Abstract
Objective:
We previously demonstrated that monosodium glutamate (MSG) consumption increases trimethylamine (TMA) level in the renal tissue as well as dimethylamine and methylamine levels in urine of rats, suggesting the effects of MSG on humans. To better define the findings, we investigated whether MSG consumption alters serum trimethylamine N-oxide (TMAO) level, and as a consequence, induces kidney injury in the rat model.
Methods:
Adult male Wistar rats (n=40) were randomized to be fed with a standard diet (control group) or a standard diet with 0.5, 1.5 or 3.0 g% MSG corresponding to 7, 21, or 42 g/day in 60 kg man, respectively in drinking water (MSG-treated groups), or a standard diet with 3.0 g% MSG in drinking water which was withdrawn after 4 weeks (MSG-withdrawal group). Blood and urine samples were collected to analyze the TMAO levels using 1H NMR and markers of kidney injury. Fecal samples were also collected for gut microbiota analysis.
Results:
We found serum TMAO levels increased and urinary TMAO excretion decreased during MSG consumption, in parallel with the increase of the neutrophil gelatinase-associated lipocalin (NGAL) excretion which subsided with the withdrawal of MSG. The fecal 16S rRNA analysis during MSG consumption showed gut microbiota changes with a consistent suppression of Akkermansia muciniphila, a mucin producing bacteria, but not of TMA-producing bacteria.
Conclusions:
Our findings suggested that prolonged high dose MSG consumption may cause TMAO accumulation in the blood via reduction of renal excretion associated with acute kidney injury. The mechanisms by which MSG reduced TMAO excretion require further investigation.
Keywords: Monosodium glutamate, Trimethylamine N-oxide, Kidney injury, Akkermansia muciniphila, gut microbiota
1. INTRODUCTION
Monosodium glutamate (MSG) is a common food additive widely used throughout the globe as a flavor enhancer and it has been listed in a category of generally recognized as safe by the US Food and Drug Administration [1]. Nonetheless, based on the data from our group and others raised question about the effects of MSG consumption on metabolic disorders including weight gain [2,3], hypertension [4] and metabolic syndrome [5]. These observations have not been entirely recapitulated by others [6,7]. Thus, the mechanistic studies are needed using established animal models such as the adult male Wistar rats fed with an MSG-added standard diet.
Long-term MSG consumption causes renal structural and functional changes and electrolyte imbalance [8] with renal oxidative stress observed by proteomic analysis [9]. Animals given MSG for a short period showed that MSG is an alkalinizing agent [10] disturbing liver and kidney metabolic pathways, and caused the elevation of dimethylamine and (mono)methylamine in urine and elevation of trimethylamine (TMA) in the kidney tissue [11]. TMA is a tertiary aliphatic amine produced by TMA-producing bacteria [12], especially from the Firmicutes and Proteobacteria phyla [13] in the intestinal lumen from dietary carnitine, choline, and choline-containing compounds. Trimethylamine N-oxide (TMAO), a stable product of TMA may causes the damage in the heart [14,15] and kidney[16–18], particularly through tubulointerstitial fibrosis and collagen deposition [18] which is prevented by inhibition of TMAO production [16] or hemodialysis [17].
Based on these observations, we sought out the evidence of MSG-associated changes connecting kidney injury, TMAO levels in serum, urine and kidney tissue, as well as TMA-producing gut bacteria.
2. MATERIALS AND METHODS
2.1. Chemicals and Animals
Food-grade MSG (Ajinomoto, Tokyo, Japan) and analytical grade chemicals including trimethylamine N-oxide (TMAO) (Sigma Aldrich, St. Louis, USA), sodium trimethylsilyl-[2,2,3,3-2H4]-propionate (TSP) (Santa Cruz Biotechnology, Dallas, USA) and creatinine (Sigma, St. Louis, USA) were used in the study. Forty-adult male Wistar rats at the age of 13 weeks were obtained from the Northeast Laboratory Animal Center (NELAC), Khon Kaen University and acclimatized prior to the experiment. The rats were housed individually in an animal room maintained at 23 ± 2°C and humidity 30–60% with a 12h light/dark cycle. The study was approved by the Institutional Animal Care and Use Committee of Khon Kaen University under the Ethics of Animal Experimentation of the National Research Council of Thailand (IACUC-KKU-92/64).
2.2. Animal study
Adult male Wistar rats were fed standard rat chow diet and drinking water ad libitum and divided into five groups with 8 rats in each as follows: 1) control group fed a standard diet and normal drinking water; 2) low MSG consumption group (L-MSG) fed a standard diet and 0.5 g% MSG in drinking water; 3) medium MSG consumption group (M-MSG) fed a standard diet and 1.5 g% MSG in drinking water; 4) high MSG consumption group (H-MSG) fed a standard diet and 3.0 g% MSG in drinking water; 5) MSG-withdrawal group (W-MSG) fed a standard diet with and without 3.0 g% MSG in drinking water for 4 and 2 consecutive weeks to investigate the long-term and short-term withdrawal effects (Figure 1). Metabolic cages were used for the collection of 24-hour urine and fecal samples every two weeks and samples were stored at −20°C until analyzed. At the end of the treatment, rats were sacrificed using carbon dioxide inhalation, blood was withdrawn by cardiac puncture and kidney were dissected and fixed in 10% formalin buffer for H&E and Masson’s Trichrome staining.
Figure 1. Experimental design.
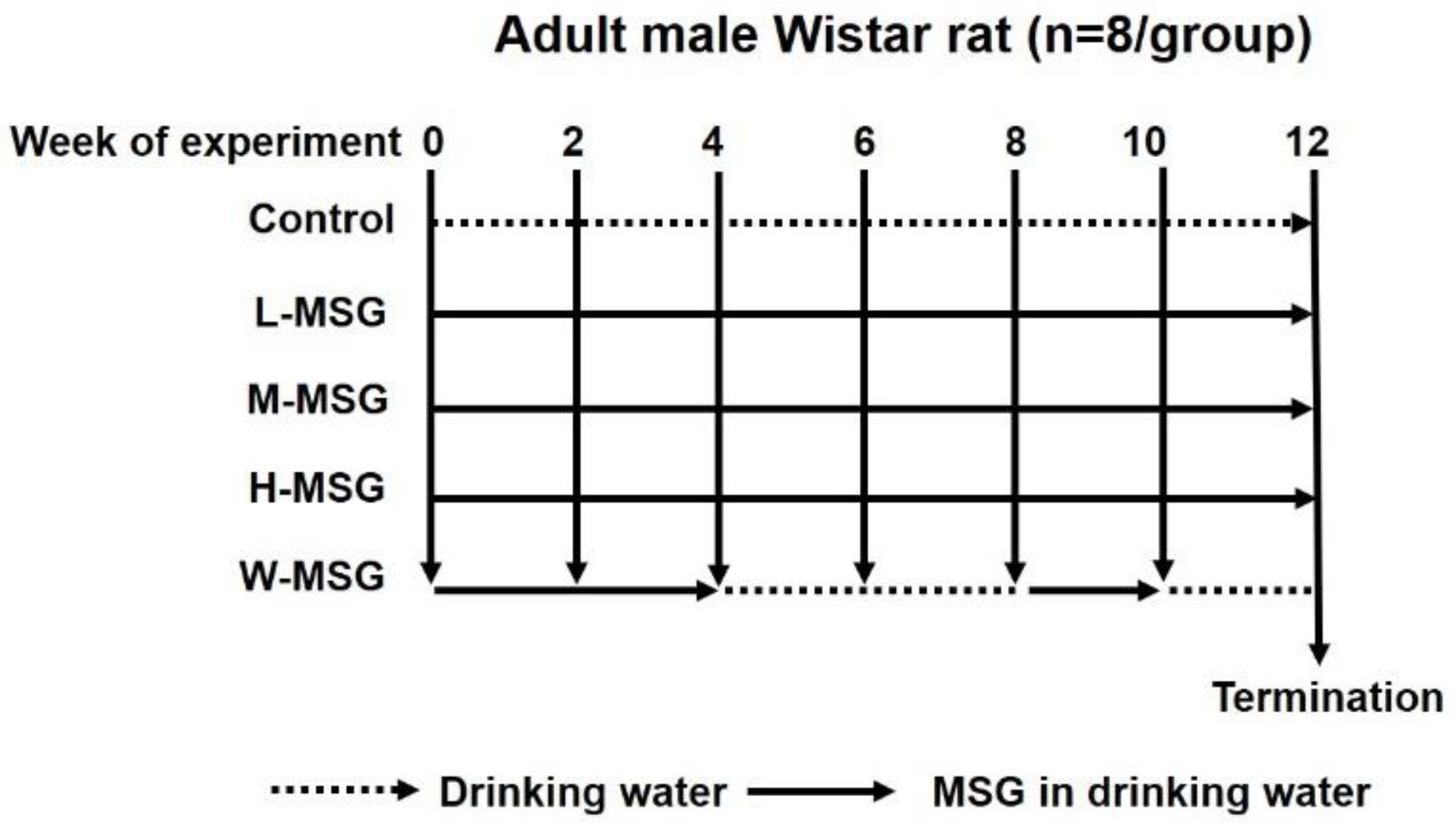
Adult male Wistar rats were divided into five groups (n=8 in each); 1) control group fed a standard diet and normal drinking water; 2) low MSG consumption group (L-MSG) fed a standard diet and 0.5 g% MSG in drinking water; 3) medium MSG consumption group (M-MSG) fed a standard diet and 1.5 g% MSG in drinking water; 4) high MSG consumption group (H-MSG) fed a standard diet and 3.0 g% MSG in drinking water; 5) MSG-withdrawal group (W-MSG) fed a standard diet with and without 3.0 g% MSG in drinking water for 4 and 2 consecutive weeks.
2.3. TMAO Determination
We determined TMAO in serum and urine samples using a 400 MHz 1H Nuclear Magnetic Resonance (NMR) spectrometer (Bruker, Massachusetts, United States) at the temperature of 298.1K using TSP as an internal standard and its peak (δ1H=0.00) to calibrate the spectra. The concentration of TMAO in urine sample was calculated using the software SMA (simple mixture analysis), MNOVA (Mestralab Research, Bajo, Spain). The overlapping taurine triplet in urine was subtracted using the formula ((I1-I2-I3)/NN1 * CCF). TMAO excretion was calculated by multiplying TMAO concentration in urine with total urine volume per day. The concentration of TMAO in serum was calculated using a software qNMR, MNOVA, Mestralab.
2.4. Markers of renal function
Serum creatinine was determined by Jaffe method [19] using UV/Visible spectrophotometer, Ultrospec 3100 pro (Amersham Biosciences, Uppsala, Sweden) and creatinine clearance was calculated by the formula ((Urine creatinine concentration X urine volume) / (serum creatinine X collection time, minutes)). Urine neutrophil gelatinase-associated lipocalin (NGAL) was measured by enzyme-linked immunosorbent assay, Rat Lipocalin-2 ELISA kit (Abcam, United Kingdom), according to the manufacturer’s instruction. NGAL release was calculated by multiplying the concentration with urine volume per day.
2.5. Histopathology of kidney
The paraffin-embedded tissue blocks of rat kidneys were processed according to the routine histopathology system and the sections were sliced at 4 μm thickness. Sections were stained with Haematoxylin & Eosin (H&E) and observed under a light microscope (Primo Star, Zeiss). To observe the fibrosis, kidney sections were stained with Masson’s Trichrome, scanned by ScanScope slide scanner (Aperio) and interpreted using Aperio ImageScope Version 12.4.3.5008.
2.6. Gut microbiota analysis
Bacterial genomic DNA was extracted using the QiAamp Power Fecal Pro DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The eluted DNA was used for 16S ribosomal RNA (rRNA) gene sequencing. The quantity and quality of genomic DNA was checked by measuring with a Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, USA) and agarose gel electrophoresis. For each genomic DNA sample, the 16S rRNA gene was amplified targeting the variable region of V3-V4 using primers: forward primer: V3 (5’ TCGTCG GCAG CGTCAGATGTG TATAAGAGAC AGCCTACGGG NGGCWGCAG) and reverse primer: V4 (5’GTCTCGTGG GCTCGGAGATGTGTATAAGAGACAGGACTACH VGGGT ATCTA ATCC). The 16S rRNA sequencing library was prepared and FLASH (1.2.11) (http://ccb.jhu.edu/software/FLASH/, accessed on 3 April 2021) was used to merge paired-end reads. CD-HIT-OTU (http://weizhongli-lab.org/cd-hit-otu/, accessed on 3 April 2021) and rDNA Tools were used to filter and trim raw reads, pick error-free reads and clustering. Based on 100% similarity, QIIME-UCLUST Sequencing was performed for classification of OTUs (Operational Taxonomic Units) with 97% threshold identity. Sharing ≥ 97% similarity of sequences was set as same OTU. Ribosomal Database Project (RDP) was used for the alignment of representative OTU sequences and reference NCBI databases were used and compared for classification of taxonomy. Quantitative Insights Into Microbial Ecology (QIIME) was used for the output of a classification of reads at several taxonomic levels-kingdom, phylum, class, order, family, genus, and species.
2.7. Statistical analysis
Continuous variables are expressed as mean ± standard error of mean (SEM). For the comparison of data among four groups, one-way analysis of variance (ANOVA) with post-hoc LSD test was used. For the comparison of data between before and after MSG treatment, Wilcoxon signed-rank test was used. The significance level was considered as p < 0.05. The analyses were shown using GraphPad Prism version 9.31 (GraphPad Software, La Jolla, CA, USA) and IBM SPSS for Windows (version 22, SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. MSG increases water intake and urine output
Throughout the experiment, food and water intake were recorded individually on a daily basis, and the body weight and urine volume were monitored every two weeks. One rat from control group was excluded because of an illness during the experiment. The effects of MSG on food intake, body weight, water intake, and urine output are summarized in Figure 2. MSG consumption exerted no marked effect on food intake (Figure 2A) and body weight among five groups (Figure 2B). However, we observed that MSG consumption induced increase of water intake (Figure 2C) and corresponding increase of urine output (Figure 2D).
Figure 2.
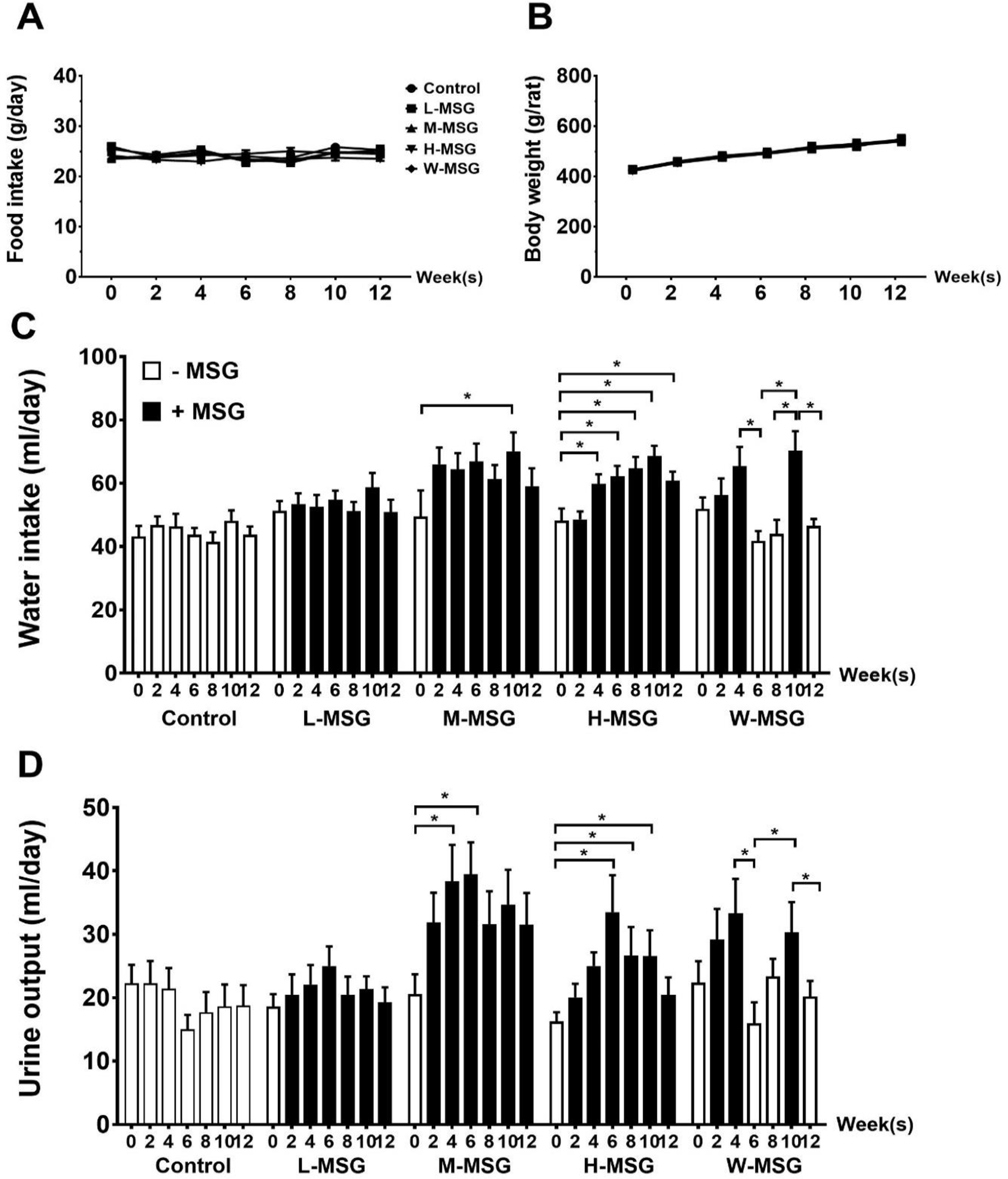
Effects of MSG consumption on food intake (A), body weight (B), water intake (C), urine output (D) of rats at various time-points during 12 weeks of the experiment. Data are shown as mean ± SEM and p-values were calculated by one-way ANOVA with post-hoc LSD tests (*p < 0.05). Control group (n=7), L-MSG group (n=8), M-MSG group (n=8), H-MSG group (n=8), W-MSG group (n=8).
3.2. MSG increases serum TMAO level and reduces urine TMAO excretion
To explore whether MSG consumption affects serum TMAO levels, serum and urine TMAO were measured and the results are summarized in Figure 3. The H-MSG group showed significantly higher serum TMAO levels compared to other groups at week 4 (Figure 3A), and, although statistically not significant, the same tendency was observed at week 12 (Figure 3C). The increase of serum TMAO level corresponded to the decrease of TMAO excretion in urine which was relatively lower in H-MSG group compared to other groups (Figure 3B, D). Then, serum and urine TMAO levels of the withdrawal group were compared to control and H-MSG groups at the same timepoint which corresponding to the same age of animals (Figure 4). Serum TMAO levels of H-MSG group were relatively higher compared to MSG-withdrawal group and reached statistically significant at week 4 and 12 (Figure 4A). These results are corresponded to the decrease of TMAO excretion in H-MSG group compared to MSG-withdrawal group (Figure 4B).
Figure 3.
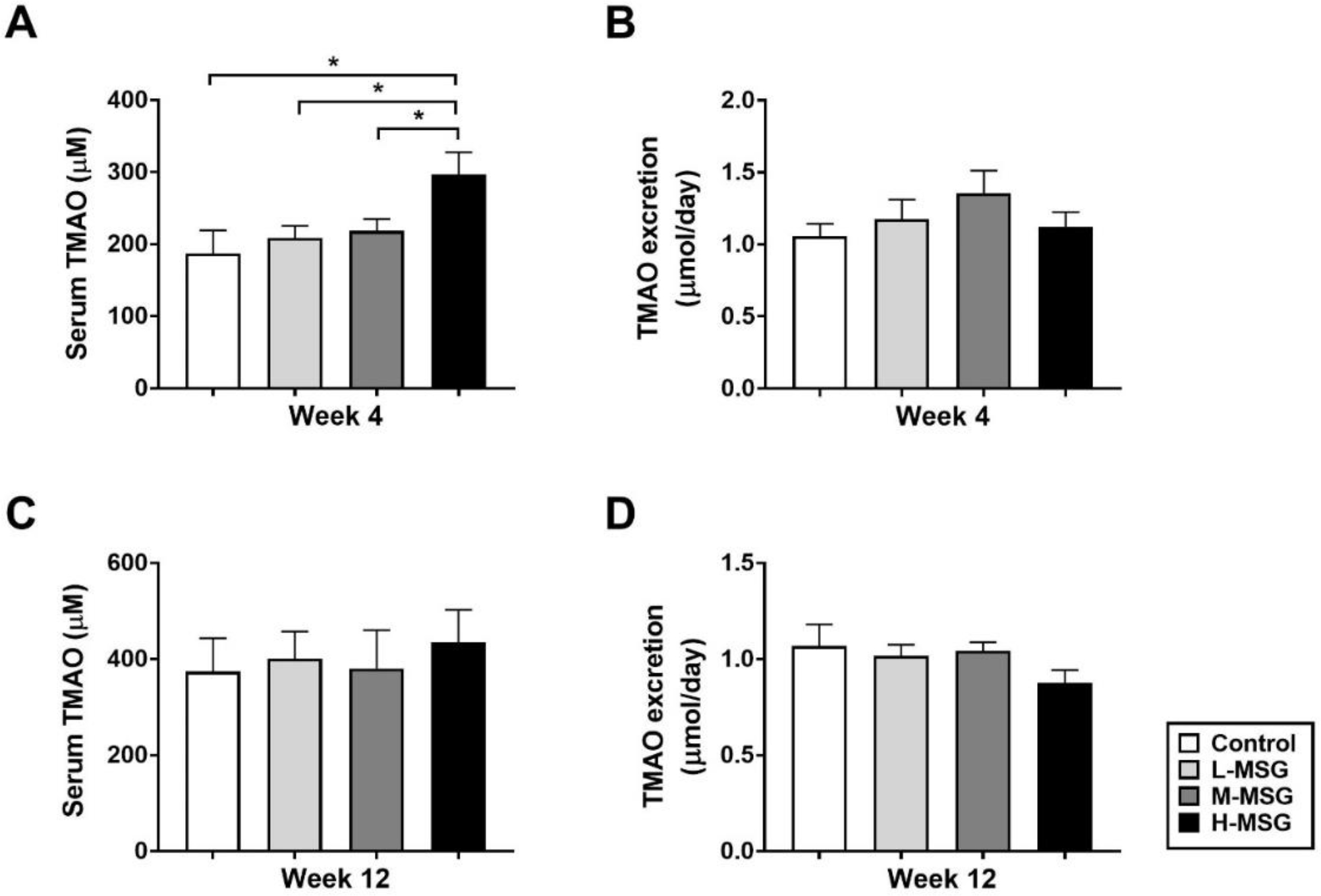
Dose and time responses of MSG consumption on TMAO levels in serum (A), urine (B) at week 4 and in serum (C) and urine (D) at week 12 among four groups of rats. Data are shown as mean ± SEM and p-values were calculated by one-way ANOVA with post-hoc LSD tests (*p < 0.05). Control group (n=7), L-MSG group (n=8), M-MSG group (n=8), H-MSG group (n=8).
Figure 4.
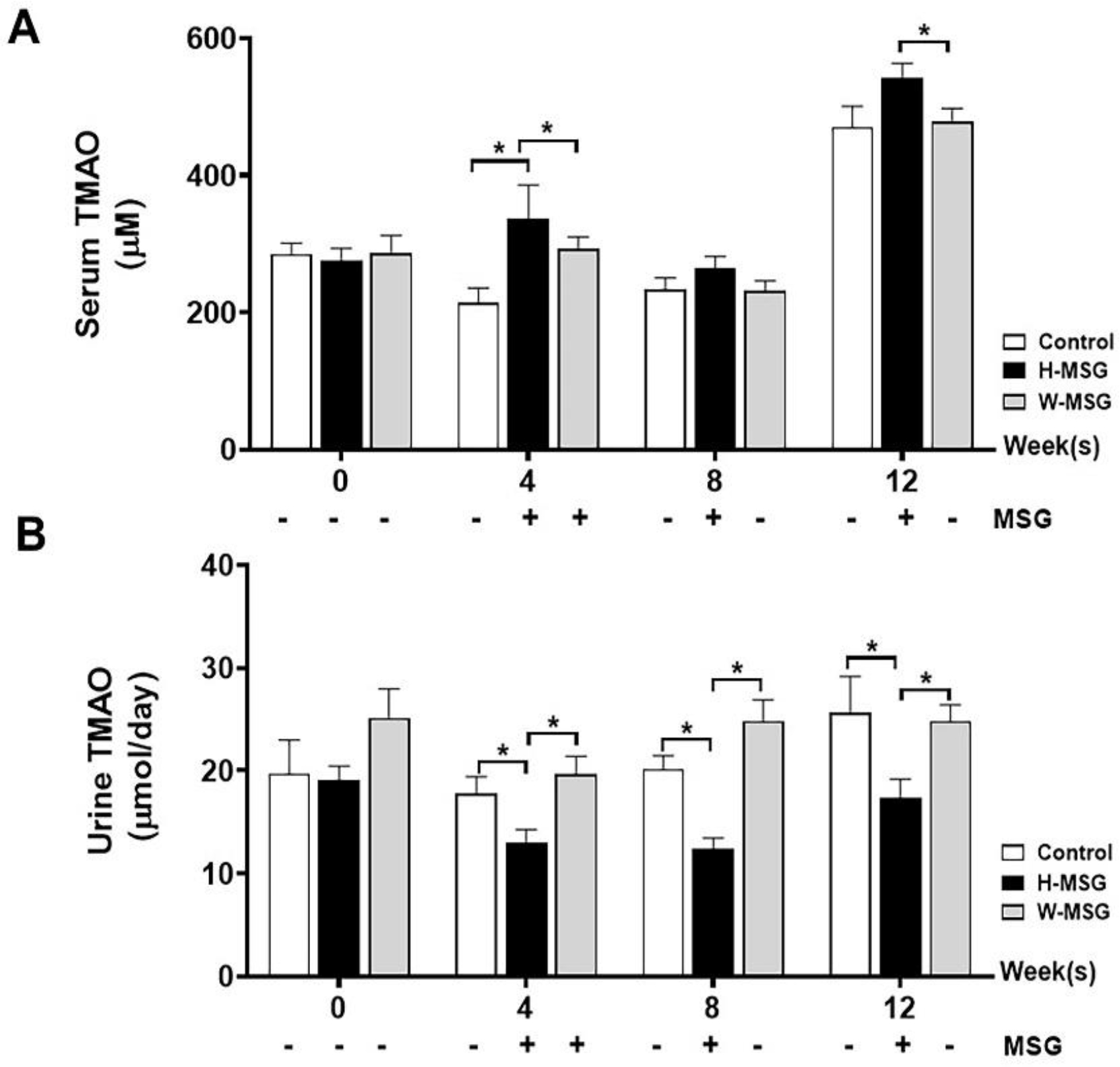
Withdrawal effects of MSG consumption on serum (A) and urine (B) TMAO levels at week 0, 4, 8, 12 of experiment. Data are shown as mean ± SEM and p-values were calculated by one-way ANOVA with post-hoc LSD tests (*p < 0.05). Control group (n=7), H-MSG group (n=8), and W-MSG group (n=8).
3.3. MSG induces acute kidney injury
The effects of MSG supplementation on the kidney were examined by comparing the histopathology of the kidney tissues (H&E, Masson’s Trichrome), kidney weight, serum creatinine, creatinine clearance, and NGAL release. Histopathological examination indicated the congested glomeruli with red blood cells in the capillary vessels, vascular congestion, cytoplasmic vacuolation in tubules, perivascular dilatation, mesangial hypercellularity in all groups. No significant changes were seen in between control and MSG treated animals (Figure 5A–D). Using Masson’s Trichrome staining, no significant difference in tubulointerstitial fibrosis was observed in between the control (Figure 5E) and MSG-treated group (Figure 5F). However, the infiltration of mononuclear inflammatory cells in interstitial space of renal tubules was observed in H-MSG group which was rarely observed in controls.
Figure 5.
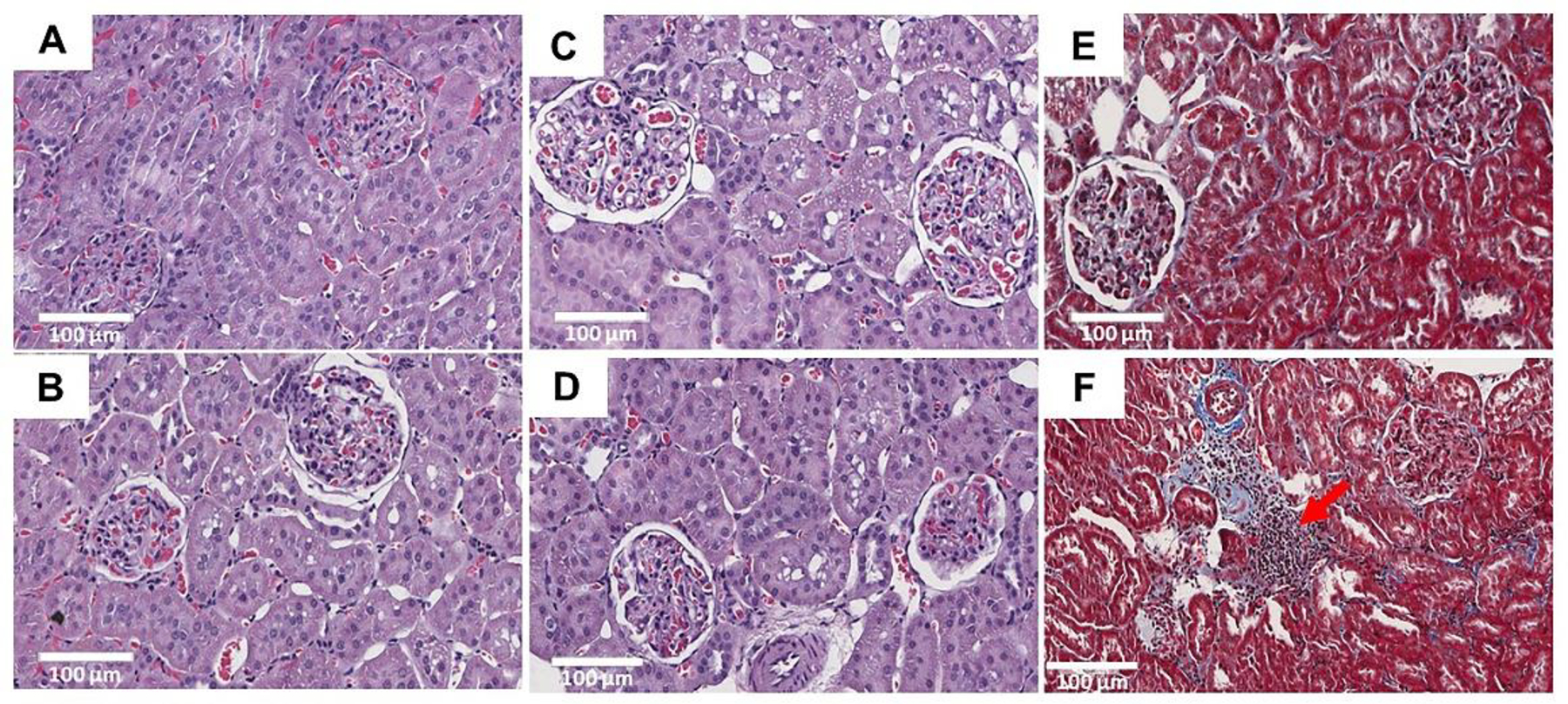
H&E and trichrome staining of kidney sections (20x) in twelve weeks of experimental animals. No significant changes were observed between control (A) and H-MSG group (B, C, D). No significant differences in interstitial fibrosis was seen in between control (E) and H-MSG group (F) animals. Marked infiltration of mononuclear inflammatory cells (red arrow) in the interstitial space of renal tubules were observed in H-MSG group compared to control.
In addition, H-MSG group had significantly higher kidney weight compared to the control group (p = 0.001) or to the L-MSG group (p = 0.012) (Figure 6A). Serum creatinine was significantly higher in the H-MSG group compared to the control group (p = 0.038) or to the M-MSG group (p = 0.032) (Figure 6B) without a parallel difference in creatinine clearance values across the four groups (Figure 6C). The NGAL release in the MSG-treated groups; L-MSG, M-MSG, and H-MSG were relatively higher but not reached statistically significant compared to the control group (Figure 6D). To a greater extent, we determined the withdrawal effect of MSG on kidney injury in MSG-withdrawal animals at different timepoints (week 0, 4, 8, 10, and 12; Figure 6E). We found that NGAL release was significantly higher in MSG-treated weeks (week 4 and 10) compared to MSG-untreated weeks (week 0, 8, and 12).
Figure 6.
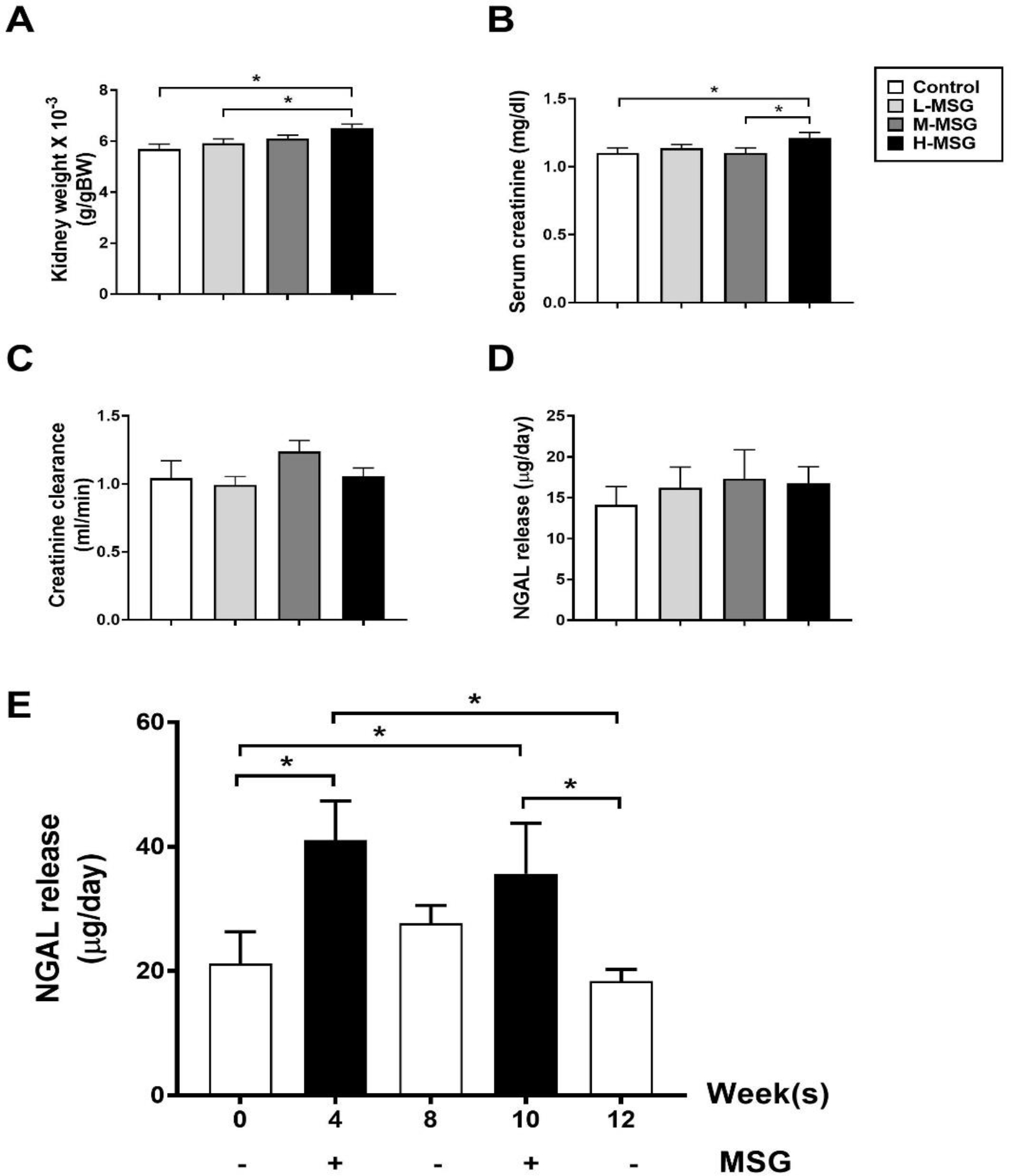
Markers for kidney injury including kidney weight (A), serum creatinine (B), creatinine clearance (C), and NGAL release (D) among four groups of rats at 12 weeks, and NGAL release (E) in MSG-withdrawal group at different time-point during the 12 weeks of the study. Data are shown as mean ± SEM and p-values are calculated by one-way ANOVA with post-hoc LSD tests (*p < 0.05). Control group (n=7), L-MSG group (n=8), M-MSG group (n=8), H-MSG group (n=8).
3.4. Gut microbiota analysis
To determine the effects of MSG consumption on gut microbiota changes, 16S rRNA sequencing on fecal samples from MSG-withdrawal rats at various time points was performed and observed 8 phyla, 17 classes, 23 orders, 47 families, 133 genera and 202 species of bacteria in total (Figure 7). The most abundant phylum was Firmicutes (68.46%), followed by Bacteroides (26.72%), and Verrucomicrobia (1.51%). At the phylum level, Firmicutes and Verrucomicrobia showed the broadest changes with MSG, albeit in opposite direction (Figure 7A). In fact, after MSG consumption Firmicutes increased (p = 0.043) while Verrucomicrobia significantly decreased (p = 0.028) compared to the withdrawal weeks. At the class level, Bacilli, Clostridia, and Erysipelotrichia (among Firmicutes), Bacteroidia (among Bacteroides), and Verrucomicrobiae (among Verrucomicrobia) were abundant in the population (Figure 7B) with elevated Bacilli and decreased Erysipelotrichia during MSG supplementation. Similar trends were observed at the order and family levels (Figure 7C–D) while at the genus level, Lactobacilli and Limosilactobacilli increased during MSG treatment (Figure 7E) and reached statistical difference for the Lactobacillus intestinalis species (Figure 7F). No significant changes with MSG were observed for the Bacteroides phylum through the species level while a significant suppression of Verrucomicrobia abundance (p = 0.028) starting at phylum to species levels accompanied MSG treatment. At the species level, Lactobacillus intestinalis and Akkermansia muciniphila changed with MSG supplementation, as shown in Figure 8. Of note, no significant change in TMA-producing bacteria correlated with MSG consumption.
Figure 7.
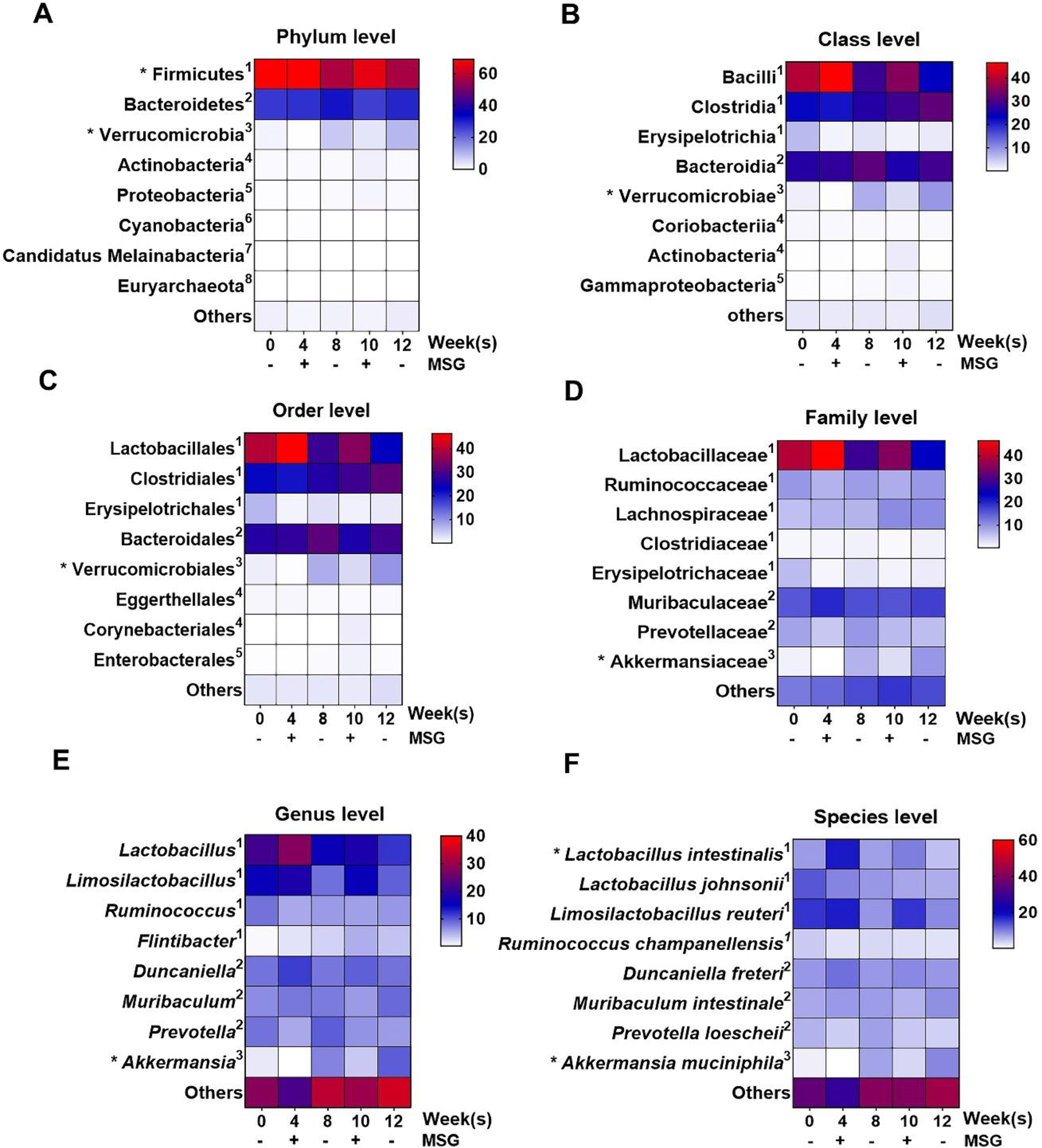
The relative operational taxonomic unit (OTU) abundance at various levels in fecal samples of MSG-withdrawal group (n=6) is shown by heatmap. The color key corresponds to the percent relative abundance in the gut microbiota at each expression level and * shows significant change. 1,2,3,4,5,6,7,8 represents the corresponding phylum.
Figure 8.
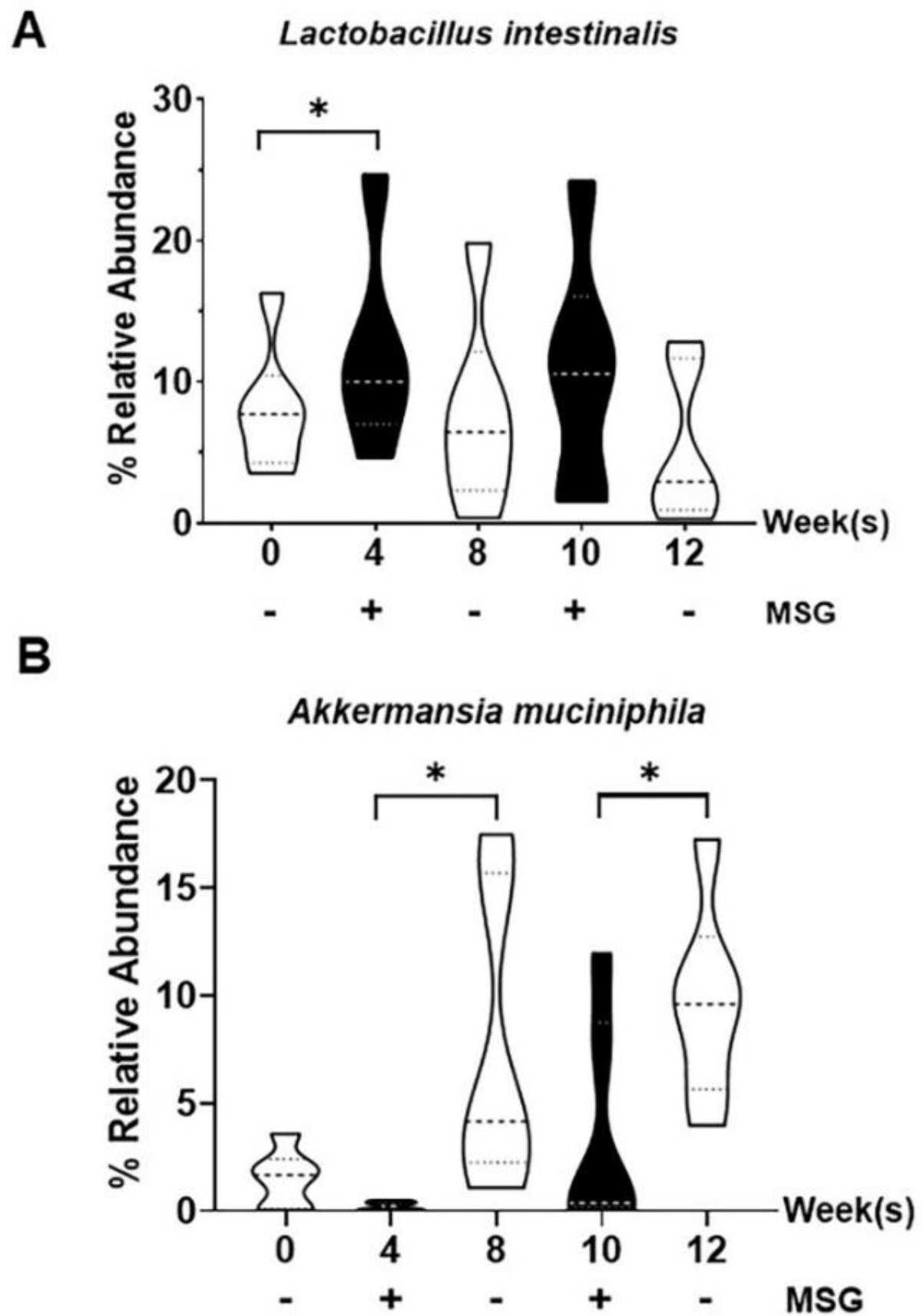
Relative operational taxonomic unit (OTU) abundance at species levels in fecal samples of the MSG-withdrawal group (n=6). Data are shown as mean ± SEM and p-values were calculated by Wilcoxon signed rank test (*p < 0.05).
4. DISCUSSION
Understanding the mechanisms by which MSG impacts renal function through the TMAO pathways is of potential clinical relevance, as previously suggested by our group and others [11,20]. To investigate this area, we used our established animal model with five experimental conditions, namely a continuous and a discontinuous (withdrawal) MSG supplementation and a control standard diet. Briefly, our data demonstrate that dietary MSG increases water intake and urine output, increases serum TMAO while inhibiting TMAO excretion, induces acute kidney injury, and reduces the abundance of Akkermansia muciniphila known to maintain the gut barrier integrity. These four observations will be discussed further.
First, while not altering the food intake MSG was associated with higher and consistent water intake and urine output (Figure 2C, D), supporting the osmotic diuretic effect of sodium in MSG [21], in agreement with previous studies [8,11]. We may envision that water intake and urinary output can be used to mirror and evaluate the amount of MSG consumption in animal models in case of ad libitum allowance. Moreover, in a clinical standpoint adequate hydration should be encouraged in subjects who follow a high-MSG diet, particularly at high temperatures.
Second, our 1H-NMR metabolomics approach revealed that MSG induced the increase of serum TMAO while reduced its renal excretion (Figure 3, 4), partly from the MSG sodium content as supported by similar changes observed with high dietary salt intake [22]. Since TMAO is produced from TMA metabolism by the gut microbiota, excreted, and secreted by the kidney within 24 hours [12,23], we evaluated its pathway at these levels. A kidney adaptive mechanism may also be involved, leading to intracellular TMAO accumulation in exchange for sodium to prevent the cell damage, as suggested by similar changes observed with loop diuretics [24] which block TMAO secretion in the renal tubules leading to its accumulation in the kidney tissue. The TMAO renal secretion takes place with the basolateral transporter mediated uptake of organic anions and cations as well as zwitterions from the blood into the renal proximal tubular cells and subsequently exported by the apical transporter through the apical membrane into urine [25,26]. The key transporter for TMAO cellular uptake is organic cation transporter 2 (OCT2) [27] while the multidrug and toxin extrusion protein 1 (MATE1) contributes the secretion of TMAO [28]. Higher plasma level and lower renal retention of TMAO were reported in Oct1/2 knockout mice [27] while low proteins and mRNA expression of renal OCT2 and MATE1 were observed in rats with acute kidney injury caused by ischemia/reperfusion [29]. NGAL, a marker of acute kidney injury, was investigated in our experimental study. Changes of NGAL are compatible with the hypothesis that the reduced TMAO excretion might be due to the inhibition of TMAO uptake or efflux by transporters and we can only speculate on a direct effect of MSG on the OCT2 and MATE2 transporters. However, the mechanism by which MSG alter TMAO transporters the dedicated studies will be required. Similarly, since the accumulation of TMAO has negative effects on cardiac function [15,30], and kidney [16–18] we encourage clinical studies on the impact of dietary MSG as a cofactor in cardiovascular and kidney diseases.
Third, urinary NGAL levels followed the MSG use or withdraw in our study thus supporting a possible acute kidney injury, also compatible with high serum creatinine levels and increased kidney weight, despite unchanged creatinine clearance, H&E and fibrosis (Figure 5, 6). This agreed with our previous observations of the effects of long-term MSG consumption [8], secondary to oxidative stress [9]. Consistent observations by others that both oral and parenteral MSG induces kidney injury [20,31,32] may have clinical implications for the dietary recommendations of patients with chronic kidney disease.
Fourth, since TMAO is modulated by the gut microbiota, we confirmed that MSG changes this crucial compartment as previously reported in animal [11,20] and human [33] studies, and report significant modifications of two major phyla, Firmicutes and Verrucomicrobia (Figure 7). Somewhat surprisingly, we did not observe the significant changes of TMAO or TMA-producing bacteria within the phylum Firmicutes (Clostridrium, Desulfitobacterium, Enterococcus, Streptococcus, Haloanearobacter, Staphylococcus) and Proteobacteria [13]. The factors known to modify the gut microbiota, including genetics, age, and the pH of colonic lumen [34–36] may be at the basis of the different findings in this and our previous study [11]. However, both experimental settings showed that MSG suppresses the abundance of beneficial bacteria in the gut, such as phylum Verrucomicrobia was previously observed in human study [33]. Suppression of Akkermansia muciniphila, a major species in phylum Verrucomycrobia found in our study (Figure 8), was also previously associated with TMAO levels [37] and directly involved in the gut barrier, mucus thickness, and gut immune response [38], ultimately with beneficial clinical associations [39,40]. The opposite effect of MSG on L. intestinalis compared to A. muciniphila may be secondary to the compensation effects to maintain the homeostasis in the host, since both bacteria exert the functional roles in gut barrier protection [38,41].
5. CONCLUSIONS
In conclusion, we submit that the evidence provided in our study supports the conclusion that MSG consumption influences animal health by modifying the gut microbiota, suppressing the excretion of TMAO, a nephrotoxin metabolite, and inducing NGAL release, a marker of acute kidney injury. Since MSG consumption remains widespread, its effects on human health need to be further explored, ideally by combining clinical, histological, and biochemical observations for which the TMAO pathway may constitute a good candidate.
ACKNOWLEDGMENTS
We would like to thank the Northeast Laboratory Animal Center (NELAC) at Khon Kaen University for animal husbandry facilities. Thanks to research institute center (RIC), Khon Kaen University for providing NMR Mnova software for data analysis. Thanks to Faculty of Science, Khon Kaen University for NMR technical support. We would like to acknowledge Professor Yukifumi Nawa for editing the MS via Publication Clinic KKU, Thailand.
FUNDING
This research article was supported by the followings funds; KKU Scholarship for ASEAN and GMS Countries’ Personal of Academic Year 2018 and the invitation research fund, Faculty of Medicine, Khon Kaen University (IN64205) to T.K. The research funding from Chronic Kidney Disease prevention in the Northeast of Thailand research group (CKDNET2562) and Fundamental Fund of Khon Kaen University (FF65), which received funding from The National Science Research and Innovation Fund (NSRF), Thailand, to U.C., S.P., and S.A. Partial support was provided by NIH-NIEHS (RIVER Award) R35 Superfund Program ES030443-01 to B.D.H.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ANIMAL ETHICS
The study was approved by the Institutional Animal Care and Use Committee of Khon Kaen University under the Ethic of Animal Experimentation of the National Research Council of Thailand (IACUC-KKU-92/64).
DATA AVAILABILITY
Data will be made available on request.
REFERENCES
- [1].Walker R, Lupien JR, 2000. The safety evaluation of monosodium glutamate. Journal of Nutrition 130(4):1049s–1052s. [DOI] [PubMed] [Google Scholar]
- [2].He K, Zhao LC, Daviglus ML, Dyer AR, Van Horn L, Garside D, et al. , 2008. Association of monosodium glutamate intake with overweight in Chinese adults: The INTERMAP study. Obesity 16(8):1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].He K, Du SF, Xun PC, Sharma S, Wang HJ, Zhai FY, et al. , 2011. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). American Journal of Clinical Nutrition 93(6):1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi ZM, Yuan BJ, Taylor AW, Dai Y, Pan XQ, Gill TK, et al. , 2011. Monosodium glutamate is related to a higher increase in blood pressure over 5 years: findings from the Jiangsu Nutrition Study of Chinese adults. Journal of Hypertension 29(5):846–853. [DOI] [PubMed] [Google Scholar]
- [5].Insawang T, Selmi C, Cha’on U, Pethlert S, Yongvanit P, Areejitranusorn P, et al. , 2012. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutrition & Metabolism 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shi ZM, Luscombe-Marsh ND, Wittert GA, Yuan BJ, Dai Y, Pan XQ, et al. , 2010. Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: findings from the Jiangsu Nutrition Study of Chinese adults. British Journal of Nutrition 104(3):457–463. [DOI] [PubMed] [Google Scholar]
- [7].Thu Hien VT, Thi Lam N, Cong Khan N, Wakita A, Yamamoto S, 2013. Monosodium glutamate is not associated with overweight in Vietnamese adults. Public Health Nutr 16(5):922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharma A, Prasongwattana V, Cha’on U, Selmi C, Hipkaeo W, Boonnate P, et al. , 2013. Monosodium Glutamate (MSG) Consumption Is Associated with Urolithiasis and Urinary Tract Obstruction in Rats. Plos One 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sharma A, Wongkham C, Prasongwattana V, Boonnate P, Thanan R, Reungjui S, et al. , 2014. Proteomic Analysis of Kidney in Rats Chronically Exposed to Monosodium Glutamate. Plos One 9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nahok K, Li JV, Phetcharaburanin J, Abdul H, Wongkham C, Thanan R, et al. , 2019. Monosodium Glutamate (MSG) Renders Alkalinizing Properties and Its Urinary Metabolic Markers of MSG Consumption in Rats. Biomolecules 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nahok K, Phetcharaburanin J, Li JV, Silsirivanit A, Thanan R, Boonnate P, et al. , 2021. Monosodium Glutamate Induces Changes in Hepatic and Renal Metabolic Profiles and Gut Microbiome of Wistar Rats. Nutrients 13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zeisel SH, Warrier M, 2017. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annual Review of Nutrition, Vol 37 37:157–181. [DOI] [PubMed] [Google Scholar]
- [13].Romano KA, Vivas EI, Amador-Noguez D, Rey FE, 2015. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. Mbio 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Senthong V, Kiatchoosakun S, Wongvipaporn C, Phetcharaburanin J, Tatsanavivat P, Sritara P, et al. , 2021. Gut microbiota-generated metabolite, trimethylamine-N-oxide, and subclinical myocardial damage: a multicenter study from Thailand. Scientific Reports 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Organ CL, Otsuka H, Bhushan S, Wang ZN, Bradley J, Trivedi R, et al. , 2016. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circulation-Heart Failure 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li L, et al. , 2020. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arteriosclerosis Thrombosis and Vascular Biology 40(5):1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S, Krempf M, et al. , 2019. Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate. Toxins 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang WHW, Wang ZN, Kennedy DJ, Wu YP, Buffa JA, Agatisa-Boyle B, et al. , 2015. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circulation Research 116(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Slot C, 1965. Plasma Creatinine Determination - a New and Specific Jaffe Reaction Method. Scandinavian Journal of Clinical & Laboratory Investigation 17(4):381-&. [DOI] [PubMed] [Google Scholar]
- [20].Pongking T, Haonon O, Dangtakot R, Onsurathum S, Jusakul A, Intuyod K, et al. , 2020. A combination of monosodium glutamate and high-fat and high-fructose diets increases the risk of kidney injury, gut dysbiosis and host-microbial co-metabolism. Plos One 15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elliot S, Goldsmith P, Knepper M, Haughey M, Olson B, 1996. Urinary excretion of aquaporin-2 in humans: A potential marker of collecting duct responsiveness to vasopressin. Journal of the American Society of Nephrology 7(3):403–409. [DOI] [PubMed] [Google Scholar]
- [22].Bielinska K, Radkowski M, Grochowska M, Perlejewski K, Huc T, Jaworska K, et al. , 2018. High salt intake increases plasma trimethylamine N-oxide (TMAO) concentration and produces gut dysbiosis in rats. Nutrition 54:33–39. [DOI] [PubMed] [Google Scholar]
- [23].Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH, 2015. Mechanism of Prominent Trimethylamine Oxide (TMAO) Accumulation in Hemodialysis Patients. Plos One 10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li DY, Wang ZN, Jia X, Yan D, Shih DM, Hazen SL, et al. , 2021. Loop Diuretics Inhibit Renal Excretion of Trimethylamine N-Oxide. Jacc-Basic to Translational Science 6(2):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].George B, You D, Joy MS, Aleksunes LM, 2017. Xenobiotic transporters and kidney injury. Advanced Drug Delivery Reviews 116:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lepist EI, Ray AS, 2016. Renal Transporter-Mediated Drug-Drug Interactions: Are They Clinically Relevant? Journal of Clinical Pharmacology 56:S73–S81. [DOI] [PubMed] [Google Scholar]
- [27].Teft WA, Morse BL, Leake BF, Wilson A, Mansell SE, Hegele RA, et al. , 2017. Identification and Characterization of Trimethylamine-N-oxide Uptake and Efflux Transporters. Molecular Pharmaceutics 14(1):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gessner A, Konig J, Fromm MF, 2018. Contribution of multidrug and toxin extrusion protein 1 (MATE1) to renal secretion of trimethylamine-N-oxide (TMAO). Scientific Reports 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matsuzaki T, Morisaki T, Sugimoto W, Yokoo K, Sato D, Nonoguchi H, et al. , 2008. Altered pharmacokinetics of cationic drugs caused by down-regulation of renal rat organic cation transporter 2 (Slc22a2) and rat multidrug and toxin extrusion 1 (Slc47a1) in ischemia/reperfusion-induced acute kidney injury. Drug Metabolism and Disposition 36(4):649–654. [DOI] [PubMed] [Google Scholar]
- [30].Chen K, Zheng XQ, Feng MC, Li DL, Zhang HQ, 2017. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Frontiers in Physiology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mahieu S, Klug M, Millen N, Fabro A, Benmelej A, Contini MD, 2016. Monosodium glutamate intake affect the function of the kidney through NMDA receptor. Life Sciences 149:114–119. [DOI] [PubMed] [Google Scholar]
- [32].Ortiz GG, Bitzer-Quintero OK, Zarate CB, Rodriguez-Reynoso S, Larios-Arceo F, Velazquez-Brizuela IE, et al. , 2006. Monosodium glutamate-induced damage in liver and kidney: a morphological and biochemical approach. Biomedicine & Pharmacotherapy 60(2):86–91. [DOI] [PubMed] [Google Scholar]
- [33].Peng QN, Huo DX, Ma CC, Jiang SM, Wang LS, Zhang JC, 2018. Monosodium glutamate induces limited modulation in gut microbiota. Journal of Functional Foods 49:493–500. [Google Scholar]
- [34].Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN, 2015. Role of the normal gut microbiota. World Journal of Gastroenterology 21(29):8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, et al. , 2016. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. Bmc Microbiology 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arnoldini M, Cremer J, Hwa T, 2018. Bacterial growth, flow, and mixing shape human gut microbiota density and composition. Gut Microbes 9(6):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Griffin LE, Djuric Z, Angiletta CJ, Mitchell CM, Baugh ME, Davy KP, et al. , 2019. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food & Function 10(4):2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C, 2017. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Practice & Research Clinical Gastroenterology 31(6):637–642. [DOI] [PubMed] [Google Scholar]
- [39].Aron RAC, Abid A, Vesa CM, Nechifor AC, Behl T, Ghitea TC, et al. , 2021. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang T, Li QQ, Cheng L, Buch H, Zhang F, 2019. Akkermansia muciniphila is a promising probiotic. Microbial Biotechnology 12(6):1109–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lim EY, Song EJ, Kim JG, Jung SY, Lee SY, Shin HS, et al. , 2021. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Beneficial Microbes 12(5):503–516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


