PURPOSE
Selumetinib can increase radioactive iodine (RAI) avidity in RAI-refractory tumors. We investigated whether selumetinib plus adjuvant RAI improves complete remission (CR) rates in patients with differentiated thyroid cancer (DTC) at high risk of primary treatment failure versus RAI alone.
METHODS
ASTRA (ClinicalTrials.gov identifier: NCT01843062) is an international, phase III, randomized, placebo-controlled, double-blind trial. Patients with DTC at high risk of primary treatment failure (primary tumor > 4 cm; gross extrathyroidal extension outside the thyroid gland [T4 disease]; or N1a/N1b disease with ≥ 1 metastatic lymph node(s) ≥ 1 cm or ≥ 5 lymph nodes [any size]) were randomly assigned 2:1 to selumetinib 75 mg orally twice daily or placebo for approximately 5 weeks (no stratification). On treatment days 29-31, recombinant human thyroid-stimulating hormone (0.9 mg)–stimulated RAI (131I; 100 mCi/3.7 GBq) was administered, followed by 5 days of selumetinib/placebo. The primary end point (CR rate 18 months after RAI) was assessed in the intention-to-treat population.
RESULTS
Four hundred patients were enrolled (August 27, 2013-March 23, 2016) and 233 randomly assigned (selumetinib, n = 155 [67%]; placebo, n = 78 [33%]). No statistically significant difference in CR rate 18 months after RAI was observed (selumetinib n = 62 [40%]; placebo n = 30 [38%]; odds ratio 1.07 [95% CI, 0.61 to 1.87]; P = .8205). Treatment-related grade ≥ 3 adverse events were reported in 25/154 patients (16%) with selumetinib and none with placebo. The most common adverse event with selumetinib was dermatitis acneiform (n = 11 [7%]). No treatment-related deaths were reported.
CONCLUSION
Postoperative pathologic risk stratification identified patients with DTC at high risk of primary treatment failure, although the addition of selumetinib to adjuvant RAI failed to improve the CR rate for these patients. Future strategies should focus on tumor genotype–tailored drug selection and maintaining drug dosing to optimize RAI efficacy.
INTRODUCTION
Thyroid surgery followed by radioactive iodine (RAI) is standard management for patients with differentiated thyroid cancer (DTC) at high and intermediate risk of disease persistence or recurrence. In the adjuvant setting, RAI is administered to consolidate complete remission (CR), although prospective trials verifying benefit have not been performed.1 Successful initial therapy is crucial because most patients with DTC who achieve remission do not experience recurrence (approximately 1%–4% over median follow-up periods of 5-10 years).2-4
CONTEXT
Key Objective
Retrospective analyses have proposed pathologic criteria for differentiated thyroid cancer that is associated with a high risk of failing to achieve complete remission (CR) with total thyroidectomy and postoperative radioactive iodine (RAI). This phase III trial evaluated whether the addition of the MEK 1/2 inhibitor selumetinib to postoperative RAI improves the CR rate for these patients.
Knowledge Generated
In a clinical trial design with a 2:1 random assignment of selumetinib versus placebo in combination with standard postoperative RAI, selumetinib did not improve the 18-month CR rate over placebo.
Relevance
Pathologic risk stratification can identify a subset of patients with differentiated thyroid cancer for whom standard thyroidectomy and RAI are largely insufficient for achieving CR. The failure of selumetinib in this study highlights the need for new strategies to improve outcomes for these high-risk patients.
Retrospective studies have identified primary tumor size/extent of invasion (> 4 cm or gross extrathyroidal extension outside the thyroid gland [T4 disease]) and lymph node status (N1a/N1b disease with ≥ 1 metastatic lymph node ≥ 1 cm or ≥ 5 lymph nodes of any size) as predictive for a high risk for failing initial therapy (unpublished analysis on the basis of studies by Tuttle et al3 and Vaisman et al4). Approximately 70% of patients whose disease met any one of these criteria failed to achieve CR with surgery and RAI, requiring more intensive surveillance, tests/evaluations, and therapies. Novel approaches to enhance RAI activity could improve outcomes for these patients.
Up to approximately 70% of DTCs harbor mutations that activate the RAS/RAF/MEK/ERK (RAS-ERK) pathway,5-9 which represses gene expression required for iodine avidity to mediate RAI-refractoriness.10,11 In mouse models of BRAF-mutant thyroid cancer, ablation of BRAF activation restored iodine avidity.12 Selumetinib is a potent and highly selective allosteric MEK 1/2 inhibitor (AZD6244, ARRY-142886)13-15 with a short half-life. A pilot clinical trial demonstrated that selumetinib increased iodine avidity and efficacy in 8/20 patients with recurrent/metastatic RAI-refractory thyroid cancer.16
To test the hypothesis that selumetinib enhances RAI efficacy in the adjuvant setting, we conducted the phase III Adjuvant Selumetinib for differentiated Thyroid cancer, Remission after RAI (ASTRA) trial to compare the CR rate achieved with adjuvant RAI in combination with placebo versus selumetinib in patients with DTC at high risk of primary treatment failure.17
METHODS
Study Design and Participants
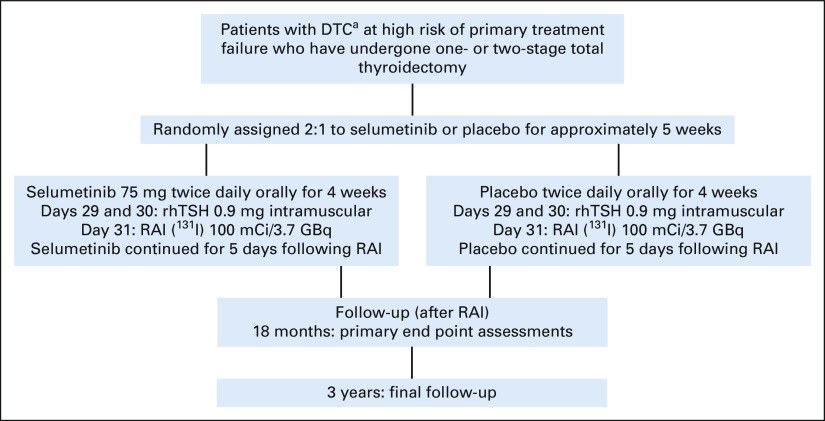
ASTRA was a phase III, randomized, placebo-controlled, double-blind trial (Fig 1) that enrolled patients from 42 sites in eight countries (Data Supplement, online only).
FIG 1.
ASTRA trial design. aIncluding papillary thyroid cancer, follicular thyroid cancer, and poorly differentiated thyroid cancer. DTC, differentiated thyroid cancer; RAI, radioactive iodine; rhTSH, recombinant human thyroid-stimulating hormone.
Eligible patients were age ≥ 18 years with pathologically confirmed DTC. Patients with anaplastic, medullary, and Hürthle cell thyroid cancers were excluded. Patients must have undergone a one- or two-stage total thyroidectomy with therapeutic neck dissection of clinically apparent metastatic lymph nodes. Patients also had to have disease with at least one of the following high-risk pathologic features: primary tumor > 4 cm; gross extrathyroidal extension (T4 disease); and at least one metastatic lymph node ≥ 1 cm or involvement of ≥ 5 lymph nodes (any size). Patients had to undergo neck ultrasound, magnetic resonance imaging (MRI) of the neck with contrast, and a computed tomography (CT) scan of the chest ≥ 4 weeks after surgery to document the absence of gross disease. Patients with antithyroglobulin antibodies were excluded.
The intention-to-treat (ITT) population included all randomly assigned patients. The safety analysis set included patients who received ≥ 1 dose(s) of randomized treatment. The treatment-compliant analysis set was a subset of the ITT population, consisting of patients who took study treatment for the crucial time period (≥ 7 consecutive days before RAI, on the day of RAI, and for 5 consecutive days after RAI).
The study was performed in accordance with ethical principles outlined by the Declaration of Helsinki and consistent with the Good Clinical Practice guidelines of the International Conference on Harmonisation, applicable regulatory requirements, and the AstraZeneca policy on Bioethics and Human Biological Samples. The Protocol (online only) was approved by local institutional review board/independent ethics committees. All patients provided written informed consent. Protocol details are in the Data Supplement.
Procedures
Patients were randomly assigned 2:1 via an interactive voice-response/web-response system to receive selumetinib or placebo (Biostatistics Group, AstraZeneca). Investigators and patients were blinded to treatment allocation.
Patients received selumetinib (AstraZeneca, Wilmington, DE) 75 mg twice daily or matched placebo (AstraZeneca) for approximately 5 weeks (typically 36 days). Therapy was started approximately 4 weeks before the planned RAI treatment (≥ 6 and ≤ 16 weeks after thyroid cancer surgery). Patients were started on a low-iodine diet ≥ 1 week before RAI and continued until the day after RAI. On days 29 and 30, patients received 0.9 mg of recombinant human thyroid-stimulating hormone (rhTSH; Thyrogen, Sanofi/Genzyme, Cambridge, MA). On day 31, all patients received RAI (131I) 100 mCi/3.7 GBq (± 10%). Patients were required to remain on treatment during the crucial time period.
Outcomes
The primary end point was CR rate at 18 months after RAI treatment in the ITT population. CR was defined as meeting the following criteria at the 18-month post-RAI time point, evaluated in three consecutive stages: stage I, serum thyroglobulin < 1 ng/mL without TSH stimulation (suppressed TG assessment by central laboratory analysis) and neck ultrasound negative for structural disease (assessed by investigator site review); stage II, following rhTSH stimulation, serum thyroglobulin level < 1 ng/mL without interfering thyroglobulin antibodies (stimulated TG assessment by central laboratory analysis) and no evidence of thyroid cancer on diagnostic whole-body scan (assessed by blinded independent central review [BICR]); and stage III, no evidence of thyroid cancer by MRI neck with gadolinium and CT chest without contrast (assessed by BICR). If patients were determined not to be in CR, subsequent stages of CR evaluation were not performed to minimize patient burden. Histopathologic evidence of thyroid cancer on a fine needle aspirate/biopsy and/or administration of additional thyroid cancer treatment after RAI (except levothyroxine) were considered failures to achieve CR. The 18-month time point was chosen on the basis of retrospective data that 94% of patients with DTC who achieve CR do so by 18 months.18
Secondary end points included assessment of clinical CR at 18 months after RAI treatment; complete and clinical remission in patients with a BRAF or NRAS mutation at 18 months after RAI treatment; safety and tolerability; and drug pharmacokinetics. Clinical CR definition was limited to standard surveillance evaluations, which includes all CR evaluations above, except MRI neck and CT chest scans. Prespecified sensitivity analyses were also performed.
Statistical Analysis
The primary analysis was of CR rate at 18 months, comparing selumetinib versus placebo with RAI in the ITT population, using a logistic regression model including treatment as the only covariate. The results were presented with the odds ratio (OR), 95% profile likelihood CI, and associated 2-sided P value. Assuming CR rates in the overall study population were 50% and 30% for the selumetinib and placebo arms, respectively, 228 patients, randomly assigned 2:1 (152 selumetinib and 76 placebo), would provide at least 80% power to show statistical significance, on the basis of a two-sided 5% significance level. Patients missing any 18-month time point assessments were categorized as not in CR.
Secondary analyses were also performed using a logistic regression model including treatment as the only covariate. For analysis of the BRAF and NRAS mutation–positive population, only patients from the ITT population with genetic samples that were positive for BRAF or NRAS mutations were included.
A sensitivity analysis for each of the primary and secondary analyses was performed using a logistic regression model including treatment and adjusted for the covariates of mutation status (ITT population only), histology status, and age, provided there were enough data points for a meaningful analysis. In addition, analyses were repeated using the treatment-compliant population, which included patients who received treatment during the crucial period.
The trial design did not include interim analyses or a data monitoring committee. This study is registered with ClinicalTrials.gov identifier: NCT01843062.
RESULTS
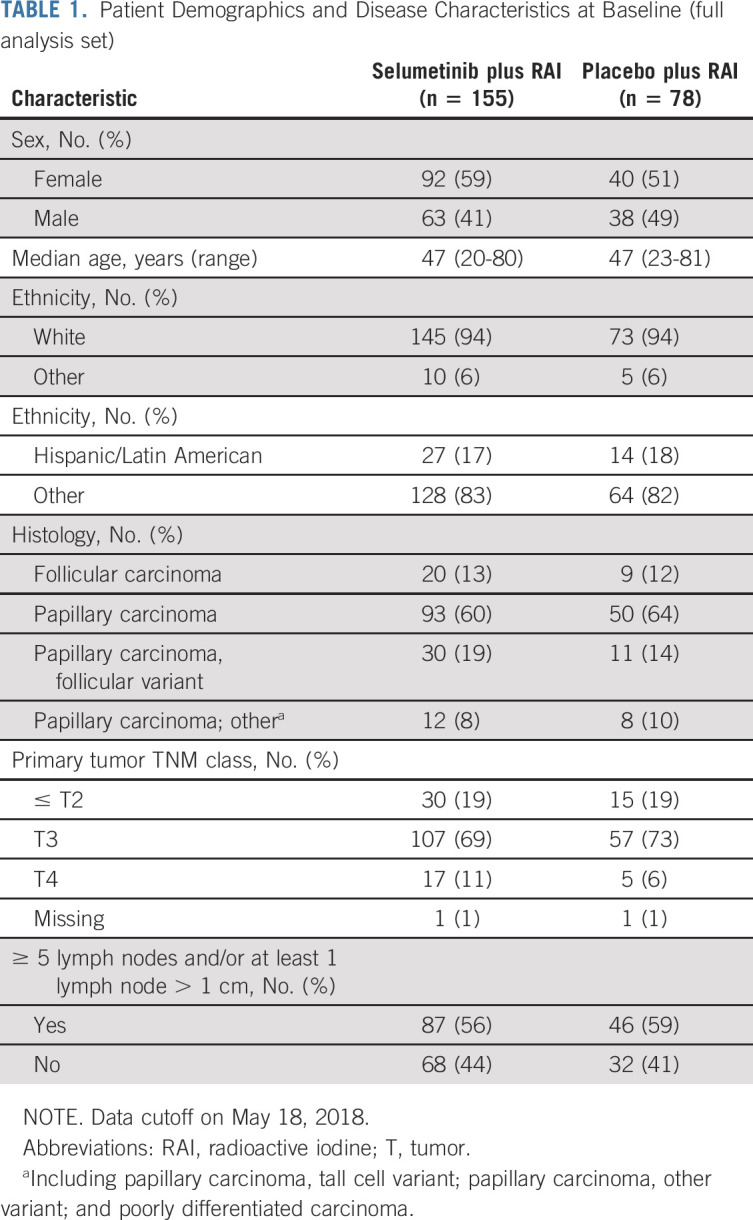
Of the 400 patients enrolled between August 27, 2013, and March 23, 2016, 233 were randomly assigned (155 [67%] in the selumetinib and 78 [33%] in the placebo arms; ITT population; Data Supplement) and 204 completed study treatment (selumetinib, n = 128/155 [83%]; placebo, n = 76/78 [97%]). Patient demographics and disease characteristics were similar between both arms and representative of the intended patient population (Table 1). These outcomes are based on the data cutoff date of May 18, 2018.
TABLE 1.
Patient Demographics and Disease Characteristics at Baseline (full analysis set)
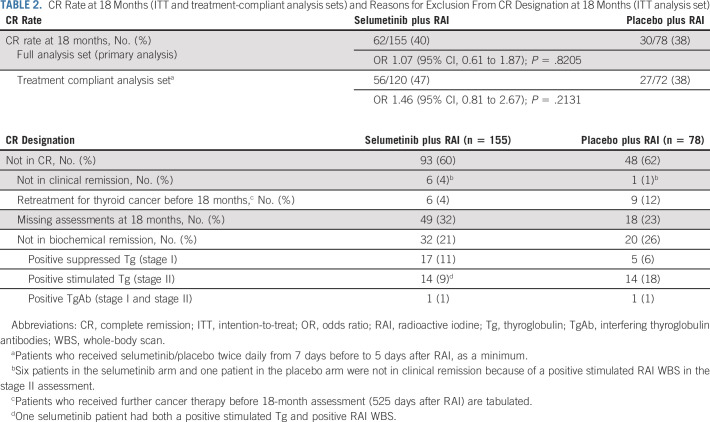
In the ITT population, there was no statistically significant improvement in CR rate at 18 months in patients receiving selumetinib plus RAI (n = 62, 40%) versus those receiving placebo plus RAI (n = 30, 38%; OR, 1.07; 95% CI, 0.61 to 1.87; P = .8205; Table 2). Reasons for patients not being in CR are summarized in Table 2. The difference in CR rate at 18 months between treatment arms was numerically greater, but not statistically significant, when the analysis was restricted to the treatment-compliant analysis set only (see the Methods section for definition of this analysis set), with 56 patients (47%) and 27 patients (38%) having CR in the selumetinib and placebo arms, respectively (OR, 1.46; 95% CI, 0.81 to 2.67; P = .2131; Table 2). A prespecified sensitivity analysis of the primary end point also found no significant difference between treatment arms in CR rate at 18 months using logistic regression analysis adjusted for mutation status, histology, and age. All patients who met 18-month clinical remission criteria (negative rhTSH-stimulated thyroglobulin/diagnostic whole-body scan and neck ultrasound) also met the primary CR criteria (which also required negative MRI neck and CT chest scans), suggesting that standard clinical surveillance is sufficient for detecting persistent or recurrent disease.
TABLE 2.
CR Rate at 18 Months (ITT and treatment-compliant analysis sets) and Reasons for Exclusion From CR Designation at 18 Months (ITT analysis set)
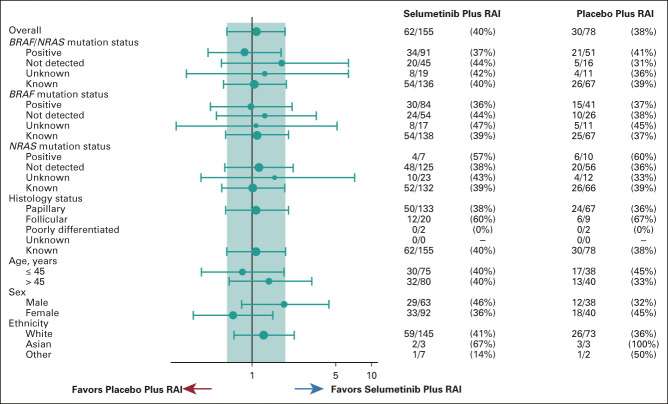
In total, 205/233 patients had BRAF genomic status known (positive or not detected); of those, 203 patients also had a known RAS mutation status (Data Supplement). For the subgroup with a BRAF (n = 125) or NRAS (n = 17) mutation, no significant difference was found for complete/clinical remission rate at 18 months for selumetinib (34/91, 37%) versus placebo (21/51, 41%; OR, 0.85; 95% CI, 0.42 to 1.73; P = .6549; Fig 2). No significant difference was found among patients with mutant BRAF (30/84, 36% with selumetinib and 15/41, 37% with placebo, OR, 0.96; 95% CI, 0.45 to 2.12; P = .9242) or wild-type BRAF (24/54, 44% with selumetinib and 10/26, 38% with placebo, OR, 1.28; 95% CI, 0.50 to 3.40; P = .6112). No other significant differences were observed in subgroup analyses of CR rate at 18 months when patients were categorized by histology, age, sex, or ethnicity (Fig 2).
FIG 2.
Forest plot of complete remission rate at 18 months by subgroup data (ITT analysis set). Population: full analysis set, data cutoff on May 18, 2018. Minimum 10 patients per treatment by factor levels are considered for calculation of OR and CI. Size of circle is proportional to the number of events. The teal band represents the 95% CI for the overall OR. When BRAF/NRAS mutations are summarized together, the status is summarized as known if (1) either BRAF or NRAS mutation is positive or (2) both BRAF and NRAS mutations are not detected. In case one mutation was not detected while the other mutation was unknown, the status is summarized as unknown. ITT, intention-to-treat; OR, odds ratio; RAI, radioactive iodine.
Overall, 147 important protocol deviations (IPDs) were captured and 95 patients (41%) had at least one IPD. Most commonly, patients were recorded to have a deviation related to procedures not being adhered to during the 18-month assessment (n = 67 [29%]; Data Supplement). The second most frequently reported IPD subgroup was consumption of < 4,320 mg (ie, 80% of the total dose) of study treatment, reported in 12% of patients overall. The mean selumetinib compliance of 84% (standard deviation 29%) of the planned study doses was lower than placebo (102%; standard deviation 10%).
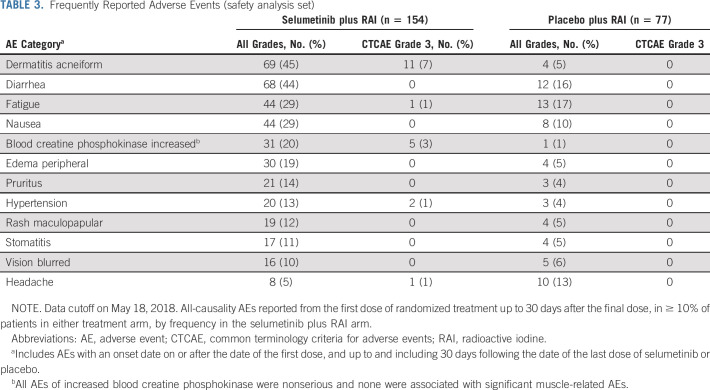
More adverse events (AEs; any grade), regardless of causality, were observed with selumetinib compared to placebo (n = 151 [98%] and [n = 58 [75%], respectively; Data Supplement). Most AEs were Common Terminology Criteria for Adverse Events grade 1 or 2. Grade 3 AEs were reported in 18% of patients receiving selumetinib and 1% of those receiving placebo (related grade ≥ 3 AEs were 16% and 0%, respectively; Data Supplement). The most frequent selumetinib grade 3 AEs were dermatitis acneiform and increased blood creatine phosphokinase (Table 3). Serious AEs (SAEs) in four patients were considered related to selumetinib (dermatitis acneiform, n = 2; drug hypersensitivity, n = 1; and gastric hemorrhage, n = 1). The drug hypersensitivity occurred 40 days after treatment initiation and 1 day after the last dose of selumetinib. Selumetinib was permanently discontinued for one of the rash SAEs and interrupted for the other rash SAE followed by dose reduction to 100 mg. All patients recovered or were recovering at data cutoff. Three patients died > 30 days after treatment: one patient in the selumetinib arm because of thyroid cancer and one case each of thyroid cancer and cerebrovascular accident in the placebo arm.
TABLE 3.
Frequently Reported Adverse Events (safety analysis set)
AEs leading to treatment discontinuation occurred in 18 patients (12%) with selumetinib and none with placebo. The most frequent AE leading to selumetinib discontinuation was dermatitis acneiform in seven patients (5%). Four patients discontinued for ophthalmologic AEs (one each of chorioretinopathy, macular detachment, macular edema, and reduced visual acuity); all recovered after drug withdrawal. Compared with placebo, more patients receiving selumetinib had dose interruptions (3% v 21%, respectively) and reductions (1% v 27%, respectively); nine patients required two selumetinib dose reductions. Only one patient in the placebo arm (1%) required a dose reduction.
DISCUSSION
In this phase III trial, the addition of selumetinib to adjuvant RAI did not significantly improve 18-month CR rate versus placebo plus RAI in patients with DTC at high risk of primary treatment failure. No selumetinib advantage was identified in the secondary analyses of CR rate in mutant BRAF/NRAS patients or clinical remission for the ITT population. However, the results are noteworthy as this is the first prospective data generated regarding the efficacy of adjuvant RAI. Without prior data to guide trial design, the study was powered on the basis of retrospective analyses,3,4 predicting a 30% CR rate in patients with DTC and the high-risk pathologic criteria used in this trial. The observation that only 38% of patients in the RAI plus placebo arm achieved CR suggests that the high-risk disease criterion successfully identified a cohort for whom standard surgery and RAI is largely insufficient for disease cure. This innovative study uses CR rate at 18 months as an end point and these results could serve as reference for future adjuvant trials.
Preclinical work has established that oncogenic mitogen-activated protein kinase (MAPK) signaling via BRAF, RAS, and other genetic alterations suppresses thyroid differentiation to diminish tumor iodide uptake and RAI efficacy.10-12,19 Our initial pilot clinical trial demonstrated that MAPK pathway inhibition with selumetinib could enhance RAI avidity and efficacy in a subset of RAI-refractory patients.16 Subsequent RAI-refractory trials with other drugs targeting MAPK signaling have confirmed this finding.20-23 Having observed these promising results in the most challenging setting of RAI-refractory disease, we hypothesized that incorporating this approach into the adjuvant setting could improve CR rates.
The reason for the lack of benefit observed with selumetinib in ASTRA may be informed by recent work identifying factors requisite for successful redifferentiation. Sustained and potent MAPK inhibition is essential for successful restoration of the thyroid-specific gene expression required for RAI avidity.22 Given that MAPK pathway inhibition and differentiation status are readily reversible phenomena in our preclinical studies, we reasoned that missed or inconsistent drug dosing could result in loss of the redifferentiation effects achieved, and planned to investigate the importance of drug compliance peri-131I administration, specifically the 7 consecutive days before RAI, on the day of RAI, and for 5 consecutive days after RAI (the crucial time period). The patients compliant with selumetinib or placebo for this period were designated the treatment-compliant set. The increased absolute difference in 18-month CR rate in favor of selumetinib for the treatment-compliant set is consistent with the hypothesis that uninterrupted treatment during this period may be important. More work is needed to definitively determine the duration and critical period of therapy necessary to optimize redifferentiation. Additionally, 21%, 27%, and 12% of selumetinib-treated patients required dose interruptions, reductions, and drug discontinuation, respectively. Difficulty maintaining full-dose selumetinib continuously suggests that drug toxicity may have compromised the ability to elicit sustained, potent MAPK pathway inhibition to enhance RAI efficacy, highlighting the potential importance of drug tolerability to achieve better outcomes in future studies.
Not all DTC oncogenic driver mutations activate MAPK signaling to the same degree. The BRAFV600E mutation induces a significantly higher MAPK transcriptional output versus RAS mutations or RET rearrangements,8 and is associated with more profound suppression of thyroid differentiation.8 These distinctions also translate to differences in susceptibility to MEK inhibition with selumetinib. In the pilot RAI-refractory selumetinib trial, only one of nine BRAFV600E-mutant patients achieved sufficient iodine uptake to warrant RAI, whereas all five NRAS-mutant patients had positive responses.16 We hypothesized that redifferentiation achieved with selumetinib may be more favorable in the adjuvant setting, regardless of BRAF status, since tumors are likely to be more differentiated and hence susceptible to MEK inhibition in this earlier disease stage. Results from ASTRA prove this is not the case as no benefit was observed with selumetinib in BRAF-mutant patients, highlighting the need for a biomarker-directed strategy. BRAF-targeted drugs are superior to MEK inhibitors for redifferentiation in BRAF-mutant, RAI-refractory patients.21,23
Our ongoing work in RAI-refractory patients also suggests that there is likely a genomic subset of patients whose tumors are refractory to RAI and all MAPK inhibitor redifferentiation strategies tested to date.24 We also have a limited understanding of how resistance to radiation, independent of the capacity to concentrate iodide, may contribute to RAI-refractoriness. For these patient subgroups, developing non–RAI-based adjuvant therapies may be required to improve outcomes. Other debated topics regarding RAI therapy not addressed by this trial include the potential importance of using higher RAI activities, implementing thyroid hormone withdrawal in lieu of rhTSH, or the impact of the theoretical sink effect (ie, minimal residual normal thyroid tissue competing RAI away from malignant cells).
Executing the novel study design internationally presented logistical challenges, resulting in protocol deviations that may have influenced outcomes. In total, 32% of patients receiving selumetinib and 23% of those receiving placebo had missing 18-month assessments that designated them as treatment failures. This observation highlights that streamlining protocol assessments and procedures should be prioritized in future studies. For assessing 18-month CR, we observed complete concordance between the CR criteria used for the primary end point and the more limited clinical remission CR definition (all evaluations, excluding the MRI neck and CT chest), verifying that the latter, simpler approach could serve as a reliable primary end point for future studies. The lack of benefit observed with selumetinib should also be considered in the context of the ambitious end points targeted. Instead of the palliative end point of partial response in recurrent/metastatic RAI-refractory studies, the goal of this trial was to increase the absolute cure rate for a high-risk population by 20% with selumetinib versus placebo. This substantial degree of benefit was judged by investigators to be necessary to justify implementing additional therapy for this patient population and to limit the trial sample size to ensure feasibility.
In conclusion, addition of selumetinib to adjuvant RAI did not sufficiently improve CR rate in patients with DTC at high risk of primary treatment failure. The study results demonstrate that risk stratification using pathologic criteria can identify patients for whom standard treatment is insufficient to elicit cure. Future efforts to enhance RAI efficacy must focus on tailoring the pharmacologic approach to tumor genotype and improving treatment tolerability/compliance to optimize clinical efficacy.
ACKNOWLEDGMENT
The authors would like to acknowledge Jon Moran, PhD, and Bernadette Tynan, MSc, of Ashfield Healthcare Communications, part of UDG Healthcare plc, Macclesfield, UK, and Robyn Fowler, PhD, of OPEN Health Communications, London, UK, for medical writing support that was funded by AstraZeneca in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Alan L. Ho
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Eisai, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia, Inxmed
Speakers’ Bureau: Medscape, Omniprex America, Novartis
Research Funding: Lilly, Genentech/Roche (Inst), AstraZeneca, Allos Therapeutics, Astellas Pharma, Ayala Pharmaceuticals, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Elevar Therapeutics, Kolltan Pharmaceuticals, Kura Oncology, Merck, Novartis, Pfizer
Travel, Accommodations, Expenses: Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, Klusa Pharma
Marek Dedecjus
Honoraria: Sanofi/Aventis, Bayer, Berlin-Chemie
Research Funding: AstraAzeneca/Merck, Exelixis
Lori J. Wirth
Consulting or Advisory Role: Merck, Loxo, Blueprint Medicines, Eisai, Lilly, Bayer, Exelixis
Researching Funding: Checkmate Pharmaceuticals (Inst), Lilly (Inst), Ayala Pharmaceuticals (Inst), Eisai (Inst)
Expert Testimony: Eisai
Other Relationship: PDS Biotechnology
R. Michael Tuttle
Research Funding: Elesta (Inst)
Fernanda Vaisman
Consulting or Advisory Role: AstraZeneca/Merck, Ipsen, Lilly, Bayer Health
Speaker’s Bureau: Bayer, AstraZeneca/Merck, Sanofi, United Medical
Lars Bastholt
Research Funding: Bristol Myers Squibb (Inst)
Andrew G. Gianoukakis
Consulting or Advisory Role: Eisai, Exelixs
Patrice Rodien
Consulting or Advisory Role: Eisai Europe
Research Funding: Pfizer (Inst)
Ralf Paschke
Consulting or Advisory Role: Bayer, Eisai
Researching Funding: Bayer (Inst)
Patents, Royalties, Other Intellectual Property: Licensing fee for a diagnostic test
Rossella Elisei
Consulting or Advisory Role: EISAI, Genezyme, Loxo, Ipsen, Bayer, Lilly
Karen So
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Danielle Carroll
Employment: AstraZeneca/MedImmune, Azeria therapeutics (I)
Leadership: Azeria therapeutics (I)
Stock and Other Ownership Interests: AstraZeneca/MedImmune, Azeria Therapeutics (I)
Honoraria: AstraZeneca (I)
Consulting or Advisory Role: Azeria therapeutics (I)
Research Funding: AstraZeneca (I)
Patents, Royalties, Other Intellectual Property: Royalties from Active Motif (I), Compositions featuring an attenuated Newcastle disease virus and methods of use for treating neoplasia, Anti-cxc chemokine-2 binding molecules and uses thereof
Tina Hovey
Employment: Phastar
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca
James A. Fagin
Honoraria: Eisai Europe
Consulting or Advisory Role: Loxo Oncology
Patents, Royalties, Other Intellectual Property: 3. MSK Ref. SK 2014-024-03 Title: Treatment of H-RAS-Driven Tumors. Application Number: 15/305,788. 3. Anti-TSHR Multispecific Antibodies and Uses Thereof, provisional patent.
No other potential conflicts of interest were reported.
See accompanying editorial on page 1847
DISCLAIMER
The sponsor (AstraZeneca) designed the trial in collaboration with the investigators (Data Supplement). Data were collected by the investigators and analyzed jointly with the sponsor. The sponsor and all authors were responsible for data interpretation and the development of the article and approval of the final version. The article was written by the corresponding author in collaboration with the coauthors, with independent medical writing assistance, supported financially by the sponsor. The authors had access to the raw data. All authors made the final decision to submit the article for publication.
PRIOR PRESENTATION
Presented in part at the 88th Annual Meeting of the American Thyroid Association, October 3-7, 2018, Washington, DC.
SUPPORT
Supported by AstraZeneca and support was received from the MSK SPORE (P50 CA172012), core grants (NIH/NCI Cancer Center Support Grant P30 CA008748, R01 CA184724, 5 R01 CA201250, R01 CA249663), and the Geoffrey Beene Cancer Research Center at Memorial Sloan Kettering Cancer Center. The authors thank all patients who participated in the trial and their families, as well as the staff and investigators at all of the ASTRA trial sites.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Deidentified participant data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Requests for data collected for this study, including individual participant data and a data dictionary defining each field in the set, will be considered in line with the above policy. In addition to the clinical study protocol, related documents (for example, the statistical analysis plan and informed consent form) can be made available.
AUTHOR CONTRIBUTIONS
Conception and design: Alan L. Ho, Lori J. Wirth, R. Michael Tuttle, William B. Inabnet III, Karen So, James A. Fagin
Administrative support: Karen So
Provision of study materials or patients: Alan L. Ho, Lori J. Wirth, R. Michael Tuttle, Jan Tennvall, Patrice Rodien, Ralf Paschke, James A. Fagin
Collection and assembly of data: Alan L. Ho, Marek Dedecjus, Lori J. Wirth, William B. Inabnet III, Jan Tennvall, Fernanda Vaisman, Lars Bastholt, Andrew G. Gianoukakis, Patrice Rodien, Ralf Paschke, Rossella Elisei, Danielle Carroll, Bhavana Thakre
Data analysis and interpretation: Alan L. Ho, Lori J. Wirth, Jan Tennvall, Fernanda Vaisman, Lars Bastholt, Andrew G. Gianoukakis, Ralf Paschke, David Viola, Karen So, Danielle Carroll, Tina Hovey, Bhavana Thakre, James A. Fagin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Selumetinib Plus Adjuvant Radioactive Iodine in Patients With High-Risk Differentiated Thyroid Cancer: A Phase III, Randomized, Placebo-Controlled Trial (ASTRA)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alan L. Ho
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Eisai, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia, Inxmed
Speakers’ Bureau: Medscape, Omniprex America, Novartis
Research Funding: Lilly, Genentech/Roche (Inst), AstraZeneca, Allos Therapeutics, Astellas Pharma, Ayala Pharmaceuticals, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Elevar Therapeutics, Kolltan Pharmaceuticals, Kura Oncology, Merck, Novartis, Pfizer
Travel, Accommodations, Expenses: Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, Klusa Pharma
Marek Dedecjus
Honoraria: Sanofi/Aventis, Bayer, Berlin-Chemie
Research Funding: AstraAzeneca/Merck, Exelixis
Lori J. Wirth
Consulting or Advisory Role: Merck, Loxo, Blueprint Medicines, Eisai, Lilly, Bayer, Exelixis
Researching Funding: Checkmate Pharmaceuticals (Inst), Lilly (Inst), Ayala Pharmaceuticals (Inst), Eisai (Inst)
Expert Testimony: Eisai
Other Relationship: PDS Biotechnology
R. Michael Tuttle
Research Funding: Elesta (Inst)
Fernanda Vaisman
Consulting or Advisory Role: AstraZeneca/Merck, Ipsen, Lilly, Bayer Health
Speaker’s Bureau: Bayer, AstraZeneca/Merck, Sanofi, United Medical
Lars Bastholt
Research Funding: Bristol Myers Squibb (Inst)
Andrew G. Gianoukakis
Consulting or Advisory Role: Eisai, Exelixs
Patrice Rodien
Consulting or Advisory Role: Eisai Europe
Research Funding: Pfizer (Inst)
Ralf Paschke
Consulting or Advisory Role: Bayer, Eisai
Researching Funding: Bayer (Inst)
Patents, Royalties, Other Intellectual Property: Licensing fee for a diagnostic test
Rossella Elisei
Consulting or Advisory Role: EISAI, Genezyme, Loxo, Ipsen, Bayer, Lilly
Karen So
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Danielle Carroll
Employment: AstraZeneca/MedImmune, Azeria therapeutics (I)
Leadership: Azeria therapeutics (I)
Stock and Other Ownership Interests: AstraZeneca/MedImmune, Azeria Therapeutics (I)
Honoraria: AstraZeneca (I)
Consulting or Advisory Role: Azeria therapeutics (I)
Research Funding: AstraZeneca (I)
Patents, Royalties, Other Intellectual Property: Royalties from Active Motif (I), Compositions featuring an attenuated Newcastle disease virus and methods of use for treating neoplasia, Anti-cxc chemokine-2 binding molecules and uses thereof
Tina Hovey
Employment: Phastar
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca
James A. Fagin
Honoraria: Eisai Europe
Consulting or Advisory Role: Loxo Oncology
Patents, Royalties, Other Intellectual Property: 3. MSK Ref. SK 2014-024-03 Title: Treatment of H-RAS-Driven Tumors. Application Number: 15/305,788. 3. Anti-TSHR Multispecific Antibodies and Uses Thereof, provisional patent.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Haugen BR, Alexander EK, Bible KC, et al. : 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1-133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagna MG, Maino F, Cipri C, et al. : Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol 165:441-446, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Tuttle RM, Tala H, Shah J, et al. : Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341-1349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaisman F, Momesso D, Bulzico DA, et al. : Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) 77:132-138, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Cohen Y, Xing M, Mambo E, et al. : BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 95:625-627, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Beal S, Sheiner L, Boeckmann A, et al. : NONMEM User’s Guide. ICON Development Solutions, Ellicott City, MD, 2009 [Google Scholar]
- 7.Soares P, Trovisco V, Rocha AS, et al. : BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 22:4578-4580, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network : Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676-690, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura ET, Nikiforova MN, Zhu Z, et al. : High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454-1457, 2003 [PubMed] [Google Scholar]
- 10.Durante C, Puxeddu E, Ferretti E, et al. : BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab 92:2840-2843, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Knauf JA, Ma X, Smith EP, et al. : Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res 65:4238-4245, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chakravarty D, Santos E, Ryder M, et al. : Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest 121:4700-4711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh TC, Marsh V, Bernat BA, et al. : Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res 13:1576-1583, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Banerji U, Camidge DR, Verheul HM, et al. : The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res 16:1613-1623, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Denton CL, Gustafson DL: Pharmacokinetics and pharmacodynamics of AZD6244 (ARRY-142886) in tumor-bearing nude mice. Cancer Chemother Pharmacol 67:349-360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho AL, Grewal RK, Leboeuf R, et al. : Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368:623-632, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuttle RM, Alzahrani AS: Risk stratification in differentiated thyroid cancer: From detection to final follow-up. J Clin Endocrinol Metab 104:4087-4100, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padovani RP, Robenshtok E, Brokhin M, et al. : Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid 22:778-783, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Hu S, Hou P, et al. : Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res 13:1341-1349, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Jaber T, Waguespack SG, Cabanillas ME, et al. : Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab 103:3698-3705, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn LA, Sherman EJ, Baxi SS, et al. : Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab 104:1417-1428, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagarajah J, Le M, Knauf JA, et al. : Sustained ERK inhibition maximizes responses of BrafV600E thyroid cancers to radioiodine. J Clin Invest 126:4119-4124, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothenberg SM, McFadden DG, Palmer EL, et al. : Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res 21:1028-1035, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Saqcena M, Leandro-Garcia LJ, Maag JLV, et al. : SWI/SNF complex mutations promote thyroid tumor progression and insensitivity to redifferentiation therapies. Cancer Discov 11:1158-1175, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Requests for data collected for this study, including individual participant data and a data dictionary defining each field in the set, will be considered in line with the above policy. In addition to the clinical study protocol, related documents (for example, the statistical analysis plan and informed consent form) can be made available.