn K.; Oroz-Guinea I.; Brundiek H.; Dorr M.; Bornscheuer U. T. Alteration of Chain Length Selectivity of Candida Antarctica Lipase A by Semi-Rational Design for the Enrichment of Erucic and Gondoic Fatty Acids. Adv. Synth Catal 2018, 360 (21), 4115–4131. 10.1002/adsc.201800889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejaldre L.; Lemay-St-Denis C.; Perez Lopez C.; Sancho Jodar F.; Guallar V.; Pelletier J. N. Known Evolutionary Paths Are Accessible to Engineered SS-Lactamases Having Altered Protein Motions at the Timescale of Catalytic Turnover. Front. Mol. Biosci. 2020, 7, 599298. 10.3389/fmolb.2020.599298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna S.; Jimenez-Oses G.; Noey E. L.; Houk K. N. Molecular Dynamics Explorations of Active Site Structure in Designed and Evolved Enzymes. Acc. Chem. Res. 2015, 48 (4), 1080–1089. 10.1021/ar500452q. [DOI] [PubMed] [Google Scholar]
- Rodrigues J. V.; Bershtein S.; Li A.; Lozovsky E. R.; Hartl D. L.; Shakhnovich E. I. Biophysical Principles Predict Fitness Landscapes of Drug Resistance. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (11), E1470–8. 10.1073/pnas.1601441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. I.; Hart K. M.; Sibbald C. A.; Frederick T. E.; Jimah J. R.; Knoverek C. R.; Tolia N. H.; Bowman G. R. Prediction of New Stabilizing Mutations Based on Mechanistic Insights from Markov State Models. ACS Central Science 2017, 3 (12), 1311–1321. 10.1021/acscentsci.7b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoi I.; Antoniou D.; Schwartz S. D. Incorporating Fast Protein Dynamics into Enzyme Design: A Proposed Mutant Aromatic Amine Dehydrogenase. J. Phys. Chem. B 2017, 121 (30), 7290–7298. 10.1021/acs.jpcb.7b05319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten R.; Liu L.; Kenner L. R.; Clarkson M. W.; Mavor D.; Tawfik D. S.; Kern D.; Fraser J. S. Rescue of Conformational Dynamics in Enzyme Catalysis by Directed Evolution. Nat. Commun. 2018, 9 (1), 1314. 10.1038/s41467-018-03562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente A. I.; Viña-Gonzalez J.; Mateljak I.; Monza E.; Lucas F.; Guallar V.; Alcalde M. Enhancing Thermostability by Modifying Flexible Surface Loops in an Evolved High-redox Potential Laccase. AIChE J. 2020, 66 (3), e16747. 10.1002/aic.16747. [DOI] [Google Scholar]
- Schenkmayerova A.; Pinto G. P.; Toul M.; Marek M.; Hernychova L.; Planas-Iglesias J.; Daniel Liskova V.; Pluskal D.; Vasina M.; Emond S.; Dörr M.; Chaloupkova R.; Bednar D.; Prokop Z.; Hollfelder F.; Bornscheuer U. T.; Damborsky J. Engineering the Protein Dynamics of an Ancestral Luciferase. Nat. Commun. 2021, 12 (1), 3616. 10.1038/s41467-021-23450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiha K. de G.; Angeli R.; de Oliveira S. D.; Almeida R. V. Are Lipases Still Important Biocatalysts? A Study of Scientific Publications and Patents for Technological Forecasting. PLoS One 2015, 10 (6), e0131624. 10.1371/journal.pone.0131624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R. A.; Brady D.; Bode M. L. The Hitchhiker’s Guide to Biocatalysis: Recent Advances in the Use of Enzymes in Organic Synthesis. Chem. Sci. 2020, 11 (10), 2587–2605. 10.1039/C9SC05746C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger K.-E.; Eggert T. Lipases for Biotechnology. Curr. Opin. Biotechnol. 2002, 13 (4), 390–397. 10.1016/S0958-1669(02)00341-5. [DOI] [PubMed] [Google Scholar]
- Reetz M. T. Lipases as Practical Biocatalysts. Curr. Opin Chem. Biol. 2002, 6 (2), 145–150. 10.1016/S1367-5931(02)00297-1. [DOI] [PubMed] [Google Scholar]
- Villeneuve P.; Muderhwa J. M.; Graille J.; Haas M. J. Customizing Lipases for Biocatalysis: A Survey of Chemical, Physical and Molecular Biological Approaches. J. Mol. Catal., B Enzym. 2000, 9 (4–6), 113–148. 10.1016/S1381-1177(99)00107-1. [DOI] [Google Scholar]
- Kundys A.; Białecka-Florjańczyk E.; Fabiszewska A.; Małajowicz J. Candida Antarctica Lipase B as Catalyst for Cyclic Esters Synthesis, Their Polymerization and Degradation of Aliphatic Polyesters. J. Polym. Environ 2018, 26 (1), 396–407. 10.1007/s10924-017-0945-1. [DOI] [Google Scholar]
- Anderson E. M.; Larsson K. M.; Kirk O. One Biocatalyst–Many Applications: The Use of Candida Antarctica B-Lipase in Organic Synthesis. Biocatal. Biotransformation 1998, 16 (3), 181–204. 10.3109/10242429809003198. [DOI] [Google Scholar]
- Kirk O.; Christensen M. W. Lipases from Candida Antarctica: Unique Biocatalysts from a Unique Origin. Org. Process Res. Dev. 2002, 6 (4), 446–451. 10.1021/op0200165. [DOI] [Google Scholar]
- Domínguez De María P.; Carboni-Oerlemans C.; Tuin B.; Bargeman G.; Van Der Meer A.; Van Gemert R. Biotechnological Applications of Candida Antarctica Lipase A: State-of-the-Art. J. Mol. Catal., B Enzym. 2005, 37 (1–6), 36–46. 10.1016/j.molcatb.2005.09.001. [DOI] [Google Scholar]
- Monteiro R. R. C.; Virgen-Ortiz J. J.; Berenguer-Murcia Á.; Da Rocha T. N.; Dos Santos J. C. S.; Alcántara A. R.; Fernandez-Lafuente R. Biotechnological Relevance of the Lipase A from Candida Antarctica. Catal. Today 2021, 362, 141. 10.1016/j.cattod.2020.03.026. [DOI] [Google Scholar]
- Martínez-Martínez M.; Coscolín C.; Santiago G.; Chow J.; Stogios P. J.; Bargiela R.; Gertler C.; Navarro-Fernández J.; Bollinger A.; Thies S.; Méndez-García C.; Popovic A.; Brown G.; Chernikova T. N.; García-Moyano A.; Bjerga G. E. K.; Pérez-García P.; Hai T.; Del Pozo M. V.; Stokke R.; Steen I. H.; Cui H.; Xu X.; Nocek B. P.; Alcaide M.; Distaso M.; Mesa V.; Peláez A. I.; Sánchez J.; Buchholz P. C. F.; Pleiss J.; Fernández-Guerra A.; Glöckner F. O.; Golyshina O. V.; Yakimov M. M.; Savchenko A.; Jaeger K.-E.; Yakunin A. F.; Streit W. R.; Golyshin P. N.; Guallar V.; Ferrer M. Determinants and Prediction of Esterase Substrate Promiscuity Patterns. ACS Chem. Biol. 2018, 13 (1), 225–234. 10.1021/acschembio.7b00996. [DOI] [PubMed] [Google Scholar]
- Robles-Machuca M.; Del Campo M. M.; Camacho-Ruiz M. A.; Ordaz E.; Zamora-Gonzalez E. O.; Muller-Santos M.; Rodriguez J. A. Comparative Features between Recombinant Lipases CALA-like from U. Maydis and CALA from C. Antarctica in Thermal Stability and Selectivity. Biotechnol. Lett. 2019, 41 (2), 241–252. 10.1007/s10529-018-2630-4. [DOI] [PubMed] [Google Scholar]
- Ericsson D. J.; Kasrayan A.; Johansson P.; Bergfors T.; Sandstrom A. G.; Backvall J.-E.; Mowbray S. L. X-Ray Structure of Candida Antarctica Lipase A Shows a Novel Lid Structure and a Likely Mode of Interfacial Activation. J. Mol. Biol. 2008, 376, 109–119. 10.1016/j.jmb.2007.10.079. [DOI] [PubMed] [Google Scholar]
- Sandstrom A. G.; Wikmark Y.; Engström K.; Nyhlén J.; Bäckvall J.-E. Combinatorial Reshaping of the Candida Antarctica Lipase A Substrate Pocket for Enantioselectivity Using an Extremely Condensed Library. Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (1), 78–83. 10.1073/pnas.1111537108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglia D.; Ebert M. C. C. J. C.; Mugford P. F.; Pelletier J. N. Enzyme Engineering: A Synthetic Biology Approach for More Effective Library Generation and Automated High-Throughput Screening. PLoS One 2017, 12 (2), e0171741. 10.1371/journal.pone.0171741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglia D.; Alejaldre L.; Ouadhi S.; Rousseau O.; Pelletier J. N. Holistic Engineering of Cal-A Lipase Chain-Length Selectivity Identifies Triglyceride Binding Hot-Spot. PLoS One 2019, 14 (1), e0210100. 10.1371/journal.pone.0210100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann M.; Juhl P. B.; Pleiss J. Structural Classification by the Lipase Engineering Database: A Case Study of Candida Antarctica Lipase A. BMC Genomics 2010, 11, 123. 10.1186/1471-2164-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyyssölä A.; Miettinen H.; Kontkanen H.; Lille M.; Partanen R.; Rokka S.; Järvenpää E.; Lantto R.; Kruus K. Treatment of Milk Fat with Sn-2 Specific Pseudozyma Antarctica Lipase A for Targeted Hydrolysis of Saturated Medium and Long-Chain Fatty Acids. Int. Dairy J. 2015, 41, 16–22. 10.1016/j.idairyj.2014.09.003. [DOI] [Google Scholar]
- Zorn K.; Oroz-Guinea I.; Bornscheuer U. T. Strategies for Enriching Erucic Acid from Crambe Abyssinica Oil by Improved Candida Antarctica Lipase A Variants. Process Biochem 2019, 79, 65–73. 10.1016/j.procbio.2018.12.022. [DOI] [Google Scholar]
- SÁ A. G. A.; Meneses A. C. de; Araújo P. H. H. de; Oliveira D. de A Review on Enzymatic Synthesis of Aromatic Esters Used as Flavor Ingredients for Food, Cosmetics and Pharmaceuticals Industries. Trends Food Sci. Technol. 2017, 69, 95–105. 10.1016/j.tifs.2017.09.004. [DOI] [Google Scholar]
- Schmitt J.; Brocca S.; Schmid R. D.; Pleiss J. Blocking the Tunnel: Engineering of Candida Rugosa Lipase Mutants with Short Chain Length Specificity. Protein Eng. Des. Sel. 2002, 15 (7), 595–601. 10.1093/protein/15.7.595. [DOI] [PubMed] [Google Scholar]
- Klein R. R.; King G.; Moreau R. A.; Haas M. J. Altered Acyl Chain Length Specificity of Rhizopus Delemar Lipase through Mutagenesis and Molecular Modeling. Lipids 1997, 32 (2), 123–130. 10.1007/s11745-997-0016-1. [DOI] [PubMed] [Google Scholar]
- Brundiek H.; Padhi S. K.; Kourist R.; Evitt A.; Bornscheuer U. T. Altering the Scissile Fatty Acid Binding Site of Candida Antarctica Lipase A by Protein Engineering for the Selective Hydrolysis of Medium Chain Fatty Acids. Eur. J. Lipid Sci. Technol. 2012, 114, 1148–1153. 10.1002/ejlt.201200106. [DOI] [Google Scholar]
- Yang J.; Koga Y.; Nakano H.; Yamane T. Modifying the Chain-Length Selectivity of the Lipase from Burkholderia Cepacia KWI-56 through in Vitro Combinatorial Mutagenesis in the Substrate-Binding Site. Protein Eng. Des Sel 2002, 15 (2), 147–152. 10.1093/protein/15.2.147. [DOI] [PubMed] [Google Scholar]
- Panizza P.; Cesarini S.; Diaz P.; Rodriguez Giordano S. Saturation Mutagenesis in Selected Amino Acids to Shift Pseudomonas Sp. Acidic Lipase Lip I.3 Substrate Specificity and Activity. Chem. Commun. (Camb) 2015, 51 (7), 1330–1333. 10.1039/C4CC08477B. [DOI] [PubMed] [Google Scholar]
- Gaskin D. J.; Romojaro A.; Turner N. A.; Jenkins J.; Vulfson E. N. Alteration of Lipase Chain Length Specificity in the Hydrolysis of Esters by Random Mutagenesis. Biotechnol. Bioeng. 2001, 73 (6), 433–441. 10.1002/bit.1077. [DOI] [PubMed] [Google Scholar]
- Brocca S.; Secundo F.; Ossola M.; Alberghina L.; Carrea G.; Lotti M. Sequence of the Lid Affects Activity and Specificity of Candida Rugosa Lipase Isoenzymes. Protein Sci. 2003, 12 (10), 2312–2319. 10.1110/ps.0304003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarossa G.; Lafranconi P. G.; Alquati C.; DeGioia L.; Alberghina L.; Fantucci P.; Lotti M. Mutations in the “Lid” Region Affect Chain Length Specificity and Thermostability of a Pseudomonas Fragi Lipase. FEBS Lett. 2005, 579 (11), 2383–2386. 10.1016/j.febslet.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Syal P.; Verma V. V.; Gupta R. Targeted Mutations and MD Simulations of a Methanol-Stable Lipase YLIP9 from Yarrowia Lipolytica MSR80 to Develop a Biodiesel Enzyme. Int. J. Biol. Macromol. 2017, 104, 78–88. 10.1016/j.ijbiomac.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Yu X. W.; Zhu S. S.; Xiao R.; Xu Y. Conversion of a Rhizopus Chinensis Lipase into an Esterase by Lid Swapping. J. Lipid Res. 2014, 55 (6), 1044–1051. 10.1194/jlr.M043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miton C. M.; Tokuriki N. How Mutational Epistasis Impairs Predictability in Protein Evolution and Design: How Epistasis Impairs Predictability in Enzyme Evolution. Protein Sci. 2016, 25 (7), 1260–1272. 10.1002/pro.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Rocha C. G.; Li A.; D’Amore L.; Hoebenreich S.; Sanchis J.; Lubrano P.; Ferla M. P.; Garcia-Borràs M.; Osuna S.; Reetz M. T. Pervasive Cooperative Mutational Effects on Multiple Catalytic Enzyme Traits Emerge via Long-Range Conformational Dynamics. Nat. Commun. 2021, 12 (1), 1621. 10.1038/s41467-021-21833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundiek H. B.; Evitt A. S.; Kourist R.; Bornscheuer U. T. Creation of a Lipase Highly Selective for Trans Fatty Acids by Protein Engineering. Angew. Chem., Int. Ed. 2012, 51, 412–414. 10.1002/anie.201106126. [DOI] [PubMed] [Google Scholar]
- Laible M.; Boonrod K. Homemade Site Directed Mutagenesis of Whole Plasmids. JoVE 2009, (27), 1135. 10.3791/1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J.; Russell D. W. Preparation and Transformation of Competent E. Coli Using Calcium Chloride. CSH Protoc 2006, 2006 (1), pdb.prot3932. 10.1101/pdb.prot3932. [DOI] [PubMed] [Google Scholar]
- Studier F. Protein Production by Auto-Induction in High-Density Shaking Cultures. Protein. Expr. Purif. 2005, 41, 207–234. 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Schindelin J.; Arganda-Carreras I.; Frise E.; Kaynig V.; Longair M.; Pietzsch T.; Preibisch S.; Rueden C.; Saalfeld S.; Schmid B.; Tinevez J.-Y.; White D. J.; Hartenstein V.; Eliceiri K.; Tomancak P.; Cardona A. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9 (7), 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madadkar-Sobhani A.; Guallar V. PELE Web Server: Atomistic Study of Biomolecular Systems at Your Fingertips. Nucleic Acids Res. 2013, 41 (W1), W322. 10.1093/nar/gkt454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli K. W.; Vitalis A.; Alcantara R.; Guallar V. PELE: Protein Energy Landscape Exploration. A Novel Monte Carlo Based Technique. J. Chem. Theory Comput 2005, 1 (6), 1304–1311. 10.1021/ct0501811. [DOI] [PubMed] [Google Scholar]
- Cossins B. P.; Hosseini A.; Guallar V. Exploration of Protein Conformational Change with PELE and Meta-Dynamics. J. Chem. Theory Comput. 2012, 8 (3), 959–965. 10.1021/ct200675g. [DOI] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual Molecular Dynamics. J. Mol. Graph 1996, 14 (1), 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Chovancova E.; Pavelka A.; Benes P.; Strnad O.; Brezovsky J.; Kozlikova B.; Gora A.; Sustr V.; Klvana M.; Medek P.; Biedermannova L.; Sochor J.; Damborsky J. CAVER 3.0: A Tool for the Analysis of Transport Pathways in Dynamic Protein Structures. PLoS Comput. Biol. 2012, 8 (10), e1002708. 10.1371/journal.pcbi.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes L. E.; Löwendahl A. C.; Kazlauskas R. J. Quantitative Screening of Hydrolase Libraries Using PH Indicators: Identifying Active and Enantioselective Hydrolases. Chem. Eur. J. 1998, 4 (11), 2324–2331. . [DOI] [Google Scholar]
- Reyes-Duarte D.; Coscolin C.; Martinez-Martinez M.; Ferrer M.; Garcia-Arellano H. Functional-Based Screening Methods for Detecting Esterase and Lipase Activity against Multiple Substrates. Methods Mol. Biol. 2018, 1835, 109–117. 10.1007/978-1-4939-8672-9_4. [DOI] [PubMed] [Google Scholar]
- Müller J.; Sowa M. A.; Dörr M.; Bornscheuer U. T. The Acyltransferase Activity of Lipase CAL-A Allows Efficient Fatty Acid Esters Formation from Plant Oil Even in an Aqueous Environment. Eur. J. Lipid Sci. Technol. 2015, 117 (12), 1903–1907. 10.1002/ejlt.201500292. [DOI] [Google Scholar]
- Müller J.; Sowa M. A.; Fredrich B.; Brundiek H.; Bornscheuer U. T. Enhancing the Acyltransferase Activity of Candida Antarctica Lipase A by Rational Design. ChemBioChem. 2015, 16 (12), 1791–1796. 10.1002/cbic.201500187. [DOI] [PubMed] [Google Scholar]
- Stoytcheva M.; Montero G.; Zlatev R.; Leon J. a.; Gochev V. Analytical Methods for Lipases Activity Determination: A Review. CAC 2012, 8 (3), 400–407. 10.2174/157341112801264879. [DOI] [Google Scholar]
- Svendsen A.; Vind J.; Pathar S.; Borch K.. Lipolytic Enzyme Variants. WO/2008/040738, 2008.
- Bornscheuer U.; Brundiek H.; Evitt A.; Saß S.; Bönisch F.; Kourist R.. Lipase Variants Having Increased Enzyme Specificity or Enhanced Trans-Selectivity, and Methods of Use. WO2012146935, November 1, 2012.
- Hatzakis N. S. Single Molecule Insights on Conformational Selection and Induced Fit Mechanism. Biophys. Chem. 2014, 186, 46–54. 10.1016/j.bpc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Michel D. Conformational Selection or Induced Fit? New Insights from Old Principles. Biochimie 2016, 128–129, 48–54. 10.1016/j.biochi.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Beyraghdar Kashkooli A.; Manzano D.; Pateraki I.; Richard L.; Kolkman P.; Lucas M. F.; Guallar V.; de Vos R. C. H.; Franssen M. C. R.; van der Krol A.; Bouwmeester H. Kauniolide Synthase Is a P450 with Unusual Hydroxylation and Cyclization-Elimination Activity. Nat. Commun. 2018, 9 (1), 4657. 10.1038/s41467-018-06565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streltsov V. A.; Luang S.; Peisley A.; Varghese J. N.; Ketudat Cairns J. R.; Fort S.; Hijnen M.; Tvaroška I.; Ardá A.; Jiménez-Barbero J.; Alfonso-Prieto M.; Rovira C.; Mendoza F.; Tiessler-Sala L.; Sánchez-Aparicio J.-E.; Rodríguez-Guerra J.; Lluch J. M.; Maréchal J.-D.; Masgrau L.; Hrmova M. Discovery of Processive Catalysis by an Exo-Hydrolase with a Pocket-Shaped Active Site. Nat. Commun. 2019, 10 (1), 2222. 10.1038/s41467-019-09691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S.; Santiago G.; Cea-Rama I.; Fernandez-Lopez L.; Coscolín C.; Modregger J.; Ressmann A. K.; Martínez-Martínez M.; Marrero H.; Bargiela R.; Pita M.; Gonzalez-Alfonso J. L.; Briand M. L.; Rojo D.; Barbas C.; Plou F. J.; Golyshin P. N.; Shahgaldian P.; Sanz-Aparicio J.; Guallar V.; Ferrer M. Genetically Engineered Proteins with Two Active Sites for Enhanced Biocatalysis and Synergistic Chemo- and Biocatalysis. Nat. Catal 2020, 3 (3), 319–328. 10.1038/s41929-019-0394-4. [DOI] [Google Scholar]
- Sirén S.; Dahlström K. M.; Puttreddy R.; Rissanen K.; Salminen T. A.; Scheinin M.; Li X.-G.; Liljeblad A. Candida Antarctica Lipase A-Based Enantiorecognition of a Highly Strained 4-Dibenzocyclooctynol (DIBO) Used for PET Imaging. Molecules 2020, 25 (4), 879. 10.3390/molecules25040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









