Abstract
Flagellin from various species of gram-negative bacteria activates monocytes to produce proinflammatory cytokines. We have analyzed the pathway by which Salmonella enteritidis flagellin (FliC) activates murine and human monocyte/macrophage-like cell lines. Since lipopolysaccharide (LPS), the principal immune stimulatory component of gram-negative bacteria, is known to signal through Toll-like receptor 4 (TLR4), we tested the possibility that FliC also signals via TLR4. When murine HeNC2 cells were stimulated with LPS in the presence of a neutralizing anti-TLR4 monoclonal antibody, tumor necrosis factor alpha (TNF-α) and nitric oxide (NO) production were markedly reduced. In contrast, FliC-mediated TNF-α and NO production were minimally affected by the anti-TLR4 antibody. Furthermore, FliC, unlike LPS, stimulated TNF-α production in the TLR4 mutant cell line, GG2EE, indicating that TLR4 is not essential for FliC-mediated signaling. To test the possibility that FliC signals via another TLR, we measured FliC-mediated activation of interleukin-1 (IL-1) receptor-associated kinase (IRAK), a central component in IL-1R/TLR signaling. FliC induced IRAK activation in HeNC2 and GG2EE cells as well as in the human promonocytic cell line THP-1. IRAK activation was rapid in HeNC2 cells, with maximal activity observed after 5 min of treatment with FliC. In addition, FliC-mediated IRAK activation exhibited the same concentration dependence as was demonstrated for the induction of TNF-α. These results represent the first demonstration of IRAK activation by a purified bacterial protein and strongly suggest that a TLR distinct from TLR4 is involved in the macrophage inflammatory response to FliC.
Activation of the innate immune response by bacteria is mediated by a group of molecules that have collectively been termed modulins (12). These surface-associated molecules are produced by a broad array of microbial pathogens and include lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acid, lipoarbinomannan, lipoproteins, and lipopeptides. Monocytes and macrophages respond to modulins by producing proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 (12). The production of cytokines is a critical event in the development of protective innate immune responses and is also involved in the initiation of adaptive immune responses. However, excessive production of cytokines has also been implicated in the pathological events associated with the septic response in patients with severe bacterial infections.
We and others have demonstrated that flagella from a number of gram-negative bacterial species induce proinflammatory cytokine production by human monocytes and monocyte-like cell lines (7, 8, 35). Using genetic and biochemical approaches, it was demonstrated that the major structural protein of flagella, flagellin (FliC), was the component required for the induction of cytokine synthesis (7). In a subsequent study, it was found that purified recombinant FliC from Salmonella enteriditis, Salmonella enterica serovar Typhimurium, and Pseudomonas aeruginosa were potent inducers of cytokine synthesis in human monocytes and THP-1 cells, a human promonocytic cell line (19). Half-maximal responses were obtained with concentrations of FliC in the range of 15 to 30 pM. Recently, it was reported that purified flagellin from enteroaggregative Escherichia coli induces IL-8 production by intestinal epithelial cells (28).
An important feature shared by many bacterial modulins is that they signal monocytes and macrophages via proteins in the IL-1 receptor/Toll-like receptor (IL-1R/TLR) family (1). Proteins in the IL-1R/TLR family are grouped based on conserved sequences in their cytoplasmic domains that are required for signal transduction (9, 10). Upon activation of the IL-1R and TLRs several common events are triggered, including recruitment and activation of the IL-1R-associated kinase (IRAK) via the adaptor protein MyD88, formation of a complex between IRAK and TNF receptor-associated factor 6, and activation of NF-κB and other factors required for transcription of proinflammatory cytokine genes (5, 20, 22, 33, 37). IL-1R/TLR family proteins belong to a larger group of innate immune system receptors recently termed pattern-recognition receptors (14). Pattern-recognition receptor ligands, which include the previously mentioned bacterial modulins, display molecular patterns unique to microbial pathogens and have thus been termed PAMPs (pathogen-associated molecular patterns). LPS, the prototype gram-negative PAMP, requires TLR4 to exert its biological effects (6, 13, 23, 24), whereas a diverse group of PAMPs including gram-positive lipoteichoic acid and peptidoglycan (25), lipoproteins and lipopeptides from various bacterial species (3, 18, 30), and mycobacterial lipoarabinomannan (31) appear to require TLR2. To date, at least 10 different TLR proteins have been described, suggesting the existence of additional TLR ligands.
In view of the involvement of TLRs in signaling by LPS and other PAMPs, we explored the possibility that FliC, a gram-negative bacterial protein, might also utilize a TLR to exert its biological effects. Our results indicate that FliC does utilize a Toll-like receptor signaling pathway as demonstrated by its ability to activate IRAK. However, FliC does not require TLR4, suggesting that an additional TLR(s) is important in the innate response to gram-negative bacteria.
MATERIALS AND METHODS
Cell culture and biological reagents.
The C3H/HeN-derived macrophage cell line HeNC2 (15) was kindly provided by A. Ding (Cornell University Medical College, New York, N.Y.). The C3H/HeJ-derived macrophage cell line GG2EE (4) was obtained from G. Cox (National Cancer Institute, Frederick, Md.). THP-1 cells were purchased from the American Type Culture Collection (Rockville, Md.). Cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, glutamine, and 50 μg of gentamicin/ml (complete medium). The monoclonal antibody MTS510, which recognizes murine TLR4 in complex with MD-2 (2), was generously provided by Kensuke Miyake (Department of Immunology, Saga Medical School, Nabeshima, Saga, Japan). Monoclonal antibody 3ZD, which recognizes murine and human IL-1β (16), was obtained from the National Cancer Institute Biological Response Modifiers Repository. Polyclonal anti-IRAK antibody was obtained from Upstate Biotechnology Corp. Purified recombinant FliC from S. enteriditis was prepared as previously described (19). LPS was removed from FliC preparations by passage through a polymyxin B column according to the manufacturer's instructions (Detoxi-Gel; Pierce Chemical Co.). Salmonella serovar Typhimurium LPS was obtained from Sigma Chemical Co.
Induction and measurement of TNF-α and NO production.
Cells were seeded in 24-well tissue culture dishes at 106 cells/ml in complete medium containing the indicated concentrations of FliC. After 24 or 48 h, the level of TNF-α or nitric oxide (NO) produced was determined. TNF-α levels in culture supernatants of HeNC2 and GG2EE cells were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc., Minneapolis, Minn.). In the experiments with THP-1 cells, 0.5% Triton X-100 was added to each culture and the total TNF-α content (cell associated and released) was measured using a commercial ELISA kit (Abraxis, Hatboro, Pa.). The production of NO by murine cell lines was determined by measuring NO2− in culture supernatants as previously described (15).
IRAK assay.
IRAK activity in cell lysates was measured as previously described (17) using [γ32P]ATP and myelin basic protein as a substrate. Reaction products were subject to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the bands were visualized by autoradiography. The intensity of 32P-labeled myelin basic protein bands was quantitated using a Molecular Dynamics phosphorimaging system. IRAK protein was detected by Western blotting using polyclonal anti-IRAK antibody.
RESULTS
Activation of HeNC2 cells by FliC.
As a first step in the analysis of the FliC signaling mechanism, we evaluated the effect of recombinant FliC on TNF-α and NO production by HeNC2 cells. To ensure that we would be measuring responses induced by FliC and not contaminating LPS, a polymyxin B resin was used to remove LPS. This treatment reduced the LPS content of the FliC preparation from approximately 20 μg/ml to 1 ng/ml as measured by the Limulus amebocyte assay. Since the concentration of the FliC stock preparation was in the range of 10−6 M and the concentrations used for assays were in the range of 10−12 to 10−9 M, the maximal level of LPS in the cultures was less than 1 pg/ml. At this concentration, LPS had little if any effect in our assays.
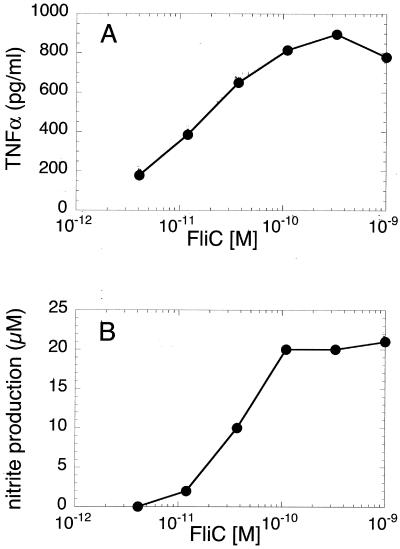
Like human monocytes and THP-1 cells (19), HeNC2 cells were extremely sensitive to stimulation by FliC. As shown in Fig. 1, TNF-α and NO production were maximally stimulated at concentrations below 10−9 M. Generally, 50% of the maximal response was achieved with FliC concentrations in the range of 10 to 40 pM.
FIG. 1.
FliC-induced activation of HeNC2 cells. HeNC2 cells were cultured with increasing concentrations of FliC. (A) TNF-α in culture supernatants was measured after 24 h by ELISA. The amount of TNF-α produced in unstimulated control cultures was subtracted from each value. Similar results were obtained in three independent experiments. (B) NO production was assessed after 48 h by measuring the amount of nitrite, a stable byproduct of NO. The values represent the mean of duplicate determinations (standard deviation, <5%) from two independent experiments.
FliC-induced macrophage activation does not require TLR4.
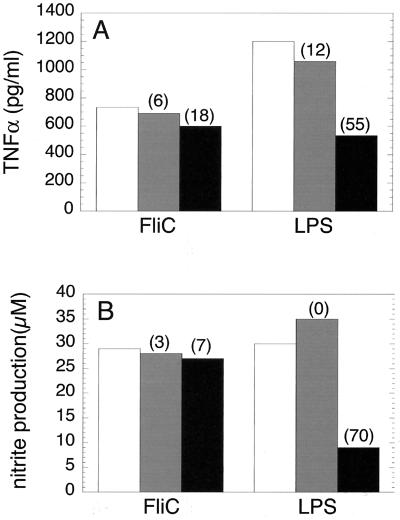
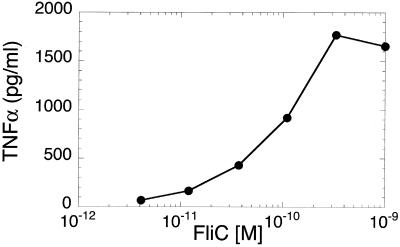
In view of the critical role of TLR4 in the innate immune response to gram-negative bacteria (23, 24), we tested the possibility that S. enteriditis FliC might, like LPS, utilize a TLR4-dependent signaling pathway to activate macrophages. Thus, HeNC2 cells were incubated with FliC in the presence and absence of a neutralizing anti-murine TLR4 monoclonal antibody (MTS510) that blocks LPS-induced cytokine production in murine macrophages (2). Although MTS510 markedly reduced TNF-α and NO production in response to LPS (55 and 70% inhibition, respectively), it had little effect on FliC-induced production of these mediators; the effect of MTS510 on FliC activity was not reproducibly different than that obtained with the control monoclonal antibody, 3ZD (Fig. 2 and data not shown). These results indicate that in contrast to LPS, FliC does not appear to utilize a TLR4-dependent pathway. However, we felt that it was important to use an additional approach to strengthen the experimental basis for this conclusion. Therefore, we tested the effect of FliC on GG2EE cells. This macrophage cell line was derived from the C3H/HeJ mouse and exhibits the same phenotype of LPS hyporesponsiveness that is due to a mutation in the TLR4 gene (13, 23, 24). As shown in Fig. 3, GG2EE cells produced TNF-α in response to FliC, with 50% of the maximal response observed at approximately 10−10 M FliC. As previously reported (15), LPS at 1 ng/ml failed to stimulate significant TNF-α production by GG2EE cells (135 ± 71 pg/ml; n = 3). In conjuction with results obtained with the anti-TLR4 monoclonal antibody, the data presented in Fig. 3 provide strong evidence that TLR4 is not required for FliC-induced signal transduction.
FIG. 2.
Anti-TLR4 monoclonal antibody does not block the stimulatory effect of FliC. HeNC2 cells were cultured in the presence of 3 × 10−11 M FliC with no antibody added (open bars), with 20 μg of anti-IL-1β antibody 3ZD/ml (gray bars), or with 20 μg of anti-TLR4 antibody MTS510/ml (black bars). (A) TNF-α production after 24 h. (B) NO production after 48 h. The values represent the mean of duplicate determinations (standard deviation, <5%). Similar results were obtained in three independent experiments. The percent inhibition relative to controls in which no antibody was added is indicated in parentheses.
FIG. 3.
FliC induces TNF-α production in LPS-hyporesponsive GG2EE cells. GG2EE cells were cultured with the indicated concentrations of FliC for 24 h. The amount of TNF-α in culture supernatants was measured by ELISA. Similar results were obtained in three independent experiments.
FliC induces IRAK activation.
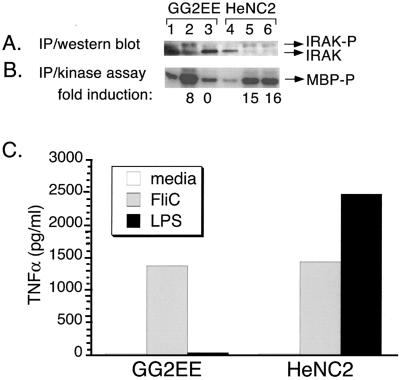
Although our results do not support a role for TLR4 in FliC signaling, it was still possible that FliC utilizes another TLR. To test this possibility, we measured the activation of IRAK, a central component in TLR signaling, in response to FliC. The murine macrophage-like cell lines HeNC2 and GG2EE were incubated with or without FliC for 15 min, and the level of IRAK catalytic activity (as measured by phosphorylation of the synthetic substrate myelin basic protein) as well as IRAK protein expression was measured. For comparative purposes, another set of cultures was stimulated with LPS. As shown in Fig. 4B, FliC induced a very pronounced increase in IRAK activity in GG2EE and HeNC2 cells (lanes 2 and 5) relative to unstimulated cells (lanes 1 and 4). In contrast, LPS (1 ng/ml) had no effect on the LPS-hyporesponsive GG2EE cells but did induce significant IRAK activity in the LPS-responsive HeNC2 cells (lanes 3 and 6). Kinase activity correlated with a decrease in the size of the 80-kDa IRAK band and the appearance of a more slowly migrating species (Fig. 4A, lanes 2, 5, and 6). The latter band presumably is phosphorylated IRAK. Since phosphorylated IRAK is rapidly degraded, this form of the protein is detected at reduced levels relative to the unactivated protein (36). In each case, the activation of IRAK corresponded with the production of TNF-α (Fig. 4C).
FIG. 4.
FliC activates IRAK in HeNC2 and GG2EE cells. HeNC2 and GG2EE cells (107 cells per sample) were cultured in the absence (lanes 1 and 4) or presence of 10−9 M FliC (lanes 2 and 5) or 1 ng of LPS/ml (lanes 3 and 6) for 15 min at 37°C. (A) IRAK was immunoprecipitated (IP) from cell lysates using anti-IRAK antibody, and the protein was visualized by Western blotting using the same antibody. (B) IRAK kinase activity in the same cell lysates was measured by in vitro kinase assay using myelin basic protein as a substrate (MBP-P). The fold induction of IRAK activity relative to the unstimulated control is indicated below each lane. (C) TNF-α production in culture supernatants after 24 h. Similar results were obtained in three independent experiments.
Recently, Li et al. demonstrated that IRAK is activated by LPS in the human promonocytic cell line THP-1 and that the response is maximal after approximately 60 min (17). To determine if FliC also activates IRAK in human cells, we incubated THP-1 cells in the presence or absence of FliC for 60 min prior to measuring IRAK activity and protein expression. The level of TNF-α was also measured after culturing the cells for 24 h. As shown in Fig. 5, FliC activated IRAK in THP-1 cells in a concentration-dependent manner. A complete dose-response curve is shown in Table 1. As with the murine cells, the appearance of a more slowly migrating species of IRAK was detected in samples exhibiting significant kinase activity (Fig. 5, lanes 3 and 4 versus lane 1).
FIG. 5.
FliC activates IRAK in the human promonocytic THP-1 cell line. THP-1 cells (5 × 106 cells per sample) were cultured for 1 h in the absence (lane 1) or presence of 10−11 M FliC (lane 2), 10−10 M FliC (lane 3), or 10−9 M FliC (lane 4). (A) Western blot of IRAK expression. (B) IRAK kinase activity. Similar results were obtained in three independent experiments.
TABLE 1.
Dose-dependent activation of IRAK and TNF-α production in THP-1 cellsa
| FliC (M) | IRAK activity (fold induction)b | TNF-α (pg/ml)c |
|---|---|---|
| 4.1 × 10−12 | <2.0 | 46 |
| 1.2 × 10−11 | <2.0 | 153 |
| 3.7 × 10−11 | 2.2 | 1,182 |
| 1.2 × 10−11 | 4.0 | 5,705 |
| 3.3 × 10−10 | 7.7 | 11,641 |
| 1.0 × 10−9 | 6.7 | 11,334 |
One of two experiments with similar results is shown.
IRAK activity in THP-1 cell lysates after 1 h of treatment with FliC. Values indicate the fold induction relative to unstimulated controls.
TNF-α produced after 24 h of treatment with FliC as measured by ELISA. The mean of duplicate determinations is shown. The amount of TNF-α produced by unstimulated cells is subtracted from each value.
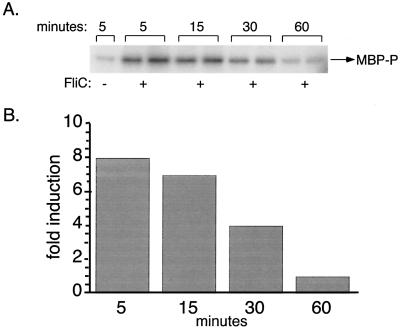
Kinetics of FliC-induced IRAK activation.
Swantek et al. recently reported that LPS activates IRAK in murine peritoneal macrophages within 7.5 min of treatment (29). Similar to the effect of LPS, the activation of IRAK in response to FliC was quite rapid. Maximal kinase activity was observed after 5 min of treatment and decreased steadily thereafter (Fig. 6). By 60 min, the level of kinase activity was only slightly above the level detected in cultures of unstimulated cells. Time course experiments with THP-1 cells also revealed a rapid induction of IRAK, with significant kinase activity observed within 5 min of the addition of FliC (data not shown).
FIG. 6.
Time course of FliC-induced IRAK activation in HeNC2 cells. (A) HeNC2 cells (107 cells per sample) were cultured in the absence or presence of 10−9 M FliC. Cultures with FliC added were prepared in duplicate. Cells were lysed at the indicated time points, and IRAK activity was measured by in vitro kinase assay. (B) Fold induction of IRAK activity relative to unstimulated controls.
DISCUSSION
Our results demonstrate that FliC is a potent stimulant for murine and human monocytes and macrophages. Half-maximal stimulation of cytokine and NO production occurs with concentrations of FliC in the pM range (Fig. 1) (19). Furthermore, our results indicate that similar concentrations of FliC induce a very rapid and marked increase in IRAK activity (Fig. 4 to 6), providing an important insight into the mechanism by which FliC exerts its effect on macrophages.
IRAK is a central component of the signal transduction pathway triggered upon activation of IL-1R/TLR family proteins (20, 33). In IL-1-responsive cells, IRAK is recruited to the type 1 IL-1R, multiphosphorylated, and almost completely degraded within 10 min of treatment with IL-1 (36). In mouse peritoneal macrophages, IRAK activity is detected within 7.5 min of treatment with LPS and declines rapidly after 15 min (29). Similarly, we showed that FliC activates IRAK catalytic activity in murine macrophages rapidly, with peak activity observed after 5 min of treatment. It was recently found that FliC induces a substantial increase in TNF-α mRNA levels in THP-1 cells within 15 min after addition to monocytic cells (F. Ciacci-Woolwine and S. B. Mizel, unpublished observations). Thus the observation that FliC activates IRAK within 5 min is consistent with the hypothesis that this process is a critical step in a signaling cascade that results in the relatively rapid expression of proinflammatory cytokines.
As demonstrated previously for LPS (17) and IL-1 (36), we observed a reduction in the level of the 80-kDa IRAK protein coincident with the appearance of a weak, more slowly migrating species in lysates of cells which exhibited significant in vitro kinase activity (see Fig. 4). The appearance of a higher molecular weight species of IRAK in response to IL-1 stimulation was shown to be the result of IRAK phosphorylation (36). It was further demonstrated that phosphorylated IRAK is rapidly degraded in IL-1-stimulated cells (36). Although not a formal demonstration, our results suggest that as documented for LPS and IL-1, transmission of a signal by FliC involves phosphorylation and degradation of IRAK.
Since IRAK activation is a hallmark of the signal transduction pathway linked to the Toll-like receptor family of proteins, our results strongly suggest that FliC signals macrophages via a TLR. To date, a diverse group of bacterial cell surface molecules (PAMPs) has been shown to signal macrophages via TLRs. LPS, the prototype bacterial PAMP, requires TLR4 for signal transduction (23, 24). TLR2 is the receptor utilized by gram-positive lipoteichoic acid and peptidoglycan (25), mycobacterial lipoarabinomannan (31), and a wide array of lipoproteins and lipopeptides (3, 18, 30). The latter group of PAMPs has been shown to require the N-terminal lipid moiety for biologic activity (12). In addition, it was reported recently that TLR9 is the receptor that transduces a signal in response to bacterial DNA (11). In view of the available data regarding TLR ligands, our results demonstrating that recombinant, LPS-free FliC activates IRAK are of particular importance, as no study to date has reported the activation of a TLR signaling pathway by a purified bacterial protein.
Given that at least 10 distinct TLRs have been described to date, and due to limited reagent availability for most of these proteins, identification of the specific TLR utilized by FliC was beyond the scope of the present study. However, a neutralizing anti-TLR4 monoclonal antibody inhibited LPS-induced TNF-α and NO production by HeNC2 cells but had little effect on FliC-induced responses, suggesting that FliC signals through a TLR other than TLR4. Furthermore, in direct contrast to the effects of LPS, FliC induced the activation of IRAK and TNF-α production in C3H/HeJ-derived GG2EE cells. These results further substantiate the conclusion that FliC utilizes a signaling receptor that is distinct from TLR4, the receptor utilized by LPS. The possibility that FliC mediates its effects via TLR2 seems unlikely as it does not appear that TLR2 is involved in the innate response to gram-negative bacteria. Specifically, it was recently demonstrated that expression of a dominant negative mutant of TLR2 in murine macrophages inhibited TNF-α production in response to Staphylococcus aureus and gram-positive cell wall products but not in response to Salmonella serovar Minnesota or LPS (32). Interestingly, however, Sebastiani et al. have mapped a Salmonella-susceptibility locus on murine chromosome 1 to a region containing the tlr5 gene (26). Thus, TLR5, which like TLR4 and TLR2 is expressed by myelomonocytic cells (21), is a candidate receptor for FliC. Clearly, additional studies will be necessary to identify the receptor(s) that participates in FliC-mediated signaling. In any case, our data suggest that in addition to TLR4, another TLR(s) is likely to be important in the recognition of gram-negative bacteria by cells of the innate immune response. In this regard, it will also be of interest to determine if, like LPS, FliC requires an initial binding receptor analogous to CD14 (34) or if FliC directly interacts with a TLR. The latter scenario has yet to be demonstrated for any TLR studied to date. Since CD14-negative U937-derived U38 cells respond to flagella (8), it is unlikely that CD14 is involved in the response to FliC.
The effects of FliC on macrophages are quantitatively and qualitatively similar to the well-documented effects of LPS, suggesting that gram-negative flagellin is likely to play an important and previously unrecognized role in the innate immune response to gram-negative bacteria. Since intact flagella (8, 35) as well as the released soluble form of flagellin (7, 19) are active, it is likely that this bacterial modulin may contribute to localized as well as disseminated responses to gram-negative pathogens. FliC may be of particular importance during the course of infections in the gastrointestinal tract, since lamina propria macrophages in the intestinal mucosa do not express CD14 and are LPS nonresponsive (27). Nevertheless, gram-negative enteric pathogens such as Salmonella serovar Enteriditis induce cytokine production and inflammation in the intestinal mucosa. Future studies directed at identifying the surface receptor(s) for FliC and further characterizing the intracellular events triggered by the interaction of FliC with macrophages should provide important insights into the mechanisms by which gram-negative bacteria activate innate immunity.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI-38670 (to S.B.M) and by the Signal Transduction Mechanisms and Cell Function training program, grant CA-09422, from the National Institutes of Health (M.A.M).
REFERENCES
- 1.Aderem A, Ulevitch R J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis A O, Yang R, Mark M, Sugget S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor 2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 4.Blasi E, Radzioch D, Durum S K, Varesio L. A murine macrophage cell line, immortalized by v-raf and v-myc oncogenes, exhibits normal macrophage functions. Eur J Immunol. 1987;17:1491–1498. doi: 10.1002/eji.1830171016. [DOI] [PubMed] [Google Scholar]
- 5.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 6.Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor 4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 7.Ciacci-Woolwine F, Blomfield I C, Richardson S H, Iyer N P, Mizel S B. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect Immun. 1998;66:1127–1134. doi: 10.1128/iai.66.3.1127-1134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciacci-Woolwine F, McDermott P F, Mizel S B. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect Immun. 1999;67:5176–5185. doi: 10.1128/iai.67.10.5176-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay N J, Keith F J. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 10.Heguy A, Baldari C T, Macchia G, Telford J L, Melli M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila Toll protein are essential for IL-1R signal transduction. J Biol Chem. 1992;267:2605–2609. [PubMed] [Google Scholar]
- 11.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 12.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 14.Janeway C A, Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 15.Jin F, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 16.Lew W, Oppenheim J J, Matsushima K. Analysis of the suppression of IL-1alpha and IL-1beta production in human peripheral blood mononuclear adherent cells by a glucocortocoid hormone. J Immunol. 1988;140:1895–1902. [PubMed] [Google Scholar]
- 17.Li L, Cousart S, Hu J, McCall C E. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J Biol Chem. 2000;275:23340–23345. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- 18.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G I, Finberg R W, Carroll J D, Espevik T, Ingalls R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 19.McDermott P F, Ciacci-Woolwine F, Snipes J A, Mizel S B. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun. 2000;68:5525–5529. doi: 10.1128/iai.68.10.5525-5529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C A., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 21.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, Veer C, Penton-Rol G, Ruco L P, Allavena P, Mantovani A. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 22.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:612–615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 23.Poltorak A, He X, Smirnova I, Liu M Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4. J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 26.Sebastiani D, Leveque G, Lariviere L, Laroche L, Skamene E, Gross P, Malo D. Cloning and characterization of the murine Toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics. 2000;64:230–240. doi: 10.1006/geno.2000.6115. [DOI] [PubMed] [Google Scholar]
- 27.Smith P D, Janoff E N, Mosteller-Barnum M, Merger M, Orenstein J M, Kearney J F, Graham M F. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Methods. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 28.Steiner T S, Nataro J P, Poteet-Smith C E, Smith J A, Guerrant R L. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Investig. 2000;105:1769–1777. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swantek J L, Tsen M F, Cobb M H, Thomas J A. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol. 2000;164:4301–4306. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi O, Kaufman A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt P F, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 31.Underhill D M, Ozinsky A, Smith K D, Aderem A. Toll-like receptor 2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Underhill D M, Ozinsky A, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 33.Wesche H, Henzel W J, Shillinglaw W, Cao Z. MyD88: an adaptor that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 34.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 35.Wyant T L, Tanner M K, Sztein M B. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamin T, Miller D K. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- 37.Yang R, Mark M, Gurney A L, Godowski P J. Signaling events induced by lipopolysaccharide-activated Toll-like receptor 2. J Immunol. 1999;163:639–643. [PubMed] [Google Scholar]