Abstract
The increasing need for joint replacement surgeries, musculoskeletal repairs, and orthodontics worldwide prompts emerging technologies to evolve with healthcare’s changing landscape. Metallic orthopaedic materials have a shared application history with the aerospace industry, making them only partly efficient in the biomedical domain. However, suitability of metallic materials in bone tissue replacements and regenerative therapies remains unchallenged due to their superior mechanical properties, eventhough they are not perfectly biocompatible. Therefore, exploring ways to improve biocompatibility is the most critical step toward designing the next generation of metallic biomaterials. This review discusses methods of improving biocompatibility of metals used in biomedical devices using surface modification, bulk modification, and incorporation of biologics. Our investigation spans multiple length scales, from bulk metals to the effect of microporosities, surface nanoarchitecture, and biomolecules such as DNA incorporation for enhanced biological response in metallic materials. We examine recent technologies such as 3D printing in alloy design and storing surface charge on nanoarchitecture surfaces, metal-on-metal, and ceramic-on-metal coatings to present a coherent and comprehensive understanding of the subject. Finally, we consider the advantages and challenges of metallic biomaterials and identify future directions.
Keywords: Implants, Metals, Alloys, Biocompatibility, 3D Printing, additive manufacturing
1.0. Introduction
Orthopaedic disorders affect millions worldwide due to traumatic injuries, advanced age, developmental and degenerative diseases, and other causes1. The primary treatment for these conditions often requires invasive surgical interventions involving metallic implants to substitute for or supplement the affected bone. Historically, when considering fracture fixation, these surgeries have often been a two-step surgical process; the first is to place the implant for fixation, and the second is to remove the device post-healing to allow more normal bone remodeling and implant complications. To reduce such secondary surgeries, innovation in metallic devices has spearheaded the field of dental and orthopaedic load-bearing implantation for the past several decades. Recently, advanced materials research in implantable metallic devices for bone disorders has significantly influenced human life quality, especially with the population’s median age increasing. Improved metals and metal alloys have consistently been used in biomedical applications due to their exceptional corrosion and fatigue resistance, high strength-to-weight ratio, longevity, and general reliability. However, these devices’ practical usage still has several challenges concerning acquiring and retaining permanent biological fixation at the implant site to avoid revision surgeries. Current biomaterials research aims to induce and accelerate early-stage bone tissue ingrowth around the implants. In addition to wound healing properties, these implants must have adequate biomechanical characteristics and biocompatibility to be considered permanent fixation devices. The definition of biocompatibility, although controversial, suggests the successful interaction of living biological tissue in apposition with nonliving materials. Recently, there have been significant changes in the perspective of biocompatibility, not only for passive implant materials but also for active tissue-engineering materials. The two majorly accepted principles of biocompatibility are biosafety and bio-functionality (or promotion of body function). Biocompatibility of an orthopaedic implant can be further sub-classified into 1) mechanical biocompatibility in terms of proper placement and stability of the implant at the surgery site, 2) biological compatibility in terms of tissue-material interaction, 3) histocompatibility in terms of adverse inflammatory and immune response, and blood compatibility. This is defined as the implant material’s ability to bond with the host tissue and promote an affected region’s healing. However, most metals and alloys currently employed in medical devices were initially developed by the aerospace industry for their mechanical properties and not biological compatibility. Therefore, metallic implants are inert in a physiological environment, which induces biocompatible behavior, an essential factor in determining the efficient application of such implants.
The importance of biocompatibility lies in minimizing the inherent limitations of metallic implants. Most importantly, these include ensuring that the body does not experience an adverse effect due to the implant (e.g., stress shielding) or antagonistic tissue-material interaction leading to fibrous tissue formation, eventually disrupting implant stability. This would guarantee that the device is implanted at the surgery site long enough to initiate a favorable biological response from the host. For implantable and fracture fixation devices, the material’s surface is the first and foremost factor that guides a device’s efficiency. This is important because the surface comes in direct contact with the host’s physiological surroundings; therefore, a device’s surface should be appropriately constructed and efficacious. Another essential factor is mechano-transduction, which relates to the device’s bulk properties, but this becomes a guiding force in the later part of implantation and usually follows surface interaction.
A significant proportion of implant usage is driven by elderly patients with other conditions, such as obesity and stress, and lifestyle changes and product innovation cases. Indeed, multiple clinical confirmations today exemplify the safety and efficacy of metallic implants for bone disorders. According to the Centers for Disease Control and Prevention (CDC), in 2010, over 40 million people in the United States were aged 65 and above, constituting 13% of the total population. The aged population in 2030 is expected to be twice as large, increasing to ~72 million and making up nearly 20% of the total U.S. population. The growing older population is expected to drive the dental, reconstructive arthroplasty, and craniomaxillofacial implant markets.2. The worldwide biomedical implant market is segmented by orthopaedic implants, cardiovascular stents, dental prosthesis, ophthalmic devices, neurostimulation grafts/, and others, and by origins such as allograft, autograft, xenograft, and synthetic graft. This review article focuses on classifying metallic implants by material types and how the materials science aspects of bulk and surface of metallic biomaterials are modified to enhance biocompatibility, i.e., biological responses such as early-stage osseointegration as well as new bone formation.
2.0. Metallic biomaterials and their applications in orthopedics and dentistry
A global surge in road accidents leading to trauma cases and continuously increasing demand for minimally invasive surgeries are favorably driving the need for the next generation of metallic biomaterials. Based on the market segmentation, titanium is expected to hold the largest market share due to its optimal biocompatibility, high strength-to-weight ratio, excellent fatigue, and corrosion resistance compared to other metals, stainless steel, and cobalt-chromium alloys. The orthopaedic biomaterials segment is expected to register a comparatively higher compound annual growth rate (CAGR) up to 2025 due to the growing elderly population and the rising prevalence of chronic diseases, including osteoarthritis and osteoporosis. Asia-Pacific is the most significant contributor to this growing market due to its large patient population, rising trauma cases, developing healthcare awareness, and improving infrastructure. The current generation of metallic implants available in the market is fabricated and modified based on their niche applications shown in Figure 1. A comprehensive representation of bulk and surface modifications is necessary for improving biocompatibility.
Figure 1.
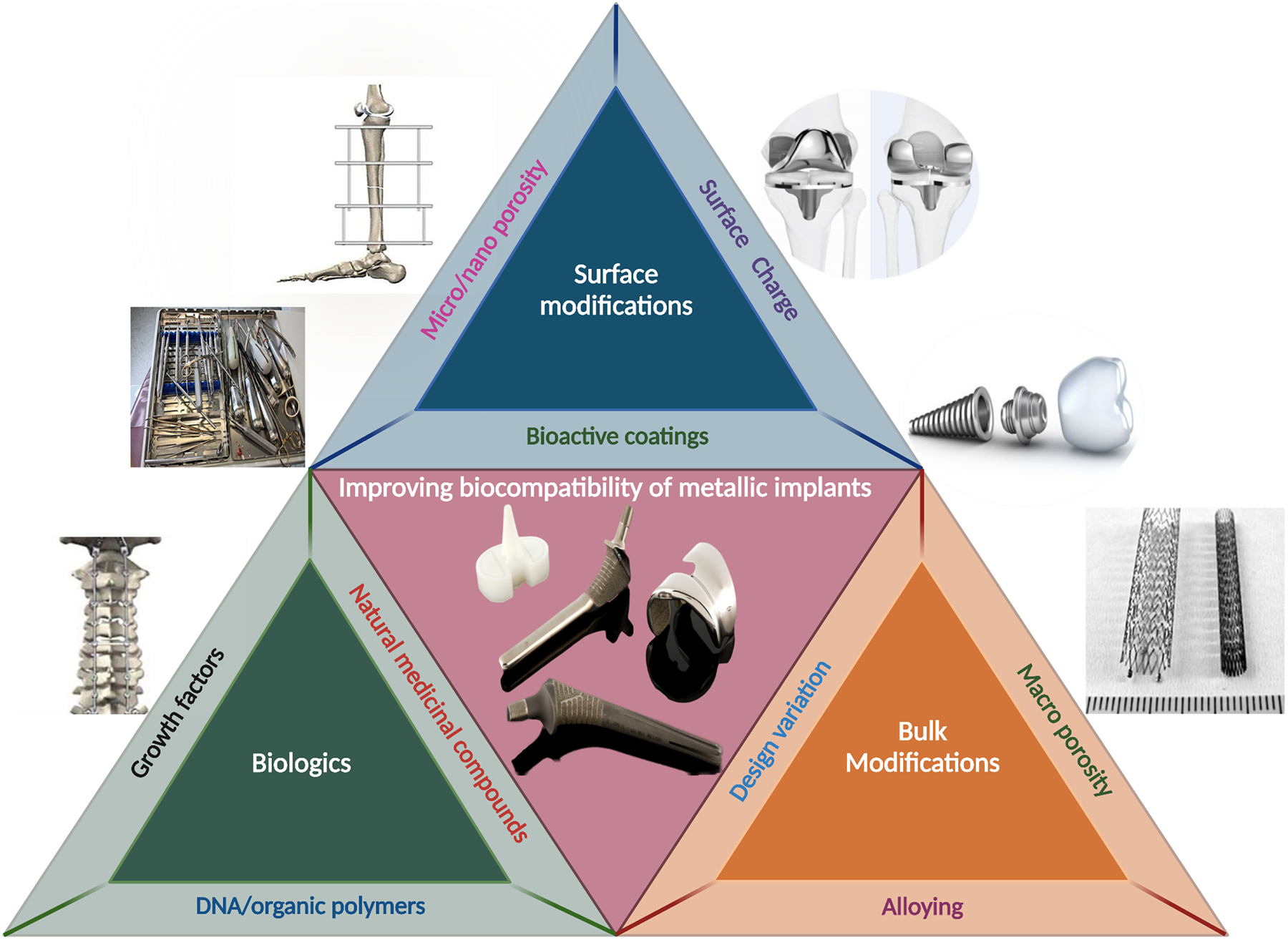
Interdependence of bulk modification and surface modification of metallic biomedical materials towards comprehensively improving biocompatibility. Representation of the current generation of some metallic implants used in the biomedical devices industry.
2.1. Metallic implants for load-bearing applications
Load-bearing implants such as total hip and knee arthroplasties are constructed out of metals with a high strength-to-weight ratio, good mechanical compatibility with bone, and excellent fatigue and fracture resistance for withstanding constant multi-axial loads. Therefore, the intrinsic mechanical properties of the bulk metals are a critical consideration for load-bearing applications. Young’s modulus, ultimate tensile strength, and fracture toughness define load-bearing implants’ mechanical compatibility. Additionally, the measure of fatigue strength in this context is equally paramount and serves as the baseline for design engineers’ fall on the historical success of forged Ti6Al4V or cast CoCrMo alloys to design new implants industry. Although fatigue strength is an intrinsic property, implants could be designed to have much lower fatigue resistance. The implication that only an increase in cross-section could successfully address the bio-mechanical loads and convince Regulatory bodies to deem these implants as safe based on just mechanical tests renders this parameter unwarranted to be used as a baseline. One of the primary reasons that the majority of the industry has chosen to stay with known alloys is the unknown risk of introducing new materials, proven by the limited examples in support of non-titanium based alloy in orthopedic except a few such as Oxinium (Zirconium Niobium alloy from Smith and Nephew), Roxolid (15 % zirconium and 85 % titanium).
CoCrMo, stainless steel, and titanium alloys have much higher Young’s modulus than the cortical bone. The significant difference in Young’s modulus may result in the implant bearing most of the load-bearing hip or knee implant load. Due to unbalanced load distribution, the surrounding bone bears less load, leading to adverse biological responses such as atrophy and cracks around the implant site, commonly known as the “stress-shielding effect.” Furthermore, bone degeneration at the surgery site may result in implant loosening, which may require revision surgery. Naturally, metallic load-bearing implants are desired to have a closer modulus to human cortical bone. Extensive research has been undertaken to remedy this difference or “mismatch” in modulus to fabricate metallic implants with lower modulus to enhance osseointegration and better load distribution in vivo. Among different options, introducing porosity in the bulk implant structure has been studied the most to lower effective Young’s modulus3–11. Additionally, alloying high-modulus metals like CoCrMo and Ti to reduce Young’s modulus has also been studied by various research groups12–18. Many examples of beta titanium alloys may be biocompatible and much lower Young’s Modulus. Unfortunately, though there has been some research on these13,14,19–23, adoption in clinical use is relatively low or scarce.
2.2. Metallic implants for articulating surfaces
Implants customized for load-bearing articulating surfaces are fabricated primarily focusing on their wear and corrosion resistance, maintaining a low friction coefficient. However, adverse tissue reactions, including implant loosening, remained a primary concern due to bio-tribo-corrosion and related metal ion release in vivo. Although exact reasons behind such aseptic loosening have not yet been fully understood, research and surveys show micro-particulate byproducts from corrosion and wear-induced damage in an artificial joint are of significant concern24–26. The initial performance efficiency of joint replacement devices is measured as a function of their potential for osseointegration, bone healing, and particle leaching susceptibility due to corrosion and severe wear. This initiates the body’s natural defense mechanism through macrophages to eliminate the foreign material27. The biological byproducts, substances, and cellular debris released during this inflammatory reaction contribute to bone erosion surrounding the implant, potentially resulting in implant loosening. Therefore, to address the problems related to corrosion and wear resistance of metallic implants, a significant reduction in the use of older CoCrMo femoral heads or conventional polyethylene liners is seen in recent years, which are replaced by novel bearings such as highly cross-linked polyethylene, ceramic femoral heads, and ceramic-on-ceramic articulations28,29. An implant’s longevity in the human body is primarily determined by fatigue strength, corrosion resistance, and wear properties. However, corrosion and wear debris cause severe adverse responses from the articulating surfaces of large joint implants, which gets further complicated due to the issue of fretting corrosion on modular implants – whether it is the issue of CoCrMo head on the taper junction on Ti6Al4V hip stem or the junction of proximal body of the Ti6Al4V hip stem with the distal section of the Ti6Al4V stem. These taper junctions are mechanically mated during surgery – though machined to a very high finish and tolerance, there is always a certain amount of micromotion leading to fretting corrosion and the unavoidable crevice where crevice corrosion can start, therefore guaranteeing revision surgeries.
2.3. Metallic implants in dentistry
Everyday dental health and hygiene challenges are periodontal diseases and trauma, leading to partial or complete edentulism’s unfortunate predicament. Traditionally these problems were dealt with indirectly or by directly fixed restorations of crowns and bridges or a removable prosthesis with a mixture of precision attachments and other innovative designs. However, some of these solutions were either very invasive and/or cumbersome, like the ‘three-unit bridge’ where a significant loss of dental material for the middle tooth. Some of these remedies were challenging to work with as there was little to no soft and hard tissue support to make these feasible for daily activities like eating a regular meal. Naturally, this led to developing a new idea to try and create a means to improve upon the dental prosthetic’s retention and function for the patient. These new endosseous dental implants were fabricated with metals with excellent corrosion resistance and quickly stabilized at the implantation site. Such properties are essential for these implants because the local environment is exposed to enzymes and acids from food, which corrode the implant’s surface. Moreover, quick stabilization is required to ensure faster patient healing and rehabilitation. The innovations in dental implantology were primarily from a material design perspective. The metal structures’ surface was either machined or textured using 1) acid or laser etching, 2) blasted to form titanium oxide, and 3) hydroxyapatite-coated, sintered, or porous titanium plasma sprayed.
For decades, these metals have been used in clinical applications with bulk and surface modifications to elicit a favorable biological response and their mechanical compatibility. However, most of these metals have been historically borrowed from the aerospace industry for their favorable mechanical properties that align with the biomedical industry’s demands. Figure 2 represents some critical structural and functional modifications employed on metallic biomedical devices to enhance their biocompatibility. Namely, those modifications include – (1) addition of porosity for better biological fixation in vivo; (2) addition of surface charge for faster healing; (3) modification of bulk materials chemistry to reduce modulus and/or improve inherent biocompatibility of the alloy; (4) addition of bioactive calcium phosphate coatings to improve implant-tissue integration in vivo and (5) addition of biologics on the surface of the metallic implants for faster healing. The following sections of this review will focus on those modifications that improve metallic devices’ biocompatibility in greater detail.
Figure 2.
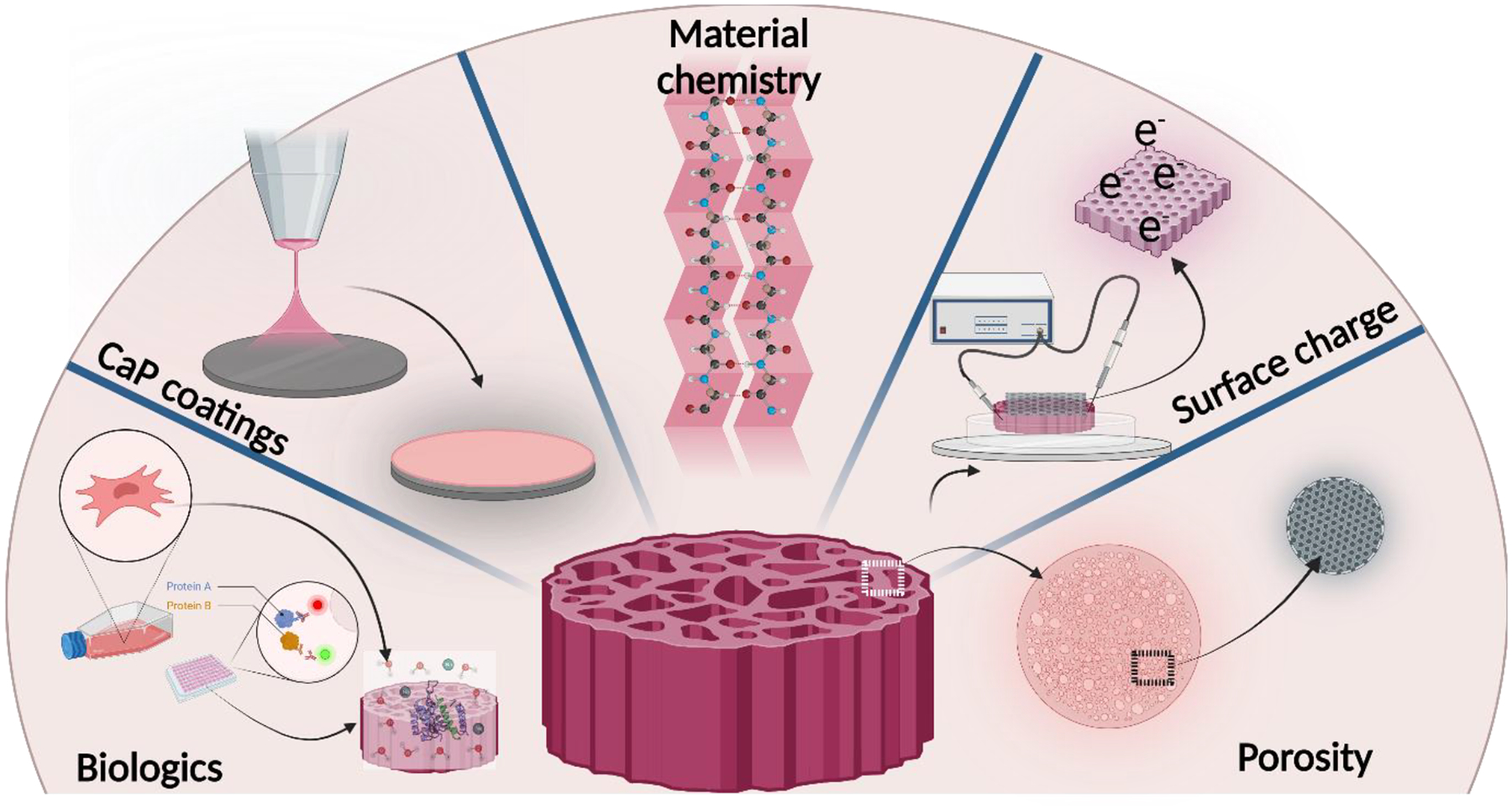
Common bulk and surface modification approach of metallic implants to improve biocompatibility.
3.0. Addition of porosity to improve the biocompatibility of metallic biomaterials
It is generally accepted that implants’ porosity has an essential role in biological fixation during the early stages of osseointegration. Historically, the industry made use of sintered beads or wire mesh pads on the implant surface. These structures were inherently smooth to both the touch and likely to osteoblasts. Further, sintering on titanium alloys resulted in grain growth and notches due to point contacts of the beads or line contact of the wire pads. Though these implants’ clinical success, there have been multiple beads shedding examples in clinical use. This was countered by spraying Ti on Ti implants to create a random porous structure. The substrate was kept metallurgically cool to prevent grain growth. The presence of interconnected three-dimensional (3D) pores in the implant helps promote cell adhesion, mechanical interlocking between host tissue and scaffold via bone ingrowth, and transport of nutrients and metabolic waste. However, porous structures must also have sufficient strength to withstand in vivo stresses at the application site until newly formed bone shows ingrowth into the porous volume, creating mechanical interlocking with the implant surface. Pore size, volume fraction porosity, pore-pore interconnectivity, and pore shape strongly influence implants’ mechanical properties. Figure 3 shows some commercially available porous metal implants and their real-world application in the orthopaedic industry.
Figure 3.

Commercially available porous metallic orthopedic devices. A. Arcam designed hip cup using electron beam melting (EBM) technology44, B. Lattice-structured implant prototype, additively manufactured by Imperial College London45, C. Alphaform produced 3D printed titanium alloy bone-implant using EOSINT M 280 for the reconstruction of the hip bone for a cancer patient in 201446.
3.1. Processing porous metal implants
One of the primary characteristics of metallic orthopedic implants is their structure and shape correlation with the patients’ defect site or the target bone. Porous implants are better suited for such a role due to the advantage of good bone in-growth through the implants’ open porous geometry, which leads to faster osseointegration and healing. Until recently, fabrication of orthopaedic implant structures was accomplished using Computer Numerical Control (CNC) based methods that employed powder metallurgy principles in processes such as gas foaming47–51, plasma spraying52–55, sintering56–58, vapor deposition59–61, combustion synthesis62–64, and fiber mesh structures65–67. However, these conventional methods only allowed for the fabrication of implants with moderately complex geometry. To achieve higher efficiency in the design and fabrication of complicated parts where patient-customization is important, additive manufacturing (AM) methods based on computed tomography (CT) or magnetic resonance imaging (MRI) images became popular in designing individual implants68,69. In contrast to conventional methods, additive manufacturing technologies allow the fabrication of complex-shaped parts with predefined and customizable external shapes and internal architectures in a highly efficient way70,71 in addition to high degrees of geometric flexibility, low production times, high material utilization rates, and true near-net-shape capability, enabling the production of complex-shaped parts which cannot be readily fabricated through conventional manufacturing techniques72. AM has experienced over 20 years of development and is now one of the most quickly developing advanced manufacturing technologies in the world73.
In porous metallic orthopaedic implants and scaffolds, additive manufacturing has been believed to be driving the recent advances in this field. Numerous additive manufacturing technologies (described in Table 3) have been researched and commercially implemented to fabricate patient-specific orthopaedic load-bearing porous metallic implants.74–76 In other orthopaedic applications, such as scaffolds to facilitate bone tissue engineering, considerable advancements have been made by additive manufacturing technology. Porous Ti scaffolds with surface modifications to improve bioactivity, porous Mg scaffolds have shown promising, and much desired, biodegradable properties, etc., have been researched to a great extent77,78.
Table 3.
enlists the primary manufacturing methods, pore characteristics, and key findings in porous metals in orthopedic applications
| Manufacturing method | Materials | Pore architecture | Key Findings |
|---|---|---|---|
| 3D fabrication | Ti6Al4V | Cross patterned hatching |
|
| Electron Beam Melting (EBM) | Ti6Al4V | Variation in shapes of the unit-cell | |
| Selective Laser Melting (SLM) | Ti6Al4V |
|
|
| EBM | Cobalt-chrome alloys | Variation in shapes of the unit-cell |
|
| SLM | Nitinol | Unit-cell variation along with gyroid cross-hatching |
|
| Liquid metal dealloying | TiZr alloys | Removal of magnesium after dealloying using etchants |
|
| Titanium wire space holder approach (TWSH) | Porous magnesium | Immersion of TWSH into molten magnesium followed by etching to remove Ti |
|
Broadly, additive manufacturing of porous metal structures could be accomplished using powder-bed-based or direct energy-based methods. Powder-bed-based methods could be further classified into Binder-jetting based and powder-fusion-based processes (Fig 4. a. i), and the directed energy deposition-based methods could either be used for metal powders or filaments (Fig. 4. a. ii). The porosity of the structures made of either process could be tailored to obtain up to 80% porous structures. The microstructure and the mechanical properties of either process could be altered by altering the process parameters such as distance from the substrate, energy input, size of the part to be built, orientation, and location. The SEM image of the microstructure of the porous Ti alloy structures built using SLM and EBM is shown in (Fig. 4. b. ii, iii, and iv). The SEM images of surface microstructures from either method indicate small spherical metal particles from unmolten metal particles sintered at the surface.79,80
Figure 4. a. Schematic representation of metal implant additive manufacturing techniques,
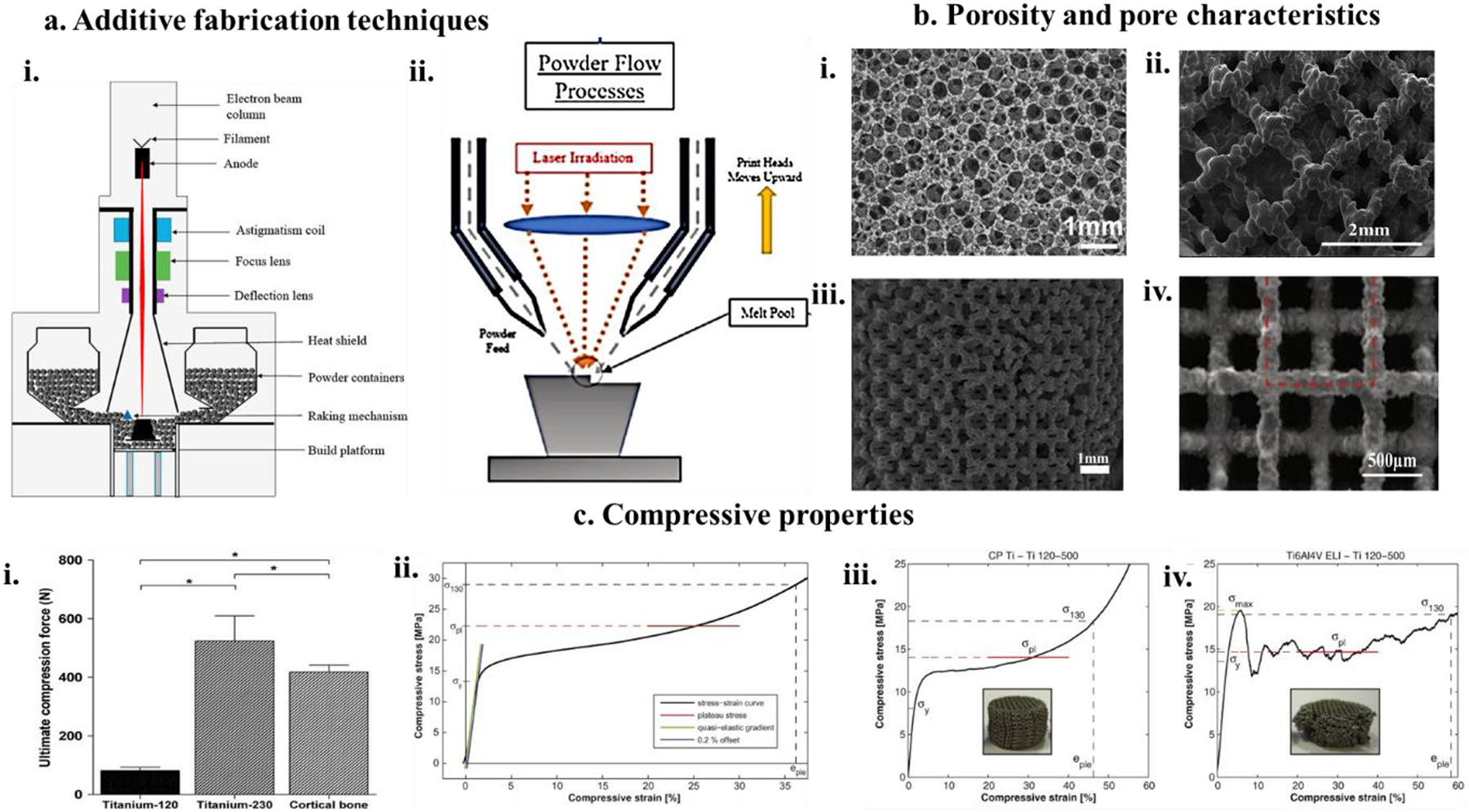
i) Powder Bed Fusion (PBF) implant fabrication involves the use of an electron beam or high energy laser to melt powder particles on a metal powder bed to fabricate the desired shape and structure of implants81, ii) Directed Energy Deposition (DED) fabrication involves metal powder flow through multiple nozzles and simultaneously being exposed to a high energy laser beam which forms a metal melt pool on the substrate gradually taking shape through layer-wise deposition of molten metal and rapid cooling81. b. Pore properties of fabricated metal structures, i) SEM micrograph of 80% porous Ti sponge sheet fabricated using slurry foaming showing similar pore characteristics as cancellous bone82. ii) Shows the interconnected pore structure of a Ti metal cage 3D printed using electron beam melting technology with a trabecular pore diameter of 413 ± 78 μm. Pores > 300 μm are suitable for bone ingrowth and fusion with metal cage83, iii) Selective laser molten (SLM) porous Ti structures with 230 μm strut size and 500 μm pore size84, iv) SLM fabricated 80% porous Ta implants85. c. Compressive properties of additively manufactured porous metal structures for biomedical applications i) porous Ti with 230 μm strut size and 68% porosity86, ii) Ta structures with 80% porosity85, iii and iv) CpTi and Ti6al4V-ELI structures with 120 μm and 500 μm pore size, respectively87.
On the other hand, porosity could be introduced in 2 ways, individually or together: using optimized process parameters to introduce inter-particle porosity or using appropriate tool paths to introduce porosity. Thus, very high control over the porosity and the pore morphology could be attained by Directed Energy deposition techniques (DED). The total energy input to the melt pool could be determined by altering the process parameters, which facilitates partial melting of the metal particles, and these surface melted particles join at the particle-particle interface leaving some inter-particle porosity. The toolpath-based porosity is because of the altering thickness of and the gap between the metal rods. Introducing porosity produces parts with functionally graded porosity or the porosity’s exact internal architecture. The schematic for the fabrication of porous parts using DED has been shown in (Fig 4. a. ii). Additionally, additive fabrication of porous structures gives precision control over pore size, shape, and geometry and provides the flexibility of mechanical property alteration such as compressive stress, compressive strain, and elastic modulus. Fig. 4. c. i–iv shows examples of volume fraction porosity and strut size dependence of porous metal implants’ compressive properties.
3.2. Biological response of porous metal structures
Biological response as a measure of biocompatibility of porous metal structures is based on mainly two types of physiological response, cytocompatibility evaluation in a static in vitro cell culture medium where the cells are directly grown and proliferated on the surface of the implants and biological response from a more dynamic in vivo living animal model. Both these characterization techniques are equally important in determining the biocompatibility of any biomaterial qualitatively and quantitatively. Researchers have studied in vitro response of porous metallic implants in various ways. Cellular growth, proliferation, morphology, and viability depend on the chemistry of the sample’s surface, pore size, morphology, interconnectivity, and pore distribution. However, the literature on in vitro and in vivo cellular response of porous metal implants is primarily divided into evaluations based on volume fraction porosity and pore geometry. 27% volume fraction porosity in Ti and 27% and 45% volume fraction in porosity Ta have been shown to exhibit a considerable growth and adhesion of osteoblasts (OB) implant surface at day 11 of culture (Fig. 5. a. i). Ti surfaces showed more rounded OB morphology and underdeveloped filopodia than Ta at early stages4. However, Ta’s difference in volume fraction porosity did not contribute to cell viability, which was corroborated through focal adhesion measurement using vinculin immunostaining assay (Fig. 5. b. ii and iii). In addition to volume fraction porosity, it is believed that higher surface area due to smaller pores and rough morphology of pores are beneficial for cell growth and better cell attachment favoring bone tissue ingrowth in vitro52,97. However, contradictory results have also been concluded in several studies showing smaller pores (~100μm) lead to favorable chondrocyte attachment to the surface under hypoxic conditions as opposed to direct inset of osteoblastogenesis in larger pores (<300 μm). In contrast, in an in vivo study, a porous Ti plate with pore sizes of 50 to 125 μm implantation in the rabbit femur concluded that bone ingrowth in porous metallic structures is independent of pore size under non-load-bearing conditions. However, this study involved assessing osteonal structures ingrown into the implant’s pores. Osteons are bone structures typically structurally ranging in nanoscale, which can be a potential reason for the unbiased observation of pore size dependency98. The effect of porosity on cell growth’s vitality after a 14-day culture on laser-formed porous Ti6Al4V alloy99 and live/dead cell assay showed that the vital cells had extensively grown throughout the sample with minimal isolated disrupted individuals. The direct laser forming process for the fabrication of porous Ti6Al4V samples did not introduce any undesirable biological properties100–102. Selective laser melting (SLM)-based fabrication of metallic structures showed pore shape and size’s contribution to the proliferation and morphology of human periosteum-derived cells (hPDC)103. The porosities for the study were designed in variations based on geometrical shapes (triangular (T), hexagonal (H), and rectangular (R)) as well as sizes (pores with 500μm and 1000 μm diameters). Overall, the DNA observed on the samples with 500μm pores was higher than that on the 1000μm samples compared to structures with lower porosities based on cell-growth media. Pore shape was a determining factor, with hexagonal pores showing higher cell bridging efficiency than triangular and rectangle-shaped pores based on the surface area. However, studies have refuted the pore shape dependency of cell attachment based on physical phenomena such as surface tension104,105. The surface tension of a curved pore is lower than that of pores with sharp edges, which has been deemed the primary reason for higher cellular affinity. Previously reported in vitro observations on porous Ta structures revealed a similar pore size effect on cell migration and growth4. Furthermore, human adipose-derived stem cells (hADSCs) were found to attach to porous Trabecular Titanium (TT) surface and subsequently proliferated and differentiated to acquire an osteoblast-like phenotype (Fig. 5. b. ii). These cells were further analyzed to produce proteins such as fibronectin, osteocalcin, osteonectin, and decorin, which are unique protein expressions indicating ECM formation. These results suggested the potential implementation of porous TT scaffolds in bone regeneration106,107.
Figure 5. a. Cell-materials interaction as a function of cellular morphology investigation,
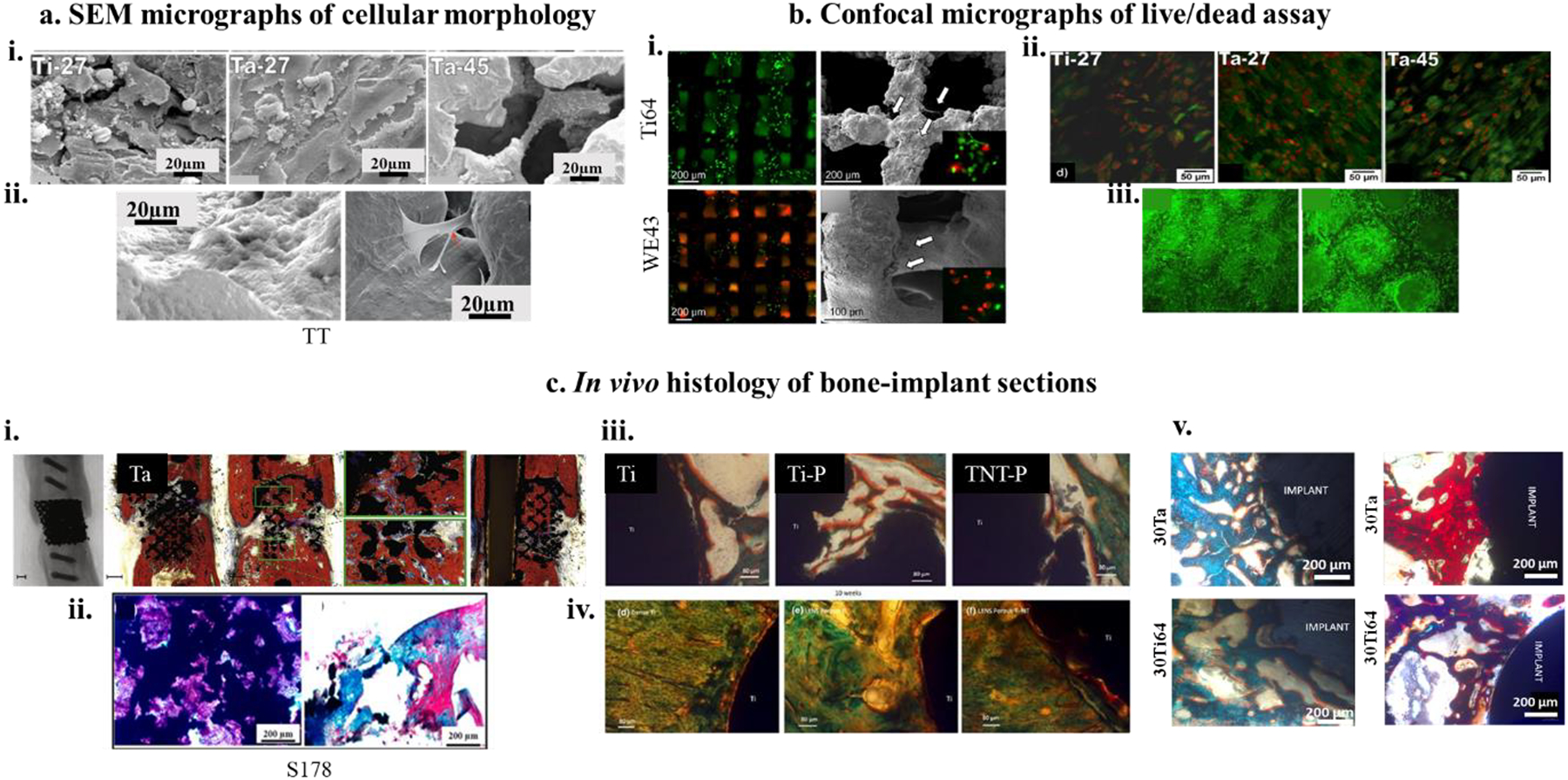
i) SEM micrographs of hFOB morphology on DED processed 27% porous Ti and Ta and 45% porous Ta at day 11, showing Ta-27 has the most optimized surface for cell-material interaction with cellular confluence as well as excellent proliferation4 ii) shows highly efficient focal adhesion of human adipose-derived stem cells (hADCs) on trabecular tantalum (TT) surface106. b) Fluorescence assay of dead and living cells on biocompatible metal surfaces. i) (Top left and bottom left) show fluorescence micrographs of Ti64 and Mg alloy (WE43) at 4h after seeding MG-63 osteoblast-like cells as compared to 24h fluorescence images (Top right and bottom right113) ii and iii) ALP protein expression for hFOB cells on 27% porous Ti and Ta and 45% porous Ta has shown through fluorescence micrographs. In contrast to SEM micrographs, Ta-27 did not show strong ALP expression at day 114. c) In vivo bone formation and osseointegration analyses, i) X-ray and histological images of open porous SLM-processed Ta revealing a fair amount of osseous growth for both the porous Ta specimens with almost 100% bridging of the defect85, ii) H&E (left) and Masson-Goldner (right) stained bone sections after 12 weeks of implantation of Ti64 implants with 178 μm pore size showing new bone formation around the porous metal implant,122 iii and iv) Photomicrograph showing the histology images after 4 weeks (top row) and 10 weeks (bottom row) for Ti, porous Ti (Ti-P) and porous Ti with nanotube surface (TNT-P) where signs of osteoid like new bone formation could be seen in orange/red color. Modified Masson Goldner’s trichrome staining method was used143,v) In vivo biological response from tantalum parts fabricated using direct energy deposition (DED) showing early-stage osseointegration at 5 weeks (left column) as a function of designed porosities and extended new bone formation at 12 weeks (right column)37.
A prominent metallic shape memory alloy in clinical practice for over two decades now is nickel-titanium, also known as nitinol (NiTi). NiTi has seen the most prolonged and most extensive history of application in dentistry. However, other clinical applications such as orthopedics, maxillofacial, and cardiovascular have also developed the use of NiTi in recent times108–110. Recent research to explore the field of porous NiTi in the light of in vitro biological response. Evaluation of cellular behavior of fibroblasts L-929 on porous NiTi (pore size – 400μm–900μm) showed healthy cell growth within the pores of the specimens and around the edges111.
The degradation of a porous metallic sample in the biological environment is significant as porosity on the sample increases the total surface area and increases the risks of metal ion leaching. Moreover, the chemistry of the surface oxides and the possible reactions with the physiological fluids significantly impact the porous structure’s applicability as a load-bearing implant. For example, porous magnesium’s in vitro degradation behavior was observed through the tendency to degrade at a controlled rate depending on their porosity with the non-toxic degradation product MgCl2112. However, cell-material interaction between OB and the degradable magnesium surface did not depend on degradation rates; instead, it showed favorable and enhanced cellular activity104,113. Thus, such materials could find an application at sites where a biodegradable implant structure with considerable mechanical strength is beneficial, such as bone defects, bone, and cartilage tissue engineering, substituting materials of lower mechanical strength such as hydroxyapatite (HA), natural polymers such as collagen and chitin, etc.114–116. Porosity plays an essential role by increasing the specimen’s surface area for higher protein adsorption, leading to enhanced corrosion resistance. Porous Mg surfaces can aid increased cell adhesion and proliferation and be a good candidate for biodegradable metallic bone implants. In vitro cytotoxicity assay with MG-63 osteoblast-like cells on porous Mg scaffolds (WE43) and Ti64 exhibited the scaffolds’ initial toxicity compared to Ti64 specimens. On direct contact with untreated Mg surfaces, cell lysis was observed in the first 4h. However, after 48h treatment of immersion in physiological serum, both surfaces showed cellular viability even after 24h of direct contact (Fig. 5. b. i).
In the case of in vivo studies, implants are manufactured and surgically placed into mice, rabbits, cats, dogs, pigs, etc. After a specific time interval, implants are harvested and subjected to histological examinations using different staining methods unique to structures such as osteoid, trabecular bone, collagen, etc. The morphology of new bone formation and interface integration is typically studied using optical or confocal or scanning electron microscopy (SEM), tomography, or radiography to learn about layer-wise bone formation in a 3D plane117–122. In vivo studies help reveal bone tissue development into and around the implants in live subjects, a more accurate evaluation of implant structures’ performance, particularly for longer-term applications. Characterizing the onset and rate of bone-remodeling in vivo can help develop the next generation of implants with higher integrity, mechanical compatibility, and enhanced tissue-material interactions123.
Ti6Al4V implants with a pore size of 178 μm (S178) have shown that the lamellar bone formation also increased with an increase in porosity, characterized by the depth of bone penetration into the implant. Highly porous implants are beneficial for faster healing in vivo; for example, at 4 weeks post-implantation, S178 and S279 specimens exhibited 26.8% and 31.5% bone volume/defect volume, respectively (Fig. 5. c. ii). In vivo bone formation in porous Ti mesh with 70 volume% porosity with three different pore sizes 188, 313, and 390 μm showed a much-pronounced porosity towards bone ingrowth at 12 weeks. Several in vitro studies have investigated the dependency of pore size of bone formation onto and into an implant, concluding that pore sizes ranging between 100–500 μm can aid in in vivo osteoinduction97,124,125. Within this range, pore sizes between 300–400 μm are optimal for osteoblast proliferation, differentiation as well as neo-vascularization within the pores of the implants; 200 μm pores to aid in early osseointegration, and pores >300 μm can aid in oxygen diffusion and nutrient transport that is needed for enhanced vascularization and new bone ingrowth97,126,127. A similar trend was observed with porous Ti implants with pore sizes 300, 600, and 900 μm (P300, P600, and P900 implants)128. With increased porosity in the P600 and P900 specimens, vascularization significantly increased, which led to enhanced bone ingrowth. This was attributed to the positive effect of increased curvature and surface area of larger pores. SLM fabricated porous Ti64 with different strut sizes, 120 μm, and 230 μm, resulting in different volume fraction porosities of 88% and 68% revealing bone growth adjacent to the bony implant proliferation into the pores in general86. The titanium-120 and titanium-230 specimens showed enhanced bone fixation compared to the empty controls, which exhibited reduced bridging of the defects and loss of fixation. In contrast, Bandyopadhyay et al.129 optimized volume porosity in porous Ti6Al4V implants to 23–32% and showed Young’s modulus to vary between 7 and 60GPa. The modulus of the LENS™ processed implants was observed to be close to that of cortical bone. Specimens with 25% porosity exhibited higher calcium content in vivo after 6 weeks of implantation. In addition, 25% porous specimens also revealed the highest bone tissue formation and surface bonding with the implants (Fig. 5. c. iii and iv).
Even though Ta has been used as a biomaterial since the 1940s, its use has been limited and compartmentalized. However, porous tantalum implants have shown better and quicker bone tissue in-growth and higher shear strength for in vivo implantation into distal femurs of dogs for 52 weeks130–136; it was observed that regardless of the pore size, the new bone tissue in growth rates has been consistently high, and it was observed that samples with large pore sizes had more significant initial bone ingrowth with implants of smaller pore sizes showing complete ingrowth. In contrast, in vivo bone ingrowth ability of porous Ta implants fabricated using the SLM technique with 500μm pore size and ~80% porosity137 revealed significant osseous growth for both the porous Ta specimens at 12 -weeks in Wistar rats. A detailed evaluation of individual longitudinal cross-sections showed better bone ingrowth for specimen 2, with almost 100% bridging of the critical-sized defect (Fig. 5. c. i). In vivo efficacy of orthopaedic implants also depends on the implant and host bone tissue’s initial surface reactions. These biochemical interactions depend on the implant’s surface characteristics such as roughness, hydrophilicity, pH dependency, and microstructure138–142.
Any physical alteration of the implant surface based on these factors can largely expedite bone binding to the implant surface. One of these techniques is rendering Ti implants’ surface with a nanoarchitecture in titania nanotubes138,144–146 to overcome the bioinert nature of Ti147. Such nanoarchitectured surfaces, in addition to customized complex porous geometry fabrication, using additive manufacturing cumulatively led to better bone-bonding due to a combinatory effect of biological fixation of bone into the pores and a bioactive fixation at the surface at both 5- and 10-weeks post-implantation (Fig. 5. c. iii and iv)143. A similar synergistic effect of inducing porosity in metallic bone implants combined with nanotubes resulting in enhanced osteoconductivity and improved interfacial mechanical properties for clinical use was reported based on a comparative study between 30% porous Ti64 and Ta implants66 (Fig. 5. c. v).
Overall pore geometry, pore size, and characteristics govern the mechanical and biological properties of orthopedic implants. It cannot be determined if cell-material interactions follow a singular trend or pattern based on pore characteristics. For example, osteoblastic precursor cells require at least 200μm pores for effective bone ingrowth and bonding.52. Cellular growth and cell morphology show characteristic dependence on pore sizes above and below 500 μm with the observed bridging of pores<500 μm and increasing along the strut of 3D printed Ti6Al4V structures > 700 μm99. None of these extensive studies were able to determine the extent of cell growth into the pores. These findings suggest that pore geometry and pore size are critical factors that play a crucial role in the cellular bridging of pores and inward cell migration proving the pivotal function of good scaffold design in additive bone-implant fabrication. While it is crucial to describe the osteoblastic activity, vascularization into the porous structure is another avenue well explored toward determining metals’ biocompatibility in biomedical applications148–151. Vascularization is essential as the porous structure’s depth increases (especially as research moves into AM-based implant design and manufacture). If there is no or limited vasculature, the advancing front of the bone cells could stop and recede. Therefore, this review presents a comprehensive table 5 enlisting a broader classification of porous materials, based on pore characteristics and fabrication methods, commonly employed in porous implant fabrications and their mechanical and biological response in vitro and in vivo for a better comparative understanding.
Table 5.
enlists the mechanical properties and biological response of common metallic orthopedic materials based on pore characteristics and processing.
| Porosity | Materials | Pore Characteristics | Processing & Mechanical properties | Key Findings | Remarks | |
|---|---|---|---|---|---|---|
| In vitro | In vivo | |||||
| MICROPOROSITY | CpTi Intervertebral cage82 |
80% v/v porosity and 300 μm pore diameter | Slurry foaming method Compressive strength=19.4Mpa Young’s Modulus=3.2GPa |
In vivo The direct bone-bonding ability of porous Ti cage with sheep lumbar spine. Circumvents the use of bone grafts |
The sample size was less considering a large animal model. The study period was 4 months, which is insufficient to determine the quality of bone growth and long-term effect |
|
| Ti6Al4V interference screw152 |
15.4% residual porosity | Powder bed direct laser sintering |
In vivo Better bone-tendon-implant integration Higher ultimate failure load |
Long term significance is not presented The study period should include early and late time points for more dynamic understanding. |
||
| CpTi porous scaffolds153 |
70% v/v porosity 500 μm pore size |
Metal laser melting Compressive yield stress ranged between 257MPa-395MPa based on the shape of the unit cell Young’s modulus ranged from 4.5GPa-5.4GPa. |
In vitro Human bone marrow mesenchymal stem cells showed good cytocompatibility on the scaffold surface Cellular differentiation and mineralization were observed after 7 and 21 days, respectively. In vivo Diamond crystal lattice showed better osteogenesis in vivo at 8 weeks Overall porous structure performed better under push-out force than solid scaffolds |
Comprehensive assessment of biomechanical functions of porous scaffolds It can be a bone graft substitute due to different topological designs |
||
| Ti lumbar spine implants154 |
57% porosity 200 μm pore size |
Powder metallurgy route with the space holder Interconnected porosity yield strength~60 MPa maximum strength~170 MPa 30 % of strain up to fracture |
In vitro Apatite formation on porous Ti surfaces In vivo Higher osseointegration with bone formation up to 64% for porous structures |
In vitro is not presented, which could provide important information on cellular morphologies based on pore size. | ||
| 3D printed porous Ti cage83 | Pore size ~600μm | EBM fabrication Young’s modulus ~20 GPa compressive strength ~38 GPa |
In vivo Sheep cervical implantation At 6 months bone contact rate was 58% |
Porous cage structure effectively avoids micromotion in vivo Long-term fusion effects are lacking. |
||
| Ti Templates155 |
Pore size~90 μm Interpore distance~ 200 μm Subsurface porosity~9 μm |
Regenerative amplified Ti: sapphire laser at 800nm 90 μm surface exhibited contact angle of ~88° 9 μm pore surface-displayed~84° Surfaces had high hydrophobicity |
In vitro Human skeletal stem cells were seeded 90 μm pore surfaces showed not only pore bridging but also cells penetrating up to 25 μm depth of the 100 μm conical pores early osteogenic differentiation was also reported |
Early differentiation resulted in interrupted proliferation and migration Long-term in vivo efficacy can be hindered Overall, microporous and micro rough surfaces can be used as peri-implant wound healing |
||
4.0. Influence of electrical charge on the biocompatibility of metals
Localized charge distribution at the electrostatic surface of a biomaterial defines biological interactions depending on surface charge polarity. Different surfaces exhibit the variable extent of cellular adsorption depending on individual predisposition towards cell-binding proteins and physiological ions, which lead to discrete cellular response and bone bonding. The biomedical device’s surface polarity often works with human bone’s innate biological direct current (DC) potential. Development of bone bio-potential can be either mechanistically driven under stress from a mechanical load or strain-related potential.
4.1. Biological potential of natural bone
Natural bone’s biological potential, also known as bio-potential, is an endogenous electrical potential in tissue structures that moderate cells’ normal growth and cellular structures156,157. Endogenous electrical potentials are classified into two categories: Strain-related potentials (SRP) and bio-potential (BP). SRPs stem from the piezoelectric properties of specific osseous structures such as collagen, which result in ion migration between tissues through the characteristic porosities of cancellous bone. On the other hand, BP results from the continuous remodeling of osseous structures vis-à-vis potential that develops across newly formed membranes in osteoid tissues, extracellular matrix formation, and osteoclast resorption leading to ionization release158,159. As mentioned above, these electrical signals work in tandem toward natural bone’s physical development but can be interrupted due to trauma or injury. Significant modifications of endogenous potentials have been observed under extraneous injury, where injury potential takes over the injured tissue’s normal bio-potential. There is often a spike in DC potential in such conditions, which lasts longer than usual until the wound is healed. DC potential in natural bone results from ion migration between the injured tissue and the intact surrounding tissue, which results in the current generation spanning over hundreds of microns. Voltage differences of 10–100 mV/cm have been measured in injured soft tissues, spanning hundreds of microns160–162. Studies have shown how injury potentials can differ based on the specific injury’s characteristics leaning towards a selective healing process based on either SRP or BP or a parallel functioning. Zhao et al. studied the activation of specific cell signaling pathways, such as the Src pathway associated with epithelial cell migration, and determining wound healing in a rat cornea163. They have also found that specific mechanistic cues such as nanoarchitecture surfaces and external electric fields164 can drive epithelial cell growth in bovine eyes by activating biological pathways. This is a befitting example of how SRP and BP can initiate unique wound healing mechanisms and work together, leading to a synergistic outcome.
More elaborately, it is possible to determine the exact characteristics or driving characteristics of the endogenous electrical potential for both SRPs and BPs. As discussed earlier, bone remodeling mainly depends on mechanical forces, generally the outcomes of extraneous stress or mechanical load165,166. It has been observed that bone tissue under stress is inclined to grow and multiply, whereas its absence leads to untimely resorption167. Due to trauma and injury, the stress applied to bone tissue has been characterized as negative polarity, leading to bone growth. In contrast, the bone under resorption has been evaluated to have an overall positive polarity. On a cellular level, bone tissue fronts with a high level of osteoblast activity and proliferation show negative polarization, while osteoclast resorption fronts have been observed to have an overall positive polarization170. Therefore, when it comes to electrical stimulation for bone growth, a natural biological feedback mechanism comes into play168,169. Of note, owing to similar chemical properties like bone, calcium phosphates have been researched for their piezoelectric properties171–173. This is particularly beneficial in hydroxyapatite-coated metallic implants under stress conditions that influence the charge toward bone-building cellular response. Additionally, external charge storage in hydroxyapatite has been explored in a similar context174–176.
4.2. Surface charge storage and corrosion potential
Over the years, several significant types of research have been driven by the influence of electrical stimulation on bone growth. Studies evaluating the electrical potential applied to repair bone trauma or stimulating selective cell differentiation pathways have proven successful177–180. Some studies have reported enhancing bone morphogenetic proteins (BMP-2,4,5,6) and higher calcium deposition in the extracellular matrix on the application of electrical stimuli177,178,181,182. These studies were based on the knowledge of induced polarities at specific locations in the bone due to trauma/ injury (as discussed earlier). Even though an overall constructive outcome has been suggested in several studies in terms of faster healing or accelerated osseointegration, there is still a significant knowledge gap in the evidential understanding of molecular pathways that play a role under electrical stimuli. However, recently, there have been several attempts to enhance the biological response of osseous tissue by storing electrical charge on the surface of metallic implants either through surface modification followed by electrical polarization or through corrosion of the implant in surface potential. Of course, there is also the possibility to apply an electrical voltage at the implant surface. In fact, there are commercially available implants to assist with bone fusion that make use of electrical stimuli183.
Development oxide surfaces on Ti implants have been extensively shown to reduce water contact angles and enhance surface hydrophilicity. However, recent research on polarizing these oxide layers has successfully stored negative charge on the surface through space-charge polarization. Yamazaki et al. have shown that electrical polarization of micro-arc oxidized Ti surfaces can store negative charges (~ 2.7μC/cm2) through ion migration of oxygen vacancies, which significantly reduces de-ionized water contact angles surface more hydrophilic for favorable biological response184. The dielectric properties of TiO2 at room temperature are 63.72, which is close to that of water (i.e., 80), giving Ti an added advantage from the perspective of cell-material interaction, meaning other than a physiological solution that interacts with the metallic implant, it is proteins that interface with the implant. Protein conformation on the implant’s surface determines the signal to cells and cellular interaction. Furthermore, these surfaces were tested against osteoblast-like MG63 cells, which enhanced cellular attachment, growth, and proliferation on the negatively polarized surfaces185. More recently, Bandyopadhyay et al. reported charge storage in nanotube-modified titanium oxide surfaces by novel bridging of nanostructures’ enhanced efficiency in storing capacitive charge with the higher surface to volume ratio imparted by nanotubes on the surface186. They have reported a negative surface charge of ~ 37mC/cm2, which was a magnitude higher than other values reported in the literature and attributed to the nanostructured surfaces’ effect. These nanotubes modified Ti implants were evaluated in vivo in a rat distal femur model and exhibited a 92% increase in newly formed bone area compared to the control commercially pure Ti surfaces.
Metallic devices for bone applications exposed to ion-rich electrolytes in the physiological system establish a fundamental reaction cell. During electrochemical responses, the progression of ions and electrons can prompt heavy potentials between the metallic electrode and the electrolyte. These currents are commonly used to quantify the reaction pace since they are directly identified with the metal leaching. Thus, small pits form on the surface due to corrosion reactions can affect its mechanical stability, reducing the device’s longevity and causing unexpected failure187–189.
4.3. External electrical stimulation
External electrical stimulation in bone repair is one of the popular treatment methods involving cell growth using external factors because of some non-invasive application methods. However, there is not much literature on these treatment methods since these are not specific to a localized physiological site. Three primary ways of external electrical stimulation for bone repair include applying DC, alternative current (AC) generated capacitive stimuli, and electromagnetically induced electrical field. DC stimulation is one of the exceptions in such therapies since this is an invasive approach. The cells are subjected to DC/faradic stimulation to enhance and accelerate growth directly on the implant’s surface or applied to the cell growth media. Usually, a maximum DC field of 50μA/cm2 is applied, positively affecting the cell-material interaction and enhancing cell proliferation190. Cellular differentiation through an expression of markers specific to osteoblast differentiation has also been reported in the literature due to DC stimulation178. However, most studies have applied the stimuli to the cell culture media instead of directly to the implanted metal device. This establishes a fixed current flow between the applied cathode and the anode, which aids in cell growth in vitro191. Despite the efficacy of such electrical stimulation, the electrode products that get generated due to electrochemical currents have been seen to have enhanced and adverse effects on cell response.
In contrast, capacitive stimulation involves the application of alternating current in a noninvasive over-the-skin approach. These cases mostly use a voltage-frequency combination of a maximum 50V at 60–200,000 Hz, which generates an adequate field strength of 0.1–5 V/m192,193. The capacitive stimulation is primarily applied externally to the body with plates placed at the implantation site externally. However, some studies have reported direct media contact of these electrodes without touching the implant in vitro. This therapy approach has shown effective results both in vitro and in vivo179,181,194,195. However, a significant concern in the capacitive stimulation approach towards bone healing is patient compliance regarding topical irritation due to high voltage and frequency. Additionally, since this approach is non-invasive, specific measurements at the surgery site, such as local current density, cannot be quantified, affecting biological response prediction. Another similar non-invasive therapeutic approach is inductive stimulation, which involves generating an electric field using electromagnets, which have been shown to influence cellular response and expression of genetic markers190. Studies involving osteoblast-like MG63 cells as well as mesenchymal stem cells (MSCs) have reported upregulation of specific osteoinductuve markers such as BMP-2, TGF-β1, osteocalcin as well as collagen synthesis and early onset of osteoblast maturation and differentiation under the influence of the electromagnetic field. However, similar to the capacitive approach, the application of inductive current is a non-specific external therapy that can adversely affect tissues in the surrounding area of the injury site.
5.0. Influence of materials chemistry on the biocompatibility of metals
The chemistry of a material is an essential factor in deciding its biocompatibility. Material chemistry signifies the properties of the metals that influence their interaction with their surroundings. These can range from multiple metals’ influence in an alloy to chemical interactions on a molecular level to some metals’ inherent tendencies to oxidize197.
5.1. β-Titanium alloys
As discussed earlier, untreated Ti surfaces are bioinert, meaning that these surfaces in a metallic orthopedic implant are inherently incapable of eliciting any kind of biological response from the host tissue. Therefore, surface modification techniques enhance the implants’ surface bioactivity, which leads to faster bioactive bone fixation. However, these metallic implants’ drawbacks have a large difference in elastic modulus compared to the bone, making them even mechanically unsuitable for orthopedic application. One way of circumventing said drawback is by fabricating phase alloys of the parent metal by introducing phase stabilizing components. For example, Ti metal has two distinct phases α and β, while CpTi is predominantly α-phase Ti, Ti6Al4V on the other hand, has vanadium as a β-phase stabilizer, which makes it a α+β type alloy. The simple introduction of vanadium in Ti enhances mechanical properties such as fatigue resistance, strength, etc. These α+β types of alloys exhibited higher strength and better corrosion properties than α alloys for long-term applications. However, vanadium and aluminum’s introduction was questionable because of their inherent toxicity from free ion leaching to the human body. Aluminum in excessive quantities and continuous exposure have been reported to cause Alzheimer’s disease. Therefore, other α+β alloys emerged in the research community, such as Ti-6Al-7Nb, which exhibited an elastic modulus of 110GPa not significantly different from Ti6Al4V (modulus ~ 112GPa)198,199. There remained a vast difference between the modulus of these metals and human cortical bone (~30GPa). At this point β type Ti alloys were developed and introduced in the orthopedic and dental application field. These alloys had a significant advantage over the dual-phase alloys because the β phase stabilizers that were used in the alloy system, such as molybdenum (Mo), tantalum (Ta), and Zirconium (Zr), were not only non-toxic to the human body but also exhibited lower elastic modulus thereby improving mechanical biocompatibility with bone200,201. For example, the elastic modulus of Ti–24Nb–4Zr–8Sn (46–55 GPa)202is notably lower than that of other Ti alloys, which allows for β-type alloys to have a considerably important position in the biomedical industry. Over the years, various other fabrication routes were implemented for β-type Ti alloys like Ti–15Mo203–206, Ti–Nb–Ta21,207, Ti–24Nb–4Zr–8Sn22,208,209, Ti–35Nb–2Ta–3Zr19,20,210,211, Ti–35Nb–5Ta–7Zr212,213, Ti–30Nb–4Sn214,215, Ti–35Nb216–218, Ti–15Nb–3Mo–3Zr–2Sn219,220, and so on. Therefore, many investigations of processing β-type Ti alloys have focused on improving their properties and tailoring their microstructures. The most recent improvement in the fabrication of β-type Ti alloys has been coupled with designing porous implants to reduce the distinction in elastic modulus and derive better biocompatibility. So far, various fabrication methods for porous materials have been implemented, such as sintering, investment casting, and rapid prototyping221,222. However, two major strategies are often used in porous β-type Ti alloy implant fabrication - powder metallurgy (PM) and additive manufacturing (AM). For porous implants, the PM process requires careful management of pores’ dimension and morphology, influencing biological fixation, osseointegration, and fatigue223,224. In current years, porous Ti alloys are designed by using PM for biomedical applications. Among various PM routes, spark plasma sintering and the space holder approach are generally used for producing porous Ti and Ti alloys. AM techniques have many advantages, including a short manufacturing cycle, easy machining, and better inventory utilization225. On the other hand, AM aids in preparing components with complicated geometry quickly and accurately, which is way more efficient than the ordinary subtractive manufacturing method226,227. Through years of evaluation and study, AM has been determined to produce Ti and Ti alloy implants using laser or electron beam to melt metal powders based on a computer-aided design (CAD). Recently, AM-fabricated porous Ti alloy structures attracted attention; selective laser melting (SLM) and electron beam melting (EBM) are proven to be efficient in producing high-efficiency metal parts228. Usually, α + β-type Ti alloys have toxic adverse effects leading to the development of β-type Ti alloys and their corresponding biocompatibility investigation. For example, comparison of biocompatibility between Ti–26Nb and Ni–49.2Ti, showed that Ti–26Nb is less cytotoxic229. Additionally, Ti–19Zr–10Nb–1Fe alloy has comparable cytocompatibility with the Ni–Ti alloy however higher hemocompatibility230. The enhanced biocompatibility of β-type alloys is due to the absence of toxic alloying elements. Current investigations on biocompatibility of β-type Ti alloys are still nascent.
In contrast, due to Ti alloys’ biological inertness, fibrous tissue can form around the implant198. Such a defensive reaction is inevitable for all sorts of bioinert β-type Ti alloys, which are non-toxic, safe, but no longer bioactive. Although β-type Ti alloys are free of toxic alloying elements, enhancing osseointegration is regarded as essential. Generally, surface modification to enhance the bioactivity of Ti alloys is employed, such as alkali coating remedies on Ti–29Nb–13Ta–4.6Zr with the aid of electrochemical, hydrothermal, or blended processes for specific times, and the outcomes have been considered to exhibit regardless of the techniques or parameters, the surface of Ti–29Nb–13Ta–4.6Zr is favorable for mesh-like apatite formation231. Also, sol-gel synthesized calcium phosphate/TiO2 coatings on Ti‒29Nb‒13Ta‒4.6Zr exhibited substantial bioactivity enhancement because calcium phosphate and TiO2 are notably bioactive to bone cells232. Besides inorganic coatings, natural coatings (or layers) have received sizeable attention. Recently, immobilized extracellular matrix (ECM) proteins on CpTi and Ti–6Al–4V showed more significant bioactivity for human mesenchymal cells with similar consequences observed in different coatings233,234. Unfortunately, there is not enough literature about the natural coatings on β-type Ti alloys. However, due to the massive success of natural coatings on other types of Ti alloys, β-type Ti alloys with bioactive coatings are expected to be a future for biomedical Ti alloys.
5.2. Tantalum, Tantalum oxide coatings, and Tantalum alloys
Alloys of metals such as tantalum (Ta) can provide better biocompatibility because of Ta chemistry and its inherent tendency to form an oxide layer on the surface. However, studies show that Ta alone does not contribute to any biological response from the body238. In that case, the biocompatibility of Ta is exclusively attributed to its non-cytotoxicity in a physiological environment238. Historically Ta has found its use in secondary medical applications, majorly as an FDA’s class II biomedical device, as mentioned in Table 2. Since then, Ta has been extensively used as a metal coating on metallic devices such as Ti implants and carbon composites36,239,240. Since Ti implants and ceramic coated metal implants are routinely applied in the orthopaedic industry, these form a standard for comparison for any new material that can be potentially introduced into the industry. Therefore, comparing Ta coatings with Ti substrate and hydroxyapatite (HA) coated Ti implants can provide a realistic picture of Ta’s applicability in FDA’s class III medical devices. It has been shown that Ta coatings on Ti implants exhibit 6 times higher osteoblast (OB) response than just Ti surfaces evaluated through living cell density using MTT assays36. The higher cell response is attributed to the increased surface energy due to coating Ta on Ti implant surfaces, making the surface more hydrophilic and favorable for cellular interactions. Additionally,36Balla et al. have also compared the biocompatibility of the same Ta-coated structures fabricated using laser-based additive manufacturing methods with hydroxyapatite (HA) coated Ti surfaces through radio frequency plasma spray coating. While Ta coatings exhibited a 6-fold increase in live OB density than the Ti substrate, OB density was comparable for Ta coatings with HA-coated Ti surfaces. This was further corroborated through confocal microscopy through fluorescence intensity observation for vinculin expression, indicating the Ta coated surfaces’ better biocompatibility than the industry standards. Several studies have evaluated Ta coatings’ biocompatibility from a corrosion resistance perspective. Zhou et al.241 fabricated Ta coated nitinol (NiTi) substrates using plasma immersion ion implantation followed by magnetron deposition (PIIID) technique to form an optimized 3.3micron coating of Ta. Auger electron spectroscopy results showed excellent bonding strength of the Ta coating on NiTi surfaces through the formation of intermetallics. This was concluded to contribute to the enhanced corrosion resistance of the coated substrates. Furthermore, electrochemical analysis through potentiodynamic polarization exhibited a higher passive anodic current, which substantiates their finding of the Ta coatings’ enhanced corrosion resistance. Other studies have examined the corrosion resistance properties of tantalum oxide layers (Ta-O) formed over magnesium and Ti6Al4V substrates. Jin et al.242 observed that a 4.8micron thick layer of Ta-O showed an active anodic current, which was ~500 times smaller than an untreated surface. Moreover, magnesium corrosion products from ZK60 alloys in untreated surfaces in vitro hindered cell proliferation compared to Ta-coated surfaces. They have also concluded that Ta coating moderate pH imbalances in the substrate’s immediate surroundings mainly result from magnesium leaching. Ta-O coatings were also examined by Rahmati et al.243 on Ti6Al4V substrates. The coating was formed using plasma vapor deposition at 100°C to achieve the highest adhesion strength, which was also observed to have increased the surface by 88.5% compared to the same group’s high adhesion strength after thermal treatment at 500°C244. However, both studies concluded that higher adhesion strength and high coating hardness make Ta-O coatings better suited for biomedical implant applications.
Table 2.
enlists the primary metals that are used in the biomedical and orthopaedic industry at present.
| Metallic materials | Application areas | Advantages | Disadvantages | |
|---|---|---|---|---|
| SS 316L 30 | Martensitic | Bone curettes, hemostats, orthodontic pliers, scalpels, root elevators, dental burs | Good short-term corrosion resistance. Low cost of fabrication Easily machinable |
Can corrode in long term application Stress shielding due to high modulus Ni and Cr toxicity leading to allergic reactions and inflammations |
| Ferritic | Solid instrument handles, pins, and fasteners | |||
| Austenitic | Hypodermic needles, sterilizing units, thoracic retractors, canulae | |||
| Duplex | Not used in the biomedical field yet | |||
| CpTi 31 | Mostly non-load bearing applications for their corrosion resistance: Pace-makers Ventricular assist devices Dental implants Maxillofacial and craniofacial implants Screws for spinal applications |
Excellent corrosion resistance for their spontaneous oxide layer formation Excellent biocompatibility Easy to weld and machine Relatively low Young’s modulus |
Heat strengthening is difficult Low strength at ambient temperature Low shear strength |
|
| Ti64 and Ti64-ELI 31 | Total hip arthroplasty (THA) and Total Knee Arthroplasty (TKA) Femoral stems Fracture fixation devices (plates and screws) |
Load bearing application It can be strengthened by heat treatment Relatively low Young’s modulus |
Lower biocompatibility than CpTi Lower corrosion resistance than CpTi. Low wear resistance |
|
| Cobalt-chrome alloys 30,32 | Permanent implants Femoral heads |
Higher long-term corrosion resistance Higher fatigue resistance Higher wear resistance Better castability |
Expensive processing due to machining difficulties High modulus leading to stress shielding Ni and Cr toxicity |
|
| Magnesium alloys 33–35 | Cardiovascular stents, Resorbable implants |
Modulus close to human bone ~40GPa Naturally occurring element in the human body Similar resorption kinetics with the healing period Similar tensile strength as human bone. |
Resorbable metal unfit for load-bearing applications Soft and malleable |
|
| Tantalum 4,18,36,37 | Suturing wires for tendons38 Clips for ligation of anastomase39 Staples for abdominal surgery40 Reconstructive craniofacial surgery41 Coating other metals36 |
Excellent corrosion resistance Excellent biocompatibility |
Comparatively heavier metal Soft and ductile metal Evidence of local sarcomas and toxicity to alveolar cells42,43 |
|
Based on its exceptional biocompatibility, there has been a surge of interest in fabricating standalone orthopaedic implants from Ta. However, the intrinsic material properties of Ta pose a hindrance to successful fabrication. Ta is a dense metal (densitŷ 16 g/cc, compared to titanium ~4.2 g/cc and Stellite (CoCr alloy)~ 8.6 g/cc) that is soft and ductile; therefore, a bulk implant fashioned out of Ta might not withstand the patient’s regular activities. It has a very high melting temperature of 3200K, which is nearly impossible to reach in conventional furnaces; therefore, there is a lack of effective processing/fabrication methods. In addition to processing difficulties, there is a price point drawback. Ta is almost 10 times more expensive than an equivalent weight of Ti. Recently, Bandyopadhyay et al.37 fabricated porous Ta implants using Laser Engineered Net Shaping (LENS™), an additive manufacturing method with high-energy laser beams to melt the fine metal powders to form local melt pools and, in turn, form 3D structures layer-wise. Their findings suggested the feasibility of processing pure Ta structures under similar processing parameters as pure Ti. Not only do their results suggest novel, easier processability of Ta structures, but in vivo results also showed exceptional biological performance of Ta over an extended period in terms of osteoid tissue formation around the implant. More recently, Ta has been alloyed with other metals such as Ti and Mo to reduce processing difficulties and modulate mechanical properties to make it better suited for commercial applications16,18,245,246. Such alloying helps elicit an amalgamation of properties that coherently work towards improving metal alloy implants’ biocompatibility.245Zhou et al. compared the mechanical and biological properties of Ti-Ta alloys with that of pure Ti and found that Ta can simultaneously enhance strength and reduce the elastic modulus of Ti alloys. Ta not only provides better corrosion resistance to Ti, which improves its biocompatibility. However, in terms of cellular interaction, Ti-Ta alloys perform comparably to pure Ti. Ta’s contribution towards reducing elastic modulus and increasing strength is based on the weight% of Ta present in the alloy. Interestingly, ZimmerBiomet has Trabecular Metal implants that essentially vapor deposition of Tantalum on carbon scaffold. Such implants have been in clinical use for over 20 years with great clinical success. Table 5 details Ti-Ta alloy processing, material characteristics, and key findings on the biological response.
5.3. Nickel-Titanium (NiTi) alloys
The NiTi alloys classify as shape memory alloys due to their ability to return to their predefined dimensions after elastic deformation under specific external stimuli. The alteration in these alloy systems’ shape/dimension is often reversible and has been termed pseudoelasticity. The shape-memory effect in NiTi alloys was first discovered by Buehler et al. circa 1960. Among other metal alloy systems in the same classification, it was observed that NiTi systems show the best performance as shape-memory alloys due to their superelasticity, stable fatigue performance, and good corrosion resistance. These overall workable qualities make NiTi a desirable candidate in the biomedical performance of shape-memory alloys257. NiTi alloys showed excellent corrosion resistance to brine, which expedited their application in the biomedical field, from orthopaedic clinical trials in the ‘80s to large-scale commercial vascular stent application258,259. Corrosion resistance is one of NiTi alloys’ biocompatibility primary yardsticks since these implants are subjected to various physiological environments. Moreover, these alloys’ shape memory properties depend on temperature, which can accelerate corrosion behavior in metals. Therefore, the analysis of NiTi alloys’ corrosion resistance in different testing conditions provides an extensive understanding of these alloys’ applicability. NiTi alloys were found to have better corrosion resistance than Co-Cr-Mo and SS316L alloys when exposed to Hank’s solution and a 0.9% NaCl solution 37°C260–262. However, NiTi alloys were less corrosion-resistant than Ti when subjected to 0.9% NaCl solution at 37°C263. The corrosion behavior in most of these alloys depends on forming spontaneous oxide films on the surface when exposed to an oxidative environment such as a highly ionic solution. These oxide films on Ti, SS316L and NiTi alloys prevent ions from interacting with the metal implant surface, reducing metal leaching. However, the capacity to form an unreactive passive oxide layer on the surface is completely dependent on the makeup of the material and its chemical compositions. For example, it was found that NiTi releases a higher amount of toxic Ni ions in simulated body fluid (SBF) compared to SS316L (austenitic). Similarly, the passivated film properties formed on NiTi surfaces were inferior to those on Ti6Al4V surfaces264. In addition to corrosion resistance in acellular in vitro conditions, evaluating these properties under in vivo dynamic conditions is essential. Studies in large animal models show that NiTi plates did not result in localized corrosion and systemic organ accumulation of Ni ions when tested in dogs for 17 months265. However, some corrosion was observed in a sheep model study where iliac artery stents were placed in the sheep for more than a year. In this case, the corrosion product was analyzed as an oxide of Ti, which is deemed toxic compared to Ni ions. Moreover, this also served as indirect proof of spontaneous surface passivation in an in vivo physiological environment266.
Studying the corrosion resistance of metal alloys is critical to better understand their influence on cell-material interactions. Therefore, evaluating NiTi alloys’ cellular performance in vitro is necessary to understand their biocompatibility. Although minimal literature on NiTi alloys’ cell-material interaction elicits a response from a wide range of human-derived cells, some reported results are exciting and essential. Human fetal lung fibroblasts responded positively to NiTi alloys compared to SS316L or Co-Cr alloys; however, cell growth was significantly reduced267. On the other hand, L-929 fibroblasts showed cytotoxicity for NiTi and Co-Cr alloys more than SS316L268. It was observed that human peripheral blood lymphocytes showed no cytotoxicity or genotoxic activity when exposed to NiTi alloys, strongly supporting its candidacy as a biologically safe implant material109,269. In orthopaedic implant applications where NiTi alloys were directly in contact with bone cells, it was concluded that this implant material was well tolerated by osteosarcoma ROS-17 cells, osteoblasts, and osteoblast-like MG63 cells with or without surface modifications such as plasma-treatment, oxidation, or nanoscale surface modifications270–274.
The worldwide application of NiTi in practical orthopaedic implantation has been hindered because of a lack of knowledge of the material’s biocompatibility. However, based on the published studies, it can be concluded that NiTi is biocompatible in vitro and in vivo, with special cases such as vascular stents requiring surface modification for long-term applications. The FDA recently approved a biomedical device for bone anchoring, which has a part fashioned out of NiTi, which remains to follow. As the research community advances new alloy formulations, it is imperative to understand that corrosion and ion leaching risks can manifest many years after implantation. Orthopedic device manufacturers would be hesitant to adopt alloys that could show ion release much later as many thousands of patients in the interim would have been implanted with such questionable alloys.
5.4. Metal and alloys in cardiovascular applications
With the advancement of research and market demands, implantable biomaterials are surgically implanted as a temporary fixture that can degrade over time while allowing surrounding tissue to regenerate and integrate into the structure, eventually replacing the structure as a whole. In such a situation, the most obvious choice for a metallic implant is magnesium (Mg). It is a microelement already present in the human physiological system and is deemed non-toxic. Studies with magnesium as early as the 1930’s show excellent resorbable and biocompatible properties of magnesium fixation devices275. Furthermore, magnesium has an elastic modulus similar to that of human bone (10–30 GPa), and also their controllable corrosion rate in physiological media makes them an excellent candidate for resorbable metallic implants. When exposed to air, the magnesium implants form a passivated magnesium oxide surface, which imparts its corrosion resistance. However, magnesium corrodes in a saline environment in human physiology, significantly reduces its lifetime, and leads to its resorbability276. The magnesium application in preclinical studies in cardiovascular stents is useful with minimal inflammatory response and complete bio-resorption within two months of placement277–281.
Nevertheless, in bone implant applications with less nutrient transport, hydrogen evolution resulting from magnesium resorption remains an issue282. Animal studies of magnesium implants have shown it to be disrupted by such hydrogen evolution where gas bubbles had to be removed using puncture procedures276,283. Therefore, the corrosion of magnesium remains a drawback in its successful application. This drawback has been overcome by alloying magnesium with other metals, primarily calcium (Ca) and zinc (Zn). A small addition of calcium, typically between 0.8 and 1.0%, has been shown to reduce corrosion effectively. However, higher calcium leads to a larger scale, irregular corrosion275. Zn is most commonly alloyed with magnesium to improve yield strength effectively. One of magnesium alloys’ practical features for orthopedic applications is their Young’s modulus of ~40GPa275,284, close to the bone (10–30 GPa). Zn addition to magnesium leads to a reduction in hydrogen gas formation282. Zn2+ ions in the solution near a bulk material tend to be removed as Zn2+ ions compete with the Mg2+ ions to form Zn(OH)2, which ultimately decreases the amount of H2 gas. As third-generation metallic biomaterials, tissue engineering applications have fundamentally determined magnesium composites’ advancement as degradable biomaterials. Various issues should precede any clinical applications, among which hydrogen formation is the most tested. A detailed review of metals in a stent and other cardiovascular applications has been recently published, which explains both resorbable and non-resorbable stent materials with a focus on 1) materials (metals and polymers), 2) properties (bioresorbable or permanent), 3) function (drug-eluting and non-drug eluting), 4) structural (bare and surface modified), 5) characteristics (self-expanding or balloon expanding)285. In light of this, the present article will not extensively elaborate on cardiovascular materials. However, since bioresorbable metals are considered the next generation, it is important to look at cardiovascular devices.
Traditionally, the non-degradable or permanent stents are designed using cobalt-chrome alloys (Abbott Vascular), stainless steel (SS) (OrbusNeich), or platinum-chromium alloys (Boston Scientific), which are already in clinical use. Degradable or resorbable metals Mg, Fe, and Zn alloys are most preferred for stent design primarily because of their less permanent residence in the human physiological system. Among these, only Mg alloy stents (CE Mark) are manufactured commercially, while Fe and Zn alloys are still developing and are far from clinically efficient in such applications. Although mechanically SS stents are superior in tensile properties with higher fatigue and creep strength, Mg and Zn have similar tensile strength compared to one another, with Mg having a slightly higher creep strength over Zn. In terms of elongation rate, which is a focus for stent application, Zn lies between Mg and SS. Even though all three of these metals meet the minimum required mechanical strength as stent materials, Mg and Zn need proper alloying strategies or post-fabrication thermal treatments to make them mechanically more biocompatible for stability in their niche application286. Recently, new stoichiometric Zn alloys such as Zn-lithium (Zn-Li), Zn-Li-Mg, and Zn-Li-Manganese (Zn-Li-Mn) have shown comparable mechanical properties to medical grade Ti. In addition to being mechanically similar to Ti, these Zn alloy stents have the advantage of bioresorption, eliminating the continuous presence of foreign material in human physiology. These materials are still in their nascent research and evaluation stages. However, the validation of a process such as alloying resulting in superior mechanical compatibility of resorbable metals like Zn proves the importance and relevance of alloying as a way to improve biocompatibility even for the next generation of metallic implants287. Additionally, multiple functionalities can be imparted to Mg and Zn-based stents over SS, which could be inherent, such as the antibacterial characteristic of Zn as a material that provides inherent infection prevention (both contact and non-contact killing) or drug-eluting features Mg stents. One such drug-eluting stent called Magmaris- DREAMS 2G (2nd generation Magmaris) is currently in human trials. These are Mg-based stents precisely fabricated from WE43 alloy and have shown favorable safety profile and efficient performance in human trials for up to 12 months288. Similarly, a certain kind of iron-based sirolinium eluting stent called IBS (Iron bioresorbable coronary scaffold system) has been researched in vivo in a pig model. These stents exhibit comparable efficiency to the cobalt-chromium everolimus-eluting stent (EES) (XIENCE prime) in terms of stenosis and histology, which has the advantage of being resorbable in the long run289. However, late stenosis and/or thrombosis remain a challenge for most of these resorbable metallic stents and require further evaluation along with finding ways to make these more biocompatible to reduce recurring incidences of moderate inflammation and moderate to severe neointimal hyperplasia285.
6.0. Influence of bioactive ceramic coatings on the biocompatibility of metals
Most orthopaedic implants are primarily made of metals but are limited by problems associated with their inherent bio-inert nature. Thus, metallic implants coated with calcium phosphates (CaP) gained more importance due to improved tissue integration. Natural bone is a composite of about 70% calcium carbonate apatites, and the rest is collagenous proteins296. Therefore, bone structure mimicking composite coatings has recently emerged as a topic of interest in metal implants’ surface functionalization. In this regard, several synthetic and non-synthetic (nature-derived) biomolecule-CaP coatings have been explored because composites provide superior properties compared to their counterparts. For example, collagen composites can compensate for the low fracture toughness of CaPs and improve the implants’ fixation ability at the surgery site297. Adding growth factors such as BMP-2 and TGF-β to CaP coatings has significantly improved osteoconduction and bone regeneration through sustained release and higher stability through immobilization298. The scientific ceramic research community recently focused on natural medicinal compounds (NMCs) with bone regeneration and antibacterial properties299,300. These NMCs are mostly loaded or immobilized on the surface of CaP-coated metal implants for sustained release applications in vitro and in vivo. However, before discussing CaP coating applications, there must be an understanding of different calcium phosphates used in biomedical applications and their effect in vitro and in vivo.
Several classes of CaPs are used as standalone materials for biomedical applications or as precursors for more chemically stable CaPs for coating applications. This review article section will focus primarily on four categories of CaPs. The first categories are octacalcium phosphates (OCP) with a Ca/P ratio of 1.33: stable at physiological pH and temperature, and hydroxyapatite precursor (HA) alkaline conditions. OCPs have improved in vitro cytocompatibility of murine fibroblasts, osteocytes, and osteoblasts, and osseous regeneration in rabbit cranial bone301–304. As mentioned, HA is the next category derivative of OCP and has shown widespread application in the biomaterial industry. HA is the mineral form of calcium apatites found in nature and has a Ca/P ratio of 1.67. HA has a slow resorption rate, is bioactive, and is a conductor of osteoblast proliferation, making it the major component of teeth and bones and the prime coating material for dental and orthopedic implants. In vitro assessment of cell-material interaction for HA has shown its cytocompatibility in sheep tibial osteoblast cells, rat osteocytes, and human skin cell lines305–307. Furthermore, there is evidence of broader potential biocompatibility of HA in animal models, such as effective bone regeneration in the mandible of pigs, rabbit femurs, dorsal muscle in dogs, and sheep femur/tibia308–310. α-Tricalcium phosphates (α-TCP) and β-Tricalcium phosphates (β-TCP) are the next relevant categories of CaPs that are applied in medical-grade devices. Both α-TCP and β-TCP have a Ca/P ratio of 1.5 and higher resorption rates than HA. However, α-TCP is less stable in a physiological environment than its counterpart leading to resorption times lower than new bone formation. Therefore, these CaPs are widely used as bone cement in a metallic component or in conjunction with HA311. Individually α-TCP and β-TCP have shown positive biological responses as materials exclusive of their application drawbacks. For example, both have exhibited adequate cytocompatibility in mouse calvaria-derived osteoblasts as primary human osteoblasts and osteoblast-like cells312–314. However, α-TCP is more efficient in mesenchymal cell proliferation315 than β-TCP, which favors endothelial cells. Although not much in vivo assessment is available on these CaPs regarding their biological response apart from bone regeneration evidence in the cranial and calvarial bone of rats and rabbits316–318.
Among the numerous available HA coating methods, like sputter coating, plasma spray deposition, pulsed laser deposition, and electrophoretic deposition, plasma spray deposition is considered one of the most commercially viable methods319,320. Though it offers the advantages of high deposition rate, bulk production, strong interfacial strength, the associated problems with phase decomposition and amorphous phase formation are harmful to the bonding strength and decrease the longevity of the coating in vivo321. Recent efforts by various implant manufacturers have also included solution-based deposition techniques where the implant is dipped in a calcium phosphates solution and using electrochemical techniques to modulate surface pH. This results in a precipitation of calcium phosphate on the surface of the implant. Zimmer Biomet BoneMaster (HA), Bonit (brushite) by DOT are some of those examples. Stryker has Peri-Apatite HA, which is HA from supersaturated solution. For the fabrication of an excellent coating, substrates usually need high surface energy to make the coating material easy to adhere to and form a strong interface. Various substrate surface modification techniques have been researched to improve the coating crystallinity and incorporate inorganic ions as dopants, enhancing osteointegration and angiogenesis305,322. Osseointegration is the measure of a biocompatible material to induce osseous integration at the bone-implant interface. It is generally accepted that CaP-based materials would favor osseointegration due to their close chemical compatibility with the natural structure of bone; therefore, applying CaPs as coating materials for metallic load-bearing orthopedic implants is beneficial for shorter healing times and defect corrections. Surgical procedures involving medical device implantation induce stress factors in the bone, which inadvertently leads to a local decrease in bony tissues’ pH—the more acidic pH results in the implant’s surface corrosion as coatings. Therefore, immediately after implantation, there is an increase in calcium and phosphate ions in the local surrounding tissues and body fluids that get reprecipitated once the pH is balanced in apatites (most commonly, hydroxycarbonate apatites). An apatite surface has been known to induce several precursor protein attachments, leading to osteoblast proliferation and differentiation through chemotaxis. The dissolution-precipitation process is influenced by the CaP coatings’ functionalization with favorable doping agents such as strontium, silicon, magnesium, etc300. Moreover, dopants’ addition leads to better cellular interaction with the implant surface through a synergistic effect300.
Material compatibility between substrate and coating is important in employing a specific coating technique and in vitro and in vivo biological assessment. The biological applicability depends heavily on the area of application and individual CaPs used for that specific application. For example, coating a metal implant with a corrosion-resistant CaP is ideal for a dental implant; however, that might not be the primary concern for coating an implant for a knee arthroplasty component. Therefore, the following section summarizes coating material, techniques, and biological responses for the major candidates in the orthopaedic metal industry, such as Ti6Al4V (Ti64), commercially pure Ti (CpTi), stainless steel (SS 316L), and magnesium.
Ti6Al4V is one of the most widely used metals for load-bearing orthopedic applications and has been coated with more than one kind of CaPs for biomedical applications. For example, biomimetic coatings of OCP, anhydrous calcium phosphates (ACP), and carbonate apatites (CA) on Ti64 have shown effective osteoclast inhibition in bone-marrow cells of mice for OCP and CA coatings; however, there was no observed osteoclast inhibition for the ACP coatings323. CA has shown excellent stromal cell attachment with extended filopodia in vitro and enhanced calcification in subcutaneous implantation in rats324,325. Plasma spray coating of α-TCP and HA on Ti64 has shown accelerated bone mineralization in trabecular implantation for HA; however, α-TCP coatings have shown slower bone healing lacunae formation over 5 months of implantation326–330. Electrodeposition of HA, fluorinated HA, and CA on metal substrates like Ti64, CpTi, and Mg alloys such as AZ91 have enhanced biological properties in vitro and in vivo. HA+OCP coating on Mg alloys and pure HA coatings on Ti64 has shown enhanced induction of new bone formation and more excellent bone apposition, leading to higher osseointegration in rabbit femurs canine trabecular bones, respectively327,331. Fluorinated HA has a better biological; response than HA on Mg alloys (Mg-6Zn) and AZ91 towards excellent proliferation and differentiation of human stromal cells in vitro and evidence of reducing trochanteric inflammation in rabbits in vivo332,333. On the other hand, CpTi coated with HA, and fluorinated HA has shown an improved biological affinity for MC3T3-E1 osteoblast-like cells with increased alkaline phosphates activity. However, an in vivo report is available for soft tissue application for HA-coated CpTi in dorsal pouches of rats showing mineralization of collagenous tissue306,334. Crystalline HA coating on CpTi has increased new bone formation activity compared to amorphous HA coating335.
An essential aspect of CaP coatings in orthopaedic and dental implants is their ability to perform as carriers for immobilized drugs with specific functionality. Drugs can be synthetic such as alendronate and bisphosphonates, both of which aid in faster bone healing, or natural such as curcumin, allicin, vitamins, etc. These drugs are usually entrapped to have favorable effects on osteoblast and osteoclast activities or their anti-inflammatory response. CaP-coated implants have the flexibility to carry antibacterial drugs or doped ions, which are efficient in preventing or treating secondary infections. Infection prevention is one of the most widespread applications of CaP coatings. CaP coatings were shown to possess antibacterial properties such as fluorinated HA compared to pure HA coating336. It was observed that pure HA coating was selectively effective against some bacterial strains; however, fluorinated HA showed efficacy against a broader range of bacterial strains. Furthermore, elemental dopant addition to CaP coatings has been extensively explored with dopants with antibacterial properties such as silver, copper, zinc, etc.299,300,337,338. Therefore, CaP based composite coatings could be applied as a carrier for bioactive agents in addition to antibacterial molecules.
7.0. Influence of biological functionalization on the biocompatibility of metals
The biological functionalization of metallic implants involves using organic molecules in the bone to initiate surface-level bioactivity, which either works individually or in combination with enhancing the implant’s biocompatibility. These organic components mainly involve molecules such as collagen or peptide sequences that are native to bone342,343. In addition to bone growth factors344, preclinical studies explore structural reinforcing components such as DNA and bone mineralizing enzymes345,346. The biomolecule functionalization has been chiefly reported using one of three primary methods vis-à-vis physisorption, physical entrapment, and covalent bonding, which helps to immobilize the molecule on the surface of Ti implants347. Another class of biological functionalization stems from nature-inspired surface modifications such as polydopamine coatings on Ti, isolated from the foot of mussels348. The polydopamine coatings serve as an efficient platform for better adhesion of cells and subsequently provide an osteoinductive and osteoconductive surface348. Further functionalization of such polydopamine coatings has been explored with arginyl-glycyl-aspartic acid (RGD) and bone morphogenic protein-2 (BMP-2) and has shown to promote mesenchymal stem cell differentiation and mineralization to osteoblasts.
An important yardstick for the biocompatibility of materials for medical devices is their compatibility with blood. This yardstick is often used to analyze and characterize a material’s tendency to adversely affect blood contact and result in blood clotting. A biomaterial tested for good blood compatibility is particularly interesting in applications such as vascular grafts, stents, heart valves, etc.349. For example, vascular stents are mostly made of metallic materials that are electropositive. These come into contact with blood components such as naturally electronegative platelets, forming a thrombus known as a clot350–352. The process involves complicated surface-mediated reactions influenced by material properties such as wettability, chemistry, etc. Therefore, surface functionalization to improve blood compatibility is an essential factor in enhancing biocompatibility and polydopamine from mussels, which functions as a protein coating on the surface of stents and has been shown to promote endothelial cell proliferation- an essential factor in the efficient functioning of stents. The polydopamine coatings also function as a carrier platform for other biomolecules, such as drugs and growth factors, synergistically improving blood compatibility353,354. Moreover, natural anti-blood-clotting agents such as heparin, prostaglandin, and other enzymes have been used to functionalize surfaces of medical devices with anti-coagulation properties. Interestingly, endothelial cells have also been used to functionalize these surfaces where the cells function individually and independently of the material.
Another essential aspect of a metallic medical device’s biocompatibility is that surface functionalization plays a prime role in infection control. Primary infection during surgical procedures is typically avoided by following proper sanitation methods and topical agents such as betadine. However, secondary infection is much more difficult to control and often leads to revision surgeries. Secondary infections commonly occur in dental implants and fracture fixation devices in bacterial invasion and biofilm formation355. Biofilms are organic polymeric extracellular secretions formed by colonies of micro-organisms that lead to cellular surfaces’ passivation and antibacterial molecules’ inactivation. Biofilms are one of the more difficult infections to treat356. However, two strategies have proven to be efficient in treating biofilms: developing a biofilm-resistant surface using bactericidal coatings that work in either contact or non-contact (by releasing antibacterial agents) and functionalizing the surface of the device to make it anti-adhesive towards bacterial attachment357,358. Synthetic approaches toward coating these medical implants with bactericidal agents have been extensively undertaken over the last decade. Evidence of fracture fixation components like intramedullary nails coated with gentamycin-loaded D-poly-lactic acid reveals effective antibacterial results in clinical studies conducted over 6 months359. Besides, several bioinspired coating techniques have also been reported based on their bactericidal properties. These include antimicrobial proteins, enzymes that break down bacterial cells and inhibit cell-to-cell communication in biofilms360,361. Nature-derived essential oils with antibacterial properties are also being explored recently, such as aromatic components of rosemary, oregano, and thyme362. A biomaterial used in medical devices having a bacteria-resistant surface is equally important as having an osteogenic surface. Therefore, these two properties should ideally work in tandem. BMP-2 and vascular endothelial growth factor (VEGF) has been reported to successfully promote osteoblast proliferation and differentiation in conjugation with chitosan-coated on Ti surfaces. Chitosan is an antibacterial agent that inhibits bacterial colony formation363,364.
As research continues in this field, the community must understand the regulatory hurdles that implant manufacturers must address before large-scale adoption. The current surgical protocol uses broad-spectrum systemic antibiotics to counter any immediate infecting bacteria. Implant material engineered to address infecting bacteria may be anti-fouling where the surface is designed to hinder bacteria’s attachment, i.e., prevent biofilm formation but is still amenable to protein and osteoblast attachment for usual osseointegration. The second approach could be using leachants that actively address planktonic bacteria. In this regard, the leachant is more like a drug whose metabolism and longevity must be addressed. Clinical trials of implants with anti-fouling or antibacterial properties that do not need antibacterial drugs post-implantation may be challenging to conduct.
8.0. Current challenges and future direction
8.1. Perspective on manufacturing metallic implants
Even though metallic biomaterials have been considered an effective and long-term solution for skeletal implantology, many challenges still need careful attention in the coming days. Due to the inherent bioinert nature of metallic biomaterials, improving biocompatibility is always a critical issue. However, besides improving biocompatibility, metal ion sensitivity, infection control, early-stage osseointegration, lowering stiffness, and introducing bio-resorbability are critical concerns for the next generation of metallic implant materials. Traditionally orthopedic implants were made from forging and casting. The adoption of additive manufacturing or 3D Printing is relatively recent365. However, there have been additively manufactured implants in the market over the last 10 years comprised of bulky parts. The adoption is now changing into more customized implants, making this a £1B industry with an upward and forward trajectory into the future. Specifically, regarding orthopaedics, there are specific relevant questions: What is the rush now? What is the unmet need? And why 3D printing? If we look at arthroplasty, what are the two main challenges, 1) Material loss at the bearing, bearing failure, bearing wear, fracture of ceramic implants over time, and wear of the polyethylene? Those tend to be the concerns surrounding arthroplasty. However, the other important area is 2) implant loosening, whether aseptic (non-infectious) or septic loosening. While our understanding of metallic implants and associated biocompatibility has come a long way, we must also recognize that early success was in a patient population that got implants later in their life; they lived a mostly sedentary lifestyle and rarely needed a revision. However, now the patient population wants to enjoy a more physically active lifestyle after implant surgery. Therefore, the assessment of the material’s biocompatibility should ideally be comprehensive and include a whole range of characterization such as
biomechanical compatibility with the host bone,
structural compatibility with the local microstructure of the implant location,
hemocompatibility for vascular applications,
infection resistance at the surgery/ implant site, etc.
These are vast ongoing research avenues in the biomedical device industry mainly because the perfectly inert orthopaedic implant still does not exist. All implants fail at some point or in some way, no matter the material. Therefore, the question is, what are we looking for at the bone/ metal interface?366 The answer to which can provide us with an everlasting/ lifelong implant, but we are unfortunately still far away from that which makes biocompatibility as relevant today as it was in the last century. To accomplish the goal of longer implant life in vivo, we must look beyond the usual materials and design for implants. This section offers the authors’ perspective overview of those issues and future directions to mitigate them.
8.2. The unmet need and current challenges
Regarding the unmet need in complex orthopaedic reconstruction, revision surgeries are the primary concern. As the science of arthroplasty becomes much more mature, for example, more and more patients have had hip replacements in the last 40 years, and there are still reports of many more failures. Going back to the understanding that an ideal implant still does not exist, some implants fail in the intermediate to long-term, and there are early failures that are not typically associated with the implants but are related to patient biology and surgeon factors. Previously, the general convention in the arthroplasty surgery was that the patient is fitted to limited designs and sizes of implants. This approach could potentially lead to suboptimal reconstruction, subsequent failure, revision surgery, and associated pain and disability. Additionally, this increases the risk of infection from multiple surgeries (statistically > 1%). On the other hand, a more futuristic outlook in the last 40 years is that the implant is made to fit the patient and has proven to be a better model for higher stability and lower failure rates. The trajectory of patient-tailored 3DP metal implants around 2007 was only 6 available designs on the market. This era marked a slow commercial adoption or transfer of this model. However, in 2021, at least 25 designs of 3DP acetabular cups have been adopted from different manufacturers. Finally, a new area of the medical devices industry is patient-specific 3D printing technology. Metal 3D printing is the primary platform for load-bearing implants where structural modification such as micro-porosities promotes better integration between metal and bone, thus enhancing biocompatibility. Zimmer-Biomet and Stryker have received FDA clearance for several 3D printed products, such as augments for revision surgery, acetabular shells, tibial trays, and ankle fusion systems. Treatments and strategies that decrease time and costs and give enhanced and customized results are used to gain popularity as technological innovations shape it.
3DP metallic implants are undoubtedly one of the most popular currents and futuristic technologies, but there still lie some challenges in all practical application purposes. Closer interrogation into this technology raises the question of whether we can manufacture parts using 3DP comparable to conventional implants in terms of production value and efficiency in some cases. For example, well-functioning conventional Ti implants have shown to release blood Ti levels under 4 ppb, but bulkier 3DP Ti implants that are directly implanted without post-processing show 10 times increase in blood ppb levels. Therefore, the current climate in orthopaedic technologies still guarantees further biocompatibility analysis, mediation, and toxicity mitigation.
Of note, unlike the Mg, Fe, and Zn alloys, these devices are the immediate future of the orthopedic implant industry since they are out of the research stage and are already in the extensive clinical trials or FDA approval stages. However, the fact that not only implants fabricated from bare metals such as Ti and Co-Cr fail due to material complications, but devices such as ITAP and POP that have ceramic or porous coatings also fail to perform in clinical applications prompt the need to focus on further developing novel materials with modified chemistry or bulk properties to circumvent the risks associated with coating applications. Among different metal ions, Ni, Co, and Cr are the three most common ions implicated in clinical studies. Both SS 316L and CoCrMo contain Ni and Cr. Co is the main element in CoCrMo alloy. Unfortunately, there is no easy solution for replacing these metal ions with other metals or alloys. However, new alloy design is generally not pursued in biomedical devices for generations due to the need for extensive experimental capabilities, a lot of experimental data for regulatory pathways, and a high probability of failure. Alloy mills have no incentive to explore new formulations as there is a high chance of failure or adoption in the orthopedic community. What would be the supply source if a company wants to explore and identify a formulation? Without a mill churning this alloy out on a large scale, the cost of this single-user alloy would be prohibitive. However, with 3D printing, alloy design is becoming common due to the ease of melting and creating new alloys on a small scale in a metal additive manufacturing set-up. It is anticipated that more research will be focused on alloy design for metallic implants in the coming days, and alloys without Ni, Co, and Cr can be innovated for human use without compromising clinical performance.
Infection is also a significant concern for fracture management devices and all metallic implants. In most cases, if an infection is not mitigated early, biofilms can form on the implant surface, necessitating revision surgery. Such revision surgeries are complex, compromise the remaining bone and soft tissue, are expensive, and cause significant pain and disability to patients for long periods. In a related area, namely amputees getting prosthetic devices, a successfully osseointegrated device significantly improves the quality of life by 17%376. This improvement in quality of life is a function of carefully measured outcomes in the efficacy and safety of the process. According to a case study performed by Van Eck et al. (2015) in a cohort for lower extremity amputation resulted in >20% implant removal due to stability issues (6%), fractures (9%), breakage (31%), and infection (41%–100%). Such high infection rates in clinical scenarios feed back into the removal rates of osseointegration devices. The challenge in such a case is the failure to identify the exact reason behind implant failure (in most cases, infection) because these procedures are heavily biased on externally prescribed antibiotics/painkillers, which make the implant seem efficacious377. The idea of designing new alloys that can minimize infection risks in vivo is not new but implementing such an idea has become easier with 3D printing. It is anticipated that more alloys will be innovated and manufactured in the coming years to protect patients from infection and biofilm formation without causing local toxicity.
Another critical area is designing implants to promote early-stage osseointegration. We have discussed many currently pursued approaches; some have already seen the clinical application. Perhaps, the porous metal coating is the most significant among them. Surface charge and alloy design are other approaches that show excellent results in improving biocompatibility and early-stage osseointegration. Finally, although good mechanical properties are the main differentiator for metallic devices from polymeric or ceramic-based implants, it is essential to remember that high stiffness is still a concern. Minimizing stiffness in metallic implant materials with new alloys without sacrificing their fatigue resistance or strength will open up new applications, particularly for young patients in whom the implant is expected to stay longer in vivo. However, as mentioned earlier, a complex procedure such as joint replacement requires a certain degree of preparation in a clinical situation, pre-op such as administration of drugs like acetaminophen, Celebrex that help preemptively reduce tissue inflammation and post-op pain. The challenge is determining if the implant’s material has an innate inflammatory response from the local tissue. Similarly, administering liposomal bupivacaine or sub-periosteal injection and early ambulation to prevent blood clots heavily bias material analysis for specific outcomes. Therefore, technology is moving towards more functional materials that can provide multiple secondary benefits along with their primary objective of bone replacement. Orthobiologics involve using the natural regenerative mechanisms of bone and the human body to functionalize external implants, such as implant materials combined with growth factors, stem cells, and engineered fractions isolated from autologous blood. Innovations in drug-eluting bone scaffolds have spearheaded the infection control side of orthopedic implantation. Several large companies in the industry have emerged with practical applications of orthobiologics.
8.3. Looking towards the future
The future perspective of orthopaedic engineering includes balancing innovation and safety because the current feedback loop is very long. Therefore, long-term post-market surveillance is necessary to mitigate such long wait times and better tools to image bony defects and match the material. Designing custom implants with porosities strut size to fit the anatomy of individual patients is an essential step in the future to implant a bioactive material into a biologically inactive defect/trauma site. This requires preemptive computation, which can only be successfully implemented if the gap between FEA modeling and biomechanically fitting the implant via surgery can be significantly narrowed. Biofunctionalized, bioadaptive and smart implants are some of the essential pillars of the next generation orthopaedic engineering. However, let’s investigate these technologies more intricately, for example. Regenerative medicine, such as stem cell or gene therapy, is proving to be efficacious for the haeling of long bone fractures in preclinical fracture healing models (where metallic devices are primarily used). These therapeutic strategies also require a scaffold system for their efficient delivery to the defect location, which necessitates focusing on the scaffold’s biocompatibility to carry stem cells or isolated genes at the fracture site. Smart implants, on the other hand, are usually pressure sensing or stress-determining additions to metal bionic limbs or long bone implants that make it easier to monitor the longevity of the implant. Smart orthopaedics—a combination of conventional methods and top-of-the-line innovation—will be the industry’s future. However, that does not discredit the material assessment for the actual implant and its biocompatibility.
Overall, the building blocks of the next generation of orthopedic implants are-
Personalized implants
If we move toward more personalized implants, the entire process becomes more stable.
The trend is already toward personalized instrumentation or guidance systems to better fit an off-shelf implant, but the next progression is the personalized instrument to match personalized implants. Particularly post-pandemic, the obvious next step is meeting the need for antimicrobial surfaces for tooling/ surgeries.
Closed Loop Process
As devices tend to become more personalized and the entire build envelope must be qualified, the need for closed-loop feedback will become necessary.
Build parameters must be qualified within a geometric and process range based on in situ feedback.
Clear Regulatory Pathway
A clear regulatory pathway will be required for patient-matched implants. The entire process will be considered, from segmentation through design to manufacture.
Deep understanding of the processing and characterization of the manufacturing approach will be needed to pave the pathway.
Although it seems mature, the field of metallic biomaterials is at an inflection point due to the infusion of new technologies, high demand from physically active younger patients, and a growing population worldwide. We hope our review will help established and young scientists understand the current issues and contribute to this field in the coming years to help improve the quality of life for all orthopedic, fracture management, and dental implant patients.
Figure 6. a. Different mechanisms of storing electrical charge on implant surfaces to enhance biocompatibility,
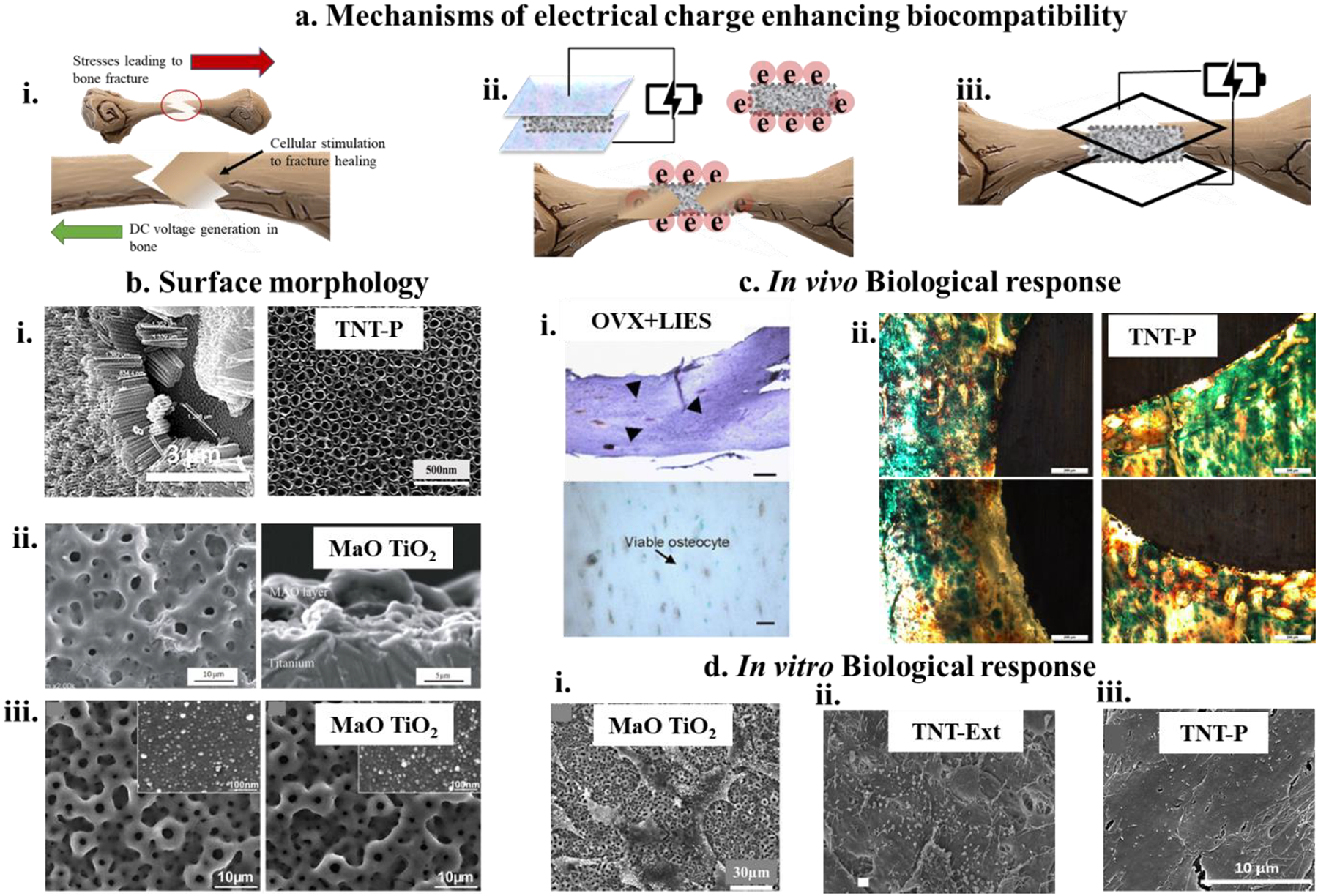
i) through the natural DC electrical potential of bone, which results as a trigger response from stresses applied on the bone, ii) through external electrothermal polarization, which induces capacitive electrical charge storage on the surface of the implant, iii) through external electrical triggers post-implantation with the electrical charge applied in situ at the surgery site. b. surface characteristics of the implant favorable towards electrical charge storage, i) nanoarchitecture Ti surface with TiO2 nanotubes which show capacitive potential for charge storage ~ 40mC/cm2 186. ii and iii) Micro arc oxidized (MaO) Ti surfaces with TiO2 nanoarchitecture for electrical charge storage185 c. In vivo bone formation and osseointegration in rat femur model, i) Endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) in ovariectomized (OVX) rats exposed to low-intensity electrical stimulation (LIES) showed similar eNOS and iNOS expressions for both in the OVX rats195, ii) optical micrographs for polarized nanotube (TNT-P) showing higher osteoids like bone as well as mineralized bone formations at 5-week post implantation186, d. In vitro cell-materials interaction, i) SaO2 cells cultured on MaO-TiO2 surface showing well-attached cells with flattened, spread out morphologies with numerous filopodial extensions196, ii) SEM micrographs of osteoblasts cultured on implants exposed to external 15 V stimulation on anodized nanotubular titanium191, iii) well-flattened and proliferated osteoblasts on polarized TNT surfaces at 7 days post-culture186.
Figure 7. a. Morphological analyses of β-Ti alloys,
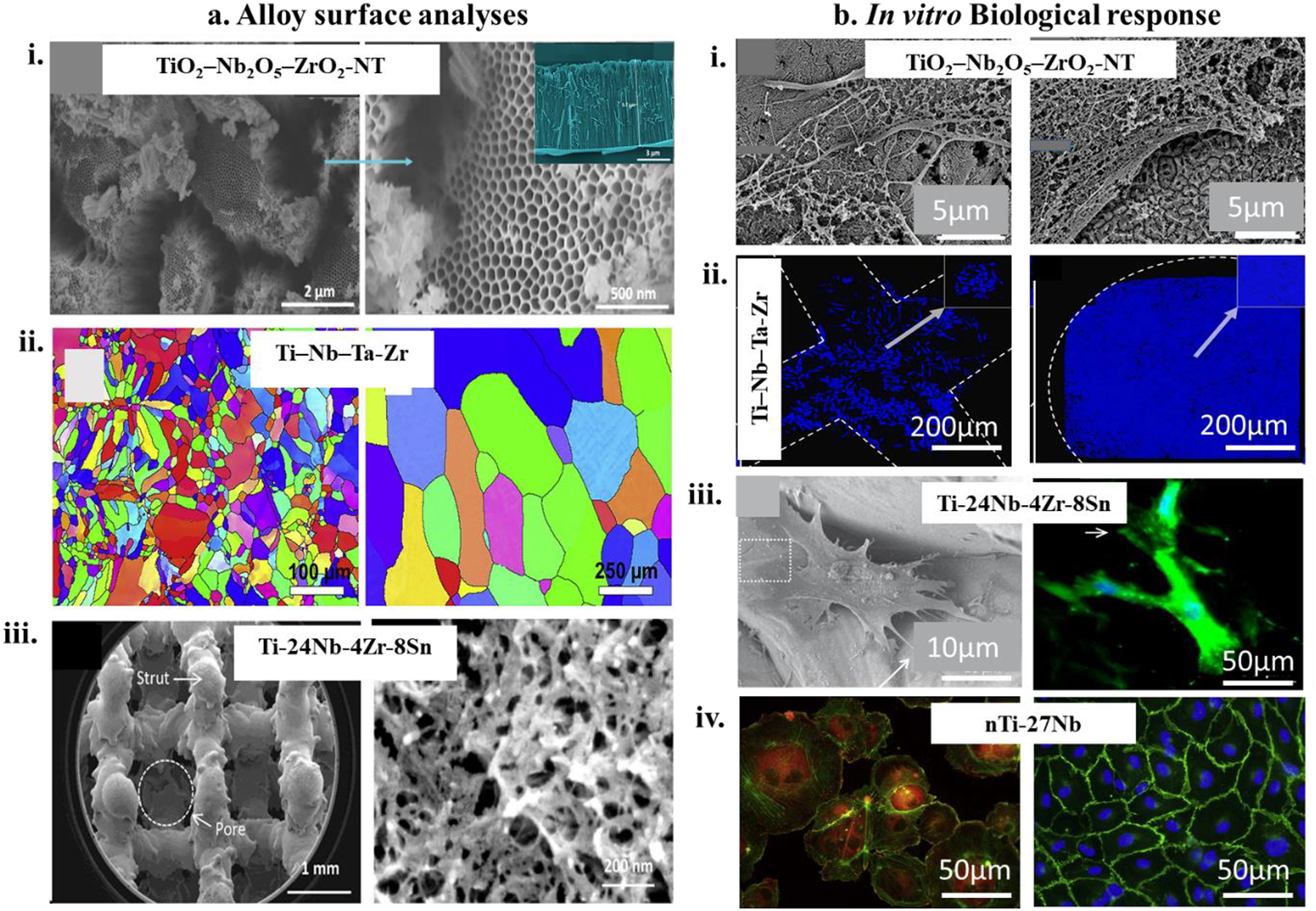
i) Low (left) and high (right) magnification SEM micrographs of TiO2-Nb2O5-ZrO2-nanotube (NT) surfaces revealing non-uniform size distribution of nanotubes (inset), with a diameter ranging between 33 and 76 nm235. ii) Electron backscattered micrograph of SLM printed TNTZ (Ti-Nb-Ta-Zr) alloy (left) and as-cast TNTZ (right), mainly revealing β-Ti phase with a minority of α’ phase and grain size almost nine times smaller than as-cast TNTZ23. iii) Optical micrograph (left) of EBM printed Ti2448 (Ti-24Nb-4Zr-8Sn) implants showing pore (800–900 μm) and strut (350 μm) characteristics, (right) shows the surface morphology of type I collagen immobilized Ti2448 implants with much smaller pores (10–100nm) due to partial coverage due to collagen immobilization236. b. In vitro cell-material interactions of β-Ti alloys, i) SEM micrographs of spreading, attachment and proliferation of osteoblast-like Saos-2 cells on TiO2-Nb2O5-ZrO2-NT indicating a positive enhancement in the biocompatibility of these surfaces compared to Ti35Zr28Nb surfaces235 , ii) fluorescence images of 72h cultured mouse fibroblast cells (L929) on rhombic dodecahedron (RD) printed TNTZ (left), and body diagonal (BD) printed TNTZ (right) showing more uniform cell spreading on BD structures23 iii) human bone marrow-derived mesenchymal stem cells (hBMSC) adhesion and spreading with long filopodial extensions on the type I collagen immobilized Ti2448 surface (left), corroborating focal adhesion observed from immunofluorescence image of the same surface (right)236, iv) nitride Ti27Nb alloy shows actin cytoskeleton, and focal adhesion of endothelial progenitor cells (left) 2h post culture, (right) shows immunofluorescence vascular endothelial cell cadherin marker on nTi27Nb surface after 5days of culture237.
Figure 8. a. Surface morphologies of Ti-Ta alloys,

i, ii and iii) show SEM micrographs of Ti-25Ta surfaces with acicular α’ and α” platelet microstructure for SLM fabricated (i)10 and DED fabricated (iii)253 Ti-25Ta alloys, while (ii) shows more β-phase stabilization for Ti-25Ta alloys248, iv) exhibits equiaxed microstructure containing both α” and ω phases254,v) shows Ti-35Ta microstructure, which was subjected to heat treatment to achieve homogenized distribution of Ta255. b. In vitro cell-material interaction of Ti-Ta alloy surfaces, i) human adipose-derived stem cells show well-attached morphology and proliferation on Ti-30Ta surfaces nanotube architecture256, ii) well proliferated osteoblast cells on Ti-25Ta surface with uniform surface area coverage and layering shows enhanced biocompatibility of this alloys253. c, In vivo implantation of Ti-Ta alloys, i and ii) show implant-bone apposition of Ti-Ta alloys from a radiograph of rabbit femurs and CT-scan of rat femurs respectively253. d, Biological response in a dynamic in vivo environment, i) showing optical micrographs of Ti-10Ta and Ti-25Ta bone-implant sections from rat femur implantation revealing enhanced osteoid formation (red areas) in NT modified alloy surfaces while (ii) corroborates the results exhibiting higher trabecular bone formation at 7 weeks in rabbit femur implantation253.
Figure 9. a. mechanism of stent placement.
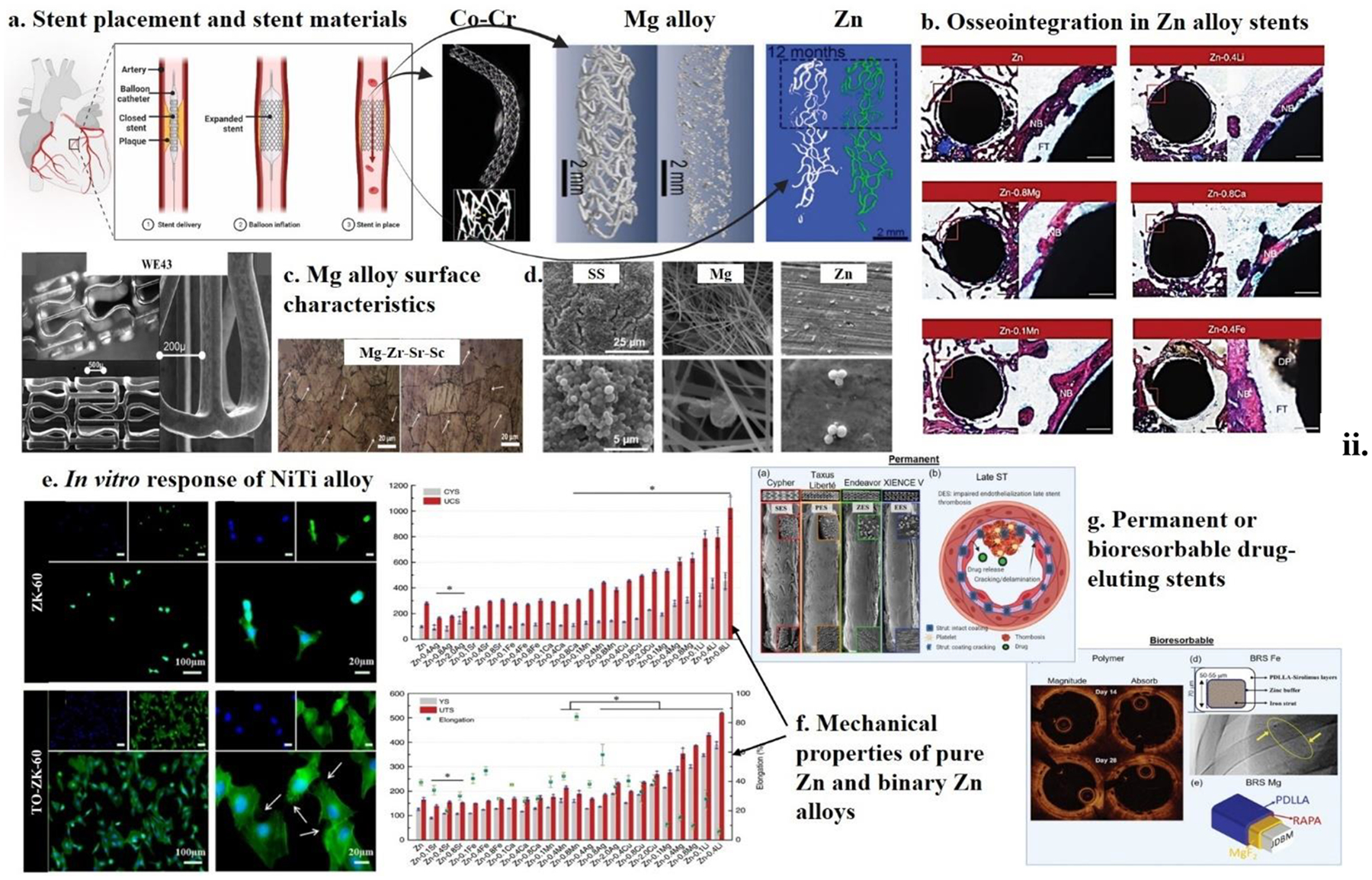
for percutaneous coronary intervention showing Co-Cr multi-vision link stents (commonly used permanent device) compared to bioresorbable Mg alloy and pure Zn devices (degraded in 12 months)290–292. b. In vivo osteogenesis and osseointegration profile for binary Zn alloy materials at 8 weeks post-implantation. Zn-Li, Zn-Mg, and Zn-Ca showed the most efficient performance in terms of Zn degradation in load-bearing sites287. c. surface characteristics of Mg alloys for bone implants, (top left) optical micrograph of the expandable tubular Mg alloy stent showing characteristic cross-linked circumferential noose- shaped structures. (Bottom and right): Electron microscopy at low and high magnification, respectively280, Optical micrographs of Mg alloys surfaces consisting of Mg-0.6Zr-0.5Sr-2Sc (left) and Mg-0.6Zr-0.5Sr-3Sc (right), which showed enhanced biocompatibility due to the addition of Sc>1284. d. antibacterial performance of SS, Mg, and Zn implants showing highest contact killing for Zn. e. in vitro cytocompatibility of Mg alloys, (top) and (bottom) shows confocal immunofluorescence images of MC3T3-E1 cells on untreated Mg alloy ZK60 surface (top) as compared to higher and enhanced cell viability on Ta suboxide functionalized ZK60 surface (TO-ZK60) in (bottom)242. f. The mechanical properties of binary Zn alloy materials compared to pure Zn show that Zn-Li alloys exhibit better stability. g. Permanent and bioresorbable (BRS)-based drug-eluting stents (DES). (a) in vivo SEM images of permanent (Co-Cr)-DES compared highlighting everolimus-eluting stent (EES) over its competitors293, (b) late stenosis as a result of the molecular level effect of strut malapposition, (c) 28-day CT images showing high embedding and low protrusion and area of uncovered struts of Magnitude-BRS as compared to the Absorb-BVS (bioresorbable vascular scaffolds)294 (d) a nitrided-Fe DES289 and (e) a PDLLA (poly DL-lactic acid)-carrying Rapamycin coated Mg-JDBM stent295.
Figure 10. a. popular mechanisms of effective ceramic coatings on metal surfaces,
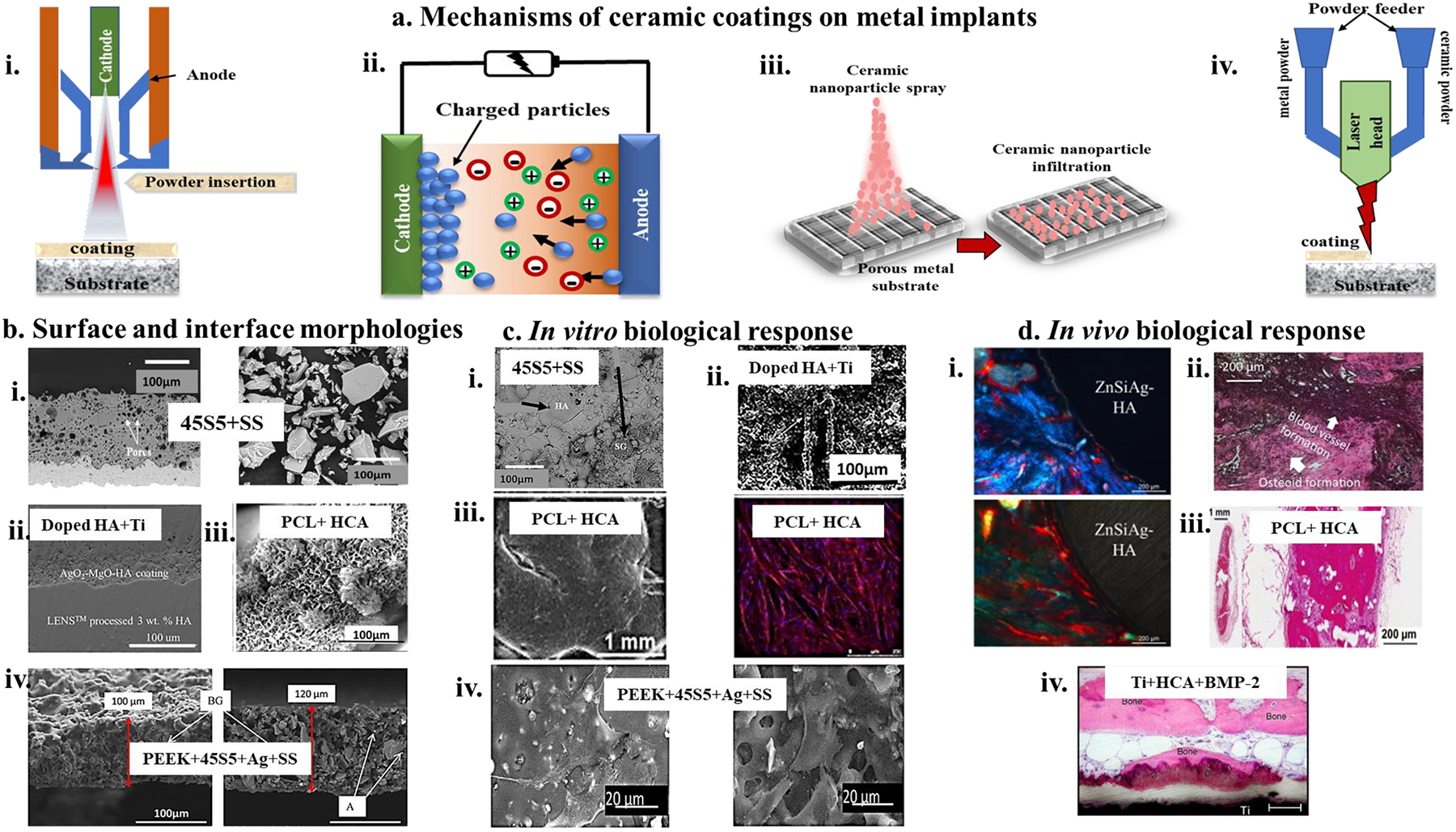
i) Plasma-spray coating molten ceramic powder particles onto the surface of a heated material to-be-coated. The ceramic powder is injected into the high-temperature plasma, ii) Electrophoretic deposition technique involves the deposition of charged ceramic particles in a colloidal solution onto a conducting metal surface, iii) infiltration process involves the use of nanoscale ceramic particles into porous metal network resulting in entrapment of the ceramic nanoparticles and coating the metal, iv) DED additive manufacturing technique involves premixing ceramic and metal powders to coat metal surfaces under high-temperature laser melting and rapid solidification. b, Morphologies of coating surfaces and coating-substrate interfaces, i) (left) interfacial SEM micrograph and (right) micrograph of the particle size distribution of 45S5 bioglass coated on stainless steel (AISI 304) with the fine dry ground (DR) particle size less than 63μm. DR63 powder coating revealed fewer cracks and pores at the interface due to decreased temperature gradient during cooling339, ii) Interfacial microstructure of Ag-oxide and Mg-oxide doped HA-coated on DED fabricated Ti+3%HA substrate showed well-bonded coating with the substrate with no cracks and delamination299, iii) CaP deposition using immersion in simulated body fluid on polycaprolactone (PCL) coated metal substrate reveals nanotextured HCA like layer formation on PCL strands305, iv) Cross-section microstructure of 45S5 and silver particles embedded in PEEK coated on SS316L, shows uniform co-deposition of 45S5 and Ag particles and homogenous microstructure from SEM (left) as well as a backscattered image (right)340. c, in vitro cellular activity on coated metal surfaces, i) 45S5 coating on AISI 304 substrate shows the formation of a uniform layer of HA after 7-day immersion in SBF, indicating enhanced bioactivity339, ii) Doped HA coating on DED processed Ti64 surfaces shows not only well-attached osteoblasts but also differentiation of osteoblast at day 11 on these increased biocompatible coated metals299, iii) At week-8, HCA coated PCL strands shows cell sheet formation around each strand of PCL when exposed to a basal media305, iv) PEEK and bioglass composite on SS316L exhibited well-spread MG63 osteoblast-like cell on the surface at 4 days after incubation, with no evident detrimental effect from Ag addition in the coating340, d, in vivo dynamic biological response, i) Tissue integration within the doped HA coating on Ti64 can be seen in week 10 with ZnSiAg HA-coated Ti64 indicating better osseointegration. The higher osteoid formation is seen with HA; however, ZnSiAg-HA has a higher total bone formation300, ii) H&E staining of rat explant section reveals new bone formation and angiogenesis for HA-coated Ti64 with natural medicinal compound curcumin doping in the HA matrix341, iii) Mineralized bone filling pores of HA+ PCL coated scaffolds at 8 weeks implantation in athymic rats305, iv) High-magnification image of Ti discs coated with HCA and BMP-2, showing bone tissue in direct contact with the coating and within the surrounding connective tissue298.
Figure 11. a. Mechanisms of immobilization of biomolecules on metal implant surfaces,
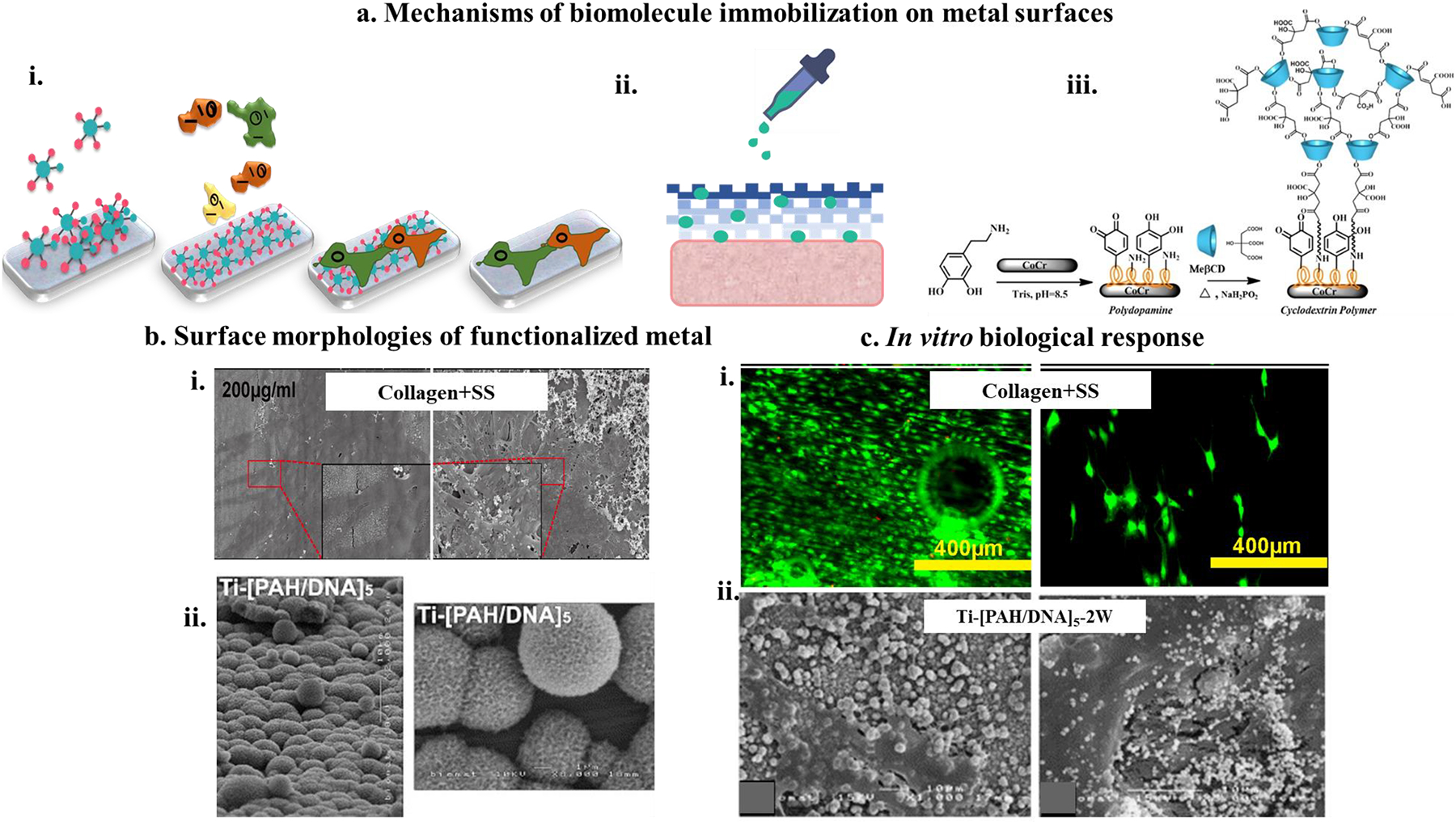
i) Physical adsorption of proteins where the proteins get adsorbed on a hydrophobic surface eventually modifying the surface characteristic to hydrophilic and favorable for cellular attachment resulting in cascades of cell-material interactions, ii) physical entrapment of biomolecule on porous metal surfaces where the biomolecule gets entrapped in the pores of the metal structure and gets released over time, iii) covalent binding of biomolecules on the surface of metals leading to specific functionalization of the metal surface to enhance biocompatibility354 b, surface characteristics of metal functionalized with biomolecules, i) Rat tail type I collagen immobilized on Mg surface(AZ31) showing collagen self-assembly structure varying with collagen concentration. At 200μg/ml, the collagen coating showed longer thin fibrils forming a multilayer network structure343, ii) Polycationic electrolytes (PAH) coupled with polyanionic salmon DNA were immobilized on Ti surfaces showed spheroid-like deposition of biomolecules on the surface of these materials aiding in cellular interactions345 c, In vitro biological response of biomolecule coated metal surfaces, i) (left and right) shows MC3T3 cells growing and proliferating on AZ31 and Mg alloys respectively with an increased number of living cells on AZ31 surface at day 7343, ii) osteoblast-like cell growth on PAH/DNA immobilized Ti surface at day 4 (left) and day 16 (right) respectively. On day 16, a large formation of collagen fibrils, mineralized globules, and extracellular matrix on the treated surfaces was observed345.
Table 1.
lists some of the mechanical properties of four dominant metallic biomaterials - stainless steel (SS 316L), commercially pure titanium (CpTi), α-β titanium alloy (Ti6Al4V), and cobalt-based alloy CoCrMo.
| Materials | Young’s Modulus (GPa) | Ultimate tensile strength (MPa) | Fracture toughness |
|---|---|---|---|
| SS 316L | 200 | 500 – 1000 | ~100 |
| Ti6AL4V | 104 – 113 | 900 | ~80 |
| Cp-Ti | 110 | ||
| CoCrMo | 240 | 900 – 1500 | ~100 |
| Cortical bone | 10 – 30 | 130 – 150 | 2–12 |
Table 6:
Ti-Ta alloy processing, material characteristics, and key findings on the biological response.
| Citation | Materials | Characteristics | Processing | Key Findings | Remarks |
|---|---|---|---|---|---|
| Huang et al. (2020) 247 | Ti-0Ta, Ti-10Ta, Ti-30Ta, Ti-50Ta |
Ti-50Ta alloy had 60% porosity for in vitro analysis | Selective Laser Melting (SLM) |
Material –
In vitro
|
|
| N/A | |||||
| Zhao et al. (2019) 248 | Ti-6Ta, Ti-12Ta, Ti-18Ta, Ti-25Ta |
Selective Laser Melting (SLM) |
Material -
Biocompatibility evaluation was done via corrosion resistance analysis of the Ti-Ta alloys. Ti-25Ta alloy formed Ta2O5/TiO2 passive layers |
|
|
| -N/A- | |||||
| -N/A- | |||||
| Fuerst et al. (2015) 249 | Pure Ta, Ti-20Ta, Ti-80Ta |
|
Laser Powder Deposition (LPD) |
Biological -
|
No in vivo assessment was done. |
| |||||
| de Souza, K. A., & Robin, A. (2003) 250 | Ti-20Ta, Ti-40Ta, Ti-60Ta, Ti-80Ta |
Conventional method- arc melting |
Material-
|
No biological assessment was done. | |
| N/A | |||||
| N/A | |||||
| Zhou, Y. L., & Niinomi, M. (2008) 251 | Ti-50Ta | Conventional method- arc melting |
Material-
|
No biological assessment was done. | |
| N/A | |||||
| N/A | |||||
| Zhou, Y. L., Niinomi, M., & Akahori, T. (2004) 18 | Ti-10Ta, Ti-20Ta, Ti-30Ta, Ti-40Ta, Ti-50Ta Ti-60Ta, Ti-70Ta, Ti-80Ta |
Conventional method- arc melting |
Material-
|
No biological assessment was done. | |
| N/A | |||||
| N/A | |||||
| Mareci, D., Chelariu, R., Gordin, D. M., Ungureanu, G., & Gloriant, T. (2009) 252 | Ti-30Ta, Ti-40Ta, Ti-50Ta Ti-60Ta |
Levitation melting |
Corrosion study
|
No biological assessment was done. | |
| I. Mitra et al. (2021) 253 | CpTi, Ti-10Ta, Ti-25Ta, Pure Ta |
|
Directed Energy Deposition using LENS™ |
Material -
In vitro
|
|
Table 7.
summarizes a few of the notable orthopedic devices being introduced into the market and their approval status. Most of these devices are not yet FDA approved but are commercially available in Europe and Australia367.
| Bone-implant interface | Product name | Composition | Manufacturer | Application | Approval status |
|---|---|---|---|---|---|
| Threaded | Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA) Implant System | Ti and Ti alloys | Integrum AB | Transfemoral Transtibial Transhumeral Transradial Thumb Digital |
Approved in EU, Australia, TF, FDA approved |
| Press fit | Integral Leg Prosthesis (ILP) | Co-Cr | Orthodynamics GMBH | Transfemoral Transtibial Transhumeral |
EU, Australia, Not FDA approved |
| Osseointegrated prosthetic limb (OPL) | Ti | Permedica s.p.a | Transfemoral Transtibial |
EU, Australia, not FDA approved | |
| Intraosseous transcutaneous amputation prosthesis (ITAP) | Ti+Hydroxyapatite | Stanmore implants worldwide/Stryker | Transfemoral Transhumeral |
In clinical trials in UK, CE Mark, Not FDA approved | |
| Keep walking Advanced | Tequir S.L. | Transfemoral | In development | ||
| POP | Porous Ti coating on Ti | DJO global | Transfemoral | In 10 patient, FDA early feasibility trail | |
| Compression/pin lock | COMPRESS | Zimmer Biomet | Transfemoral Transhumeral |
Custom |
Table 8.
shows biomedical devices listed in Table 7 and case studies on patient follow up range, reason for failure and recalls. Most cases failed due to implant infection while some also resulted in femoral fractures288.
| Complications | |||||||
|---|---|---|---|---|---|---|---|
| Number | Follow-up (range) | Aseptic failure (%) | Septic failure (%) | Infection (%) | Femoral Fracture (%) | ||
| OPRA | Hagberg et al368 | 18 | 24 mo | 5.6 | 0 | Superficial: 11.2 | 0 |
| Tillander et al369 | 39 | 39 mo | 2.6 | 2.6 | Superficial: 19.5 | 0 | |
| Branemark et al370 | 51 | 24 mo | 5.9 | 2.0 | Superficial: 54.9 Deep: 2 |
5.9 | |
| Branemark et al371 | 51 | 60 mo | 5.9 | 2.0 | Superficial: 66.7 Deep: 21.6 |
NR | |
| ILP | Aschoff (2010)372 | 37 | NR | 7.7 | 2.6 | Superficial: 36 | 0 |
| Van de Meent at al373 | 22 | 12 mo | 0 | 0 | Superficial: 36.4 | 0 | |
| Juhnke et al374 | 1st and 2nd gen: 30 3rd gen: 39 |
32.4 mo | 3.3 | 3.3 | Superficial: 76.7 Deep: 3.3 |
10 3rd gen: 5.6 |
|
| Al Muderis at al375 | 86 | 34 mo | 3.5 | 0 | Superficial: 33.7 | 3.5 | |
| OPL | Al Muderis at al376 | 51 | 21.5 mo | 3.9 | 0 | Superficial: 35.3 Deep: 5.9 |
7.8 |
| Al Muderis at al376 | 22 | 12 mo | 0 | 0 | Superficial: 54.5 | 0 | |
| POP | Agarwal (2018)377 | 10 | 12 mo | 10 | 0 | Superficial: 20 | 10 |
Scope of this review.
This article evaluates multiple approaches to improving the biocompatibility of next-generation metallic implants. Metals that are most commonly used, such as titanium (Ti), β- Ti alloys, tantalum (Ta), binary and ternary alloys of Ti, Nitinol along with Mg, Zn, and other alloys, are selectively covered in the review as a function of relevant modification techniques to improve biocompatibility and their applications. Biomedical devices fabricated using these metals and techniques currently used in a commercial capacity or clinical trials/ FDA approval stages are considered next-generation implants from the perspective of this article. Since the implant’s surface is the first contact with native bone at the defect site and, eventually, the implant’s material plays a role in healing, the authors have focused this article more on biocompatible surfaces and new alloy designs.
Acknowledgements
The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01 AR067306-01 (PI - Bandyopadhyay) and R01 AR078241-01 (PI - Bandyopadhyay). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
All raw data for this study has been presented in this manuscript.
References
- [1].Beveridge M, Howard A The burden of orthopaedic disease in developing countries JBJS, 86 (2004), pp. 1819–1822 [DOI] [PubMed] [Google Scholar]
- [2].Orthopedic devices research revenue | key players market share & position. Advance Market Analytics https://www.advancemarketanalytics.com/reports/66050-global-orthopedic-devices-market-1.
- [3].Arabnejad S, et al. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints Acta Biomater, 30 (2016), pp. 345–356 [DOI] [PubMed] [Google Scholar]
- [4].Balla VK, Bodhak S, Bose S, Bandyopadhyay A Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties Acta Biomater, 6 (2010), pp. 3349–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bandyopadhyay A, Krishna BV, Xue W, Bose S Application of laser engineered net shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants J Mater Sci - Mater Med, 20 (2009), p. 29. [DOI] [PubMed] [Google Scholar]
- [6].Greiner C, Oppenheimer SM, Dunand DC High strength, low stiffness, porous NiTi with superelastic properties Acta Biomater, 1 (2005), pp. 705–716 [DOI] [PubMed] [Google Scholar]
- [7].Bandyopadhyay A, Ghosh S, Boccaccini AR, Bose S 3D printing of biomedical materials and devices J Mater Res, 36 (2021), pp. 3713–3724, 10.1557/s43578-021-00407-y [DOI] [Google Scholar]
- [8].Krishna BV, Bose S, Bandyopadhyay A Low stiffness porous Ti structures for load-bearing implants Acta Biomater, 3 (2007), pp. 997–1006 [DOI] [PubMed] [Google Scholar]
- [9].Krishna BV, Xue W, Bose S, Bandyopadhyay A Engineered porous metals for implants JOM, 60 (2008), pp. 45–48 [Google Scholar]
- [10].Bose S, Robertson S, Bandyopadhyay A, et al. Surface Modification of Biomaterials and Biomedical Devices using Additive Manufacturing Acta Biomater, 66 (2018), pp. 6–22, 10.1016/j.actbio.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang B, et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction Mater Des, 152 (2018), pp. 30–39 [Google Scholar]
- [12].Bidaux J-E, Closuit C, Rodriguez-Arbaizar M, Zufferey D, Carreño-Morelli E Metal injection moulding of low modulus Ti–Nb alloys for biomedical applications Powder Metall, 56 (2013), pp. 263–266 [Google Scholar]
- [13].Brailovski V, et al. Bulk and porous metastable beta Ti–Nb–Zr (Ta) alloys for biomedical applications Mater Sci Eng C, 31 (2011), pp. 643–657 [Google Scholar]
- [14].Goodman SB, Davidson JA, Fornasier VL, Mishra AK Histological response to cylinders of a low modulus titanium alloy (Ti-13Nb-13Zr) and a wear resistant zirconium alloy (Zr-2.5 Nb) implanted in the rabbit tibia J Appl Biomater, 4 (1993), pp. 331–339 [Google Scholar]
- [15].Liu Y, et al. Powder metallurgical low-modulus Ti–Mg alloys for biomedical applications Mater Sci Eng C, 56 (2015), pp. 241–250 [DOI] [PubMed] [Google Scholar]
- [16].Niinomi M, Nakai M, Hieda J Development of new metallic alloys for biomedical applications Acta Biomater, 8 (2012), pp. 3888–3903 [DOI] [PubMed] [Google Scholar]
- [17].Okulov IV, et al. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications J Mech Behav Biomed Mater, 65 (2017), pp. 866–871 [DOI] [PubMed] [Google Scholar]
- [18].Zhou YL, Niinomi M, Akahori T Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications Mater Sci Eng A, 371 (2004), pp. 283–290 [Google Scholar]
- [19].Gu H, et al. Microstructure evolution and electrochemical properties of TiO 2/Ti-35Nb-2Ta-3Zr micro/nano-composites fabricated by friction stir processing; 2019.
- [20].Hafeez N, et al. Mechanical behavior and phase transformation of β-type Ti-35Nb-2Ta-3Zr alloy fabricated by 3D-Printing; 2019.
- [21].Inaekyan K, et al. Comparative study of structure formation and mechanical behavior of age-hardened Ti–Nb–Zr and Ti–Nb–Ta shape memory alloys Mater Charact, 100 (2015), pp. 65–74 [Google Scholar]
- [22].Liu YJ, Li XP, Zhang LC, Sercombe TB Processing and properties of topologically optimised biomedical Ti–24Nb–4Zr–8Sn scaffolds manufactured by selective laser melting Mater Sci Eng A, 642 (2015), pp. 268–278 [Google Scholar]
- [23].Luo JP, et al. Additively manufactured biomedical Ti-Nb-Ta-Zr lattices with tunable Young’s modulus: Mechanical property, biocompatibility, and proteomics analysis Mater Sci Eng C, 114 (2020), Article 110903. [DOI] [PubMed] [Google Scholar]
- [24].Lennox DW, Schofield BH, McDonald DF, Riley LH Jr A histologic comparison of aseptic loosening of cemented, press-fit, and biologic ingrowth prostheses Clin Orthop Relat Res, 171–191 (1987) [PubMed] [Google Scholar]
- [25].Lombardi JA, Mallory TH, Vaughn BK, Drouillard P Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium-alloy modular femoral heads J Bone Joint Surg. American, 71 (1989), pp. 1337–1342 [PubMed] [Google Scholar]
- [26].Schweizer A, Riede U, Maurer TB, Ochsner PE Ten-year follow-up of primary straight-stem prosthesis (MEM) made of titanium or cobalt chromium alloy Arch Orthop Trauma Surg, 123 (2003), pp. 353–356 [DOI] [PubMed] [Google Scholar]
- [27].Papageorgiou I, et al. Macrophages detoxify the genotoxic and cytotoxic effects of surgical cobalt chrome alloy particles but not quartz particles on human cells in vitro Mutation Res/Fundamental Mol Mech Mutagenesis, 643 (2008), pp. 11–19 [DOI] [PubMed] [Google Scholar]
- [28].Isik M, Avila JD, Bandyopadhyay A Alumina and tricalcium phosphate added CoCr alloy for load-bearing implants Addit Manuf, 36 (2020), Article 101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Avila JD, Alrawahi Z, Bose S, Bandyopadhyay A Additively manufactured Ti6Al4V-Si-hydroxyapatite composites for articulating surfaces of load-bearing implants Addit Manuf, 34 (2020), Article 101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Davis JR. Handbook of materials for medical devices; 2003.
- [31].Lütjering G, Williams JC Titanium Springer Science & Business Media (2007)
- [32].Cancer I. A. for R. on. Surgical implants and other foreign bodies. IARC monographs on the Evaluation of carcinogenic risks to humans 74, (1999). [PMC free article] [PubMed] [Google Scholar]
- [33].Böstman OM Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three-to nine-year follow-up study J Bone Joint Surg. British, 80 (1998), pp. 333–338 [DOI] [PubMed] [Google Scholar]
- [34].Hofmann D, Entrialgo-Castaño M, Kratz K, Lendlein A Knowledge-based approach towards hydrolytic degradation of polymer-based biomaterials Adv Mater, 21 (2009), pp. 3237–3245 [DOI] [PubMed] [Google Scholar]
- [35].Xu L, et al. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy Biomaterials, 30 (2009), pp. 1512–1523 [DOI] [PubMed] [Google Scholar]
- [36].Balla VK, Banerjee S, Bose S, Bandyopadhyay A Direct laser processing of a tantalum coating on titanium for bone replacement structures Acta Biomater, 6 (2010), pp. 2329–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bandyopadhyay A, Mitra I, Shivaram A, Dasgupta N, Bose S Direct comparison of additively manufactured porous titanium and tantalum implants towards in vivo osseointegration Addit Manuf, 28 (2019), pp. 259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brumme S, Löwicke GW The use of tantalum wire as a suture material Zeitschrift Fur Experimentelle Chirurgie, Transplantation, Und Kunstliche Organe: Organ Der Sektion Experimentelle Chirurgie Der Gesellschaft Fur Chirurgie Der DDR, 22 (1989), pp. 308–313 [PubMed] [Google Scholar]
- [39].Bando S The experimental and clinical studies on the anastomosis of the intercostal nerve to the splanchnic nerve by means of metal tantalum foil (an operative procedure on the so-called abdominal neurosis) Kobe Ika Daigaku Kiyo, 26 (1964), p. 98. [PubMed] [Google Scholar]
- [40].Kalinina TV The fate of tantalum clamps in anastomoses of the digestive tract Khirurgiia, 44 (1968), p. 97. [PubMed] [Google Scholar]
- [41].Andrews AH Jr, Tamari MJ Treatment of anterior laryngeal stenosis by tantalum plate implant Ill Med J, 103 (1953), p. 175. [PubMed] [Google Scholar]
- [42].Oppenheimer BS, Oppenheimer ET, Danishefsky I, Stout AP, Willhite M Carcinogenic effect of metals in rodents Cancer Res, 16 (1956), pp. 439–441 [PubMed] [Google Scholar]
- [43].Matthay RA, et al. Tantalum oxide, silica and latex: effects on alveolar macrophage viability and lysozyme release Invest Radiol, 13 (1978), pp. 514–518 [DOI] [PubMed] [Google Scholar]
- [44].About Arcam | GE Additive. https://www.ge.com/additive/who-we-are/about-arcam.
- [45].Renishaw showcases metal AM implants to American Academy of Orthopaedic Surgeons. Metal Additive Manufacturing https://www.metal-am.com/renishaw-showcases-metal-implants-american-academy-orthopaedic-surgeons/ (2018).
- [46].Alphaform Produces Metal 3D Printed Hip Implant For Boy With Bone Cancer. http://www.prototypetoday.com/eos/alphaform-produces-metal-3d-printed-hip-implant-for-boy-with-bone-cancer.
- [47].Beals JT, Thompson MS Density gradient effects on aluminium foam compression behaviour J Mater Sci, 32 (1997), pp. 3595–3600 [Google Scholar]
- [48].JOACHIM L Influence of imperfections on effective properties of cellular solids. Porous and Cellular Materials for Structural Applications 3. [Google Scholar]
- [49].Sanders W, Gibson LJ Reduction in Young’s modulus of aluminum foams due to cell wall curvature and corrugation MRS Online Proceedings Library Archive, 521 (1998) [Google Scholar]
- [50].Sang H, Kenny LD, Jin I. Process for producing shaped slabs of particle stabilized foamed metal; 1994.
- [51].Surace R, De Filippis LAC, Niini E, Ludovico AD, Orkas J Morphological investigation of foamed aluminum parts produced by melt gas injection Adv Mater Sci Eng, 2009 (2009) [Google Scholar]
- [52].Xue W, Krishna BV, Bandyopadhyay A, Bose S Processing and biocompatibility evaluation of laser processed porous titanium Acta Biomater, 3 (2007), pp. 1007–1018 [DOI] [PubMed] [Google Scholar]
- [53].Hahn H, Palich W Preliminary evaluation of porous metal surfaced titanium for orthopedic implants J Biomed Mater Res, 4 (1970), pp. 571–577 [DOI] [PubMed] [Google Scholar]
- [54].Yang YZ, et al. Preparation of graded porous titanium coatings on titanium implant materials by plasma spraying J Biomed Mater Res: Official J Soc Biomater, The Japanese Soc Biomater, Aust Soc Biomater Korean Soc Biomater, 52 (2000), pp. 333–337 [DOI] [PubMed] [Google Scholar]
- [55].Thieme M, et al. Titanium powder sintering for preparation of a porous functionally graded material destined for orthopaedic implants J Mater Sci - Mater Med, 12 (2001), pp. 225–231 [DOI] [PubMed] [Google Scholar]
- [56].Rausch G, Banhart J Making cellular metals from metals other than aluminum Wiley-VCH Verlag, Weinheim, Germany: (2002) [Google Scholar]
- [57].Oh I-H, Nomura N, Masahashi N, Hanada S Mechanical properties of porous titanium compacts prepared by powder sintering Scr Mater, 49 (2003), pp. 1197–1202 [Google Scholar]
- [58].Oh I-H, Nomura N, Hanada S Microstructures and mechanical properties of porous titanium compacts prepared by powder sintering Mater Trans, 43 (2002), pp. 443–446 [Google Scholar]
- [59].Adell R, Branemark BO, Branemark PI, Breine U Intra-osseous anchorage of dental prostheses: II. Review of clinical approaches Plast Reconstr Surg, 49 (1972), p. 102. [DOI] [PubMed] [Google Scholar]
- [60].Bobyn JD. Characterization of a new porous tantalum biomaterial for reconstructive orthopaedics. Scientific Exhibit, Proc of AAOS, Anaheim, CA, 1999 (1999). [Google Scholar]
- [61].Zardiackas LD, et al. Structure, metallurgy, and mechanical properties of a porous tantalum foam J Biomed Mater Res: Official J Soc Biomater, Japanese Soc Biomater, Aust Soc Biomater Korean Soc Biomater, 58 (2001), pp. 180–187 [DOI] [PubMed] [Google Scholar]
- [62].Munir ZA, Anselmi-Tamburini U Self-propagating exothermic reactions: the synthesis of high-temperature materials by combustion Mater Sci Rep, 3 (1989), pp. 279–365 [Google Scholar]
- [63].Yi HC, Moore JJ Combustion synthesis of TiNi intermetallic compounds J Mater Sci, 24 (1989), pp. 3456–3462 [Google Scholar]
- [64].Zhang X, Ayers RA, Thorne K, Moore JJ, Schowengerdt F Combustion synthesis of porous materials for bone replacement Biomed Sci Instrum, 37 (2001), pp. 463–468 [PubMed] [Google Scholar]
- [65].Martell JM, Jacobs JJ, Rosenberg AG, Maley M, Galante JO Primary total hip reconstruction with a titanium fiber-coated prosthesis inserted without cement J Bone Joint Surg. American, 75 (1993), pp. 554–571 [DOI] [PubMed] [Google Scholar]
- [66].Galante J, Rostoker W Fiber metal composites in the fixation of skeletal prosthesis J Biomed Mater Res, 7 (1973), pp. 43–61 [DOI] [PubMed] [Google Scholar]
- [67].Ducheyne P In vitro corrosion study of porous metal fibre coatings for bone ingrowth Biomaterials, 4 (1983), pp. 185–191 [DOI] [PubMed] [Google Scholar]
- [68].Bechtold JE Application of computer graphics in the design of custom orthopedic implants Orthop Clin N Am, 17 (1986), pp. 605–612 [PubMed] [Google Scholar]
- [69].Bandyopadhyay A, Bose S, Narayan R Translation of 3D printed materials for medical applications MRS Bull, 1–10 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Attar H, et al. Selective laser melting of in situ titanium-titanium boride composites: Processing, microstructure and mechanical properties Acta Mater, 76 (2014), pp. 13–22 [Google Scholar]
- [71].Bandyopadhyay A, Ciliveri S, Bose S Metal additive manufacturing for load-bearing implants J Indian Inst Sci, 1–24 (2022) [Google Scholar]
- [72].Thijs L, Verhaeghe F, Craeghs T, Van Humbeeck J, Kruth J-P A study of the microstructural evolution during selective laser melting of Ti–6Al–4V Acta Mater, 58 (2010), pp. 3303–3312 [Google Scholar]
- [73].Gu DD, Meiners W, Wissenbach K, Poprawe R Laser additive manufacturing of metallic components: materials, processes and mechanisms Int Mater Rev, 57 (2012), pp. 133–164 [Google Scholar]
- [74].Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A Additive manufacturing of biomaterials Prog Mater Sci, 93 (2018), pp. 45–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ryan G, Pandit A, Apatsidis D Fabrication methods of porous metals for use in orthopaedic applications Biomaterials, 27 (2006), pp. 2651–2670 [DOI] [PubMed] [Google Scholar]
- [76].Ciliveri S, Bandyopadhyay A Influence of strut-size and cell-size variations on porous Ti6Al4V structures for load-bearing implants J Mech Behav Biomed Mater, 126 (2022), Article 105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bose S, Roy M, Bandyopadhyay A Recent advances in bone tissue engineering scaffolds Trends Biotechnol, 30 (2012), pp. 546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhuang H, Han Y, Feng A Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds Mater Sci Eng C, 28 (2008), pp. 1462–1466 [Google Scholar]
- [79].Sallica-Leva E, Jardini AL, Fogagnolo JB Microstructure and mechanical behavior of porous Ti–6Al–4V parts obtained by selective laser melting J Mech Behav Biomed Mater, 26 (2013), pp. 98–108 [DOI] [PubMed] [Google Scholar]
- [80].Hrabe NW, Heinl P, Flinn B, Körner C, Bordia RK Compression-compression fatigue of selective electron beam melted cellular titanium (Ti-6Al-4V) J Biomed Mater Res B Appl Biomater, 99B (2011), pp. 313–320 [DOI] [PubMed] [Google Scholar]
- [81].Bandyopadhyay A, Mitra I & Bose S 3D Printing for Bone Regeneration. Current Osteoporosis Reports pp. 18 (5), pp. 505–514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yamada K, et al. A preclinical large animal study on a novel intervertebral fusion cage covered with high porosity titanium sheets with a triple pore structure used for spinal fusion Eur Spine J: Official Publ Eur Spine Soc, Eur Spinal Deformity Soc, and the Eur Section of the Cervical Spine Res Soc, 24 (2015), pp. 2530–2537 [DOI] [PubMed] [Google Scholar]
- [83].Li P, et al. A novel 3D printed cage with microporous structure and in vivo fusion function J Biomed Mater Res A, 107 (2019), pp. 1386–1392 [DOI] [PubMed] [Google Scholar]
- [84].Yavari SA, et al. Fatigue behavior of porous biomaterials manufactured using selective laser melting Mater Sci Eng C, 33 (2013), pp. 4849–4858 [DOI] [PubMed] [Google Scholar]
- [85].Wauthle R, et al. Additively manufactured porous tantalum implants Acta Biomater, 14 (2015), pp. 217–225 [DOI] [PubMed] [Google Scholar]
- [86].Van der Stok J, et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects J Orthop Res, 31 (2013), pp. 792–799 [DOI] [PubMed] [Google Scholar]
- [87].Wauthle R, Ahmadi SM, Yavari SA, Mulier M, Zadpoor AA Revival of pure titanium for dynamically loaded porous implants using additive manufacturing Mater Sci Eng C, 54 (2015), pp. 94–100 [DOI] [PubMed] [Google Scholar]
- [88].Kelly CN, Miller AT, Hollister SJ, Guldberg RE, Gall K Design and structure–function characterization of 3D printed synthetic porous biomaterials for tissue engineering Adv Healthc Mater, 7 (2018), p. 1701095. [DOI] [PubMed] [Google Scholar]
- [89].Ahmadi SM, et al. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells J Mech Behav Biomed Mater, 34 (2014), pp. 106–115 [DOI] [PubMed] [Google Scholar]
- [90].Zhang XY, Fang G, Leeflang S, Zadpoor AA, Zhou J Topological design, permeability and mechanical behavior of additively manufactured functionally graded porous metallic biomaterials Acta Biomater, 84 (2018), pp. 437–452 [DOI] [PubMed] [Google Scholar]
- [91].Liu Y, Sing SL, Lim RXE, Yeong WY, Goh BT Preliminary investigation on the geometric accuracy of 3D printed dental implant using a monkey maxilla incisor model Int J Bioprinting, 8 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cheng A, et al. Laser sintered porous Ti–6Al–4V implants stimulate vertical bone growth Ann Biomed Eng, 45 (2017) [DOI] [PubMed] [Google Scholar]
- [93].Shah FA, et al. Long-term osseointegration of 3D printed CoCr constructs with an interconnected open-pore architecture prepared by electron beam melting Acta Biomater, 36 (2016), pp. 296–309 [DOI] [PubMed] [Google Scholar]
- [94].Habijan T, et al. The biocompatibility of dense and porous Nickel-Titanium produced by selective laser melting Mater Sci Eng C, 33 (2013), pp. 419–426 [DOI] [PubMed] [Google Scholar]
- [95].Okulov IV, et al. Open porous dealloying-based biomaterials as a novel biomaterial platform Mater Sci Eng C, 88 (2018), pp. 95–103 [DOI] [PubMed] [Google Scholar]
- [96].Jiang G, He G A new approach to the fabrication of porous magnesium with well-controlled 3D pore structure for orthopedic applications Mater Sci Eng C, 100 (2014), pp. 317–320 [DOI] [PubMed] [Google Scholar]
- [97].Karageorgiou V, Kaplan D Porosity of 3D biomaterial scaffolds and osteogenesis Biomaterials, 26 (2005), pp. 5474–5491 [DOI] [PubMed] [Google Scholar]
- [98].Itälä AI, Ylänen HO, Ekholm C, Karlsson KH, Aro HT Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits J Biomed Mater Res: An Official J Soc Biomater, Japanese Soc Biomater, and The Aust Soc Biomater the Korean Soc Biomater, 58 (2001), pp. 679–683 [DOI] [PubMed] [Google Scholar]
- [99].Hollander DA, et al. Structural, mechanical and in vitro characterization of individually structured Ti–6Al–4V produced by direct laser forming Biomaterials, 27 (2006), pp. 955–963 [DOI] [PubMed] [Google Scholar]
- [100].Williams DF Implants in dental and maxillofacial surgery Biomaterials, 2 (1981), pp. 133–146 [DOI] [PubMed] [Google Scholar]
- [101].Puleo DA, Holleran LA, Doremus RH, Bizios R Osteoblast responses to orthopedic implant materials in vitro J Biomed Mater Res, 25 (1991), pp. 711–723 [DOI] [PubMed] [Google Scholar]
- [102].Kua C, Piolettia DP, Browneb M, Gregsonb PJ. Effect of different Ti–6Al–4V surface treatments on osteoblasts behaviour; 2000. [DOI] [PubMed]
- [103].Hrabe N, Quinn T Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti–6Al–4V) fabricated using electron beam melting (EBM), Part 2: Energy input, orientation, and location Mater Sci Eng A, 573 (2013), pp. 271–277 [Google Scholar]
- [104].Reilly GC, Engler AJ Intrinsic extracellular matrix properties regulate stem cell differentiation J Biomech, 43 (2010), pp. 55–62 [DOI] [PubMed] [Google Scholar]
- [105].Rumpler M, Woesz A, Dunlop JW, van Dongen JT, Fratzl P. The effect of geometry on three-dimensional tissue growth. [DOI] [PMC free article] [PubMed]
- [106].Regis M, Marin E, Fedrizzi L, Pressacco M Additive manufacturing of Trabecular Titanium orthopedic implants MRS Bull, 40 (2015), pp. 137–144 [Google Scholar]
- [107].Gastaldi G, et al. Human adipose-derived stem cells (hASCs) proliferate and differentiate in osteoblast-like cells on trabecular titanium scaffolds J Biomed Mater Res A, 94 (2010), pp. 790–799 [DOI] [PubMed] [Google Scholar]
- [108].Assad M, Lemieux N, Rivard CH, Yahia L Comparative in vitro biocompatibility of nickel-titanium, pure nickel, pure titanium, and stainless steel: genotoxicity and atomic absorption evaluation Biomed Mater Eng, 9 (1999), pp. 1–12 [PubMed] [Google Scholar]
- [109].Wever DJ, Veldhuizen AG, Sanders MM, Schakenraad JM, Van Horn JR Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy Biomaterials, 18 (1997), pp. 1115–1120 [DOI] [PubMed] [Google Scholar]
- [110].Berger-Gorbet M, Broxup B, Rivard C, Yahia L Biocompatibility testing of NiTi screws using immunohistochemistry on sections containing metallic implants J Biomed Mater Res: An Official J Soc Biomater The Japanese Soc Biomater, 32 (1996), pp. 243–248 [DOI] [PubMed] [Google Scholar]
- [111].Rhalmi S, et al. Hard, soft tissue and in vitro cell response to porous nickel-titanium: A biocompatibility evaluation Biomed Mater Eng, 9 (1999), pp. 151–162 [PubMed] [Google Scholar]
- [112].Li Y, et al. Additively manufactured biodegradable porous magnesium Acta Biomater, 67 (2018), pp. 378–392 [DOI] [PubMed] [Google Scholar]
- [113].Gomes ME, Ribeiro AS, Malafaya PB, Reis RL, Cunha AM A new approach based on injection moulding to produce biodegradable starch-based polymeric scaffolds: morphology, mechanical and degradation behaviour Biomaterials, 22 (2001), pp. 883–889 [DOI] [PubMed] [Google Scholar]
- [114].Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone Biomaterials, 17 (1996), pp. 175–185 [DOI] [PubMed] [Google Scholar]
- [115].Borden M, El-Amin SF, Attawia M, Laurencin CT Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair Biomaterials, 24 (2003), pp. 597–609 [DOI] [PubMed] [Google Scholar]
- [116].Rønningen H, Solhelm LF, Langeland N Invasion of bone into porous fiber metal implants in cats Acta Orthop Scand, 55 (1984), pp. 352–358 [DOI] [PubMed] [Google Scholar]
- [117].Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial J Bone Joint Surg, 81 (1999), pp. 907–914 [DOI] [PubMed] [Google Scholar]
- [118].Hofmann AA, Bloebaum RD, Bachus KN Progression of human bone ingrowth into porous-coated implants: Rate of bone ingrowth in humans Acta Orthop Scand, 68 (1997), pp. 161–166 [DOI] [PubMed] [Google Scholar]
- [119].Zou X, et al. Bone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigs Spine J, 4 (2004), pp. 99–105 [DOI] [PubMed] [Google Scholar]
- [120].Chen J, et al. Correlation of in vivo bone formation capability and in vitro differentiation of human bone marrow stromal cells J Med Dent Sci, 52 (2005), pp. 27–34 [PubMed] [Google Scholar]
- [121].Kapat K, et al. Influence of porosity and pore-size distribution in Ti6Al4 V foam on physicomechanical properties, osteogenesis, and quantitative validation of bone ingrowth by micro-computed tomography. https://pubs.acs.org/doi/full/10.1021/acsami.7b13960 (2017) doi: 10.1021/acsami.7b13960. [DOI] [PubMed] [Google Scholar]
- [122].An YH, Martin KL. Handbook of histology methods for bone and cartilage. (Springer, 2003). [Google Scholar]
- [123].Hulbert S Potential of ceramic materials as permanently implantable skeletal prostheses J Biomed Mater Res, 4 (1970), pp. 433–456 [DOI] [PubMed] [Google Scholar]
- [124].Cyster I, et al. The influence of dispersant concentration on the pore morphology of hydroxyapatite ceramics for bone tissue engineering Biomaterials, 26 (2005), pp. 697–702 [DOI] [PubMed] [Google Scholar]
- [125].TSURUGA E, TAKITA H, ITOH H, WAKISAKA Y & KUBOKI Y PORE SIZE OF POROUS HYDROXYAPATITE AS THE CELL-SUBSTRATUM CONTROLS BMP-INDUCED OSTEOGENESIS. Journal of biochemistry 121, 317–324 (1997). [DOI] [PubMed] [Google Scholar]
- [126].GOTZ H Effect of Surface Finish on the Osseointegration of Laser-treated Titanium Alloy Implants. Biomaterials 25, 4057–4064 (2004). [DOI] [PubMed] [Google Scholar]
- [127].Taniguchi N, et al. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment Mater Sci Eng C, 59 (2016), pp. 690–701 [DOI] [PubMed] [Google Scholar]
- [128].Bandyopadhyay A, et al. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants Acta Biomater, 6 (2010), pp. 1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Camron HU, Pilliar RM, Macnab I The rate of bone ingrowth into porous metal J Biomed Mater Res, 10 (1976), pp. 295–302 [DOI] [PubMed] [Google Scholar]
- [130].Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone Clin Orthop Relat Res, 263–270 (1980) [PubMed] [Google Scholar]
- [131].Tanzer M, Harvey E, Kay A, Morton P, Bobyn JD Effect of noninvasive low intensity ultrasound on bone growth into porous-coated implants J Orthop Res, 14 (1996), pp. 901–906 [DOI] [PubMed] [Google Scholar]
- [132].Bobyn JD Features of biologically fixed devices Orthopedic Basic Science (1994) [Google Scholar]
- [133].Galante J, Rostoker W, Lueck R, Ray r.d. Sintered fiber metal composites as a basis for attachment of implants to bone JBJS, 53 (1971), pp. 101–114 [PubMed] [Google Scholar]
- [134].Pilliar RM, Cameron HU, Macnab I Porous surface layered prosthetic devices Biomed Eng, 10 (1975), pp. 126–131 [PubMed] [Google Scholar]
- [135].Pilliar RM Powder metal-made orthopedic implants with porous surface for fixation by tissue ingrowth Clin Orthop Relat Res, 42–51 (1983) [PubMed] [Google Scholar]
- [136].Rho J-Y, Kuhn-Spearing L, Zioupos P Mechanical properties and the hierarchical structure of bone Med Eng Phys, 20 (1998), pp. 92–102 [DOI] [PubMed] [Google Scholar]
- [137].Albrektsson T, et al. The interface zone of inorganic implantsIn vivo: Titanium implants in bone Ann Biomed Eng, 11 (1983), pp. 1–27 [Google Scholar]
- [138].Amin Yavari S, et al. Bone regeneration performance of surface-treated porous titanium Biomaterials, 35 (2014), pp. 6172–6181 [DOI] [PubMed] [Google Scholar]
- [139].Kulkarni M, Mazare A, Schmuki P, Iglič A Biomaterial surface modification of titanium and titanium alloys for medical applications Nanomedicine, 111 (2014), p. 111 [Google Scholar]
- [140].Vlacic-Zischke J, Hamlet SM, Friis T, Tonetti MS, Ivanovski S The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFβ/BMP signalling in osteoblasts Biomaterials, 32 (2011), pp. 665–671 [DOI] [PubMed] [Google Scholar]
- [141].Schwarz F, et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants J Biomed Mater Res Part B: Appl Biomater: An Official J Soc Biomater, Japanese Soc Biomater, and The Aust Soc Biomater Korean Soc Biomater, 88 (2009), pp. 544–557 [DOI] [PubMed] [Google Scholar]
- [142].Bandyopadhyay A, et al. In vivo response of laser processed porous titanium implants for load-bearing implants Ann Biomed Eng, 45 (2017), pp. 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bjursten LM, et al. Titanium dioxide nanotubes enhance bone bonding in vivo J Biomed Mater Res A, 92 (2010), pp. 1218–1224 [DOI] [PubMed] [Google Scholar]
- [144].Das K, Bose S, Bandyopadhyay A TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell–materials interaction J Biomed Mater Res A, 90 (2009), pp. 225–237 [DOI] [PubMed] [Google Scholar]
- [145].Das K, Bandyopadhyay A, Bose S Biocompatibility and in situ growth of TiO2 nanotubes on Ti using different electrolyte chemistry J Am Ceram Soc, 91 (2008), pp. 2808–2814 [Google Scholar]
- [146].Tan AW, Pingguan-Murphy B, Ahmad R, Akbar SA Review of titania nanotubes: fabrication and cellular response Ceram Int, 38 (2012), pp. 4421–4435 [Google Scholar]
- [147].Li S, et al. Fabrication of open-cellular (porous) titanium alloy implants: osseointegration, vascularization and preliminary human trials Sci. China Mater, 61 (2018), pp. 525–536 [Google Scholar]
- [148].Lewallen EA, et al. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants Tissue Eng B Rev, 21 (2014), pp. 218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Matena J, et al. SLM Produced Porous Titanium Implant Improvements for Enhanced Vascularization and Osteoblast Seeding Int J Mol Sci, 16 (2015), pp. 7478–7492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Bai F, et al. The Correlation Between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study Tissue Eng A, 16 (2010), pp. 3791–3803 [DOI] [PubMed] [Google Scholar]
- [151].Tsai P-I, et al. Improvement of bone-tendon fixation by porous titanium interference screw: A rabbit animal model Journal of Orthopaedic Research®, 36 (2018), pp. 2633–2640 [DOI] [PubMed] [Google Scholar]
- [152].Wang H, et al. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: A biomechanical evaluation J Mech Behav Biomed Mater, 88 (2018), pp. 488–496 [DOI] [PubMed] [Google Scholar]
- [153].Caparrós C et al. Bioactive macroporous titanium implants highly interconnected J Mater Sci - Mater Med, 27 (2016), p. 151. [DOI] [PubMed] [Google Scholar]
- [154].Tang D, Yang LY, Ou KL, Oreffo RO Repositioning titanium: An in vitro evaluation of laser-generated microporous, microrough titanium templates as a potential bridging interface for enhanced osseointegration and durability of implants Front Bioeng Biotechnol, 5 (2017), p. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].FERRIER J, ROSS SM, KANEHISA J & AUBIN JE Osteoclasts and Osteoblasts Migrate in Opposite Directions in Response to a Constant Electrical Field. [DOI] [PubMed]
- [156].Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M Asymmetries in H/K-ATPase and Cell Membrane Potentials Comprise a Very Early Step in Left-Right Patterning Cell, 111 (2002), pp. 77–89 [DOI] [PubMed] [Google Scholar]
- [157].Guzelsu N, Demiray H Electromechanical properties and related models of bone tissues: A review Int J Eng Sci, 17 (1979), pp. 813–851 [Google Scholar]
- [158].Rubinacci A, Black J, Brighton CT, Friedenberg ZB Changes in bioelectric potentials on bone associated with direct current stimulation of osteogenesis J Orthop Res, 6 (1988), pp. 335–345 [DOI] [PubMed] [Google Scholar]
- [159].Borgens RB, Jaffe LF, Cohen J Large and persistent electrical currents enter the transected lamprey spinal cord Proc. Natl. Acad. Sci. USA, 77 (1980), pp. 1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Faes TJC, van der Meij HA, de Munck JC, Heethaar RM The electric resistivity of human tissues (100 Hz–10 MHz): a meta-analysis of review studies Physiol. Meas, 20 (1999), pp. R1–R10 [DOI] [PubMed] [Google Scholar]
- [161].McCaig CD, Rajnicek AM, Song B, Zhao M Controlling Cell Behavior Electrically: Current Views and Future Potential PNAS, 105 (2008), pp. 16608–16613 [DOI] [PubMed] [Google Scholar]
- [162].Zhao M et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-g and PTEN. (2006). [DOI] [PubMed]
- [163].Rajnicek AM, Foubister LE, McCaig CD Prioritising guidance cues: Directional migration induced by substratum contours and electrical gradients is controlled by a rho/cdc42 switch Dev Biol, 312 (2007), pp. 448–460 [DOI] [PubMed] [Google Scholar]
- [164].Burr D Effects of biomechanical stress on bones in animals Bone, 30 (2002), pp. 781–786 [DOI] [PubMed] [Google Scholar]
- [165].Hou B, Fukai N, Olsen BR Mechanical force-induced midpalatal suture remodeling in mice Bone (New York, NY), 40 (2007), pp. 1483–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Duncan RL, Turner CH Mechanotransduction and the functional response of bone to mechanical strain Calcif Tissue Int, 57 (1995), pp. 344–358 [DOI] [PubMed] [Google Scholar]
- [167].Bassett CAL & Becker RO Generation of Electric Potentials by Bone in Response to Mechanical Stress. Science 137, 1063–1064 (1962). [DOI] [PubMed] [Google Scholar]
- [168].BECKER RO Bioelectric factors controlling bone structure. Bone biodynamics (1964). [Google Scholar]
- [169].Norton LA, Hanley KJ & Turkewicz J Bioelectric perturbations of bone. Research directions and clinical applications. The Angle orthodontist 54, 73 (1984). [DOI] [PubMed] [Google Scholar]
- [170].Bowen CR, Gittings JP, Turner IG, Baxter F, Chaudhuri JB Dielectric and piezoelectric properties of hydroxyapatite-BaTiO3 composites Applied Physics Letter, 89 (2006), Article 132906 [Google Scholar]
- [171].Silva CC, et al. Collagen–hydroxyapatite films: piezoelectric properties Mater Sci Eng B, 86 (2001), pp. 210–218 [Google Scholar]
- [172].Tofail SAM, Gandhi AA, Gregor M, Bauer J Electrical properties of hydroxyapatite Pure Appl Chem, 87 (2015), pp. 221–229 [Google Scholar]
- [173].Dhal J, Fielding G, Bose S, Bandyopadhyay A Understanding bioactivity and polarizability of hydroxyapatite doped with tungsten J Biomed Mater Res B Appl Biomater, 100B (2012), pp. 1836–1845 [DOI] [PubMed] [Google Scholar]
- [174].Bodhak S, Bose S, Bandyopadhyay A Surface Modification and Electro-thermal Polarisation for Bone Tissue Engineering Electrically Active Materials for Medical Devices 103–114, (IMPERIAL COLLEGE PRESS; (2016), 10.1142/9781783269877_0008 [DOI] [Google Scholar]
- [175].Bodhak S, Bose S, Bandyopadhyay A Electro-thermal Polarisation of Hydroxyapatite Ceramics and Coatings for Bone Tissue Engineering Applications Electrically Active Materials for Medical Devices 115–134, (IMPERIAL COLLEGE PRESS; (2016), 10.1142/9781783269877_0009 [DOI] [Google Scholar]
- [176].Wang Q, et al. Osteogenesis of electrically stimulated bone cells mediated in part by calcium ions Clin Orthop Relat Res (1998), pp. 259–268 [PubMed] [Google Scholar]
- [177].Kim IS, et al. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res, 1763 (2006), pp. 907–916 [DOI] [PubMed] [Google Scholar]
- [178].Brighton CT, Hunt RM Ultrastructure of electrically induced osteogenesis in the rabbit medullary canal J Orthop Res, 4 (1986), p. 27. [DOI] [PubMed] [Google Scholar]
- [179].Shafer DM, Rogerson K, Norton L, Bennett J The effect of electrical perturbation on osseointegration of titanium dental implants. A preliminary study J Oral Maxillofac Surg, 53 (1995), pp. 1063–1068 [DOI] [PubMed] [Google Scholar]
- [180].WANG Z, CLARK CC & BRIGHTON CT Up-Regulation of Bone Morphogenetic Proteins in Cultured Murine Bone Cells with Use of Specific Electric Fields. (2006). [DOI] [PubMed]
- [181].Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ Stimulation of Growth Factor Synthesis by Electric and Electromagnetic Fields Clinical Orthopaedics and Related Research®, 419 (2004), pp. 30–37 [DOI] [PubMed] [Google Scholar]
- [182].SpF® Implantable Spinal Fusion Stimulators | SpF Spinal Fusion Stimulator by Zimmer Biomet. https://www.zimmerbiomet.com/medical-professionals/spine/product/spf-spinal-fusion-stimulator.html.
- [183].Ma C, et al. Electrically polarized micro-arc oxidized TiO2 coatings with enhanced surface hydrophilicity Acta Biomater, 8 (2012), pp. 860–865 [DOI] [PubMed] [Google Scholar]
- [184].Nagai A, et al. Response of osteoblast-like MG63 cells to TiO2 layer prepared by micro-arc oxidation and electric polarization J Eur Ceram Soc, 32 (2012), pp. 2647–2652 [Google Scholar]
- [185].Bandyopadhyay A, Shivaram A, Mitra I, Bose S Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration Acta Biomater, 96 (2019), pp. 686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Mudali UK, Sridhar TM, Eliaz N, Raj B Failures of stainless steel orthopedic devices-causes and remedies Corros Rev, 21 (2003), pp. 231–268 [Google Scholar]
- [187].Papakyriacou M, Mayer H, Pypen C, Plenk H Jr, Stanzl-Tschegg S Effects of surface treatments on high cycle corrosion fatigue of metallic implant materials Int J Fatigue, 22 (2000), pp. 873–886 [Google Scholar]
- [188].Teoh SH Fatigue of biomaterials: a review Int J Fatigue, 22 (2000), pp. 825–837 [Google Scholar]
- [189].Bodamyali T, Kanczler JM, Simon B, Blake DR, Stevens CR Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels Biochem Biophys Res Commun, 264 (1998), pp. 657–661 [DOI] [PubMed] [Google Scholar]
- [190].Ercan B, Webster TJ The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces Biomaterials, 31 (2010), pp. 3684–3693 [DOI] [PubMed] [Google Scholar]
- [191].Lorich DG, et al. Biochemical pathway mediating the response of bone cells to capacitive coupling Clin Orthop Relat Res, 246–256 (1998) [PubMed] [Google Scholar]
- [192].Shigino T, Ochi M, Hirose Y, Hirayama H, Sakaguchi K Enhancing Osseointegration by Capacitively Coupled Electric Field: A Pilot Study on Early Occlusal Loading in the Dog Mandible Int J Oral Maxillofac Implants, 16 (2001) [PubMed] [Google Scholar]
- [193].Brighton c. Signal transaction in electrically stimulated bone cells Journal of Bone and Joint Surgery (American version), 83 (2001), pp. 1514–1523 [DOI] [PubMed] [Google Scholar]
- [194].Lirani-galvão R, et al. Low-Intensity Electrical Stimulation Counteracts the Effects of Ovariectomy on Bone Tissue of Rats: Effects on Bone Microarchitecture, Viability of Osteocytes, and Nitric Oxide Expression Calcif Tissue Int, 84 (2009), p. 502. [DOI] [PubMed] [Google Scholar]
- [195].Han Y, Chen D, Sun J, Zhang Y, Xu K UV-enhanced Bioactivity and Cell Response of Micro-Arc Oxidized Titania Coatings Acta Biomater, 4 (2008), pp. 1518–1529 [DOI] [PubMed] [Google Scholar]
- [196].Bandyopadhyay A, et al. Alloy design via additive manufacturing: Advantages, challenges, applications and perspectives Mater Today, 52 (2022), pp. 207–224, 10.1016/j.mattod.2021.11.026 [DOI] [Google Scholar]
- [197].Zhang L-C, Chen L-Y A review on biomedical titanium alloys: recent progress and prospect Adv Eng Mater, 21 (2019), p. 1801215 [Google Scholar]
- [198].Abdel-Hady GM, Niinomi M Biocompatibility of Ti-alloys for long-term implantation J Mech Behav Biomed Mater, 20 (2013), p. 407. [DOI] [PubMed] [Google Scholar]
- [199].Carman A, et al. Role of alloying elements in microstructure evolution and alloying elements behaviour during sintering of a near-β titanium alloy Mater Sci Eng A, 528 (2011), pp. 1686–1693 [Google Scholar]
- [200].Calin M, Zhang LC, Eckert J Tailoring of microstructure and mechanical properties of a Ti-based bulk metallic glass-forming alloy Scr Mater, 57 (2007), pp. 1101–1104 [Google Scholar]
- [201].Zhang L, Klemm D, Eckert J, Hao YL, Sercombe T Manufacture by selective laser melting and mechanical behavior of a biomedical Ti-24Nb-4Zr-8Sn alloy Scr Mater, 65 (2011), pp. 21–24 [Google Scholar]
- [202].Babilas D, et al. On the electropolishing and anodic oxidation of Ti-15Mo alloy Electrochim Acta, 205 (2016), pp. 256–265 [Google Scholar]
- [203].Nag S, Banerjee R, Fraser HL Microstructural evolution and strengthening mechanisms in Ti–Nb–Zr–Ta, Ti–Mo–Zr–Fe and Ti–15Mo biocompatible alloys Mater Sci Eng C, 25 (2005), pp. 357–362 [DOI] [PubMed] [Google Scholar]
- [204].Banerjee S, Naik UM Plastic instability in an omega forming Ti-l5% Mo alloy Acta Mater, 44 (1996), pp. 3667–3677 [Google Scholar]
- [205].Nag S, Banerjee R, Stechschulte J, Fraser HL Comparison of microstructural evolution in Ti-Mo-Zr-Fe and Ti-15Mo biocompatible alloys J Mater Sci - Mater Med, 16 (2005), pp. 679–685 [DOI] [PubMed] [Google Scholar]
- [206].Hussein AH, Mohamed A-H-G, Gouda MK Biocompatibility of new Ti–Nb–Ta base alloys Mater Sci Eng C, 61 (2016), pp. 574–578 [DOI] [PubMed] [Google Scholar]
- [207].Bai Y, Li SJ, Prima F, Hao YL, Yang R Electrochemical corrosion behavior of Ti–24Nb–4Zr–8Sn alloy in a simulated physiological environment Appl Surf Sci, 258 (2012), pp. 4035–4040 [Google Scholar]
- [208].Liu YJ et al. Electron beam melted beta-type Ti-24Nb-4Zr-8Sn porous structures with high strength-to-modulus ratio.
- [209].Wang L, Xie L, Lv Y, Zhang L-C, Chen L Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing Acta Mater, 131 (2017), p. 499e510 [Google Scholar]
- [210].Zhang T, et al. Microstructure and Mechanical Properties of Ti-35Nb-2Ta-3Zr Alloy by Laser Quenching Front. Mater, 6 (2019), p. 318, 10.3389/fmats [DOI] [Google Scholar]
- [211].Zou LM, Yang C, Long Y, Xiao ZY, Li YY Fabrication of biomedical Ti–35Nb–7Zr–5Ta alloys by mechanical alloying and spark plasma sintering Powder Metall, 55 (2012), p. 65 [Google Scholar]
- [212].Yang K, Wang J, Tang H, Li Y Additive manufacturing of in-situ reinforced Ti–35Nb–5Ta–7Zr (TNTZ) alloy by selective electron beam melting (SEBM) J Alloy Compd, 826 (2020), Article 154178 [Google Scholar]
- [213].Salvador CA, Lopes ES, Ospina CA, Caram R Orthorhombic martensite formation upon aging in a Ti-30Nb-4Sn alloy Mater Chem Phys, 183 (2016), p. 238 [Google Scholar]
- [214].FANTON L, HAYAMA A. de O., CARAM R & FOGAGNOLO JB Texture development in cold deformed and recrystallized Ti–30Nb–4Sn alloy and its effects on hardness and young’s modulus. Advanced Engineering Materials. [Google Scholar]
- [215].Wang JC, et al. Selective laser melting of Ti–35Nb composite from elemental powder mixture: Microstructure, mechanical behavior and corrosion behavior Mater Sci Eng A, 760 (2019), pp. 214–224 [Google Scholar]
- [216].Karre R, Niranjan MK, Dey SR First principles theoretical investigations of low Young’s modulus beta Ti-Nb and Ti-Nb-Zr alloys compositions for biomedical applications Korean J Couns Psychother, 50 (2015), p. 52. [DOI] [PubMed] [Google Scholar]
- [217].Veríssimo NC et al. Characterization of the photoactivity of nanotube layers grown on Ti–35Nb and Ti–35Nb–4Sn alloys. (2016).
- [218].Liu YJ, Zhang YS & Zhang LC Transformation-induced plasticity and high strength in beta titanium alloy manufactured by selective laser melting. (2019).
- [219].Bai XF, Zhao YQ, Jia ZQ, Zhang YS, Li B Grain Boundary Character Distribution of TLM Titanium Alloy During Deformation JMEP, 25 (2016), pp. 2236–2244 [Google Scholar]
- [220].Li Y et al. New Developments of Ti-Based Alloys for Biomedical Applications. [DOI] [PMC free article] [PubMed]
- [221].Yang C, et al. Bimodal titanium alloys with ultrafine lamellar eutectic structure fabricated by semi-solid sintering Acta Mater, 132 (2017), p. 491e502 [Google Scholar]
- [222].Torres Y, Pavo J, Nieto I, Rodriguez J Conventional powder metallurgy process and characterization of porous titanium for biomedical applications Metall Mater Trans B, 42 (2011), p. 891 [Google Scholar]
- [223].Wang H, Fang ZZ, Sun P A critical review of mechanical properties of powder metallurgy titanium Int J Powder Metall, 46 (2010), pp. 45–57 [Google Scholar]
- [224].Attar H, Ehtemam-Haghighi S, Soro N, Kent D, Dargusch MS Additive manufacturing of low-cost porous titanium-based composites for biomedical applications: Advantages, challenges and opinion for future development J Alloy Compd, 827 (2020), Article 154263 [Google Scholar]
- [225].Zhang L-C, Attar H Selective laser melting of titanium alloys and titanium matrix composites for biomedical applications: a review Adv Eng Mater, 18 (2016), pp. 463–475 [Google Scholar]
- [226].Qin P, et al. Resemblance in corrosion behavior of selective laser melted and traditional monolithic β Ti-24Nb-4Zr-8Sn alloy ACS Biomater Sci Eng, 5 (2018), pp. 1141–1149 [DOI] [PubMed] [Google Scholar]
- [227].Bai Y, Gai X, Li S, Zhang L-C. Improved corrosion behaviour of electron beam melted Ti-6Al–4V alloy in phosphate buffered saline. (2017).
- [228].McMahon RE, et al. comparative study of the cytotoxicity and corrosion resistance of nickel–titanium and titanium–niobium shape memory alloys Acta Biomater (2012) [DOI] [PubMed] [Google Scholar]
- [229].Xue P, Li Y, Li K, Zhang D, Zhou C Superelasticity, corrosion resistance and biocompatibility of the Ti–19Zr–10Nb–1Fe alloy Mater Sci Eng. C, Biomimetic Mater, Sens Syst, 50 (2015), pp. 179–186 [DOI] [PubMed] [Google Scholar]
- [230].Takematsu E, Katsumata K, Okada K, Niinomi M, Matsushita N Bioactive surface modification of Ti–29Nb–13Ta–4.6 Zr alloy through alkali solution treatments Mater Sci Eng. C, Biomimetic Mater, Sens Syst, 62 (2016), pp. 662–667 [DOI] [PubMed] [Google Scholar]
- [231].DİKİCİ B, Niinomi M, Topuz M, Koc S & NAKAI M Synthesis of biphasic calcium phosphate (BCP) coatings on beta-type titanium alloys reinforced with rutile-TiO2 compounds: adhesion resistance and in-vitro corrosion. (2018).
- [232].Morra M, Cassinelli C, Cascardo G, Bollati D, R.R.y. Baena Gene expression of markers of osteogenic differentiation of human mesenchymal cells on collagen I-modified microrough titanium surfaces J Biomed Mater Res A, 96 (2011), pp. 449–455 [DOI] [PubMed] [Google Scholar]
- [233].Hoene A, et al. In vivo investigation of the inflammatory response against allylamine plasma polymer coated titanium implants in a rat model Acta Biomater, 6 (2010), pp. 676–683 [DOI] [PubMed] [Google Scholar]
- [234].Qadir M Effect of anodized TiO2–Nb2O5–ZrO2 nanotubes with different nanoscale dimensions on the biocompatibility of Ti35Zr28Nb alloy Nanostructured surface modification for enhanced biocompatibility of Ti-based implant materials, 169 (2020) [DOI] [PubMed] [Google Scholar]
- [235].Liu CF, Li SJ, Hou WT, Hao YL, Huang HH Enhancing corrosion resistance and biocompatibility of interconnected porous beta-type Ti-24Nb-4Zr-8Sn alloy scaffold through alkaline treatment and type I collagen immobilization Appl Surf Sci, 476 (2019), pp. 325–334 [Google Scholar]
- [236].Ion R, et al. In vitro study of human endothelial progenitor cells behaviour on nitrided Ni-free Ti–27Nb alloy Prog Nat Sci: Mater Int, 29 (2019), pp. 466–471 [Google Scholar]
- [237].Black J Biologic performance of tantalum Clin Mater, 16 (1994), pp. 167–173 [DOI] [PubMed] [Google Scholar]
- [238].Balla VK, Bose S, Davies NM, Bandyopadhyay A Tantalum—A bioactive metal for implants JOM, 62 (2010), pp. 61–64 [Google Scholar]
- [239].Eriksen S et al. Tantalum coated carbon-carbon composite material for surgical implants. in Medical Device Materials II: Proceedings of the Materials & Processes for Medical Devices Conference 245–250 (2004). [Google Scholar]
- [240].Zhou Y, et al. Tantalum coated NiTi alloy by PIIID for biomedical application Surf Coat Technol, 228 (2013), pp. S2–S6 [Google Scholar]
- [241].Jin W, et al. Corrosion resistance and cytocompatibility of tantalum-surface-functionalized biomedical ZK60 Mg alloy Corros Sci, 114 (2017), pp. 45–56 [Google Scholar]
- [242].Rahmati B, et al. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility Ceram Int, 42 (2016), pp. 466–480 [Google Scholar]
- [243].Rahmati B, et al. Enhancing the adhesion strength of tantalum oxide ceramic thin film coating on biomedical Ti–6Al–4V alloy by thermal surface treatment Ceram Int, 41 (2015), pp. 13055–13063 [Google Scholar]
- [244].Zhou Y-L, Niinomi M, Akahori T, Nakai M, Fukui H Comparison of Various Properties between Titanium-Tantalum Alloy and Pure Titanium for Biomedical Applications Mater. Trans, 48 (2007), pp. 380–384 [Google Scholar]
- [245].Sing SL, Yeong WY, Wiria FE Selective laser melting of titanium alloy with 50 wt% tantalum: Microstructure and mechanical properties J Alloy Compd, 660 (2016), pp. 461–470 [Google Scholar]
- [246].Huang S, Sing SL, Delooze G, Wilson R, Yeong WY Laser powder bed fusion of titanium-tantalum alloys: Compositions and designs for biomedical applications J Mech Behav Biomed Mater, 103775 (2020) [DOI] [PubMed] [Google Scholar]
- [247].Zhao D et al. Improvement on mechanical properties and corrosion resistance of titanium-tantalum alloys in-situ fabricated via selective laser melting.
- [248].Fuerst J, Medlin D, Carter M, Sears J, Vander G Voort LASER additive manufacturing of titanium-tantalum alloy structured interfaces for modular orthopedic devices JOM, 67 (2015), pp. 775–780 [Google Scholar]
- [249].de Souza KA, Robin A Preparation and characterization of Ti-Ta alloys for application in corrosive media Mater Lett, 57 (2003), pp. 3010–3016 [Google Scholar]
- [250].Zhou Y-L, Niinomi M Microstructures and mechanical properties of Ti–50 mass% Ta alloy for biomedical applications J Alloy Compd, 466 (2008), pp. 535–542 [Google Scholar]
- [251].Mareci D, Chelariu R, Gordin D-M, Ungureanu G, Gloriant T Comparative corrosion study of Ti–Ta alloys for dental applications Acta Biomater, 5 (2009), pp. 3625–3639 [DOI] [PubMed] [Google Scholar]
- [252].Mitra I, et al. 3D Printing in alloy design to improve biocompatibility in metallic implants Mater Today, 45 (2021), pp. 20–34, 10.1016/j.mattod.2020.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Chen G, Yin J, Zhao S, Tang H, Qu X Microstructure and tensile properties of a Ti-28Ta alloy studied by transmission electron microscopy and digital image correlation Int J Refract Metal Hard Mater, 81 (2019), pp. 71–77 [Google Scholar]
- [254].Bahador A, Kariya S, Umeda J, Hamzah E, Kondoh K Tailoring Microstructure and Properties of a Superelastic Ti-Ta Alloy by Incorporating Spark Plasma Sintering with Thermomechanical Processing J Mater Eng Perform, 28 (2019), pp. 3012–3020 [Google Scholar]
- [255].Capellato P, et al. Coated Surface on Ti-30Ta Alloy for Biomedical Application: Mechanical and in-vitro Characterization Mater Res, 23 (2020) [Google Scholar]
- [256].Elahinia MH, Hashemi M, Tabesh M, Bhaduri SB Manufacturing and processing of NiTi implants: a review Prog Mater Sci, 57 (2012), pp. 911–946 [Google Scholar]
- [257].Matsumoto K, Tajima N, Kuwahara S Correction of scoliosis with shape-memory alloy Nihon Seikeigeka Gakkai zasshi, 67 (1993), pp. 267–274 [PubMed] [Google Scholar]
- [258].Ryhänen J Biocompatibility evaluation of nickel-titanium shape memory metal alloy Oulun yliopisto; (1999) [Google Scholar]
- [259].Speck KM, Fraker AC Anodic polarization behavior of Ti-Ni and Ti-6A 1–4 V in simulated physiological solutions J Dent Res, 59 (1980), pp. 1590–1595 [DOI] [PubMed] [Google Scholar]
- [260].Wever DJ, et al. Electrochemical and surface characterization of a nickel–titanium alloy Biomaterials, 19 (1998), pp. 761–769 [DOI] [PubMed] [Google Scholar]
- [261].Platt JA, et al. Corrosion behavior of 2205 duplex stainless steel Am J Orthod Dentofac Orthop, 112 (1997), pp. 69–79 [DOI] [PubMed] [Google Scholar]
- [262].Sarkar NK, Redmond W, Schwaninger B, Goldberg AJ The chloride corrosion behaviour of four orthodontic wires J Oral Rehabil, 10 (1983), pp. 121–128 [DOI] [PubMed] [Google Scholar]
- [263].Rondelli G Corrosion resistance tests on NiTi shape memory alloy Biomaterials, 17 (1996), pp. 2003–2008 [DOI] [PubMed] [Google Scholar]
- [264].Castleman LS, Motzkin SM, Alicandri FP, Bonawit VL, Johnson AA Biocompatibility of nitinol alloy as an implant material J Biomed Mater Res, 10 (1976), pp. 695–731 [DOI] [PubMed] [Google Scholar]
- [265].Cragg AH, De Jong SC, Barnhart WH, Landas SK, Smith TP Nitinol intravascular stent: results of preclinical evaluation Radiology, 189 (1993), pp. 775–778 [DOI] [PubMed] [Google Scholar]
- [266].Williams DF Biocompatibility of Clinical Implant Mtls CRC-Press; (1981) [Google Scholar]
- [267].Assad M, et al. Assays of cytotoxicity of the Nickel-Titanium shape memory alloy Ann Chir, 48 (1994), pp. 731–736 [PubMed] [Google Scholar]
- [268].Assad M, Yahia LH, Rivard CH, Lemieux N In vitro biocompatibility assessment of a nickel–titanium alloy using electron microscopy in situ end-labeling (EM-ISEL) Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials, 41 (1998), pp. 154–161 [DOI] [PubMed] [Google Scholar]
- [269].Kapanen A, et al. Behaviour of nitinol in osteoblast-like ROS-17 cell cultures Biomaterials, 23 (2002), pp. 645–650 [DOI] [PubMed] [Google Scholar]
- [270].Yeung KWK, et al. Corrosion resistance, surface mechanical properties, and cytocompatibility of plasma immersion ion implantation–treated nickel-titanium shape memory alloys J Biomed Mater Res Part A: An Official J Soc Biomater, The Japanese Soc Biomater, Aust Soc Biomater Korean Soc Biomater, 75 (2005), pp. 256–267 [DOI] [PubMed] [Google Scholar]
- [271].Yeung KWK, et al. Nitrogen plasma-implanted nickel titanium alloys for orthopedic use Surf Coat Technol, 201 (2007), pp. 5607–5612 [Google Scholar]
- [272].Michiardi A, Engel E, Aparicio C, Planell JA, Gil FJ Oxidized NiTi surfaces enhance differentiation of osteoblast-like cells Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 85 (2008), pp. 108–114 [DOI] [PubMed] [Google Scholar]
- [273].Liu XM, et al. Nano-scale surface morphology, wettability and osteoblast adhesion on nitrogen plasma-implanted NiTi shape memory alloy J Nanosci Nanotechnol, 9 (2009), pp. 3449–3454 [DOI] [PubMed] [Google Scholar]
- [274].Salahshoor M, Guo Y Biodegradable orthopedic magnesium-calcium (MgCa) alloys, processing, and corrosion performance Materials, 5 (2012), pp. 135–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Staiger MP, Pietak AM, Huadmai J, Dias G Magnesium and its alloys as orthopedic biomaterials: A review Biomaterials, 9 (2006), pp. 1728–1734 [DOI] [PubMed] [Google Scholar]
- [276].Heublein B, et al. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart, 89 (2003), pp. 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Erne P, Schier M, Resink TJ The road to bioabsorbable stents: reaching clinical reality? Cardiovasc Interv Radiol, 29 (2006), pp. 11–16 [DOI] [PubMed] [Google Scholar]
- [278].Erbel R, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial Lancet, 369 (2007), pp. 1869–1875 [DOI] [PubMed] [Google Scholar]
- [279].Di Mario C, et al. Drug-eluting bioabsorbable magnesium stent J Interv Cardiol, 17 (2004), pp. 391–395 [DOI] [PubMed] [Google Scholar]
- [280].Peeters P, Bosiers M, Verbist J, Deloose K, Heublein B Preliminary results after application of absorbable metal stents in patients with critical limb ischemia J Endovasc Ther, 12 (2005), pp. 1–5 [DOI] [PubMed] [Google Scholar]
- [281].Zberg B, Arata ER, Uggowitzer PJ, Löffler JF Tensile properties of glassy MgZnCa wires and reliability analysis using Weibull statistics Acta Mater, 57 (2009), pp. 3223–3231 [Google Scholar]
- [282].Witte F, et al. In vivo corrosion of four magnesium alloys and the associated bone response Biomaterials, 26 (2005), pp. 3557–3563 [DOI] [PubMed] [Google Scholar]
- [283].Munir K, Lin J, Wen C, Wright PFA, Li Y Mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Sc alloys for biodegradable implant applications Acta Biomater, 102 (2019), pp. 493–507 [DOI] [PubMed] [Google Scholar]
- [284].Cockerill I, See CW, Young ML, Wang Y, Zhu D Designing better cardiovascular stent materials: A learning curve Adv Funct Mater, 31 (2021), p. 2005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Fu J, et al. Evolution of metallic cardiovascular stent materials: a comparative study among stainless steel, magnesium and zinc Biomaterials, 230 (2020), Article 119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Yang H, et al. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications Nat Commun, 11 (2020), pp. 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Garcia-Garcia HM, et al. In vivo serial invasive imaging of the second-generation drug-eluting absorbable metal scaffold (Magmaris—DREAMS 2G) in de novo coronary lesions: insights from the BIOSOLVE-II first-in-man trial Int J Cardiol, 255 (2018), pp. 22–28 [DOI] [PubMed] [Google Scholar]
- [288].Zheng J-F, et al. Preclinical evaluation of a novel sirolimus-eluting iron bioresorbable coronary scaffold in porcine coronary artery at 6 months Cardiovascular Interventions, 12 (2019), pp. 245–255 [DOI] [PubMed] [Google Scholar]
- [289].Mori H, Kutys R, Romero M, Virmani R, Finn AV Metallic coronary stents: is there a relationship between stent fracture and hypersensitivity? J Am Coll Cardiol Intv, 10 (2017), pp. 1175–1177 [DOI] [PubMed] [Google Scholar]
- [290].Zhang J, et al. The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: A 20-month study Acta Biomater, 69 (2018), pp. 372–384 [DOI] [PubMed] [Google Scholar]
- [291].Yang H, et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model Biomaterials, 145 (2017), pp. 92–105 [DOI] [PubMed] [Google Scholar]
- [292].Joner M, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents J Am Coll Cardiol, 52 (2008), pp. 333–342 [DOI] [PubMed] [Google Scholar]
- [293].Cheng Y, et al. In vitro mechanical behavior and in vivo healing response of a novel thin-strut ultrahigh molecular weight poly-L-lactic acid sirolimus-eluting bioresorbable coronary scaffold in normal swine Int J Cardiol, 286 (2019), pp. 21–28 [DOI] [PubMed] [Google Scholar]
- [294].Shi Y, et al. In vitro and in vivo degradation of rapamycin-eluting Mg-Nd-Zn-Zr alloy stents in porcine coronary arteries Mater Sci Eng C, 80 (2017), pp. 1–6 [DOI] [PubMed] [Google Scholar]
- [295].Bose S, Vahabzadeh S, Banerjee D, Ke D Enhanced osteogenic protein expression on human osteoblast-osteoclast co-culture system using doped hydroxyapatite plasma coatings for orthopedic and dental applications Mater Today Commun, 21 (2019), p. 100534, 10.1016/j.mtcomm.2019.05.010 [DOI] [Google Scholar]
- [296].Fan Y, Duan K, Wang R A composite coating by electrolysis-induced collagen self-assembly and calcium phosphate mineralization Biomaterials, 26 (2005), pp. 1623–1632 [DOI] [PubMed] [Google Scholar]
- [297].Liu Y, De Groot K, Hunziker EB BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model Bone, 36 (2005), pp. 745–757 [DOI] [PubMed] [Google Scholar]
- [298].Bose S, Sarkar N Natural medicinal compounds in bone tissue engineering Trends Biotechnol, 38 (2020), pp. 404–417, 10.1016/j.tibtech.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Vu AA, Bose S Natural antibiotic oregano in hydroxyapatite-coated titanium reduces osteoclastic bone resorption for orthopedic and dental applications ACS Appl Mater Interfaces, 12 (2020), pp. 52383–52392, 10.1021/acsami.0c14993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].MIHAILESCU I et al. Calcium phosphate thin films synthesized by pulsed laser deposition: Physico-chemical characterization and in vitro cell response. Applied surface science 248, 344–348 (2005). [Google Scholar]
- [301].Liu y. Influence of calcium phosphate crystal assemblies on the proliferation and osteogenic gene expression of rat bone marrow stromal cells Biomaterials, 28 (2007), pp. 1393–1403 [DOI] [PubMed] [Google Scholar]
- [302].Bigi a., et al. Human osteoblast response to pulsed laser deposited calcium phosphate coatings Biomaterials, 26 (2005), pp. 2381–2389 [DOI] [PubMed] [Google Scholar]
- [303].Wiltfang J, Merten H, Schlegel K, Schultze-Mosgau S, Kloss F Degradation characteristics of α and β tri-calcium-phosphate (TCP) in minipigs J Biomed Mater Res, 63 (2002), pp. 115–121 [DOI] [PubMed] [Google Scholar]
- [304].Vaquette C, Ivanovski S, Hamlet SM, Hutmacher DW Effect of culture conditions and calcium phosphate coating on ectopic bone formation Biomaterials, 34 (2013), pp. 5538–5551 [DOI] [PubMed] [Google Scholar]
- [305].Lopez-Heredia MA, et al. Rapid prototyped porous titanium coated with calcium phosphate as a scaffold for bone tissue engineering Biomaterials, 29 (2008), pp. 2608–2615 [DOI] [PubMed] [Google Scholar]
- [306].Sandeman SR, et al. The in vitro corneal biocompatibility of hydroxyapatite coated carbon mesh Biomaterials, 30 (2009), pp. 3143–3149 [DOI] [PubMed] [Google Scholar]
- [307].Chu TM Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures Biomaterials, 23 (2002), pp. 1283–1293 [DOI] [PubMed] [Google Scholar]
- [308].Yuan H, et al. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics Biomaterials, 20 (1999), pp. 1799–1806 [DOI] [PubMed] [Google Scholar]
- [309].PORTER A, PATEL N, SKEPPER J, BEST S & BONFIELD W Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 24, 4609–4620 (2003). [DOI] [PubMed] [Google Scholar]
- [310].Avila JD, Stenberg K, Bose S, Bandyopadhyay A Hydroxyapatite reinforced Ti6Al4V composites for load-bearing implants Acta Biomater, 123 (2021), pp. 379–392, 10.1016/j.actbio.2020.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Mayr-Wohlfart U, Fiedler J, Günther K-P, Puhl W, Kessler S Proliferation and differentiation rates of a human osteoblast-like cell line (SaOS-2) in contact with different bone substitute materials Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 57 (2001), pp. 132–139 [DOI] [PubMed] [Google Scholar]
- [312].Cai S, et al. Fabrication and biological characteristics of β-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass J Mater Sci - Mater Med, 20 (2009), pp. 351–358 [DOI] [PubMed] [Google Scholar]
- [313].Sous m., et al. Cellular biocompatibility and resistance to compression of macroporous β-tricalcium phosphate ceramics Biomaterials, 19 (1998), pp. 2147–2153 [DOI] [PubMed] [Google Scholar]
- [314].Herten M, et al. Surface-and nonsurface-dependent in vitro effects of bone substitutes on cell viability Clin Oral Invest, 13 (2009), pp. 149–155 [DOI] [PubMed] [Google Scholar]
- [315].Yamada M, Shiota M, Yamashita Y, Kasugai S Histological and histomorphometrical comparative study of the degradation and osteoconductive characteristics of α-and β-tricalcium phosphate in block grafts J Biomed Mater Res B Appl Biomater, 82 (2007), pp. 139–148 [DOI] [PubMed] [Google Scholar]
- [316].Shirasu N Bone formation in a rat calvarial defect model after transplanting autogenous bone marrow with β-tricalcium phosphate Acta Histochem, 112 (2010), pp. 270–277 [DOI] [PubMed] [Google Scholar]
- [317].Dorozhkin SV Calcium orthophosphate coatings on magnesium and its biodegradable alloys Acta Biomater, 10 (2014), pp. 2919–2934 [DOI] [PubMed] [Google Scholar]
- [318].Bose S, Ke D, Vu AA, Bandyopadhyay A, Goodman SB Thermal oxide layer enhances crystallinity and mechanical properties for plasma-sprayed hydroxyapatite biomedical coatings ACS Appl Mater Interfaces, 12 (2020), pp. 33465–33472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].de Jonge LT, Leeuwenburgh SC, Wolke JG, Jansen JA Organic–inorganic surface modifications for titanium implant surfaces Pharm Res, 25 (2008), pp. 2357–2369 [DOI] [PubMed] [Google Scholar]
- [320].Barrere F, et al. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 67 (2003), pp. 655–665 [DOI] [PubMed] [Google Scholar]
- [321].Leeuwenburgh S, Layrolle P, Barrere F, de Bruijn J, Schoonman J Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro J Biomed Mater Res, 56 (2001), pp. 208–215 [DOI] [PubMed] [Google Scholar]
- [322].Wang J, Layrolle P, Stigter M, de Groot K Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: physicochemical characteristics and cell attachment Biomaterials, 25 (2004), pp. 583–592 [DOI] [PubMed] [Google Scholar]
- [323].Barrere F, van der Valk C, Dalmeiier RJ, van Blitterswijk C, de Groot K In vitro and in vivo degradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants J Biomed Mater Res, 64 (2003), pp. 378–387 [DOI] [PubMed] [Google Scholar]
- [324].Klein C, de K Groat Long-term in viva study of plasma-sprayed coatings on titanium alloys of tetracalcium phosphate, hydroxyapa-tite and a-tricalcium phosphate Biomaterials, 15 (1994) [DOI] [PubMed] [Google Scholar]
- [325].Spector M, Hobbs LW Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy Biomaterials, 27 (2006), pp. 4192–4203 [DOI] [PubMed] [Google Scholar]
- [326].Roy M, Bandyopadhyay A, Bose S Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants Surf Coat Technol, 205 (2011), pp. 2785–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Roy M, Krishna BV, Bandyopadhyay A, Bose S Laser processing of bioactive tricalcium phosphate coating on titanium for load-bearing implants Acta Biomater, 4 (2008), pp. 324–333 [DOI] [PubMed] [Google Scholar]
- [328].Vahabzadeh S, Roy M, Bandyopadhyay A, Bose S Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications Acta Biomater, 17 (2015), pp. 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Chen S, et al. In vivo degradation and bone response of a composite coating on Mg–Zn–Ca alloy prepared by microarc oxidation and electrochemical deposition J Biomed Mater Res B Appl Biomater, 100 (2012), pp. 533–543 [DOI] [PubMed] [Google Scholar]
- [330].Razavi M, Fathi M, Savabi O, Vashaee D, Tayebi L In vivo assessments of bioabsorbable AZ91 magnesium implants coated with nanostructured fluoridated hydroxyapatite by MAO/EPD technique for biomedical applications Mater Sci Eng C, 48 (2015), pp. 21–27 [DOI] [PubMed] [Google Scholar]
- [331].Li J, et al. In vitro responses of human bone marrow stromal cells to a fluoridated hydroxyapatite coated biodegradable Mg–Zn alloy Biomaterials, 31 (2010), pp. 5782–5788 [DOI] [PubMed] [Google Scholar]
- [332].Wang J, Chao Y, Wan Q, Zhu Z, Yu H Fluoridated hydroxyapatite coatings on titanium obtained by electrochemical deposition Acta Biomater, 5 (2009), pp. 1798–1807 [DOI] [PubMed] [Google Scholar]
- [333].Ter Brugge PJ, Wolke JGC, Jansen JA Effect of calcium phosphate coating crystallinity and implant surface roughness on differentiation of rat bone marrow cells J Biomed Mater Res, 60 (2002), pp. 70–78 [DOI] [PubMed] [Google Scholar]
- [334].Ge X, et al. Antibacterial coatings of fluoridated hydroxyapatite for percutaneous implants J Biomed Mater Res A, 95 (2010), pp. 588–599 [DOI] [PubMed] [Google Scholar]
- [335].Su Y, et al. Development and characterization of silver containing calcium phosphate coatings on pure iron foam intended for bone scaffold applications Mater Des, 148 (2018), pp. 124–134 [Google Scholar]
- [336].Huang Y, et al. Osteoblastic cell responses and antibacterial efficacy of Cu/Zn co-substituted hydroxyapatite coatings on pure titanium using electrodeposition method RSC Adv, 5 (2015), pp. 17076–17086 [Google Scholar]
- [337].Cabedo MV, et al. 45S5 bioactive glass coatings by atmospheric plasma spraying obtained from feedstocks prepared by different routes J Mater Sci, 49 (2014) [Google Scholar]
- [338].Seuss S, Heinloth M, Boccaccini AR Development of bioactive composite coatings based on combination of PEEK, bioactive glass and Ag nanoparticles with antibacterial properties Surf Coat Technol, 100 (2016), pp. 100–105 [Google Scholar]
- [339].Bose S, Sarkar N, Banerjee D Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration Mater Today Chem, 8 (2018), pp. 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Hinderer S, Layland SL, Schenke-Layland K ECM and ECM-like materials—biomaterials for applications in regenerative medicine and cancer therapy Adv Drug Deliv Rev, 97 (2016), pp. 260–269 [DOI] [PubMed] [Google Scholar]
- [341].Zhao N, Zhu D Collagen Self-Assembly on Orthopedic Magnesium Biomaterials Surface and Subsequent Bone Cell Attachment PLoSO, 9 (2014), p. e110420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Ronga M, et al. Clinical applications of growth factors in bone injuries Experience with BMPs. (2013) [DOI] [PubMed]
- [343].van den Beucken J, et al. Multilayered DNA coatings: In vitro bioactivity studies and effects on osteoblast-like cell behavior Acta Biomater, 3 (2007), pp. 587–596 [DOI] [PubMed] [Google Scholar]
- [344].Douglas T, et al. Enzymatic mineralization of gellan gum hydrogel for bone tissue-engineering applications and its enhancement by polydopamine JOURNAL OF TISSUE ENGINEERING AND REGENERATIVE MEDICINE, 8 (2014), pp. 906–918 [DOI] [PubMed] [Google Scholar]
- [345].D.E. Jonge L Organicinorganic surface modifications for titanium implant surfaces Pharm Res, 25 (2008), pp. 2357–2369 [DOI] [PubMed] [Google Scholar]
- [346].Lee H, Dellatore SM, Miller WM, Messersmith PB Mussel-Inspired Surface Chemistry for Multifunctional Coatings Science, 318 (2007), pp. 426–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS Polymeric scaffolds in tissue engineering application: a review International journal of polymer science, 2011 (2011) [Google Scholar]
- [348].Campos CA, et al. Bioresorbable vascular scaffolds in the clinical setting Interv Cardiol, 5 (2013), pp. 639–646 [Google Scholar]
- [349].Ma J, Thompson M, Zhao N, Zhu D Similarities and differences in coatings for magnesium-based stents and orthopaedic implants. Journal of Orthopaedic Translation, 2 (2014), p. 118e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Ma J, Zhao N, Betts L & Zhu D Bio-Adaption between Magnesium Alloy Stent and the Blood Vessel: A Review. (2015). [DOI] [PMC free article] [PubMed]
- [351].Yang Z, et al. Mussel-inspired coating of polydopamine directs endothelial and smooth muscle cell fate for re-endothelialization of vascular devices Adv Healthc Mater, 1 (2012), p. 548. [DOI] [PubMed] [Google Scholar]
- [352].Sobocinski J, et al. Mussel inspired coating of a biocompatible cyclodextrin based polymer onto CoCr vascular stents ACS Appl Mater Interfaces, 6 (2014), pp. 3575–3586 [DOI] [PubMed] [Google Scholar]
- [353].Donlan RM, Costerton JW Biofilms: Survival mechanisms of clinically relevant microorganisms Clinical microbiology reviews (Print), 15 (2002), pp. 147–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Hook AL, et al. Combinatorial discovery of polymers resistant to bacterial attachment Nat Biotechnol, 30 (2012), p. 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Zhao L, Chu PK, Zhang Y, Wu Z Antibacterial Coatings on Titanium Implants J Biomed Mater Res B Appl Biomater, 91 (2009), pp. 470–480 [DOI] [PubMed] [Google Scholar]
- [356].Tiller J Designing surfaces that kill bacteria on contact PNAS, 98 (2001), pp. 5981–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Fuchs T, Stange R, Schmidmaier G, Raschke MJ The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study Arch Orthop Trauma Surg, 131 (2011), pp. 1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Holmberg KV, et al. Bio-inspired stable antimicrobial peptide coatings for dental applications Acta Biomater (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Pavlukhina SV, et al. Noneluting enzymatic antibiofilm coatings ACS Appl Mater Interfaces, 4 (2012), pp. 4708–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Burt S Essential oils: their antibacterial properties and potential applications in foods: a review Int J Food Microbiol, 94 (2004), pp. 223–253 [DOI] [PubMed] [Google Scholar]
- [361].Shi Z, Neoh KG, Kang ET, Poh CK, Wang W Surface Functionalization of Titanium with Carboxymethyl Chitosan and Immobilized Bone Morphogenetic Protein-2 for Enhanced Osseointegration Biomacromolecules, 6 (2009), pp. 1603–1611 [DOI] [PubMed] [Google Scholar]
- [362].Hu X et al. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. (2010). [DOI] [PubMed]
- [363].Attarilar S, et al. 3D Printing technologies in metallic implants: A thematic review on the techniques and procedures International Journal of Bioprinting, 7 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Graves SE, et al. The Australian orthopaedic association national joint replacement registry Med J Aust, 180 (2004), pp. S31–S34 [DOI] [PubMed] [Google Scholar]
- [365].Hoyt BW, Walsh SA, Forsberg JA Osseointegrated prostheses for the rehabilitation of amputees (OPRA): results and clinical perspective Expert Rev Med Devices, 17 (2020), pp. 17–25 [DOI] [PubMed] [Google Scholar]
- [366].Hagberg K, Brånemark R, Gunterberg B, Rydevik B Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up Prosthet Orthot Int, 32 (2008), pp. 29–41 [DOI] [PubMed] [Google Scholar]
- [367].Tillander J, Hagberg K, Hagberg L, Brånemark R Osseointegrated titanium implants for limb prostheses attachments: infectious complications Clinical Orthopaedics and Related Research®, 468 (2010), pp. 2781–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Brånemark R, et al. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: A prospective study of 51 patients The bone & joint journal, 96 (2014), pp. 106–113 [DOI] [PubMed] [Google Scholar]
- [369].Brånemark RP, Hagberg K, Kulbacka-Ortiz K, Berlin Ö, Rydevik B Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications J Am Acad Orthop Surg, 27 (2019), p. e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Aschoff HH, Kennon RE, Keggi JM, Rubin LE Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device JBJS, 92 (2010), pp. 180–186 [DOI] [PubMed] [Google Scholar]
- [371].Van de Meent H, Hopman MT, Frölke JP Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses Arch Phys Med Rehabil, 94 (2013), pp. 2174–2178 [DOI] [PubMed] [Google Scholar]
- [372].Juhnke D-L, Beck JP, Aschoff HH Fifteen years of experience with Integral-Leg-Prosthesis: Cohort study of artificial limb attachment system J Rehabil Res Dev, 52 (2015), p. 407. [DOI] [PubMed] [Google Scholar]
- [373].Al Muderis M, Khemka A, Lord SJ, Van de Meent H, Frölke JPM Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study JBJS, 98 (2016), pp. 900–909 [DOI] [PubMed] [Google Scholar]
- [374].Al Muderis M, Lu W, Tetsworth K, Bosley B, Li JJ Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: the Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study BMJ Open, 7 (2017), p. e013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Agarwal J, et al. Development of a percutaneous prosthesis for transfemoral amputees, the Utah experience Plastic and Reconstructive Surgery-Global Open, 6 (2018), pp. 95–96 [Google Scholar]
- [376].Frossard L, Berg D, Merlo G, Quincey T, Burkett B Cost comparison of socket-suspended and bone-anchored transfemoral prostheses JPO: J Prosthetics Orthotics, 29 (2017), pp. 150–160. [Google Scholar]
- [377].Van Eck CF, McGough RL Clinical outcome of osseointegrated prostheses for lower extremity amputations: a systematic review of the literature Curr Orthopaedic Practice, 26 (2015), pp. 349–357 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data for this study has been presented in this manuscript.


