Abstract
Background
The association between cancer types and specific bleeding events in patients with atrial fibrillation has been scarcely investigated. Also, the performance of bleeding risk scores in this high‐risk subgroup of patients is unclear. We investigated the rate of any bleeding, intracranial hemorrhage, major bleeding, and gastrointestinal bleeding according to cancer types in patients with atrial fibrillation. We also tested the predictive value of HAS‐BLED, ATRIA, and ORBIT bleeding risk scores.
Methods and Results
Observational retrospective cohort study including hospitalized patients with atrial fibrillation and cancer from the French National Hospital Discharge Database (Programme de Medicalisation des Systemes d'Information) from January 2010 to December 2019. Major bleeding was defined according to Bleeding Academic Research Consortium definitions. Patients with HAS‐BLED ≥3, ATRIA ≥5, or ORBIT ≥4 were classified as at high bleeding risk. Receiver operating characteristic analysis for each score against any bleeding, major bleeding, gastrointestinal bleeding, and intracranial hemorrhage was performed. Areas under the curve (AUCs) were then compared. We included 399 344 patients. Mean age was 77.9±10.2 years, and 63.2% were men. The highest intracranial hemorrhage rates were found in leukemia (1.89%/year), myeloma (1.52%/year), lymphoma and liver (1.45%/year), and pancreas cancer (1.41%/year). Receiver operating characteristic analysis showed that ORBIT score predicted best for any bleeding. In addition, ORBIT score ≥4 had the highest predictivity for major bleeding (AUC, 0.805), followed by HAS‐BLED ≥3 and ATRIA ≥5 (AUCs, 0.716 and 0.700, respectively). HAS‐BLED and ORBIT performed best for intracranial hemorrhage (AUCs, 0.744 and 0.742 for continuous scores, respectively), better than ATRIA (AUC, 0.635). For gastrointestinal bleeding, ORBIT ≥4 had the highest predictivity (AUC, 0.756), followed by the HAS‐BLED ≥3 (AUC, 0.702) and ATRIA ≥5 (AUC, 0.662).
Conclusions
Some cancer types carry a greater bleeding risk in patients with atrial fibrillation. The identification and management of modifiable bleeding risk factors is crucial in these patients, as well as to flag up high bleeding risk patients for early review and follow‐up.
Keywords: ATRIA, atrial fibrillation, bleeding, cancer, HAS‐BLED, intracranial hemorrhage, ORBIT
Subject Categories: Atrial Fibrillation, Quality and Outcomes, Risk Factors
Nonstandard Abbreviations and Acronyms
- GB
gastrointestinal bleeding
- ICH
intracranial hemorrhage
- IR
incidence rate
- MB
major bleeding
Clinical Perspective.
What Is New?
Our results provide novel insights on the predictive value of 3 commonly used bleeding risk scores (namely, HAS‐BLED, ORBIT, and ATRIA scores) in patients with atrial fibrillation and cancer, showing that HAS‐BLED and ORBIT performed best for intracranial hemorrhage (areas under the curve, 0.744 and 0.742, respectively), better than ATRIA (area under the curve, 0.635), with similar results for any and major bleedings.
What Are the Clinical Implications?
Some cancer types are associated with an excess risk of major bleeding and intracranial hemorrhage, especially leukemia, myeloma, lymphoma, and liver and pancreas cancer, suggesting that a careful bleeding risk stratification in patients with atrial fibrillation diagnosed with these cancers should be performed to tailor antithrombotic therapy and to reduce the risk of bleeding.
The prevalence of atrial fibrillation (AF) is rapidly increasing with the aging of the general population. 1 Recent data on >8 million people showed that the prevalence of AF in the elderly population, aged ≥65 years, is up to 12.7%. 2 Most elderly patients with AF have multiple cardiovascular comorbidities, 3 accounting for the increased mortality risk associated with this arrhythmia. 4 However, a substantial proportion of deaths in the population with AF is not attributable to the effect of cardiovascular risk factors 5 but relates to noncardiovascular causes, such as renal failure and cancer. 6
Both AF and cancer incidences are aging related, explaining the rapidly increasing proportion of elderly patients with AF surviving cancer and living with both these conditions. 7 , 8 Previous studies showed that the coexistence of cancer complicates the prognosis of patients with AF by increasing all‐cause and cardiovascular mortality. 9 In addition, cancer may increase the risk of major bleeding (MB) and intracranial hemorrhage (ICH) in AF, 10 , 11 , 12 which is associated with an increased risk of mortality. 13 , 14 However, the risks of bleeding may vary according to cancer site, as well as the interplay of nonmodifiable and modifiable bleeding risk factors, but data on this aspect are sparse. In addition, clinical characteristics of patients with cancer and AF experiencing MB, gastrointestinal bleeding (GB), and ICH are not well described.
Another still open issue is the limited validation of existing bleeding risk scores used for patients with AF and no cancer. A recent network metanalysis showed some differences on the predictivity of bleeding risk scores in the general population with AF, 15 whereas an independent systematic review and evidence appraisal found that the HAS‐BLED score had the best predictive value, 16 including for ICH prediction. 17 The appropriate use of bleeding risk scores is to draw attention to modifiable bleeding risk factors for mitigation, as well as to flag up patients with high bleeding risk for early review and follow‐up. 18
In a large cohort of nearly 400 000 patients with AF and cancer, our aim was to investigate the risk of MB, GB, and ICH according to cancer types, and second, to determine the predictive value of 3 commonly used bleeding risk scores: HAS‐BLED, 19 ATRIA, 20 and ORBIT. 21
METHODS
Data are presented as per the Reporting of Studies Conducted Using Observational Routinely Collected Data guidelines. The authors declare that all supporting data are available within the article and its supplemental files. This longitudinal cohort study was based on a national hospitalization database in France covering hospital care across the entire population. In France, each hospital discharge, whether from a public or a private hospital, must be registered in the National Hospital Discharge Database (Programme de Medicalisation des Systemes d'Information). A standardized discharge summary is collected for every hospital stay in France based on the diagnosis and procedures codes, inspired by the US Medicare system. Since 2004, each hospital's budget has been linked to the medical activity described in this specific program, which compiles discharge abstracts related to all admissions for inpatients in the 1546 French health care facilities, and the International Classification of Diseases, Tenth Revision (ICD‐10) is used to code discharge diagnoses. A unique patient identification number makes it possible to link multiple hospital stays corresponding to a single patient without revealing his or her identity. Data for all patients admitted with AF in France from January 2010 to December 2019 were collected from the National Hospital Discharge Database using the annually updated versions of the ICD‐10 for the years 2010 to 2019 (Table S1). All medical procedures are recorded according to the national nomenclature, Classification Commune des Actes Medicaux. The reliability of National Hospital Discharge Database data has already been assessed and used previously to study patients with AF and stroke. 22 The National Hospital Discharge Database does not contain data on anticoagulants.
The medical information contained in the database is anonymous and protected by professional confidentiality. Consequently, ethics review was not required. Patient consent was not sought. The study was conducted retrospectively, patients were not involved in its conduct, and there was no impact on their care. This type of study was approved by the institutional review board of the Pole Coeur Thorax Vaisseaux from the Trousseau University Hospital (Tours, France) on December 1, 2015, and registered as a clinical audit. Procedures for data collection and management were approved by the Conseil National de l'Informatique et des Libertés, the independent national ethics committee protecting human rights in France, which ensures that all information is kept confidential and anonymous (authorization No. 1749007). From January 2010 to December 2019, 2 435 541 adults (aged ≥18 years) were hospitalized with a diagnosis of AF (code I48) as the principal diagnosis (ie, the condition justifying hospital admission), a related diagnosis (ie, potential chronic disease or health state during the hospital stay), or an associated diagnosis (ie, comorbidity or associated complication). For each hospital stay, combined diagnoses at discharge were obtained.
Cancer was defined by the specific ICD‐10 indicating its site; patients may have experienced >1 cancer type during follow‐up. Furthermore, metastatic cancer definition included any primary cancer location in metastatic phase.
Bleeding Risk Score Calculation
The ATRIA bleeding risk was calculated according to the original work by Fang et al 20 as follows: anemia, severe renal disease (eg, dialysis), age ≥75 years, prior bleeding, and hypertension. High bleeding risk was defined as an ATRIA score ≥5. The HAS‐BLED score was developed from the European Heart Survey database, 19 including uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse. Labile international normalized ratio was unavailable, as in most administrative databases using ICD‐10 codes, and it was scored 0 point. Patients were classified at high risk when the HAS‐BLED score was ≥3. The ORBIT score was derived from the ORBIT‐AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) 21 and included older age ≥75 years, anemia, bleeding history, and chronic kidney disease. Treatment with antiplatelet drugs was scored 0 as this information is lacking in the administrative data set.
Use of medication was identified from a 1 of 97 permanent random sample of the complete French nationwide claims database (Echantillon Généraliste de Bénéficiaires: general sample of health care beneficiary, which has been previously used to study patients with AF in France 23 ) for patients with same inclusion criteria as those in the present analysis. Patients were considered to be included in a treatment group if they received a treatment from that class of drugs for ≥60 days within 6 months after enrollment.
Follow‐Up and Definition of Outcomes
The follow‐up started at the date of hospitalization, and all bleeding events occurring during the in‐hospital stay or during follow‐up after discharge were recorded. We defined MB using the Bleeding Academic Research Consortium definitions. 24 Major Bleeding Academic Research Consortium bleeding was defined as bleeding with a reduction in the hemoglobin level of at least 20 g/L, or with transfusion of at least 1 unit of blood, or symptomatic bleeding in a critical area or organ (eg, intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome) or bleeding that causes death.
Any bleeding definition included MB with the addition of other bleeding (see Table S1 for ICD‐10 codes).
Statistical Analysis
Qualitative variables were described using counts and percentages, and continuous quantitative variables were described as mean and SD. Comparisons were made using parametric or nonparametric tests, as appropriate. The Student t test or Wilcoxon rank sum test was used for comparing values between 2 independent groups, and the χ 2 test was used to compare categorical data. Standardized differences were calculated as the difference in means or proportions divided by a pooled estimate of the SD and multiplied by 100. A standardized difference of >10 was consider as significant.
Incidence rates (IRs) with 95% CIs were calculated for bleeding events and by univariable Cox proportional hazards regression models to calculate the relative hazard ratio (HR) and 95% CI for each clinical variable. Different Cox proportional hazards models were used to identify independent characteristics associated with the occurrence of each clinical outcome.
Nonparametric receiver operating characteristic (ROC) curves were constructed, and Harrell C indexes (ie, area under the curve [AUC]) were calculated to investigate the predictive value of HAS‐BLED, ATRIA, and ORBIT scores. The ROC curve was plotted by computing the sensitivity and specificity using each value of the rating variable as a possible cut point (the cutoffs of HAS‐BLED score ≥3 [versus <3], ATRIA score ≥5 [versus <5], and ORBIT score ≥4 [versus <4] were used on the basis of the original derivation work of each score). The ROC curves were then compared using the DeLong test.
Decision‐curve analysis was used to quantify the clinical usefulness of the prediction models according to previously reported methods. 25 , 26
In all analyses, P values are 2 sided, and P<0.05 was considered statistically significant. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc, Cary, NC) and STATA 16.0 (Stata Corp, College Station, TX).
RESULTS
Characteristics of the Study Population
Mean age was 77.9±10.2 years, and 63.2% were men. Clinical characteristics of the study cohort are reported in Table 1.
Table 1.
Characteristics of Patients With AF and Cancer Experiencing Different Bleeding Type
| Characteristic | All patients (n=399 344) | Any bleeding | Standardized difference | Major bleeding | Standardized difference | ICH | Standardized difference | GB | Standardized difference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | ||||||
| (n=273 949) | (n=125 395) | (n=356 087) | (n=43 257) | (n=391 800) | (n=7544) | (n=379 319) | (n=20 025) | ||||||
| Age, y | 77.9±10.2 | 77.8±10.3 | 78.0±9.9 | 2.0 | 77.9±10.3 | 77.4±9.5 | −4.9 | 77.8±10.2 | 78.9±9.0 | 11.1 | 77.9±10.2 | 77.7±9.3 | −2.2 |
| Sex, men | 252 300 (63.2) | 165 965 (60.6) | 86 335 (68.9) | 17.4 | 221 927 (62.3) | 30 373 (70.2) | 16.7 | 247 350 (63.1) | 4950 (65.6) | 5.2 | 238 543 (62.9) | 13 757 (68.7) | 12.3 |
| CHA2DS2VASc score | 3.4±1.5 | 3.4±1.5 | 3.6±1.5 | 14.9 | 3.4±1.5 | 3.5±1.5 | 3.1 | 3.4±1.5 | 3.6±1.5 | 11.1 | 3.4±1.5 | 3.5±1.5 | 2.4 |
| Hypertension | 255 733 (64.0) | 168 692 (61.6) | 87 041 (69.4) | 16.5 | 226 397 (63.6) | 29 336 (67.8) | 8.9 | 250 679 (64.0) | 5054 (67.0) | 6.3 | 242 285 (63.9) | 13 448 (67.2) | 6.9 |
| Diabetes | 89 768 (22.5) | 58 058 (21.2) | 31 710 (25.3) | 9.7 | 78 661 (22.1) | 11 107 (25.7) | 8.4 | 87 906 (22.4) | 1862 (24.7) | 5.3 | 84 763 (22.3) | 5005 (25.0) | 6.2 |
| Heart failure | 114 243 (28.6) | 89 893 (32.8) | 49 397 (39.4) | 13.7 | 123 523 (34.7) | 15 767 (36.4) | 3.7 | 136 749 (34.9) | 2541 (33.7) | −2.6 | 132 067 (34.8) | 7223 (36.1) | 2.6 |
| Dilated cardiomyopathy | 23 910 (6.0) | 15 415 (5.6) | 8495 (6.8) | 4.8 | 21 095 (5.9) | 2815 (6.5) | 2.4 | 23 455 (6.0) | 455 (6.0) | 0.2 | 22 616 (6.0) | 1294 (6.5) | 2.1 |
| Coronary artery disease | 98 260 (24.6) | 60 498 (22.1) | 37 762 (30.1) | 18.4 | 85 874 (24.1) | 12 386 (28.6) | 10.3 | 96 352 (24.6) | 1908 (25.3) | 1.6 | 92 744 (24.5) | 5516 (27.5) | 7.1 |
| Previous myocardial infarction | 17 643 (4.4) | 10 556 (3.9) | 7087 (5.7) | 8.5 | 15 613 (4.4) | 2030 (4.7) | 1.5 | 17 361 (4.4) | 282 (3.7) | −3.5 | 16 793 (4.4) | 850 (4.2) | −0.9 |
| Previous PCI | 16 692 (4.2) | 9716 (3.5) | 6976 (5.6) | 9.7 | 14 619 (4.1) | 2073 (4.8) | 3.3 | 16 392 (4.2) | 300 (4.0) | −1.0 | 15 819 (4.2) | 873 (4.4) | 0.9 |
| Previous CABG | 1965 (0.5) | 7456 (2.7) | 6139 (4.9) | 11.4 | 11 553 (3.2) | 2042 (4.7) | 7.6 | 13 308 (3.4) | 287 (3.8) | 2.2 | 12 723 (3.4) | 872 (4.4) | 5.2 |
| Vascular disease | 77 224 (19.3) | 46 334 (16.9) | 30 890 (24.6) | 19.1 | 67 478 (18.9) | 9746 (22.5) | 8.8 | 75 774 (19.3) | 1450 (19.2) | −0.3 | 72 987 (19.2) | 4237 (21.2) | 4.8 |
| Dyslipidemia | 95 820 (24.0) | 60 946 (22.2) | 34 874 (27.8) | 4.5 | 84 273 (23.7) | 11 547 (26.7) | 7.0 | 93 950 (24.0) | 1870 (24.8) | 1.9 | 90 626 (23.9) | 5194 (25.9) | 4.7 |
| Ischemic stroke | 22 593 (5.7) | 14 597 (5.3) | 7996 (6.4) | 4.5 | 20 382 (5.7) | 2211 (5.1) | −2.7 | 22 001 (5.6) | 592 (7.8) | 8.9 | 21 634 (5.7) | 959 (4.8) | −4.1 |
| Previous ICH | 7948 (2.0) | 0 (0.0) | 7948 (6.3) | 36.8 | 6620 (1.9) | 1328 (3.1) | 7.8 | 7948 (2.0) | 0 (0.0) | −20.3 | 7686 (2.0) | 262 (1.3) | −5.6 |
| Lung disease | 87 569 (21.9) | 56 481 (20.6) | 31 088 (24.8) | 10.0 | 78 476 (22.0) | 9093 (21.0) | −2.5 | 86 266 (22.0) | 1303 (17.3) | −12.0 | 83 380 (22.0) | 4189 (20.9) | −2.6 |
| COPD | 55 891 (14.0) | 35 707 (13.0) | 20 184 (16.1) | 8.7 | 49 775 (14.0) | 6116 (14.1) | 0.5 | 55 102 (14.1) | 789 (10.5) | −11.0 | 53 061 (14.0) | 2830 (14.1) | 0.4 |
| Liver disease | 23 265 (5.8) | 13 435 (4.9) | 9830 (7.8) | 12.0 | 20 478 (5.8) | 2787 (6.4) | 2.9 | 22 893 (5.8) | 372 (4.9) | −4.0 | 21 966 (5.8) | 1299 (6.5) | 2.9 |
| Thyroid diseases | 39 955 (10.0) | 27 088 (9.9) | 12 867 (10.3) | 1.2 | 35 844 (10.1) | 4111 (9.5) | −1.9 | 39 217 (10.0) | 738 (9.8) | −0.8 | 38 028 (10.0) | 1927 (9.6) | −1.4 |
| Inflammatory disease | 30 925 (7.7) | 18 506 (6.8) | 12 419 (9.9) | 11.4 | 27 204 (7.6) | 3721 (8.6) | 3.5 | 30 433 (7.8) | 492 (6.5) | −4.8 | 29 318 (7.7) | 1607 (8.0) | 1.1 |
| Cognitive impairment | 33 205 (8.3) | 22 416 (8.2) | 10 789 (8.6) | 1.5 | 30 697 (8.6) | 2508 (5.8) | −10.9 | 32 580 (8.3) | 625 (8.3) | −0.1 | 32 063 (8.5) | 1142 (5.7) | −10.7 |
| Abnormal renal function | 35 344 (8.9) | 19 855 (7.2) | 15 489 (12.4) | 17.2 | 30 544 (8.6) | 4800 (11.1) | 8.5 | 34 653 (8.8) | 691 (9.2) | 1.1 | 33 374 (8.8) | 1970 (9.8) | 3.6 |
| Modifiable risk factors | |||||||||||||
| Smoker | 41 307 (10.3) | 26 117 (9.5) | 15 190 (12.1) | 8.3 | 36 788 (10.3) | 4519 (10.4) | 0.4 | 40 712 (10.4) | 595 (7.9) | −8.7 | 39 386 (10.4) | 1921 (9.6) | −2.6 |
| Obesity | 60 134 (15.1) | 38 312 (14.0) | 21 822 (17.4) | 9.4 | 52 982 (14.9) | 7152 (16.5) | 4.5 | 59 088 (15.1) | 1046 (13.9) | −3.5 | 56 831 (15.0) | 3303 (16.5) | 4.2 |
| Alcohol‐related diagnoses | 27 438 (6.9) | 16 316 (6.0) | 11 122 (8.9) | 11.1 | 23 986 (6.7) | 3452 (8.0) | 4.8 | 26 976 (6.9) | 462 (6.1) | −3.1 | 25 825 (6.8) | 1613 (8.1) | 4.8 |
| Sleep apnea syndrome | 22 752 (5.7) | 14 456 (5.3) | 8296 (6.6) | 5.7 | 20 037 (5.6) | 2715 (6.3) | 2.7 | 22 326 (5.7) | 426 (5.6) | −0.2 | 21 496 (5.7) | 1256 (6.3) | 2.6 |
| Anemia | 113 132 (28.3) | 63 363 (23.1) | 65 535 (52.3) | 63.0 | 108 026 (30.3) | 20 872 (48.3) | 37.3 | 126 743 (32.3) | 2155 (28.6) | −8.2 | 121 047 (31.9) | 7851 (39.2) | 15.3 |
| Denutrition | 59 919 (15.0) | 37 815 (13.8) | 22 104 (17.6) | 10.5 | 54 524 (15.3) | 5395 (12.5) | −8.2 | 59 094 (15.1) | 825 (10.9) | −12.4 | 57 544 (15.2) | 2375 (11.9) | −9.7 |
| Bleeding risk scores | |||||||||||||
| HAS‐BLED score | 2.5±1.2 | 2.1±1.0 | 3.4±1.0 | 127.5 | 2.4±1.1 | 3.6±1.0 | 109.0 | 2.5±1.2 | 3.5±1.0 | 95.3 | 2.5±1.1 | 3.5±1.0 | 98.7 |
| HAS‐BLED score ≥3 | 195 516 (49.0) | 90 653 (33.1) | 104 863 (83.6) | 119.4 | 157 667 (44.3) | 37 849 (87.5) | 102.4 | 188 888 (48.2) | 6628 (87.9) | 93.9 | 178 044 (46.9) | 17 472 (87.3) | 95.0 |
| ATRIA score | 4.6±2.7 | 3.8±2.5 | 6.3±2.5 | 101.8 | 4.3±2.6 | 7.0±2.3 | 107.6 | 4.6±2.7 | 5.8±2.5 | 46.3 | 4.5±2.7 | 6.7±2.5 | 84.9 |
| ATRIA score ≥5 | 186 308 (46.7) | 97 184 (35.5) | 89 124 (71.1) | 76.4 | 150 725 (42.3) | 35 583 (82.3) | 90.4 | 181 860 (46.4) | 4448 (59.0) | 25.3 | 170 800 (45.0) | 15 508 (77.4) | 70.5 |
| ORBIT score | 2.6±1.8 | 1.7±1.3 | 4.4±1.2 | 213.5 | 2.3±1.7 | 4.8±1.1 | 174.2 | 2.5±1.8 | 4.0±1.2 | 97.2 | 2.5±1.8 | 4.6±1.2 | 142.6 |
| ORBIT score ≥4 | 122 960 (30.8) | 30 576 (11.2) | 92 384 (73.7) | 163.3 | 86 082 (24.2) | 36 878 (85.3) | 155.4 | 118 552 (30.3) | 4408 (58.4) | 59.1 | 107 072 (28.2) | 15 888 (79.3) | 119.4 |
AF indicates atrial fibrillation; ATRIA, anemia, severe renal disease (eg, dialysis), age ≥75 years, prior bleeding, and hypertension; CABG, coronary artery bypass grafting; CHA2DS2‐VASc congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 and sex category (female); COPD, chronic obstructive pulmonary disease; GB, gastrointestinal bleeding; HAS‐BLED, uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse; ICH, intracranial hemorrhage; ORBIT, older age ≥75 years, anemia, bleeding history, chronic kidney disease, and treatment with antiplatelet drugs; and PCI, percutaneous coronary intervention.
Overall, 43 257 patients had an MB event: of these, there were 7544 ICH events and 20 025 GB events (Table 1). Table 1 shows clinical characteristics of patients who experienced a bleeding event. Patients with bleeding of any type were more commonly men, had hypertension, had diabetes, and had abnormal renal function (Table 1). Patients with ICH were older than those without, whereas those with MB or GB tended to be younger compared with patients without bleeding. The prevalence of heart failure, liver disease, and anemia was higher in patients with MB and GB but lower in the ICH group.
In a random sample of 26 046 patients from the Echantillon Généraliste de Bénéficiaires, 8987 (34.0%) were treated with vitamin K antagonists, and 5115 (19.4%) were treated with a direct oral anticoagulant (Table S2). Patients with AF and cancer had a lower use of anticoagulant drugs compared with those without (22.5% versus 36.1% [P<0.0001] for vitamin K antagonists; 14.5% versus 20.2% [P<0.0001] for direct oral anticoagulants).
Modifiable Bleeding Risk Factors
Patients with ICH had a lower prevalence of almost all modifiable risk factors, including obesity, alcohol, anemia, and denutrition (Table 1). Conversely, patients with MB and GB had a higher prevalence of dyslipidemia, obesity, alcohol use, sleep apnea, and anemia (Table 1). Patients with GB had a lower prevalence of smoking and denutrition than those who did not bleed (Table 1).
Incidence of Bleeding According to Cancer Location
During a mean follow‐up of 2.0 years, the IR of any bleeding was 25.60%/year, MB was 8.41%/year, GB was 3.61%/year, and ICH was 1.33%/year. Figure 1 left top panel shows the IR for any bleeding according to cancer site (numbers are reported in Table S3); cancers at highest risk were liver (44.64%/year), pancreas (41.3%/year), bladder (40.78%/year), gastric (40.69%/year), and metastatic (40.24%/year).
Figure 1. Incidence rates (and 95% CIs) of any bleeding (left top panel), major bleeding (right top panel), GI bleeding (right lower panel), and ICH (left lower panel), according to the cancer site. GI indicates gastrointestinal; and ICH, intracranial hemorrhage.
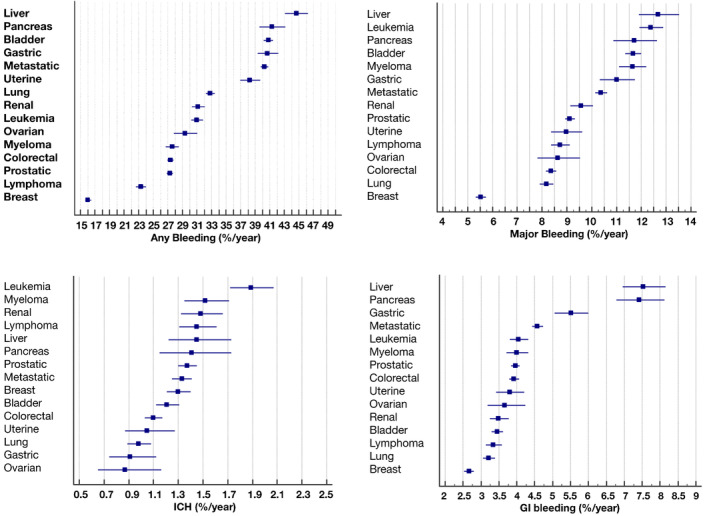
MB (Figure 1 right top panel) was more frequent in liver (12.68%/year), leukemia (12.39%/year), pancreas (11.71%/year), bladder (11.67%/year), and myeloma (11.64%/year). The highest IR of ICH (Figure 1 left lower panel) was found in leukemia (1.89%/year), myeloma (1.52%/year), lymphoma and liver (1.45%/year), and pancreas cancer (1.41%/year). The IR of GB (Figure 1 right lower panel) was highest in liver (7.54%/year), pancreas (7.42%/year), and gastric (5.51%/year) cancer.
Performance of Bleeding Risk Scores
All 3 bleeding risk scores were higher in patients experiencing a bleeding event (Table 1). Although the proportion of patients categorized as “high risk” was similar for the 3 scores for MB, this figure was significantly higher for the HAS‐BLED score in patients with ICH (87.9% compared with 59.0% for ATRIA and 58.4% for ORBIT) and GB (87.3% compared with 77.4% for ATRIA and 79.3% for ORBIT) (Table 1).
Intracranial Hemorrhage
The high‐risk HAS‐BLED score category had the strongest association with ICH (HR, 5.803) when compared with both ATRIA and ORBIT scores (Table 2). ROC curve analysis confirmed that HAS‐BLED score had the highest predictive value (AUC, 0.698) (Table 3). Comparison of AUCs confirmed a better predictive value of the HAS‐BLED score over the ORBIT and ATRIA scores (P<0.0001 for all comparisons) (Table 4). We then investigated the association of HAS‐BLED score with ICH, according to each cancer type (Table 5). The AUC for the HAS‐BLED was generally good and >0.70 in all cancer types for continuous values, whereas it was slightly lower when we used high‐risk categorization (HAS‐BLED ≥3) for liver, bladder, and renal cancers (Table 5).
Table 2.
Univariable Cox Regression Analysis for HAS‐BLED, ATRIA, and ORBIT Scores for Bleeding Outcomes in Patients With AF and Cancer
| Variable | Hazard ratio (95% CI) | |||
|---|---|---|---|---|
| Any bleeding | Major bleeding | ICH | GB | |
| HAS‐BLED score (for each point) | 1.82 (1.81–1.83) | 1.98 (1.97–2.00) | 1.78 (1.75–1.82) | 1.84 (1.82–1.86) |
| HAS‐BLED score ≥3 (vs <3) | 5.00 (4.93–5.08) | 6.58 (6.39–6.77) | 5.80 (5.42–6.22) | 5.74 (5.50–5.98) |
| ATRIA score (for each point) | 1.25 (1.25–1.26) | 1.37 (1.37–1.38) | 1.13 (1.12–1.14) | 1.28 (1.27–1.28) |
| ATRIA score ≥5 (vs <5) | 2.88 (2.84–2.91) | 5.37 (5.24–5.51) | 1.47 (1.40–1.54) | 3.62 (3.50–3.74) |
| ORBIT score (for each point) | 1.86 (1.85–1.86) | 2.15 (2.14–2.17) | 1.47 (1.45–1.49) | 1.86 (1.84–1.88) |
| ORBIT score ≥4 (vs <4) | 6.70 (6.62–6.79) | 13.33 (12.98–13.69) | 2.58 (2.46–2.70) | 7.45 (7.20–7.71) |
AF indicates atrial fibrillation; ATRIA, anemia, severe renal disease (eg, dialysis), age ≥75 years, prior bleeding, and hypertension; GB, gastrointestinal bleeding; HAS‐BLED, uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse; ICH, intracranial hemorrhage; and ORBIT, older age ≥75 years, anemia, bleeding history, chronic kidney disease, and treatment with antiplatelet drugs.
Table 3.
ROC Curves for Different Outcomes in Patients With AF and Cancer
| Area under the curve (95% CI) | ||||
|---|---|---|---|---|
| Variable | Any bleeding | Major bleeding | ICH | GB |
| HAS‐BLED score (continuous) | 0.809 (0.808–0.810) | 0.774 (0.772–0.776) | 0.744 (0.740–0.748) | 0.752 (0.749–0.755) |
| HAS‐BLED score ≥3 | 0.753 (0.751–0.754) | 0.716 (0.714–0.718) | 0.698 (0.694–0.702) | 0.702 (0.699–0.704) |
| ATRIA score (continuous) | 0.768 (0.766–0.769) | 0.777 (0.774–0.779) | 0.635 (0.629–0.641) | 0.728 (0.725–0.731) |
| ATRIA score ≥5 | 0.678 (0.676–0.680) | 0.700 (0.698–0.702) | 0.563 (0.557–0.568) | 0.662 (0.659–0.665) |
| ORBIT score (continuous) | 0.918 (0.917–0.918) | 0.870 (0.869–0.871) | 0.742 (0.738–0.745) | 0.825 (0.822–0.827) |
| ORBIT score ≥4 | 0.813 (0.811–0.814) | 0.805 (0.804–0.807) | 0.641 (0.635–0.646) | 0.756 (0.753–0.758) |
AF indicates atrial fibrillation; ATRIA, anemia, severe renal disease (eg, dialysis), age ≥75 years, prior bleeding, and hypertension; GB, gastrointestinal bleeding; HAS‐BLED, uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse; ICH, intracranial hemorrhage; ORBIT, older age ≥75 years, anemia, bleeding history, chronic kidney disease, and treatment with antiplatelet drugs; and ROC, receiver operating characteristic.
Table 4.
Comparison of AUCs for Bleeding Risk Scores
| Variable | Any bleeding | P value | Major bleeding | P value | ICH | P value | GB | P value |
|---|---|---|---|---|---|---|---|---|
| AUC difference (95% CI) | AUC difference (95% CI) | AUC difference (95% CI) | AUC difference (95% CI) | |||||
| HAS‐BLED score ≥3 vs ATRIA score ≥5 | 0.075 (0.073 to 0.077) | <0.0001 | 0.016 (0.014 to 0.018) | <0.0001 | 0.136 (0.133 to 0.138) | <0.0001 | 0.040 (0.037 to 0.042) | <0.0001 |
| HAS‐BLED score ≥3 vs ORBIT score ≥4 | −0.060 (−0.062 to 0.058) | <0.0001 | −0.089 (−0.091 to 0.087) | <0.0001 | 0.057 (0.055 to 0.059) | <0.0001 | −0.054 (−0.056 to 0.052) | <0.0001 |
| ATRIA score ≥5 vs ORBIT score ≥4 | −0.135 (−0.136 to 0.133) | <0.0001 | −0.106 (−0.108 to 0.104) | <0.0001 | −0.078 (−0.080 to 0.076) | <0.0001 | −0.094 (−0.095 to 0.092) | <0.0001 |
ATRIA indicates anemia, severe renal disease (eg, dialysis), age ≥75 years, prior bleeding, and hypertension; AUC, area under the curve; GB, gastrointestinal bleeding; HAS‐BLED, uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse; ICH, intracranial hemorrhage; and ORBIT, older age ≥75 years, anemia, bleeding history, chronic kidney disease, and treatment with antiplatelet drugs.
Table 5.
C‐Statistics for HAS‐BLED Score Against ICH, According to Each Cancer Type
| Type of cancer | No. of patients (descending order) | C‐statistic (95% CI) for ICH | |
|---|---|---|---|
| HAS‐BLED score continuous | HAS‐BLED score ≥3 | ||
| All cancers | 399 344 | 0.74 (0.74–0.75) | 0.70 (0.69–0.70) |
| Metastatic cancer | 97 606 | 0.75 (0.73–0.76) | 0.71 (0.69–0.72) |
| Prostatic cancer | 62 710 | 0.72 (0.71–0.73) | 0.67 (0.67–0.68) |
| Colorectal cancer | 54 755 | 0.74 (0.73–0.75) | 0.69 (0.68–0.70) |
| Lung cancer | 49 737 | 0.75 (0.73–0.76) | 0.70 (0.68–0.72) |
| Breast cancer | 38 699 | 0.80 (0.79–0.81) | 0.75 (0.74–0.76) |
| Bladder cancer | 32 342 | 0.69 (0.67–0.71) | 0.64 (0.63–0.65) |
| Leukemia | 19 148 | 0.70 (0.68–0.72) | 0.66 (0.65–0.68) |
| Lymphoma | 17 986 | 0.74 (0.72–0.76) | 0.71 (0.69–0.72) |
| Renal cancer | 13 294 | 0.71 (0.68–0.74) | 0.64 (0.63–0.66) |
| Myeloma | 13 081 | 0.71 (0.69–0.74) | 0.67 (0.64–0.69) |
| Liver cancer | 9261 | 0.69 (0.65–0.73) | 0.61 (0.59–0.63) |
| Gastric cancer | 8470 | 0.75 (0.71–0.79) | 0.70 (0.67–0.74) |
| Pancreas cancer | 8409 | 0.78 (0.75–0.82) | 0.74 (0.71–0.77) |
| Uterine cancer | 7670 | 0.74 (0.70–0.78) | 0.69 (0.66–0.72) |
| Ovarian cancer | 4494 | 0.79 (0.73–0.84) | 0.73 (0.68–0.78) |
HAS‐BLED indicates uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse; and ICH, intracranial hemorrhage.
Major Bleeding
The ORBIT score showed the strongest association with MB, with an HR of 13.326 for the high‐risk group, followed by HAS‐BLED and ATRIA (Table 2). After the ORBIT, HAS‐BLED score had the second highest HR of 6.575 (Table 2). ROC curve analysis showed that all 3 scores had good predictive values (AUC, >0.7), with the highest AUC for the high‐risk ORBIT score category (AUC, 0.805) (Table 3). Comparison of AUCs for bleeding risk scores showed a stepwise improvement in performance when ATRIA was compared with HAS‐BLED, and then ORBIT (Table 4).
Gastrointestinal Bleeding
HAS‐BLED and ORBIT scores showed a similar association with GB and were analyzed as continuous values, but the high‐risk ORBIT score category was strongly associated with GB (Table 2). ROC curve analysis showed that all 3 scores had good predictive values (AUC, >0.7), with high‐risk ORBIT score having the highest predictive value (AUC, 0.756) (Table 3). Comparison of AUCs for bleeding risk scores showed a stepwise improvement in performance when ATRIA was compared with HAS‐BLED, and then ORBIT (Table 4).
Any Bleeding
All 3 scores were associated with the “any bleeding” outcome, for each point and when categorized as “high risk” (Table 2). ROC curve analysis showed that HAS‐BLED and ORBIT scores had good predictive values (AUC, >0.7) (Table 3). Comparison of AUCs for bleeding risk scores showed a stepwise improvement in performance when ATRIA was compared with HAS‐BLED, and then ORBIT (Table 4). Decision‐curve analysis (Figure 2) showed an overall clinical benefit of using ORBIT score for the prediction of any bleeding.
Figure 2. Performances of scores in predicting bleeding events with decision‐curve analysis: net number of true positives gained using different models compared with no model at a range of threshold probabilities.
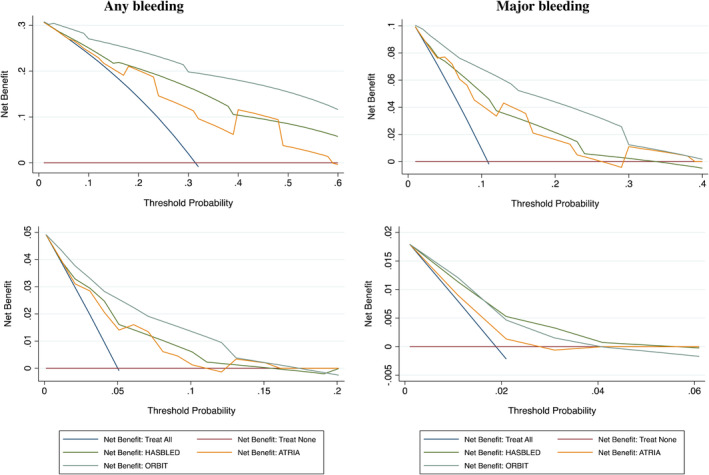
Right top panel: any bleeding; left top panel: major bleeding; right lower panel: gastrointestinal bleeding; left lower panel: intracranial hemorrhage. ATRIA indicates anemia, severe renal disease (eg, dialysis), age ≥75 years, prior bleeding, and hypertension; HAS‐BLED, uncontrolled hypertension (systolic blood pressure >160 mm Hg), abnormal kidney (dialysis or transplant)/liver function (ie, cirrhosis), previous stroke, bleeding history or predisposition, elderly age (≥65 years), and drug (antiplatelet, nonsteroidal anti‐inflammatory drugs)/alcohol abuse; and ORBIT, older age ≥75 years, anemia, bleeding history, chronic kidney disease, and treatment with antiplatelet drugs.
DISCUSSION
This is the first large cohort study investigating the incidence of different bleeding complications in a large sample of patients with AF and cancer, according to cancer location. We also tested in this high‐risk group of patients the performance of 3 bleeding risk scores commonly used in the population with AF. We found that the risks of MB, GB, and ICH were considerably increased in some types of cancers, despite a lower use of anticoagulant drugs. We also found that the predictive value of the HAS‐BLED, ORBIT, and ATRIA scores varied according to the outcome considered.
The analysis of clinical characteristics of patients with AF and cancer showed some important differences according to the type of bleeding. Indeed, bleeding rates varied by cancer type, which implies that cancer is not a yes/no diagnosis (or statistical adjustment in some studies) 10 , 11 , 27 when determining the potential for serious bleeding during clinical evaluation or risk prediction. Also, although patients with MB and GB shared some clinical features, such as a high prevalence of heart failure, liver disease, and anemia and a relatively younger age, these factors were conversely less prevalent in those experiencing ICH, who were also older than patients without ICH. In particular, patients with ICH had a lower prevalence of modifiable risk factors, implying that bleeding risk in these patients is more difficult to address and may be driven by other factors, such as cerebral amyloid angiopathy, moyamoya disease, and coagulopathies.
The rate of ICH, which is the most serious and disabling bleeding complication in patients with AF, reported in the present study is remarkably higher than that previously observed in patients with AF and without cancer. Thus, in the ROCKET‐AF (Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), the rate of ICH was 0.67%/year. 28 Similarly, in the warfarin arm of the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulant Therapy) trial, the rate of ICH was 0.76%/year and was reduced to 0.22%/year in patients on dabigatran. 29 This incidence was a bit higher in a real‐world study, reaching 0.8%/year. 30 In our study, we found that the risk of ICH exceeded >1%/year in several types of cancers, with those at highest risk (>1.4%/year) having leukemia, myeloma, renal, lymphoma, liver, and pancreas cancers. The choice of using or not using oral anticoagulation in these patients should be carefully evaluated as it may not give a clear benefit compared with the risk of bleeding and bleeding‐related complications.
The analysis of the incidence of non‐ICH bleeding according to the cancer location identified some types of cancer showing a disproportionally high risk of bleeding. Above all, liver cancer was associated with an increased risk of any bleeding, MB, and GB, followed by pancreas, bladder, gastric, and hematological cancers. The risk of bleeding in these cancers even exceeded that observed in the pooled group of metastatic cancers of any origin. Conversely, breast and lung cancer showed the lowest risk of MB and GB. This finding, weighted with an increased risk of ischemic stroke in patients with breast 9 (IR, 2.6%/year) and lung cancer,31 may warrant the need for anticoagulant therapy in these patients.
Another novel finding of our study relies on the investigation of the predictive value of 3 commonly used bleeding risk scores, such as the HAS‐BLED, ORBIT, and ATRIA scores, in patients with AF and cancer. Despite recent guidelines that suggested a risk factor–based approach to the evaluation of bleeding risk in the population with AF, mainly aimed at identifying modifiable risk factors, 32 the use of scores may be helpful for the rapid identification of patients at higher bleeding risk, but more important, to address modifiable bleeding risk factors for mitigation, as well as to flag up patients with high bleeding risk for early review and follow‐up. 33 In this context, we found that a high proportion of patients with AF and cancer are classified at high risk of bleeding according to risk scores: 49.0% with the HAS‐BLED, 46.7% with the ATRIA, and 30.8% with the ORBIT. These figures are much higher compared with patients with AF and no cancer, such as 36%, 14%, and 7%, for the 3 scores, respectively, in a large cohort of patients with similar age and clinical characteristics. 34 In particular, the HAS‐BLED score categorized more patients as high risk, especially for ICH, compared with ORBIT and ATRIA scores, which is in keeping with a previous report in patients without cancer. 35 This finding suggests an increased bleeding risk conferred by the presence of cancer.
All the 3 scores showed a modest predictive value for ICH, with C indexes of nearly 0.70. Among all, the HAS‐BLED score performed better than others for ICH prediction (the principal and most serious bleeding outcome), whereas the ATRIA score showed unsatisfactory discriminative value. Of note, the AUC of the HAS‐BLED score was 0.698, which is higher than that reported in noncancer patients with AF (C index, 0.64). 34 , 35 When analyzed according to cancer type, the HAS‐BLED score had broadly similar predictive value for ICH (≈0.7). The association between HAS‐BLED score and ICH overall is in keeping with a previous study in noncancer patients with AF from the AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) trial that showed a C‐index of 0.75. 17 For non‐ICH bleeding events, the ORBIT score showed the best predictivity for MB and GB (C indexes of 0.805 and 0.756, respectively). These statistical differences need to be put into clinical and practical perspectives, whereby bleeding risk scores need to first address modifiable bleeding risk factors (and some [eg, ORBIT score] have mostly nonmodifiable bleeding risks and are poorly calibrated 18 ); and second, to flag up patients with high bleeding risk for early review and follow‐up. This appropriate use of bleeding risk scores is evidence based, being associated with lower MB at 1 year, and an increase in oral anticoagulation uptake in a prospective trial. 36
A comprehensive strategy to stratify bleeding risk in patients with AF and cancer may rely on the use of different bleeding risk scores, according to the outcome of interest and based on cancer location. Indeed, the use of the HAS‐BLED score may turn useful for assessing ICH bleeding risk, but the ORBIT score has better predictive value for MB.
Limitations
Limitations of this study include the lack of data on anticoagulant therapy, which affects the risk of bleeding. In particular, anticoagulation could be an important confounder given that it is associated with both the exposure variables (the bleeding risk scores) and the outcome (bleeding). Indeed, patients with AF with high bleeding risk according to clinical scores may be less likely prescribed anticoagulation agents after the diagnosis of cancer. However, baseline data provided by most “real‐life” studies and registries do not reflect changes of anticoagulant treatment occurring during follow‐up, thus providing inconsistent associations. Data from the Medicare database showed that up to 30% of patients with AF have their anticoagulant drug discontinued after the diagnosis of cancer 37 for several reasons, including surgery, radiotherapy, and chemotherapy. Thus, data from the subgroup of patients showed that <40% of patients were taking oral anticoagulants, confirming previous findings showing that cancer is a major reason for underprescription of anticoagulation in patients with AF, who are often left untreated. 38
Also, data on antineoplastic drugs, which may increase the risk of bleeding interacting with oral anticoagulants, may help to understand the risk of bleeding in these patients. We also do not have data on surgery that may increase the risk of bleeding.
A limitation of the study relies on its retrospective design with its intrinsic potential biases. However, it was also based on administrative data obtained and manually filled by physicians and administrators, and coding is linked to reimbursement and is regularly checked, therefore ensuring a good quality of data. The observational design of the analysis leaves a risk of residual confounding factors. However, the large sample of the study population is likely to be representative of the general French population. Finally, a proportion of asymptomatic patients with AF may not have been detected.
In conclusion, patients with AF and cancer represent a group of patients at high risk of bleeding in whom the clinical management should balance thrombotic and bleeding risk. Different types of cancer confer different risks of bleeding, and the ability to identify those at particularly high risk of ICH may help clinicians in the choice of the most appropriate therapeutic strategy.
Sources of Funding
None.
Disclosures
Dr Lip: consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees are received personally. All other authors have no
Supporting information
Table S1–S3
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.026388
Drs Lip and Fauchier are joint senior authors.
Presented in part at the ESC Congress 2022 in Barcelona, Spain, August 26 to 29, 2022, and published in abstract form (Eur Heart J. 2022:43;ehac544.620 or https://doi.org/10.1093/eurheartj/ehac544.620).
For Sources of Funding and Disclosures, see page 11.
References
- 1. Chung MK, Refaat M, Shen WK, Kutyifa V, Cha YM, Di Biase L, Baranchuk A, Lampert R, Natale A, Fisher J, et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. 2020;75:1689–1713. doi: 10.1016/j.jacc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 2. Lip GYH, Tran G, Genaidy A, Marroquin P, Estes C, Harrelll T. Prevalence/incidence of atrial fibrillation based on integrated medical/pharmacy claims, and association with co‐morbidity profiles/multi‐morbidity in a large US adult cohort. Int J Clin Pract. 2021; 75:e14042. doi: 10.1111/ijcp.14042 [DOI] [PubMed] [Google Scholar]
- 3. Violi F, Loffredo L, Carnevale R, Pignatelli P, Pastori D. Atherothrombosis and oxidative stress: mechanisms and management in elderly. Antioxid Redox Signal. 2017;27:1083–1124. doi: 10.1089/ars.2016.6963 [DOI] [PubMed] [Google Scholar]
- 4. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, Breithardt G, Singer DE, Hankey GJ, Hacke W, et al. Cause of death and predictors of all‐cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc. 2016;5:e002197. doi: 10.1161/JAHA.115.002197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez‐Outes A, Lagunar‐Ruiz J, Terleira‐Fernandez AI, Calvo‐Rojas G, Suarez‐Gea ML, Vargas‐Castrillon E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508–2521. doi: 10.1016/j.jacc.2016.09.944 [DOI] [PubMed] [Google Scholar]
- 6. Fauchier L, Samson A, Chaize G, Gaudin AF, Vainchtock A, Bailly C, Cotte FE. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart. 2015;2:e000290. doi: 10.1136/openhrt-2015-000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu G, Versteeg HH, Verschoor AJ, Trines SA, Hemels MEW, Ay C, Huisman MV, Klok FA. Atrial fibrillation and cancer – an unexplored field in cardiovascular oncology. Blood Rev. 2019;35:59–67. doi: 10.1016/j.blre.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 8. Menichelli D, Vicario T, Ameri P, Toma M, Violi F, Pignatelli P, Pastori D. Cancer and atrial fibrillation: epidemiology, mechanisms, and anticoagulation treatment. Prog Cardiovasc Dis. 2021;66:28–36. doi: 10.1016/j.pcad.2021.04.004 [DOI] [PubMed] [Google Scholar]
- 9. Pastori D, Marang A, Bisson A, Menichelli D, Herbert J, Lip GY, Fauchier L. Cancer and thromboembolism, mortality and bleeding in 2,435,541 atrial fibrillation patients: a nationwide cohort study. Cancer. 2021;127:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA, Sritara P, Mercuri MF, Kamphuisen PW, Antman EM, et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF – TIMI 48 trial. J Am Heart Assoc. 2018;7:e008987. doi: 10.1161/JAHA.118.008987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA, Hacke W, Halperin JL, Hankey GJ, Mahaffey KW, et al. Efficacy and safety of rivaroxaban vs. warfarin in patients with non‐valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes. 2019;5:145–152. doi: 10.1093/ehjqcco/qcy040 [DOI] [PubMed] [Google Scholar]
- 12. D'Souza M, Carlson N, Fosbol E, Lamberts M, Smedegaard L, Nielsen D, Torp‐Pedersen C, Gislason G, Schou M. CHA2DS2‐VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–658. doi: 10.1177/2047487318759858 [DOI] [PubMed] [Google Scholar]
- 13. Pani A, Pastori D, Senatore M, Romandini A, Colombo G, Agnelli F, Scaglione F, Colombo F. Clinical and pharmacological characteristics of elderly patients admitted for bleeding: impact on in‐hospital mortality. Ann Med. 2020;52:413–422. doi: 10.1080/07853890.2020.1808238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuohn LR, Leasure AC, Acosta JN, Vanent K, Murthy SB, Kamel H, Matouk CC, Sansing LH, Falcone GJ, Sheth KN. Cause of death in spontaneous intracerebral hemorrhage survivors: multistate longitudinal study. Neurology. 2020;95:e2736–e2745. doi: 10.1212/WNL.0000000000010736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang G, Xie Q, Ma L, Hu K, Zhang Z, Mu G, Cui Y. Accuracy of HAS‐BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: a network meta‐analysis. J Thromb Haemost. 2020;18:791–801. doi: 10.1111/jth.14692 [DOI] [PubMed] [Google Scholar]
- 16. Borre ED, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, Sharan L, Allen LaPointe NM, Yapa R, Davis JK, et al. Predicting thromboembolic and bleeding event risk in patients with non‐valvular atrial fibrillation: a systematic review. Thromb Haemost. 2018;118:2171–2187. doi: 10.1055/s-0038-1675400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS‐BLED bleeding risk‐prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60:861–867. doi: 10.1016/j.jacc.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 18. Proietti M, Romiti GF, Vitolo M, Potpara TS, Boriani G, Lip GYH. Comparison of HAS‐BLED and ORBIT bleeding risk scores in AF patients treated with NOACs: a report from the ESC‐EHRA EORP‐AF general long‐term registry. Eur Heart J Qual Care Clin Outcomes. 2022; 8:778–786. doi: 10.1093/ehjqcco/qcab069 [DOI] [PubMed] [Google Scholar]
- 19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the euro heart survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 20. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin‐associated hemorrhage: the ATRIA (anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, Kowey PR, Mahaffey KW, Chang P, Fonarow GC, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36:3258–3264. doi: 10.1093/eurheartj/ehv476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li YG, Bisson A, Bodin A, Herbert J, Grammatico‐Guillon L, Joung B, Wang YT, Lip GYH, Fauchier L. C2 HEST score and prediction of incident atrial fibrillation in poststroke patients: a French Nationwide study. J Am Heart Assoc. 2019;8:e012546. doi: 10.1161/JAHA.119.012546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fauchier L, Bisson A, Bodin A, Herbert J, Angoulvant D, Danchin N, Cottin Y. Outcomes in patients with acute myocardial infarction and new atrial fibrillation: a nationwide analysis. Clin Res Cardiol. 2021;110:1431–1438. doi: 10.1007/s00392-021-01805-2 [DOI] [PubMed] [Google Scholar]
- 24. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 25. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi: 10.1186/1472-6947-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Melloni C, Dunning A, Granger CB, Thomas L, Khouri MG, Garcia DA, Hylek EM, Hanna M, Wallentin L, Gersh BJ, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the ARISTOTLE trial. Am J Med. 2017;130:1440–1448.e1. doi: 10.1016/j.amjmed.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 28. Hankey GJ, Stevens SR, Piccini JP, Lokhnygina Y, Mahaffey KW, Halperin JL, Patel MR, Breithardt G, Singer DE, Becker RC, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45:1304–1312. doi: 10.1161/STROKEAHA.113.004506 [DOI] [PubMed] [Google Scholar]
- 29. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747 [DOI] [PubMed] [Google Scholar]
- 30. Roldan V, Marin F, Manzano‐Fernandez S, Gallego P, Vilchez JA, Valdes M, Vicente V, Lip GY. The HAS‐BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2‐VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2013;62:2199–2204. doi: 10.1016/j.jacc.2013.08.1623 [DOI] [PubMed] [Google Scholar]
- 31. Pastori D, Menichelli D, Bucci T, Violi F; Pignatelli P and group A‐As . Cancer‐specific ischemic complications in elderly patients with atrial fibrillation: data from the prospective ATHERO‐AF study. Int J Cancer. 2020;147:3424–3430. doi: 10.1002/ijc.33179 [DOI] [PubMed] [Google Scholar]
- 32. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 33. Lip GY, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14:1711–1714. doi: 10.1111/jth.13386 [DOI] [PubMed] [Google Scholar]
- 34. Yao X, Gersh BJ, Sangaralingham LR, Kent DM, Shah ND, Abraham NS, Noseworthy PA. Comparison of the CHA2DS2‐VASc, CHADS2, HAS‐BLED, ORBIT, and ATRIA risk scores in predicting non‐vitamin K antagonist oral anticoagulants‐associated bleeding in patients with atrial fibrillation. Am J Cardiol. 2017;120:1549–1556. doi: 10.1016/j.amjcard.2017.07.051 [DOI] [PubMed] [Google Scholar]
- 35. Lip GYH, Skjoth F, Nielsen PB, Kjaeldgaard JN, Larsen TB. The HAS‐BLED, ATRIA, and ORBIT bleeding scores in atrial fibrillation patients using non‐vitamin K antagonist oral anticoagulants. Am J Med. 2018;131:574.e13–574.e27. [DOI] [PubMed] [Google Scholar]
- 36. Guo Y, Lane DA, Chen Y; Lip GYH and m AFAIITi . Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA‐II randomized trial. Am J Med. 2020;133:1195–1202. e2 [DOI] [PubMed] [Google Scholar]
- 37. Chen S, Hellkamp A, Alhanti B, Melloni C, Piccini JP, Khouri MG, Granger G, Pokorney S. Patterns of anticoagulation use in atrial fibrillation and active cancer: observations from medicare. Circulation. 2021;144. doi: 10.1161/circ.144.suppl_1.12118 [DOI] [Google Scholar]
- 38. Szpotowicz A, Gorczyca I, Jelonek O, Uzieblo‐Zyczkowska B, Maciorowska M, Wojcik M, Blaszczyk R, Kaplon‐Cieslicka A, Gawalko M, Budnik M, et al. Why did all patients with atrial fibrillation and high risk of stroke not receive oral anticoagulants? Results of the polish atrial fibrillation (POL‐AF) registry. J Clin Med. 2021;10. doi: 10.3390/jcm10194611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3


