ABSTRACT
Macroautophagy/autophagy defects are a risk factor for inflamatory bowel disease (IBD), but the mechanism remains unclear. We previously demonstrated that conditional whole-body deletion of the essential Atg7 (autophagy related 7) gene in adult mice (atg7Δ/Δ) causes specific tissue damage and shortens lifespan to three months primarily due to neurodegeneration with surprisingly no disturbing effects on the intestine. In contrast, we recently found that conditional whole-body deletion of other essential autophagy genes, Atg5 or Rb1cc1/Fip200 (atg5Δ/Δ or rb1cc1Δ/Δ), cause death within five days due to rapid inhibition of autophagy, elimination of intestinal stem cells, and loss of barrier function in the ileum. atg5Δ/Δ mice lose PDGFRA/PDGFRα+ mesenchymal cells (PMCs) and WNT signaling essential for stem cell renewal. Depletion of aspartate and nucleotides in atg5Δ/Δ ileum was revealed by novel mass-spectrometry imaging (MALDI-MSI), consistent with metabolic insufficiency underlying PMCs loss. The difference in the autophagy gene knockout phenotypes is likely due to distinct kinetics of autophagy loss because gradual whole-body atg5 deletion extends lifespan, phenocopying deletion of Atg7 or Atg12. Therefore, we established that autophagy is required for ileum PMC metabolism, stem cell maintenance and mammalian survival. PMC loss caused by autophagy deficiency may therefore contribute to IBD.
KEYWORDS: Autophagy, IBD, intestinal stem cells, Pdgfrα+ mesenchymal cells, WNT signaling
Macroautophagy (hereafter autophagy) requires the formation of double-membrane-bound vesicles called autophagosomes to sequester cytoplasmic proteins, organelles and bacteria as cargos. The major protein complexes involved in autophagosome formation include: the initiation complex ULK1 (unc-51 like autophagy activating kinase 1)-RB1CC1/FIP200 (RB1 inducible coiled-coil 1)-ATG13 (autophagy related 13)-ATG101, the nucleation complex BECN1 (beclin 1)-PIK3C3/VPS34 (phosphatidylinositol 3-kinase catalytic subunit type 3)-ATG14-NRBF2 (nuclear receptor binding factor 2), the ATG12–ATG5-ATG16L1 (autophagy related 16 like 1) complex (formation requires ATG7 and ATG10) and the Atg8-family proteins including MAP1LC3/LC3 (microtubule associated protein 1 light chain 3; formation requires ATG4, ATG7 and ATG3). In general, the ATG12–ATG5-ATG16L1 complex is recruited to the phagophore membrane, where it facilitates LC3 lipidation required for membrane association, expansion and formation of mature autophagosomes. Therefore, Atg5, Atg7 and Rb1cc1 are all essential for formation of functional autophagosomes and autophagy.
Autophagy is dramatically induced under nutrient deprivation and required for metabolic homeostasis and survival. Conditional, whole-body deletion of Atg7 in adult mice (atg7Δ/Δ) causes susceptibility to infection, liver and brain damage, muscle wasting and loss of white adipose tissue with their lifespan decreased to three months primarily due to neurodegeneration. Moreover, fasting in atg7Δ/Δ but not wild-type mice is lethal due to hypoglycemia. Both phenotypes are in part through activation of TRP53. Autophagy-deficient intestine functions normally despite slight apoptosis induced by TRP53 in stem cells and some abnormalities in Paneth cells, which was surprising given that ATG16L1 mutations are associated with IBDs.
Autophagy also plays a critical role in sustaining cancer metabolism. In tumor cells, autophagy provides amino acids, especially glutamine, to maintain pools for the tricarboxylic acid cycle, aspartate, and nucleotides. Therefore, autophagy is essential in intracellular nutrient scavenging, particularly during starvation, which guarantees adequate substrate supply to support essential metabolic functions.
Because Atg7, Atg5 and Rb1cc1 are all essential autophagy genes, we sought to assess whether loss of Atg5 or Rb1cc1 would have similar phenotype as loss of Atg7. We recently reported that conditional whole-body deletion of Atg5 (atg5Δ/Δ) or Rb1cc1 (rb1cc1Δ/Δ) cause ileum destruction with loss of barrier function and mice survive less than five days due to rapid loss of autophagy associated with metabolic impairment in the ileum, specifically in PDGFRA+ mesenchymal cells (PMCs), indicating the essential role of autophagy in PMC survival and intestinal homeostasis, the loss of function in which may contribute to the pathogenesis of IBDs [1] (Figure 1A).
Figure 1.
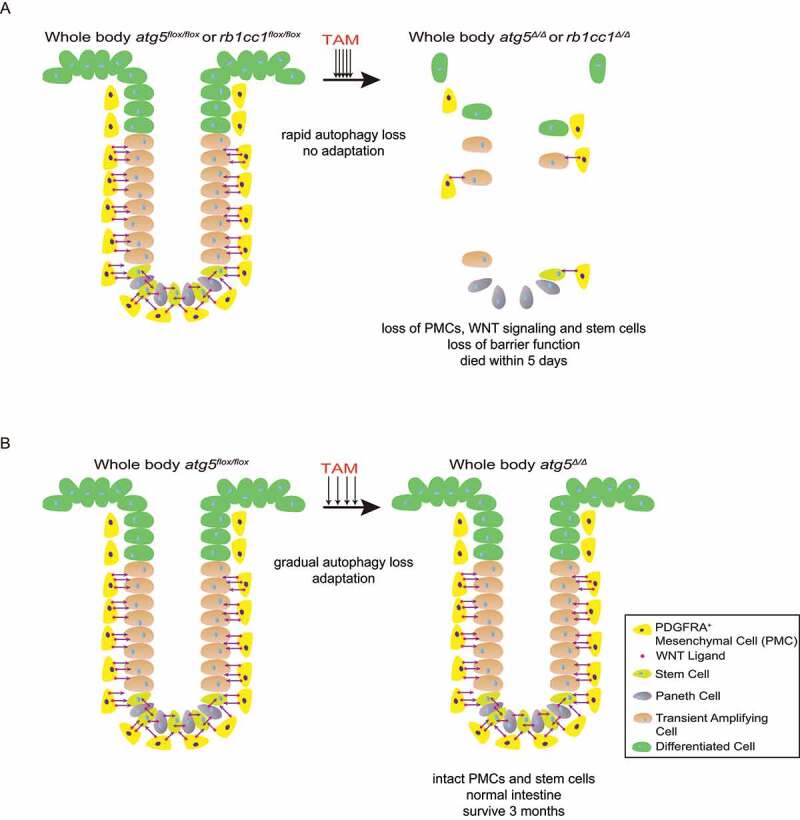
Autophagy in PMCs is required to maintain intestinal homeostasis and mammalian survival. (A) Fast deletion of Atg5 or Rb1cc1 causes depletion of PMCs and WNT signaling failure, resulting in rapid death due to loss of stem cells and barrier function. (B) Slow deletion of Atg5 allows adaptation and rescues the lethality.
We demonstrated the phenotypes of these essential autophagy gene losses by utilizing a genetically engineered mouse models (GEMM) for conditional, whole-body atg5, rb1cc1 or atg7 deletion in adult mice by administration of tamoxifen (TAM) to activate ubiquitously expressed Cre recombinase for five consecutive days (fast deletion). In contrast to atg7Δ/Δ mice that tolerate loss of ATG7 for up to three months in the fed state, atg5Δ/Δ and rb1cc1Δ/Δ mice show rapid lethality within five days with singularly specific damage to the ileum, loss of intestinal stem cells and transient amplifying cells with failure of WNT signaling essential for their survival, and loss of barrier function. The loss of ileum stem cells is likely due to the accelerated kinetics of loss of autophagy. However, atg5 deletion using epithelium-specific Vil1 (villin 1)-Cre does not cause these phenotypes, indicating that the essential nature of WNT for stem cell maintenance originates outside the epithelium. We further found that PMCs, a special cell type in the mesenchymal layer surrounding the epithelium that are important in maintaining WNT and BMP (bone morphogenetic protein) gradients for intestinal homeostasis, are depleted in the atg5Δ/Δ ileum. atg5Δ/Δ ileum displays globally decreased aspartate and increased nucleotide degradation revealed by matrix-assisted laser desorption ionization coupled to mass spectrometry imaging (MALDI-MSI). Therefore, we concluded that autophagy is critical for ileum metabolism in general while PMCs may be extremely sensitive to these metabolic alterations upon autophagy loss, which compromises PMC survival and thereby WNT signaling essential for stem cell survival and intestinal homeostasis (Figure 1A).
Because acute whole-body deletion of Atg5 and Rb1cc1 causes lethality, we tested whether gradual loss of autophagy could allow adaptation and survival by administering TAM once per week for four weeks (slow deletion) instead of fast deletion. Interestingly, loss of Atg5 following slow deletion does not affect the normal intestine architecture, which maintains intact PMCs and stem cells. atg5Δ/Δ mice generated by slow deletion die after several months from neurodegeneration similar to atg7Δ/Δ and atg12Δ/Δ mice. Thus, slow whole-body deletion of Atg5 overcomes the lethality and resembles atg7Δ/Δ mice due to gradual loss and retention of some autophagy function (Figure 1B).
In summary, our recent work illustrated that autophagy is required for PMC metabolism and survival essential for intestinal stem cell maintenance and mouse survival. Acute deletion of essential autophagy genes Atg5 and Rb1cc1 with rapid kinetics of autophagy function loss accelerate death due to a deleterious ileum phenotype caused by comprised PMC survival and WNT failure essential for stem cell survival. Alternatively, slow deletion of Atg5 allows adaptation and rescues the PMC and intestinal homeostasis, and these mice die of neurodegeneration similar to atg7Δ/Δ and atg12Δ/Δ mice. Thus, our data indicate that altering the kinetics of autophagy inhibitors in therapies can potentially avoid the side effects. Notably, PMCs are significantly decreased in IBDs as it progresses to an advanced stage in patient samples. As such, our findings provide a possible IBD development mechanism: decreased autophagy in PMCs in IBD results in failure of WNT signaling and stem cell survival, whereas restoration of autophagy and WNT signaling might be potential therapies.
Funding Statement
This work was supported by the NIH grants R01 CA130893, CA188096 and CA163591 and the Ludwig Princeton Branch of the Ludwig Institute for Cancer Research at Princeton University (to E.W.).
Disclosure statement
E.W. declares financial interest in Forma Therapeutics and Vescor Therapeutics. Y.Y. declare no competing interests.
Reference
- [1].Yang Y, Gomez M, Marsh T, et al. Autophagy in PDGFRα+ mesenchymal cells is essential for intestinal stem cell survival. Proc Natl Acad Sci U S A. 2022;119(21):e2202016119. [DOI] [PMC free article] [PubMed] [Google Scholar]


