PURPOSE
CD19-targeted chimeric antigen receptor (CAR)–modified T cells demonstrate unprecedented responses in B-cell acute lymphoblastic leukemia (B-ALL); however, relapse remains a substantial challenge. Short CAR T-cell persistence contributes to this risk; therefore, strategies to improve persistence are needed.
METHODS
We conducted a pilot clinical trial of a humanized CD19 CAR T-cell product (huCART19) in children and young adults with relapsed or refractory B-ALL (n = 72) or B-lymphoblastic lymphoma (n = 2), treated in two cohorts: with (retreatment, n = 33) or without (CAR-naive, n = 41) prior CAR exposure. Patients were monitored for toxicity, response, and persistence of huCART19.
RESULTS
Seventy-four patients 1-29 years of age received huCART19. Cytokine release syndrome developed in 62 (84%) patients and was grade 4 in five (6.8%). Neurologic toxicities were reported in 29 (39%), three (4%) grade 3 or 4, and fully resolved in all cases. The overall response rate at 1 month after infusion was 98% (100% in B-ALL) in the CAR-naive cohort and 64% in the retreatment cohort. At 6 months, the probability of losing huCART19 persistence was 27% (95% CI, 14 to 41) for CAR-naive and 48% (95% CI, 30 to 64) for retreatment patients, whereas the incidence of B-cell recovery was 15% (95% CI, 6 to 28) and 58% (95% CI, 33 to 77), respectively. Relapse-free survival at 12 and 24 months, respectively, was 84% (95% CI, 72 to 97) and 74% (95% CI, 60 to 90) in CAR-naive and 74% (95% CI, 56 to 97) and 58% (95% CI, 37 to 90) in retreatment cohorts.
CONCLUSION
HuCART19 achieved durable remissions with long-term persistence in children and young adults with relapsed or refractory B-ALL, including after failure of prior CAR T-cell therapy.
INTRODUCTION
Immunotherapy using T cells engineered to express a chimeric antigen receptor (CAR) targeting CD19 has proven transformational in the treatment of multiply relapsed and chemorefractory B-cell acute lymphoblastic leukemia (B-ALL).1,2 Response rates as high as 93% and durable remissions have been demonstrated in these leukemias once thought to be incurable.3-11 Nevertheless, relapse remains an obstacle to cure in a substantial proportion of patients, surpassing 50% in some studies.10,12 Relapse after CAR T-cell therapy results from two overarching mechanisms: loss of CAR T-cell persistence allowing for relapse of residual leukemia, and escape from CAR T-cell killing primarily through antigen loss. CD19+ relapses, which account for up to 78% of relapses after CD19 CAR T cells, frequently involve loss of CAR T-cell surveillance because of short persistence.7,10,11,13 The PLAT-02 trial demonstrated that loss of B-cell aplasia, which is indicative of loss of CD19 targeting by CAR T cells, significantly increased the risk of relapse with a hazard ratio of 3.5 (95% CI, 1.0 to 11.9; P = .04).7 Outcomes after relapse are quite poor for pediatric patients who relapse after tisagenlecleucel, with < 50% becoming long-term survivors.14 Strategies to reduce the risk of relapse and to treat relapse after CAR T-cell therapy are urgently needed.
CONTEXT
Key Objective
We developed a humanized CD19 chimeric antigen receptor (CAR) T-cell product (huCART19) with the goal of providing an effective treatment for relapse as a result of poor CAR T-cell persistence. In this pilot clinical trial, we assessed the safety, feasibility, persistence, and efficacy of huCART19 in children and young adults with relapsed or refractory B-cell lymphoblastic leukemia or lymphoma, including patients previously treated with CAR T cells with demonstrated poor persistence.
Knowledge Generated
We treated 74 patients with huCART19 and demonstrated a similar safety profile to murine CD19 CAR T-cell products. We also reported high response rates, long-term persistence, and durable remissions among both patients without (CAR-naive) and with (retreatment) prior CAR exposure.
Relevance
Relapse is a major challenge after CD19 CARs. Strategies to reduce relapse risk and to treat relapse are urgently needed. Our findings suggest that huCART19 may represent an approach to reduce relapse risk through improved persistence and treat patients for whom previous CAR T-cell therapy had failed.
Several factors affecting CAR T-cell persistence are known, including costimulatory domain, baseline T-cell repertoire, and underlying disease.15,16 Other factors are hypothesized such as CAR immunogenicity. Most CARs in clinical development contain single-chain variable fragment (scFv) domains derived from mouse monoclonal antibodies; therefore, antimurine immune responses could lead to rejection, and CAR-specific T-cell responses have been demonstrated in a handful of cases.11 Humanization of CAR components is theorized to bypass immune-mediated rejection targeting the murine domain, potentially leading to improved persistence. We developed a CAR-containing a humanized anti-CD19 scFv domain and a 4-1BB costimulatory domain (huCART19) with the goal of overcoming potential barriers to prolonged persistence. Here, we report the results of a first-in-human pilot clinical trial assessing the safety, feasibility, persistence, and efficacy of huCART19 in children and young adults with relapsed or refractory B-ALL or B-lymphoblastic lymphoma (B-LLy), including patients previously treated with CAR T cells with demonstrated poor persistence.
METHODS
Patients and Study Design
We conducted a single-arm, pilot study of huCART19 in children and young adults with relapsed or refractory CD19+ B-ALL or B-LLy (ClinicalTrials.gov identifier: NCT02374333). The trial was conducted in accordance with the declaration of Helsinki and was approved by the institutional review board of the Children's Hospital of Philadelphia. Patients or their guardians provided written informed consent. Patients 1-29 years of age with or without prior exposure to a CAR T-cell product were enrolled in retreatment and CAR-naive cohorts, respectively. Patients without prior CAR exposure were eligible for the CAR-naive cohort if they had documented CD19 expression and met one of the following indications: (1) second or greater relapse, (2) relapse after allogeneic hematopoietic stem-cell transplantation (alloHSCT), (3) refractory disease defined as having not achieved a minimal residual disease (MRD)-negative and/or CSF-negative remission (for B-ALL) or radiographic remission (for B-LLy) after ≥ 2 chemotherapy regimens or cycles of frontline therapy or one cycle of reinduction therapy for patients in first relapse, or (4) ineligible for alloHSCT. Patients with prior CAR exposure were required to have documented CD19 expression after the prior CAR and meet one of the following indications to be eligible: (1) partial or no response to prior CAR, (2) CD19+ relapse, or (3) early B-cell recovery, defined as occurring within 6 months of prior CAR infusion. The initial target dose for the first 48 huCART19 products infused was 3 × 107 cells/kg with an acceptable range of 3 × 105 to 3 × 107 cells/kg and a maximum total dose of 1.5 × 109 cells for patients ≥ 50 kg. After an amendment to base the dose on transduced cells, the target dose for the remaining 26 products infused was 6 × 106 huCART19 cells/kg with an acceptable range of 2 × 105 to 6 × 106 huCART19 cells/kg and a maximum dose of 5 × 108 huCART19 cells. Additional experimental details are included in the Data Supplement (online only).
Assessments and End Points
The primary end points included safety and feasibility of huCART19 infusion and the duration of huCART19 persistence. Cytokine release syndrome (CRS) was graded according to the Penn scale.17 Other adverse events, including neurologic toxicities, were captured using the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03). Secondary end points included the overall response rate (ORR, defined as the rate of either complete remission [CR] or complete remission with incomplete count recovery [CRi]) at day 28 after infusion, the MRD-negative CR/CRi rate, defined as bone marrow blasts < 0.01% of mononuclear cells by multiparameter flow cytometry at the University of Washington, relapse-free survival (RFS), event-free survival (EFS), overall survival (OS), and exploratory cytokine analyses. Additional details are available in the Data Supplement.
Statistical Analysis
Standard descriptive statistics were calculated for patient and disease characteristics, adverse events, ORR, and cytokine profiling. RFS, EFS, and OS were evaluated using Kaplan-Meier methods. Time to relapse was also evaluated using a cumulative incidence analysis, treating alloHSCT and other anticancer therapy as competing risks. Loss of huCART19 persistence and time to B-cell recovery were evaluated using the cumulative incidence method; treating alloHSCT, alternative therapy, relapse, or huCART19 reinfusion as competing risks for loss of persistence; and CD19− relapse, receipt of alternative therapy and death as competing risks for B-cell recovery. Patients were censored at huCART19 infusion (for B-cell recovery) or last follow-up. The association of B-cell recovery with RFS was evaluated with an extended version of the Kaplan-Meier method that treated B-cell recovery as a time-varying covariate.18,19 In an exploratory analysis, the cumulative incidence of B-cell recovery at 6 months in the huCART19 CAR-naive cohort was compared with a historical cohort treated with murine CTL019 on our phase I trial (ClinicalTrials.gov identifier: NCT01626495) using Gray's test.3,4,20 Additional details are provided in the Data Supplement.
RESULTS
Patients
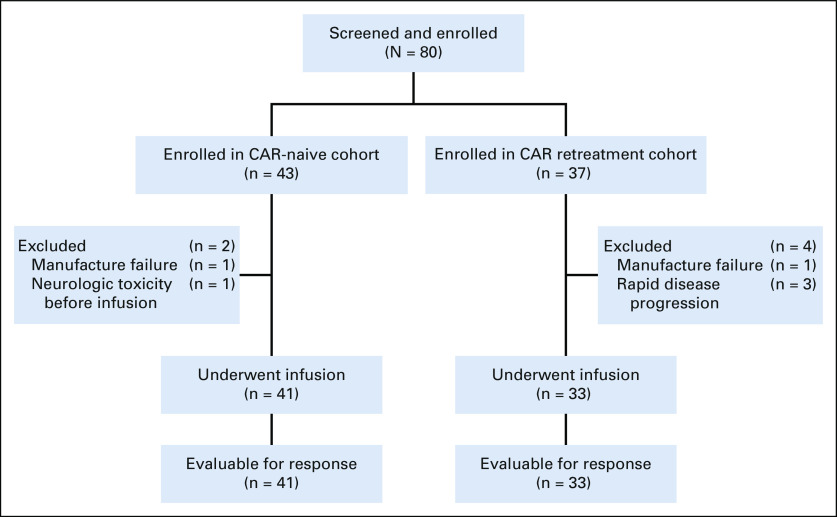
A total of eighty patients with relapsed or refractory B-ALL or B-LLy were screened and enrolled between March 2014 and November 2018 (Fig 1). Of those, 74 were infused, including 41 (B-ALL, n = 39; B-LLy, n = 2) in the CAR-naive cohort and 33 (B-ALL, n = 33) in the retreatment cohort. The median dose of huCART19 cells per kilogram was 6.0 × 106 (range, 1.0 × 106 to 9.7 × 106) in CAR-naive and 5.5 × 106 (range, 1.2 × 106 to 9.7 × 106) in retreatment patients; 65 (88%) products met the target dose.
FIG 1.
CONSORT diagram. In addition to the excluded patients detailed, a repeat manufacture attempt failed because of poor cell growth for a patient with a CD19+ relapse after initial treatment with huCART19 in the retreatment cohort. CAR, chimeric antigen receptor; huCART19, humanized CD19 CAR T-cell product.
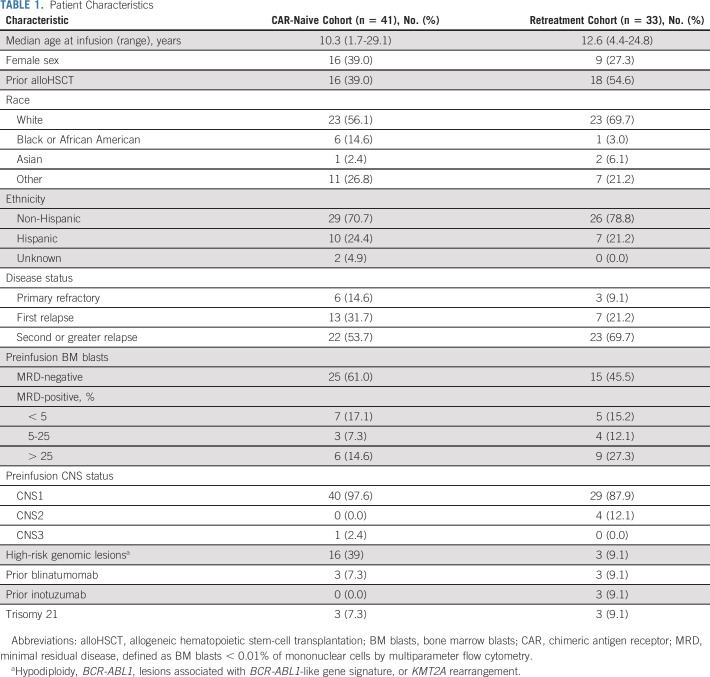
The median age at infusion was 10.3 (range, 1.7-29.1) and 12.6 (range, 4.4-24.8) years in the CAR-naive and retreatment cohorts, respectively (Table 1). The majority of patients were in second or greater relapse (CAR-naive, 53.7%; retreatment, 69.7%), whereas 14.6% of CAR-naive and 9.1% of retreatment patients had primary refractory disease. Prior immunotherapy included alloHSCT in 46% (CAR-naive, 39%; retreatment, 55%), blinatumomab in 8.1% (CAR-naive, 7.3%; retreatment, 9.1%), and inotuzumab in 4.1% (CAR-naive, 0%; retreatment, 9.1%) of patients. In addition, all 33 retreatment patients received previous murine-derived, CD19-targeted CAR T cells (investigational CTL019, n = 18; commercial tisagenlecleucel, n = 7; other product, n = 8; and multiple products, n = 3). Indications for retreatment included CD19+ relapse (n = 15, 45%), B-cell recovery (n = 16, 48%), or no response to prior CAR T cells (n = 2, 6.1%). Twelve patients previously received a reinfusion of the murine CAR T-cell product.
TABLE 1.
Patient Characteristics
Toxicity
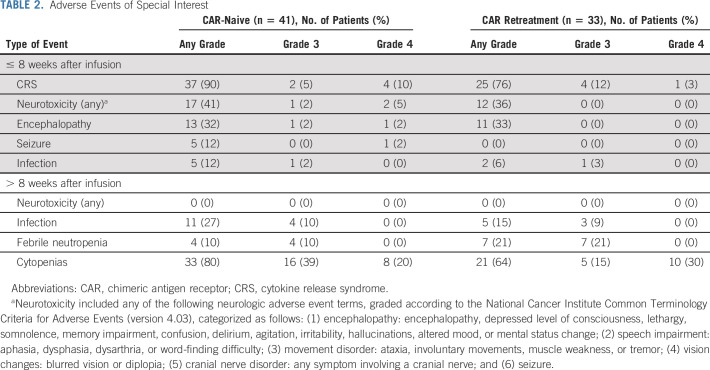
The overall rates of serious adverse events (SAEs) and grade 3 or 4 SAEs within 8 weeks of infusion were, respectively, 76% and 71% in CAR-naive and 61% and 52% in retreatment patients (Data Supplement). The most common SAEs were CRS, febrile neutropenia, and encephalopathy (Table 2). There were no treatment-related deaths and all treatment-related adverse events were reversible, except for grade 3 or 4 persistent cytopenias present beyond 8 weeks in 59% and 45% of CAR-naive and retreatment patients, respectively.
TABLE 2.
Adverse Events of Special Interest
Cytokine Release Syndrome
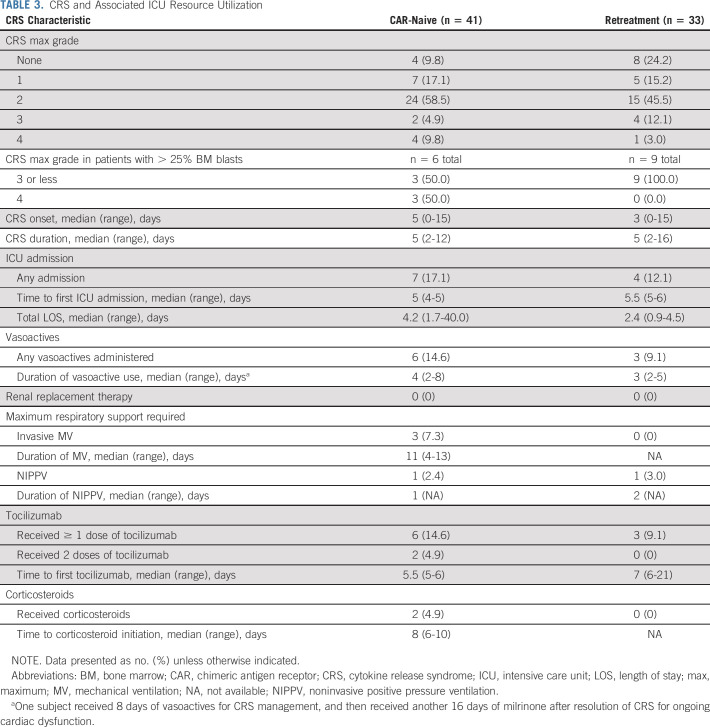
Among CAR-naive patients, 37 (90%) developed CRS and four (9.8%) developed grade 4 (Penn scale) CRS (Table 3).17 Median time to CRS onset was 5 days (range, 0-15 days) after infusion and median CRS duration was 5 days (range, 2-12 days). Six (15%) patients received tocilizumab and two (5%) received corticosteroids for CRS management. No other anticytokine therapy was administered. Of the seven patients admitted to the intensive care unit (ICU), six required vasoactive medications for a median of 4 days (range, 2-8 days) and three required invasive mechanical ventilation for a median of 11 days (range, 4-13 days). One patient received 8 days of vasoactive medications for CRS management, and then received an additional 16 days of milrinone for ongoing cardiac dysfunction after resolution of CRS.
TABLE 3.
CRS and Associated ICU Resource Utilization
Among retreatment patients, 25 (76%) developed CRS and one (3.0%) developed grade 4 CRS (Table 3). Median time to CRS onset was 3 days (range, 0-15 days) after infusion, and median CRS duration was 5 days (range, 2-16 days). Three (9.1%) patients received tocilizumab for CRS management; none received corticosteroids. Of the four patients admitted to the intensive care unit, three required vasoactive medications for a median of 3 days (range, 2-5 days); none required invasive mechanical ventilation. There were no episodes of grade 5 CRS in either cohort and all episodes of CRS fully resolved.
Patients with severe CRS had higher peak levels of C-reactive protein (CRP), ferritin, and interleukin (IL)-6, and lower trough levels of fibrinogen compared with patients without severe CRS (Data Supplement). A nonsignificant trend toward higher peak levels of granulocyte-macrophage colony-stimulating factor, IL-2R, and IL-8 was observed in grade 4 CRS. For patients with severe CRS, median time to peak CRP was 6 days (range, 3-7 days), to peak ferritin was 7 days (range, 7-8 days), and to trough fibrinogen was 9.5 days (range, 7-11 days). The Data Supplement displays levels of these serum biomarkers over the first 21 days after infusion.
Neurotoxicity
Neurologic toxicities were observed in 17 (41%) CAR-naive patients and 12 (36%) retreatment patients (Table 2). The most common neurologic adverse event was encephalopathy, observed in 24 (32%) patients (grade 3, n = 1; grade 4, n = 1). Seizure was observed in five patients, status epilepticus requiring midazolam infusion and dexamethasone in one patient, and one patient required intubation for apnea after benzodiazepine administration. All neurologic toxicities fully resolved, and there was no ongoing or new neurotoxicity attributed to huCART19 after 8 weeks. No cerebral edema was observed.
Toxicity After Reinfusion
Twelve CAR-naive and 10 retreatment patients received ≥ 1 reinfusion because of concern for loss of huCART19 persistence within 6 months of initial infusion. Of those, one (8%) CAR-naive and two (20%) retreatment patients had ≥ 1 SAE within 30 days of reinfusion. The SAEs observed were CRS (grade 2, n = 3), encephalopathy (grade 2, n = 2), febrile neutropenia (grade 3, n = 3), catheter-related infection (grade 3, n = 3), dehydration (grade 3, n = 1), and vomiting (grade 3, n = 1).
Efficacy: CAR-Naive Cohort
In the CAR-naive cohort, the CR/CRi rate was 98% (40 out of 41) overall and 100% (39 out of 39) in B-ALL at day 28 after infusion (Data Supplement). All responses were also MRD-negative. One patient with B-LLy, classified as nonresponse, was MRD-negative in the bone marrow at day 28 with resolution of multiple bone lesions by PET but with one residual bone lesion and activity in the mediastinum, which resolved by month 4, whereas the PET-avid bone lesion persisted. The patient proceeded to alloHSCT without biopsy to confirm disease. The median duration of follow-up was 34.6 months (range, 11.6-49.9 months) after infusion. Among the 40 patients who achieved CR/CRi, RFS was 84% (95% CI, 72 to 97) at 12 months and 74% (95% CI, 60 to 90) at 24 months after infusion (Fig 2A). The cumulative incidence of relapse was 15% (95% CI, 6 to 28) and 23% (95% CI, 11 to 38) at 12 and 24 months, respectively (Data Supplement). RFS estimates were higher in patients with baseline bone marrow blasts < 5% than in those with ≥ 5% blasts (P < .001, Data Supplement); RFS at 12 months was 93% (95% CI, 84 to 100) versus 50% (95% CI, 24 to 100). Among CR/CRi patients, 12 experienced a relapse before receiving additional anticancer therapy; six were CD19+, four were CD19−, and two had both CD19+ and CD19− leukemic cells. Two additional patients received new cancer therapy for the emergence of CD19− MRD. Four patients underwent alloHSCT while in remission, including one with B-cell recovery within 6 months after infusion. EFS was 82% (95% CI, 70 to 95) at 12 months and 72% (95% CI, 58 to 88) at 24 months (Fig 2B). OS was 90% (95% CI, 82 to 100) at 12 months and 88% (95% CI, 78 to 98) at 24 months (Fig 2C). Median RFS, EFS, and OS were not reached.
FIG 2.
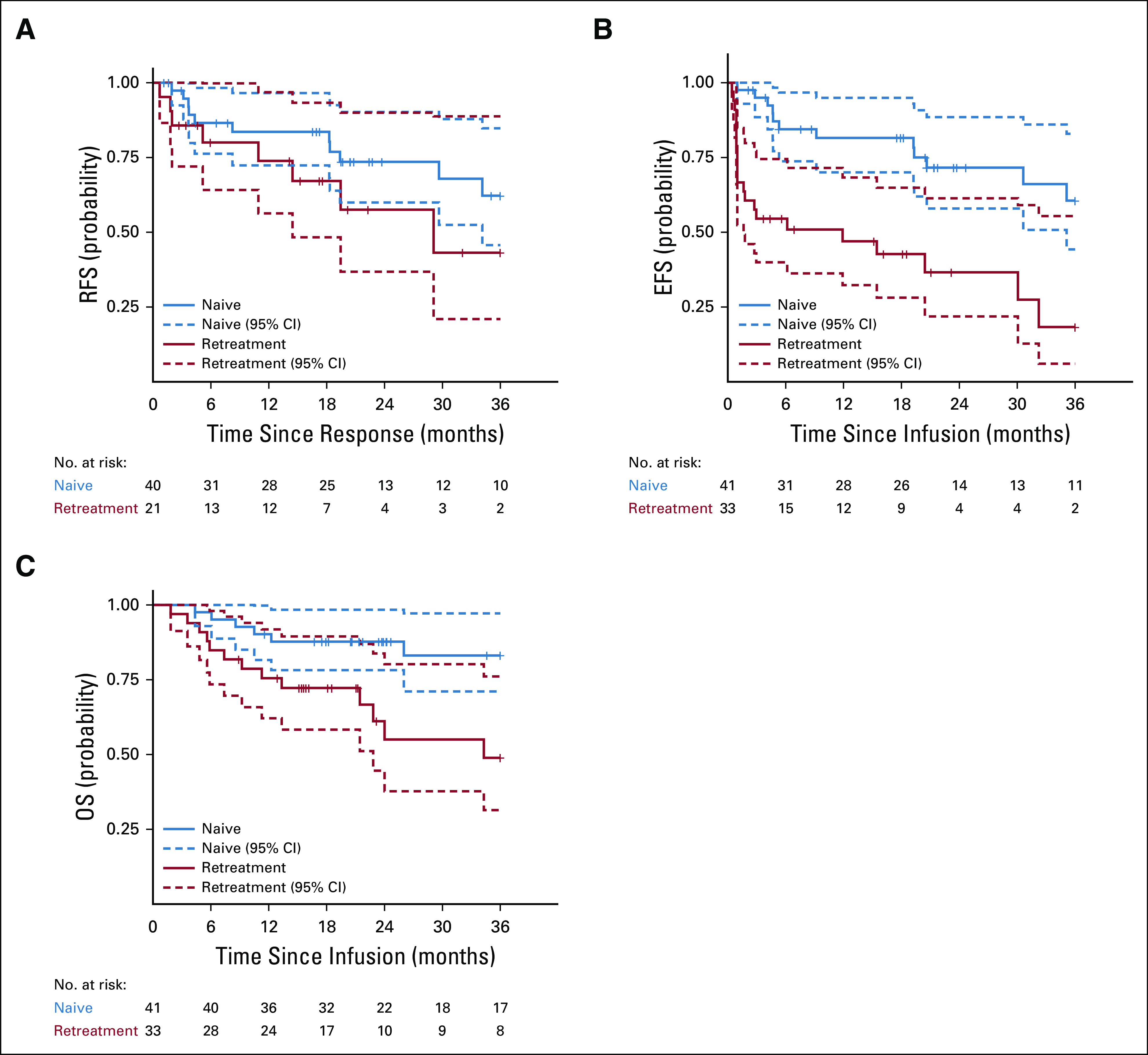
RFS, EFS, and OS. (A) RFS by cohort, defined as the time from onset of remission to relapse in the patients who achieved a complete response. Data were censored for allogeneic hematopoietic stem-cell transplant (CAR-naive, n = 4; retreatment, n = 1) or other alternative therapy during remission (CAR-naive, n = 2; retreatment, n = 4). (B) EFS by cohort, defined as the time from huCART19 infusion to the earliest of the following events: no response (CAR-naive, n = 1; retreatment, n = 12), relapse after achieving a complete response (CAR naive, n = 12; retreatment, n = 8), or development of a subsequent malignancy (retreatment, n = 1). Data were censored for alternative therapy or allogeneic hematopoietic stem-cell transplant during remission. (C) OS by cohort, defined as the time from huCART19 to date of death from any cause. Seven CAR-naive and 14 retreatment patients died during the follow-up period. Tick marks indicate the time of censoring. CAR, chimeric antigen receptor; EFS, event-free survival; huCART19, humanized CD19 CAR T-cell product; OS, overall survival; RFS, relapse-free survival.
Efficacy: Retreatment Cohort
In the retreatment cohort, the CR/CRi rate was 79% (26 out of 33) at day 28 after infusion; however, five patients were classified as having no biologic response because B-cell aplasia was not established. Therefore, the ORR was 64% (21 out of 33), of which 86% were MRD-negative (Data Supplement). CD19 expression was retained in five and decreased in two patients not in CR at day 28. The median duration of follow-up was 21.2 months (range, 8.9-55.0 months) after infusion. Among the 21 patients who achieved CR/CRi with B-cell aplasia, RFS was 74% (95% CI, 56 to 97) at 12 months and 58% (95% CI, 37 to 90) at 24 months after infusion (Fig 2A). The cumulative incidence of relapse was 24% (95% CI, 8 to 44) and 36% (95% CI, 15 to 59) at 12 and 24 months, respectively (Data Supplement). RFS rates were not significantly different between patients with baseline bone marrow blasts < 5% versus those with ≥ 5% blasts (P = .28, Data Supplement); RFS at 12 months was 74% (95% CI, 53 to 100) versus 71% (95% CI, 45 to 100). Among CR/CRi patients, eight experienced a relapse before receiving additional anticancer therapy. All relapses were CD19+, but one also had a minor subset of CD19− leukemic cells. One patient who had undergone alloHSCT twice before huCART19 infusion received new cancer therapy for development of treatment-related acute myeloid leukemia that was determined to be donor-derived. Testing for CAR transgene showed no evidence of CAR in the expanded leukemic clone. In addition, two patients received new cancer therapy for the emergence of MRD, and one patient received new cancer therapy because of B-cell recovery within 6 months of infusion. One patient underwent alloHSCT while in remission because of early B-cell recovery. EFS was 47% (95% CI, 32 to 68) at 12 months and 37% (95% CI, 22 to 61) at 24 months (Fig 2B). OS was 76% (95% CI, 62 to 92) at 12 months and 55% (95% CI, 38 to 80) at 24 months (Fig 2C). Median RFS, EFS, and OS were 29 months (95% CI, 14 to not reached), 12 months (95% CI, 1.6 to not reached), and 34 months (95% CI, 23 to not reached), respectively.
HuCART19 Expansion and Persistence
HuCART19 expansion and persistence was monitored by quantitative polymerase chain reaction for the transgene and flow cytometry for CAR surface expression. CAR was detected by quantitative polymerase chain reaction and flow cytometry through the last measurement, 2 years and 1 year after infusion, respectively (Figs 3A-3D). To determine the probability of losing huCART19 persistence by 6 months, we performed a cumulative incidence analysis, treating alloHSCT, other anticancer therapy, relapse, and reinfusion as competing risks. The 6-month cumulative incidence of loss of flow-detectable persistence was 27% (95% CI, 14 to 41) in the CAR-naive cohort and 48% (95% CI, 30 to 64) in the retreatment cohort (Figs 3A and 3B).
FIG 3.
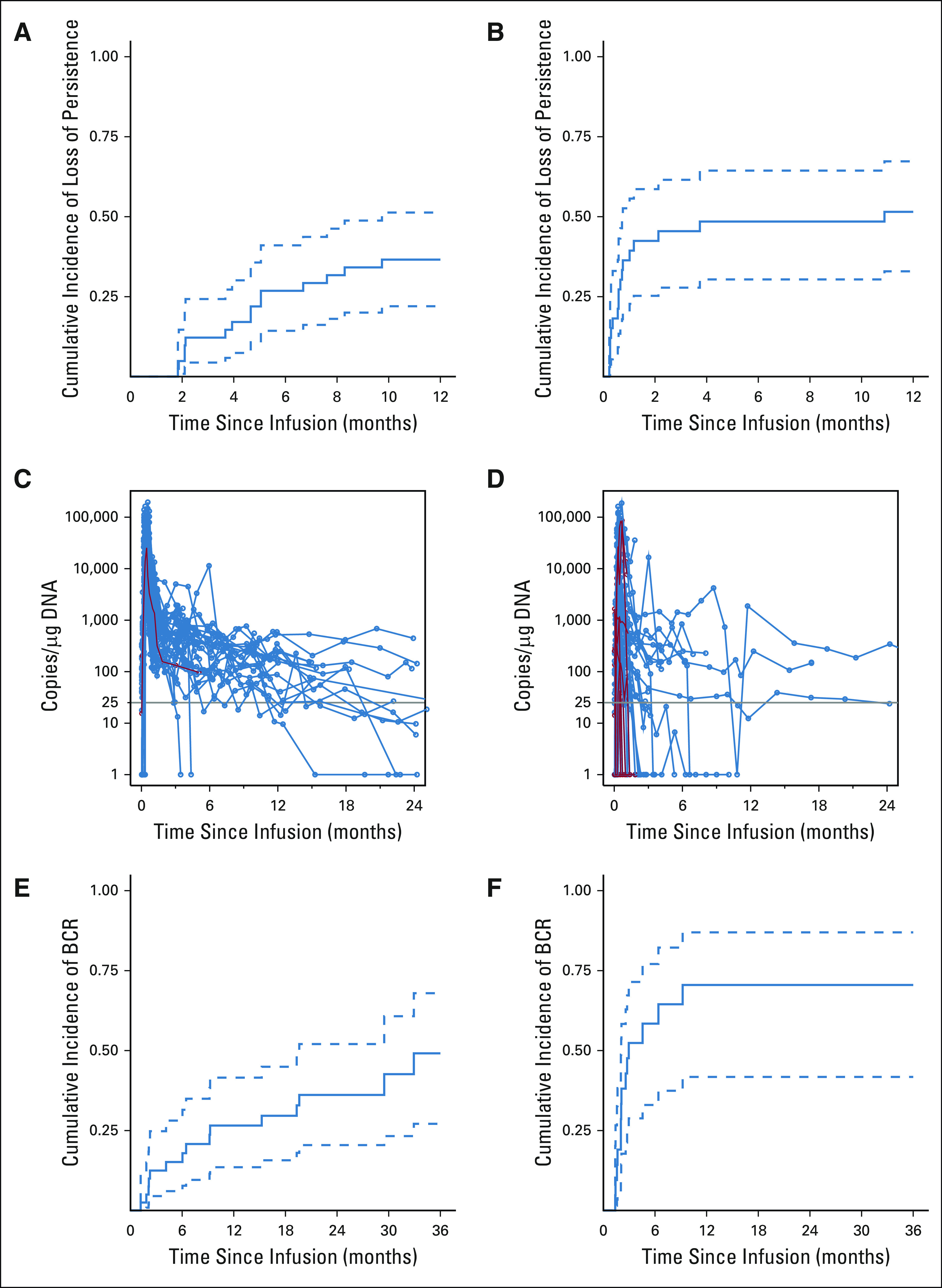
Persistence of huCART19. (A and B) Cumulative incidence curves of the time to the first confirmed negative measurement of huCART19 in the (A) CAR-naive and (B) retreatment cohorts, as measured by flow cytometry, in the peripheral blood and bone marrow. Confirmed negative was defined as two consecutive measurements < 0.1% huCART19-positive cells in CD3-positive cells without two or more subsequent positive measurements. Receipt of alternative therapy, relapse, and huCART19 reinfusion were considered competing risks, and data were censored at last follow-up if no event occurred. (C and D) Measurements of huCART19 gene-modified T cells in peripheral blood as measured by quantitative real-time PCR assay in the (C) CAR-naive and (D) retreatment cohorts. The horizontal line at 25 copies/µg DNA represents the lower limit of quantification of this assay. Data on patients who did not have a response are shown in red. (E and F) Cumulative incidence curves of the time to either detection of ≥ 3% CD19+ lymphocytes in peripheral blood samples by means of flow cytometry or CD19+ relapse in the (E) CAR-naive and (F) retreatment cohorts. Data were censored at the time of huCART19 reinfusion or last follow-up. CD19− relapse, receipt of alternative therapy, and death were treated as competing risks. Dashed lines represent 95% CIs. BCR, B-cell recovery; CAR, chimeric antigen receptor; huCART19, humanized CD19 CAR T-cell product; PCR, polymerase chain reaction.
B-Cell Aplasia
B-cell aplasia was followed as a marker of functional persistence of CAR T cells. In patients with CR/CRi with biologic response, B-cell aplasia was observed by day 28. The cumulative incidence of B-cell recovery by 6 months after infusion was 15% (95% CI, 6 to 28) in CAR-naive and 58% (95% CI, 33 to 77) in retreatment patients (Figs 3E and 3F). Twelve CAR-naive and 10 retreatment patients received ≥ 1 reinfusion for return of circulating B cells (CAR-naive, n = 8, retreatment, n = 8) or CD19+ hematogones (CAR-naive, n = 4; retreatment, n = 2) in the bone marrow within 6 months of initial infusion.
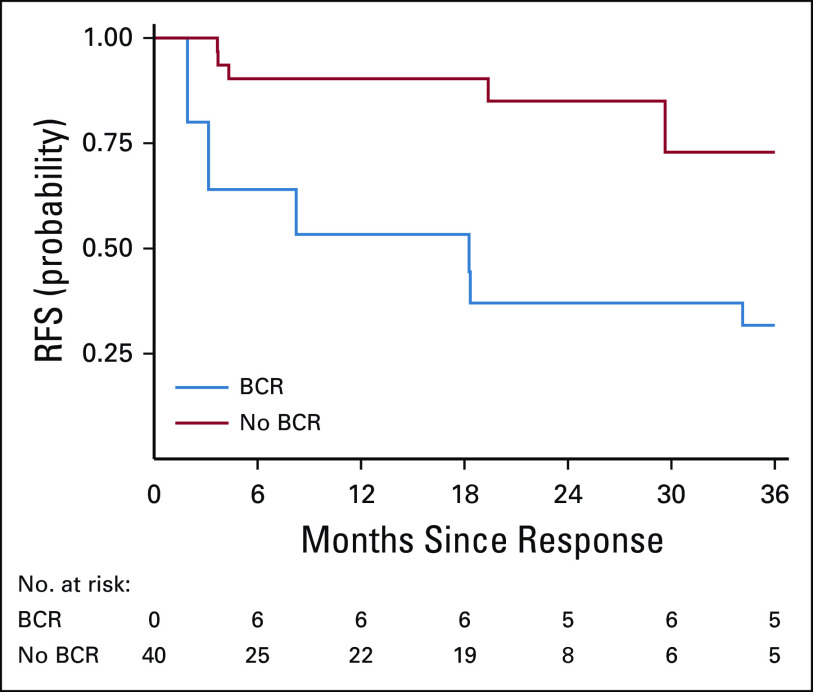
When evaluating B-cell recovery as a time-varying covariate for RFS, we observed significantly worse RFS associated with B-cell recovery (P = .011, Fig 4) in the CAR-naive cohort. Six patients experienced B-cell recovery within 6 months of infusion. Of those, two experienced a CD19+ relapse and four remained in remission at last follow-up (three received ≥ 1 reinfusion and one underwent alloHSCT). Nine patients had B-cell recovery more than 6 months after infusion; two experienced a subsequent CD19+ relapse, and seven remained in remission at last follow-up (four received ≥ 1 reinfusion and three without further therapy). An additional four patients developed a CD19+ relapse (at 9, 19, 31, and 37 months) without preceding B-cell recovery.
FIG 4.
Effect of BCR on RFS. RFS, defined as the time from onset of remission to relapse in the patients who achieved a complete response, in CAR-naive patients, with BCR treated as a time-varying covariate. BCR, B-cell recovery; CAR, chimeric antigen receptor; RFS, relapse-free survival.
In an exploratory analysis, we compared the time to B-cell recovery in the huCART19 CAR-naive cohort to a historical cohort treated with murine CTL019 on our phase I trial.3,4 There was a trend toward a lower cumulative incidence of B-cell recovery within 6 months of infusion in the huCART19 cohort compared with the CTL019 cohort (15% [95% CI, 6 to 28] v 29% [95% CI, 17 to 41]; P = .15), although not statistically significant.
DISCUSSION
Despite high rates of response to CD19-targeted CAR T-cell therapies having a transformative impact on the treatment of relapsed or refractory pediatric B-ALL, poor CAR T-cell persistence remains a problem in at least 25% of patients, limiting the potential for durable remission.3,6,7,10,11 We demonstrate that huCART19 can induce deep and durable remissions without further therapy, in children and young adults with relapsed or refractory B-ALL, including in patients who experienced poor persistence with a murine CAR T-cell product.
Poor CAR T-cell persistence is associated with an increased risk of relapse in B-ALL, a finding that this study also supports.7 Persistence differs among CAR T-cell products and disease treated; however, there are also differences within populations treated with the same product. We developed a humanized CD19 CAR based on the backbone of CTL019 (now known as tisagenlecleucel), with the hypothesis that it would have a lower risk of immunogenicity than a murine-based CAR and show improved persistence.
We show that huCART19 is efficacious in B-ALL, with a CR/CRi rate of 100% in CAR-naive B-ALL patients and durable remission achieved without further therapy. The RFS at 12 and 24 months, 84% and 74%, respectively, is among the highest reported to date. In addition, a comparison of this cohort with a historical population treated with the murine product, CTL019, suggested improved CAR T-cell persistence, as measured by the incidence of B-cell recovery by 6 months. It is important to note, however, that the difference was not statistically significant and the comparison had limitations, including small sample size, unmatched populations for potential confounders, including disease burden and prior therapies, and largely nonoverlapping treatment periods. Nevertheless, the potential for improved persistence with huCART19, which may be associated with a decreased risk of relapse, warrants further study.
Leukemia recurrence after CAR T-cell therapy can be extremely difficult to treat, with few options when numerous prior therapies have not been successful, particularly when alloHSCT has failed.14,21 Reinfusion of murine CAR T-cell products has been attempted, with varying rates of success.7,11,22,23 We show that huCART19 can produce initial responses in 64% of patients treated for CD19+ relapse, early B-cell recovery, or nonresponse after prior murine CAR T cells. Although poor persistence is more likely to recur in this population, durable remissions in most were observed, with 12- and 24-month RFS estimates of 74% and 58%, respectively. Possibly contributing to remission durability, 10 patients received repeat infusions. Future analyses of the contribution to durability of response from reinfusions are planned. This study demonstrates that huCART19 can achieve response, long-term persistence, and durable remission in a substantive fraction of patients with a history of poor persistence with prior CAR T-cell therapy.
The toxicity profile of huCART19 was similar to that reported for CTL019 or tisagenlecleucel and other CD19 CAR T-cell products.5-8 Although the rate of grade 4 CRS was lower (6.8%) than has been reported on previous CD19 CAR trials, it should be noted that the distribution of preinfusion disease burden on this trial confounds interpretation of this lower rate as 54% of patients were MRD-negative preinfusion. The cytokine profile observed in the first month after huCART19 infusion largely mirrored that observed with CTL019.3,5,24 However, differences in cytokine levels between grade 4 CRS and all other grades only reached statistical significance for IL-6, likely related to the small sample size for grade 4 CRS. Nevertheless, the pattern of cytokine elevations was similar to our previous report, with granulocyte-macrophage colony-stimulating factor and IL-8 showing a trend toward differential elevations in grade 4 CRS, in addition to IL-6.24 We characterized several markers of inflammation and coagulopathy during the clinical course of CRS, showing that CRP charts the clinical course, whereas ferritin lags behind. In patients with grade 4 CRS, fibrinogen falls late in the course, often as CRS is resolving or has resolved. The fibrinogen drop can be precipitous and lead to an increased risk of bleeding; therefore, the finding that this occurs late in the CRS course lends important information to guide clinical management.
The current study has some limitations that should be considered in interpreting the results. Although decreased immunogenicity is hypothesized for a humanized CAR, evidence to support that hypothesis has not been shown in this study or others. Moreover, it is difficult to compare CAR T-cell persistence and toxicities between huCART19 and the murine product, CTL019, in a nonrandomized trial because of differences in disease burden and treatment periods between the trial cohorts, and inadequate power to detect statistically significant differences. In addition, small sample sizes limited subgroup analyses.
In this first characterization of the safety and efficacy of huCART19, we report a similar safety profile to other CD19 CARs, high response rates, and durable remissions without further therapy in children and young adults with relapsed or refractory B-ALL. Importantly, durable remissions were achieved in patients for whom previous CAR T-cell therapy had failed. These encouraging outcomes led to an ongoing phase II trial (ClinicalTrials.gov identifier: NCT03792633) in B-ALL, including indications not previously studied. Future research will be aimed at studying immunogenicity, comparing CRS risk in high disease burden, and confirming efficacy and improved persistence.
ACKNOWLEDGMENT
The authors thank Elizabeth McBride, Denise Gallagher, Amy Marshall, and Avery Gaymon for clinical trial operations support; Vanessa Gonzalez, Farzana Nazimuddin, Brett Menchel, Fang Chen, Natalka Koterba, Irina Kulikovskaya, and Minnal Gupta for correlative assays; Yongping Wang and members of the Cell and Gene Therapy Laboratory for cell processing; and members of the Clinical Cell and Vaccine Production Facility for manufacturing and testing of the huCART19 cell product.
David M. Barrett
Travel, Accommodations, Expenses: Therakos
David T. Teachey
Consulting or Advisory Role: Sobi
Research Funding: Novartis, Beam Therapeutics, NeoImmuneTech
Colleen Callahan
Consulting or Advisory Role: Novartis
Speakers' Bureau: Peerview
Susan R. Rheingold
Employment: OptiNose
Stock and Other Ownership Interests: OptiNose
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer
Richard Aplenc
Expert Testimony: Vorys
Pamela A. Shaw
Patents, Royalties, Other Intellectual Property: I am part of a patent owned by UPenn and currently licensed to Novartis for an algorithm that predicts severe cytokine release syndrome for the CART 19 therapy. I receive 10% of the licensing fees
Edward Pequignot
Patents, Royalties, Other Intellectual Property: As part of Penn's role in the FDA-approval of CAR-T therapy, and for my part as an employee of Penn involved in their research in CAR-T, I have received royalties of approximately $300 in US dollars over the past 2 years
Jennifer L. Brogdon
Employment: Novartis Institutes for BioMedical Research
Stock and Other Ownership Interests: Novartis Institutes for BioMedical Research
Donald L. Siegel
Research Funding: Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: A patent, owned by the Trustees of the University of Pennsylvania, on which I am listed as an inventor is licensed to Alexion Pharmaceuticals. I receive royalties as stipulated in the University of Pennsylvania Faculty Handbook
Expert Testimony: Regeneron
Megan M. Davis
Leadership: Cellares Corporation
Consulting or Advisory Role: Tmunity Therapeutics Inc
Research Funding: Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: Novartis Institutes for Biomedical Research—royalties and milestones for patents/knowhow, Tmunity Therapeutics—royalties andmilestones from patents/knowhow
Simon F. Lacey
Consulting or Advisory Role: Gilead Sciences
Research Funding: Tmunity Therapeutics Inc, Cabaletta Bio, Novartis Institutes for BioMedical Research
Patents, Royalties, Other Intellectual Property: Patents and IP related to CTL019 (Kymriah) assigned by the University of Pennsylvania to Novartis
Elizabeth O. Hexner
Consulting or Advisory Role: Blueprint Medicines, ABIM Subspecialty Board
Research Funding: Blueprint Medicines, Tmunity Therapeutics Inc
Gerald B. Wertheim
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Bruce L. Levine
Stock and Other Ownership Interests: Tmunity Therapeutics Inc
Honoraria: Novartis, Terumo
Consulting or Advisory Role: Avectas, Ori Biotech, Vycellix, Immuneel Therapeutics, In8bio, Patheon/ThermoFisher Viral Vector Services
Research Funding: Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: Intellectual property and patents in the field of cell and gene therapy
Travel, Accommodations, Expenses: Avectas, Terumo
Carl H. June
Leadership: AC Immune
Stock and Other Ownership Interests: Celldex, Tmunity Therapeutics Inc, Cabaletta Bio, Carisma Therapeutics, DeCART Therapeutics, Bluesphere Bio, Cellares, ZIOPHARM Oncology, Decheng Capital, Posieda Therapeutics, Verisma
Honoraria: Pfizer
Consulting or Advisory Role: Celldex, Viracta Therapeutics, Cabaletta Bio, Carisma Therapeutics, Kiadis Pharma, WIRB-Copernicus Group, Janssen Oncology
Research Funding: Novartis, Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: IP licensed to Novartis; Royalties paid to University of Pennsylvania, Office of Naval Research; IP and patent royalties, IP licensed to Tmunity
Stephan A. Grupp
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, Cellular Biomedicine Group, TCR2 Therapeutics, Humanigen, Roche, Adaptimmune, Alimera Sciences, Cabaletta Bio, CRISPR Therapeutics/Vertex
Research Funding: Novartis, Kite/Gilead, Servier, Jazz Pharmaceuticals, Vertex
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent
Expert Testimony: Juno Therapeutics
Shannon L. Maude
Consulting or Advisory Role: Novartis, Wugen Inc
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis, Kite, a Gilead company, Wugen Inc
No other potential conflicts of interest were reported.
SUPPORT
Supported, in part, by a clinical trial award funded by a research alliance between the University of Pennsylvania and Novartis Pharmaceuticals and the Children's Hospital of Philadelphia Frontier Program.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: David M. Barrett, Susan R. Rheingold, Jennifer L. Brogdon, Donald L. Siegel, Bruce L. Levine, Carl H. June, Stephan A. Grupp, Shannon L. Maude
Financial support: David T. Teachey, Carl H. June, Stephan A. Grupp, Shannon L. Maude
Administrative support: Edward Pequignot, Anne Chew, Stephan A. Grupp, Shannon L. Maude
Provision of study materials or patients: Susan R. Rheingold, Richard Aplenc, Andrew D. Fesnak, Donald L. Siegel, Simon F. Lacey, Bruce L. Levine, Stephan A. Grupp, Shannon L. Maude
Collection and assembly of data: Regina M. Myers, Allison Barz Leahy, Colleen Callahan, Christina C. Fasano, Susan R. Rheingold, Lisa Wray, Richard Aplenc, Diane Baniewicz, Edward Pequignot, Kelly D. Getz, Andrew D. Fesnak, Donald L. Siegel, Megan M. Davis, Chelsie Bartoszek, Simon F. Lacey, Elizabeth O. Hexner, Anne Chew, Gerald B. Wertheim, Bruce L. Levine, Shannon L. Maude
Data analysis and interpretation: Regina M. Myers, Yimei Li, Allison Barz Leahy, David M. Barrett, David T. Teachey, Susan R. Rheingold, Amanda DiNofia, Richard Aplenc, Hongyan Liu, Pamela A. Shaw, Edward Pequignot, Kelly D. Getz, Jennifer L. Brogdon, Megan M. Davis, Chelsie Bartoszek, Simon F. Lacey, Elizabeth O. Hexner, Bruce L. Levine, Stephan A. Grupp, Shannon L. Maude
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David M. Barrett
Travel, Accommodations, Expenses: Therakos
David T. Teachey
Consulting or Advisory Role: Sobi
Research Funding: Novartis, Beam Therapeutics, NeoImmuneTech
Colleen Callahan
Consulting or Advisory Role: Novartis
Speakers' Bureau: Peerview
Susan R. Rheingold
Employment: OptiNose
Stock and Other Ownership Interests: OptiNose
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer
Richard Aplenc
Expert Testimony: Vorys
Pamela A. Shaw
Patents, Royalties, Other Intellectual Property: I am part of a patent owned by UPenn and currently licensed to Novartis for an algorithm that predicts severe cytokine release syndrome for the CART 19 therapy. I receive 10% of the licensing fees
Edward Pequignot
Patents, Royalties, Other Intellectual Property: As part of Penn's role in the FDA-approval of CAR-T therapy, and for my part as an employee of Penn involved in their research in CAR-T, I have received royalties of approximately $300 in US dollars over the past 2 years
Jennifer L. Brogdon
Employment: Novartis Institutes for BioMedical Research
Stock and Other Ownership Interests: Novartis Institutes for BioMedical Research
Donald L. Siegel
Research Funding: Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: A patent, owned by the Trustees of the University of Pennsylvania, on which I am listed as an inventor is licensed to Alexion Pharmaceuticals. I receive royalties as stipulated in the University of Pennsylvania Faculty Handbook
Expert Testimony: Regeneron
Megan M. Davis
Leadership: Cellares Corporation
Consulting or Advisory Role: Tmunity Therapeutics Inc
Research Funding: Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: Novartis Institutes for Biomedical Research—royalties and milestones for patents/knowhow, Tmunity Therapeutics—royalties andmilestones from patents/knowhow
Simon F. Lacey
Consulting or Advisory Role: Gilead Sciences
Research Funding: Tmunity Therapeutics Inc, Cabaletta Bio, Novartis Institutes for BioMedical Research
Patents, Royalties, Other Intellectual Property: Patents and IP related to CTL019 (Kymriah) assigned by the University of Pennsylvania to Novartis
Elizabeth O. Hexner
Consulting or Advisory Role: Blueprint Medicines, ABIM Subspecialty Board
Research Funding: Blueprint Medicines, Tmunity Therapeutics Inc
Gerald B. Wertheim
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Bruce L. Levine
Stock and Other Ownership Interests: Tmunity Therapeutics Inc
Honoraria: Novartis, Terumo
Consulting or Advisory Role: Avectas, Ori Biotech, Vycellix, Immuneel Therapeutics, In8bio, Patheon/ThermoFisher Viral Vector Services
Research Funding: Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: Intellectual property and patents in the field of cell and gene therapy
Travel, Accommodations, Expenses: Avectas, Terumo
Carl H. June
Leadership: AC Immune
Stock and Other Ownership Interests: Celldex, Tmunity Therapeutics Inc, Cabaletta Bio, Carisma Therapeutics, DeCART Therapeutics, Bluesphere Bio, Cellares, ZIOPHARM Oncology, Decheng Capital, Posieda Therapeutics, Verisma
Honoraria: Pfizer
Consulting or Advisory Role: Celldex, Viracta Therapeutics, Cabaletta Bio, Carisma Therapeutics, Kiadis Pharma, WIRB-Copernicus Group, Janssen Oncology
Research Funding: Novartis, Tmunity Therapeutics Inc
Patents, Royalties, Other Intellectual Property: IP licensed to Novartis; Royalties paid to University of Pennsylvania, Office of Naval Research; IP and patent royalties, IP licensed to Tmunity
Stephan A. Grupp
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, Cellular Biomedicine Group, TCR2 Therapeutics, Humanigen, Roche, Adaptimmune, Alimera Sciences, Cabaletta Bio, CRISPR Therapeutics/Vertex
Research Funding: Novartis, Kite/Gilead, Servier, Jazz Pharmaceuticals, Vertex
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent
Expert Testimony: Juno Therapeutics
Shannon L. Maude
Consulting or Advisory Role: Novartis, Wugen Inc
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis, Kite, a Gilead company, Wugen Inc
No other potential conflicts of interest were reported.
REFERENCES
- 1.DiNofia AM, Maude SL: Chimeric antigen receptor T-cell therapy clinical results in pediatric and young adult B-ALL. Hemasphere 3:e279, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers RM, Dolan JG, Teachey DT: Chimeric antigen receptor T cell therapy for pediatric and young adult B cell acute lymphoblastic leukemia. Expert Rev Clin Immunol 16:1029-1042, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Teachey DT, Rheingold SR, et al. : Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol 34, 2016. (suppl; abstr 3011) [Google Scholar]
- 5.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner RA, Finney O, Annesley C, et al. : Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129:3322-3331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran KJ, Margossian SP, Kernan NA, et al. : Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134:2361-2368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp SA, Kalos M, Barrett D, et al. : Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509-1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Rivière I, Gonen M, et al. : Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378:449-459, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turtle CJ, Hanafi LA, Berger C, et al. : CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126:2123-2138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majzner RG, Mackall CL: Tumor antigen escape from car t-cell therapy. Cancer Discov 8:1219-1226, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Sun Q, Liang X, et al. : Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. 10:2664, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanski HE, Verneris MR, Eaton A, et al. : Post-relapse outcomes following tisagenlecleucel: Poor survival, despite current salvage therapies: Results from the Pediatric Real World CAR Consortium (PRWCC). Transpl Cell Ther 27:S117-S118, 2021 [Google Scholar]
- 15.Kalos M, Levine BL, Porter DL, et al. : T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3:95ra73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N, Perazzelli J, Grupp SA, et al. : Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med 8:320ra3, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Porter D, Frey N, Wood PA, et al. : Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol 11:35, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snapinn SM, Jiang Q, Iglewicz B: Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 59:301-307, 2005 [Google Scholar]
- 19.Jann B, Zürich E: Stata tip 8: Splitting time-span records with categorical time-varying covariates. Stata J 4:221-222, 2004 [Google Scholar]
- 20.Gray RJ: A class of $K$-Sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 21.Schultz L: Chimeric antigen receptor T cell therapy for pediatric B-ALL: Narrowing the gap between early and long-term outcomes. Front Immunol 11:1985, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maude SL, Barrett DM, Rheingold SR, et al. : Efficacy of retreatment with humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in children with relapsed ALL. J Clin Oncol 34, 2016. (suppl; abstr 3007) [Google Scholar]
- 23.Gauthier J, Bezerra ED, Hirayama AV, et al. : Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B cell malignancies. Blood 137:323-335, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teachey DT, Lacey SF, Shaw PA, et al. : Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 6:664-679, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]