Abstract
Background
Mechanical circulatory support devices, such as the intra‐aortic balloon pump (IABP) and Impella, are often used in patients on veno‐arterial extracorporeal life support (VA‐ECLS) with cardiogenic shock despite limited supporting clinical trial data.
Methods and Results
Hospitalizations for cardiogenic shock from 2016 to 2018 were identified from the National Inpatient Sample. Trends in the use of VA‐ECLS with and without an IABP or Impella were assessed semiannually. Multivariable logistic regression and general linear regression evaluated the association of Impella and IABP use with in‐hospital outcomes. Overall, 12 035 hospitalizations with cardiogenic shock and VA‐ECLS were identified, of which 3115 (26%) also received an IABP and 1880 (16%) an Impella. Use of an Impella with VA‐ECLS substantially increased from 10% to 18% over this period (P<0.001), whereas an IABP modestly increased from 25% to 26% (P<0.001). In‐hospital mortality decreased 54% to 48% for VA‐ECLS only, 61% to 58% for VA‐ECLS with an Impella, and 54% to 49% for VA‐ECLS with an IABP (P<0.001 each). Most (57%) IABPs or Impellas were placed on the same day as VA‐ECLS. After adjustment, there were no differences in in‐hospital mortality or length of stay with the addition of an IABP or Impella compared with VA‐ECLS alone.
Conclusions
From 2016 to 2018 in the United States, use of an Impella and IABP with VA‐ECLS significantly increased. More than half of Impellas and IABPs were placed on the same day as VA‐ECLS, and the use of a second mechanical circulatory support device did not impact in‐hospital mortality. Further studies are needed to decipher the optimal timing and patient selection for this growing practice.
Keywords: cardiogenic shock, Impella, intra‐aortic balloon pump, mechanical circulatory support
Subject Categories: Treatment, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- CS
cardiogenic shock
- IABP
intra‐aortic balloon pump
- MCS
mechanical circulatory support
- NIS
National Inpatient Sample
- SNF
skilled nursing facility
- VA‐ECLS
veno‐arterial extracorporeal life support
Clinical Perspective
What Is New?
Use of an Impella and intra‐aortic balloon pump with veno‐arterial extracorporeal life support have drastically increased in the United States from 2016 to 2018; however, the seemingly complimentary use of a second mechanical support device with veno‐arterial extracorporeal life support is not associated with a commensurate impact on in‐hospital mortality.
What Are the Clinical Implications?
Many have concluded that an Impella or intra‐aortic balloon pump may counteract the deleterious increased afterload from veno‐arterial extracorporeal life support.
Although use of these devices is increasing mostly on the same day as veno‐arterial extracorporeal life support, whether and when to use an Impella or intra‐aortic balloon pump is still unclear and warrants further investigation.
Despite significant advancement in treatment modalities, cardiogenic shock (CS) continues to be associated with significant (≈50%) mortality. 1 At present, early revascularization of the culprit lesion in those with CS secondary to acute myocardial infarction (AMI) is the only treatment known to be associated with better outcomes. It is important to identify other treatments that can help improve outcomes in patients across the spectrum of CS. 2 Mechanical circulatory support (MCS) with veno‐arterial extracorporeal life support (VA‐ECLS) has been shown to improve survival after cardiopulmonary resuscitation among patients with refractory cardiac arrest 3 ; however, its use in patients with CS is not based on evidence from randomized controlled trials. 4
Although VA‐ECLS may help in providing temporary support as a bridge to a durable left ventricular (LV) assist device or heart transplant, its use comes at the hemodynamic cost of increased LV afterload secondary to retrograde aortic blood flow. In patients with CS, increased LV afterload can result in delayed myocardial recovery, myocardial ischemia, pulmonary congestion, and increased predilection to arrhythmias and thrombotic events secondary to hemostasis. 5 , 6 Consequently, many percutaneous assist devices, such as an intra‐aortic balloon pump (IABP) and Impella (Abiomed), as well as surgical interventions including central cannulation of the left atrium, left ventricle, and axillary artery have been proposed as potential LV unloading or LV venting mechanisms to balance the increased afterload with VA‐ECLS. Although this approach is promising based on the pathophysiology, clinical trials in this critically ill population have proven to be difficult 7 ; as such, data on outcomes to support real‐world usefulness remain limited from small scale studies. 8 , 9 Accordingly, we designed the present study to assess the contemporary trends in use and outcomes of VA‐ECLS support with and without a second MCS device in CS using a nationwide, representative cohort.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. The Healthcare Cost and Utilization Project National Inpatient Sample (NIS) is the largest publicly available, all‐payer, nationally representative database. It constitutes a 20% random sample of all community hospital discharges in the United States unweighted and contains >7 million hospitalizations in 2018; weighted, it includes ≈35 million hospitalizations. It contains a complex sampling design with provided weights that allow for calculation of unbiased, population‐level national estimates. 10 Patient data in the NIS are deidentified, and therefore, no institutional review board approval was necessary for the present study. Written informed consent was not required; the study had proper ethical oversight throughout.
Hospitalizations containing a diagnosis of CS with VA‐ECLS use from 2016 to 2018 were identified from the NIS using International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10) codes. Comorbidities, inpatient complications, and procedures were similarly assigned using ICD‐9 and ICD‐10 codes, and are detailed in Table S1–S5. Hospitalizations for individuals aged <18 years or those with missing age or mortality data were excluded (Figure 1). Identified hospitalizations were stratified into those who underwent VA‐ECLS with the use of additional MCS devices, such as an Impella and IABP, versus those with VA‐ECLS alone. Relative timing of VA‐ECLS and second MCS devices were determined in units of days. Hospitalizations were further stratified by sex.
Figure 1. Inclusion and exclusion criteria.
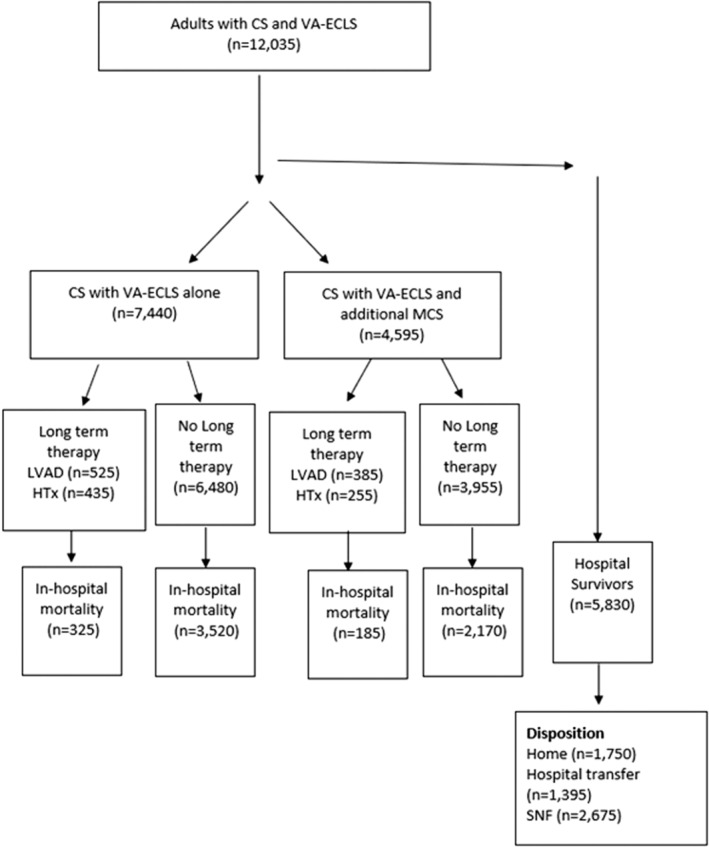
CS indicates cardiogenic shock; HTx, heart transplant; LVAD, left ventricular assist device; MCS, mechanical circulatory support; SNF, skilled nursing facility; and VA‐ECLS, veno‐arterial extracorporeal life support.
Statistical Analysis
Descriptive statistics were used to examine distributions of continuous variables and frequencies of categorical variables; baseline characteristics were compared between groups using the Kruskal‐Wallis test for continuous variables and Wald χ2 test for categorical variables, where appropriate. Poisson regression adjusting for age and Charlson Comorbidity Index evaluated trends in use of VA‐ECLS, use of second MCS devices (Impella or IABP) with VA‐ECLS, and in‐hospital mortality in 6‐month intervals from 2016 to 2018. Charlson Comorbidity Index was calculated using Quan et al's 11 adaptation of the Deyo et al 12 method; age and Charlson Comorbidity Index were treated as restricted quadratic splines. The primary outcome of interest was all‐cause in‐hospital mortality. Secondary outcomes included discharge to skilled nursing facility (SNF) and length of stay in days. Unadjusted and multivariable logistic regression models examined associations of concomitant use of an Impella or IABP and VA‐ECLS with in‐hospital mortality and discharge to an SNF compared with VA‐ECLS alone; multivariable general linear models were used to examine association with length of stay. Multivariable models were adjusted for age, Charlson Comorbidity Index, atrial fibrillation, hypertension, diabetes, chronic obstructive pulmonary disease, primary insurance, year, income quartile of the patient's zip code, and hospital bed size. Finally, median time to VA‐ECLS and use of an additional MCS device were examined; outcomes in patients who received a second MCS device at least 1 day before VA‐ECLS and at least 1 day after VA‐ECLS were compared with those who received VA‐ECLS and an additional MCS device on the same day. We conducted a sensitivity analysis examining the above primary and secondary outcomes including only patients who received a second MCS device within 48 hours of VA‐ECLS, consistent with prior studies. 8 , 13 We further performed a subgroup analysis among patients where AMI was in the primary (Diagnosis 1 or DX1) or first secondary (Diagnosis 2 or DX2) position, to examine patients with CS secondary to AMI.
Complex sampling design and weights were accounted for in all analyses using SAS survey packages and %SURVEYGENMOD for Poisson regression; cluster variables included unique hospital identifier and year. 14 All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Overall, 460 040 hospitalizations (92 008 unweighted observations) for CS were identified from 2016 to 2018, of which 12 035 (3%) were treated with VA‐ECLS. Of these, 4595 (38%) were treated with a second MCS device during the hospitalization, including 3115 with an IABP (26%) and 1880 (16%) with an Impella. There were 3910 (32%) women with CS and VA‐ECLS, of which 455 (12%) received an Impella in addition to VA‐ECLS, and 840 (21%) received an IABP. In comparison, 1425 (18%) men received an Impella in addition to VA‐ECLS, and 2275 (28%) received an IABP. Those who had a second MCS device were older, more likely to be men, White, to have coronary artery disease, heart failure, diabetes, chronic kidney disease, and hypertension (Table 1). They were also more likely to have had AMI during the hospitalization (52% versus 28%, P<0.001) and had similar use of destination therapy such as placement of a durable LV assist device and heart transplant (6% versus 6%, P=0.50). Use of VA‐ECLS, an IABP, and an Impella were more common in large‐volume hospitals.
Table 1.
Baseline Characteristics of Adults With Cardiogenic Shock and VA‐ECLS From 2016 to 2018
| Variable | VA‐ECLS only, N=7440 | VA‐ECLS+Impella, N=1880 (16%) | VA‐ECLS+intra‐aortic balloon pump, N=3115 (26%) |
|---|---|---|---|
| Age, y, median (IQR) | 56 (45–65) | 58 (51–64) | 60 (52–68) |
| Women, n (%) | 2715 (36) | 455 (24) | 840 (27) |
| Race and ethnicity, n (%) | |||
| Non‐Hispanic White | 4340 (63) | 1110 (65) | 1925 (68) |
| Non‐Hispanic Black | 1210 (18) | 220 (13) | 370 (13) |
| Hispanic | 530 (8) | 200 (12) | 260 (9) |
| Other, including Asian/Pacific Islander, Native American | 785 (11) | 190 (11) | 270 (10) |
| Charlson score, median (IQR) | 2.06 (1.29–3.50) | 2.31 (1.41–3.82) | 2.43 (1.46–3.81) |
| Comorbidities, n (%) | |||
| Atrial fibrillation | 2425 (33) | 595 (32) | 1140 (37) |
| Chronic kidney disease | 1615 (22) | 440 (23) | 800 (26) |
| Chronic obstructive pulmonary disease | 1080 (14) | 245 (13) | 450 (14) |
| Coronary artery disease | 3455 (46) | 1455 (77) | 2245 (72) |
| Diabetes | 1185 (25) | 615 (33) | 930 (30) |
| Heart failure | 4770 (64) | 1410 (75) | 2345 (75) |
| Hypertension | 4145 (56) | 1150 (61) | 1965 (63) |
| Peripheral arterial disease | 305 (4) | 125 (7) | 165 (5) |
| Valvular heart disease | 1725 (23) | 335 (18) | 870 (28) |
| Acute myocardial infarction | 2050 (28) | 1110 (59) | 1555 (50) |
| Percutaneous coronary intervention | 505 (25) | 655 (59) | 670 (43) |
| Coronary artery bypass grafting | 290 (14) | 145 (13) | 465 (30) |
| Primary insurance, n (%) | |||
| Medicaid/Medicare | 3910 (53) | 840 (45) | 1605 (52) |
| Private | 3075 (42) | 895 (48) | 1275 (41) |
| Other/self‐pay | 425 (6) | 140 (8) | 225 (7) |
| Household income, n (%) | |||
| Low | 1955 (27) | 435 (24) | 685 (22) |
| Medium | 1815 (25) | 455 (25) | 780 (26) |
| High | 1885 (26) | 450 (24) | 805 (26) |
| Highest | 1675 (23) | 505 (27) | 785 (26) |
| Hospital size, n (%) | |||
| Small | 210 (2) | 35 (2) | 120 (4) |
| Medium | 810 (11) | 305 (16) | 365 (12) |
| Large | 6420 (86) | 1540 (82) | 2630 (84) |
| Length of stay, d, median (IQR) | 14.34 (4.80–29.62) | 8.55 (2.31–25.62) | 14.05 (5.37–27.50) |
| Destination therapy, n (%) | 945 (13) | 195 (10) | 465 (15) |
| Heart transplant | 435 (6) | 50 (3) | 210 (7) |
| Durable left ventricle assist device | 525 (7) | 145 (8) | 260 (8) |
Trends in VA‐ECLS, Impella, and IABP
Hospitalizations with CS and VA‐ECLS increased from 1585 in the first half of 2016 to 2335 in the latter half of 2018 (P<0.001). Use of an Impella in addition to VA‐ECLS substantially increased from 10% to 18% over this period (P<0.001), whereas use of an IABP modestly increased from 25% to 26% (P<0.001) (Figure 2). In the last 6 months of 2018, 41% of adults with cardiogenic shock on VA‐ECLS were treated with a second MCS device, with 26% receiving an IABP, 16% receiving an Impella, and 3% undergoing both.
Figure 2. Trends in hospitalizations for cardiogenic shock with VA‐ECLS and VA‐ECLS+second MCS from 2016 to 2018 by 6‐month intervals.
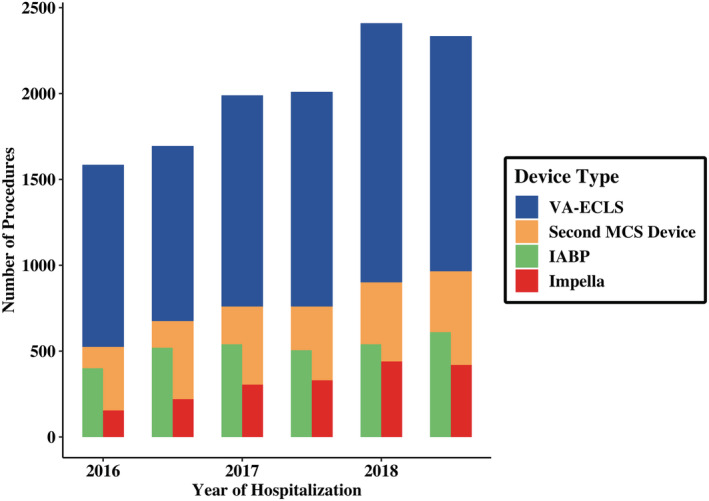
IABP indicates intra‐aortic balloon pump; MCS, mechanical circulatory support; and VA‐ECLS, veno‐arterial extracorporeal life support.
Hospitalizations with CS and VA‐ECLS increased from 1135 to 1500 in men and from 450 to 835 in women; use of a second MCS device also increased from 36% to 41% for men and from 27% to 42% for women, largely driven by significant increases in Impella use (men: from 11% to 19%, women: from 7% to 17%) (P<0.001 for each). IABP use remained highly variable and relatively clinically unchanged for both men and women (Table 2).
Table 2.
Use of VA‐ECLS, VA‐ECLS With an Impella, and VA‐ECLS With an Intra‐Aortic Balloon Pump Semiannually From 2016 to 2018, Stratified by Sex
| 2016 (January–June) | 2016 (July–December) | 2017 (January–June) | 2017 (July–December) | 2018 (January–June) | 2018 (July–December) | P value, trend* | |
|---|---|---|---|---|---|---|---|
| Men, n (%) | |||||||
| VA‐ECLS | 1135 | 1125 | 1320 | 1410 | 1625 | 1500 | <0.001 |
| VA‐ECLS+any second MCS device | 405 (36) | 495 (44) | 565 (43) | 615 (44) | 695 (43) | 615 (41) | <0.001 |
| VA‐ECLS+Impella | 125 (11) | 175 (16) | 215 (16) | 270 (19) | 350 (22) | 280 (19) | <0.001 |
| VA‐ECLS+intra‐aortic balloon pump | 295 (26) | 385 (34) | 415 (31) | 410 (29) | 405 (25) | 365 (24) | <0.001 |
| Women, n (%) | |||||||
| VA‐ECLS | 450 | 570 | 670 | 600 | 785 | 835 | <0.001 |
| VA‐ECLS+any second MCS device | 120 (27) | 180 (32) | 195 (29) | 145 (24) | 205 (26) | 350 (42) | <0.001 |
| VA‐ECLS+Impella | 30 (7) | 45 (8) | 90 (14) | 60 (10) | 90 (12) | 140 (17) | <0.001 |
| VA‐ECLS+intra‐aortic balloon pump | 105 (23) | 135 (24) | 125 (19) | 95 (16) | 135 (17) | 245 (29) | <0.001 |
MCS indicates mechanical circulatory support; and VA‐ECLS, veno‐arterial extracorporeal life support.
Adjusted for age and Charlson Comorbidity Index.
Outcomes in VA‐ECLS, Impella, and IABP
In‐hospital all‐cause mortality decreased from 54% to 48% over the course of the study in those undergoing VA‐ECLS only, and from 56% to 52% in those undergoing VA‐ECLS plus an Impella or IABP (P<0.001 for both). Mortality in those receiving an Impella decreased from 61% to 58% and from 54% to 49% in those receiving an IABP (P<0.001 for both) (Figure 3). Stratified by sex, in‐hospital all‐cause mortality decreased from 54% to 50% for men and from 52% to 43% for women (P<0.001 for each).
Figure 3. Temporal trends of in‐hospital mortality in cardiogenic shock admissions receiving VA‐ECLS therapy and VA‐ECLS+second mechanical circulatory support device by 6‐month intervals.
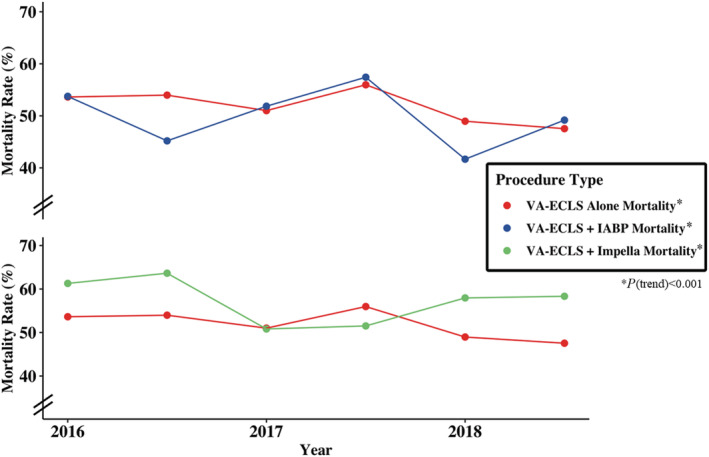
IABP indicates intra‐aortic balloon pump; and VA‐ECLS, veno‐arterial extracorporeal life support.
After multivariable adjustment, there were no differences in mortality or length of stay with use of an IABP with VA‐ECLS and use of an Impella with VA‐ECLS compared with VA‐ECLS alone. Both use of an Impella with VA‐ECLS and use of an IABP with VA‐ECLS were associated with decreased odds of discharge to an SNF compared with VA‐ECLS alone (Figure 4; Table S2).
Figure 4. Adjusted odds of in‐hospital outcomes by use of an Impella or IABP with VA‐ECLS compared with VA‐ECLS alone.
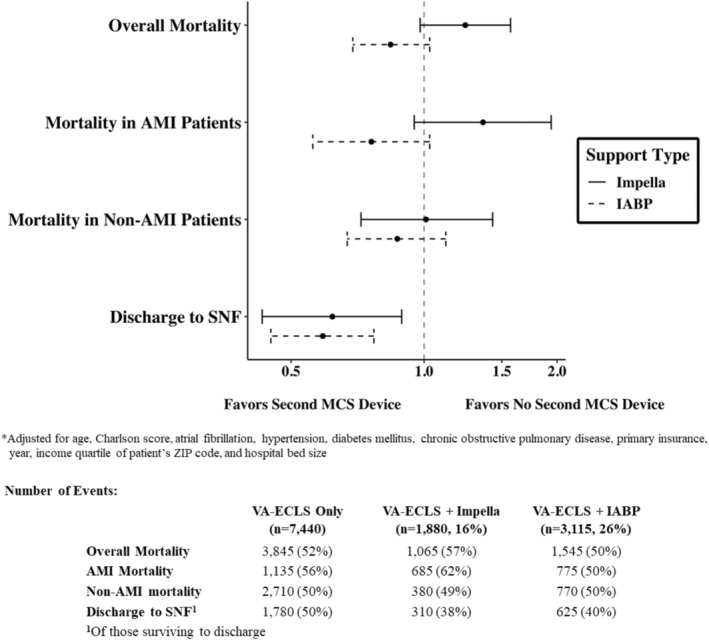
AMI indicates acute myocardial infarction; IABP, intra‐aortic balloon pump; MCS, mechanical circulatory support; SNF, skilled nursing facility; and VA‐ECLS, veno‐arterial extracorporeal life support.
Timing of Second MCS Device
VA‐ECLS was placed on a median hospital day 0.53 (interquartile range [IQR], 0–4.11). In those undergoing a second MCS device, additional support (an IABP or Impella) was placed a median of 0.42 days (IQR, 0–4.13) after VA‐ECLS. A majority (2280 [57%]) of additional MCS devices were placed on the same day as VA‐ECLS; 1190 (29%) were placed ≥1 day before VA‐ECLS, and 565 (14%) were placed ≥1 day after VA‐ECLS (Figure 5). After multivariable adjustment, in‐hospital mortality and discharge to an SNF did not differ in those undergoing VA‐ECLS and a second MCS device >24 hours before or after VA‐ECLS compared with same day use. Use of a second MCS device >24 hours before VA‐ECLS and >24 hours after VA‐ECLS were both associated with an increased length of stay (Table S3).
Figure 5. Timing of second MCS device (Impella or IABP) relative to VA‐ECLS.
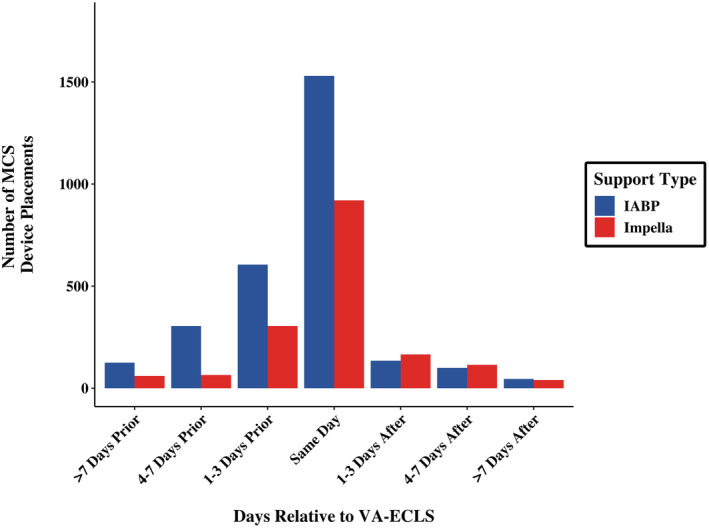
IABP indicates intra‐aortic balloon pump; MCS, mechanical circulatory support; and VA‐ECLS, veno‐arterial extracorporeal life support.
Sensitivity Analyses
Including only adults who underwent a second MCS device within 48 hours of VA‐ECLS, 2895 (28%) hospitalizations for CS and VA‐ECLS included a second MCS device, of which 1190 (12%) were an Impella and 1900 (18%) were an IABP. Impella use increased over the course of the study (8% of all hospitalizations with VA‐ECLS to 10%, P<0.001), whereas IABP use declined from 18% to 11% (P=0.03). Mortality decreased from 52% to 46% overall (P<0.001), but modestly increased from 50% to 53% in those with a second MCS device (P<0.001). After multivariable adjustment, use of an Impella with VA‐ECLS was not associated with a difference in mortality compared with VA‐ECLS alone; however, it was associated with decreased odds of discharge to an SNF and decreased length of stay. Use of an IABP with VA‐ECLS was associated with decreased odds of in‐hospital mortality (Table S4).
In the subgroup analysis of hospitalizations with CS complicating AMI, use of VA‐ECLS increased from 465 in the first half of 2016 to 745 in the latter half of 2018 (P<0.001). Use of an IABP increased from 33% to 37%, and use of an Impella similarly increased from 17% to 30% over this period (P<0.001 for both). Mortality during this period in those undergoing VA‐ECLS alone increased from 61% to 63% (P<0.001) but decreased from 62% to 59% (P<0.001) for those undergoing VA‐ECLS and a second MCS device. After multivariable adjustment, use of an Impella with VA‐ECLS was associated with modestly increased odds of in‐hospital mortality but decreased length of stay in those surviving to discharge. Odds of in‐hospital mortality and SNF discharge did not differ with an IABP plus VA‐ECLS compared with VA‐ECLS alone, and length of stay did not differ for either subgroup (Table S5).
DISCUSSION
In this study of a nationally representative sample of adults hospitalized with CS in the United States from 2016 to 2018, 38% of those undergoing VA‐ECLS also underwent a second MCS device; 26% had IABP placement, and 16% underwent Impella placement. Use of VA‐ECLS overall increased over the study period, as did use of both Impella and IABP as second MCS devices. In‐hospital mortality decreased with time in those with VA‐ECLS alone, VA‐ECLS with an Impella, and VA‐ECLS with an IABP. Use of an IABP and Impella with VA‐ECLS both decreased odds of SNF discharge; neither were associated with decreased odds of in‐hospital mortality or length of stay; however, IABP trended toward a mortality benefit. More than half of second MCS devices were placed on the same day as VA‐ECLS, and variation from this timing did not affect in‐hospital mortality (Figure 6). The present study provides important contemporary data on increasing use of VA‐ECLS and LV support devices such as an IABP and an Impella in adults with CS, in the absence of clinical trial data and updated guidelines.
Figure 6. Use of an Impella and IABP with VA‐ECLS for cardiogenic shock in the United States from 2016 to 2018.
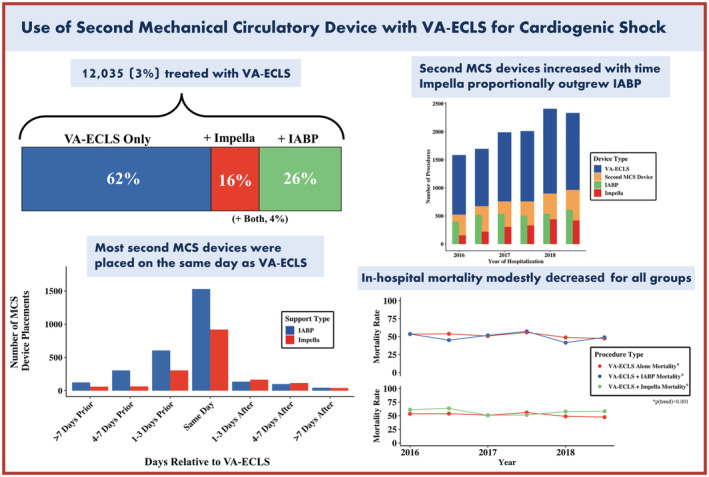
MCS indicates mechanical circulatory support; IABP, intra‐aortic balloon pump; and VA‐ECLS, veno‐arterial extracorporeal life support.
Despite advancements in management modalities, CS continues to carry significant morbidity and mortality. 1 , 15 Timely use of mechanical circulatory support devices in certain cases can be a life‐saving intervention, serving as a bridge to recovery or to destination therapy with durable mechanical circulatory support or heart transplantation. 16 , 17 Our study found use of VA‐ECLS in CS significantly increased from 2016 to 2018, and recent use of VA‐ECLS from 2016 to 2018 was considerably higher (≈4000 per year) compared with prior studies from 2004 to 2016, which reported an average of ≈1150 per year. 18
Increased afterload during VA‐ECLS can cause increased LV pressure and decreased stroke volume. It is likely that increased wall stress can lead to additional myocardial ischemia, which has been hypothesized to delay myocardial recovery, increase pulmonary congestion, and predispose patients to arrythmias and pulmonary thrombi. 19 , 20 Consequently, many LV venting/unloading strategies, including an Impella/IABP, respectively, have been used to offset this limitation of VA‐ECLS use. 6 , 21 , 22 , 23 Although the premise of using such a strategy is physiologically promising, data on outcomes have been limited to small observational studies and a few registries. 8 , 9 , 24 Use of a second MCS device for LV venting/unloading has demonstrated mortality benefit in observational studies compared with VA‐ECLS alone 25 ; however, randomized trials with VA‐ECLS and cardiogenic shock do not exist, and several trials exploring MCS devices in AMI have proven to be challenging. 7 , 26 Although our present study is limited in determining whether an IABP or Impella were placed for the purpose of LV venting, it suggests that even in the absence of trial data and supporting guidelines, physicians are increasingly adopting a strategy of using a second MCS device. An Impella and IABP were most frequently used in the setting of CS complicating AMI, consistent with populations studied in the IABP‐SHOCK (Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock) and the IMPRESS (Impella Versus Intra‐Aortic Balloon Pump in Acute Myocardial Infarction Complicated by Cardiogenic Shock) trials. Although Impella did not demonstrate a mortality benefit and saw a modestly increased odds of in‐hospital mortality, in a sensitivity analysis, IABP use trended toward decreased odds of in‐hospital mortality in the present study. The variation in mortality results in our study and previous observational studies may be explained by the heterogeneity in the study population, but warrants further investigation.
Aside from causes of CS, timing of implementation of a second MCS device is a particularly important consideration. In our nationally representative sample from 2016 to 2018, more than half of second MCS devices were placed on the same day as VA‐ECLS, and the relative timing of an Impella or IABP did not impact in‐hospital mortality. Use of an early or concurrent Impella or IABP with VA‐ECLS is consistent with positive results from prior studies; specifically, a meta‐analysis of 62 observational studies found decreased mortality with LV venting and VA‐ECLS compared with VA‐ECLS alone only in those with early (<12 hours from VA‐ECLS) support with an Impella or IABP. 27 Early additional support may prevent LV hypertension and distension, which is inversely proportional to myocardial recovery. 20 Timing of implementation in many cases is affected by multiple factors, including type of device (Impella versus IABP), indication (high risk percutaneous coronary intervention, LV venting, need for additional hemodynamic support), and clinical setting (preemptive LV venting versus bailout strategy). In some cases, VA‐ECLS may represent an escalation in level of MCS in a patient who already had an IABP or Impella in place, and in such patients, the first device would be left in place. In other cases, an IABP or Impella may be used as a bailout strategy when complications of LV loading with VA‐ECLS have set in. Although our study is limited in delineating the underlying clinical scenario in which a second MCS device is placed, it did demonstrate that outcomes do not differ by timing of placement for all comers. Different VA‐ECLS modalities, such as left atrial VA‐ECLS, have emerged as possible alternatives to unload LV without addition of a second MCS, which could prevent the associated mechanical and vascular complications.
Our study does have limitations. First, given the nature of the NIS, we were unable to determine the clinical scenario in which a second MCS device was deployed. We were also unable to determine duration of Impella/IABP, and it is thus possible that some patients with a second MCS device had that device removed during VA‐ECLS cannulation, and it was not left in place as a vent. Second, the NIS is reliant on documentation with ICD‐9 and ICD‐10 coding. Thus, coding differences may exist between participating hospitals, and certain covariates that may be of interest could not be obtained. Also, because of nonspecific codes, we were unable to identify other venting techniques such as atrial septostomy. Third, the NIS does not identify individual patients or their hospitalizations, and therefore we could not examine patient‐level longitudinal trends. Also, because we relied on an ICD procedure coding system for identification of VA‐ECLS and LV venting with an Impella or IABP, we could not determine days on VA‐ECLS as an end point. Furthermore, because the NIS reports days of procedures, we were limited to describe relative timing of VA‐ECLS and a second MCS device in units of days instead of hours as previous studies have. 27 The retrospective nature of these data, however, precluded causal inference and could not exclude treatment and selection biases or identify provisional versus immediate unloading strategies. Collectively, these observational data suggest that LV unloading is not a benign therapy, and further work is required to evaluate efficacy, safety, and the requisite clinical baseline risk to potentially unload the LV with a second MCS. Finally, interinstitutional practices for durable ventricular assist device or transplant might affect the decision pathway and outcomes in this registry.
CONCLUSIONS
In this nationally representative study of adults hospitalized with CS in the United States from 2016 to 2018, hospitalizations with VA‐ECLS therapy significantly increased, as did use of an Impella and IABP with VA‐ECLS. More than half of Impellas and IABPs were placed on the same day as VA‐ECLS, although relative timing did not impact in‐hospital mortality. The use of a second MCS device, such as an Impella or IABP, with VA‐ECLS was not associated with differences in in‐hospital mortality compared with VA‐ECLS alone; however, both were associated with decreased odds of SNF discharge. Despite limited clinical trial/guideline support, use of an Impella and IABP with VA‐ECLS is significantly increasing in the United States; further studies are needed to decipher the optimal timing and patient selection to improve outcomes in this critically ill population.
Sources of Funding
The authors received no financial support for the research, authorship, or publication of this article.
Disclosures
Dr Ricciardi receives consulting and speaker support from Abiomed. Dr Qamar reports receiving institutional grant support from Novo Nordisk, NorthShore Auxiliary Research Scholar Fund, NorthShore CardioDiabetes Pilot Grant, and fees for educational activities from the American College of Cardiology, Society for Vascular Medicine, Society for Cardiovascular Angiography and Interventions, Janssen and Janssen, Pfizer, Medscape, and the Clinical Exercise Physiology Association. The remaining authors report no disclosures, financial or otherwise.
Supporting information
Tables S1–S5
Presented in part at Cardiovascular Research Technologies (CRT) 2022 in Washington, DC, February 26–March 1, 2022, and published in abstract form (J Am Coll Cardiol Intv. 2022;15[4_Supplement]:S52–S53).
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025216
For Sources of Funding and Disclosures, see page 10.
See Editorial by Baran and Brozzi
References
- 1. Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–1230. doi: 10.1093/eurheartj/ehv051 [DOI] [PubMed] [Google Scholar]
- 3. Chen Y‐S, Lin J‐W, Yu H‐Y, Ko W‐J, Jerng J‐S, Chang W‐T, Chen W‐J, Huang S‐C, Chi N‐H, Wang C‐H, et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7 [DOI] [PubMed] [Google Scholar]
- 4. Becher PM, Schrage B, Sinning CR, Schmack B, Fluschnik N, Schwarzl M, Waldeyer C, Lindner D, Seiffert M, Neumann JT, et al. Venoarterial extracorporeal membrane oxygenation for cardiopulmonary support. Circulation. 2018;138:2298–2300. doi: 10.1161/CIRCULATIONAHA.118.036691 [DOI] [PubMed] [Google Scholar]
- 5. High mortality associated with intracardiac and intrapulmonary thromboses after cardiopulmonary bypass | SpringerLink. Available at: https://link.springer.com/article/10.1007/s00540‐011‐1253‐x. Accessed April 15, 2021. [DOI] [PubMed]
- 6. Koeckert MS, Jorde UP, Naka Y, Moses JW, Takayama H. Impella LP 2.5 for left ventricular unloading during venoarterial extracorporeal membrane oxygenation support. J Cardiac Surg. 2011;26:666–668. doi: 10.1111/j.1540-8191.2011.01338.x [DOI] [PubMed] [Google Scholar]
- 7. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJS, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, et al. Percutaneous mechanical circulatory support versus intra‐aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:278–287. doi: 10.1016/j.jacc.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 8. Akanni OJ, Takeda K, Truby LK, Kurlansky PA, Chiuzan C, Han J, Topkara VK, Yuzefpolskaya M, Colombo PC, Karmpaliotis D, et al. EC‐VAD: combined use of extracorporeal membrane oxygenation and percutaneous microaxial pump left ventricular assist device. ASAIO J. 2019;65:219–226. doi: 10.1097/MAT.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 9. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, et al. Concomitant implantation of Impella® on top of veno‐arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–412. doi: 10.1002/ejhf.668 [DOI] [PubMed] [Google Scholar]
- 10. Healthcare Cost and Utilization Project (HCUP) . HCUP NIS Database Documentation. Agency for Healthcare Research and Quality; 2022. [PubMed] [Google Scholar]
- 11. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8.. [DOI] [PubMed] [Google Scholar]
- 13. Patel SM, Lipinski J, Al‐Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, et al. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019;65:21–28. doi: 10.1097/MAT.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 14. Da Silva AR. %SURVEYGENMOD Macro: an Alternative to Deal with Complex Survey Design for the GENMOD Procedure. SAS Global Forum 2017, Orlando, FL. [Google Scholar]
- 15. Vallabhajosyula S, Prasad A, Bell MR, Sandhu GS, Eleid MF, Dunlay SM, Schears GJ, Stulak JM, Singh M, Gersh BJ, et al. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail. 2019;12:e005929. doi: 10.1161/CIRCHEARTFAILURE.119.005929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American heart Assocation, the cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention*. J Am Coll Cardiol. 2015;65:e7–e26. doi: 10.1016/j.jacc.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 17. Combes A, Leprince P, Luyt C‐E, Bonnet N, Trouillet J‐L, Léger P, Pavie A, Chastre J. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7 [DOI] [PubMed] [Google Scholar]
- 18. Chung M, Zhao Y, Strom JB, Shen C, Yeh RW. Extracorporeal membrane oxygenation use in cardiogenic shock: impact of age on in‐hospital mortality, length of stay, and costs. Crit Care Med. 2019;47:e214–e221. doi: 10.1097/CCM.0000000000003631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66:2663–2674. doi: 10.1016/j.jacc.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 20. Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, Topkara VK, Abrams D, Brodie D, Colombo PC, et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017;63:257–265. doi: 10.1097/MAT.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 21. Yang F, Jia Z, Xing J, Wang Z, Liu Y, Hao X, Jiang C, Wang H, Jia M, Hou X. Effects of intra‐aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med. 2014;12:106. doi: 10.1186/1479-5876-12-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh‐Kucukarslan G, Raad M, Al‐Darzi W, Cowger J, Brice L, Basir MB, O'Neill WW, Alaswaad K, Eng MH. Hemodynamic effects of left‐atrial venous arterial extra‐corporeal membrane oxygenation (LAVA‐ECMO). ASAIO J. 2022;68:e148–e151. doi: 10.1097/MAT.0000000000001628 [DOI] [PubMed] [Google Scholar]
- 23. Lüsebrink E, Massberg S, Orban M. The multiple options of left atrial and ventricular venting during veno‐arterial extra‐corporeal membrane oxygenation: practical considerations. Eur Heart J. 2021;42:2399–2400. doi: 10.1093/eurheartj/ehaa1073 [DOI] [PubMed] [Google Scholar]
- 24. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, Multicenter Cohort Study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, Di Santo P, Mathew R, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 26. Intraaortic balloon support for myocardial infarction with cardiogenic shock | NEJM. Available at: https://www.nejm.org/doi/full/10.1056/nejmoa1208410. Accessed August 26, 2021. [DOI] [PubMed]
- 27. Al‐Fares AA, Randhawa VK, Englesakis M, McDonald MA, Nagpal AD, Estep JD, Soltesz EG, Fan E. Optimal strategy and timing of left ventricular venting during veno‐arterial extracorporeal life support for adults in cardiogenic shock. Circulation Heart Fail. 2019;12:e006486. doi: 10.1161/CIRCHEARTFAILURE.119.006486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


