To the Editor:
Early reports of patients with hypoxemia and coronavirus disease (COVID-19) pneumonia exhibiting little respiratory distress have prompted the suggestion that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in a unique respiratory pathophysiology (1). One hypothesis to explain the apparent disconnect between severe hypoxemia and the reported absence of dyspnea is a blunted hypoxic ventilatory response (HVR).
We therefore sought to test the hypothesis that patients with hypoxemia and COVID-19 have reduced ventilation compared with healthy control subjects. As part of a cross-sectional study of gas exchange in patients with early COVID-19 pneumonia on presentation to the hospital, we measured mean alveolar partial pressure of CO2 (PaCO2), which represents the inverse of alveolar ventilation (a), and related it to the severity of hypoxemia as measured by PaO2. Published healthy subject data relating PaCO2 to PaO2 under normoxic and acute hypoxic conditions (2–4) were used to assess whether a levels of patients with COVID-19 were in the expected range for the severity of hypoxemia, thus inferring the ventilatory response of these patients.
Methods
The protocol was approved by the Swedish Ethical Review Authority (diary no. 2020-02966).
Subjects
Thirty spontaneously breathing symptomatic patients admitted to Danderyd Hospital, Stockholm, Sweden, who were ⩾18 years of age, had a positive PCR result for COVID-19, and had SaO2 levels of <96% were included. Patients unable to maintain constant Vt and breathing frequency over the data collection period (of several breaths) were excluded. All patients gave written informed consent.
Protocol
PaO2 and PaCO2 were measured from an arterial blood sample collected over two or three steady-state breaths while the patient was breathing ambient air. Immediately before collecting the blood sample, exhaled CO2 concentrations and gas flow were measured at 100 Hz at the mouth (Oxycon Pro; Vyaire Medical [5]), and, after adjustment for analyzer lag, mean PaCO2 was determined as the average of three separate breaths. Measured PaO2 and PaCO2 were corrected to body temperature (6), and exhaled gas measurements were also temperature adjusted using the Antoine equation.
Data analysis
We calculated the term 40/PaCO2 (indicating the a relative to that which would be present in the same patient had PaCO2 been normal at 40 mm Hg). This term, abbreviated to arel, was plotted against PaO2.
Results
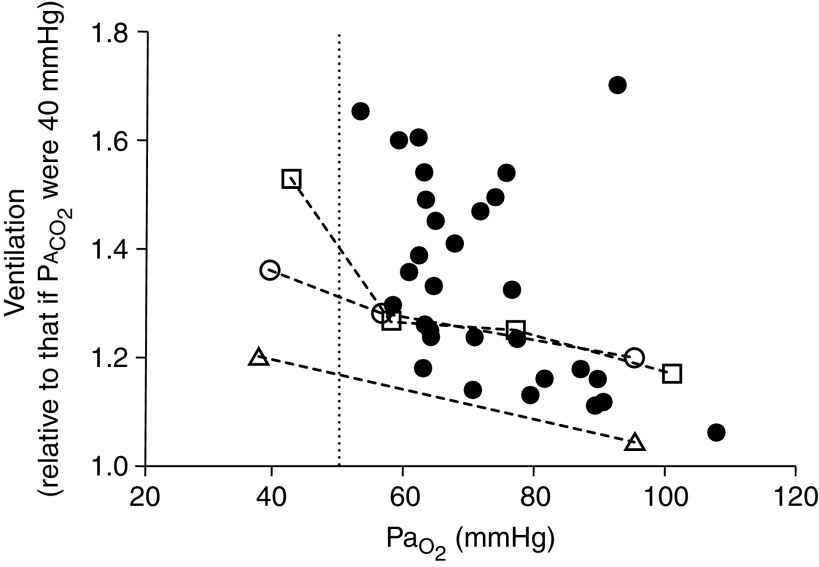
Data were collected from 22 males and 8 females, aged 23–85 years (mean ± SD, 50.7 ± 15.0 yr). All subjects had mildly symptomatic COVID-19 pneumonia; the majority were tachypneic (respiratory rate, 21.8 ± 7.2 breaths/min; range, 9–38), 22 had dyspnea, and most were febrile at the time of testing (body temperature, 38.0 ± 1.0°C; range, 36.5–40°C). No patient required ICU admission. Exhaled CO2 was collected between 2 and 100 seconds (mean ± SD, 35 ± 10 s) before the arterial blood gas sample. PaO2 ranged from 52.9 to 107.5 mm Hg (mean ± SD, 72.0 ± 12.7 mm Hg), arterial oxygen saturation ranged from 89% to 99% (mean ± SD, 94 ± 2%), PaCO2 ranged from 27.8 to 46.8 mm Hg (mean ± SD, 36.3 ± 4.6 mm Hg), and arel ranged from 1.1 to 1.7 (mean ± SD, 1.3 ± 0.2). Approximately 50% of patients had arel values that were in broad agreement with normal values (Figure 1). For all remaining patients, arel was greater than expected from the normal data. Most importantly, in no patient was arel lower than that seen in healthy subjects at any PaO2.
Figure 1.
PaO2 plotted against 40/alveolar partial pressure of CO2 (PaCO2), indicating alveolar ventilation (a) relative to that which would be present in the same patient had PaCO2 been normal at 40 mm Hg (n = 30; solid circles). In no patient was relative a less than 1.0 or less than that calculated for healthy young adult subjects from Wagner and colleagues (2) (open circles, dashed lines representing 7 males and 1 female; mean ± SD age, 29.8 ± 6.1 yr), Hammond and colleagues (3) (open triangles, dashed lines representing 10 males; mean ± SD age, 22.0 ± 1.2 yr), and Torre-Bueno and colleagues (4) (open squares, dashed lines representing 9 males; mean ± SD age, 26.0 ± 6.0 yr). PaCO2 for historical control subjects was calculated using measured PaCO2, and the multiple inert gas elimination technique was used to measure a/ inequality to estimate the arterial–alveolar difference. Vertical dotted line represents PaO2 = 50 mm Hg.
Discussion
Our findings demonstrate that in this group of 30 patients with acute symptomatic COVID-19, a was normal or increased at any PaO2 as compared with that in healthy subjects exposed to acute hypoxia. Contrary to our hypothesis, no patient had evidence of reduced or blunted ventilation. Notably, all patients had PaO2 > 50 mm Hg, the nominal level below which hypoxia-driven dyspnea and ventilation increase rapidly in healthy subjects (Figure 1) (7). To our knowledge, these findings represent the first report with data showing that ventilatory responsiveness in spontaneously ambient air–breathing patients with COVID-19 is normal or increased and not decreased. A strength of our study is that our patient data are based on a direct, noninvasive measurement of PaCO2 from exhaled gas analysis, thus providing a surrogate measurement of a that is obtainable at the bedside in a clinical infectious disease setting.
Limitations
This is an observational cross-sectional study, and we are unable to determine the mechanism contributing to the observed arel data. Potential mechanisms influencing ventilatory drive in COVID-19 pneumonia include the following: 1) genetically determined differences in HVR (8, 9); 2) sustained hypoxemia over a period of hours to days, resulting in an increase in HVR (via the hypoxia-inducible factor hydroxylase system) (10); 3) SARS-CoV-2 invasion of the carotid body or central nervous system, resulting in direct changes in ventilatory response (8); and 4) other disease-related but not COVID-19–specific factors affecting ventilatory control (e.g., sensory receptor inputs, fever, anxiety, pain, inflammation) or respiratory mechanics. Additional studies would be required to establish the roles of these contributing factors.
We recruited spontaneously breathing, symptomatic, hospitalized patients with COVID-19 who were judged by their caregivers to be safe while breathing ambient air for the few minutes of the study, and our findings may not be generalizable to other COVID-19 disease stages or severities. We did not perform classical HVR protocols, with control of inhaled gases and direct measurement of ventilation in each individual, because of logistical challenges in a highly infectious acute disease setting. Our data consist of a single sample for each patient, and we do not know where each subject is operating in their intrinsic HVR relationship. We also have not included concurrent healthy or non–COVID-19 pneumonia control subjects and have used historical control subjects from three prior physiological studies in which healthy young subjects were studied over the same range of PaO2 levels as encountered in our patients with COVID-19.
Conclusions
Patients with acute COVID-19 (spontaneously breathing ambient air) do not have depressed a relative to their degree of hypoxemia. Indeed, some patients have relatively high ventilatory levels. These findings do not support the concept of impaired HVR in COVID-19 pneumonia.
Footnotes
Supported by the NIH (A.M.).
Author Contributions: K.K., C.E.F., T.A., and A.M. designed the study and prepared the manuscript. P.H. designed the study, collected and analyzed data, and prepared the manuscript. G.H. designed the study and collected and analyzed data. G.K.P. and P.D.W. designed the study, analyzed data, and prepared the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202109-2025LE on February 7, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med . 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol (1985) . 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- 3. Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during normobaric hypoxic exercise. J Appl Physiol (1985) . 1986;61:1749–1757. doi: 10.1152/jappl.1986.61.5.1749. [DOI] [PubMed] [Google Scholar]
- 4. Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE, Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol (1985) . 1985;58:989–995. doi: 10.1152/jappl.1985.58.3.989. [DOI] [PubMed] [Google Scholar]
- 5. Carter J, Jeukendrup AE. Validity and reliability of three commercially available breath-by-breath respiratory systems. Eur J Appl Physiol . 2002;86:435–441. doi: 10.1007/s00421-001-0572-2. [DOI] [PubMed] [Google Scholar]
- 6. Bradley AF, Severinghaus JW, Stupfel M. Effect of temperature on PCO2 and PO2 of blood in vitro. J Appl Physiol . 1956;9:201–204. doi: 10.1152/jappl.1956.9.2.201. [DOI] [PubMed] [Google Scholar]
- 7. Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol (1985) . 2003;94:141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- 8. Swenson KE, Ruoss SJ, Swenson ER. The pathophysiology and dangers of silent hypoxemia in COVID-19 lung injury. Ann Am Thorac Soc . 2021;18:1098–1105. doi: 10.1513/AnnalsATS.202011-1376CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weil JV. Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol . 2003;135:239–246. doi: 10.1016/s1569-9048(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 10. Hodson EJ, Nicholls LG, Turner PJ, Llyr R, Fielding JW, Douglas G, et al. Regulation of ventilatory sensitivity and carotid body proliferation in hypoxia by the PHD2/HIF-2 pathway. J Physiol . 2016;594:1179–1195. doi: 10.1113/JP271050. [DOI] [PMC free article] [PubMed] [Google Scholar]