PURPOSE
The PALLAS study investigated whether the addition of palbociclib, an oral CDK4/6 inhibitor, to adjuvant endocrine therapy (ET) improves invasive disease-free survival (iDFS) in early hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) breast cancer. In this analysis, we evaluated palbociclib exposure and discontinuation in PALLAS.
METHODS
Patients with stage II-III HR+, HER2– disease were randomly assigned to 2 years of palbociclib with adjuvant ET versus ET alone. The primary objective was to compare iDFS between arms. Continuous monitoring of toxicity, dose modifications, and early discontinuation was performed. Association of baseline covariates with time to palbociclib reduction and discontinuation was analyzed with multivariable competing risk models. Landmark and inverse probability weighted per-protocol analyses were performed to assess the impact of drug persistence and exposure on iDFS.
RESULTS
Of the 5,743 patient analysis population (2,840 initiating palbociclib), 1,199 (42.2%) stopped palbociclib before 2 years, the majority (772, 27.2%) for adverse effects, most commonly neutropenia and fatigue. Discontinuation of ET did not differ between arms. Discontinuations for non–protocol-defined reasons were greater in the first 3 months of palbociclib, and in the first calendar year of accrual, and declined over time. No significant relationship was seen between longer palbociclib duration or ≥ 70% exposure intensity and improved iDFS. In the weighted per-protocol analysis, no improvement in iDFS was observed in patients receiving palbociclib versus not (hazard ratio 0.89; 95% CI, 0.72 to 1.11).
CONCLUSION
Despite observed rates of discontinuation in PALLAS, analyses suggest that the lack of significant iDFS difference between arms was not directly related to inadequate palbociclib exposure. However, the discontinuation rate illustrates the challenge of introducing novel adjuvant treatments, and the need for interventions to improve persistence with oral cancer therapies.
BACKGROUND
The use of adjuvant endocrine therapy (ET) has substantially improved outcomes for patients diagnosed with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) breast cancer1; however, up to 30% of patients experience cancer recurrence. There have been many efforts to improve outcomes through the addition of novel adjuvant agents. Palbociclib, a CDK4/6 inhibitor, is approved for the treatment of advanced HR+, HER2– breast cancer in combination with ET in the first-line and pretreated settings.2-5 The PALLAS trial (NCT02513394) is a global randomized, phase III, open-label trial designed to evaluate palbociclib in the adjuvant setting. Between September 1, 2015, and November 30, 2018, 5,760 patients with stage II-III HR+, HER2– breast cancer were randomly assigned 1:1 to receive 2 years of palbociclib with ongoing adjuvant ET versus ET alone, with a primary end point of invasive disease-free survival (iDFS). At the second interim analysis (IA2) with 23.7 months of follow-up, the Independent Data Monitoring Committee (IDMC) determined that a futility boundary was crossed for the primary end point. Patients in the palbociclib + ET arm stopped palbociclib, and all patients were moved to long-term follow-up with ongoing ET. The IA2 results were subsequently published, showing no improvement in iDFS with the addition of palbociclib.6 Data from the final analysis are awaited.
CONTEXT
Key Objective
The phase III PALLAS trial evaluated the addition of 2 years of the CDK4/6 inhibitor palbociclib to standard adjuvant endocrine therapy in patients with early breast cancer; notably, about 40% of PALLAS patients randomly assigned to palbociclib discontinued therapy early. This analysis examines predictors of palbociclib discontinuation, and whether palbociclib exposure was correlated with clinical outcomes.
Knowledge Generated
Most early palbociclib discontinuations in PALLAS were because of drug-related neutropenia; neither longer duration of therapy with palbociclib nor greater palbociclib exposure intensity predicted improved clinical outcomes with the drug. Therefore, the results of the parent PALLAS trial cannot be explained by inadequate exposure to palbociclib.
Relevance
Development of novel agents in the adjuvant oncology setting can be challenging. Methods to reduce drug toxicity, and interventions to improve patient and provider communication and support, may help optimize persistence with emerging oral therapies and maximize the chance of detecting drug benefit.
At the time of the IA2 data report, 42% of patients in the palbociclib + ET arm had stopped palbociclib prematurely, including 27% stopping for toxicity.6 The ability to remain on any oral agent in the adjuvant breast cancer setting may be challenging for a variety of reasons, including side effects, drug schedule, or perceived therapeutic benefit versus cancer risk.7 Early discontinuation limits drug exposure, potentially interfering with efficacy.8 Given the observed rates of early discontinuation in PALLAS, an in-depth analysis was conducted to evaluate predictors of drug exposure, as well as any potential impact of drug exposure on the primary end point of iDFS.
METHODS
Study Population and Procedures
The IA2 data set was used in this analysis, with a cutoff of January 9, 2020. Eligibility criteria were previously described.6 Patients randomly assigned to palbociclib + ET were initiated on palbociclib at a starting dose of 125 mg daily, in a 3 weeks on/1 week off schedule. Patients randomly assigned to ET alone continued on their ongoing selected ET. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The research protocol was approved by local or central institutional review boards or ethic committees, and all participants provided written informed consent. The trial was monitored throughout by an international IDMC. The study Protocol (online only) is available in the Data Supplement.
All patients in PALLAS were evaluated monthly for the first 3 months of protocol therapy, with complete blood count and chemistries every 2 weeks for the first 8 weeks. Subsequently, the patients were seen for examination and laboratory testing every 3 months for the first 2 years of the trial (Treatment Phase). Dose modifications were made for protocol-defined toxicities. Notably, uncomplicated grade 3 neutropenia required holding palbociclib until recovery to grade 2 or less, with resumption at the same dose level for the first occurrence. Dose reductions were required for each subsequent occurrence, and discontinuation of palbociclib therapy was required upon occurrence of a fourth episode of neutropenia.
The frequency of early discontinuation of protocol therapy was monitored continuously through the time of data cutoff. Rates of early palbociclib discontinuation (defined as stopping palbociclib before completion of the planned 2 year course) were presented every 6 months to the IDMC. Additionally, reasons for early discontinuation were categorized into protocol-defined reasons (ie, recurrent grade 3 neutropenia as described or other persistent toxicity despite dose modification, surgical delays, and iDFS event) versus non–protocol-defined reasons (ie, patient/provider decision to discontinue without protocol-defined toxicity, noncompliance, and withdrawal of consent). Reasons for discontinuation for each patient were adjudicated by the study leadership team in real time on regular calls, and were not captured as part of the clinical trial database. Efforts were made during the course of the trial to reduce non–protocol-related discontinuation, including patient and provider education and support through regular country-specific webinars and lectures, as well as periodic global letters to patients.
At the time of IA2 in May 2020, on the basis of the futility outcome and IDMC recommendations, patients in the palbociclib + ET arm were instructed to stop taking palbociclib and continue ET, with ongoing monitoring and survival follow-up per study guidance.
Statistical Analysis
Treatment persistence and iDFS by exposure analyses were carried out in the safety population defined as all patients with at least one dose of treatment. Patients were assigned to the palbociclib + ET group if at least one dose of palbociclib was received, and to the ET alone group otherwise. Early discontinuation of palbociclib was defined as stopping drug before the planned 2-year duration for any reason (eg, because of iDFS event, patient noncompliance, toxicity, etc). Notably, the analyses presented in this paper were not affected by the IA2 IDMC recommendation to discontinue palbociclib for patients still receiving therapy, as this decision occurred after data cutoff.
Multivariable Fine and Gray (competing risk) models including patient demographic and clinicopathologic factors were used to explore time to palbociclib dose reductions, with competing risk being discontinuation of palbociclib, and time to palbociclib early discontinuation because of adverse events (AEs) and other reasons, with competing risks being discontinuation because of an iDFS event or completion of palbociclib per protocol. Patients still on drug at the data cutoff were censored at the date of their last intake.
Persistence (ie, time on drug) as well as exposure intensity (ie, number of actual intake days divided by the number of expected intake days for the planned 26 cycles/2 years of therapy) of palbociclib were evaluated at landmark time points of 6, 12, 18, and 24 months and classified into either intake duration < versus ≥ the given landmark, or intensity < 70% versus ≥ 70% (which was approximately the observed median intensity). Association of those groups with time to iDFS was analyzed using univariable and multivariable (adjusted for T-stage, N-stage, histologic grade, and progesterone receptor) Cox regression models. Of note, this analysis was only carried out within the palbociclib + ET arm to avoid potential bias introduced by the different potential reasons for discontinuation with a targeted agent (ie, palbociclib) versus ET alone.
Finally, a naive (ie, unweighted) per-protocol efficacy analysis, including only protocol-adherent patients, comparing palbociclib + ET versus ET alone was performed. The protocol-adherent subset included all patients who were still taking palbociclib at data cutoff, or who discontinued because of iDFS event, medical monitor decision, or protocol-defined reasons (eg, palbociclib discontinuation because of AEs at the 75 mg level). In addition, to deal with imbalanced groups, an inverse probability treatment weighted approach was used in the same subset of patients. In this approach, patients were weighted using a propensity score on the basis of a model including race, ethnicity, region, N-stage, T-stage, histologic grade, progesterone receptor, prior chemotherapy, and Eastern Cooperative Oncology Group performance status (ECOG PS) at baseline. The results with stabilized weights are shown.
Analyses were carried out using SAS software, version 9.4. The results with two-sided P values < .05 were considered statistically significant.
RESULTS
At the time of data cutoff, the study analysis population, defined as those who initiated protocol therapy, was 5,743 patients, with 2,840 initiating palbociclib on the palbociclib + ET arm, and 2,903 on the ET alone arm (Data Supplement, online only). Treatment status at time of IA2 and reasons for early discontinuation of palbociclib and ET have previously been reported.6 Notably, 1,199 patients (42.2%) stopped palbociclib before the planned 2-year duration, with 772 (27.2%) stopping for AEs (combined protocol-defined and non–protocol-defined; Table 1). Of those stopping for toxicity, the most common reasons for discontinuation were neutropenia (461, 59.7%) and fatigue (71, 9.2%; Data Supplement). In a post hoc analysis, rates of palbociclib early discontinuation on the basis of competing risk analysis are estimated to be 17.9% (95% CI, 16.5 to 19.3) at 6 months, 30.0% (95% CI, 28.3 to 31.7) at 12 months, 38.0% (95% CI, 36.2 to 39.8) at 18 months, and 44.9% (95% CI, 42.9 to 46.8) at 24 months. In a multivariable Fine and Gray analysis, baseline variables associated with higher early discontinuation rates included older age, Asian race (but represented by very small numbers), lower anatomic stage, and lower tumor grade (Fig 1, Data Supplement). Of note, the rate of ET discontinuation was not significantly different between the two arms (6.9% for palbociclib + ET v 6.3% for ET alone).
TABLE 1.
Palbociclib + ET Patient Status
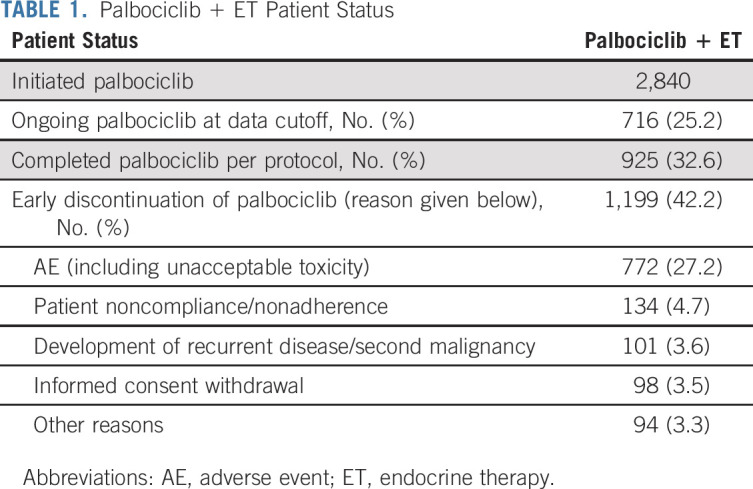
FIG 1.
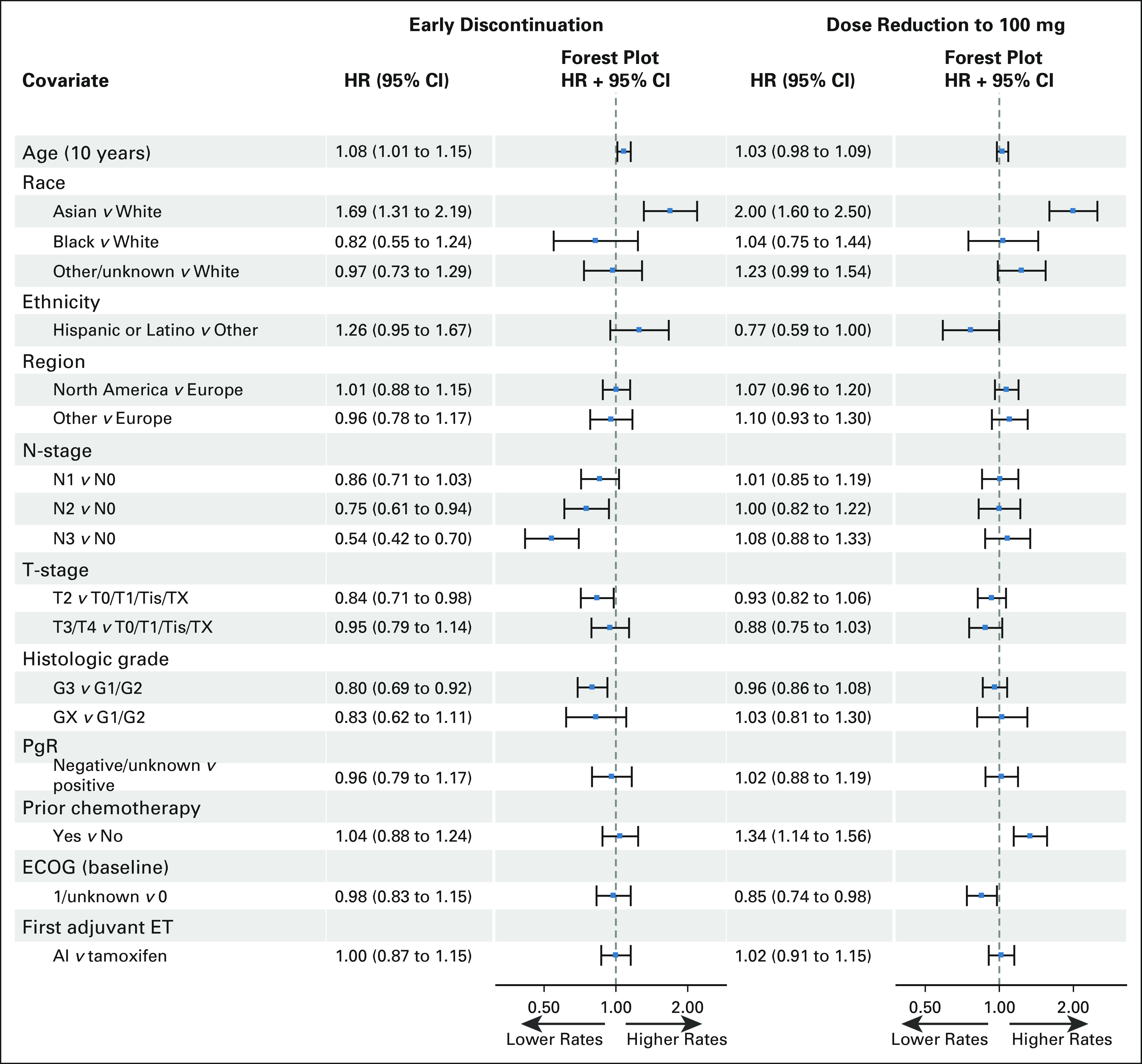
Association of baseline variables with palbociclib discontinuation and dose reduction to 100 mg. Individual forest plots can be found in the Data Supplement. AI, aromatase inhibitor: ECOG, Eastern Cooperative Oncology Group; ET, endocrine therapy; G, grade; HR, hazard ratio; PgR, progesterone receptor; Tis, carcinoma in situ; Tx, primary tumor cannot be assessed.
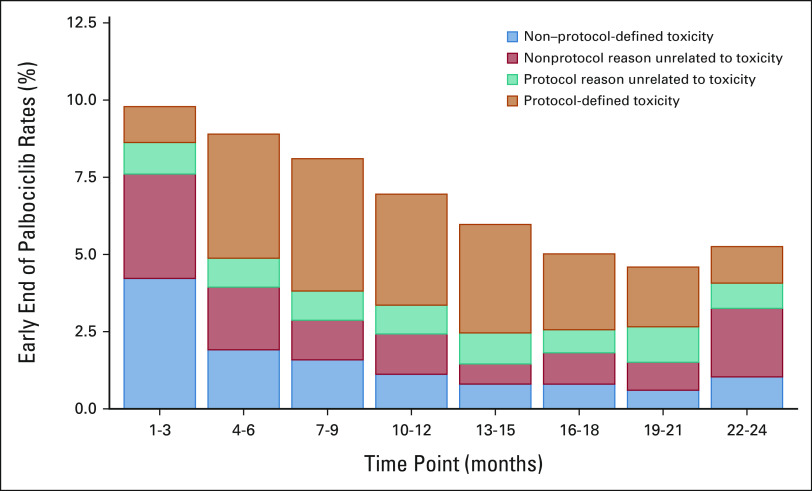
As reviewed at the final IDMC meeting, the fraction of patients stopping palbociclib for non–protocol-defined reasons was qualitatively greatest in the first 3 months of drug exposure, and then decreased over the subsequent months of therapy, with protocol-defined toxicity being the predominant cause for discontinuation (Fig 2). The rate of palbociclib early discontinuation for non–protocol-defined toxicity was greatest in the first year of study accrual and declined over the subsequent years (Data Supplement).
FIG 2.
Reasons for early palbociclib discontinuation, categorized by central study management teams as protocol-defined or non–protocol-defined, for palbociclib + ET patients by 3-month time points as presented to the IDMC in May 2020. The percentages in the 3-month periods refer to the number of patients who were still receiving palbociclib treatment up to the respective period. aThe increased rate of early discontinuation for nonprotocol reasons unrelated to toxicity in months 22-24 reflects administrative reasons early in the course of the study. ET, endocrine therapy; IMDC, Independent Data Monitoring Committee.
A total of 1,549 (55%) patients required palbociclib dose reduction to 100 mg, and 928 (32.7%) required further reduction to 75 mg, at some point during treatment. In the first 3 months, dose reductions to 100 mg occurred in 953 (33.6%) patients, and to 75 mg in 154 (5.4%). Among 772 patients discontinuing because of toxicity, only 476 (62%) were at a dose level of 75 mg at the time of discontinuation, suggesting that some discontinuations occurred without maximum dose reduction. In multivariable analysis, Asian race, non-Hispanic ethnicity, prior chemotherapy exposure, and lower ECOG PS were associated with higher rates of palbociclib dose reduction from the starting dose of 125 mg to 100 mg (Fig 1), with similar findings for dose reduction from 100 mg to 75 mg.
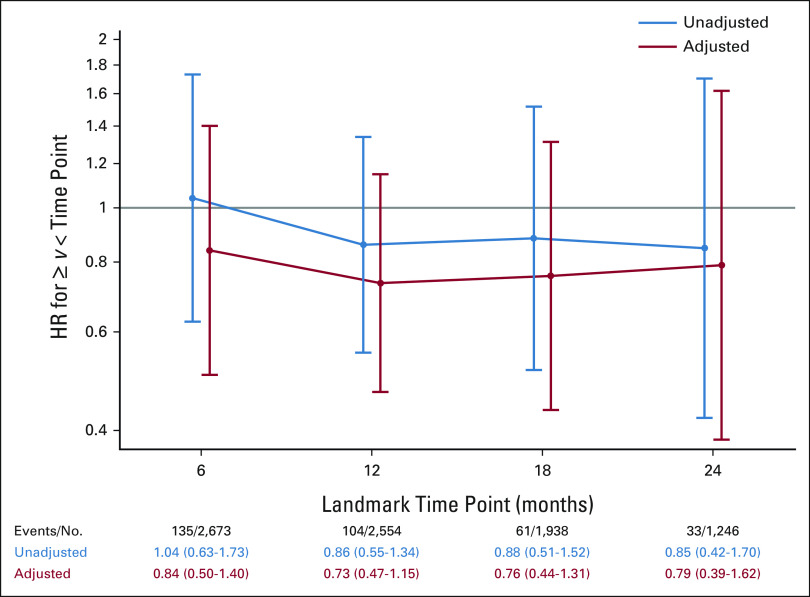
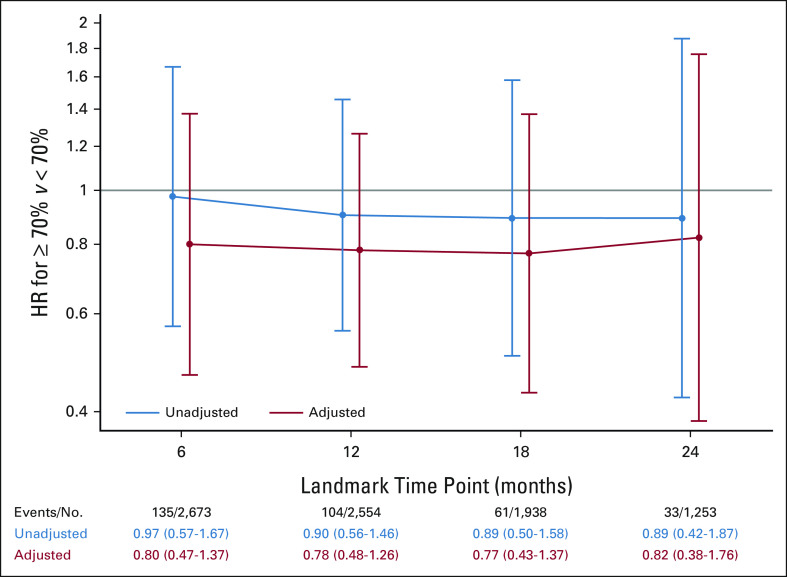
No significant relationship was seen between longer palbociclib duration and improved iDFS (Fig 3), although the results are limited by low numbers of events. The median palbociclib exposure intensity for the palbociclib + ET arm was 69.6% (quartile 1 = 34.6%, quartile 3 = 95.4%). Palbociclib + ET patients with ≥ 70% exposure intensity did not show a significant improvement in iDFS when compared to patients with < 70% exposure intensity (Fig 4); again, analysis is limited by low number of events. A similar analysis evaluating relative dose intensity and iDFS also did not show a significant improvement (Data Supplement).
FIG 3.
HR for palbociclib intake duration (≥ or < the given landmark) up to 6, 12, 18, and 24 months (HR < 1 suggests that longer palbociclib duration is associated with improved iDFS). HR, hazard ratio; iDFS, invasive disease-free survival.
FIG 4.
HR for palbociclib exposure intensity (≥ or < 70%) up to 6, 12, 18, and 24 months (HR < 1 suggests that ≥ 70% exposure intensity is associated with improved iDFS). HR, hazard ratio; iDFS, invasive disease-free survival.
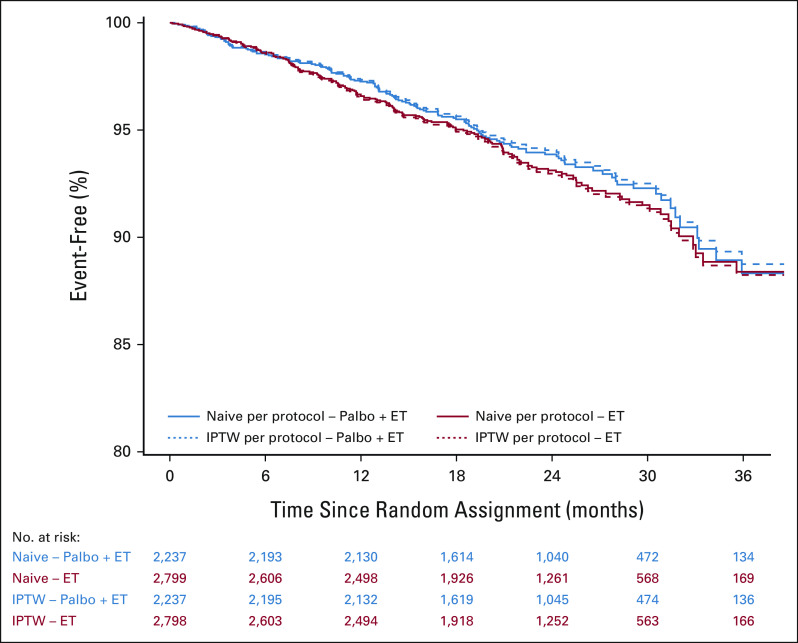
A per-protocol analysis of iDFS was performed comparing palbociclib + ET versus ET alone to evaluate outcomes exclusively in adherent patients (ie, patients without non–protocol-defined discontinuation, n = 5,035). In the weighted analysis balancing groups on baseline characteristics (Fig 5), no improvement in iDFS was observed in patients receiving palbociclib + ET versus those receiving ET alone (hazard ratio 0.89; 95% CI, 0.72 to 1.11).
FIG 5.
Per-protocol analysis of iDFS in palbociclib + ET versus ET alone in adherent patients (ie, without non–protocol-defined discontinuation). Naive analysis is a simple, unadjusted comparison between arms; IPTW analysis is a comparison between arms after balancing groups on baseline characteristics (see Statistical Analysis). ET, endocrine therapy; iDFS, invasive disease-free survival; IPTW, inverse probability treatment weighted; Palbo, palbociclib. The results of the PALLAS trial of the CDK4/6 inhibitor palbociclib as adjuvant therapy for HR+, HER2– breast cancer cannot be explained by inadequate exposure to palbociclib; neither longer duration of palbociclib therapy nor greater drug exposure intensity predicted improved clinical outcomes. The development of novel agents in the adjuvant oncology setting can be challenging, and highlights the need for effective interventions to help improve persistence with oral cancer therapies.
DISCUSSION
In the PALLAS trial evaluating adjuvant palbociclib in combination with ET,6 completing 2 full years of palbociclib therapy using the protocol-defined dosing schedule and guidance on dose reduction and discontinuation was challenging. Using the IA2 data set, early discontinuation was associated with older age, Asian race (a very small subset of the study population), lower anatomic stage, and lower tumor grade. Palbociclib dose reduction was associated with Asian race, non-Hispanic ethnicity, prior chemotherapy exposure, and lower ECOG PS. In an analysis evaluating any relationship between exposure intensity and clinical outcome, higher exposure intensity was not significantly associated with iDFS.
In preparation for the design of PALLAS, a pilot phase II study was conducted to evaluate the feasibility of administering 2 years of adjuvant palbociclib + ET.9 In this study, 31% of patients discontinued palbociclib early, most commonly for protocol-defined toxicity, which was within the statistical expectations used in the trial design. It was noted that some discontinuations were non–protocol-defined, eg, patients stopping because of anxiety about toxicity. Active measures to encourage persistence, including patient and provider education, were launched, and a 50% reduction in non–protocol-defined discontinuations was subsequently observed. Similar measures were adopted into the real-time trial management for PALLAS. Consequently, the drop in discontinuations because of non–protocol-defined reasons as shown in the Data Supplement may reflect the beneficial impact of these interventions, as well as greater provider familiarity with managing palbociclib toxicities. Given the results of this presented analysis, it is not clear that further reductions in early palbociclib discontinuation would have had an impact on overall trial results. However, the observation that active monitoring and management of discontinuations was beneficial may inform the design of trials of novel agents in the adjuvant breast cancer setting.
Early palbociclib discontinuation occurred in the PALLAS trial for several reasons. Most commonly, patients discontinued palbociclib therapy for protocol-mandated reasons, such as persistent neutropenia despite dose reduction. The protocol guidance for management of neutropenia was designed to be conservative in the adjuvant survivorship setting. It is possible this conservative trial guidance, or fear of infectious complications in the curative setting, may have increased the rate of early discontinuation for toxicity, especially in regions less familiar with palbociclib management. However, in contrast to chemotherapy, the immune system remains intact with palbociclib-associated neutropenia, demonstrated in the metastatic trials where very few serious infections were observed despite at least 50% of patients experiencing grade 3/4 neutropenia.3,4 It has also been demonstrated that dose reductions do not appear to lead to decreased efficacy from palbociclib,10 and neutropenia may even be a pharmacodynamic marker of treatment efficacy.11 This information provides reassurance to patients and providers regarding the safety of palbociclib despite observed rates of neutropenia.
It is important to put the rates of discontinuation observed in PALLAS into the context of oral medications used in the adjuvant breast cancer setting. Despite the remarkable benefits gained from the use of adjuvant ET, it is well established that rates of adherence to ET decline over the years of administration, highlighting the challenge for cancer survivors to take long-term well-tolerated oral medications.12,13 Of note, rates of discontinuation of adjuvant ET in PALLAS were low and not affected by exposure to or discontinuation of palbociclib.
Multiple adjuvant experiences have demonstrated the challenge for higher-risk patients to receive extended courses of novel agents in the adjuvant setting. The UNIRAD trial found that the addition of a planned 2 years of the mTOR inhibitor everolimus did not improve iDFS in patients with HR+, HER2– breast cancer receiving ongoing adjuvant ET.14 In this trial, 53.4% of patients were unable to complete the planned therapy, with 35.3% stopping for toxicity. In ECOG 5103, a study evaluating adjuvant chemotherapy with or without bevacizumab for HER2– breast cancer, 71% of patients were unable to complete a planned 1 year of maintenance bevacizumab.15 The International Breast Cancer Study Group Trial 22-00 studied the addition of 1 year of maintenance adjuvant metronomic oral cyclophosphamide and methotrexate in HR+ disease. Despite the favorable toxicity profile of this regimen, up to 30% of patients stopped cyclophosphamide and methotrexate early, 23% for toxicity or patient decision.16 It is not known in these trials whether early discontinuation affected overall study results. Although reasons for early discontinuation varied trial by trial, and are likely multifactorial, this pattern demonstrates the challenges of maintaining patients on additional agents in the adjuvant setting.
Early discontinuation has been observed in other adjuvant trials of CDK4/6 inhibitors in the adjuvant setting. In the PENELOPE-B study, which randomly assigned patients with residual HR+, HER2– breast cancer after preoperative chemotherapy to 1 year of palbociclib versus placebo in combination with ongoing ET, 17.5% of patients stopped palbociclib early, 3% because of toxicity.17 Notably, in PALLAS, 30% of all patients had discontinued adjuvant palbociclib at 1 year. Dose modification guidance used in PENELOPE-B was less conservative than that used in PALLAS, with greater flexibility to maintain dose after recovery of uncomplicated neutropenia. At IA2 of the MONARCH-E study, which randomly assigned patients with high-risk HR+, HER2– breast cancer to 2 years of adjuvant abemaciclib in combination with adjuvant ET versus ET alone, 16.6% of patients were reported to have stopped abemaciclib early because of toxicity. However, the total number of discontinuations has not been reported, and 73% of patients were still receiving therapy at the time of study report at a median follow-up of 15.5 months.18 Notably, within a high-risk clinical cohort in PALLAS resembling the MONARCH-E population, at 1 year of palbociclib therapy, a similar rate of 18% of patients had discontinued palbociclib for toxicity. Reasons for the variability in discontinuation rates among these CDK4/6 trials is not clear, but may reflect differences in toxicity profiles, dose modification structures, or time since exposure to prior therapies including chemotherapy or radiation.
Adherence to and persistence with study medication were also measured in PALLAS using drug diaries and a validated patient-reported assessment tool to measure nonadherence. These patient-level analyses will contribute to further understanding of the patient experience on the trial, and evaluate any relationship between adherence with baseline clinicopathologic variables or specific toxicity.
A limitation of this analysis is the lack of comprehensive classification of discontinuations. For discontinuations not clearly related to protocol-defined toxicity, it was not always clear why a patient stopped study medication. A number of patients who stopped for non–protocol-related reasons were classified as either patient choice or provider choice. Efforts were made during the trial to identify patterns for discontinuation with a goal to reduce non–protocol-defined discontinuation, whether by reported reason, site, or country. However, precise granularity could not be achieved, and a greater number of discontinuations related to toxicity or other tolerability issues may have existed. Additionally, the power of this analysis is limited by the number of events in the IA2 data set; however, testing will be repeated with a greater number of events at the time of final analysis.
In conclusion, despite higher rates of discontinuation than anticipated, extensive analyses suggest that the lack of significant difference between PALLAS study arms observed at IA2 was not directly related to inadequate palbociclib exposure. However, the higher-than-anticipated rates of discontinuation, also seen within other adjuvant CDK4/6 inhibitor trials, illustrate the importance of understanding patient and provider challenges with novel therapies in this setting, and the need for effective interventions to help improve persistence with oral cancer therapies. Continued efforts may provide not only more accurate trial end point assessment, but also the best chance for patients to benefit from an intervention.
ACKNOWLEDGMENT
This manuscript is dedicated to the memory of Dr Bella Kaufman, a PALLAS coinvestigator and member of the PALLAS Steering Committee. Dr Kaufman was an outstanding scientist and an exceptional advocate for clinical trials. Her work led to substantial progress in the field of breast cancer research, positively affecting the lives of patients worldwide.
The PALLAS trial is cosponsored by the Alliance Foundation and the Austrian Breast Cancer Study Group, in collaboration with the Eastern Cooperative Oncology Group, the National Surgical Adjuvant Breast and Bowel Project, the German Breast Group, and the Breast International Group, with funding from Pfizer. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors thank the patients, their families, and all caregivers who have been participating in the PALLAS trial; the investigators and staff of the global research teams; and the support of the Alliance Foundation Trials (https://acknowledgments.alliancefound.org) and the Austrian Breast and Colorectal Cancer Study Group (https://www.abcsg.com). The authors would like to acknowledge Timothy K. Erick, PhD, for writing and editorial assistance, and Kaitlyn T. Bifolck, BA, for editorial and submission assistance in the preparation of this manuscript. Both are full-time employees of Dana-Farber Cancer Institute.
Erica L. Mayer
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Gilead Sciences
Research Funding: Pfizer (Inst)
Christian Fesl
Research Funding: Pfizer (Inst)
Dominik Hlauschek
Research Funding: Pfizer (Inst)
Laura Garcia-Estevez
Consulting or Advisory Role: Daiichi Sankyo/Astra Zeneca, Palex, Seattle Genetics
Research Funding: Roche/Genentech (Inst)
Nicholas Zdenkowski
Honoraria: Roche, Pfizer, Eisai
Consulting or Advisory Role: Lilly, AstraZeneca, Eisai
Research Funding: Roche (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Novartis
Kathy D. Miller
This author is the Senior Deputy Editor of Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Genentech/Roche, Athenex, AstraZeneca, Bristol Myers Squibb/Celgene
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Seattle Genetics (Inst), Pfizer (Inst), Astex Pharmaceuticals (Inst), British Biotech (Inst), CytomX Therapeutics (Inst), Alphamab (Inst)
Marija Balic
Consulting or Advisory Role: Amgen, AstraZeneca, Daiichi Sankyo/Astra Zeneca, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Samsung
Speakers' Bureau: Amgen, AstraZeneca, Daiichi Sankyo/Astra Zeneca, Lilly, Novartis, Pierre Fabre, Pfizer, Roche, Seattle Genetics
Research Funding: Lilly (Inst), Novartis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: MSD
Ingrid A. Mayer
Consulting or Advisory Role: Novartis, AstraZeneca, Lilly, Genentech, GlaxoSmithKline, Immunomedics, Macrogenics, Pfizer, AbbVie, Seattle Genetics, Puma Biotechnology, Cyclacel, Blueprint Medicines, Sanofi
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst)
David Cameron
Consulting or Advisory Role: Lilly (Inst), Novartis (Inst), Novartis (Inst), Research Triangle Institute RTI Health Solutions (Inst), Daiichi Sankyo (Inst), Prima BioMed (Inst), Merck Sharp & Dohme (Inst), Zymeworks (Inst), Eisai (Inst), Puma Biotechnology (Inst), Pfizer (Inst), Oncolytics (Inst), Roche (Inst), Roche (Inst), Samsung Bioepis (Inst), Seattle Genetics (Inst), Synthon (Inst), Clarity Pharmaceuticals (Inst), Bexon/Zymeworks (Inst), Sanofi (Inst)
Research Funding: Roche (Inst), Novartis (Inst), AstraZeneca (Inst)
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
José Juan Ponce Lorenzo
Honoraria: Seattle Genetics, Novartis, Pfizer, AstraZeneca/Daiichi Sankyo, Lilly, Roche
Consulting or Advisory Role: Seattle Genetics, Novartis, AstraZeneca/Daiichi Sankyo, Roche
Tufia C. Haddad
Research Funding: Takeda (Inst)
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo, Lilly Japan, Kyowa Hakko Kirin, Taiho Pharmaceutical
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin, Novartis
Research Funding: MSD (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nihonkayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Sanofi (Inst)
Matthew Goetz
Consulting or Advisory Role: Lilly, bioTheranostics, Genomic Health, Novartis, Eisai, Sermonix Pharmaceuticals, Context Therapeutics, Pfizer, Biovica
Research Funding: Lilly (Inst), Pfizer (Inst), Sermonix Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Methods and Materials for Assessing Chemotherapy Responsiveness and Treating Cancer, Methods and Materials for Using Butyrylcholinesterases to Treat Cancer, Development of Human Tumor Xenografts from Women with Breast Cancer Treated with Neoadjuvant Chemotherapy (Inst)
Travel, Accommodations, Expenses: Lilly
Fatima Cardoso
Consulting or Advisory Role: Roche, Novartis, Pfizer, AstraZeneca, Teva, Astellas Pharma, Merus, Celgene, Eisai, Daiichi Sankyo, Genentech, Merck Sharp & Dohme, Sanofi, Pierre Fabre, Macrogenics, Amgen, GE Healthcare, GlaxoSmithKline, Mylan, Mundipharma, Seattle Genetics, Samsung Bioepis, Medscape, Prime Oncology
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Tiffany A. Traina
Consulting or Advisory Role: Genentech/Roche, Pfizer, AstraZeneca, Merck, Puma Biotechnology, Athenex, Daiichi Sankyo, Ionis Pharmaceuticals, Seattle Genetics, Eisai, Exact Sciences, Foundation Medicine, Ayala Pharmaceuticals, Gilead Sciences, Blueprint Medicines, Ellipses Pharma, Fuji Pharma, ITeos Therapeutics, Agendia
Research Funding: Eisai (Inst), Pfizer (Inst), Novartis (Inst), Innocrin Pharma (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Immunomedics (Inst), Genentech/Roche (Inst), Daiichi Sankyo (Inst), Carrick Pharm (Inst), Ayala Pharmaceuticals (Inst)
Urs Breitenstein
Consulting or Advisory Role: AstraZeneca (Inst), Elie Lilly (Inst), Novartis (Inst), Pierre Fabre (Inst), Roche (Inst)
Kerstin Ackerl
Research Funding: Pfizer (Inst)
Otto Metzger Filho
Honoraria: Grupo Oncoclinicas, Roche
Research Funding: Susan G. Komen for the Cure (Inst), Pfizer (Inst), Roche/Genentech (Inst), Eisai (Inst), Cascadian Therapeutics (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Grupo Oncoclinicas
Karin Zehetner
Research Funding: Pfizer (Inst)
Kadine Solomon
Employment: Alliance Foundation Trials
Stock and Other Ownership Interests: Pfizer, Merck, Moderna Therapeutics
Sarra El-Abed
Employment: Astellas Pharma (I), argenx (I)
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Pfizer (Inst)
Kathy Puyana Theall
Employment: Pfizer (I)
Stock and Other Ownership Interests: Pfizer (I)
Honoraria: Pfizer
Travel, Accommodations, Expenses: Pfizer
Dongrui Ray Lu
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Amylou Dueck
Patents, Royalties, Other Intellectual Property: Royalties from licensing fees for a patient symptom questionnaire (MPN-SAF)
Michael Gnant
Employment: Sandoz (I)
Honoraria: Amgen, Novartis, AstraZeneca, Lilly
Consulting or Advisory Role: Daiichi-Sankyo, Veracyte, Tolmar, LifeBrain, Lilly
Angela DeMichele
Research Funding: Pfizer (Inst), Genentech (Inst), Calithera Biosciences (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the virtual San Antonio Breast Cancer Symposium, December 8-11, 2020.
SUPPORT
Supported by Pfizer, Inc.
CLINICAL TRIAL INFORMATION
ClinicalTrials.gov identifier: NCT02513394; EudraCT number 2014-005181-30. The PALLAS trial is also ABCSG-42, AFT-05, and BIG-14-03.
M.G. and A.D. shared last authorship.
DATA SHARING STATEMENT
Pseudonymized individual participant data will be made available after completion of the study (ie, end of the Follow-Up phase) at the latest; the specific data made available will depend on the data needed to answer the question in the application. Other documents that will be available include the master informed consent form, the study Protocol and amendments, and the PALLAS Policy for Access to Study Data or Surplus Samples for Research Projects not related to the Protocol (Policy). We will share data with researchers whose proposed use of the data has been approved according to the PALLAS Policy for Access to Study Data or Surplus Samples for Research Projects not related to the Protocol (Policy), and whose research purpose is in line and approved according to the Policy. Excluded research include a research project for the benefit of any commercial or for-profit entity to develop (1) any product intended for use in the cure, mitigation, treatment, or prevention of disease in man or other animals (Therapeutic Product), or (2) any diagnostic product intended for the use in connection with any Therapeutic Product, whose primary method of action is modulation of the target known as CDK 4/6. This restriction does not prevent or preclude development of other diagnostic product development. Proposals should be directed to pallas.proposals@abcsg.at and pallas_aft@alliancefoundationtrials.org to request access. Data will be made available after approval of a research proposal according to the Policy and with a signed Data Transfer Agreement.
AUTHOR CONTRIBUTIONS
Conception and design: Erica L. Mayer, Christian Fesl, Dominik Hlauschek, Harold J. Burstein, Kathy D. Miller, Ingrid A. Mayer, Eric P. Winer, Matthew Goetz, Kerstin Ackerl, Otto Metzger Filho, Karin Zehetner, Amylou Dueck, Michael Gnant, Angela DeMichele
Administrative support: Dominik Hlauschek, Gunda Pristauz-Telsnigg, Otto Metzger Filho, Sarra El-Abed
Provision of study materials or patients: Harold J. Burstein, Nicholas Zdenkowski, Viktor Wette, Kathy D. Miller, Marija Balic, Tufia C. Haddad, Hiroji Iwata, Matthew Goetz, Fatima Cardoso, Otto Metzger Filho, Michael Gnant, Angela DeMichele
Collection and assembly of data: Erica L. Mayer, Dominik Hlauschek, Harold J. Burstein, Nicholas Zdenkowski, Viktor Wette, Kathy D. Miller, Marija Balic, Ingrid A. Mayer, Diana Lake, Gunda Pristauz-Telsnigg, Hiroji Iwata, Matthew Goetz, Dhanusha Sabanathan, Urs Breitenstein, Kerstin Ackerl, Otto Metzger Filho, Kadine Solomon, Amylou Dueck, Michael Gnant, Angela DeMichele
Data analysis and interpretation: Erica L. Mayer, Christian Fesl, Dominik Hlauschek, Laura Garcia-Estevez, Harold J. Burstein, Nicholas Zdenkowski, Ingrid A. Mayer, David Cameron, José Juan Ponce Lorenzo, Tufia C. Haddad, Lois Shepherd, Matthew Goetz, Fatima Cardoso, Tiffany A. Traina, Urs Breitenstein, Otto Metzger Filho, Sarra El-Abed, Kathy Puyana Theall, Dongrui Ray Lu, Amylou Dueck, Michael Gnant
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Treatment Exposure and Discontinuation in the PALbociclib CoLlaborative Adjuvant Study of Palbociclib With Adjuvant Endocrine Therapy for Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Early Breast Cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Erica L. Mayer
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Gilead Sciences
Research Funding: Pfizer (Inst)
Christian Fesl
Research Funding: Pfizer (Inst)
Dominik Hlauschek
Research Funding: Pfizer (Inst)
Laura Garcia-Estevez
Consulting or Advisory Role: Daiichi Sankyo/Astra Zeneca, Palex, Seattle Genetics
Research Funding: Roche/Genentech (Inst)
Nicholas Zdenkowski
Honoraria: Roche, Pfizer, Eisai
Consulting or Advisory Role: Lilly, AstraZeneca, Eisai
Research Funding: Roche (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Novartis
Kathy D. Miller
This author is the Senior Deputy Editor of Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Genentech/Roche, Athenex, AstraZeneca, Bristol Myers Squibb/Celgene
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Seattle Genetics (Inst), Pfizer (Inst), Astex Pharmaceuticals (Inst), British Biotech (Inst), CytomX Therapeutics (Inst), Alphamab (Inst)
Marija Balic
Consulting or Advisory Role: Amgen, AstraZeneca, Daiichi Sankyo/Astra Zeneca, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Samsung
Speakers' Bureau: Amgen, AstraZeneca, Daiichi Sankyo/Astra Zeneca, Lilly, Novartis, Pierre Fabre, Pfizer, Roche, Seattle Genetics
Research Funding: Lilly (Inst), Novartis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: MSD
Ingrid A. Mayer
Consulting or Advisory Role: Novartis, AstraZeneca, Lilly, Genentech, GlaxoSmithKline, Immunomedics, Macrogenics, Pfizer, AbbVie, Seattle Genetics, Puma Biotechnology, Cyclacel, Blueprint Medicines, Sanofi
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst)
David Cameron
Consulting or Advisory Role: Lilly (Inst), Novartis (Inst), Novartis (Inst), Research Triangle Institute RTI Health Solutions (Inst), Daiichi Sankyo (Inst), Prima BioMed (Inst), Merck Sharp & Dohme (Inst), Zymeworks (Inst), Eisai (Inst), Puma Biotechnology (Inst), Pfizer (Inst), Oncolytics (Inst), Roche (Inst), Roche (Inst), Samsung Bioepis (Inst), Seattle Genetics (Inst), Synthon (Inst), Clarity Pharmaceuticals (Inst), Bexon/Zymeworks (Inst), Sanofi (Inst)
Research Funding: Roche (Inst), Novartis (Inst), AstraZeneca (Inst)
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
José Juan Ponce Lorenzo
Honoraria: Seattle Genetics, Novartis, Pfizer, AstraZeneca/Daiichi Sankyo, Lilly, Roche
Consulting or Advisory Role: Seattle Genetics, Novartis, AstraZeneca/Daiichi Sankyo, Roche
Tufia C. Haddad
Research Funding: Takeda (Inst)
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo, Lilly Japan, Kyowa Hakko Kirin, Taiho Pharmaceutical
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin, Novartis
Research Funding: MSD (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nihonkayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Sanofi (Inst)
Matthew Goetz
Consulting or Advisory Role: Lilly, bioTheranostics, Genomic Health, Novartis, Eisai, Sermonix Pharmaceuticals, Context Therapeutics, Pfizer, Biovica
Research Funding: Lilly (Inst), Pfizer (Inst), Sermonix Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Methods and Materials for Assessing Chemotherapy Responsiveness and Treating Cancer, Methods and Materials for Using Butyrylcholinesterases to Treat Cancer, Development of Human Tumor Xenografts from Women with Breast Cancer Treated with Neoadjuvant Chemotherapy (Inst)
Travel, Accommodations, Expenses: Lilly
Fatima Cardoso
Consulting or Advisory Role: Roche, Novartis, Pfizer, AstraZeneca, Teva, Astellas Pharma, Merus, Celgene, Eisai, Daiichi Sankyo, Genentech, Merck Sharp & Dohme, Sanofi, Pierre Fabre, Macrogenics, Amgen, GE Healthcare, GlaxoSmithKline, Mylan, Mundipharma, Seattle Genetics, Samsung Bioepis, Medscape, Prime Oncology
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Tiffany A. Traina
Consulting or Advisory Role: Genentech/Roche, Pfizer, AstraZeneca, Merck, Puma Biotechnology, Athenex, Daiichi Sankyo, Ionis Pharmaceuticals, Seattle Genetics, Eisai, Exact Sciences, Foundation Medicine, Ayala Pharmaceuticals, Gilead Sciences, Blueprint Medicines, Ellipses Pharma, Fuji Pharma, ITeos Therapeutics, Agendia
Research Funding: Eisai (Inst), Pfizer (Inst), Novartis (Inst), Innocrin Pharma (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Immunomedics (Inst), Genentech/Roche (Inst), Daiichi Sankyo (Inst), Carrick Pharm (Inst), Ayala Pharmaceuticals (Inst)
Urs Breitenstein
Consulting or Advisory Role: AstraZeneca (Inst), Elie Lilly (Inst), Novartis (Inst), Pierre Fabre (Inst), Roche (Inst)
Kerstin Ackerl
Research Funding: Pfizer (Inst)
Otto Metzger Filho
Honoraria: Grupo Oncoclinicas, Roche
Research Funding: Susan G. Komen for the Cure (Inst), Pfizer (Inst), Roche/Genentech (Inst), Eisai (Inst), Cascadian Therapeutics (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Grupo Oncoclinicas
Karin Zehetner
Research Funding: Pfizer (Inst)
Kadine Solomon
Employment: Alliance Foundation Trials
Stock and Other Ownership Interests: Pfizer, Merck, Moderna Therapeutics
Sarra El-Abed
Employment: Astellas Pharma (I), argenx (I)
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Pfizer (Inst)
Kathy Puyana Theall
Employment: Pfizer (I)
Stock and Other Ownership Interests: Pfizer (I)
Honoraria: Pfizer
Travel, Accommodations, Expenses: Pfizer
Dongrui Ray Lu
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Amylou Dueck
Patents, Royalties, Other Intellectual Property: Royalties from licensing fees for a patient symptom questionnaire (MPN-SAF)
Michael Gnant
Employment: Sandoz (I)
Honoraria: Amgen, Novartis, AstraZeneca, Lilly
Consulting or Advisory Role: Daiichi-Sankyo, Veracyte, Tolmar, LifeBrain, Lilly
Angela DeMichele
Research Funding: Pfizer (Inst), Genentech (Inst), Calithera Biosciences (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group : Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Crown JP, Lang I, et al. : The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol 16:25-35, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Martin M, Rugo HS, et al. : Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925-1936, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Turner NC, Ro J, Andre F, et al. : Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373:209-219, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Turner NC, Slamon DJ, Ro J, et al. : Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379:1926-1936, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Mayer EL, Dueck AC, Martin M, et al. : Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 22:212-222, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Henry NL, Azzouz F, Desta Z, et al. : Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30:936-942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman DL, Shao T, Kushi LH, et al. : Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529-537, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer EL, DeMichele A, Rugo HS, et al. : A phase II feasibility study of palbociclib in combination with adjuvant endocrine therapy for hormone receptor-positive invasive breast carcinoma. Ann Oncol 30:1514-1520, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Bartlett CH, Schnell P, et al. : Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 21:1165-1175, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAndrew NP, Dickson MA, Clark AS, et al. : Early treatment-related neutropenia predicts response to palbociclib. Br J Cancer 123:912-918, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershman DL, Kushi LH, Shao T, et al. : Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28:4120-4128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partridge AH, LaFountain A, Mayer E, et al. : Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556-562, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Bachelot T, Dalenc F, Chabaud S, et al. : Efficacy of everolimus in patients with HR+/HER2- high risk early stage breast cancer. Ann Oncol 32:574-575, 2021 [Google Scholar]
- 15.Miller KD, O’Neill A, Gradishar W, et al. : Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node–positive and high-risk lymph node–negative breast cancer (E5103). J Clin Oncol 36:2621-2629, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colleoni M, Gray KP, Gelber S, et al. : Low-dose oral cyclophosphamide and methotrexate maintenance for hormone receptor-negative early breast cancer: International Breast Cancer Study Group Trial 22-00. J Clin Oncol 34:3400-3408, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loibl S, Marme F, Martin M, et al. : Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—The Penelope-B trial. J Clin Oncol 39:1518-1530, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Johnston SRD, Harbeck N, Hegg R, et al. : Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 38:3987-3998, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pseudonymized individual participant data will be made available after completion of the study (ie, end of the Follow-Up phase) at the latest; the specific data made available will depend on the data needed to answer the question in the application. Other documents that will be available include the master informed consent form, the study Protocol and amendments, and the PALLAS Policy for Access to Study Data or Surplus Samples for Research Projects not related to the Protocol (Policy). We will share data with researchers whose proposed use of the data has been approved according to the PALLAS Policy for Access to Study Data or Surplus Samples for Research Projects not related to the Protocol (Policy), and whose research purpose is in line and approved according to the Policy. Excluded research include a research project for the benefit of any commercial or for-profit entity to develop (1) any product intended for use in the cure, mitigation, treatment, or prevention of disease in man or other animals (Therapeutic Product), or (2) any diagnostic product intended for the use in connection with any Therapeutic Product, whose primary method of action is modulation of the target known as CDK 4/6. This restriction does not prevent or preclude development of other diagnostic product development. Proposals should be directed to pallas.proposals@abcsg.at and pallas_aft@alliancefoundationtrials.org to request access. Data will be made available after approval of a research proposal according to the Policy and with a signed Data Transfer Agreement.