Abstract
Dopaminergic neurons in the substantia nigra pars compacta (SNc) differentially degenerate in Parkinson’s Disease, with the ventral region degenerating more severely than the dorsal region. Compared with the dorsal neurons, the ventral neurons in the SNc have distinct dendritic morphology, electrophysiological characteristics, and circuit connections with the basal ganglia. These characteristics shape information processing in the ventral SNc and structure the balance of inhibition and disinhibition in the striatonigral circuitry. In this paper, I review foundational studies and recent work comparing the circuitry of the ventral and dorsal SNc neurons and discuss how loss of the ventral neurons early in Parkinson’s Disease could affect the overall balance of inhibition and disinhibition of dopamine signals.
Keywords: Dopamine, Parkinson’s disease, SNc, Striatum, Inhibition, Basal ganglia, Dendrites, Disinhibition, Circuit mapping, Dendritic morphology, Substantia nigra, Striatonigral, Nigrostriatal
1. Introduction
Dopaminergic neurons differentially degenerate in Parkinson’s Disease (PD). While dopaminergic neurons in the substantia nigra pars compacta (SNc) degenerate more severely than the dopaminergic neurons in the ventral tegmental area (VTA) (Dauer and Przedborski, 2003; Alberico et al., 2015), there is also differential degeneration within the SNc (Yamada et al., 1990; Damier et al., 1999; Kordower et al., 2013; Fearnley and Lees, 1991; Kamath et al., 2022). These findings and results from animal models of PD indicate that one subset of SNc dopaminergic neurons are more vulnerable to degeneration, while another subset is resilient. These vulnerable and resilient SNc dopaminergic neurons differ in their anatomical location, molecular identity, and projection patterns. Specifically, the vulnerable SNc dopaminergic neurons are located in the ventral region (Yamada et al., 1990; McRitchie et al., 1997; Damier et al., 1999; Kamath et al., 2022), express aldehyde dehydrogenase 1a1 (Aldh1a1) (Liu et al., 2014; Poulin et al., 2014), do not express the calcium buffering protein calbindin (Gerfen et al., 1987a; Yamada et al., 1990; Lavoie and Parent, 1991; Aguila et al., 2021), and project to the dorsal striatum (Gerfen et al., 1987b; Matsuda et al., 2009; Poulin et al., 2018; Prensa and Parent, 2001; Wu et al., 2019), while the resilient population are located in the dorsal region of the SNc and display the opposite molecular profile (Fig. 1).
Fig. 1. Diagram of the ventral and dorsal tier of the substantia nigra pars compacta (SNc).
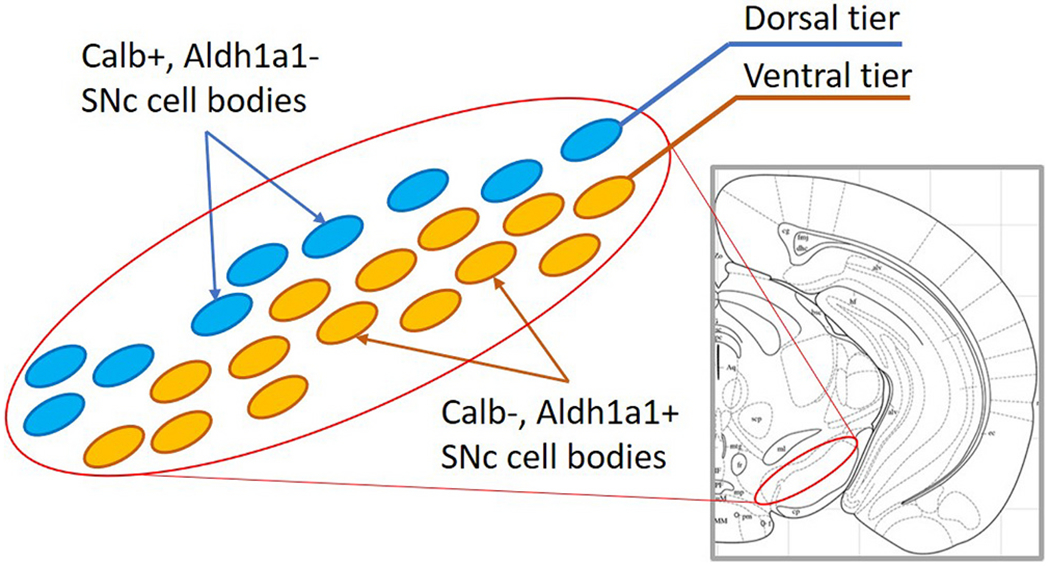
Yellow ovals represent the calbindin-negative, Aldh1a1-positive ventral tier dopaminergic neurons that are more vulnerable to degeneration. Blue ovals represent the dorsal tier calbindin-positive, Aldh1a1-negative dopaminergic neurons that are more resilient. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Foundational publications and recent studies comparing the vulnerable and resilient SNc populations have identified differences in intrinsic currents and synaptic connectivity, shedding light on how these subpopulations process information and how pathological conditions can cause imbalances in the basal ganglia network. The intrinsic properties of dopaminergic neurons and their relevance to Parkinson’s Disease have been extensively covered (Surmeier et al., 2010; Morikawa and Paladini, 2011; Brichta and Greengard, 2014; Canavier et al., 2016; Philippart et al., 2016; Duda et al., 2016; Gantz et al., 2018; Giguère et al., 2018; Gonzalez-Rodriguez et al., 2020; Carmichael et al., 2021; Ortner, 2021). This review will focus on the inhibitory and disinhibitory circuits that modulate the activity of the vulnerable and resilient dopaminergic neurons. Understanding the differences between vulnerable and resilient dopaminergic populations is important not only for identifying factors that may increase risk of neurodegeneration, but also for defining the type of information processing that is lost early in Parkinson’s Disease.
2. Morphology of vulnerable SNc neurons shapes their incoming synaptic input
The vulnerable dopaminergic neurons located in the ventral tier of the SNc contain two distinct types of dendrites. “SNc dendrites” project along the dopaminergic cell body layer, while “SNr dendrites” project ventrally into the GABAergic SNr nucleus (Fig. 2). This overall structure of the SNc neurons has been noted in several early studies (Björklund and Lindvall, 1975; Oertel et al., 1982; Jimenez-Castellanos and Graybiel, 1987; Triarhou and Ghetti, 1989), and one study has reported that the more ventrally-located a neuron is within the SNc, the more likely it is to have an SNr dendrite (Henny et al., 2012).
Fig. 2. Dendritic morphology of ventral SNc neurons.
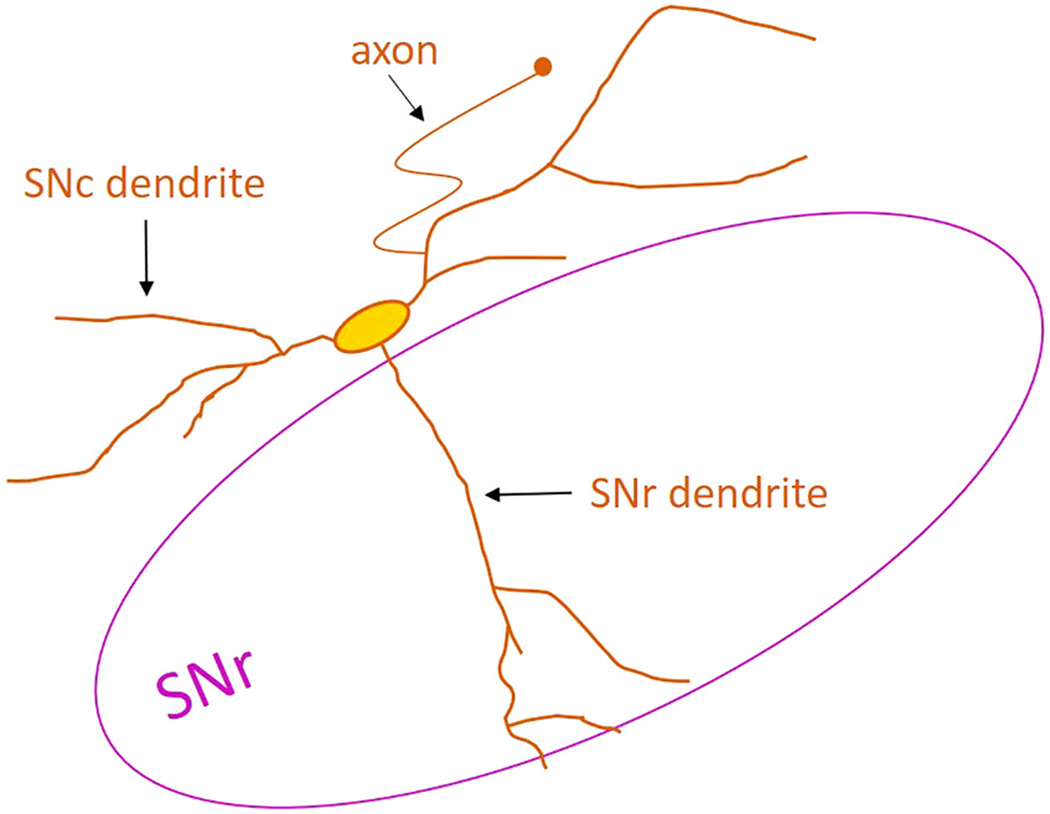
The ventrally-located SNc dopaminergic neurons have two distinct dendrite types: SNc dendrites projecting along the substantia nigra pars compacta cell body layer and SNr dendrites projecting ventrally into the GABAergic substantia nigra pars reticulata. The axon-bearing dendrite is most often an SNc dendrite.
The SNr dendrites are thought to be specialized dendrites with a distinct role compared to the SNc dendrites. However, the difference in function is unclear. The dopaminergic SNr dendrite may be a site of strong dendrodendritic dopamine release (Robertson and Robertson, 1989), but direct evidence for this idea has not yet been published. Another possibility is that computations performed by the SNr dendrite are different from those performed by the SNc dendrite. One idea is that the SNr dendrite is optimized for processing inhibitory input. Inhibitory synapses are proportionally denser on the SNr dendrite than on the SNc dendrite (Henny et al., 2012), and the long, unbranching morphology of the SNr dendrite facilitates communication of slow inhibitory signals to the soma (Evans et al., 2020). On the other hand, the SNc dendrites may be optimized for processing excitatory signals. In dopaminergic neurons, the axon often comes off of a dendrite, rather than issuing from the soma (Grace and Bunney, 1983). In 70% of SNc dopaminergic neurons, the axon-bearing dendrite is an SNc dendrite, while the SNr dendrites are only axon-bearing in 18% of cells (Evans et al., 2020). The axon-bearing dendrite is in an ‘electrotonically privileged’ position, meaning that it transmits incoming excitatory input more effectively than non-axon-bearing dendrites (Häusser et al., 1995), and excitatory synaptic activity on the axon-bearing dendrite can evoke action potentials that do not propagate to the non-axon bearing dendrites (Gentet and Williams, 2007). The axon-bearing dendrite has a higher expression of the hyperpolarization-activated cation current (Ih) that the non-axon bearing dendrite, which may result in increased excitability of this dendrite (Engel and Seutin, 2015). Finally, increased dendritic branches between the soma and the axon initial segment are associated with increased pacemaking frequency (Moubarak et al., 2019). The SNc dendrites branch close to the soma, resulting in more numerous small dendritic compartments (Evans et al., 2020). Small dendritic compartments can amplify excitatory synaptic inputs through supralinear integration (Spruston, 2008), and because these compartments are close to the axon and soma, they are likely to be particularly effective at generating action potential output. Therefore, even though the density of excitatory synaptic inputs onto the SNc and SNr dendrites is similar (Henny et al., 2012), the input is likely to be more effective when located on the SNc dendrite. Together, the evidence suggests that SNr dendrites readily transmit inhibitory synaptic input, while SNc dendrites are optimized to transmit excitatory synaptic input.
3. Striatonigral control of dopaminergic neurons
Direct pathway spiny projection neurons in the dorsal striatum project to the midbrain (Kemp and Powell, 1971; Ribak et al., 1980; Wassef et al., 1981; Gerfen, 1985; Smith and Bolam, 1991) inhibiting both SNr GABAergic neurons (Grace and Bunney, 1985; Hikosaka et al., 1993; Connelly et al., 2010; Chuhma et al., 2011) and SNc dopaminergic neurons (Grace and Bunney, 1985; Paladini et al., 1999; Brazhnik et al., 2008; Lerner et al., 2015; McGregor et al., 2019; Evans et al., 2020). Because GABAergic SNr neurons also inhibit dopaminergic SNc neurons (Grace and Bunney, 1979; Tepper et al., 1995; Rizzi and Tan, 2019; Ambrosi and Lerner, 2021), the striatonigral connection has the potential to both inhibit and disinhibit SNc activity (Fig. 3). These findings have led to the question: Does striatal activity increase dopaminergic activity or decrease it? While this question is the subject of ongoing research (Paladini and Tepper, 1999; Mailly et al., 2003; Lobb et al., 2011; Paladini and Tepper, 2016; Yang et al., 2018; Ambrosi and Lerner, 2021), it is likely that striatal activity will inhibit dopaminergic neurons in some situations, and disinhibit dopaminergic neurons in others. There are several possible scenarios that could make sense of the striatum’s ability to both inhibit and disinhibit the dopaminergic neurons of the SNc. Here I describe the current data supporting and contradicting four specific hypotheses for how striatonigral circuitry may function. These hypotheses are not mutually exclusive and all four frameworks could play a role in shaping dopamine signals.
Fig. 3. Canonical pathway for striatonigral inhibition and disinhibition.
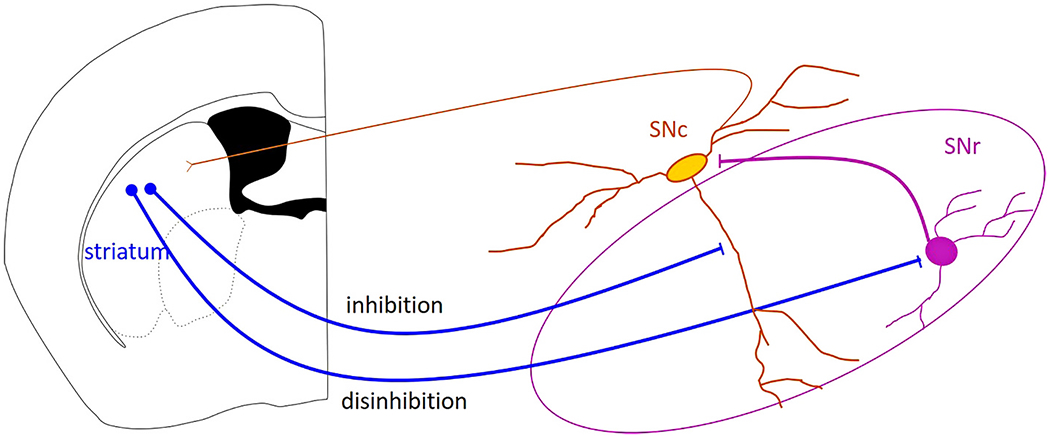
Striatal projection neurons can directly inhibit substantia nigra pars compacta (SNc) dopaminergic neurons, and can disinhibit them by inhibiting substantia nigra pars reticulata (SNr) GABAergic neurons. The inhibition and disinhibition of the SNc neurons will shape the dopamine signal sent to the striatum through SNc dopaminergic axons.
Hypothesis 1. Neurons located in striosomes (striatal patches) directly inhibit SNc neurons, while neurons located in the matrix disinhibit SNc neurons (Fig. 4).
Fig. 4. Hypothesis 1: Striosome inhibits and matrix disinhibits dopaminergic neurons of the substantia nigra pars compacta (SNc).
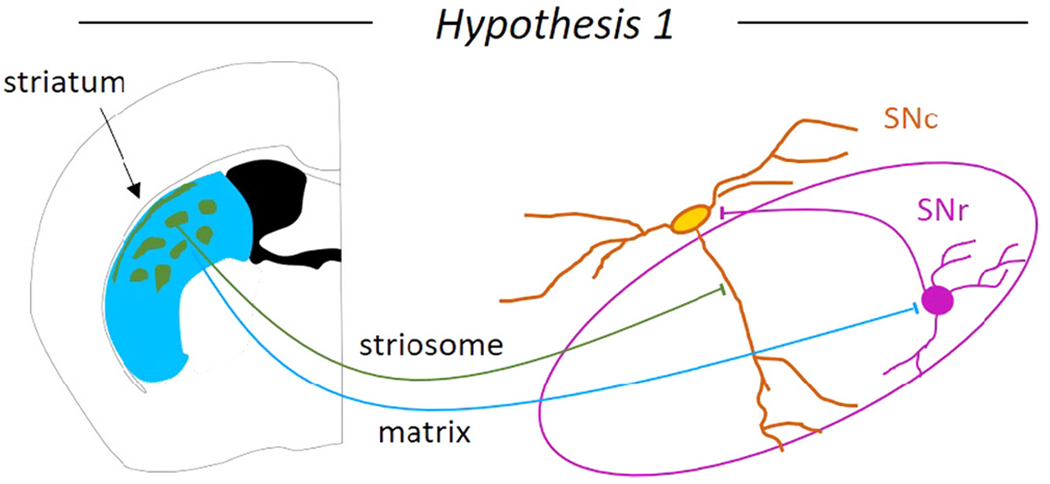
The two striatal compartments, the striosomes and the matrix may exert opposing control of the SNc dopaminergic neurons. The striosomes are thought to directly inhibit the SNc dopaminergic neurons on the SNr dendrite, while the matrix is thought to disinhibit the SNc dopaminergic neurons through inhibition of substantia nigra pars reticulata (SNr) GABAergic neurons.
The dorsal striatum can be divided into the striosome (mu-opioid receptor-positive) and matrix (calbindin-positive) compartments (Graybiel et al., 1981; Gerfen, 1984). Both compartments contain direct pathway spiny projection neurons that project to the midbrain. The idea that striosome neurons inhibit while the matrix neurons disinihibit midbrain dopaminergic neurons first came from anatomical evidence that the striosomes send axons to the dopaminergic SNc, while the matrix sends axons to the GABAergic SNr (Gerfen, 1985; Fujiyama et al., 2011; Watabe-Uchida et al., 2012), but see (Smith et al., 2016). Striosomal axons form columns in the primate SNr (Smith and Parent, 1986; Lévesque and Parent, 2005) and tightly wrap around the SNr dendrite of the ventral dopaminergic neurons forming ‘striosome-dendron bouquets’ in mice (Crittenden et al., 2016; Matsushima and Graybiel, 2020). These striosome-dendron bouquets are enriched in pre-synaptic GABA release markers (GAD1/GAD2), D2 dopamine receptors, and cannabinoid (CB1) receptors (Crittenden et al., 2016; Davis et al., 2018). Electrophysiological recordings show that within this bouquet structure, the striosomes selectively inhibit the SNr dendrites of the dopaminergic neurons by recruiting local GABAA and GABAB receptors (Evans et al., 2020). In vivo dopamine measurements show that activation of striosomal fibers reduces dopamine release in the striatum (Nadel et al., 2021), indicating that even with local circuitry intact, the overall effect of striosome stimulation is to inhibit dopaminergic activity.
The evidence that neurons in the striatal matrix disinhibit the dopaminergic neurons is less clear. The axons from the matrix do not participate in striosome-dendron bouquets (Crittenden et al., 2016), but retrograde rabies viral tracing from dopaminergic neurons has shown both few (Watabe-Uchida et al., 2012) and many (Smith et al., 2016) labeled matrix neurons. Optogenetic stimulation of striatal matrix axons only weakly inhibits the dopaminergic SNc neurons when compared to stimulation of striosomal axons (Evans et al., 2020). Similarly, the connection from the striosomes to the SNc is of a similar strength to the connection from the entire striatum to the SNc. However, the entire striatum was more strongly connected to the GABAergic neurons of the SNr than the striosomes alone (McGregor et al., 2019). This finding suggests that the striatal matrix inhibits the SNr neurons more strongly than the striosomes do. However, the strength of the synaptic connection from the striatal matrix neurons to the SNr GABAergic neurons has not been directly tested or directly compared to the striosome-SNr connection. In addition, the relative strength of the SNr GABAergic input to the SNc dopaminergic neurons is unknown and may depend on striatal subregion (Ambrosi and Lerner, 2021). Therefore, the hypothesis that the striosomes inhibit and the matrix neurons disinhibit dopaminergic neurons is well-supported, with only a few experiments required to fully confirm it.
Hypothesis 2. The striatum inhibits ventral tier SNc neurons, but disinhibits dorsal tier SNc neurons (Fig 5).
Fig. 5. Hypothesis 2: the ventral tier neurons of the substantia nigra pars compacta (SNc) are inhibited by the striatum, while the dorsal tier neurons of the SNc are disinhibited.
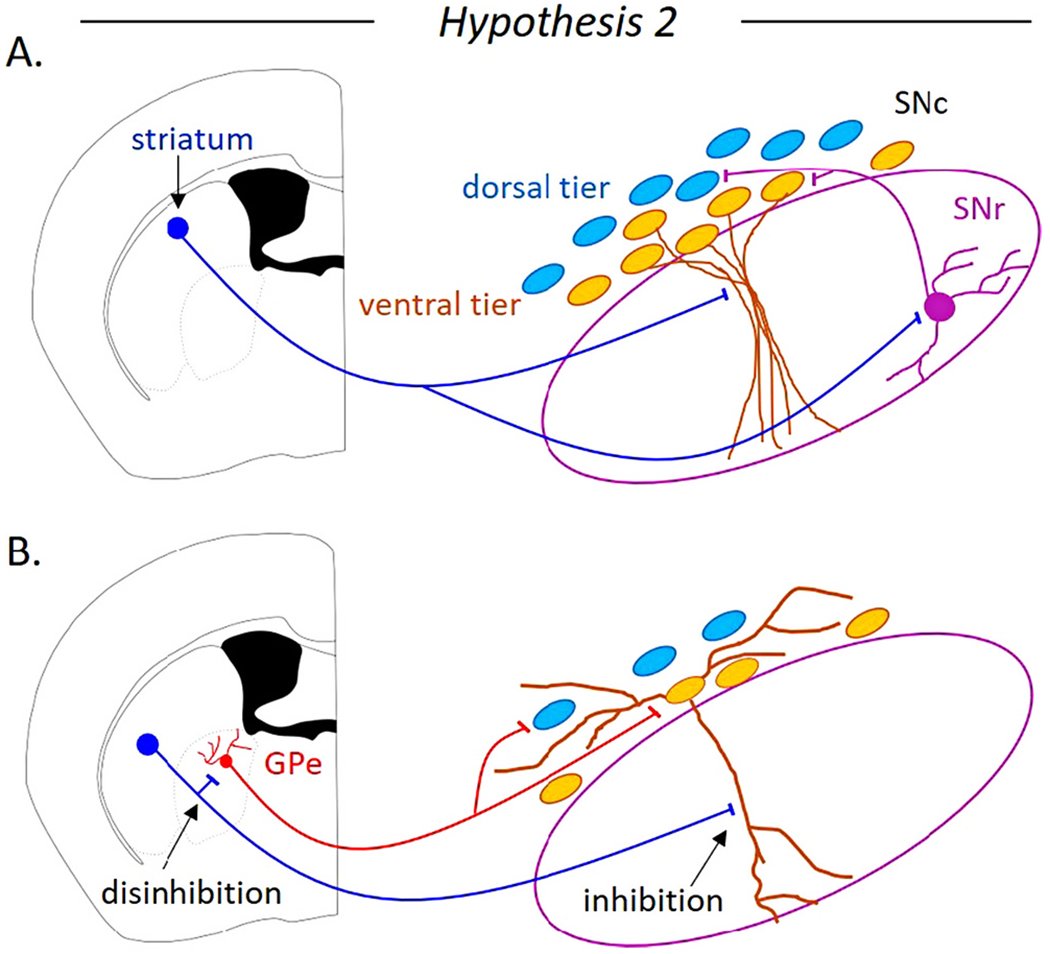
A. The striatal direct pathway may inhibit the ventrally-located SNc neurons and disinhibit the dorsally-located SNc neurons through the GABAergic neurons of the substantia nigra pars reticulata (SNr). B. A non-canonical pathway for striatonigral disinhibition is through the globus pallidus external segment (GPe). The striatal direct pathway spiny projection neurons inhibit a subpopulation of GPe neurons known to directly inhibit dopaminergic SNc neurons.
The vulnerable ventral tier neurons of the SNc receive strong inhibition from the striatum. Anatomical studies show that axons from the dorsal striatum predominately project to the ventral SNc (Gerfen, 1985; Hedreen and DeLong, 1991; Lynd-Balta and Haber, 1994; Maurin et al., 1999; Haber et al., 2000; Crittenden et al., 2016; Wu et al., 2019). Calcium imaging and electrophysiological recordings show that the striatum makes stronger functional synaptic connections onto the ventral tier neurons than onto the dorsal tier SNc neurons (Evans et al., 2020). Therefore, it is clear that the vulnerable, ventral tier SNc neurons are more strongly inhibited by the striatum than the resilient, dorsal tier SNc neurons. Because the dorsal tier receives weak direct striatal inhibition, these neurons are more likely to be disinhibited by striatal activation. In this framework, the inhibitory and disinhibitory circuit could be coming from either the same striatal neurons or separate neural populations. For example, hypothesis 1 and hypothesis 2 could both be true if the striosomes inhibited the ventral tier and the matrix disinhibited the dorsal tier. While hypothesis 2 is well-supported, a key piece of information that has yet to be experimentally determined is the relative strength of SNr GABAergic synapses onto the ventral and dorsal tier SNc neurons.
An interesting, but mostly unexplored possibility is that the dorsal tier may be disinhibited through pathways not involving the SNr. One alternative pathway is through the external globus pallidus (GPe). Striatal direct pathway neurons selectively inhibit the Lhx6-positive (Spix et al., 2021) and Npas1-positive (Cui et al., 2021) population of GPe neurons. While there is some overlap between these GPe subtypes (Abecassis et al., 2020), the Lhx6-positive neurons have been shown to inhibit both the dorsal and ventral tier SNc neurons selectively activating GABAA receptors and undergoing strong short-term synaptic depression (Evans et al., 2020). The striatal direct pathway inhibiting both the Lhx6-GPe neurons and the ventral tier SNc neurons could create a situation where the ventral dopaminergic neurons are inhibited, but the dorsal tier neurons are disinhibited (Fig. 5). Striatal activation of GABAB receptors inhibits the ventral tier neurons so strongly that it may overcome any potential disinhibition through the GPe with the overall result being inhibition of the ventral tier. However, because the striatum does not strongly inhibit dorsal SNc neurons, the overall effect on the dorsal tier would be disinhibition through the GPe. Further experiments are needed to determine the extent to which significant disinhibition of dorsal dopaminergic neurons occurs through direct pathway inhibition of Lhx6-positive or Npas1-positive GPe neurons.
Hypothesis 3. Brief striatal activity disinhibits while sustained striatal activity inhibits dopaminergic neurons (Fig 6).
Fig. 6. Hypothesis 3: The timing of the striatal activity determines whether the dopamine neurons of the substantia nigra pars compacta (SNc) are inhibited or disinhibited.
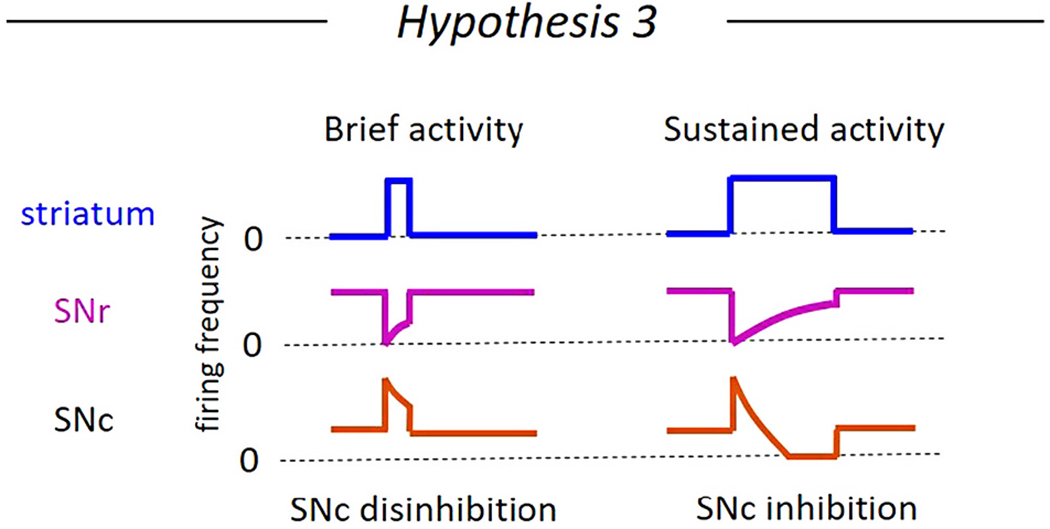
Brief increases in striatal projection neuron firing frequency briefly inhibits the substantia nigra pars reticulata (SNr) GABAergic neurons to disinhibit SNc dopaminergic neurons (left). Sustained increases in striatal projection neuron firing could result in short term synaptic depression between the striatum and the SNr neurons or between the SNr neurons and the SNc, resulting in a short disinhibition followed by a period of inhibition in the SNc dopaminergic neurons.
In vivo experiments stimulating the striatum and recording action potentials from dopaminergic neurons show that single pulses and trains of striatal activation result in complex responses including excitatory, early inhibitory, and late inhibitory components, with larger stimulation amplitudes and longer trains of activation associated with inhibitory responses (Grace and Bunney, 1985; Brazhnik et al., 2008). These multicomponent responses reflect the complex inhibitory and disinhibitory circuitry between the striatum and the dopaminergic neurons. To determine whether striatal activation time course influences the inhibition vs disinhibition state of the dopaminergic neurons, the characteristics of the synaptic connections from the striatum to the GABAergic SNr neurons and to the dopaminergic SNc neurons must be understood.
The synaptic connection from the striatum to the SNc dopaminergic neurons and from the striatum and the SNr GABAergic neurons appear to display different temporal characteristics, suggesting the possibility that distinct patterns of striatal activity would differentially cause inhibition vs disinhibition in the dopaminergic SNc neurons. The striatonigral synapses on the SNc dopaminergic neurons become stronger over time during sustained activity. Specifically, the striosome-SNc connection includes facilitating GABAA-mediated currents and a slow-developing GABAB-mediated current (Evans et al., 2020). By contrast, the striosome-SNr connections show short term depression and do not include a GABAB-mediated component (Faust, 2017). This suggests that a period of brief striatal activity would only weakly inhibit the SNc dopaminergic neurons, but would strongly inhibit the SNr GABAergic neurons resulting in a brief period of dopaminergic disinhibition. On the other hand, a sustained period of striatal activity would strongly inhibit the SNc dopaminergic neurons while only weakly inhibiting the SNr GABAergic neurons, resulting in overall inhibition of dopaminergic neurons (Fig. 6). However, the evidence that the striatal input to the SNr and SNc differ in their time course has been specific to the activation of striosomes. Electrical activation of the striatonigral connection (including both striosome and matrix connections) in brain slices has been shown to cause facilitating inhibitory synaptic currents on GABAergic SNr neurons (Wallmichrath and Szabo, 2002; Connelly et al., 2010; de Jesús Aceves et al., 2011), and it is not yet known whether the striatum, as a whole, activates the GABAB receptors located on SNr neurons (Boyes and Bolam, 2003). Therefore, additional experiments are needed to conclusively determine which patterns of striatal activity will promote inhibition vs disinhibition of the dopaminergic neurons.
A second factor to consider is the connection between the SNr GABAergic neurons and the SNc dopaminergic neurons. For the striatum to effectively disinhibit the dopaminergic neurons, the SNr GABAergic neurons receiving striatal input must tonically inhibit the SNc. Historically, it has been difficult to isolate and characterize the direct synaptic connection between the SNr neurons and the dopaminergic neurons because many inhibitory axons from the striatum and the globus pallidus are intermixed with the SNr GABAergic cell bodies. Therefore, electrical stimulation within the SNr (Hajós and Greenfield, 1994; Saitoh et al., 2004) will not selectively activate SNr inputs to the SNc. However, recent work using optogenetics and slice electrophysiology has shown that genetically- and anatomically-defined subsets of SNr neurons selectively inhibit SNc dopaminergic neurons (Rizzi and Tan, 2019; Ambrosi and Lerner, 2021). This connection appears to be mediated by GABAA receptors and to display short term synaptic depression. However, a comprehensive analysis of the short-term plasticity and the receptors activated at this connection has not been published. Therefore, the hypothesis that brief striatal activation disinhibits while sustained striatal activation inhibits SNc dopaminergic neurons is somewhat supported, but key experiments are needed to draw a firm conclusion. These experiments include applying trains of optical stimulation to channel rhodopsin-expressing SNr neurons while recording from SNc neurons. This would determine the extent to which the SNr-SNc connection undergoes short-term synaptic depression of facilitation.
Hypothesis 4. Striatal inhibition promotes rebound dopamine activity (Fig. 7).
Fig. 7. Hypothesis 4: Striatal inhibition of substantia nigra pars compacta (SNc) dopaminergic neurons generates rebound activity.
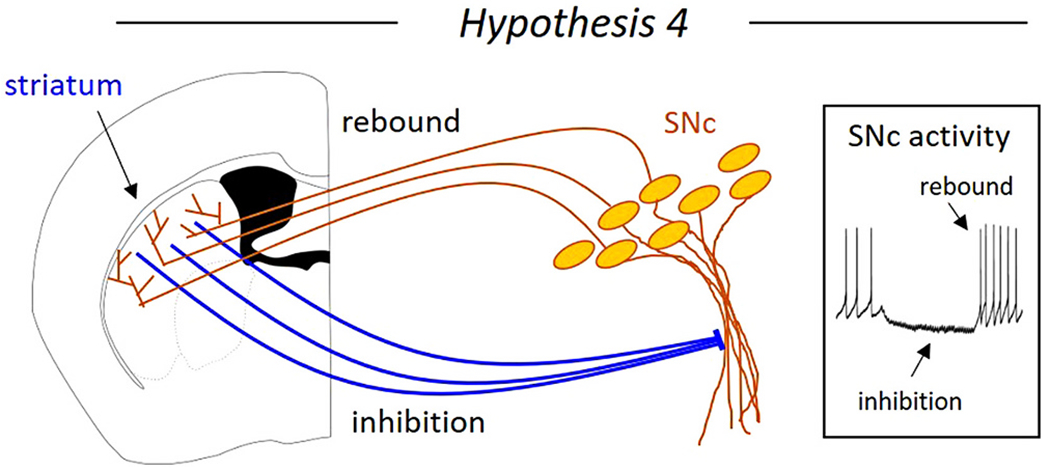
Striatal inhibition of SNc neurons recruits intrinsic rebound mechanisms in the ventral SNc neurons, causing increased dopaminergic neuron activity when inhibition is released.
Another way that striatal input to dopaminergic neurons can cause a type of inhibitory and disinhibitory activity is by recruiting intrinsic rebound mechanisms in the dopamine neurons and causing a pause-rebound firing pattern. Dopaminergic neurons are often inhibited during aversive stimuli and rebound firing may act as a safety or relief signal after aversive events (Oleson et al., 2012; Lee et al., 2016; Schultz, 2019). Unlike the dorsal tier SNc neurons, the vulnerable ventral tier SNc neurons highly express channels recruited by inhibition such as hyperpolarization-dependent cation channels (Neuhoff et al., 2002) and T-type calcium channels (Evans et al., 2017). Because these channels require membrane potential hyperpolarization to be engaged, they facilitate rebound firing upon release of inhibition. However, not all forms of inhibition can recruit these rebound currents. GABAA receptor activation inhibits dopaminergic neurons via chloride ion channels, which shunt current, but do not strongly hyperpolarize. This is because dopaminergic neurons have a high (−62 to −69 mV) reversal potential for chloride (Grace and Bunney, 1985; Gulácsi et al., 2003). By contrast, GABAB receptor activation strongly hyperpolarizes dopamine neuron membrane potential through the activation of g-protein dependent inwardly rectifying potassium (GIRK) channels (Beckstead and Williams, 2007; Koyrakh et al., 2005) and blocking sodium leak channels (Philippart and Khaliq, 2018). The striatonigral connection from the striosomes to the ventral tier SNc recruits GABAB receptors and can generate pause-rebound dopamine activity through the striosome-dendron bouquet connection (Evans et al., 2020). Because these ventral tier dopamine neurons project back to the striatum (Gerfen et al., 1987a; Jiménez-Castellanos and Graybiel, 1989; Prensa and Parent, 2001; Matsuda et al., 2009; Poulin et al., 2018; Wu et al., 2019), this circuit represents a mechanism by which the striatal neurons might control the dopamine release back onto themselves (Fig. 7). Because this dopamine signal directly follows striatal activity, it would be precisely timed with respect to striatal neuron action potentials and may serve as a plasticity signal (Yagishita et al., 2014; Shindou et al., 2019) and be critical for habit and motor skill learning (Carmichael et al., 2021). While ventral tier SNc neurons were once thought to selectively innervate striosomes (Gerfen et al., 1987a; Jiménez-Castellanos and Graybiel, 1989; Prensa and Parent, 2001), more recent work suggests that these neurons do heavily innervate striosomes, but also innervate the striatal matrix (Matsuda et al., 2009; Wu et al., 2019). However, future work is needed to determine whether dopaminergic SNc neurons connect back to the same striosomal neurons that inhibit them forming a closed loop, or to adjacent striatal regions, forming an open loop, ascending spiral, or descending spiral (Haber et al., 2000; Belin and Everitt, 2008).
Pause-rebound activity has been observed in dopamine neurons in vivo (Brischoux et al., 2009; Wang and Tsien, 2011) and in fluorescent measurements of dopamine release in the striatum (Gut et al., 2022). Pause-rebound activity is particularly strong in ventral SNc neurons in monkeys (Fiorillo et al., 2013a, 2013b). However, additional experiments are needed to determine whether striatal activity causes this pause-rebound in vivo. Because multiple striatal neurons are likely to synapse on each dopaminergic neuron (Lévesque and Parent, 2005), striatal activity would likely need to be synchronized to generate pause-rebound firing. Future work is required to determine the extent to which striatonigral connectivity governs the pause-rebound activity observed in the ventral SNc in vivo.
4. Potential importance of the bouquet structure
The striatonigral connection may communicate information in a highly specialized way due to the unique striosome-dendron bouquet structure. This structure is characterized by tightly packed dopaminergic dendrites with thin striatal axons wrapping around them. The parallel dopaminergic dendrites in close proximity to each other may facilitate dendrodendritic communication between SNc neurons, while the high-density striatal axons may facilitate dopamine signaling from the SNc neurons to the pre-synaptic striatal terminals.
Dendrodendritic communication between SNc dopaminergic neurons has been observed for over 40 years (Groves et al., 1975; Cheramy et al., 1981). When depolarized, midbrain dopaminergic neurons can release dopamine from their dendrites and activate D2 dopamine receptors on nearby neurons (Beckstead et al., 2004; Gantz et al., 2013) and on themselves (Hikima et al., 2021). This D2 receptor activation inhibits the dopaminergic neuron through the activation of G-protein coupled inwardly rectifying potassium (GIRK) channels (Beckstead and Williams, 2007; Koyrakh et al., 2005), and through the inhibition of the sodium leak channel (NALCN) (Philippart and Khaliq, 2018), similar to GABAB activation. An early study evaluating dopaminergic dendrites with electron microscopy postulated that different dendrites would serve different roles, specifically hypothesizing that there were ‘presynaptic dendrites’ which receive few axodendritic contacts and whose role would be primarily to release dopamine (Groves and Linder, 1983). Recent work shows that the dopaminergic SNr dendrites contain the mRNA necessary for local translation of dopamine transmission machinery (Hobson et al., 2022). Another recent study using dopamine-sensitive nanofilm and cultured dopaminergic neurons observed that some dopaminergic dendrites released dopamine, while others did not (Bulumulla et al., 2022). Because dendrodendritic communication between dopaminergic neurons depends on dopamine transporters, but does not depend strongly on dopamine diffusion (Ford et al., 2010; Condon et al., 2021), the D2 dopamine receptors activated by somatodendritic release of dopamine must be in close proximity to the release sites. In the bouquet structure, the dopaminergic dendrites can run parallel to each other for long distances (100 s of microns) and each dendrite is within a few microns of several others (Crittenden et al., 2016). Not only are these bouquet-participating dendrites in close proximity, but their long, unbranched nature can facilitate slow inhibitory signals reaching the soma to inhibit action potential firing (Evans et al., 2020). Therefore, the dendron bouquets may be sites of particularly effective dendrodendritic inhibition between SNc dopaminergic neurons.
The bouquet structure may also facilitate dendroaxonic communication between the dopaminergic dendrites and presynaptic striatal terminals. Striatal axons express presynaptic D1 dopamine receptors at their synaptic connections with both dopaminergic and non-dopaminergic neurons (Caillé et al., 1996; Hattori et al., 1979). When activated, these D1 receptors potentiate GABA release (Cameron and Williams, 1993; Radnikow and Misgeld, 1998). In the striosome-dendron bouquet, dendritically released dopamine has the potential to enhance striatal GABAergic inhibition onto the bouquet-participating ventral tier SNc neurons. The axonal configuration of the bouquet may also shape the way that the striatum inhibits the dopaminergic neurons. Striatonigral axons are fine processes with minimal boutons and are densely packed in striosome-dendron bouquets (Crittenden et al., 2016; Evans et al., 2020; Matsushima and Graybiel, 2020). Multiple striatal neurons are likely to participate in each bouquet (Lévesque and Parent, 2005). Striato-nigral axons have a slow conduction velocity (~0.7 m/s) (Connelly et al., 2010), and their synaptic signal builds up over time (Evans et al., 2020). These characteristics may facilitate the transmission of sustained signals to the dopaminergic neurons and may enhance the extra-synaptic activation of GABAA and GABAB receptors on dopaminergic dendrites. However, more studies are needed to understand how the bouquet structure affects neurotransmitter release dynamics and the degree of synchronization in striatonigral axons.
5. Inhibition and disinhibition in pathological conditions
In pathological conditions, the circuitry controlling inhibition and disinhibition of dopaminergic neurons can be disrupted. In Parkinson’s Disease when the ventral, more vulnerable SNc neurons begin to degenerate, the striosome-dendron bouquet structure and the striatonigral connection may be disrupted. The evidence for dendritic disruption in PD includes human and model animal studies. In PD patients, dopaminergic dendrites in the SNr are truncated and display reduced spine density (Patt et al., 1991). The MitoPark mouse model shows morphological disruptions to dendritic arbors (Lynch et al., 2018), and mutations in the leucine-rich repeat kinase (LRRK2), implicated in inherited Parkinson’s Disease, alter dendritic morphology in culture (MacLeod et al., 2006). A 6-hydroxydopamine (6-OHDA) lesion model of PD shows that the ventral tier, calbindin-negative SNc neurons with SNr dendrites are selectively lost compared to unlesioned controls (Gerfen et al., 1987a). Importantly, the SNr dendrites of the dopaminergic neurons appear to be lost in the LRRK2 (R1441G) mutation mouse model of PD (Li et al., 2009) and in an alpha-synuclein seeding model of PD (Paumier et al., 2015). These studies did not determine whether the SNr dendrites were selectively degenerated relative to the SNc dendrites or examine the extent to which the SNr dendrite loss was simply due to the loss of ventral tier SNc neurons. Regardless of whether the SNr dendrites are lost because the cells containing them are lost or because SNr dendrites degenerate relative to the other dendrites, these findings suggest that the dendrite-selective striatonigral connection would be disrupted in Parkinson’s Disease.
The degeneration of the dopaminergic dendrites in the SNr may have additional consequences due to the reduced dendritic release of dopamine within the SNr. Dopamine release in the SNr can directly modulate SNr GABAergic output (Zhou et al., 2009), and acute blockade of dopamine receptors within the SNr results in a bursting pattern similar to that observed in Parkinsonian conditions (de Jesús Aceves et al., 2011). Dopamine neuron loss results in an upregulation of D1 receptors on striatonigral terminals which increases their sensitivity to dopamine and enhances their dopamine-dependent inhibitory output (Ding et al., 2015). A recent study selectively knocked out Ndufs2 from dopaminergic neurons to model progressive degeneration in PD and showed that the loss of dendritic dopamine release was critical for the development of Parkinsonian motor symptoms in the experimental animals (González-Rodríguez et al., 2021). Previous work has shown that the dopamine precursor commonly used to treat PD L-dihydroxyphenylalanine (L-DOPA) increases dopamine release in the striatum of dopamine depleted (6-OHDA) mice for only a short time, but increases dendritic dopamine release in the midbrain for the duration of the behavioral effects (Robertson and Robertson, 1989). Similarly, these behavioral effects (L-DOPA induced rotational behavior) were more effectively reduced by dopamine receptor antagonists infused into the SNr than by the same antagonists infused into the striatum. Together these results implicate dendritic dopamine release in the symptoms in PD, and suggest that the loss of dopaminergic dendrites in the SNr could directly impair basal ganglia function by disrupting midbrain dopamine signaling.
Because the ventral tier SNc neurons degenerate first in PD and these neurons preferentially receive direct inhibition from the striosomes, the connection between the striatum and the SNc dopaminergic neurons may undergo a functional shift from inhibition to disinhibition in PD (Fig. 8). The early degeneration of the ventral tier and the loss of the SNr dendrites would reduce direct inhibition from the striatum. However, based on hypothesis 2, the remaining dopaminergic neurons in the dorsal tier could continue to be disinhibited by striatal activity. Therefore, in this pathological condition, the disinhibited neurons would outnumber the inhibited neurons, shifting the overall balance of the striatonigral connection to disinhibition. Interestingly, in rodent models of PD, striatonigral neurons show increased excitability (Fieblinger et al., 2014), the striatonigral connection to the GABAergic SNr neurons shows a higher probability of GABA release due to the loss of GABAB-mediated presynaptic inhibition (Borgkvist et al., 2015), and the frequency and amplitude of spontaneous inhibitory post-synaptic currents (IPSCs) are increased in SNr GABAergic neurons (Faynveitz et al., 2019). Therefore, striatal disinhibition of the dopaminergic neurons through the SNr could be enhanced under dopamine-depletion conditions. Together, the disrupted inhibition pathway and the potentially-enhanced disinhibition pathway could play a role in the progression of PD pathology, resulting in hyper-excitability of the remaining dopaminergic neurons (Kovacheva et al., 2021) and potentially increasing their susceptibility to neurodegeneration.
Fig. 8. Hypothetical imbalance between inhibition and disinhibition of SNc dopaminergic neurons under pathological conditions.
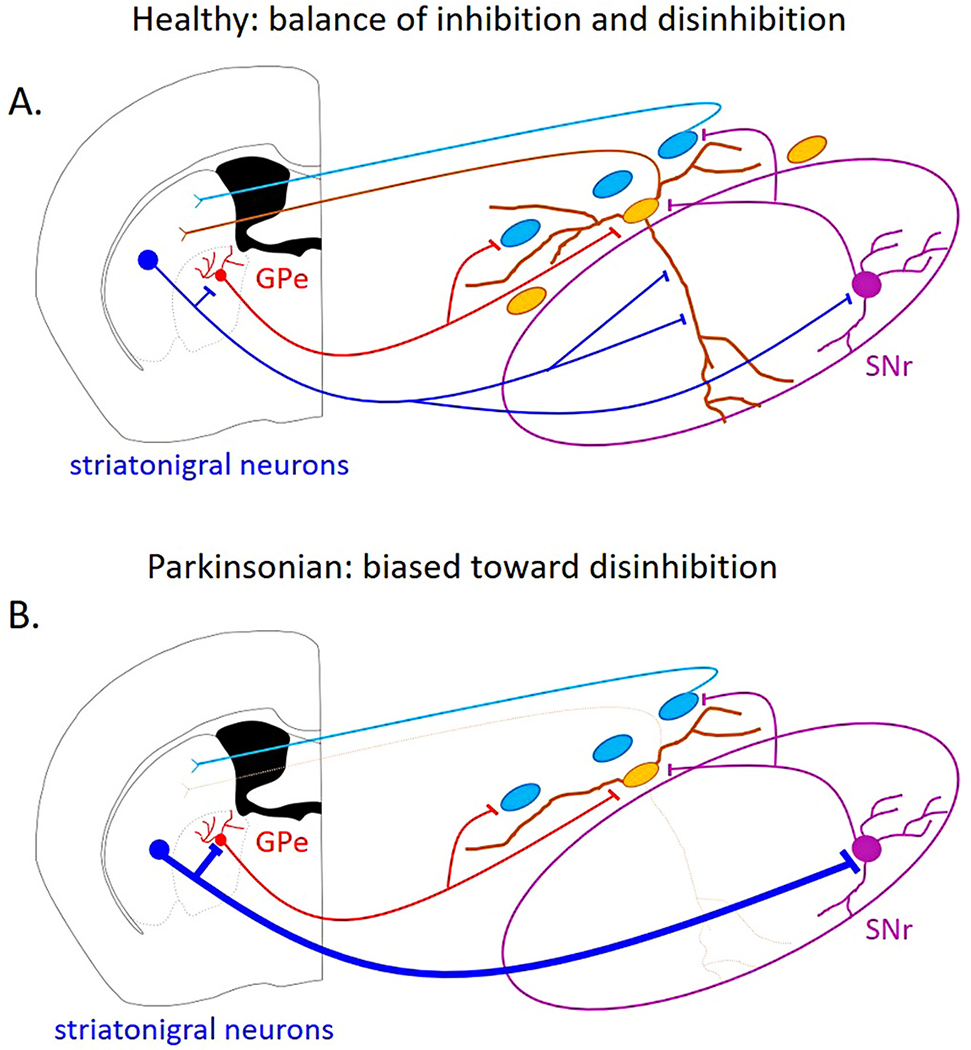
A. In the healthy condition, the balance of inhibition and disinhibition of the substantia nigra pars compacta (SNc) dopaminergic neurons is intact. B. Under Parkinsonian conditions, when the ventral SNc neurons have degenerated, direct striatonigral inhibition onto the SNc neurons is reduced, while disinhibitory pathways may be strengthened.
The information processing that is lost early in PD as a result of the selective degeneration of the ventral tier is just beginning to be understood. A study selectively lesioning the ventral tier, Aldh1a1-positive, SNc neurons showed that lesioned mice did not appear clearly Parkinsonian and could walk around an open field without significant impairment, but had severe impairments in motor learning as assessed by the accelerating rotarod task (Wu et al., 2019). Similarly, selective lesions of the striosomes (the striatal structures that selectively inhibit the ventral tier SNc neurons) do not cause movement impairments, but do impair stimulus-response and habit learning (Jenrette et al., 2019; Nadel et al., 2020). These studies suggest that the striatum-nigrastriatum loop involving the striosomes and ventral tier SNc neurons is critical for habit and motor learning, and predict that such learning would be impaired early in the time course of PD. However, addition studies using progressive models of PD are critical because compensatory mechanisms might develop as the ventral SNc neurons degenerate and these compensatory mechanisms could alter the inhibition and disinhibition circuitry over the course of the disease.
6. Summary
In this review, I have summarized the previous findings supporting four distinct hypotheses for the striatonigral control of dopaminergic signaling and have outlined some of the key experiments needed to fully confirm them. These four hypotheses are not mutually exclusive. Indeed, all four modes of inhibition and disinhibition could act in concert to govern dopaminergic output. The morphological characteristics of the SNc neurons and the distinct striosome-dendron bouquet structure shapes the dopaminergic response to striatal activity. The degeneration of the ventral tier SNc neurons in Parkinson’s Disease may disrupt the balance of inhibition and disinhibition from the striatum to the SNc, resulting in increased activity of the surviving dopaminergic neurons. This increase in activity could exacerbate the neurodegenerative processes occurring in Parkinson’s Disease.
Acknowledgements
Thanks to Megan Beaver and Paul Kramer for feedback on versions of this manuscript.
Funding
This manuscript was supported by BRAIN initiative NIH grant R00NS112417 and APDA grant 2021APDA00RG00000209666-R13 awarded to Rebekah C. Evans.
Footnotes
Declaration of Competing Interest
None.
References
- Abecassis ZA, Berceau BL, Win PH, García D, Xenias HS, Cui Q, Pamukcu A, Cherian S, Hernández VM, Chon U, et al. , 2020. Npas1+-Nkx2.1+ neurons are an integral part of the Cortico-pallido-cortical loop. J. Neurosci 40, 743–768. 10.1523/JNEUROSCI.1199-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila J, Cheng S, Kee N, Cao M, Wang M, Deng Q, Hedlund E, 2021. Spatial RNA sequencing identifies robust markers of vulnerable and resistant human midbrain dopamine neurons and their expression in Parkinson’s disease. Front. Mol. Neurosci 14, 699562 10.3389/fnmol.2021.699562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberico SL, Cassell MD, Narayanan NS, 2015. The vulnerable ventral tegmental area in Parkinson’s disease. Basal Ganglia 5, 51–55. 10.1016/j.baga.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi P, Lerner TN, 2021. Striatonigrostriatal circuit architecture for disinhibition of dopamine signaling. BioRxiv. 10.1101/2021.06.22.449416, 2021.06.22.449416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT, 2007. Long-term depression of a dopamine IPSC. J. Neurosci 27, 2074–2080. 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT, 2004. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42, 939–946. 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ, 2008. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57, 432–441. 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O, 1975. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 83, 531–537. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Avegno EM, Wong MY, Kheirbek MA, Sonders MS, Hen R, Sulzer D, 2015. Loss of Striatonigral GABAergic presynaptic inhibition enables motor sensitization in parkinsonian mice. Neuron 87, 976–988. 10.1016/j.neuron.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes J, Bolam JP, 2003. The subcellular localization of GABA(B) receptor subunits in the rat substantia nigra. Eur. J. Neurosci 18, 3279–3293. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, Shah F, Tepper JM, 2008. GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo. J. Neurosci 28, 10386–10398. 10.1523/JNEUROSCI.2387-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta L, Greengard P, 2014. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Front. Neuroanat 8, 152. 10.3389/fnana.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA, 2009. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U. S. A 106, 4894–4899. 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulumulla C, Krasley AT, Walpita D, Beyene AG, 2022. Visualizing synaptic dopamine efflux with a 2D nanofilm. BioRxiv. 10.1101/2022.01.19.476937, 2022.01.19.476937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé I, Dumartin B, Bloch B, 1996. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 730, 17–31. 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT, 1993. Dopamine D1 receptors facilitate transmitter release. Nature 366, 344–347. 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Canavier CC, Evans RC, Oster AM, Pissadaki EK, Drion G, Kuznetsov AS, Gutkin BS, 2016. Implications of cellular models of dopamine neurons for disease. J. Neurophysiol 116, 2815–2830. 10.1152/jn.00530.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael K, Evans RC, Lopez E, Sun L, Kumar M, Ding J, Khaliq ZM, Cai H, 2021. Function and regulation of ALDH1A1-positive nigrostriatal dopaminergic neurons in motor control and Parkinson’s disease. Front. Neural Circuits 15, 644776. 10.3389/fncir.2021.644776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J, 1981. Dendritic release of dopamine in the substantia nigra. Nature 289, 537–542. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S, 2011. Functional connectome of the striatal medium spiny neuron. J. Neurosci 31, 1183–1192. 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AF, Robinson BG, Asad N, Dore TM, Tian L, Williams JT, 2021. The residence of synaptically released dopamine on D2 autoreceptors. Cell Rep. 36, 109465 10.1016/j.celrep.2021.109465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly WM, Schulz JM, Lees G, Reynolds JNJ, 2010. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J. Neurosci 30, 14854–14861. 10.1523/JNEUROSCI.3895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Tillberg PW, Riad MH, Shima Y, Gerfen CR, Curry J, Housman DE, Nelson SB, Boyden ES, Graybiel AM, 2016. Striosome-dendron bouquets highlight a unique striatonigral circuit targeting dopamine-containing neurons. Proc. Natl. Acad. Sci. U. S. A 113, 11318–11323. 10.1073/pnas.1613337113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Du X, Chang IYM, Pamukcu A, Lilascharoen V, Berceau BL, García D, Hong D, Chon U, Narayanan A, et al. , 2021. Striatal direct pathway targets Npas1 + pallidal neurons. J. Neurosci 41, 3966–3987. 10.1523/JNEUROSCI.2306-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM, 1999. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. J. Neurol 122 (Pt 8), 1437–1448. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S, 2003. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909. 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davis MI, Crittenden JR, Feng AY, Kupferschmidt DA, Naydenov A, Stella N, Graybiel AM, Lovinger DM, 2018. The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS One 13, e0191436. 10.1371/journal.pone.0191436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesús Aceves J, Rueda-Orozco PE, Hernández R, Plata V, Ibañez-Sandoval O, Galarraga E, Bargas J, 2011. Dopaminergic presynaptic modulation of nigral afferents: its role in the generation of recurrent bursting in substantia nigra pars reticulata neurons. Front. Syst. Neurosci 5, 6. 10.3389/fnsys.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou F-M, 2015. Nigral dopamine loss induces a global upregulation of presynaptic dopamine D1 receptor facilitation of the striatonigral GABAergic output. J. Neurophysiol 113, 1697–1711. 10.1152/jn.00752.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda J, Pötschke C, Liss B, 2016. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of substantia nigra dopaminergic neurons in health and Parkinson’s disease. J. Neurochem 139 (Suppl. 1), 156–178. 10.1111/jnc.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Seutin V, 2015. High dendritic expression of Ih in the proximity of the axon origin controls the integrative properties of nigral dopamine neurons. J. Physiol 593, 4905–4922. 10.1113/JP271052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RC, Zhu M, Khaliq ZM, 2017. Dopamine inhibition differentially controls excitability of substantia nigra dopamine neuron subpopulations through T-type calcium channels. J. Neurosci 37, 3704–3720. 10.1523/JNEUROSCI.0117-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RC, Twedell EL, Zhu M, Ascencio J, Zhang R, Khaliq ZM, 2020. Functional dissection of basal ganglia inhibitory inputs onto substantia nigra dopaminergic neurons. Cell Rep. 32, 108156 10.1016/j.celrep.2020.108156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, 2017. Influence of the Neostriatal Patch System on the Prediction-Based Coding of Midbrain Dopaminergic Neurons. Rutgers University - Graduate School - Newark. [Google Scholar]
- Faynveitz A, Lavian H, Jacob A, Korngreen A, 2019. Proliferation of inhibitory input to the substantia Nigra in experimental parkinsonism. Front. Cell. Neurosci 13, 417. 10.3389/fncel.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ, 1991. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain J. Neurol 114 (Pt 5), 2283–2301. 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M, Greengard P, Cenci MA, et al. , 2014. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat. Commun 5, 5316. 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Yun SR, Song MR, 2013a. Diversity and homogeneity in responses of midbrain dopamine neurons. J. Neurosci 33, 4693–4709. 10.1523/JNEUROSCI.3886-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Song MR, Yun SR, 2013b. Multiphasic temporal dynamics in responses of midbrain dopamine neurons to appetitive and aversive stimuli. J. Neurosci 33, 4710–4725. 10.1523/JNEUROSCI.3883-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PEM, Williams JT, 2010. Control of extracellular dopamine at dendrite and axon terminals. J. Neurosci 30, 6975–6983. 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T, 2011. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur. J. Neurosci 33, 668–677. 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Bunzow JR, Williams JT, 2013. Spontaneous inhibitory synaptic currents mediated by a G protein-coupled receptor. Neuron 78, 807–812. 10.1016/j.neuron.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Ford CP, Morikawa H, Williams JT, 2018. The evolving understanding of dopamine neurons in the substantia nigra and ventral tegmental area. Annu. Rev. Physiol 80, 219–241. 10.1146/annurev-physiol-021317-121615. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Williams SR, 2007. Dopamine gates action potential backpropagation in midbrain dopaminergic neurons. J. Neurosci 27, 1892–1901. 10.1523/JNEUROSCI.5234-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, 1984. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311, 461–464. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, 1985. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J. Comp. Neurol 236, 454–476. 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Thibault J, 1987a. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J. Neurosci 7, 3935–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J, 1987b. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci 7, 3915–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère N, Burke Nanni S, Trudeau L-E, 2018. On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front. Neurol 9, 455. 10.3389/fneur.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez P, Zampese E, Surmeier DJ, 2020. Selective neuronal vulnerability in Parkinson’s disease. Prog. Brain Res 252, 61–89. 10.1016/bs.pbr.2020.02.005. [DOI] [PubMed] [Google Scholar]
- González-Rodríguez P, Zampese E, Stout KA, Guzman JN, Ilijic E, Yang B, Tkatch T, Stavarache MA, Wokosin DL, Gao L, et al. , 2021. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 599, 650–656. 10.1038/s41586-021-04059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1979. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur. J. Pharmacol 59, 211–218. 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1983. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–2. Action potential generating mechanisms and morphological correlates. Neuroscience 10, 317–331. 10.1016/0306-4522(83)90136-7. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1985. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 333, 271–284. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW, Yoneoka ES, Elde RP, 1981. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience 6, 377–397. 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- Groves PM, Linder JC, 1983. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp. Brain Res 49, 209–217. [DOI] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Young SJ, Rebec GV, 1975. Self-inhibition by dopaminergic neurons. Science 190, 522–528. [DOI] [PubMed] [Google Scholar]
- Gulácsi A, Lee CR, Sík A, Viitanen T, Kaila K, Tepper JM, Freund TF, 2003. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. J. Neurosci 23, 8237–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut NK, Yilmaz D, Kondabolu K, Huerta-Ocampo I, Mena-Segovia J, 2022. Selective Inhibition of Goal-Directed Actions in the Mesencephalic Locomotor Region, 2022.01.18.476772. 10.1101/2022.01.18.476772. [DOI] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR, 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Greenfield SA, 1994. Synaptic connections between pars compacta and pars reticulata neurones: electrophysiological evidence for functional modules within the substantia nigra. Brain Res. 660, 216–224. 10.1016/0006-8993(94)91292-0. [DOI] [PubMed] [Google Scholar]
- Hattori T, McGeer PL, McGeer EG, 1979. Dendro axonic neurotransmission. II. Morphological sites for the synthesis, binding and release of neurotransmitters in dopaminergic dendrites in the substantia nigra and cholinergic dendrites in the neostriatum. Brain Res. 170, 71–83. 10.1016/0006-8993(79)90941-7. [DOI] [PubMed] [Google Scholar]
- Häusser M, Stuart G, Racca C, Sakmann B, 1995. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron 15, 637–647. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, DeLong MR, 1991. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J. Comp. Neurol 304, 569–595. 10.1002/cne.903040406. [DOI] [PubMed] [Google Scholar]
- Henny P, Brown MTC, Northrop A, Faunes M, Ungless MA, Magill PJ, Bolam JP, 2012. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat. Neurosci 15, 613–619. 10.1038/nn.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikima T, Lee CR, Witkovsky P, Chesler J, Ichtchenko K, Rice ME, 2021. Activity-dependent somatodendritic dopamine release in the substantia nigra autoinhibits the releasing neuron. Cell Rep. 35, 108951 10.1016/j.celrep.2021.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Miyashita N, 1993. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp. Brain Res 95, 457–472. 10.1007/BF00227139. [DOI] [PubMed] [Google Scholar]
- Hobson BD, Kong L, Angelo MF, Lieberman OJ, Mosharov EV, Herzog E, Sulzer D, Sims PA, 2022. Subcellular and regional localization of mRNA translation in midbrain dopamine neurons. Cell Rep. 38, 110208 10.1016/j.celrep.2021.110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenrette TA, Logue JB, Horner KA, 2019. Lesions of the patch compartment of dorsolateral striatum disrupt stimulus-response learning. Neuroscience 415, 161–172. 10.1016/j.neuroscience.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM, 1987. Subdivisions of the primate substantia nigra pars compacta detected by acetylcholinesterase histochemisty. Brain Res. 437, 349–354. 10.1016/0006-8993(87)91650-7. [DOI] [PubMed] [Google Scholar]
- Jiménez-Castellanos J, Graybiel AM, 1989. Compartmental origins of striatal efferent projections in the cat. Neuroscience 32, 297–321. 10.1016/0306-4522(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Kamath T, Abdulraouf A, Burris SJ, Langlieb J, Gazestani V, Nadaf NM, Balderrama K, Vanderburg C, Macosko EZ, 2022. Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease. Nat. Neurosci 25, 588–595. 10.1038/s41593-022-01061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM, Powell TP, 1971. The site of termination of afferent fibres in the caudate nucleus. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 262, 413–427. 10.1098/rstb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT, 2013. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain J. Neurol 136, 2419–2431. 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacheva L, Shin J, Farassat N, Roeper J, 2021. Kv4.3 channel downregulation mediates chronic post-lesional pacemaker acceleration in surviving dopamine substantia nigra neurons. In: BioRxiv. 10.1101/2021.08.09.455657, 2021.08.09.455657. [DOI] [Google Scholar]
- Koyrakh L, Luján R, Colón J, Karschin C, Kurachi Y, Karschin A, Wickman K, 2005. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J. Neurosci 25, 11468–11478. 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Parent A, 1991. Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport 2, 601–604. [DOI] [PubMed] [Google Scholar]
- Lee JC, Wang LP, Tsien JZ, 2016. Dopamine rebound-excitation theory: putting brakes on PTSD. Front. Psychiatry 7, 163. 10.3389/fpsyt.2016.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, et al. , 2015. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647. 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Parent A, 2005. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc. Natl. Acad. Sci. U. S. A 102, 11888–11893. 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, et al. , 2009. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci 12, 826–828. 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yu J, Ding J, Xie C, Sun L, Rudenko I, Zheng W, Sastry N, Luo J, Rudow G, et al. , 2014. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J. Clin. Invest 124, 3032–3046. 10.1172/JCI72176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Wilson CJ, Paladini CA, 2011. High-frequency, short-latency disinhibition bursting of midbrain dopaminergic neurons. J. Neurophysiol 105, 2501–2511. 10.1152/jn.01076.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WB, Tschumi CW, Sharpe AL, Branch SY, Chen C, Ge G, Li S, Beckstead MJ, 2018. Progressively disrupted somatodendritic morphology in dopamine neurons in a mouse Parkinson’s model. Mov. Disord. Off. J. Mov. Disord. Soc 33, 1928–1937. 10.1002/mds.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN, 1994. Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J. Comp. Neurol 345, 562–578. 10.1002/cne.903450407. [DOI] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A, 2006. The familial parkinsonism gene LRRK2 regulates neurite process morphology. Neuron 52, 587–593. 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Deniau J-M, 2003. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J. Neurosci 23, 5247–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T, 2009. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci 29, 444–453. 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A, Graybiel AM, 2020. Combinatorial developmental controls on striatonigral circuits. Cell Rep. 31, 107778 10.1016/j.celrep.2020.107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin Y, Banrezes B, Menetrey A, Mailly P, Deniau JM, 1999. Three-dimensional distribution of nigrostriatal neurons in the rat: relation to the topography of striatonigral projections. Neuroscience 91, 891–909. [DOI] [PubMed] [Google Scholar]
- McGregor MM, McKinsey GL, Girasole AE, Bair-Marshall CJ, Rubenstein JLR, Nelson AB, 2019. Functionally distinct connectivity of developmentally targeted striosome neurons. Cell Rep. 29, 1419–1428.e5. 10.1016/j.celrep.2019.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie DA, Cartwright HR, Halliday GM, 1997. Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson’s disease. Exp. Neurol 144, 202–213. 10.1006/exnr.1997.6418. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA, 2011. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience 198, 95–111. 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubarak E, Engel D, Dufour MA, Tapia M, Tell F, Goaillard J-M, 2019. Robustness to axon initial segment variation is explained by somatodendritic excitability in rat substantia nigra dopaminergic neurons. J. Neurosci 39, 5044–5063. 10.1523/JNEUROSCI.2781-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel JA, Pawelko SS, Copes-Finke D, Neidhart M, Howard CD, 2020. Lesion of striatal patches disrupts habitual behaviors and increases behavioral variability. PLoS One 15, e0224715. 10.1371/journal.pone.0224715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel JA, Pawelko SS, Scott JR, McLaughlin R, Fox M, Ghanem M, van der Merwe R, Hollon NG, Ramsson ES, Howard CD, 2021. Optogenetic stimulation of striatal patches modifies habit formation and inhibits dopamine release. Sci. Rep 11, 19847. 10.1038/s41598-021-99350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J, 2002. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J. Neurosci 22, 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Tappaz ML, Berod A, Mugnaini E, 1982. Two-color immunohistochemistry for dopamine and GABA neurons in rat substantia nigra and zona incerta. Brain Res. Bull 9, 463–474. 10.1016/0361-9230(82)90155-1. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF, 2012. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci 32, 14804–14808. 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner NJ, 2021. Voltage-gated Ca2+ channels in dopaminergic substantia nigra neurons: therapeutic targets for neuroprotection in Parkinson’s disease? Front. Synaptic Neurosci 13, 636103 10.3389/fnsyn.2021.636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM, 1999. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synap. N. Y. N 32, 165–176. . [DOI] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM, 2016. Chapter 17 - neurophysiology of substantia nigra dopamine neurons: modulation by GABA and glutamate. In: Steiner H, Tseng KY (Eds.), Handbook of Behavioral Neuroscience. Elsevier, pp. 335–360. [Google Scholar]
- Paladini CA, Celada P, Tepper JM, 1999. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience 89, 799–812. 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- Patt S, Gertz HJ, Gerhard L, Cervós-Navarro J, 1991. Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: a Golgi study. Histol. Histopathol 6, 373–380. [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, et al. , 2015. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis 82, 185–199. 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippart F, Khaliq ZM, 2018. Gi/o protein-coupled receptors in dopamine neurons inhibit the sodium leak channel NALCN. ELife 7. 10.7554/eLife.40984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippart F, Destreel G, Merino-Sepúlveda P, Henny P, Engel D, Seutin V, 2016. Differential somatic Ca2+ channel profile in midbrain dopaminergic neurons. J. Neurosci 36, 7234–7245. 10.1523/JNEUROSCI.0459-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J-F, Zou J, Drouin-Ouellet J, Kim K-YA, Cicchetti F, Awatramani RB, 2014. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 9, 930–943. 10.1016/j.celrep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J-F, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan CS, Dombeck DA, Deisseroth K, Awatramani R, 2018. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci 21, 1260–1271. 10.1038/s41593-018-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensa L, Parent A, 2001. The nigrostriatal pathway in the rat: a single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J. Neurosci 21, 7247–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U, 1998. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J. Neurosci 18, 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Roberts E, 1980. GABAergic nerve terminals decrease in the substantia nigra following hemitransections of the striatonigral and pallidonigral pathways. Brain Res. 192, 413–420. 10.1016/0006-8993(80)90893-8. [DOI] [PubMed] [Google Scholar]
- Rizzi G, Tan KR, 2019. Synergistic nigral output pathways shape movement. Cell Rep. 27, 2184–2198.e4. 10.1016/j.celrep.2019.04.068. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA, 1989. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci 9, 3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh K, Isa T, Takakusaki K, 2004. Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats. Eur. J. Neurosci 19, 2399–2409. 10.1111/j.0953-816X.2004.03337.x. [DOI] [PubMed] [Google Scholar]
- Schultz W, 2019. Recent advances in understanding the role of phasic dopamine activity. F1000Research 8. 10.12688/f1000research.19793.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindou T, Shindou M, Watanabe S, Wickens J, 2019. A silent eligibility trace enables dopamine-dependent synaptic plasticity for reinforcement learning in the mouse striatum. Eur. J. Neurosci 49, 726–736. 10.1111/ejn.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bolam JP, 1991. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience 44, 45–73. 10.1016/0306-4522(91)90250-r. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A, 1986. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience 18, 347–371. 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Smith JB, Klug JR, Ross DL, Howard CD, Hollon NG, Ko VI, Hoffman H, Callaway EM, Gerfen CR, Jin X, 2016. Genetic-based dissection unveils the inputs and outputs of striatal patch and matrix compartments. Neuron 91, 1069–1084. 10.1016/j.neuron.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spix TA, Nanivadekar S, Toong N, Kaplow IM, Isett BR, Goksen Y, Pfenning AR, Gittis AH, 2021. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science 374, 201–206. 10.1126/science.abi7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, 2008. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci 9, 206–221. 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J, 2010. Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium 47, 175–182. 10.1016/j.ceca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR, 1995. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J. Neurosci 15, 3092–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triarhou LC, Ghetti B, 1989. The dendritic dopamine projection of the substantia nigra: phenotypic denominator of weaver gene action in hetero- and homozygosity. Brain Res. 501, 373–381. 10.1016/0006-8993(89)90654-9. [DOI] [PubMed] [Google Scholar]
- Wallmichrath I, Szabo B, 2002. Cannabinoids inhibit striatonigral GABAergic neurotransmission in the mouse. Neuroscience 113, 671–682. 10.1016/s0306-4522(02)00109-4. [DOI] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ, 2011. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS One 6, e17047. 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M, Berod A, Sotelo C, 1981. Dopaminergic dendrites in the pars reticulata of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience 6, 2125–2139. 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N, 2012. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873. 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wu J, Kung J, Dong J, Chang L, Xie C, Habib A, Hawes S, Yang N, Chen V, Liu Z, et al. , 2019. Distinct connectivity and functionality of aldehyde dehydrogenase 1a1-positive nigrostriatal dopaminergic neurons in motor learning. Cell Rep. 28, 1167–1181.e7. 10.1016/j.celrep.2019.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GCR, Urakubo H, Ishii S, Kasai H, 2014. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620. 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, Baimbridge KG, McGeer EG, 1990. Relative sparing in Parkinson’s disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res. 526, 303–307. [DOI] [PubMed] [Google Scholar]
- Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S, 2018. Nucleus Accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 97, 434–449.e4. 10.1016/j.neuron.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F-W, Jin Y, Matta SG, Xu M, Zhou F-M, 2009. An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci 29, 10424–10435. 10.1523/JNEUROSCI.4402-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


