ABSTRACT
Cancer is often caused by the immune system's inability to deal with malignant cells and allows them to progress and proliferate. Emerging cancerous cells constantly evade the immune system, and as a result, these cancerous cells acquire more mutations and exhibit the deadliest characteristics among malignant tumors. The importance of understanding tumor immunology, particularly the functions of tumor antigens and the immunosuppressive tumor microenvironment, is highlighted by the effectiveness of cancer immunotherapy therapies. Many innovative immunotherapy drugs that effectively battle cancer have been produced since the 1980s. At present, in cancer treatment, immunotherapy appears as a paradigm that targets immune checkpoints of tumor cells such as CTLA-4, PD-1, and monoclonal antibodies (MABs), although the treatment of cancer is classified into non-specific and specific types. Specific types define the antibody targeting cell receptors as a new cancer treatment modality. For a number of malignancies, checkpoint inhibitors, MABs, and their derivatives have become standard-of-care therapy. Other immunotherapy techniques, such as most cancer vaccines and cell-based therapies, are still in the experimental stage. Many new immunotherapy techniques and agents are being explored and evaluated in clinical trials, which is a good thing. Thus, this review discusses the role of checkpoint inhibitors and MABs in the treatment of tumor cells. Moreover, these findings help us to understand the mechanism of action of this class of therapeutics and provide support for the management of cancer treatment.
Keywords: Cancer, checkpoint inhibitors, CTLA-4, immunotherapeutic, monoclonal antibodies
INTRODUCTION
Cancer has the highest mortality rate and occurs in almost every part of the world. The Cancer Journal of Clinician predicts that approximately two million new cancer cases will occur in the United States alone in 2022, as well as 0.6 million people will die due to cancer.[1] Cancer is a multifactorial disorder and many hallmarks of cancer have already been identified. The most notable hallmark of cancer is a compromised immune system. The development of cancer, in many cases, is due to the inability of the immune system to deal with malignant cells. Different immune-based therapies are part of the current treatment plan, which has led to the discovery of new immunotherapeutic agents.[2] There are two basic approaches to modulating the immune system. First, it stimulates the patient's own immune system, which can commandeer against cancer cells. Second, it modulates the immune system by providing in-vitro processed immune system proteins and cells against cancer cells, and immuno-suppression.[3]
Paul Ehrlich proposed around 100 years ago that antibodies could be used to selectively target tumors. With the introduction of Hybridoma technology in 1975, the production of monoclonal antibodies (MABs) became possible. Initially, these antibodies were derived from mice, and they have immunogenic properties as well as a poor ability to induce immunogenic responses, which limits their application.[4] As antibody engineering progressed, the development of chimeric, humanized, and fully human MAB became possible, as it was mostly devoid of issues associated with Hybridoma Technique. MABs behave similarly to natural antibodies in that they target a specific (antigen) protein present on the surface of cancer cells.[5] MAB therapy, a cancer treatment method that blocks important pathways vital to tumor cell proliferation and survival, has revolutionized cancer treatment. In addition, some MAB can be used as bio-specific antibodies that attach to effector cells or conjugates and antigens via their fragment antigen-binding (Fab) domains. Tumor-specific MABs can direct or indirect immune response, which ultimately leads to cell death.[6] MAB suppresses angiogenesis and inhibits signaling pathways, resulting in immune-mediated cytotoxicity and cell death.[6,7,8] The immune system recognizes and kills cancer cells by various mechanisms, such as antibody-dependent cell-mediated cytotoxicity, though rapid growth of cancer cells can be checked by inhibiting receptors involved in cell growth. Cancer cells are selectively targeted to deliver radio-immunotherapy. We can diagnose cancer earlier, which helps in its better therapeutic management, and cytotoxic drugs can be directly delivered to cancer cells.[9]
Tumor-specific MABs are now an important therapeutic option in the treatment of leukaemia, breast cancer, colorectal cancer, and head and neck cancers (HNCs).[10,11,12,13] A modest response rate of 8–10% is observed when these MABs are used as a single treatment in advanced stage, severely pretreated, and recurrent illness. Response rate increases to more than 30% when these MABs are combined with traditional chemotherapy and/or radiotherapy. This can also be used against auto-immune B and T cells when their functions are altered, resulting in disease [Table 1]. Currently, the FDA has approved more than 100 MABs for various diseases, but they have a limited number of targets (PD1/PDL1, CD20, TNF, HER2, CGRP/CGRP, VEGF/VEGFR, IL-6/IL-6R, IL-2, p19, EGFR, CD19). Immune checkpoints are the primary regulators of the immune system, assisting normal cells in maintaining an equilibrium of activity. The “immunological brakes” rely on many checkpoints.[14,15] Every type of cancer has numerous genetic and epigenetic modifications in different sets of antigens of the immune system. The immune system distinguishes cancerous cells from normal cells by using these immune antigens. The immune checkpoint is the regulatory balance between the co-stimulatory and inhibitory signals of the T-cell receptor. Antigen identification by the T-cell receptor triggers the T-cell response (TCR).[16,17] The immune checkpoint in normal cells plays a key role in the inhibition of autoimmunity by maintaining self-tolerance. Cancer altered the expression of immune checkpoint proteins. These alterations give tumor cells an advantage over the immune system. In this review, we will look at the target-specific MABs-based immunotherapy options available right now, as well as their mechanisms of action.
Table 1.
List of monoclonal antibodies approved by the FDA for cancer treatment
| Name of monoclonal antibody | Type of cancer treatment |
|---|---|
| Epidermal Growth Factor Receptor (EGFR) | |
| Amivantamab | non-small cell lung cancer (NSCLC) |
| Cetuximab | metastatic colorectal cancer and head and neck cancer |
| Nimotuzumab | squamous cell carcinoma, head and neck cancer, nasopharyngeal cancer, glioma |
| Panitumumab | colorectal cancer |
|
| |
| VEGF | |
|
| |
| Bevacizumab | colorectal cancer, glioblastoma, and breast cancer |
| Ramucirumab | gastric cancer or gastro-esophageal junction (GEJ) adenocarcinoma |
|
| |
| Programmed Cell Death Protein -1 (PD-1) | |
|
| |
| Nivolumab | unresectable or metastatic melanoma |
| Pembrolizumab | unresectable or metastatic solid tumor with certain genetic anomalies melanoma and other cancers |
| Cemiplimab | metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC |
|
| |
| Programmed death-ligand 1 (PD-L-1) | |
|
| |
| Atezolizumab | urothelial carcinoma, NSCLC, triple-negative breast cancer, small cell lung cancer, and hepatocellular carcinoma |
| Avelumab | Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma |
| Durvalumab | certain types of bladder and lung cancer |
|
| |
| Human Epidermal Growth Factor Receptor 2 (HER2) | |
|
| |
| Ertumaxomab | breast cancer, etc. |
| Margetuximab | breast cancer |
| Pertuzumab | cancer |
| Trastuzumab | breast cancer |
| Trastuzumab duocarmazine | breast cancer |
| Trastuzumab emtansine | breast cancer |
|
| |
| CD20 | |
|
| |
| Rituximab | chronic lymphocytic leukemia |
| Human or humanized anti-CD20 antibodies | B-cell non-Hodgkin’s lymphoma |
| Ibritumomab | treatment of non-Hodgkin’s lymphoma |
|
| |
| CD-30 | |
|
| |
| Brentuximab (+mono methyl auristatin E) | Hodgkin’s lymphoma |
|
| |
| CD52 | |
|
| |
| Alemtuzumab | chronic lymphocytic leukemia |
|
| |
| CTLA-4 | |
|
| |
| Ipilimumab (Yervoy) | Melanoma |
Immune checkpoint inhibitor
T cells are the primary therapeutic target for the immune checkpoint inhibitor. Manipulating Immune Check Points increases the immune system's ability to recognize and kill cells that asseverate antigens by CD8+effector T cells (CTLs). Controlling these checkpoints activates CD4+helper T cells, combining adaptive and innate effector pathways. These can act as co-stimulatory and inhibitory agonists, amplifying antigen-specific T-cell responses. Tumor immune evasion can occur by inducing immune tolerance and resistance to immune effector cell destruction.[18] Tumors manipulate their microenvironment through the release of cytokines, chemokines, and other soluble factors. This process, also known as “immuno-editing,” assists tumors in evolving mechanisms to evade the host's immune response. Inhibiting the CTLA-4 and PD-1 checkpoints boosts the immune response in cancer, and these are well-studied cancer targets.[15,19]
CYTOTOXIC T LYMPHOCYTE-ASSOCIATED PROTEIN 4 (CTLA-4)
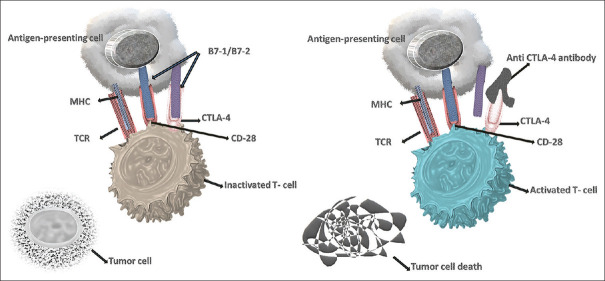
It is a significant discovery in the field of cancer therapeutics, and James P. Allison was awarded the Nobel Prize in 2018. It was the first immune-checkpoint receptor to be studied in a clinical setting. CLTA4 regulates the early phases of T-cell activation and stabilizes the T-cell co-stimulatory receptor CD28.[20,21,22] CD28 is the most potent co-stimulatory receptor present on the cell surface of T-cells and its ligands, B71 and B72, are expressed on antigen-presenting cells [Figure 1], activates toll-like receptors when microbes are present. Anti-CD28 antibodies have also been shown to suppress T-cell activation and proliferation. CTLA4 shares structural and molecular similarities with CD28, and both genes are located on chromosome 2. (2q33.2).[23,24,25,26,27] CTLA4 blocks T-cell activation by directly inhibiting CD28, competing for co-stimulatory ligands, blocking immunological conjugate formation, and recruiting inhibitory effectors. CTLA4 outperforms CD28 due to its higher affinity for CD80 and CD86, resulting in T-cell suppression.[28,29,30,31] Cancerous cells up-regulate the expression of CTLA-4 on the T cells, which directly inhibits the T-cell activation in response to tumor cells.[14,15] Intracellular vesicles release CTLA4 at the immunological synapse to directly counter CD28 activation.[32]
Figure 1.
Blockage of CTLA-4 pathway by anti-CTLA-4: CTLA4 present on the T-cell and bound to B7-1/B7-2 of Antigen Presenting Cells (APC), though the higher expression of CTLA4 on T-cells results in inactivated T-cells and tumor growth continue. When anti-CTLA-4 was introduced, it bound to CTLA4 on the surface of T cells
Patients with stage IV advanced oral and maxillofacial cancers are treated with ex-vivo activated CTL. It shows the good clinical efficacy of adoptive immunotherapy. During the lymphocyte assay, patients infused with lymphocytes show a significant increase in CD8+ T cells and a decrease in CD4+ T cells when compared with un-stimulated peripheral blood mononuclear cells (PBMCs). Elevated levels of IFN-γ were observed in infused lymphocytes as compared to PBMCs or tumor cells. Tumor regression was observed in the patients who received CTL infusion but did not go for surgery. Significant diminution of the tumor size was observed in patients who CTL therapy. Recently therapies targeting immune system checkpoints are emerging.[33] Many therapeutic agents are now being developed to target checkpoints and keep the immune system well informed. The commercial name of the CTLA-4 drug is Ipilimumab (Yervoy), which targets the T-lymphocyte immune checkpoint CTLA4. The prime goals of this checkpoint inhibitor therapy are to perk up the survival rate, better manage the symptoms, and enhance the quality of life. The histology of the patients who received CTL therapy shows infiltrated lymphocytes. Recurrent disease and/or new disease lesions were not seen in individuals who underwent CTL treatment as adjuvant therapy. This means that CTL activation using autologous tumor cells as immunogens may be able to fight a number of different cancers.[34]
PD-1
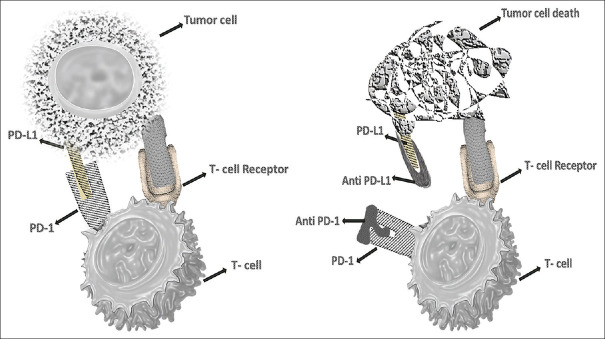
The Immune Checkpoint PD-1 is also up-regulated in cancer patients. T-cell activity in peripheral tissue is inhibited to prevent an inflammatory response to infection and to minimize autoimmunity.[35,36,37,38,39] It also inhibits T-cell function, allowing the tumor to evade the immune system. T-cells induce expression of PD1, which then inhibits T-cell activation by inhibiting kinases involved in T-cell activation through SHP2 phosphatase.[35,36] Increased PD1 expression inhibits the T-Cell response 'stop signal’, affecting the extent of T-cell–APC or T-cell–target cell contact [Figure 2].[40] When PD1/PDL2 is combined with other conventional treatments, it improves response and overcomes the class resistance problem.[41] In the presence of ligand, PD1, like CTLA4, is highly expressed in TReg cells and promotes proliferation.[42] TReg cells help the tumor to inflate and lead to suppression of the immune response. The PD1 pathway may also boost anti-tumor immune responses by lowering intra-tumoral TReg cell numbers and/or suppressive activity. The exact method by which CTLA4 inhibits T-cell activation is uncertain, but activation of protein phosphatases, PTPN11 and PP2A, has been postulated.
Figure 2.
Blockage of PD-1/PDL-1 pathway by anti-PD-1/PDL-1: PD-1 expressed on the activated T cells. The receptor-ligand interaction induces an inhibitory antitumor activity. Whereas the administration of anti-PD1 counter the inhibitory anti-tumor response and unleash the T-cell activity by upregulating the T-cell activation and proliferation by enhancing effector function and subsequent tumor cell death
Moreover, the commercially available PD-L1 inhibitors are Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab (Imfinzi), Pembrolizumab (Keytruda), Nivolumab (Opdivo), and Cemiplimab (Libtayo). These drugs are administered via intravenously (IV). Anti-programmed death-ligand-1 (PD-L1), as well as T-cell checkpoint inhibitors CTAL-4 and PD1, have transformed the treatment of patients with melanoma, lung cancer, renal cell carcinoma, and bladder cancer, among other cancers.[43,44,45,46] As the first immunotherapeutic gene therapy for B-cell cancers, autologous T-cells that were changed to express chimeric antigen receptor (CAR)-T cells that recognize CD-19 were given the green light.[47] According to research, immune-checkpoint inhibitors improve chemotherapeutic response.[48]
Lymphocyte activation gene 3 (LAG3)
The powerful effects of CTLA4 and PD-1-induced immunotherapy treat cancer patients, though a large number of patients with various tumor types do not respond to the therapy.[49] Therefore, an alternative regimen is required for the efficacy of the therapy. The lymphocyte activation gene-3 (LAG3) is an immune checkpoint analogous to PD-1. LAG3 express on NK cells activated T cells, dendritic, and B cells with an MHC-II interaction. LAG3 upregulation prevents autoimmunity with a structure similar to CD4 cells.[50] Nonetheless, the constant exposure of antigen in tumor cells results in LAG3 overexpression with a contribution to a state of exhaustion manifested in impaired proliferation and cytokine production.[51] The LAG3 inhibitor receptor shows a potential cancer therapy with anti-PDL-1 and suppresses the tumor-mediated immune.[52] In melanoma (B16-F10) and fibrosarcoma (Sa1N), LAG3 is co-expressed with PD1 on tumor-infiltrating CD4+ and CD8+ T cells. The major tumor-infiltrating lymphocyte (TIL) population in CT26 colon cancer is CD8+ T cells that express both LAG3 and PD1, resulting in hypofunction.[53,54]
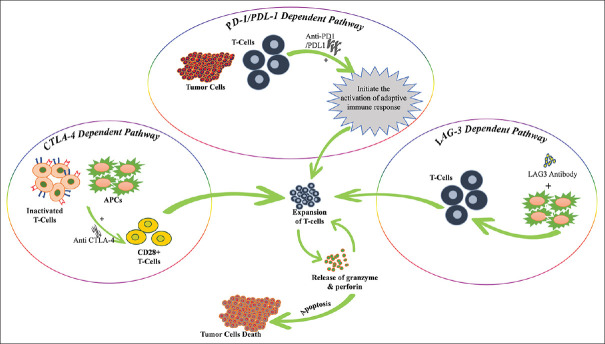
Clinical evidence has demonstrated a synergistic interaction between LAG3 and PD1. Tumor samples were analyzed for inhibitor receptor expression and their effect on intratumoral T-cell activity, paving the way for combinational immunotherapeutic regimens [Figure 3].
Figure 3.
PD-1/PDL-1-dependent pathway: PD-1 expressed on the activated T cells. The receptor-ligand interaction induces an inhibitory antitumor activity. Whereas the administration of anti-PD1 counter the inhibitory anti-tumor response and unleash the T-cell activity by upregulating the T-cell activation and proliferation by enhancing effector function. Therefore, the T-cell receptor binds with the MHC molecule on APC and releases the perforin and granzyme for tumor death. CTLA-4-dependent pathway: Anti CTLA4: the anti-CTLA4 blocked the CTLA4, when bound with the Fc receptor on APC and induce antibody-dependent cellular toxicity. Though the higher expression of CTLA4 on CD4+, CD25+, and T regulatory cells make them prone to anti-CTLA4 induced cellular toxicity. Anti- CTLA-4 can binds to CTLA4 on the surface of Treg cells, preventing it from counter-regulating T-cell activation via CD28-mediated co-stimulatory pathways. By blocking CTLA4 on the surface of activated conventional T cells, -CTLA4 can increase T-cell responses. LAG-3-dependent pathway: The activity of LAG3: the activation of dendritic cells depends on the soluble LAG3 followed by MHC class II molecule signaling in lipid rafts microdomain. Furthermore, the mature dendritic cells induce the production of IL-12 and expression of CCR7 which allows migrating in the T-cells region followed by activation of T cells. The maturation and differentiation of T cells followed by tumor cell death
Target-specific monoclonal antibodies
Over two decades, we established the phenomenal characteristics and potential of MABs, which are certainly used in the treatment and selection of infectious diseases. After binding to target antigen epitopes, MABs have antitumor activity. However, the antitumor activity of MAB has been accomplished by conjugation among MAB and effector molecules, which leads to cell death by the internalization and downregulation of signaling in the tumor microenvironment.[55] Thus, the specificity of MAB against the antigen allows them to be useful antitumor agents. The MAB follows the various strategies for cancer treatment, that is, alteration of host response, distribution of cytotoxic phenomena, and retargeting of the cellular immune response toward cancer.[56] The binding of antigen and MAB on tumor cells and previously Fc receptor of immune cells identify the cell-bound MAB in altered host response.[57] The receptor cross-linking upregulates the cytotoxic phenomenons which prompt the tumor cell apoptosis.[58] MABs used in cancer immunotherapy suppress the proliferation of tumor cells by blocking the specific downstream pathways. Although there are two classes of MAB identified based on whether they contain medicines or radioactive substances. The first class of MABs is self-acting, non-conjugated, naked MABs that bind to antigens on cancer cells, such as alemtuzumab and trastuzumab. The second type of MAB is conjugated MABs, which use chemotherapeutic medicines or radioactive particles as targets to deliver these MABs to cancer cells.[59] Drug conjugated MABs include gemtuzumab ozogamicin, and radioactive conjugated MABs include ibritumomab thiuxetan. Anti-EGFR medicines include MABs, tyrosine kinase inhibitors (TKIs), vaccine-based immune therapy, and antisense therapies. On the other hand, MABs and TKIs have been shown to be effective anti-EGFR therapies in clinical trials.[60,61]
EGFR-targeted monoclonal antibody
The epidermal growth factor receptor (EGFR) is a HER family tyrosine kinase receptor. The PI3K/AKT, RAS/RAF/MEK/ERK, and PLC/PKC pathways are all activated when a ligand binds to receptors. EGFR activation by gene amplification, protein overexpression, or mutation has been linked to several human epithelial malignancies (e.g., NSCLC, CRC, glioblastoma, and breast cancer). Thus, targeting EGFR as a cancer therapeutic technique has been researched for two decades.[62] The antitumor effect of EGFR MABs is mediated by specialized structures with various roles. Cetuximab, panitumumab, nimotuzumab, and necitumumab are the four main EGFR MABs currently authorized for clinical use.
Cetuximab
Cetuximab is a recombinant monoclonal antibody that targets epidermal growth factor receptor (EGFR), human epidermal growth factor receptor (HER-1), and c-ErbB-1. Cetuximab reduces cell growth by competitively hindering the binding of EGFR and other ligands, which finally leads to apoptosis by blocking the phosphorylation and activation of receptor-associated kinases. It has been successfully used in the treatment of several kinds of cancer, such as colorectal cancer, recurrent or metastatic head and neck squamous-cell carcinoma (HNSCC).[63,64,65,66]
Panitumumab
The mechanism of panitumumab is similar to that of cetuximab. Both drugs are competitive inhibitors of EGFR.[67] Panitumumab is currently the go to drug for the management of metastatic colorectal cancer.[68] Cetuximab and panitumumab are only effective in patients with metastatic colorectal cancer who have wild-type K-ras and not mutated K-ras (about 40% of patients).[69]
Vascular endothelial growth factor (VEGF)-targeted monoclonal antibodies
MABs that target the vascular endothelial growth factor (VEGF) pathway have been shown to be a valuable supplement to cancer treatment. VEGF-A, a member of the VEGF family, has been shown to have a role in angiogenesis.
Bevacizumab
It is a recombinant, humanised monoclonal antibody that acts as an angiogenesis inhibitor by targeting VEGF. It is used for the treatment of non-small cell lung cancer (NSCLC), colorectal cancer, glioblastoma, and breast cancer. Bevacizumab inhibits VEGF and prevents it from interacting with endothelial receptors, Flt-1, and KDR.[70] then results in inhibition of angiogenesis (endothelial proliferation and formation of new blood vessels).[70,71,72]
Ramucirumab
Ramucirumab is used as monotherapy or in conjunction with paclitaxel in advanced gastric or GEJ adenocarcinoma following one line of therapy. The response mechanism is a VEGFR-2 (vascular endothelial growth factor receptor-2) inhibitor licenced by the Food and Drug Administration (FDA). Ramucirumab did not show efficacy in a randomly selected first-line treatment patient cohort.
Cluster of differentiation (CD)
CD molecules are cell surface molecules that aid in cell identification and provide immunophenotyping targets for cells. In terms of physiology, CD molecules can work in a variety of ways, frequently acting as receptors or ligands crucial to the cell. A signal cascade is frequently started, changing the cell's behavior.[73] Many CD molecules act as targets in cancer therapeutics, for example CD-52, CD-20, CD-30.
CD-52-targeted monoclonal antibodies
CD-52-targeted MABs bind to CD52 antigens, causing cell-mediated cytotoxicity that is dependent on complement and antibodies. Patients with B-cell chronic lymphocytic leukaemia are treated with CD52 MABs (B-CLL). Alemtuzumab, which targets the lymphocyte antigen CD52, is currently used to treat chronic lymphocytic leukaemia (CLL). It is made up of 12 amino acids and is a polypeptide glycoprotein. Alemtuzumab recruits immune cells to destroy cancer cells.[74]
CD-20-targeted monoclonal antibodies
Ibritumomab targets another lymphocyte antigen, CD20, and directs radioactivity to the B cells. It is used for the treatment of non-Hodgkin's lymphoma.[75]
CD-30-targeted monoclonal antibodies
Brentuximab in combination with monomethyl auristatin E targets the lymphocyte antigen CD30. It is used for the treatment of Hodgkin's lymphoma.
CONCLUSION AND FUTURE PERSPECTIVE
MABs are very important tools for treating many diseases. More than 100 MABs have been approved by the FDA for use. But targets are limited to these MABs. Currently, most of the MABs are given in combination either with other MABs or with chemo/radiotherapy. This shows that challenges for successful immune checkpoint inhibition remain, such as identifying clear biomarkers and effective combinatorial techniques, growing resistance, and the cost-effectiveness ratio of these medicines. Combining existing and new immune checkpoint inhibitors and developing predictive biomarkers may significantly improve immunotherapy's success. A holistic strategy is required for the most effective combination of immunotherapies that can overcome resistance mechanisms.
Authorship contribution
Significant contributions to the work's conception or design, as well as its acquisition; Drafting or critically reviewing the work for essential intellectual substance.
Ethical approval
Not applicable because human subjects were not included in this study. Therefore, this study did not require approval ethics committee.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Mayank Jain is gratefully acknowledged the University Grant Commission to provide the Maulana Azad National Fellowship.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: Potential therapeutic approaches. J Immunol Res. 2016;2016:4273943. doi: 10.1155/2016/4273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol. 2005;174:2453–5. [PubMed] [Google Scholar]
- 5.Rapidis AD, Wolf GT. Immunotherapy of head and neck cancer: Current and future considerations. J Oncol. 2009;2009:346345. doi: 10.1155/2009/346345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–35. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med. 2008;359:613–26. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 8.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalanjiam V, Gopika Manoharan GM. A new alternative cancer treatment modality: Immunotherapy. SRM J Res Dent Sci. 2015;6:175–80. [Google Scholar]
- 10.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 11.Curran D, Giralt J, Harari PM, Ang KK, Cohen RB, Kies MS, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol. 2007;25:2191–7. doi: 10.1200/JCO.2006.08.8005. [DOI] [PubMed] [Google Scholar]
- 12.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 13.Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–65. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer. 2011;11:805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 17.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 18.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 19.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz RH. Costimulation of T lymphocytes: The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 22.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, et al. Cloning of B7-2: A CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–11. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 24.Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 25.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262:905–7. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 26.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87:5031–5. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 29.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–5. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 31.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chikuma S. CTLA-4, an essential immune-checkpoint for T-cell activation. In: Yoshimura A, editor. Emerging Concepts Targeting Immune Checkpoints in Cancer and Autoimmunity. Cham: Springer International Publishing; 2017. pp. 99–126. [Google Scholar]
- 34.Ohtani T, Yamada Y, Furuhashi A, Ohmura Y, Nakamura S, Kato H, et al. Activated cytotoxic T-lymphocyte immunotherapy is effective for advanced oral and maxillofacial cancers. Int J Oncol. 2014;45:2051–7. doi: 10.3892/ijo.2014.2599. [DOI] [PubMed] [Google Scholar]
- 35.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 39.Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19:813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 40.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleh K, Khalifeh-Saleh N, Kourie HR. Is it possible to rechallenge with PD-1/PD-L1 inhibitors after progression? Immunotherapy. 2018;10:345–7. doi: 10.2217/imt-2017-0180. [DOI] [PubMed] [Google Scholar]
- 42.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning YM, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, et al. FDA approval summary: Atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy. Oncologist. 2017;22:743–9. doi: 10.1634/theoncologist.2017-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 47.McNeel DG. Therapeutic cancer vaccines: How much closer are we? BioDrugs. 2018;32:1–7. doi: 10.1007/s40259-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleh K, Khalifeh-Saleh N, Kourie HR, Nasr F, Chahine G. Do immune checkpoint inhibitors increase sensitivity to salvage chemotherapy? Immunotherapy. 2018;10:163–5. doi: 10.2217/imt-2017-0153. [DOI] [PubMed] [Google Scholar]
- 49.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solinas C, Migliori E, De Silva P, Willard-Gallo K. LAG3: The biological processes that motivate targeting this immune checkpoint molecule in human cancer. Cancers (Basel) 2019;11:1213. doi: 10.3390/cancers11081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi Y, Chen L, Liu Q, Kong X, Fang Y, Wang J. Research progress concerning dual blockade of lymphocyte-activation gene 3 and programmed death-1/programmed death-1 ligand-1 blockade in cancer immunotherapy: Preclinical and clinical evidence of this potentially more effective immunotherapy strategy. Front Immunol. 2020;11:563258. doi: 10.3389/fimmu.2020.563258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zahavi D, Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel) 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–70. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via fcgamma receptor-mediated cross-linking. J Immunol. 2016;197:807–13. doi: 10.4049/jimmunol.1501351. [DOI] [PubMed] [Google Scholar]
- 59.Corraliza-Gorjon I, Somovilla-Crespo B, Santamaria S, Garcia-Sanz JA, Kremer L. New strategies using antibody combinations to increase cancer treatment effectiveness. Front Immunol. 2017;8:1804. doi: 10.3389/fimmu.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morelli MP, Cascone T, Troiani T, Tuccillo C, Bianco R, Normanno N, et al. Anti-tumor activity of the combination of cetuximab, an anti-EGFR blocking monoclonal antibody and ZD6474, an inhibitor of VEGFR and EGFR tyrosine kinases. J Cell Physiol. 2006;208:344–53. doi: 10.1002/jcp.20666. [DOI] [PubMed] [Google Scholar]
- 61.Yun J, Lee SH, Kim SY, Jeong SY, Kim JH, Pyo KH, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 2020;10:1194–209. doi: 10.1158/2159-8290.CD-20-0116. [DOI] [PubMed] [Google Scholar]
- 62.Cai WQ, Zeng LS, Wang LF, Wang YY, Cheng JT, Zhang Y, et al. The latest battles between EGFR monoclonal antibodies and resistant tumor cells. Front Oncol. 2020;10:1249. doi: 10.3389/fonc.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfeiffer P, Nielsen D, Bjerregaard J, Qvortrup C, Yilmaz M, Jensen B. Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol. 2008;19:1141–5. doi: 10.1093/annonc/mdn020. [DOI] [PubMed] [Google Scholar]
- 64.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 65.Mehra R, Cohen RB, Burtness BA. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol. 2008;6:742–50. [PMC free article] [PubMed] [Google Scholar]
- 66.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 67.Lynch DH, Yang XD. Therapeutic potential of ABX-EGF: A fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol. 2002;29:47–50. doi: 10.1053/sonc.2002.31522. [DOI] [PubMed] [Google Scholar]
- 68.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 69.Takamura N, Hombrados I, Tanigawa K, Namba H, Nagayama Y, de Verneuil H, et al. Novel point mutation in the uroporphyrinogen III synthase gene causes congenital erythropoietic porphyria of a Japanese family. Am J Med Genet. 1997;70:299–302. doi: 10.1002/(sici)1096-8628(19970613)70:3<299::aid-ajmg16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 70.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 71.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 72.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 73.Chan J, Ng C, Hui P. A simple guide to the terminology and application of leucocyte monoclonal antibodies. Histopathology. 1988;12:461–80. doi: 10.1111/j.1365-2559.1988.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 74.Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26:3644–53. doi: 10.1038/sj.onc.1210380. [DOI] [PubMed] [Google Scholar]
- 75.Witzig TE. Yttrium-90-ibritumomab tiuxetan radioimmunotherapy: A new treatment approach for B-cell non-Hodgkin's lymphoma. Drugs Today (Barc) 2004;40:111–9. doi: 10.1358/dot.2004.40.2.799423. [DOI] [PubMed] [Google Scholar]