Abstract
Introduction
We sought to compare cost and safety outcomes of patients who received a kidney transplant and bilateral nephrectomy in either a simultaneous or staged approach.
Methods
We reviewed all adult patients with autosomal dominant polycystic kidney disease (ADPKD) who received a kidney transplant and underwent bilateral nephrectomy between 2008 and 2019. Patients were divided into two groups: staged (nephrectomy prior to transplant) and simultaneous (nephrectomy at the time of transplant). The primary outcome was cumulative cost of nephrectomy and transplantation ($CAD). We analyzed several secondary outcomes, including 90-day Clavien-Dindo complication rates.
Results
A total of 114 patients with ADPKD received a kidney transplant over 11 years. Of these, 28 patients underwent both nephrectomy and transplantation (10 staged, 18 simultaneous). More patients in the simultaneous group had a living donor transplant (83% vs. 0%, p<0.001). Creatinine clearance at one year/last followup did not differ between groups (p=0.12). With similar overall complication rates between groups, the transfusion rate was also similar between groups (simultaneous 50% vs. staged 40%, p=0.91). Total cost was lower in the simultaneous group ($23 775.33 CAD vs. $35 048.83 CAD, p<0.001), largely owing to a longer total length of stay in the staged group as compared to the simultaneous group (8.1 vs. 14.5 days, p<0.001).
Conclusions
These data suggest that a simultaneous approach to bilateral nephrectomy and kidney transplantation provides potential cost savings with no adverse outcomes. This provides a rationale to investigate simultaneous nephrectomy and transplantation in the deceased donor setting.
KEY MESSAGES
Simultaneous bilateral nephrectomy and kidney transplantation has a similar adverse event profile to a staged nephrectomy/transplant approach.
Cost savings may be realized with a simultaneous approach.
While a simultaneous approach is typically reserved for patients with a living donor, well-selected deceased donor kidney recipients should also be considered.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) affects approximately one in 1000 people, with up to 50% of individuals requiring renal replacement therapy by age 59.1,2 Both kidney and cyst volumes can increase exponentially over time,3 leading to a number of symptoms necessitating bilateral nephrectomy. Commonly accepted indications for nephrectomy include abdominal pain attributable to mass effect, early satiety, cyst bleeding/hematuria, and recurrent cyst infections.4 Lack of space to place a transplant graft has been recognized as a relative indication for nephrectomy, with one study finding that kidney size greater than 21.5 cm predicts the need for bilateral nephrectomy prior to kidney transplantation.5
Open bilateral nephrectomy has been the standard approach for several decades, but there are a number of publications describing minimally invasive techniques.6–12 While the need for bilateral nephrectomy prior to transplant is not disputed, there remains debate regarding the optimal sequencing of bilateral nephrectomy. Some have argued for a simultaneous approach (nephrectomy and transplant in the same sitting) in order to avoid an anephric state, with subsequent issues surrounding fluid management, conversion to hemodialysis, and the risk of sensitization after blood transfusion.4,13 Others advocate for a staged approach (nephrectomy first followed by kidney transplant at different sittings) out of concern for the magnitude of both a nephrectomy and transplant in a single sitting and implications on perioperative recovery.14 A number of studies have been performed demonstrating an acceptable complication profile for each approach.14–16
Logistical issues, such as organizing a living donor or mobilizing overnight operative resources, are important factors in decision-making, but are less well-described in the literature. In particular, there is a paucity of data on the cost implication of each approach. To that end, we conducted a review of patients who underwent either a simultaneous or staged nephrectomy and kidney transplantation. We hypothesized that there would be no cost difference between groups, with no significant difference in functional outcomes and 90-day perioperative complications.
Methods
Study design and patient population
All patients with ADPKD who underwent bilateral native nephrectomy either prior to, or at the time of, kidney transplantation from January 2008 to December 2019 at an academic tertiary care hospital in Ontario, Canada, were reviewed. Patients were included in the study if they were adults (>18 years of age), had an indication for bilateral native nephrectomy, and underwent a kidney transplant either simultaneously or following bilateral native nephrectomy. We excluded pediatric patients, those who underwent a multiorgan transplant, and those who underwent nephrectomy at an outside institution. Patients were divided into two groups: the staged group (STG) underwent nephrectomy followed by kidney transplantation at a separate sitting, whereas the simultaneous group (SIM) underwent both bilateral nephrectomy and kidney transplantation at the same sitting.
Cost analysis
We obtained cost data from the hospital finance department for fiscal years 2016–2019 inclusive, as accurate data was not available prior to 2016. Reported costs encompassed all hospital-related expenses and were grouped into major cost centers. These included food services, inpatient care (ward/ICU/monitored unit costs), operative services, labs, medical imaging, and pharmacy (including immunosuppressive medications). The cumulative cost for the STG group included both the nephrectomy and kidney transplant inpatient stays. We analyzed all available cost data and compared cumulative costs, as well as per-day costs between groups. Physicians’ fees and outpatient costs were not captured in this analysis. All costs are reported in Canadian dollars (CAD; $1 CAD = $0.75 USD approximately).
Outcomes of interest
Additional outcomes of interest for this study included 90-day Clavien-Dindo (CD) surgical complication rates,17 patient demographics, operative time (skin incision to closure), estimated blood loss (surgeon-reported), change in hemoglobin (g/L; preoperative to postoperative), transfusion rate, length of stay, immunological parameters and complications, transplant type, and creatinine at one year and last followup. In the STG group, all outcomes were reported as an aggregate of both the nephrectomy and the kidney transplant procedures in order to accurately compare both groups.
Data collection and statistical analysis
Ethics approval for this study was obtained from the Western University Research Ethics Board (REB# 100030). Data were collected from the hospital electronic health record in anonymized fashion. Descriptive statistics were used to define means and frequencies for primary and secondary outcomes. T-test, Mann-Whitney U, and Chi-squared were used to compare outcomes between the STG and SIM groups. All statistical analysis was completed using SPSS (Version 26, IBM). Alpha was set at 0.05 for the purpose of determining significance.
Surgical technique: Nephrectomy and kidney transplant
Bilateral native nephrectomy was performed via midline incision by one of two urologic surgeons at our center. In the STG group, kidney transplantation was performed via Gibson incision in the left or right lower quadrant at a separate sitting. If patients had a potential living donor, they were also offered simultaneous bilateral nephrectomy and transplantation. This procedure would coincide with the living donor nephrectomy operation. In the SIM group, the kidney was placed in an extraperitoneal pocket in the right or left iliac fossa through the same midline incision. Vascular anastomoses were performed to the external iliac vessels in most cases. A standard Lich-Grégoire ureteral reimplantation was then performed. Immunosuppression induction consisted of methylprednisolone and either basiliximab (at the time of transplant and postoperative day 4), or rabbit anti-thymocyte globulin (total target dose 5–6 mg/kg given over 3–5 days). Postoperative immunosuppression generally consisted of prednisone, mycophenolate sodium, and tacrolimus.
Results
Population characteristics
We identified 114 patients with ADPKD who underwent kidney transplantation between January 2008 and December 2019. After reviewing their electronic chart, we identified 30 patients who had a bilateral nephrectomy and kidney transplant. Two patients were excluded, as one patient underwent nephrectomy at an outside institution and one patient had bilateral nephrectomy at the time of major colorectal surgery. Patient demographics are included in Table 1. Patients in the SIM group were more likely to receive a pre-emptive transplant (simultaneous 9/18 vs. staged 0/10, p<0.001) and were more likely to receive a kidney from a living donor (simultaneous 15/18 vs. staged 0/10, p<0.001).
Table 1.
Patient demographics
| Simultaneous group | Staged group | p | |
|---|---|---|---|
| n | 18 | 10 | |
| Age in years | 56 (8.7) | 57 (8.4) | 0.68 |
| Dialysis modality | |||
| Pre-emptive | 9 (50%) | 0 (0%) | <0.001 |
| PD | 5 (28%) | 0 (0%) | |
| HD | 4 (22%) | 10 (10%) | |
| Kidney allograft type | |||
| LD | 15 (83%) | 0 (0%) | <0.001 |
| NDD | 1 (6%) | 4 (40%) | |
| DCD | 2 (11%) | 6 (60%) | |
| SCD | 18 (100%) | 8 (80%) | 0.12 |
| ECD | 0 (0%) | 2 (20%) | |
| Native kidney maximum length (cm) | 22.7 | 24.4 | 0.13 |
| Indication for nephrectomy | |||
| Space/extension below iliac crest | 18 | 8 | |
| Renal mass | 0 | 1 | |
| Symptomatic (pain, bleeding, etc.) | 0 | 1 | |
DCD: donation after cardiac death; ECD: expanded criteria donor; HD: hemodialysis: LD: living donor; NDD: neurological determination of death; PD: peritoneal dialysis; SCD: standard criteria donor.
Cost comparison
Figure 1 demonstrates the comparison of total cumulative costs, as well as a breakdown of major reporting cost centers. The STG group had a significantly higher total cost than the SIM group ($35 048.83 vs. $23 775.33, p<0.001), while mean cost per day was lower in the STG group ($2742.12/day vs. $3301.75/day; p=0.02). The STG group had higher inpatient care costs ($13 538 vs. $7944, p=0.002), as well as operative services costs ($10 107 vs. $7391, p<0.001).
Figure 1.
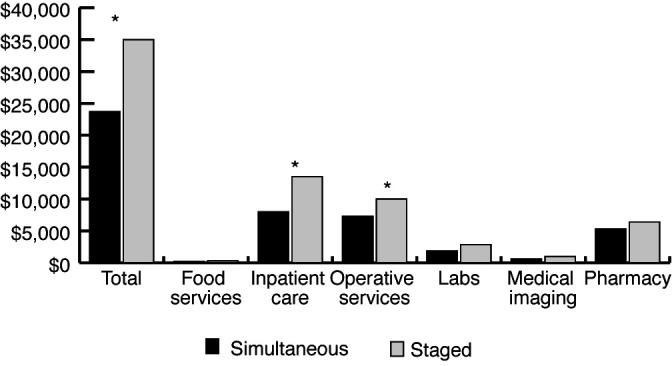
Cumulative inpatient costs ($CAD) for simultaneous (dark) and staged (light) groups. Reported as total costs, as well as cost broken down by major cost center. Total cost was significantly higher in the staged group ($35 048.83 vs. $23 775.33, p<0.001). Costs were similarly higher for inpatient care and operative services. *p<0.05.
Additional outcomes of interest
Figure 2 depicts total complication rates, as well as complications by CD grade for both groups. There was no significant difference in 90-day CD complications between the SIM and STG groups (78% vs. 70%, p=0.67). The overall complication rate appeared to be driven primarily by need for blood transfusion, which is classified as a CD 2 event. There was no significant difference in high-grade complications (CD >2) between groups (p=0.45) (Figure 2). In the SIM group, 1/18 patients (6%) required postoperative admission to the intensive care unit (ICU) (CD 4a). In the STG group, 2/10 (20%) patients’ arteriovenous fistulae thrombosed requiring either radiological (CD 3a) or surgical (CD 3b) intervention, and one patient (10%) required postoperative admission to the ICU (CD 4a).
Figure 2.
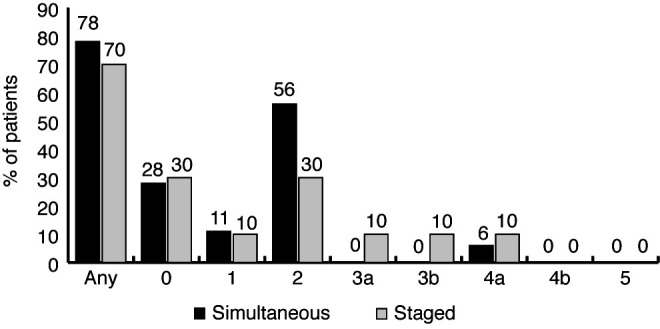
90-day complications for simultaneous (dark) and staged (light) groups stratified by Clavien-Dindo (CD) grade. There were no significant differences between groups. CD grade 3 or greater complications are considered severe.
Surgical outcomes are outlined in Table 2. Overall, 9/18 (50%) patients in the SIM group and 4/10 (40%) patients in the STG group required a blood transfusion in the perioperative period. Transfusions were handled as an aggregate of the nephrectomy and transplant phases in the STG group to facilitate comparison. Overall, patients in the SIM group had a shorter length of stay than those in the STG group (8.7 vs. 14.5, p<0.001).
Table 2.
Surgical outcomes in simultaneous and staged groups
| Simultaneous | Staged | p | |||
|---|---|---|---|---|---|
|
| |||||
| Nephrectomy | Transplant | Combined | |||
| OR time, minutes (±1 SD) | 338 (59) | 196 (45) | 186 (25) | 382 (56) | 0.06 |
| Length of stay, days (±1 SD) | 8.1 (3.7) | 5.9 (2.3) | 8.6 (2) | 14.5 (3.2) | <0.001 |
| Hemoglobin change and transfusions | |||||
| Preoperative Hgb, g/L (±1 SD) | 109 (13) | 120 (21) | 113 (8) | 0.4 | |
| Nadir Hgb, g/L (±1 SD) | 73 (9) | 80 (11) | 75 (7) | 0.76 | |
| Change in Hgb, g/L (± 1 SD) | −35 (11) | −39 (17) | −39 (7) | ||
| Transfusion rate (%) | 9 (50%) | 3 (30%) | 3(30%) | 4 (40%) | 0.91 |
| Units transfused | |||||
| 0 | 9 (50%) | 7 (70%) | 7 (70%) | 4 (40%) | 0.63 |
| 1 | 2 (11%) | 1 (10%) | 0 (0%) | 1 (10%) | |
| 2 | 6 (33%) | 1 (10%) | 3 (30%) | 4 (40%) | |
| 3 | 0 (0%) | 1 (10%) | 0 (0%) | 1 (10%) | |
| 4 | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | |
Hbg: hemoglobin; OR: operating room; SD: standard deviation.
Immunological and graft functional data are presented in Table 3. Of note, the SIM group had a lower discharge creatinine (151 mmol/L vs. 376 mmol/L, p<0.001) and no instances of delayed graft function (DGF). The STG group had a significantly higher DGF rate (70% vs. 0%, p<0.001). This is an expected finding given that the majority of patients in the SIM group underwent a living donor kidney transplant. At one year, there was no difference in serum creatinine between groups (p=0.12).
Table 3.
Transplant immunological and functional outcomes
| Simultaneous | Staged | p | |||
|---|---|---|---|---|---|
|
| |||||
| Nephrectomy | Transplant | Combined | |||
| cPRA % (SD) | 0 (29.75) | 24.6 (76.3) | 46.7 (80.5) | 0.11 | |
| Immunosuppression induction agent (in addition to methylprednisolone) | 8.1 (3.7) | 5.9 (2.3) | 8.6 (2) | 14.5 (3.2) | <0.001 |
| Basiliximab, n (%) | 12 (67%) | 4 (40%) | 0.24 | ||
| r-ATG, n (%) | 6 (33%) | 6 (60%) | |||
| DGF, n (%) | 0 (0%) | 7 (70%) | <0.001 | ||
| Serum creatinine at discharge mmol/L (SD) | 151 (115) | 376 (173) | <0.001 | ||
| Serum sreatinine at 1 year mmol/L (SD) | 113 (28) | 127 (15) | 0.12 | ||
| Acute rejection n (%) | 0 (0%) | 0 (0%) | |||
cPRA: cumulative panel reactive antibodies; DGF: delayed graft function (dialysis within 7 days post-transplant); r-ATG: rabbit antithymocyte globulin; SD: standard deviation.
Discussion
In this study, there was a significantly lower cumulative cost for patients who underwent a simultaneous bilateral nephrectomy and kidney transplantation compared to those who underwent a staged approach ($23 775.33 CAD vs. $35 048.83 CAD). This appeared to be largely driven by two factors.
Firstly, there was a reduction in inpatient care costs for the SIM group that is likely attributable to a decreased cumulative length of stay. Secondly, there was a reduction in operative service costs for the SIM group despite no difference in cumulative operative time. This is expected, since two separate sittings in the operating room would entail duplication of certain fixed costs (disposables, instrument processing, etc.). The patient groups had several key differences that could influence cost, including donor type (living vs. deceased) and the manner in which the operating room was used. Those patients receiving living donor transplant were booked during elective operative time, whereas deceased donor transplants were performed during emergency operative time. Interestingly, there was no difference in cost between the SIM group and the transplant phase of the STG group ($23 775.33 vs. $23 648.86 CAD); however, the influence of the higher proportion of pre-emptive and living donors in the SIM group would favorably affect inpatient costs with regards to this cost comparison. Despite differences between donor type, to our knowledge, this is the first study to report a cost comparison between the simultaneous and staged approaches.
Cumulative length of stay was shorter in the SIM group (8.1 days vs. 14.5 days, p<0.001). Other series of simultaneous nephrectomy and transplant via open incision report similar length of stays between seven and 12 days.12,15,18 Interestingly, there was no difference in length of stay when comparing the SIM group with the transplant phase of the STG group (8.1 days vs. 8.6 days). This suggests that the increased length of stay in the STG group is entirely due to the separate bilateral nephrectomy phase (5.9 days). Ahmad and colleagues report a similar finding in a small subset of patients who underwent a staged approach and had a cumulative length of stay of 14.6 days.18
Complication rates vary in the literature and are reported to occur in up to 60% of cases;6,16,18–20 however, reported rates are highly influenced by definition of complication, kidney size, and open vs. laparoscopic approach. We chose to use the CD classification system to ensure uniformity in reporting.17 Due to our methodology, the overall complication rate was high in our series (78% vs. 70%; SIM vs. STG) but was not significantly different between groups. Reassuringly, high-grade complications (CD ≥3) were less common in both groups, with 2/4 high-grade complications arising from thrombosed arteriovenous fistulae likely due to patient positioning (CD 3a/3b). The remaining two high-grade complications were ICU admissions for postoperative hemodynamic support (CD 4a). There were no perioperative mortalities. The overall complication rate in this study appears to be largely driven by lower-grade complications (CD <3), such as blood transfusion (50% vs. 40%, SIM vs. STG). Transfusion rates are variably reported between 10% and 90%,6,15,16 with the laparoscopic approach carrying a lower risk of blood loss and transfusion than traditional open procedures.12 Our transfusion rate appears to be in line with reported series of open bilateral nephrectomy, whether simultaneous or staged.
The major weakness of our study is that there was a significant difference in donor type between groups. The SIM group was heavily weighted towards living donors (83%), whereas the STG group was made up exclusively of deceased donors (60% donation after cardiac death [DCD], 40% neurological determination of death [NDD]). This is a result of logistical concerns dictating standard local practice. Given resource constraints in using after-hours operative time (nursing staff, anesthesia, theatre availability), the transplant team had avoided simultaneous nephrectomy and transplant in patients receiving a deceased donor kidney. The results of this study provide support to pursue simultaneous nephrectomy and transplant for patients receiving a kidney from a deceased donor at our center.
A common concern regarding the simultaneous approach is the hemodynamic effect bilateral nephrectomy would have on the transplanted kidney secondary to fluid shifts perioperatively. 14 Grodstein and colleagues reported a 4.4% arterial thrombosis rate in their simultaneous nephrectomy and transplant cohort. This may be partially explained by a higher incidence of multiple renal arteries, some of which required back-table reconstruction.16 Other series, including our own, have not found an increased risk of thrombosis with the simultaneous approach.4,15 Furthermore, there did not appear to be any functional consequences in the SIM group, including no episodes of DGF, and renal function was excellent at both the time of discharge and at one year or last followup.
There appears to be controversy as to whether a staged procedure may result in increased sensitization, particularly in those patients who receive a blood transfusion. Grodstein and colleagues reported a significant increase in % panel reactive antibody (PRA) in patients undergoing a staged approach, while others have not shown an increase in sensitization.16,21 Despite the need for blood transfusion, we did not find a statistically significant increase in %PRA between the nephrectomy and transplant phases of the STG group (24.6% vs. 46.7%; pre- vs. post-nephrectomy), although with greater numbers, a significant difference may become apparent. While our study only captures patients who were able to proceed with a successful kidney transplant, we are not aware of any patients who underwent bilateral nephrectomy and were unable to receive a kidney transplant as a result of sensitization from blood transfusion.
The effect of surgeon fatigue is also an important consideration in these cases, as bilateral nephrectomy increases case time significantly. We were unable to identify any literature evaluating the effect of fatigue on graft or patient outcomes in this setting. Our center typically employs a two-attending surgeon approach for the transplantation portion of the operation in order to reduce operative time and surgeon cognitive load.
Limitations
There are several important limitations present within our study. This was a retrospective cohort study conducted at a single center and is subject to all the inherent biases associated with this design. There was a significant difference in baseline donor type between groups, which could have significant effects on graft function, postoperative complications, length of stay, and cumulative inpatient costs. There was heavy weighting of living donor kidneys within the simultaneous group, which would be expected to reduce rates of DGF within this population. Finally, cost data was only available for inpatient care during specific fiscal years. As well, we did not capture costs associated with outpatient care, including dialysis in the STG group between nephrectomy and transplant. If dialysis costs were included, this would increase the cost of pursuing a STG approach.
Conclusions
To our knowledge this is the first study reporting a cost comparison between staged and simultaneous approaches to bilateral native nephrectomy and kidney transplantation for ADPKD. Our data suggest that simultaneous bilateral nephrectomy and kidney transplantation could carry cost savings with no increase in perioperative morbidity or threat to graft function, particular in a living donor cohort. This study provides a basis for further exploration of a simultaneous approach in the deceased donor transplant population.
Acknowledgements
The authors would like to thank the following individuals for their contributions to this study: Dr. Shahid Aquil and Wendy Taylor.
Footnotes
This paper has been peer-reviewed.
Competing interests: The authors do not report any competing personal or financial interests related to this work.
Visit https://www.cua.org/UROpedia to complete the multiple-choice questionnaire associated with this article. This program is an Accredited Self-Assessment Program (Section 3) as defined by the Maintenance of Certification Program of The Royal College of Physicians & Surgeons of Canada, and approved by the Canadian Urological Association. You may claim a maximum of 1 hour of credit.
References
- 1.Parfrey PS, Bear JC, Morgan J, et al. The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1085–90. doi: 10.1056/NEJM199010183231601. [DOI] [PubMed] [Google Scholar]
- 2.Menezes LF, Germino GG. The pathobiology of polycystic kidney disease from a metabolic viewpoint. Nat Rev Nephrol. 2019;15:735–49. doi: 10.1038/s41581-019-0183-y. [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 4.Fuller TF, Brennan TV, Feng S, et al. End stage polycystic kidney disease: Indications and timing of native nephrectomy relative to kidney transplantation. J Urol. 2005;174:2284–8. doi: 10.1097/01.ju.0000181208.06507.aa. [DOI] [PubMed] [Google Scholar]
- 5.Cristea O, Yanko D, Felbel S, et al. Maximal kidney length predicts need for native nephrectomy in ADPKD patients undergoing renal transplantation. Can Urol Assoc J. 2014;8:278–82. doi: 10.5489/cuaj.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrol N, Prieto M. Simultaneous hand-assisted laparoscopic bilateral native nephrectomy and kidney transplantation for patients with large polycystic kidneys. Urology. 2020;146:271–7. doi: 10.1016/j.urology.2020.06.090. [DOI] [PubMed] [Google Scholar]
- 7.Martin AD, Mekeel KL, Castle EP, et al. Laparoscopic bilateral native nephrectomies with simultaneous kidney transplantation. BJU Int. 2012;110:E1003–7. doi: 10.1111/j.1464-410X.2012.11379.x. [DOI] [PubMed] [Google Scholar]
- 8.Wisenbaugh ES, Tyson MD, 2nd, Castle EP, et al. Massive renal size is not a contraindication to a laparoscopic approach for bilateral native nephrectomies in autosomal dominant polycystic kidney disease (ADPKD) BJU Int. 2015;115:796–801. doi: 10.1111/bju.12821. [DOI] [PubMed] [Google Scholar]
- 9.Bansal RK, Kapoor A. Laparoscopic nephrectomy for massive polycystic kidney disease: Updated technique and outcomes. Can Urol Assoc J. 2014;8:341–5. doi: 10.5489/cuaj.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binsaleh S, Luke PP, Nguan C, et al. Comparison of laparoscopic and open nephrectomy for adult polycystic kidney disease: Operative challenges and technique. Can J Urol. 2006;13:3340–5. https://pubmed.ncbi.nlm.nih.gov/17187698/ [PubMed] [Google Scholar]
- 11.Luke PP, Spodek J. Hand-assisted laparoscopic resection of the massive autosomal dominant polycystic kidney. Urology. 2004;63:369–72. doi: 10.1016/j.urology.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Guo P, Xu W, Li H, et al. Laparoscopic nephrectomy vs. open nephrectomy for patients with autosomal dominant polycystic kidney disease: A systematic review and meta-analysis. PLoS One. 2015;10:e0129317. doi: 10.1371/journal.pone.0129317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skauby MH, Øyen O, Hartman A, et al. Kidney transplantation with and without simultaneous bilateral native nephrectomy in patients with polycystic kidney disease: A comparative retrospective study. Transplantation. 2012;94:383–8. doi: 10.1097/TP.0b013e31825812b9. [DOI] [PubMed] [Google Scholar]
- 14.Ismail HR, Flechner SM, Kaouk JH, et al. Simultaneous vs. sequential laparoscopic bilateral native nephrectomy and renal transplantation. Transplantation. 2005;80:1124–7. doi: 10.1097/01.tp.0000179109.51593.87. [DOI] [PubMed] [Google Scholar]
- 15.Kramer A, Sausville J, Haririan A, et al. Simultaneous bilateral native nephrectomy and living donor renal transplantation are successful for polycystic kidney disease: The University of Maryland experience. J Urol. 2009;181:724–8. doi: 10.1016/j.juro.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Grodstein EI, Baggett N, Wayne S, et al. An evaluation of the safety and efficacy of simultaneous bilateral nephrectomy and renal transplantation for polycystic kidney disease: A 20-year experience. Transplantation. 2017;101:2774–9. doi: 10.1097/TP.0000000000001779. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad SB, Inouye B, Phelan MS, et al. Live donor renal transplant with simultaneous bilateral nephrectomy for autosomal dominant polycystic kidney disease is feasible and satisfactory at long-term followup. Transplantation. 2016;100:407–15. doi: 10.1097/TP.0000000000000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chebib FT, Prieto M, Jung Y, et al. Native nephrectomy in renal transplant recipients with autosomal dominant polycystic kidney disease. Transplant Direct. 2015;1:e43. doi: 10.1097/TXD.0000000000000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veroux M, Zerbo D, Basile G, et al. Simultaneous native nephrectomy and kidney transplantation in patients with autosomal dominant polycystic kidney disease. PLoS One. 2016;11:e0155481. doi: 10.1371/journal.pone.0155481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellini MI, Charalmpidis S, Brookes P, et al. Bilateral nephrectomy for adult polycystic kidney disease does not affect the graft function of transplant patients and does not result in sensitization. Biomed Res Int. 2019;2019:7423158. doi: 10.1155/2019/7423158. [DOI] [PMC free article] [PubMed] [Google Scholar]


