Abstract
Background
ATP‐sensitive potassium channels are inhibited by ATP and open during metabolic stress, providing endogenous myocardial protection. Pharmacologic opening of ATP potassium channels with diazoxide preserves myocardial function following prolonged global ischemia, making it an ideal candidate for use during cardiac surgery. We hypothesized that diazoxide would reduce myocardial stunning after regional ischemia with subsequent prolonged global ischemia, similar to the clinical situation of myocardial ischemia at the time of revascularization.
Methods and Results
Swine underwent left anterior descending occlusion (30 minutes), followed by 120 minutes global ischemia protected with hyperkalemic cardioplegia±diazoxide (N=6 each), every 20 minutes cardioplegia, then 60 minutes reperfusion. Cardiac output, time to wean from cardiopulmonary bypass, left ventricular (LV) function, caspase‐3, and infarct size were compared. Six animals in the diazoxide group separated from bypass by 30 minutes, whereas only 4 animals in the cardioplegia group separated. Diazoxide was associated with shorter but not significant time to wean from bypass (17.5 versus 27.0 minutes; P=0.13), higher, but not significant, cardiac output during reperfusion (2.9 versus 1.5 L/min at 30 minutes; P=0.05), and significantly higher left ventricular ejection fraction at 30 minutes (42.5 versus 15.8%; P<0.01). Linear mixed regression modeling demonstrated greater left ventricular developed pressure (P<0.01) and maximum change in ventricular pressure during isovolumetric contraction (P<0.01) in the diazoxide group at 30 minutes of reperfusion.
Conclusions
Diazoxide reduces myocardial stunning and facilitates separation from cardiopulmonary bypass in a model that mimics the clinical setting of ongoing myocardial ischemia before revascularization. Diazoxide has the potential to reduce myocardial stunning in the clinical setting.
Keywords: animals; cardiopulmonary bypass; diazoxide; heart arrest, induced; myocardial stunning; swine; ventricular function, left
Subject Categories: Ischemia, Ion Channels/Membrane Transport, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- CPB
cardiopulmonary bypass
- dp/dt max
maximum change in ventricular pressure during isovolumetric contraction
- dp/dt min
maximum change in ventricular pressure during isovolumetric relaxation
- KATP
ATP potassium
- LVDP
left ventricular developed pressure
The underlying mechanism for the cardioprotective action of ATP potassium (KATP) channel openers remains elusive. It has been proposed that diazoxide and other KATP channel openers provide benefit via a mechanism of mitochondrial matrix expansion. 1 , 2 , 3 Opening of a mitochondrial KATP would result in K+ influx from the cytosol into the mitochondria 4 and mitochondrial swelling, suggesting a potential basis for mitochondrial KATP‐dependent changes in mitochondrial and cellular volume secondary to stress. We have shown that diazoxide does not provide cardioprotection via sarcolemmal KATP channels, 5 although it requires the KATP channel subunit sulfonylurea receptor 1 5 and requires the inhibition of succinate dehydrogenase. 6
We have demonstrated that KATP channel openers prevent the detrimental consequences of various stresses in isolated animal and human myocytes. 7 , 8 , 9 , 10 We have uniquely linked myocyte volume with contractility (demonstrating an inverse relationship following stress), and thus identified a cellular etiology (loss of volume homeostasis) as one mechanism of myocardial stunning. 7 , 11 Similarly, we developed isolated mouse heart and intact porcine models of prolonged global ischemia mimicking the clinical situation of global ischemia during cardiac surgery and demonstrated that diazoxide provides additional cardioprotection when added to traditional hyperkalemic cardioplegia versus cardioplegia alone. 12 , 13 Using these models, we have characterized this myocardial protection and demonstrated the potential for KATP channel opener use in humans undergoing cardiac surgery.
The majority of patients who undergo cardiac surgery with cardiopulmonary bypass (CPB) with global ischemia (during cross‐clamp application) exhibit myocardial stunning despite efficient electromechanical arrest. 14 The myocardium is subject to the added stresses of global ischemia with exposure to hypothermic, hyperkalemic cardioplegia (known to independently result in reduced myocyte contractility and function). 15 Myocardial stunning following these insults requires inotropic and mechanical support following cardiac surgery. Pharmacologic KATP channel openers such as diazoxide may have the potential to prevent stunning, improve patient outcomes, and reduce health care costs.
The hypothesis for the current study was that the addition of diazoxide to hypothermic hyperkalemic cardioplegia would reduce myocardial stunning after 30 minutes of left anterior descending artery (LAD) occlusion (regional ischemia), followed by 2 hours of global ischemia compared with cardioplegia alone. The large animal model used uniquely simulates the situation of a patient presenting to the operating room for coronary revascularization with ongoing myocardial ischemia.
Methods
Operative Protocol
The Johns Hopkins Animal Care and Use Committee approved of this study, and animal care was conducted according to their guidelines. Eighteen American Yorkshire swine (38–43 kg) were included: 9 male and 9 female. The operative protocol is represented in Figure 1. Animals were sedated using intramuscular telazol (4.4 mg/kg), ketamine (2.2 mg/kg), and xylazine (2.2 mg/kg) and intubated as previously described. 13 After intubation, anesthesia was maintained with isoflurane. Systemic temperature was monitored with a rectal probe and maintained between 35 and 37 °C by using a warm water blanket.
Figure 1. Operative protocol and time points of data acquisition in a porcine model of 30 minutes of regional ischemia followed by 2 hours of global ischemia.

Animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (N=6) or cardioplegia with diazoxide (N=6). After baseline data acquisition, the LAD was occluded for 30 minutes, cardiopulmonary bypass was then instituted, the aortic cross clamp was placed, and cardioplegia ± diazoxide was administered. During 2 hours of aortic cross‐clamp time, low‐dose hyperkalemic cardioplegia alone was administered every 20 minutes in the retrograde catheter. After 2 hours of global ischemia and removal of the aortic cross clamp and LAD occlusion, efforts to wean from cardiopulmonary bypass were initiated and contractility was assessed throughout 1 hour of reperfusion. CPB indicates cardiopulmonary bypass; CPG, cardioplegia; dp/dt min, maximum change in ventricular pressure during isovolumetric relaxation; dp/dt max, maximum change in ventricular pressure during isovolumetric contraction; LAD, left anterior descending artery; LV, left ventricle; TEE, transesophageal echocardiogram; VF, ventricular fibrillation; and VT, ventricular tachycardia.
The left common carotid artery and internal jugular vein were cannulated for blood pressure monitoring and administration of fluids and medications. After sternotomy, 300 U/kg of heparin was given. Pacing wires were placed in the right atrium for pacing at 95 bpm. After confirming adequate heparinization (>480 activated clotting time [ACT]), the aorta was cannulated. Antegrade and retrograde cardioplegia catheters were inserted in the aorta and in the coronary sinus, respectively, for cardioplegia delivery. The LAD was occluded just distal to the second diagonal branch using a vessel loop and a tourniquet. Adequate occlusion was confirmed by visualization of a change of color in the apex (Figure 2). After 20 minutes of regional ischemia, a dual‐stage venous cannula was placed in the right atrial appendage. After 30 minutes of regional ischemia, CPB was initiated and the aorta was cross clamped for 2 hours. All researchers performing each experiment remained blinded to group allocation. Hypothermic hyperkalemic blood cardioplegia (modified St. Thomas solution, 106 mEq K/L) was delivered in an antegrade (500 mL) and then retrograde fashion (500 mL). Animals were randomly assigned to receive an induction dose of cardioplegia with diazoxide (500 μmol/L) or cardioplegia alone (control group). The dose used was determined by a dose‐response curve in prior work and is the same that has been tested in previous mouse and swine experiments. 12 , 13 , 16 Low‐dose (62 mEq K/L) retrograde hypothermic hyperkalemic cardioplegia (300 mL) was administered every 20 minutes throughout 2 hours of global ischemia in both groups. After 2 hours of ischemia, lidocaine (100 mg) and magnesium sulfate (2.5 mg) were administered, the aortic cross clamp was removed after appropriate deairing maneuvers, and the tourniquet on the LAD was removed. Animals were then weaned off CPB using norepinephrine (0.01–0.40 μg/kg per min), phenylephrine boluses (0.2 mg), or defibrillation as needed. After data collection and 1 hour of reperfusion, potassium was administered intravenously under anesthesia for euthanasia. Cardiectomy was performed, and the left ventricle was sectioned and stained with tetrazolium chloride to assess myocardial viability.
Figure 2. Representative experiment demonstrating the location of the left anterior descending coronary artery occlusion in a porcine model of 30 minutes of regional ischemia followed by 2 hours of global ischemia.
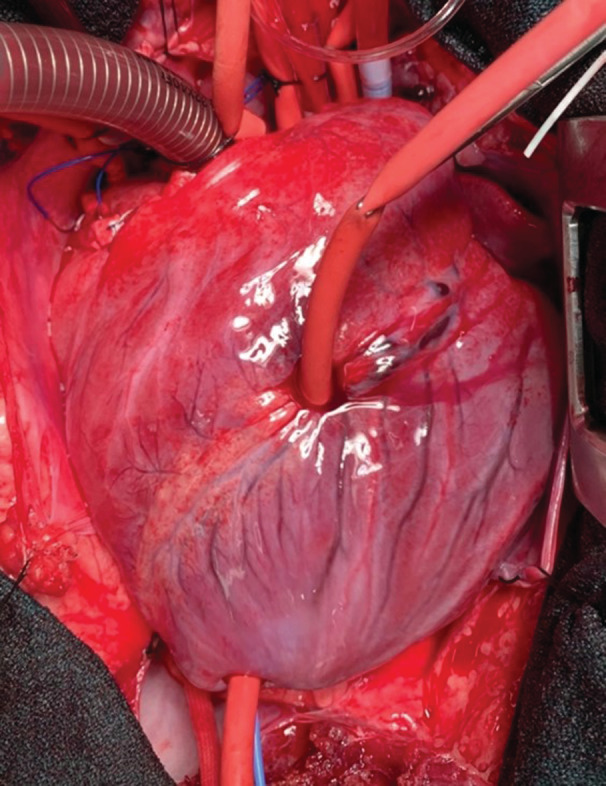
The left anterior descending artery was occluded using a vessel loop and a tourniquet, distal to the second diagonal branch. Adequate occlusion was confirmed by visualization of a change of color in the apex, as demonstrated in this figure. The vessel loop was removed at the time of cross‐clamp removal.
Data Acquisition
Arterial blood gas and electrolytes were monitored every 15 minutes. Coronary sinus samples were taken at baseline and after 15, 30 and 60 minutes of reperfusion. Myocardial oxygen consumption and cardiac output were calculated using the Fick principle. 17 , 18
Intraventricular pressure was measured using a catheter (Millar Inc., Houston, TX), recorded using LabChart Pro (ADInstruments, Sydney, Australia) and were processed with a blood pressure module (MLS370/8, ADInstruments). Left ventricular developed pressure (LVDP), maximum change in ventricular pressure during isovolumetric relaxation (dp/dt min), and maximum change in ventricular pressure during isovolumetric contraction (dp/dt max) were calculated.
Transesophageal echocardiogram was performed by a blinded cardiac anesthesiologist using a transesophageal sonographic system (Vivid E9 XD Clear and 6VT‐D 4D probe, General Electric, Boston, MA). Measurements were recorded at 3 time points: baseline (before LAD occlusion), after 30 minutes of reperfusion, and after 60 minutes of reperfusion. Left ventricular ejection fraction (LVEF), right and left heart dimensions and mitral velocities were recorded. A blinded cardiologist interpreted the echocardiographic readings.
Serum samples were collected throughout the experiment, and levels of myoglobin and troponin I were quantified at baseline and after 15, 30, and 60 minutes of reperfusion. A small biopsy of the left ventricle was taken from the apex at baseline. Subsequent biopsies were taken after 2 hours of global ischemia and after 60 minutes of reperfusion from a region affected by the LAD occlusion (apex) and from a nonaffected region for apoptosis (caspase‐3 measurement). Biopsies were fixed in formalin and immunostained.
Upon completion of each experiment, hearts were excised and irrigated with Krebs‐Hensleit buffer. The left ventricle was excised, weighed, and sectioned into 5 slices from apex to base. The slices were immersed in a tetrazolium chloride buffer at 37 °C for 15 minutes for myocardial viability assessment as previously reported. 13 , 19
Statistical Analysis
Data from experimental groups were compared using unpaired 2‐sided Student's t test or Mann–Whitney rank‐sum test, as appropriate. A mixed linear regression model was conducted on measurements derived from the intraventricular pressure catheter (LVDP, dp/dt min, and dp/dt max). Because of the large variation in pressure measurements within groups, a mixed linear regression model was used to account for these differences. Level of significance was set to P<0.05 for all statistical tests. Analyses were performed using STATA software version 17/SE (StataCorp LLC, College Station, TX).
Results
Ability to Wean From CPB Following 30 Minutes of Regional Ischemia and 2 Hours of Global Ischemia
Six pigs were excluded for the following reasons: presence of severe pericarditis (n=2), technical difficulties (n=3), and incomplete data capture (n=1). Six control swine and 6 diazoxide group swine were included in the study, with an equal distribution of male to female, and a mean weight of 41.8 kg.
Mean systemic temperature during bypass (control, 32.3 °C; diazoxide, 32.4 °C; P=0.58) and upon reperfusion (control, 36.9 °C; diazoxide, 36.5 °C; P=0.42) was similar between groups.
Animals in the diazoxide group required fewer defibrillations after cross clamp removal, although this was not statistically different between groups (mean number of defibrillations: diazoxide, 5.3; control, 7.5; P=0.60; mean number of joules: diazoxide, 122.5; control, 195.8; P=0.49). Animals in the diazoxide group received less phenylephrine (2.76 versus 4.27 mg; P=0.41) and more norepinephrine (635.8 versus 329.6 μg; P=0.11) than the control group, but the differences were not statistically significant.
After 15 minutes of reperfusion, 3 animals in the diazoxide group and 1 animal in the control group were able to wean from CPB. After 30 minutes of reperfusion, all animals in the diazoxide group (n=6) and 4 in the control group weaned from CPB. After 60 minutes, all animals in both groups were off CPB (n=12) (Figure 3). The mean time to wean from CPB in the diazoxide group was less than that in the control group, but not statistically significant (mean, 17.5 minutes; SD, 6.89; SEM, 2.81 versus 27 minutes; SD, 13.92; SEM, 5.08, respectively; P=0.13).
Figure 3. Number of animals that successfully weaned from cardiopulmonary bypass over time.

In a porcine model of 30 minutes of regional ischemia, followed by 2 hours of global ischemia, animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (red line, N=6) or cardioplegia with diazoxide (blue line, N=6). After 15 minutes of reperfusion, only 1 cardioplegia animal and 3 diazoxide animals had successfully weaned from cardiopulmonary bypass. By 60 minutes of reperfusion, all animals successfully weaned from cardiopulmonary bypass. There were no statistically significant differences in mean time to wean from cardiopulmonary bypass between groups using Student's t test. CPB indicates cardiopulmonary bypass; and RP, reperfusion.
Myocardial Function Following 30 Minutes of Regional Ischemia and 2 Hours of Global Ischemia
Cardiac Output
Mean cardiac output was higher with diazoxide but not significantly different between groups at baseline or after 15, 30, and 60 minutes of reperfusion (diazoxide group,1.77, 2.85, 3.19 L/min versus the control group, 0.39, 1.54, 2.46 L/min). There were also no significant differences in percentage change from baseline at any time point. (Table S1, Figure 4).
Figure 4. Cardiac output and percent change in cardiac output throughout reperfusion in a porcine model of 30 minutes of regional ischemia, followed by 2 hours of global ischemia.

Animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (red, N=6) or cardioplegia with diazoxide (blue, N=6). Each dot represents an individual animal. The lower and upper borders of each box represent the lower and upper quartile, respectively. The middle line represents the median, and the cross represents the mean. Cardiac output was calculated using the Fick principle at baseline, before regional and global ischemia, and throughout 1 hour of reperfusion. There were no significant differences in mean cardiac output between groups using Student's t test. A, Cardiac output represented in liters per minute. B, Cardiac output represented as percent change from baseline. RP indicates reperfusion.
Myocardial Oxygen Consumption
Myocardial oxygen consumption was not different between groups at baseline, after 15 or 30 minutes of reperfusion. After 60 minutes of reperfusion, mean myocardial oxygen consumption was higher in the control group compared with diazoxide (4.49 versus 3.03; P=0.03) (Tables S1 through S3). When comparing percentage change from baseline, the difference between groups was not significant (27% versus 39%; P=0.51) (Figure 5).
Figure 5. Myocardial oxygen consumption was significantly higher in the control cardioplegia group after 60 minutes of reperfusion in a porcine model of 30 minutes of regional ischemia, followed by 2 hours of global ischemia.

Animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (red, N=6) or cardioplegia with diazoxide (blue, N=6). Each dot represents an individual animal. The lower and upper borders of each box represent the lower and upper quartile, respectively. The middle line represents the median, and the cross represents the mean. Myocardial oxygen consumption was calculated using the Fick principle at baseline, before localized and global ischemia, and throughout 1 hour of reperfusion. Statistical comparisons using Student's t test. A, Myocardial oxygen consumption represented in mL/min. B, Myocardial oxygen consumption represented as percent change from baseline. RP indicates reperfusion.
Left Ventricular Ejection Fraction
Mean LVEF was not different between groups at baseline. After 30 minutes of reperfusion, LVEF was 27% higher in the diazoxide group compared with the control (42.5% ± 16.05 and 15.8% ±12.42, respectively; P=0.009). By 60 minutes of reperfusion, there was no statistically significant difference in LVEF between groups (Figure 6). There were no statistically significant differences between groups in other transesophageal echocardiography measured parameters (Table S2).
Figure 6. Diazoxide was associated with improved LVEF at 30 minutes of reperfusion in a porcine model of 30 minutes regional ischemia followed by 2 hours global ischemia.

Using a porcine model of 30 minutes of regional ischemia, followed by 2 hours of global ischemia, animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (red, N=6) or cardioplegia with diazoxide (blue, N=6). Each dot represents an individual animal (N=6 in each group). The lower and upper borders of each box represent the lower and upper quartile, respectively. The middle line represents the median, and the cross represents the mean. Left ventricular ejection fraction was measured at baseline, before regional and global ischemia, and throughout 1 hour of reperfusion by transesophageal echocardiography. Statistical comparisons using Student's t test. A, Left ventricular ejection fraction represented as a percentage. B, Left ventricular ejection fraction represented as percent change from baseline. C, LVEF represented over time. LVEF indicates left ventricular ejection fraction; and RP, reperfusion.
Left Ventricular Pressures
Because of catheter malpositioning, only 5 animals in the diazoxide group had 30‐ and 60‐minute data, and only 4 animals in the control group had measurements at 60 minutes of reperfusion. LVDP, dp/dt min, and dp/dt max were not different between groups at baseline. There were no significant differences between groups in the average dp/dt max, dp/dt min, and LVDP at any time point using Student's t tests (Figure 7).
Figure 7. Left ventricular pressures were similar between groups in a porcine model of 30 minutes of regional ischemia, followed by 2 hours of global ischemia.

Animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (red, N=6) or cardioplegia with diazoxide (blue, N=6). A pressure catheter was inserted into the left ventricle, and pressure was monitored at baseline and throughout reperfusion. The lower and upper borders of each box represent the lower and upper quartile, respectively. The middle line represents the median, and the cross represents the mean. Statistical comparisons using Student's t‐test. A, dp/dt min, maximum change in ventricular pressure during isovolumetric relaxation. B, dp/dt max, maximum change in ventricular pressure during isovolumetric contraction. C, left ventricular developed pressure, calculated as the difference between left ventricular systolic pressure and left ventricular diastolic pressure. LV indicates left ventricle; and RP, reperfusion.
Linear mixed regression modeling demonstrated that animals treated with diazoxide had 1026 mm Hg greater dp/dt max and 24 mm Hg greater LVDP after 30 minutes of reperfusion (P=0.001 and 0.008, respectively) compared with controls. There were no significant differences between groups in dp/dt min.
Cardiac Enzymes, Pro‐Apoptotic Markers, and Infarct Size Following 30 Minutes of Regional Ischemia and Subsequent 2 Hours of Global Ischemia
There were no differences in the number of apoptotic cells stained with caspase‐3 between groups at baseline or after reperfusion (Table S3). Mean myoglobin and troponin I levels were within a normal range in both groups at all time points and were not significantly different between groups (Table S3).
Infarct Size
Left ventricular weight at the end of the experiment was not different between groups, with a mean weight of 139.85 g in the diazoxide group and 139.65 g in the control group (P=0.98). Infarct size was not different between groups (diazoxide, 97.11%; control, 97.70%; P=0.83) (Figure 8).
Figure 8. Infarct size in representative sections from apex to base of a heart in the diazoxide group.

In a porcine model of 30 minutes of regional ischemia, followed by 2 hours of global ischemia, animals were randomly assigned to a control group of hyperkalemic cardioplegia alone (N=6) or cardioplegia with diazoxide (N=6). Hearts underwent 30 minutes left anterior descending coronary artery occlusion followed by 2 hours of global ischemia. Upon completion of each experiment, cardiectomy was performed and the left ventricle was sliced into 5 sections. The heart was submerged in a tetrazolium chloride buffer for 15 minutes for myocardial viability assessment. Infarct is visible in the anterior wall, consistent with left anterior descending occlusion. Infarct size was not different between groups.
Discussion
Cardiac surgery is performed with excellent outcomes despite the routine use of hypothermia and electromechanical arrest to provide a quiet and bloodless field. This type of arrest remains the best method known to reduce myocardial oxygen consumption, and it is accomplished using a diverse range of solutions to protect the myocardium during a global ischemic period. 20 Unfortunately, cardiac surgery inflicts this global ischemic episode on an already compromised myocardium. In addition, exposure to hypothermic, hyperkalemic cardioplegia provides an additional insult, as it is known to result in myocyte swelling and reduced contractility in vitro. 1 , 15 It is not surprising that the majority of patients suffer from myocardial stunning after cardiac surgery. 21 , 22 Such stunning results in the need for inotropic and mechanical circulatory support, and novel strategies to reduce myocardial stunning are needed. Encouraged by our previous results using KATP channel opener diazoxide to reduce myocardial stunning in a porcine model of prolonged global ischemia, 13 we hypothesized that diazoxide would reduce myocardial stunning in a porcine model designed to mimic the situation of a patient undergoing cardiac surgery with ongoing myocardial ischemia.
Using a model of 30 minutes of regional (LAD occlusion) followed by 120 minutes of global ischemia, we found no differences in need for defibrillation after cross‐clamp removal or the level of inotropic drugs needed to wean from CPB between the control group (hypothermic hyperkalemic cardioplegia) and the diazoxide group. However, with equivalent inotropic medications, the diazoxide group had higher LVEF, LVDP, and dp/dt max at 30 minutes of reperfusion compared with the control group, suggesting reduced myocardial stunning. The control group had higher myocardial oxygen consumption at 60 minutes, which may be attributable to increased oxygen extraction, although this requires further study.
There were no differences in myoglobin, troponin, pro‐apoptotic markers, or infarct size between groups, likely because of the excellent myocardial protection provided by both groups and because both groups underwent the same regional ischemic episode (LAD occlusion) before CPB and cardioplegia administration. In addition, peak myoglobin and troponin may not have occurred by 60 minutes of reperfusion.
Others have demonstrated that the addition of KATP channel opener diazoxide (50 μmol/L) resulted in reduced regional necrosis and apoptosis and mitochondrial damage using a porcine model of acute infarction followed by 30 minutes of global ischemia. 23 Interestingly, the addition of diazoxide had no effect on systolic and diastolic function. We propose that this is attributable to the smaller dose of diazoxide (50μM) and the shorter global ischemic period (30 minutes) that is not clinically relevant. In a prolonged global ischemia mouse model, we demonstrated superior diastolic protection with hypothermic hyperkalemic cardioplegia with diazoxide compared with cardioplegia alone. 12 In humans, diazoxide demonstrated improved cardiac function and reduced markers of injury compared with controls when given as a preconditioning agent before CPB (not as a component of cardioplegia) 24 or as a component of warm cardioplegia, in 2 small preliminary human trials of otherwise healthy patients with normal LVEF, no concomitant diseases, and only stable angina. 25 However, diazoxide has not been seriously considered as an additive to hypothermic cardioplegia combined with prolonged global ischemia in humans. To provide preclinical data, we designed this clinically relevant porcine model to inform future potential trials in humans. These results suggest that diazoxide may represent a potential pharmacologic method to reduce myocardial stunning after a regional ischemic episode with a subsequent global ischemic period. The exploitation of diazoxide or other KATP channel openers to reduce myocardial stunning following cardiac surgery could provide significant impact as hypothermic hyperkalemic cardioplegia is widely used, patients undergoing cardiac surgery are now older and sicker, and surgeries are more complex and associated with longer global ischemia times. 26 , 27 , 28 , 29 , 30 , 31
Study Limitations
There are several limitations to this study. This study used healthy, young swine with no known comorbidities in contrast with patients undergoing coronary artery bypass grafting who are more likely to be older and have severe coronary artery disease and other comorbidities. Despite the fact that many patients undergo percutaneous intervention for acute ischemia before coronary artery bypass grafting, the current model was created to evaluate the effect of diazoxide administered in a clinically relevant method after a regional ischemic episode (an induction dose of diazoxide in hypothermic, hyperkalemic cardioplegia, followed by subsequent cardioplegia doses without diazoxide, during a long ischemic period). Finally, while the sample size and power calculation is consistent with data from previous swine studies and large animal studies from our laboratory, the study is likely underpowered because of the small number.
Sources of Funding
This work was supported by the American Heart Association Grant‐in‐Aid 16GRNT31170 and philanthropic funding from the Magic That Matters Foundation (J.S.L), the Martin and Vera Kohn Research Fellowship in Cardiac Surgery (A.K.V.) and the Hugh R. Sharp Research Fellowship in Cardiac Surgery (A.K.V.).
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
The authors thank Natalie Elwarner, Randolph Franks, and Chad Wierschke for their perfusion services; Gayane Yenokyan for biostatistics consulting; Rosmi Thomas for the quantification of apoptotic myocytes; Fernanda Carrizo Velazquez and Sam Das for the myoglobin and troponin quantification; and Charles Steenbergen for his assistance with the myocardial viability assessment.
This work was presented at the American Heart Association Scientific Sessions, November 13–15, 2021.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007;292:C157–C163. doi: 10.1152/ajpcell.00272.2006 [DOI] [PubMed] [Google Scholar]
- 2. Olson TM, Terzic A. Human K(ATP) channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010;460:295–306. doi: 10.1007/s00424-009-0771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper PE, Reutter H, Woelfle J, Engels H, Grange DK, Van Haaften G, Van Bon BW, Hoischen A, Nichols CG. Cantú syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat. 2014;35:809–813. doi: 10.1002/humu.22555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noma A. ATP‐regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0 [DOI] [PubMed] [Google Scholar]
- 5. Sellitto AD, Maffit SK, Al‐Dadah AS, Zhang H, Schuessler RB, Nichols CG, Lawton JS. Diazoxide maintenance of myocyte volume and contractility during stress: evidence for a non‐sarcolemmal K(ATP) channel location. J Thorac Cardiovasc Surg. 2010;140:1153–1159. doi: 10.1016/j.jtcvs.2010.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anastacio MM, Kanter EM, Makepeace C, Keith AD, Schuessler RB, Nichols CG, Lawton JS. Cardioprotective mechanism of diazoxide involves the inhibition of succinate dehydrogenase. Ann Thorac Surg. 2013;95:2042–2050. doi: 10.1016/j.athoracsur.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizutani S, Prasad SM, Sellitto AD, Schuessler RB, Damiano RJ, Lawton JS. Myocyte volume and function in response to osmotic stress: observations in the presence of an adenosine triphosphate‐sensitive potassium channel opener. Circulation. 2005;112(9 Suppl):I219–I223. doi: 10.1161/CIRCULATIONAHA.104.523746 [DOI] [PubMed] [Google Scholar]
- 8. Mizutani S, Al‐Dadah AS, Bloch JB, Prasad SM, Diodato MD, Schuessler RB, Damiano RJ Jr, Lawton JS. Hyperkalemic cardioplegia‐induced myocyte swelling and contractile dysfunction: prevention by diazoxide. Ann Thorac Surg. 2006;81:154–159. doi: 10.1016/j.athoracsur.2005.06.057 [DOI] [PubMed] [Google Scholar]
- 9. Maffit SK, Sellitto AD, Al‐Dadah AS, Schuessler RB, Damiano RJ, Lawton JS. Diazoxide maintains human myocyte volume homeostasis during stress. J Am Heart Assoc 2012;1:jah3‐e000778. doi: 10.1161/JAHA.112.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al‐Dadah AS, Voeller RK, Schuessler RB, Damiano RJ, Lawton JS. Maintenance of myocyte volume homeostasis during stress by diazoxide is cardioprotective. Ann Thorac Surg. 2007;84:857–862. doi: 10.1016/j.athoracsur.2007.04.103 [DOI] [PubMed] [Google Scholar]
- 11. Prasad SM, Al‐Dadah AS, Byrd GD, Flagg TP, Gomes J, Damiano RJ Jr, Nichols CG, Lawton JS. Role of the sarcolemmal adenosine triphosphate‐sensitive potassium channel in hyperkalemic cardioplegia‐induced myocyte swelling and reduced contractility. Ann Thorac Surg. 2006;81:148–153. doi: 10.1016/j.athoracsur.2005.06.055 [DOI] [PubMed] [Google Scholar]
- 12. Makepeace CM, Suarez‐Pierre A, Kanter EM, Schuessler RB, Nichols CG, Lawton JS. Superior diastolic function with K ATP channel opener diazoxide in a novel mouse Langendorff model. J Surg Res. 2018;227:186–193. doi: 10.1016/j.jss.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suarez‐Pierre A, Lui C, Zhou X, Kearney S, Jones M, Wang J, Thomas RP, Gaughan N, Metkus TS, Brady MB, et al. Diazoxide preserves myocardial function in a swine model of hypothermic cardioplegic arrest and prolonged global ischemia. J Thorac Cardiovasc Surg. 2022;163:e385–e400. doi: 10.1016/j.jtcvs.2020.08.069 [DOI] [PubMed] [Google Scholar]
- 14. Breisblatt WM, Stein KL, Wolfe CJ, Follansbee WP, Capozzi J, Armitage JM, Hardesty RL. Acute myocardial dysfunction and recovery: a common occurrence after coronary bypass surgery. J Am Coll Cardiol. 1990;15:1261–1269. doi: 10.1016/s0735-1097(10)80011-7 [DOI] [PubMed] [Google Scholar]
- 15. Anastacio MM, Kanter EM, Keith AD, Schuessler RB, Nichols CG, Lawton JS. Inhibition of succinate dehydrogenase by diazoxide is independent of the ATP‐sensitive potassium channel subunit sulfonylurea type 1 receptor. J Am Coll Surg. 2013;216:1144–1149. doi: 10.1016/j.jamcollsurg.2013.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janjua MB, Makepeace CM, Anastacio MM, Schuessler RB, Nichols CG, Lawton JS. Cardioprotective benefits of adenosine triphosphate‐sensitive potassium channel opener diazoxide are lost with administration after the onset of stress in mouse and human myocytes. J Am Coll Surg. 2014;219:803–813. doi: 10.1016/j.jamcollsurg.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Stokoe J, Konstantinov IE, Edgell D, Cheung MMH, Kharbanda RK, Redington AN. Continuous measurement of oxygen consumption during cardiopulmonary bypass: description of the method and in vivo observations. Ann Thorac Surg. 2004;77:1671–1677. doi: 10.1016/j.athoracsur.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 18. Davies G, Hess D, Jebson P. Continuous fick cardiac output compared to continuous pulmonary artery electromagnetic flow measurement in pigs. Anesthesiology. 1987;66:805–809. doi: 10.1097/00000542-198706000-00015 [DOI] [PubMed] [Google Scholar]
- 19. Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, et al. The NHLBI‐Sponsored Consortium for preclinicAl assESsment of cARdioprotective Therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct‐sparing interventions in mice, rabbits, and pigs. Circ Res. 2015;116:572–586. doi: 10.1161/CIRCRESAHA.116.305462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali JM, Miles LF, Abu‐Omar Y, Galhardo C, Falter F. Global cardioplegia practices: results from the global cardiopulmonary bypass survey. J Extra Corpor Technol. 2018;50:83–93. [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen G, Borger MA, Weisel RD, Rao V. Intraoperative myocardial protection: current trends and future perspectives. Ann Thorac Surg. 1999;68:1995–2001. doi: 10.1016/s0003-4975(99)01026-7 [DOI] [PubMed] [Google Scholar]
- 22. Shigematsu S, Sato T, Abe T, Saikawa T, Sakata T, Arita M. Pharmacological evidence for the persistent activation of ATP‐sensitive K+ channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation. 1995;92:2266–2275. doi: 10.1161/01.cir.92.8.2266 [DOI] [PubMed] [Google Scholar]
- 23. Wakiyama H, Cowan DB, Toyoda Y, Federman M, Levitsky S, McCully JD. Selective opening of mitochondrial ATP‐sensitive potassium channels during surgically induced myocardial ischemia decreases necrosis and apoptosis. Eur J Cardiothorac Surg. 2002;21:424–433. doi: 10.1016/s1010-7940(01)01156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deja MA, Malinowski M, Gołba KS, Kajor M, Lebda‐Wyborny T, Hudziak D, Domaradski W, Szurlek D, Bonczyk A, Biernat J, et al. Diazoxide protects myocardial mitochondria, metabolism, and function during cardiac surgery: a double‐blind randomized feasibility study of diazoxide‐supplemented cardioplegia. J Thorac Cardiovasc Surg. 2009;137:997–1004.e2. doi: 10.1016/j.jtcvs.2008.08.068 [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Wei M, Kuukasjärvi P, Laurikka J, Jarvinen O, Rinne T, Liisa E, Tarkka M. Novel pharmacological preconditioning with diazoxide attenuates myocardial stunning in coronary artery bypass grafting. Eur J Cardiothorac Surg. 2003;24:967–973. doi: 10.1016/s1010-7940(03)00438-x [DOI] [PubMed] [Google Scholar]
- 26. Baumgartner WA, Burrows S, del Nido PJ, Gardner TJ, Goldberg S, Gorman RC, Letsou GV, Mascette A, Michler RE, Puskas JD, et al. Recommendations of the National Heart, Lung, and Blood Institute Working Group on future direction in cardiac surgery. Circulation. 2005;111:3007–3013. doi: 10.1161/CIRCULATIONAHA.104.530154 [DOI] [PubMed] [Google Scholar]
- 27. Raza S, Deo SV, Kalra A, Zia A, Altarabsheh SE, Deo VS, Mustafa RR, Younes A, Rao SV, Markowitz AH. Stability after initial decline in coronary revascularization rates in the United States. Ann Thorac Surg. 2019;108:1404–1408. doi: 10.1016/j.athoracsur.2019.03.080 [DOI] [PubMed] [Google Scholar]
- 28. Mariscalco G, Rosato S, Serraino GF, Maselli D, Dalén M, Airaksinen JKE, Reichart D, Zanobini M, Onorati F, De Feo M, et al. Prior percutaneous coronary intervention and mortality in patients undergoing surgical myocardial revascularization: results from the E‐CABG (European Multicenter Study on Coronary Artery Bypass Grafting) with a systematic review and meta‐analysis. Circ Cardiovasc Interv. 2018;11. doi: 10.1161/CIRCINTERVENTIONS.117.005650 [DOI] [PubMed] [Google Scholar]
- 29. O'Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC Jr, Lobdell KW, et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 2‐statistical methods and results. Ann Thorac Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 30. Barakate M. Coronary artery bypass grafting (CABG) after initially successful percutaneous transluminal coronary angioplasty (PTCA): a review of 17 years experience. Eur J Cardio‐Thorac Surg. 2003;23:179–186. doi: 10.1016/s1010-7940(02)00764-9 [DOI] [PubMed] [Google Scholar]
- 31. Taggart DP, Thomas B, Lecture F. Coronary artery bypass grafting is still the best treatment for multivessel and left main disease, but patients need to know. Ann Thorac Surg. 2006;82:1966–1975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


