Abstract
Background
Persons with HIV have a higher prevalence of coronary artery disease compared with their HIV‐negative counterparts. Earlier identification of subclinical atherosclerosis may provide a greater opportunity for cardiovascular disease risk reduction. We investigated coronary cross‐sectional area (CorCSA) by noncontrasted computed tomography imaging as a noninvasive measure of arterial remodeling among virally suppressed persons with HIV.
Methods and Results
We assessed 105 persons with HIV with a spectrum of cardiometabolic health. All participants underwent computed tomography imaging to assess the mean corCSA of the proximal left anterior descending artery and 28 participants underwent additional coronary computed tomography angiography. Partial Spearman rank correlations adjusted for cardiovascular disease risk factors were used to assess relationships of corCSA with anthropometric measurements, HIV‐related factors, and plasma cytokines. Mean corCSA measured by noncontrast computed tomography and coronary computed tomography angiography were strongly correlated (ρ=0.91, P<0.0001). Higher mean corCSA was present in those with coronary artery calcium (P=0.005) and it correlated with participants' atherosclerotic cardiovascular disease risk score (ρ=0.35, P=0.01). After adjusting for established cardiovascular disease risk factors, we observed an inverse relationship between corCSA and CD4+ T‐cell count (ρ=−0.2, P=0.047). Removal of age from the model strengthened the relationships between corCSA and antiretroviral therapy duration (from ρ=0.19, P=0.08 to ρ=0.3, P=0.01). CorCSA was also inversely correlated with plasma IL‐10 (ρ=−0.25, P=0.03) but had no relationship with IL‐6 (ρ=0.11, P=0.4) or IL‐1β (ρ=0.08, P=0.5).
Conclusions
Positive coronary arterial remodeling, an imaging marker of subclinical atherosclerosis, is associated with a lower CD4 T‐cell count, lower circulating IL‐10, and possibly a longer antiretroviral therapy duration in persons with HIV.
Registration
Clinicaltrials.gov; Unique identifier: NCT04451980.
Keywords: cardiovascular diseases, coronary arterial calcium, coronary artery disease, cross‐sectional area, glycated hemoglobin A, interleukin‐10, interleukin‐6
Subject Categories: Biomarkers, Clinical Studies, Inflammation, Translational Studies, Vascular Biology
Nonstandard Abbreviations and Acronyms
- CorCSA
coronary cross‐sectional area
- IL‐1β
interleukin 1β
- IL‐6
interleukin 6
- IL‐10
interleukin 10
- PWH
persons with HIV
Clinical Perspective.
What Is New?
Coronary cross‐sectional area, a measure of positive coronary arterial remodeling, can be reliably measured using noncontrast computed tomography imaging and is strongly correlated with computed tomography angiogram measurements.
Low CD4+ T‐cell count and low plasma IL‐10 in persons with HIV is associated with positive arterial remodeling.
What Are the Clinical Implications?
In persons with HIV on antiretroviral therapy with low CD4+ T‐cell counts, the risk of cardiovascular disease may be higher and needs to be considered when stratifying risk.
Where coronary artery calcium may not be a reliable measure of subclinical atherosclerosis in persons with HIV because of soft plaque predominance, coronary cross‐sectional area measured on noncontrast computed tomography imaging may be an additional measure that needs to be studied further.
Persons with HIV (PWH) have a 2‐fold increased risk of cardiovascular disease (CVD) compared with the general population, as well as a higher prevalence of noncalcified plaque. 1 , 2 , 3 , 4 , 5 , 6 , 7 Calcified plaque is measured using cardiac computed tomography with a noncontrast imaging technique and the coronary artery calcium (CAC) score has been demonstrated to be an independent predictor of near‐term clinical events for multiple race–ethnic groups, sex, and during early adult life. 8 Previous studies have demonstrated that the amount of calcified plaque is strongly correlated with cardiac events, 9 predicts the incidence, 10 , 11 and assesses the prevalence 12 of CVD in the general population 13 , 14 , 15 ; but although the CAC score is correlated with total coronary plaque calcified and noncalcified histology, 16 the CAC score is not able to directly measure noncalcified plaque. 9 Alternative imaging with coronary computed tomography angiography (CCTA) can assess the luminal diameter, noncalcified plaque burden, and arterial remodeling, but this imaging modality is recommended in the general population when there is suspicion of CVD and not as a routine screening measure.
Noncalcified plaque accumulation is accompanied by compensatory arterial enlargement, a process described by Glagov et al in 1987. 17 This compensatory, positive remodeling in arteries with plaque buildup initially maintains the luminal area because of the expansion of the external arterial diameter despite plaque accumulation. 18 A study of HIV‐positive men in the MACS (Multicenter AIDS cohort study) found that HIV‐positive men were more likely to have positive arterial remodeling on CCTA imaging compared with HIV‐negative men even after adjustment for demographic and coronary artery disease risk factors. 19 A similar study also using CCTA showed a higher prevalence of positively remodeled plaque in HIV‐positive compared with HIV‐negative men. 20
The coronary artery cross‐sectional area (corCSA) can be measured on noncontrast CT images with the limitation that the lumen cannot be defined secondary to the lack of intravascular contrast. The external CorCSA enlarges in the setting of positive arterial wall remodeling and thus is comparable to CCTA and intravascular ultrasound. Furthermore, it can be accurately measured on submillimeter thick, noncontrast CT images. 21 Unlike CCTA, which involves identifying plaques and measuring the vessel diameter enlargement through the use of contrast, corCSA measures the wall‐to‐wall dimensions using similar high‐resolution ECG‐gated images without the addition of intravenous contrast. Given the increased likelihood of noncalcified plaque among PWH with atherosclerotic disease, which often would not be detected on standard CAC scoring, the measurement of corCSA from the same imaging approach may be valuable as a noninvasive risk stratification tool in the HIV population. In this study, we measured CorCSA of the proximal left anterior descending artery (LAD) from noncontrast CAC imaging among nondiabetic, prediabetic, and diabetic PWH. A subset of participants had noncontrasted CT imaging and CCTA performed during the same examination. We hypothesized that corCSA, as a marker of arterial remodeling and the potential presence of noncalcified plaque, would be associated with other CVD risk factors. Demonstrating the feasibility of measuring CorCSA and its association with established markers of CVD and related disease may support CorCSA as a new imaging biomarker of importance to help identify drivers of CVD in PWH.
MATERIALS AND METHODS
All the data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Participants
We enrolled 105 PWH with a range of metabolic and cardiovascular health from the Vanderbilt Comprehensive Care Clinic between August 2017 and June 2018. Participants were recruited as nondiabetic (hemoglobin A1c [HbA1c] <5.7% and/or fasting blood glucose [FBG] <100 mg/dL), prediabetic (HbA1c 5.7% to 6.4% and/or FBG 100 to 125 mg/dL) or diabetic (HbA1c ≥6.5% and/or FBG≥126 mg/dL, and/or those on antidiabetic medications). This classification was maintained throughout this article when discussing the demographics of the cohort. In participants who had discrepancy between HbA1c and FBG, FBG was used to classify patients, because HBA1c is known to sometimes underestimate blood glucose in PWH. Participants were on ART for at least 18 months with sustained virologic suppression (serum HIV‐1 RNA quantification <400 copies/mL) and a CD4+ T‐cell count >350 cells/μL for the previous 12 months. We excluded individuals with known inflammatory or rheumatologic conditions, heavy alcohol use (>11 drinks per week), and self‐reported cocaine, amphetamine, or nonprescribed opiate use. Anthropometric measurements including body mass index (BMI), waist circumference, hip circumference, and CVD‐related laboratory values were measured within 1 week of CT imaging (Table 1). Atherosclerotic cardiovascular disease risk was calculated using the American College of Cardiology Risk calculator. 22 The study was approved by the Vanderbilt University Medical Center Institutional Review Board. All participants provided written informed consent. The investigators carried out the study following the guidelines of the United States Department of Health and Human Services. This study is registered on clinicaltrials.gov (NCT04451980).
Table 1.
Clinical Demographics Stratified by Metabolic Status
| HIV‐positive individuals | N | Nondiabetic, N=37 | Prediabetic, N=36 | Diabetic, N=32 | Combined, N=105 | Test statistic |
|---|---|---|---|---|---|---|
| Age, y | 105 | 45 [37–52] | 44 [37–57] | 54 [48–58] | 48 [38–56] | 0.007* |
| Race: White | 0.6524/37 | 0.5018/36 | 0.4414/32 | 0.5356/105 | 0.4 | |
| Race: Black | 0.3011/37 | 0.4215/36 | 0.4715/32 | 0.3941/105 | ||
| Sex: Male | 105 | 0.8130/37 | 0.7828/36 | 0.6922/32 | 0.7680/105 | 0.5 |
| Systolic blood pressure, mm Hg | 92 | 131 [122–140] | 130 [120–139] | 132 [118–140] | 130 [120–140] | 0.9 |
| Hypertension: Yes | 105 | 0.6524/37 | 0.5919/32 | 0.6122/36 | 0.6265/105 | 1.0 |
| Tobacco use: Yes | 103 | 0.3011/37 | 0.3111/36 | 0.103/30 | 0.2425/103 | 0.1 |
| Statin use: Yes | 98 | 0.258/32 | 0.3312/36 | 0.5717/30 | 0.3837/98 | 0.03* |
| Anthropometric measurements | ||||||
| Hip circumference, cm | 103 | 107 [104–116] | 110 [103–117] | 104 108 123 | 109 [104–118] | 0.7 |
| Body mass index, kg/m2 | 105 | 31 [28–35] | 32 [29–35] | 33 [30–39] | 32 [29–36] | 0.05* |
| Waist circumference, cm | 103 | 104 [95–109] | 106 [93–113] | 110 [107–120] | 107 [98–113] | <0.001* |
| Body surface area, cm2 | 105 | 2.2 [2.0–2.2] | 2.2 [2.0–2.3] | 2.2 [2.1–2.4] | 2.2 [2.0–2.3] | 0.3 |
| Laboratory values | ||||||
| Fasting blood glucose, mg/dL | 103 | 90 [82–95] | 111 [106–117] | 174 [129–234] | 108 [91–129] | <0.001* |
| Hemoglobin A1c, % | 104 | 5.3 [5.0–5.4] | 5.6 [5.2–5.9] | 6.8 [6.2–8.8] | 5.5 [5.2–6.0] | <0.001* |
| Total cholesterol, mg/dL | 105 | 173 [154–202] | 180 [166–214] | 175 [150–196] | 175 [157–202] | 0.6 |
| HDL, mg/dL | 105 | 45 [35–55] | 40 [34–50] | 42 [34–46] | 42 [34–51] | 0.6 |
| LDL, mg/dL | 103 | 96 [85–122] | 111 [93–127] | 90 [81–105] | 99 [83–120] | 0.05* |
| Triglycerides, mg/dL | 105 | 74 96 161 | 90 145 211 | 119 174 272 | 83 127 214 | 0.004* |
| Creatinine, mg/dL | 105 | 1.0 [0.9–1.1] | 1.0 [0.8–1.2] | 1.0 [0.8–1.1] | 1.0 [0.9–1.1] | 0.6 |
| HsCRP, mg/dL | 104 | 2.7 [1.4–4.9] | 2.9 [1.1–4.0] | 3.0 [2.1–7.2] | 2.9 [1.4–4.6] | 0.5 |
| HIV laboratory values | ||||||
| CD4 at ART start, cells/mL | 102 | 533 [389–645] | 422 [288–572] | 399 [241–718] | 465 [296–643] | 0.4 |
| Duration on ART, y | 103 | 6.7 [4.4–11.7] | 7.1 [3.2–10.5] | 8.9 [5.2–16.1] | 7.2 [4.2–12.6] | 0.2 |
| CD4 count, cells/mL | 105 | 813 [683–944] | 786 [627–964] | 952 [732–1172] | 840 [673–1044] | 0.05* |
| CD4 count, % | 105 | 37 [31–40] | 34 [29–38] | 40 [36–47] | 37 [31–42] | <0.001* |
| Median CD4, cells/mL† | 105 | 775 [628–912] | 810 [664–949] | 855 [719–1087] | 821 [670–947] | 0.4 |
| Mean CD4, cells/mL† | 105 | 809 [632–914] | 796 [661–923] | 843 [729–1134] | 819 [670–939] | 0.4 |
| Hepatitis C positivity: yes | 105 | 0.083/37 | 0.083/36 | 0.124/32 | 0.1010/105 | 0.8 |
| CD8 at enrollment, cells/mL | 104 | 792 [620–1053] | 826 [660–1211] | 810 [674–1033] | 802 [660–1071] | 0.9 |
| White blood cells, cells/mL | 102 | 6.4 [5.1–7.9] | 7.6 [6.5–8.7] | 6.7 [6.0–8.3] | 6.8 [5.8–8.6] | 0.09 |
| Cell‐associated HIV RNA, copies/mL | 45 | 1342 [517–3280] | 992 [375–2116] | 1075 [435–2932] | 0.2 | |
| Cell‐associated HIV DNA, copies/mL | 45 | 630 [235–1419] | 424 [248–1169] | 466 [234–1362] | 0.9 | |
| Computed tomography measurements | ||||||
| CAC>0 | 105 | 0.031/37 | 0.3312/36 | 0.4414/32 | 0.2627/105 | <0.001* |
| CAC total, Au | 104 | 0.2±1.4 | 99.7±325.3 | 328.3±943.3 | 132.5±559.7 | <0.001* |
| CAC risk group: | 105 | 0.005* | ||||
| No CAC Au, 0 | 0.9736/37 | 0.6724/36 | 0.5317/32 | 0.7377/105 | ||
| Low CAC Au, 1–100 | 0.031/37 | 0.176/36 | 0.165/32 | 0.1112/105 | ||
| Moderate CAC Au, 101–300 | 0.000/37 | 0.114/36 | 0.124/32 | 0.088/105 | ||
| High CAC Au, >300 | 0.000/37 | 0.062/36 | 0.165/32 | 0.077/105 | ||
| corCSA ROI a, cm2 | 105 | 0.22 [0.19–0.26] | 0.24 [0.18–0.29] | 0.26 [0.20–0.31] | 0.23 [0.19–0.29] | 0.1 |
| corCSA ROI b, cm2 | 105 | 0.19 [0.16–0.23] | 0.22 [0.18–0.25] | 0.23 [0.17–0.28] | 0.21 [0.17–0.26] | 0.08 |
| corCSA ROI c, cm2 | 105 | 0.18 [0.14–0.20] | 0.18 [0.14–0.25] | 0.21 [0.16–0.25] | 0.19 [0.14–0.24] | 0.04* |
| Mean CorCSA, cm2 | 105 | 0.17 [0.19–0.23] | 0.17 [0.22–0.26] | 0.18 [0.23–0.28] | 0.17 [0.21–0.26] | 0.08 |
N is the number of non‐missing values. Tests used: Kruskal–Wallis test for continuous variables; Pearson χ2 test for categorical variables; and Wilcoxon test (cell‐associated HIV RNA/DNA). ART indicates antiretroviral therapy; Au, Agatston units; CAC, coronary artery calcium; CorCSA, coronary cross‐sectional area; HDL, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein cholesterol; and ROI, region of interest.
Statistical significance defined as P<0.05.
Median and mean CD4 T‐cell counts over the duration of therapy at the Vanderbilt Comprehensive Care Clinic.
Laboratory Testing
Laboratory testing obtained during this study included FBG, HbA1c, total cholesterol, low‐density lipoprotein cholesterol (LDL), high‐density lipoprotein cholesterol, triglycerides, high‐sensitivity C‐reactive protein, CD4+ T‐cell count at the start of therapy, current CD4+ T‐cell count, current CD8+ T‐cell counts, total white blood cells, and plasma cytokines.
Cell‐Associated HIV DNA and RNA Quantification
HIV reservoir activity and size were assessed by quantifying the cell‐associated HIV‐1 RNA and cell‐associated HIV‐1 DNA. 23 Intracellular RNA and DNA were isolated from cryopreserved peripheral blood mononuclear cells using the AllPrep DNA/RNA Mini Kit (Qiagen). Cellular integrity for RNA analysis was assessed by the measurement of total extracted RNA and evaluation of the IPO‐8 housekeeping gene. 24 Unspliced cell‐associated HIV‐1 RNA and total cell‐associated HIV‐1 DNA were quantified using a real‐time polymerase chain reaction approach with primers/probes targeting conserved regions of HIV LTR/gag as previously described. 25
CT Imaging
A noncontrast ECG‐gated CT of the thorax was performed using a Siemens Somatom Force multidetector scanner (Erlangen, Germany) or GE Healthcare Revolution CT (Waukesha, WI). CorCSA was measured on an Apple MacPro workstation using OsiriX (OsiriX, Pixmeo, Geneva, Switzerland) and an in‐house, customized program. 26 Coronary cross‐sections were measured in the proximal left anterior descending artery (LAD; American Heart Association coronary segment 6) in 3 sequential locations from proximal to distal as shown (Figure 1A). To measure corCSA, the technician identified the ostium of the LAD and traced the centerline of the vessel on 0.6‐mm‐thick CT slices. The proximal 10 mm of the LAD was identified, 3 equidistant points marked, diameter measured externally from outer wall to outer wall, and the area measured in cm2. The mean corCSA of the 3 sections was calculated. Total CAC (Agatston units) was measured in epicardial coronary arteries as previously reported. 27 This included the LAD, right coronary artery, left coronary artery (main), and left circumflex coronary artery.
Figure 1. Mean corCSA is higher with coronary calcium and correlated with ASCVD risk.
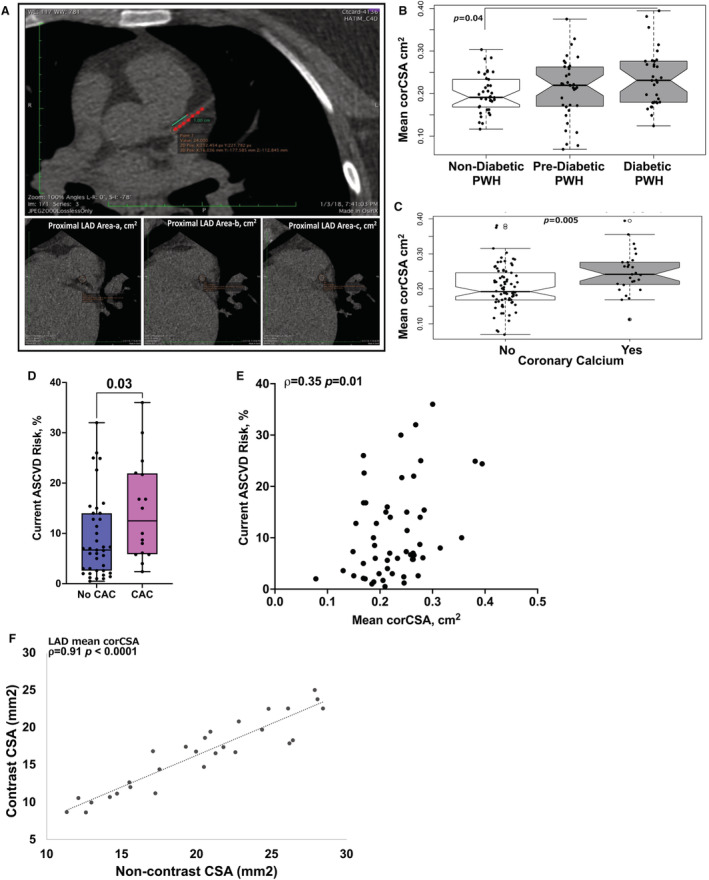
Representative computed tomography image (CT) demonstrating the technique for measuring coronary cross‐sectional area (corCSA) of the proximal left anterior descending coronary artery (LAD) using previously published methods. The proximal 1.0 cm of the LAD was identified, at 3 equidistant points as labeled a, b, and c. The corCSA includes the area of the wall of the vessel as well as the lumen. Mean corCSA is the average of all 3 areas (A). Notched box plots showing mean corCSA by diabetes status (B) and by quantifiable calcium on CT imaging (C). Box plots showing current atherosclerotic cardiovascular disease (ASCVD) risk score in persons with HIV (PWH) with and without coronary artery calcium (CAC) (D) and correlation between ASCVD risk score and corCSA (E). Correlation plot of corCSA measurements obtained by noncontrast CT and coronary computed tomography angiography imaging (n=28). Analyses were performed using Kruskal–Wallis test (B), Mann–Whitney U tests (C and D), and Spearman rank correlation (E and F).
Coronary CT Angiography
We obtained CCTAs on 28 of the 105 participants in this study. Before all contrast scans, a prospectively ECG‐triggered Calcium Scoring scan was performed as above. CCTAs were performed using whole heart scans or GE Healthcare Revolution CT (Waukesha, WI). A β‐blocker (metoprolol) was administered to participants whose heart rates were >75 beats per minute, and a cardiac gating with multiphase reconstruction and motion correction was utilized (Snapshot Freeze). Patients with acute or chronic kidney injury, and/or allergy to iodinated contrast were excluded.
Plasma Cytokine Measurements
All plasma cytokines were measured using a standard multiple immunoassay panel (MesoScale, Rockville, MD) as previously published. 28
Statistical Analysis
We calculated the medians and 25th and 75th percentiles of continuous variables and proportions of categorical variables. We first compared the clinical and demographic characteristics among PWH by metabolic status using the Kruskal–Wallis test or Wilcoxon rank‐sum test for continuous variables and Pearson χ2 test for categorical variables. Mean CorCSA was compared between groups using Kruskal–Wallis or Wilcoxon rank‐sum tests. We assessed the relationships between corCSA and clinical demographic measures using scatter plots and partial Spearman rank correlations. Partial Spearman's correlation was our preferred method of analysis because it results in a single number between −1 and 1 with a simple interpretation. Additionally, it allowed us to examine Spearman's correlation after adjusting for covariates and to compare directly to the unadjusted version to understand the impact of adjusting for covariates. Correlations between mean corCSA and anthropometric measurements, laboratory measurements, and HIV factors were also assessed using Spearman rank.
Covariate‐adjusted estimates of partial Spearman rank correlations were calculated using probability‐scale residuals from cumulative probability models with logit link functions; adjustment variables included potentially confounding CVD risk factors. 29 We first performed a partial Spearman rank correlation analysis that included all PWH adjusted for hypertension, sex, smoker status, statin use, and CAC. Additional analysis that was performed to investigate the relationship with HIV‐related factors did not include LDL, or high‐density lipoprotein cholesterol as covariates because our unadjusted correlation analysis suggested that there was no relationship between LDL or high‐density lipoprotein cholesterol with mean corCSA in this cohort. Also including them in the model did not change the strength of the associations. Among the nondiabetic HIV‐positive participants alone, partial Spearman correlation analysis was adjusted for hypertension, sex, smoker status, and statin use. Analysis of corCSA and HIV‐related factors were adjusted for age, HbA1c, hypertension, sex, smoker status, BMI, statin use, and CAC in all PWH. We performed similar analyses that were stratified by diabetes status and adjusted for age, HbA1c, BMI, and sex in nondiabetic PWH. We added CAC to the analysis of prediabetic and diabetic PWH. Analyses were performed using R versions 3.6.1 and 4.0.2. (http://www.R‐project.org) and Graph Pad Prism software.
RESULTS
Clinical Demographics of PWH
A total of 37 nondiabetic, 36 prediabetic, and 32 diabetic PWH were included in the analysis. The cohort was 24% female, 39% Black, with a median age of 48 years, current CD4 T‐cell count of 840 cells/mL, and ART duration of 7 years (Table 1). PWH with diabetes were older compared with nondiabetic and prediabetic PWH but had a similar distribution of race and sex. Metabolic syndrome measures including FBG, triglyceride levels, waist circumference, and BMI were generally higher in PWH with diabetes except for LDL, which was lower in PWH with diabetes, because of statin therapy (Table 1). The CD4+ T‐cell count, reported as absolute count (P=0.05) and percentage at the time of study enrollment (P<0.001) differed by metabolic status (Table 1). Females with HIV had a higher BMI (P=0.003), higher percentage of current CD4+ T cells (P=0.02), and lower HIV cell‐associated DNA (P=0.03) and RNA (P=0.01) than males with HIV (Table S1).
corCSA as a Measure of Subclinical Cardiovascular Disease in PWH
When comparing by diabetes status, PWH with diabetes had higher mean corCSA compared with participants without diabetes (Figure 1B, Table 1). Not surprisingly, we detected CAC in a higher proportion in PWH with diabetes and those who were prediabetic compared with PWH without diabetes, P<0.001 (Table 1). Stratification by CAC in PWH showed larger mean corCSA measurements in PWH with detectable coronary calcium on CT, P=0.005 (Figure 1C). Mean corCSA did not differ by sex (P=0.7) (Table 2). We also found that atherosclerotic cardiovascular disease risk score in this cohort was higher in persons with CAC (Figure 1D) and correlated with the corCSA measurements (Figure 1E). Moreover, mean corCSA measurements in noncontrast CT imaging were strongly correlated with CCTA measurements performed on the same day (Table 2, Figure 1F).
Table 2.
Noncontrast CT and Contrast CT Measures of Proximal LAD CSA (mm2) in OsiriX (n=28 Paired Studies Done Same Day)
| Measure | Noncontrast CT | Contrast CT | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Median | 25th, 75th percentiles | Mean (SD) | Median | 25th, 75th percentiles | |
| Area‐a | 21.4 (5.6) | 20.8 | 17.3, 24.9 | 17.6 (5.5) | 18.1 | 12.7, 21.9 |
| Area‐b | 20.2 (5.9) | 18.6 | 16.1, 25.2 | 16.2 (4.8) | 17.0 | 11.5, 19.3 |
| Area‐c | 18.8 (5.6) | 19.0 | 14.7, 22.1 | 15.4 (4.9) | 14.9 | 11.2, 20.1 |
| Mean | 20.1 (5.2) | 20.5 | 15.6, 24.6 | 16.4 (4.8) | 16.9 | 11.7, 19.6 |
Area‐a, b, c represent the CSA of 3 equidistant points measured 1 cm proximal of the LAD. CSA indicates cross‐sectional area; CT, computed tomography; LAD, left anterior descending coronary artery; and SD, standard deviation.
Association Between Mean corCSA and Established CVD Risk Factors Among PWH
Among all 105 PWH included in this study, independent of metabolic status, mean corCSA was significantly correlated with BMI (ρ=0.23, P=0.016), waist circumference (ρ=0.32, P<0.001), age (ρ=0.32, P<0.001), and HbA1c (ρ=0.29, P<0.01) with no significant relationship between mean corCSA, weight, height, LDL, high‐density lipoprotein cholesterol, triglycerides, and creatinine (Figure 2). After adjusting for hypertension, sex, smoking status, statin use, and CAC prevalence (in all PWH, n=105), there remained a significant correlation between mean corCSA with age (ρ=0.25, P<0.05), and waist circumference (ρ=0.22, P<0.05) (Figure 3A). When stratified by diabetes groups, mean corCSA remained correlated with age (ρ=0.43, P<0.05) in PWH without diabetes (Figure 3B). We did not adjust for CAC prevalence in PWH without diabetes, because only 1 participant in this subgroup had CAC on imaging. There was no significant correlation observed in PWH who were prediabetic and those who diabetic in the fully adjusted model (Figure 3C).
Figure 2. Mean coronary cross‐sectional area (corCSA) correlation with cardiovascular disease risk factors.
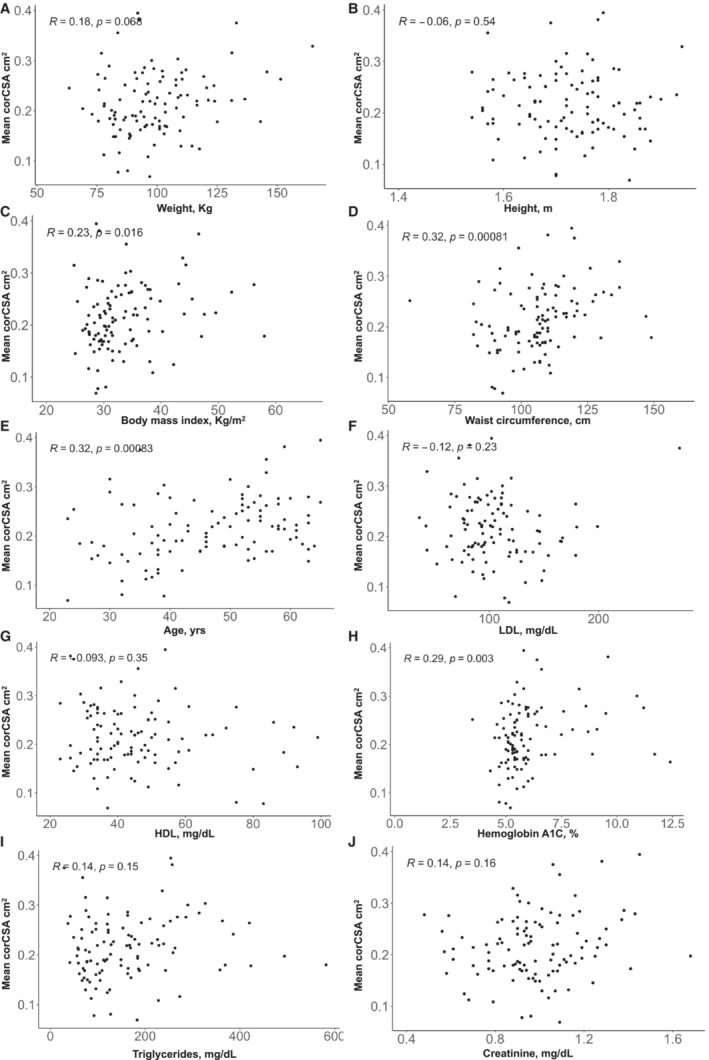
Unadjusted Spearman rank correlation plots of mean corCSA and weight (A), height (B), body mass index (C), waist circumference (D), age (E), low‐density lipoprotein cholesterol (LDL) (F), high‐density lipoprotein cholesterol (HDL) (G), hemoglobin A1c (H), triglycerides (I), and creatinine (J). Statistical analyses were performed using Spearman rank correlation.
Figure 3. Mean coronary cross‐sectional area (corCSA) correlates with age and waist circumference in adjusted models.
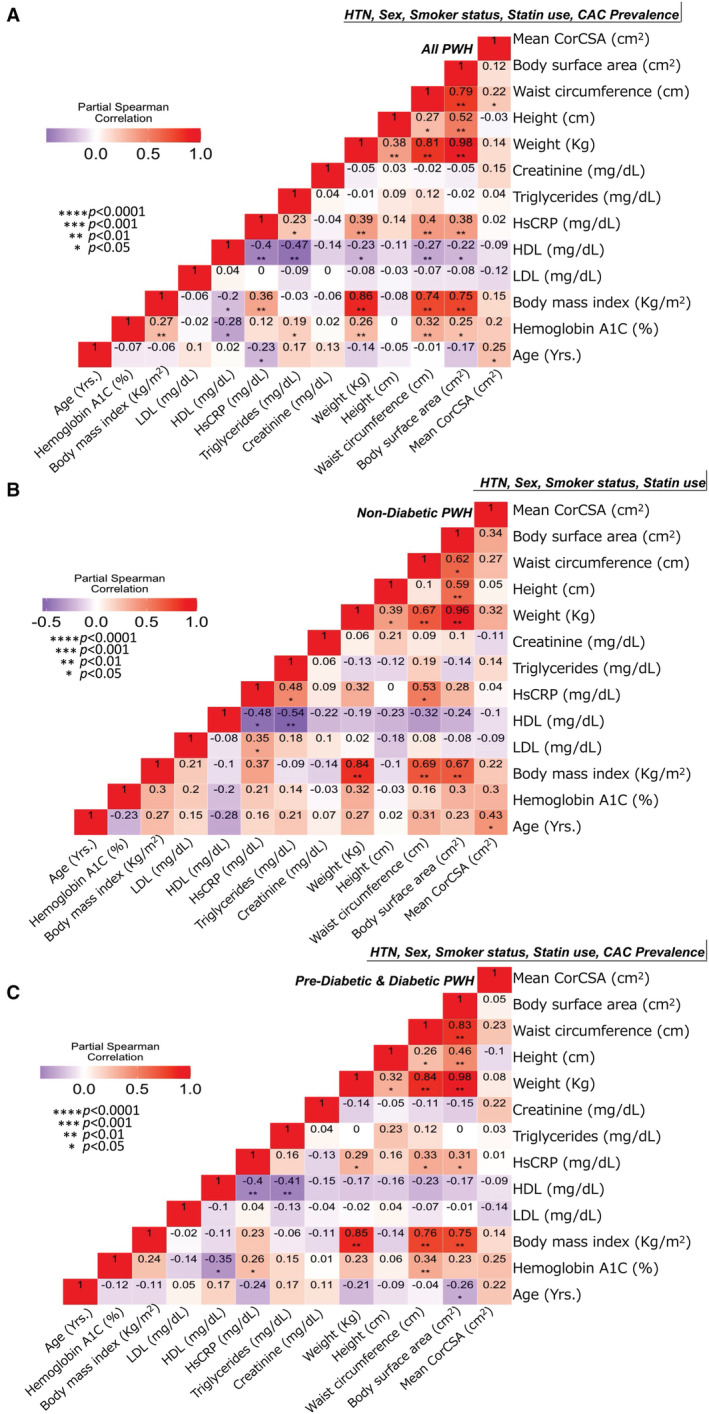
Partial Spearman rank correlation analysis adjusted for hypertension (HTN), smoker status, sex, statin use, and coronary artery calcium (CAC) prevalence in all persons with HIV (PWH) (n=105) (A), PWH without diabetes (n=38) (B), and PWH who were prediabetic/diabetic (n=68) (C). HDL indicates high‐density lipoprotein cholesterol; HsCRP, high‐sensitivity C‐reactive protein; and LDL, low‐density lipoprotein cholesterol.
Mean corCSA Is Associated With HIV‐Related Variables That Vary With Diabetes
We evaluated the relationship between mean corCSA and HIV‐related measures using unadjusted Spearman rank correlations. Duration of ART was positively correlated with mean corCSA (P=0.004) among all PWH and strongest in PWH without diabetes (Figure 4A) and males (Figure 4B). We also considered that older PWH were more likely to have had HIV for a longer period of time. We calculated the “HIV age” of each participant as the duration of time on ART (years) divided by their age (yrs.). There was a correlation between mean corCSA and HIV age that was not statistically significant (P=0.05) in the entire group but significant in PWH without diabetes (P=0.044) (Figure 4C) and HIV‐positive men (P=0.048) (Figure 4D). Other HIV‐related measurements that included nadir CD4 T‐cell count (P=0.57, Figure 4E) and current CD4+ T‐cell count (P=0.067, Figure 4F) were not significant in all PWH. The current CD4+ T‐cell count was negatively correlated with mean corCSA in PWH with diabetes (P=0.048) and PWH who were prediabetic (P=0.044). Although cell‐associated RNA and DNA quantification was perfomed in a small subset of participants, there was a positive correlation between mean corCSA and cell‐associated HIV RNA (P=0.05) and DNA (P=0.04) in PWH with diabetes (Figure 4G through 4H).
Figure 4. Mean coronary cross‐sectional area (corCSA) positively correlates with duration of antiretroviral therapy (ART), cell‐associated HIV RNA/DNA, and negatively with CD4 T cell count.
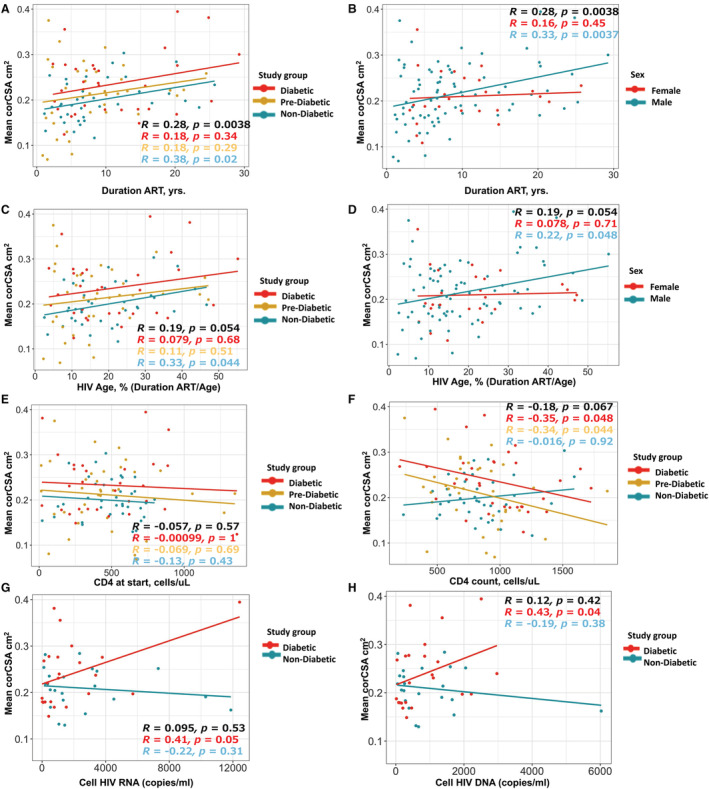
Unadjusted correlation plots of mean corCSA and duration of ART in all persons with HIV (PWH) by diabetes status (A) and sex (B). Similar correlation plot of mean corCSA and HIV age (a calculation of the duration of ART divided by age at study enrollment) stratified by metabolic status (C) and sex (D). Mean corCSA was also measured against CD4 T‐cell count at the start of therapy (E), CD4 at study enrollment (F), cell‐associated HIV RNA (G), and cell‐associated HIV DNA (H). Spearman rank test was used for statistical analysis. Note that the correlations are rank‐based whereas the slopes are based on the original data, and therefore, they may not always be in the same direction (eg, Spearman's correlation=−0.016 for those who were nondiabetic in (F), but slightly positive slope with Pearson's correlation=0.17, P=0.33).
We performed a partial Spearman correlation analysis of all PWH adjusted for CVD risk factors including age, HbA1c, hypertension, waist circumference, sex, smoker status, statin use, and CAC prevalence. We observed a negative correlation between mean corCSA and the current CD4 T‐cell count (ρ=−0.2, P=0.047) in all PWH (Figure 5A). Removal of age strengthened the relationships between mean corCSA with HIV age (ρ=0.25, P=0.03) and ART duration (ρ=0.3, P=0.01), while CD4 T‐cell count remained the same (ρ=−0.2, P=0.049) (Figure 5B).
Figure 5. Mean coronary cross‐sectional area (corCSA) is correlated with antiretroviral therapy (ART) duration and the current CD4 T‐cell count.
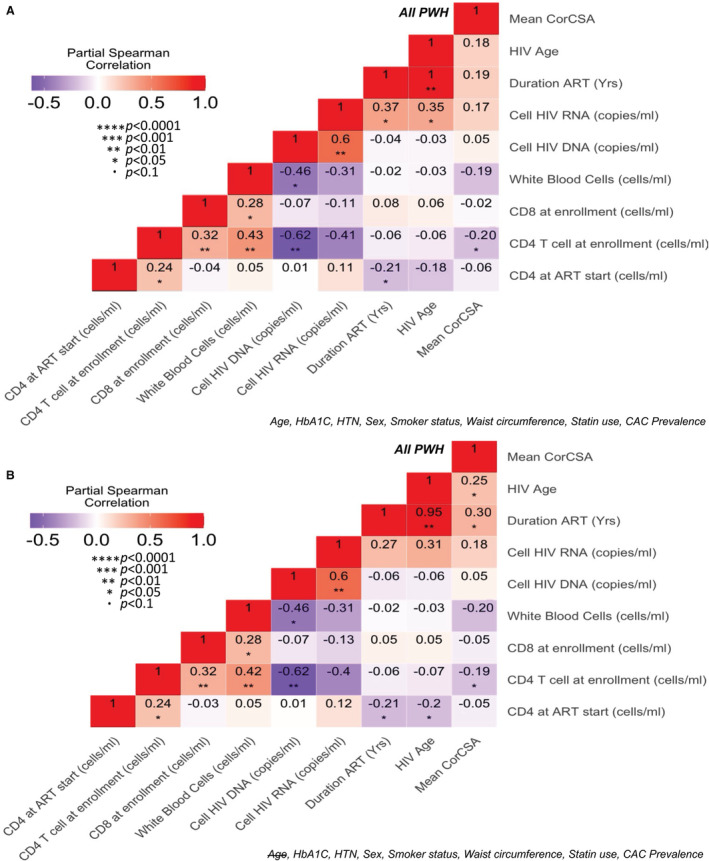
Partial Spearman correlation analysis adjusted for cardiovascular disease risk factors (A), and with age removed from the model (B). CAC indicates coronary artery calcium; CVD, cardiovascular disease; HbA1c, hemoglobin A1c; HTN, hypertension; and PWH, persons with HIV.
When we stratified the analysis by diabetes status adjusted for age, HbA1c, waist circumference, and sex only, there was no significant relationship between mean corCSA and HIV‐related factors in PWH without diabetes (Figure 6A). Duration of ART (ρ=0.37, P=0.02), HIV age (ρ=0.32, P=0.07), but not current CD4 T‐cell count were correlated with mean corCSA with the exclusion of age in PWH without diabetes (Figure 6B). This was different in PWH who were prediabetic and those with diabetes adjusted for age, HbA1c, waist circumference, hypertension, sex, and CAC. We observed a negative correlation between corCSA and the current CD4 T‐cell count (ρ=−0.28, P=0.03), and no significance with the white blood cell count (ρ=−0.25, P=0.08), (Figure 6C). Significance after the exclusion of age was higher for the white blood cell count (ρ=−0.31, P=0.02), unchanged for the current CD4 T‐cell count (ρ=−0.28, P=0.02), but not significant for the duration of ART (ρ=0.21, P=0.15) (Figure 6D).
Figure 6. Mean coronary cross‐sectional area (corCSA) correlation with HIV risk factors differs by diabetes among all PWH.
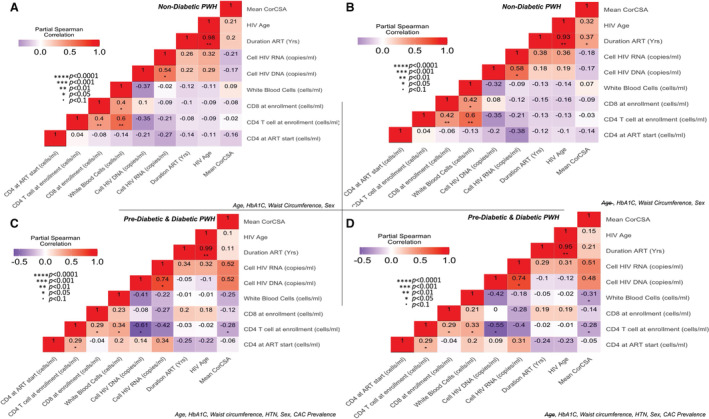
Partial Spearman correlation analysis adjusted for HbA1c, sex, and waist circumference in PWH without diabetes with (A) and without age (B). A similar analysis in PWH with prediabetes and diabetes adjusted for HbA1c, waist circumference, HTN, sex, and CAC prevalence with (C) and without age (D). ART indicates antiretroviral therapy; CAC, coronary artery calcium; HbA1c, hemoglobin A1c; HTN, hypertension; and PWH, persons with HIV.
IL‐10 Is Inversely Related to Arterial Remodeling
We measured several plasma cytokines related to macrophage activation including IL‐1b, IL‐6, tumor necrosis factor‐α, growth differentiation factor 15, and IL‐10. There were no significant differences in the plasma cytokine levels by diabetes status except for growth differentiation factor 15 (Figure 7A). Growth differentiation factor 15 was higher in PWH with diabetes (P<0.0001). We also measured differences in plasma cytokines between individuals with and without CAC on imaging. Growth differentiation factor 15 (P<0.001), interferon‐γ (P=0.03), and sCD163 (P=0.04) were higher in participants with CAC (Table 3). Partial Spearman rank adjusted for CVD risk factors including age, sex, HbA1c, hypertension, BMI, smoker status, statin use, and CAC showed an inverse association between IL‐10 and corCSA (ρ=−0.25, P=0.02) among all PWH (Figure 7B). When we stratified this by diabetes status, the correlation between corCSA and plasma IL‐10 in nondiabetic (P=0.11), prediabetic, and diabetic PWH (P=0.05) was not statistically significant (Figure 7C through 7D).
Figure 7. Mean coronary cross‐sectional area (corCSA) is negatively associated with plasma IL‐10.
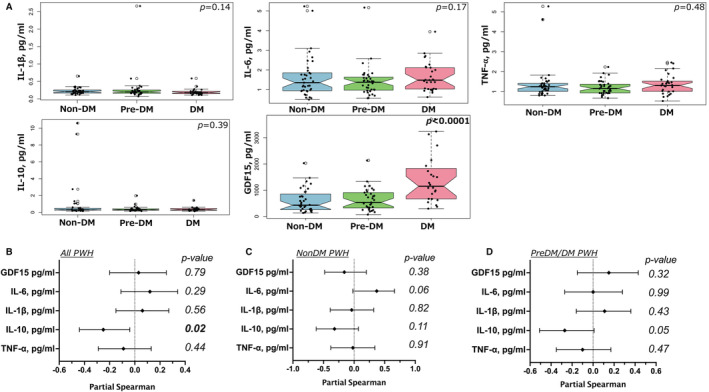
Box plots showing plasma cytokines from nondiabetic, prediabetic combined with diabetic PWH (A). Forest plot showing the adjusted (age, sex, HbA1c, HTN, waist circumference, smoker status, statin use, and the presence of CAC) correlation between corCSA and cytokines in all PWH (B), PWH without diabetes adjusted for age, HbA1c, sex, and waist circumference (C), and PWH who were prediabetic/diabetic adjusted for age, HbA1c, sex, waist circumference, and the presence of CAC (D). Mann–Whitney test and partial Spearman rank test were used for statistical analysis. CAC indicates coronary artery calcium; HbA1c, hemoglobin A1c; HTN, hypertension; and PWH, persons with HIV.
Table 3.
Inflammatory Cytokines of PWH Stratified by Coronary Calcium Prevalence
| N | No coronary calcium N=78 | Coronary calcium N=27 | Combined N=105 | P value | |
|---|---|---|---|---|---|
| GDF‐15 pg/mL | 93 | 512 [293–928] | 1155 [658–1662] | 656 [347–1088] | <0.001* |
| IL‐1β pg/mL | 100 | 0.20 [0.17–0.24] | 0.16 [0.14–0.24] | 0.19 [0.16–0.24] | 0.1 |
| IL‐6 pg/mL | 102 | 1.38 [0.94–1.81] | 1.48 [1.05–1.76] | 1.41 [0.99–1.80] | 0.4 |
| IL‐10 pg/mL | 102 | 0.31 [0.25–0.45] | 0.27 [0.23–0.39] | 0.30 [0.24–0.44] | 0.2 |
| IFN‐γ pg/mL | 102 | 5.4 [4.1–8.2] | 6.5 [5.8–11.7] | 6.0 [4.3–9.4] | 0.03* |
| TNF‐α pg/mL | 102 | 1.23 [1.00–1.38] | 1.41 [1.00–1.60] | 1.26 [1.00–1.46] | 0.1 |
N is the number of nonmissing values. Test used: Wilcoxon test. GDF‐15 indicates growth differentiation factor 15; IFN‐γ, interferon‐γ; PWH, persons with HIV; and TNF‐α, tumor necrosis factor α.
Statistical significance defined as P<0.05.
DISCUSSION
In this study, mean corCSA was used as a measure of positive arterial remodeling from noncontrast CT imaging and suggests that independent of diabetes, and after adjusting for traditional CVD risk factors, PWH on ART for a longer duration may have larger arteries, which could partly be from noncalcified plaque and inflammation of the vessel related to other processes including HIV and co‐infection with other viruses such as cytomegalovirus. 30 This would be consistent with a previous study using CCTA in PWH, which showed that positive remodeling was significantly greater when there was mixed plaque with or without stenosis compared with calcified plaque. 19 Mean corCSA measured by noncontrast CT imaging was associated with traditional risk factors, the strongest being age and HbA1c. When we adjusted for traditional CVD risk factors, we observed that ART duration and current CD4+ T‐cell count were modestly associated with corCSA.
Unlike previous studies, our cohort included PWH with a spectrum of metabolic disease and used noncontrast CT imaging. 19 , 20 There were notable differences in significance between nondiabetic and prediabetic/diabetic PWH, which is likely confounded by nontraditional variables that we did not include in our models. Stratification of our analysis by diabetes status revealed that corCSA was positively correlated with ART duration in PWH without diabetes but not in PWH who were prediabetic or diabetic despite the latter 2 groups having larger corCSA. Based on the clinical demographics, this could not be attributed to a difference in ART duration by metabolic status. Another variable, CD4 T‐cell count at the time of the study, was different by metabolic status (P=0.05) and correlated modestly with higher corCSA in prediabetic and diabetic PWH only. These differences by metabolic disease suggest that other factors related to diabetes may alter the circulating immune cell profile, which may be important. Residual cardiovascular risk in PWH after adjustment for CVD risk is thought to be caused by multiple factors including inflammation. 28 , 30 A persistent HIV viral reservoir, its effect on CD4 T‐cell counts, and other chronic viral co‐infections may be important. The relationships between mean corCSA, ART duration, and CD4 T cells suggest that HIV status and the effect of the virus on CD4 T cells may contribute to coronary arterial remodeling in PWH and CVD risk.
IL‐10 is a pleiotropic hemopoietic cytokine that is broadly expressed by cells of both the innate and adaptive immune system, and largely functions as a regulatory cytokine. 31 Some studies in HIV‐negative populations have reported a positive correlation between plasma IL‐10 and increased risk of cardiovascular events. 32 , 33 Other studies in PWH have reported an increase in the likelihood of noncalcified coronary plaque with lower plasma IL‐10 levels, 34 and lower carotid intima thickness with higher levels of IL‐10 in PWH. 35 In our study, mean corCSA was inversely correlated with plasma IL‐10 while there was no statistical difference in the plasma IL‐10 levels in those with and without CAC. Diabetes status did not seem to change the direction of the relationship between corCSA and IL‐10, although significance was lost because of a smaller number of participants in the metabolic subgroups. Further studies are needed to understand mechanisms, because administering IL‐10 is not likely to be an intervention in PWH, because of the possible effect on HIV viral persistence.
As the life expectancy of PWH increases, there has been an increased prevalence of diabetes, hypertension, dyslipidemia, and other comorbidities predisposing to CVD. 36 Rates of myocardial infarction or coronary heart disease in PWH are higher with approximately 2‐fold increased relative risk compared with HIV‐negative controls. 7 , 37 , 38 , 39 , 40 , 41 , 42 This increased risk remained even when controlled for potential confounders such as socioeconomic status or viral load. 30 This study provides an additional clinical end point that could be used in research studies aimed at understanding mechanisms that drive CVD in PWH. This is because mean corCSA is affected by noncalcified coronary plaque and calcified coronary plaque and may be used to revisit studies where noncontrast CT imaging was obtained but only CAC was included in the analysis.
This study has several limitations including the cross‐sectional design, which precludes the assessment of causality. We focused on PWH with and without diabetes and had an unequal number of males and females with 80 males and 25 females, which may limit the generalizability of our study. Furthermore, with our cohort we are not able to differentiate the influence of HIV alone or ART. For the latter, future studies that recruit HIV‐negative participants on HIV pre‐exposure prophylaxis would serve as an important control to address the effect of ART in individuals where HIV is not a factor. A lack of a matched HIV‐negative control group with and without diabetes would have added great value to understanding how HIV‐related factors relate to corCSA. Future studies with larger cohorts and HIV‐negative control groups are needed to further elucidate not only the differences and applicability of each study but also answer whether mean corCSA is comparable with positive remodeling measurements using CCTA to stratify the HIV‐risk factors of CVD compared with CAC.
CONCLUSIONS
CAC alone may not capture the risk of subclinical atherosclerosis in PWH where noncalcified plaque is predominant and additional risk factors, above the traditional, significantly increase the risk of CVD. Our study shows that current CD4+ T cell and plasma IL‐10 are negatively associated with corCSA. Although CCTA is an alternative used to capture additional characteristics of subclinical CVD, the strong correlation between corCSA measured by noncontrast CT imaging and CCTA suggests that mean corCSA may be an additional tool that can be utilized to stratify CVD risks in PWH while avoiding the need for intravenous contrast as well as additional radiation exposure. This measure can also be applied to existing studies with archived noncontrast CTs. Future larger studies linking CCTA and noncontrast CT imaging with inflammatory markers will provide a better understanding of possible targets to improve outcomes in PWH.
Sources of Funding
This work was funded by NIH grants K23 100700 (JK), R01 DK112262 (JRK and CNW), HL131977 (JB), K23 HL156759 (CNW), Doris Duke Clinical Scientist Development Award 2021193 (CNW), Burroughs Wellcome Fund 1021480 (CNW), and the Tennessee Center for AIDS Research grant P30 AI110527 (SAM). The funding authorities had no role in study design; data collection, analysis, or interpretation; the decision to publish; or preparation of the manuscript.
Disclosures
Dr Celestine Wanjalla is on the ViiV Healthcare advisory Board for Fostemsavir.
Supporting information
Table S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025768
For Sources of Funding and Disclosures, see page 14.
References
- 1. Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc. 2019;8:e012241. doi: 10.1161/JAHA.119.012241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So‐Armah K, Freiberg MS, Lloyd‐Jones DM. Patterns of cardiovascular mortality for HIV‐infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117:214–220. doi: 10.1016/j.amjcard.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha‐Filho JA, Nasir K, Grinspoon SK. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV‐infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soares C, Samara A, Yuyun MF, Echouffo‐Tcheugui JB, Masri A, Morrison AR, Lin N, Wu WC, Erqou S. Coronary artery calcification and plaque characteristics in people living with HIV: a systematic review and meta‐analysis. J Am Heart Assoc. 2021;10:e019291. doi: 10.1161/JAHA.120.019291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr JJ, Jr Jacobs DR, Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, Goff DC Jr. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American Heart Association/American College of Cardiology Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 11. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210 [DOI] [PubMed] [Google Scholar]
- 12. Terry JG, Carr JJ, Tang R, Evans GW, Kouba EO, Shi R, Cook DR, Vieira JL, Espeland MA, Mercuri MF, et al. Coronary artery calcium outperforms carotid artery intima‐media thickness as a noninvasive index of prevalent coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2005;25:1723–1728. doi: 10.1161/01.ATV.0000173418.42264.19 [DOI] [PubMed] [Google Scholar]
- 13. Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena MG, et al. Coronary aging in HIV‐infected patients. Clin Infect Dis. 2009;49:1756–1762. doi: 10.1086/648080 [DOI] [PubMed] [Google Scholar]
- 14. Kingsley LA, Cuervo‐Rojas J, Muñoz A, Palella FJ, Post W, Witt MD, Budoff M, Kuller L. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: multicenter AIDS cohort study. AIDS. 2008;22:1589–1599. doi: 10.1097/QAD.0b013e328306a6c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mangili A, Gerrior J, Tang AM, O'Leary DH, Polak JK, Schaefer EJ, Gorbach SL, Wanke CA. Risk of cardiovascular disease in a cohort of HIV‐infected adults: a study using carotid intima‐media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43:1482–1489. doi: 10.1086/509575 [DOI] [PubMed] [Google Scholar]
- 16. Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron‐beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157 [DOI] [PubMed] [Google Scholar]
- 17. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204 [DOI] [PubMed] [Google Scholar]
- 18. Schoenhagen P, Nissen SE, Tuzcu EM. Coronary arterial remodeling: from bench to bedside. Curr Atheroscler Rep. 2003;5:150–154. doi: 10.1007/s11883-003-0088-9 [DOI] [PubMed] [Google Scholar]
- 19. Miller PE, Haberlen SA, Metkus T, Rezaeian P, Palella F, Kingsley LA, Witt MD, George RT, Jacobson LP, Brown TT, et al. HIV and coronary arterial remodeling from the Multicenter AIDS Cohort Study (MACS). Atherosclerosis. 2015;241:716–722. doi: 10.1016/j.atherosclerosis.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV‐infected men. AIDS. 2013;27:1263–1272. doi: 10.1097/QAD.0b013e32835eca9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papayannis A, Duprez D, Ge Y, Terry J, Kabagambe E, Lloyd‐Jones D, Lima J, Venkatesh BA, Kishi S, Carr J, et al. Coronary artery structural remodeling by computed tomography and echocardiographic left ventricular mass changes over the next 5 years: the coronary artery risk development in young adults (cardia) study. J Am Coll Cardiol. 2017;69:1604. doi: 10.1016/S0735-1097(17)34993-8 [DOI] [Google Scholar]
- 22. ACC . American College of Cardiology. ASCVD estimator plus (https://tools.acc.org/ascvd‐risk‐estimator‐plus/#!/calculate/estimate). https://tools.acc.org/ascvd‐risk‐estimator‐plus/#!/calculate/estimate. 2022.
- 23. Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, Kuritzkes DR, Lederman MM, Para M, Gandhi RT. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016;30:343–353. doi: 10.1097/QAD.0000000000000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ledderose C, Heyn J, Limbeck E, Kreth S. Selection of reliable reference genes for quantitative real‐time PCR in human T cells and neutrophils. BMC Res Notes. 2011;4:427. doi: 10.1186/1756-0500-4-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malnati MS, Scarlatti G, Gatto F, Salvatori F, Cassina G, Rutigliano T, Volpi R, Lusso P. A universal real‐time PCR assay for the quantification of group‐M HIV‐1 proviral load. Nat Protoc. 2008;3:1240–1248. doi: 10.1038/nprot.2008.108 [DOI] [PubMed] [Google Scholar]
- 26. Carr JJ, Ge Y, Kabagambe EK, Terry JG, Lloyd‐Jones D, Papayannis A, Duprez DA, Rana JS, Sidney S, Jacobs DR. Coronary artery structural remodeling by computed tomography and cardiovascular disease; the coronary artery risk development in young adults. Circulation. 2017;135(Suppl 1):AP375. doi: 10.1161/circ.135.suppl_1.p375 [DOI] [Google Scholar]
- 27. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t [DOI] [PubMed] [Google Scholar]
- 28. Wanjalla CN, Mashayekhi M, Bailin S, Gabriel CL, Meenderink LM, Temu T, Fuller DT, Guo L, Kawai K, Virmani R, et al. Anticytomegalovirus CD4+ T cells are associated with subclinical atherosclerosis in persons with HIV. Arterioscler Thromb Vasc Biol. 2021;41(4):1459–1473. doi: 10.1161/ATVBAHA.120.315786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Q, Li C, Wanga V, Shepherd BE. Covariate‐adjusted Spearman's rank correlation with probability‐scale residuals. Biometrics. 2018;74:595–605. doi: 10.1111/biom.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol. 2019;16:745–759. doi: 10.1038/s41569-019-0219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 32. Lakoski SG, Liu Y, Brosnihan KB, Herrington DM. Interleukin‐10 concentration and coronary heart disease (CHD) event risk in the estrogen replacement and atherosclerosis (ERA) study. Atherosclerosis. 2008;197:443–447. doi: 10.1016/j.atherosclerosis.2007.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welsh P, Murray HM, Ford I, Trompet S, de Craen AJ, Jukema JW, Stott DJ, McInnes IB, Packard CJ, Westendorp RG, et al. Circulating interleukin‐10 and risk of cardiovascular events: a prospective study in the elderly at risk. Arterioscler Thromb Vasc Biol. 2011;31:2338–2344. doi: 10.1161/ATVBAHA.111.231795 [DOI] [PubMed] [Google Scholar]
- 34. Fourman LT, Saylor CF, Cheru L, Fitch K, Looby S, Keller K, Robinson JA, Hoffmann U, Lu MT, Burdo T, et al. Anti‐inflammatory interleukin 10 inversely relates to coronary atherosclerosis in persons with human immunodeficiency virus. J Infect Dis. 2020;221:510–515. doi: 10.1093/infdis/jiz254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Desvarieux M, Boccara F, Meynard JL, Bastard JP, Mallat Z, Charbit B, Demmer RT, Haddour N, Fellahi S, Tedgui A, et al. Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV‐infected never‐smokers independent of antiretroviral therapy. AIDS. 2013;27:2603–2614. doi: 10.1097/QAD.0b013e3283634819 [DOI] [PubMed] [Google Scholar]
- 36. Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res. 2006;4:79–85. doi: 10.2174/157016206775197664 [DOI] [PubMed] [Google Scholar]
- 37. Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV‐infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012 [DOI] [PubMed] [Google Scholar]
- 38. Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case‐control study using Québec's public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–253. doi: 10.1097/QAI.0b013e31821d33a5 [DOI] [PubMed] [Google Scholar]
- 39. Klein D, Hurley LB, Quesenberry CP, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV‐1 infection? J Acquir Immune Defic Syndr. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002 [DOI] [PubMed] [Google Scholar]
- 40. Lang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D, French Hospital Database on HIV‐ANRS CO4 . Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. doi: 10.1097/QAD.0b013e328339192f [DOI] [PubMed] [Google Scholar]
- 41. Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sørensen HT, Gerstoft J. Ischemic heart disease in HIV‐infected and HIV‐uninfected individuals: a population‐based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285 [DOI] [PubMed] [Google Scholar]
- 42. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


